Abstract
COVID-19 is a novel virus which causes a variety of clinical manifestations in the body, some of which are yet to be discovered. The main aim of our study is to highlight the neurological manifestations of COVID-19 as it is still new to the medical world, and to emphasize the fact that the physicians have to be wary of the possibility that patients affected by COVID-19 can present with encephalitis. Only a few studies are available so far regarding the neurological manifestations of this novel virus which highlights the need for this study. We present a case series of 4 patients who were found to have COVID-19 encephalitis. There is still no disease-defining test for diagnosis so the mainstay of diagnosis is exclusion of all the common causes of encephalitis. Brain magnetic resonance imaging and cerebrospinal fluid analysis performs an ancillary in the diagnostic tools. Our study also supports the use of IV tocilizumab (4–8 mg/kg) and IV methylprednisolone (0.5–2 mg/kg) as possible treatment options with good results, as the patients described in our case series responded well to these medications.
Keywords: Case report, Corticosteroids, COVID-19, Encephalitis, Tocilizumab, Neurological manifestations
Introduction
COVID-19 has a wide variety of clinical manifestations and signs and symptoms involving various systems of the body. This novel infection has been associated with acute respiratory distress syndrome, thromboembolic syndrome, severe metabolic syndromes, severe acute tubular necrosis, electrolyte abnormalities, neurologic syndromes, and cardiac events, including myocarditis and arrhythmias [1]. The first case of COVID-19-associated viral encephalitis was reported in March 2020, and through genome sequencing of the cerebrospinal fluid (CSF), COVID-19’s presence was identified [2]. The neurological manifestations of COVID-19 can include either nonspecific symptoms like headache, dizziness, taste and olfactory sensation disturbance, or specific syndromes like encephalitis, acute transverse myelitis, meningitis, or stroke [3]. These can be caused by either direct invasion of the cells or indirectly due to inflammatory response and cytokine storm [4].
Our primary interest is that of encephalitis in COVID-19-affected patients. The presentation of COVID-19 encephalitis is similar to other causes of encephalitis, with patients showing features of fever, confusion, seizures, and focal neurological signs. It can be diagnosed by magnetic resonance imaging (MRI) of the brain and CSF polymerase chain reaction (PCR) for the COVID-19 genome. Various treatments, including intravenous steroids, immunoglobulins, and rituximab, have been tried with variable outcomes [5]. Herein, we present a case series of 5 patients with COVID-19 encephalitis managed with intravenous tocilizumab and methylprednisolone.
Case Presentation
Patient 1
Patient 1 was a 48-year-old male who presented with sore throat, cough, and shortness of breath for 12 days. Also, he had fever and confusion for 3 days, and an episode of seizure. His past medical history, personal history, and drug history were unremarkable. On arrival, he had a temperature of 103°F, blood pressure of 120/75 mm Hg, pulse rate of 103 bpm, respiratory rate of 16/min, and a GCS score of 11/15. His systemic examination was unremarkable except for bilateral crepitations on chest auscultation. He had no neck stiffness or focal neurologic deficits.
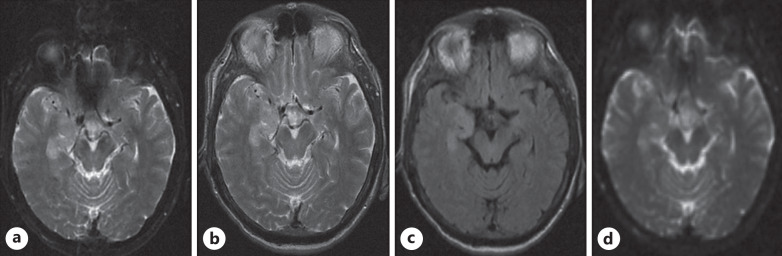
His laboratory findings are mentioned in Table 1. The patient was suspected of having encephalitis, so an MRI of the brain and lumbar puncture were ordered. MRI of the brain showed gyral swelling and hyperintensities in the right mesial temporal lobe, suggestive of encephalitis (shown in Fig. 1). The findings of the lumbar puncture are given in Table 2. Also, we did an autoimmune workup, including anti-NMDA receptor antibodies, anti-Ro antibodies, anti-La antibodies, ANCA antibodies, and anti-Hu antibodies, which were negative. These investigations were carried out to rule out the common causes of encephalitis. As the patient had respiratory symptoms, we did a chest X-ray, which showed bilateral homogenous peripheral opacities. A nasal swab COVID-19 PCR was ordered, which was positive. The patient was suspected of having COVID-19-related encephalitis, so a CSF COVID-19 PCR was done, but it was negative.
Table 1.
Summary of the laboratory investigations of all the cases
| Laboratory investigation | Unit | Reference value | Case 1 | Case 2 | Case 3 | Case 4 |
|---|---|---|---|---|---|---|
| WBC | Cells/mm3 | 4,000–11,000 | 13,000 | 17,000 | 14,000 | 16,000 |
| CRP | mg/mL | 0–5 | 87 | 102 | 68 | 112 |
| D dimers | ng/mL | 0–200 | 810 | 1,318 | 984 | 1,118 |
| Ferritin | ng/mL | 30–400 | 1,245 | 865 | 1,311 | 754 |
| LDH | U/L | 140–280 | 564 | 653 | 486 | 560 |
| Random blood sugar | mg/dL | 120–140 | 119 | 112 | 108 | 102 |
| Blood culture | Negative | Negative | Negative | Negative | ||
| Urine culture | Negative | Negative | Negative | Negative | ||
| Urine toxicology | Negative | Negative | Negative | Negative | ||
| HIV antibodies | Negative | Negative | Negative | Negative | ||
| Rapid plasma reagin | Negative | Negative | Negative | Negative |
Fig. 1.
a, b Axial T2Wl demonstrates gyral swelling and hyperintensities in the right mesial temporal lobe, in particular the uncus and part of the hippocampus consistent with encephalitis. c Axial FLAIR demonstrates hyperintensities in the same areas. d Axial ADC map shows facilitated diffusion in the right mesial temporal lobe.
Table 2.
Summarizing the CSF findings of all the cases
| Spinal fluid | Units | Reference value | Case 1 | Case 2 | Case 3 | Case 4 |
|---|---|---|---|---|---|---|
| Color | Colorless | Colorless | Colorless | Colorless | Colorless | |
| WBC | Cells/mm3 | 0–5 | 57 | 76 | 63 | 59 |
| Lymphocytes | % | 81 | 79 | 86 | 77 | |
| Neutrophils | % | 19 | 21 | 14 | 23 | |
| RBC | Cells/mm3 | 0 | 342 | 423 | 297 | 400 |
| Proteins | mg/dL | 15–45 | 80 | 95 | 73 | 65 |
| Glucose | mg/dL | 40–70 | 76 | 64 | 61 | 52 |
| Glucose as percentage of serum glucose | % | 50–70 | 63 | 57 | 56 | 51 |
| Opening pressure | cm H2O | 5–20 | 14 | 18 | 12 | 16 |
| Gram stain | Negative | Negative | Negative | Negative | Negative | |
| HSV 1 and 2 PCR | Negative | Negative | Negative | Negative | Negative | |
| Cryptococcal antigen | Negative | Negative | Negative | Negative | Negative | |
| CMV PCR | Negative | Negative | Negative | Negative | Negative | |
| VDRL | Negative | Negative | Negative | Negative | Negative | |
| COVID-19 PCR | Negative | Negative | Positive | Negative | Negative |
Based on clinical evaluation and investigation reports, we made a diagnosis of COVID-19-associated encephalitis. He was treated with a single dose of IV tocilizumab 400 mg (4–8 mg/kg) followed by IV methylprednisolone 1 g (0.5–2 mg/kg) for 5 days. The patient’s condition improved and was then discharged. On follow-up after 1 month, the patient was doing well, and there were no residual neurological signs.
Patient 2
Patient 2 was a 63-year-old female with complaints of fever and confusion for 2 days and 3 episodes of seizures in the last 24 h. Also, she had nasal stuffiness, rhinorrhea, sore throat, and body aches for the last 10 days. She was a known case of hypertension and poorly controlled type 2 diabetes mellitus. Her personal history and drug history were unremarkable. She had a temperature of 102.6°F, blood pressure of 105/72 mm Hg, pulse rate of 102 bpm and a GCS of 11/15. Physical examination revealed throat hyperemia and lung crepitations on the left side. She had no neck stiffness or focal neurologic deficits.
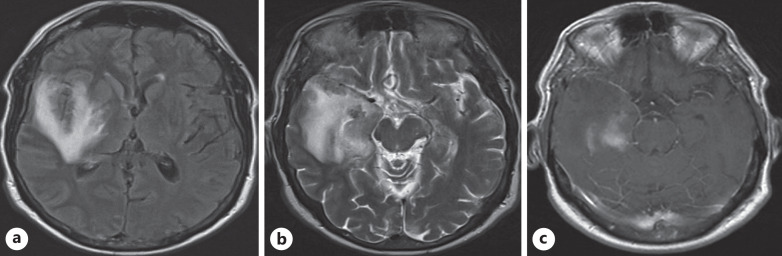
Her laboratory findings are mentioned in Table 1. The patient was suspected of having encephalitis, so an MRI of the brain and lumbar puncture were ordered. MRI of the brain was suggestive of encephalitis (shown in Fig. 2). The findings of the lumbar puncture are given in Table 2. Also, we did an autoimmune workup, including anti-NMDA receptor antibodies, anti-Ro antibodies, anti-La antibodies, ANCA antibodies, and anti-Hu antibodies, which were negative. These investigations were carried out to rule out the common causes of encephalitis. A chest X-ray was done, which showed bilateral opacities, which were more on the left side. A nasal swab COVID-19 PCR was ordered, and it was positive. The patient was suspected of having COVID-19 encephalitis, so a CSF COVID-19 PCR was ordered, and it came back positive.
Fig. 2.
a Axial FLAIR demonstrates hyperintensities involving the right mesial temporal lobe, insular cortex, and basal ganglia with axial T2WI (b) showing hyperintensities consistent with encephalitis. c Post-contrast T1WI shows patchy areas of enhancement in the mesial temporal lobe.
Based on clinical evaluation and investigation reports, we made a diagnosis of COVID-19-associated encephalitis. She was then treated with a single dose of IV tocilizumab 400 mg (4–8 mg/kg) followed by IV methylprednisolone 1 g (0.5–2 mg/kg) for 5 days. The patient failed to show any improvement. Another dose of IV tocilizumab was repeated, and methylprednisolone 1 g IV was continued for another 5 days. The patient’s condition improved and was then discharged. On follow-up after 1 month, the patient was doing well, and there were no residual neurological signs.
Patient 3
Patient 3 was a 38-year-old male with the complaints of shortness of breath and chest pain for the last 12 days. Also, he had complaints of fever and drowsiness for the last 4 days. His past medical history, personal history, and drug history were unremarkable. He had a temperature of 101.3°F, blood pressure of 120/75 mm Hg, pulse rate of 96 bpm, respiratory rate of 18/min, and a GCS of 10/15. His physical examination was unremarkable apart from bilateral chest crepitations. There was no neck stiffness or focal neurologic deficits.
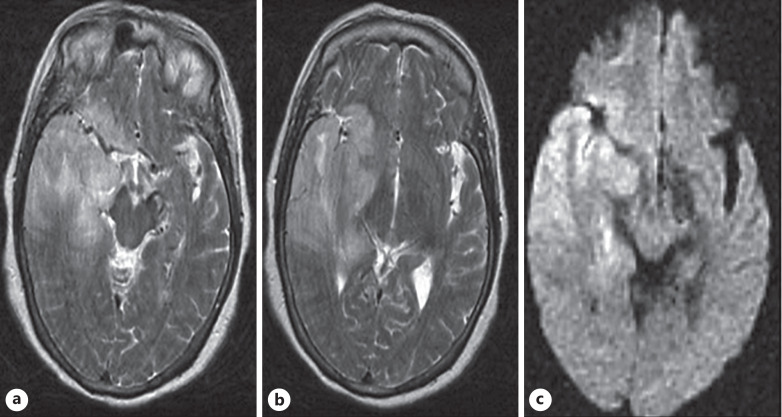
His laboratory findings are mentioned in Table 1. The patient was suspected of having encephalitis, so an MRI of the brain and lumbar puncture were ordered. MRI of the brain showed signs suggestive of encephalitis (shown in Fig. 3). The findings of the lumbar puncture are given in Table 2. Also, we did an autoimmune workup, including anti-NMDA receptor antibodies, anti-Ro antibodies, anti-La antibodies, ANCA antibodies, and anti-Hu antibodies, which were negative. These investigations were carried out to rule out the common causes of encephalitis. His chest X-ray showed bilateral opacities, and nasal swab COVID-19 PCR came out positive. He was suspected of having COVID-19 encephalitis, so CSF COVID-19 PCR was also ordered, but it came back negative.
Fig. 3.
a, b Axial T2WI shows gyral inflammation and hyperintensities involving the right temporal lobe, posterior aspect of the temporal lobe, and right basal ganglia with restriction of diffusion on the corresponding axial DWI image (c). There is a subtle mass effect on the brainstem with mild leftward midline shift.
Based on clinical evaluation and investigation reports, we made a diagnosis of COVID-19-associated encephalitis. He was then treated with a single dose of IV tocilizumab 400 mg (4–8 mg/kg) followed by IV methylprednisolone 1 g (0.5–2 mg/kg) for 5 days. The patient responded very well to this treatment and was then discharged. On follow-up after 1 month, the patient was doing well, and there were no residual neurological signs.
Patient 4
Patient 4 was a 57-year-old female with complaints of sore throat and body ache for 10 days. She also had high-grade fever and confusion for the last 2 days. She was a known case of hypertension, asthma, and well-controlled diabetes. Her personal history and drug history were unremarkable. She had a temperature of 104.6°F, blood pressure of 125/75 mm Hg, pulse rate of 98 bpm, respiratory rate of 19/min, and a GCS score of 10/15. Physical examination revealed throat hyperemia and left-side chest crepitations with bilateral expiratory wheezes. There was no neck stiffness or focal neurologic deficits.
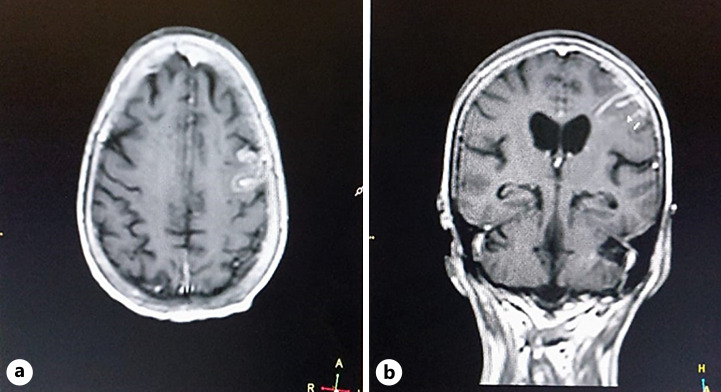
Her laboratory findings are mentioned in Table 1. The patient was suspected of having encephalitis, so an MRI of the brain and lumbar puncture were ordered. MRI of the brain was suggestive of encephalitis (Fig. 4). The findings of the lumbar puncture are given in Table 2. Also, we did an autoimmune workup, including anti-NMDA receptor antibodies, anti-Ro antibodies, anti-La antibodies, ANCA antibodies, and anti-Hu antibodies, which were negative. These investigations were carried out to rule out the common causes of encephalitis. Her chest X-ray showed bilateral opacities, more on the left side. A nasal swab COVID-19 PCR was ordered, and it came back positive. The clinical picture pointed toward a diagnosis of encephalitis, so CSF COVID-19 PCR was also ordered, but it came back negative. The rest of the systemic examinations were unremarkable.
Fig. 4.
Axial post-contrast T1WI (a) and coronal post-contrast T1WI (b) show abnormal areas of enhancement involving the sulci of the left parietal lobe with prominent localized meningeal enhancement.
Based on clinical evaluation and investigation reports, we made a diagnosis of COVID-19-associated encephalitis. The patient was then treated with a single dose of IV tocilizumab 400 mg (4–8 mg/kg) followed by IV methylprednisolone 1 g (0.5–2 mg/kg) for 5 days. The patient responded very well to this treatment and was then discharged. On follow-up after 1 month, the patient was doing well, and there were no residual neurological signs.
Discussion
The primary effect of COVID-19 on the human body is on the respiratory system, but it can also inflict certain neurological manifestations. Although neurologic manifestations of the disease may be common, encephalitis is still rare and not frequently reported. A study has documented that 36% of patients with respiratory distress caused by COVID-19 had some form of neurological manifestations [6]. COVID-19 can affect the nervous system in different ways, which are classified into three main categories: CNS symptoms or diseases (headache, dizziness, impaired consciousness, ataxia, acute cerebrovascular disease, and epilepsy), peripheral nervous system symptoms (hypogeusia, hyposmia, hypoplasia, and neuralgia), and skeletal muscular symptoms [7]. Interestingly, the occurrence of neurological manifestations in COVID-19-affected patients depends upon the severity of the disease; the greater the severity, the greater the likelihood of having the neurological manifestations [2].
COVID-19 virus gets attached to the ACE-2 receptor, and after internalization of the virus into the cells, the RNA of the virus is released, leading to translation and replication [8]. After entering the cells, the COVID-19 virus can damage the cells via two main mechanisms: immune-mediated damage due to cytokine storm or severe hypoxia due to pneumonia and acute respiratory distress syndrome [9]. The encephalopathy associated with COVID-19 can result from either direct damage caused by the virus, an inflammatory response, or a post-infection autoimmune process.
The diagnosis of COVID-19 encephalitis is usually very challenging because of the transient dissemination of the virus in the CSF and very low titers, which do not allow virus detection [6]. A similar pattern was also observed in our case series, with only 1 out of 4 patients having a positive CSF COVID-19 PCR, and the mainstay of diagnosis was the exclusion of all other important causes of encephalitis. Based on the WHO definition, SARS-CoV-2 found in respiratory or other non-CNS samples with the exclusion of other possible causes is sufficient for diagnosing COVID-19 encephalitis [6]. MRI brain can also aid in diagnosing COVID-19 encephalitis, and a wide range of MRI findings can be seen in COVID-19-related encephalopathy. These include leptomeningeal enhancement, ischemic strokes, and cortical fluid-attenuated inversion recovery (FLAIR) signals [10]. The pattern described in our patients, demonstrating the involvement of the temporal lobes (and in 1 case, the insula) is very similar to those described in herpes virus type 1 encephalitis and in autoimmune encephalitis, which should be ruled out with appropriate laboratory investigations. Also, limbic encephalitis can present with similar clinical picture and MRI findings of hyperintensities on FLAIR and T2WI in the medial temporal lobes [11]. Herpes simplex encephalitis is indicated by cortical and subcortical temporal lobe hyperintensity on T2 and FLAIR weighted images, which is unilateral before developing into an asymmetric bilateral type [12]. Various other pathologies related to demyelination, endothelial lesions, and cytokine release syndrome can be observed in critically ill patients with COVID-19 with brain MRI demonstrating hyperintensity on T2WI with restricted diffusion in the subcortical and deep white matter, with small punctate hemorrhagic foci [13].
The primary treatment for COVID-19 encephalitis is supportive. However, various treatments like intravenous immunoglobulins, steroids, tocilizumab, and rituximab have been tried with variable outcomes [5]. A study by Narain et al. [14] revealed improved survival in COVID-19 patients. Hence, in our case series, we used IV tocilizumab 400 mg (4–8 mg/kg), and a short course of IV methylprednisolone (0.5–2 mg/kg). This resulted in drastic improvement in the patient’s outlook, showing its efficacy as a treatment option. Neurological dysfunction may be persistent even after the acute illness has resolved, and in a case series, almost one-third of the patients were cognitively impaired at discharge and follow-up [15]. In our case series, the patients had improved significantly at discharge and had no residual neurological damage on follow-up after 1 month. The CARE Checklist has been completed by the authors for this case report, attached as online supplementary material (for all online suppl. material, see https://doi.org/10.1159/000530926).
Conclusion
COVID-19 can cause various neurological manifestations, and physicians must be wary of them. There are no disease-defining imaging findings on MRI of the brain and the yield of PCR analysis of CSF is low. Hence, physicians should rule out common causes of encephalitis before reaching a diagnosis of COVID-19 encephalitis. Though data regarding long-term sequelae of COVID-19 encephalitis are limited, clinicians should ensure timely follow-up to detect any complications. Our study showed that complete recovery is possible with timely intervention. Though further studies are warranted to determine the effectiveness of the current proposed treatment for COVID-19 encephalitis, our study showed promising results for IV tocilizumab (4–8 mg/kg) and IV methylprednisolone (0.5–2 mg/kg) use in these patients.
Statement of Ethics
Ethical approval is not required for this study in accordance with local/national guidelines. Written informed consent was obtained from the patients for publication of the details of their medical case and any accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding was received.
Author Contributions
Muhammad Hammad Sharif, Madeeha Khaleeque, Asad Ali Khan, Muhammad Hassan Jan, Atif Ahmed, Nida Latif, Abdul Qadir, Muhammad Hanif, and Amjid Iqbal performed conceptualization, writing – original draft, and writing – review and editing of the manuscript. Asad Ali Khan was involved in conceptualization, supervision, writing – original draft, and writing – review and editing of the manuscript.
Funding Statement
No funding was received.
Data Availability Statement
This manuscript has all the data relevant to this case report included. Further inquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. Liu K, Chen Y, Lin R, Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J Infect. 2020;80(6):14–8. 10.1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Desai I, Manchanda R, Kumar N, Tiwari A, Kumar M. Neurological manifestations of coronavirus disease 2019: exploring past to understand present. Neurol Sci. 2021;42(3):773–85. 10.1007/s10072-020-04964-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W, et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12(4):372. 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ghosh R, Dubey S, Finsterer J, Chatterjee S, Ray BK. SARS-CoV-2-Associated acute hemorrhagic, necrotizing encephalitis (AHNE) presenting with cognitive impairment in a 44-year-old woman without comorbidities: a case report. Am J Case Rep. 2020;21:e925641. 10.12659/AJCR.925641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haider A, Siddiqa A, Ali N, Dhallu M. COVID-19 and the brain: acute encephalitis as a clinical manifestation. Cureus. 2020;12(10):e10784. 10.7759/CUREUS.10784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mao L, Wang M, Chen S, He Q, Chang J, Hong C, et al. Neurological manifestations of hospitalized patients with COVID-19 in wuhan, China: a retrospective case series study. 2020. (in press). 10.1101/2020.02.22.20026500. [DOI]
- 8. Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995–8. 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 9. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–4. 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kandemirli SG, Dogan L, Sarikaya ZT, Kara S, Akinci C, Kaya D, et al. Brain MRI findings in patients in the intensive care unit with COVID-19 infection. Radiology. 2020;297(1):E232–5. 10.1148/radiol.2020201697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pereira FV, Jarry VM, Castro JTS, Appenzeller S, Reis F. Pediatric inflammatory demyelinating disorders and mimickers: how to differentiate with MRI? Autoimmun Rev. 2021;20(5):102801. 10.1016/J.AUTREV.2021.102801. [DOI] [PubMed] [Google Scholar]
- 12. Bisinotto HS, Jarry VM, Reis F. Clinical and radiological aspects of bilateral temporal abnormalities: pictorial essay. Radiol Bras. 2021;54(2):115–22. 10.1590/0100-3984.2019.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sabino JV, Gonçales ESL, Reis F. COVID-19-associated leukoencephalopathy and brain microhemorrhages. Rev Soc Bras Med Trop. 2021;54:e0469. 10.1590/0037-8682-0469-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Narain S, Stefanov DG, Chau AS, Weber AG, Marder G, Kaplan B, et al. Comparative survival analysis of immunomodulatory therapy for coronavirus disease 2019 cytokine storm. Chest. 2021;159(3):933–48. 10.1016/J.CHEST.2020.09.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382(23):2268–70. 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This manuscript has all the data relevant to this case report included. Further inquiries can be directed to the corresponding author.