Abstract
The aim of the study was to report a case of orbital perivascular epithelioid cell tumor (PEComa) in a known diagnosed patient of tuberous sclerosis and retinal astrocytic hamartoma. 43-year-old female presented with rapid progressive painful proptosis in the left eye, also reported new mass growing in her upper back. The patient past medical history is significant for left renal angiomyolipoma and multiple bilateral lung cysts of which she underwent right nephrectomy and lung biopsy, respectively. The lung biopsy turned diagnostic for lymphangiomyomatosis. On external examination, the left eye was grossly proptotic with hypoglobus. A typical butterfly distribution of sebaceous adenoma was noted across the patient cheeks and nose. Visual acuity in the right eye was 20/20 and the left eye, 20/25. Funduscopic examination identified type 1, 2, and 3 retinal astrocytic hamartomas. MRI brain and orbit was significant for a lesion arising from the lateral orbital wall with extensive bone destruction, displacing the left optic nerve medially. CT chest showed left extrathoracic mass had same radiological features as the orbital lesion; thus, an incisional biopsy performed on the former was diagnostic for PEComa with atypical features. This is the first observed case of PEComa in a known diagnosed patent with TS and retinal astrocytic hamartoma. The association of tuberous sclerosis complex and orbital PEComa is rarely and poorly reported in the literature compared to extraocular PEComa.
Keywords: Tuberous sclerosis, Orbital perivascular epithelioid cell tumor, Retinal astrocytic hamartoma
Introduction
Tuberous sclerosis complex (TSC) was first reported as a disease characterized by facial angiofibroma in 1835 and had been classically considered to have a triad of “epilepsy, mental retardation, and adenoma sebaceous” [1]. However, TSC is presently considered a neurocutaneous syndrome, manifesting with benign hamartomas in many vital organs of the body. It is caused by an alteration in one of two genes, tuberous sclerosis complex genes 1 and 2 (TSC1 and TSC2 genes) [2]. In this condition, hamartomas develop in the skin and other organs such as the brain, kidney, lungs, and heart.
The most common ocular feature of TSC is retinal astrocytic hamartomas (RAHs) [3], and their presence is one of the primary criteria for diagnosing TSC [4]. RAHs can be divided into different types using multimodal imaging, and different types seem to have a statistically significant association with systemic findings of various severity [5, 6]. As the ophthalmological findings of RAHs are associated with specific systemic manifestations, the ophthalmologist’s role becomes important in estimating systemic disorders and prognosis.
Perivascular epithelioid cell tumor (PEComa) is an uncommon soft tissue tumor forming around the blood vessels of different organs like the uterus and heart [6]. Systemic PEComa has been reported in patients with TSC as it shares genetic abnormalities [7]. However, orbital PEComa was never reported in patients diagnosed with tuberous sclerosis. We hereby present the first case of orbital PEComa in a patient diagnosed with TSC and correlate it with the types of RAHs identified.
Case Report
A 43-year-old female patient was referred to ophthalmology services at Cleveland Clinic Abu Dhabi for further evaluation of a rapid progressive painful proptosis in the left eye. The patient also reported a new mass growing in her upper back.
Her medical history was significant for fatiguability and shortness of breath since 2015 due to a low hemoglobin count of 6 g/dL from a right kidney hemorrhage, requiring multiple embolizations, and numerous left kidney cysts. A renal biopsy was diagnostic for renal angiomyolipoma. Simultaneously, multiple bilateral lung cysts were found in the imaging studies, and a lung biopsy was sent for pathology and returned diagnostic for lymphangiomyomatosis. Based on her clinical findings and biopsies, a diagnosis of TSC was confirmed in 2015. Four months before her ophthalmology consultation, she underwent right nephrectomy for renal angiomyolipoma.
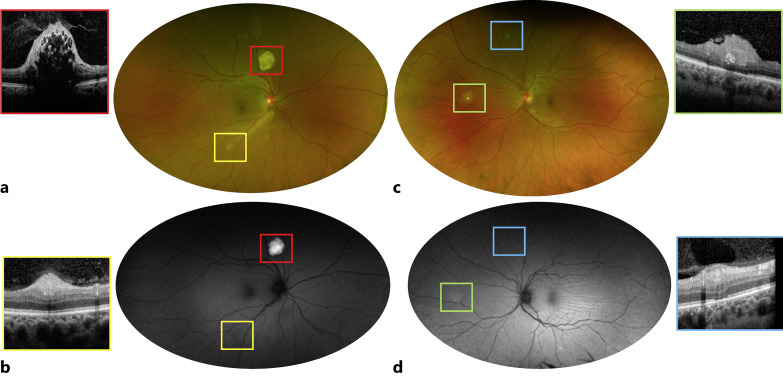
On external examination, the left eye was grossly proptotic and displaced downward with eyelid ptosis. Also, typical butterfly distribution of sebaceous adenoma was noted across her cheeks and nose. A clinical exam revealed visual acuity of 20/20 in the right eye and 20/25 in the left eye. Pupils were symmetrical with normal reactivity to light and near. Sensorimotor testing showed 4 restricted ocular motility in all gaze of the left eye. On dilated examination, the right eye (Fig. 1a, red square) demonstrated an elevated whitish lesion superonasal to the optic nerve with hyper-autofluorescence features (Fig. 1b, red square) and SD-OCT mulberry-like appearance, consistent with type 3 RAHs (Fig. 1, red square) [5, 6]. In addition, multiple white slightly elevated lesions, hyper-reflective at the level of the RNLF on SD-OCT (Fig. 1, yellow square) and iso-autofluorescent (Fig. 1b, yellow square), were present along the vascular arcades in the mid-periphery of the right eye, consistent with type 1 RAHs [5, 6]. The left eye exam (Fig. 1c) was remarkable for a nasal elevated, yellow lesion above the RNFLs (Fig. 1, green square) with minimal calcification, compatible with type 2 RAH, [6] and one type 1 lesion in the superior periphery. Choroidal folds were visible on examination of the left fundus at the level of the macula.
Fig. 1.
Bilateral retinal findings in a 43-year-old female patient with TSC. Fundus photo of the right eye (a) reveals a calcified, mulberry-shaped type 3 RAH superior to the nerve (red square) with hyper-autofluorescence (b) compatible with internal calcifications. Multiple, opaque, smaller lesions are visible along the inferior arcade (a), type 1 RAHs limited to the RNFL (yellow square) and iso-autofluorescent (b). c, d These solitary and flat type 1 RAHs are also present in the left eye (blue square); a nasal type 2 RAH is present in the left eye (c, green square) with elevation and some degree of retina traction (green square) and mild hyper-autofluorescence (d, green square).
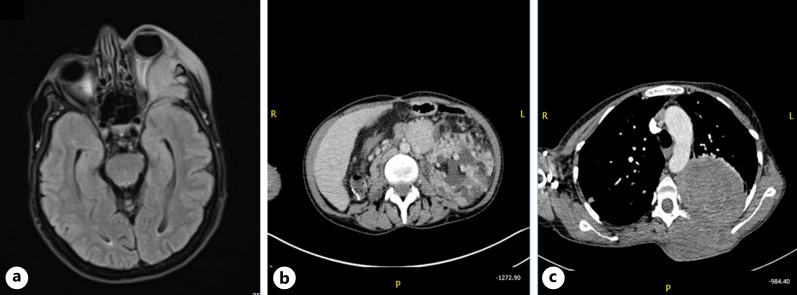
MRI brain and orbit was significant for a lesion arising from the lateral orbital wall with extensive bone destruction, displacing the left optic nerve medially and pushing the globe anteriorly (Fig. 2a). Her CT abdomen showed left renal enlargement, angiomyolipoma, and polycystic kidney disease (Fig. 2b). Her chest CT showed a large left lung mass with the intrathoracic and extrathoracic component, multiple bilateral nodules and cysts with small left pleural effusion, and left apical pneumothorax with multiple bone lytic lesions (Fig. 2c). As the left extrathoracic mass had the same radiological features as the orbital lesion, an incisional biopsy was performed on the former as it carried less risk of complications. The biopsy was positive for a morphological diagnosis of a PEComa with atypical features. The core biopsy of left thoracic mass showed fragments of tissue occupied by tumor. The tumor comprised sheets of epithelioid cells with occasional high-grade nuclei with hyperchromatic and prominent nucleoli. The cytoplasm was clear to granular eosinophils with noticeable cell borders. Abundant necrosis and numerous mitotic figures were also seen. On immunochemistry, tumor cells were strongly and diffusely positive for HMB45, melan A, vimentin, and nucleus positive for TFE3. The cells were negative for the following markers: CAM 5.2, pancytokeratin, synaptophysin, PAX8, CD68, and CAIX.
Fig. 2.
Systemic imaging studies of a 43-year-old female with TSC. MRI brain and orbit (a) shows left eye proptosis due to aggressive solid enhancing heterogenous infiltrative lesion arising from the lateral orbital wall with extensive bone destruction of left medial cranial fossa and extending into the orbital canal, displacing the left optic nerve medially. There is compressive mass effect on the left inferior rectus muscle as well as the left superior rectus muscle. Her CT abdomen (b) shows left renal enlargement, angiomyolipoma, and polycystic kidney disease. Her chest CT (c) shows a large left lung mass that on incisional biopsy was positive for a morphological diagnosis of a PEComa.
A multidisciplinary meeting involving neurosurgery, pulmonology, and oncology recommended to proceed with radiotherapy to left orbit as a first step. Given the aggressive nature of the disease, the orbital mass deemed surgically unresectable without exenteration. Another recommendation was to do neoadjuvant systemic therapy to shrink the left orbital mass before any surgery can be done, but the orbital mass was not considered highly responsive to available systemic therapies, and the expected results on tumor shrinkage are unknown. The patient agreed to proceed with palliative radiation to the orbit. After the third cycle, the patient passed away.
Discussion
Tuberous sclerosis is a genetic condition that can target different parts of the body, resulting in hamartomatous lesions of varying degrees [2]. Approximately half the patients with tuberous sclerosis have RAHs [3, 4] and as such these retinal tumors have become diagnostic criteria for TSC. Four morphological types of astrocytic hamartomas can be recognized on multimodal imaging, according to Pichi et al. [5]. Type 1 RAH is a solitary flat mass limited to the RNFL on SD-OCT and iso-autofluorescent; type 2 is an elevated lesion with a small degree of retinal disorganization and displaying some retinal traction; type 3 is a calcified mushroom-shaped lesion with internal “moth-eaten” optically empty spaces that are hyper-autofluorescent; and type 4 is a dome-shaped mass with a single large cavity blocking the underlying autofluorescence. Recently, Mutolo et al. [6] have modified the classification of Pichi et al. [5]. They divided type 2 into two groups, type 2a and type 2b, based on the tumor’s height and intratumoral appearance. Type 2a is relatively flat with a homogeneous intratumoral aspect, and type 2b is highly protruded with a heterogeneous intratumoral aspect.
It is important to classify RAHs correctly as early studies [5, 6, 8] correlated each type of RAHs with systemic manifestations of TSC. For example, there seems to be a strong association between type 2 lesions and cutaneous fibrous plaques, type 3 lesions and subependymal giant-cell astrocytomas, and type 4 lesions and pulmonary lymphangiomyomatosis [5].
Our patient had three different types of RAHs (type 1, 2, and 3) and these mirrored the severity of her TSC manifestations. Our patient is the first case to report and confirm the association between orbital PEComa and TSC.
Extraocular PEComa associated with TSC has been well reported in the literature. Jank et al. [9] reported 2 patients with TSC presenting with PEComa liver lesions histologically; also, Maebayashi et al. [10] reported 2 patients with tuberous sclerosis who were diagnosed with hepatic PEComa. Interestingly, in a clinicopathologic study of 26 cases of PEComa, only a tiny minority (9%) of soft tissue and gynecologic PEComa was TSC associated [11].
The association between orbital PEComa and TSC is rarely and poorly reported in the literature. Moreover, only a few cases of PEComa and their malignant potential have been described in the eye and orbit [12, 13]. Nair et al. [12] and Feu-Basilio et al. [13] reported a case of 9-year-old child with large orbital PEComa and 28 years old man with extremely rare orbital TFE3-rearranged PEComa, respectively, with no signs or family history of tuberous sclerosis. Another case was reported of 54 years old male with a slowly progressing painless mass of the right temporal lower lid diagnosed as begin PEComa with no history of TSC [14]. Furusato et al. [15] described clinicopathologic features of ocular PEComa, and neither patient had documented evidence of TSC.
Generally, PEComa is related to the genetic alteration of TSC due to losses of TSC1 (9q34) or TSC2 (16p13.3) genes. It has been postulated that TSC genes regulate the Rhheb/mTOR/p70S6K pathway. Kenerson et al. [16] have recently demonstrated increased levels of phospho-p70S6K, a marker of mTOR activity, in sporadic AMLs. The associated reduced phospho-AKT expression is consistent with the disruption of the TSC1/2 function. Similar findings were obtained by analyzing extrarenal PEComas [17]. Furthermore, a high frequency of syndromic and sporadic PEComas has a loss of function mutations in TSC1 or TSC2 genes. By analyzing several case reports, Folpe et al. [11] proposed a tentative classification of PEComas as “benign,” “of uncertain malignant potential,” and “malignant” based on tumor size (<5 cm or >5 cm), growth pattern (infiltrative or not), nuclear grade (low or high), presence or absence of necrosis, and mitotic activity.
Conclusion
We hereby present the first case of an orbital PEComa associated with TSC and further highlight the correlation between the variety of RAHs and the severity of the clinical manifestations of the disease. This case further emphasizes the role of the ophthalmologist in the multidisciplinary care team of patients with TSC. The CARE Checklist has been completed by the authors for this case report, attached as online supplementary material at https://doi.org/10.1159/000530036.
Statement of Ethics
Ethical approval is not required for this case report in accordance with Cleveland Clinic Ethics Committee guidelines. Written informed consent was obtained from the patient’s next of kin for publication of the details of their medical case and any accompanying images.
Conflict of Interest Statement
The following authors have no financial disclosures: S.H.A., A.A.A., K.A., and F.P.
Funding Sources
No funding or grant support.
Author Contribution
S.H.A. and A.A.A.: data collection, literature review, and manuscript drafting. K.A. and F.P.: manuscript editing and final approval. All authors attest that they meet the current ICMJE criteria for authorship.
Funding Statement
No funding or grant support.
Data Availability Statement
All data generated or analyzed during this study are included in this article and the supporting files. Further inquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. Rayer PFO. Traite theorique et pratique des maladies de la peau. 2nd ed. Paris: JB Baillier; 1835. [Google Scholar]
- 2. Wataya-Kaneda M, Uemura M, Fujita K, Hirata H, Osuga K, Kagitani-Shimono K, et al. Tuberous sclerosis complex: recent advances in manifestations and therapy. Int J Urol. 2017;24(9):681–91. 10.1111/iju.13390. [DOI] [PubMed] [Google Scholar]
- 3. Robertson DM. Ophthalmic manifestations of tuberous sclerosis. Ann N Y Acad Sci. 1991;615:17–25. 10.1111/j.1749-6632.1991.tb37744.x. [DOI] [PubMed] [Google Scholar]
- 4. Rowley SA, O’Callaghan FJ, Osborne JP. Ophthalmic manifestations of tuberous sclerosis: a population based study. Br J Ophthalmol. 2001;85(4):420–3. 10.1136/bjo.85.4.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pichi F, Massaro D, Serafino M, Carrai P, Giuliari GP, Shields CL, et al. Retinal astrocytic hamartoma: optical coherence tomography classification and correlation with tuberous sclerosis complex. Retina. 2016;36(6):1199–208. 10.1097/IAE.0000000000000829. [DOI] [PubMed] [Google Scholar]
- 6. Mutolo MG, Marciano S, Benassi F, Pardini M, Curatolo P, Emberti Gialloreti L. Optical coherence tomography and infrared images of astrocytic hamartomas not revealed by funduscopy in tuberous sclerosis complex. Retina. 2017;37(7):1383–92. 10.1097/IAE.0000000000001373. [DOI] [PubMed] [Google Scholar]
- 7. Thway K, Fisher C. PEComa: morphology and genetics of a complex tumor family. Ann Diagn Pathol. 2015;19(5):359–68. 10.1016/j.anndiagpath.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 8. Kato A, Obana A, Gohto Y, Seto T, Sasano H. Optic coherence tomography appearances of retinal astrocytic hamartoma and systemic features in tuberous sclerosis of Japanese patients. Eur J Ophthalmol. 2019;29(3):330–7. 10.1177/1120672118787441. [DOI] [PubMed] [Google Scholar]
- 9. Janks M, Heaford A, Deheragoda M, Hadzic ND. Hepatic perivascular epithelioid cell tumours in children with tuberous sclerosis. BMJ Case Rep. 2020;13(12):e236288. 10.1136/bcr-2020-236288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maebayashi T, Abe K, Aizawa T, Sakaguchi M, Ishibashi N, Abe O, et al. Improving recognition of hepatic perivascular epithelioid cell tumor: case report and literature review. World J Gastroenterol. 2015;21(17):5432–41. 10.3748/wjg.v21.i17.5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol. 2005;29(12):1558–75. 10.1097/01.pas.0000173232.22117.37. [DOI] [PubMed] [Google Scholar]
- 12. Nair AG, Gore SS, Ganvir AY, Adulkar NG, Gopinathan I, Murthy AK, et al. Giant perivascular epithelioid cell tumor of the orbit: a clinicopathological analysis and review of the literature. Ocul Oncol Pathol. 2018;4(5):272–9. 10.1159/000484425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feu-Basilio S, Matas J, Dotti-Boada M, Toll A, Larque AB, Pigem R, et al. Orbital TFE3-rearranged perivascular epithelioid cell tumor: a case report and review of the literature. Am J Dermatopathol. 2021;43(12):e263–66. 10.1097/DAD.0000000000002023. [DOI] [PubMed] [Google Scholar]
- 14. Guthoff R, Guthoff T, Mueller-Hermelink HK, Sold-Darseff J, Geissinger E. Perivascular epithelioid cell tumor of the orbit. Arch Ophthalmol. 2008;126(7):1009–11. 10.1001/archopht.126.7.1009. [DOI] [PubMed] [Google Scholar]
- 15. Furusato E, Cameron JD, Newsom RW, Fujishiro T, Kojima T, Specht CS, et al. Ocular perivascular epithelioid cell tumor: report of 2 cases with distinct clinical presentations. Hum Pathol. 2010;41(5):768–72. 10.1016/j.humpath.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 16. Kenerson H, Folpe AL, Takayama TK, Yeung RS. Activation of the mTOR pathway in sporadic angiomyolipomas and other perivascular epithelioid cell neoplasms. Hum Pathol. 2007;38(9):1361–71. 10.1016/j.humpath.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martignoni G, Pea M, Reghellin D, Zamboni G, Bonetti F. PEComas: the past, the present and the future. Virchows Arch. 2008;452(2):119–32. 10.1007/s00428-007-0509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and the supporting files. Further inquiries can be directed to the corresponding author.