Abstract
We present 2 cases of sutureless 25-gauge pars plana vitrectomy and fluid-gas exchange, in which incorrect gas concentrations likely led to elevated intraocular pressures and retrobulbar gas. Combined removal of orbital gas with anterior orbitotomy and pars plana vitrectomy was performed in the first case to address expanding intraocular and retrobulbar gas resulting from a suspected error in gas dilution. Vitreous and orbital gas removal by needling was effective in the second case. In patients with elevated intraocular pressure and orbital gas accumulation after vitrectomy, combined intraocular and orbital decompressions were effective in optimizing clinical outcomes. There is no consensus regarding the best management of orbital gas after vitrectomy. We propose that a multidisciplinary technique should be considered, when available.
Keywords: Retrobulbar gas, Orbital decompression, Small-gauge pars plana vitrectomy, Vitreoretinal surgery
Introduction
Perfluoropropane (C3F8) and sulfur hexafluoride (SF6) gases are common internal tamponade agents used in routine vitreoretinal surgery. Intraocular gas expansion has been reported to lead to gas migration into the anterior chamber, subretinal, intracranial, subconjunctival, and retrobulbar spaces [1–6]. Pure intravitreal C3F8 quadruples and pure SF6 gas doubles in 3–4 days [1, 7]. Incorrect concentration of diluted gas is generally thought to be related to a suspected error in gas mixture or the presence of highly soluble nitrous oxide-based anesthesia allowing for rapid gas expansion of the gas/air mixture. Retrobulbar gas is rare in the era of microincisional vitrectomy but can occur in the setting of incomplete sclerotomy closures [8]. Potential complications of expandable gases include increased intraocular pressure (IOP), vision loss, and orbital compartment syndrome [9]. We herein describe 2 patients in whom retrobulbar gas following vitreoretinal surgery required urgent orbital gas removal. Neither of these patients received nitrous oxide-based anesthesia during vitreoretinal surgery, and neither case had evidence of a retrobulbar hemorrhage at the time of regional anesthesia. A retrospective case review of two clinical cases was performed. The methods were conducted in compliance with the tenants of the Declaration of Helsinki. The CARE Checklist has been completed by the authors for this case report, attached as online supplementary material (for all online suppl. material, see https://doi.org/10.1159/000530401).
Case 1
A 63-year-old female developed right eyelid swelling since postoperative day (POD) 1, following sutureless 25-gauge pars plana vitrectomy and C3F8 gas (intended to be 10% by the referring provider) for macular hole repair in the right eye, under monitored anesthesia care. Emergency department evaluation on POD5 resulted in treatment for suspected orbital cellulitis with oral amoxicillin-clavulanate, prednisone, acetazolamide, and lateral canthotomy/cantholysis. The patient was seen at our facility on POD6 due to progressive, painful proptosis. IOP was 55 mm Hg in the right eye (OD) and 18 mm Hg in the left eye (OS). Visual acuities (VAs) were counting fingers OD and 20/40 OS. The pupil OD was poorly reactive. Extraocular motions OD were restricted in all directions. Corneal haze, subconjunctival gas, and periorbital crepitus were present OD (Fig. 1). The anterior chamber was shallow. There was no retinal detachment or choroidals on B-scan.
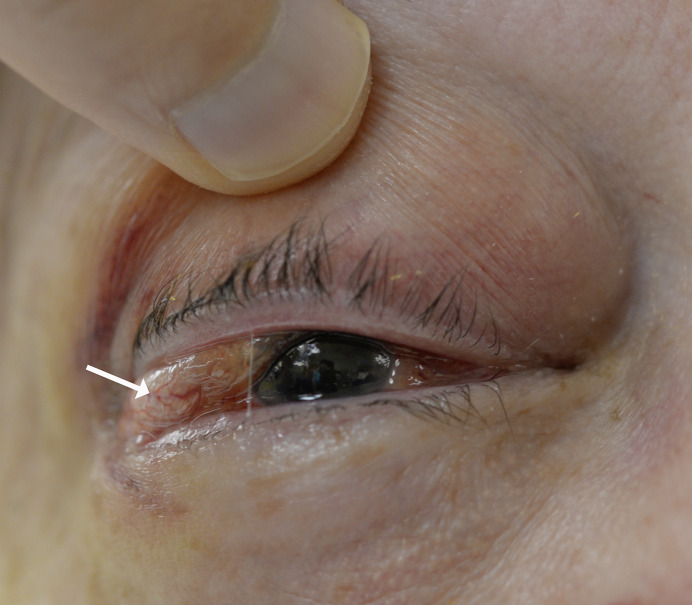
Fig. 1.
External photograph showing subconjunctival gas (arrow) and periorbital edema, clinically consistent with extraocular gas expansion into the orbit and periorbital tissues.
Topical glaucoma drops and a vitreous tap removing 0.3 cc of gas decreased the IOP to 30 mm Hg OD. An orbital CT demonstrated the presence of intra- and extraconal orbital gas, proptosis, and no subperiosteal abscess (Fig. 2a, b). A bedside orbital gas removal by needle tap was not believed to be adequate to evacuate the gas in the setting of significant periocular soft tissue swelling. The following day, the patient underwent a right anterior orbitotomy with gas removal, lower eyelid canthotomy with cantholysis, followed by suturing of the original sclerotomies, a 25-gauge sutured pars plana vitrectomy, and evacuation of intraocular gas. Detailed technique is shown in the online supplementary video and described in online Supplementary Materials section.
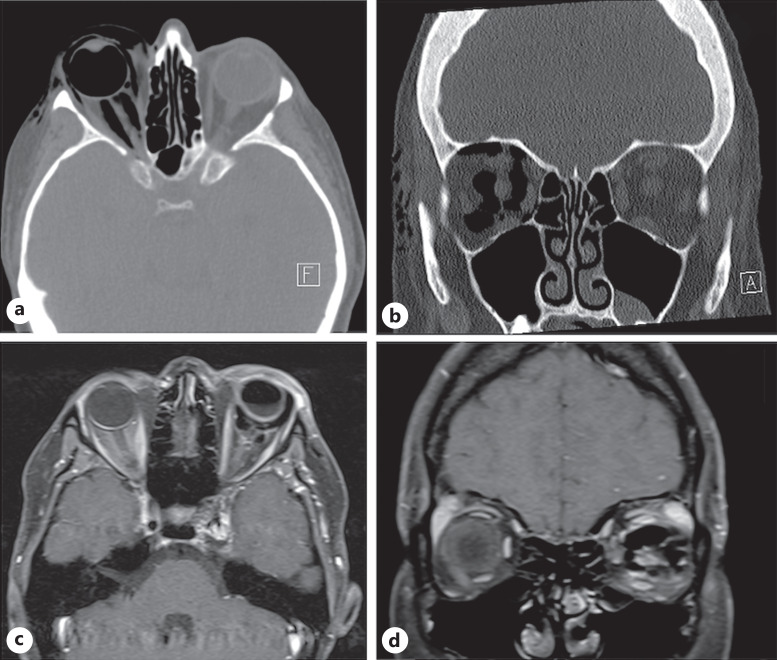
Fig. 2.
Axial (a) and coronal (b) views of orbital CT show extensive right-sided orbital gas and complete vitreous gas fill, with intra- and extraconal gas and tomographic evidence of proptosis in case 1. There was extensive orbital and periorbital emphysema dissecting and expanding between most of the tissue planes in the nondependent right orbit. Nerve compression resulted from the retrobulbar mass effect from this gas, which led to marked proptosis of the right globe on the CT scan. There were no bony fractures. Axial (c) and coronal (d) section of the orbital MRI shows an intraocular gas meniscus following a vitreous gas removal by needle expression, as well as intraconal gas, which was subsequently managed with needle expression of orbital gas until the IOP normalized to palpation, in case 2. MRI, magnetic resonance imaging.
On POD1, IOP was 16 mm Hg OD and the macular hole appeared closed. At 4 months of follow-up, best-corrected VA improved to 20/20 OD after cataract surgery. The cup-to-disk ratio was 0.6 OD and 0.3 OS.
Case 2
A 29-year-old female with a left-sided traumatic cavernous-carotid fistula, status post-coiling, developed ocular ischemic syndrome as well as a tractional retinal detachment, hyphema, and vitreous hemorrhage OS, requiring multiple intravitreal anti-vascular endothelial growth factor injections and panretinal photocoagulation treatments. She underwent sutureless 25-gauge pars plana vitrectomy, membrane peeling, endolaser, fluid-air exchange, and presumed 20% SF6 gas injection OS, under monitored anesthesia care without immediate surgical complications.
On POD1, VA was hand motions and IOP was 51 mm Hg OS. Vitreous gas removal by needle tap reduced the IOP to 11 mm Hg, and glaucoma drops were started. On POD2, IOP was 40 mm Hg OS with proptosis and limited extraocular motions, concerning for orbital compartment syndrome. Hertel exophthalmometry was 17 and 21 mm OD and OS, respectively. An urgent magnetic resonance imaging of the brain and orbits revealed the presence of intraconal gas (Fig. 2c, d). An orbital gas removal using a 25-gauge needle on an open syringe through the inferior fornix, similar to a retrobulbar block, reduced the IOP to 8 mm Hg and Hertel to 18 mm OS. The needle was inserted in the fornix until the orbital and ocular pressures normalized by palpation. The IOP was maintained at 22 mm Hg on glaucoma drops 3 weeks later, and VA improved to 20/200 OS. The patient was lost to follow-up for 2 months and returned with IOP of 41 mm Hg due to neovascular glaucoma and tractional retinal detachment OS. She underwent pars plana vitrectomy, membrane peeling, silicone oil injection, and cyclophotocoagulation. Final VA OS 8 months later was light perception, and IOP was 13.
Discussion
Complications related to intraocular gas tamponades used in vitreoretinal surgery occur in conjunction with dilutional errors or general anesthesia using nitrous oxide, the latter of which did not apply to our patients [1, 9]. A high clinical suspicion for errors in gas dilution is needed to make an accurate diagnosis, and this error may be mitigated by implementing a time-out prior to gas exchange. In case 1, there was a full vitreous gas fill 6 days after macular hole repair, even though the half-life of 10% C3F8 is 6.5 days [1]. Therefore, it was suspected that a more concentrated gas than the reported 10% was used. Roth and Ballintine [1] described a similar case of vitreous gas expansion into retrobulbar spaces after sutureless 23-gauge macular hole surgery. Incorrectly diluted 16% C3F8 led to an IOP of 86 mm Hg and limited extraocular motility. The authors managed the complication with surgical decompression of the globe, although their technique was not detailed [1].
Orbital cellulitis was on the differential diagnosis in over 31% of 16 patients in the largest published series of orbital emphysema following vitreoretinal surgery by Enriquez et al. [2]. Orbital emphysema after vitrectomy has also been described in the setting of disrupted pressurized infusion devices in globe penetration [10] and following fluid-air exchange in patients with orbital fracture [2, 11]. The patient in case 1 was initially suspected of orbital cellulitis, and the patient was sent to the emergency department for urgent management. Kumar et al. [3] published a similar case of retrobulbar gas masquerading as orbital cellulitis in a patient treated with incorrectly diluted 16% perfluoroethane gas following a rhegmatogenous retinal detachment repair with sutureless 23-gauge pars plana vitrectomy. It was only after review of the magnetic resonance imaging by the referring physician that cellulitis was deemed less likely, given the presence of retrobulbar and intraorbital gas and the absence of sinus disease or endophthalmitis.
The literature details various management strategies for orbital emphysema, including external pressure patching of the eye, trans-palpebral drainage or needling, hyperbaric oxygen therapy to eliminate nitrogen from tissues, and surgical decompression [2, 4, 7]. Despite these individual case reports, the literature lacks consistent management guidelines regarding the superiority of different treatment modalities and final visual outcomes. Vitreous gas removal alone does not address the rise in orbital pressure resulting from expanding retrobulbar gas. Orbital needling, a minimally invasive decompression technique utilized in case 2, is useful in select cases to reduce the proptosis and IOP associated with orbital emphysema [12–14]. Managing orbital gas through a multidisciplinary intraocular- and orbital-surgical approach can be effective when adequate IOP reduction cannot be achieved conservatively. This approach likely optimized visual outcome in our cases; case 1 achieved an excellent visual outcome, while case 2 had visual potential limited by other ocular comorbidities.
In the era of sutureless vitrectomy with microincisional instrumentation, prompt management of complications related to retrobulbar gas migration is important in reducing visual morbidity. Final VAs following orbital gas removal in the Enriquez et al. [2] study ranged from 20/40 to no light perception, with nearly 63% experiencing an objective decline in best-corrected VA and over 74% having a final vision of 20/200 or worse. Visual field defects and nerve fiber layer damage occur after retrobulbar gas, caused by vascular compromise and direct compressive damage to the optic nerve [1, 6].
In conclusion, retrobulbar gas following vitreoretinal surgery can cause postoperative IOP elevation and may resemble orbital cellulitis. High clinical suspicion and orbital imaging are needed for early diagnosis and management. We show that combined intraocular and orbital gas removal using needling or surgical methods can be effective treatment options.
Statement of Ethics
Written informed consent was obtained from the patients for publication of the details of their medical case and any accompanying images. No identifying information was included in the contents of this manuscript. This retrospective review of patient data did not require ethical approval, in accordance with local/national guidelines.
Conflict of Interest Statement
None of the authors report any conflicts of interest as it pertains to the content of this manuscript. Noy Ashkenazy: Alimera Sciences (advisory board), DORC Surgical (lecture, honorarium). Carl J. Danzig: DORC Surgical (consultant). Sara P. Read, Michelle M. Maeng ORCID RELNO, Harry W. Flynn Jr., Thomas A. Albini: none.
Funding Sources
This study was supported by NIH Center Core Grant P30EY014801 (Bethesda, MD, USA) and the Research to Prevent Blindness Unrestricted Grant (GR004596) to the University of Miami.
Author Contributions
Noy Ashkenazy: conception and design, data collection, analysis and interpretation of data, writing the manuscript, and critical revision of the manuscript. Carl J. Danzig: data collection and critical revision of the manuscript. Andrew J. Rong: conception and design, analysis and interpretation of data, and critical revision of the manuscript. Sarah P. Read and Harry W. Flynn Jr.: analysis and interpretation of data, and critical revision of the manuscript. Michelle M. Maeng: critical revision of the manuscript. Thomas A. Albini: conception and design, critical revision of the manuscript, and supervision.
Funding Statement
This study was supported by NIH Center Core Grant P30EY014801 (Bethesda, MD, USA) and the Research to Prevent Blindness Unrestricted Grant (GR004596) to the University of Miami.
Data Availability Statement
All data that support the findings of this study are included in this article and its online supplementary material files. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary Material
Supplementary Material
References
- 1. Roth D, Ballintine SD. Retrobulbar gas after macular hole surgery: a case report. Retin Cases Brief Rep. 2013;7(3):188–92. 10.1097/ICB.0b013e31827cee14. [DOI] [PubMed] [Google Scholar]
- 2. Enriquez AB, Wheelock-Gutierrez L, Golzarri MF, Planella S, Salcedo-Villanueva G, Salcedo-Casillas G, et al. Unilateral orbital emphesema secondary to vitreoretinal surgery. Ophthalmol Retina. 2020;4(7):708–19. 10.1016/j.oret.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 3. Kumar N, Tsangaris P, Haynes RJ. Intra-orbital gas following sutureless small-gauge (23-gauge) vitrectomy masquerading as orbital cellulitis. Eye. 2014;28(10):1263–4. 10.1038/eye.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iniesta-Sanchez D, Romero-Caballero F, Aguirre-Alvarado A, Rebollo-Hurtado V, Velez-Montoya R. Management of orbital emphysema secondary to rhegmatogenous retinal detachment repair with hyperbaric oxygen therapy. Am J Ophthalmol Case Rep. 2016;1:26–30. 10.1016/j.ajoc.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnson T, Johnson MW. Pathogenic implications of subretinal gas migration through pits and atypical colobomas of the optic nerve. Arch Ophthalmol. 2004;122(12):1793–800. 10.1001/archopht.122.12.1793. [DOI] [PubMed] [Google Scholar]
- 6. Harris J, Han IC, Sachdeva MM, Zhang AY, Zebardast N. Post-operative intracranial gas migration with optic nerve infiltration and atrophy following retinal detachment repair. Am J Ophthalmol Case Rep. 2020;20:100920. 10.1016/j.ajoc.2020.100920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aziz A, Nagendran S, Perry M, Lee V. Orbital emphysema with loss of vision following undiluted intravitrel perfluoropropane administration. Adv Ophthalmol Vis Syst. 2019;9(2):53–4. 10.15406/aovs.2019.09.00345. [DOI] [Google Scholar]
- 8. Kanclerz P, Grzybowski A. Case series of inappropriate concentration of intraocular sulfur hexafluoride. Case Rep Ophthalmol. 2018;9(2):405–10. 10.1159/000492746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kanclerz P, Grzybowski A. Complications associated with the use of expandable gases in vitrectomy. J Ophthalmol. 2018;2018:8606494. 10.1155/2018/8606494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chak G, Joseph JM, Tao JP. Needle decompression of acute orbital emphysema: case report with video. Br J Ophthalmol. 2012;96(10):1346–7, 1360. 10.1136/bjophthalmol-2012-302129. [DOI] [PubMed] [Google Scholar]
- 11. Ahmad B, Barakat MR, Feldman M, Singh RP. Bilateral subcutaneous emphysema from pressurized infusion during pars plana vitrectomy: a case report. Retin Cases Brief Rep. 2012;6(1):22–4. 10.1097/ICB.0b013e3181f98cea. [DOI] [PubMed] [Google Scholar]
- 12. Damasceno E, Damasceno N, Horowitz S, Rodrigues MM. Emphysema following vitrectomy with fluid-gas exchange: description of a rare complication. Clin Ophthalmol. 2014;8:401–3. 10.2147/OPTH.S56083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Singh M, Phua VM, Sundar G. Sight-threatening orbital emphysema treated with needle decompression. Clin Exp Ophthalmol. 2007;35(4):386–7. 10.1111/j.1442-9071.2007.01494.x. [DOI] [PubMed] [Google Scholar]
- 14. Lin CY, Tsai CC, Kao SC, Kau HC, Lee FL. Needle decompression in a patient with vision-threatening orbital emphysema. Taiwan J Ophthalmol. 2016;6(2):93–5. 10.1016/j.tjo.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data that support the findings of this study are included in this article and its online supplementary material files. Further inquiries can be directed to the corresponding author.