Abstract
Objective
Little is known about how lower respiratory tract infections (LRTIs) before chronic obstructive pulmonary disease (COPD) are associated with future exacerbations and mortality. We investigated this association in patients with COPD in England.
Methods
Clinical Practice Research Datalink Aurum, Hospital Episode Statistics and Office of National Statistics data were used. Start of follow-up was patient’s first ever COPD diagnosis date and a 1-year baseline period prior to start of follow-up was used to find mild LRTIs (general practice (GP) events/no antibiotics), moderate LRTIs (GP events+antibiotics) and severe LRTIs (hospitalised). Patients were categorised as having: none, 1 mild only, 2+ mild only, 1 moderate, 2+ moderate and 1+ severe. Negative binomial regression modelled the association between baseline LRTIs and subsequent COPD exacerbations and Cox proportional hazard regression was used to investigate mortality.
Results
In 215 234 patients with COPD, increasing frequency and severity of mild and moderate LRTIs were associated with increased rates of subsequent exacerbations compared with no recorded LRTIs (1 mild adjusted IRR 1.16, 95% CI 1.14 to 1.18, 2+ mild IRR 1.51, 95% CI 1.46 to 1.55, 1 moderate IRR 1.81, 95% CI 1.78 to 1.85, 2+ moderate IRR 2.55, 95% CI 2.48 to 2.63). Patients with 1+ severe LRTI (vs no baseline LRTIs) also showed an increased rate of future exacerbations (adjusted IRR 1.75, 95% CI, 1.70 to 1.80). This pattern of association was similar for risk of all-cause and COPD-related mortality; however, patients with 1+ severe LRTIs had the highest risk of all-cause and COPD mortality.
Conclusion
Increasing frequency and severity of LRTIs prior to COPD diagnosis were associated with increasing rates of subsequent exacerbations, and increasing risk of all-cause and COPD-related mortality.
Keywords: COPD epidemiology
WHAT IS ALREADY KNOWN ON THIS TOPIC
Exacerbations of chronic obstructive pulmonary disease (COPD) have been associated with future exacerbations and mortality, but little is known about how lower respiratory tract infections (LRTIs) before a COPD diagnosis are associated with exacerbations risk and mortality after diagnosis.
WHAT THIS STUDY ADDS
This study found that increasing frequency and severity of LRTIs prior to COPD diagnosis are associated with increased rate of future exacerbations of COPD and increased risk of mortality after COPD diagnosis.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study emphasises the importance of identifying LRTIs early in order to treat and prevent subsequent COPD outcomes and improve the lives of people with COPD.
Introduction
Acute exacerbations of chronic obstructive pulmonary disease (COPD) are worsening of COPD symptoms (breathlessness, cough, and sputum volume and purulence) beyond normal day-to-day variation. Approximately 50% to 80% of patients with COPD experience at least one exacerbation in a given year, and even a single moderate exacerbation increases the risk of future multiple exacerbation events.1 2–4 Exacerbations have also been associated with other clinical outcomes including accelerated lung function decline, reduced quality of life,and mortality.5–7
Lower respiratory tract infections (LRTIs) are important to consider when thinking about a diagnosis of COPD. Following the National Institute for Health and Care Excellence (NICE) COPD guidelines, a diagnosis should be suspected in people who experience frequent winter bronchitis (ie, multiple LRTIs) or present with symptoms such as breathlessness, chronic cough, sputum production or wheeze.8 It is therefore possible that individuals may have several LRTI events prior to COPD diagnosis. How frequently these events occur and how they are managed may relate to future COPD exacerbation events. This is important to understand, as it is recognised that current COPD exacerbations are related to future exacerbation events, and LRTIs prior to diagnosis may impact clinical burden, healthcare resource utilisation and the exacerbation pathway following diagnosis.9 10 To date, most published literature reports on prevalent COPD cohorts or incident cohorts focusing on what happens after diagnosis, but not prior to diagnosis.
We aimed to quantify the association between frequency and severity of LRTIs prior to COPD diagnosis and future exacerbations and mortality in a newly diagnosed COPD cohort. The goal of the analysis was to provide an evidence-based resource to guide healthcare professionals as they endeavour to determine which patients may be at greatest risk of frequent exacerbations or exacerbations early on in their disease who could possibly be targeted with more aggressive COPD management, to prevent the downwards spiral of future exacerbations.
Methods
An observational cohort study was conducted in the UK analysing deidentified routinely collected electronic healthcare data.
Study design and participants
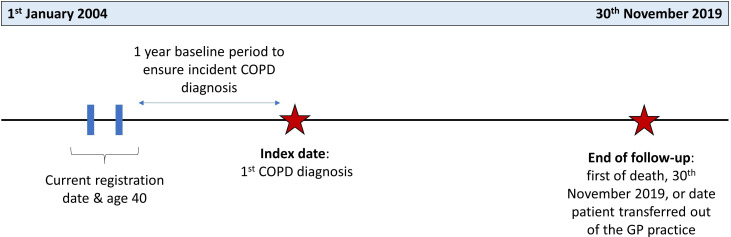
This EXAcerbations of COPD and their OutcomeS (EXACOS-UK) study used Clinical Practice Research Datalink (CPRD) Aurum data, a primary care database of patients registered at general practices (GPs) in England. Linked mortality data from the Office for National Statistics (ONS), socioeconomic data from the Index of Multiple Deprivation and secondary care data from Hospital Episode Statistics (HES) were provided for this study by CPRD. People were included if they met the following criteria: (1) aged 40 or older; (2) current or ex-smokers; (3) any data recorded in CPRD Aurum from 1 January 2004 onwards. After all these inclusion criteria were satisfied, patients were included in the cohort if a first ever validated diagnostic code of COPD was identified ≥1 year later,11 the date of this diagnosis being the index date. This was so that patients had at least 1 year of baseline data prior to COPD diagnosis but after all other criteria were satisfied. Follow-up was from index date until the 30 November 2019, death or the date at which the patient transferred to a non-CPRD contributing practice, whichever came first (figure 1).
Figure 1.
Study design for newly diagnosed COPD cohort. Patients were followed up from index date to the end of follow-up date. Outcome events were identified during this follow-up period. Baseline lower respiratory tract infections were identified in the baseline period prior to index date. COPD, chronic obstructive pulmonary disease; GP, general practice.
Exposure
The exposure of interest was frequency and severity of LRTIs identified during baseline. LRTIs were deemed: (1) mild if the GP visit was not associated with any antibiotic prescription; (2) moderate if the GP visit was associated with a prescription of respiratory antibiotics for 5–14 days; (3) severe if they required hospitalisation with internationl classification of disease (ICD)-10 codes for LRTI or pneumonia. LRTIs are usually treated with antibiotics alone and therefore a prescription of oral corticosteroids (OCS) was not included in our definition. However, the proportion treated with both was described.
Events that were recorded within 2 weeks of each other were assumed to be the same event. Baseline LRTI frequency and severity was categorised into one of the six mutually exclusive categories: none, 1 mild event (and no moderate or severe), ≥2 mild events (and no moderate or severe), 1 moderate event (any mild event and no severe), ≥2 moderate events (any mild events and no severe) and ≥1 severe events (any mild or moderate events).
Outcomes
Study outcomes were measured between index date and the end of follow-up. These included postdiagnosis exacerbations of COPD (moderate or severe) and mortality. Moderate exacerbation events were defined as events recorded in the GP using the combination of exacerbation diagnosis codes, coprescription of antibiotics plus OCS for 5–14 days and recorded symptoms based on a previous validated definition.11 Severe exacerbation events were defined as COPD and LRTI events leading to a hospitalisation as recorded in HES.12 Events that were recorded within 2 weeks of each other were assumed to be the same event. All-cause mortality, COPD-related mortality and cardiovascular disease (CVD)-related mortality were extracted from the ONS mortality database and identified using the underlying ICD-10 cause of death code.
Statistical analysis
Analyses were performed in STATA V.17. Baseline characteristics were defined prior to COPD diagnosis. These included age, sex, smoking status (current or ex-smoker), body mass index, history of depression, anxiety disorder, gastro-oesophageal reflux disease, lung cancer, myocardial infarction, heart failure and stroke, current asthma, socioeconomic deprivation, Medical Research Council (MRC) dyspnoea score, Global Initiative for Chronic Obstructive Lung Disease (GOLD)-defined airflow obstruction and current elevated blood eosinophil level (using threshold of 300 cells/µL). See online supplemental material p.2 for further details on baseline characteristic definitions. These characteristics were described overall and by LRTI exposure subgroup using mean (SD) for continuous measures and numbers (%) for categorical measures. COPD medication prescription in the year after COPD diagnosis (including combination of long and short-acting bronchodilators and inhaled corticosteroids) was described.
thorax-2022-219039supp002.pdf (60.8KB, pdf)
Negative binomial regression was used to investigate the association between baseline LRTI frequency and severity and the number of subsequent exacerbations expressed as incidence rate ratio (IRR) and its 95% CI (any type of exacerbation and separately for moderate and severe exacerbations). Person-time was defined as the time from start of follow-up to end of follow-up. Cox regression was used to investigate the association between baseline LRTI frequency and severity and all-cause, COPD-related, and CVD-related mortality. Adjusted models were adjusted for baseline characteristics listed. Missing values in baseline variables were coded as ‘missing’ and included in the final models (‘missing’ categories were not used as reference categories). No imputation was performed.
Sensitivity analyses
Sensitivity analyses were repeated using a different classification of baseline LRTIs in which mild and moderate LRTIs were grouped together, that is, irrespective of any antibiotics prescription as GPs may have different thresholds for choosing to prescribe antibiotics and antibiotics may not reflect the severity of the event.13 The following baseline LRTI categories were used: none, 1 primary care recorded LRTI, ≥2 primary care recorded LRTIs, ≥3 primary care recorded LRTIs, ≥1 secondary care recorded LRTIs (ie, severe events). In addition, we repeated our main analysis using complete case analysis for variables that were not missing.
Results
Overall, 215 234 patients newly diagnosed with COPD were included (online supplemental figure S1). In the baseline year, 158 074 (73.4%) patients had no LRTIs, 18 081 (8.4%) had 1 mild LRTI, 6133 (2.8%) had ≥2 mild LRTIs, 19 787 (9.2%) had 1 moderate LRTI, 6227 (2.9%) had ≥2 moderate LRTIs and 6932 (3.2%) had ≥1 severe LRTIs that required hospitalisation. Of those patients who had 1 moderate LRTI, 9.3% were also treated with OCS and 19.6% of patients with 2+ moderate were also treated with OCS.
thorax-2022-219039supp001.pdf (147.5KB, pdf)
Baseline characteristics of patients are detailed in table 1. Compared with patients with mild or moderate LRTIs, those with at least one severe LRTI in the year prior to COPD diagnosis tended to be older, were more likely to be ex-smokers, to have more breathlessness as per higher MRC scores ≥4 (although missing data concerned one third of the population on average) and more patients had a history of heart failure. Compared with patients with none, or only mild LRTIs, patients with moderate or severe LRTIs at baseline were more likely to have worse airflow obstruction on COPD diagnosis—with 20%–26% of patients with non-missing data having GOLD grade 3 or 4—and to have current asthma.
Table 1.
Baseline characteristics of newly diagnosed patients with COPD categorised by baseline LRTI frequency and severity
| Overall | None | 1 mild | ≥2 mild | 1 moderate | ≥2 moderate | ≥1 severe | |
| Patient, n (%) | 215 234 (100) | 158 074 (73.4) | 18 081 (8.4) | 6133 (2.8) | 19 787 (9.2) | 6227 (2.9) | 6932 (3.2) |
| Median study follow-up in years (Q1–Q3) | 4.4 (1.9–7.8) | 4.5 (2.0–7.9) | 3.1 (1.5–5.7) | 2.9 (1.3–5.2) | 6.4 (3.3–9.3) | 6.5 (3.3–9.5) | 2.4 (0.9–4.8) |
| Mean age (SD) | 66.9 (11.4) | 66.6 (11.3) | 67.4 (11.5) | 67.8 (11.6) | 66.9 (11.2) | 67.2 (11.4) | 71.5 (12.0) |
| Sex | |||||||
| Male | 114 738 (53.3) | 86 166 (54.5) | 8998 (49.8) | 2921 (47.6) | 9991 (50.5) | 2915 (46.8) | 3747 (54.1) |
| Female | 100 496 (46.7) | 71 908 (45.5) | 9083 (50.2) | 3212 (52.4) | 9796 (49.5) | 3312 (53.2) | 3185 (46.0) |
| Smoking status | |||||||
| Ex-smoker | 107 787 (50.1) | 84 066 (53.2) | 9407 (52.0) | 3274 (53.4) | 10 508 (53.1) | 3324 (53.4) | 4128 (59.6) |
| Current smoker | 107 447 (49.9) | 74 008 (46.8) | 8674 (48.0) | 2859 (46.6) | 9279 (46.9) | 2903 (46.6) | 2804 (40.5) |
| GOLD grade of airflow limitation | |||||||
| 1 | 51 151 (23.8) | 38 450 (24.3) | 4370 (24.2) | 1441 (23.5) | 4627 (23.4) | 1336 (21.5) | 927 (13.4) |
| 2 | 78 736 (36.6) | 57 432 (36.3) | 6757 (37.4) | 2287 (37.3) | 7793 (39.4) | 2537 (40.7) | 1930 (27.8) |
| 3 | 24 985 (11.6) | 17 764 (11.2) | 2086 (11.5) | 724 (11.8) | 2705 (13.7) | 859 (13.8) | 847 (12.2) |
| 4 | 3814 (1.8) | 2781 (1.8) | 282 (1.6) | 99 (1.6) | 361 (1.8) | 138 (2.2) | 153 (2.2) |
| Missing | 56 548 (26.3) | 41 647 (26.4) | 4586 (25.4) | 1582 (25.8) | 4301 (21.7) | 1357 (21.8) | 3075 (44.4) |
| mMRC score | |||||||
| 1 | 34 191 (15.9) | 25 687 (16.3) | 3201 (17.7) | 1000 (16.3) | 2854 (14.4) | 779 (12.5) | 670 (9.7) |
| 2 | 59 658 (27.7) | 43 767 (27.7) | 5580 (30.9) | 1967 (32.1) | 5297 (26.8) | 1579 (25.4) | 1468 (21.2) |
| 3 | 32 330 (15.0) | 22 858 (14.5) | 3118 (17.2) | 1161 (18.9) | 2938 (14.9) | 1001 (16.1) | 1254 (18.1) |
| 4 | 13 249 (6.2) | 9050 (5.7) | 1319 (7.3) | 487 (7.9) | 1188 (6.0) | 434 (7.0) | 771 (11.1) |
| 5 | 2559 (1.2) | 1652 (1.1) | 279 (1.5) | 94 (1.5) | 235 (1.2) | 78 (1.3) | 221 (3.2) |
| Missing | 73 247 (34.0) | 55 060 (34.8) | 4584 (25.4) | 1424 (23.2) | 7275 (36.8) | 2356 (37.8) | 2548 (36.8) |
| Myocardial infarction | 15 062 (7.0) | 10 599 (6.7) | 1367 (7.6) | 467 (7.6) | 1408 (7.1) | 491 (7.9) | 730 (10.5) |
| Stroke | 17 311 (8.0) | 12 381 (7.8) | 1553 (8.6) | 588 (9.6) | 1399 (7.1) | 458 (7.4) | 932 (13.4) |
| Heart failure | 9136 (4.2) | 6045 (3.8) | 951 (5.3) | 335 (5.5) | 726 (3.7) | 303 (4.9) | 776 (11.2) |
| Lung cancer | 412 (0.2) | 285 (0.2) | 42 (0.2) | <20 | 42 (0.2) | <20 | <20 |
| Asthma | 45 751 (21.3) | 33 480 (21.2) | 3540 (19.6) | 1285 (21.0) | 4505 (22.8) | 1745 (28.0) | 1196 (17.3) |
| GORD | 31 904 (14.8) | 22 403 (14.2) | 3154 (17.4) | 1217 (19.8) | 3005 (15.2) | 1067 (17.1) | 1058 (15.3) |
| Anxiety disorder | 46 184 (21.5) | 32 399 (20.5) | 4625 (25.6) | 1678 (27.4) | 4409 (22.3) | 1571 (25.2) | 1502 (21.7) |
| Depression | 55 323 (25.7) | 39 000 (24.7) | 5358 (29.6) | 1990 (32.5) | 5320 (26.9) | 1839 (29.5) | 1816 (26.2) |
| BMI group (in kg/m2) | |||||||
| <19 | 8395 (3.9) | 5996 (3.8) | 757 (4.2) | 226 (3.7) | 735 (3.7) | 247 (4.0) | 434 (6.3) |
| 19–24 | 62 392 (29.0) | 46 015 (29.1) | 5126 (28.4) | 1662 (27.1) | 5919 (29.9) | 1725 (27.7) | 1945 (28.1) |
| 25–29 | 64 885 (30.2) | 48 313 (30.6) | 5314 (29.4) | 1802 (29.4) | 5863 (29.6) | 1877 (30.1) | 1716 (24.8) |
| ≥30 | 58 796 (27.3) | 42 687 (27.0) | 5251 (29.0) | 1897 (30.9) | 5325 (26.9) | 1781 (28.6) | 1855 (26.8) |
| Missing | 20 766 (9.7) | 15 063 (9.5) | 1633 (9.0) | 546 (8.9) | 1945 (9.8) | 597 (9.6) | 982 (14.2) |
| IMD quintiles | |||||||
| 1 (least deprived) | 34 469 (16.0) | 25 774 (16.3) | 2744 (15.2) | 896 (14.6) | 3148 (15.9) | 909 (14.6) | 998 (14.4) |
| 2 | 38 946 (18.1) | 28 881 (18.3) | 3.122 (17.3) | 1036 (16.9) | 3589 (18.1) | 1111 (17.8) | 1207 (17.4) |
| 3 | 42 090 (19.6) | 31 054 (19.7) | 3436 (19.0) | 1206 (19.7) | 3837 (19.4) | 1202 (19.3) | 1355 (19.6) |
| 4 | 45 775 (21.3) | 33 531 (21.2) | 3913 (21.6) | 1325 (21.6) | 4120 (20.8) | 1335 (21.4) | 1551 (22.4) |
| 5 (most deprived) | 53 797 (25.0) | 38 714 (24.5) | 4852 (26.8) | 1669 (27.2) | 5076 (25.7) | 1669 (26.8) | 1817 (26.2) |
| Missing | 157 (0.1) | 120 (0.1) | 14 (0.1) | <5 | 17 (0.1) | <5 | <5 |
| Blood eosinophil levels | |||||||
| <300 cells/µL | 83 244 (38.7) | 60 574 (38.3) | 7377 (40.8) | 2659 (43.4) | 7340 (37.1) | 2329 (37.4) | 2965 (42.8) |
| ≥300 cells/µL | 37 720 (17.5) | 26 885 (17.0) | 3408 (18.9) | 1272 (20.7) | 3611 (18.3) | 1206 (19.4) | 1338 (19.3) |
| Missing | 94 270 (43.8) | 70 615 (44.7) | 7296 (40.4) | 2202 (35.9) | 8826 (44.7) | 2692 (43.2) | 2629 (37.9) |
The following baseline variables were identified at or close to index date and were identified as ‘current’: age, smoking status, IMD, BMI, mMRC, GOLD, asthma, eosinophil level. The following baseline variables were recorded any time prior to index date: depression, anxiety, GORD, lung cancer, myocardial infarction, heart failure, stroke.
BMI, body mass index; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; GORD, gastro-oesophageal reflux disease; IMD, Index of Multiple Deprivation; LRTI, lower respiratory tract infection; mMRC, modified Medical Research Council.
COPD medications prescribed in the first year after COPD diagnosis are detailed in table 2. Compared with patients with no LRTI, those with 2 or more mild LRTIs, 1 moderate LRTI, or 2 or more moderate LRTIs were more frequently prescribed COPD-related medications (67.6% vs between 72.8% and 82.1%) after initial diagnosis, whereas patients who had at least one severe LRTI (ie, required hospitalisation) were less frequently prescribed COPD-related medications (37.8%).
Table 2.
COPD medications in the year after COPD diagnosis in patients categorised by baseline LRTI frequency and severity
| Overall N=2 15 234 |
None n=1 58 074 | 1 mild n=18 081 | ≥2 mild n=6133 | 1 moderate n=19 787 | ≥2 moderate n=6227 | ≥1 severe 6932 |
|
| Any COPD medications | 148 655 (69.1) | 106 778 (67.6) | 12 583 (69.6) | 4467 (72.8) | 15 403 (77.8) | 5109 (82.1) | 2617 (37.8) |
| Maintenance inhaled therapies | |||||||
| Any COPD maintenance medications | 125 540 (58.3) | 89 637 (56.7) | 10 690 (59.1) | 3832 (62.5) | 13 022 (65.8) | 4485 (72.0) | 3874 (55.9) |
| No COPD maintenance medications | 89 694 (41.7) | 68 437 (43.3) | 7391 (40.9) | 2301 (37.5) | 6765 (34.2) | 1742 (28.0) | 3058 (44.1) |
| LABA monotherapy | 4096 (1.9) | 3062 (1.9) | 285 (1.6) | 93 (1.5) | 446 (2.3) | 146 (2.3) | 64 (0.9) |
| LAMA monotherapy | 20 328 (9.4) | 14 896 (9.4) | 1912 (10.6) | 604 (9.9) | 1802 (9.1) | 497 (8.0) | 617 (8.9) |
| ICS monotherapy | 14 243 (6.6) | 10 783 (6.8) | 943 (5.2) | 264 (4.3) | 1563 (7.9) | 491 (7.9) | 199 (2.9) |
| LABA/ICS dual therapy | 38 284 (17.8) | 27 590 (17.5) | 1073 (5.9) | 1055 (17.2) | 4214 (21.3) | 1549 (24.9) | 947 (13.7) |
| LABA/LAMA dual therapy | 8837 (4.1) | 6476 (4.1) | 1073 (5.9) | 388 (6.3) | 438 (2.2) | 104 (1.7) | 358 (5.2) |
| LAMA/ICS dual therapy | 3137 (1.5) | 2234 (1.4) | 242 (1.3) | 89 (1.5) | 360 (1.8) | 131 (2.1) | 81 (1.2) |
| Triple therapy | 36 615 (17.0) | 24 596 (15.6) | 3306 (18.3) | 1339 (21.8) | 4199 (21.2) | 1567 (25.2) | 1608 (23.2) |
| Short-acting therapies | |||||||
| SABA | 114 354 (90.1) | 81 630 (90.2) | 10 029 (93.1) | 3606 (93.3) | 11 806 (87.5) | 3942 (86.4) | 3341 (91.6) |
| SAMA | 2448 (1.9) | 1770 (2.0) | 157 (1.5) | 55 (1.4) | 320 (2.4) | 107 (2.3) | 39 (1.1) |
| SAMA/SABA | 10 060 (7.9) | 7122 (7.9) | 583 (5.4) | 206 (5.3) | 1368 (10.1) | 514 (11.3) | 267 (7.3) |
Any COPD medications includes any maintenance COPD medications or any short-acting therapies. COPD maintenance therapies include any combination of LABA, LAMA, ICS. Triple therapy=LABA/LAMA/ICS. Short-acting therapies include SABA, SAMA. Numbers are n(%).
COPD, chronic obstructive pulmonary disease; ICS, inhaled corticosteroids; LABA, long-acting beta agonist; LAMA, long-acting muscarinic antagonist; LRTI, lower respiratory tract infection; SABA, short-acting beta agonists; SAMA, short-acting muscarinic antagonists.
Baseline LRTIs and future exacerbations of COPD
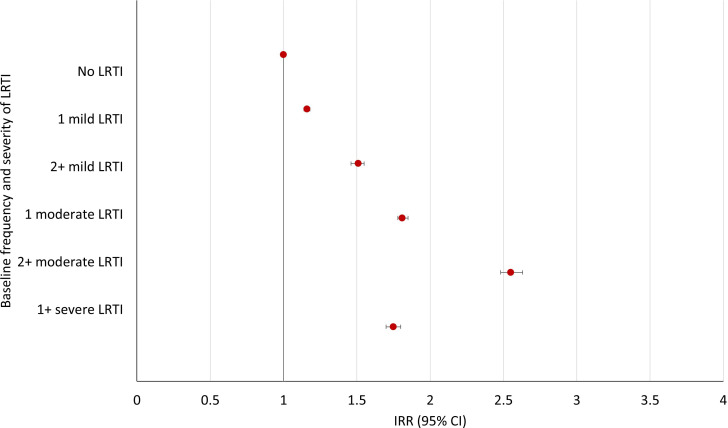
A total of 948 576 COPD exacerbation events were recorded over a median follow-up of 4.4 years (IQR 1.9–7.9) in the population. Compared with patients with no LRTI in the year prior to COPD diagnosis, patients with 1 moderate LRTI or ≥2 moderate LRTIs had higher incidence rates of future exacerbations of COPD of any severity: adjusted IRR 1.81 (95% CI, 1.78 to 1.85) and 2.55 (95% CI, 2.48 to 2.63), respectively (figure 2, online supplemental table S1). This higher incidence rate was also found for patients with severe LRTIs at baseline, however, with a smaller magnitude of association: adjusted IRR 1.75 95% CI, 1.70 to 1.801. Patients with 1 or ≥2 mild LRTIs had higher incidence rates compared with those with no LRTIs but also to a lower extent: adjusted IRR 1.16 95% CI, 1.14 to 1.18 and adjusted IRR 1.51, 95% CI, 1.46 to 1.55, respectively.
Figure 2.
Incidence rate ratios (IRRs) of at least one acute exacerbation of chronic obstructive pulmonary disease (COPD) following COPD diagnosis by baseline frequency and severity of lower respiratory tract infection (LRTI).
A similar pattern of association was seen with future moderate exacerbations of COPD (online supplemental table S2); however, the strongest association was seen between baseline severe LRTIs and future severe exacerbations (adjusted IRR 3.02 95% CI, 2.87 to 3.18; online supplemental table S3).
Baseline LRTIs and mortality
A total of 51 669 (24%) of 215 234 patients with COPD died during follow-up, the highest risk being for the baseline severe LRTI group (figure 3, online supplemental table S4) (adjusted HR: 1.92 95% CI, 1.84 to 1.99), followed by ≥2 moderate and 1 moderate baseline LRTI (adjusted IRR: 1.22 95% CI, 1.17 to 1.27 and 1.10 95% CI, 1.07 to 1.13, respectively). An association was seen between 1 or ≥2 mild baseline LRTIs but to a lesser extent: adjusted HR 1.11 95% CI, 1.07 to 1.15, and 1.14 95% CI, 1.08 to 1.22, respectively.
Figure 3.
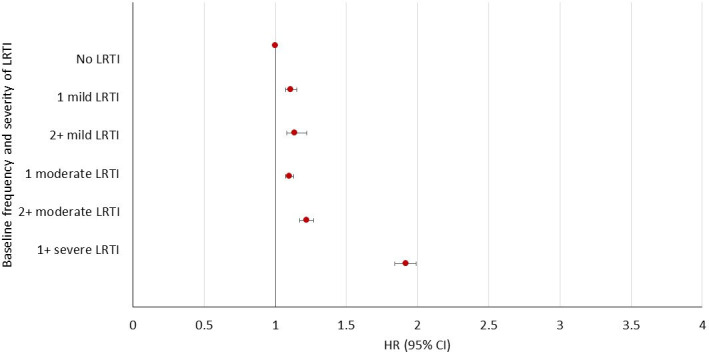
HRs for all-cause mortality following chronic obstructive pulmonary disease diagnosis by baseline frequency and severity of lower respiratory tract infection (LRTI).
In terms of COPD and CVD-related mortality, compared with no baseline LRTIs, the strongest association was seen in patients with≥1 severe LRTI (adjusted HR, 2.32 95% CI, 2.15 to 2.50 and IRR 1.68 95% CI, 1.55 to 1.82, respectively) (online supplemental tables S5 and S6).
Sensitivity analyses
When baseline LRTI events were grouped into the following categories: no LRTI, 1 primary care recorded LRTI, 2 primary care recorded LRTIs, ≥3 primary care recorded LRTIs, and ≥1 hospitalised LRTI, all categories were significantly associated with future exacerbations of COPD compared with no baseline LRTIs (online supplemental table S6). When analyses were repeated using complete case analysis for non-missing covariates, results remained consistent (online supplemental table S7).
Discussion
We explored the association between LRTIs requiring antibiotics in primary care or not, as well as those requiring hospitalisation in the year prior to a COPD diagnosis and the rate of subsequent moderate or severe exacerbation in a population-based cohort.
In our cohort, over a quarter of people had evidence of an LRTI in the year prior to COPD diagnosis. All of these patients were at higher rate of having subsequent moderate, and/or severe exacerbations of COPD in the near term after diagnosis (median follow-up of 4 years). To our knowledge, no studies have investigated whether respiratory infection events prior to COPD diagnosis are associated with an increase in a patient’s rate of subsequent exacerbations of COPD or mortality.1 9 14–17 According to guidelines, COPD should be investigated in people with frequent bronchitis. These events in themselves may help to understand COPD progression and ultimately help to treat patients with COPD more effectively.8 The results of our study illustrate the importance of LRTIs in the course of COPD.
Patients with one mild LRTI in 12 months prior to incident COPD diagnosis had the lowest rate of subsequent exacerbations compared with patients with increased frequency and severity at baseline. It is possible that one mild LRTI event in primary care that is not treated with antibiotics is extremely mild and misclassification may exist between mild LRTIs and events occurring but not recorded by the GP. We also saw a difference in associations between mild and moderate primary care events in terms of subsequent moderate exacerbations of COPD, whereby LRTI events treated in primary care were more strongly associated with future moderate COPD exacerbation events. This was not seen with future severe exacerbations of COPD, whereby severe LRTI events were most strongly associated with future severe exacerbations followed by two or more mild or moderate LRTI. This suggests that other than severe LRTI, increased frequency of any type of baseline LRTI is also important in relation to future severe exacerbations of COPD.
The association between exacerbations of COPD and increased risk of future exacerbations is well known.4 9 In addition, recent studies suggest that the presence of moderate exacerbations of COPD during a year, even a single one, are associated with future risk of exacerbations.2–4 Our study expands on this result by highlighting the importance of even a single LRTI requiring antibiotics prior to first COPD diagnosis, in the estimation of the future rate of COPD exacerbations.
When LRTIs were categorised into levels of frequency and severity, we observed that the rate of subsequent moderate COPD exacerbations (ie, managed in primary care settings) was the highest for patients with moderate LRTIs prior to COPD diagnosis, while the rate of subsequent severe exacerbations (ie, managed in the hospital) was the highest for patients with severe LRTIs, which may reflect the underlying severity of COPD on diagnosis. Indeed, the group of patients who were diagnosed after severe LRTIs or multiple LRTIs had worse airflow limitation, dyspnoea, and were older suggesting that COPD had existed for a longer period and that the formal diagnosis of COPD was made at a later stage. This suggests a missed opportunity for an earlier diagnosis in people experiencing frequent or severe LRTI. Another plausible explanation could be residual confounding related to healthcare utilisation and quality of care, as patients with severe LRTIs at baseline had the lowest proportion of any COPD therapies. Finally, it is possible that some patients who have LRTIs, or COPD exacerbations, go to the emergency room instead of the GP.18
In terms of mortality, we found that increased frequency and severity of baseline LRTI were associated with increased rate of all-cause and COPD-related mortality but only multiple moderate LRTIs and severe LRTIs were associated with CVD-related mortality.
A previous study investigated exacerbations of COPD in the year following diagnosis and future risk of exacerbations and mortality, namely in incident COPD.9 This study defined GP related exacerbations as exacerbation of COPD events or LRTIs, a prescription of antibiotics, OCS or both, for 5–14 days, or exacerbation-related symptoms combined with prescription for antibiotics and/or OCS. Authors found that increasing frequency of GP-related exacerbations in the year following COPD diagnosis was associated with increased risk of future exacerbations of COPD after COPD diagnosis, which is similar to the results of our sensitivity analysis. The main difference, however, was that we included events using a different definition for LRTIs. Our definition did not include the use of OCS nor did it include events recorded as exacerbations of COPD in order to identify LRTI events which general practitioners would have recorded prior to COPD diagnosis. Despite this, we found that the vast proportion of events treated with a respiratory antibiotic were also treated with a course of OCS before a clinical COPD diagnosis was given. This suggests that GPs may suspect COPD; however, they do not formally diagnose these patients until a short while after. In addition, we expanded on previous work by showing that increasing frequency and severity of LRTIs are associated with future exacerbations of COPD, all-cause mortality and COPD-related mortality and that in patients with LRTIs, multiple moderate or any severe LRTIs had similar CVD-related risk of death than those who had no LRTIs at baseline.
Studies of this type have a number of limitations. First, while severe exacerbations were identified by hospitalised events, we cannot be sure that these events are severe events in the clinical sense. It is possible that some patients who exacerbate go to the emergency room instead of the GP and are in turn hospitalised in the absence of outpatient management. Indeed, access to GPs in the UK is becoming increasingly difficult.18 In addition, we included LRTI events as LRTI/pneumonia recorded events with or without antibiotic prescriptions; however, it is possible that events might have been missed if they were coded as exacerbations of COPD. Similarly, antibiotic prescriptions for LRTI events will depend on whether the infection is viral or bacterial based on the general practitioner’s judgement. In the same way that prior exacerbations are associated with subsequent exacerbation events, it could be argued that LRTI events are associated with subsequent exacerbation events after a diagnosis of COPD. Alternatively, it could be that in fact the LRTI events were themselves exacerbations of COPD (just unknown as a diagnosis of COPD had not been made), as those with LRTI events had lower lung function at COPD diagnosis. However, as these events occurred prior to a COPD diagnosis, they will have been managed differently to an exacerbation in a diagnosed patient with COPD, for example, prednisolone will not have been prescribed. Despite this, bacterial infections or bacterial coinfections are associated with increased symptoms and can be more severe in patients with COPD.19 In addition, residual confounding is likely to exist as we were unable to account for variables such as environmental factors and time-varying confounders such as COPD medication over follow-up. Lastly, this study investigated the association between events that occurred prior to a COPD diagnosis and COPD-related events that occurred after COPD diagnosis and does not imply causation. While LRTI events might have occurred earlier than in the year prior to COPD diagnosis, the events that occur close to a diagnosis are important in order to understand how better management of LRTI events or earlier diagnosis could improve COPD-related outcomes in the future.
Conclusion
The frequency and severity of LRTI events prior to first COPD diagnosis are associated with future increased rates of exacerbations of COPD, all-cause mortality and COPD-related mortality. Overall, this study suggests that LRTIs prior to COPD diagnosis may be indicative of subsequent increased rate of exacerbations and increased risk of mortality and should be taken seriously by healthcare professionals.
Acknowledgments
The authors would also like to thank Precil Varghese, formerly of Biopharmaceuticals Medical, Respiratory and Immunology, AstraZeneca, USA, for his early contributions to this study.
Footnotes
Contributors: HW, CN, HM and JKQ planned the study. HW conducted the study. HW, CN and JKQ wrote the manuscript. AR, TM, YX, EDN and HM provided study feedback.JKQ is guarantor.
Funding: This study was funded by AstraZeneca (D5980R00023).
Competing interests: JKQ has received grants from The Health Foundation, MRC, GSK, Bayer, BI, AUK-BLF, Chiesi, and AZ, and personal fees for advisory board participation or speaking fees from GlaxoSmithKline, Boehringer Ingelheim, AstraZeneca and Bayer. HW has nothing to disclose. CL, AR, HM, TM and YX are employees of AstraZeneca and holds stock and/or options in the company. EDN is a former employee of AstraZeneca, but was employed by AstraZeneca at the time of the study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. Data are available on request from the CPRD. Their provision requires the purchase of a license, and this license does not permit the authors to make them publicly available to all.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This work is based in part on data from the CPRD obtained under license from the UK Medicines and Healthcare products Regulatory Agency. The data is provided by patients and collected by the National Health Service as part of their care and support. The interpretation and conclusions contained in this study are those of the authors alone. A protocol for this research was approved by the Independent Scientific Advisory Committee (ISAC) for Medicines and Healthcare products Regulatory Agency Database Research (protocol number 20_103R) and the approved protocol was made available to the journal in which this research is published, and to the reviewers during peer review. Generic ethical approval for observational research using the CPRD with approval from ISAC has been granted by a Health Research Authority Research Ethics Committee (East Midlands—Derby, REC reference number 05/MRE04/87). The interpretation and conclusions contained in this study are those of the authors alone.
References
- 1. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010;363:1128–38. 10.1056/NEJMoa0909883 [DOI] [PubMed] [Google Scholar]
- 2. Haughney J, et al. The long-term clinical and economic impact of COPD exacerbations: an observational study (SHERLOCK). Europ Res J 2020;56:4910. [Google Scholar]
- 3. Vogelmeier CF, Diesing J, Kossack N, et al. COPD exacerbation history and impact on future exacerbations - 8-year retrospective observational database cohort study from Germany. [DOI] [PMC free article] [PubMed]
- 4. Whittaker H, et al. Frequency and severity of exacerbations of COPD associated with future risk of exacerbations and mortality: a UK routine health care data study. [DOI] [PMC free article] [PubMed]
- 5. Miravitlles M, Ferrer M, Pont A, et al. Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up study. Thorax 2004;59:387–95. 10.1136/thx.2003.008730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Donaldson GC, Seemungal TAR, Bhowmik A, et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57:847–52. 10.1136/thorax.57.10.847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005;60:925–31. 10.1136/thx.2005.040527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. NICE . Chronic obstructive pulmonary disease in over 16s: diagnosis and management (ed Excellence, N.I.f.H.a.C.), 2019. [PubMed] [Google Scholar]
- 9. Rothnie KJ, Müllerová H, Smeeth L, et al. Natural history of chronic obstructive pulmonary disease exacerbations in a general practice-based population with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2018;198:464–71. 10.1164/rccm.201710-2029OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pasquale MK, Sun SX, Song F, et al. Impact of exacerbations on health care cost and resource utilization in chronic obstructive pulmonary disease patients with chronic bronchitis from a predominantly Medicare population. Int J Chron Obstruct Pulmon Dis 2012;7:757–64. 10.2147/COPD.S36997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rothnie KJ, Müllerová H, Hurst JR, et al. Validation of the recording of acute exacerbations of COPD in UK primary care electronic healthcare records. PLoS One 2016;11:e0151357. 10.1371/journal.pone.0151357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rothnie KJ, Müllerová H, Thomas SL, et al. Recording of hospitalizations for acute exacerbations of COPD in UK electronic health care records. Clin Epidemiol 2016;8:771–82. 10.2147/CLEP.S117867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Palin V, Mölter A, Belmonte M, et al. Antibiotic prescribing for common infections in UK general practice: variability and drivers. J Antimicrob Chemother 2019;74:2440–50. 10.1093/jac/dkz163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sadatsafavi M, Xie H, Etminan M, et al. The association between previous and future severe exacerbations of chronic obstructive pulmonary disease: updating the literature using robust statistical methodology. PLoS One 2018;13:e0191243. 10.1371/journal.pone.0191243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hartley BF, Barnes NC, Lettis S, et al. Risk factors for exacerbations and pneumonia in patients with chronic obstructive pulmonary disease: a pooled analysis. Respir Res 2020;21:5. 10.1186/s12931-019-1262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Müllerová H, Shukla A, Hawkins A, et al. Risk factors for acute exacerbations of COPD in a primary care population: a retrospective observational cohort study. BMJ Open 2014;4:e006171. 10.1136/bmjopen-2014-006171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Margüello MS, Garrastazu R, Ruiz-Nuñez M, et al. Independent effect of prior exacerbation frequency and disease severity on the risk of future exacerbations of COPD: a retrospective cohort study. NPJ Prim Care Respir Med 2016;26:16046. 10.1038/npjpcrm.2016.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baker M, Jeffers H. Continuity of care in modern day general practice. London: Royal College of General Practitioners, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fau FH, Jungblut S. The influence of virus infections on the course of COPD. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
thorax-2022-219039supp002.pdf (60.8KB, pdf)
thorax-2022-219039supp001.pdf (147.5KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. Data are available on request from the CPRD. Their provision requires the purchase of a license, and this license does not permit the authors to make them publicly available to all.