Abstract
Purpose
To assess the 10-year incidence of open-angle glaucoma (OAG) and its associations in an adult Chinese population.
Methods
Longitudinal observational population-based study. Out of 4439 participants aged 40+ years participating in the Beijing Eye Study in 2001, 2695 individuals (60.7%) were re-examined in 2011, while 397 participants had died (8.5%).
Results
Incident OAG was found in 75 participants among 2494 individuals free of glaucoma at baseline. The 10-year OAG incidence (mean: 3.0%; 95% CI 2.5 to 3.5) increased from 1.8% (95% CI 1.3 to 2.4) in individuals aged 40–49 years, to 5.9% (95% CI 3.1 to 9.6) in participants aged 70+ years. OAG incidence was highest in the high myopia group (13.3%±6.3%, OR: 7.3; 95% CI 3.3 to 16.3), followed by the moderately myopic group (8.1%±4.3%, OR: 4.2; 95% CI 2.0 to 8.8) and the low myopic group (6.2%±2.8%, OR: 3.2; 95% CI 1.7 to 5.8), as compared with the emmetropic/hyperopic group (2.1%±0.8%). In multivariable analysis, higher OAG incidence was associated with older age (OR: 1.06; 95% CI 1.03 to 1.09), longer axial length (OR: 1.72; 95% CI 1.45 to 2.05), higher intraocular pressure (IOP) in 2001 (OR: 1.18; 95% CI 1.08 to 1.29), higher vertical cup/disc ratio (VCDR) (OR: 60.8; 95% CI 6.7 to 556) and thinner central corneal thickness (CCT) (OR: 0.98; 95% CI 0.97 to 0.99).
Conclusions
In a 10-year follow-up, high myopia was a major risk factor for the development of OAG with a 7.3-fold risk increase as compared with emmetropic eyes. Higher age, IOP, VCDR and thinner CCT were additionally related with an increased OAG incidence. The findings may be of importance to clinical protocols and screening strategies.
Keywords: glaucoma, epidemiology
Key messages.
What is already known about this subject?
Data on the long-term incidence and risk factors of open-angle glaucoma (OAG) has not been reported in Chinese people.
What are the new findings?
Based on the population-based longitudinal Beijing Eye Study, the 10-year incidence of OAG was 3.0%, which was comparable with previous studies applied in other ethnicities. High myopia was found to be its major risk factor, with a 7.3-fold increased incidence as compared with emmetropic eyes.
How might these results change the focus of research or clinical practice?
The findings should be of importance to clinical protocols and screening strategies.
Introduction
Glaucoma is globally one of the leading causes of irreversible vision impairment and blindness.1 While numerous studies have examined the prevalence of glaucoma in various world regions and countries, only few investigations addressed the incidence of glaucoma and its risk factors.2 3 In the Barbados Eye Study, the 9-year incidence of open-angle glaucoma (OAG) was 4.4% in individuals of African ethnicity and aged 40+ years.4 The Rotterdam Eye Study reported on a 5-year incidence of 1.2% for probable OAG, and of 0.6% for definite OAG in white participants older than 55 years.5 In the study population of the Australian Blue Mountain Eye Study, the 10-year cumulative incidence was 3.4% for residents aged 49+ years.6 In individuals of Bai ethnicity in Yunnan, South China, the 5-year incidence of OAG was 1.3%.7 Data on the OAG incidence in the Han Chinese population of China have been lacking so far. The previous longitudinal studies had limitations such that some of them covered only a 5-year period, and that the list of ocular and systemic parameters, for which associations with the OAG incidence were examined, was relatively small. In particular, the relationship between the development of OAG and myopia and high myopia and the optic disc size have not fully been explored yet. We therefore conducted this study to assess the incidence of OAG and its risk factors over 10-year period in a population-based Chinese population.
Methods
The Beijing Eye Study is a population-based longitudinal investigation carried out in the region Greater Beijing.8 9 First conducted in 2001, the survey was repeated in 2006 and 2011 with inviting all participants of the survey of 2001.
All individuals participating in the study underwent a structured interview and medical and ophthalmic examinations. The latter included uncorrected visual acuity, automatic refractometry (Auto Refractometer AR-610, Nidek, Tokyo, Japan) and slit lamp examination of the anterior segment. Photography of the optic nerve head and macula (fundus camera Type CR6-45NM; Canon Inc., Tokyo, Japan) was performed after pupil dilation. The visual field was examined by frequency-doubling perimetry using the screening programme C-20-1 (Zeiss-Humphrey, Dublin, California, USA) for all participants, and additionally by Octopus perimetry (TOP Strategy, Haag-Streit International, Koeniz, Switzerland) for glaucoma suspects at the baseline examination. Intraocular pressure (IOP) was measured using a non-contact pneumotonometer (CT-60; Topcon, Tokyo, Japan). Three measurements were taken, and the mean of the three measurements was taken for the further statistical analysis. In the follow-up examinations in 2006 and 2011, the procedures were applied in a similar manner. In the year 2011, spectral domain optical coherence tomography (OCT) (Spectralis, Heidelberg Engineering, Heidelberg, Germany) of the optic nerve head and macula, and ocular biometry (Lenstar 900 Optical Biometer, Haag-Streit, Koeniz, Switzerland) for measurement of the central corneal thickness (CCT), corneal curvature radius, anterior chamber depth, lens thickness and total axial length, were additionally performed for all participants.
The IOP readings were corrected for their dependence on CCT and the central corneal curvature radius using the following formula:
This formula was based on the linear regression analysis with IOP as dependent variable and CCT and corneal curvature radius as independent variables, performed in a previous study.10 The anterior segment was imaged by slit-lamp adapted OCT (Heidelberg Engineering) in a dark room. The anterior chamber image was evaluated by a single reviewer (YXW). An angle was defined as open if there was no contact between the peripheral iris and any part of the angle wall anterior to the scleral spur, while an angle was defined as closed if the peripheral iris touched the peripheral cornea or trabecular meshwork.11
As described previously, glaucomatous optic neuropathy was defined by absolute criteria each of which were sufficient for the diagnosis of glaucoma, and by relative criteria.12 The absolute criteria included a notch in the neuroretinal rim in the temporal inferior region and/or the temporal superior region, so that the inferior-superior-nasal-temporal rule of the neuroretinal rim shape was not fulfilled (in eyes with an optic cup sufficiently large to allow an assessment of the neuroretinal shape), localised retinal nerve layer defects which could not be explained by any other cause than glaucoma, and an abnormally large cup in relation to the size of the optic disc.13 14 Relative criteria for the diagnosis of glaucomatous optic neuropathy were a neuroretinal rim which was markedly thinner in the inferior disc region compared with the superior disc region, even if the smallest part of the neuroretinal rim was located in the temporal horizontal disc region; a diffuse decrease in the visibility of the retinal nerve fibre layer (particularly in eyes with small optic discs), if the background pigmentation of the eye allowed an assessment of the retinal nerve fibre layer and if there was no other reason than glaucoma for the retinal nerve fibre layer loss; a marked diffuse thinning and/or focal thinning of the retinal arteries if there was no other reason than glaucoma for retinal vessel thinning; and an optic disc haemorrhage, if there was no other reason for a disc bleeding such as retinal vessel occlusions. If none of the absolute glaucoma criteria were fulfilled, the diagnosis of glaucoma required that at least two relative criteria had to be fulfilled, among them had to be a suspicious neuroretinal rim shape in eyes with an optic cup large enough for the assessment of the rim shape; or at least two relative criteria had to be positive including the occurrence of an optic cup in a small optic disc which usually would not show cupping. This glaucoma definition was similar, but not identical with the glaucoma definition recommended by the International Society of Geographical and Epidemiological Ophthalmology (ISGEO).15 Reason for not applying the ISGEO definition was a limitation of the ISGEO-based glaucoma definition, that is, that cup/disc diameter ratio-based parameters were considered to indicate glaucoma if they were ≥97.5th percentile for the normal population. This implies that a priori the prevalence of glaucoma would be roughly 2.5%. Using digital fundus photographs, the assessment of glaucomatous optic neuropathy was carried out by two senior graders (YXW, JBJ). In case of disagreement, the optic disc photographs were reassessed up to three times, until eventually both graders agreed on the diagnosis. Besides, the vertical cup/disc ratio (VCDR) was measured by a single observer (YXW).
Flicker chronoscopy was used to evaluate changes in the appearance of the optic nerve and retinal nerve fibre layer, suggesting the new development of, or incident, glaucoma, if the criteria for glaucomatous optic neuropathy were fulfilled.16 17 The fundus photos which were centred on the optic disc or on the macula and which were taken at baseline and after 10 years, were colocalised and aligned in a flicker manner using an automatic alignment software (Daheng Imaging, Beijing, China). Incident glaucoma was defined as absence of glaucoma at baseline in the year 2001 and presence of structural glaucomatous changes in the optic nerve head and retinal nerve fibre layer in 2011, such as neuroretinal rim loss and relevant retinal nerve fibre layer defects. Development or enlargement of parapapillary beta zone supported the diagnosis of incident glaucoma, however, its sole presence without being associated with neuroretinal rim loss and retinal nerve fibre layer defects was not sufficient for the diagnosis of glaucoma. The flicker chronoscopy of the fundus photos was carried out in a masked manner by a panel of experienced examiners (HY, YXW, JBJ). In case of a disagreement, the optic disc photographs were reassessed up to three times, until eventually all three graders agreed on the diagnosis.
The statistical analysis was performed using a statistical software package (SPSS for Windows, V.25.0, IBM-SPSS). Based on spherical equivalent (SE) of the refractive error at baseline, the whole study population was subdivided into an emmetropic/hyperopic group (SE: >−1 dioptre), minor myopic group (SE: ≤−1 and >−3 dioptres), moderately myopic group (SE: ≤−3 and >−6 dioptres), and a highly myopic group (SE: ≤−6 dioptres). For pseudophakic or aphakic eyes at baseline, the data of axial length was used for the definition of refraction. Emmetropia/hyperopia, minor myopia, moderate myopia and high myopia was defined as an axial length <24 mm, 24–25 mm, 25.01–26 mm and >26 mm, respectively.18 19 The refractive error of the right eye was considered for each study participant. If the ophthalmic data of the right eye was unavailable or in the case of a unilateral OAG in the left eye, the refractive error of the left eye was taken into account. The incidence of OAG was assessed as frequency (percentage) and its 95% CI. Using the χ2 test, we assessed the statistical significance of differences in the follow-up rates among the emmetropic/hyperopic, low myopic, moderately myopic and highly myopic groups. Associations between the OAG incidence and other ocular and systemic parameters were evaluated in a binary regression analysis, first in univariate mode, and followed by a multivariable analysis. All p values were two-sided and considered statistically significant when they were <0.05.
Results
Among the 4439 participants of the baseline Beijing Eye Study in 2001, 2695 participants were re-examined 10 years later (follow-up rate: 60.7% among all participants, or 66.4% of all living participants of the baseline examination), while 379 participants had died (8.5%) and 1347 (30.3%) had moved away or refused to re-participate in the study. The participants of the 10 year follow-up study were younger (54.6±9.8 years vs 58.7±11.3 years; p<0.001) as compared with those who were not followed (table 1). Among the 130 participants with high myopia at baseline, 81 were re-examined in 2011 (follow-up rate: 62.3%). The follow-up rate in the highly myopic group was not significantly associated with age (p=0.99) or gender (p=0.79), and did not differ significantly (χ2, p=0.71) from the overall follow-up rate (table 1).
Table 1.
The 10-year follow-up status of participants of the Beijing Eye Study 2001/2011
| Participants | Non-participants | P value | Follow-up rate (%) | Follow-up rate in live participants (%) | ||
| All | n | 2695 | 1744 (died 379) | 60.7 | 66.4 | |
| Age (years) | 54.6±9.8 | 58.7±11.3 | <0.001 | |||
| Gender (male/female) | 1139/1556 | 955/789 | 0.051 | |||
| Spherical equivalent (D) | −0.35±2.09 | −0.40±2.33 | 0.48 | |||
| Emmetropia (>−1 D) |
n | 2229 | 1339 (died 271) | 62.5 | 67.6 | |
| Age (years) | 54.4±9.8 | 58.0±10.9 | <0.001 | |||
| Gender (male/female) | 939/1290 | 603/736 | 0.09 | |||
| Low myopia (≤−1 and >−3 D) |
n | 258 | 188 (died 48) | |||
| Age (years) | 55.1±9.9 | 59.7±11.5 | <0.001 | 57.8 | 64.8 | |
| Gender (male/female) | 112/146 | 89/99 | 0.41 | |||
| Moderate myopia (≤−3 D and >−6 D) |
n | 123 | 102 (died 16) | 54.7 | 58.9 | |
| Age (years) | 56.4±8.9 | 58.4±10.5 | 0.14 | |||
| Gender (male/female) | 58/65 | 58/44 | 0.15 | |||
| High myopia (≤−6 D) |
n | 81 | 49 (died 7) | 62.3 | 65.9 | |
| Age (years) | 57.0±9.7 | 56.9±11.1 | 0.99 | |||
| Gender (male/female) | 30/51 | 17/32 | 0.79 |
D, dioptre.
After excluding 148 (5.5%) glaucoma patients diagnosed at the baseline examination and after excluding those individuals without available or assessable fundus images taken either at baseline or at the follow-up (n=53; 2.0%), the study eventually included 2494 participants (92.5%) free of glaucoma at baseline. Out these 2494 individuals, 48 (1.9%) were pseudophakic at baseline, and an additional 67 (2.7 %) participants underwent cataract surgery during the study period. Out of 2494 individuals at baseline, 75 participants had developed OAG in 2011, with a 10-year incidence of 3.0% (95% CI 2.5 to 3.5). It ranged from 1.8% in individuals aged 40–49 years, to 5.9% in individuals aged 70+ years (online supplemental table 1). Using the census data of Beijing from 2001 to correct for population demographic considerations,20 the age-standardised and sex-standardised OAG incidence was 3.0% as well.
bjophthalmol-2021-320644supp001.pdf (29.9KB, pdf)
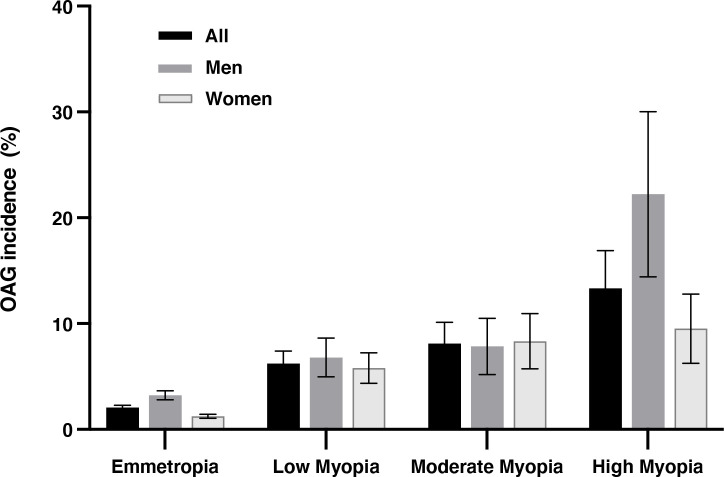
The OAG incidence was highest in high myopia group (13.3%±6.3% (95% CI 6.2 to 22.7)), followed by the moderately myopic group (8.1%±4.3% (95% CI 4.1 to 13.3)), the minor myopia group (6.2%±2.8% (95% CI 3.8 to 9.1)) and the emmetropic/hyperopic group (2.1%±0.8% (95% CI 1.7 to 2.5)). In comparing with emmetropic eyes, the OAG incidence in low myopia, moderate myopia and high myopia had a relative risk factor of 3.2, 4.2 and 7.3, after correcting the effect of all the other risk factors (p<0.01) (table 2) (figure 1).
Table 2.
the 10 year incidence of open-angle glaucoma in the groups with emmetropia, low myopia, moderate myopia and high myopia group in the Beijing Eye Study 2001/2011
| Men+women | Men | Women | |||||||
| n | % (95% CI) |
OR (95% CI) |
n | % (95% CI) |
OR (95% CI) |
n | % (95% CI) |
OR (95% CI) |
|
| Emmetropia (>−1 D) |
2082 | 2.07 (1.66 to 2.52) |
Reference | 870 | 3.22 (2.38 to 4.18) |
Reference | 1212 | 1.24 (0.88 to 1.66) |
Reference |
| Low myopia (≤−1 and >−3 D) |
241 | 6.22 (3.84 to 9.13) |
3.15 (1.72 to 5.76)* |
103 | 6.80 (3.16 to 11.68) |
2.19 (0.93 to 5.15)† |
138 | 5.80 (2.92 to 9.58) |
4.91 (2.04 to 11.8)* |
| Moderate myopia (≤−3 and >−6 D) |
111 | 8.11 (4.12 to 13.28) |
4.18 (1.99 to 8.82)* |
51 | 7.84 (2.54 to 15.71) |
2.56 (0.86 to 7.6)† |
60 | 8.33 (3.12 to 15.74) |
7.26 (2.55 to 20.68)* |
| High myopia (≤−6 D) |
60 | 13.33 (6.19 to 22.67) |
7.3 (3.27 to 16.29)* |
18 | 22.22 (6.61 to 43.66) |
8.59 (2.66 to 27.77)* |
42 | 9.52 (2.98 to 19.24) |
8.40 (2.66 to 26.51)* |
*OR significant (all p<0.001).
†OR not significant (p=0.07 for low myopia; p=0.09 for moderate myopia).
D, dioptre.
Figure 1.
The 10-year incidence of open-angle glaucoma (OAG) in emmetropia/hyperopic (>−1 dioptre), low myopia: ≤−1 and >−3 dioptres), moderate myopia (≤−3 and >−6 dioptres) and high myopia ≤−6 dioptres) in the Beijing Eye Study 2001/2011. The bars show the incidence and its 95% CI.
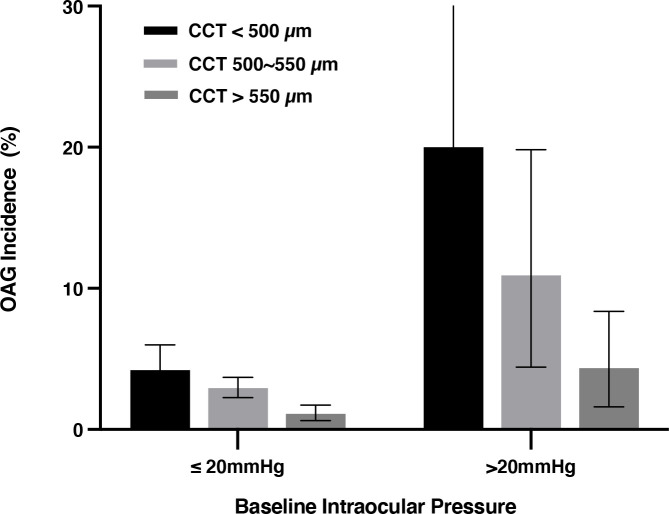
In univariate logistic regression analysis, a higher OAG incidence was significantly (all p<0.05) related with the systemic parameters of older age, male gender, urban region of habitation, higher serum concentration of C reactive protein and higher prevalence of chronic kidney disease, and with the ophthalmological parameters of more myopic refractive error (figure 1), higher cylindrical refractive error, longer axial length, lower CCT, deeper anterior chamber depth, larger optic disc area, smaller neuroretinal rim area, higher VCDR, larger parapapillary beta zone, higher IOP in 2001 and 2011 (corrected for CCT), longer disc-fovea distance, larger disc-fovea angle, higher degree and more marked progression of fundus tessellation, higher prevalence of nuclear cataract in 2001, higher prevalence of epiretinal membranes in 2001, and higher prevalence of retinal vein occlusions in 2011 (online supplemental table 2) (figures 1 and 2).
Figure 2.
The 10-year incidence of open-angle glaucoma (OAG) in eyes with intraocular pressure ≤20 mm Hg and >20 mm Hg, stratified by central corneal thickness (CCT) grades. The bars show the incidence and its 95% CI.
bjophthalmol-2021-320644supp002.pdf (52.2KB, pdf)
Parameters with a significance of p<0.05 were included in the multivariable analysis. Since the CCT-corrected IOP in 2001 was significantly related with the incidence of OAG, the IOP at baseline and CCT were both included in the multivariable analysis at the first step. Parameters with a variance inflation factor >5 were removed due to collinearity (including refractive error, cylindrical refractive error, optic disc area, optic rim area and anterior chamber depth).21 After dropping step by step parameters which missed a statistical significance (p>0.05), we reached the final model, in which a higher OAG incidence was associated (Nagelkerke R2=0.17) with older age (OR: 1.06, 95% CI 1.03 to 1.09), longer axial length (OR: 1.72, 95% CI 1.45 to 2.05), higher intraocular pressure in 2001 (OR: 1.18, 95% CI 1.08 to 1.29), higher vertical CDR (OR: 60.8, 95% CI 6.7 to 556.1) and thinner CCT (OR: 0.98, 95% CI 0.97 to 0.99) (all p<0.001) (model #1). Male gender was marginally associated with a higher incidence (p=0.075) (table 3) (figures 1 and 2).
Table 3.
Associations (multivariable analysis) between the incidence of open-angle glaucoma and ocular and systemic parameter in the Beijing Eye Study 2001/2011
| Coefficient B | P value | OR | 95% CI of OR | |
| Model 1 | ||||
| Age (years) | 0.06 | <0.001 | 1.06 | 1.03 to 1.09 |
| Axial length (mm) | 0.55 | <0.001 | 1.72 | 1.45 to 2.05 |
| CCT (µm) | −0.02 | <0.001 | 0.98 | 0.97 to 0.99 |
| IOP (mm Hg) | 0.17 | <0.001 | 1.18 | 1.08 to 1.29 |
| VCDR | 4.11 | <0.001 | 60.8 | 6.65 to 566 |
| Model 2 | ||||
| Age (years) | 0.05 | <0.001 | 1.05 | 1.03 to 1.08 |
| Axial length (mm) | 0.55 | <0.001 | 1.73 | 1.45 to 2.06 |
| CCT (µm) | −0.02 | <0.001 | 0.98 | 0.97 to 0.99 |
| IOP (mm Hg) | 0.16 | 0.001 | 1.17 | 1.07 to 1.29 |
| VCDR | 4.30 | <0.001 | 73.6 | 7.32, 740 |
| Epiretinal membrane 2001 | 0.17 | 0.044 | 1.19 | 1.01, 1.40 |
| RVO 2011 | 3.32 | 0.001 | 27.7 | 3.62, 212 |
Parameters with unmarked time means baseline parameters (2001).
CCT, central corneal thickness; IOP, intraocular pressure; RVO, retinal vein occlusion; VCDR, vertical cup disc ratio.
When the comorbidities were additionally considered, a higher prevalence of epiretinal membranes in 2001 (OR: 1.19, 95% CI 1.02 to 1.38; p=0.025) and a higher prevalence of retinal vein occlusions in 2011 (OR: 4.62, 95% CI 1.76 to 12.10; p=0.002) were associated with a higher OAG incidence (Nagelkerke R2=0.20) (model #2) (table 3).
Discussion
In our population-based 10-year follow study of Chinese aged 40+ years, the risk of developing OAG (mean: 3.0%) increased by a factor of 1.72 for each mm higher axial length. Highly myopic eyes as compared with emmetropic/hyperopic eyes had a 7.3-fold risk increase for the development of OAG. OAG additionally increased by a factor of 1.18 for each mm Hg higher IOP at baseline, by a factor of 6.1 for each 1/10 increase in VCD, in addition to a factor of 1.06 for each year higher age. It decreased by a factor of 0.98 for each µm thicker CCT. Male gender was marginally associated with a higher OAG incidence.
The results obtained in our study agree with findings made in previous investigations. The incidence rate of 3.0% found in our study was similar to the rate of 3.4% for the participants of the Blue Mountain Eye Study with an age of 49+ years,6 It was higher than the OAG incidence reported for the Rotterdam Eye Study with a 5-year incidence of 1.2% for probable OAG and of 0.6% for definite OAG in white individuals with an age of more than 55 years.5 It was lower than the 9-year follow-up of individuals of African ethnicity aged 40+ years in the Barbados Eye Study with the incidence of 4.4%.4 Taking into account the length of follow-up, the OAG incidence was lower in our study population than in the Los Angeles Latino Eye Study population, in which the 4-year incidence rate of OAG was 2.3%.22 The OAG incidence of 3% in our 10-year follow-up study was also similar to the rate of 1.3% found in a 5-year follow-up study of individuals of Bai ethnicity in South China.7
The association between higher OAG incidence and longer axial length, in particular high axial myopia with an axial length of more than 26 mm, agrees with cross-sectional studies in which high axial myopia has been a major factor associated with the prevalence of OAG.23 24 In our study, the presence of high axial myopia, defined by an axial length of >26 mm, increased the risk of developing OAG by a factor of 13.7 (95% CI 6.44 to 29.0) in the multivariable analysis. It conforms with the results of a hospital-based study on highly myopic patients in whom the prevalence of glaucomatous or glaucoma-like optic neuropathy markedly increased with longer axial length, with values higher than 40% in eyes with an axial length of ≥30 mm.25 In the latter study, a large parapapillary delta was an additional factor associated with a higher prevalence of glaucomatous or glaucoma-like optic neuropathy, a variable, which was not examined in the present study. Reasons for the increase in OAG incidence with longer axial length may be axial elongation-related anatomical changes in the optic nerve head including an elongation and thinning of the lamina cribrosa, an enlargement of Bruch’s membrane opening, and a lengthening and thinning of the peripapillary choroidal border tissue and of the peripapillary scleral flange.26 One has additionally discussed that the elongation of the disc-fovea distance, parallel to the development and enlargement of parapapillary gamma zone, may lead, through a stretching of the papillo-macular retinal nerve fibres, to a non-glaucomatous type of optic nerve damage.27
The observation of a positive correlation between higher OAG incidence and larger optic disc size in the univariate analysis (OR: 1.69 (95% CI 1.12 to 2.56; p=0.01) agrees with previous cross-sectional hospital-based studies such as the investigation by Nagaoka and colleagues, who found an increase in the prevalence of OAG by a factor of 3.2 in eyes with large optic discs (>3.79 mm2) than in normal-sized discs or small discs (<1.51 mm2) after adjusting for age (table 3).28 The finding that optic disc size was not significantly (p=0.28) related with the OAG incidence within the non-highly myopic group of our study population agrees with previous studies in which the size of optic disc was not related with the OAG prevalence.26 It supports the differentiation of primary macrodiscs in non-highly myopic eyes and without an abnormally high OAG prevalence (and incidence), and secondarily enlarged macrodiscs in highly myopic eyes with an increase in the incidence and prevalence of OAG.26
A higher OAG incidence in our study population was associated with a thinner CCT, in agreement with the Early Manifest Glaucoma Trial and other investigations.29 Although a thin CCT has thus been shown to be risk factor for the development of OAG in the setting of our study, it has remained unclear whether it is structural risk factor or a diagnostic risk factor. It may be discussed that eyes with a thin cornea and an underestimation of the IOP were less intensively treated than eyes with a thick cornea and an overestimation of the IOP. According to previous studies it has not been shown that a thin cornea is directly or indirectly associated with an increased susceptibility for glaucomatous optic nerve damage at a given IOP. Histomorphometric studies did not show an association between corneal thickness and the thickness of the lamina cribrosa, optic disc size, axial length or other structural variables which may be associated with a higher glaucoma susceptibility.
The frequency of disc haemorrhages was not related with the OAG incidence in our study population, although numerous studies have revealed a correlation between disc haemorrhages and a higher rate of progression of glaucoma.26 It can be discussed that a single examination at study end may not have been sufficient to show up a statistically significant association between disc haemorrhages and the development of OAG.
When the results of our study are discussed, its limitations have to be taken into account. First, the participation rate in our survey was 66.4% of the survivors after a 10-year period, a figure lower than the rates in the 10-year follow-up of the Blue Mountains Eye Study (75.6%) and of the Beaver Dam Eye Study (82.9%). The cause for the lower follow-up rate in our investigation was the relatively high mobility of the population in the Greater Beijing area. Since the reason to move (eg, construction of a new airport) might have been independent of the general health condition of the individual, it might not have induced a major bias into the study. Second, perimetry could not be used as basis for the detection of glaucoma at study end. In that context, one may take into account that early glaucoma can be present with a statistically normal visual field test result. Third, the IOP correction formula based on CCT and corneal curvature was derived from a Chinese population, and may thus not be universally applicable. In addition, biomechanical parameters were not taken into account. Fourth, 67 individuals underwent cataract surgery in the study period, and the surgery-associated change in the anterior chamber might have affected the assessment of the anterior chamber angle.
In conclusion, in our 10-year follow-up, the risk factors of developing OAG (mean: 3.0%) were longer axial length at baseline, higher IOP, older age, thinner CCT and higher VCDR at baseline. High myopia was a major risk factor for the development of OAG with a 7.3-fold risk increase as compared with emmetropic/hyperopic eyes.
Footnotes
Contributors: YXW: study design, application, analysis, writing; HY: organisation, photo-reading, quality control; CCW: photo-reading support, database preparation; analysis; LX: study design, database; WW: quality control; data acquisition; JBJ: study design, writing, analysis. Guarantor: YXW
Funding: National Natural Science Foundation of China (#81570835); Beijing Municipal of Health Reform and Development Project (#2019-4).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Not applicable.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and ethical approval for this study was obtained from Ethics Committee of Beijing Institute of Ophthalmology, Beijing Tongren Hospital according to the Declaration of Helsinki. Participants gave informed consent to participate in the study before taking part.
References
- 1. GBD 2019 Blindness and Vision Impairment Collaborators, Vision Loss Expert Group of the Global Burden of Disease Study . Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to vision 2020: the right to sight: an analysis for the global burden of disease study. Lancet Glob Health 2021;9:e144–60. 10.1016/S2214-109X(20)30489-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tham Y-C, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 2014;121:2081–90. 10.1016/j.ophtha.2014.05.013 [DOI] [PubMed] [Google Scholar]
- 3. Bourne RRA, Taylor HR, Flaxman SR, et al. Number of people blind or visually impaired by glaucoma worldwide and in world regions 1990 - 2010: a meta-analysis. PLoS One 2016;11:e0162229. 10.1371/journal.pone.0162229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leske MC, Wu SY, Honkanen R, et al. Nine-Year incidence of open-angle glaucoma in the Barbados eye studies. Ophthalmology 2007;114:1058–64. 10.1016/j.ophtha.2006.08.051 [DOI] [PubMed] [Google Scholar]
- 5. de Voogd S, Ikram MK, Wolfs RCW, et al. Incidence of open-angle glaucoma in a general elderly population: the Rotterdam study. Ophthalmology 2005;112:1487–93. 10.1016/j.ophtha.2005.04.018 [DOI] [PubMed] [Google Scholar]
- 6. Wang JJ, Mitchell P, Smith W. Is there an association between migraine headache and open-angle glaucoma? findings from the blue Mountains eye study. Ophthalmology 1997;104:1714–9. 10.1016/s0161-6420(97)30075-x [DOI] [PubMed] [Google Scholar]
- 7. Pan C-W, Yang W-Y, Hu D-N, et al. Longitudinal cohort study on the incidence of primary open-angle glaucoma in BAI Chinese. Am J Ophthalmol 2017;176:127–33. 10.1016/j.ajo.2017.01.008 [DOI] [PubMed] [Google Scholar]
- 8. Wang YX, Zhang JS, You QS, et al. Ocular diseases and 10-year mortality: the Beijing eye study 2001/2011. Acta Ophthalmol 2014;92:e424–8. 10.1111/aos.12370 [DOI] [PubMed] [Google Scholar]
- 9. Yan YN, Wang YX, Yang Y, et al. Ten-Year progression of myopic maculopathy: the Beijing eye study 2001-2011. Ophthalmology 2018;125:1253–63. 10.1016/j.ophtha.2018.01.035 [DOI] [PubMed] [Google Scholar]
- 10. Wang YX, Xu L, Wei WB, et al. Intraocular pressure and its normal range adjusted for ocular and systemic parameters. The Beijing eye study 2011. PLoS One 2018;13:e0196926. 10.1371/journal.pone.0196926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nolan WP, See JL, Chew PTK, et al. Detection of primary angle closure using anterior segment optical coherence tomography in Asian eyes. Ophthalmology 2007;114:33–9. 10.1016/j.ophtha.2006.05.073 [DOI] [PubMed] [Google Scholar]
- 12. Wang YX, Xu L, Yang H, et al. Prevalence of glaucoma in North China: the Beijing eye study. Am J Ophthalmol 2010;150:917–24. 10.1016/j.ajo.2010.06.037 [DOI] [PubMed] [Google Scholar]
- 13. Jonas JB, Gusek GC, Naumann GO. Optic disc, cup and neuroretinal rim size, configuration and correlations in normal eyes. Invest Ophthalmol Vis Sci 1988;29:1151–8. [PubMed] [Google Scholar]
- 14. Jonas JB, Budde WM, Panda-Jonas S. Ophthalmoscopic evaluation of the optic nerve head. Surv Ophthalmol 1999;43:293–320. 10.1016/S0039-6257(98)00049-6 [DOI] [PubMed] [Google Scholar]
- 15. Foster PJ, Buhrmann R, Quigley HA, et al. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol 2002;86:238–42. 10.1136/bjo.86.2.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chee R-I, Silva FQ, Ehrlich JR, et al. Agreement of flicker chronoscopy for structural glaucomatous progression detection and factors associated with progression. Am J Ophthalmol 2013;155:983–90. 10.1016/j.ajo.2013.01.005 [DOI] [PubMed] [Google Scholar]
- 17. Leske MC, Heijl A, Hyman L, et al. Early manifest glaucoma trial: design and baseline data. Ophthalmology 1999;106:2144–53. 10.1016/s0161-6420(99)90497-9 [DOI] [PubMed] [Google Scholar]
- 18. van ALPHEN G. On emmetropia and ametropia. Opt Acta 1961;142:1–92. [PubMed] [Google Scholar]
- 19. Xu L, Wang YX, Wang S, et al. Definition of high myopia by parapapillary atrophy. The Beijing eye study. Acta Ophthalmol 2010;88:e350–1. 10.1111/j.1755-3768.2009.01770.x [DOI] [PubMed] [Google Scholar]
- 20. Yu X . Beijing statistical Yearbook 2001. Beijing, China: China Statistics Press, 2001: 68–76. [Google Scholar]
- 21. Craney TA, Surles JG. Model-Dependent variance inflation factor cutoff values. Qual Eng 2002;14:391–403. 10.1081/QEN-120001878 [DOI] [Google Scholar]
- 22. Varma R, Wang D, Wu C, et al. Four-year incidence of open-angle glaucoma and ocular hypertension: the Los Angeles Latino eye study. Am J Ophthalmol 2012;154:315–25. 10.1016/j.ajo.2012.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chihara E, Liu X, Dong J, et al. Severe myopia as a risk factor for progressive visual field loss in primary open-angle glaucoma. Ophthalmologica 1997;211:66–71. 10.1159/000310760 [DOI] [PubMed] [Google Scholar]
- 24. Xu L, Wang Y, Wang S, et al. High myopia and glaucoma susceptibility the Beijing eye study. Ophthalmology 2007;114:216–20. 10.1016/j.ophtha.2006.06.050 [DOI] [PubMed] [Google Scholar]
- 25. Jonas JB, Weber P, Nagaoka N, et al. Glaucoma in high myopia and parapapillary delta zone. PLoS One 2017;12:e0175120. 10.1371/journal.pone.0175120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang YX, Panda-Jonas S, Jonas JB. Optic nerve head anatomy in myopia and glaucoma, including parapapillary zones alpha, beta, gamma and delta: histology and clinical features. Prog Retin Eye Res 2021;83:100933. 10.1016/j.preteyeres.2020.100933 [DOI] [PubMed] [Google Scholar]
- 27. Jonas RA, Yan YN, Zhang Q, et al. Elongation of the disc-fovea distance and retinal vessel straightening in high myopia in a 10-year follow-up of the Beijing eye study. Sci Rep 2021;11:9006. 10.1038/s41598-021-88579-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nagaoka N, Jonas JB, Morohoshi K, et al. Glaucomatous-Type optic discs in high myopia. PLoS One 2015;10:e0138825. 10.1371/journal.pone.0138825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology 2007;114:1965–72. 10.1016/j.ophtha.2007.03.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjophthalmol-2021-320644supp001.pdf (29.9KB, pdf)
bjophthalmol-2021-320644supp002.pdf (52.2KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Not applicable.