Abstract
Diphthamide (DPH), a conserved amino acid modification on eukaryotic translation elongation factor eEF2, is synthesized via a complex, multi-enzyme pathway. While DPH is non-essential for cell viability and its function has not been resolved, diphtheria and other bacterial toxins ADP-ribosylate DPH to inhibit translation. Characterizing Saccharomyces cerevisiae mutants that lack DPH or show synthetic growth defects in the absence of DPH, we show that loss of DPH increases resistance to the fungal translation inhibitor sordarin and increases –1 ribosomal frameshifting at non-programmed sites during normal translation elongation and at viral programmed frameshifting sites. Ribosome profiling of yeast and mammalian cells lacking DPH reveals increased ribosomal drop-off during elongation, and removal of out-of-frame stop codons restores ribosomal processivity on the ultralong yeast MDN1 mRNA. Finally, we show that ADP-ribosylation of DPH impairs the productive binding of eEF2 to elongating ribosomes. Our results reveal that loss of DPH impairs the fidelity of translocation during translation elongation resulting in increased rates of ribosomal frameshifting throughout elongation and leading to premature termination at out-of-frame stop codons. We propose that the costly, yet non-essential, DPH modification has been conserved through evolution to maintain translational fidelity despite being a target for inactivation by bacterial toxins.
Graphical Abstract
Graphical Abstract.

INTRODUCTION
The diphthamide (DPH; 2-[3-carboxyamido-3-(trimethylammonio)propyl] histidine) is a conserved histidine modification found in archaeal and eukaryotic translation elongation factor eEF2 (1) (Supplementary Figure S1A). Like its bacterial ortholog EF-G, eEF2 promotes translocation of tRNA and mRNA on the ribosome following peptide bond formation during the translation elongation cycle (2). The DPH is synthesized on eEF2 in a multistep process catalyzed by seven different proteins that have been conserved through evolution (1,3) (Supplementary Figure S1A). Despite this conservation, the genes catalyzing DPH synthesis are non-essential in yeast (4) (Supplementary Figure S1B) and in mammalian cell culture (4,5), though mice lacking DPH are non-viable (6–8).
Consistent with the non-essential nature of DPH, substitution of thirteen different amino acids in place of His699, the site of the DPH modification on yeast eEF2, maintains cell viability but prevents DPH modification (9). The key known role of DPH is to serve as a target for ADP ribosylation (ADPR) by various bacterial toxins including diphtheria toxin, resulting in cell death (reviewed in 3). Biochemical studies have yielded conflicting results on the impact of ADP-ribosylation on eEF2 function. While ADP-ribosylation clearly impairs the ability of eEF2 to promote translation and the modification does not affect GTP or GDP binding to eEF2 nor the binding of eEF2 to vacant 80S ribosomes (10); whether ADP-ribosylation impairs productive binding of eEF2 to elongating ribosomes remains unclear (11,12). High-resolution cryo-EM and X-ray structures of 80S ribosome–eEF2 complexes showed that DPH interacts directly with mRNA or codon-anticodon bases in the decoding center of the ribosome (13–17), leading to proposals that DPH facilitates proper translocation by breaking decoding interactions between conserved rRNA bases and the codon-anticodon duplex in the A site of the ribosome and by preventing mRNA slippage during translocation.
Further supporting a role for DPH in translocation, previous studies reported that loss of DPH increases ribosomal frameshifting on programmed ribosomal frameshifting (PRF) sites linking the gag and pol open reading frames (ORFs) of the yeast L-A virus and of HIV (18–20). A fraction of ribosomes, after translating the gag ORF, shift to the -1 reading frame on a slippery sequence that allows tRNA anticodons to pair with the mRNA codons in overlapping reading frames to translate the pol ORF. This -1 PRF is facilitated by a downstream pseudoknot structure, and a similar –1 PRF is also required for synthesis of the gag-pol fusion protein in SARS-CoV-2 (21). Loss of DPH was found to increase frameshifting on the L-A and HIV programmed sites by ∼1.5–2-fold (18,19). Recently, it was proposed that similar slippery sequences and downstream secondary structures in cellular mRNAs promote -1 frameshifting in a manner suppressed by DPH (22). Based on these studies, it has been proposed that the function of DPH is to promote translational fidelity; however, it is not clear whether the insights obtained from studies employing slippery sites are applicable to general translation elongation and thus fully account for the conservation of DPH.
Intrigued by the evolutionary conservation of a modification that confers susceptibility to a pathogen, yet is non-essential for cell viability, we sought to identify the function of DPH. Characterizing eEF2 mutants that show a heightened requirement for DPH and mutants that lack DPH due to loss of the modification enzymes or mutation of the modified residue, we show that loss of DPH markedly increased -1 ribosomal frameshifting at both programmed viral frameshifting sites containing slippery codons as well as at non-programmed sites. Ribosome profiling of yeast and mammalian cells lacking DPH reveals a global impairment of ribosomal processivity during elongation with increased rates of ribosomal drop-off as the ribosomes progress down mRNAs, and removal of out-of-frame stop codons suppresses this ribosome loss of processivity defect in these cells lacking DPH. Our data reveal that loss of DPH impairs the fidelity of translocation during translation elongation resulting in increased rates of ribosomal frameshifting and premature termination at out-of-frame stop codons.
MATERIALS AND METHODS
Yeast strains and plasmids
Yeast strains and plasmids used in this study are presented in Tables 1 and 2, respectively.
Table 1.
Yeast strains used in this study
| Name | Genotype | Source |
|---|---|---|
| BY4741 | MATa his3-Δ1 leu2-Δ0 met15-Δ0 ura3-Δ0 | Yeast Deletion Collection |
| F2000 | MATa his3-Δ1 leu2-Δ0 met15-Δ0 ura3-Δ0 dph2::KanMX | Yeast Deletion Collection |
| TKY918 (J1158) | MATα ade2 leu2 ura3 his3 trp1 eft1::HIS3 eft2::TRP1 p[EFT2-6xHis URA3 2μ] | (60) |
| TKY675 (J1319) | MATα ade2 leu2 ura3 his3 trp1 eft1::HIS3 eft2::TRP1 p[EFT2-6xHis LEU2 CEN] | (61) |
| TKY742 (J1245) | MATα ade2 leu2 ura3 his3 trp1 eft1::HIS3 eft2::TRP1 p[EFT2-6xHis-H699N LEU2 CEN] | (19) |
| J1239 | MATα ade2 leu2 ura3 his3 trp1 eft1::HIS3 eft2::TRP1 PGAL1-DPH2::KanMX p[EFT2-6xHis URA3 2μ] | This study |
| J1287 | MATα ade2 leu2 ura3 his3 trp1 eft1::HIS3 eft2::TRP1 dph2Δ::KanMX p[EFT2-6xHis URA3 2μ] | This study |
| J1321 | MATα ade2 leu2 ura3 his3 trp1 eft1::HIS3 eft2::TRP1 dph2Δ::KanMX p[EFT2-6xHis LEU2 CEN] | This study |
| J1504 | MATα ade2 leu2 ura3 his3 trp1 eft1::HIS3 eft2::TRP1 upf1Δ p[EFT2-6xHis LEU2 CEN] | This study |
| J1505 | MATα ade2 leu2 ura3 his3 trp1 eft1::HIS3 eft2::TRP1 upf1Δ p[EFT2-6xHis-H699N LEU2 CEN] | This study |
| J1576 | MATα ade2 leu2 ura3 his3 trp1 eft1::HIS3 eft2::TRP1 MDN1-13Myc-KanMX6 p[EFT2-6xHis URA3 2μ] | This study |
| J1578 | MATα ade2 leu2 ura3 his3 trp1 eft1::His3 eft2::TRP1 DYN1-13Myc-KanMX6 p[EFT2-6xHis URA3 2μ] | This study |
| Y4512 (J1633) | W303 (MATa ade2-1 his3-11,15 leu2-3 112 trp1-1 ura3-1) rea1::KanMX6 p[YCG-YLR106C] | (62) |
| J1640 | W303 (MATa ade2-1 his3-11,15 leu2-3 112 trp1-1 ura3-1) rea1::NatMX4 p[YCG-YLR106C] | This study |
| J1641 | W303 (MATa ade2-1 his3-11,15 leu2-3 112 trp1-1 ura3-1) rea1::NatMX4 dph2::KanMX6 p[YCG-YLR106C] | This study |
| yMN2/DBY12394 | MATα ura3Δ leu2Δ0::ACT1pr-Z3EV-NatMX | (24) |
| J1634 | MATα ura3Δ leu2Δ0::ACT1pr-Z3EV-NatMX pC6310[UBI-DTA, URA3] | This study |
| J1636 | MATα ura3Δ leu2Δ0::ACT1pr-Z3EV-NatMX dph2Δ pC6310[UBI-DTA, URA3] | This study |
Table 2.
Plasmids used in this study
| Name | Description | Source |
|---|---|---|
| pTKB612 (pC4002) | EFT2-6xHis LEU2 CEN | (61) |
| pC5533 | EFT2-6xHis-R180S LEU2 CEN | This study |
| pC5111 | YCplac33-DPH2 URA3 CEN | This study |
| pC6725 | -1 ribosomal frameshifting site of SARS-CoV-2 in LacZ gene, URA3 CEN (from pJD204.-1) | This study |
| pC6726 | In-frame control for pC6725 | This study |
| pC6727 | -1 ribosomal frameshifting site of HIV in LacZ gene, URA3 CEN (from pJD204.-1) | This study |
| pC6728 | In-frame control for pC6727 | This study |
| pJD204.-1 (pC5610) | URA3 CEN (-1 ribosomal frameshifting site of L-A virus in N-terminal region of LacZ gene) | (63) |
| pJD204.+1 (pC5609) | URA3 CEN (+1 ribosomal frameshifting site of Ty1 in LacZ gene) | (63) |
| pJD204.0 (pC5608) | URA3 CEN (in-frame control for pC5610 and pC5609) | (63) |
| pC6500 | LacZ gene -1 frame to AUG start codon (no programmed frameshifting site) URA3 CEN (from pJD204.-1) | This study |
| pC6501 | LacZ gene + 1 frame to AUG start codon (no programmed frameshifting site) URA3 CEN (from pJD204.-1) | This study |
| pC6502 | LacZ gene 0 frame to AUG start codon (in-frame control for C6500 and C6501) URA3 CEN | This study |
| pJD375 (pC3752) | pYDL-control (Firefly luciferase 0 frame to Renilla luciferase URA3 CEN) | (25) |
| pC6499 | Firefly luciferase -1 frame to Renilla luciferase in pJD375 URA3 CEN | This study |
| pC6498 | Firefly luciferase + 1 frame to Renilla luciferase in pJD375 URA3 CEN | This study |
| pC3752 | Firefly luciferase 0 frame to Renilla luciferase in pJD375 URA3 CEN (in-frame control for C6499 and C6498) | This study |
| pEKD0209 (pC4310) | pPGK1-Renilla-GluGAA10-firefly URA3 2μ | (26) |
| pC5380 | Renilla ΔG62 reporter URA3 2μ (Deletion of firefly luciferase gene from pEDK0209) | This study |
| pC5379 | In-frame control for pC5380 | This study |
| pC6205 | pSynoYAC0-MDN1-WT HIS3 CEN (pSynoYAC0 from LifeSct) | This study |
| pC6160 | pSynoYAC0-MDN1-LS HIS3 CEN (Low out-of-frame Stop codons, synthesized by LifeSct, Rockville, Maryland) | This study |
| p2luc (pC5150) | Dual (Renilla-Firefly) luciferase reporter | (64) |
| pC6615 | -1 ribosomal frameshifting site from SARS-CoV-2 in p2luc | This study |
| pC6616 | In-frame control for pC6615 | This study |
| pC6619 | -1 ribosomal frameshifting site from HIV in p2luc | This study |
| pC6620 | In-frame control for pC6619 | This study |
| pRS415-REA1 (pC6334) | pRS415 REA1 LEU2 CEN | (62) |
| pRS415-rea1-7 (pC6335) | pRS415 rea1-7 LEU2 CEN | (62) |
| pRS415-rea1-21 (pC6336) | pRS415 rea1-21 LEU2 CEN | (62) |
| pTAP-REA1 (pC6337) | pTAP-REA1 LEU2 CEN | (62) |
| pTAP-rea1-DTS (pC6339) | pTAP-rea1-DTS LEU2 CEN | (62) |
| pMN3 (pC6157) | GFP with modified GAL1 promoter (GCGTGGGCG), URA3 CEN | (24) |
| pC6310 | Ubi-DTA in pMN3 | This study |
| pC4464 | pET15b 6xHis-DTA | Gerald Marsischky & R. John Collier, Harvard Medical School |
Screening of eEF2 mutants
Plasmid pC4002 (pEFT2-6xHis LEU2 CEN) was randomly mutated by passaging through the bacterial mutator strain XL1-Red (Agilent). The mutated plasmids were introduced into the yeast strain J1239 in which DPH2 expression is under control of the galactose-inducible GAL1 promoter. Strain J1239 was generated by directing a kanMX4:PGAL1 promoter cassette (23) to integrate at DPH2. Transformants of J1239 showing normal growth on yeast synthetic galactose (SGal) medium, where DPH2 is expressed and eEF2 is modified with DPH, but slow growth on synthetic dextrose (SD) medium, where DPH2 expression is repressed and eEF2 lacks DPH, were chosen for further analysis. Plasmids were rescued from 36 candidates and introduced into the dph2Δ strain J1287. In this second screen only 3 mutants (R180S, I529N and D810V) were confirmed to confer a slow-growth phenotype in the absence of DPH.
Expression of Ubi-DTA in yeast
To avoid possible toxicity due to leaky expression of DTA under repressing conditions, the ubiquitin (Ubi) coding sequence was appended onto the N-terminus of DTA generating a Ubi-DTA fusion (see Supplementary Table S3). The Ubi-DTA coding sequence was inserted in the plasmid pMN3 (24), downstream of a modified GAL1 promoter containing binding sites for the artificial steroid-responsive transcription factor Z3EV. The plasmid was introduced into yeast strain yMN2/DBY12394 that expresses Z3EV or into its isogenic dph2Δ derivative J1636. Expression of Ubi-DTA was induced by addition of β-estradiol.
LacZ reporter assay
Yeast strains were grown in 20 ml SD (with supplements) medium to A600 = 0.5–1.0. After harvesting, cell pellets were suspended in 0.2 ml Breaking Buffer (100 mM Tris–HCl pH 8.0, 20% glycerol, 1 mM β-mercaptoethanol, 1 mM 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF) and 1× Complete Protease Inhibitor [Roche]). A 50% cell suspension volume of glass beads was added, and cells were broken by vigorous mixing on a vortex for 10 min at 4°C. Following clarification, 0.05 ml cell lysate was mixed with 0.95 ml Z buffer (60 mM Na2HPO4.7H2O, 40 mM NaH2PO4.H2O, 10 mM KCl, 1 mM MgSO4, 50 mM β-mercaptoethanol) and incubated at 28°C for 5 min. When high levels of β-galactosidase activity were anticipated, 0.01 ml lysate was mixed with 0.99 ml Z buffer. Reactions were started by adding 0.2 ml 2-nitrophenyl β-d-galactopyranoside (ONPG) solution (4 mg/ml in Z buffer) and incubated at 28°C. Reactions were stopped by adding 0.5 ml 1 M Na2CO3. Protein concentrations of samples were determined, and β-galactosidase activities (Miller units) were calculated using the formula: units = A420 × 1.7/(0.0045 × [P] × Vol × Time), where [P] = protein concentration in mg/ml; Vol = sample volume (ml) added to the reaction; and Time = time in minutes.
Luciferase reporter assay for yeast
Luciferase assays were performed according to a published protocol (25) with some modifications. Yeast transformants were grown in 2 ml SD medium (with necessary supplements) to A600 = 0.5–1.0. Cells were harvested by centrifugation, washed once with 1 ml ice-cold 1× PBS (pH 7.4) with 1 mM AEBSF, and then suspended in 0.3 ml of the same buffer. Cell suspensions were lysed with glass beads and then clarified as described for the LacZ reporter assays. Luciferase activities were determined using either the Renilla Luciferase Assay System (Promega) or the Dual-Luciferase Assay System (Promega) and measured on a Centro XS3 LB 960 plate reader (Berthold Technologies). For analysis of the Renilla-ΔG62 reporter, a derivatize of pEKD0209 (26) lacking the firefly luciferase coding region, activities were normalized to total protein levels. Frameshift efficiencies were calculated as a percentage of the in-frame control.
Luciferase assay for CHO K1 cells
CHO K1 WT and dph2 mutant cells (4,5) were maintained in DMEM/F-12 media (ThermoFisher Scientific) supplemented with 10% FBS (GIBCO) and penicillin/streptomycin (Quality Biological). Cells were incubated 37°C in 5% CO2. At the start of the experiment 4 × 106 cells were placed in 10-cm plates and incubated overnight to 70% confluence. Cells were then trypsinized transfected using Lipofectamine 2000 reagent (Invitrogen). For transfection, 20 ng of reporter plasmid suspended in 2 μl H2O was mixed with 0.2 μl Lipofectamine 2000 (Invitrogen) and 22.8 μl Opti-MEM (GIBCO) to a final volume of 25 μl. Mixtures were dispensed in the wells of white 96-well half-area plates (Costar), and 2 × 104 cells suspended in 25μl DMEM/F-12 media were added to each well. After 24 h, cells were lysed in 25 μl passive lysis buffer (Promega), and luciferase activities were determined by a Dual Luciferase Stop and Glo Reporter assay system using reagents (27) modified as described previously (28). Relative light units were measured on a CentroXS3 LB960 microplate luminometer fitted with two injectors (Berthold Technologies). For analysis, firefly activity was first normalized to Renilla luciferase activity and then these values for the frameshift reporters were compared to the respective in-frame control reporter to calculate percent frameshifting.
Preparation of ribosome profiling library
Ribosome profiling libraries were prepared according to the protocols of McGlincy (29) with some modifications. Yeast strains were grown in 500 ml SC medium to A600 = 0.5–1.0. For steroid induction of Ubi-DTA, 100 nM β-estradiol was added to the culture of yeast cells at A600 ∼0.2, and cultures were incubated for an additional 4 h for the WT (DPH2) strain or 2 h for the dph2Δ strain. Cells were collected by filtration, frozen in liquid nitrogen with Lysis Buffer (20 mM Tris–HCl pH 7.4, 150 mM NaCl, 5 mM MgCl2, 1 mM DTT, 100 mg/ml cycloheximide (CHX), 1% v/v Triton X-100 and 2 U/μl Turbo DNase [Invitrogen]). In addition to CHX, tigecycline (TIG, 100 μg/ml), as described by Wu et al. (30), was also added to Lysis Buffer for experiments examining ADP-ribosylated eEF2. Frozen cells were lysed using a freezer mill (SPEX SamplePrep Freezer/Mill 6870). Powdered frozen cells were thawed in ice, transferred to a 50 ml falcon tube, and spun at 3000 g for 5 min at 4°C. The lysate was further cleared by centrifugation at 20 000 g for 20 min at 4°C. Aliquots (A260 = 100) of the lysate were frozen in dry ice and stored at −80°C.
For profiling of mammalian cell lines, CHO K1 WT and dph2 mutant cells were grown as described above for the luciferase assay and harvested at ∼70% confluence. After removing the media from the dish, the adherent cells were lysed with 0.5 ml of Lysis Buffer A (per each 150 mm diameter dish) and then spun at 20 000 g for 10 min at 4°C to recover the supernatant.
For ribosome profiling, 60 μg total RNA was incubated with 1.5 μl RNase I (Lucigen, 10 U/μl) in 0.2 ml of reaction volume for 45 min at 25°C with gentle agitation. Reactions were then loaded on 0.8 ml 1M sucrose cushions and subjected to ultracentrifugation at 90 000 rpm for 1 h at 4°C in a Beckman TLA-120.2 rotor. The ribosome pellet was resuspended in 300 μl TRI Reagent (Zymo Research), and the RNA was extracted using a Direct-zol RNA MiniPrep kit (Zymo Research). The extracted RNA was then separated on 15% TBE–urea polyacrylamide gel (Bio-Rad) and fragments 15–35 nucleotides in length were eluted from the gel. The eluted RNA samples were dephosphorylated and then ligated to 5′-pre-adenylated sample-barcoded linkers. Excess unligated linkers were removed using 5′ Deadenylase (NEB) and RecJ exonuclease (Lucigen). The barcoded RNA samples were pooled and then purified using Oligo Clean & Concentrator (Zymo Research). rRNAs were depleted from the eluted RNA samples using QIAseq FastSelect Kit (Qiagen). RNA samples were reverse transcribed using ProtoScript II (NEB) with the reverse transcription primer NI-802 (5′-/5Phos/NNAGATCGGAAGAGCGTCGTGTAGGGAAAGAG/iSp18/GTGACTGGAGTTCAGACGTGTGCTC). The PCR-generated library was sequenced on an Illumina HiSeq2500 (single-end 50 cycle) or an Illumina NovaSeq (single-end 100 cycle) by the DNA Sequencing and Genomics Core at NHLBI, NIH (Bethesda, MD).
For RNA-seq library preparation, 20 μg total RNA was incubated with 10X Fragmentation Reagent (Invitrogen) at 70°C for 15 min, and the reaction was stopped by adding 10% (v/v) of Stop Solution (Invitrogen). RNA Fragments were separated by electrophoresis on a 15% TBE–urea polyacrylamide gel (Bio-Rad) and fragments of 25–34 nucleotides in length (for single-end 50 cycles) or 40–60 nucleotides in length (for single-end 100 cycles) were eluted. RNA fragments were further processed for cDNA library preparation as described above for ribosome profiling.
Processing of fastq sequencing data
The DNA sequencing results were processed by first removing the adapter sequences from the fastq sequences using cutadapt (version 2.8, (31)) with the following parameter: -a AGATCGGAAGAGCAC -m 28 -q 10 –discard-untrimmed. If the DNA samples have sample barcodes and were mixed during the preparation, then the mixed sequences were separated according to the sample barcode. PCR duplicates were removed from DNA sample having UMI (unique molecular identifier) sequences using a custom python script (python 3.9.4). Noncoding RNAs (ncRNA database downloaded from https://useast.ensembl.org/info/data/ftp/index.html) were removed from the sequences by bowtie (version 1.1.2, (32)) with the following parameters: -v 3. The remaining fastq sequences were aligned to the transcriptome using bowtie with the following parameters: -S -v 2 -y -a -m 1 –best –strata –norc. For the yeast transcriptome, the most abundant and longest form of transcripts were selected from the transcriptome data (33) (a bed file constructed by Nick Ingolia, first used in (34), and available upon request); the lengths of the 5′UTR, CDS and 3′UTR for the prominent transcript from each yeast gene are presented in Supplementary Table S4. As some genes in the bed file lack annotated 3′ UTRs, the 3′ end of each transcript was extended by appending the next 25 nt for each transcript as determined from the yeast genome sequence. For analysis of the CHO K1 cell data, CDS sequences for all protein coding transcripts of CHOK1GS_HDv1 (downloaded from https://useast.ensembl.org/info/data/ftp/index.html) were used for alignments and bowtie was used with the same parameters as listed above for yeast.
Analysis of ribosome fraction in each reading frame
To analyze the fraction of ribosomal reads in each of the three reading frames, ribosomal protected fragments (RPF) of 27 or 28 nt in length were aligned to the yeast transcriptome (or aligned to MDN1 or MDN1-LS) and assigned to the 0, –1 or +1 reading frame based on the CDS of each gene and a 12-nt offset from the first position of A-site codon to the 3′ end of the RPF. To avoid possible complications of altered sizes of RPF for initiating and terminating ribosomes, the first 50 codons after each AUG start codon and the last 10 codons before each stop codon were not included for the analysis. Fractions of reads in each reading frame in all genes were plotted on a boxplot drawn with a custom R script using ggplot2 (version 3.3.3).
Measurement of ribosomal drop-off rate
Ribosomal drop-off rate was determined from ribosome profiling data as shown in Supplementary Figure S2A. Briefly, all CDS were aligned at the AUG start codon and then divided into bins of 120 nt. Each ribo-seq read was allocated to the appropriate bin and the number of reads from each bin was normalized to the ribosome read density on the same gene. The medians of the total normalized reads in each bin were plotted versus the bin number (x-axis), and the ribosomal drop-off rate was obtained from the slope of the line determined by linear regression. Ribosomal drop-off rate per codon was calculated as 1 – (1 – R)1/40 where R is drop-off rate per bin (35). Bootstrapping was performed, using the R boot package (version 1.3-24) with default options, to obtain the distribution of the ribosomal drop-off rates and associated errors (script in Supplementary Table S5).
Analysis of ribosome occupancies on A- and P-site codon pairs
Ribosomal occupancy on each possible A- and P-site codon pairs was analyzed using the 26–28 nt RPFs from the ribosome profiling library prepared in the presence of CHX. The A-site codon was assigned based on a 12 nt offset from the 3′ end of the RPF. To define the enrichment of ribosome reads mapping to each of the possible A- and P-site codon pairs, the RPF reads were first normalized by the ribosome density on the respective gene. Then, the sum of all normalized reads mapping to each codon pair was further normalized by the abundance of the respective codon pair in the genome.
Polysome profile analysis
For polysome analysis of yeast expressing Ubi-DTA, strains were grown in SC medium to A600 = 0.2 and then incubated an additional 4 h for WT strain (DPH2) or 2 h for dph2Δ strain after addition of 100 nM β-estradiol. Cells were processed and polysomes were analyzed as previously described except CHX was omitted in some experiments (36). Briefly, cultures were transferred to 500 ml centrifuge bottles containing 30% bottle volume of ice pellets. Cells were then collected by centrifugation at 5000 rpm at 4°C for 10 min in a Sorvall F10S-4 × 1000 rotor. Cell pellets were suspended in 0.5 ml Polysome Buffer (20 mM Tris–HCl [pH 7.5], 50 mM KCl, 10 mM MgCl2, 1 mM DTT, 1× Complete Protease Inhibitor cocktail [Roche]). Following addition of an equal volume of glass beads, cells were broken by 5 cycles of vigorous mixing on a vortex for 1 min followed by 1 min on ice. Lysates were clarified by centrifugation and then ∼10 A260 units of the whole cell extracts (WCEs) were layered on 7–47% sucrose gradients prepared in 20 mM Tris–HCl (pH 7.5), 50 mM KCl, 10 mM MgCl2 and 1 mM DTT. After centrifugation in a Beckman SW41 rotor for 2.5 h at 39 000 rpm, gradients were fractionated with continuous absorbance monitoring using a BioComp Fractionator (BioComp Instruments Ltd, Canada).
Purification of elongation factor eEF2
A poly-histidine tagged version of elongation factor eEF2 was purified from yeast strain TKY675 for eEF2-WT and from J1287 for eEF2 lacking DPH modification, as described previously (37). Briefly, cells were grown in 5 l YPD medium to A600 = 2.0, harvested, and then suspended in 200 ml Lysis Buffer C (50 mM potassium phosphate [pH 7.6], 500 mM KCl, 1 mM DTT, 10 mM imidazole and 1× Complete protease inhibitor EDTA-free [Roche]). Cells were broken using a bead beater (BioSpec Products), and the lysate was cleared by centrifugation at 27 000 × g for 30 min. The clarified lysate was gently mixed with 2 ml Ni-NTA resin for 1 h at 4°C. The resin was then sequentially washed with 10 vol of Lysis Buffer C, 5 vol of Lysis Buffer C containing 20 mM imidazole, and then the His-tagged proteins were eluted in Lysis Buffer C containing 500 mM imidazole. The eluted proteins were dialyzed against Lysis Buffer C containing 100 mM KCl and applied to a HiTrap Q column (GE Healthcare). eEF2 proteins were eluted with a gradient to 1 M KCl in the Buffer C. eEF2 fractions were confirmed by SDS-PAGE, pooled, and dialyzed against Storage Buffer A (20 mM Tris–HCl [pH 7.5], 100 mM potassium acetate, 0.1 mM magnesium acetate, 2 mM DTT and 10% glycerol). Aliquots of the purified protein were stored at –80°C.
Purification of DTA
Escherichia coli BL21(DE3) pLysS cells transformed with pET15b-6xHis-DTA (kindly provided by Gerald Marsischky and R. John Collier, Harvard Medical School) were grown in 500 ml LB medium to A600 = 0.6, and then 0.5 mM IPTG was added to the culture to induce DTA expression for 3 h at 37°C. Cells were harvested and suspended in Lysis Buffer D (50 mM sodium phosphate [pH 7.7], 300 mM NaCl, 6 mM β-mercaptoethanol, 10 mM imidazole). Cells were broken by sonication, and the lysate was cleared of unbroken cells by centrifugation. The clarified lysate was gently mixed with 1 ml Ni-NTA resin (Qiagen) for 1 h at 4°C. The resin was then sequentially washed with 5 vol of the Lysis Buffer D, 5 vol of Lysis Buffer D containing 20 mM imidazole, and then the His-tagged DTA proteins were eluted in the Lysis Buffer D containing 500 mM imidazole. The eluted proteins were dialyzed against Storage Buffer B (20 mM Tris–HCl [pH 7.3], 100 mM KCl, 0.5 mM EDTA, 1 mM DTT and 10% glycerol). After concentration the protein solution was aliquoted and stored at –80°C.
Preparation of ADP-ribosylated eEF2
eEF2 was ADP-ribosylated by mixing 50 μl of 10 μM eEF2 solution, 43 μl of 20 mM Tris–HCl pH 7.5, 1 μl 1M DTT (final 10 mM), 1 μl 5 mM NAD+ (final 50 μM) and 5 μl 2 mg/ml DTA, and incubating the reaction for 30 min at 30°C. Next, the reaction was dialyzed against 20 mM Tris–HCl (pH 7.5), 100 mM potassium acetate and 2 mM DTT, and then concentrated using a microcon YM 50. The reaction products were analyzed by ElectroSpray-Ionization Quadrupole-Time-of-Flight Mass Spectrometry (ESI QTOF MS) to determine the yield of ADP-ribosylated eEF2 (Supplementary Figure S3D).
In vitro reconstituted translation elongation assay
Preparation of 40S and 60S ribosomal subunits was performed according to published protocols (37). Translation initiation factors eIF1, eIF1A, eIF2, eIF5 and eIF5B, and translation elongation factors eEF1A and eEF3 were purified as described previously (37,38). Preparation and aminoacylation of yeast initiator tRNAiMet, tRNAPhe and tRNALys were performed as described previously (37). Assembly of 80S initiation complexes on uncapped model mRNAs was performed as previously described (38). The coding sequence of the model mRNA encoding Met-Phe-Phe-Phe-Lys-stop was AUGUUCUUCUUCAAAUAA. The coding sequence of the model mRNA encoding Met-Phe-Lys-Phe-stop was AUGUUCAAAUUCUAA. Final concentrations of components in the assays were: 4 nM 80S initiation complex, 200 nM eEF1A, 0–100 nM eEF2, 200 nM eEF3, 1 μM aminoacyl tRNA, 1 mM GTP-Mg2+, 1 mM ATP-Mg2+ in 1× Recon Buffer (30 mM HEPES pH 7.5, 100 mM potassium acetate, 1 mM magnesium acetate, 1 mM spermidine and 2 mM DTT). The progress of peptide formation was examined by electrophoretic thin-layer chromatography (TLC) as described previously (39). In assays containing sordarin (Sigma, S1442-5MG), the drug was added at a final concentration of 1 μM.
RESULTS
Genetic screen for eEF2 mutants showing synthetic growth defects in the absence of DPH
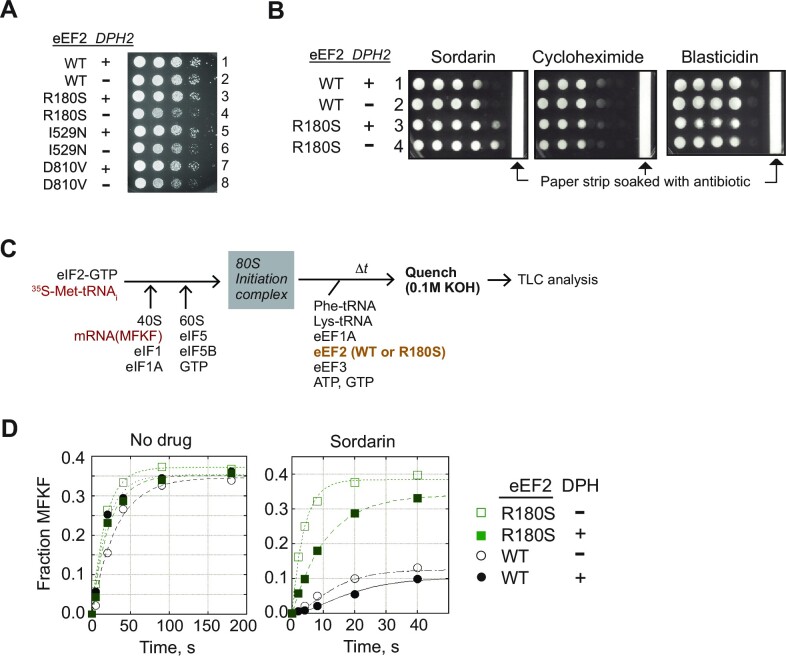
The DPH modification in eEF2 is not essential for yeast cell growth and a dph2Δ strain, which completely lacks the DPH modification (19), grows at a rate comparable to an isogenic WT strain (Supplementary Figure S1B). In contrast, in competitive growth assays yeast lacking DPH are outcompeted by wild-type (WT) cells (40, and Supplementary Figure S1B, C), demonstrating that DPH is beneficial to yeast. To gain insights into the role of DPH, we performed a genetic screen to identify eEF2 mutants that display a synthetic growth defect in the absence of DPH. A plasmid containing the EFT2 gene encoding yeast eEF2 was subjected to random mutagenesis and then introduced into the yeast eft2Δ strain J1239 that carries the essential EFT2 gene on a URA3 plasmid. In J1239, expression of DPH2 is under the control of the galactose-inducible GAL1 promoter. Following selection for loss of the WT EFT2 plasmid (plasmid shuffling) from the transformants, colonies were screened for growth on galactose media where DPH2 is expressed and on glucose media where DPH2 expression is repressed. Colonies showing normal growth on galactose media, indicating that the plasmid-encoded eEF2 is functional when it contains DPH, but slow growth on glucose media, indicating that the eEF2 mutant is defective when it lacks DPH, were selected for further analysis. The EFT2 plasmids were isolated from the candidates and then introduced into isogenic WT and dph2Δ strains to confirm the synthetic growth defect of the EFT2 mutants in strains lacking DPH. From an initial screen of ∼105 yeast transformants, three eEF2 mutants were isolated with the single mutations: R180S, I529N, and D810V, respectively. Notably, the eEF2-R180S mutant displayed the most distinct synthetic growth defect in the absence of DPH (Figure 1A, compare R180S-DPH2 and R180S-dph2Δ).
Figure 1.
eEF2 mutants showing synthetic growth phenotype with dph2Δ mutant. (A) Serial-dilution spotting assay of yeast with the indicated eEF2 and DPH2 alleles on minimal SD medium. The plate was incubated at 30°C for 3 days. (B) Antibiotic sensitivity of the indicated yeast strains was tested by spotting equivalent amounts of the strains on SD medium and then placing a drug-soaked paper strip adjacent to the final column of spots. The plates were incubated at 30°C for 3 days. (C) Scheme for reconstituted in vitro translation elongation assay. (D) Fractions of MFKF peptide synthesis obtained in yeast in vitro reconstituted elongation assays supplemented with solvent (no drug) or sordarin [1 μM] and containing the indicated forms of eEF2 with or without the DPH modification were plotted. Results are representative of three independent experiments.
Sordarins are anti-fungal drugs that inhibit protein synthesis by targeting eEF2 (41,42). Previous studies have shown that elimination of DPH by deleting any of the seven genes required for its synthesis confers sordarin resistance in S. cerevisiae (43,44). Consistent with these earlier reports and as shown in Figure 1B, adph2Δ strain grew slightly better than an isogenic WT strain on media containing a diffused gradient of sordarin (row 2 versus row 1). In addition to loss of DPH, sordarin resistance has also been linked to mutations in EFT2, including substitution of Gly for R180 (42), the same residue that is mutated in one of the three DPH-sensitive mutants (Figure 1A). In accord with this previous report (42), the eEF2-R180S mutation conferred a significant sordarin-resistant growth phenotype both in the presence (row 3) and absence (row 4) of DPH. The resistance of the eEF2-R180S and dph2Δ mutants to the growth inhibitory effects of sordarin is specific as the mutants grew like an isogenic WT control strain on media containing the translation inhibitors cycloheximide or blasticidin (Figure 1B).
Based on the results of assays examining eEF2 function in whole-cell yeast extracts (41,42) as well as structural studies of eEF2 with sordarin and/or an 80S ribosome (17,45,46), sordarins are thought to prevent eEF2 release following GTP hydrolysis and translocation (47). To explore the mechanism of sordarin resistance by the eEF2-R180S and DPH biosynthesis mutants, we conducted in vitro reconstituted translation elongation assays employing WT and mutant forms of eEF2. After assembling 80S initiation complexes that include [35S]Met-tRNAiMet on mRNAs encoding Met-Phe-Lys-Phe-Stop (MFKF), elongation reactions were started by adding a mix of eEF1A, eEF3, Phe-tRNAPhe, Lys-tRNALys, and WT or mutant forms of eEF2 (schematic in Figure 1C). The yield of final product (MFKF peptide) was monitored by electrophoretic thin-layer chromatography. As shown in the Figure 1D, in the absence of sordarin (left panel), neither the R180S mutation nor the loss of DPH in eEF2 significantly affected the yield of MFKF peptide, indicating that these mutations do not significantly impair the translocation activity of eEF2 required for model peptide synthesis. As expected, sordarin strongly inhibited MFKF peptide formation in assays containing WT eEF2 (Figure 1D, right panel, dark-filled circle). In contrast, peptide synthesis in assays containing the eEF2-R180S mutant (green filled square) was largely resistant to sordarin. To examine the effects of DPH in the in vitro assays, WT eEF2 and eEF2-R180S were purified from a dph2Δ strain that lacks the modification (19). In the presence of sordarin, peptide yields in assays containing WT eEF2 lacking DPH were slightly higher than the yields in assays containing the modified factor (Figure 1D, right panel, open versus filled circles), consistent with the improved growth in the presence of sordarin of the dph2Δ strain versus the WT strain (Figure 1B, row 2 versus row 1). Peptide synthesis assays containing unmodified eEF2-R180S showed the greatest yields in the presence of sordarin (Figure 1D, right panel, open squares), approaching the yields observed in the absence of the drug. Based on the model that sordarin blocks eEF2 domain movements required to release the factor from the post-translocation ribosome, the loss of DPH and the R180S mutation might confer sordarin resistance by weakening eEF2 binding to the ribosome. Consistent with this hypothesis, the R180 residue maps to a ribosomal binding interface where eEF2 contacts the ribosomal P-stalk protein Rpp0/uL10 (Supplementary Figure S1D). Interestingly, in addition to EFT2, sordarin-resistant mutations in S. cerevisiae were previously mapped in RPP0 (encoding Rpp0/uL10) (48), raising the possibility that the eEF2–Rpp0 interface plays an important role in eEF2 binding to the ribosome.
Loss of DPH increases non-programmed ribosomal frameshifting
To further explore the function of DPH, we asked if DPH differentially affects translocation of distinct tRNAs on the ribosome. Using a published set of dual-luciferase reporters containing repeats of 10 identical codons between the upstream Renilla and downstream firefly luciferase open reading frames (26), that previously revealed the inhibitory effect of consecutive ArgCGA codons (26) and the important role of eIF5A in translating polyproline sequences (38), we examined whether loss of DPH affected expression from reporters containing specific codon repeats. Whereas the firefly to Renilla luciferase ratio in the WT and dph2Δ strain was comparable for nearly all the reporters, we repeatedly observed an increase in the ratio for the reporter containing 10 repeats of the Glu(GAA) codon. Further analysis of this reporter revealed that the DPH sensitivity was not due to the codon repeats, but instead due to the accidental deletion of a single nucleotide, G62, in the Renilla ORF (Renilla-ΔG62, Supplementary Table S1A). Ribosomes translating Renilla-ΔG62 are predicted to switch to the -1 frame after codon 21, incorporate five amino acids encoded in the -1 frame, and then terminate at a stop codon (Supplementary Table S1A).
To further characterize the impact of DPH and eEF2 on Renilla-ΔG62 translation, matching single ORF Renilla and Renilla-ΔG62 reporters (Figure 2A) were assayed in DPH2+ or dph2Δ strains expressing WT eEF2 or eEF2-R180S. In WT cells, Renilla-ΔG62 reporter activity was less than 0.02% of the in-frame control (Figure 2A). Whereas the dph2Δ and the eEF2-R180S single mutants increased Renilla-ΔG62 reporter activity by 1.7- and 2.1-fold, respectively, the activity increased around 2.6-fold in the eEF2-R180S, dph2Δ double mutant (Figure 2A). As there are no apparent slippery sites for PRF in the 5′ region of the Renilla luciferase ORF (Supplementary Table S1A), we hypothesize that the eEF2-R180S mutation combined with the loss of DPH increased the frequency of random, non-programmed, frameshifting and enabled more ribosomes to synthesize functional luciferase from the mutant Renilla-ΔG62 reporter. To further explore the impact of loss of DPH on non-programmed frameshifting, we examined the mutation of His699 in yeast eEF2, the site of the DPH modification, to Asn (eEF2-H699N). This mutation was previously shown to not affect the growth of yeast on rich medium, but to confer resistance to diphtheria toxin, consistent with the loss of DPH (19). As shown in Figure 2A, the eEF2-H699N mutant increased Renilla-ΔG62 luciferase activity by 2.8-fold compared to strains expressing WT eEF2. Because the eEF2-H699N mutation had a more distinct and consistent impact on frameshifting than deletion of DPH2, perhaps reflecting loss of both DPH and the precursor histidine side chain in the eEF2-H699N mutant, the eEF2-H699N mutant was used to further study how loss of DPH affects translation.
Figure 2.
Enhanced non-programmed frameshifting in yeast lacking DPH. (A) Luciferase activity from out-of-frame Renilla luciferase reporter lacking residue G62 compared to corresponding WT reporter. Alleles of eEF2 and DPH2 were indicated. (B) Percent non-programmed frameshifting on lacZ reporters in the -1 or + 1 frame relative to the AUG start codon compared to inframe control. Error bars in (A, B) denote SD; ****P ≤ 10−4, ***P ≤ 10−3, **P ≤ 10−2, *P ≤ 0.05, ns > 0.05 (Student's two-tailed t test; n ≥ 4, assayed at least twice).
Based on this serendipitous discovery for the role of DPH in maintaining translational fidelity and preventing non-programmed ribosomal frameshifting, we generated additional reporters to explore the impact of DPH on non-programmed frameshifting. Matching reporters were generated in which the AUG start codon was in-frame or in the –1 or + 1 frame relative to the lacZ ORF (Figure 2B and Supplementary Table S1B). In WT cells the basal activity of both the –1 (∼0.1%) and +1 (∼0.05%) reporters relative to the in-frame control was very low, consistent with high translational fidelity in cells expressing WT eEF2 (Figure 2B). The dph2Δ mutation marginally increased expression from –1 and + 1 frameshift reporters, while the loss of DPH in the eEF2-H699N mutant specifically increased β-galactosidase activity from the –1 frameshift reporter by 3-fold (Figure 2B), as was also seen using dual-luciferase reporter assays (Supplementary Figure S1E).
Consistent with previous reports showing that loss of DPH increases frameshifting on the –1 programmed ribosomal frameshifting (PRF) sites on the yeast L-A virus and the HIV mRNAs (18,19), we observed increased frameshifting in the dph2Δ strain using PRF reporters containing an AUG start codon followed by the SARS-CoV-2, HIV, or L-A virus PRF site and then the lacZ ORF in the –1 frame relative to the AUG start codon (Supplementary Figure S1F and Supplementary Table S1D). Whereas deletion of DPH2 increased frameshifting by 1.5–2-fold on the three reporters, the eEF2-H699N mutation increased PRF by >3-fold (Supplementary Figure S1F). The loss of DPH specifically enhanced -1 PRF, as neither the dph2Δ nor the eEF2-H699N mutation significantly increased frameshifting on a reporter containing the +1 PRF site from the yeast Ty1 retrotransposon (Supplementary Figure S1F). Taken together, these reporter assays reveal that loss of DPH relaxes translational fidelity and leads to increased programmed and non-programmed ribosomal frameshifting.
Termination at out-of-frame stop codons increases ribosomal drop-off in cells lacking DPH
Increased rates of random frameshifting in cells lacking DPH are expected to result in loss of ribosomal processivity during elongation, as the frameshifted ribosomes terminate at out-of-frame stop codons and fail to progress to the cognate stop codon. To assess ribosome processivity, we performed ribosome profiling on yeast expressing WT eEF2 or eEF2-H699N. The rate of ribosome disengagement or drop-off from the mRNA—not to be confused with the distinct process of peptidyl-tRNA drop-off (reviewed in (49)—was determined by monitoring ribosome read density across all genes (35). Yeast ORF sequences were divided into bins of 120-nts in length, and the number of RPF reads in each bin for a particular gene was normalized to the read density for the entire gene (Supplementary Figure S2A). The median values of normalized reads from all genes decreased with higher bin number (Figure 3A), indicating that a fraction of ribosomes was disengaging from mRNAs during elongation. The ribosomal drop-off rate was calculated by linear regression of the plot of median read number versus bin number using bin numbers 5–40 (600–4800 nts) (Supplementary Figure S2A). In WT cells ribosomal drop-off was very rare, occurring once in ∼104 codons (Figure 3B), and the rate increased 2-fold in the eEF2-H699N mutant (Figures 3A–B). This increase in ribosomal drop-off was not due to premature termination triggering nonsense-mediated mRNA decay (NMD), as the increased ribosomal drop-off was maintained following deletion of UPF1 to block NMD (see (50) (Figure 3B)). Moreover, RNAseq analysis confirmed comparable RNA levels in the WT and eEF2-H699N mutant strain regardless of gene size (Supplementary Figure S2D).
Figure 3.
Increased ribosomal drop-off in the absence of DPH due to termination at out-of-frame stop codons. (A) Plot of normalized median read counts versus bin number (see Supplementary Figure S2A for detailed scheme; bin size = 40 codons). Two WT (blue) and two eEF2-H699N mutant (purple) replicates are shown. (B) Medians and 95% confidence intervals of ribosomal drop-off rate obtained by linear regression and bootstrapping of data from dashed box in (A); ****P ≤ 10−4 (Student's two-tailed t test; n = 104, analyzed in two replicates). (C) Western blot of C-terminal Myc-epitope tagged Mdn1 and Dyn1 probed using anti-Myc tag antibodies; eIF2α loading control was probed using polyclonal antiserum. (D) Normalized ribosomal reads on the MDN1-WT or MDN1-LS (low-stop allele lacking most out-of-frame stop codons) mRNAs in the indicated strains. (E) Sum of ribosomal reads per 1.2-kb bin from (D) was normalized and plotted versus bin number. (F) Fractions of ribosomes on 0, –1 or + 1 reading frame on MDN1-WT or MDN1-LS mRNAs in WT or eEF2-H699N mutant strain.
The loss of ribosomal processivity in cells lacking DPH is predicted to impair production of longer proteins. Myc-epitope tags were inserted at the C-terminus of the chromosomal MDN1 (also known as REA1) (4910 amino acids) and DYN1 (4092 amino acids) genes. As shown in Figure 3C, the levels of both proteins were substantially decreased in the eEF2-H699N mutant. Consistent with the reduced levels of Mdn1 in cells lacking DPH, deletion of DPH2 exacerbated growth defects associated with missense mutations in MDN1 (Supplementary Figure S2B). This defect in synthesis of long proteins might account for the embryonic lethal phenotype in mice lacking DPH (6–8).
Consistent with the reduced levels of Mdn1 in the eEF2-H699N mutant, ribosome profiling revealed a dramatic loss of ribosomal reads at the 3′ end of the nearly 15-kb MDN1 ORF in the eEF2-H699N mutant versus the WT strain (Figures 3D, E) that was not due to loss of the MDN1 mRNA (Supplementary Figures S2E-F). To test the hypothesis that the decreased ribosomal processivity in the strain lacking DPH was due to ribosomal frameshifting and termination at out-of-frame stop codons, we constructed an MDN1 low stop (MDN1-LS) mutant allele by introducing synonymous codon substitutions that eliminated all 506 stop codons in the –1 frame and all but 28 of the 358 stop codons in the +1 frame (Supplementary Figure S2C and Supplementary Table S2). As shown in Figures 3D–E, removal of the out-of-frame stop codons suppressed the ribosomal drop-off defect in the eEF2-H699N mutant without affecting the MDN1 mRNA (Supplementary Figures S2E, F). Moreover, the fraction of ribosomal reads mapping to the –1 and +1 frames was increased on the MDN1-LS allele in the eEF2-H699N mutant (Figure 3F). Taken together, these results support the hypothesis that loss of DPH reduces the fidelity of translation elongation with increased frameshifting and termination at out-of-frame stop codons (Figure 4A).
Figure 4.
DPH controls frameshifting and ribosome drop-off in mammalian cells. (A) Model depicting stochastic frameshifting in absence of DPH that triggers termination at out-of-frame stop codons leading to enhanced ribosomal drop-off. (B) Dual luciferase reporter assay analyzing frameshifting relative to inframe control on SARS-CoV2 or HIV PRF sites in CHO K1 WT or dph2 cell line. Error bars denote SD; ****P ≤ 10−4 (Student's two-tailed t test; n = 6, assayed in duplicate). (C) Plot of normalized median read counts versus bin number for two WT replicates (blue) and two dph2 mutants (purple). Bin size = 40 codons. (D) Medians and 95% confidence intervals of ribosomal drop-off rate obtained by linear regression and bootstrapping of data in dashed box in (C); ****P ≤ 10−4 (Student's two-tailed t test; n = 104, analyzed in two replicates).
Loss of ribosomal processivity in mammalian cells lacking DPH
The DPH modification is conserved in many archaea and nearly all eukaryotes (1). To determine whether DPH contributes to translational fidelity in mammals, ribosomal frameshifting was assessed in WT and dph2 mutant derivatives of the Chinese Hamster Ovary cell line CHO-K1 (4,5). As shown in Figure 4B, loss of DPH increased -1 frameshifting on both the SARS-CoV-2 and HIV PRF sites in the mammalian cell line, consistent with a previous report that loss of DPH increased -1 PRF in mammalian cells (18). Ribosome profiling revealed an increased rate of ribosomal drop-off in dph2 mutant cells (Figures 4C, D), demonstrating that loss of DPH alone was sufficient to increase ribosomal drop-off and indicating that DPH has a conserved role across eukaryotes to enhance translational fidelity.
ADP-ribosylation of DPH impairs functional binding of eEF2 to the ribosome
ADP-ribosylation of DPH by diphtheria and other bacterial toxins inhibits translation and causes cell death (reviewed in 3); however, the mechanism of translational inhibition is not clearly understood. Expression of the catalytic diphtheria toxin fragment A (DTA) in yeast impaired translation elongation with an increase in the polysome to 80S monosome ratio (Figure 5A), resembling the impact of translation elongation inhibitor cycloheximide (Supplementary Figure S3A, (51)). Ribosome profiling revealed a nearly 4-fold increase in the ratio of large RPFs (26 to 29 nt), indicating a filled A site (20), to small (19 to 21 nt) RPFs, representing a vacant A site (20), upon ADPR of eEF2 (∼3.8 in WT to ∼14.3 with ADPR-eEF2) (Figure 5B and Supplementary Figure S3B), consistent with a defect in translocation upon ADPR of eEF2 (Figure 5C). The ribosomal occupancies for the majority of A- and P-site codon pairs were not altered upon ADPR of eEF2, indicating that ADPR of eEF2 equally affects all ribosomal translocations (Supplementary Figures S3C).
Figure 5.
ADP-ribosylation of eEF2 by diphtheria toxin DTA impairs translocation. (A) Polysome profile analysis (-cycloheximide, CHX) of yeast before or after steroid-induced expression of Ubi-DTA (Supplementary Table S3). Polysome to monosome (P/M) ratio and positions of 40S, 60S and 80S ribosomes and polysomes are indicated. (B) Distribution of RPF lengths from ribosome profiling of strains containing eEF2-WT (dph2Δ, two replicates in green) or ADP-ribosylated eEF2 (ADPR-eEF2; two replicates in blue) and processed in the presence of CHX and tigecycline (TIG). (C) Schematic of translation elongation showing ribosomal complex with vacant A site that generates short RPF (Complex 1) and the steps of elongation inhibited by cycloheximide (CHX), tigecycline (TIG) and ADP-ribosylation of eEF2. (D) Fractions of MFFFK peptide synthesis in yeast in vitro reconstituted elongation assays with indicated amounts of eEF2-WT or ADPR-eEF2 were plotted. Results are representative of three independent experiments.
To further define how ADPR of eEF2 impacts translocation, unmodified and ADPR forms of eEF2 were examined using in vitro reconstituted translation elongation assays. Similar to the schematic depicted in Figure 1C, 80S initiation complexes were prepared on mRNA coding for Met-Phe-Phe-Phe-Lys (MFFFK). Synthesis of this peptide requires multiple rounds of eEF2-catalyzed ribosomal translocation before base-catalyzed release of the completed pentapeptide. Whereas WT eEF2 readily promoted peptide synthesis, higher concentrations of ADPR-eEF2 were required to show comparable levels of MFFFK formation. Thus, 25 nM ADPR-eEF2 yielded similar levels of peptide synthesis as 6.25 nM of the WT factor (Figure 5D, Supplementary Figure S3E). The restoration of peptide synthesis by increasing the concentration of ADPR-eEF2 indicates, as suggested previously (12), that ADPR inhibits protein synthesis by decreasing the binding affinity of eEF2 to ribosomal elongation complexes (Figure 5C).
DISCUSSION
The conservation of DPH among eukaryotes and many archaea requires the conservation of seven gene products required to synthesize the modification. Despite DPH serving as a target for inactivation of translation by pathogens, the conservation of the modification indicates that DPH has an important function. Thus, it was surprising that DPH is non-essential in yeast and in mammalian cells in culture. Previous studies linked DPH to translational fidelity based on increased levels of frameshifting on viral programmed -1 frameshifting sites in cells lacking DPH (18,19). These viral frameshift sites consist of a slippery sequence, a string of nucleotides that permits pairing of the P- and A-site tRNAs in both the 0 and -1 reading frames, followed by downstream secondary structure, typically a stem-loop or pseudoknot (52). Recently, it was suggested that DPH has been conserved in evolution to prevent frameshifting on cryptic slippery sites upstream of secondary structures that occur by chance in cellular mRNAs (22). Dysregulation of the TOR pathway in cells lacking DPH was attributed to spurious frameshifting on cryptic programmed sites in mRNAs encoding activators of TORC1 signaling (22). Our results expand on these findings and reveal that lack of DPH increases frameshifting genome-wide including at sites that lack slippery sequences, thus revealing a critical role for DPH in maintaining the fidelity of translation elongation.
The serendipitous finding that lack of DPH elevated the expression of a Renilla luciferase reporter containing an accidental frameshift mutation early in the CDS, in a region lacking in slippery sequences, reveals that DPH supports proper frame maintenance throughout elongation. Ribosome profiling data further support this conclusion. First, we observed increased frequency of ribosome drop-off in the absence of DPH in both yeast (Figure 3A, B) and mammalian cells (Figure 4C, D). Second, the increased rate of ribosome drop-off on the MDN1 mRNA was suppressed by removing out-of-frame stop codons (Figure 3D, E), consistent with the idea that ribosome frameshifting followed by termination at out-of-frame stop codons accounts for the ribosomal drop-off defect observed in cells lacking DPH. Notably, the previous bioinformatic analysis to detect cryptic frameshift sites in the yeast genome, that was used to link DPH to the TOR pathway (22), did not identify any sites on the MDN1 mRNA, indicating that the lack of DPH enhances frameshifting throughout elongation and not just at select cryptic frameshifting sites. Taken together, our results reveal that the DPH modification on eEF2 has been retained through evolution to enhance the fidelity of translation elongation and prevent spurious frameshifting. Though not essential in yeast or in mammalian cells in culture, loss of DPH impaired production of long proteins (Figure 3C) and showed synthetic growth defects in yeast with mutations in the long Mdn1/Rea1 protein (Supplementary Figure S2B). Thus, even at a low rate, spurious frameshifting can prove deleterious, and we propose that the impaired production of long proteins might account for the embryonic lethal phenotype in mice lacking DPH (6–8).
Our study provides new insights into two mechanisms of eEF2 regulation. First, the identification of synthetic growth defects in mutants lacking DPH and carrying the eEF2-R180S mutation prompted us to examine the inhibition of eEF2 by the fungal-specific antibiotic sordarin. Sordarin, a tetracyclic diterpene glycoside (47), inhibits translation by blocking eEF2-catalyzed GTP hydrolysis and translocation (53). Sordarin-resistant mutations in eEF2 were mapped throughout the protein including in the GTP-binding domain and domains III-V (42). One sordarin-resistant mutation, R180G, maps to the same residue as the synthetic mutant, R180S, identified in our studies (Figure 1), (42). Interestingly, loss of diphthamide also confers resistance to sordarin (43). Additional sordarin-resistant mutations have been mapped to the ribosomal P stalk protein uL10 (also called P0) (48,54). In recent structures of eEF2 bound to the yeast 80S ribosome (14,17), the R180 residue in eEF2 is in close proximity to uL10 residues T134, Q137 and T143 that when mutated confer resistance to sordarin (48). Accordingly, we hypothesize that the R180 mutations and uL10 mutations destabilize the same eEF2–80S interface, and that loss of DPH might act similarly to confer sordarin resistance by weakening the interaction between eEF2 and the ribosome. Notably, peptide synthesis catalyzed by the eEF2-R180S mutant protein lacking DPH is strongly resistant to sordarin (Figure 1D). While sordarin has been proposed to inhibit translation by either preventing release of eEF2 from the post-translocation complex (42) or by inhibiting translocation (53), the ability of the eEF2-R180S mutant lacking DPH to efficiently promote translation in the presence of sordarin favors the former model with the mutations weakening eEF2 binding to the ribosome and thereby enabling the factor to release from the post-translocation complex.
Second, we examined the mechanism by which ADP-ribosylation of DPH by diphtheria toxin impairs translation. Whereas diphtheria toxin is a highly potent inhibitor of translation, with as few as one molecule per cell thought to sufficient to inhibit cell growth (55), the mechanism by which ADP-ribosylation impairs the function of eEF2 has been unclear. Our studies support earlier work (19,51) and show that expression of diphtheria toxin in yeast cells impairs translation and cell growth (Figure 5A and Supplementary Figure S3A). Moreover, the enrichment of 26–29 nt ribosome protected mRNA fragments in cells expressing diphtheria toxin (Figure 5B and Supplementary Figure S3B) indicates that ribosomes are accumulating in a pre-translocation state with the A site occupied. Conflicting results have been reported regarding the impact of ADP-ribosylation on eEF2 binding to vacant or translating ribosomes (10–12). Focusing on the functional interaction of eEF2 with translating ribosomes using in vitro reconstituted translation assays, we found that ∼4-fold higher concentrations of ADP-ribosylated eEF2 was required to stimulate peptide synthesis to the same extent as the factor lacking ADP-ribosylation (Figure 5D). As >85% of the eEF2 protein used in the assay was ADP-ribosylated (Supplementary Figure S3D), the translocation activity is unlikely to be due to the presence of unmodified factor. Thus, we conclude that ADP-ribosylation impairs translation by reducing the affinity of eEF2 for translating ribosomes.
A recent high resolution crystal structure of a yeast translocation complex with eEF2 bound in the A site of an 80S ribosome and two tRNAs bound in unique hybrid A/P and P/E states (14), provides structural insights into the role of DPH. The DPH modification is proposed to trigger unlocking of the rRNA decoding nucleotides (A1755, A1756 in yeast, A1492, A1493 in bacteria) from the codon-anticodon duplex in the A site, thereby lowering the energy barrier for translocation. Moreover, DPH is proposed to stabilize the codon-anticodon duplex during translocation from the A- to the P-site. This chaperone function of DPH is mediated by direct interactions between the DPH side chain and the codon-anticodon duplex and by electrostatic interactions with the phosphate backbones of mRNA and rRNA (14). Loss of these interactions in cells lacking DPH would be expected destabilize the codon-anticodon duplex during translocation, thereby accounting for the increased rates of frameshifting on both programmed and non-programmed sites in cells lacking DPH.
In bacteria, which lack DPH, the tip of domain IV of EF-G, the ortholog of eEF2, is extended to enable interaction with aminoacyl-tRNA in the ribosomal A site (56,57). As noted above, similar interactions have been observed for the DPH residue in eEF2 (13–17)(Supplementary Figure S1G). Mutation of conserved residues at the tip of domain IV of EF-G, like loss of DPH on eEF2, increases ribosomal frameshifting (58,59), indicating a common fidelity function for the tip of domain IV in eEF2 and EF-G. So, why have eukaryotes conserved seven genes through evolution to synthesize DPH and confer sensitivity to diphtheria and other bacterial toxins? We propose that the retention of DPH in eukaryotes, despite an alternative structure working in bacteria, indicates that the fidelity advantage offered by DPH outweighs the inhibitory costs associated with ADPR of the residue by bacterial toxins.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Alan Hinnebusch, Jon Lorsch, Nick Guydosh and members of their labs for helpful discussions; Stephen Leppla, Ira Pastan and Uli Brinkmann for providing DPH mutant cell lines; Scott McIsaac for the steroid-inducible yeast strain and plasmids, Jon Dinman for frameshift reporter plasmids, Gerald Marsischky and R. John Collier for the DTA expression plasmid, and the NHLBI Biochemistry and DNA Sequencing Cores for analyses.
Contributor Information
Byung-Sik Shin, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD 20892, USA.
Ivaylo P Ivanov, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD 20892, USA.
Joo-Ran Kim, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD 20892, USA.
Chune Cao, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD 20892, USA.
Terri G Kinzy, Department of Biochemistry and Molecular Biology, Rutgers, The State University of New Jersey, Piscataway, NJ 08854, USA.
Thomas E Dever, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD 20892, USA.
Data Availability
Sequencing data were deposited in the GEO database under the accession number GSE176169. Images of TLC results from in vitro reconstituted translation elongation assays, plates from yeast cell growth assays, and films from western blot analyses were deposited in the Mendeley database at: https://data.mendeley.com/drafts/5tr9hmm7×9. R scripts for calculation of ribosomal drop-off rate and for drawing boxplots are shown Supplementary Table S5.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Intramural Research Program of the National Institutes of Health; Eunice Kennedy ShriverNational Institute of Child Health and Human Development (NICHD) [HD001010 to T.E.D.]; Intramural Targeted Anti-COVID-19 (ITAC) Award. Funding for open access charge: Intramural Research Program of the National Institutes of Health.
Conflict of interest statement. None declared.
REFERENCES
- 1. Narrowe A.B., Spang A., Stairs C.W., Caceres E.F., Baker B.J., Miller C.S., Ettema T.J.G.. Complex evolutionary history of translation elongation factor 2 and diphthamide biosynthesis in archaea and parabasalids. Genome. Biol. Evol. 2018; 10:2380–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dever T.E., Dinman J.D., Green R.. Translation elongation and recoding in eukaryotes. Cold Spring Harb. Perspect. Biol. 2018; 10:a032649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schaffrath R., Abdel-Fattah W., Klassen R., Stark M.J.. The diphthamide modification pathway from Saccharomyces cerevisiae–revisited. Mol. Microbiol. 2014; 94:1213–1226. [DOI] [PubMed] [Google Scholar]
- 4. Liu S., Milne G.T., Kuremsky J.G., Fink G.R., Leppla S.H.. Identification of the proteins required for biosynthesis of diphthamide, the target of bacterial ADP-ribosylating toxins on translation elongation factor 2. Mol. Cell. Biol. 2004; 24:9487–9497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moehring J.M., Moehring T.J., Danley D.E.. Posttranslational modification of elongation factor 2 in diphtheria toxin-resistant mutants of CHO-K1 cells. Proc. Natl. Acad. Sci. U.S.A. 1980; 77:1010–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen C.M., Behringer R.R.. Ovca1 regulates cell proliferation, embryonic development, and tumorigenesis. Genes Dev. 2004; 18:320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu S., Wiggins J.F., Sreenath T., Kulkarni A.B., Ward J.M., Leppla S.H.. Dph3, a small protein required for diphthamide biosynthesis, is essential in mouse development. Mol. Cell. Biol. 2006; 26:3835–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Webb T.R., Cross S.H., McKie L., Edgar R., Vizor L., Harrison J., Peters J., Jackson I.J.. Diphthamide modification of eEF2 requires a J-domain protein and is essential for normal development. J. Cell Sci. 2008; 121:3140–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kimata Y., Kohno K.. Elongation factor 2 mutants deficient in diphthamide formation show temperature-sensitive cell growth. J. Biol. Chem. 1994; 269:13497–13501. [PubMed] [Google Scholar]
- 10. Jorgensen R., Yates S.P., Teal D.J., Nilsson J., Prentice G.A., Merrill A.R., Andersen G.R.. Crystal structure of ADP-ribosylated ribosomal translocase from Saccharomyces cerevisiae. J. Biol. Chem. 2004; 279:45919–45925. [DOI] [PubMed] [Google Scholar]
- 11. Davydova E.K., Ovchinnikov L.P.. ADP-ribosylated elongation factor 2 (ADP-ribosyl-EF-2) is unable to promote translocation within the ribosome. FEBS. Lett. 1990; 261:350–352. [DOI] [PubMed] [Google Scholar]
- 12. Nygard O., Nilsson L.. Reduced ribosomal binding of eukaryotic elongation factor 2 following ADP-ribosylation. Difference in binding selectivity between polyribosomes and reconstituted monoribosomes. Biochim. Biophys. Acta. 1985; 824:152–162. [DOI] [PubMed] [Google Scholar]
- 13. Abeyrathne P.D., Koh C.S., Grant T., Grigorieff N., Korostelev A.A.. Ensemble cryo-EM uncovers inchworm-like translocation of a viral IRES through the ribosome. Elife. 2016; 5:e14874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Djumagulov M., Demeshkina N., Jenner L., Rozov A., Yusupov M., Yusupova G.. Accuracy mechanism of eukaryotic ribosome translocation. Nature. 2021; 600:543–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flis J., Holm M., Rundlet E.J., Loerke J., Hilal T., Dabrowski M., Burger J., Mielke T., Blanchard S.C., Spahn C.M.T.et al.. tRNA translocation by the eukaryotic 80S ribosome and the impact of GTP hydrolysis. Cell Rep. 2018; 25:2676–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murray J., Savva C.G., Shin B.S., Dever T.E., Ramakrishnan V., Fernandez I.S.. Structural characterization of ribosome recruitment and translocation by type IV IRES. Elife. 2016; 5:e13567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pellegrino S., Demeshkina N., Mancera-Martinez E., Melnikov S., Simonetti A., Myasnikov A., Yusupov M., Yusupova G., Hashem Y.. Structural insights into the role of diphthamide on elongation factor 2 in mRNA reading-frame maintenance. J. Mol. Biol. 2018; 430:2677–2687. [DOI] [PubMed] [Google Scholar]
- 18. Liu S., Bachran C., Gupta P., Miller-Randolph S., Wang H., Crown D., Zhang Y., Wein A.N., Singh R., Fattah R.et al.. Diphthamide modification on eukaryotic elongation factor 2 is needed to assure fidelity of mRNA translation and mouse development. Proc. Natl. Acad. Sci. U.S.A. 2012; 109:13817–13822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ortiz P.A., Ulloque R., Kihara G.K., Zheng H., Kinzy T.G.. Translation elongation factor 2 anticodon mimicry domain mutants affect fidelity and diphtheria toxin resistance. J. Biol. Chem. 2006; 281:32639–32648. [DOI] [PubMed] [Google Scholar]
- 20. Zhang H., Quintana J., Utkur K., Adrian L., Hawer H., Mayer K., Gong X., Castanedo L., Schulten A., Janina N.et al.. Translational fidelity and growth of Arabidopsis require stress-sensitive diphthamide biosynthesis. Nat. Commun. 2022; 13:4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kelly J.A., Olson A.N., Neupane K., Munshi S., San Emeterio J., Pollack L., Woodside M.T., Dinman J.D.. Structural and functional conservation of the programmed -1 ribosomal frameshift signal of SARS coronavirus 2 (SARS-CoV-2). J. Biol. Chem. 2020; 295:10741–10748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang Y., Lin Z., Zhu J., Wang M., Lin H.. Diphthamide promotes TOR signaling by increasing the translation of proteins in the TORC1 pathway. Proc. Natl. Acad. Sci. U.S.A. 2021; 118:e2104577118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Longtine M.S., McKenzie A. III, Demarini D.J., Shah N.G., Wach A., Brachat A., Philippsen P., Pringle J.R. Additonal modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998; 14:953–961. [DOI] [PubMed] [Google Scholar]
- 24. McIsaac R.S., Oakes B.L., Wang X., Dummit K.A., Botstein D., Noyes M.B.. Synthetic gene expression perturbation systems with rapid, tunable, single-gene specificity in yeast. Nucleic Acids Res. 2013; 41:e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harger J.W., Dinman J.D.. An in vivo dual-luciferase assay system for studying translational recoding in the yeast Saccharomyces cerevisiae. RNA. 2003; 9:1019–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Letzring D.P., Dean K.M., Grayhack E.J.. Control of translation efficiency in yeast by codon-anticodon interactions. RNA. 2010; 16:2516–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dyer B.W., Ferrer F.A., Klinedinst D.K., Rodriguez R.. A noncommercial dual luciferase enzyme assay system for reporter gene analysis. Anal. Biochem. 2000; 282:158–161. [DOI] [PubMed] [Google Scholar]
- 28. Ivanov I.P., Shin B.S., Loughran G., Tzani I., Young-Baird S.K., Cao C., Atkins J.F., Dever T.E.. Polyamine control of translation elongation regulates start site selection on antizyme inhibitor mRNA via ribosome queuing. Mol. Cell. 2018; 70:254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McGlincy N.J., Ingolia N.T.. Transcriptome-wide measurement of translation by ribosome profiling. Methods. 2017; 126:112–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu C.C., Zinshteyn B., Wehner K.A., Green R.. High-resolution ribosome profiling defines discrete ribosome elongation states and translational regulation during cellular stress. Mol. Cell. 2019; 73:959–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011; 17:10–12. [Google Scholar]
- 32. Langmead B., Trapnell C., Pop M., Salzberg S.L.. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009; 10:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pelechano V., Wei W., Steinmetz L.M.. Extensive transcriptional heterogeneity revealed by isoform profiling. Nature. 2013; 497:127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martin-Marcos P., Zhou F., Karunasiri C., Zhang F., Dong J., Nanda J., Kulkarni S.D., Sen N.D., Tamame M., Zeschnigk M.et al.. eIF1A residues implicated in cancer stabilize translation preinitiation complexes and favor suboptimal initiation sites in yeast. Elife. 2017; 6:e31250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sin C., Chiarugi D., Valleriani A.. Quantitative assessment of ribosome drop-off in E. coli. Nucleic Acids Res. 2016; 44:2528–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shin B.S., Dever T.E.. Molecular genetic structure-function analysis of translation initiation factor eIF5B. Methods Enzymol. 2007; 429:185–201. [DOI] [PubMed] [Google Scholar]
- 37. Shin B.S., Katoh T., Gutierrez E., Kim J.R., Suga H., Dever T.E.. Amino acid substrates impose polyamine, eIF5A, or hypusine requirement for peptide synthesis. Nucleic Acids Res. 2017; 45:8392–8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gutierrez E., Shin B.S., Woolstenhulme C.J., Kim J.R., Saini P., Buskirk A.R., Dever T.E.. eIF5A promotes translation of polyproline motifs. Mol. Cell. 2013; 51:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eyler D.E., Green R.. Distinct response of yeast ribosomes to a miscoding event during translation. RNA. 2011; 17:925–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hawer H., Utkur K., Arend M., Mayer K., Adrian L., Brinkmann U., Schaffrath R.. Importance of diphthamide modified EF2 for translational accuracy and competitive cell growth in yeast. PLoS One. 2018; 13:e0205870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dominguez J.M., Martin J.J.. Identification of elongation factor 2 as the essential protein targeted by sordarins in Candida albicans. Antimicrob. Agents Chemother. 1998; 42:2279–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Justice M.C., Hsu M.J., Tse B., Ku T., Balkovec J., Schmatz D., Nielsen J.. Elongation factor 2 as a novel target for selective inhibition of fungal protein synthesis. J. Biol. Chem. 1998; 273:3148–3151. [DOI] [PubMed] [Google Scholar]
- 43. Botet J., Rodriguez-Mateos M., Ballesta J.P., Revuelta J.L., Remacha M.. A chemical genomic screen in Saccharomyces cerevisiae reveals a role for diphthamidation of translation elongation factor 2 in inhibition of protein synthesis by sordarin. Antimicrob. Agents Chemother. 2008; 52:1623–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Uthman S., Bar C., Scheidt V., Liu S., ten Have S., Giorgini F., Stark M.J., Schaffrath R.. The amidation step of diphthamide biosynthesis in yeast requires DPH6, a gene identified through mining the DPH1-DPH5 interaction network. PLoS Genet. 2013; 9:e1003334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jorgensen R., Ortiz P.A., Carr-Schmid A., Nissen P., Kinzy T.G., Andersen G.R.. Two crystal structures demonstrate large conformational changes in the eukaryotic ribosomal translocase. Nat. Struct. Biol. 2003; 10:379–385. [DOI] [PubMed] [Google Scholar]
- 46. Spahn C.M., Gomez-Lorenzo M.G., Grassucci R.A., Jorgensen R., Andersen G.R., Beckmann R., Penczek P.A., Ballesta J.P., Frank J.. Domain movements of elongation factor eEF2 and the eukaryotic 80S ribosome facilitate tRNA translocation. EMBO J. 2004; 23:1008–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shao Y., Molestak E., Su W., Stankevic M., Tchorzewski M.. Sordarin- an anti-fungal antibiotic with a unique modus operandi. Br. J. Pharmacol. 2022; 179:1125–1145. [DOI] [PubMed] [Google Scholar]
- 48. Justice M.C., Ku T., Hsu M.J., Carniol K., Schmatz D., Nielsen J.. Mutations in ribosomal protein L10e confer resistance to the fungal-specific eukaryotic elongation factor 2 inhibitor sordarin. J. Biol. Chem. 1999; 274:4869–4875. [DOI] [PubMed] [Google Scholar]
- 49. Muller C., Crowe-McAuliffe C., Wilson D.N.. Ribosome rescue pathways in bacteria. Front Microbiol. 2021; 12:652980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kervestin S., Jacobson A.. NMD: a multifaceted response to premature translational termination. Nat. Rev. Mol. Cell Biol. 2012; 13:700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mateyak M.K., Kinzy T.G.. ADP-ribosylation of translation elongation factor 2 by diphtheria toxin in yeast inhibits translation and cell separation. J. Biol. Chem. 2013; 288:24647–24655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Atkins J.F., Loughran G., Bhatt P.R., Firth A.E., Baranov P.V.. Ribosomal frameshifting and transcriptional slippage: from genetic steganography and cryptography to adventitious use. Nucleic Acids Res. 2016; 44:7007–7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dominguez J.M., Gomez-Lorenzo M.G., Martin J.J.. Sordarin inhibits fungal protein synthesis by blocking translocation differently to fusidic acid. J. Biol. Chem. 1999; 274:22423–22427. [DOI] [PubMed] [Google Scholar]
- 54. Santos C., Ballesta J.P.. Role of the ribosomal stalk components in the resistance of Aspergillus fumigatus to the sordarin antifungals. Mol. Microbiol. 2002; 43:227–237. [DOI] [PubMed] [Google Scholar]
- 55. Yamaizumi M., Mekada E., Uchida T., Okada Y.. One molecule of diphtheria toxin fragment A introduced into a cell can kill the cell. Cell. 1978; 15:245–250. [DOI] [PubMed] [Google Scholar]
- 56. Ramrath D.J., Lancaster L., Sprink T., Mielke T., Loerke J., Noller H.F., Spahn C.M.. Visualization of two transfer rnas trapped in transit during elongation factor G-mediated translocation. Proc. Natl. Acad. Sci. U.S.A. 2013; 110:20964–20969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhou J., Lancaster L., Donohue J.P., Noller H.F.. How the ribosome hands the A-site tRNA to the P site during EF-G-catalyzed translocation. Science. 2014; 345:1188–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Peng B.Z., Bock L.V., Belardinelli R., Peske F., Grubmuller H., Rodnina M.V.. Active role of elongation factor G in maintaining the mRNA reading frame during translation. Sci. Adv. 2019; 5:eaax8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Niblett D., Nelson C., Leung C.S., Rexroad G., Cozy J., Zhou J., Lancaster L., Noller H.F.. Mutations in domain IV of elongation factor EF-G confer -1 frameshifting. RNA. 2021; 27:40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ortiz P.A., Kinzy T.G.. Dominant-negative mutant phenotypes and the regulation of translation elongation factor 2 levels in yeast. Nucleic Acids Res. 2005; 33:5740–5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jorgensen R., Carr-Schmid A., Ortiz P.A., Kinzy T.G., Andersen G.R.. Purification and crystallization of the yeast elongation factor eEF2. Acta. Crystallogr. D Biol. Crystallogr. 2002; 58:712–715. [DOI] [PubMed] [Google Scholar]
- 62. Ulbrich C., Diepholz M., Bassler J., Kressler D., Pertschy B., Galani K., Bottcher B., Hurt E.. Mechanochemical removal of ribosome biogenesis factors from nascent 60S ribosomal subunits. Cell. 2009; 138:911–922. [DOI] [PubMed] [Google Scholar]
- 63. Harger J.W., Meskauskas A., Nielsen J., Justice M.C., Dinman J.D.. Ty1 retrotransposition and programmed +1 ribosomal frameshifting require the integrity of the protein synthetic translocation step. Virology. 2001; 286:216–224. [DOI] [PubMed] [Google Scholar]
- 64. Grentzmann G., Ingram J.A., Kelly P.J., Gesteland R.F., Atkins J.F.. A dual-luciferase reporter system for studying recoding signals. RNA. 1998; 4:479–486. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequencing data were deposited in the GEO database under the accession number GSE176169. Images of TLC results from in vitro reconstituted translation elongation assays, plates from yeast cell growth assays, and films from western blot analyses were deposited in the Mendeley database at: https://data.mendeley.com/drafts/5tr9hmm7×9. R scripts for calculation of ribosomal drop-off rate and for drawing boxplots are shown Supplementary Table S5.