Abstract
In Turkey, diphtheria-tetanus-acellular pertussis-inactivated poliovirus and Haemophilus influenzae type b (DTaP-IPV-Hib) vaccine has been administered to all children in the second, fourth, sixth and 18th months within the scope of the national vaccination programme. Here we present a rare case of fixed drug eruption (FDE) that occurred as a result of the administration of a pentavalent DTaP-IPV-Hib combined vaccine in a 4-month-old girl. There was no history of taking any other medication before or when the lesion appeared. The lesion responded well to 1 week of topical methylprednisolone aceponate cream application and regressed within 1 week, leaving mild hyperpigmentation. Few cases of FDE have been reported occurring after administration of various vaccines and it is extremely rare in children. To our knowledge, this is the first reported case of FDE developing in an infant after the combined pentavalent DTaP-IPV-Hib vaccine.
Keywords: drug-related side effects and adverse reactions, allergy and immunology, clinical medicine, case reports, pediatrics, primary health care, dermatology, public health
Background
Fixed drug eruption (FDE) is a delayed type of hypersensitivity reaction that usually begins as solitary, well-demarcated, round or oval, slightly itchy macules that turn into erythematous oedematous plaques, regress with hyperpigmentation, and often recur in the same place when exposed to the culprit drug.1 Although FDE has been reported in all ages, it is rarely observed in children. Furthermore, few cases of FDE have been reported following various vaccines. We present a rare case of a FDE that occurred as a result of the administration of a pentavalent DTaP-IPV-Hib combined vaccine, Pentaxim, produced by Sanofi Pasteur in a 4-month-old girl (see online supplementary table S1 for Pentaxim ingredients).
ejhpharm-2021-002875supp001.pdf (55.6KB, pdf)
Case presentation
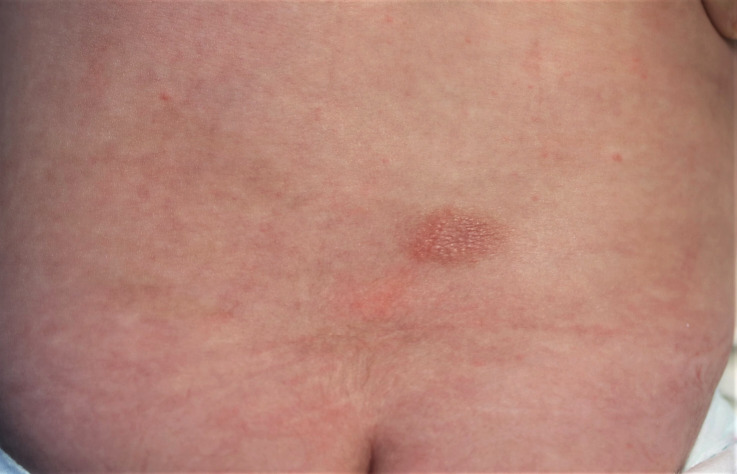
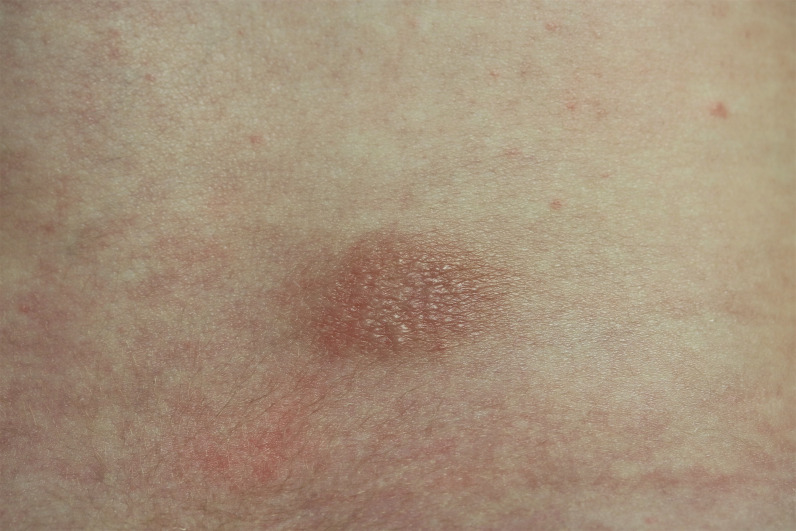
A 4-month-old girl presented with a 2×3 cm diameter, oval, pale erythematous, slightly raised patch lesion on the waist region, accompanied by several 1–2 mm papules on and around the central lesion (figures 1 and 2). The mother reported that this lesion appeared 2–3 weeks after the combined pentavalent DTaP-IPV-Hib vaccine was administered when the baby was 2 months old, and it was erythematous in the beginning, its colour later turning pale brown. Two days after the second dose of combined pentavalent vaccine administered in the fourth month, the present lesion became apparent with an increase of erythema and the formation of papules.
Figure 1.
Oval, erythematous patch lesion on the waist region that occurred during the second recurrence of fixed drug eruption.
Figure 2.
Closer view of the lesion: single, oval, mildly swollen, pale erythematous, slightly raised patch measuring 2×3 cm with several 1–2 mm papules on and around the central lesion.
Investigations
There was no history of taking any other medication before or when the lesion appeared. A clinical diagnosis of FDE was made. The second combined pentavalent DTaP-IPV-Hib vaccine in our patient served as a systemic provocation test and was accepted as positive. Due to the young age of the patient, we did not perform a skin biopsy. In this child, FDE was attributed to the combined pentavalent DTaP-IPV-Hib vaccine. The adverse event associated with this vaccine was confirmed by the Naranjo algorithm, with a total score of 8 representing a “probable adverse drug reaction”.2
Treatment
No lesions were observed in any other region. We explained our clinical definition to the parents and made recommendations. The lesion responded well to 1 week of topical methylprednisolone aceponate cream application and regressed within 1 week, leaving mild hyperpigmentation.
Outcome and follow-up
Since the vaccine must be repeated in the sixth and 18th months to provide full protection and FDE is an innocent reaction, the patient’s parents were informed that the remaining doses of the vaccine could continue. Delayed reactions such as FDE are usually self-limiting conditions that do not contraindicate the administration of future booster doses of the same vaccine.3 Considering that it is an innocent reaction that is classically solitary and repeats in the same localisation, and that it will have the same clinical course in subsequent reactions, the patient’s parents were advised not to attend the hospital during pandemic conditions unless the patient developed a widespread rash.
Discussion
In Turkey, the combined pentavalent DTaP-IPV-Hib vaccine has been administered to all children in the second, fourth, sixth and 18th months within the scope of the national vaccination programme since 2008. Skin reactions caused by vaccines consist of immediate and delayed types of hypersensitivity reactions to one or more vaccine components. Combined pentavalent DTaP-IPV-Hib vaccine contains diphtheria toxoid, tetanus toxoid, pertussis toxoid, fibrous haemagglutinin, inactive poliovirus type 1, inactive poliovirus type 2, inactive poliovirus type 3, Haemophilus influenzae type b together with polysaccharide tetanus protein and aluminium hydroxide as an adjuvant.
FDE is thought to be caused by local induction of CD8+ T cells by the suspected drug, which have a phenotype similar to effector memory T cells and are mainly responsible for epidermal and dermal damage.4 There are a limited number of reported cases of FDE following influenza, human papillomavirus and yellow fever vaccines.5–9 Common components between the vaccines in previously reported cases and DTaP-IPV-Hib vaccine in our case were haemagglutinin,5 7 9 formaldehyde5 7 9 and aluminium hydroxide.6 To our knowledge, this is the first reported case of FDE developing in an infant after the combined pentavalent DTaP-IPV-Hib vaccine. Similar to a previously proposed mechanism, protein components in the vaccine, other vaccine ingredients or cross-reactive proteins within the epidermis may have triggered the hypersensitivity reaction by inducing T cells.10
Adverse reactions may be due to the vaccine excipient and active component (whole organisms or parts of organisms, toxoids) of the vaccine or may be related to host immunodeficiency. Immune-mediated side effects associated with vaccination include IgE mediated (immediate-type), immune complex, T-cell mediated, pseudoallergic and autoimmune/inflammatory types.11 We describe an infant who developed FDE as a cutaneous T-cell-mediated delayed-type hypersensitivity reaction probably to vaccine components. In patients who developed severe urticaria and angioedema, positive skin- and serum-specific IgE test results to tetanus and diphtheria toxoids have been identified following booster injections of toxoid-containing vaccines.12 Rarely, anaphylactic reactions to toxoids have been reported.13 14 Combined pentavalent DTaP-IPV-Hib vaccine contains aluminium hydroxide, tromethamol, formaldehyde and phenoxyethanol as preservatives. The most common clinical manifestation of the reaction to aluminium in vaccines is the development of painful, pruritic nodules at the injection site.15 Also, a few cases of localised or generalised dermatitis have been reported.16 Patients with hypersensitivity to aluminium-containing vaccines usually show a positive patch testing to aluminium.17 Cases of systemic allergic contact dermatitis18 and eczematous dermatitis on the hands19 have been reported with positive patch testing after administration of formaldehyde-containing vaccines. An 18-month-old patient who developed eczematous dermatitis less than 24 hours after two separate administrations of DTaP vaccine was reported and a patch test was positive for phenoxyethanol.20 Since eczematous reactions are T-cell-mediated like FDE, we suggest that our case may have developed FDE against one of these three preservatives in the combined pentavalent DTaP-IPV-Hib vaccine. The actual culprit component can be determined by performing a patch test with all the individual components of the vaccine at standard concentrations. However, a patch test was not performed because the patient was aged under 1 year and the reaction was self-limiting and of a benign nature.
Erythema multiforme, eosinophilic cellulitis, erythema nodosum and anaphylactic reaction after diphtheria, pertussis, tetanus and polio vaccination have been described in a few case reports.21–24 Although FDE caused by the combined pentavalent vaccine is probably rare, clinicians should be aware of this possibility.
Learning points.
This is the first published case report of a fixed drug reaction (FDE) in an infant after the combined DTaP-IPV-Hib pentavalent vaccine.
We emphasise that due to the benign nature of FDE, the patient may be re-vaccinated with the same vaccine.
It is important for both paediatricians and dermatologists to be aware that such a reaction may occur in order to manage parental concerns.
Footnotes
Contributors: SSE: approval of the final version of the manuscript; critical literature review; data collection, analysis and interpretation; manuscript critical review; preparation and writing of the manuscript. FC: approval of the final version of the manuscript; critical literature review; data analysis and interpretation; manuscript critical review; preparation and writing of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Parental/guardian consent obtained.
References
- 1. Lee AY. Fixed drug eruptions. incidence, recognition, and avoidance. Am J Clin Dermatol 2000;1:277–85. 10.2165/00128071-200001050-00003 [DOI] [PubMed] [Google Scholar]
- 2. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239–45. 10.1038/clpt.1981.154 [DOI] [PubMed] [Google Scholar]
- 3. McNeil MM, DeStefano F. Vaccine-associated hypersensitivity. J Allergy Clin Immunol 2018;141:463–72. 10.1016/j.jaci.2017.12.971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shiohara T, Mizukawa Y, Teraki Y. Pathophysiology of fixed drug eruption: the role of skin-resident T cells. Curr Opin Allergy Clin Immunol 2002;2:317–23. 10.1097/00130832-200208000-00005 [DOI] [PubMed] [Google Scholar]
- 5. García-Doval I, Rosón E, Feal C, et al. Generalized bullous fixed drug eruption after influenza vaccination, simulating bullous pemphigoid. Acta Derm Venereol 2001;81:450–1. 10.1080/000155501317208534 [DOI] [PubMed] [Google Scholar]
- 6. Pyl J, Aerts O, Siozopoulou V, et al. Bullous fixed drug eruption following human papilloma virus vaccination. J Eur Acad Dermatol Venereol 2020;34:e697–8. 10.1111/jdv.16377 [DOI] [PubMed] [Google Scholar]
- 7. Byrd RC, Mournighan KJ, Baca-Atlas M, et al. Generalized bullous fixed-drug eruption secondary to the influenza vaccine. JAAD Case Rep 2018;4:953–5. 10.1016/j.jdcr.2018.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sako EY, Rubin A, Young LC. Localized bullous fixed drug eruption following yellow fever vaccine. J Am Acad Dermatol 2014;70:e113–4. 10.1016/j.jaad.2013.11.040 [DOI] [PubMed] [Google Scholar]
- 9. Al-Mutairi N, Al-Fouzan A, Nour-Eldin O. Fixed drug eruption due to influenza vaccine. J Cutan Med Surg 2004;8:16–18. 10.1177/120347540400800104 [DOI] [PubMed] [Google Scholar]
- 10. Katoulis AC, Liakou A, Bozi E, et al. Erythema multiforme following vaccination for human papillomavirus. Dermatology 2010;220:60–2. 10.1159/000254898 [DOI] [PubMed] [Google Scholar]
- 11. Fritsche PJ, Helbling A, Ballmer-Weber BK. Vaccine hypersensitivity-update and overview. Swiss Med Wkly 2010;140:238–46. [DOI] [PubMed] [Google Scholar]
- 12. Ponvert C, Scheinmann P, Karila C. L’allergie aux vaccins associés chez l’enfant. Une étude de 30 cas fondée sur les tests cutanés lecture immédiate, semi-retardée et retardée, sur les dosages des anticorps spécifiques et sur les injections de rappel. Rev Fr Allergol Immunol Clin 2001;41:701–11. [Google Scholar]
- 13. Cody CL, Baraff LJ, Cherry JD, et al. Nature and rates of adverse reactions associated with DTP and DT immunizations in infants and children. Pediatrics 1981;68:650–60. [PubMed] [Google Scholar]
- 14. Pollock TM, Morris J. A 7-year survey of disorders attributed to vaccination in North West Thames region. Lancet 1983;1:753–7. 10.1016/S0140-6736(83)92037-8 [DOI] [PubMed] [Google Scholar]
- 15. García-Patos V, et al. Persistent subcutaneous nodules in patients hyposensitized with aluminum-containing allergen extracts. Arch Dermatol 1995;131:1421–4. 10.1001/archderm.131.12.1421 [DOI] [PubMed] [Google Scholar]
- 16. Cox NH, Moss C, Forsyth A. Allergy to non-toxoid constituents of vaccines and implications for patch testing. Contact Dermatitis 1988;18:143–6. 10.1111/j.1600-0536.1988.tb04500.x [DOI] [PubMed] [Google Scholar]
- 17. Bergfors E, Björkelund C, Trollfors B. Nineteen cases of persistent pruritic nodules and contact allergy to aluminium after injection of commonly used aluminium-adsorbed vaccines. Eur J Pediatr 2005;164:691–7. 10.1007/s00431-005-1704-1 [DOI] [PubMed] [Google Scholar]
- 18. Kuritzky LA, Pratt M. Systemic allergic contact dermatitis after formaldehyde-containing influenza vaccination. J Cutan Med Surg 2015;19:504–6. 10.1177/1203475415582306 [DOI] [PubMed] [Google Scholar]
- 19. Ring J. Exacerbation of eczema by formalin-containing hepatitis B vaccine in formaldehyde-allergic patient. The Lancet 1986;328:522–3. 10.1016/S0140-6736(86)90397-1 [DOI] [PubMed] [Google Scholar]
- 20. Vogt T, Landthaler M, Stolz W. Generalized eczema in an 18-month-old boy due to phenoxyethanol in DPT vaccine. Contact Dermatitis 1998;38:50–1. 10.1111/j.1600-0536.1998.tb05644.x [DOI] [PubMed] [Google Scholar]
- 21. Kaur S, Handa S. Erythema multiforme following vaccination in an infant. Indian J Dermatol Venereol Leprol 2008;74:251–3. 10.4103/0378-6323.41373 [DOI] [PubMed] [Google Scholar]
- 22. AM Y, Ito S, Leibson T. Pediatric Wells syndrome (eosinophilic cellulitis) after vaccination: a case report and review of the literature. Pediatr Dermatol 2018;35:e262–4. [DOI] [PubMed] [Google Scholar]
- 23. Cohen PR. Combined reduced-antigen content tetanus, diphtheria, and acellular pertussis (tdap) vaccine-related erythema nodosum: case report and review of vaccine-associated erythema nodosum. Dermatol Ther 2013;3:191–7. 10.1007/s13555-013-0035-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martín-Muñoz MF, Pereira MJ, Posadas S, et al. Anaphylactic reaction to diphtheria-tetanus vaccine in a child: specific IgE/IgG determinations and cross-reactivity studies. Vaccine 2002;20:3409–12. 10.1016/S0264-410X(02)00228-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ejhpharm-2021-002875supp001.pdf (55.6KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.