Summary
Background
Currently, melatonin is used to treat children and adolescents with insomnia without knowing the full extent of the short-term and long-term consequences. Our aim was to provide clinicians and guideline panels with a systematic assessment of serious—and non-serious adverse events seen in continuation of melatonin treatment and the impact on pubertal development and bone health following long-term administration in children and adolescents with chronic insomnia.
Methods
We searched PubMed, Embase, Cinahl and PsycINFO via Ovid, up to March 17, 2023, for studies on melatonin treatment among children and adolescents (aged 5–20 years) with chronic insomnia. The language was restricted to English, Danish, Norwegian, and Swedish. Outcomes were non-serious adverse events and serious adverse events assessed 2–4 weeks after initiating treatment and pubertal development and bone health, with no restriction on definition or time of measurement. Observational studies were included for the assessment of long-term outcomes, and serious and non-serious adverse events were assessed via randomised studies. The certainty of the evidence was assessed using Grades of Recommendation, Assessment, Development and Evaluation (GRADE). The protocol is registered with the Danish Health Authority.
Findings
We identified 22 randomised studies with 1350 patients reporting on serious—and non-serious adverse events and four observational studies with a total of 105 patients reporting on pubertal development. Melatonin was not associated with serious adverse events, yet the number of patients experiencing non-serious adverse events was increased (Relative risk 1.56, 95% CI 1.01–2.43, 17 studies, I2 = 47%). Three studies reported little or no influence on pubertal development following 2–4 years of treatment, whereas one study registered a potential delay following longer treatment durations (>7 years). These findings need further evaluation due to several methodological limitations.
Interpretation
Children who use melatonin are likely to experience non-serious adverse events, yet the actual extent to which melatonin leads to non-serious adverse events and the long-term consequences remain uncertain. This major gap of knowledge on safety calls for caution against complacent use of melatonin in children and adolescents with chronic insomnia and for more research to inform clinicians and guideline panels on this key issue.
Funding
The Danish Health Authority. The Parker Institute, Bispebjerg and Frederiksberg Hospital, supported by the Oak Foundation.
Keywords: Melatonin, Children and adolescents, Safety, Long-term effects
Research in context.
Evidence before this study
The increased use of melatonin in children and adolescents, involving both off-label use and self-prescriptions, underlines the need for sufficient short-term and long-term safety data. However, systematic reviews on safety in children and adolescents are scarce and with current reviews largely focusing on narrative summaries. This hinders a general overview of the extent of adverse events in the young population.
Added value of this study
Our synthesis of the evidence shows, of moderate certainty, that children and adolescents treated with melatonin due to chronic insomnia are likely to experience non-serious adverse events, yet the actual extent of non-serious adverse events in the young population needs further assessment. Evidence of very low certainty indicates that the impact on pubertal development may rely on the duration of treatment. These findings are for now, however, only speculative. No studies were identified on bone health.
Implications of all the available evidence
The major gap of knowledge on safety identified through this analysis calls for caution against complacent use of melatonin in children and adolescents with chronic insomnia and for more research on this key issue.
Introduction
In children, sleep is vital for healthy development, optimal growth, emotional regulation, and mental health.1,2 Moreover, sleep influences the reconstruction, restoration and functioning of the brain and other tissues, including strengthening the immune system, improving cardiovascular functioning, and managing energy regulation.3 The consequences of chronic insomnia are serious and far-reaching, and among children the negative social, psychological, and physiological outcomes of sleep deficiency may persist into adulthood.4 Therefore, focus on promoting quality sleep remains an important public health issue already early in life, especially considering the worldwide high prevalence of insomnia among children.5
Non-pharmacological interventions are first-line treatment of insomnia in children. Several non-pharmacological interventions have been proposed, with the overarching aim of introducing low-risk, non-invasive strategies with few side effects to promote healthy sleep.6, 7, 8, 9, 10 When non-pharmacological strategies have been tested, potential pharmacological therapies, such as melatonin may be considered. Registry and survey data from Scandinavia and Northern America show that melatonin supplementation is widely used among children with a substantial and a noteworthy increase in paediatric users over the last two decades, making melatonin a commonly used treatment in children.11, 12, 13, 14 The increased use of melatonin in children and adolescents, involving both off-label use and self-prescriptions, underlines the need for safety data, however systematic reviews on safety in children and adolescents are scarce and with current reviews largely focusing on narrative summaries. This hinders a general overview of the extent of adverse events in the young population.15, 16, 17, 18, 19, 20 In the European Union (EU), melatonin has had a marketing for use in the adult population since 2007 and for children with specific psychiatric disorders since 2018.21 Thus, the potential long-term consequences are largely unknown. According to the European assessment report,22 a potential long-term adverse event of melatonin treatment is the risk of delayed puberty, mainly due to concerns that melatonin treatment may disturb the gradual hormonal decline in melatonin plasma concentrations detected before the onset of puberty in normal development. Numerous systematic and narrative reviews have discussed the evidence, with some advocating for and others against such an association.23, 24, 25, 26, 27, 28 Another element of pubertal physiology is growth spurts, which may be considered a particular vulnerable period regarding bone health, the achieved final height and fracture risk.29, 30, 31, 32 Disturbances in melatonin regulation during pubertal development may adversely influence bone mineralisation, e.g., via the effects on pituitary and gonadal function, but beneficial effects of melatonin on bone metabolism, including both antiresorptive and anabolic effects, have also been suggested from in vitro studies and studies conducted in the elderly.33,34 In a cross-sectional study among 100 healthy girls aged 9–15 years, night urine melatonin excretion was positively correlated with osteocalcin levels, a biochemical marker for bone formation (osteoblast activity), but circulating concentrations of melatonin was not correlated with bone mineral content or bone mineral density.35 Whether long-term melatonin treatment among children and adolescents with chronic insomnia influences bone health is at this point uncertain.
Therefore, the aim of this systematic review was to provide clinicians and guideline panels with estimates on serious—and non-serious adverse events seen in continuation of melatonin treatment and the impact on pubertal development and bone health following long-term administration in children and adolescents with chronic insomnia. Rigorous standardised methods were used, including the Grades of Recommendation, Assessment, Development and Evaluation (GRADE)36 and the guidelines of the Cochrane collaboration. This review question is a part of national clinical recommendations on the use of melatonin for children and adolescents, published by the Danish Health Authority in November 2022.
Methods
A prespecified protocol in Danish was registered and approved by management from the Department of Evidence Based Medicine at the Danish Health Authority on December 21, 2021 and is publicly available on the Danish Health Authority website at https://www.sst.dk/da/Udgivelser/2022/NKA_-Behandling-med-melatonin-ved-soevnforstyrrelser-hos-boern-og-unge. Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) were followed in the reporting of this systematic review37 (provided in the Supplementary).
Eligibility criteria
The a priori eligibility criteria follow the Population, Intervention, Comparison and Outcome (PICO) scheme.38 The population eligible for inclusion was children and adolescents (5–20 years of age) with chronic insomnia. The age band was chosen based on registry data showing that prescribed melatonin has increased substantially among those 5 years and above within the last decade, and children younger than 5 years who are being prescribed melatonin has remained stable in the same period. The intervention consisted of melatonin, with no restrictions on treatment dosage, duration of treatment, time of consumption or release formular. The comparison was either no treatment (or placebo) or non-pharmacological interventions. Outcomes were non-serious adverse events and serious adverse events assessed 2–4 weeks after initiating treatment and pubertal development and bone health, with no restriction on definition or time of measurement.
Information sources and search strategy
A systematic search was performed in three separate steps. A search for observational studies was performed in Medline, EMBASE, and PsycINFO via Ovid up to February 7th, 2023. A search for randomised studies was performed in Medline, EMBASE, Cinahl and PsycINFO via Ovid up to February 6th, 2023. Finally, a restricted search on adverse events, which included all types of studies, was performed in Medline, EMBASE and PsycINFO up to March 17th, 2023. The search included words with medical subject headings and free-text word. There was no restriction on date of publication, yet language was confined to English and Scandinavian languages. The search strategies are found in the Supplementary information. Observational studies were included for the assessment of long-term outcomes, whereas serious—and non-serious adverse events were assessed by means of randomised studies.
Selection process
The search was merged in RefWorks, and duplicates were removed, after which title and abstracts were imported to the Covidence software for final screening and selection. The title and abstracts were assessed by one reviewer (HKA or HEC), and if the abstract was classified as “include”, the study would proceed to the full-text review. Two reviewers (HKA, HEC or MNH) independently screened the studies that proceeded to full-text review according to the eligibility criteria (the PICO scheme described above). Any discrepancies were resolved through a consensus discussion. Conference abstracts were considered if data were not published elsewhere.
Data collection process, data items and bias assessment
Information extracted from each individual study included details on the study design, diagnosis, age of the participants, length of treatment, dosage, as well as information on the control condition and outcomes of interest. In Covidence, data extraction was performed in duplicate and independently by two reviewers (HEC and HKA). Risk of bias assessment by means of ROBINS-I39 for observational studies and Cochrane risk of bias tool for randomised studies40 was subsequently done in duplicate and independently (MNH, HEC and HKA). Discrepancy was resolved through discussion. Study investigators of the included studies were not contacted in case of missing data nor contacted regarding confirming data. Journal article(s), conference abstract(s), trial protocol or trial registry record were obtained as sources to inform the data extraction and the risk of bias assessment.
Statistical analyses
Serious–and non-serious events were estimated using relative risk (RR) and 95% confidence interval (CI). Forest plots were made in RevMan 5, version 5.3 (Cochrane collaboration), using inverse variance random-effects models assuming that the different studies are related in the intervention effects (follow some distribution), but are estimating different. Sensitivity analyses were performed using risk difference (RD) meta-analysis (RD; 95% CI) when there were zero events in both the intervention and control groups. Post hoc meta-regression analyses were performed on non-serious adverse events (log risk ratio) and the covariates mean age (years), sex (% females), duration (weeks), release type (slow or fast), and dose (mg) with Restricted Maximum Likelihood [REML] estimation. Back-transformation was used for ease of interpretation. Meta-regression analysis on serious adverse events were deemed infeasible, due to lack of data. Meta-regression analyses were performed in Stata (version 14.2).
For long-term outcome data, we undertook a narrative synthesis of outcomes obtained in the included studies, as a meta-analysis was deemed infeasible, due to clinical and methodological heterogeneity. To ensure rigorous and transparent reporting of the narrative synthesis, the 2020 “synthesis without meta-analysis” (SWiM) guideline was followed.41
Certainty assessment
The certainty of evidence was evaluated using the GRADE approach.36
Role of funding source
The Danish Health Authority was involved in all major steps of this study, including the study design, data extraction and–analysis, as well as results interpretation.
Results
Study selection
Serious–and non-serious adverse events
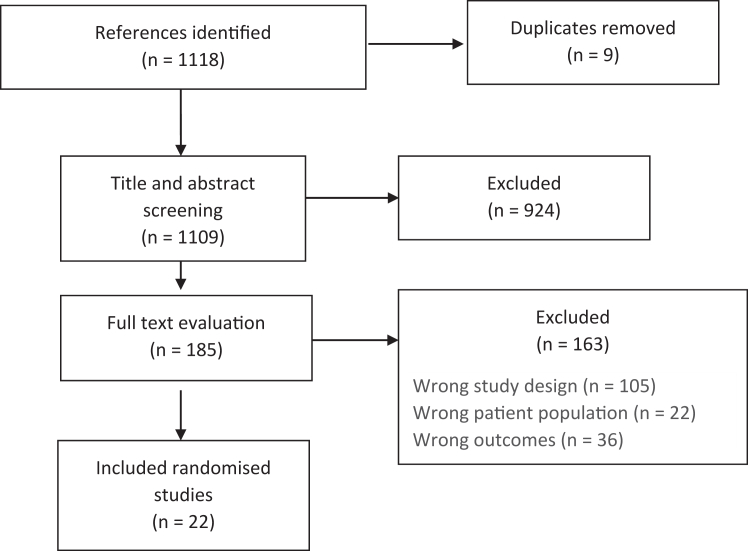
We identified 1109 references following the search for randomised studies. A total of 1087 studies did not meet inclusion and were excluded; 924 were excluded at title and abstract level, and 185 at full text level (Fig. 1). Studies were excluded due to wrong study design (105 studies), wrong outcomes (36 studies) or wrong patient population (n = 22). The list of excluded studies at full text level is provided in the Supplementary. Finally, a total of 22 randomised studies reporting on adverse events were included.
Fig. 1.
PRISMA flowchart for the screening of randomised studies.
Long-term outcome data
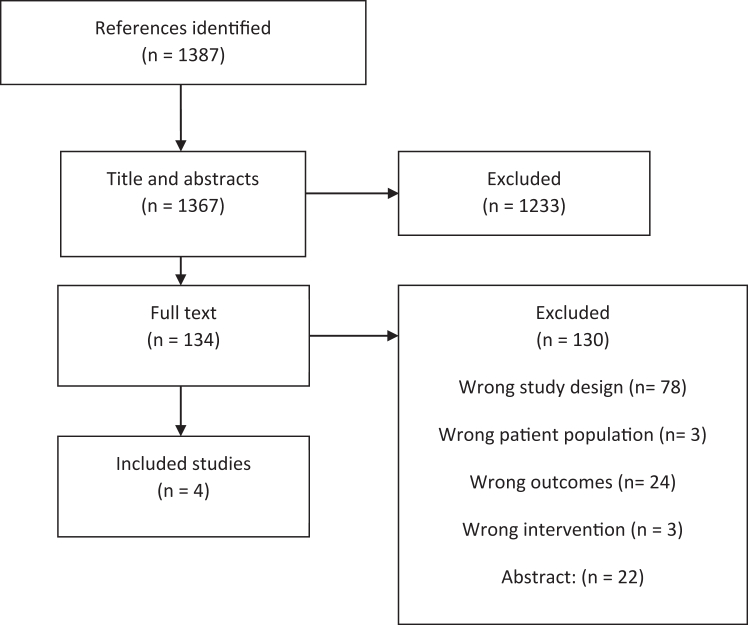
We identified 1387 references in the search for observational studies. A total of 1383 studies did not meet the inclusion criteria and were excluded; 1233 studies were excluded at title and abstract level, and 130 studies at full text level (Fig. 2). Most studies were excluded due to wrong study design (78 studies) or wrong outcomes (24 studies). The list of excluded studies at full text level is provided in Supplementary. Finally, a total of four observational studies were included.
Fig. 2.
PRISMA flowchart for the screening of observational studies.
The restricted search on adverse events provided with 1749 studies, of which 1695 were excluded on title and abstract level and 54 at full text level. This restricted search did not provide with any further relevant studies, which had not already been identified in the two initial searches mentioned above. The flowchart and list of excluded studies is provided in the Supplementary.
Study characteristics
An overview of study characteristics can be found in Table 1 for the randomised controlled studies and in Table 2 for the observational studies. The 22 randomised studies reporting on serious and non-serious adverse events are based on a broad population consisting of 1350 participants with neurodevelopmental disorders,42, 43, 44 epilepsy,45 sleep–wake cycle disorder,46 tuberous sclerosis complex,47 fragile X syndrome/autism,48 autism spectrum disorder,49, 50, 51, 52 attention deficits hyperactive disorder (ADHD),53,54 atopic dermatitis,55,56 concussion with sleep disorders,57 Rett syndrome,58 Idiopathic Chronic Sleep Onset insomnia59, 60, 61, 62 and Delayed Sleep Phase disorder.63 The age of the participants ranged from 1 to 24 years. The melatonin dose varied from 0.5 to 15 mg, and the duration of treatment from 1 week to 3 months.
Table 1.
Characteristics of the included randomised controlled trials.
| Study/Country | Diagnosis | Age range/Average age (SD or 95% CI) | Sex (male %)/ethnicity | Melatonin type/route of administration | Dosage | Duration of treatment |
|---|---|---|---|---|---|---|
| Appleton 2012 United Kingdom |
Neurodevelopmental disorders | 3–15 years of age/8.8 years (2.9) | Melatonin group: 70% males Placebo group: 63% males No information on ethnicity |
Immediate release melatonin. Oral administration or nasogastric feeding tube or gastrostomy feeding tube if the patient was not able to feed orally | 0.5–12 mg | 12 weeks |
| Jain 2015 United States |
Epilepsy | 6–11 years of age/8.4 years (1.3) | Total: 70% males Total: 90% caucasian |
Sustained release melatonin Oral administration |
9 mg | 4 weeks |
| Jan 2000′ Saudi Arabia |
Sleep-wake cycle disorder | 1–11 years/5.4 years (no SD provided) | No information | No information | / | / |
| Dodge 2001 United States |
Developmental disabilities | 1–12 years of age/7.4 years (no SD provided) | No information | No information on melatonin type Oral administration |
5 mg | 4 weeks |
| Hancock 2005 United Kingdom |
Tuberous sclerosis complex | 1–19 years of age/no mean provided | No information | Immediate release melatonin Oral administration |
5–10 mg | 2 weeks |
| Wirojanan 2009 United States |
Fragile X syndrome/Autisme | 2–15 years of age/5.47 years (3.6) | Total: 88% males No information on ethnicity |
No information on melatonin type Oral administration |
3 mg | 2 weeks |
| Wright 2011 United Kingdom |
Autisme spectrum disorder | 3–16 years of age/9 years (2.9) | Total: 80% males No information on ethnicity |
Immediate release melatonin Oral administration |
10 mg | 3 months |
| Cortesi 2012 Italy |
Autisme spectrum disorder | 4–10 years of age Melatonin group: 6.8 years (0.9) Placebo group: 6.3 years (1.2) |
Melatonin group: 82% males Placebo group 84% males Melatonin group: 100% Caucasian Placebo group: 96% Caucasian |
Sustained release melatonin Oral administration |
/ | 12 weeks |
| Gringas 2017 United States |
Autisme spectrum disorder | 2–17 years of age/8.7 years (4.15) | Melatonin group: 45% males Placebo group: 47% males Melatonin group: 40% not Hispanic or latino Placebo group: 49% not Hispanic or latino |
Sustained release melatonin Oral administration |
2–5 mg | 13 weeks |
| Van der Heiden 2007 The Netherlands |
ADHD | 6–12 years of age Melatonin group: 9.1 years (2.3) Placebo group: 9.3 years (1.8) |
Melatonin group: 35% males Placebo group: 43% males No information on ethnicity |
Immediate release melatonin Oral administration |
3 or 6 mg | 4 weeks |
| Weiss 2006 Canada |
ADHD | 6–14 years of age/10.29 years (no SD provided) | Total: 90.9% males Total: 87.9% Caucasian |
Immediate release melatonin Oral administration |
5 mg | 30 days |
| Hayashi 2021 Japan |
Autisme spectrum disorder | 6–15 years of age/11.2 years (2.5) | Total: 61.7% males No information on ehtnicity |
No information on melatonin type Oral administration (granuels) |
1–4 mg | 14 days |
| Taghavi Ardakani 2018 Iran |
Atopic dermatitis | 6–12 years of age Melatonin group: 8.9 years (2.1) Placebo group: 8.4 years (2.2) |
Melatonin group: 45.7% males Placebo group: 51.4% males No information on ethnicity |
Immediate release melatonin Oral administration |
6 mg | 6 weeks |
| Chang 2016 Taiwan |
Atopic dermatitis | 1–18 years of age/7.5 years (3.7) | Melatonin group: 54% females Placebo group: 42% females No information on ethnicity |
Immediate release melatonin Oral administration |
3 mg | 4 weeks |
| Barlow 2021 Australia |
Postconcussion + sleep disabilities | 8–18 years of age Melatonin group 3 mg: 13.7 years (12.7–14.7) Melatonin group 10 mg: 14.2 years (13.3–15.2) Placebo group: 14.2 years (12.9–15.5) |
Melatonin group 10 mg: 64% females Melatonin group 3 mg: 62% females Placebo group: 59% females No information on ethnicity |
Controlled release melatonin Oral administration |
3 mg or 10 mg | 2 weeks |
| McArthur 1998 United states |
Rett syndrome | Average age 10.1 years (no SD provided) | Total: 100% females | Immediate release melatonin Oral administration |
2.5–7.5 mg | 4 weeks |
| Wasdell 2008 The Netherlands |
Neurodevelopmental disorders | 2–18 years of age/7.38 years | Total: 62% males No information on ethnicity |
Controlled release melatonin Oral administration |
5–15 mg | 10 days |
| Eckerberg 2012 Sweden |
Idiopathic Chronic Sleep onset insomnia | 14–19 years of age (no average provided) | Total: 48% males No information on ethnicity |
Immediate release melatonin Oral administration |
1 mg | 2 weeks |
| Smits 2001 The Netherlands |
Idiopathic Chronic Sleep onset insomnia | 6–12 years of age (no average provided) | Total: 70% males No information on ethnicity |
Immediate release melatonin Oral administration |
5 mg | 4 weeks |
| Smits 2003 The Netherlands |
Idiopathic Chronic Sleep onset insomnia | 6–12 years of age/9.2 years (2.1) | Total: 70% males No information on ethnicity |
Immediate release melatonin Oral administration |
5 mg | 4 weeks |
| Van Geijlswijk 2010 The Netherlands |
Idiopathic Chronic Sleep onset insomnia | 6–12 years of age Melatonin group 0.05 mg/kg: 9.5 years (1.8) Melatonin group 0.1 mg/kg: 8.9 years (1.4) Melatonin group 0.15 mg/kg: 8.7 years (1.4) Placebo group: 8.7 years (2.8) |
Total: 85% males No information on ethnicity |
No information | Mean dosage 1.60/2.91/4.39 mg | 1 week |
| Wilhelmsen-langeland 2013 Norway |
Delayed sleep phase disorder | 16–24 years of age Melatonin group: 21.2 years (2.7) Placebo group: 20.8 years (3.4) |
Total: 30% males No information on ethnicity |
Immediate release melatonin Oral administration |
3 mg | 4 weeks |
SD: Standard deviation, CI: Confidence interval; mg: Milligram; ADHD: Attention deficits hyperactive disorder.
Table 2.
Characteristics of the included observational studies.
| Reference, country/region | Study design | Population and diagnosis | Age of assessment, sex (% female), Ethnicity | Outcomes/Assessment method | Dosage, type | Length of treatment | Comparator | Comments |
|---|---|---|---|---|---|---|---|---|
| Carr et al., 2007, The Netherlands | Follow-up study of Wasdell 2008 (RCT) | 41 participants with neurodevelopmental disability (NDD). Included mental retardation, cerebral palsy, epilepsy, visual impairment (none completely blind) and autistic spectrum disorders. | Median age 8.6 years (range 4.8–19.3 years), Sex: 31.7, Ethnicity: not reported | Pubertal development. Assessment method not stated. | Mean dose 10.4 mg (SD 5.2) (range 5–15 mg), beaded sustained-release (1 mg fast and 4 mg controlled release) and later melatonin supplier replaced controlled-release melatonin (5 mg) with fast release (5 mg) formulation | Mean 4.3 years (range 2–12 years) | Not reported | The median age of the onset of puberty was 11.5 years (range 2—15 years). Precocious puberty developed in five children who had severe NDD, all prior to the MT therapy, at ages 2, 3, 4, 6 and 7 years. In the others with signs of puberty the onset was age appropriate (mean (±S.D.) age: 13.4 (±1.4) years). |
| Malow et al., 2021, United States and Europe | Follow-up study of Gringas (2017) (RCT) | 31 participants with Autism Spectrum Disorder (96%) and Smith-Magenis syndrome | Mean age 9 years (SD 4.2). Range 2–17 years. Sex: 25, Ethnicity: Not Hispanic or Latino (66.7%), Hispanic or Latino (20.0%), Other (13.3%) | Pubertal development. Assessed by a Physician in children≥8 years using Tanner pubertal staging score. | Range 2–10 mg, PedPRM | Mean 1.4 years (Range 3 days∗ to 2 years) | Control population matched on age and sex (Nilsson et al., 2001) | The study shows no delay in sexual maturation after 2 yr of continuous use of prolonged release melatonin. |
| vanGeijlswijk et al., 2011, The Netherlands | Follow-up study of vanGeijlswik 2010 (RCT) | 19 participants with Chronic idiopathic childhood sleep onset insomnia | Mean age 12.0 years (range 8.6–15.7 years), Sex: Group 1: 44, Group 2: 74, Group 3: 44, Group 4: 65 Ethnicity: not reported | Pubertal development. Written interview sent to the participants including three Tanner score questions. Self-reported | Mean dose 2.7 mg (range 0.3–10 mg), type not reported | Mean 3.1 years (range 1.0–4.6 years) | Control population matched on age and sex (Mul et al., 2001) | Puberty onset, as assessed by Tanner scores, seems to be undisturbed after 3.1 yr of exogenous melatonin usage. |
| Zwart et al., 2017, The Netherlands | Follow-up study of vanGeijlswik 2010 and 2011 (RCT) | 33 participants with Chronic idiopathic childhood sleep onset insomnia | Mean age 19.6 years (range 16.7–23.2 years), Sex: 57.6, Ethnicity: not reported | Pubertal development. Online questionnaire. Participants asked to indicate whether they felt their timing of pubertal development was any earlier or later than their peers. | Range 0.5–5 mg, type not reported | Mean duration of treatment 10.8 years. Overall average duration of treatment was 7.1 years. | Control population (Bratberg et al., 2007) | The perceived timing of pubertal development suggested a tendency towards delayed puberty. 31.3% of the participants experienced their pubertal timing as late. |
RCT: Randomised controlled trial: yr: Year; SD: Standard deviation; mg: Milligram; MT: Melatonin; NDD: Neurodevelopmental disorder.
The four observational studies investigating the effect of long-term melatonin treatment on pubertal development in 105 patients, consisted of a population who at enrolment were diagnosed with either neurodevelopmental disorders, autism or Smith-Magenis syndrome, or chronic Idiopathic Childhood Sleep Onset insomnia.27,28,64,65 Three of the studies are follow-up studies of earlier randomised studies.44,51,62 The study by Zwart et al. 201765 is a follow-up of the already included follow-up study.62 The age of the participants when starting melatonin varied from 2 to 18 years, and with the age at assessment ranging from 8.6 to 19.6 years of age. The average duration of treatment was between 1.4 and 10.8 years, and melatonin doses of 0.5–15 mg were used. Two studies examined pubertal development as measured by the Tanner score (either clinician assessed- or self-reported) compared to pubertal development in the general population in the Netherlands.27,28 In the two remaining studies, pubertal development was evaluated based on interviews provided by telephone or online. The basis of comparison was either a Norwegian background population or not reported.64,65
Synthesis
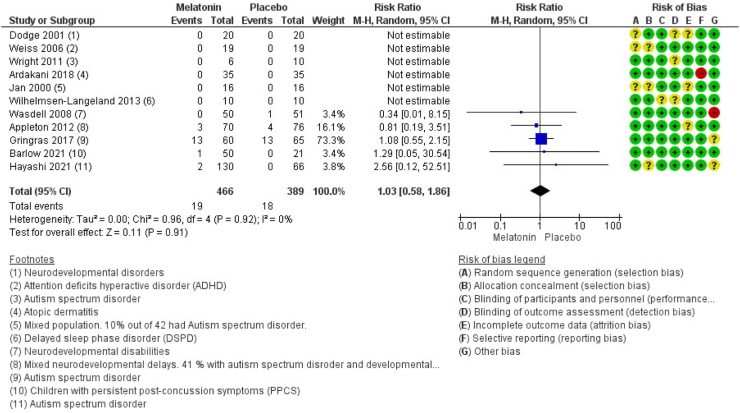
Eleven studies encompassing 855 participants reported on the number of patients experiencing serious adverse events.42, 43, 44,46,49,51,52,54,55,57,63 The results show that treatment with melatonin is most likely not associated with serious adverse events (RR 1.03 (CI 95% 0.58–1.86), I2 = 0%, and (RD 0.00 (CI 95% −0.02 to 0.02), I2 = 0%), moderate certainty) (Fig. 3 and Supplementary). An overview of the type of serious adverse events reported in the eleven randomised studies is found in the Supplementary.
Fig. 3.
Forest plot of the effect of melatonin versus placebo on serious adverse events (p = 0.92). CI: Confidence interval; ADHD: Attention deficits hyperactive disorder; DSPD: Delayed sleep phase disorder; PPCS: Persistent post-concussion symptoms; M–H: Mantel–Haenszel test.
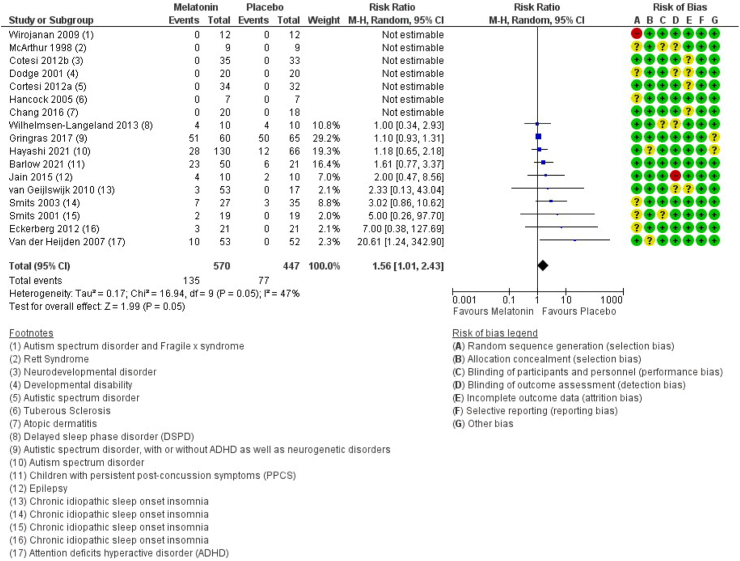
Seventeen studies encompassing 1017 participants reported on the number of patients experiencing non-serious adverse events.43,45,47,48,50, 51, 52, 53,56, 57, 58, 59, 60, 61, 62, 63 The results show that the number of patients experiencing non-serious adverse events is increased following intake of melatonin (RR 1.56 (CI 95% 1.01–2.43), I2 = 47%, and RD 0.06 (CI 95% 0.01–0.10), I2 = 36%, moderate certainty) (Fig. 4 and Supplementary). Of the 17 included studies, nine of these specified various non-serious adverse events, such as headache, nausea, red cheeks, red earlobes, sore/red eyes, fatigue/drowsiness, dizziness, vomiting, influenza symptoms/infections, change in mood/cognition, musculoskeletal pain and gastrointestinal problems (see overview in Supplementary). The effect of melatonin on non-serious adverse events was unaffected by age, sex, duration, release type, and dose (Supplementary).
Fig. 4.
Forest plot of the effect of melatonin versus placebo on non-serious adverse events (p = 0.05). CI: Confidence interval; ADHD: Attention deficits hyperactive disorder; DSPD: Delayed sleep phase disorder; PPCS: Persistent post-concussion symptoms; M–H: Mantel–Haenszel test.
It was not possible to make a meta-analysis for pubertal development due to the format the data was given in. The results are therefore described narratively. In the study by Malow et al., 2021 (n = 31) and Carr et al., 2017 (n = 41), no delay in pubertal development was found after an average of 2 and 4.3 years of continuous use of melatonin, respectively.28,64 In the study by van Geijlswijk et al., 2011, pubertal onset seemed to be undisturbed after 3.1 years of melatonin treatment (n = 19).27 However, when the same population was later evaluated in the study by Zwart et al., 2018, a tendency towards delayed pubertal timing was observed. At this point, the participants had received treatment with melatonin for an average of 7.1 years.65
No studies were identified that reported on bone mineral density or risk of fractures.
Certainty of evidence
Certainty in the evidence regarding serious as well as non-serious adverse events as reported in randomised studies is moderate due to an imprecise effect estimate (wide confidence interval). The risk of bias evaluation is seen in the forest plots (Figs. 3 and 4). For long-term outcome data assessed in observational studies, the certainty is very low, due to serious risk of bias (serious risk of bias due to confounding, serious risk of bias due to deviations from the intended interventions, serious risk of bias due to missing data and serious risk of bias in measurement of outcomes) (Table 3) and risk of imprecision (few patients included). The control group for two of the observational studies is based on other previously published material.
Table 3.
Risk of bias (ROBINS-I) of the included observational studies.
| Author/year | Bias due to confounding | Bias in the selection of participants into the study | Bias in classification of interventions | Bias due to deviations from the intended interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported results | Overall bias |
|---|---|---|---|---|---|---|---|---|
| Carr 200764 | NI | Low | Low | Serious | Low | Serious | Low | Serious |
| Malow 202128 | Serious | Low | Low | NI | Serious | Serious | Low | Serious |
| vanGeijlswijk 201127 | Serious | Low | Low | NI | Serious | Serious | Low | Serious |
| Zwart 201765 | Serious | Low | Low | Serious | NI | Serious | Low | Serious |
NI: No information.
Discussion
Our systematic review that followed the standardised and transparent GRADE method revealed that treatment with melatonin in children and adolescents is not associated with an increase in serious adverse events such as death, hospitalisation, or significant disability/incapacity. However, we found that it is likely to experience a range of non-serious adverse events. The certainty of the evidence for non-serious adverse events was downgraded to moderate due to a wide confidence interval, indicating some level of uncertainty regarding the extent to which melatonin leads to non-serious adverse events. On this notion, the discrepancy across studies is noteworthy, as some studies reported a substantial number of adverse events, whereas others reported no adverse events in either the melatonin or placebo group. The current assessment of serious—and non-serious adverse events is based on few patients and with the majority of studies performed in a population with an underlying disorder, which may hinder the possibility to identify any subtle effects. The identified studies only rarely performed a systematic evaluation of adverse events, and with even fewer studies providing with a full report on the frequency and type of adverse events that occurred throughout the trial period. Overall, this scarcity of data indicates that adverse events in children and adolescents may be underreported and/or insufficiently investigated and thus further studies on safety in children and adolescents are highly needed.
Current evidence reports that melatonin treatment may have little or no influence on later pubertal development after 2–4 years of treatment, whereas one study showed a trend towards a delay in pubertal development in participants who on average had been treated with melatonin for 7.1 years.65 This may indicate that the length of treatment is essential when it comes to the impact on pubertal development in young individuals. These findings are for now, however, only speculative as the certainty of evidence is very low, as results are based on few patients, and with a serious risk of bias due to risk of confounding, incomplete follow-up and a highly questionable validity on how pubertal development was measured. Only one of the identified studies made use of a physician-rated Tanner score and with results compared to a group of peers published elsewhere.28 For the remaining studies, the impact on development was assessed based on either a telephone interview or questionnaire with the parents or participants.62,64,65 As such, apart from one study, the current findings in large rely on subjective rather than objective evaluations of pubertal development, which inevitably introduces a concern in the robustness of these results. The age of the participants when initiating melatonin varied from 2 to 18 years, thus including both prepubescent and postpubescent participants. To better address the impact on pubertal development, objective measures in solely prepubescent participants is needed.
Melatonin is associated with the overall bone remodelling process through indirect and direct stimulatory actions on both osteoblasts and osteoclasts and potentially also through effects on the gonadal hormone axis (pituritary-gonodal),66 and thus is considered to hold the potential of long-term bone health and clinical prevention of bone-related diseases. Bearing this in mind, this may also be the reason that the literature on melatonin and bone metabolism, up until now, has primarily focused on the therapeutic role of melatonin in osteoporosis prevention and treatment.67, 68, 69, 70, 71 From our review we can confirm that the role of melatonin on paediatric and pubertal bone development still needs to be elucidated, as we did not identify any studies that reported on the association between long-term melatonin treatment and bone outcomes in our search for literature.
This systematic review was conducted in accordance with the current highest standards within systematic review methodology, including a pre-specified protocol, a highly sensitive search strategy, the screening, data extraction and risk of bias assessment were conducted by two independent review authors, and the certainty in the evidence was considered during interpretation of the results.36 The major limitation of this systematic review is that the planned meta-analysis on long-term outcomes was not deemed possible, due to small number of included studies with very heterogeneous reporting of results. Therefore, we cannot at this point provide a reliable and robust effect estimate of long-term melatonin treatment. The risk of bias assessment of the included studies was limited by what was reported, and thus we may have overestimated risk of bias. Trial registries, observational studies or grey literature were not assessed in the evaluation of serious—and non-serious adverse events.
In the Scandinavian countries, melatonin is a prescription medication, and we have access to public data sources to inform on use through the national prescription registers.13,14,72 Although we acknowledge that information on the incidence of central precocious puberty, premature thelarche and premature adrenarche, as well as fracture events are available in the national patient registries, routine pubertal development assessment or dual-energy x-ray absorptiometry (DXA) scans are unfortunately not available in the health registries. Collected cohort data may be insufficiently powered to study such associations, so new cohorts should be established with the aim of exploring melatonin treatment from a life course perspective.
Taken together, melatonin is a commonly used treatment in children and adolescents with insomnia, and therefore concerns have been raised regarding the short-term and long-term adverse consequences. Patients are likely to experience non-serious adverse events, however the actual extent to which melatonin leads to non-serious adverse events in the young population and the impact on pubertal development remains uncertain. No studies were identified on bone health. This major gap of knowledge on short-term and long-term safety of melatonin treatment in children and adolescents calls for cautious use and for more research to inform clinicians and guideline panels on this key issue.
Contributors
The authors confirm contribution to the paper as follows: study design: all authors, data collection: HEC, HKA, MNH; analysis of results: HEC, HKA, MNH; interpretation of results: all authors; draft of manuscript: MNH, HEC and HKA. All authors reviewed and approved the final version of the manuscript. HEC, HKA and MNH accessed and verified the underlying data.
Data sharing statement
Data and all other relevant materials are publicly available either at the Danish Health Authority website (www.sst.dk) or upon request to the corresponding author.
Declaration of interests
LB is a member of the Danish medication reimbursement committee. AV has previously received honoraria for lectures at AGB pharma, Takeda & Medice, and holds stocks at Novo Nordisk. All other authors declare no competing interests. Statements of conflicts of interests can be found for all members of the guideline panel, the external reviewer of the national clinical guideline, the reference–and project group at the Danish Health Authority website (www.sst.dk).
Acknowledgments
We would like to thank the reference group, guideline panel and the secretary of the “National clinical recommendation for the use of melatonin in children and adolescents with chronic insomnia” published by the Danish Health Authority. The Parker Institute, Bispebjerg and Frederiksberg Hospital, is supported by a core grant from the Oak Foundation (OCAY-18-774-OFIL).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102083.
Appendix A. Supplementary data
References
- 1.Lollies F., Schnatschmidt M., Bihlmeier I., Genuneit J., In-Albnon T., Holtmann M., et al. Associations of sleep and emotion regulation processes in childhood and adolescence–a systematic review, report of methodological challenges and future directions. Sleep Sci. 2022;15(4):490–514. doi: 10.5935/1984-0063.20220082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilhite K., Booker B., Huang B.H., et al. Combinations of physical activity, sedentary behavior, and sleep and their associations with physical, psychological, and educational outcomes in children and adolescents: a systematic review. Am J Epidemiol. 2022;192:665. doi: 10.1093/aje/kwac212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Julian V., Haschke F., Fearnbach N., et al. Effects of movement behaviors on overall health and appetite control: current evidence and perspectives in children and adolescents. Curr Obes Rep. 2022;11(1):10–22. doi: 10.1007/s13679-021-00467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medic G., Wille M., Hemels M.E. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;9:151–161. doi: 10.2147/NSS.S134864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mindell J.A., Sadeh A., Kwon R., Goh D.Y. Cross-cultural differences in the sleep of preschool children. Sleep Med. 2013;14(12):1283–1289. doi: 10.1016/j.sleep.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Beresford B., McDaid C., Parker A., et al. Pharmacological and non-pharmacological interventions for non-respiratory sleep disturbance in children with neurodisabilities: a systematic review. Health Technol Assess. 2018;22(60):1–296. doi: 10.3310/hta22600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scantlebury A., McDaid C., Dawson V., et al. Non-pharmacological interventions for non-respiratory sleep disturbance in children with neurodisabilities: a systematic review. Dev Med Child Neurol. 2018;60(11):1076–1092. doi: 10.1111/dmcn.13972. [DOI] [PubMed] [Google Scholar]
- 8.Keogh S., Bridle C., Siriwardena N.A., et al. Effectiveness of non-pharmacological interventions for insomnia in children with Autism Spectrum Disorder: a systematic review and meta-analysis. PLoS One. 2019;14(8) doi: 10.1371/journal.pone.0221428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Händel M.N., Cardoso I., von Bülow C., et al. Fracture risk reduction and safety by osteoporosis treatment compared with placebo or active comparator in postmenopausal women: systematic review, network meta-analysis, and meta-regression analysis of randomised clinical trials. BMJ. 2023;381:e068033. doi: 10.1136/bmj-2021-068033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Z.R., Shi L.J., Deng M.H. Efficacy of cognitive behavioral therapy in children and adolescents with insomnia: a systematic review and meta-analysis. Braz J Med Biol Res. 2018;51(6) doi: 10.1590/1414-431X20187070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Black L.I., Clarke T.C., Barnes P.M., Stussman B.J., Nahin R.L. Use of complementary health approaches among children aged 4-17 years in the United States: national Health Interview Survey, 2007-2012. Natl Health Stat Rep. 2015;(78):1–19. [PMC free article] [PubMed] [Google Scholar]
- 12.The Danish Health Data Authority Medstat.dk. 2021. [Ref Type: Online Source] [Google Scholar]
- 13.Wesselhoeft R., Rasmussen L., Jensen P.B., et al. Use of hypnotic drugs among children, adolescents, and young adults in Scandinavia. Acta Psychiatr Scand. 2021;144(2):100–112. doi: 10.1111/acps.13329. [DOI] [PubMed] [Google Scholar]
- 14.Bliddal M., Kildegaard H., Rasmussen L., et al. Melatonin use among children, adolescents, and young adults: a Danish nationwide drug utilization study. Eur Child Adolesc Psychiatry. 2022:1–9. doi: 10.1007/s00787-022-02035-1. [DOI] [PubMed] [Google Scholar]
- 15.Abdelgadir I.S., Gordon M.A., Akobeng A.K. Melatonin for the management of sleep problems in children with neurodevelopmental disorders: a systematic review and meta-analysis. Arch Dis Child. 2018;103(12):1155–1162. doi: 10.1136/archdischild-2017-314181. [DOI] [PubMed] [Google Scholar]
- 16.Wei S., Smits M.G., Tang X., et al. Efficacy and safety of melatonin for sleep onset insomnia in children and adolescents: a meta-analysis of randomized controlled trials. Sleep Med. 2020;68:1–8. doi: 10.1016/j.sleep.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Parker A., Beresford B., Dawson V., et al. Oral melatonin for non-respiratory sleep disturbance in children with neurodisabilities: systematic review and meta-analyses. Dev Med Child Neurol. 2019;61(8):880–890. doi: 10.1111/dmcn.14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Besag F.M.C., Vasey M.J., Lao K.S.J., Wong I.C.K. Adverse events associated with melatonin for the treatment of primary or secondary sleep disorders: a systematic review. CNS Drugs. 2019;33(12):1167–1186. doi: 10.1007/s40263-019-00680-w. [DOI] [PubMed] [Google Scholar]
- 19.Foley H.M., Steel A.E. Adverse events associated with oral administration of melatonin: a critical systematic review of clinical evidence. Complement Ther Med. 2019;42:65–81. doi: 10.1016/j.ctim.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Hoebert M., van der Heijden K.B., van Geijlswijk I.M., Smits M.G. Long-term follow-up of melatonin treatment in children with ADHD and chronic sleep onset insomnia. J Pineal Res. 2009;47(1):1–7. doi: 10.1111/j.1600-079X.2009.00681.x. [DOI] [PubMed] [Google Scholar]
- 21.European Medicines Agency (2018) 2023. Slenyto. Slenyto | European medicines agency (europa.eu) [Ref Type: Report] [Google Scholar]
- 22.EMA. Assessment report Slenyto International non-proprietary name: melatonin Procedure No.26 July 2018 EMA/556280/2018.Committee for Medicinal Products for Human Use (CHMP)EMEA/H/C/004425/0000 Note Assessment report as adopted by the CHMP with all information of a commercially confidential nature deleted. 26-7-2018. [Ref Type: Report]. 2018.
- 23.Kennaway D.J. What do we really know about the safety and efficacy of melatonin for sleep disorders? Curr Med Res Opin. 2022;38(2):211–227. doi: 10.1080/03007995.2021.2000714. [DOI] [PubMed] [Google Scholar]
- 24.Kennaway D.J. Potential safety issues in the use of the hormone melatonin in paediatrics. J Paediatr Child Health. 2015;51(6):584–589. doi: 10.1111/jpc.12840. [DOI] [PubMed] [Google Scholar]
- 25.Goldman R.D., Bongiorno P.B., Olcese J.M., Witt-Enderby P.A., Shatkin J.P. Myths and evidence regarding melatonin supplementation for occasional sleeplessness in the pediatric population. Pediatr Ann. 2021;50(9):e391–e395. doi: 10.3928/19382359-20210823-01. [DOI] [PubMed] [Google Scholar]
- 26.Boafo A., Greenham S., Alenezi S., et al. Could long-term administration of melatonin to prepubertal children affect timing of puberty? A clinician's perspective. Nat Sci Sleep. 2019;11:1–10. doi: 10.2147/NSS.S181365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Geijlswijk I.M., Mol R.H., Egberts T.C., Smits M.G. Evaluation of sleep, puberty and mental health in children with long-term melatonin treatment for chronic idiopathic childhood sleep onset insomnia. Psychopharmacology (Berl) 2011;216(1):111–120. doi: 10.1007/s00213-011-2202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malow B.A., Findling R.L., Schroder C.M., et al. Sleep, growth, and puberty after 2 years of prolonged-release melatonin in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2021;60(2):252–261. doi: 10.1016/j.jaac.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hedstrom E.M., Svensson O., Bergstrom U., Michno P. Epidemiology of fractures in children and adolescents. Acta Orthop. 2010;81(1):148–153. doi: 10.3109/17453671003628780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khosla S., Melton L.J., III, Dekutoski M.B., Achenbach S.J., Oberg A.L., Riggs B.L. Incidence of childhood distal forearm fractures over 30 years: a population-based study. JAMA. 2003;290(11):1479–1485. doi: 10.1001/jama.290.11.1479. [DOI] [PubMed] [Google Scholar]
- 31.Handel M.N., Heitmann B.L., Abrahamsen B. Nutrient and food intakes in early life and risk of childhood fractures: a systematic review and meta-analysis. Am J Clin Nutr. 2015;102(5):1182–1195. doi: 10.3945/ajcn.115.108456. [DOI] [PubMed] [Google Scholar]
- 32.Handel M.N., Frederiksen P., Osmond C., Cooper C., Abrahamsen B., Heitmann B.L. Prenatal exposure to vitamin D from fortified margarine and risk of fractures in late childhood: period and cohort results from 222,000 subjects in the D-tect observational study. Br J Nutr. 2017;117(6):872–881. doi: 10.1017/S000711451700071X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amstrup A.K., Sikjaer T., Heickendorff L., Mosekilde L., Rejnmark L. Melatonin improves bone mineral density at the femoral neck in postmenopausal women with osteopenia: a randomized controlled trial. J Pineal Res. 2015;59(2):221–229. doi: 10.1111/jpi.12252. [DOI] [PubMed] [Google Scholar]
- 34.Amstrup A.K., Sikjaer T., Mosekilde L., Rejnmark L. Melatonin and the skeleton. Osteoporos Int. 2013;24(12):2919–2927. doi: 10.1007/s00198-013-2404-8. [DOI] [PubMed] [Google Scholar]
- 35.Cirmanova V., Zofkova I., Kasalicky P., et al. Hormonal and bone parameters in pubertal girls. Physiol Res. 2017;66(Suppl 3):S419–S424. doi: 10.33549/physiolres.933733. [DOI] [PubMed] [Google Scholar]
- 36.Guyatt G., Oxman A.D., Akl E.A., et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 37.Page M.J., Moher D., Bossuyt P.M., et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guyatt G.H., Oxman A.D., Kunz R., et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64(4):395–400. doi: 10.1016/j.jclinepi.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Sterne J.A.C., Hernán M.A., Reeves B.C., et al. ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higgins J.P., Altman D.G., Gotzsche P.C., et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campbell M., McKenzie J.E., Sowden A., et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:l6890. doi: 10.1136/bmj.l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Appleton R.E., Jones A.P., Gamble C., et al. The use of MElatonin in children with neurodevelopmental disorders and impaired sleep: a randomised, double-blind, placebo-controlled, parallel study (MENDS) Health Technol Assess. 2012;16(40):239. doi: 10.3310/hta16400. [DOI] [PubMed] [Google Scholar]
- 43.Dodge N.N., Wilson G.A. Melatonin for treatment of sleep disorders in children with developmental disabilities. J Child Neurol. 2001;16(8):581–584. doi: 10.1177/088307380101600808. [DOI] [PubMed] [Google Scholar]
- 44.Wasdell M.B., Jan J.E., Bomben M.M., et al. A randomized, placebo-controlled trial of controlled release melatonin treatment of delayed sleep phase syndrome and impaired sleep maintenance in children with neurodevelopmental disabilities. J Pineal Res. 2008;44(1):57–64. doi: 10.1111/j.1600-079X.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- 45.Jain S.V., Horn P.S., Simakajornboon N., et al. Melatonin improves sleep in children with epilepsy: a randomized, double-blind, crossover study. Sleep Med. 2015;16(5):637–644. doi: 10.1016/j.sleep.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jan M.M. Melatonin for the treatment of handicapped children with severe sleep disorders. Pediatr Neurol. 2000;23(3):229–232. doi: 10.1016/s0887-8994(00)00188-0. [DOI] [PubMed] [Google Scholar]
- 47.Hancock E., O'Callaghan F., English J., Osborne J.P. Melatonin excretion in normal children and in tuberous sclerosis complex with sleep disorder responsive to melatonin. J Child Neurol. 2005;20(1):21–25. doi: 10.1177/08830738050200010301. [DOI] [PubMed] [Google Scholar]
- 48.Wirojanan J., Jacquemont S., Diaz R., et al. The efficacy of melatonin for sleep problems in children with autism, fragile X syndrome, or autism and fragile X syndrome. J Clin Sleep Med. 2009;5(2):145–150. [PMC free article] [PubMed] [Google Scholar]
- 49.Wright B., Sims D., Smart S., et al. Melatonin versus placebo in children with autism spectrum conditions and severe sleep problems not amenable to behaviour management strategies: a randomised controlled crossover trial. J Autism Dev Disord. 2011;41(2):175–184. doi: 10.1007/s10803-010-1036-5. [DOI] [PubMed] [Google Scholar]
- 50.Cortesi F., Giannotti F., Sebastiani T., Panunzi S., Valente D. Controlled-release melatonin, singly and combined with cognitive behavioural therapy, for persistent insomnia in children with autism spectrum disorders: a randomized placebo-controlled trial. J Sleep Res. 2012;21(6):700–709. doi: 10.1111/j.1365-2869.2012.01021.x. [DOI] [PubMed] [Google Scholar]
- 51.Gringras P., Nir T., Breddy J., Frydman-Marom A., Findling R.L. Efficacy and safety of pediatric prolonged-release melatonin for insomnia in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2017;56(11):948–957. doi: 10.1016/j.jaac.2017.09.414. [DOI] [PubMed] [Google Scholar]
- 52.Hayashi M., Mishima K., Fukumizu M., et al. Melatonin treatment and adequate sleep hygiene interventions in children with autism spectrum disorder: a randomized controlled trial. J Autism Dev Disord. 2022;52(6):2784–2793. doi: 10.1007/s10803-021-05139-w. [DOI] [PubMed] [Google Scholar]
- 53.van der Heijden K.B., Smits M.G., Van Someren E.J., Ridderinkhof K.R., Gunning W.B. Effect of melatonin on sleep, behavior, and cognition in ADHD and chronic sleep-onset insomnia. J Am Acad Child Adolesc Psychiatry. 2007;46(2):233–241. doi: 10.1097/01.chi.0000246055.76167.0d. [DOI] [PubMed] [Google Scholar]
- 54.Weiss M.D., Wasdell M.B., Bomben M.M., Rea K.J., Freeman R.D. Sleep hygiene and melatonin treatment for children and adolescents with ADHD and initial insomnia. J Am Acad Child Adolesc Psychiatry. 2006;45(5):512–519. [PubMed] [Google Scholar]
- 55.Taghavi A.A., Farrehi M., Sharif M.R., et al. The effects of melatonin administration on disease severity and sleep quality in children with atopic dermatitis: a randomized, double-blinded, placebo-controlled trial. Pediatr Allergy Immunol. 2018;29(8):834–840. doi: 10.1111/pai.12978. [DOI] [PubMed] [Google Scholar]
- 56.Chang Y.S., Lin M.H., Lee J.H., et al. Melatonin supplementation for children with atopic dermatitis and sleep disturbance: a randomized clinical trial. JAMA Pediatr. 2016;170(1):35–42. doi: 10.1001/jamapediatrics.2015.3092. [DOI] [PubMed] [Google Scholar]
- 57.Barlow K.M., Kirk V., Brooks B., et al. Efficacy of melatonin for sleep disturbance in children with persistent post-concussion symptoms: secondary analysis of a randomized controlled trial. J Neurotrauma. 2021;38(8):950–959. doi: 10.1089/neu.2020.7154. [DOI] [PubMed] [Google Scholar]
- 58.McArthur A.J., Budden S.S. Sleep dysfunction in Rett syndrome: a trial of exogenous melatonin treatment. Dev Med Child Neurol. 1998;40(3):186–192. doi: 10.1111/j.1469-8749.1998.tb15445.x. [DOI] [PubMed] [Google Scholar]
- 59.Eckerberg B., Lowden A., Nagai R., Akerstedt T. Melatonin treatment effects on adolescent students' sleep timing and sleepiness in a placebo-controlled crossover study. Chronobiol Int. 2012;29(9):1239–1248. doi: 10.3109/07420528.2012.719962. [DOI] [PubMed] [Google Scholar]
- 60.Smits M.G., van Stel H.F., Van der Heijden K., Meijer A.M., Coenen A.M., Kerkhof G.A. Melatonin improves health status and sleep in children with idiopathic chronic sleep-onset insomnia: a randomized placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2003;42(11):1286–1293. doi: 10.1097/01.chi.0000085756.71002.86. [DOI] [PubMed] [Google Scholar]
- 61.Smits M.G., Nagtegaal E.E., van der Heijden J., Coenen A.M., Kerkhof G.A. Melatonin for chronic sleep onset insomnia in children: a randomized placebo-controlled trial. J Child Neurol. 2001;16(2):86–92. doi: 10.1177/088307380101600204. [DOI] [PubMed] [Google Scholar]
- 62.van Geijlswijk I.M., van der Heijden K.B., Egberts A.C., Korzilius H.P., Smits M.G. Dose finding of melatonin for chronic idiopathic childhood sleep onset insomnia: an RCT. Psychopharmacology (Berl) 2010;212(3):379–391. doi: 10.1007/s00213-010-1962-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilhelmsen-Langeland A., Saxvig I.W., Pallesen S., et al. A randomized controlled trial with bright light and melatonin for the treatment of delayed sleep phase disorder: effects on subjective and objective sleepiness and cognitive function. J Biol Rhythms. 2013;28(5):306–321. doi: 10.1177/0748730413500126. [DOI] [PubMed] [Google Scholar]
- 64.Carr R., Wasdell M.B., Hamilton D., et al. Long-term effectiveness outcome of melatonin therapy in children with treatment-resistant circadian rhythm sleep disorders. J Pineal Res. 2007;43(4):351. doi: 10.1111/j.1600-079X.2007.00485.x. [DOI] [PubMed] [Google Scholar]
- 65.Zwart T.C., Smits M.G., Egberts T.C.G., Rademaker C.M.A., van Geijlswijk I.M. Long-term melatonin therapy for adolescents and young adults with chronic sleep onset insomnia and late melatonin onset: evaluation of sleep quality, chronotype, and lifestyle factors compared to age-related randomly selected population cohorts. Healthcare (Basel) 2018;6(1):23. doi: 10.3390/healthcare6010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Munmun F., Witt-Enderby P.A. Melatonin effects on bone: implications for use as a therapy for managing bone loss. J Pineal Res. 2021;71(1) doi: 10.1111/jpi.12749. [DOI] [PubMed] [Google Scholar]
- 67.Li T., Jiang S., Lu C., et al. Melatonin: another avenue for treating osteoporosis? J Pineal Res. 2019;66(2) doi: 10.1111/jpi.12548. [DOI] [PubMed] [Google Scholar]
- 68.Lu X., Yu S., Chen G., et al. Insight into the roles of melatonin in bone tissue and bone‑related diseases (Review) Int J Mol Med. 2021;47(5):82. doi: 10.3892/ijmm.2021.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cardinali D.P., Ladizesky M.G., Boggio V., Cutrera R.A., Mautalen C. Melatonin effects on bone: experimental facts and clinical perspectives. J Pineal Res. 2003;34(2):81–87. doi: 10.1034/j.1600-079x.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 70.Tian Y., Ming J. The role of circadian rhythm in osteoporosis; a review. Front Cell Dev Biol. 2022;10 doi: 10.3389/fcell.2022.960456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maria S., Witt-Enderby P.A. Melatonin effects on bone: potential use for the prevention and treatment for osteopenia, osteoporosis, and periodontal disease and for use in bone-grafting procedures. J Pineal Res. 2014;56(2):115–125. doi: 10.1111/jpi.12116. [DOI] [PubMed] [Google Scholar]
- 72.Furu K., Wettermark B., Andersen M., Martikainen J.E., Almarsdottir A.B., Sorensen H.T. The Nordic countries as a cohort for pharmacoepidemiological research. Basic Clin Pharmacol Toxicol. 2010;106(2):86–94. doi: 10.1111/j.1742-7843.2009.00494.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.