Abstract
Purpose
Severe asthma (SA) is characterized by persistent airway inflammation and remodeling, followed by lung function decline. The present study aimed to evaluate the role of tissue inhibitor of metalloproteinase-1 (TIMP-1) in the pathogenesis of SA.
Methods
We enrolled 250 adult asthmatics (54 with SA and 196 with non-SA) and 140 healthy controls (HCs). Serum TIMP-1 levels were determined by enzyme-linked immunosorbent assay. The release of TIMP-1 from airway epithelial cells (AECs) in response to stimuli as well as the effects of TIMP-1 on the activations of eosinophils and macrophages were evaluated in vitro and in vivo.
Results
Significantly higher levels of serum TIMP-1 were noted in asthmatics than in HCs, in the SA group than in non-SA group, and in the type 2 SA group than in non-type 2 SA group (P < 0.01 for all). A negative correlation between serum TIMP-1 and FEV1% values (r = −0.400, P = 0.003) was noted in the SA group. In vitro study demonstrated that TIMP-1 was released from AECs in response to poly I:C, IL-13, eosinophil extracellular traps (EETs) and in coculture with eosinophils. TIMP-1-stimulated mice showed eosinophilic airway inflammation, which was not completely suppressed by steroid treatment. In vitro and in vivo functional studies showed that TIMP-1 directly activated eosinophils and macrophages, and induced the release of EETs and macrophages to polarize toward M2 subset, which was suppressed by anti-TIMP-1 antibody.
Conclusions
These findings suggest that TIMP-1 enhances eosinophilic airway inflammation and that serum TIMP-1 may be a potential biomarker and/or therapeutic target for type 2 SA.
Keywords: Asthma, TIMP-1, inflammation, airway remodeling, eosinophils, macrophages, epithelial cells
INTRODUCTION
Asthma is a chronic airway inflammatory disease presenting various phenotypes and endotypes.1 Asthma endotypes are clinically stratified into type 2 and non-type 2 asthma based on type 2 biomarkers (total eosinophil count [TEC], sputum eosinophils, and total immunoglobulin E [IgE]).1,2 Type 2 asthma is characterized by allergic mechanisms through the activation of T helper 2 (Th2) lymphocytes and/or by non-allergic mechanisms through the activation of group 2 innate lymphoid cells (ILC2), leading to the overproductions of type 2 cytokines with eosinophilia.2,3 Severe asthma (SA) is characterized by recurrent asthma exacerbations, persistent blood/sputum eosinophilia, and low lung function even on maintenance medication (including inhaled corticosteroids [ICS] and long-acting beta-agonist [LABA]).4,5 There are unmet needs for identifying prognostic markers and new targets for SA in clinical practice.1,4
Airway epithelial cells (AECs) are the first-line defense cells to communicate between external and internal respiration,6,7,8 and AECs-derived cytokines (e.g., interleukin [IL]-33 and thymic stromal lymphopoietin [TSLP]) induce the activation of immune cells such as eosinophils, mast cells, and macrophages.9 Various mediators released from immune cells (vascular endothelial growth factor [VEGF], IL-13, and eosinophil extracellular traps [EETs]) induce tight junction disruption and epithelial-mesenchymal transition process, contributing to airway obstruction and remodeling in SA.10,11,12,13,14 Therefore, AECs-derived cytokines could be critical biomarkers and/or therapeutic targets for SA.
Tissue inhibitor of metalloproteinase-1 (TIMP-1) is a member of the TIMP family, inhibiting metalloproteinases in airway that lead to the accumulation of extracellular matrix components. AECs are the major source of TIMP-1 in response to various stimuli.15,16,17,18 High levels of TIMP-1 have been shown to be involved in lower FEV1% in asthma, chronic obstructive pulmonary disease, and allergic rhinitis.19,20,21 Regarding its relationship with matrix metallopeptidase 9 (MMP-9) in asthma, increased MMP-9/TIMP-1 ratio was associated with airway neutrophilia,22 while decreased ratio (due to increased TIMP-1 level) was related to lower lung function.23 Therefore, we hypothesized that TIMP-1 released from AECs may enhance airway inflammation and remodeling through interacting with type 2 immune cells (including eosinophils, mast cells, and macrophages). This study aimed to investigate 1) the clinical significance of TIMP-1 in association with clinical parameters in SA, 2) TIMP-1 production from AECs under various stimuli, 3) the effect of TIMP-1 on eosinophils/macrophages in the context of activation and EET formation in vitro, and 4) the effects of TIMP-1 and anti-TIMP-1 antibody on airway inflammation in vivo.
MATERIALS AND METHODS
Study subjects
This study enrolled 250 asthmatics (age ≥ 18 years) and 140 healthy controls (HCs, age ≥ 18 years) from Ajou University Hospital (Suwon, Korea). Asthma was defined according to the Global Initiative for Asthma (GINA) 2020 guideline; the diagnosis of SA was confirmed according to the American Thoracic Society/European Respiratory Society guideline.24 We excluded patients having 1) autoimmune diseases, 2) systemic inflammatory diseases, or 3) who were using or had used type 2 biologics within 180 days.
Atopy was determined by a positive skin prick test for at least one of the following common inhalant allergens: Dermatophagoides pteronyssinus, Dermatophagoides farinae, cat, dog, cockroach, tree pollen mixture, grass pollen mixture, mugwort, ragweed, and Alternaria spp. Lung function parameters were assessed using spirometry.25 TEC, sputum eosinophils and neutrophils (%) were calculated as previously described.26 The ImmunoCAP system was used to determine total IgE levels in sera (Thermo Fisher Scientific, Waltham, MA, USA). Patients with type 2 asthma were defined according to TEC (greater than or equal to 150 cells/µL) and/or sputum eosinophils (greater than or equal to 2%) and/or serum total IgE (greater than or equal to 150 kU/L).2,27 All the study subjects submitted informed consent, and this study was approved by the Institutional Review Board of Ajou University Hospital (AJIRB-GEN-SMP-13-108, AJIRB-BMR-SUR-15-498).
The production of TIMP-1 from AECs
Two human AECs (type II alveolar epithelial cell line [A549] and primary small airway epithelial cells [SAECs]) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). A549 cells were cultured in RPMI-1640 medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, Gibco), and 1% penicillin-streptomycin (Gibco). SAECs were cultured in AEC basal medium (ATCC) plus bronchial epithelial cell growth kit (ATCC), 1% penicillin-streptomycin, and 25 ng/mL amphotericin B (Sigma-Aldrich, St. Louis, MO, USA).
SAECs (1 × 105) or A549 cells (2 × 105) were stimulated with poly I:C (1 or 10 µg/mL; Sigma-Aldrich), IL-13 (10 or 100 ng/mL; R&D Systems, Minneapolis, MN, USA), or phorbol myristate acetate (PMA)-induced EETs (100 ng/mL) for 24 hours or cocultured with 5 × 105 peripheral blood eosinophils (PBEs) from asthmatics for 18 hours. AECs were pretreated with 1 or 10 µg/mL dexamethasone (Dex; Sigma-Aldrich) for 30 minutes to evaluate its suppressive effect. Supernatants were collected for ELISA.
Human immune cell isolation
Human immune cells, such as PBEs and classical monocytes, were isolated from peripheral bloods of asthmatics (SA and non-SA) using Eosinophil Isolation Kit and Pan Monocyte Isolation Kit (Miltenyi Biotec Inc., Auburn, CA, USA) as previously described.28 The cells were rested in RPMI-1640 supplemented with 10% heat-inactivated FBS and 1% penicillin-streptomycin for 30 minutes before the following experiments.
The effects of TIMP-1 on human PBEs
PBEs (1 × 106) were stimulated with 100 ng/mL human TIMP-1 protein (R&D Systems) in RPMI-1640 medium supplemented with 2% FBS and 1% penicillin-streptomycin in a time-dependent manner. In some conditions, cells were pretreated with 1 µg/mL Dex or 1 µg/mL anti-CD63 antibody (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 30 minutes, followed by 100 ng/mL TIMP-1 for 1 hour (for reactive oxygen species [ROS] quantification), 3 hours (for ELISA and migration assay), and 6 hours (for confocal laser scanning microscopy).
To measure the extracellular levels of ROS, PBEs were stained with 2'7′ dichlorofluorescein diacetate (H2DCFDA) (Life Technologies, Eugene, OR, USA) for 30 minutes before the stimulated process. For migration assay, isolated PBEs were stained with 2 µmol calcium aceto-methyl ester (Life Technologies) and then pretreated with Dex or anti-CD63 antibody for 30 minutes. Cells were seeded on the upper chamber with a 3.0-µm-pore trans-well plate (Neuro Probe, Gaithersburg, MD, USA), while phenol red-free RPMI medium containing 100 ng/mL TIMP-1 was added to the lower chamber, and then the trans-well plate was incubated for 3 hours at 37°C. ROS in supernatants was read at 480 nm for excitation wavelengths and at 520 nm for emission wavelengths under a fluorescence microplate reader (Synergy HT; BioTek Instrument, Inc., Winooski, VT, USA).
To detect the levels of EETs released from TIMP-1-stimulated PBEs, 5 × 106 cells were suspended in phenol red-free RPMI with 2% FBS and antibiotics, and then seeded on 24-well plate and stimulated with 100 ng/mL TIMP-1 for 6 hours. Next, cells were gently washed 3 times with phosphate buffered saline (PBS). The wells were added 500 µL phenol red-free RPMI plus 1 U/mL micrococcal nuclease (Thermo Fisher Scientific) and incubated for 20 minutes at 37°C, followed by centrifugation at 300 g at 4°C for 10 minutes to remove cell debris. The supernatants were used to detect the levels of dsDNA using Quanti-iTTM PioGreen® dsDNA kits (Invitrogen, Paisley, UK).
The effects of TIMP-1 on human mast cell line (LAD-2)
LAD-2 cells were provided by the National Institute of Allergy and Infectious Diseases (Bethesda, MD, USA) and cultured in StemPro-34 medium (Life Technologies) supplemented with 2 mM L-glutamine (Gibco), 1% penicillin-streptomycin, and 100 ng/mL recombinant human stem cell factor (R&D Systems). LAD-2 cells (5 × 104 cells) were stimulated with 10 ng/mL human simultaneous biotinylated-IgE (BioPorto Diagnostics, Hellerup, Denmark) with or without 100 ng/mL TIMP-1 in serum-free StemPro-34 medium plus 1% penicillin-streptomycin for 24 hours. To create cross-linking of 2 IgE molecules on LAD-2 cells, 100 ng/mL streptavidin-horseradish peroxidase conjugate having the ability to bind to biotinylated proteins was added to supernatants for 6 hours before harvesting. In some conditions, cells were pretreated with 1 µg/mL Dex for 30 minutes. Supernatants were collected for ELISA.
The effects of TIMP-1 on human macrophages
Human macrophages were derived from classical monocytes as previously reported.29 Briefly, isolated monocytes (1 × 106) were maintained in 1mL RPMI-1640 supplemented with 10% heat-inactivated FBS and 1% penicillin-streptomycin for 7 days to polarize toward human macrophages. Subsequently, cells were stimulated with TIMP-1 for 3 days in a dose-dependent manner (1, 10, and 100 ng/mL). In some conditions, cells were pretreated with 1 µg/mL Dex or 1 µg/mL anti-CD63 for 30 minutes, followed by 100 ng/mL TIMP-1 for 3 days. Supernatants and cells were then collected for ELISA and western blotting/flow cytometry, respectively.
The effects of TIMP-1 on airway inflammation and remodeling in vivo
Female 6-week-old BALB/c mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA) and maintained under specific pathogen-free conditions. All experiments were approved by the Institutional Animal Care and Use Committee of Ajou University (IACUC 2021-0007). To assess the effect of TIMP-1 on airway inflammation, mice were stimulated with TIMP-1 (Supplementary Fig. S1). Mice were intranasally administered with 5 µg/kg mouse TIMP-1 protein (R&D Systems) for 7 days (TIMP-1 group). As controls, mice were intranasally stimulated with PBS (PBS group). To evaluate the effect of Dex treatment on the effects of TIMP-1, mice were treated with 1 mg/kg Dex (TIMP-1/Dex group) for 30 minutes before TIMP-1 stimulation. Mice were sacrificed 24 hours after the last stimulation.
To validate the role of TIMP-1 in airway inflammation and remodeling, the mouse model with secondary ovalbumin (OVA) challenge was established in this study (Supplementary Fig. S2), which is a chronic allergic asthma mouse model (representing severe allergic asthma), where 2% OVA exposure was repeated and the airway presents eosinophilia accumulation and chronic pulmonary remodeling.30 Mice were randomly divided into 4 groups (n = 5 for each group): 1) the OVA-sensitized and PBS-challenged mice (OVA/PBS/PBS); 2) the OVA-sensitized, challenged, and isotype-treated group (OVA/OVA/Iso); 3) the OVA-sensitized, challenged, and Dex-treated group (OVA/OVA/Dex); and 4) the OVA-sensitized, challenged, and anti-TIMP-1-treated group (OVA/OVA/Anti-TIMP-1). On days 1 and 14, mice were intraperitoneally sensitized with 10 μg/mL OVA (Sigma-Aldrich) with Imject™ Alum Adjuvant (Thermo Fisher Scientific). On days 28–30 and 43–45, the mice were challenged with 2% OVA for 30 minutes using an ultrasonic nebulizer (KTMED Inc., Seoul, Korea). For the treated-mice groups, mice were intranasally treated with 50 µg/kg anti-TIMP-1 (R&D Systems) or intraperitoneally injected with 1 mg/kg Dex before the challenge. Mice were assayed 24 hours after the final challenge.
The degree of airway hyperresponsiveness (AHR) to inhaled methacholine (Sigma-Aldrich) was examined for the chronic allergic asthma mouse model using the FlexiVent System (SCIREQ, Montreal, Canada). To evaluate the degree of airway inflammation and remodeling, bronchoalveolar lavage fluid (BALF) and lung tissues were collected for ELISA and western blotting, respectively. BALF was collected by washing with PBS plus 1% bovine serum albumin (BSA, Sigma-Aldrich) through a cannula and centrifuged at 1,200 rpm, for 5 minutes and at 4°C. The fixed tissues were sectioned at 5-µm thickness. Hematoxylin, eosin and Masson’s trichrome staining were conducted for the lung tissues to investigate the levels of immune cell infiltration and collagen, respectively.
To analyze CD marker expression, single cells were isolated after pulmonary tissue homogenization for flow cytometry assay. The homogenization of the whole lung tissue was processed in RPMI-1640 medium with collagenase/hyaluronidase (StemCell Technologies Inc., Biotech & Pharma, Vancouver, BC, Canada) and DNase I (StemCell).31
ELISA
ELISA kits were used as follows: human IL-5, IL-13, IL-33, TIMP-1, MMP-9, and VEGF (R&D Systems); mouse MMP-9, TIMP-1, VEGF, IL-5, IL-13, and IL-33 were measured according to the manufacturer’s instructions (R&D Systems). Human eosinophil-derived neurotoxin (EDN) from SKIMS-BIO (Seoul, Korea) and mouse EDN from MyBioSource (Biotech & Pharma) were measured according to the manufacturer’s instructions.
Western blotting
To detect protein expression, antibodies were used as follows: major basic protein (MBP), CD68, and CD163 from Abcam (Cambridge, MA, USA); E-cadherin and phosphorylated form of phosphatidylinositol 3-kinases (PI3K) from Cell Signaling (Danvers, MA, USA); CD63 and β-actin from Santa Cruz Biotechnology; glyceraldehyde 3-phosphate dehydrogenase from Proteintech (Rosemont, IL, USA).
Flow cytometry
Human macrophages or murine single cells from the lung tissues were blocked with the Fc Receptor Binding Inhibitor Polyclonal Antibody (Thermo Fisher Scientific) for 20 minutes before the staining procedure. The eBioscience™ Intracellular Fixation & Permeabilization Buffer Set (Carlsbad, CA, USA) was used in the process of intracellular staining.
Human macrophages: 1 × 106 single cells were extracellularly stained with antibodies in fluorescent activated cell sorting (FACS) buffer (2% FBS in PBS) as follows: fluorochrome-conjugated anti-CD206 and anti-CD11c antibodies. Then, cells were intracellularly stained with anti-CD68 antibody. Human M2 macrophages were defined as CD68+CD11c−CD206+ cells.
Mouse macrophages: 1 × 106 single cells were extracellularly stained with antibodies in FACS buffer as follows: fluorochrome-conjugated anti-CD45, anti-F4/80, anti-CD206, and anti-CD11c antibodies. Mouse M2 macrophages were defined as F4/80+CD45+CD11c−CD206+ cells.
Mouse ILC2: 1 × 106 single cells from the lung tissues were extracellularly stained with antibodies as follows: fluorochrome-conjugated anti-CD45, anti-Lineage, anti-CD278, and anti-CD90.2 antibodies. Mouse ILC2 cells were defined as CD45+Lineage−CD90.2+CD278+ cells.
All monoclonal antibodies were purchased from BD Biosciences (San Diego, CA, USA). Stained cells were analyzed with BD FACSCantoTM II (BD Biosciences), and graphs were produced using FlowJo software. Cell viability was achieved above 99.0% in the procedure of dissociation or isolation as determined by using the fixable viability dye eFlourTM 780 purchased from eBioscience (San Diego, CA, USA). Individual isotype controls were used to exclude nonspecific binding. Flow cytometry for the evaluations of human macrophage and mouse ILC2 are shown in Supplementary Fig. S3.
Immunofluorescence with confocal microscope
For the detection of EETs, PBEs were stained with EDN, CD63, and 4’,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich) as previously described.32 Cells were blocked with 5% BSA in 10% normal donkey serum (Abcam) and incubated overnight with appropriate primary antibodies.
For the detection of inflammatory levels in murine lung tissues, the fixed tissues were deparaffinated and rehydrated. The slides were blocked with 5% BSA in 10% normal donkey serum (Abcam) at room temperature for 1 hour and stained with MBP, CD63, and DAPI.29
After primary antibody incubation, cells/slides were stained with Alexa Flour 488-conjugated donkey anti-rabbit and 594-conjugated donkey anti-mouse (Thermo Fisher Scientific) for 1 hour. The slides were stained with DAPI (1:1,000) for 5 minutes. Fluorescent images were acquired using confocal laser scanning microscopy at the Three-Dimensional Immune System Imaging Core Facility (LSM710; Cal Zeiss Microscopy GmbH, Jena, Germany).
Statistical analysis
IBM SPSS for Windows, version 22 was used to analyze clinical data (SPSS Inc., Chicago, IL, USA). GraphPad Prism version 8.4.3 was also used to analyze the findings of the experiment (GraphPad Software Inc., San Diego, CA, USA). Regarding continuous variables, multiple groups were compared by using the Kruskal-Wallis test with Dunn’s post hoc test or one-way analysis of variance with the Bonferroni post hoc test. To compare 2 groups, the Mann-Whitney U test was used. Regarding categorical variables, the Pearson’s χ2 test was used. The correlations between TIMP-1 levels and other clinical parameters were evaluated using Spearman correlation. Receiver operating characteristic (ROC) curve analysis was performed to discriminate type 2 SA from non-type 2 SA. Data are presented as median with interquartile range or mean ± standard deviation. Significant differences were set at P < 0.05. Illustrative images were created with Servier Medical Art (https://smart.servier.com/), licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
RESULTS
Clinical characteristics and serum cytokine levels of the SA and non-SA groups
The demographics of the study subjects are presented in Supplementary Table S1. When compared to HCs, asthmatics were older, and had higher prevalence of atopy and levels of serum total IgE (P = 0.001 for all). The SA group had lower FEV1% and FVC% (P = 0.002 for FEV1% and P = 0.001 for FVC%), and higher TEC (P = 0.042) than the non-SA group, while no differences were noted in sputum eosinophils and neutrophils between the 2 groups.
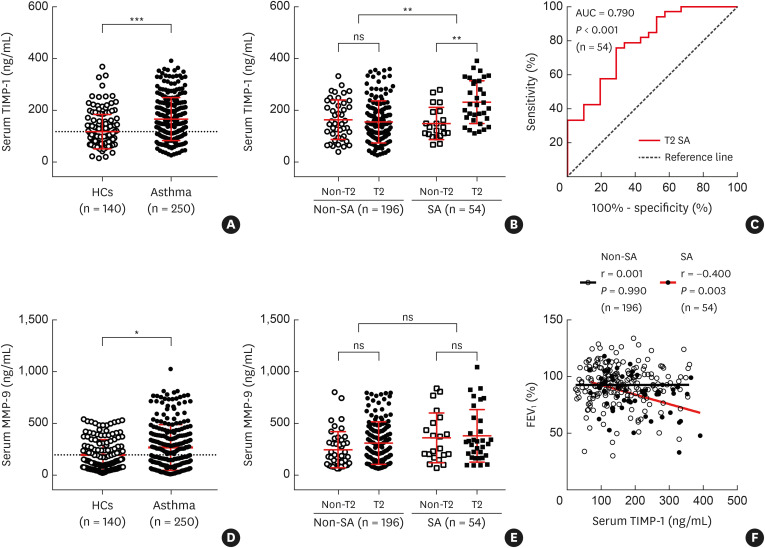
MMP-9 activates cytokines, such as tumor necrosis factor-alpha, VEGF, transforming growth factor-β1 and IL-1β, and upregulates tissue remodeling in collaboration with TIMP-1 in SA.20,23,33 Severe asthmatics had higher levels of serum EDN.34,35 Serum VEGF was suggested as a marker for acute asthma exacerbation.36 The present study demonstrated higher levels of serum TIMP-1 in asthmatics (median [interquartile range], 147.7 [104.2–221.0] ng/mL) than in HCs (91.8 [71.5–144.7], P < 0.001; Supplementary Table S1, Fig. 1A), in the SA group (186.3 [124.2–268.4] ng/mL) than in the non-SA group (144.6 [94.3–209.5] ng/mL, P = 0.001; Supplementary Table S1, Fig. 1B), and in the type 2 SA group (220.4 [164.7–312.4] ng/mL) than in the non-type 2 SA group (133.9 [103.7–194.6] ng/mL, P < 0.01; Fig. 1B). In addition, the ROC curve analysis showed a cutoff level of serum TIMP-1 (165.2 ng/mL) for differentiating type 2 SA from non-type 2 SA (area under the curve: 0.790, sensitivity: 75.8%, specificity: 71.4%, P < 0.001; Fig. 1C). Serum MMP-9 levels were significantly higher in asthmatics (208.7 [78.9–388.7] ng/mL) than in HCs (135.5 [69.8–298.2] ng/mL, P = 0.016), no difference was observed between the SA and non-SA groups (Supplementary Table S1, Fig. 1D and E). Serum TIMP-1 levels had a negative correlation with FEV1% values in the SA group (r = −0.400, P = 0.003), but not in the non-SA group (P = 0.990; Fig. 1F). The serum VEGF levels were not different between asthmatics (71.0 [33.8–133.5] pg/mL) and HCs (109.0 [80.4–185.6] pg/mL, P > 0.05), significantly higher VEGF level was noted in the SA group (114.6 [62.9–170.6] pg/mL) than in the non-SA group (66.2 [29.9–122.2] pg/mL, P = 0.005; Supplementary Table S1).
Fig. 1. Increased levels of serum TIMP-1 in patients with SA. Comparisons of serum TIMP-1 levels between (A) HCs (n = 140) and asthmatic patients (n = 250), between (B) patients with SA and those with non-SA, and between T2 and non-T2. Asthmatics were grouped into non-T2 non-SA (n = 45), T2 non-SA (n = 151), non-T2 SA (n = 21), and T2 SA (n = 33). (C) The receiver operating characteristic curve of serum TIMP-1 levels for discriminating the patients with T2 SA from those with non-T2 SA. Comparisons of serum MMP-9 levels between (D) HCs and asthmatic patients as well as (E) patients with SA and those with non-SA. Data are presented as median with interquartile range. (F) Correlations between serum TIMP-1 levels and FEV1% in patients with SA or non-SA. Data are presented as Spearman correlation coefficient r.
SA, severe asthma; HCs, healthy controls; T2, type 2; ns, not significant; FEV1, forced expiratory volume in the first second; MMP-9, matrix metallopeptidase 9; TIMP-1, tissue inhibitor of metalloproteinase-1; AUC, area under the curve.
*P < 0.05, **P < 0.01, and ***P < 0.001 by Mann-Whitney U test or Kruskal-Wallis test with Dunn’s post hoc test.
Asthmatics were classified into the TIMP-1-high and TIMP-1-low groups at the cutoff value (the mean plus 2 standard deviations of HCs, 250.5 ng/mL), and the laboratory parameters were compared between the 2 groups as shown in Supplementary Table S2. The TIMP-1-high group was older and had significantly lower FEV1%, higher prevalence of type 2 SA, and higher levels of serum MMP-9, VEGF, and EDN than in the TIMP-1-low group (P < 0.05 for all). No differences were found in TEC or sputum eosinophils and neutrophils (%) between the 2 groups (P > 0.05 for all). Moreover, the predictability of each parameter for SA was analyzed by using univariate and multivariate logistic regression analyses (Supplementary Table S3). TEC and serum levels of MMP-9 and TIMP-1 were identified to be significant parameters associated with the phenotype of SA in univariate analysis (P = 0.04 for TEC, P = 0.02 for MMP-9, and P = 0.001 for TIMP-1); however, multivariate analysis demonstrated that serum TIMP-1 levels remained a significant parameter for predicting SA (odds ratio [95% confidence interval], 1.012 [1.004–1.021], P = 0.002).
TIMP-1 release from AECs
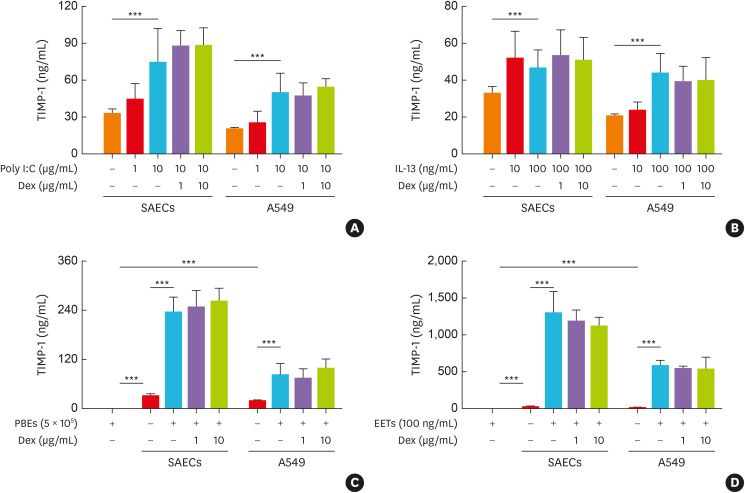
While poly I:C and IL-13 were related type 2 asthma, lipopolysaccharide (LPS) and IL-17A were the key factors for the induction of non-type 2 asthma.7 The present study demonstrated that TIMP-1, but not MMP-9, was mainly released from AECs in response to poly I:C and IL-13 (P < 0.001; Fig. 2A and B, Supplementary Fig. S4A and B), but not released from LPS- or IL-17A-stimulated AECs (data not shown). Eosinophilia and high EET-forming eosinophils have been reported to be key findings in SA.4 When AECs were cocultured with PBEs from asthmatics or stimulated with PMA-induced EETs, significantly higher levels of TIMP-1, but not MMP-9, were released from AECs (P < 0.001 for all; Fig. 2C and D, Supplementary Fig. S4C and D). The TIMP-1 levels released from AECs in response to poly I:C, IL-13, EETs, and in coculture with eosinophils were not suppressed by Dex treatment (Fig. 2).
Fig. 2. The production of TIMP-1 from human AECs. The production of TIMP-1 from AECs (SAECs and A549) in response to (A) poly I:C, (B) IL-13, and in coculture with (C) PBEs as well as (D) in response to EETs (n = 6 for each group). Data are presented as mean ± standard deviation.
AECs, airway epithelial cells; A549, type II alveolar epithelial cell line; IL, interleukin; Dex, dexamethasone; EETs, eosinophil extracellular traps; PBEs, peripheral blood eosinophils; SAECs, primary small airway epithelial cells; TIMP-1, tissue inhibitor of metalloproteinase-1.
***P < 0.001 by one-way analysis of variance with the Bonferroni post hoc test.
The effects of TIMP-1 on PBEs
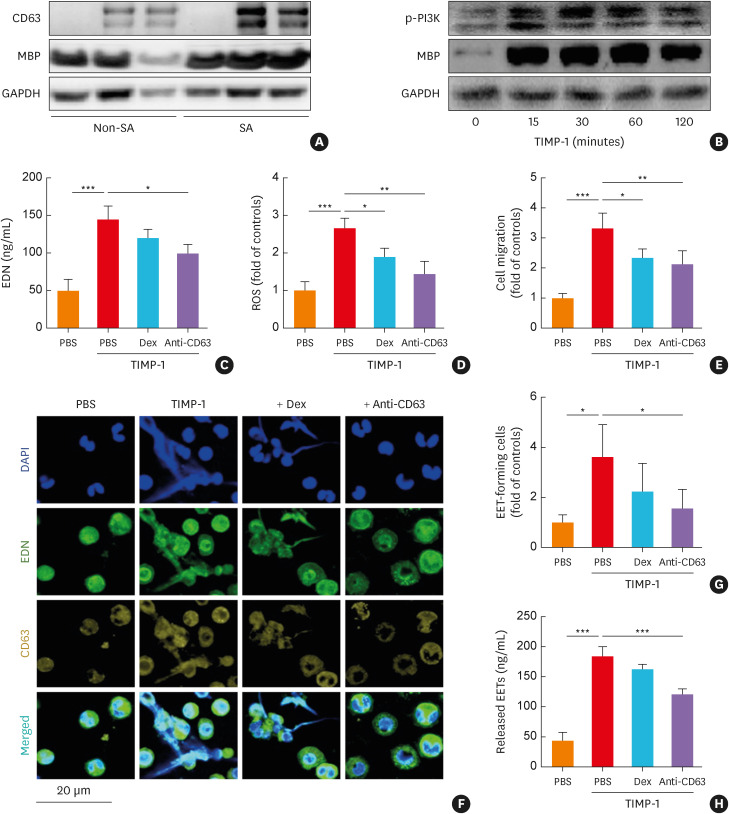
As CD63 receptor (the key receptor of TIMP-1) expressed on resting and activated eosinophils,37 its expression in eosinophils was compared between the SA and non-SA groups (Fig. 3A). As a result, more significant expressions of CD63 and MBP were observed in the SA group than in the non-SA group. Moreover, potential mechanisms by which TIMP-1 activates PBEs were evaluated. The expression of phosphorylation form of PI3K and MBP in PBEs (from asthmatics) were increased in a time-dependent manner after TIMP-1 stimulation (Fig. 3B). When PBEs were incubated with TIMP-1, significantly higher levels of EDN and ROS releases were noted (P < 0.001 for both; Fig. 3C and D). TIMP-1 induced the recruitment of eosinophils as evaluated by trans-well migration assays (P < 0.001; Fig. 3E). Furthermore, TIMP-1 significantly induced the formation of EETs in severe asthmatics as observed by confocal assays (Fig. 3F and G). When quantification of EETs levels was observed by the PicoGreen assay, TIMP-1 induced greater production of EETs (P < 0.001; Fig. 3H). Anti-CD63 or Dex treatment markedly suppressed TIMP-1-induced eosinophil recruitment and ROS release (P < 0.05 for both; Fig. 3D and E). However, the levels of EDN and EETs were reduced by anti-CD63 antibody, but not by Dex treatment (Fig. 3C and F-H), suggesting that TIMP-1 may contribute to steroid insensitivity in the EET-mediated airway inflammation in SA.
Fig. 3. The effects of TIMP-1 on human PBEs. (A) The protein expressions of CD63 and MBP in PBEs from asthmatics according to asthma severity. (B) The effect of TIMP-1 on the phosphorylation of PI3K in a time-dependent manner. TIMP-1 induced the release of (C) EDN and (D) ROS with (E) eosinophil recruitment (n = 6 for each group). (F, G) The levels of EETs were evaluated using confocal microscopy. Cells were stained with EDN (green), CD63 (yellow), and DAPI (blue). Scale bar, 20 µm. (H) The levels of released EETs were evaluated by measuring dsDNA concentrations using PicoGreen assay (n = 6 for each group). Data are presented as mean ± standard deviation.
PBEs, peripheral blood eosinophils; DAPI, 4’,6-diamidino-2-phenylindole; Dex, dexamethasone; EETs, eosinophil extracellular traps; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MBP, major basic protein; PI3K, phosphatidylinositol 3-kinases; ROS, reactive oxygen species; SA, severe asthma; TIMP-1, tissue inhibitor of metalloproteinase-1; EDN, eosinophil-derived neurotoxin.
*P < 0.05, **P < 0.01, and ***P < 0.001 by one-way analysis of variance with the Bonferroni post hoc test.
The effect of TIMP-1 on LAD-2 cells
Because IgE-dependent mechanism induces surface expression of CD63 on mast cell surface,38 we evaluated the effects of TIMP-1 on mast cell activation. The combination of TIMP-1 and IgE induced significantly greater release of IL-5, IL-13, and IL-33 from LAD-2 cells compared to IgE alone (P < 0.05 for all; Supplementary Fig. S5A-C). In addition, TIMP-1 alone significantly induced IL-13 release from LAD-2 cells (Supplementary Fig. S5B).
The effects of TIMP-1 on human macrophages
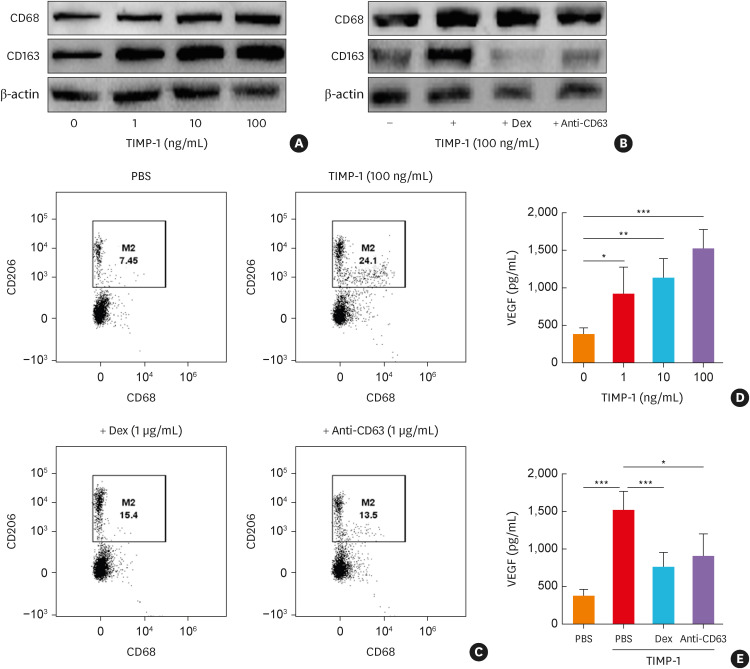
Macrophage activation and polarization toward M2 subset contribute to chronic airway inflammation and remodeling in the pathogenesis of type 2 asthma.39 The present study evaluated the effects of TIMP-1 on macrophage polarization by applying western blotting and flow cytometry using M2 markers (CD68+CD11c−CD206+ cells). The increased expression of CD68 and CD163 and percentage of M2 macrophage markers were found, when human macrophages were stimulated with TIMP-1 (Fig. 4A-C). When macrophages were treated with anti-CD63 or Dex, TIMP-1-induced M2 marker expressions (by flow cytometry) were significantly decreased (24.1% vs. 15.4% for Dex and 24.1% vs. 13.5% for anti-CD63; Fig. 4C). Moreover, VEGF levels released from M2 macrophages were increased in response to TIMP-1 in a dose-dependent manner, which was suppressed by Dex and anti-CD63 (Fig. 4D and E).
Fig. 4. The effect of TIMP-1 on human macrophages. (A) The protein expressions of CD68 (macrophage maturation marker) and CD163 (M2 macrophage marker) from macrophages in response to TIMP-1 in a dose-dependent manner. (B) The effects of anti-CD63 antibody treatment on TIMP-1-stimulated macrophages. (C) Macrophage polarization was evaluated by flow cytometry. The levels of VEGF from macrophages in response to TIMP-1 in (D) a dose-dependent manner or with (E) the presence of anti-CD63 treatment (n = 6 for each group). Data are presented as mean ± standard deviation.
Dex, dexamethasone; TIMP-1, tissue inhibitor of metalloproteinase-1; VEGF, vascular endothelial growth factor; PBS, phosphate buffered saline.
*P < 0.05, **P < 0.01, and ***P < 0.001 by one-way analysis of variance with the Bonferroni post hoc test.
The effects of TIMP-1 on airway inflammation in mice
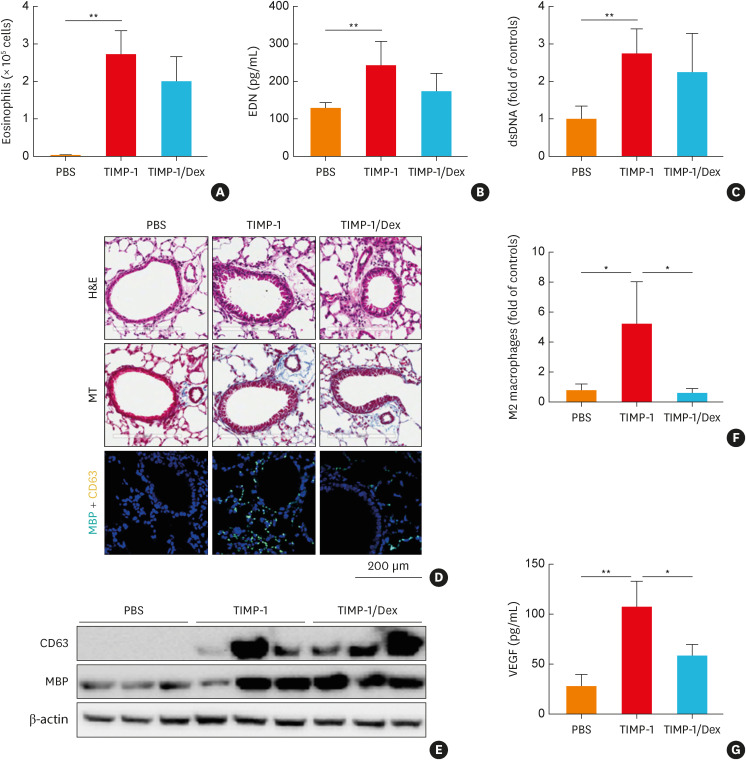
To validate the effects of TIMP-1 on airway inflammation in vivo, mouse TIMP-1 protein was used to stimulate the murine airways. The optimal dose of TIMP-1 was determined in the preliminary study, in which 5 µg/kg TIMP-1 treatment was able to induce eosinophil recruitment, EDN production, and MBP expression (Supplementary Fig. S6). After 7-day stimulation, eosinophils (but not neutrophils) were significantly trafficked and recruited to the murine lung tissues in the TIMP-1 group compared to the PBS group (Fig. 5A). In addition, TIMP-1-stimulated mice showed significantly higher levels of EDN and dsDNA in the BALF with increased expression of MBP and CD63 in the lung tissues compared to the PBS group, which were not suppressed by Dex (Fig. 5B-E). Increased immune cell infiltration, collagen overproduction, and higher percentage of M2 macrophages in the lung tissues as well as higher levels of VEGF in the BALF were noted in the TIMP-1 group compared to the PBS group (Fig. 5D and F-G).
Fig. 5. The effects of TIMP-1 on airway inflammation in vivo. (A) Eosinophils in the BALF. The levels of (B) EDN and (C) dsDNA in the BALF. (D) Lung histology stained with H&E and MT staining. Immunofluorescence staining for DAPI (nuclear, blue), MBP (turquoise), and CD63 (yellow). Scale bar, 200 µm. (E) The expressions of CD63 and MBP in the lung tissues. (F) M2 macrophage counts in the lung tissues were obtained by flow cytometry. (G) The levels of VEGF in the BALF (n = 5 for each group). Data are presented as mean ± standard deviation.
BALF, bronchoalveolar lavage fluid; DAPI, 4’,6-diamidino-2-phenylindole; Dex, dexamethasone; EDN, eosinophil-derived neurotoxin; H&E, hematoxylin and eosin; MT, Masson’s trichrome; MBP, major basic protein; TIMP-1, tissue inhibitor of metalloproteinase-1; VEGF, vascular endothelial growth factor; PBS, phosphate buffered saline.
*P < 0.05 and **P < 0.01 by one-way analysis of variance with the Bonferroni post hoc test.
When type 2 cytokines and ILC2 count were evaluated, the number of ILC2 was significantly higher in the lung tissues of the TIMP-1 group than in the PBS group (Supplementary Fig. S1B). Significantly higher levels of IL-5 and IL-13 were noted in the BALF of the TIMP-1 group than in the PBS group (P < 0.001 for all; Supplementary Fig. S1C and D). To evaluate the direct effects of TIMP-1 on ILCs, ILCs isolated from HCs were treated with/without TIMP-1 for 3 days. However, TIMP-1 failed to stimulate ILC activation and polarization (data not shown), suggesting the effects of TIMP-1 on ILC2 may be mediated by EET formation rather than direct activation.
The effects of anti-TIMP-1 treatment in vivo
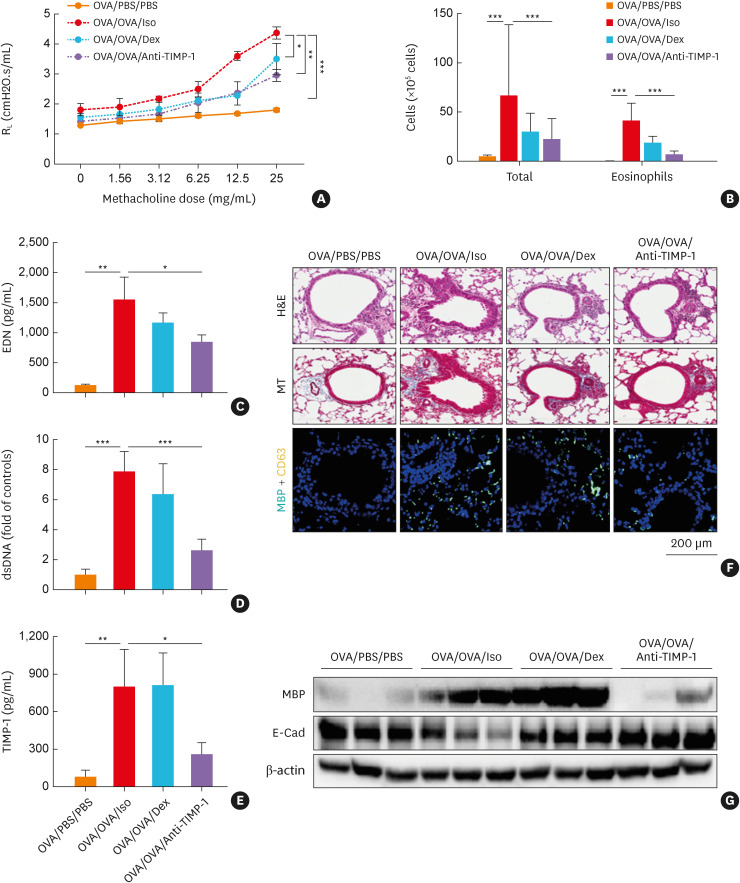
TIMP-1 was released from AECs in response to poly I:C and IL-13 in vitro. Increased levels of TIMP-1 in the BALF of 2 mouse models, the poly I:C-infected acute allergic asthma mouse model and the chronic allergic asthma mouse model, were evaluated.29,30 The present study used a mouse model of chronic allergic asthma, because 1) the levels of TIMP-1 were higher in the chronic allergic asthma mouse model than in the poly I:C-infected acute allergic asthma (data not shown); and 2) high TIMP-1 levels were related to chronic inflammatory diseases.20,21,40 The AHR, eosinophil count, EDN, and released dsDNA levels in the BALF were significantly higher in the OVA/OVA/Iso group, which were suppressed by anti-TIMP-1 treatment (Fig. 6A-D). Higher levels of TIMP-1 were noted in the OVA/OVA/Iso group and positively correlated with the levels of IL-33 (Fig. 6E, Supplementary Fig. S2F). Dex treatment markedly reduced MMP-9 secretion in the BALF but did not affect TIMP-1 levels (Fig. 6E, Supplementary Fig. S2E). An increased number of double-positive cells (CD63 plus MBP) was noted in the peri-bronchial area of the OVA/OVA/Iso group (Fig. 6F and G). Moreover, the expression of E-cadherin was downregulated in the OVA/OVA/Iso group, which was restored by Dex and anti-TIMP-1 treatment (Fig. 6G).
Fig. 6. The effects of anti-TIMP-1 antibody treatment in a mouse model of chronic allergic asthma. (A) Airway hyperresponsiveness. (B) Total cells and eosinophils in the BALF. The levels of (C) EDN, (D) dsDNA, and (E) TIMP-1 in the BALF (n = 5 for each group). Data are presented as mean ± standard deviation. (F) Lung histology stained with H&E and MT staining. Immunofluorescence staining for DAPI (nuclear, blue), MBP (turquoise), and CD63 (yellow). Scale bar, 200 µm. (G) The expressions of MBP and E-Cad in the lung tissues.
BALF, bronchoalveolar lavage fluid; DAPI, 4’,6-diamidino-2-phenylindole; Dex, dexamethasone; EDN, eosinophil-derived neurotoxin; H&E, hematoxylin and eosin; MT, Masson’s trichrome; MBP, major basic protein; RL, resistance to airflow across the lung; OVA, ovalbumin; TIMP-1, tissue inhibitor of metalloproteinase-1; E-Cad, E-cadherin; PBS, phosphate buffered saline.
*P < 0.05, **P < 0.01, and ***P < 0.001 by one-way analysis of variance with the Bonferroni post hoc test.
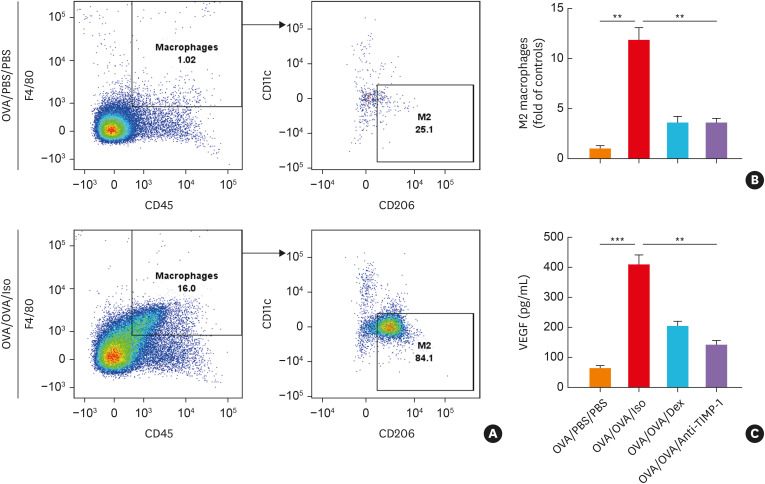
The percentages of M2 macrophage were significantly higher in the OVA/OVA/Iso group than in the OVA/PBS/PBS group (Fig. 7A). Anti-TIMP-1 or Dex significantly suppressed OVA-induced M2 macrophage in the lung tissues and VEGF levels in the BALF (Fig. 7B and C). The percentage of pulmonary ILC2 and levels of IL-5 and IL-13 in the BALF and were significantly higher in the OVA/OVA/Iso group, which were markedly suppressed by anti-TIMP-1 treatment (Supplementary Fig. S2B-D).
Fig. 7. The effects of anti-TIMP-1 antibody treatment on mouse macrophages. (A) The percentage of macrophage count (CD45+F4/80+ cells) and M2 macrophages (CD11c−CD206+ macrophages) measured by flow cytometry. (B) The degree of M2 macrophage count in lung tissue. (C) The levels of VEGF in the BALF (n = 5 for each group). Data are presented as mean ± standard deviation.
BALF, bronchoalveolar lavage fluid; Dex, dexamethasone; OVA, ovalbumin; TIMP-1, tissue inhibitor of metalloproteinase-1; VEGF, vascular endothelial growth factor; PBS, phosphate buffered saline.
**P < 0.01 and ***P < 0.001 by one-way analysis of variance with the Bonferroni post hoc test.
DISCUSSION
Although there have been a few studies reporting the effect of TIMP-1 on airway remodeling in asthma,41,42,43 the role of TIMP-1 has not been fully understood in SA. The present study demonstrated the mechanism by which TIMP-1 enhances type 2 airway inflammation in SA. The SA group had higher serum TIMP-1 levels than non-SA group with a negative correlation between serum TIMP-1 and FEV1% values. Asthmatics with type 2 SA had higher serum TIMP-1 levels than those with non-type 2 SA. Asthmatics with higher serum TIMP-1 levels had higher levels of serum EDN and VEGF. The in vitro and in vivo studies demonstrated that TIMP-1 could induce eosinophil activation, EET formation, and M2 polarization, enhancing persistent eosinophilic airway inflammation, which was not suppressed by Dex. These findings suggest that serum TIMP-1 may be a potential biomarker and/or a therapeutic target for type 2 SA.
Airway and blood eosinophilia have been shown to closely correlate with frequent asthma exacerbations and lung function decline in SA; identification of serum markers for predicting the phenotype of SA is required.1,4 Previous studies reported that sputum TIMP-1 levels were significantly higher in patients with SA than in those with non-SA.42 Sputum TIMP-1 has been suggested as a useful biomarker for classifying GINA 4/5 phenotypes in asthmatics.43 The present study replicated significantly higher serum TIMP-1 levels in SA (especially in type 2 SA) than in non-SA. The in vitro studies supported TIMP-1-induced eosinophil activation and EET release through the CD63/PI3K signaling axis, contributing to AHR and steroid resistance.44 ,45 EETs in SA directly activated eosinophils (autocrine function) and stimulated AECs to release alarmins (IL-33 and TSLP), contributing to type 2 airway inflammation in SA.13,46 Taken together, TIMP-1 could enhance eosinophilic airway inflammation via the TIMP-1-CD63-eosinophils-EETs axis, contributing to the phenotype of type 2 SA.
Patients with severe eosinophilic airway inflammation showed progressive airway remodeling,6,44 ,45 and released mediators, such as IL-13 and VEGF, involved in vascular proliferation and airway remodeling.6,8 In addition, activated mast cells and macrophages (expressing CD63 receptors) are closely associated with eosinophil activation and airway remodeling in SA.38,47 Mast cell activation through IgE-dependent mechanisms induces high levels of type 2 inflammatory cytokines (IL-4, IL-5, and IL-13). The present study demonstrated that: 1) TIMP-1 stimulated mast cells to release IL-13; 2) TIMP-1 enhanced IgE-induced mast cell degranulation with the release of IL-5 and IL-33. IL-5 stimulates eosinophils to induce recruitment and activation as well as longer survival.48 IL-13 is essential in chronic airway remodeling processes (via increasing mucus overproduction, goblet cell metaplasia, and smooth muscle hypertrophy); additionally, IL4- and IL13-induced macrophage polarization toward M2 subset releases proinflammatory cytokines (transglutaminase 2, CCL17, and CCL22), enhancing eosinophilic inflammation and Th2 cell recruitment in patients with SA.49,50 The present study demonstrated that TIMP-1 could induce M2 macrophage polarization, as evaluated in both human and mouse macrophages, and trigger airway remodeling in association with VEGF released from macrophages.50 These findings indicate that TIMP-1 may indirectly up-regulate airway remodeling through activated mast cells and macrophages.
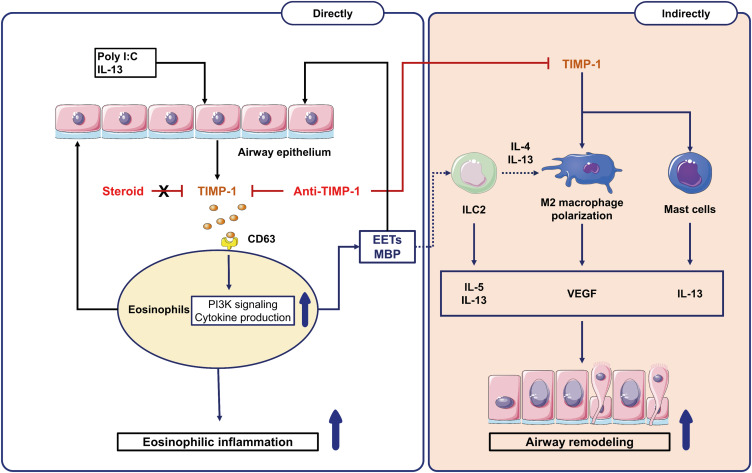
Patients with SA are not adequately controlled with current anti-inflammatory medications (ICS plus LABA) and are at high risk of steroid resistance, requiring additional controllers including biologics. Down-regulation of EET formation is a potential therapeutic target for SA.14,35 In the present study, severe asthmatics who had maintained medium-to-high doses of ICS plus LABA were enrolled for ex vivo studies; therefore, their eosinophils may not have been sensitive to current steroid treatment. However, in the current in vivo model asthma, Dex could not suppress TIMP-1, and eosinophil activation markers, such as EDN and EETs, in the BALF, while anti-TIMP-1 treatment could markedly suppress eosinophilic activation markers as well as M2 macrophages. Taken together, anti-TIMP-1 antibody (compared to steroid) may provide additional benefits via suppressing eosinophilic inflammation and preventing airway remodeling in type 2 SA as summarized in Fig. 8.
Fig. 8. Available mechanisms of TIMP-1 in type 2 SA. The exogenous (poly I:C) and endogenous factors (IL-13, eosinophils, and EETs) stimulate airway epithelial cells to release TIMP-1. The left panel showed TIMP-1-induced eosinophilic inflammation through (1) directly activating eosinophils (MBP release) and (2) indirectly activating EET formation (through CD63/PI3K signaling) in SA, which were suppressed by anti-TIMP-1 antibody (not by steroid). The right panel showed TIMP-1-induced airway remodeling via releasing proinflammatory cytokines (IL-13 and VEGF) from activated ILC2, M2 macrophages, mast cells, and eosinophils.
EETs, eosinophil extracellular traps; ILC2, group 2 innate lymphoid cells; MBP, major basic protein; TIMP-1, tissue inhibitor of metalloproteinase-1; PI3K; phosphatidylinositol 3-kinases; VEGF, vascular endothelial growth factor; IL, interleukin; SA, severe asthma.
The present study has some limitations. First, although a significant association was found between serum TIMP-1 and EDN levels in asthmatics, no direct correlations were observed between serum TIMP-1 levels and the degree of blood/sputum eosinophilia, which may be attributed to the limited number of asthmatic subjects having high levels of serum TIMP-1. Secondly, our asthma mouse model represented the phenotype of chronic allergic asthma; however, it showed EETs-mediated eosinophilic airway inflammation, validating the effect of TIMP-1 and anti-TIMP-1 antibody on severe type 2 inflammation. Thirdly, further studies are needed to validate the exact role of TIMP-1 in a large cohort of adult asthmatics according to specific endotypes.
In conclusion, TIMP-1 could enhance eosinophilic airway inflammation, inducing airway remodeling and lung function decline in SA, suggesting that serum TIMP-1 may be a potential marker for predicting type 2 SA characterized by persistent eosinophilic inflammation and poor clinical outcomes.
ACKNOWLEDGMENTS
This work was supported by the Korea Health Technology R&D Project (grant No. HR16C0001) and by Korea Basic Science Institute (National research Facilities and Equipment Center) for the research using confocal laser scanning microscopy (LSM710) funded by the Ministry of Education (grant No. 2019R1A6C1010003).
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interests.
SUPPLEMENTARY MATERIALS
Characteristics of the study subjects
Comparison of demographic characteristics between the high-TIMP-1 and low-TIMP-1 groups
Univariate and multivariate logistic regression for predicting severe asthma
The effects of TIMP-1 on the airway inflammation in vivo. (A) Experimental protocol for TIMP-1 stimulation in vivo. (B) The percentage of ILC2 count (CD45+Lineage−CD90.2+CD278+ cells) in the lung tissues was detected by fluorescent activated cell sorting and depicted by graph (n = 5 for each group). (C, D) The levels of IL-5 and IL-13 in the bronchoalveolar lavage fluid (n = 5 for each group). Data are presented as mean ± standard deviation.
The effects of anti-TIMP-1 antibody on airway inflammation in the chronic allergic asthma mouse model. (A) Experimental protocol for anti-TIMP-1 treatment in the chronic allergic asthma mouse model. (B) The percentage of ILC2 count (CD45+Lineage-CD90.2+CD278+ cells) in the lung tissues was detected by fluorescent activated cell sorting and depicted by graph (n = 5 for each group). The levels of (C) IL-5, (D) IL-13, and (E) MMP-9 in the BALF (n = 5 for each group). Data are presented as mean ± standard deviation. (F) Correlations between TIMP-1 and IL-33 levels in BALF. Data are presented as Spearman correlation coefficient r.
Flow cytometry gating strategy for immunophenotyping. (A) Flow cytometric plots of human M2 macrophages (CD68+CD11c−CD206+ cells). (B) Flow cytometric plots of group 2 innate lymphoid cells count (CD45+Lineage−CD90.2+CD278+ cells) from single cells of the murine lung tissues.
The production of MMP-9 from human AECs. The production of MMP-9 from AECs (SAECs and A549) in response to (A) poly I:C, (B) IL-13, and in co-culture with (C) PBEs as well as (D) in response to EETs (n = 6 for each group). Data are presented as mean ± standard deviation.
The effects of TIMP-1 on cytokine release from LAD-2 cells. The levels of (A) IL-5, (B) IL-13, and (C) IL-33 from LAD-2 cells in response to TIMP-1 alone or in combination with cross-linking IgE (n = 6 for each group). Data are presented as mean ± standard deviation.
The effects of TIMP-1 on the airway inflammation in vivo in a dose-dependent manner. (A) TIMP-1 induced an increase in eosinophil count in the BALF (n = 3 for each group) in a dose-dependent manner (0.05, 0.5, and 5 µg/kg). (B) The levels of EDN in the BALF (n = 3 for each group). (C) The expression of MBP in the lung tissues. Data are presented as mean ± standard deviation.
References
- 1.Lee Y, Quoc QL, Park HS. Biomarkers for severe asthma: lessons from longitudinal cohort studies. Allergy Asthma Immunol Res. 2021;13:375–389. doi: 10.4168/aair.2021.13.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woo SD, Yang EM, Jang J, Lee Y, Shin YS, Ye YM, et al. Serum-free immunoglobulin E: a useful biomarker of atopy and type 2 asthma in adults with asthma. Ann Allergy Asthma Immunol. 2021;127:109–115.e1. doi: 10.1016/j.anai.2021.03.023. [DOI] [PubMed] [Google Scholar]
- 3.Nair P, Surette MG, Virchow JC. Neutrophilic asthma: misconception or misnomer? Lancet Respir Med. 2021;9:441–443. doi: 10.1016/S2213-2600(21)00023-0. [DOI] [PubMed] [Google Scholar]
- 4.Lee Y, Park Y, Kim C, Lee E, Lee HY, Woo SD, et al. Longitudinal outcomes of severe asthma: real-world evidence of multidimensional analyses. J Allergy Clin Immunol Pract. 2021;9:1285–1294.e6. doi: 10.1016/j.jaip.2020.09.055. [DOI] [PubMed] [Google Scholar]
- 5.Park SY, Kang SY, Song WJ, Kim JH. Evolving concept of severe asthma: transition from diagnosis to treatable traits. Allergy Asthma Immunol Res. 2022;14:447–464. doi: 10.4168/aair.2022.14.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winkler T, Frey U. Airway remodeling: shifting the trigger point for exacerbations in asthma. J Allergy Clin Immunol. 2021;148:710–712. doi: 10.1016/j.jaci.2021.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Hellings PW, Steelant B. Epithelial barriers in allergy and asthma. J Allergy Clin Immunol. 2020;145:1499–1509. doi: 10.1016/j.jaci.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lloyd CM, Robinson DS. Allergen-induced airway remodelling. Eur Respir J. 2007;29:1020–1032. doi: 10.1183/09031936.00150305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brusselle GG, Maes T, Bracke KR. Eosinophils in the spotlight: eosinophilic airway inflammation in nonallergic asthma. Nat Med. 2013;19:977–979. doi: 10.1038/nm.3300. [DOI] [PubMed] [Google Scholar]
- 10.Türkeli A, Yilmaz Ö, Karaman M, Kanik ET, Firinci F, İnan S, et al. Anti-VEGF treatment suppresses remodeling factors and restores epithelial barrier function through the E-cadherin/β-catenin signaling axis in experimental asthma models. Exp Ther Med. 2021;22:689. doi: 10.3892/etm.2021.10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agache I, Eguiluz-Gracia I, Cojanu C, Laculiceanu A, Del Giacco S, Zemelka-Wiacek M, et al. Advances and highlights in asthma in 2021. Allergy. 2021;76:3390–3407. doi: 10.1111/all.15054. [DOI] [PubMed] [Google Scholar]
- 12.Choi Y, Lee DH, Trinh HKT, Ban GY, Park HK, Shin YS, et al. Surfactant protein D alleviates eosinophil-mediated airway inflammation and remodeling in patients with aspirin-exacerbated respiratory disease. Allergy. 2019;74:78–88. doi: 10.1111/all.13458. [DOI] [PubMed] [Google Scholar]
- 13.Choi Y, Kim YM, Lee HR, Mun J, Sim S, Lee DH, et al. Eosinophil extracellular traps activate type 2 innate lymphoid cells through stimulating airway epithelium in severe asthma. Allergy. 2020;75:95–103. doi: 10.1111/all.13997. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Cho YS. Eosinophil extracellular traps pave the way for the identification of novel therapeutics in severe asthma. Allergy Asthma Immunol Res. 2022;14:441–443. doi: 10.4168/aair.2022.14.5.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park CS, Kim TB, Moon KA, Bae YJ, Lee HR, Jang MK, et al. Chlamydophila pneumoniae enhances secretion of VEGF, TGF-β and TIMP-1 from human bronchial epithelial cells under Th2 dominant microenvironment. Allergy Asthma Immunol Res. 2010;2:41–47. doi: 10.4168/aair.2010.2.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, McCluskey K, Fujii K, Wahl LM. Differential regulation of monocyte matrix metalloproteinase and TIMP-1 production by TNF-alpha, granulocyte-macrophage CSF, and IL-1 beta through prostaglandin-dependent and -independent mechanisms. J Immunol. 1998;161:3071–3076. [PubMed] [Google Scholar]
- 17.Hemmerlein B, Johanns U, Halbfass J, Böttcher T, Heuser M, Radzun HJ, et al. The balance between MMP-2/-9 and TIMP-1/-2 is shifted towards MMP in renal cell carcinomas and can be further disturbed by hydrogen peroxide. Int J Oncol. 2004;24:1069–1076. [PubMed] [Google Scholar]
- 18.Weng W, Hu Z, Pan Y. Macrophage extracellular traps: current opinions and the state of research regarding various diseases. J Immunol Res. 2022;2022:7050807. doi: 10.1155/2022/7050807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sivakoti K, Chaya SK, Jayaraj BS, Lokesh KS, Veerapaneni VV, Madhunapantula S, et al. Evaluation of inflammatory markers MMP-2 and TIMP-1 in asthma. Eur Respir J. 2018;52:PA5044 [Google Scholar]
- 20.Mercer PF, Shute JK, Bhowmik A, Donaldson GC, Wedzicha JA, Warner JA. MMP-9, TIMP-1 and inflammatory cells in sputum from COPD patients during exacerbation. Respir Res. 2005;6:151. doi: 10.1186/1465-9921-6-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giannelli G, Erriquez R, Iannone F, Marinosci F, Lapadula G, Antonaci S. MMP-2, MMP-9, TIMP-1 and TIMP-2 levels in patients with rheumatoid arthritis and psoriatic arthritis. Clin Exp Rheumatol. 2004;22:335–338. [PubMed] [Google Scholar]
- 22.Cundall M, Sun Y, Miranda C, Trudeau JB, Barnes S, Wenzel SE. Neutrophil-derived matrix metalloproteinase-9 is increased in severe asthma and poorly inhibited by glucocorticoids. J Allergy Clin Immunol. 2003;112:1064–1071. doi: 10.1016/j.jaci.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Chaudhuri R, McSharry C, Brady J, Grierson C, Messow CM, Spears M, et al. Low sputum MMP-9/TIMP ratio is associated with airway narrowing in smokers with asthma. Eur Respir J. 2014;44:895–904. doi: 10.1183/09031936.00047014. [DOI] [PubMed] [Google Scholar]
- 24.Holguin F, Cardet JC, Chung KF, Diver S, Ferreira DS, Fitzpatrick A, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2020;55:1900588. doi: 10.1183/13993003.00588-2019. [DOI] [PubMed] [Google Scholar]
- 25.Morris JF. Spirometry in the evaluation of pulmonary function. West J Med. 1976;125:110–118. [PMC free article] [PubMed] [Google Scholar]
- 26.Pham DL, Kim SH, Losol P, Yang EM, Shin YS, Ye YM, et al. Association of autophagy related gene polymorphisms with neutrophilic airway inflammation in adult asthma. Korean J Intern Med. 2016;31:375–385. doi: 10.3904/kjim.2014.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim BK, Park SY, Ban GY, Kim MA, Lee JH, An J, et al. Evaluation and management of difficult-to-treat and severe asthma: an expert opinion from the Korean Academy of Asthma, Allergy and Clinical Immunology, the working group on severe asthma. Allergy Asthma Immunol Res. 2020;12:910–933. doi: 10.4168/aair.2020.12.6.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quoc QL, Choi Y, Thi Bich TC, Yang EM, Shin YS, Park HS. S100A9 in adult asthmatic patients: a biomarker for neutrophilic asthma. Exp Mol Med. 2021;53:1170–1179. doi: 10.1038/s12276-021-00652-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bich TCT, Quoc QL, Choi Y, Yang EM, Trinh HKT, Shin YS, et al. Serum amyloid A1: a biomarker for neutrophilic airway inflammation in adult asthmatic patients. Allergy Asthma Immunol Res. 2022;14:40–58. doi: 10.4168/aair.2022.14.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu JN, Suh DH, Trinh HK, Chwae YJ, Park HS, Shin YS. The role of autophagy in allergic inflammation: a new target for severe asthma. Exp Mol Med. 2016;48:e243. doi: 10.1038/emm.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Lucía Finkel P, Sherwood C, Saranchova I, Xia W, Munro L, Pfeifer CG, et al. Serum free culture for the expansion and study of type 2 innate lymphoid cells. Sci Rep. 2021;11:12233. doi: 10.1038/s41598-021-91500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luu Quoc Q, Cao Thi Bich T, Kim SH, Park HS, Shin YS. Administration of vitamin E attenuates airway inflammation through restoration of Nrf2 in a mouse model of asthma. J Cell Mol Med. 2021;25:6721–6732. doi: 10.1111/jcmm.16675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee Y, Lee JH, Yang EM, Kwon E, Jung CG, Kim SC, et al. Serum levels of eosinophil-derived neurotoxin: a biomarker for asthma severity in adult asthmatics. Allergy Asthma Immunol Res. 2019;11:394–405. doi: 10.4168/aair.2019.11.3.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi Y, Le Pham D, Lee DH, Lee SH, Kim SH, Park HS. Biological function of eosinophil extracellular traps in patients with severe eosinophilic asthma. Exp Mol Med. 2018;50:1–8. doi: 10.1038/s12276-018-0136-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han H, Kim Y, Mo H, Choi SH, Lee K, Rim YA, et al. Preferential stimulation of melanocytes by M2 macrophages to produce melanin through vascular endothelial growth factor. Sci Rep. 2022;12:6416. doi: 10.1038/s41598-022-08163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahmudi-Azer S, Downey GP, Moqbel R. Translocation of the tetraspanin CD63 in association with human eosinophil mediator release. Blood. 2002;99:4039–4047. doi: 10.1182/blood.v99.11.4039. [DOI] [PubMed] [Google Scholar]
- 38.Köberle M, Kaesler S, Kempf W, Wölbing F, Biedermann T. Tetraspanins in mast cells. Front Immunol. 2012;3:106. doi: 10.3389/fimmu.2012.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- 40.Mori S, Pawankar R, Ozu C, Nonaka M, Yagi T, Okubo K. Expression and roles of MMP-2, MMP-9, MMP-13, TIMP-1, and TIMP-2 in allergic nasal mucosa. Allergy Asthma Immunol Res. 2012;4:231–239. doi: 10.4168/aair.2012.4.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung YS, Liu XW, Chirco R, Warner RB, Fridman R, Kim HR. TIMP-1 induces an EMT-like phenotypic conversion in MDCK cells independent of its MMP-inhibitory domain. PLoS One. 2012;7:e38773. doi: 10.1371/journal.pone.0038773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luu QQ, Cao TBT, Shin YS, Yang EM, Moon JY, Park HS. Sputum antinuclear antibody serves as a biomarker for severe asthma. Allergy. 2021;76:3832–3835. doi: 10.1111/all.15086. [DOI] [PubMed] [Google Scholar]
- 43.Suzukawa M, Ohta K, Fukutomi Y, Hashimoto H, Endo T, Abe M, et al. Classifications of moderate to severe asthma phenotypes in Japan and analysis of serum biomarkers: a nationwide cohort study in Japan (NHOM Asthma Study) Allergol Int. 2023;72:63–74. doi: 10.1016/j.alit.2022.06.002. [DOI] [PubMed] [Google Scholar]
- 44.Athari SS. Targeting cell signaling in allergic asthma. Signal Transduct Target Ther. 2019;4:45. doi: 10.1038/s41392-019-0079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Germic N, Hosseini A, Yousefi S, Karaulov A, Simon HU. Regulation of eosinophil functions by autophagy. Semin Immunopathol. 2021;43:347–362. doi: 10.1007/s00281-021-00860-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsu AT, Gottschalk TA, Tsantikos E, Hibbs ML. The role of innate lymphoid cells in chronic respiratory diseases. Front Immunol. 2021;12:733324. doi: 10.3389/fimmu.2021.733324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bradding P, Walls AF, Holgate ST. The role of the mast cell in the pathophysiology of asthma. J Allergy Clin Immunol. 2006;117:1277–1284. doi: 10.1016/j.jaci.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 48.Marone G, Granata F, Pucino V, Pecoraro A, Heffler E, Loffredo S, et al. The intriguing role of interleukin 13 in the pathophysiology of asthma. Front Pharmacol. 2019;10:1387. doi: 10.3389/fphar.2019.01387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abdelaziz MH, Abdelwahab SF, Wan J, Cai W, Huixuan W, Jianjun C, et al. Alternatively activated macrophages; a double-edged sword in allergic asthma. J Transl Med. 2020;18:58. doi: 10.1186/s12967-020-02251-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu L, Qin Y, Cai Z, Tian Y, Liu X, Li J, et al. Effective-components combination improves airway remodeling in COPD rats by suppressing M2 macrophage polarization via the inhibition of mTORC2 activity. Phytomedicine. 2021;92:153759. doi: 10.1016/j.phymed.2021.153759. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of the study subjects
Comparison of demographic characteristics between the high-TIMP-1 and low-TIMP-1 groups
Univariate and multivariate logistic regression for predicting severe asthma
The effects of TIMP-1 on the airway inflammation in vivo. (A) Experimental protocol for TIMP-1 stimulation in vivo. (B) The percentage of ILC2 count (CD45+Lineage−CD90.2+CD278+ cells) in the lung tissues was detected by fluorescent activated cell sorting and depicted by graph (n = 5 for each group). (C, D) The levels of IL-5 and IL-13 in the bronchoalveolar lavage fluid (n = 5 for each group). Data are presented as mean ± standard deviation.
The effects of anti-TIMP-1 antibody on airway inflammation in the chronic allergic asthma mouse model. (A) Experimental protocol for anti-TIMP-1 treatment in the chronic allergic asthma mouse model. (B) The percentage of ILC2 count (CD45+Lineage-CD90.2+CD278+ cells) in the lung tissues was detected by fluorescent activated cell sorting and depicted by graph (n = 5 for each group). The levels of (C) IL-5, (D) IL-13, and (E) MMP-9 in the BALF (n = 5 for each group). Data are presented as mean ± standard deviation. (F) Correlations between TIMP-1 and IL-33 levels in BALF. Data are presented as Spearman correlation coefficient r.
Flow cytometry gating strategy for immunophenotyping. (A) Flow cytometric plots of human M2 macrophages (CD68+CD11c−CD206+ cells). (B) Flow cytometric plots of group 2 innate lymphoid cells count (CD45+Lineage−CD90.2+CD278+ cells) from single cells of the murine lung tissues.
The production of MMP-9 from human AECs. The production of MMP-9 from AECs (SAECs and A549) in response to (A) poly I:C, (B) IL-13, and in co-culture with (C) PBEs as well as (D) in response to EETs (n = 6 for each group). Data are presented as mean ± standard deviation.
The effects of TIMP-1 on cytokine release from LAD-2 cells. The levels of (A) IL-5, (B) IL-13, and (C) IL-33 from LAD-2 cells in response to TIMP-1 alone or in combination with cross-linking IgE (n = 6 for each group). Data are presented as mean ± standard deviation.
The effects of TIMP-1 on the airway inflammation in vivo in a dose-dependent manner. (A) TIMP-1 induced an increase in eosinophil count in the BALF (n = 3 for each group) in a dose-dependent manner (0.05, 0.5, and 5 µg/kg). (B) The levels of EDN in the BALF (n = 3 for each group). (C) The expression of MBP in the lung tissues. Data are presented as mean ± standard deviation.