Abstract
Purpose
Cluster analyses on inflammatory markers of chronic rhinosinusitis (CRS) in Asians from multicenter data are lacking. This multicenter study aimed to identify the endotypes of CRS in Koreans and to evaluate the relationship between the endotypes and clinical parameters.
Methods
Nasal tissues were obtained from patients with CRS and controls who underwent surgery. The endotypes of CRS were investigated by measuring interleukin (IL)-5, interferon (IFN)-γ, IL-17A, IL-22, IL-1β, IL-6, IL-8, matrix metalloproteinase-9, eotaxin-3, eosinophil cationic protein, myeloperoxidase (MPO), human neutrophil elastase (HNE), periostin, transforming growth factor-β1, total immunoglobulin E (IgE), and staphylococcal enterotoxin (SE)-specific IgE. We performed hierarchical cluster analysis and evaluated the phenotype, comorbidities, and Lund-Mackay computed tomography (LM CT) score in each cluster.
Results
Five clusters and 3 endotypes were extracted from 244 CRS patients: cluster 1 had no upregulated mediators compared to the other clusters (mild mixed inflammatory CRS); clusters 2, 3, and 4 had higher concentrations of neutrophil-associated mediators including HNE, IL-8, IL-17A, and MPO (T3 CRS); and cluster 5 had higher levels of eosinophil-associated mediators (T2 CRS). SE-specific IgE was undetectable in T3 CRS and had low detectable levels (6.2%) even in T2 CRS. The CRS with nasal polyps (CRSwNP) phenotype and LM CT scores showed no significant differences between T2 and T3 CRS, while the incidence of comorbid asthma was higher in T2 CRS than T3 CRS. In T3 clusters, higher levels of neutrophilic markers were associated with disease severity and CRSwNP phenotype.
Conclusions
In Koreans, there is a distinct T3 CRS endotype showing a high proportion of CRSwNP and severe disease extent, along with T2 CRS.
Keywords: Asians, biomarker, cluster analysis, nasal polyps, sinusitis, multicenter study, surgery
INTRODUCTION
Chronic rhinosinusitis (CRS) is an inflammatory disease of the sinonasal mucosa with duration of at least 12 weeks; its prevalence is 12% in the United States,1 10.9% in Europe,2 and 7%–8.4% in Korea.3,4 CRS is a vastly heterogeneous disorder with a wide spectrum of inflammatory patterns. Moreover, the immunological features of CRS vary across different geographic locations and racial backgrounds, further complicating the heterogeneity of CRS.5,6,7 Studies in Caucasian patients have demonstrated that CRS with nasal polyps (CRSwNP) is characterized by eosinophilic and type 2 (T2) inflammation, with the expression of interleukin (IL)-4, IL-5, IL-13, and immunoglobulin E (IgE), whereas type 1 (T1) inflammation with interferon (IFN)-γ expression and type 3 (T3) inflammation with IL-17A expression predominate in CRS without nasal polyps (CRSsNP).8,9,10,11,12,13 In contrast, approximately half of the Asian CRSwNP patients demonstrate non-eosinophilic inflammation involving T1 and T3 inflammation as well as neutrophilic inflammation.14,15,16
Given the immunological heterogeneity of CRS, several studies have performed endotyping of CRS based on inflammatory profiles using cluster analysis to identify diverse clinical and immunological features of CRS and determine therapeutic arms.5,6,15,17,18,19,20,21 Tomassen et al.21 carried out a cluster analysis of 14 inflammatory markers in 173 CRS patients in Europe and identified 10 clusters demonstrating distinct endotypes. In Chinese populations,15,18 Lou et al.18 showed that CRSwNP patients could be classified into 5 phenotypes based on the predominant presence of plasma cells, lymphocytes, neutrophils, and mixed inflammatory cells. Additionally, Liao et al.15 subclassified Chinese CRS patients into 7 clusters and documented their associations with treatment outcomes; their classification included 28 clinical indicators and 39 cellular/molecular markers as cluster variables. Although that study integrated various multidimensional characteristics of CRS, the 7 clusters did not completely reflect the pure inflammatory endotype as they included clinical parameters such as the presence of nasal polyps. In addition, cluster analyses based on multicenter data in Asia are lacking.
Considering different immunologic profiles of CRS between Western and Asian countries, further studies involving cluster analysis with inflammatory markers for Asian populations from multicenter data are warranted to achieve a better understanding of the endotypes of CRS. Hence, the present multicenter study aimed to identify endotypes of CRS in the Korean population and sought to assess the relationships between the endotype and nasal polyp phenotype, comorbid asthma, and disease extent.
MATERIALS AND METHODS
Subjects and sample collection
Healthy controls and patients with CRS were recruited from the otolaryngology-head and neck clinics at 19 institutions in Korea in this prospective multicenter study. The diagnosis of asthma was based on medical history and lung function tests, as confirmed by allergy specialists. Preoperative computed tomography (CT) scans were analyzed according to the grading system of Lund-Mackay CT (LM CT) scores.22 This study was approved by the ethics committees of all individual institutions and that of Seoul National University Hospital, Boramae Medical Center (20-2019-100), and written informed consent was obtained from all subjects before sampling. Ethmoid tissues were obtained from control subjects and CRSsNP patients, and nasal polyp tissues from CRSwNP patients. Further details are provided in the Supplementary Data S1.
Measurement of inflammatory markers in nasal tissue
We selected 16 inflammatory markers based on previous reports.21,23 The protein concentrations of tissue extracts were determined using the Pierce 660 nm Protein Assay Kit (Thermo Fisher Scientific ., Waltham, MA, USA). Enzyme-linked immunosorbent assays for IL-22 and human neutrophil elastase (HNE) were performed with commercially available kits (all from R&D Systems, Minneapolis, MN, USA). Multiplex cytokine analysis kits (IL-1β, IL-5, IL-6, IL-8, IL-17A, IFN-γ, eotaxin-3, myeloperoxidase [MPO], matrix metalloproteinase [MMP]-9, TGF-β1, and periostin) were purchased from R&D Systems, and data were collected using a Luminex 100 reader (Luminex, Austin, TX, USA). Data analysis was conducted using MasterPlex QT version 2.0 (MiraiBio, Alameda, CA, USA). The levels of total IgE, specific IgE to staphylococcal enterotoxins (SE-IgE [SEA, SEB, SEC, and TSST-1]), and eosinophil cationic protein (ECP) in nasal tissue homogenates were measured using the ImmunoCAP assay (Thermo Fisher Scientific). A SE-IgE level > 0.35 IU/mL for at least one SE-specific IgE (SEA, SEB, SEC, and TSST-1) was defined as SE-specific IgE positive. All assay procedures mentioned were run in duplicate according to the manufacturer’s protocol. All protein levels in the tissue homogenates were normalized to the concentration of total protein. Further details are provided in the Supplementary Data S1.
Statistical analysis
Sixteen cluster variables that reflect the inflammatory profiles of CRS were selected (Table 1). We performed hierarchical cluster analysis based on the correlation ratio and exploratory factor analysis (EFA) of the mixed data.24 Orthogonal rotation with the varimax method was performed, and only variables with factor loadings > 0.4 were retained. Ward’s minimum variance was used for hierarchical clustering.25 For cluster analysis of individual cases, the Euclidean distance was calculated, which allowed for both ordinal and binomial data. Next, the optimal number of clusters was determined by the NbClust R software package, which uses a variety of indices to find the optimal number of clusters ranging from 3 to 10 in a partitioning of a data set during the clustering process.26 Further details are provided in the Supplementary Data S1. All statistical analyses were conducted using R for Windows version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria). A P value less than 0.05 was considered significant.
Table 1. The inflammatory markers used as cluster variables.
| Marker | Interpretation of increased levels |
|---|---|
| IFN-γ | Type 1 inflammation |
| IL-5 | Type 2 inflammation |
| IL-17A | Type 3 inflammation |
| IL-22 | Cytokine for epithelial homeostasis |
| IL-1β | Proinflammatory action |
| IL-6 | Proinflammatory action |
| IL-8 | Neutrophilic chemotaxis |
| MPO | Neutrophilic activity marker |
| HNE | Neutrophilic activity marker |
| MMP-9 | Marker for extracellular matrix homeostasis |
| ECP | Eosinophilic activity marker |
| Eotaxin-3 | Eosinophilic chemotaxis |
| Total IgE | Adaptive immunity marker for type 2 inflammation |
| SE-IgE | Marker for superantigen effect on local mucosa |
| TGF-β1 | Fibrosis/regulatory T-cell activation |
| Periostin | Marker for tissue remodeling in type 2 inflammation |
Cutoff value of 0.35 IU/mL is given for SE-IgE that was analyzed as categorical.
IFN, interferon; IL, interleukin; MPO, myeloperoxidase; HNE, human neutrophil elastase; MMP, matrix metalloproteinase; ECP, eosinophil cationic protein; IgE, immunoglobulin E; SE, staphylococcal enterotoxin; TGF, transforming growth factor.
RESULTS
Characteristics of the study cohort
Of the 287 CRS patients and 27 control participants recruited, 244 and 27, respectively, who had complete clinical data and an adequate amount of tissue collected were enrolled in all of the intended analyses. Among the 244 CRS patients, 208 had CRSwNP (85.2%), 36 had CRSsNP (14.8%), and 20% had comorbid asthma (41/205). The demographic characteristics and inflammatory profiles of participants are demonstrated in Supplementary Table S1. Most of the inflammatory markers used in the cluster analysis had significantly higher levels in the CRSwNP or CRSsNP groups than in the control group. However, there were no significant differences in the levels of some inflammatory markers including IFN-γ, MMP-9, HNE, and periostin. In addition, the TGF-β1 level was higher in the control group than in the CRSwNP or CRSsNP groups.
Clustering outcomes
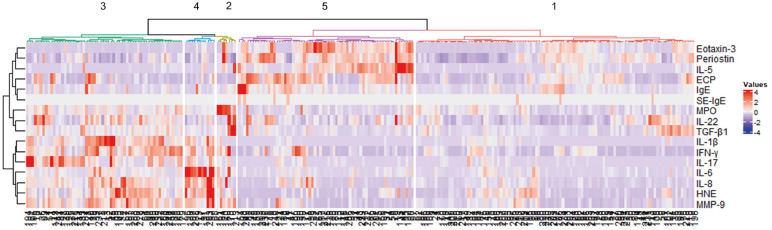
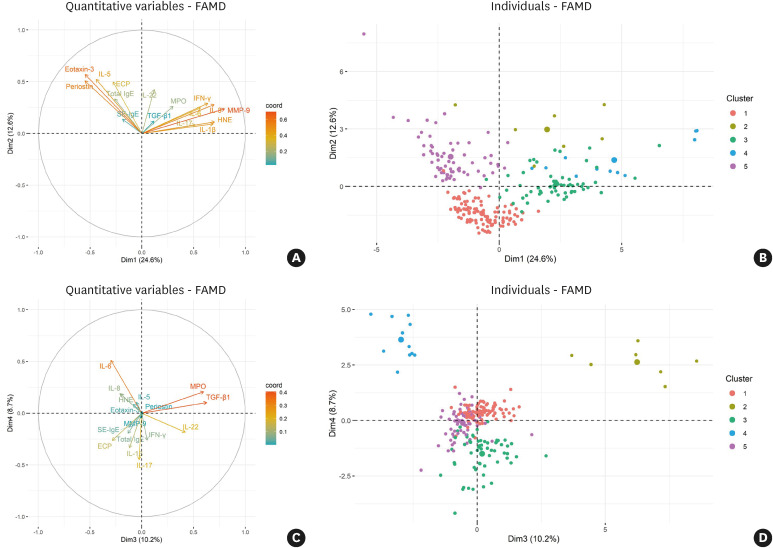
Five clusters were established as the optimal number through hierarchical clustering (Fig. 1). EFA retained 6 dimensions accounting for 68.5% of all variances in the data (Supplementary Table S2), and the first and second dimensions accounted for 37.2% of the total variance (Fig. 2A and B). Individual patient data are presented in dimensions categorized by cluster, and the clusters were separated from each other (Fig. 2B and D). A modified heatmap with inflammatory markers was generated to characterize the clusters (Fig. 3).
Fig. 1. Heatmap of the hierarchical clustering. The dendrogram on the left side shows the cluster variables, and the dendrogram on the bottom shows the 244 CRS patients in this study. Five clusters of CRS patients were obtained, as illustrated on the top of the dendrogram.
CRS, chronic rhinosinusitis; IL, interleukin; ECP, eosinophil cationic protein; IgE, immunoglobulin E; SE, staphylococcal enterotoxin; MPO, myeloperoxidase; TGF, transforming growth factor; IFN, interferon; HNE, human neutrophil elastase; MMP, matrix metalloproteinase.
Fig. 2. Exploratory factor analysis based on principal component analysis and multiple correspondence analysis. (A) The first and second dimensions accounted for 24.6% and 12.6% of total variance, respectively. Arrows are displayed for inflammatory markers used as cluster variables against the first 2 dimensions. (B) Individual patients were categorized by the cluster against the first 2 dimensions. Dots are displayed for individual patients, and they are divided into 5 colors based on the cluster. (C) The third and fourth dimensions accounted for 10.2% and 8.7% of the total variance, respectively. (D) Individual patients were categorized by the cluster against the third and fourth dimensions.
FAMD, factor analysis of mixed data; IL, interleukin; ECP, eosinophil cationic protein; IgE, immunoglobulin E; SE, staphylococcal enterotoxin; MPO, myeloperoxidase; TGF, transforming growth factor; IFN, interferon; HNE, human neutrophil elastase; MMP, matrix metalloproteinase.
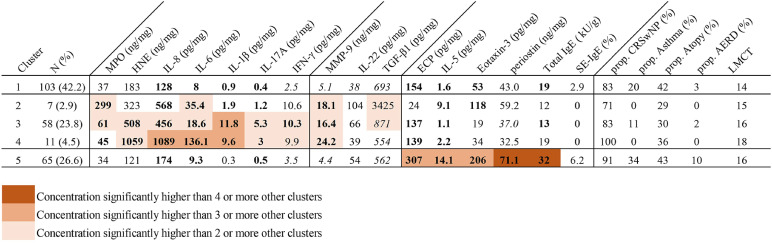
Fig. 3. Modified heat map of inflammatory markers and clinical features according to clusters. Rows indicate clusters of patients with CRS, and columns define inflammatory markers used for cluster analysis (cellular markers, chemotaxis-associated markers, and others). Mean values for each marker are provided for each cluster. Phenotypes, the presence of asthma as a comorbidity, and disease extent according to clusters are demonstrated, although these parameters were not used in the cluster analysis. To characterize clusters, a multiple-group comparison for between-cluster differences is visualized with color coding, as shown in the legend. In addition, differences in inflammatory markers between each cluster and controls were demonstrated with bold character and italic characters; bold and italic characters mean higher and lower levels than controls, respectively.
CRS, chronic rhinosinusitis; MPO, myeloperoxidase; HNE, human neutrophil elastase; IL, interleukin; IFN, interferon; MMP, matrix metalloproteinase; TGF, transforming growth factor; ECP, eosinophil cationic protein; IgE, immunoglobulin E; SE, staphylococcal enterotoxin; prop. CRSwNP, proportion of chronic rhinosinusitis with nasal polyps; AERD, aspirin exacerbated respiratory disease; LM CT, Lund-Mackay computed tomography.
Cluster 1: mild mixed inflammation (mild mixed group; n = 103, 42.2%)
Cluster 1 was the largest of the 5 clusters (42.2%). The levels of inflammatory markers were not significantly higher in this cluster than in the other clusters (Fig. 3). However, the levels of T2 (ECP, IL-5, eotaxin-3, and total IgE) and T3 (IL-17A and IL-8) inflammatory markers as well as of proinflammatory markers (IL-6, IL-1β, and MMP-9) were significantly higher in cluster 1 than in the control group.
Clusters 2, 3, and 4: T3 inflammation (T3 group; n = 76, 31.1%)
Clusters 2, 3, and 4 exhibited neutrophilic inflammation with T3 inflammatory profiles mixed with T1 inflammation and accounted for about one-third of all patients (31.1%). Cluster 2 accounted for 2.9% of all patients and showed mild T3 inflammation with the highest levels of MPO and TGF-β1. The patients in this cluster showed the lowest proportion of CRSwNP and atopy among the clusters. Cluster 3 accounted for 23.8% of all patients and the largest proportion among the T3 clusters (clusters 2, 3, and 4). Cluster 3 showed T1 inflammation as well as moderate T3 and proinflammatory profiles (IL-6, IL-1β, and MMP-9). Cluster 4 exhibited more severe T3 inflammation, characterized by higher levels of IL-8 and IL-6 than cluster 3. The proportion of CRSwNP in cluster 4 was 100%, and no patients in cluster 4 had asthma. In addition, patients in cluster 4 showed the greatest disease severity, as determined by the LM CT score, among the clusters (Supplementary Table S3). To sum up, stratification of T3 inflammation was observed, and these clusters were also correlated to the CRSwNP phenotype and disease extent.
Cluster 5: T2 inflammation (T2 group; n = 65, 26.6%)
Cluster 5, which accounted for 26.6% of all patients, showed typical T2 inflammatory profiles with elevated ECP, IL-5, eotaxin-3, and total IgE levels. The level of periostin, a marker of tissue remodeling in T2 inflammation, was also significantly higher in cluster 5 than in the other clusters and controls. The positivity of SE-specific IgE was 6.2%, which was the highest among the clusters. The proportion of CRSwNP was 91%, and this cluster had the highest proportion of asthmatic patients (34%).
Endotypes of CRS derived from cluster analysis
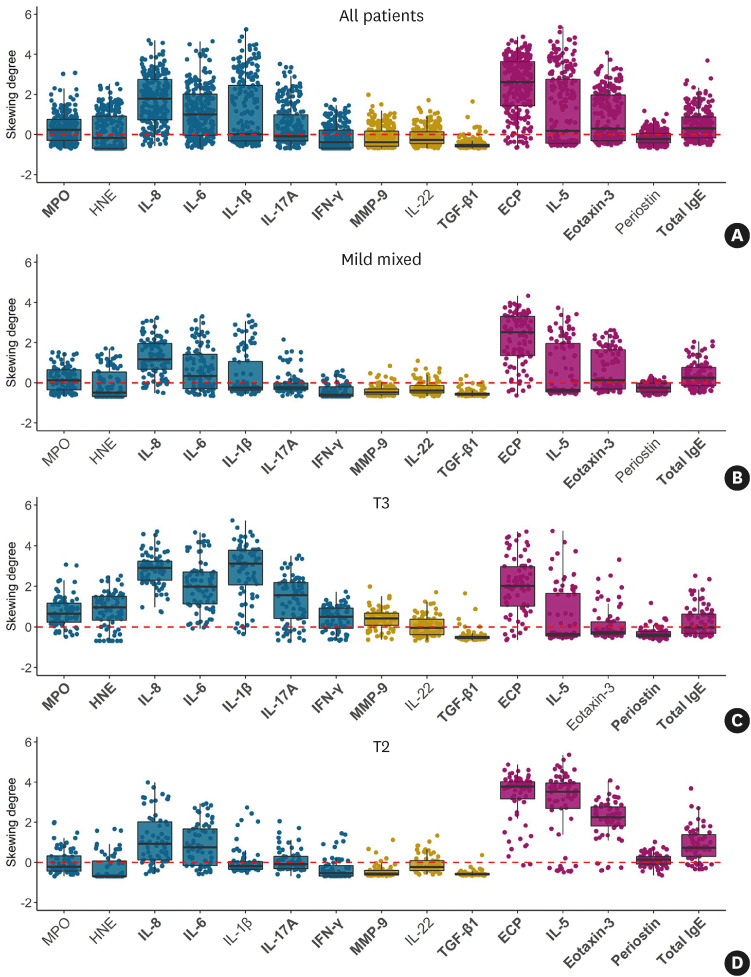
To characterize the 3 endotype groups elicited from cluster analysis, we compared the cytokine levels between these groups and controls, which were standardized using skewing degree (Fig. 4). In total CRS patients, MPO, IL-8, IL-6, IL-1β, IL-17A, ECP, IL-5, eotaxin-3, and total IgE levels were higher than in controls, while IFN-γ, MMP-9, and TGF-β1 levels were lower in total CRS patients than in controls. The mild mixed inflammation group had higher levels of IL-8, IL-6, IL-1β, IL-17A, ECP, IL-5, eotaxin-3, and total IgE than controls, whereas the mild mixed group had lower levels of IFN-γ, MMP-9, IL-22, and TGF-β1 than controls. In the T3 group, MPO, HNE, IL-8, IL-6, IL-1β, IL-17A, IFN-γ, MMP-9, ECP, IL-5, and total IgE levels were higher than in controls, while the levels of TGF-β1 and periostin were lower in the T3 group than in controls. Notably, the skewing degree of neutrophilic and proinflammatory markers (MPO, HNE, IL-8, IL-6, IL-1β, IL-17A, IFN-γ, and MMP-9) were markedly higher, and the levels of IFN-γ and MMP-9 were higher in the T3 group than in controls, unlike the mild mixed inflammation group. As expected, the T2 group showed higher levels of IL-8, IL-6, IL-17A, ECP, IL-5, eotaxin-3, periostin, and total IgE, whereas IFN-γ, MMP-9, and TGF-β1 levels were lower in the T2 group than in controls. Altogether, the T2 group showed a similar inflammatory pattern to that of the mild mixed inflammation group; however, the high skewing degree of eosinophilic markers and significantly higher periostin levels than the control group were noted. Additionally, IL-1β levels were not significantly different from those of the controls.
Fig. 4. Standardized cytokine levels in CRS patients compared to controls using skewing degree. Dots display the values for individual patients, and a boxplot was made to demonstrate the median value, interquartile range, and 95% confidence interval of skewing degree for each cytokine. Bold text on the x-axis denotes a cytokine that is significantly different from the controls in the original values. (A) All patients. (B) Mild mixed group. (C) T3 group. (D) T2 group.
CRS, chronic rhinosinusitis; T, type; MPO, myeloperoxidase; HNE, human neutrophil elastase; IL, interleukin; IFN, interferon; MMP, matrix metalloproteinase; TGF, transforming growth factor; ECP, eosinophil cationic protein; IgE, immunoglobulin E.
Next, we analyzed the demographics and clinical characteristics of patients with each endotype (Table 2). The proportion of patients with a history of asthma was higher in the T2 group than in the T3 group (P = 0.002). In addition, the T2 group had higher incidence of serum and tissue eosinophilia than the other groups. Considering the extent of disease, the LM CT scores were higher in the T3 and T2 groups than in the mild mixed inflammation group (P = 0.018 and P = 0.035, respectively). To investigate the inflammatory mediators associated with disease extent, we evaluated the correlation between the LM CT score and each cytokine level according to endotype (Supplementary Table S4). In all patients, IL-8, IL-17A, and IL-5 levels were weakly but positively correlated to the LM CT score, and IL-22 level was inversely correlated to the LM CT score. In the mild mixed inflammation group, IL-22 and TGF-β1 levels were inversely correlated to the LM CT score. IL-8 level was positively correlated to the LM CT score in the T3 group. However, no inflammatory mediators showed significant correlations to the LM CT score in the T2 group.
Table 2. Demographics and inflammatory profiles of endotype groups.
| Characteristics | Mild mixed (n = 103, 42.2%) | Type 3 (n = 76, 31.1%) | Type 2 (n = 65, 26.6%) | P value |
|---|---|---|---|---|
| Age (yr) | 51 (42–60) | 53 (36–62) | 49 (41–57) | 0.647 |
| Sex, ratio (male:female) | 82:21 | 45:31 | 43:22 | 0.010 |
| Presence of nasal polyp (%) | 85/103 (82.5) | 64/76 (84.2) | 59/65 (90.8) | 0.325 |
| Asthma (%) | 16/82 (19.5) | 5/64 (7.8) | 20/59 (33.9) | 0.001 |
| AERD (%) | 3/95 (3.2) | 1/70 (1.4) | 6/63 (9.5) | 0.088 |
| Atopy (%) | 42/101 (41.6) | 22/72 (30.6) | 27/63 (42.9) | 0.245 |
| Serum eosinophil (%) | 3.2 (1.5–5.8) | 1.7 (1.1–3.3) | 5.5 (3.0–8.5) | < 0.001 |
| Tissue eosinophil count (cells/HPF) | 4 (1–18) | 1 (0–5) | 17 (4–33) | < 0.001 |
| Revision surgery (%) | 15/103 (14.6) | 16/76 (21.1) | 9/65 (13.8) | 0.458 |
| Lund-Mackay score | 14 (10–18) | 17 (13–20) | 15 (12–21) | 0.010 |
| SNOT-22 | 32 (22–50) | 37 (25–55) | 39 (26–53) | 0.250 |
| IFN-γ (pg/mg) | 1.1 (0–4.5) | 9.7 (5.4–14.8) | 1.7 (0–4.8) | < 0.001 |
| IL-5 (pg/mg) | 0 (0–2.4) | 0 (0–1.9) | 11.7 (5.1–18.0) | < 0.001 |
| IL-17A (pg/mg) | 0 (0–0) | 3.2 (0.6–6.6) | 0 (0–0) | < 0.001 |
| IL-22 (pg/mg) | 30 (20–49) | 55.3 (31.0–84.7) | 43 (29–63) | < 0.001 |
| IL-1β (pg/mg) | 0 (0–1.0) | 7.8 (2.6–15.0) | 0 (0–0) | < 0.001 |
| IL-6 (pg/mg) | 3.2 (1.5–9.4) | 16.6 (7.0–34.1) | 4.8 (1.9–12.0) | < 0.001 |
| IL-8 (pg/mg) | 79 (48–174) | 448 (241–625) | 63 (28–185) | < 0.001 |
| MPO (ng/mg) | 31 (15–51) | 52 (34–89) | 21 (13–37) | < 0.001 |
| MMP-9 (ng/mg) | 4.1 (2.0–6.7) | 16.4 (11.6–21.1) | 2.6 (1.2–5.5) | < 0.001 |
| HNE (ng/mg) | 65 (0–299) | 458 (233–787) | 0 (0–188) | < 0.001 |
| ECP (pg/mg) | 98 (28–275) | 55 (21–166) | 350 (149–430) | < 0.001 |
| Eotaxin-3 (pg/mg) | 19 (11–88) | 12 (10–22) | 163 (105–271) | < 0.001 |
| Total IgE (kU/g) | 12 (8–24) | 8 (6–14) | 22 (11–31) | < 0.001 |
| SE-IgE (%, positivity) | 3/103 (2.9) | 0/76 (0) | 4/65 (6.2) | 0.082 |
| TGF-β1 (pg/mg) | 493 (290–734) | 705 (508–972) | 425 (312–627) | < 0.001 |
| Periostin (ng/mg) | 44.2 (25.2–60.3) | 32.8 (23.4–45.3) | 70.3 (55.2–81.7) | < 0.001 |
Value is presented as median with interquartile range. Bold text indicates statistical significance (P < 0.05).
AERD, aspirin exacerbated respiratory disease; HPF, high-power field; SNOT-22, sino-nasal outcome test-22; IFN, interferon; IL, interleukin; MPO, myeloperoxidase; HNE, human neutrophil elastase; MMP, matrix metalloproteinase; ECP, eosinophil cationic protein; IgE, total immunoglobulin E; SE, staphylococcal enterotoxin; TGF, transforming growth factor.
DISCUSSION
In this study, the inflammatory endotypes of CRS in the Korean population were derived from multicenter data by cluster analysis based on inflammatory markers, unbiased by phenotype data. Patients were clustered by the inflammatory markers, and 5 clusters were derived: one cluster with mild mixed inflammation (cluster 1), 3 clusters with T3 inflammation (clusters 2, 3, and 4), and one cluster with moderate T2 inflammation (cluster 5).
In the present study, it was found that there was a difference in inflammatory CRS endotypes between the previous cluster analysis in Europe21 and the present Korean multicenter study. The previous cluster analysis demonstrated that CRS could be categorized into non-T2, moderate, and severe T2 inflammation, with a clear increase in CRSwNP phenotype and comorbid asthma.21 Unlike that study, we showed that CRS could be classified into mild mixed, T3, and moderate T2 inflammation in the Korean population. In Western populations, T2 inflammation plays a major role in determining the inflammatory profile, and markedly eosinophilic inflammation is accompanied by substantial levels of neutrophilic inflammation in CRSwNP patients. However, CRSwNP in China and Korea revealed that only a small percentage of CRSwNP patients (4.4%–11.2%) had both high eosinophilic and neutrophilic inflammation.23,27,28 A divergence between T3 and T2 inflammation was consistently observed in CRS studies in Asian populations, including this cluster analysis,15,28,29 suggesting that both T3 and T2 inflammation may play major roles in determining the inflammatory profiles of CRS in Asian populations.
In the T3 group (clusters 2, 3, and 4), the skewing degrees of neutrophilic and proinflammatory mediators were prominent. Furthermore, elevated levels of IFN-γ, a T1 marker, were also present in the T3 group, which was not found in the mild mixed and T2 groups. The T3 group consisted of 3 clusters, and stratification of T3 inflammatory patterns was observed among these clusters (mild T3, cluster 2; moderate T3, cluster 3; and severe T3, cluster 4). Moreover, the stratification of T3 inflammation also correlated to phenotype and disease extent. Unlike our findings, in the study of Liao et al.,15 clusters derived from molecular and cellular factors did not show significant differences in a number of clinical characteristics. However, our study showed that the clusters generated by only inflammatory markers correlated to phenotypes.
In the mild mixed and T2 groups, although MPO expression is commonly consistent with HNE expression in neutrophils, our previous study revealed that MPO was upregulated in the controlled CRSwNP patients, but HNE-positive cells and HNE levels were upregulated in the refractory CRSwNP patients.23 Cluster 2, which exhibited high MPO and relatively low HNE levels, had a low proportion of CRSwNP and low LM CT scores, and cluster 4, which had relatively low MPO and high HNE levels, showed a high proportion of CRSwNP and high LM CT scores, which is consistent with the results of our previous study.23 In addition, IL-8 levels, which were associated with poor disease outcomes in the study of Liao et al.,15 were also high in cluster 4 and were significantly correlated with disease extent in the T3 groups.
The T2 group (cluster 5) was characterized by a predominant elevation in T2 inflammatory mediators. Periostin, which is known to be induced mainly by T2 cytokines,30 was also prominently increased in the T2 group. The positivity of SE-specific IgE was 6.2%, which was the highest among the clusters. In a previous multi-continent study that revealed the difference in the positivity of SE-specific IgE according to regional factors, the positivity of SE-IgE was high in European and Oceanian countries and Japan (26%–56%), but low in China (3%–4%).6 Similar to the Chinese population,6 we found that the positivity of SE-IgE was 2.9% in the overall CRS patients. It is known that the presence of SE-IgE is associated with intense eosinophilic inflammation associated with elevated IgE concentrations,31 and a previous cluster analysis21 showed that the most severe T2 cluster had a high positivity of SE-IgE (100%). Given this, we could hypothesize that the low positivity of SE-IgE in the T2 group indicates that there is a small population of individuals with severe T2 CRSwNP in Korea and China. The observation of relatively low levels of IL-5, a key cytokine in T2 inflammation, compared with CRS in Western countries6,21 may also support this idea. In the present study, comorbid asthma was most common in the T2 group (34%), but this proportion was comparable to the “IL-5 moderate-expression group” in the study of Tomassen et al.21 (27%–37%). Interestingly, 20% of patients in the mild mixed group had comorbid asthma, which was the second-highest rate among the clusters. Although the levels of eosinophilic and T2 inflammatory markers in the mild mixed group were not as markedly high as in the T2 group, levels of these markers were higher in the mild mixed group than in controls, suggesting that T2 inflammation might contribute to the relatively high proportion of comorbid asthma in the mild mixed group. Because of the absence of the severe form of T2 inflammation, we speculated that a mild form of T2 inflammation (which may be mixed with T3) was presented as cluster 1, and a moderate form of T2 inflammation was presented as cluster 5. Altogether, the predominance and severity of T2 inflammation in CRS may be less common in Asian countries than in Western countries. Future studies are warranted.
It is known that the severity of T2 inflammation plays a main role in clinical outcomes and disease severity of CRS, especially in Western countries.5,6,9,21 However, another major finding of our study is that the disease extent of the T3 group was comparable to that of the T2 group, and the proportion of CRSwNP in cluster 4 (severe T3 inflammation) was 100%, indicating that T3 inflammation may be a main driver determining disease severity and T2 inflammation. In other words, endotyping by only T2 inflammation could not reflect clinical features, such as the phenotype and disease extent, in the Korean population. Supporting our findings, our previous study showed that non-eosinophilic nasal polyps with tissue neutrophilia and eosinophilic nasal polyps without tissue neutrophilia had similar refractoriness (50% vs. 51%, respectively).23 Moreover, the presence of non-eosinophilic CRS with poor clinical outcomes in another cluster analysis in the Chinese population15 may support the important role of neutrophil-associated inflammation in severe CRS in Asians.
Furthermore, we sought to investigate whether any factors in each endotype group were significantly correlated to the extent of disease. Interestingly, IL-22 and TGF-β1 showed significant inverse correlations with the LM CT score in the mild mixed group, whereas IL-8 was positively correlated to the LM CT score in the T3 group, suggesting that biomarkers may differ according to endotype. Additionally, IL-5 was positively correlated to the LM CT score in all CRS patients, although the relationship was not significant in the T2 group. This could be explained by the absence of severe T2 inflammation in this study, which did not lead to stratification in the T2 group (cluster 5) as clearly as in the T3 group. The presence of different biomarkers according to endotype was also demonstrated in a previous cluster analysis,15 and these findings emphasize the heterogeneity of CRS and the necessity of endotype-based management.
This study has some limitations. First, the long-term clinical outcomes were not assessed. Secondly, the number of patients in cluster 2 (mild T3 group) and cluster 4 (severe T3 group) was small, and further characterization of these patients is needed. Lastly, although distinct inflammatory mediators showed significant correlations with disease extent in each endotype group, most correlation coefficients were relatively low (r < 0.3). This may be because complex interactions between inflammatory mediators determine the severity and extent of disease, rather than the effects of a single mediator.
In conclusion, distinct inflammatory endotypes were derived in our cluster analysis, which correlated with phenotype, comorbid asthma, and disease severity: mild mixed, T3, and moderate T2 inflammation. Furthermore, the divergence between T3 and T2 inflammation observed in this cluster analysis indicates that both T3 and T2 inflammation may play important roles in the pathophysiology of CRS in Asian populations. The inflammatory endotypes and their relevant clinical features in the Korean population may provide a better understanding of the pathophysiology of CRS and aid in optimizing therapeutic approaches to CRS in Asians.
ACKNOWLEDGMENTS
This work was supported by a focused clinical research grant-in-aid from the Seoul Metropolitan Government-Seoul National University (SMG-SNU) Boramae Medical Center (04-2021-0008 to D.W.K.) and a grant from the National Research Foundation of Korea (NRF-2019R1A2C208717014 to D.W.K.).
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
Methods
Demographics and inflammatory profiles of participants
Coordinates of given variables in the first 6 dimensions from the results of factor analysis for mixed data
Demographics and inflammatory profiles of the clusters
Correlation between Lund-Mackay score and each cytokine according to endotypes
References
- 1.Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: national health interview survey, 2012. Vital Health Stat 10. 2014:1–161. [PubMed] [Google Scholar]
- 2.Hastan D, Fokkens WJ, Bachert C, Newson RB, Bislimovska J, Bockelbrink A, et al. Chronic rhinosinusitis in Europe--an underestimated disease. A GA2LEN study. Allergy. 2011;66:1216–1223. doi: 10.1111/j.1398-9995.2011.02646.x. [DOI] [PubMed] [Google Scholar]
- 3.Kim YS, Kim NH, Seong SY, Kim KR, Lee GB, Kim KS. Prevalence and risk factors of chronic rhinosinusitis in Korea. Am J Rhinol Allergy. 2011;25:117–121. doi: 10.2500/ajra.2011.25.3630. [DOI] [PubMed] [Google Scholar]
- 4.We J, Lee WH, Tan KL, Wee JH, Rhee CS, Lee CH, et al. Prevalence of nasal polyps and its risk factors: Korean National Health and Nutrition Examination Survey 2009-2011. Am J Rhinol Allergy. 2015;29:e24–e28. doi: 10.2500/ajra.2015.29.4131. [DOI] [PubMed] [Google Scholar]
- 5.Cao PP, Wang ZC, Schleimer RP, Liu Z. Pathophysiologic mechanisms of chronic rhinosinusitis and their roles in emerging disease endotypes. Ann Allergy Asthma Immunol. 2019;122:33–40. doi: 10.1016/j.anai.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Zhang N, Bo M, Holtappels G, Zheng M, Lou H, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016;138:1344–1353. doi: 10.1016/j.jaci.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 7.Khalmuratova R, Shin HW. Crosstalk between mucosal inflammation and bone metabolism in chronic rhinosinusitis. Clin Exp Otorhinolaryngol. 2021;14:43–49. doi: 10.21053/ceo.2020.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bachert C, Marple B, Schlosser RJ, Hopkins C, Schleimer RP, Lambrecht BN, et al. Adult chronic rhinosinusitis. Nat Rev Dis Primers. 2020;6:86. doi: 10.1038/s41572-020-00218-1. [DOI] [PubMed] [Google Scholar]
- 9.Yao Y, Zeng M, Liu Z. Revisiting Asian chronic rhinosinusitis in the era of type 2 biologics. Clin Exp Allergy. 2022;52:231–243. doi: 10.1111/cea.14065. [DOI] [PubMed] [Google Scholar]
- 10.Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58:1–464. doi: 10.4193/Rhin20.600. [DOI] [PubMed] [Google Scholar]
- 11.Bachert C, Akdis CA. Phenotypes and emerging endotypes of chronic rhinosinusitis. J Allergy Clin Immunol Pract. 2016;4:621–628. doi: 10.1016/j.jaip.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Kim DH, Kim SW, Basurrah MA, Hwang SH. Clinical and laboratory features of various criteria of eosinophilic chronic rhinosinusitis: a systematic review and meta-analysis. Clin Exp Otorhinolaryngol. 2022;15:230–246. doi: 10.21053/ceo.2022.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bochner BS, Stevens WW. Biology and function of eosinophils in chronic rhinosinusitis with or without nasal polyps. Allergy Asthma Immunol Res. 2021;13:8–22. doi: 10.4168/aair.2021.13.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Gevaert E, Lou H, Wang X, Zhang L, Bachert C, et al. Chronic rhinosinusitis in Asia. J Allergy Clin Immunol. 2017;140:1230–1239. doi: 10.1016/j.jaci.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Liao B, Liu JX, Li ZY, Zhen Z, Cao PP, Yao Y, et al. Multidimensional endotypes of chronic rhinosinusitis and their association with treatment outcomes. Allergy. 2018;73:1459–1469. doi: 10.1111/all.13411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim DW, Yang SK. Application of biologics in treating chronic rhinosinusitis with nasal polyps in Asian populations. Clin Exp Otorhinolaryngol. 2022;15:125–126. doi: 10.21053/ceo.2022.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen CL, Yao Y, Pan L, Hu ST, Ma J, Wang ZC, et al. Common fibrin deposition and tissue plasminogen activator downregulation in nasal polyps with distinct inflammatory endotypes. J Allergy Clin Immunol. 2020;146:677–681. doi: 10.1016/j.jaci.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lou H, Meng Y, Piao Y, Zhang N, Bachert C, Wang C, et al. Cellular phenotyping of chronic rhinosinusitis with nasal polyps. Rhinology. 2016;54:150–159. doi: 10.4193/Rhino15.271. [DOI] [PubMed] [Google Scholar]
- 19.Staudacher AG, Peters AT, Kato A, Stevens WW. Use of endotypes, phenotypes, and inflammatory markers to guide treatment decisions in chronic rhinosinusitis. Ann Allergy Asthma Immunol. 2020;124:318–325. doi: 10.1016/j.anai.2020.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevens WW, Peters AT, Tan BK, Klingler AI, Poposki JA, Hulse KE, et al. Associations between inflammatory endotypes and clinical presentations in chronic rhinosinusitis. J Allergy Clin Immunol Pract. 2019;7:2812–2820.e3. doi: 10.1016/j.jaip.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomassen P, Vandeplas G, Van Zele T, Cardell LO, Arebro J, Olze H, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016;137:1449–1456.e4. doi: 10.1016/j.jaci.2015.12.1324. [DOI] [PubMed] [Google Scholar]
- 22.Hopkins C, Browne JP, Slack R, Lund V, Brown P. The Lund-Mackay staging system for chronic rhinosinusitis: how is it used and what does it predict? Otolaryngol Head Neck Surg. 2007;137:555–561. doi: 10.1016/j.otohns.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Kim DK, Kim JY, Han YE, Kim JK, Lim HS, Eun KM, et al. Elastase-positive neutrophils are associated with refractoriness of chronic rhinosinusitis with nasal polyps in an Asian population. Allergy Asthma Immunol Res. 2020;12:42–55. doi: 10.4168/aair.2020.12.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Husson F, Josse J, Le S, Mazet J. Package ‘FactoMineR’. An R package. 2016;96:698. [Google Scholar]
- 25.Murtagh F, Contreras P. Algorithms for hierarchical clustering: an overview. Wiley Interdiscip Rev Data Min Knowl Discov. 2012;2:86–97. [Google Scholar]
- 26.Charrad M, Ghazzali N, Boiteau V, Niknafs A. NbClust: an R package for determining the relevant number of clusters in a data set. J Stat Softw. 2014;61:1–36. [Google Scholar]
- 27.Pan L, Liao B, Guo CL, Liu JX, Wang H, Long XB, et al. Inflammatory features and predictors for postsurgical outcomes in patients with nasal polyps stratified by local and systemic eosinophilia. Int Forum Allergy Rhinol. 2021;11:846–856. doi: 10.1002/alr.22702. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, Li ZY, Jiang WX, Liao B, Zhai GT, Wang N, et al. The activation and function of IL-36γ in neutrophilic inflammation in chronic rhinosinusitis. J Allergy Clin Immunol. 2018;141:1646–1658. doi: 10.1016/j.jaci.2017.12.972. [DOI] [PubMed] [Google Scholar]
- 29.Ma J, Shi LL, Deng YK, Wang H, Cao PP, Long XB, et al. CD8+ T cells with distinct cytokine-producing features and low cytotoxic activity in eosinophilic and non-eosinophilic chronic rhinosinusitis with nasal polyps. Clin Exp Allergy. 2016;46:1162–1175. doi: 10.1111/cea.12758. [DOI] [PubMed] [Google Scholar]
- 30.Masuoka M, Shiraishi H, Ohta S, Suzuki S, Arima K, Aoki S, et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest. 2012;122:2590–2600. doi: 10.1172/JCI58978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bachert C, Zhang N, Holtappels G, De Lobel L, van Cauwenberge P, Liu S, et al. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J Allergy Clin Immunol. 2010;126:962–968. 968.e1–966. doi: 10.1016/j.jaci.2010.07.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods
Demographics and inflammatory profiles of participants
Coordinates of given variables in the first 6 dimensions from the results of factor analysis for mixed data
Demographics and inflammatory profiles of the clusters
Correlation between Lund-Mackay score and each cytokine according to endotypes