Abstract
In the present investigation, two fungal strains were exploited to evaluate their degradation capability on Synozol Red, Yellow, and Navy-Blue dyes which gave the utmost decolorization such as 40%, 70%, 90% by Aspergillus niger, and 36%, 73%, 87% by Trichoderma viride, respectively for 60 days. The Gas Chromatography-Mass Spectrometry (GC–MS) analysis of the decolorized dyes suggested that various compounds such as Caprolactam, Furazan-3-carboxamide, oxime, 4-amino-N, N-dimethyl, 6H-Pyrazolo[1,2-a] [1,2,4,5]tetrazine, Hexahydro-2,3-dimethyl, Benzene, 1-propenyl, Dihydroxymaleic acid, Arsenous acid, tris(trimethylsilyl) ester were produced by the fungi which helped in the removal of dyes from the wastewater. The laccase activity of the degraded dyes was proof that both of the strains positively produced the enzyme that helped in the biodegradation of carcinogenic dyes into less harmful products. The A. niger extracted laccase relative activity was 262%, 265%, and 145.7% for Synozol Yellow, Synozol Red, and Navy Blue, respectively. Similarly, laccase, obtained from T. viride, showed relative activity of 187.5% against Synozol Yellow, 215% against Synozol Red, and 202% against Navy Blue. Furthermore, the supernatant extracted from fungi-decolorized wastewater was used to check phytotoxicity on Vigna radiata, which gave excellent results. Both fungal strains, on the basis of their dye degradation potential, can be used to ameliorate wastewater contaminated with azo dyes.
Keywords: Azo dyes pollution, Aspergillus niger, Trichoderma viride, Dyes degradation, GC–MS analysis
1. Introduction
The immeasurable release of contaminants, including plastic, azo dyes, and especially heavy metals, by industries and factories is posing life-threatening perils to the environment. Many contaminants have adverse effects on the living systems, including suffocation due to plastic contamination and contaminants assemblage in the digestive tracts leading to the death of the species. Contaminants are broadly classified as; chemical contaminants (PCBs, antibiotics, metals, personal care products), physical contaminants (radiation, noise, light), biological contaminants (pollen, animal by-products, infectious agents such as bacteria, viruses, etc.) (Anwar et al., 2018).
Dyes are widely spread all around due to lots of operating textile and food industries. Textile dyes are essentially soluble in water and have many types including basic dyes, acidic dyes, reactive, and direct dyes. All of these types are recalcitrant naturally. They usually impart great damage to marine life because enough light cannot enter and there is a decrease in photosynthesis rate, hence leading to low oxygen and overall death of the life forms (Lellis et al., 2019). Food dyes are equally important because they give aesthetic color and flavor to food, attracting many consumers. Besides benefits, there are many harmful aspects such as the death of the marine life form, the immune system imbalance in humans, ADHD among kids, hypersensitivity reactions, certain organ cancers, etc (Evyaz and Yuksel, 2020).
Azo dyes have become a necessity in today’s world. They are exploited in products of everyday use such as food, medicines, the clothing industry, etc. The azo dyes contain several amine groups (R1-N = N-R2), giving toxic products in reduced form (Feng et al., 2018). Azo dyes have the most carcinogenic effects ranging from skin cancers to chromosomal aberrations in an individual (Chequer et al., 2011). Prominent azo dyes include Synozol Red, Synozol Yellow, Synozol Blue, Tetrazine, etc.
The major problem associated with the release of azo dyes in water is the carcinogenic effect on humans, plants, and animals. The negative impacts of Synozol dyes absorption include the alteration of soil chemistry leading to disturbance of the natural flora of the soil. Consequently, plants with major nutrient deficiencies will grow or have stunted growth. When consumed by animals or indirectly by humans cause serious reproductive, dermal, genetic, and neurological effects. In extreme conditions, the reaction of human serum albumin protein with Synozol dyes results in the degranulation of mast cells, releasing histamine, heparin, and serine proteases, indicating type 1 hypersensitivity (Tohamy et al., 2022).
Even when these Synozol dyes are degraded, the generated compounds are relatively toxic due to their mutagenicity and carcinogenicity. For the decolorization of such dyes, physical methods such as ion exchange chromatography, photodegradation, etc are employed. The most effective method of dye decolorization is by biological agents such as bacteria and fungi. The reason is the lack of production of harmful compounds as these living agents either adsorb or assimilate dyes into their cells (Rehman and Ilyas, 2013). This study employs two fungal strains i.e., Aspergillus niger and Trichoderma viride, to check the biodegradation of toxic dyes.
2. Materials and methods
2.1. Fungal strains and growth medium
Two fungal strains i.e., Aspergillus niger and Trichoderma viride were obtained from the First Culture Bank of Pakistan (FCBP) of the Institute of Agricultural Sciences, University of the Punjab, Lahore, Pakistan. Natural Potato Dextrose Agar (PDA) was prepared. One kg of potatoes was peeled and boiled in 1.5 l of water for 25 min until the volume reached 1200 ml, which was adjusted according to the needs of the experiment. The medium needed ‘glucose’ (2%), as a carbon source, and agar (2%) as a solidifying agent. Antibiotic such as ‘chloramphenicol’ was used to inhibit the growth of other microorganisms i.e., bacteria.
Chloramphenicol was prepared by mixing 0.25 g of the antibiotic in 9 ml ethanol (70%) and volume was made up to 10 ml with autoclaved distilled water. Potato Dextrose Broth (PDB) was used with the same recipe without agar for further cultivation of fungal strains. The media were incubated at 30 ± 2 °C for 3 days (A. niger) and 7 days (T. viride) (Figs. S1, S2).
2.2. Dye decolorization by fungi
The PDB medium (200 ml) was prepared, autoclaved, and inoculated with both fungal strains i.e., A. niger and T. viride. For inoculation, mycelial disks of both strains having a diameter of 1 cm were removed from the solid agar surface (Si et al., 2012). After incubation, 0.05% of Synozol Red, Synozol Blue, and Synozol Yellow were added separately to the medium containing fully cultivated fungal strains. The preparations were kept for almost 2 months, taken out, and centrifuged at 6,000 rpm for 5 min. The optical density of control and treated samples for Synozol Red (λ = 547 nm) (Khan et al., 2020), Synozol Yellow (λ = 510 nm), and Synozol Blue (λ = 420 nm) (Olczyk et al., 2020) were measured. The percentage of dye decolorization was calculated as:
2.3. GC–MS analysis
The Gas Chromatography-Mass Spectrometry (GC–MS) analysis of the supernatants was done for the analysis of different compounds produced by fungal strains (Ali et al., 2023).
2.4. Laccase assay
The supernatants were collected from the dye-containing culture media by centrifugation at 6000 rpm for 5 min. they were then treated with 60% ammonium sulfate, and incubated for 24 h. The mixture was centrifuged at 6,000 rpm for 5 min. Then, 1 ml of the supernatant was used as crude enzyme against the substrate, ABTS (2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic) acid) (Rehman and Ilyas, 2013).
2.5. Plant growth using fungal-treated wastewater
To determine whether fungal-treated wastewater is toxic for plant growth or not, an experiment was set up. For this, the soil was collected from the backyard of the department. Mung beans were treated with mercuric chloride (HgCl2) to get rid of any kind of contamination. Then, autoclaved distilled water was used for their washing prior to potting.
Mung bean is a member of the family of ‘legumes.’ Mung beans are used as a staple food all over the world. Many proteins in mung beans help in reducing the risks of different cancers, inflammation, and cholesterol accumulation (Du et al., 2018). The seeds were cultivated using tap water and sewage water as controls. Fungal-treated Synozol yellow, blue, and red waste waters acted as test samples. The implanted seeds were kept in sunlight for 12 h and in the dark for the next 12 h. The continuous supply of oxygen was ensured. The observations were recorded after 6 days.
2.6. Statistical analysis
Version 20.0 of the SPSS (Statistical Pack initial age for Social Sciences) software was performed for all statistical evaluations. The results were shown as the standard error of the mean, and the significance level was specified at (p < 0.05).
3. Results
3.1. Dye decolorization
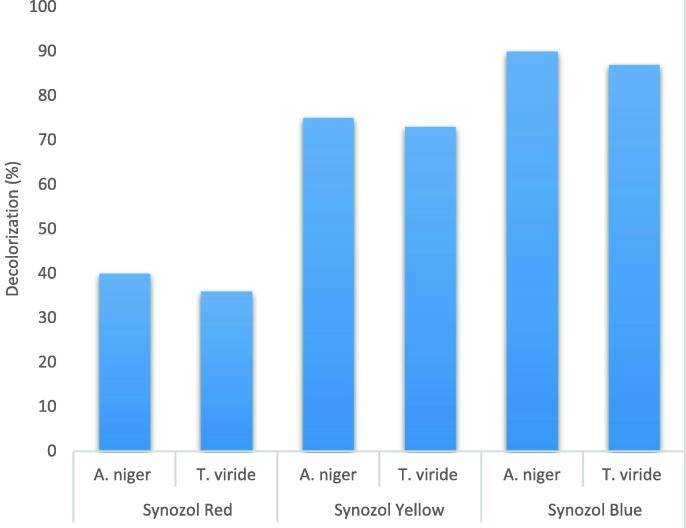
Post incubation, the media containing fungi and dye were analyzed to have a considerable color change from dark to light due to the enzymatic activity of the fungal strains (Fig. 1a,b,c). The absorbance (optical density) values of the treated samples (with dyes) showed a significant decrease as compared to the control (without dyes). Aspergillus niger was able to degrade Synozol Red, Yellow, and Navy-Blue up to 40%, 70%, and 90% within 60 days of incubation. Similarly, Trichoderma viride within 60 days of incubation showed 36%, 73%, and 87% degradation potential of Synozol Red, Yellow, and Navy-Blue (Fig. 2).
Fig. 1.
Decolorization of (a) Synozol Yellow (T. viride), (b) Synozol Red (T. viride), and (c) Synozol Red (A. niger).
Fig. 2.
Percentage decolorization of Synozol dyes contaminated wastewater by A. niger and T. viride.
3.2. Laccase assay
The laccase activity for three of the dyes i.e., Synozol Yellow, Synozol Red, and Navy Blue for A. niger and T. viride was measured by taking the optical densities. In contrast to the control for Synozol Yellow which was 0.672, the relative optical density for A. niger was 1.76 (262%), and for T. viride, the value came out to be 1.26 (187.5%). Similarly, for Synozol Red, the control’s absorbance was computed to be 0.781, while 2.07 (265%) for A. niger and 1.68 (215%) for T. viride. The evaluated value for Navy Blue’s control was 0.592, the Aspergillus and Trichoderma strains lay at 0.863 (145.7%) and 1.197 (202%), respectively.
3.3. GC–MS analysis
A number of compounds were found to be produced in the medium containing dyes by the activity of A. niger and T. viride (Fig. 3a, b; Table S1). The control and test samples were compared and various compounds i.e., 2,3-Butanediol, Caprolactam, Oleic Acid, Octadecanoic acid, Urazan-3-carboxamide, Oxime, 4-amino-N, N-dimethyl, Diglycolamine, and isopropyl benzene were produced from the degradation of dyes by the action of fungal strains (Table 1).
Fig. 3.
(a) GC–MS analysis of Synozol Yellow degraded products by T. viride(b) GC–MS analysis of Synozol Yellow degraded products by A. niger.
Table 1.
Various compounds produced of azo dyes degradation by fungal strains.
| A. niger | T. viride | |
|---|---|---|
| Synozol Red | Allantoic acid | 2,3-Butanediol |
| Hydroxyurea | Caprolactam | |
| 1,4-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester | n-Hexadecanoic acid | |
| o-Xylene | cis-Vaccenic acid | |
| MDMA methylene homolog | Oleic Acid | |
| Furazan-3-carboxamide, oxime, 4-amino-N, N-dimethyl | Octadecanoic acid | |
| Propanamide, 3-(3,4-dimethylphenylsulfonyl) | Silane, (2-methoxyphenyl) trimethyl | |
| Carbonic acid, hexadecyl prop-1-en-2-yl ester | L-Proline, N-(phenylacetyl)-, propyl ester | |
| Synozol Yellow | Guanidine, N, N-dimethyl | Benzene, 1-propenyl |
| N-Methyl-3-(methylamino)propanamide | Quinoline, 1,2,3,4-tetrahydro-1-((2-phenylcyclopropyl) sulfonyl)-, trans | |
| 6H-Pyrazolo[1,2-a][1,2,4,5] tetrazine, hexahydro-2,3-dimethyl | Carvacrol, TBDMS derivative | |
| 1-Naphthoic acid, 4-chlorophenyl ester | Heptasiloxane, hexadecamethyl | |
| Diglycolamine | 1-(2-Aminoethoxy)-2-isopropylbenzene | |
| Cathinone | Phenol, 2,2′-methylenebis[6-(1,1-dimethylethyl)-4-methyl | |
| Navy Blue | Cyclotetrasiloxane, octamethyl | Tryptamine |
| Epinephrine, (. beta.)-, 3TMS derivative | Arsenous acid, tris(trimethylsilyl) ester | |
| Benzeneethanamine, N-[(pentafluoro phenyl)methylene]-. beta.,4-bis[(trimethylsilyl)oxy] | 4-tert-Octylphenol, TMS derivative | |
| Dihydroxymaleic acid | 1,2-Bis(trimethylsilyl)benzene |
3.4. Plant growth using fungal-treated wastewater
The seeds began to germinate after 2 days. Regular watering and provision of light were maintained throughout the time of germination. After 3 days, the shoot began to appear, and on the 6th day, leaves were apparent above the shoot. The maximum growth was observed for tap water and the minimum was for Synozol Red (Fig. 4a, b).
Fig. 4.
(a) Growth of Mung bean in the absence and presence of Synozol Blue treated water by T. viride(b) and Synozol Yellow treated water by A. niger.
4. Discussion
The biodegradation of harmful and carcinogenic dyes to reduce their toxic effects is effectively achieved by the use of some fungal strains. It was reported that the decolorization ability of white rot fungi was 52%, due to the excretion of certain extracellular enzymes such as laccase, Mn-peroxidase, etc (Rodríguez et al., 1999). Many Aspergillus strains efficiently degraded Acid Blue, Disperse Red1, and Congo Red dyes (Ameen et al., 2021). The degradation of the dye, Remazol Brilliant Blue R by fungal strains Trichoderma citrinoviride, Trichoderma koningiopsis, and Pestalotiopsis sp. was reported to be 33.1%, 52.5%, and 74.8%, respectively (Syafiuddin and Fulazzaky, 2021). The biosorption and biodegradation capability of the fungi, Bjerkandera adusta resulted in 90% decolorization of dyes such as Crystal Violet, Malachite Green, etc (Gao et al., 2020).
In the current investigation of dye decolorization, two fungal strains, Aspergillus niger, and Trichoderma viride were utilized. The maximum decolorization capability was calculated to be by A. niger for the dye Synozol blue, which was 90%, and the least for Synozol yellow by T. viride, which was 36%. In another work by a researcher, the consortium of T. viride and Ralstonia pickettii was used, giving an excellent decolorization rate of 97.8% for Methylene Blue (Nabilah et al., 2023). Dimethyl yellow dye was decolorized up to 74.3% by the fungal strain Aspergillus quadrilineatus (Table 2) when the temperature and pH were kept at 30 °C and 5, respectively (Yusuf et al., 2021).
Table 2.
Azo dyes degradation potential of various fungal strains reported by researchers.
| Organism’s names | Decolorization (%) | Reference |
|---|---|---|
| Aspergillus flavus, A. fumigatus, A. flavus, A. terreus | Acid Blue – 80% Disperse Red – 83% Congo Red – 84% |
Ameen et al. (2021) |
| Aspergillus quadrilineatus | Dimethyl Yellow – 74.3% | Yusuf et al. (2021) |
| Aspergillus niger | Congo Red – 93.9% | Sing and Dwivedi (2022) |
| Bjerkandera adusta | Triphenyl Methane – 90% | Gao et al. (2020) |
| Phanerochaete chrysosporium | Direct Yellow – 82% Reactive Black – 89% Reactive Red – 94% |
Almeida et al. (2021) |
|
Gloeophyllum trabeum, Trichoderma viride |
Methylene Blue 31.50% 53.89% |
Pratiwi et al. (2021) |
| Pseudopestalotiopsis theae, Astrocystis bambusae, Trichoderma asperellum | Malachite Green 89.2% 67.6% 76.1% |
Ting et al. (2021) |
| Aspergillus niger | Synozol Blue – 90% Synozol Yellow – 75% Synozol Red – 40% |
Present study |
| Trichoderma viride | Synozol Blue – 87% Synozol Yellow – 73% Synozol Red – 36% |
Present study |
According to a study, Aspergillus sp. Omeje showed good laccase activity (Omeje et al., 2020). In one of the studies, Pleurotus ostreatus was shown to produce the highest quantity of laccase i.e., 60–80% (Kumar et al., 2011). Porodaedalea laricis was found to have laccase activity in the range of 3.29U/ml-3.32U/ml (Yi et al., 2020). Copper sulfate and ferulic acid-mediated laccase production were recorded when the test organism was Nectriella pironii. The highest range of the enzyme produced was 3,300U/l (Góralczyk-Bińkowska et al., 2020). In the present study, two fungal strains i.e., A. niger and T. viride were exploited and their laccase enzyme production capability was analyzed which came out to be 2.07 and 1.68 for both of the strains, respectively.
GC–MS analysis of a dye Disperse Red by the combination of Aspergillus sp. and Chlorella sorokiniana resulted in the formation of many products such as o-xylene, acetone, and di isobutyl phthalate, etc (Tang et al., 2019). The analysis of compounds produced by treating contaminated Alizarin Red wastewater by Trametes gibbosa resulted in acryaldehyde, and 1-butylene (Zhang et al., 2021). When Congo Red dye-contaminated water treated with Aspergillus niger was observed by GC–MS analysis, benzene propanoic acid, and benzene dicarboxylic acid were fundamentally produced as the degraded products (Singh and Dwivedi, 2022). Recent research suggests that the degraded products by the fungi, A. niger, and T. viride were Caprolactam, Diglycolamine, Tris(trimethylsilyl) ester, and many others.
The toxicity test was performed by irrigating the Lepidium sativum (Garden cress) seeds with Phanerochaete chrysosporium-treated water. The germination rate was much higher in fungal-treated plants than in untreated ones (Almeida et al., 2021). In another study, Marasmius sp. was used for treating the water polluted with Diazo Reactive dye, and the resultant product was exploited for irrigation of two seeds i.e., Phaseolus mungo (Black gram) and Sorghum vulgare (Jowar). The mean shoot and root lengths were the maximum for S. vulgare (Vantamuri and Shettar, 2020). The present examination investigated A. niger and T. viride to check phytotoxicity on Vigna radiata (Mung beans). The maximum rate of germination was observed for plants that were treated with water remediated with A. niger.
5. Conclusion
This study is a shred of evidence that the environmental pollution induced by azo dyes can be potentially reduced by using certain fungal strains. The utmost biodegradation of Synozol dyes by A. niger, and T. viride showed 90% and 87% effectivity respectively. The analysis of degraded products by GC–MS exhibited various compounds such as Caprolactam, Furazan-3-carboxamide, oxime, 4-amino-N, N-dimethyl, 6H-Pyrazolo[1,2-a] [1,2,4,5] tetrazine, Hexahydro-2,3-dimethyl, Benzene, 1-propenyl, Dihydroxymaleic acid, Arsenous acid, tris(trimethylsilyl) ester, etc. The fungal-extracted laccase relative activity was 262% and 187.5% against Synozol Yellow, 265% and 215% against Synozol Red, and 145.7% and 202% against Navy Blue. The laccase activity of both fungal strains had positive results. The negative phytotoxic effect of the fungal-treated wastewater was indicated by the germination of the Mung bean seeds. Both fungal strains use is propitious to clean up the environment contaminated with toxic pollutants, especially azo dyes.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2023.103734.
Contributor Information
Eeman Ali, Email: eeman.bs.mmg@pu.edu.pk.
Ifrah Amjad, Email: ifrah.bs.mmg@pu.edu.pk.
Abdul Rehman, Email: rehman.mmg@pu.edu.pk.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Ali S., Rehman A., Hussain S.Z., Bukhari D.A. Characterization of plastic degrading bacteria isolated from sewage wastewater. Saudi J. Biol. Sci. 2023;30(5):1–6. doi: 10.1016/j.sjbs.2023.103628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida A.P., Macrae A., Ribiero B.D., Nascimento R.P. Decolorization and detoxification of different azo dyes by Phanerochaete chrysosporium ME-446 under submerged fermentation. Braz. J. Microbiol. 2021;52:727–738. doi: 10.1007/s42770-021-00458-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameen F., Dawoud T.M., Alshehrei F., Alsamhary K., Almansob A. Decolorization of acid blue 29, disperse red 1 and congo red by different indigenous fungal strains. Chemosphere. 2021;271:1–9. doi: 10.1016/j.chemosphere.2021.129532. [DOI] [PubMed] [Google Scholar]
- Anwar, Y., Hakeem, K.R., Alharby, H.F., Alghamdi, K.M., 2018. Environmental contamination and remediation, chapter 1, emerging environmental pollutants: issues and challenges by Samia Qadeer, Muzammil Anjum, and Azeem Khalid, pp. 5-6.
- Du M., Xie J., Gong B., Xu X., Tang W., Li X., Li C., Xie M. Extraction, physicochemical characteristics, and functional properties of Mung bean protein. Food Hydrocoll. 2018;76:131–140. [Google Scholar]
- Evyaz, M., Yuksel, E., 2020. Water Chemistry, pp. 113-114 (Chapter 7).
- Feng J., Cerniglia C.E., Chen H. Toxicological significance of azo dye metabolism by human intestinal microbiota. HHS Public Access. 2018;4:568–586. doi: 10.2741/400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T., Qin D., Zuo S., Peng Y., Xu J., Yu B., Song H., Dong J. Decolorization and detoxification of triphenylmethane dyes by isolated endophytic fungus, Bjerkandera adusta SWUSI4 under non-nutritive conditions. Bioresour. Bioprocess. 2020;7(53):1–12. [Google Scholar]
- Góralczyk-Bińkowska A., Jasińska A., Długoński A., Płociński P., Długoński J. Laccase activity of the ascomycete fungus Nectriella pironii and innovative strategies for its production on leaf litter of an urban park. PLoS One. 2020;15(4):e0231453. doi: 10.1371/journal.pone.0231453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S.U., Khan H., Anwar S., Khan S., Zanoni M.V.B., Hussain S. Computational and statistical modeling for parameters optimization of electrochemical decontamination of synozol red dye wastewater. Chemosphere. 2020;253:1–9. doi: 10.1016/j.chemosphere.2020.126673. [DOI] [PubMed] [Google Scholar]
- Kumar V.V., Kirupha S.D., Periyaraman P., Sivanesan S. Screening and induction of laccase activity in fungal species and its application in dye decolorization. Afr. J. Microbiol. Res. 2011;5(11):1–8. [Google Scholar]
- Lellis B., Fávaro-Polonio C.Z., Pamphile J.A., Polonio J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Inn. 2019;3(2):275–290. [Google Scholar]
- Nabilah B., Purnomo A.S., Prasetyoko D., Rohmah A.A. Methylene blue biodecolorization and biodegradation by immobilized mixed cultures of Trichoderma viride and Ralstonia pickettii into SA-PVA-Bentonite matrix. Arab. J. Chem. 2023;16:1–12. [Google Scholar]
- Omeje K.O., Nnolim N.E., Ezema B.O., Ozioko J.N., Eze S.O.O. Synthetic dyes decolorization potential of agro-industrial waste-derived thermo-active laccase from Aspergillus species. Biocatal. Agric. Biotechnol. 2020;29:1–7. [Google Scholar]
- Pratiwi, N.I., Purnomo, A.S., Rizqi, H.D., Alkas, T.R., Nawfa, R., 2021. Biodecolorization and biotransformation of methylene blue by mixed cultures of brown-rot fungus Gloeophyllum trabeum and filamentous fungus Trichoderma viride. In: 4th International Seminar on Chemistry AIP Conf. Proc. 2349, 020076-1–020076-11; Published by AIP Publishing. 978-0-7354-4100-2/$30.00.
- Rehman A., Ilyas S. Decolorization and detoxification of Synozol red HF-6BN azo dye, by Aspergillus niger and Nigrospora sp. J. Environ. Health Sci. Eng. 2013;10(12):1–9. doi: 10.1186/1735-2746-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez E., Pickard M.A., Duhalt R.V. Industrial dye decolorization by laccases from ligninolytic fungi. Curr. Microbiol. 1999;38:27–32. doi: 10.1007/pl00006767. [DOI] [PubMed] [Google Scholar]
- Singh G., Dwivedi S.K. Mechanistic, adsorption kinetics and confirmatory study of Congo red dye removal by native fungus Aspergillus niger. Biomass Convers. Biorefin. 2022:1–19. [Google Scholar]
- Syafiuddin A., Fulazzaky M.A. Decolorization kinetics and mass transfer mechanisms of Remazol Brilliant Blue R dye mediated by different fungi. Biotechnol. Rep. 2021;29:1–14. doi: 10.1016/j.btre.2020.e00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W., Xu X., Ye B.-C., Cao P., Ali A. Decolorization and degradation analysis of Disperse Red 3B by a consortium of the fungus Aspergillus sp. XJ-2 and the microalgae Chlorella sorokiniana XJK. R. Soc. Chem. 2019;9:1–9. doi: 10.1039/c9ra01169b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting A.S.Y., Cheng C.K.W., Santiago K.A.A. Decolourization of malachite green dye by endolichenic fungi from the lichen Usnea sp. A novel study on their dye removal potential. J. King Saud Uni.-Sci. 2021;33(7):[101579]. [Google Scholar]
- Tohamy R.A., Ali S.S., Li F., Okasha K.M., Mahmoud Y.A.G., Elsamahy T., Jiao H., Fu Y., Sun J. A critical review on the treatment of dye-containing wastewater: ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022;231:1–17. doi: 10.1016/j.ecoenv.2021.113160. [DOI] [PubMed] [Google Scholar]
- Vantamuri A.B., Shettar A.K. Biodegradation of Diazo Reactive dye (Green HE4BD) by Marasmius sp. BBKAV79. Chem. Data Collect. 2020;28:1–9. [Google Scholar]
- Yi W., Hong-fei M.A., Young-jia C.A.O., Jing S.I., Bao-kai C.U.I. Medium optimization for the laccase production by white rot fungus Porodaedalea laricis and its dye decolorizing capacity. Biotechnol. Bull. 2020;36(1):45–59. [Google Scholar]
- Yusuf F., Ahmad F.A., Muhammad F., Shehu U., Yakasai H.M. Profiling the effects of pH and temperature on azo dye-decolourisation by Aspergillus quadrilineatus strain BUK_BCH_BTE1 isolated from textile effluents. Chemsearch e-J. 2021;12(2):1–6. [Google Scholar]
- Zhang J., Chi Y., Feng L. The mechanism of degradation of alizarin red by a white-rot fungus Trametes gibbosa. BMC Biotechnol. 2021;21(64):1–17. doi: 10.1186/s12896-021-00720-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further Reading
- Hauser, P. (Ed.). 2011. Advances in Treating Textile Effluent. InTech. doi: 10.5772/1039.
- Olczyk J., Sójka-Ledakowicz J., Kudzin M., Antecka A. The eco-modification of textiles using enzymatic pretreatment and new organic UV absorbers. Autex Res. J. 2021;21(3):242–251. [Google Scholar]
- Si J., Cui B.K., Dai Y.C. Decolorization of chemically different dyes by white-rot fungi in submerged cultures. Ann. Microbiol. 2013;63:1099–1108. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.