Abstract
Background
As part of its mission to improve the quality of care for women with gynecological cancers across Europe, the European Society of Gynaecological Oncology (ESGO) first published in 2017 evidence-based guidelines for the management of patients with vulvar cancer.
Objective
To update the ESGO guidelines based on the new evidence addressing the management of vulvar cancer and to cover new topics in order to provide comprehensive guidelines on all relevant issues of diagnosis and treatment of vulvar cancer.
Methods
The ESGO Council nominated an international development group comprised of practicing clinicians who provide care to vulvar cancer patients and have demonstrated leadership through their expertize in clinical care and research, national and international engagement and profile as well as dedication to the topics addressed to serve on the expert panel (18 experts across Europe). To ensure that the statements were evidence-based, new data identified from a systematic search were reviewed and critically appraised. In the absence of any clear scientific evidence, judgment was based on the professional experience and consensus of the international development group. Prior to publication, the guidelines were reviewed by 206 international practitioners in cancer care delivery and patient representatives.
Results
The updated guidelines cover comprehensively diagnosis and referral, staging, pathology, pre-operative investigations, surgical management (local treatment, groin treatment, sentinel lymph node procedure, reconstructive surgery), (chemo)radiotherapy, systemic treatment, treatment of recurrent disease (vulvar, inguinal, pelvic, and distant recurrences), and follow-up. Management algorithms are also defined.
Keywords: Vulvar and Vaginal Cancer
Introduction
Vulvar cancers are relatively uncommon, ranking as the nineteenth most common cause of cancer incidence in European women with approximatively 16 506 new cases in 2020, and affect predominatly elderly women.1 The vast majority are squamous cell carcinomas. Epidemiologic risk factors associated with vulvar cancer are notably age, human papillomavirus (HPV) infection prevalence, smoking, HIV infection, vulvar intraepithelial neoplasia, and lichen sclerosus.
As part of its mission to improve the quality of care for women with gynecological cancers across Europe, the European Society of Gynaecological Oncology (ESGO) first published in 2017 evidence-based guidelines in order to improve the management of patients with vulvar cancer within a multidisciplinary setting.2 Given the body of new evidence addressing the management of vulvar cancer, ESGO decided to update these evidence-based guidelines and moreover to cover new topics in order to provide comprehensive guidelines on all relevant issues of diagnosis and treatment in vulvar cancer.
These guidelines are intended for use by gynecological oncologists, general gynecologists, gynaecologic surgeons, radiation oncologists, pathologists, medical and clinical oncologists, radiologists, general practitioners, palliative care teams, and allied health professionals. These guidelines apply to adults over the age of 18 years with squamous cell carcinoma of the vulva. These guidelines do not address patients with other vulvar cancer histologies and do not include any economic analysis of the strategies.
Responsibilities
Even though our aim is to present the highest standard of evidence in an optimal management of patients with vulvar cancer, ESGO acknowledges that there will be broad variability in practices between the various centers worldwide and also significant differences in infrastructure, access to medical and surgical technology, and also training, medicolegal, financial, and cultural aspects that will affect the implementation of any guidelines. These guidelines are a statement of evidence and consensus of the multidisciplinary development group regarding their views and perspective of currently accepted approaches for the management of patients with vulvar cancer. Any clinician applying or consulting these guidelines is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient’s care or treatment. These guidelines make no representations or warranties of any kind whatsoever regarding their content, use, or application and disclaim any responsibility for their application or use in any way.
Methods
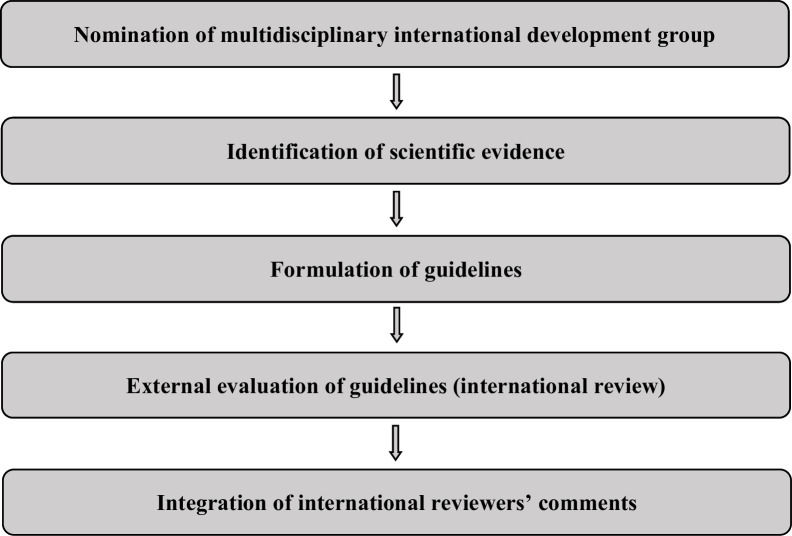
The guidelines were developed using a five-step process as defined by the ESGO Guidelines Committee (see Figure 1). The strengths of the process include creation of a multidisciplinary international development group, use of scientific evidence and international expert consensus to support the guidelines, and use of an international external review process (physicians and patients). This development process involved three meetings of the international development group, chaired by Dr Maaike H M Oonk (University Medical Center Groningen, University of Groningen, Groningen, The Netherlands).
Figure 1.
Guidelines development process.
ESGO nominated practicing clinicians who are involved in the management of patients with vulvar cancer and have demonstrated leadership through their expertize in clinical care and research, national and international engagement and profile as well as dedication to the topics addressed to serve on the expert panel. The objective was to assemble a multidisciplinary development group and it was therefore essential to include professionals from relevant disciplines (gynecological oncology and gynecology, medical, clinical and radiation oncology, pathology) to contribute to the validity and acceptability of the guidelines.
To ensure that the statements were evidence-based, the current literature was reviewed and critically appraised. A systematic literature review of relevant studies published between September 2015 and April 2022 was carried out using the MEDLINE database (see online supplemental appendix 1). The literature search was limited to publications in the English language. Priority was given to high-quality systematic reviews, meta-analyses, and randomized controlled trials, but studies of lower levels of evidence were also evaluated. The search strategy excluded editorials, letters, and in vitro studies. The reference list of each identified article was reviewed for other potentially relevant articles. Based on the collected evidence and clinical expertize, the international development group drafted guidelines for all the topics. The updated guidelines were retained if they were supported by a sufficiently high level of scientific evidence and/or when a large consensus among experts was obtained. An adapted version of the “Infectious Diseases Society of America-United States Public Health Service Grading System” was used to define the level of evidence and grade of recommendation for each of the recommendations3 (see Table 1). In the absence of any clear scientific evidence, judgment was based on the professional experience and consensus of the international development group.
Table 1.
Levels of evidence and grades of recommendations
| Levels of evidence | |
| I | Evidence from at least one large randomized, controlled trial of good methodological quality (low potential for bias) or meta-analyses of well-conducted, randomized trials without heterogeneity. |
| II | Small randomized trials or large randomized trials with a suspicion of bias (lower methodological quality) or meta-analyses of such trials or of trials with demonstrated heterogeneity. |
| III | Prospective cohort studies. |
| IV | Retrospective cohort studies or case–control studies. |
| V | Studies without control group, case reports, experts' opinions. |
| Grades of recommendations | |
| A | Strong evidence for efficacy with a substantial clinical benefit, strongly recommended. |
| B | Strong or moderate evidence for efficacy but with a limited clinical benefit, generally recommended. |
| C | Insufficient evidence for efficacy or benefit does not outweigh the risk or the disadvantages (adverse events, costs, etc.), optional. |
| D | Moderate evidence against efficacy or for adverse outcome, generally not recommended. |
| E | Strong evidence against efficacy or for adverse outcome, never recommended. |
ijgc-2023-004486supp001.pdf (294.5KB, pdf)
ESGO established a large multidisciplinary panel of practicing clinicians who provide care to patients with vulvar cancer to act as independent reviewers for the updated guidelines. These reviewers were selected according to their expertize, had to be still involved in clinical practice/research, and were from different European and non-European countries to ensure a global perspective. Patients with vulvar cancer were also included. The independent reviewers were asked to evaluate each recommendation according to its relevance and feasibility in clinical practice (only physicians), so that comprehensive quantitative and qualitative evaluations of the updated guidelines were completed. Patients were asked to evaluate qualitatively each recommendation (according to their experience, personal perceptions, etc.). Evaluations of the external reviewers (n=206) were pooled and discussed by the international development group to finalize the guidelines updating process. The list of the 206 external reviewers is available in online supplemental appendix 2.
Guidelines
General recommendations
Planning of staging investigations and treatment should be made on a multidisciplinary basis (generally at a tumor board meeting) and based on the comprehensive and precise knowledge of prognostic and predictive factors for oncological outcome, side effects, and quality of life [V, B].
Patients should be carefully counseled about the suggested diagnostic and treatment plan and potential alternatives, including risks and benefits of all options [V, B].
Treatment should be undertaken by a dedicated team of specialists in the diagnosis and management of vulvar cancers [V, B].
Enrolment of patients with vulvar cancer in clinical trials is encouraged [V, B].
Centralization of care in specialized centers and referral network is encouraged [V, B].
Supportive care and psychological support should be offered to all patients with vulvar cancer throughout their pathway [V, B].
Diagnosis and referral
Vulvar cancer is a rare disease, but incidence is increasing over the last decades, especially in women <60 years of age.4 Recently several studies have illustrated the delay in diagnosis that is often the case in patients who are referred with (the suspicion of) vulvar cancer. A study in patients from Germany showed a mean delay of vulvar cancer diagnosis ranging from 186 to 328 days.5 This was most commonly due to a misdiagnosis of vulvovaginal inflammation. To prevent this delay, women with any vulvar complaints should undergo vulvar examination in a low-threshold manner. Diagnosis of vulvar cancer is made by a punch or incision biopsy of the vulvar lesion. For accurate treatment planning (sentinel lymph node (SLN) procedure yes/no, expected uni- or bilateral lymph drainage) the localization of the primary tumor is important. Therefore, excision biopsy should be avoided. In patients with multiple vulvar lesions, all lesions should be biopsied separately to rule out multifocal disease, since patients with multifocal disease are not eligible for the SLN procedure. Because vulvar cancer is a rare disease and the outcome of, for example, the SLN procedure is related to the experience of the treating physician, treatment should be centralized in centers with adequate experience. A European study showed that patients treated in centers with low volume institutions had worse survival rates.6
Recommendations
Inspection of the vulva is indicated for women with vulvar symptoms [V, B].
Clinical drawing and/or photographs are recommended [V, B].
In any patient with suspected vulvar cancer, diagnosis should be established by a punch/incision biopsy. Excision biopsy should be avoided for initial diagnosis, as this may hinder further treatment planning [V, B].
In patients with multiple vulvar lesions, all lesions should be biopsied separately with clear documentation of mapping [V, B].
All patients with vulvar cancer should be referred to a center specialized in vulvar disease and treated by a multidisciplinary gynecological oncology team [V, B].
Staging
The International Federation of Gynecology and Obstetrics (FIGO) and the TNM classification systems are both used to stage vulvar cancer and are closely aligned.7 8 The FIGO staging system was last reviewed in 2021 by the FIGO Committee on Gynecologic Oncology since the previous 2009 FIGO classification was limited by its prognostic significance.7 The revised FIGO staging was based on an analysis of data from the National Cancer Database from 2010 to 2017.7 The revised staging classification has been simplified from eleven to eight groups (change of lymph node cut-off ≤5 mm for stage IIIA vs >5 mm for stage IIIB in 2021 compared with 2009 when the cut-off was <5 mm and ≥5 mm, respectively). Some prognostic relevant staging tumor factors were re-allocated between stage subgroups to achieve similar survival rates (non-osseous organ extension included originally in stage IVA in 2009 was downstaged to IIIA stage by 2021) (see Table 2). In 2022, the Surveillance, Epidemiology, and End Results (SEER) analysis (2010–2015) noted better survival rates for nodal (48.9%) versus non-nodal organ involvement (38.7%) in stage IIIA, while similar survival rates between nodal and non-nodal involvement (12.2% vs 14.9%) were confirmed in stage IVA.9 In addition, a new measurement of depth of invasion in vulvar cancer was introduced in FIGO 2021, which is analogous to cervical cancer, as it might better reflect prognosis, although interobserver variability between pathologists remains moderate.10–12 Based on the limited evidence, the working group advises basing treatment planning on the conventional depth of invasion measurement.
Table 2.
Comparison between revised 2021 International Federation of Gynecology and Obstetrics (FIGO) and 2017 8th version of TNM staging vulvar cancer
| Stage | Year | TNM | FIGO | FIGO (continued) | FIGO (continued) |
| Stage I | 2009 | I T1 N0M0 |
IA T1a N0M0 |
IB T1bN0M0 |
|
| Tumor confined to the vulva | IA: Tumor size ≤2 cm and stromal invasion ≤1 mm | IB: Tumor size >2 cm or stromal invasion >1 mm | |||
| Stage I | 2021 | Tumor confined to the vulva | IA: Tumor size ≤2 cm and stromal invasion ≤1 mm* | IB: Tumor size >2 cm or stromal invasion >1 mm* | |
| Stage II | 2009 | II T2 N0 M0 |
|||
| Tumor of any size with extension to the lower one-third of the urethra, lower one-third of vagina, anus with negative nodes | |||||
| Stage II | 2021 | Tumor of any size with extension to the lower one-third of the urethra, lower one-third of vagina, anus with negative nodes | |||
| Stage III | 2009 | III T1-T2 N1-N2c M0 |
IIIA T1-T2 N1 M0 |
IIIB T1-2 N2a, N2b M0 |
IIIC T1-2 N2c M0 |
| Tumor of any size, with/without extension to adjacent perineal structures (lower third of urethra, the lower third of vagina, anus) with positive inguinofemoral lymph nodes | With one lymph node metastasis (≥5 mm) or 1–2 lymph node metastases (<5 mm) | With two or more lymph node metastases (≥5 mm) or three or more lymph node metastases (<5 mm) | With positive lymph nodes with extracapsular spread | ||
| Stage III | 2021 | III T3 (non-fixed to the bone) or N+ (non-ulcerated/non-fixed) M0 |
IIIA T1-T2 N+ (≤5 mm and no extracapsular spread) M0 T3 N0/N+ (≤5 mm and no extracapsular spread) M0 |
IIIB T1-3 N+ (>5 mm and no extracapsular spread) M0 |
IIIC T1-3 N+ (extracapsular spread) M0 |
| Tumor of any size with extension to upper part of adjacent perineal structures, or with any number of non-fixed, non-ulcerated lymph nodes | Tumor of any size with disease extension to upper two-thirds of the urethra, upper two-thirds of the vagina, bladder mucosa, rectal mucosa, or regional† lymph node metastases ≤5 mm | Regional† lymph node metastases >5 mm | Regional† lymph node metastases with extracapsular spread | ||
| Stage IV | 2009 | IV T1-T3 N3 M0-M1 |
IVA T1-T2 N3 M0 T3 any N M0 |
IVB Any T any N M1 |
|
| Tumor invades adjacent structures or fixed and ulcerated lymph nodes or distant metastases | Upper urethral and/or vaginal mucosa, bladder mucosa, rectal mucosa, or is fixed to the pelvic bone. Fixed or ulcerated inguinofemoral lymph nodes | Any distant metastases, Including pelvic lymph nodes |
|||
| Stage IV | 2021 | IV T3 (fixed to the bone) or N+ (ulcerated/fixed) or M1 |
IVA Any T N+ (fixed/ulcerated) M0 T3 (fixed to the bone) any N M0 |
IVB Any T any N M1 |
|
| Tumor of any size fixed to the bone, or fixed and ulcerated lymph node metastases, or distant metastases | Disease fixed to the pelvic bone,or fixed or ulcerated regional† lymph node metastases | Distant metastases |
*Stromal invasion measured by new method; from the basement membrane of the deepest adjacent dysplatic (tumor-free) rete ridge to the deepest point of invasion.
†Inguinal and femoral lymph nodes.
M, metastasis; N, node; T, tumor.
The 2021 FIGO staging allows incorporating findings from cross-sectional imaging into vulvar cancer staging.7 The 8th editions of the TNM staging systems of the American Joint Committee on Cancer (AJCC) and the International Union Against Cancer (UICC) for the vulva were published in 2017 and apply for primary carcinomas.8 Other tumors including melanoma, mesenchymal neoplasms, and metastases are not included. A next version of TNM for vulvar cancer (9th version) in line with the 2021 FIGO classification system for vulvar cancer is expected to be available in 2023. The development group recommends using the 8th TNM classification because it more accurately reflects the status of the primary tumor and the regional lymph node and is more in line with current treatment advice.
Recommendations
Currently there is limited alignment between the 8th edition of TNM and FIGO 2021 classifications, and lack of evidence to base treatment on the FIGO 2021 staging. Therefore, TNM classification is advised [V, B].
Throughout these recommendations, advanced stage of disease is defined as clinical ≥T3 and/or ≥N2 [V, B].
The method used to determine tumor status (T), lymph node status (N), and systemic (metastasis) status (M) should be documented [V, B].
Pathology
The panel experts consider that a widespread utilization of a structured pathology dataset such as the International Collaboration on Cancer Reporting histopathology reporting guide, developed with the support of the International Society of Gynecological Pathologists, can lead not only to improved patient management but is a prerequisite for research and for international benchmarking in healthcare.13 Of note, this dataset has been developed for the pathological reporting of resection specimens of primary carcinomas of the vulva.
Vulvectomy specimen dimensions should be recorded on the pathology report in order to give the clinicians an indication as to how radical a resection has been undertaken.14 Anatomical site of a vulvar cancer must also be clearly indicated since tumors located close to the midline can be associated with bilateral or contralateral lymph node involvement. Moreover, midline/clitoral involvement is more frequently observed in HPV-independent tumors.15 16
According to WHO, the categorization of squamous cell carcinoma of the vulva into HPV-associated and HPV-independent subtypes is mandatory and requires the use of p16 immunohistochemistry.17 P16 immunohistochemistry is available in most pathology laboratories and has shown a good correlation with HPV testing; however, only the so-called “block-type” p16 staining is supportive of an association with a high-risk HPV infection.18 19 Since most HPV-independent vulvar cancers harbor TP53 mutations, pathology laboratories have increasingly used p53 immunohistochemistry. Almost all HPV-associated lesions exhibit a “wild-type” pattern of p53 immunoreactivity, while many HPV-independent tumors and precursor lesions usually exhibit an abnormal “mutation-type” immunoreactivity, which may be strongly positive or completely negative.20 21 In addition, there is emerging evidence that HPV-associated and TP53 wild-type tumors may show a better prognosis than those harboring TP53 mutations.22
Measurement of tumor dimensions and depth of invasion is essential for staging purposes. The maximum depth of tumor invasion must be accurately measured since invasion >1 mm typically results in surgical lymph node staging. Conventional measurement is taken from the most superficial dermal papilla to the deepest point of invasion. An alternative method of measuring the depth of invasion has been recently proposed: the depth of invasion is measured from the basement membrane of the adjacent dysplastic rete ridge to the deepest point of invasion.10 12 Despite this method having been shown to downstage some Stage IB tumors to IA, two retrospective studies showed an overall good prognosis in downstaged patients; however, further prospective studies are needed to validate this alternative method.
Lymphovascular space invasion (LVSI) represents an adverse prognostic factor in vulvar squamous cell carcinoma. However, its prognostic role in vulvar cancer is still poorly understood; moreover, there is substantial variability across studies in terms of diagnostic thresholds.23 Several retrospective studies also demonstrated the prognostic role of perineural invasion which is associated with shorter survival and increased risk of local recurrence based on multivariate analysis in two studies.24 25
Regarding margin status in vulvar cancer, recent studies showed no significant differences in recurrence between <8 mm and ≥8 mm tumor-free surgical margin.26 27 It is also likely that the risk of recurrence is higher for HPV-independent squamous cell carcinoma, especially when there are HPV-independent precursor lesions in the resection margins.28 29 In this regard, the pathology report must clearly indicate the presence of HPV-associated or HPV-independent precursor lesions and their relations with the surgical margins.30
Recommendations
The surgeon should secure the specimen in a way that allows accurate orientation by the pathologist. The anatomical site of a vulvar cancer should be clearly indicated. Lymph node basins and/or SLN should be sent as separate specimens [III, A].
-
The pathology reports must include [III, A]:
Specimen dimensions
Tumor dimensions
Histological type (5th edition of the WHO classification 2020)
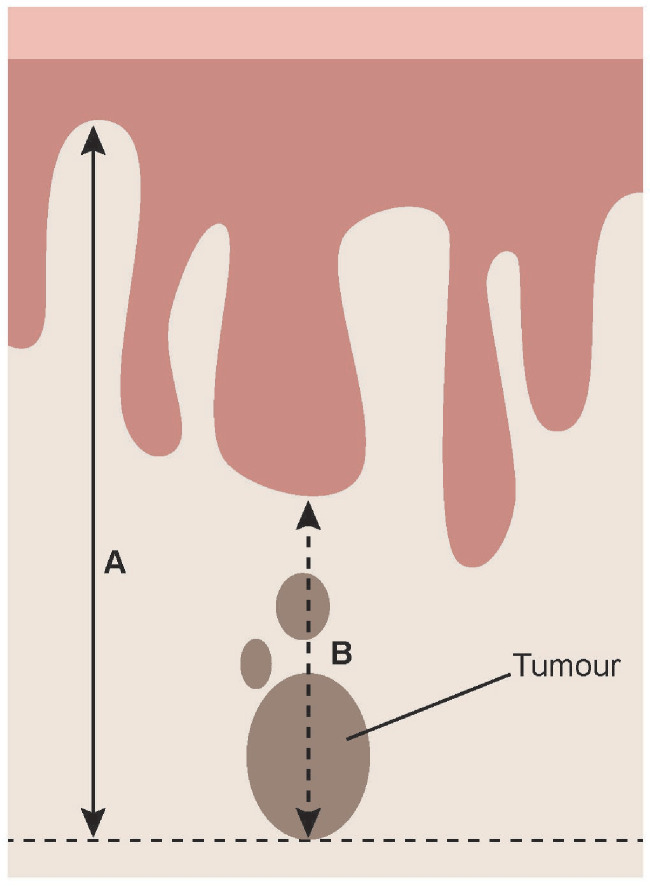
Depth of invasion (including at least A, and preferably B method) (see Figure 2)
Tumor margin status (distance to lateral and deep resection planes in millimeters)
Presence or absence of LVSI and perineural invasion
Presence or absence of pre-malignant disease, including presence in resection margins
Lymph nodes per site (total number, number of involved nodes, size of largest metastasis, extranodal extension)
Pathological staging (pTNM) for surgical specimen.
The origin/designation of all tissue blocks should be recorded (block code). This information should be documented in the pathology report and is particularly important in case of external review [IV, A].
Immunohistochemistry for p16 (surrogate marker for HPV infection) or molecular testing for HPV is mandatory to correctly classify HPV association. For HPV-independent carcinoma and for differentiated vulvar intraepithelial neoplasia, p53 immunohistochemistry is recommended [III, A].
Pathological evaluation of SLN should include at least three sections per millimeter. If the hematoxylin and eosin sections are negative, immunohistochemistry for cytokeratin should be performed [III, A].
Figure 2.
Schematic diagram showing measurement of depth of invasion in vulvar cancer. (A) Method of measurement from the adjacent most superficial dermal papilla to the deepest point of invasion. (B) Method of measurement from the basement membrane of the deepest adjacent dysplatic (tumor-free) rete ridge to the deepest point of invasion. Figure permission courtesy of Mr Norm Cyr.
Pre-operative investigations
The size, depth of the invasion, distance to the midline, histological type, and assessment of disease spread including nodal status determine the choice for primary treatment.11 12 In case of multifocal disease, the largest lesion, the lesion with the greatest depth of invasion, or the lesion closest to the midline should be evaluated as the dominant lesion to guide treatment planning.31 In HPV-independent tumors, vulvar cancer often presents as a single mass or ulcer on the labia majora or minora, close to the midline. In HPV-associated tumors, multifocal lesions and concomitant cervical neoplasia are more common. Information on involvement of the urethra, vagina, and/or anus is important for treatment planning and informing the patient. In addition to examination of the vulva, emphasis should be placed on evaluation of the vagina, cervix, and the anus due to the multifocal nature of lower anogenital squamous cell intraepithelial lesions.17 Even with normal findings, cytology and HPV test from cervix/vagina are recommended. If intraepithelial changes or invasive tumor continue into the anus, anorectoscopy is recommended and, depending on the findings, consultation with a colorectal surgeon.
For locally advanced tumors that clearly involve the median structures (urethra, vagina, or rectum) or in case of equivocal involvement regardless of primary tumor size, imaging techniques should be used to assess the extent of infiltration of deep pelvic structures (septum, urethra, bladder, vagina, anal canal, and rectum). Both ultrasound and pelvic MRI allow high soft-tissue resolution in the pelvis.32 In accordance with the European Society of Urogenital Radiology (ESUR) guidelines for vulvar cancer staging, the proposed standardized MRI protocol includes a pelvic MRI, including high-resolution T2 and T1 turbo spin echo imaging, diffusion-weighted imaging (DWI) and dynamic contrast-enhanced imaging (DCE-MRI) of the pelvis and groin for locoregional staging, and single-shot fast spin echo T2-weighted imaging and DWI of the upper abdomen to assess para-aortic LN.32 The structured reporting helps in communicating clinically relevant information to the referring physician.
Careful assessment of the regional lymph nodes by imaging, in addition to physical examination, is always required, except in T1a tumors, to determine treatment strategy and decide on surgical approach: (a) if the lymph nodes are clinically negative to investigate the presence of non-palpable metastasis and (b) if the inguinofemoral lymph nodes are clinically palpable (suspicious or bulky) to confirm the suspicion and to provide information on the distance of the metastasis from the skin and blood vessels.33–36 Ultrasound examination alone is the method of choice for pre-operative assessment of regional inguinofemoral lymph nodes if performed by a trained examiner.37–39 High-resolution ultrasound probes combined with color Doppler can show detailed changes in lymph node morphology and vascular architecture. Apart from excellent ultrasound accuracy in lymph node status determination, it is a largely available, low-cost procedure which can be integrated into the first outpatient visit.39 In addition, ultrasound-guided biopsy can be completed in a single visit.40 In 2021, the Vulvar International Tumor Analysis (VITA) group published an international consensus on ultrasound standardized terms, definitions, and methodology for evaluating inguinofemoral lymph nodes for vulvar cancer staging.37 Alternative investigations include [18F]-fluorodeoxyglucose-positron emission tomography-computed tomography (18F-FDG-PET-CT) and MRI.32 CT has a low diagnostic performance in the diagnosis of inguinofemoral lymph node metastases and is therefore not recommended for localized vulvar cancer without clinical evidence of inguinofemoral lymph node metastases.34 35 In case of isolated suspicious findings in the groin on MRI, CT, and 18F-FDG-PET-CT, subsequent correlation with ultrasound findings±ultrasound-guided biopsy is recommended in case this would alter primary treatment.
When metastatic involvement of inguinofemoral lymph nodes and/or advanced disease (≥T3) are suspected, whole-body CT with intravenous contrast and coverage of the inguinofemoral region or 18F-FDG-PET-CT should be performed to exclude pelvic lymph node involvement and the presence of other distant metastases.32 41 42 For equivocal distant metastasis, biopsy should be performed whenever possible, with preference given to tru-cut or core needle biopsy to obtain sufficient material for histological analysis, although fine-needle aspiration biopsy can be considered appropriate for small suspicious metastatic lesion (ie, pelvic, para-aortic, mediastinal lymph nodes, lung metastasis, etc.).43–45
As more than one-third of vulvar cancer cases affect elderly women (35% over 75 years), it is important to carefully assess the suitability of these patients for cancer-specific treatment, taking into account the overall life expectancy and specific goals with respect to the cancer diagnosis, before initiating extensive pre-treatment evaluation. Collaboration between a geriatric-trained clinician and oncologist in the care of an older patient with cancer is advised. Geriatric screening tools are used to identify older patients with cancer who would benefit from a comprehensive geriatric assessment.
Recommendations
Pre-operative work-up includes a medical history; general assessment and inventory of co-morbidities; frailty assessment; clinical examination; biopsy of all suspicious areas followed by pathologic review; and imaging as indicated [V, B].
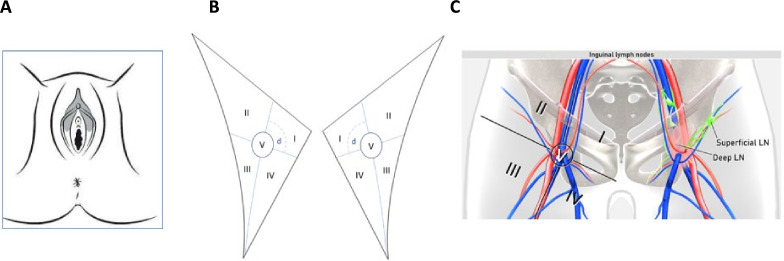
Clinical examination should document tumor site (labia majora/minora/Bartholin gland, clitoris, mons pubis, or perineum) and laterality (if relevant); tumor focality; the size of each lesion separately; the closest distance to midline and infiltration of and distance to the urethra/vagina/anus; and tumor mobility. Photograph or clinical drawing is recommended (see Figure 3) [V, B].
In advanced stage, bimanual vaginal and rectal examination should be considered [V, B].
Palpation of the inguinofemoral lymph nodes should be included to assess laterality, site, size, mobility, consistency, skin over the nodes [V, B].
Evaluation of cervix/vagina/anus including cytology and HPV test from cervix/vagina are recommended [IV, B].
For pT1a tumors (tumor ≤2 cm confined to the vulva and/or perineum, with stromal invasion ≤1 mm), no further imaging is required [III, B].
In patients considered for SLN procedure, imaging of inguinofemoral lymph nodes by ultrasound is recommended [III, B].
In all other cases, systemic staging (including pelvic lymph nodes and distant organs) by CT (chest/abdomen/pelvis) or 18F-FDG-PET-CT is recommended [III, B].
Suspicious inguinofemoral nodes (on imaging) should be assessed by ultrasound-guided fine-needle aspiration or core needle biopsy if this would alter primary treatment [III, A].
If the invasive tumor clinically involves surrounding tissues (≥T2 tumors) or if the finding is equivocal, evaluation of extra-vulvar structures (septa, urethra, bladder, vagina, cervix, and anal canal) with MRI is recommended [IV, B]. In specialized centers with an available trained ultrasound examiner, transvaginal/transrectal/perineal ultrasound can be an option in determining local staging [V, C].
Use of a structured report and a standardized imaging protocol is recommended [IV, B].
Equivocal distant metastasis should be biopsied (if possible) to avoid inappropriate treatment [V, B].
Figure 3.
Schematic drawing of the anatomy of the vulvar and inguinofemoral lymph nodes. Illustration of vulva and adjacent perineal structures for clinical drawing (A), schematic drawings of deep and superficial inguinofemoral lymph nodes, including Daseler regions, for evaluation of regional lymph nodes during clinical examination or by imaging (B). The clinical examination documents the site of the tumor (labia majora/minora/Bartholin gland, clitoris, mons pubis, or perineum) and laterality (if relevant), tumor focality, the size of each lesion separately, the closest distance to midline and infiltration of the urethra/vagina/anus, tumor mobility, and palpation of inguinofemoral lymph nodes (assessment of size, site, laterality, mobility/fixation, consistency, skin over the nodes/ulceration). The nodal status is documented either by ultrasound according to a standardized report published in 2021 by the Vulvar International Tumor Analysis (VITA) collaborative group or by MRI according to the 2021 European Society of Urogenital Radiology (ESUR) guidelines.32 37 Both modalities are documenting the size of lymph node metastasis/-es, number of lymph nodes involved, and the presence or absence of extracapsular spread. For describing the location of superficial inguinofemoral lymph nodes, virtual line drawn along femoral vein and second virtual line drawn perpendicular to first line and passing through saphenofemoral junction divide femoral triangle into: superomedial region (I); superolateral region (II); inferolateral region (III); and inferomedial region (IV). Central zone (V) is circled. Deep inguinofemoral nodes are located medial to femoral vein and cranial to lower margin of oval fossa (C). Pre-biopsy photograph is encouraged, particularly if the diagnostic phase and treatment phases are conducted in separate centers. LN, lymph node.
Surgical management
The vulvar tumor should be removed with a radical local excision. For many years, the primary aim has been to obtain tumor-free margins of at least 8 mm. Large recent studies could not confirm the relation between tumor-free margin distance and incidence of local recurrences. The evidence for the 8 mm margin is very low. The discussion on the optimal tumor-free margin in order to reduce the risk of local recurrences is still ongoing.26 29 46–53 The working group advises aiming for tumor-free margins. A pathological minimal margin of >2–3 mm seems sufficient, but the optimal margin remains to be decided. In order to achieve this, a sufficient surgical excision margin is advised; however, in case of midline tumors close to the clitorus/urethra/anus, this can compromise the margin distance.
Tumors with depth of invasion ≤1 mm do not need groin treatment. One should be aware of the fact that FIGO 2021 included a new way of measuring depth of invasion, by which a portion of the former FIGO stage IB patients will now be classified as FIGO stage IA.7
A SLN procedure is indicated in all patients with a primary unifocal tumor <4 cm with a depth of invasion >1 mm and no suspicious nodes. A bilateral inguinofemoral lymphadenectomy is indicated in patients with tumor ≥4 cm and in multifocal disease. The recommendations on inguinofemoral lymphadenectomy are unchanged since previous guidelines were published. Several studies have been published on the videoscopic approach for inguinofemoral lymphadenectomy.54–61 Studies show reduction in complications. However, no randomized or sufficiently powered prospective studies with sufficient follow-up have been performed to guarantee oncological safety. For now, this technique should only be performed within clinical trials.
Treatment of advanced-stage vulvar cancer often involves multiple treatment modalities. Primary chemoradiotherapy can prevent the need for exenterative surgery, but in some cases surgery may be the treatment of choice. Treatment planning is individualized in advanced-stage disease and depends on primary tumor characteristics and presence of regional and/or distant metastases. Also, co-morbidity and/or frailty of the patient influences treatment planning. Therefore, a multidisciplinary setting is needed to optimize treatment planning.
Several studies have been published on different reconstructive techniques for closure of large vulvar wounds.62–74 The working group concluded that no preferred technique can be recommended, but it is important to consider reconstruction in cases where wound closure will be challenging but also in those cases where reconstruction will give better cosmetic and/or functional outcome (for example, to preserve sexual functioning). Therefore, availability of reconstructive surgical skills as part of the multidisciplinary team is required in early as well as advanced-stage disease.
Recommendations
Local treatment
Radical local excision is recommended with the aim to obtain histological tumor-free margins [III, B].
Extending primary excision in a superficial fashion to include adjacent differentiated vulvar intraepithelial neoplasia is highly recommended [IV, B].
In multifocal invasive disease, radical excision of each lesion as a separate entity may be considered. Vulvectomy may be required in cases with multifocal invasion arising on a background of extensive vulvar dermatosis [IV, C].
The optimal radicality of the excision remains to be defined. It is acceptable and often desirable to limit radicality in order to preserve structure and function (eg, preservation of midline structures such as clitoris, anus, and urethra) [IV, C].
When invasive disease extends to the excision margins of the primary tumor, re-excision is the treatment of choice if feasible [III, A].
Advanced-stage patients should be evaluated in a multidisciplinary setting to determine the optimal choice and order of treatment modalities [V, B].
Groin treatment
Groin treatment should be performed for tumors >T1a (method of measurement of depth of invasion according to the 8th version of the TNM classification) [IV, B]. Surgical bilateral evaluation should be performed for non-lateralized tumors (medial border <1 cm from midline) [III, B].
For unifocal tumors <4 cm without suspicious inguinofemoral lymph nodes on clinical examination and imaging the SLN procedure is recommended [III, B].
For tumors ≥4 cm and/or in case of multifocal invasive disease, inguinofemoral lymphadenectomy by separate incisions is mandatory. In lateralized tumors at least ipsilateral inguinofemoral lymphadenectomy should be performed [III, A]. Contralateral inguinofemoral lymphadenectomy may be performed when ipsilateral lymphadenectomy has demonstrated metastatic disease [IV, C].
When lymphadenectomy is indicated, superficial and deep femoral nodes should be removed [IV, B].
Preservation of the saphenous vein is recommended [IV, C].
The optimal management of the groin for enlarged, proven metastatic nodes (inguinofemoral lymphadenectomy or isolated removal/debulking only) remains to be defined and treatment needs to be individualized [IV, C].
Reconstructive surgery
Availability of reconstructive surgical skills as part of the multidisciplinary team is required in early as well as advanced-stage disease. The type of reconstruction is based on patient/tumor characteristics and experience of the surgical team [IV, B].
SLN procedure
SLN dissection for early vulvar cancer appears safe, accurate, and cost effective.75–78 The prerequisites for SLN dissection are unchanged since the previous guidelines and would support the use of SLN dissection as an alternative to inguinofemoral lymphadenectomy for small (<4 cm), unifocal tumors without clinical suspicion of lymph node metastasis. As previously established, combination detection techniques are the most accurate in early vulvar cancer. At present, the evidence would support the combination of blue dye and Tc99m nanocolloid.75 77 Of note, this was the recommended detection method for the large GROningen International Study on Sentinel nodes in Vulvar Cancer V (GROINSS-V) study.78
There is increasing evidence to support the use of indocyanine green (ICG) as an alternative to blue dye. In their multicenter, randomized study, Deken et al demonstrated comparable efficacy in terms of sentinel node detection between isotope/ICG compared with conventional combined detection with isotope/blue dye,79 A systematic review of the literature identified 13 studies between 2011 and 2020 suggested similar detection rates for SLN to the gold standard technetium.80 The authors highlight the potential fall in detection rates in the presence of metastatic disease and highlight increased method failure in cases of obese patients and midline tumors. A more recent meta-analysis by Di Donna et al provides further evidence to support the utility of ICG in SLN dissection for early vulvar cancer.81 The use of ICG appears to be associated with a learning curve.82 Protocols for ICG use remain heterogeneous and the optimum protocol remains to be defined. At present, a combination technique including isotope and either blue dye or ICG would seem to provide the highest detection rates and proven clinical efficacy. Pre-operative lymphoscintigraphy is recommended to provide information on the location and number of sentinel nodes.
At present, evidence for the use of the SLN procedure in the case of recurrent cancer is lacking. A small study suggests the technique is feasible, but that detection rates are lower and lymphatic drainage may be unusual following previous surgery.83 This area is in need of further investigation but a comment is included in the guidelines to aid clinical practice.
The finding of metastatic disease in a SLN should prompt additional treatment to the involved inguinofemoral area. Although intra-operative frozen section may be considered, typically in an effort to avoid a second surgical procedure, caution is advised due to the potential risk in missing micrometastases on final histology and the need for accurate measurement of metastastic deposits. A retrospective institutional study published since the guideline review provides some reassurance in this regard.84
The large (n=1535) prospective, multicenter GROINSS-V II study aimed to establish the safety of replacing inguinofemoral lymphadenectomy with radiotherapy for patients with early vulvar cancer who were found to have metastasis in a SLN. Analysis of isolated groin recurrence in the first 91 patients identified the presence of macrometastatic disease (>2 mm) in nine of ten patients. The protocol was amended to allow only patients with micrometastatic (≤2 mm) disease in the sentinel node to receive inguinofemoral node radiotherapy without further surgery. Patients with macrometastatic disease were managed with inguinofemoral lymphadenectomy as standard of care with additional radiotherapy for those patients with more than one node metastasis or in whom there was extracapsular spread. The authors report a median follow-up of 24.3 months. Recurrence rates for those patients with SLN micrometastasis (≤2 mm) who received radiotherapy alone without lymphadenectomy were low (1.6%, 95% CI 0% to 3.9%) with acceptable levels of treatment-associated toxicity.85
Retrospective studies provide conflicting evidence on the safety of omitting treatment to the unaffected groin in patients with unilateral positivity at bilateral SLN dissection. Woelber et al, Nica et al, and Ignatov et al observed contralateral non-sentinel-positive node rates of 0% (0/28), 5.3% (1/19), and 0% (0/62), respectively.86–88
In contrast, data from a single institution identified contralateral positivity in 22% (4/18).89 A more recent larger (n=244), prospective study from the GROINSS-V trial group provides evidence to support the omission of further treatment to the unaffected groin, providing bilateral drainage has been identified for true midline tumors. The authors found the incidence of a non-sentinel, contralateral metastasis to be 2.9% (7/244; 95% CI 1.4% to 5.8%), comparable to the risk of groin recurrence after a negative SNL dissection.90 Caution is advised with tumors of >3 cm, the authors highlighting that the majority of non-sentinel contralateral metastasis occurred in these larger tumors.
Recommendations
The SLN procedure is recommended in patients with unifocal cancers of <4 cm, >T1a, without suspicious inguinofemoral nodes [II, B].
There are insufficient data to confirm the efficacy and safety of the SLN procedure in the case of recurrent disease [IV, C].
Use of radioactive tracer (Tc99/nanocolloid) is mandatory [II, A].
Combination detection techniques with isotope and either blue dye or ICG are recommended [II, B]. When used as part of combination technique, ICG appears more effective than blue dye in the detection of the SLN although the imaging protocol is still to be defined [II, B].
Lymphoscintigraphy is advised to enable the pre-operative identification, location, and number of SLN [III, C].
Intra-operative frozen section is optional, balancing the importance of accurate measurement of size of lymph node metastasis and increased risk of missing micrometastases on final pathology against the impact of a second surgical procedure [IV, C].
When a SLN is not found (method failure), inguinofemoral lymphadenectomy should be performed [I, A].
For tumors involving the midline, bilateral SLN detection is mandatory. When only unilateral SLN detection is achieved, contralateral inguinofemoral lymphadenectomy should be performed [I, A].
When tumor cells, both metastases and isolated tumor cells, are identified in the SLN, additional treatment to the involved inguinofemoral area is indicated [I, A].
When macrometastatic (>2 mm) disease is identified in the SLN, inguinofemoral lymphadenectomy of the affected site should be performed [I, A].
Inguinofemoral lymphadenectomy can safely be omitted in favor of radiotherapy when micrometastatic disease (≤2 mm) or isolated tumor cells are identified in the metastatic SLN [III, B].
For patients undergoing a bilateral SLN procedure, who are found to have unilateral metastasis, the incidence of contralateral metastasis is low and further treatment may be limited to the affected groin [III, B].
(Chemo)radiotherapy
Adjuvant radiotherapy/chemoradiotherapy
Adjuvant treatment after surgery for vulvar cancer is controversial.91 92 Following surgery, up to 40–50% of patients develop a local recurrence, although many are second primaries.93 The main goal of adjuvant treatment is to reduce the risk of local, and especially inguinofemoral and pelvic, recurrences, which are often fatal. In vulvar cancer, positive margins and lymph node involvement are the two most important factors for recommending adjuvant therapy.94 Post-operative radiotherapy to the vulva is recommended for all women with a positive margin where re-excision is not possible. Radiotherapy may also be considered in the setting of risk factors for local recurrence: close margins, lymphovascular or perineural invasion, large tumor size, and/or depth of invasion >5 mm.93 95–97
A recent large retrospective study (AGO-CaRE −1 study) on 360 patients with vulvar cancer pN+, with median follow-up of 17.2 months, showed that adjuvant radiation to the primary site in addition to the groins/pelvis lymph nodes results in less vulva-only recurrences (15.8%) as compared with 22.8% in patients with adjuvant radiotherapy to groins/pelvis and 25.5% with no adjuvant radiotherapy.98 The risk-reducing effect of local radiotherapy was independent of the resection margin status. Additionally, there was greater impact with adjuvant radiotherapy for HPV-related tumors than for HPV-independent tumors with median disease-free survival of 20.7 versus 17.8 months, respectively.98
Patients with SLN metastasis ≤2 mm can be treated with post-operative radiotherapy (2-year isolated groin recurrence rate of 1.6% in GROINSS-V II), as a safe alternative to inguinofemoral lymphadenectomy.85 In this study, radiotherapy had to be initiated within 6 weeks post-surgery. Radiotherapy was given to a total dose of 50 Gy in 25–28 fractions of 1.8–2 Gy, 5 fractions/week.
Patients with early-stage vulvar cancer with SLN metastasis >2 mm following SLN biopsy should undergo inguinofemoral lymphadenectomy followed by post-operative radiotherapy in case of one or more additional lymph node metastasis and/or extracapsular tumor spread; the 2-year isolated groin recurrence rate was unacceptably high (22%) with radiotherapy alone using 50 Gy in the GROINSS-V II study.85 96 Retrospective studies suggest that the addition of concurrent chemotherapy to radiotherapy may improve survival.99 100 Toxicity of radiotherapy versus chemoradiotherapy in this situation needs to be carefully considered on an individual patient basis.
A recent large database retrospective study on survival of 2779 node-positive patients (1436 1N+, 1208 with ≥2 N+) showed better survival for chemoradiotherapy compared with radiotherapy alone in both patients with one positive node and those with two or more positive nodes.100 Five-year overall survival was highest among patients with one positive node who received chemoradiotherapy (68.1%), compared with 55.9% for adjuvant external beam radiation therapy only and 46.1% for no adjuvant treatment. Survival was likewise highest among the patients with two or more positive nodes who received chemoradiotherapy (49.1%), compared with 29.4% for adjuvant external beam radiation therapy and 21.2% for no adjuvant treatment.100 However, in this analysis women with a single positive node derived a survival advantage from radiotherapy but no incremental advantage from the addition of chemotherapy to radiotherapy. The median dose in these studies was 50 Gy, which may not be an adequate microscopic dose for nodal involvement.
The ongoing GROINSS-V III study is investigating concurrent chemotherapy and radiotherapy dose escalation at the involved inguinofemoral site in case of macrometastasis in the SLN.
The optimal overall treatment time (OTT) from radical surgery to the end of adjuvant radiotherapy for squamous cell carcinomas has been found to impact treatment outcomes. In a large National Cancer Database series including 1500 patients treated with adjuvant radiotherapy, median overall survival with OTT ≤104 days was 56.1 months versus 45.4 months if ≥105 days (p=0.015).101 On multivariable Cox analysis, OTT was independently associated with a significant increased risk of death of 0.4% per additional day, as were age at diagnosis, number of metastatic nodes, the use of concurrent chemotherapy, and increasing pT/pN stage. After propensity adjustment for factors predicting a shorter OTT, OTT continued to be associated with a significant increased risk of death per additional day. Therefore, it is recommended that the interval between surgery and start of radiotherapy is maximum 8–10 weeks, the total OTT of radiotherapy (with or without chemotherapy) does not exceed 8 weeks, and that the total time to completion of adjuvant radiation following surgery should not exceed 105 days from the surgery date.101
Adjuvant radiotherapy for metastatic inguinofemoral lymphadenopathy should include the ipsilateral inguinofemoral region; and where pelvic nodes are non-suspicious on imaging, the distal part of the iliac nodes up to the iliac bifurcation. Contralateral inguinofemoral radiotherapy could be considered if the contralateral groin was not dissected and the tumor was located midline. When pelvic lymph nodes are positive, bilateral inguinofemoral and pelvic nodal region to one level above the most cranial involved lymph node should be included. When bilateral inguinofemoral lymph nodes are positive after lymphadenectomy, bilateral inguinofemoral and pelvic lymph nodes should be included.
Adjuvant radiotherapy should be performed by modern intensity-modulated radiotherapy techniques (IMRT/VMAT-like) with daily setup verification especially if a simultaneous integrated boost is used.
Elective radiation dose to the inguinofemoral and iliac regions could be a range of 45–50.4 Gy in 1.8 (–2) Gy fractions. If nodal involvement with macrometastases is present, or extracapsular extention, a inguinofemoral boost to the involved nodal area to 54–56 Gy EQD2 is recommended if no residual macroscopic disease is present (for example, with simultanous integrated boost with fraction sizes 1.8 (elective) and 2.05 or 2.1 Gy (boost) or a sequential boost). If macrometastases are found which have not been removed, simultaneous integrated boost or sequential boost to a dose of 64–66 Gy EQD2 is recommended. In the setting of enlarged pelvic nodes, a simultaneous integrated or sequential boost should be delivered with IMRT/VMAT-like techniques to 57–60 Gy (EQD2). In case of positive resection margin of the primary vulvar cancer, a boost to 60–66 Gy (EQD2) should be considered using external beam radiation therapy or image-guided adaptive brachytherapy.
Primary chemoradiotherapy
The management of patients with locally advanced vulvar cancer presents a difficult therapeutic challenge. Historically, when the disease involves the anus, rectum, rectovaginal septum, proximal urethra, or bladder, primary exenterative surgery necessitating colostomy and/or urinary diversion has been required. In an effort to decrease morbidity, the Gynecologic Oncology Group (GOG) explored the addition of neoadjuvant chemoradiotherapy to downstage the tumor and allow for less extensive surgical resection. GOG 101 and GOG 205 were two landmark clinical trials evaluating pre-operative chemoradiotherapy for locally advanced vulvar cancer.102 103 In GOG 205 patients received pre-operative chemoradiotherapy with concurrent weekly cisplatin at 40 mg/m² (up to a dose of 70 mg/m²) and radiation to a total dose of 57.6 Gy in 32 fractions to gross disease; split course was avoided.103 Definitive radical vulvectomy was performed about 6–8 weeks following completion of pre-operative chemoradiotherapy. With this higher dose, 64% of patients experienced a clinical complete response and 50% had a pathologic complete response. Of those who experienced a clinical complete response, 78% had a pathologic complete response. Treatment was generally tolerable, with the most common adverse effects being grade 3 or higher hematologic, radiation dermatitis/desquamation, pain, and gastrointestinal toxicities. A Cochrane systematic review on neoadjuvant chemoradiotherapy for advanced primary vulvar cancer selected five studies in which patients with advanced primary squamous cell carcinoma of the vulva treated with curative intent by concurrent radiotherapy and chemotherapy followed by surgery.104 Operability was achieved in 63–92% of cases in the four studies using 5-fluorouracil and cisplatin or 5-fluorouracil and mitomycin-c. In contrast, only 20% of the patients who received bleomycin were operable after chemoradiotherapy. Skin toxicity was observed in nearly all patients. Wound breakdown, infection, lymphedema, lymphorrhoea, and lymphoceles were also common. The conclusion of the authors was that neoadjuvant therapy is not justified in patients with tumors that can be adequately treated with radical vulvectomy and bilateral groin node dissection alone.
A large retrospective study based on the National Cancer Database included 1352 patients treated by radiation or chemoradiotherapy for unresectable tumor.105 Median radiation dose was 59.40 Gy. In the chemoradiotherapy cohort 62% received single-agent chemotherapy, 31% received multi-agent platinum-based regimen, and the number of agents was unknown for 7% of patients. The 5-year overall survival was significantly higher in the chemoradiotherapy group compared with radiotherapy, with significant benefit for chemoradiotherapy in stage II-IV disease.
With advancements in radiotherapy techniques and imaging for staging disease, single institutional series showed the feasibility of both dose-escalated pre-operative and definitive intensity-modulated radiotherapy-based chemoradiotherapy.106–108
In a recent series on 49 patients, the median vulva dose was 66 Gy for definitive and 59.4 Gy for pre-operative therapy. Ninety-four percent of all patients received chemotherapy, predominantly weekly cisplatin (40 mg/m²), for a median of five cycles. In this series, with dose-escalated intensity-modulated radiotherapy, clinical complete response and pathologic complete response were 76% and 70%, respectively.106 Whether a brachytherapy boost offers any advantage needs to be ascertained, although it may be an option for significant vaginal extension or deep perineal disease.97 109 In a recent prospective multi-institutional study, 52 patients with mainly T2/T3 disease were treated with 64.8 Gy total dose to tumor and 50.4 Gy to the elective nodes and capecitabine 825–1000 mg/m2 twice-daily during the 6 weeks (concomitantly during days 1–14, 22–35 and 43–49 of treatment).110 Surgery was avoided and only done in case of no complete remission after 8–12 weeks. Local control was 42% and regional control 58% at 2 years. Overall survival was 52% at 5 years.110 Acute grade 3 or more toxicity was 54% skin/mucosa and 37% pain. Late toxicity grade 3 or more occurred in 21% patients. In locally advanced vulvar cancer, definitive capecitabine-based or cisplatin-based chemotherapy should be considered as an alternative to extensive surgery, resulting in equivalent locoregional control with less long-term toxicity. Survival rates are acceptable and acute and late toxicities are manageable. Additionally, treatment breaks should be avoided, as a prolonged treatment time of >50 days was associated with higher recurrence rates.101 105
The treated volume should include the primary tumor, the vulva and bilateral inguinofemoral area, and pelvic nodes depending on extent of primary disease and nodal involvement.When pelvic lymph nodes are involved, bilateral inguinofemoral and pelvic nodes to at least one level above the most cranial involved lymph node should be included. In case of enlarged pelvic nodes, a boost through IMRT/VMAT-like techniques should be performed through simultaneously integrated or sequential boost to a EQD2 dose of 57–60 Gy, preferably using MRI-based planning.
The recommended dose to the primary tumor is 64–70 Gy EQD2 in 1.8–2 Gy per fraction, and MRI-based contouring and planning is highly recommended. Image-guided brachytherapy as a boost modality could be considered in specialized centers.
The optimal dose to involved inguinofemoral lymph nodes is controversial, but should be 60–66 Gy EQD2 to macroscopic disease while the elective nodal dose is 46–50.4 Gy in fractions of 1.8–2 Gy.
Careful management of side effects and skin toxicity with regular review, expert skin care, and adequate analgesia are essential when treating women with advanced vulvar cancers.
Recommendations
Adjuvant radiotherapy/chemoradiotherapy
-
Post-operative radiotherapy to the vulva:
When invasive disease extends to the pathological excision margins of the primary tumor, and further surgical excision is not feasible, post-operative radiotherapy to the vulva is indicated [IV, B].
In case of close but clear pathological margins with extensive LVSI, perineural involvement or lymph node involvement, post-operative vulvar radiotherapy may be considered on an individualized basis to reduce the frequency of local recurrences [IV, C].
-
Post-operative radiotherapy to the inguinofemoral region:
SLN metastasis ≤2 mm and isolated tumor cells can be treated with post-operative radiotherapy as a safe alternative to inguinofemoral lymphadenectomy with fewer long-term side effects [III, B].
-
After inguinofemoral lymphadenectomy:
Radiotherapy is recommended for cases with more than one metastatic lymph node and/or extracapsular spread [II, A].
Concurrent radiosensitizing chemotherapy should be considered [IV, B].
Target volume and dose for adjuvant (chemo)radiotherapy should be defined on individual basis according to tumor and patient characteristics [III, A].
Radiotherapy should be started as soon as possible (total time from surgery to completion of radiotherapy preferably less than 104 days).Treatment breaks should be avoided [IV, B].
Radiotherapy should be performed with intensity-modulated radiotherapy techniques [III, B].
Primary chemoradiotherapy
Primary chemoradiotherapy should be performed in a specialized gynecological radiotherapy center [V, B].
Primary chemoradiotherapy is the treatment of choice in patients with unresectable disease and should be considered for tumors which would otherwise need exenterative surgery with stoma formation [III, B].
Appropriate tumor and lymph node imaging (MRI and/or 18F-FDG-PET-CT) should be performed prior to commencing chemoradiotherapy [IV, A].
Assessment of response should be performed at 12 weeks following completion of treatment (clinically, imaging and/or biopsy if residual tumor is suspected). In case of residual disease surgery should be considered [III, B].
Treatment breaks should be avoided, as a prolonged treatment time of >50 days is associated with higher recurrence rates for primary therapy [IV, B].
Systemic treatment
Neoadjuvant chemotherapy for locally advanced disease
A pooled analysis of 12 studies, including 97 patients with stage III or IV (non-metastatic) vulvar cancer who had received neoadjuvant or definitive chemotherapy or chemoradiotherapy, showed that neoadjuvant treatment followed by surgery was associated with an increase of 5-year overall survival rate versus definitive chemoradiotherapy.111 There was no difference between chemotherapy and chemoradiotherapy in the neoadjuvant setting. Overall response and 5-year overall survival rates of chemotherapy regimens (without radiotherapy) were: paclitaxel±cisplatin (n=13) 70% and 74%, cisplatin+5-fluorouracil (n=13) 62% and 58%, and bleomycin (n=8) 62% and 53%, respectively.111 However, this analysis is biased due to selection of patients, non-randomized nature, and small sample size; response and survival must be interpreted with caution.
Therefore, neoadjuvant platinum-based chemotherapy cannot be considered as a standard treatment in vulvar cancer, and randomized clinical trials would be needed to establish its role. However, after a multidisciplinary assessment, neoadjuvant platinum-based chemotherapy may be an option for selected patients who are not eligible/fit for upfront surgery or primary chemoradiotherapy. After 3–4 cycles of chemotherapy, re-staging and re-assessment regarding definitive treatment should be performed.
Systemic treatment for metastatic or recurrent disease
Treatment options for recurrent or metastatic vulvar cancer are limited, and there is no standard of care. For this reason, the enrolment of patients with metastatic vulvar cancer in clinical trials is strongly encouraged. Best supportive care should therefore be discussed with the patient as an alternative.
No standard systemic therapy regimens exist for treating advanced or recurrent/metastatic disease not amenable to curative radiotherapy or surgery. There are very few studies exploring systemic therapies in metastatic vulvar cancer, so data from cervical cancer are usually extrapolated. Regarding studies specifically developed in vulvar cancer patients, single-agent paclitaxel was explored in a phase II trial with 31 patients diagnosed with recurrent/metastatic vulvar cancer, showing an overall response rate of 14% and a progression-free survival of 2.6 months.112 In a retrospective series of 16 patients, cisplatin+vinorelbine obtained an overall response rate of 40%, a median progression-free survival of 10 months, and a median overall survival of 19 months.113 Although the evidence for the use of platinum combinations in vulvar cancer is limited, these can be considered due to the positive results in cervical cancer.114–116 Based on these, cisplatin or carboplatin+paclitaxel would be the regimen of choice.117
The cohort of vulvar cancer patients of the KEYNOTE-158 was published recently, enrolling 101 recurrent/metastatic vulvar cancer patients treated with pembrolizumab, and thus being the largest clinical trial performed in the metastatic setting of this disease. The overall response rate was 10.9% (9.5% among the 84 patients with PD-L1-positive tumors and 28.6% among the 7 patients with PD-L1-negative tumors) with a median duration of response of 20.4 months. Median progression-free survival and overall survival were 2.1 and 6.2 months, respectively.118 The CHECKMATE-358 trial assessed the efficacy of nivolumab in patients with virus-associated tumors.119 In a report on 24 patients with recurrent/metastatic cervical (n=19) or vaginal/vulvar cancers (n=5), and with <2 prior systemic therapies, the overall response rate and disease control rates were 26% and 68% for cervical cancer and 20% and 80% for vaginal/vulvar cancers, respectively. Responses occurred regardless of tumor PD-L1 status. At the time of data cut-off, the median duration of response was not reached in the cervical cohort and was 5 months in the single responding patient in the vaginal/vulvar cohort.
In the phase III EMPOWER-CERVICAL-1 trial, which included patients with recurrent and metastatic cervical cancer in ≥2nd line, the cemiplimab arm achieved a longer overall survival and a higher overall response rate compared with the chemotherapy arm with favorable toxicity.120
In the phase III trial KEYNOTE-826, performed in patients with recurrent or metastatic cervical cancer, the addition of pembrolizumab to first-line platinum-based chemotherapy, with or without bevacizumab, showed a significant benefit in progression-free survival and overall survival, especially in patients with PD-L1-positive tumors (combined positive score (CPS)≥1).121
Therefore, some patients with metastatic vulvar cancer can achieve a significant benefit with immunotherapy, but it has no specific approval by regulatory agencies in Europe.
Targeting angiogenesis is an attractive therapeutic strategy in HPV-related tumors. In advanced vulvar cancer there are only publications of some case reports with bevacizumab combined with chemotherapy, but in cervical cancer a phase III trial (GOG-240) demonstrated a longer overall survival when bevacizumab was added to chemotherapy.122 Therefore, the addition of bevacizumab to platinum-based chemotherapy may be considered in vulvar cancer. Epidermal growth factor receptor emerged as another possible target in vulvar squamous cell carcinoma. High expression of this protein had been identified as a negative prognostic factor, also correlated to the absence of HPV.123 The epidermal growth factor receptor tyrosine kinase inhibitor erlotinib was studied in a phase II trial with a cohort of 24 metastatic vulvar cancer patients. The disease control rate was 67.5% (overall response rate 27.5% and stable disease 40%), although responses had a short duration.124
Recommendations
Neoadjuvant chemotherapy for locally advanced disease
In selected patients, not eligible/fit for upfront surgery or chemoradiotherapy, neoadjuvant platinum-based combination chemotherapy may be considered after a multidisciplinary assessment [IV, C].
Systemic treatment for metastatic or recurrent unresectable disease
Platinum-based combination chemotherapy should be considered as first-line treatment for metastatic or recurrent unresectable disease [III, B].
Although the best combination partner for platinum is unclear, cisplatin or carboplatin and-paclitaxel could be considered the preferred regimen [IV, C].
Based on cervical cancer data, the addition of pembrolizumab in cases with PD-L1 expression with CPS≥1 and/or bevacizumab to platinum-based chemotherapy may be considered for selected patients in first line, although these drugs do not have specific approval for vulvar cancer [IV, C].
After progressing to platinum-based first-line chemotherapy, there are no standard treatments. Immune checkpoint inhibitors can be considered as monotherapy [III, B]. Chemotherapy or epidermal growth factor receptor targeting inhibitors may be considered as possible alternatives, taking into account that there is no specific approval for any drug [III, C].
Follow-up
The optimum follow-up schedule for vulval cancer remains undetermined. Follow-up visits provide an important opportunity to address the long-term physical and psychological impact of vulvar cancer and its treatment. Access to specialist support services such as lymphedema clinics and psychosexual counseling can improve quality of life for survivors of this disease. From an oncological perspective, follow-up is aimed at the evaluation of treatment effect as well as the prevention and early detection of subsequent tumors. The suggested schedule is intended as a general guideline and follow-up schedules should be individualized with these aspects in mind. The typical areas for recurrences are vulva/perineum, inguinofemoral region, multiple sites, and distant metastasis in decreasing order of frequency.125 Follow-up visits should include symptom review, and examination of the vulva, skin bridge, and inguinofemoral lymph nodes. Multicentric high-grade dysplasia in the lower genital tract occurs in ~10% of patients who present with vulval cancer.126 127 Where not available at presentation, cervical/vaginal screening with HPV testing/cytology should be considered at 6–12 months following primary treatment. Detection of local recurrence at ‘routine’ surveillance visits may lead to detection at a smaller size and facilitate treatment with a curative intent.128 However, evidence is conflicting as to whether routine clinical surveillance provides earlier detection that symptom-triggered review.129
For node-negative patients treated with SLN dissection, the risk of nodal recurrence appears to be within the first 2 years after treatment and salvage therapy can be effective in this group.130 The option of ultrasound surveillance of the groins may be considered for these patients but there is a lack of proven benefit/cost-effectiveness and regional variation in clinical availability of ultrasound.131 As such it is not mandatory in this setting.
The risk of local recurrence and new primary disease persists over time with a significant incidence of recurrence at 5–10 years following initial treatment. This suggests a possible role for long-term follow-up after primary treatment for vulval cancer. The evidence is lacking as to the clinical benefit or cost-efficiency of extended follow-up. However, in addition to detecting recurrence, many patients have long-term vulval dermatoses and active management of these conditions remains an important part of long-term management. There is the potential for individualization of follow-up regimes, taking into account the known risk factors for recurrence. These include age, pre-existing vulval lichen sclerosus or dysplasia, and nodal positivity at treatment.6 29 130 132 133
Recommendations
The optimal follow-up schedule for vulvar cancer is undetermined [V, C].
The follow-up strategy should be individualized in terms of intensity, duration, and procedures, taking into account individual risk assessment [V, B].
Counseling patients about signs of recurrence and adverse short-term, long-term, and late side effects of treatment remains an important part of survivorship care [V, B].
-
After treatment with curative intent, the following follow-up schedule is suggested [V, C]:
First follow-up 6–8 weeks after the end of treatment
First 2 years, every 3–4 months
Third to fifth year, biannual/annual
Long-term surveillance may be appropriate in individuals with ongoing predisposing vulvar disease or treatment-related side effects.
Follow-up visits should include, at a minimum, a symptom review and a complete physical examination of the vulva, skin bridge, and inguinofemoral lymph nodes [V, B].
Imaging and laboratory tests should be performed only based on risks of recurrence, symptoms, or findings suggestive of recurrence and/or side effects [V, B].
Treatment of recurrent disease
Recurrent disease includes local vulvar, inguinofemoral, or distant recurrences which can occur isolated or combined. About 12–37% of women with vulvar cancer develop a recurrence within the first years, mostly within 2 years.134 In general, women with p53 mutated tumors (without HPV association) and women with involved lymph nodes have the highest recurrence risk. CT, MRI, 18F-FDG-PET-CT, or PET-MRI thorax/abdomen/pelvis are recommended to examine any abnormalities in the (previously treated) vulvar, inguinofemoral, and pubic area and to detect possible additional metastases, which may influence treatment decisions. Imaging might also be helpful in determining the feasibility of surgery.135
Local recurrence
Most recurrences occur in the vulva. While groin/distant recurrencesare are rarely observed later than 2 years after primary diagnosis, the incidence of local recurrences seems to be stable even beyond the first years. Te Grootenhuis et al calculated a 4% risk for local recurrence per year without plateauing.130 133 136 137 It is unclear if late recurrences are a true recurrence, or rather new primaries based on pre-existing inflammatory vulvar skin disorders such as lichen sclerosus.
A local recurrence is usually treated with curative intention and, if possible, by surgery, aiming on radical excision of the tumor. In women with extensive treatment at primary diagnosis (large excision or combination with radiotherapy) it is often necessary to use plastic reconstruction with flaps which seems feasible and safe with acceptable complication rates. Previous radiotherapy increases the risk for peri-operative complications.62 138 139
In advanced local recurrences and if radio(chemo)therapeutic options have previously been utilized, exenterative surgery should be considered and has shown good results with manageable morbidity. Complete excision and negative lymph nodes seem to be important prognostic factors for further survival.140–143 Of note, there is no evidence regarding the optimal surgical margin in recurrent disease. Similar treatment principles as for primary disease may apply, and existing data suggest that not close but only involved margins should be an indication for further treatment. In case of (microscopically) involved margins re-excision is recommended. If further surgery is not possible, radiotherapy is recommended.27
In isolated local recurrences there is no proven benefit of surgical staging of clinically unsuspicious groins, but analogous to primary treatment, the detection and treatment of occult inguinofemoral lymph node metastasis may improve prognosis. In women with surgically naïve groins, SLN biopsy can be considered. Despite a lack of data on oncological safety, it seems comparable to the primary situation when the tumor has been removed before the SLN biopsy. For those with a local recurrence who previously underwent a SLN, a repeated SLN procedure was shown to be feasible in one small retrospective study, although with lower detection rates. Further studies evaluating the feasibility and oncological safety of a repeated SLN biopsy in locally recurrent disease are ongoing.144 145 In general, the recommendation is to perform an inguinofemoral lymphadenectomy. No further surgical groin staging is recommended in women without suspicious nodes on imaging who already underwent inguinofemoral lymphadenectomy (with or without radiotherapy) of the groins at primary treatment.
The prognosis after an isolated local recurrence is generally good, but impaired compared with women without recurrence, with a 5-year survival of about 60%, and with an increased risk for further recurrences.130 134 146 147 In case of occult lymph node metastasis diagnosed by surgical groin staging, recommendations for post-operative radiotherapy analogous to treatment in primary disease apply. In women previously treated by inguinofemoral radiotherapy, no adjuvant therapy is recommended if complete resection of the involved nodes has been performed. When residual disease is left behind, re-irradiation (with dose-adaption) with or without concurrent chemotherapy could be considered.
Inguinofemoral nodal recurrence
In 9–38% of cases, the recurrence is localized in the inguinofemoral LN. Especially at risk are women with primary lymph node metastases.134 148 Inguinofemoral nodal recurrences tend to occur earlier than local recurrences, almost all within 2 years.125 130 The prognosis is generally poor with a 5-year survival rate of only 0–20%, and possibly dependent on previous treatment.149 150 In a small retrospective study, eight of ten women who previously had been treated by sentinel node biopsy only were still alive 22 months after complete resection of the involved lymph nodes followed by chemoradiotherapy.151
Evidence about the best treatment is scarce, with data derived from small cohorts.149–151 Best results were achieved with a combination of surgery and chemoradiotherapy.151
Distant recurrence
Isolated distant recurrences are rare and occur mostly within 2 years.137 Limited treatment options exist, and survival is dismal.112 124 134 137 152 153 Isolated distant recurrence is rare, and surgery or stereotactic radiotherapy can be considered for oligometastatic disease.
Recommendations
General recommendations
All patients with a recurrence after primary vulvar cancer should be discussed by a multidisciplinary team and treated at a specialized center [V, B].
Before treatment of recurrent disease, vulvar examination, with biopsies from all suspicious areas, is recommended. Evaluation with ultrasound, MRI, and/or CT (or 18F-FDG-PET) of the thorax/abdomen/pelvis should be performed. When suspecting nodal or distant recurrence, a biopsy is recommended if feasible [V, B].
In case of incurable recurrent disease, early palliative care referral should be offered [V, B].
Treatment of local recurrence
For treatment of vulvar recurrence, radical local excision is recommended [IV, B].
Since many vulvar recurrences could be classified as new primary disease, arising from underlying pre-malignant skin conditions, surgical groin re-staging should be considered in clinically negative inguinofemoral lymph nodes [V, B].
In case of resection of the tumor with involved margins, re-excision (if feasible) or post-operative radiotherapy is recommended [IV, B].
In locally advanced disease, definitive (chemo)radiotherapy is recommended in radiotherapy-naïve patients. In selected cases, pelvic exenteration can be considered [IV, B].
Treatment of inguinofemoral and pelvic lymph node recurrence
Preferred treatment of an inguinofemoral nodal recurrence is inguinofemoral lymphadenectomy or debulking of suspicious inguinofemoral lymph nodes, followed by (chemo)radiotherapy in radiotherapy-naïve patients [IV, B].
In case of pelvic lymph node recurrence with or without inguinofemoral lymph node recurrence, (chemo)radiotherapy is recommended [V, B]. Debulking of enlarged pelvic lymph nodes may be considered prior to commencing the treatment [V, C].
In previously irradiated women, complete resection and/or stereotactic radiotherapy can be considered for oligometastatic inguinofemoral/pelvic disease [V, B]. Systemic therapy may be an option when local therapies are not feasible [V, C].
Based on evidence from other squamous cell cancers such as cervical and anal cancer, the addition of radio-sensitizing chemotherapy to radiotherapy can be considered [V, B].
Treatment of distant recurrence
For treatment of distant metastases, systemic therapy may be considered [V, C].
Stereotactic radiotherapy or surgery can be considered for oligometastatic disease [V, C].
Supportive care
The diagnosis and treatment of vulvar cancer can have significant physical and psychological impact on women, and it is essential that the potential consequences of treatment are discussed before and following treatment. These include psychosocial concerns, lymphedema, altered sexual function and body image, and, following radiotherapy, possible altered bowel and bladder function. A structured multidisciplinary program for functional rehabilitation and holistic care should be available either in the healthcare structure itself or through well-identified referral networks.
The diagnosis of cancer and the treatment can affect quality of life and the psychosocial needs of women need to be addressed throughout their pathway.154 Women should be informed of the predicted anatomical and physiological changes prior to treatment. Sexual health should be addressed as part of the follow-up program and access to sexual rehabilitation programs should be available in the healthcare structure. Pelvic radiotherapy can result in premature menopause in younger women, and hormone replacement therapy should be considered. Topical estrogens also can be considered for any women following treatment.
The risk of developing lymphedema depends on the extent of treatment, with a low risk with SLN assessment, but the incidence ranges from 16.7% to 49.2% following inguinofemoral lymph node dissection and is significantly worse in women who have both surgery and radiotherapy.155 156 The GROINSS-V II study showed that SLN only and SLN+radiotherapy have a lower incidence than inguinofemoral lymphadenectomy with or without radiotherapy.85 Women with lower limb lymphedema can experience significant negative impact on cancer distress, self-image, quality of life, and daily activities.157 Information on how to reduce the risk of developing lymphedema should be available, including advice on exercice, weight loss, and prompt treatment of skin infections or insect bites. Those women who develop lymphedema should be referred to specialist lymphedema services for management. Lymphovascular anastomosis may be an option for women with severe symptoms, but this is only available in some specialized centers.158
Following radiotherapy, long-term changes include telangiectasia, ulceration, fibrosis, and skin thickening. There can be changes to bowel or bladder function and referral to other services including gastroenterology or urology may be required if there are persisting symptoms impacting on quality of life.
Recommendations
Dedicated supportive services should be available in any specialized center for vulvar cancer treatment [V, B].
Women should be given information about potential consequences of treatment and have multidisciplinary holistic support available at all stages of care [V, B].
Access to specialist psychosexual and psychosocial counseling services is required [V, B].
Patients should receive information on decreasing risk of lymphedema following inguinofemoral lymphadenectomy, with access to specialized lymphedema services if required [IV, B].
Palliative care
Despite treatment, vulvar cancer recurs in about 33% of cases with a 5-year survival rate after primary treatment of around 70%. Prognosisis is most unfavourable in women with primary lymph node involvement.159 160 In case of distant or inoperable recurrence, therapeutic options are limited and quality of life is poor. Data about palliative and supportive care in vulvar cancer is very limited. However, considering that squamous cell carcinoma represents the most common histotype (90% of cases), one may extrapolate data from cervical cancer experience in this challenging field.161 For almost a decade, the American Society of Clinical Oncology, WHO, and the Society of Gynecologic Oncology have recommended integrating dedicated palliative care services into oncological care early in the disease course for patients with advanced cancer.162–165 This was as a response to the randomized trial published by Temel et al, which showed that early integration of palliative care for patients with metastatic lung cancer resulted in better symptom control and quality of life, less aggressive end-of-life care, and longer overall survival.166 Subsequent studies investigating the benefits of palliative care in the gynecological and general oncology populations demonstrated improvement in symptoms, quality of life, and clarity regarding goals of care at the end of life.167–170
Some retrospective studies on cervical cancer showed that even in tertiary care centers fewer than half of patients received palliative care consultation, and those referred to palliative care were often evaluated late in their disease course.171–175 Palliative care referral was associated with fewer emergency department visits, inpatient stays, and intensive care unit admissions in the last 30 days of life. Palliative care did not affect chemotherapy or radiation administration within 14 days of death. Women evaluated by palliative care providers were less likely to die in the acute care setting.171 176 177 Among women with advanced gynecological cancer, suffering is highly prevalent and often severe and multifaceted.172 178–180 They may experience various types of symptoms that are refractory to basic palliative care, and therefore a group of international experienced experts from countries of all income levels created an augmented package of palliative care for gynecological/cervical cancer with which even refractory suffering can be relieved. The package consists of medicines, radiotherapy, surgical procedures, and psycho-oncologic therapies that require advanced or specialized training. Each item in this package should be made accessible whenever the necessary resources and expertize are available.181 182
The role of radiotherapy continues to be important due to its rapid pain relief and temporary regression with cessation of bleeding in the majority of patients. Indications include palliative treatment of local vulvar disease, lymph node metastases, and symptomatic distant metastases. Hypofractionated small-volume external beam radiation therapy can be used for treating primary disease in patients not fit for radical treatment and/or for symptomatic metastases.183–186 Different doses and fractionations can be used, including 1×8 Gy, 5×4 Gy, quadshot regimen (3.3–3.7 Gy twice-daily × 4 fractions repeated at 2–4-week intervals for a total of 12 fractions). The role of systemic agents in a palliative setting in the treatment of vulvar cancer is limited. When available, treatment in clinical studies is recommended.112
Electrochemotherapy (where available) may have a role in the palliative management of vulvar cancer, especially when other therapies are no longer applicable, and may result in improved outcome and better quality of life. Electrochemotherapy is an emerging treatment that is a feasible, easy to perform, and reproducible procedure in patients with primary or recurrent vulvar cancer who are unable to undergo surgery. Survival after 1 year in this population was 50% and resulted in improved quality of life.187–191 Subgroup analyses showed worse quality of life in patients with stable or progressive disease, posterior site, and multiple or larger than 3 cm nodules.
Recommendations
Early palliative care referral is strongly recommended as an important step towards improved symptom control and end-of-life care [III, A].
Radiotherapy is indicated for palliation of symptoms related to pelvic disease including bleeding, ulceration, pain, and/or systemic disease [IV, B].
Hypofractionated small-volume external beam radiation therapy can be used for treating primary disease in patients not fit for radical treatment or in pre-irradiated, inoperable patients [IV, B].
Palliative surgery can be considered in selected cases [IV, B].
Algorithms
Work-up
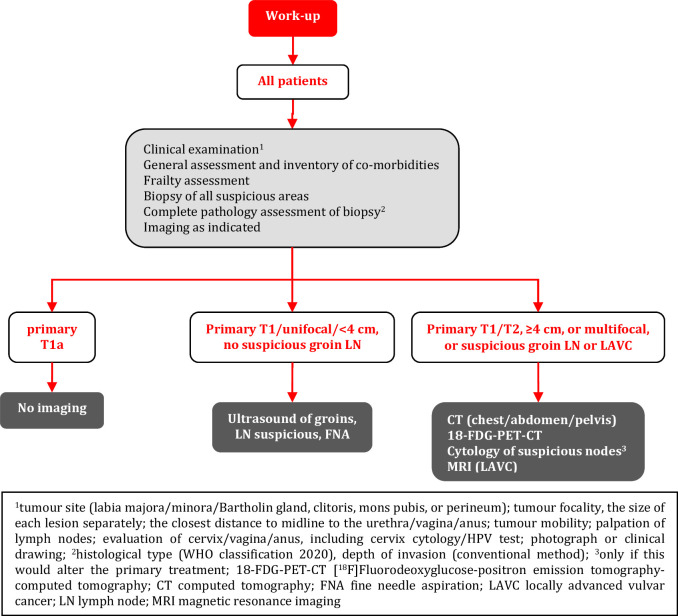
Primary treatment of early-stage vulvar cancer
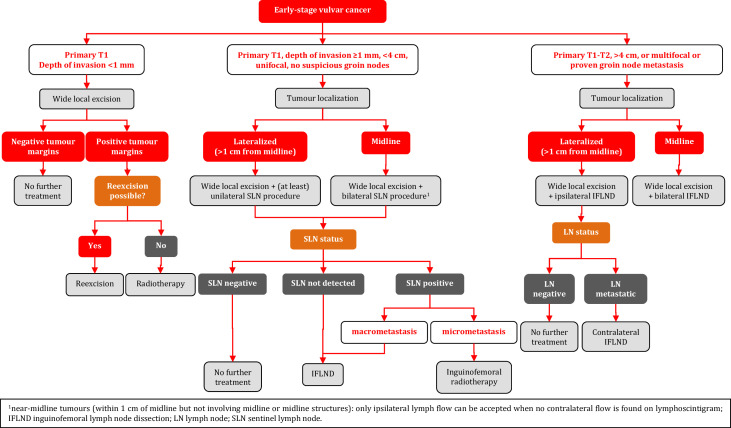
Adjuvant treatment of early-stage vulvar cancer
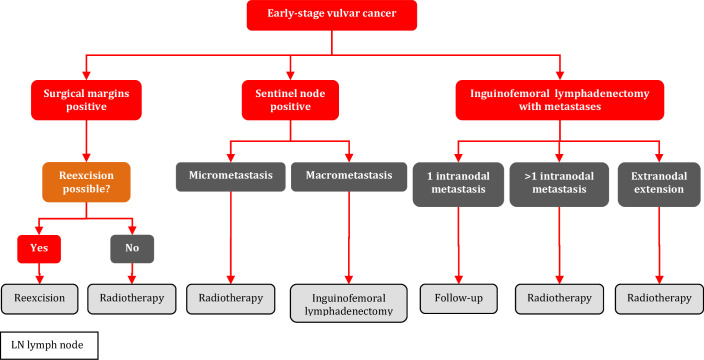
Treatment of locally advanced vulvar cancer
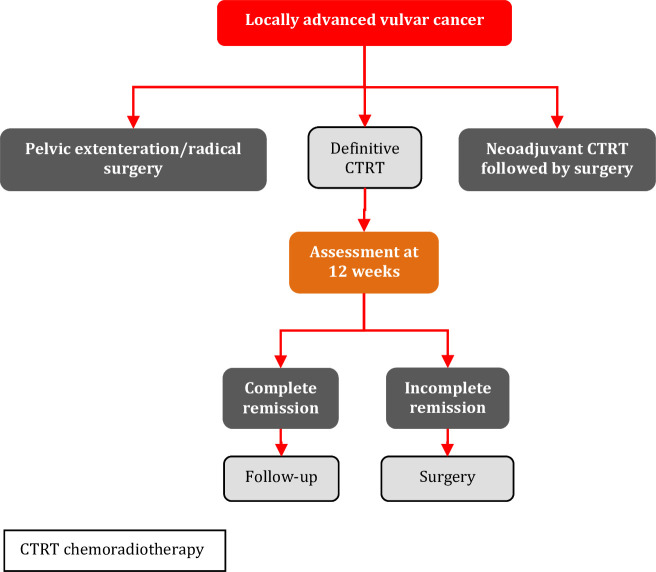
Acknowledgments
The authors thank ESGO for their support. The authors also thank the 206 international reviewers (physicians and patient representatives) for their valuable comments and suggestions. The ESGO office, especially Kamila Macku, Tereza Cicakova, and Kateřina Šibravová, provided invaluable logistical and administrative support throughout the process.
Footnotes
Contributors: The development group (including all the authors) is collectively responsible for the decision to submit for publication. MHMO (chair) and François Planchamp (methodologist) wrote the first draft of the manuscript. All the other contributors have actively given personal input, reviewed the manuscript, and have given final approval before submission. MHMO is responsible for the overall content as guarantor.
Funding: All costs relating to the development process were covered from ESGO funds. There was no external funding of the development process or manuscript production.
Competing interests: SM has reported advisory boards for AbbVie, AstraZeneca, Clovis, Eisai, GlaxoSmithKline, Hubro, MEdac, MSD, Novartis, Nykode, Novartis, Olympus, PharmaMar, Pfizer, Roche, Sensor Kinesis, Teva, Tesaro, and grants for travelling from AbbVie, AstraZeneca, Clovis, Eisai, GlaxoSmithKline, Hubro, MEdac, MSD, Novartis, Nykode, Olympus, PharmaMar, Pfizer, Roche, Sensor Kinesis, Teva, Tesaro. AR has reported institutional grants from Eisai, PharmaMar, Roche, speaker’s bureau for AstraZeneca, MSD, GlaxoSmithKline, PharmaMar, Clovis, advisory boards for AstraZeneca, Eisai, GlaxoSmithKline, PharmaMar, Clovis, and grants for travelling from AstraZeneca, GlaxoSmithKline, Clovis, and PharmaMar. AS has reported grants for travelling from Elekta, Stiftung Filantropie Österreich, and Medizinische Universität Wien. AT has reported advisory boards for MSD. LW has reported funding from MEdac Oncology, Roche Diagnostics, Hamburger KG, DKH, honoraria from Roche, Tesaro, Pfizer, GlaxoSmithKline, GynOnko Update, AstraZeneca, Teva, Omniamed, Promedicis, MSD, Eisai, Seagen, and advisory boards for MSD, GlaxoSmithKline, Roche, Eisai, and Seagen. MHMO, FP, PB, MRM, DF, CLC, EG, GG, SL, EU, VV, AvdZ, DZ, GFZ, and IZ have reported no conflicts of interest.
Provenance and peer review: Not commissioned; internally peer reviewed.
Author note: The project was initiated by ESGO, which provided administrative and meeting expenses support. The decision to develop guidelines was made by the Guidelines, Recommendations and Quality Assurance Committee of ESGO, with the approval of ESGO Council. ESGO is a nonprofit knowledgeable society.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. World Health Organization . GLOBOCAN 2020: estimated cancer incidence, mortality and prevalence worldwide in 2020. 2022. Available: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_population=continents&population=900&populations=908&key=asr&sex=2&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&include_nmsc=0&include_nmsc_other=1#collapse-group-0-0 [Accessed 08 Jun 2022].
- 2. Oonk MHM, Planchamp F, Baldwin P, et al. European Society of Gynaecological Oncology Guidelines for the Management of Patients with Vulvar Cancer. Int J Gynecol Cancer 2017;27:832–7. 10.1097/IGC.0000000000000975 [DOI] [PubMed] [Google Scholar]
- 3. Dykewicz CA, Centers for Disease Control and Prevention (U.S.), Infectious Diseases Society of America, et al. Summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clin Infect Dis 2001;33:139–44. 10.1086/321805 [DOI] [PubMed] [Google Scholar]
- 4. Bray F, Laversanne M, Weiderpass E, et al. Geographic and temporal variations in the incidence of vulvar and vaginal cancers. Int J Cancer 2020;147:2764–71. 10.1002/ijc.33055 [DOI] [PubMed] [Google Scholar]
- 5. Muigai J, Jacob L, Dinas K, et al. Potential delay in the diagnosis of vulvar cancer and associated risk factors in women treated in German gynecological practices. Oncotarget 2018;9:8725–30. 10.18632/oncotarget.23848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zapardiel I, Iacoponi S, Coronado PJ, et al. Prognostic factors in patients with vulvar cancer: the VULCAN study. Int J Gynecol Cancer 2020;30:1285–91. 10.1136/ijgc-2019-000526 [DOI] [PubMed] [Google Scholar]
- 7. Olawaiye AB, Cuello MA, Rogers LJ. Cancer of the vulva: 2021 update. Int J Gynaecol Obstet 2021;155 Suppl 1:7–18. 10.1002/ijgo.13881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brierley JD, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours, 8th ed. 2016. Available: https://www.uicc.org/resources/tnm-classification-malignant-tumours-8th-edition [Google Scholar]
- 9. Matsuo K, Klar M, Nishio S, et al. Validation of the 2021 FIGO staging schema for advanced vulvar cancer. Int J Gynecol Cancer 2022;32:474–9. 10.1136/ijgc-2021-003168 [DOI] [PubMed] [Google Scholar]
- 10. van den Einden LCG, Massuger LFAG, Jonkman JK, et al. An alternative way to measure the depth of invasion of vulvar squamous cell carcinoma in relation to prognosis. Mod Pathol 2015;28:295–302. 10.1038/modpathol.2014.103 [DOI] [PubMed] [Google Scholar]
- 11. Pouwer A-FW, Bult P, Otte I, et al. Measuring the depth of invasion in vulvar squamous cell carcinoma: interobserver agreement and pitfalls. Histopathology 2019;75:413–20. 10.1111/his.13883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Skala SL, Ebott JA, Zhao L, et al. Predictive value of an alternative strategy for measuring depth and size of stage 1 vulvar squamous cell carcinoma. J Low Genit Tract Dis 2020;24:265–71. 10.1097/LGT.0000000000000544 [DOI] [PubMed] [Google Scholar]
- 13. McCluggage WG. Carcinoma of the vulva histopathology reporting guide. In: International Collaboration on Cancer Reporting. Sydney, Australia, 2021. [Google Scholar]
- 14. Heatley MK. Dissection and reporting of the organs of the female genital tract. J Clin Pathol 2008;61:241–57. 10.1136/jcp.2007.051110 [DOI] [PubMed] [Google Scholar]
- 15. Hinten F, Molijn A, Eckhardt L, et al. Vulvar cancer: two pathways with different localization and prognosis. Gynecol Oncol 2018;149:310–7. 10.1016/j.ygyno.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 16. Hinten F, van den Einden LCG, Cissen M, et al. Clitoral involvement of squamous cell carcinoma of the vulva: localization with the worst prognosis. Eur J Surg Oncol 2015;41:592–8. 10.1016/j.ejso.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 17. Herrington C, Kim K-R, McCluggage W, et al. Tumours of the vulva. In: Female genital tumours. WHO Classification of Tumours, 5th ed, vol. 4. Lyon, France: International Agency for Research on Cancer, 2020: 419–49. [Google Scholar]
- 18. Cao H, Wang S, Zhang Z, et al. Prognostic value of overexpressed P16Ink4A in vulvar cancer: a meta-analysis. PLoS One 2016;11:e0152459. 10.1371/journal.pone.0152459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rakislova N, Clavero O, Alemany L, et al. Histological characteristics of HPV-associated and-independent squamous cell carcinomas of the vulva: a study of 1,594 cases. Int J Cancer 2017;141:2517–27. 10.1002/ijc.31006 [DOI] [PubMed] [Google Scholar]
- 20. Tessier-Cloutier B, Kortekaas KE, Thompson E, et al. Major P53 immunohistochemical patterns in in situ and invasive squamous cell carcinomas of the vulva and correlation with TP53 mutation status. Mod Pathol 2020;33:1595–605. 10.1038/s41379-020-0524-1 [DOI] [PubMed] [Google Scholar]
- 21. Kortekaas KE, Solleveld-Westerink N, Tessier-Cloutier B, et al. Performance of the pattern-based interpretation of P53 immunohistochemistry as a surrogate for TP53 mutations in vulvar squamous cell carcinoma. Histopathology 2020;77:92–9. 10.1111/his.14109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kortekaas KE, Bastiaannet E, van Doorn HC, et al. Vulvar cancer subclassification by HPV and P53 status results in three clinically distinct subtypes. Gynecol Oncol 2020;159:649–56. 10.1016/j.ygyno.2020.09.024 [DOI] [PubMed] [Google Scholar]
- 23. Julia CJ, Hoang LN. A review of prognostic factors in squamous cell carcinoma of the vulva: evidence from the last decade. Semin Diagn Pathol 2021;38:37–49. 10.1053/j.semdp.2020.09.004 [DOI] [PubMed] [Google Scholar]
- 24. Ferrari F, Forte S, Ardighieri L, et al. Multivariate analysis of prognostic factors in primary squamous cell vulvar cancer: the role of perineural invasion in recurrence and survival. Eur J Surg Oncol 2019;45:2115–9. 10.1016/j.ejso.2019.07.029 [DOI] [PubMed] [Google Scholar]
- 25. Holthoff ER, Jeffus SK, Gehlot A, et al. Perineural invasion is an independent pathologic indicator of recurrence in vulvar squamous cell carcinoma. Am J Surg Pathol 2015;39:1070–4. 10.1097/PAS.0000000000000422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Woelber L, Griebel L-F, Eulenburg C, et al. Role of tumour-free margin distance for loco-regional control in vulvar cancer-a subset analysis of the Arbeitsgemeinschaft Gynakologische Onkologie Care-1 multicenter study. Eur J Cancer 2016;69:180–8. 10.1016/j.ejca.2016.09.038 [DOI] [PubMed] [Google Scholar]
- 27. Nooij LS, van der Slot MA, Dekkers OM, et al. Tumour-free margins in vulvar squamous cell carcinoma: does distance really matter. Eur J Cancer 2016;65:139–49. 10.1016/j.ejca.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 28. McAlpine JN, Leung SCY, Cheng A, et al. Human papillomavirus (HPV)-independent vulvar squamous cell carcinoma has a worse prognosis than HPV-associated disease: a retrospective cohort study. Histopathology 2017;71:238–46. 10.1111/his.13205 [DOI] [PubMed] [Google Scholar]
- 29. te Grootenhuis NC, Pouwer AW, de Bock GH, et al. Margin status revisited in vulvar squamous cell carcinoma. Gynecol Oncol 2019;154:266–75. 10.1016/j.ygyno.2019.05.010 [DOI] [PubMed] [Google Scholar]
- 30. Nooij LS, Ter Haar NT, Ruano D, et al. Genomic characterization of vulvar (pre)cancers identifies distinct molecular subtypes with prognostic significance. Clin Cancer Res 2017;23:6781–9. 10.1158/1078-0432.CCR-17-1302 [DOI] [PubMed] [Google Scholar]
- 31. Pahmeyer C, Thangarajah F, Ratiu D, et al. Preoperative biopsies as predictor for the necessity of inguinal lymph node surgery in squamous cell carcinoma of the vulva-a retrospective tertiary center analysis. J Cancer Res Clin Oncol 2020;146:2709–12. 10.1007/s00432-020-03263-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nikolić O, Sousa FA e, Cunha TM, et al. Vulvar cancer staging: guidelines of the European Society of Urogenital Radiology (ESUR). Insights Imaging 2021;12:131. 10.1186/s13244-021-01075-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pleunis N, Pouwer AW, Ploegmakers MJ, et al. Low incidence of pulmonary metastases in vulvar cancer patients: limited value of routine chest imaging based on a cohort study. BJOG 2022;129:769–76. 10.1111/1471-0528.16636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pounds R, O’Neill D, Subba K, et al. The role of preoperative computerized tomography (CT) scan of the pelvis and groin in the management of clinically early staged vulva squamous cell carcinoma. Gynecol Oncol 2020;157:444–9. 10.1016/j.ygyno.2020.01.031 [DOI] [PubMed] [Google Scholar]
- 35. Bohlin KS, Bruno A-K, von Knorring C, et al. Accuracy of computerized tomography in the preoperative evaluation of metastases in primary vulvar cancer - a population-based study. Gynecol Oncol 2021;161:449–53. 10.1016/j.ygyno.2021.02.005 [DOI] [PubMed] [Google Scholar]
- 36. Crivellaro C, Guglielmo P, De Ponti E, et al. 18F-FDG PET/CT in preoperative staging of vulvar cancer patients: is it really effective? Medicine (Baltimore) 2017;96:e7943. 10.1097/MD.0000000000007943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fischerova D, Garganese G, Reina H, et al. Terms, definitions and measurements to describe sonographic features of lymph nodes: consensus opinion from the Vulvar International Tumor Analysis (VITA) group. Ultrasound Obstet Gynecol 2021;57:861–79. 10.1002/uog.23617 [DOI] [PubMed] [Google Scholar]
- 38. Garganese G, Fragomeni SM, Pasciuto T, et al. Ultrasound morphometric and cytologic preoperative assessment of inguinal lymph-node status in women with vulvar cancer: MorphoNode study. Ultrasound Obstet Gynecol 2020;55:401–10. 10.1002/uog.20378 [DOI] [PubMed] [Google Scholar]
- 39. Verri D, Moro F, Fragomeni SM, et al. The role of ultrasound in the evaluation of inguinal lymph nodes in patients with vulvar cancer: a systematic review and meta-analysis. Cancers 2022;14:3082. 10.3390/cancers14133082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Angelico G, Santoro A, Inzani F, et al. Ultrasound-guided FNA cytology of groin lymph nodes improves the management of squamous cell carcinoma of the vulva: results from a comparative cytohistological study. Cancer Cytopathol 2019;127:514–20. 10.1002/cncy.22154 [DOI] [PubMed] [Google Scholar]
- 41. Rufini V, Garganese G, Ieria FP, et al. Diagnostic performance of preoperative [18F]FDG-PET/CT for lymph node staging in vulvar cancer: a large single-centre study. Eur J Nucl Med Mol Imaging 2021;48:3303–14. 10.1007/s00259-021-05257-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lakhman Y, Vargas HA, Reinhold C, et al. ACR Appropriateness Criteria® staging and follow-up of vulvar cancer. J Am Coll Radiol 2021;18:S212–28. 10.1016/j.jacr.2021.02.016 [DOI] [PubMed] [Google Scholar]
- 43. Fischerova D, Cibula D, Dundr P, et al. Ultrasound-guided tru-cut biopsy in the management of advanced abdomino-pelvic tumors. Int J Gynecol Cancer 2008;18:833–7. 10.1111/j.1525-1438.2007.01015.x [DOI] [PubMed] [Google Scholar]
- 44. Zikan M, Fischerova D, Pinkavova I, et al. Ultrasound-guided tru-cut biopsy of abdominal and pelvic tumors in gynecology. Ultrasound Obstet Gynecol 2010;36:767–72. 10.1002/uog.8803 [DOI] [PubMed] [Google Scholar]
- 45. Epstein E, Van Calster B, Timmerman D, et al. Subjective ultrasound assessment, the ADNEX model and ultrasound-guided tru-cut biopsy to differentiate disseminated primary ovarian cancer from metastatic non-ovarian cancer. Ultrasound Obstet Gynecol 2016;47:110–6. 10.1002/uog.14892 [DOI] [PubMed] [Google Scholar]
- 46. Arvas M, Kahramanoglu I, Bese T, et al. The role of pathological margin distance and prognostic factors after primary surgery in squamous cell carcinoma of the vulva. Int J Gynecol Cancer 2018;28:623–31. 10.1097/IGC.0000000000001195 [DOI] [PubMed] [Google Scholar]
- 47. Baiocchi G, Mantoan H, de Brot L, et al. How important is the pathological margin distance in vulvar cancer? Eur J Surg Oncol 2015;41:1653–8. 10.1016/j.ejso.2015.09.024 [DOI] [PubMed] [Google Scholar]
- 48. Barlow EL, Jackson M, Hacker NF. The prognostic role of the surgical margins in squamous vulvar cancer: a retrospective Australian study. Cancers (Basel) 2020;12:3375. 10.3390/cancers12113375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Micheletti L, Preti M, Cintolesi V, et al. Prognostic impact of reduced tumor-free margin distance on long-term survival in FIGO stage IB/II vulvar squamous cell carcinoma. J Gynecol Oncol 2018;29. 10.3802/jgo.2018.29.e61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Milliken S, May J, Sanderson PA, et al. Reducing the radicality of surgery for vulvar cancer: are smaller margins safer? Minerva Obstet Gynecol 2021;73:160–5. 10.23736/S2724-606X.20.04743-7 [DOI] [PubMed] [Google Scholar]
- 51. Pleunis N, Leermakers MEJ, van der Wurff AA, et al. Surgical margins in squamous cell carcinoma, different for the vulva? Eur J Surg Oncol 2018;44:1555–61. 10.1016/j.ejso.2018.05.031 [DOI] [PubMed] [Google Scholar]
- 52. Raimond E, Delorme C, Ouldamer L, et al. Surgical treatment of vulvar cancer: impact of tumor-free margin distance on recurrence and survival. A multicentre cohort analysis from the Francogyn study group. Eur J Surg Oncol 2019;45:2109–14. 10.1016/j.ejso.2019.07.005 [DOI] [PubMed] [Google Scholar]
- 53. Yang J, Delara R, Ghaith S, et al. Tumor-free margins and local recurrence in squamous cell carcinoma of the vulva. Gynecol Oncol 2020;158:555–61. 10.1016/j.ygyno.2020.06.503 [DOI] [PubMed] [Google Scholar]
- 54. Jain V, Sekhon R, Giri S, et al. Robotic-assisted video endoscopic lnguinal lymphadenectomy in carcinoma vulva: our experiences and intermediate results. Int J Gynecol Cancer 2017;27:159–65. 10.1097/IGC.0000000000000854 [DOI] [PubMed] [Google Scholar]
- 55. Mohammad A, Hunter MI. Robot-assisted sentinel lymph node mapping and inguinal lymph node dissection using near-infrared fluorescence in vulvar cancer. J Minim Invasive Gynecol 2019;26:968–72. 10.1016/j.jmig.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 56. Wu Q, Gong Z, Zhao Y, et al. Video endoscopic inguinal lymphadenectomy via 3-incision lateral approach for vulvar cancers: our preliminary outcome of 37 cases. Int J Gynecol Cancer 2016;26:1706–11. 10.1097/IGC.0000000000000816 [DOI] [PubMed] [Google Scholar]
- 57. Zhang M, Chen L, Zhang X, et al. A comparative study of video endoscopic inguinal lymphadenectomy and conventional open inguinal lymphadenectomy for treating vulvar cancer. Int J Gynecol Cancer 2017;27:1983–9. 10.1097/IGC.0000000000001100 [DOI] [PubMed] [Google Scholar]
- 58. Naldini A, Rossitto C, Pacelli F, et al. The video endoscopy inguinal lymphadenectomy for vulvar cancer: a pilot study. Taiwan J Obstet Gynecol 2017;56:281–5. 10.1016/j.tjog.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 59. Le A, Xiong J, Wang Z, et al. Endoscopy-assisted inguinal lymphadenectomy in vulvar cancer. Arch Gynecol Obstet 2018;297:1277–83. 10.1007/s00404-018-4732-6 [DOI] [PubMed] [Google Scholar]
- 60. Nabavizadeh R, Petrinec B, Nabavizadeh B, et al. Inguinal lymph node dissection in the era of minimally invasive surgical technology. Urol Oncol 2023;41:1–14. 10.1016/j.urolonc.2020.07.026 [DOI] [PubMed] [Google Scholar]
- 61. Ding J, Teng P, Guan X, et al. Analysis of short-term efficacy of gasless single-port laparoscopic inguinal lymphadenectomy through vulva incision for vulvar cancer. Front Surg 2022;9. 10.3389/fsurg.2022.813711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Di Donato V, Bracchi C, Cigna E, et al. Vulvo-vaginal reconstruction after radical excision for treatment of vulvar cancer: evaluation of feasibility and morbidity of different surgical techniques. Surg Oncol 2017;26:511–21. 10.1016/j.suronc.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 63. Chang TN-J, Lee C-H, Lai C-H, et al. Profunda artery perforator flap for isolated vulvar defect reconstruction after oncological resection. J Surg Oncol 2016;113:828–34. 10.1002/jso.24233 [DOI] [PubMed] [Google Scholar]
- 64. Confalonieri PL, Gilardi R, Rovati LC, et al. Comparison of V-Y advancement flap versus lotus petal flap for plastic reconstruction after surgery in case of vulvar malignancies: a retrospective single center experience. Ann Plast Surg 2017;79:186–91. 10.1097/SAP.0000000000001094 [DOI] [PubMed] [Google Scholar]
- 65. Conri V, Casoli V, Coret M, et al. Modified gluteal fold V-Y advancement flap for reconstruction after radical vulvectomy. Int J Gynecol Cancer 2016;26:1300–6. 10.1097/IGC.0000000000000765 [DOI] [PubMed] [Google Scholar]
- 66. Gentileschi S, Caretto AA, Servillo M, et al. Feasibility, indications and complications of SCIP flap for reconstruction after extirpative surgery for vulvar cancer. J Plast Reconstr Aesthet Surg 2022;75:1150–7. 10.1016/j.bjps.2021.11.005 [DOI] [PubMed] [Google Scholar]
- 67. Gentileschi S, Servillo M, Garganese G, et al. Surgical therapy of vulvar cancer: how to choose the correct reconstruction J Gynecol Oncol 2016;27:e60. 10.3802/jgo.2016.27.e60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gentileschi S, Servillo M, Garganese G, et al. Versatility of pedicled anterolateral thigh flap in gynecologic reconstruction after vulvar cancer extirpative surgery. Microsurgery 2017;37:516–24. 10.1002/micr.30077 [DOI] [PubMed] [Google Scholar]
- 69. Giannini A, Di Donato V, D’Oria O, et al. The V-Y gluteal fold advancement flap: outcomes following radical surgery for vulvar malignancies. Int J Gynaecol Obstet 2021;152:421–4. 10.1002/ijgo.13430 [DOI] [PubMed] [Google Scholar]
- 70. Lange M, Hage JJ, van Beurden M. A prospective assessment of surgical risk factors in 114 gluteal fold flap reconstructions after oncological vulvoperineal resection. Ann Plast Surg 2017;79:53–9. 10.1097/SAP.0000000000000964 [DOI] [PubMed] [Google Scholar]
- 71. Mercut R, Sinna R, Vaucher R, et al. Triple flap technique for vulvar reconstruction. Ann Chir Plast Esthet 2018;63:343–8. 10.1016/j.anplas.2018.03.007 [DOI] [PubMed] [Google Scholar]
- 72. Nomura H, Maeda T, Usami T, et al. Vulvar reconstruction following surgery for vulvar cancer using a stepladder V-Y advancement medial thigh flap. Int J Gynecol Cancer 2015;25:1484–7. 10.1097/IGC.0000000000000518 [DOI] [PubMed] [Google Scholar]
- 73. Selçuk İ, Doğan O, Barut C, et al. Radical vulvectomy with right gluteal and left medial thigh V-Y advancement flap reconstruction. J Turk Ger Gynecol Assoc 2021;22:339–42. 10.4274/jtgga.galenos.2020.2020.0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tock S, Wallet J, Belhadia M, et al. Outcomes of the use of different vulvar flaps for reconstruction during surgery for vulvar cancer. Eur J Surg Oncol 2019;45:1625–31. 10.1016/j.ejso.2019.04.012 [DOI] [PubMed] [Google Scholar]
- 75. Hassanzade M, Attaran M, Treglia G, et al. Lymphatic mapping and sentinel node biopsy in squamous cell carcinoma of the vulva: systematic review and meta-analysis of the literature. Gynecol Oncol 2013;130:237–45. 10.1016/j.ygyno.2013.04.023 [DOI] [PubMed] [Google Scholar]
- 76. Lawrie TA, Patel A, Martin-Hirsch PPL, et al. Sentinel node assessment for diagnosis of groin lymph node involvement in vulval cancer. Cochrane Database Syst Rev 2014;2014:CD010409. 10.1002/14651858.CD010409.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Meads C, Sutton A, Małysiak S, et al. Sentinel lymph node status in vulval cancer: systematic reviews of test accuracy and decision-analytic model-based economic evaluation. Health Technol Assess 2013;17:1–216. 10.3310/hta17600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Van der Zee AGJ, Oonk MH, De Hullu JA, et al. Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J Clin Oncol 2008;26:884–9. 10.1200/JCO.2007.14.0566 [DOI] [PubMed] [Google Scholar]
- 79. Deken MM, van Doorn HC, Verver D, et al. Near-infrared fluorescence imaging compared to standard sentinel lymph node detection with blue dye in patients with vulvar cancer - a randomized controlled trial. Gynecol Oncol 2020;159:672–80. 10.1016/j.ygyno.2020.09.044 [DOI] [PubMed] [Google Scholar]
- 80. Koual M, Benoit L, Nguyen-Xuan H-T, et al. Diagnostic value of indocyanine green fluorescence guided sentinel lymph node biopsy in vulvar cancer: a systematic review. Gynecol Oncol 2021;161:436–41. 10.1016/j.ygyno.2021.01.031 [DOI] [PubMed] [Google Scholar]
- 81. Di Donna MC, Quartuccio N, Giallombardo V, et al. Detection of sentinel lymph node in vulvar cancer using (99M)Tc-labeled colloid lymphoscintigraphy, blue dye, and indocyanine-green fluorescence: a meta-analysis of studies published in 2010-2020. Arch Gynecol Obstet 2023;307:1677–86. 10.1007/s00404-022-06605-1 [DOI] [PubMed] [Google Scholar]
- 82. Prader S, du Bois A, Harter P, et al. Sentinel lymph node mapping with fluorescent and radioactive tracers in vulvar cancer patients. Arch Gynecol Obstet 2020;301:729–36. 10.1007/s00404-019-05415-2 [DOI] [PubMed] [Google Scholar]
- 83. van Doorn HC, van Beekhuizen HJ, Gaarenstroom KN, et al. Repeat sentinel lymph node procedure in patients with recurrent vulvar squamous cell carcinoma is feasible. Gynecol Oncol 2016;140:415–9. 10.1016/j.ygyno.2016.01.013 [DOI] [PubMed] [Google Scholar]
- 84. Swift BE, Tigert M, Nica A, et al. The accuracy of intraoperative frozen section examination of sentinel lymph nodes in squamous cell cancer of the vulva. Gynecol Oncol 2022;164:393–7. 10.1016/j.ygyno.2021.11.020 [DOI] [PubMed] [Google Scholar]
- 85. Oonk MHM, Slomovitz B, Baldwin PJW, et al. Radiotherapy versus inguinofemoral lymphadenectomy as treatment for vulvar cancer patients with micrometastases in the sentinel node: results of GROINSS-V II. J Clin Oncol 2021;39:3623–32. 10.1200/JCO.21.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Woelber L, Eulenburg C, Grimm D, et al. The risk of contralateral non-sentinel metastasis in patients with primary vulvar cancer and unilaterally positive sentinel node. Ann Surg Oncol 2016;23:2508–14. 10.1245/s10434-016-5114-6 [DOI] [PubMed] [Google Scholar]
- 87. Nica A, Covens A, Vicus D, et al. Sentinel lymph nodes in vulvar cancer: management dilemmas in patients with positive nodes and larger tumors. Gynecol Oncol 2019;152:94–100. 10.1016/j.ygyno.2018.10.047 [DOI] [PubMed] [Google Scholar]
- 88. Ignatov T, Gaßner J, Bozukova M, et al. Contralateral lymph node metastases in patients with vulvar cancer and unilateral sentinel lymph node metastases. Acta Obstet Gynecol Scand 2021;100:1520–5. 10.1111/aogs.14157 [DOI] [PubMed] [Google Scholar]
- 89. Winarno AS, Mondal A, Martignoni FC, et al. The potential risk of contralateral non-sentinel groin node metastasis in women with early primary vulvar cancer following unilateral sentinel node metastasis: a single center evaluation in University Hospital of Dusseldorf. BMC Womens Health 2021;21:23. 10.1186/s12905-020-01165-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Van der Kolk WL, Van der Zee AGJ, Slomovitz BM, et al. Unilateral inguinofemoral lymphadenectomy in patients with early-stage vulvar squamous cell carcinoma and a unilateral metastatic sentinel lymph node is safe. Gynecol Oncol 2022;167:3–10. 10.1016/j.ygyno.2022.07.017 [DOI] [PubMed] [Google Scholar]
- 91. Gadducci A, Aletti GD. Locally advanced squamous cell carcinoma of the vulva: a challenging question for gynecologic oncologists. Gynecol Oncol 2020;158:208–17. 10.1016/j.ygyno.2020.05.021 [DOI] [PubMed] [Google Scholar]
- 92. Tagliaferri L, Lancellotta V, Casà C, et al. The radiotherapy role in the multidisciplinary management of locally advanced vulvar cancer: a multidisciplinary VulCan team review. Cancers (Basel) 2021;13:5747. 10.3390/cancers13225747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chapman BV, Gill BS, Viswanathan AN, et al. Adjuvant radiation therapy for margin-positive vulvar squamous cell carcinoma: defining the ideal dose-response using the National Cancer Data Base. Int J Radiat Oncol Biol Phys 2017;97:107–17. 10.1016/j.ijrobp.2016.09.023 [DOI] [PubMed] [Google Scholar]
- 94. Bhatla N, Tomar S, Meena J, et al. Adjuvant treatment in cervical, vaginal and vulvar cancer. Best Pract Res Clin Obstet Gynaecol 2022;78:36–51. 10.1016/j.bpobgyn.2021.07.005 [DOI] [PubMed] [Google Scholar]
- 95. Ignatov T, Eggemann H, Burger E, et al. Adjuvant radiotherapy for vulvar cancer with close or positive surgical margins. J Cancer Res Clin Oncol 2016;142:489–95. 10.1007/s00432-015-2060-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lukovic J, Han K. Postoperative management of vulvar cancer. Int J Gynecol Cancer 2022;32:338–43. 10.1136/ijgc-2021-002463 [DOI] [PubMed] [Google Scholar]
- 97. Castelnau-Marchand P, Escande A, Mazeron R, et al. Brachytherapy as part of the conservative treatment for primary and recurrent vulvar carcinoma. Brachytherapy 2017;16:518–25. 10.1016/j.brachy.2017.01.005 [DOI] [PubMed] [Google Scholar]
- 98. Woelber L, Prieske K, Eulenburg CZ, et al. Adjuvant radiotherapy and local recurrence in vulvar cancer - a subset analysis of the AGO-Care-1 study. Gynecol Oncol 2022;164:68–75. 10.1016/j.ygyno.2021.11.004 [DOI] [PubMed] [Google Scholar]
- 99. Gill BS, Bernard ME, Lin JF, et al. Impact of adjuvant chemotherapy with radiation for node-positive vulvar cancer: a National Cancer Data Base (NCDB) analysis. Gynecol Oncol 2015;137:365–72. 10.1016/j.ygyno.2015.03.056 [DOI] [PubMed] [Google Scholar]
- 100. Rydzewski NR, Kanis MJ, Donnelly ED, et al. Role of adjuvant external beam radiotherapy and chemotherapy in one versus two or more node-positive vulvar cancer: a National Cancer Database study. Radiother Oncol 2018;129:534–9. 10.1016/j.radonc.2018.03.023 [DOI] [PubMed] [Google Scholar]
- 101. Ashmore S, Crafton SM, Miller EM, et al. Optimal overall treatment time for adjuvant therapy for women with completely resected, node-positive vulvar cancer. Gynecol Oncol 2021;161:63–9. 10.1016/j.ygyno.2021.01.003 [DOI] [PubMed] [Google Scholar]
- 102. Moore DH, Thomas GM, Montana GS, et al. Preoperative chemoradiation for advanced vulvar cancer: a phase II study of the Gynecologic Oncology Group. Int J Radiat Oncol Biol Phys 1998;42:79–85. 10.1016/s0360-3016(98)00193-x [DOI] [PubMed] [Google Scholar]
- 103. Moore DH, Ali S, Koh W-J, et al. A phase II trial of radiation therapy and weekly cisplatin chemotherapy for the treatment of locally-advanced squamous cell carcinoma of the vulva: a Gynecologic Oncology Group study. Gynecol Oncol 2012;124:529–33. 10.1016/j.ygyno.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 104. van Doorn HC, Ansink A, Verhaar-Langereis M, et al. Neoadjuvant chemoradiation for advanced primary vulvar cancer. Cochrane Database Syst Rev 2006;3:CD003752. 10.1002/14651858.CD003752.pub2 [DOI] [PubMed] [Google Scholar]
- 105. Rao YJ, Chin R-I, Hui C, et al. Improved survival with definitive chemoradiation compared to definitive radiation alone in squamous cell carcinoma of the vulva: a review of the National Cancer Database. Gynecol Oncol 2017;146:572–9. 10.1016/j.ygyno.2017.06.022 [DOI] [PubMed] [Google Scholar]
- 106. Richman AH, Vargo JA, Ling DC, et al. Dose-escalated intensity modulated radiation therapy in patients with locally-advanced vulvar cancer - does it increase response rate? Gynecol Oncol 2020;159:657–62. 10.1016/j.ygyno.2020.09.019 [DOI] [PubMed] [Google Scholar]
- 107. Beriwal S, Coon D, Heron DE, et al. Preoperative intensity-modulated radiotherapy and chemotherapy for locally advanced vulvar carcinoma. Gynecol Oncol 2008;109:291–5. 10.1016/j.ygyno.2007.10.026 [DOI] [PubMed] [Google Scholar]
- 108. Rishi A, Rollins M, Ahmed KA, et al. High-dose intensity-modulated chemoradiotherapy in vulvar squamous cell carcinoma: outcome and toxicity. Gynecol Oncol 2020;156:349–56. 10.1016/j.ygyno.2019.11.027 [DOI] [PubMed] [Google Scholar]
- 109. Mahantshetty U, Naga P, Engineer R, et al. Clinical outcome of high-dose-rate interstitial brachytherapy in vulvar cancer: a single institutional experience. Brachytherapy 2017;16:153–60. 10.1016/j.brachy.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 110. van Triest B, Rasing M, van der Velden J, et al. Phase II study of definitive chemoradiation for locally advanced squamous cell cancer of the vulva: an efficacy study. Gynecol Oncol 2021;163:117–24. 10.1016/j.ygyno.2021.07.020 [DOI] [PubMed] [Google Scholar]
- 111. Forner DM, Mallmann P. Neoadjuvant and definitive chemotherapy or chemoradiation for stage III and IV vulvar cancer: a pooled reanalysis. Eur J Obstet Gynecol Reprod Biol 2017;212:115–8. 10.1016/j.ejogrb.2017.03.030 [DOI] [PubMed] [Google Scholar]
- 112. Witteveen PO, van der Velden J, Vergote I, et al. Phase II study on paclitaxel in patients with recurrent, metastatic or locally advanced vulvar cancer not amenable to surgery or radiotherapy: a study of the EORTC-GCG (European Organisation for Research and Treatment of Cancer--Gynaecological Cancer Group). Ann Oncol 2009;20:1511–6. 10.1093/annonc/mdp043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Cormio G, Loizzi V, Gissi F, et al. Cisplatin and vinorelbine chemotherapy in recurrent vulvar carcinoma. Oncology 2009;77:281–4. 10.1159/000259259 [DOI] [PubMed] [Google Scholar]
- 114. Han SN, Vergote I, Amant F. Weekly paclitaxel/carboplatin in the treatment of locally advanced, recurrent, or metastatic vulvar cancer. Int J Gynecol Cancer 2012;22:865–8. 10.1097/IGC.0b013e31824b4058 [DOI] [PubMed] [Google Scholar]
- 115. Santeufemia DA, Capobianco G, Re GL, et al. Cisplatin-gemcitabine as palliative chemotherapy in advanced squamous vulvar carcinoma: report of two cases. Eur J Gynaecol Oncol 2012;33:421–2. [PubMed] [Google Scholar]
- 116. Monk BJ, Sill MW, McMeekin DS, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol 2009;27:4649–55. 10.1200/JCO.2009.21.8909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kitagawa R, Katsumata N, Shibata T, et al. Paclitaxel plus carboplatin versus paclitaxel plus cisplatin in metastatic or recurrent cervical cancer: the open-label randomized phase III trial JCOG0505. JCO 2015;33:2129–35. 10.1200/JCO.2014.58.4391 [DOI] [PubMed] [Google Scholar]
- 118. Shapira-Frommer R, Mileshkin L, Manzyuk L, et al. Efficacy and safety of pembrolizumab for patients with previously treated advanced vulvar squamous cell carcinoma: results from the phase 2 KEYNOTE-158 study. Gynecologic Oncology 2022;166:211–8. 10.1016/j.ygyno.2022.01.029 [DOI] [PubMed] [Google Scholar]
- 119. Naumann RW, Hollebecque A, Meyer T, et al. Safety and efficacy of nivolumab monotherapy in recurrent or metastatic cervical, vaginal, or vulvar carcinoma: results from the phase I/II Checkmate 358 trial. J Clin Oncol 2019;37:2825–34. 10.1200/JCO.19.00739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Tewari KS, Monk BJ, Vergote I, et al. Survival with cemiplimab in recurrent cervical cancer. N Engl J Med 2022;386:544–55. 10.1056/NEJMoa2112187 [DOI] [PubMed] [Google Scholar]
- 121. Colombo N, Dubot C, Lorusso D, et al. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med 2021;385:1856–67. 10.1056/NEJMoa2112435 [DOI] [PubMed] [Google Scholar]
- 122. Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet 2017;390:1654–63. 10.1016/S0140-6736(17)31607-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Growdon WB, Boisvert SL, Akhavanfard S, et al. Decreased survival in EGFR gene amplified vulvar carcinoma. Gynecol Oncol 2008;111:289–97. 10.1016/j.ygyno.2008.07.038 [DOI] [PubMed] [Google Scholar]
- 124. Horowitz NS, Olawaiye AB, Borger DR, et al. Phase II trial of erlotinib in women with squamous cell carcinoma of the vulva. Gynecol Oncol 2012;127:141–6. 10.1016/j.ygyno.2012.06.028 [DOI] [PubMed] [Google Scholar]
- 125. Maggino T, Landoni F, Sartori E, et al. Patterns of recurrence in patients with squamous cell carcinoma of the vulva. A multicenter CTF study. Cancer 2000;89:116–22. [DOI] [PubMed] [Google Scholar]
- 126. Buchanan TR, Zamorano AS, Massad LS, et al. Risk of cervical and vaginal dysplasia after surgery for vulvar intraepithelial neoplasia or cancer: a 6 year follow-up study. Gynecol Oncol 2019;155:88–92. 10.1016/j.ygyno.2019.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kuroki LM, Frolova AI, Wu N, et al. Yield of cytology surveillance after high-grade vulvar intraepithelial neoplasia or cancer. J Low Genit Tract Dis 2017;21:193–7. 10.1097/LGT.0000000000000321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Oonk MHM, de Hullu JA, Hollema H, et al. The value of routine follow-up in patients treated for carcinoma of the vulva. Cancer 2003;98:2624–9. 10.1002/cncr.11837 [DOI] [PubMed] [Google Scholar]
- 129. Nordin A, Mohammed KA, Naik R, et al. Does long-term follow-up have a role for node negative squamous carcinoma of the vulva? The Gateshead experience. Eur J Gynaecol 2001;22:36–9. [PubMed] [Google Scholar]
- 130. Te Grootenhuis NC, van der Zee AGJ, van Doorn HC, et al. Sentinel nodes in vulvar cancer: long-term follow-up of the Groningen International Study on Sentinel Nodes in Vulvar Cancer (GROINSS-V) I. Gynecol Oncol 2016;140:8–14. 10.1016/j.ygyno.2015.09.077 [DOI] [PubMed] [Google Scholar]
- 131. Pouwer AW, Mus R, IntHout J, et al. The efficacy of ultrasound in the follow up after a negative sentinel lymph node in women with vulvar cancer: a prospective single-centre study. BJOG 2018;125:1461–8. 10.1111/1471-0528.15341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Gonzalez Bosquet J, Magrina JF, Gaffey TA, et al. Long-term survival and disease recurrence in patients with primary squamous cell carcinoma of the vulva. Gynecol Oncol 2005;97:828–33. 10.1016/j.ygyno.2005.03.006 [DOI] [PubMed] [Google Scholar]
- 133. Röhrmoser K, Ignatov A, Gerken M, et al. Risk factors and temporal patterns of recurrences in patients with vulvar cancer: implications for follow-up intervals and duration. J Cancer Res Clin Oncol 2023;149:803–10. 10.1007/s00432-022-03954-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Nooij LS, Brand FAM, Gaarenstroom KN, et al. Risk factors and treatment for recurrent vulvar squamous cell carcinoma. Crit Rev Oncol Hematol 2016;106:1–13. 10.1016/j.critrevonc.2016.07.007 [DOI] [PubMed] [Google Scholar]
- 135. Albano D, Bonacina M, Savelli G, et al. Clinical and prognostic (18)F-FDG PET/CT role in recurrent vulvar cancer: a multicentric experience. Jpn J Radiol 2022;40:66–74. 10.1007/s11604-021-01173-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Te Grootenhuis NC, Pouwer A-F, de Bock GH, et al. Prognostic factors for local recurrence of squamous cell carcinoma of the vulva: a systematic review. Gynecol Oncol 2018;148:622–31. 10.1016/j.ygyno.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 137. Prieske K, Haeringer N, Grimm D, et al. Patterns of distant metastases in vulvar cancer. Gynecol Oncol 2016;142:427–34. 10.1016/j.ygyno.2016.07.009 [DOI] [PubMed] [Google Scholar]
- 138. Zhang W, Zeng A, Yang J, et al. Outcome of vulvar reconstruction in patients with advanced and recurrent vulvar malignancies. BMC Cancer 2015;15:851. 10.1186/s12885-015-1792-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Aviki EM, Esselen KM, Barcia SM, et al. Does plastic surgical consultation improve the outcome of patients undergoing radical vulvectomy for squamous cell carcinoma of the vulva? Gynecol Oncol 2015;137:60–5. 10.1016/j.ygyno.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 140. Hannes S, Nijboer JM, Reinisch A, et al. Abdominoperineal excisions in the treatment regimen for advanced and recurrent vulvar cancers-analysis of a single-centre experience. Indian J Surg 2015;77:1270–4. 10.1007/s12262-015-1273-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. de Gregorio N, de Gregorio A, Ebner F, et al. Pelvic exenteration as ultimate ratio for gynecologic cancers: single-center analyses of 37 cases. Arch Gynecol Obstet 2019;300:161–8. 10.1007/s00404-019-05154-4 [DOI] [PubMed] [Google Scholar]
- 142. Forner DM, Lampe B. Exenteration in the treatment of stage III/IV vulvar cancer. Gynecol Oncol 2012;124:87–91. 10.1016/j.ygyno.2011.09.014 [DOI] [PubMed] [Google Scholar]
- 143. Westin SN, Rallapalli V, Fellman B, et al. Overall survival after pelvic exenteration for gynecologic malignancy. Gynecol Oncol 2014;134:546–51. 10.1016/j.ygyno.2014.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. van Doorn HC, Oonk MHM, Fons G, et al. Sentinel lymph node procedure in patients with recurrent vulvar squamous cell carcinoma: a proposed protocol for a multicentre observational study. BMC Cancer 2022;22. 10.1186/s12885-022-09543-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Zach D, Kannisto P, Stenström Bohlin K, et al. Can we extend the indication for sentinel node biopsy in vulvar cancer? A nationwide feasibility study from Sweden. Int J Gynecol Cancer 2020;30:402–5. 10.1136/ijgc-2019-000938 [DOI] [PubMed] [Google Scholar]
- 146. Woelber L, Eulenburg C, Kosse J, et al. Predicting the course of disease in recurrent vulvar cancer - a subset analysis of the AGO-Care-1 study. Gynecol Oncol 2019;154:571–6. 10.1016/j.ygyno.2019.07.009 [DOI] [PubMed] [Google Scholar]
- 147. Tantipalakorn C, Robertson G, Marsden DE, et al. Outcome and patterns of recurrence for International Federation of Gynecology and Obstetrics (FIGO) stages I and II squamous cell vulvar cancer. Obstet Gynecol 2009;113:895–901. 10.1097/AOG.0b013e31819b413f [DOI] [PubMed] [Google Scholar]
- 148. Woelber L, Eulenburg C, Choschzick M, et al. Prognostic role of lymph node metastases in vulvar cancer and implications for adjuvant treatment. Int J Gynecol Cancer 2012;22:503–8. 10.1097/IGC.0b013e31823eed4c [DOI] [PubMed] [Google Scholar]
- 149. Cormio G, Loizzi V, Carriero C, et al. Groin recurrence in carcinoma of the vulva: management and outcome. Eur J Cancer Care (Engl) 2010;19:302–7. 10.1111/j.1365-2354.2008.01011.x [DOI] [PubMed] [Google Scholar]
- 150. Wagenaar HC, Colombo N, Vergote I, et al. Bleomycin, methotrexate, and CCNU in locally advanced or recurrent, inoperable, squamous-cell carcinoma of the vulva: an EORTC Gynaecological Cancer Cooperative Group study. Gynecol Oncol 2001;81:348–54. 10.1006/gyno.2001.6180 [DOI] [PubMed] [Google Scholar]
- 151. Frey JN, Hampl M, Mueller MD, et al. Should groin recurrence still be considered as a palliative situation in vulvar cancer patients?: a brief report. Int J Gynecol Cancer 2016;26:575–9. 10.1097/IGC.0000000000000637 [DOI] [PubMed] [Google Scholar]
- 152. Woolderink JM, de Bock GH, de Hullu JA, et al. Patterns and frequency of recurrences of squamous cell carcinoma of the vulva. Gynecol Oncol 2006;103:293–9. 10.1016/j.ygyno.2006.03.010 [DOI] [PubMed] [Google Scholar]
- 153. Ott PA, Bang Y-J, Piha-Paul SA, et al. T-cell-inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J Clin Oncol 2019;37:318–27. 10.1200/JCO.2018.78.2276 [DOI] [PubMed] [Google Scholar]
- 154. Malandrone F, Bevilacqua F, Merola M, et al. The impact of vulvar cancer on psychosocial and sexual functioning: a literature review. Cancers (Basel) 2021;14:63. 10.3390/cancers14010063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Huang J, Yu N, Wang X, et al. Incidence of lower limb lymphedema after vulvar cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e8722. 10.1097/MD.0000000000008722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Berger J, Scott E, Sukumvanich P, et al. The effect of groin treatment modality and sequence on clinically significant chronic lymphedema in patients with vulvar carcinoma. Int J Gynecol Cancer 2015;25:119–24. 10.1097/IGC.0000000000000311 [DOI] [PubMed] [Google Scholar]
- 157. Carter J, Huang HQ, Armer J, et al. GOG 244 - The Lymphedema and Gynecologic cancer (LeG) study: the impact of lower-extremity lymphedema on quality of life, psychological adjustment, physical disability, and function. Gynecol Oncol 2021;160:244–51. 10.1016/j.ygyno.2020.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Sharkey AR, King SW, Ramsden AJ, et al. Do surgical interventions for limb lymphoedema reduce cellulitis attack frequency. Microsurgery 2017;37:348–53. 10.1002/micr.30115 [DOI] [PubMed] [Google Scholar]
- 159. Woelber L, Trillsch F, Kock L, et al. Management of patients with vulvar cancer: a perspective review according to tumour stage. Ther Adv Med Oncol 2013;5:183–92. 10.1177/1758834012471699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Crosbie EJ, Slade RJ, Ahmed AS. The management of vulval cancer. Cancer Treat Rev 2009;35:533–9. 10.1016/j.ctrv.2009.01.005 [DOI] [PubMed] [Google Scholar]
- 161. Weinberg D, Gomez-Martinez RA. Vulvar cancer. Obstet Gynecol Clin North Am 2019;46:125–35. 10.1016/j.ogc.2018.09.008 [DOI] [PubMed] [Google Scholar]
- 162. Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol 2012;30:880–7. 10.1200/JCO.2011.38.5161 [DOI] [PubMed] [Google Scholar]
- 163. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2017;35:96–112. 10.1200/JCO.2016.70.1474 [DOI] [PubMed] [Google Scholar]
- 164. World Health Organization, Worldwide Palliative Care Alliance . Global Atlas of Palliative Care at the End of Life. 2014. Available: https://www.iccp-portal.org/system/files/resources/Global_Atlas_of_Palliative_Care.pdf [Google Scholar]
- 165. Landrum LM, Blank S, Chen L, et al. Comprehensive care in gynecologic oncology: the importance of palliative care. Gynecol Oncol 2015;137:193–202. 10.1016/j.ygyno.2015.02.026 [DOI] [PubMed] [Google Scholar]
- 166. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733–42. 10.1056/NEJMoa1000678 [DOI] [PubMed] [Google Scholar]
- 167. Lefkowits C, Binstock AB, Courtney-Brooks M, et al. Predictors of palliative care consultation on an inpatient gynecologic oncology service: are we following ASCO recommendations? Gynecol Oncol 2014;133:319–25. 10.1016/j.ygyno.2014.02.031 [DOI] [PubMed] [Google Scholar]
- 168. Lefkowits C, Teuteberg W, Courtney-Brooks M, et al. Improvement in symptom burden within one day after palliative care consultation in a cohort of gynecologic oncology inpatients. Gynecol Oncol 2015;136:424–8. 10.1016/j.ygyno.2014.12.030 [DOI] [PubMed] [Google Scholar]
- 169. Nitecki R, Diver EJ, Kamdar MM, et al. Patterns of palliative care referral in ovarian cancer: a single institution 5 year retrospective analysis. Gynecol Oncol 2018;148:521–6. 10.1016/j.ygyno.2018.01.018 [DOI] [PubMed] [Google Scholar]
- 170. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 2008;300:1665–73. 10.1001/jama.300.14.1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Bercow AS, Nitecki R, Haber H, et al. Palliative care referral patterns and measures of aggressive care at the end of life in patients with cervical cancer. Int J Gynecol Cancer 2021;31:66–72. 10.1136/ijgc-2020-001812 [DOI] [PubMed] [Google Scholar]
- 172. Doubova SV, Pérez-Cuevas R. Association of supportive care needs and quality of patient-centered cancer care with depression in women with breast and cervical cancer in Mexico. Psychooncology 2021;30:591–601. 10.1002/pon.5608 [DOI] [PubMed] [Google Scholar]
- 173. Hoppenot C, Littell RD, DeEulis T, et al. Top ten tips palliative care clinicians should know about caring for patients with cervical cancer. J Palliat Med 2021;24:438–42. 10.1089/jpm.2021.0006 [DOI] [PubMed] [Google Scholar]
- 174. Kim YJ, Munsell MF, Park JC, et al. Retrospective review of symptoms and palliative care interventions in women with advanced cervical cancer. Gynecol Oncol 2015;139:553–8. 10.1016/j.ygyno.2015.09.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Aeckerle S, Moor M, Pilz LR, et al. Characteristics, treatment and prognostic factors of patients with gynaecological malignancies treated in a palliative care unit at a university hospital. Oncol Res Treat 2013;36:642–8. 10.1159/000355642 [DOI] [PubMed] [Google Scholar]
- 176. Paulsen T, Liland H, Myklebust TÅ, et al. Early referral to a palliative team improves end-of-life care among gynecological cancer patients: a retrospective, population-based study. Int J Gynecol Cancer 2022;32:181–8. 10.1136/ijgc-2021-002898 [DOI] [PubMed] [Google Scholar]
- 177. Sompratthana T, Phoolcharoen N, Schmeler KM, et al. End-of-life symptoms and interventions among women with gynecologic cancers in a tertiary-care hospital in Thailand. Int J Gynecol Cancer 2019:ijgc-2019-000338. 10.1136/ijgc-2019-000338 [DOI] [PubMed] [Google Scholar]
- 178. Krakauer EL, Kwete X, Kane K, et al. Cervical cancer-associated suffering: estimating the palliative care needs of a highly vulnerable population. JCO Glob Oncol 2021;Oncol7:862–72. 10.1200/GO.21.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Atreya S. Referral patterns of gynecological cancer patients to a palliative medicine unit: a 2 years retrospective analysis. Indian J Palliat Care 2017;23:409–12. 10.4103/IJPC.IJPC_77_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Maguire R, Kotronoulas G, Simpson M, et al. A systematic review of the supportive care needs of women living with and beyond cervical cancer. Gynecol Oncol 2015;136:478–90. 10.1016/j.ygyno.2014.10.030 [DOI] [PubMed] [Google Scholar]
- 181. Krakauer EL, Kane K, Kwete X, et al. Augmented package of palliative care for women with cervical cancer: responding to refractory suffering. JCO Glob Oncol 2021;7:886–95. 10.1200/GO.21.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Krakauer EL, Kane K, Kwete X, et al. Essential package of palliative care for women with cervical cancer: responding to the suffering of a highly vulnerable population. JCO Glob Oncol 2021;7:873–85. 10.1200/GO.21.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. van Lonkhuijzen L, Thomas G. Palliative radiotherapy for cervical carcinoma, a systematic review. Radiother Oncol 2011;98:287–91. 10.1016/j.radonc.2011.01.009 [DOI] [PubMed] [Google Scholar]
- 184. Kim DH, Lee JH, Ki YK, et al. Short-course palliative radiotherapy for uterine cervical cancer. Radiat Oncol J 2013;31:216. 10.3857/roj.2013.31.4.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Hata M. Radiation therapy for patients with bone metastasis from uterine cervical cancer: its role and optimal radiation regimen for palliative care. Anticancer Res 2018;38:1033–40. 10.21873/anticanres.12319 [DOI] [PubMed] [Google Scholar]
- 186. George R, Rai B. Practical aspects of palliative care & palliative radiotherapy in incurable cervical cancer. Indian J Med Res 2021;154:262–6. 10.4103/ijmr.IJMR_1642_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Perrone AM, Cima S, Pozzati F, et al. Palliative electro-chemotherapy in elderly patients with vulvar cancer: a phase II trial. J Surg Oncol 2015;112:529–32. 10.1002/jso.24036 [DOI] [PubMed] [Google Scholar]
- 188. Corrado G, Cutillo G, Fragomeni SM, et al. Palliative electrochemotherapy in primary or recurrent vulvar cancer. Int J Gynecol Cancer 2020;30:927–31. 10.1136/ijgc-2019-001178 [DOI] [PubMed] [Google Scholar]
- 189. Tranoulis A, Georgiou D, Founta C, et al. Use of electrochemotherapy in women with vulvar cancer to improve quality-of-life in the palliative setting: a meta-analysis. Int J Gynecol Cancer 2020;30:107–14. 10.1136/ijgc-2019-000868 [DOI] [PubMed] [Google Scholar]
- 190. Perrone AM, Ravegnini G, Miglietta S, et al. Electrochemotherapy in vulvar cancer and cisplatin combined with electroporation. Cancers 2021;13:1993. 10.3390/cancers13091993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Perrone AM, Ferioli M, Argnani L, et al. Quality of life with vulvar carcinoma treated with palliative electrochemotherapy: the ELECHTRA (ELEctroCHemoTherapy vulvaR cAncer) study. Cancers (Basel) 2021;13:1622. 10.3390/cancers13071622 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ijgc-2023-004486supp001.pdf (294.5KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.