Abstract
The Timber Rattlesnake (Crotalus horridus) is the largest pit viper in the Northern United States and is the prominent venomous snake species indigenous to the bluff land habitats of the Upper Mississippi River Valley (UMRV). Conservation of C. horridus in this geographic region not only preserves the ecosystem's biodiversity and ecological balance, but also assures the continued study of their biomedically important venoms/toxins. Field studies of C. horridus biology and natural history performed from 1985 to 2015 in southeastern Minnesota and western Wisconsin along the Mississippi River showed populations have declined. Consequently, the implementation of improved conservation measures afforded the species protective status in both states. Historically, accounts of Timber Rattlesnake bites in the UMRV have been sparse, and medical consequences of envenomation have had limited documentation. However, in recent decades cases of envenomation by C. horridus have continued to occur. Retrospective analysis of clinical toxinology consultations documented from 1982 to 2020 on cases of envenomation by C. horridus in the UMRV revealed a very low incidence of bites annually and revealed that their venom can induce a rapid and precipitous decline in platelets.
Keywords: Crotalus horridus, Crotaline, Ecology, Envenomation, Human-snake conflict, Thrombocytopenia
Graphical abstract
Highlights
-
•
Crotalus horridus research in the Upper Mississippi River Valley (UMRV) showed populations have declined from 1982 to 2020.
-
•
A novel genetic maternal haplotype appears to have been discovered in the Upper Mississippi River Valley.
-
•
Crotalus horridus envenomation (29 cases) was documented in the Upper Mississippi River Valley from 1982 to 2020.
-
•
Crotalus horridus cases of envenomation appear to have declined in the Upper Mississippi River Valley.
-
•
Thrombocytopenia was the most frequent systemic complication of Crotalus horridus envenomation in the UMRV.
1. Introduction
The Timber Rattlesnake (Crotalus horridus) (Fig. 1), originally described by Linnaeus (1758) is indigenous to the eastern half of the United States and is one of the few rattlesnake species in North America that can cause life-threatening and uncommon fatal envenomations (Gallagher et al., 2023; Brown et al., 2022; Carroll et al., 1997; Joslin et al., 2014; Kitchens et al., 1987; Langley, 2009; Morgan et al., 2004). C. horridus was the first rattlesnake species encountered by early settlers during their forays into the wilderness of northeastern North America (Palmer, 1992). The species once ranged from southeastern Canada south to Florida and westward to Minnesota and south to Texas (Clark et al., 2003). The Upper Mississippi River Valley (UMRV) is a region of the Mississippi River encompassed by western Wisconsin on the river's eastern shoreline and Minnesota on its western shoreline. C. horridus primarily live in the bluff land habitat adjacent to the river corridor. Timber Rattlesnake presence in the region was first reported by Father Hennepin in 1680 during his voyages up the Mississippi River (Schoerger, 1967-68). The snake has been known by several descriptive common names; Banded Rattlesnake, Bluff Rattler, Velvet Tail, Mountain and Rock Rattlesnake, and the Midewankaton Sioux Indians called them “sin-tah-da” (yellow rattlesnakes) (Schoerger, 1967-68). Native American Indians and European settlers obviously had physical encounters with Timber Rattlesnakes in the UMRV; however, historically, confirmed cases of envenomation have been minimally documented (Babcock, 1928; Palmer, 1992; Schoerger, 1967-68). It's been over six centuries since the arrival of European settlers in the region, and despite multiple human inflicted insults to habitat and human persecution, the species has managed to survive (Babcock, 1928; Clurman, 1989; Sajdak and Berg, 2005). In Minnesota and Wisconsin monetary bounties were paid well into the 20th century for the killing of timber rattlesnakes. Thousands of snakes killed, combined with the loss of habitat from agricultural and human encroachment resulted in significantly depleted populations (Keyler, 2021a, 2021b; Oldfield and Keyler, 1989; Sajdak and Berg, 2005). In the final decade of the 20th century C. horridus was afforded protected status in the UMRV states of Minnesota and Wisconsin (Keyler, 2021a, 2021b). Unfortunately, despite their current protected status predation by humans has continued, and humans are uncommonly bitten by timber rattlesnakes, which likely leads to fear influencing people's negative perspectives and behavior (Eiksund, 2009). Consequently, C. horridus populations have significantly declined throughout their range. Multiple surveys by academic biologists during the 1900s and early 2000s in 37 US states have revealed that C. horridus has been extirpated from the states of Delaware, Maine, Michigan, and Rhode Island, and has been determined to be extinct in Canada (Ontario and Quebec) since the 1950s (Breisch et al., 2021). The preservation of venomous snake species is important for their substantial contributive roles in the maintenance of balance and diversity in ecosystems, functioning as essential predators responsible for control of local small mammal populations. Importantly, and relevant to toxinology, their venoms provide an array of toxins that serve as effective scaffolds for drug design and development and are valuable in biomedical and scientific research (Peigneur and Tytgat, 2018; Clark et al., 2019; El-Aziz et al., 2019).
Fig. 1.
Adult male Timber Rattlesnake (Crotalus horridus; Viperidae: Crotalinae) from Houston County, Minnesota. Geographic range in North America and Upper Mississippi River Valley (outlined).
A limited number of C. horridus envenomations to humans in the UMRV showed pronounced thrombocytopenia (abnormally low platelet levels), a documented important medical complication following Timber Rattlesnake envenomation in the eastern US (Schmaier and Coleman 1980; Bond and Burkhart, 1997; Odeleye et al., 2004; Trautman and Pizon, 2023). This venom-induced effect may be attributable to the platelet activator, crotalocytin, present in the venom of C. horridus from the northeastern US (Schmaier and Coleman 1980). Interestingly, thrombocytopenia has been reportedly absent in envenomated patients treated in parts of the southeastern United States (U.S.) where the species is commonly identified as the Canebrake Rattlesnake (Carroll et al., 1997; Gold et al., 2004; Kerns and Tomaszewski, 2001). Thus, the study of C. horridus venom composition has shown intraspecific and geographical variability (Straight et al., 1991; Glenn and Straight, 1994; Wang et al., 2010). The documentation of this medical complication involving envenomation cases in the UMRV (the extreme northwestern portion of C. horridus geographic range) has been minimal with only three bites by Timber Rattlesnakes having been reported in Minnesota, and 15 rattlesnake bites in Wisconsin in the mid-twentieth century, respectively (Parrish, 1965a, 1965b). The case data reported here confirm C. horridus envenomations in the UMRV have occurred in recent years in Minnesota and Wisconsin and provides current documentation of thrombocytopenia being an important venom-induced pathophysiological effect in this geographic region.
Reported here are extensions of C. horridus research regarding the biology, conservation, and natural history of the species, and cases of envenomation in the UMRV of Minnesota and Wisconsin from the closing decades of the 20th century and beginning of the 21st century (Cochran, 2008; Keyler, 2008, 2021a, 2021b).
2. Methods
2.1. Biology
From 1985 to 2015 the author studied the geographic distribution and biology of C. horridus in the UMRV of Minnesota and Wisconsin with funding from federal and state research grants (United States Fish and Wildlife Service; Minnesota Nongame Wildlife Program; Lois Almon Small Grants Research Program, Wisconsin Academy of Arts and Letters). Field surveys were completed on foot from sunrise until dark from April through October in six southeastern Minnesota counties and four western counties of Wisconsin that were immediately adjacent to the Mississippi River (Keyler, 2021a, 2021b; Oldfield and Keyler, 1989). Survey dates were selected to coincide with the snakes approximated active season, ranging from potential spring emergence in April through ingress for hibernation in October. Survey sites were selected based on personal communications with older retired C. horridus bounty hunters, prior C. horridus observations and reports from private landowners, and state natural resource department personnel. Additional survey sites were selected based on C. horridus habitat features (Brown, 1993). Data regarding C. horridus hibernation ingress and egress dates, number of snakes studied, demographics, and mitochondrial DNA haplotype were collected. Snakes were captured with FurMont Snake Hooks (Furhman Diversified, Inc., LaPorte, Texas, USA), and were restrained in a clear plexiglass Restraining Tube (Midwest Tongs, Inc., Greenwood, Missouri, USA) for general physical examination and sex determination with the use of Sexing Probes (Midwest Tongs, Inc., Greenwood, Missouri, USA). A caudal vein blood sample (0.1 mL) was collected by intravenous puncture (snakes were not anesthetized due to sampling in remote field habitat) with a 22-gauge needle for genetic haplotype (mitochondrial DNA - mtDNA) determination. Tissue was harvested from a separate single fresh road killed specimen. Samples were preserved in lysis buffer and maintained at room temperatures. Genotypic assays were performed at the BEECS Genetic Analysis Lab, University of Florida, Gainesville, Florida. Mitochondrial haplotypes from two individuals were assembled and their mtDNA sequences compared to known haplotypes from 123 C. horridus specimens from across the species range. Analysis was per the methods of Clark (Clark et al., 2003). All specimens manually processed for biological measurements and sample collection and were released at the site of capture following processing. Treatment of all snakes were per Guidelines for Use of Live Amphibians and Reptiles in Field Research (Society for the Study of Amphibians and Reptiles; http://www.asih.org/publications).
2.2. Conservation
The author has been directly involved with the development of State and federal government conservation recovery plans (Breisch et al., 2021; Keyler, 2021a, 2021b; Timber Rattlesnake Recovery Team, 2009). Documents were reviewed for details regarding conservation measures achieved and the conservation status of Timber Rattlesnakes in Minnesota and Wisconsin, and economic bounties paid on Timber Rattlesnakes. Public education seminars to landowners with C. horridus habitat and snakes on their property were presented.
2.3. Envenomation
Crotalus horridus documented envenomation cases from the author's toxicology consultation records (1982–2020) from Hennepin County Medical Center, Minneapolis, Minnesota, USA, and the University of Minnesota, Minneapolis, Minnesota were retrospectively reviewed. Snake identification was confirmed in all cases by the author by actual visual inspection of the responsible snake, or visual inspection by other expert herpetologists. Case details and data reported were extracted from records that involved direct toxicology consultation with the author with Minnesota and Wisconsin emergency departments and hospitals.
Data collected included the geographic location where the snakebite occurred in Minnesota and Wisconsin, victim age, sex, anatomical location of bite, previous medical history including previous snakebite or antivenom exposure, and alcohol consumption around the time of the bite. Activity as to how the bite occurred and circumstances of the bite were also recorded. Symptoms were not scored using snakebite severity score criteria, but a dry bite with no envenomation was defined by the presence of fang marks and absence of symptoms other than mild erythema from fang puncture localized trauma, no pain, no swelling, and no progression or development of any symptoms over an observation period from 4 to 24 h. Clinical envenomation was evidenced by fang marks, in association with progressive swelling, ecchymosis, and pain, with alterations in platelet counts. In patients requiring antivenom therapy the number of vials administered and type of antivenom were documented. IgG antivenom (IgG AV) administered was Antivenin (Crotalidae) Polyvalent (equine) - ICP; Wyeth Laboratories, Marietta, PA, USA) a polyspecific antivenom derived using the venoms of Crotalus adamanteus (Eastern diamondback rattlesnake), Crotalus atrox (Western diamondback rattlesnake), Crotalus durissus terrificus (Tropical rattlesnake), and Bothrops atrox (Fer-de-lance) as immunizing venoms. Fab antivenom (Fab AV) administered was Crotalidae polyvalent immune Fab (ovine) - CroFab®; BTG International, West Conshohocken, PA, USA) is a polyspecific antivenom derived using the venoms of Crotalus adamanteus (Eastern diamondback rattlesnake), Crotalus atrox (Western diamondback rattlesnake), Crotalus scutulatus (Mojave rattlesnake), and Agkistrodon piscivorus (Cottonmouth or Water Moccasin) as immunizing venoms. Decision as to which antivenom was used was limited by the fact that IgG AV was the only antivenom FDA approved and available for use until 2001 when Fab AV entered the market. However, Fab AV was not routinely stocked in hospital pharmacies in the UMRV region until previous lots of IgG AV were exhausted in 2008-9, at which time Fab AV use began. Additional complications such as infection or surgical intervention, and the duration of hospitalization were also recorded.
Platelet counts were selected as a monitoring parameter for severity of envenomation and its progression. Rationale for the focus on platelets is based on prior studies that showed C. horridus venom has a pronounced aggregating and consumptive effect on platelets (Odeleye et al., 2004; Schmaier and Colman, 1980). Significant thrombocytopenia is a common feature in patients envenomed by C. horridus in the northeastern USA where the species is indigenous (Bond and Burkhart, 1997; Gold et al., 2004; Trautman and Pizon, 2023). Platelet counts were performed on venous blood drawn immediately after patient presentation to the emergency department as well as prior to the administration of antivenom or other interventions. Values reported for each patient were confirmed with the respective accredited hospital laboratories that performed the platelet counts. The decline in platelet counts and the time post envenomation of this venom-induced effect were documented. Thrombocytopenia was defined as a platelet count ≤150 × 103/mm3.
3. Results
3.1. Biology
A total of 37 sites were surveyed in six southeastern Minnesota counties (1985–2015) with 121 C. horridus observed at 18 sites. Survey of 16 sites in four western Wisconsin counties (1988–2020) yielded the presence of only 53 C. horridus at 9 sites. The active season, based on the earliest egress and ingress dates, ranged from 18 April to 16 October. The male:female sex ratio (n = 31 m, n = 36 f) approximated unity, sexual dimorphism showed adult males being larger than females (mean body mass M 974 g v. 586 g respectively; mean body length from tip of snout to base of rattle was 110 cm v. 90 cm respectively). The largest specimen was 1.4 m (4.5 ft) in length (2.2 kg). Genetic haplotype determined from maternal mtDNA was designated (E) and was the most common as evidenced in specimens (n = 6) from two Minnesota counties. Findings from two different specimens from a single Minnesota county support the presence of a potentially new 21st haplotype representing the first documentation of this haplotype. Geographic distribution, habitat, and population number findings; collectively confirm a significant reduction in all these parameters compared to historical records. Fragmentation of habitat and associated populations, of what was once contiguous habitat that allowed for gene flow between populations has appeared to be a potential factor that has contributed to population declines.
3.2. Conservation
The author personally spoke at the Minnesota State Legislature, Minneapolis, Minnesota in early 1989 and presented data confirming the need for the removal of the economic incentive to kill C. horridus in the state. The author spoke at a county legislative meeting in LaCrosse, Wisconsin in 1991 presenting data supporting the need for protection of C. horridus in the state. Rattlesnake Bounty systems were repealed in 1975 in Wisconsin and 1989 in Minnesota. Threatened Species and protected status was formally instated via legislative action in 1996 in Minnesota. The snake became a protected wild animal in 1998 in Wisconsin via legislative action, and currently is considered a Species of Greatest Conservation Need and Special Concern. The implementation of these legal statutes made it illegal to capture, possess, or kill C. horridus unless there is endangerment to life. Habitat protection and restoration has been accomplished in the Minnesota counties that border the Mississippi River following the development of the Timber Rattlesnake Recovery Plan, April 2009. River bluff C. horridus and their habitats in Wisconsin have ongoing studies to support the protection and management of critical habitat. Collectively, the encroachment of human developments (housing, golf courses, paved roads, increased automobile traffic) and human predation by many individuals who see the snake as a dangerous threat to human life continue to threaten the potential long-term survival of the species in the Upper Mississippi River Valley. Education at all levels and of private landowners who have Timber Rattlesnake habitat and snakes on their property has been an important step toward the maintaining existing populations. The completion of conservation action plans for states with Upper Mississippi River shorelines have also been completed (Peterson and Sealy, 2021; Timber Rattlesnake Recovery Team, 2009).
3.3. Envenomation
The cases reported here may not represent all C. horridus bites and envenomations that occurred in the Upper Mississippi River Valley of Minnesota and Wisconsin from 1982 to 2020, as toxicology consultation by the author was likely not requested in all cases. However, the data presented were derived from the largest known collection of confirmed cases in the region.
3.3.1. Envenomation epidemiology and victim demographics
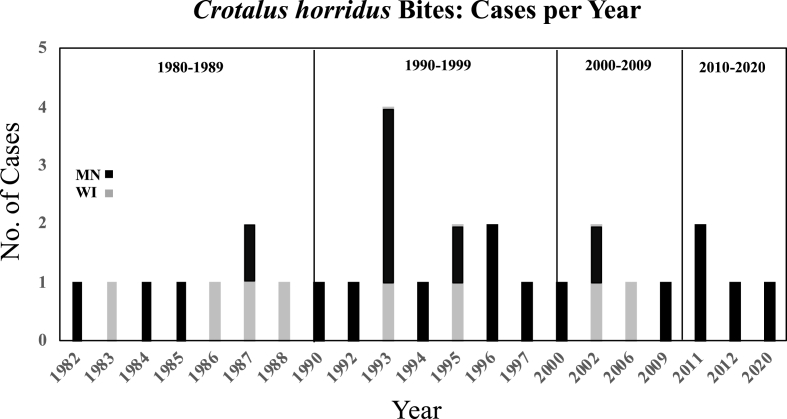
A total of 29 cases of Timber Rattlesnake bites to humans (21 Minnesota and 8 Wisconsin), were consulted on by the author over the 38-year interval from 1982 to 2020. The data collected during these four decades revealed a low incidence for both states. Other than four bites that occurred in 1993, there were only one or two consults provided per annum. There was also a decline in the number of bites during the first two decades of the 21st century (Fig. 2). Circumstances of bites involved victims 16–52 years of age who were engaged in various indoor and outdoor activities (Table 1). The majority (86%) of envenomations involved males, with bare handling by males having accounted for 31% of bites to males (Fig. 3). Geographic locality of bite occurrence reflects approximately one quarter of bites were sustained in a metropolitan area, all involved males and bare handling with three victims having consumed alcohol, all of which had a prior history of venomous snakebites (Fig. 3). The 20–29 and 30-39-year-old age decade individuals represented the majority of individuals bitten (Fig. 3).
Fig. 2.
Chronology of Crotalus horridus bites: Minnesota (MN) and Wisconsin (WI), Upper Mississippi river Valley (1982–2020).
Table 1.
Age Range, Gender, and Activity at the time of the bite: Timber Rattlesnake Bite Victims in the Upper Mississippi River Valley (1982–2020).
| State | Victim Age Range (yrs) | Gender | (n) | Activity |
|---|---|---|---|---|
| Minnesota | 16–52 | F | 1 | Hiking |
| M | 9 | Bare handling | ||
| M | 6 | Farming activities accidental | ||
| M | 4 | Driving, fishing, photography | ||
| M | 1 | Telephone lineman | ||
| Wisconsin | 16–51 | F | 3 | Hiking, mowing*, stepped on |
| M | 3 | Farming activities accidental | ||
| M | 1 | Bare handling | ||
| M | 1 | Searching for golf ball |
*Pregnant, F = female, M = male.
Fig. 3.
Timber Rattlesnake bite victim demographics in the Upper Mississippi River Valley (1982–2020): ^3 male victims with history of prior bites (2 Crotalus horridus; 1 Agkistrodon contortrix); *3 male victims with alcohol intoxication and bare handling the snake (ethanol levels unconfirmed).
3.3.2. Clinical manifestations of envenomation
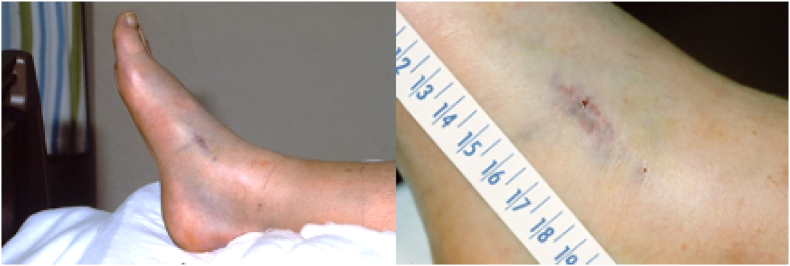
Clinically, as observed in the ED of eleven different hospitals, 26 victims had positive evidence of fang punctures, and the most frequent observed signs and symptoms were pain, swelling, and ecchymosis (Fig. 4, Table 2). Two individuals chose not to go to a medical facility as fangs had only penetrated the thickness of a leather boot and barely grazed the skin of the first victim, and despite the presence of fang punctures in a second victim no apparent symptoms developed. As such, 27 human victims of confirmed C. horridus bites were seen in a medical facility with 9 having been released from the ED the same day, 2 signed out against medical advice, and 15 required hospital admission (13 treated with antivenom) (Table 2). Neurotoxic symptoms were not observed in any patients. From 1982 through 2002, IgG AV was used in the treatment of ten patients in doses ranging from 4 to 20 vials, and from 2011 to 2020 three patients were treated with Fab AV doses ranging from 10 to 22 vials. Two patients had surgery based on the observation of extensive swelling, one prior to IgG AV treatment (not per toxicology consultation), and the other at 4 h after IgG AV treatment; 9 h post envenomation (compartment pressures not measured). The length of hospital stay for the admitted thirteen patients ranged from 2 to 12 days. A single fatality resulted from a severe intravenous envenomation that was confirmed postmortem, revealing that two fang punctures had penetrated the popliteal vein. The case was further complicated by significantly delayed transport from a rural location; the victim expired prior to arrival at the hospital.
Fig. 4.
Fang punctures, ecchymosis, and swelling following an envenoming bite from a 1.2 m Crotalus horridus on the right foot. The victim was a golfer who was bitten while searching for his lost golf ball in the fairway: Upper Mississippi River Valley, Pierce County, Wisconsin.
Table 2.
Signs, symptoms, thrombocytopenia, antivenom use and length of hospital admission: Upper Mississippi river valley (1982–2020).
|
Signs-Symptoms, Lab Abnormalities |
No. Patients | Antivenom (# vials) | Length of Admission (days) |
|---|---|---|---|
| FP | 2 | 0 | Did not go to hospital |
| FP | 2 | 0 | Same Day - left AMA |
| FP, Dry bites | 9 | 0 | Same Day ER discharge |
| FP, R, C, I | 1 | 0 | 10 - Infection – Antibiotics |
| FP, P, S, I | 1 | Unk | Unk – Streaking up arm – Antibiotics |
| FP, P, S, E |
1 |
0 |
0 - Fatality – intravenous bite |
|
IgG | |||
| FP, P, S, E | 1 | 4 | 2 |
| FP, P, S, E | 1 | 5 | 2 |
| FP, P, S, E | 1 | 10 | 2 |
| FP, P, S, E, T | 1 | 10 | 3 |
| FP, P, S, E, T | 1 | 10 | 11 Persistent thrombocytopenia |
| FP, P, S, E, T | 1 | 10 | 3 |
| FP, P, S, E, T | 1 | 10 | 3 |
| FP, P, S, E, T | 1 | 10 | 7+ Surgery (hand-forearm bite) |
| FP, P, S, E | 1 | 13 | 12 Surgery (hand bite) |
| FP, P, S, E, T |
1 |
20 |
5 |
|
Fab | |||
| FP, P, S, E, T | 1 | 10 | 3 |
| FP, P, S, E, T | 1 | 18 | 11 Recurrent thrombocytopenia |
| FP, P, S, E, T | 1 | 22 | 5 |
AMA = against medical advice, FP = fang punctures, P = pain; R = redness, S = swelling, E = ecchymosis.
I = infection, T = thrombocytopenia, C = cellulitis.
IgG = aWyeth Antivenin (Crotalidae) Polyvalent; Fab = Crotalidae polyvalent immune fab (ovine).
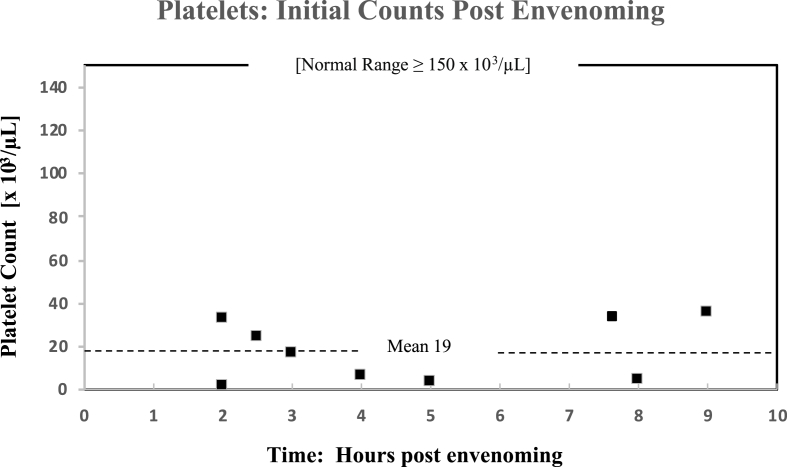
Thrombocytopenia was confirmed in the laboratory investigations obtained from 9/15 patients on ED admission. All the nine patient platelet counts were <40 × 103/mm3 (>73% reduction based on a normal range ≥150 × 103/mm3). The mean reduction in platelets (n = 9) was 19 × 103/mm3 (range 2–40 × 103/mm3). The lowest platelet count obtained in the initial investigations was 2 × 103/mm3 (≈90% reduction); the patient had been bitten <2 h prior to presentation (Fig. 5). One patient had a prolonged course of recurrent thrombocytopenia, which was likely related with the use of Fab AV. Another patient that was treated with IgG AV demonstrated persistent thrombocytopenia, which is uncommon with the use of whole IgG AV. Both patients required 11+ days for resolution of thrombocytopenia. Two patients had surgical interventions performed in addition to antivenom treatment (Table 2).
Fig. 5.
Crotalus horridus venom-induced precipitous platelet decline (thrombocytopenia) in nine individual patients following presentation to the ED and prior to any treatment: Upper Mississippi River Valley (1982–2020). Each plot represents an individual patient's platelet count at the time the sample was drawn post envenoming.
4. Discussion
4.1. Biology and conservation
Coexistence between humans and rattlesnakes is complex, and the Timber Rattlesnake continues to face serious long-term survival challenges across its geographic range (Brown et al., 2007; Martin et al., 2008; Palmer, 1992). The UMRV is the northwestern limitof the geographic range of C. horridus (Furman, 2007). The climactic extremes of this region are challenging for survival and are compounded by the increasing presence of humans and their related activities causing loss and fragmentation of habitat in the UMRV. Collectively, these pressures have significantly reduced the ability of the species to regionally recover historically recorded distribution and population levels. Unfortunately, the snakes do not alter their historical movement patterns to avoid roads (Sajdak and Berg, 2005; Reinert et al., 2011). Although observations of C. horridus swimming are rare it has been observed swimming across the Mississippi between states in the southern portion of its range (George, 2010). Additionally, climate change and fungal disease have become emerging threats (Clark et al., 2011; Lorch et al., 2016). Surveys in the UMRV during the recent decades reported here show that the distribution of C. horridus has been reduced by approximately 50%. Collectively, studies have revealed that C. horridus is continuing to lose its foothold in the Upper Mississippi River Valley (Cochran, 2008; Keyler, 2021a, 2021b; Sajdak and Berg, 2005). The numbers of snakes at multiple preferred habitat sites surveyed appear to have declined compared to historical accounts (personal communication with retired bounty hunters) and bounty records revealed that large numbers of C. horridus were killed purely for direct economic reasons. During a 15-year period 28,685 snakes were bountied from 1967 to 1982 in Minnesota, and in a single Wisconsin county 10,918 were killed for bounty in 1964 and 12,038 were bountied in 1965 (Keyler, 2021a; Keyler, 2021b; Schoerger, 1967-68). Although conservation measures protecting the species have been implemented, the long-term survival of C. horridus in the UMRV bluff lands is precarious due to human activities that continue to reduce unaltered natural habitat and collateral losses from human persecution.
Information regarding the genetics of C. horridus is limited (Clark et al., 2003; Hall et al., 2013; Bushar et al., 2014), and that of UMRV populations is nearly non-existent. Fragmentation of habitat has disrupted the physical interaction of snakes that should occur between different populations along the Mississippi River, and as such gene flow is lost with the consequent loss of genetic diversity (Clark et al., 2008). The “E” haplotype is one of 16 haplotypes that comprise the E lineage, which is the most common lineage west of the Mississippi River. A 21st haplotype that is different than the 20 known mitochondrial DNA (mtDNA) haplotypes was discovered in Minnesota; however, further sampling is needed to determine the abundance and geographic distribution of female C. horridus that harbor this specific mtDNA (Clark et al., 2003). It may potentially be an important contributor to enriching genetic diversity for the species in the region. However, it's contribution to diversity is lost if the snakes are unable to have contact and exchange genetics between fragmented ‘island’ populations that are separated by great distances due to loss of contiguous habitat (habitat favorable for prey, foraging, hibernation, reproduction, logging operations, or heavily trafficked roads) (Brown, 2016; Bushar et al., 2014; Clark et al., 2008, 2011; Reinert et al., 2011). Future genetic research with C. horridus in the Upper Mississippi River Valley would provide for further development of species protection strategies; identify C. horridus in the region that possess novel venom properties; emphasize the implications and significance of genetic diversity in relation to the importance of contiguous favorable habitat, and assure the maintenance of a diverse and sustainable ecosystem within the UMRV.
4.2. Epidemiology and victim demographics
Crotalus horridus is one of only two venomous snake species native in the UMRV; the second species being the Eastern massasauga (Sistrurus catenatus) (Keyler, 2008). The incidence of Timber Rattlesnake envenomations in the UMRV of Minnesota and Wisconsin has been reportedly low (Parrish, 1965a, 1965b; Seifert et al., 2009). Substantial risk of rattlesnake bites would not be anticipated in the UMRV given the rarity of C. horridus and infrequent encounters with humans because of the tendency of this species to inhabit habitats with limited accessibility. Remarkably, the risk of a venomous snakebite in the UMRV is quite low at 1–2/annum, while in contrast there were >20,000 car/motorcycle collisions with deer during 2017 (78 suffering incapacitating injuries and 9 fatalities) (Wisconsin Department of Transportation, 2017). The UMRV cases reported here showed that males (86%) and adults >20 yrs of age were victim characteristics consistent with those reported for venomous snakebite victims in the U.S. in general, 77% males and 70% adults, respectively (Seifert et al., 2009). From an international perspective, similar victim characteristics with males have also been reported in Australia, suggesting that young male victims exhibit more risky behaviors (Welton et al., 2017). Interesting, is the decline in the number of consulted cases by decade from 1982 to 2020. Although, it is quite possible the author was not contacted regarding every case of C. horridus envenomation, the downward trend over the 38-year period probably reflects the loss of C. horridus habitat from human encroachment into snake habitat and human predation, and the apparent decline in C. horridus population numbers despite protective measures. Consequently, there were fewer human-snake encounters, and fewer snakebite victims.
4.3. Clinical manifestations of envenomation
In 1997 the phenomenon of profound thrombocytopenia as a specific hematologic consequence of C. horridus envenomation was reported in a series of cases from the northeastern U.S. (Bond and Burkhart, 1997). This venom-induced medical complication has also been reported to result from envenomation by species of several other genera such as Daboia siamensis, Macrovipera lebetina, and Bothrops asper (Alape-Girón et al., 2009; Abukamar et al., 2022; Zhang et al., 2022). In the UMRV during the late 20th century a limited number of cases have been noted (Keyler, 2008). Current documentation of early thrombocytopenia following C. horridus envenomation as reported here has now demonstrated the consistency of this symptom in cases of C. horridus envenomation in the UMRV, expanding the total number of documented cases into the 21st century (Fig. 5). Interestingly, based on multiple clinical observations of patients envenomed in some southeastern regions of the U.S. thrombocytopenia does not appear to develop in envenomed patients, but rather neurotoxic symptoms were reported predominant (Carroll et al., 1997; Madey et al., 2013). Thus, geographic intraspecific variations in venom composition can be correlated with pathophysiological clinical complications of envenomation that are observed in defined geographic locales (Bond and Burkhart, 1997: Carroll et al., 1997; Clark et al., 2015; Glenn and Straight, 1994). Historically, intraspecific venom variation in C. horridus was reported by Minton (1967), and geographic variation in other rattlesnake species venom composition has been reported in Crotalus atrox and Crotalus scutulatus, Crotalus pyrrhus, and Crotalus helleri ranging across the southern and southwestern U.S. (Minton, 1967; Minton and Weinstein, 1986; Glenn and Straight, 1989; Sunagar et al., 2014; Cochran et al., 2019). More recent genetic phenotypic studies have further illustrated the intraspecific differences in C. horridus venom throughout most of its range (Rokyta et al., 2013, 2015).
C. horridus venom contains hemorrhagic and neurotoxic properties that vary depending on the geographic range of different C. horridus populations (Rokyta et al., 2015; Soto et al., 1989). A specific C. horridus venom toxin, crotalocytin, is a unique serine protease and potent platelet activator that induces platelet aggregation contributing to a consumptive coagulopathy (Schmaier and Colman, 1980; Offerman et al., 2003; Odeleye et al., 2004). Although this venom toxin has not specifically been confirmed present in C. horridus of the UMRV, given the evidence of mtDNA maternal linkage with C. horridus from the northeastern U.S. in conjunction with the rapid development of thrombocytopenia observed clinically in envenomated patients in both geographic regions, crotalocytin is a venom component in C. horridus of the UMRV that potentially contributes to the pronounced thrombocytopenia (Bond and Burkhart, 1997; Clark et al., 2003; Trautman and Pizon, 2023).
Timber Rattlesnake envenomation has been reported to result in both prolonged and recurrent thrombocytopenia (Gold et al., 2004; Odeleye et al., 2004; Trautman and Pizon, 2023). This complication was observed in two cases in which the author was consulted (Table 2). In the case with persistent thrombocytopenia, the 32-year-old male had received a bite that at 2 h post bite appeared as only scratches on the dorsum of his right hand. On ED admission at 2 h post bite his platelets were 33 × 103/mm3. He was treated with 10 vials of IgG AV and six units of platelets. Despite these treatments the platelet values fluctuated from 14 × 103/mm3 (nadir) to 47 × 103/mm3 during hospitalization without any evidence of spontaneous bleeding. The patient was discharged from the hospital on day eleven with a platelet value of 147 × 103/mm3. Management of the case with recurrent thrombocytopenia was confounded due to varied consultations from different sources, but the case clearly illustrates the persistence of recurrent thrombocytopenia. The second case involved a 28-year-old male that was bitten on the ankle (medial malleolus) when he stepped on the snake and the bite appeared as two deep fang punctures. Platelets were 206 × 103/mm3 at 2100 h on the evening of ED admission and then dropped by late the next morning to 137 × 103/mm3. He was administered a total of 10 vials of Fab AV and discharged on day three, platelets 161 × 103/mm3. The patient was readmitted on day four post bite with persistently declining platelets (nadir 5 × 103/mm3) and was administered fourteen more vials of antivenom over the next four days. Following a total of 28 vials of Fab AV he was discharged on day 8 (not per toxicology consult), platelets 17 × 103/mm3. Close outpatient follow-up with daily platelet level monitoring showed that platelet levels did not return to normal until fifteen days post envenomation (141 × 103/mm3). Interestingly, in either case, the INR never exceeded 1.1, PTT >30 s, and fibrinogen was not <193 mg/dL. These cases illustrate how rapidly circulating platelets can decline following UMRV Timber Rattlesnake envenomation and how recalcitrant the venom-induced thrombocytopenia was to IgG and Fab antivenoms. The recurrence of venom-induced effects after Fab AV therapy has been previously reported and believed to be due to the more rapid clearance of Fab in contrast to venom components (Boyer et al., 2001). Other explanations for the lack of therapeutic response to antivenom may be due to inadequate dosing, antivenom antibody/venom antigen pharmacokinetic differences, lack of antibody recognition of venom antigen-toxins, aggregated platelet sequestration at the bite site, or possible immune response against antivenom antibodies. Whether thrombocytopenia alone in the absence of other altered hematological parameters is a complication of serious medical significance is uncertain. Importantly, some patients may have comorbidities such as a history of cerebral or cardiovascular infarcts, dyscrasias, peripheral vascular disease, or vasculitis. Abnormally low platelet levels may be associated with variably elevated risks of bleeding in the absence of the added risks due to trauma. It is worth noting that the data for all nine thrombocytopenic patients reported here with ecchymosis showed no clinical evidence of active bleeding.
4.4. Surgery and timber rattlesnake envenomation
Surgical intervention to prevent or relieve compartment syndrome complications in treating pit viper (family Viperidae, subfamily Crotalinae) envenomated patients has been controversial and there continues to be a lack of consensus regarding its beneficial role in the medical management of envenomations, including C. horridus envenomated patients (Gold et al., 2003; Corneille et al., 2006; Hardy and Zamudio, 2006; Cumpston, 2011; Toschlog et al., 2013). Although more recent reports in general do not support the implementation of surgical intervention it is still occasionally performed despite the lack of formal clinical trial evidence (Cumpston, 2011; Toschlog et al., 2013). In cases of crotaline envenoming compartment pressures may be transiently elevated by venom-induced myositis, which has been shown to respond favorably to appropriate antivenom therapy (Lee et al., 2019; Cumpston, 2011). Serial measurements are essential in order to identify rare patients that develop compartment syndrome (Toschlog et al., 2013). Reported here are two cases in which surgical intervention was performed (Table 2). One patient was bitten in the webbing between the index finger and thumb. Given the inaccuracy of any form of pressure measurements in this anatomical region the initiation of surgery was based on the surgeon's assessment of the swollen hand and clinical judgement. It is unfortunate that antivenom had not been administered first as it may have prevented the unnecessary surgery. In the second case the patient was bitten on the finger and lost consciousness from excess alcohol consumption, with his arm pressed under his torso for 13 h. His arm was badly swollen to the axilla, and ecchymotic. Again, the decision to perform a fasciotomy of the arm was based on clinical judgement in the absence of intra-compartmental pressure measurements. Initial administration of antivenom may have precluded the implementation of surgery that was based on subjective observations, thereby eliminating the increased risk of myonecrosis and potential permanent disability (Toschlog et al., 2013; Cumpston, 2011). However, prior administration of antivenom may not always preclude the need for surgery in extremity envenomated patients if subfascial compartment pressures are excessively elevated (Hardy and Zamudio, 2006; Razavi et al., 2022; Toschlog et al., 2013).
4.5. Fatalities and Crotalus horridus
Fatalities from venomous snakes are extremely rare in the U.S. with a reported five fatalities having occurred annually between 1979 and 2004 (Langley, 2009). Historically, human fatalities from Timber Rattlesnake envenomation have been even more scarce with only sporadic single cases documented (Gallagher et al., 2023; Brown et al., 2022; Carroll et al., 1997; Joslin et al., 2014; Kitchens et al., 1987). Typically, the victims have been involved in “rattlesnake roundups” or religious activities, and more rarely involved professional herpetologists (Gallagher et al., 2023; Brown et al., 2022; Carroll et al., 1997; Joslin et al., 2014; Kitchens et al., 1987; Langley, 2009; Morgan et al., 2004). Prior to 1880 in Wisconsin only twelve fatalities from snakebite were reported (Schoerger, 1967-68). In Minnesota a single fatality was reported in a local newspaper in 1868 (Red Wing Argus, 1868). In the UMRV of Minnesota and Wisconsin no fatalities were reported from 1950 to 59, and the case-fatality rate was less than 0.05% (Parrish, 1965a, 1965b). Tragically, a more recent C. horridus fatality, previously reported and included here, occurred in Wisconsin (1983) and was the consequence from a confirmed intravenous bite. This was a severe envenomation further complicated by a significant delay in transport to a medical facility to another state, and details regarding the case have been legally protected (Keyler, 2008).
5. Conclusions
The future of Crotalus horridus in the UMRV is dependent on halting the destruction of the rattlesnake's habitat, elimination of deliberate killing by humans, and the enforcement of established legal protection measures against illegal human activities. The fear of sustaining a bite from a wild C. horridus in the region is highly exaggerated; however, there will likely be incidental circumstances where humans are bitten due to inappropriate interactions with the snake. When bites have occurred in the UMRV, envenomation by C. horridus has been shown to cause a severe and rapid thrombocytopenia that did not readily resolve with IgG or Fab antivenom therapy. Collectively, the findings reported here show Crotalus horridus is an integral species of the UMRV ecosystem, and a species of medical importance, possessing a venom of biomedical interest.
Ethical statement
No wild snakes were harmed during biological and conservation field work studies. Handling and processing of live venomous snakes were per the author's permit via the Minnesota Department of Natural Resources, Division of Nongame Wildlife.
CRediT authorship contribution statement
D.E. Keyler: Writing – original draft, Project administration, Methodology, Investigation, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Daniel E Keyler reports was provided by University of Minnesota Twin Cities. Past guest editor for Toxicon special issue.
Acknowledgements
The author is most grateful for the knowledge and field experiences shared over decades of time with the late Dr. Philip A. Cochran of Saint Mary's University, Minnesota, Dr. Barney Oldfield, Hesperus, Colorado, and the late W.H. (Marty) Martin of Harpers Ferry, West Virginia.
Handling Editor: Ray Norton
Data availability
The data that has been used is confidential.
References
- Abukamar A., Abudalo R., Odat M., Al-Sarayreh M., Issa M.B., Momanie A. Arabian Levantine viper bite induces thrombocytopenia - a case report. J Med Life. 2022;15:867–870. doi: 10.25122/jml-2021-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alape-Girón A., Flores-Díaz M., Sanz L., et al. Studies on the venom proteome of Bothrops asper: perspectives and applications. Toxicon. 2009;54:938–948. doi: 10.1016/j.toxicon.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Babcock H.L. vol. II. Bulletin of the Antevenin Institute of America; 1928. pp. 77–78. (Notes on the Treatment of Rattlesnake Bites during Colonial Times in Massachusetts). [Google Scholar]
- Bond G.R., Burkhart K.K. Thrombocytopenia following timber rattlesnake envenomation. Ann. Emerg. Med. 1997;30:40–44. doi: 10.1016/s0196-0644(97)70108-2. [DOI] [PubMed] [Google Scholar]
- Boyer L.V., et al. Recurrence phenomena after immunoglobulin therapy for snake envenomations: part 2. Guidelines for clinical management with crotaline Fab antivenom. Ann. Emerg. Med. 2001;37:156–201. doi: 10.1067/mem.2001.113134. [DOI] [PubMed] [Google Scholar]
- Breisch A.R., Martin W.H., Sealy J.B., Peterson C.E., Possardt E., editors. The Timber Rattlesnake, Life History, Distribution, Status, and Conservation Action Plan. Partners with Amphibian and Reptile Conservation Technical Publication CAP-1. Amphibian and Reptile Conservancy, Inc. Nashville, TN; 2021. [Google Scholar]
- Brown W.S. Society for the Study of Amphibians and Reptiles; 1993. Biology, Status, and Management of the Timber Rattlesnake (Crotalus horridus): a Guide for Conservation. Herpetol. Circ. 22. [Google Scholar]
- Brown W.S., Kery M., Hines J.E. Survival of Timber Rattlesnakes (Crotalus horridus) estimated by capture-recapture models in relation to age, sex, color morph, time, and birthplace. Copeia. 2007;3:656–671. [Google Scholar]
- Brown W.S. Lifetime reproduction in a northern metapopulation of Timber Rattlesnakes (Crotalus horridus) Herpetologica. 2016;72:331–342. [Google Scholar]
- Brown W.S., Sealy J.B., Keyler D.E. W.H. “Marty” Martin (1941-2022): rattlesnake field biologist extraordinaire. Herpetol. Rev. 2022;53:766–769. [Google Scholar]
- Bushar L.M., Aborde C.C.B., Gao S., Gonzalez M.V., Hoffman J.A., Massaro I.K., Savitzky A.H., Reinert H.K. Genetic structure of Timber Rattlesnake (Crotalus horridus) populations: physiographic influences and conservation implications. Copeia. 2014;4:694–706. [Google Scholar]
- Carroll R.R., Hall E.L., Kitchens C.S. Canebrake rattlesnake envenomation. Ann. Emerg. Med. 1997;30:45–48. doi: 10.1016/s0196-0644(97)70109-4. [DOI] [PubMed] [Google Scholar]
- Clark A.M., Moler E.E., Possardt A.H., Savitsky A.H., Brown W.S., Bowen B.W. Phylogeography of the Timber Rattlesnake (Crotalus horridus) based on mtDNA sequences. J. Herpetol. 2003;37:145–154. [Google Scholar]
- Clark G.C., Casewell N.R., Elliott C.T., Harvey A.L., Jamieson A.G., Strong P.N., Turner A.D. Friends or foes? Emerging impacts of biological toxins. Trends Biochem. Sci. 2019;44:365–379. doi: 10.1016/j.tibs.2018.12.004. [DOI] [PubMed] [Google Scholar]
- Clark R.F., O'Connell C.W., Villano J.H., Kim A., Ly B.T. Sever recurrent coagulopathy following crotaline envenomation refractory to maintenance dosing of antivenom. Am. J. Emerg. Med. 2015;33 doi: 10.1016/j.ajem.2014.11.047. 856e856-e5. [DOI] [PubMed] [Google Scholar]
- Clark R.W., Brown W.S., Stechert R., Zamudio K.R. Integrating individual behaviour and landscape genetics: the population structure of timber rattlesnake hibernacula. Mol. Ecol. 2008;17:719–730. doi: 10.1111/j.1365-294X.2007.03594.x. [DOI] [PubMed] [Google Scholar]
- Clark R.W., Marchand M.N., Clifford B.J., Stechert R., Stephens S. Decline of an isolated timber rattlesnake (Crotalus horridus) population: interactions between climate change, disease, and loss of genetic diversity. Biol. Conserv. 2011;144:886–891. [Google Scholar]
- Clurman C. Governing, the States and Localities. Publication of the Congressional Quarterly, Inc.; 1989. If snakes had PACs, they couldn't have done better. August 15. [Google Scholar]
- Corneille M.G., Larson S., Stewart R.M., Dent D., Myers J.G., et al. A large single-center experience with treatment of patients with crotalid envenomations: outcomes with and evolution of antivenin therapy. Am. J. Surg. 2006;192:848–852. doi: 10.1016/j.amjsurg.2006.08.056. [DOI] [PubMed] [Google Scholar]
- Cochran P.A. In: Hayes W.K., Beaman K.R., Cardwell M.D., Bush S.P., editors. vol. 48. Loma Linda University Press; Loma Linda, California: 2008. Phenology of Timber Rattlesnakes (Crotalus horridus) in southern Minnesota: implications for conservation; pp. 441–446. (The Biology of Rattlesnakes). [Google Scholar]
- Cochran C., Hax S., Hayes W.K. Case reports of envenomation and venom composition differences between two Arizona populations of the Southwestern Speckled Rattlesnake, Crotalus pyrrhus (Cope, 1867) Toxicon. 2019;171:29–34. doi: 10.1016/j.toxicon.2019.09.022. [DOI] [PubMed] [Google Scholar]
- Cumpston K.L. Is there a role for fasciotomy in Crotalinae envenomations in North America? Clin. Toxicol. 2011;49:351–365. doi: 10.3109/15563650.2011.597032. [DOI] [PubMed] [Google Scholar]
- Eiksund S.A. Geographical perspective on driving attitudes and behaviour among young adults in urban and rural Norway. Saf. Sci. 2009;47:529–536. doi: 10.1016/j.ssci.2008.07.034. [DOI] [Google Scholar]
- El-Aziz T.M.A., Soares A.G., Stockand J.D. Snake venoms in drug discovery: valuable therapeutic tools for life saving. Toxins. 2019;11:1–25. doi: 10.3390/toxins11100564.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman J. University Press of New England; Lebanon, New Hampshire: 2007. Timber Rattlesnakes in Vermont and New York: Biology, History, and the Fate of an Endangered Species; p. 207. [Google Scholar]
- Gallagher T., Roberts S., Silva-Sanchez C., Sutton L., Laventure K. The use of serum protein analysis in the diagnosis of fatal envenomation via Crotalus horridus (timber rattlesnake) J. Forensic Sci. 2023;68:711–715. doi: 10.1111/1556-4029.15213. [DOI] [PubMed] [Google Scholar]
- George S.G. Tmber rattlesnake (Crotalus horridus) swims the Mississippi river. ICRF Reptiles & Amphibians. 2010;17:40–41. [Google Scholar]
- Glenn J.L., Straight R.C. Intergradation of two different venom population of the Mojave Rattlesnake (Crotalus scutulatus scutulatus) in Arizona. Toxicon. 1989;27:411–418. doi: 10.1016/0041-0101(89)90203-1. [DOI] [PubMed] [Google Scholar]
- Glenn J.L., Straight R.C. Regional variation in the presence of canebrake toxin in Crotalus horridus venom. Comp. Biochem. Physiol. 1994;107C:337–346. doi: 10.1016/1367-8280(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Gold B.S., Barrish R.A., Dart R.C., Silverman R.P., Bochicchio G.V. Resolution of compartment syndrome after rattlesnake envenomation utilizing non-invasive measures. J. Emerg. Med. 2003;24 doi: 10.1016/s0736-4679(02)00762-x. 284-283. [DOI] [PubMed] [Google Scholar]
- Gold B.S., Barrish R.A., Rudman M.S. Refractory thrombocytopenia despite treatment for rattlesnake envenomation. N. Engl. J. Med. 2004;350:1912–1913. doi: 10.1056/NEJM200404293501824. [DOI] [PubMed] [Google Scholar]
- Hall J.B., Cobb V.A., Cahoon A.B. The complete mitochondrial DNA sequence of Crotalus horridus (timber rattlesnake) Mitochondrial DNA. 2013;24:94–96. doi: 10.3109/19401736.2012.722999. [DOI] [PubMed] [Google Scholar]
- Hardy D.L., Sr., Zamudio K.R. Compartment syndrome, fasciotomy, and neuropathy after rattlesnake envenomation: aspects of monitoring and diagnosis. Wild. Env. Med. 2006;17:36–40. doi: 10.1580/1080-6032(2006)17[36:csfana]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Joslin J.D., Marraffa J.M., Singh H., Mularella J. Incidence and characteristics of snakebite envenomations in the New York State between 2000 and 2010. Wild. Env. Med. 2014;25:289–294. doi: 10.1016/j.wem.2014.01.017. [DOI] [PubMed] [Google Scholar]
- Kerns K., Tomaszewski C. Airway obstruction following canebrake rattlesnake envenomation. J. Emerg. Med. 2001;29:377–380. doi: 10.1016/s0736-4679(01)00315-8. [DOI] [PubMed] [Google Scholar]
- Keyler D.E. In: Hayes W.K., Beaman K.R., Cardwell M.D., Bush S.P., editors. vol. 49. Loma Linda University Press; Loma Linda, California: 2008. Timber rattlesnake (Crotalus horridus) envenomations in the upper Mississippi River Valley; pp. 569–580. (The Biology of Rattlesnakes). [Google Scholar]
- Keyler D.E. In: The Timber Rattlesnake: Life History, Distribution, Status, and Conservation Action Plan. Amphibian and Reptile Conservancy. Breisch A.R., Martin W.H., Sealy J.B., Peterson C.E., editors. , Inc. Nashville, TN; USA: 2021. Minnesota; pp. 218–226. [Google Scholar]
- Keyler D.E. In: The Timber Rattlesnake: Life History, Distribution, Status, and Conservation Action Plan. Amphibian and Reptile Conservancy. Breisch A.R., Martin W.H., Sealy J.B., Peterson C.E., editors. , Inc. Nashville, TN; USA: 2021. Wisconsin; pp. 446–453. [Google Scholar]
- Kitchens C.S., Hunter S., Van Mierop L.H.S. Severe myonecrosis in a fatal case of envenomation by the canebrake rattlesnake (Crotalus horridus atricaudatus) Toxicon. 1987;25:455–458. doi: 10.1016/0041-0101(87)90080-8. [DOI] [PubMed] [Google Scholar]
- Langley R.L. Deaths from reptile bites in the United States, 1979-2004. Clin. Toxicol. 2009;47:44–47. doi: 10.1080/15563650801968313. [DOI] [PubMed] [Google Scholar]
- Lee P.-H., Mao Y.-C., Liu P.-Y., et al. Snakebite (Protobothrops mucrosquamatus)-related myositis. J. Formos. Med. Assoc. 2019;118:1168–1169. doi: 10.1016/j.jfma.2019.03.013. [DOI] [PubMed] [Google Scholar]
- Linnaeus C. Systema Naturae per Regna Tri Naturae, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis. 1758, vol. 1 (10th revised ed.). Holmiae: (Laurentii Salvii). P. 214 – via the Internet Archive.
- Lorch J.M., Knowles S., Lankton J.S., et al. Snake fungal disease: an emerging threat to wild snakes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016;371(1709) doi: 10.1098/rstb.2015.0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madey J.J., Price A.B., Dobson J.V., Stickler D.E., McSwain S.D. Facial diplegia, pharyngeal paralysis, and ophthalmoplegia after a timber rattlesnake envenomation. Pediatr. Emerg. Care. 2013;29:1213–1216. doi: 10.1097/PEC.0b013e3182aa472f. [DOI] [PubMed] [Google Scholar]
- Martin W.H., Brown W.S., Possardt E., Sealy J.B. In: Hayes W.K., Beaman K.R., Cardwell M.D., Bush S.P., editors. vol. 40. Loma Linda University Press; Loma Linda, California: 2008. Biological variation, management units, and conservation action plan for the Timber rattlesnake (Crotalus horridus) pp. 447–462. (The Biology of Rattlesnakes). [Google Scholar]
- Minton S.A. In: Animal Toxins. Russel F.E., Saunders P.R., editors. Permagon Press; New York: 1967. Observation on toxicity and antigenic makeup of venoms from juvenile snakes. [Google Scholar]
- Minton S.A., Weinstein S.A. Geographic and ontogenic variation in venom of the western diamondback rattlesnake (Crotalus atrox) Toxicon. 1986;24:71–80. doi: 10.1016/0041-0101(86)90167-4. [DOI] [PubMed] [Google Scholar]
- Morgan B.W., Lee C., Damiano L., Whitlow K., Geller R. Reptile envenomation 20-year mortality as reported by US medical examiners. South. Med. J. 2004;97:642–644. doi: 10.1097/00007611-200407000-00006. [DOI] [PubMed] [Google Scholar]
- Odeleye A.A., Presley A.E., Passwater M.E., Mintz P.D. Rattlesnake venom-induced thrombocytopenia. Ann. Clin. Lab. Sci. 2004;34:467–470. [PubMed] [Google Scholar]
- Offerman S.R., Barry J.D., Schneir A., Clark R.F. Biphasic rattlesnake venom-induced thrombocytopenia. J. Emerg. Med. 2003;24:289–293. doi: 10.1016/s0736-4679(02)00763-1. [DOI] [PubMed] [Google Scholar]
- Oldfield B.L., Keyler D.E. Survey of timber rattlesnake (Crotalus horridus) distribution along the Mississippi river in western Wisconsin. Trans. Wis. Acad. Sci. Arts Lett. 1989;77:27–35. [Google Scholar]
- Palmer T. Ticknor & Fields; NY, New York: 1992. Landscape with Reptile: Rattlesnakes in an Urban World. [Google Scholar]
- Parrish H.M. Rarity of snakebites in Minnesota. Minn. Med. 1965;48:1071–1076. [Google Scholar]
- Parrish H.M. Frequency of snakebites in Wisconsin. Wis. Med. J. 1965;64:216–220. [PubMed] [Google Scholar]
- Peigneur S., Tytgat J. Toxins in drug discovery and pharmacology. Toxins. 2018;10:126. doi: 10.3390/toxins10030126.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson C.E., Sealy J.B. In: The Timber Rattlesnake: Life History, Distribution, Status, and Conservation Action Plan. Amphibian and Reptile Conservancy. Breisch A.R., Martin W.H., Sealy J.B., Peterson C.E., editors. , Inc. Nashville, TN; USA: 2021. Threats and conservation actions; pp. 81–98. [Google Scholar]
- Razavi S.A., Stewart C.M., Nogee D.P., Geller R.J., Seiler J.G. Upper extremity crotalid envenomation: a review of incidence and recent trends in management of snakebites. J .Hand. Surg. Am. 2022 doi: 10.1016/j.jhsa.2022.04.016. [DOI] [PubMed] [Google Scholar]
- Red Wing Argus . Red Wing; Minnesota: 1868. Death from Rattlesnake Bite. [Google Scholar]
- Reinert H.K., Munroe W.F., Brennen C.E., Rach M.N., Pelesky S., Bushar L.M. Response of Timber Rattlesnakes to commercial logging operations. J. Wildl. Manag. 2011;75:19–29. [Google Scholar]
- Rokyta D.R., Wray K.P., Margres M.J. The genesis of an exceptionally lethal venom in the timber rattlesnake (Crotalus horridus) revealed through comparative venom-gland transcriptomics. BMC Genom. 2013;14:394. doi: 10.1186/1471-2164-14-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokyta D.R., Wray K.P., McGivern J.J., Margres M.J. The transcriptomic and proteomic basis for the evolution of a novel venom phenotype within Timber Rattlesnake (Crotalus horridus) Toxicon. 2015;98:34–48. doi: 10.1016/j.toxicon.2015.02.015. [DOI] [PubMed] [Google Scholar]
- Sajdak R., Berg C. Rattlesnakes on the bluffs: Wisconsin timber rattlesnakes. Iguana. 2005;12:90–97. [Google Scholar]
- Schmaier A.H., Coleman R.W. Crotalocytin: characterization of the Timber Rattlesnake platelet activating protein. Blood. 1980;56:1020–1028. [PubMed] [Google Scholar]
- Schoerger A.W. Rattlesnakes in early Wisconsin. Trans. Wis. Acad. Sci. Arts Lett. 1967-68;56:27–34. [Google Scholar]
- Seifert S.A., Boyer L.V., Benson B.E., Rogers J.J. AAPCC database characterization of native U.S. venomous snakebite exposures, 2001-2008. Clin. Toxicol. 2009;47:327–335. doi: 10.1080/15563650902870277. [DOI] [PubMed] [Google Scholar]
- Soto J.G., Perez J.C., Lopez M.M., Martinez M., Quintanilla-Hernandez T.B., Santa- Hernandez M., et al. Comparative enzymatic study of HPLC-fractionated Crotalus venoms. Comp. Biochem. Physiol. 1989;93:847–855. doi: 10.1016/0305-0491(89)90056-4. [DOI] [PubMed] [Google Scholar]
- Straight R.C., Glenn J.L., Wolt T.B., Wolfe M.C. Regional differences in content of small basic peptide toxins in the venoms of Crotalus adamanteus and Crotalus horridus. Comp. Biochem. Physiol. 1991;100B:51–58. doi: 10.1016/0305-0491(91)90083-p. [DOI] [PubMed] [Google Scholar]
- Sunagar K., Undheim E.A., Scheib H., et al. Intraspecific venom variation in the medically significant Southern Pacific Rattlesnake (Crotalus oreganus helleri): biodiscovery, clinical and evolutionary implications. J. Proteonomics. 2014;99:68–83. doi: 10.1016/j.jprot.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Timber Rattlesnake Recovery Team . Minnesota Department of Natural Resources, Division of Ecological Resources; Saint Paul, MN: 2009. Timber Rattlesnake Recovery Plan (Crotalus horridus) p. 47. [Google Scholar]
- Toschlog E.A., Bauer C.R., Hall E.L., Dart R.C., Khatri V., Lavonas E.J. Surgical considerations in the management of pit viper snake envenomation. J. Am. Coll. Surg. 2013;217:726–735. doi: 10.1016/j.jamcollsurg.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Trautman W., Pizon A. Severe, persistent thrombocytopenia in Crotalus horridus envenomation despite antivenom: a retrospective review. Toxicon. 2023 doi: 10.1016/j.toxicon.2023.107029. 2023. [DOI] [PubMed] [Google Scholar]
- Wang Y.-M., Parmelee J., Guo Y.-W., Tsai I.-H. Absence of phospholipase A2 in most Crotalus horridus venom due to translation blockage: comparison with Crotalus horridus atricaudatus venom. Toxicon. 2010;56:93–100. doi: 10.1016/j.toxicon.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Welton R.E., Liew D., Braitberg G. Incidence of fatal snake bite in Australia: a coronial based retrospective study (2000-2016) Toxicon. 2017;131:11–15. doi: 10.1016/j.toxicon.2017.03.008. [DOI] [PubMed] [Google Scholar]
- Wisconsin Department of Transportation [WDOT] 2017. Motor Vehicle-Deer Crashes 2017.https://wisconsindot.gov/Documents/safety/education/crash-data/deerfacts-2017.pdf [Google Scholar]
- Zhang C., Zhang Z., Liang E., et al. Platelet desialylation is a novel mechanism and therapeutic target in Daboia siamensis and Agkistrodon halys envenomation-induced thrombocytopenia. Molecules. 2022;27:7779. doi: 10.3390/molecules27227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.