Abstract
The largest study on cyclophosphamide pharmacokinetics in dialysis patients comprises of 6 subjects. In the 2 decades since these data were obtained, dialyser membranes, treatment intensities, and treatment duration have changed considerably making new pharmacokinetic studies desirable. We aimed to readdress the pharmacokinetics of cyclophosphamide in a 74-year-old critically ill male suffering from ANCA-associated vasculitis. Due to an acute-on-chronic kidney injury, he underwent intermittent (IHD) and prolonged intermittent kidney replacement therapy (PIKRT). IHD was started 7 h after end of a cyclophosphamide infusion with a blood/dialysate flow of 300 mL/min for 255 min, followed by PIKRT with a blood/dialysate flow of 140 mL/min for 540 min, both using a 1.3 m2 polysulphone high-flux dialyser (F60S, Fresenius Medical Care). Peak concentration of cyclophosphamide was 20.2 mg/L. Using IHD and PIKRT serum concentration of cyclophosphamide decreased to 1.2 mg/L after IHD and to <0.1 mg/L after PIKRT with dialyser-clearances of 153.0 mL/min and 84.9 mL/min, respectively. Total recovery of cyclophosphamide, calculated from the collected dialysate, was 57.5 mg (7.7% of administered dose) for IHD and was 8.3 mg (1.1% of administered dose) for PIKRT. By using IHD with a high-flux dialyser cyclophosphamide could be eliminated. Remaining cyclophosphamide should be eliminated by PIKRT. Hence, even in the absence of renal function a dose >50% of the recommended for patient with normal renal function may be applied, as complete elimination of the parent drug by modern dialysis is feasible.
Keywords: Drug dosing, Cyclophosphamide, Pharmacokinetics, Dialysate, Cancer
Introduction
The alkylating agent cyclophosphamide is the mainstay of therapy for many haematological and oncological diseases; to name a few: non-Hodgkin’s lymphoma and multiple myeloma. Moreover, cyclophosphamide is frequently used for immunosuppression in autoimmune diseases like ANCA-associated vasculitis (AAV) and systemic lupus erythematosus. Despite the high frequency of chronic kidney disease (CKD) as well as acute kidney injury (AKI) requiring dialysis, dosing of cyclophosphamide is based on anecdotal and sometimes controversial data [1–4]. This is best epitomized by the fact that the largest pharmacokinetic study on cyclophosphamide dosing in dialysis patients comprises of 6 patients [5]. Therefore, a global initiative called for more drug dosing and pharmacokinetic studies in patients with AKI and CKD [6].
As dialyser membranes and treatment intensities have changed over the last decades, the few pharmacokinetic studies available may even lead to inadequate dosing of cyclophosphamide. Thus, we aimed to readdress the pharmacokinetics of cyclophosphamide in a patient suffering from AAV using both, intermittent (IHD) and prolonged intermittent kidney replacement therapy (PIKRT) due to AKI on CKD. In addition to the blood levels, we analysed drug removal by as pre- and post-dialyser blood levels as well as the eliminated amount in the total spent dialysate using the GENIUS dialysis system as described previously [7].
Case Report
A 74-year-old Caucasian male (Table 1) with Pneumocystis jirovecii pneumonia was admitted to our medical intensive care unit for progressive respiratory failure. He had been undergoing cyclophosphamide pulse therapy for relapse of a known AAV. Kidney replacement therapy was required due to AKI in CKD. Due to a Pneumocystis jirovecii pneumonia, he received treatment with co-trimoxazole turning negative for Pneumocystis jirovecii [8] so that immunosuppressive treatment with cyclophosphamide pulse therapy was continued (360 mg/m2; i.e., 72% of standard dose). To obtain maximum therapeutic but minimum side effect of cyclophosphamide, we used IHD followed by PIKRT both with a polysulphone high-flux dialyser (F60S, surface area 1.3 m2, Fresenius Medical Care). IHD was started 7 h after end of cyclophosphamide infusion with a blood/dialysate flow of 300 mL/h for 255 min, followed by PIKRT with a blood/dialysate flow of 140 mL/h for 540 min. Dialyser clearance was calculated from concentrations before (Cin) and after (Cout) the dialysis membrane as with plasma flow in (Flin) and out (Flout) of the dialyser estimated by blood flow, haematocrit, and ultrafiltration rate. All samples were centrifuged at 1,300 g for 10 min at 4°C. Plasma was separated and stored at −80°C until analysis. After fluid extraction (sodium carbonate/diethylether) and derivatization with trifluoracetic acid anhydride, the amount of cyclophosphamide was quantified by gas chromatography (Agilent GC 7890A) with mass spectrometric detection (Agilent MS 5975C) in the selected ion-monitoring mode. Measuring range of cyclophosphamide was from 0.005 (limit of detection) up to 200 mg/L with an intra-assay imprecision of 4.5%. For calibration, Ifosphamide was used as an internal standard.
Table 1.
Key patient characteristics and treatment variables
| Gender | Male |
| Age | 74 years |
| Height | 186 cm |
| Weight | 84 kg |
| Cyclophosphamide | |
| Standard dosage | 500 mg/m2 |
| Dialysis dosage | 72% of 500 mg/m2 = 360 mg/m2 |
| Time between end of infusion and start of IHD | 410 min |
| Duration of IHD | 255 min |
| Time between IHD and PIKRT | 30 min |
| Duration of PIKRT | 540 min |
| Peak concentration | 1.2 mg/L |
| Concentration after PIKRT | <0.1 mg/L |
| Concentration after IHD | 20.2 mg/L |
| Total amount of removed cyclophosphamide, mg | 65.8 |
| IHD | 57.5 |
| PIKRT | 8.3 |
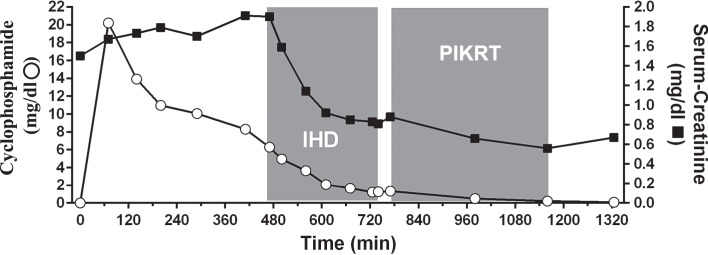
Serum peak concentration after end of infusion was 20.21 mg/L. Using IHD and PIKRT cyclophosphamide concentration decreased to 1.2 mg/L and <0.1 mg/L, respectively (Fig. 1).
Fig. 1.
Serum levels of creatinine and cyclophosphamide over time in a patient with AKI undergoing intermittent haemodialysis (IHD) and prolonged intermittent kidney replacement therapy (PIKRT) after intravenous administration of cyclophosphamide. Duration of treatment is represented by the grey areas. Open circles represent cyclophosphamide concentration and full squares represent the creatinine concentration.
During IHD and PIKRT dialyser-clearance for cyclophosphamide was 153.0 mL/min and 84.9 mL/min, which corresponds to the range of creatinine clearance (213.0 mL/min for IHD, 117.7 mL/min for PIKRT). Total recovery of cyclophosphamide, calculated from the collected dialysate, was 57.5 mg after IHD and 8.3 mg after PIKRT, respectively.
Conclusion
Cyclophosphamide, a drug with a small molecular weight of 279 D and negligible protein binding of 0–10%, is eliminated by renal excretion to 5–25%. Hence, the half-life in patients with end-stage renal disease (CKD5) is up to 10 h as compared to 3–12 h in subjects with normal renal function. Therefore, in addition to age, dosing recommendations advise reducing the standard dose up to 50% in patients on dialysis. We could show elimination of cyclophosphamide by IHD with a high-flux dialyser and subsequent PIKRT also using a high-flux dialyser.
Previous studies showed that over a 3 h dialysis with a 1.3 m2 polysulfone low-flux membrane 22% of the drug was removed, as estimated by spot dialysate samples. In our patient, only ∼9% of the administered amount was recovered in the total collected dialysate of both, the IHD and the PIKRT. Nonetheless, we achieved a sufficient decrease of cyclophosphamide serum levels so that the 72% of standard dosage lead to a desirable leucocyte nadir of 2.9 G/L on day 11 after administration of cyclophosphamide. This indicates that starting the dialysis procedure about 7 h after the end of the cyclophosphamide infusion allows distribution and metabolization of the cyclophosphamide on one hand while shortening the otherwise prolonged half-life in dialysis patients on the other hand. Why could an extended half-life be harmful for the patient? Cyclophosphamide is a prodrug and serves as surrogate marker for active, cytotoxic, and inactive metabolites formed by extensive hepatic metabolism. With their protein binding of around 50% removal by dialysis using conventional high-flux dialysers is limited [3]. Therefore, it is of utmost importance to remove the unmetabolized cyclophosphamide from bloodstream in a timely manner to avoid disproportionately high side effects. Hence, even in the absence of renal function a dose >50% of the recommended for patient with normal renal function may be applied, as complete elimination of the parent drug, i.e., cyclophosphamide, by modern dialysis is feasible. This is in line with a recent strategy that advocates a dialysis after the administration of chemotherapeutic drugs to enhance their elimination after maximum effect, rather than decreasing the dose and compromising on the potential therapeutic benefit [9].
Our data do also raise concern that a dialysis session after a (reduced) dose of cyclophosphamide could lead to treatment failure due to removal of cyclophosphamide. This theoretical problem might especially arise in the setting of intravenous pulse therapy.
Cyclophosphamide is eliminated by high-flux dialysis, allowing and necessitating a higher cyclophosphamide dose than previously reported for patients undergoing haemodialysis. The CARE Checklist has been completed by the authors for this case report, attached as supplementary Material (for all online suppl. material, see https://doi.org/10.1159/000531129).
Statement of Ethics
Ethical issues (including plagiarism, data fabrication, double publication) have been completely observed by the authors. Written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images. Ethical approval is not required for this study in accordance with the guidelines of the IRB of the Hannover Medical School.
Conflict of Interest Statement
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding Sources
None of the authors received any funding for this study.
Author Contributions
Gernot Beutel and Jan T. Kielstein treated the patient. Catherina Lück wrote the case report. W. Nikolaus Kühn-Velten performed the cyclophosphamide measurements. Jan T. Kielsrein made the graph. Catherina Lück, Gernot Beutel, W. Nikolaus Kühn-Velten, and Jan T. Kielstein revised the manuscript.
Funding Statement
None of the authors received any funding for this study.
Data Availability Statement
All data generated or analysed during this study are included in this article and its online supplementary material files. Further enquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. Eden G, Kuhn-Velten WN, Hafer C, Kielstein JT. Enhanced elimination of cyclophosphamide by high cut-off haemodialysis: single-dose pharmacokinetics in a patient with cast nephropathy. BMJ Case Rep. 2018. pii: bcr-2017-221735. 10.1136/bcr-2017-221735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Juma FD, Rogers HJ, Trounce JR. Effect of renal insufficiency on the pharmacokinetics of cyclophosphamide and some of its metabolites. Eur J Clin Pharmacol. 1981;19(6):443–51. 10.1007/BF00548589. [DOI] [PubMed] [Google Scholar]
- 3. Moore MJ. Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet. 1991;20(3):194–208. 10.2165/00003088-199120030-00002. [DOI] [PubMed] [Google Scholar]
- 4. Perry JJ, Fleming RA, Rocco MV, Petros WP, Bleyer AJ, Radford JE Jr, et al. Administration and pharmacokinetics of high-dose cyclophosphamide with hemodialysis support for allogeneic bone marrow transplantation in acute leukemia and end-stage renal disease. Bone Marrow Transpl. 1999;23(8):839–42. 10.1038/sj.bmt.1701646. [DOI] [PubMed] [Google Scholar]
- 5. Haubitz M, Bohnenstengel F, Brunkhorst R, Schwab M, Hofmann U, Busse D. Cyclophosphamide pharmacokinetics and dose requirements in patients with renal insufficiency. Kidney Int. 2002;61(4):1495–501. 10.1046/j.1523-1755.2002.00279.x. [DOI] [PubMed] [Google Scholar]
- 6. Matzke GR, Aronoff GR, Atkinson AJ Jr, Bennett WM, Decker BS, Eckardt KU, et al. Drug dosing consideration in patients with acute and chronic kidney disease-a clinical update from Kidney Disease: improving Global Outcomes (KDIGO). Kidney Int. 2011;80(11):1122–37. 10.1038/ki.2011.322. [DOI] [PubMed] [Google Scholar]
- 7. Kielstein JT, Stadler M, Czock D, Keller F, Hertenstein B, Radermacher J. Dialysate concentration and pharmacokinetics of 2F-Ara-A in a patient with acute renal failure. Eur J Haematol. 2005;74(6):533–4. 10.1111/j.1600-0609.2005.00439.x. [DOI] [PubMed] [Google Scholar]
- 8. Clajus C, Kuhn-Velten WN, Schmidt JJ, Lorenzen J, Pietsch D, Beutel G, et al. Cotrimoxazole plasma levels, dialyzer clearance and total removal by extended dialysis in a patient with acute kidney injury: risk of under-dosing using current dosing recommendations. BMC Pharmacol Toxicol. 2013;14:19. 10.1186/2050-6511-14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hann A, Nosalski E, Hermann PC, Egger J, Seufferlein T, Keller F. Chemotherapeutic agents eligible for prior dosing in pancreatic cancer patients requiring hemodialysis: a systematic review. Clin Nephrol. 2018;90(2):125–41. 10.5414/CN109327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this article and its online supplementary material files. Further enquiries can be directed to the corresponding author.