Abstract
The high recurrence rate of cystine lithiasis observed in cystinuria patients highlights the need for new therapeutic options to address this chronic disease. There is growing evidence of an antioxidant defect in cystinuria, which has led to test antioxidant molecules as new therapeutic approaches. In this study, the antioxidant l-Ergothioneine was evaluated, at two different doses, as a preventive and long-term treatment for cystinuria in the Slc7a9−/− mouse model. l-Ergothioneine treatments decreased the rate of stone formation by more than 60% and delayed its onset in those mice that still developed calculi. Although there were no differences in metabolic parameters or urinary cystine concentration between control and treated mice, cystine solubility was increased by 50% in the urines of treated mice. We also demonstrate that l-Ergothioneine needs to be internalized by its transporter OCTN1 (Slc22a4) to be effective, as when administrated to the double mutant Slc7a9−/−Slc22a4−/− mouse model, no effect on the lithiasis phenotype was observed. In kidneys, we detected a decrease in GSH levels and an impairment of maximal mitochondrial respiratory capacity in cystinuric mice that l-Ergothioneine treatment was able to restore. Thus, l-Ergothioneine administration prevented cystine lithiasis in the Slc7a9−/− mouse model by increasing urinary cystine solubility and recovered renal GSH metabolism and mitochondrial function. These results support the need for clinical trials to test l-Ergothioneine as a new treatment for cystinuria.
Keywords: Cystinuria, l-Ergothioneine, Cystine lithiasis, Antioxidant, Oxidative stress, Treatment
Graphical abstract
Highlights
-
•
l-Ergothioneine treatment prevents cystine lithiasis in the Slc7a9−/− mouse model.
-
•
l-Ergothioneine increases cystine solubility in the urine of treated mice.
-
•
Renal and hepatic GSH levels are decreased in the Slc7a9−/− mouse model.
-
•
Mitochondrial respiration is impaired in the kidneys of the Slc7a9−/− mouse model.
-
•
l-Ergothioneine treatment restores GSH pools and mitochondrial function.
1. Introduction
Cystinuria is one of the urolithiasis disease with the worst reported quality of life in patients [1,2]. It is caused by mutations in the SLC3A1 and SLC7A9 genes [3,4], which encode the cystine and dibasic amino acid transport system b0,+, expressed in the intestine and renal proximal tubules [5]. At the intestinal level, no clinical effect is observed as plasma concentrations of these amino acids are normal or slightly lower in cystinuria patients [6]. However, defects in renal reabsorption lead to the hyperexcretion of cystine, lysine, arginine and ornithine [7,8]. The low solubility of cystine at physiological urine pH induces its precipitation and calculi formation, which is the main clinical manifestation of cystinuria [9]. Cystine urolithiasis accounts for 1–2% of urinary tract calculi in adults and 6–8% in children [10,11].
About 94% of patients develop cystine stones during their lifetime and, of these, 75% suffer from them bilaterally [12,13]. Recurrent renal calculi episodes causing obstructive uropathy and repeated urological interventions can result in chronic kidney disease [14,15]. Therefore, cystinuria patients require intensive follow-up to preserve their renal function. First-line treatments are conservative measures focused on preventing stone formation: hydration therapy, dietary recommendations and urine alkalinization [16]. When these approaches fail, thiol drugs as d-penicillamine or tiopronin are administered to dissolve the stones by forming cysteine-drug products, which are up to 50-fold more soluble in urines [16]. However, thiol drugs have side effects that must be monitored and lead to treatment discontinuation [17,18].
New therapeutic approaches for cystinuria are being investigated, ranging from thiol drugs as bucillamine (NCT02942420), diuretic drugs as Tolvaptan [19], or crystal growth inhibitors as l-cystine dimethylester [20] and l-cystine bis(N′-methylpiperazide) [21,22]. In addition, antioxidant molecules are being considered as a potential treatment as they showed stone growth inhibitory properties, although the mechanism by which they act remains unknown. α-Lipoic acid showed to prevent cystine stone formation by increasing urinary cystine solubility in a cystinuria mouse model [23] and a clinical trial is underway to prove its effectiveness in patients (NTC02910531). Likewise, Salvianolic acid B and Selenium have been described to reduce cystine crystals deposition in mouse models and cystinuria patients [24,25].
In this line, we have recently identified the antioxidant l-Ergothioneine (L-Erg) as a modulator of cystine lithiasis as the double mutant Slc7a9−/−Slc22a4−/− mouse model (cystinuria model lacking the L-Erg transporter), has shown an increased rate of stone-former mice [26]. In addition, the urinary ratio of the metabolite S-Methyl-l-Ergothioneine (S-Met-L-Erg) to L-Erg proved to be a marker to discriminate stone-former from non-stone-former cystinuric mice [26]. L-Erg is an intracellular antioxidant molecule with cytoprotective and anti-inflammatory effects which role associated with pathologies has been described in several organs [[27], [28], [29], [30]], including the kidney [[31], [32], [33]]. It is a natural thiol molecule synthesized by cyanobacteria [34], mycobacteria [35] and non-yeast-fungi [36], that animals must absorb from diet. However, although animals cannot synthesize L-Erg, the existence of a specific transporter (OCTN1-SLC22A4) in most tissues that ensures its absorption and retention in the body [37], L-Erg long half-life [38] and its ability to accumulate in injured tissues [39], suggest that L-Erg has an important physiological role.
Based on the above-described findings and for its thiol-antioxidant properties, we have tested L-Erg treatment to prevent cystine lithiasis in the Slc7a9−/− mouse model and assessed its mechanism of action.
2. Methods
2.1. Mice treatments
Mice colonies were maintained in a specific pathogen-free animal facility. All protocols were approved by the Animal Experimentation Ethics Committee of IDIBELL (AAALAC accredited facility, B9900010). Homozygous Slc7a9−/− [40] and Slc7a9−/−Slc22a4−/− [26] mice on a C57Bl/6J genetic background were assigned randomly to control or L-Erg treated groups with balanced sex distribution and housed with ad libitum access to food and drinking water.
L-Erg was in-house synthesized by the SIMChem service or provided by Tetrahedron (www.tetrahedron.fr). Three different treatments were administered to Slc7a9−/− mice: 1-month treatment in adult mice to test doses (n = 8, mixed sexes), low-dose (16 mg/kg/day) treatment since weaning to 7 months of age (n = 40, mixed sexes) and high-dose (200 mg/kg/day) treatment since weaning to 4 months of age (n = 55, mixed sexes). Low-dose L-Erg treatment was also supplied to Slc7a9−/−Slc22a4−/− mice since weaning and up to 4 months of age (n = 22, mixed sexes). L-Erg concentration in the drinking water was adjusted per cage weekly according to mouse weight and water intake. Each treatment had its own control group with the same number of mice.
2.2. Cystine calculi detection by X-ray
To detect the stone onset and follow up its progression in vivo, X-ray images were taken monthly using an IVIS Lumina XR Series III (Caliper LifeScience–Vertex Technics, Hopkinton, MA, USA) as described in Ref. [26] and were analyzed using Living Image® Software.
2.3. Sample collection
Individual mice urine was collected using metabolic cages for 4 days at the beginning and the end of treatments. Urinary pH was determined using the 52 09 pH electrode (CRISON, Barcelona, Spain) and urines were kept at −80 °C until further analysis. Mice were sacrificed on the last day in the metabolic cages by intracardiac puncture and kidneys, livers and stones were removed.
2.4. L-Erg and S-Met-L-Erg determination
L-Erg and S-Met-L-Erg determination in the urines of the test experiment was carried out as previously described in Ref. [26]. The analysis of L-Erg and S-Met-L-Erg in the urines of low and high-dose treatment was performed on an Agilent 1290 Infinity II UHPLC system (Agilent Technologies, Santa Clara, USA) coupled to a 6500 QTRAP mass spectrometer equipped with Ion Drive Turbo V ion source LC-MS/MS system (Sciex, Framingham, MA, USA). L-Erg and S-Met-L-Erg concentration was normalized by urinary creatinine, determined with the Creatinine Assay Kit (Sigma-Aldrich, MA, USA).
2.5. Cystine analysis
Cystine precipitation assay was performed as described in Ref. [23]. Briefly, 400 μL of a supersaturated l-cystine solution (4 mM) were added to: i) 100 μL of a pool of Slc7a9−/− mice urine, ii) 100 μL of a pool of Slc7a9−/− mice urine supplemented with L-Erg or S-Met-L-Erg 500 μM (final concentration), or iii) 100 μL of a pool of L-Erg treated Slc7a9−/− mice urine (4 replicates per condition). Then, samples were vortexed and left at 4 °C for 96 h to allow cystine precipitation. After that, samples were centrifuged at 4000 rpm for 20 min and supernatants were discarded. The precipitate obtained was dissolved in 1.5 mL of ultrapure H2O. Cystine concentration of pellets and mice urines was determined by UPLC-MS/MS as described by Ref. [41].
2.6. Electron microscopy
Cystine stones were split into two parts to analyze the outer surface and the inner layers of stones. Stone fragments were fixed with silicone, in its corresponding spatial orientation, to the 1 cm in diameter support element. No sample coating was needed as the analyses were carried out under low vacuum. Crystal morphology was evaluated using a Scanning Electron Microscopy (SEM) Quanta 200 3D (FEI Company™). The SEM was coupled with energy-dispersive spectroscopy (EDS) (Thermo Fisher UltraDry, 30 mm2) allowing the simultaneous analysis of stone elemental composition. Stone images were acquired with magnification ranging from 100 to 1000 and an image resolution of 512 × 340. Nine sections were analyzed for each stone.
2.7. GSH and GSSG determination
To determine kidney and liver intracellular reduced glutathione (GSH) and oxidized glutathione (GSSG) levels, 100 mg of pulverized tissue were homogenized in 400 μL of PBS-N-Ethylmaleimide (NEM) buffer (10 mM). To induce protein precipitation, perchloric acid was added at 4% final concentration, and samples were centrifuged at 10,000 rpm, 15 min at 4 °C. Supernatants were transferred to a new tube for analysis and pellets were resuspended in 100 μL of NaOH (1 M) to obtain the total protein content by BCA Protein Assay Kit (ThermoScientific, MA, USA). Then, GSH and GSSG levels were analyzed by UPLC-MS/MS as described in Ref. [42]. Data were normalized by grams of total protein content.
2.8. Mitochondrial respirometry
Mitochondrial respiration was measured in fresh renal biopsies by high-resolution respirometry using an Oxygraph-2k (Oroboros® Instrument Gmbh Corp) following the substrate-uncoupler-inhibitor titration (SUIT-008) protocol described in Refs. [43,44]. Fresh kidneys were cut into small pieces and were permeabilized for 30 min at 4 °C in 2 mL of ice-cold saponin solution (0.05 mg saponin/mL BIOPS). After a 5 min wash with MiR05 buffer, 1.5 mg of tissue were introduced in the O2k chamber to achieve a 0.75 mg/mL tissue concentration. Leak state of uncoupled respiration was assessed by adding malate (2 mM) and pyruvate (10 mM). Complex I oxidative phosphorylation was measured by adding ADP (5 mM). Then, cytochrome c (10 mM) was added to check the integrity of the outer mitochondrial membrane. NADH linked pathway respiration was evaluated by adding glutamate (10 mM) and, NADH and succinate linked pathways, adding succinate (10 mM). Maximal uncouple respiration was determined by following titration of carbonylcyanide-4-(trifluoromethoxy)-phenyl-hydrazone (FCCP). Finally, complex I and complex III were inhibited by the sequential addition of rotenone (0.5 μM) and antimycin A (2.5 μM), and residual oxygen consumption was quantified to subtract this value of the other respiratory states.
2.9. Mitochondrial respiratory chain enzymatic activities
Frozen kidneys were thawed in ice-cold mannitol (200 μL every 50 mg of tissue), cut into tiny pieces and homogenized with a Teflon plunger motor-driven homogenizer. After centrifuging the homogenates for 20 min at 650×g and 4 °C, supernatants were kept, and pellets were resuspended and homogenized again on ice-cold mannitol. Then, total protein quantification was performed using the BCA protein assay and samples were diluted to a final concentration of 2 mg/mL with mannitol. Complex II, complex IV and Citrate Synthase activities were assessed spectrophotometrically as described in Ref. [45]. Complex II and complex IV activities were normalized by Citrate Synthase activity and expressed as nmol/min/mg protein.
2.10. Statistical analysis
The unpaired non-parametric Wilcoxon-Mann-Whitney test was used to assess differences between independent group samples. Data are presented as mean values ± SEM and P values below 0.05 were considered statistically significant. Data analyses and figure design were performed with RStudio version 3.6.0. R packages used were openxlsx, dplyr, ggpubr, tidyr, devtools, and ggplot2.
3. Results
3.1. Determination of L-Erg dose
One-month treatment with L-Erg was performed in adult mice to evaluate whether metabolic parameters could be affected by L-Erg administration and to study L-Erg and S-Met-L-Erg urinary bioavailability testing two different doses. Hence, the drinking water of treated mice was supplemented by 15 or 60 mg/L of L-Erg. After that time, no significant variations in metabolic parameters such as weight, water intake, urinary excretion and pH were observed (Fig. 1a–d). Nevertheless, L-Erg and S-Met-L-Erg urinary concentrations increased significantly at both tested doses (Fig. 1e–f). However, that increase was shown to be nonproportional as in the 15 mg/L group L-Erg and S-Met-L-Erg levels increased 3.4-fold and 5.4, respectively, and in the 60 mg/mL group, the increase was 150.0-fold and 13.4-fold, respectively (Fig. 1g). The administration of 60 mg/L of L-Erg, corresponding to a dose of 16.3 ± 6.6 mg/kg/day in mice (Fig. 1h), was chosen for subsequent experiments as it resulted in a higher urinary concentration of L-Erg and S-Met-L-Erg.
Fig. 1.
L-Erg treatment for 1 month increases L-Erg and S-Met-L-Erg urinary concentration without affecting metabolic parameters. Comparison of (A) mice weight, (B) water intake normalized by mice body surface area, (C) urinary excretion normalized by body surface area, and (D) urinary pH values recorded in metabolic cages before and after administration of 15 or 60 mg/L of L-Erg. (E) L-Erg and (F) S-Met-L-Erg urinary concentration in μM before and after administration of 15 mg/L or 60 mg/L of L-Erg. (G) Ratio between post and pre-treatment levels of L-Erg and S-Met-L-Erg in the urine of treated mice. E, F and G graphs are shown using a logarithmic scale for visualization purposes. (H) Calculated L-Erg mean dose during the 1-month L-Erg treatment taking into consideration L-Erg drinking water concentration, mice body weight and water intake. The dashed black line indicates the 16 mg/kg/day dose. Each dot represents an individual mouse, and the bars indicate the mean ± SEM. p-value is shown at the top if significant after Mann-Whitney-Wilcoxon test (***p < 0.001).
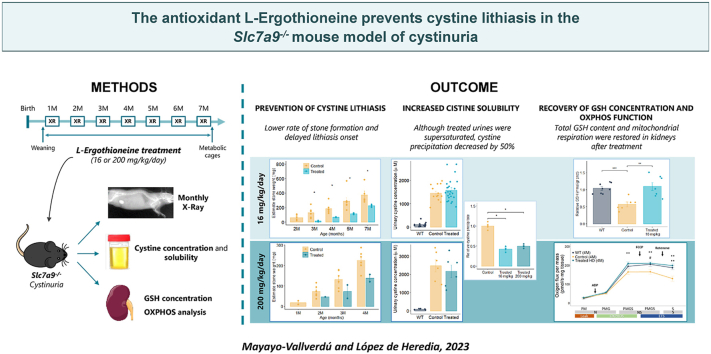
3.2. Long-term L-Erg treatment prevents cystine lithiasis and delays its onset
Since cystinuria patients have an early onset and recurrent stone episodes, L-Erg was administered to mice before stone formation as a preventive and sustained treatment: from weaning to 7 months of age (Fig. 2a). L-Erg concentration in the drinking water was adjusted weekly according to mouse weight and water intake to administer a dose of 16 mg/kg/day throughout the treatment (Supplementary Fig. S1). The 6-months treated group showed above 60% decrease in stone-former mice (relative risk = 0.375) (Fig. 2b), independently of mice sex (Supplementary Fig. S2). In addition, through the X-ray follow-up, a 2-month delay in stone onset and growth evolution was observed in the treated group (Fig. 2c). Post-mortem stone weight of the treated group was also significantly lower compared to control group (Fig. 2d).
Fig. 2.
Long-term L-Erg treatment reduces and delays stone formation in theSlc7a9−/−mouse model. (A) Timeline of preventive L-Erg (16 mg/kg/day) treatment from weaning to seven months of age in Slc7a9−/− mice. (B) Evolution of the rate of stone-former mice in control and treated groups (N = 40 per group). (C) Comparison of the monthly stone weight evolution in control and treated stone-former mice. The stone weight follow-up was estimated by X-ray images. (D) Post-mortem stone weight in control and treated stone-former mice after L-Erg treatment. Each dot represents an individual mouse, and the bars indicate the mean ± SEM. p-value is shown at the top if significant after Mann-Whitney-Wilcoxon test (*p < 0.05). M = Month of age, XR = X-ray.
3.3. L-Erg treatment increases urinary cystine solubility without affecting metabolic parameters or chelating cysteine
To study L-Erg mechanism of action, urinary parameters related to other cystinuria treatment approaches were evaluated. No significant differences were observed when comparing the urinary excretion volume and pH levels of treated and control mice (Fig. 3a–b), revealing that L-Erg does not act as a diuretic or urinary alkalinizing drug. Then, to evaluate if L-Erg could be chelating cysteine, an in vitro assay combining different concentrations of L-Erg and cysteine was performed, but no L-Erg-cysteine dimers were detected by LC/MS-MS. Moreover, after L-Erg treatment, no differences in urinary cystine concentration were observed between treated and control mice, with both urines being highly concentrated compared to WT mice and confirming that L-Erg does not chelate cysteine (Fig. 3c). Then, urinary cystine solubility was assessed both ex vivo and in vitro by a cystine precipitation assay. After adding a supersaturated cystine solution to control and treated urines, the cystine precipitate was significantly lower in treated urines (Fig. 3d). Thus, although treated urines contained high amounts of cystine, L-Erg treatment decreased its precipitation which could explain the observed effect on stone formation. Next, cystine stones were analyzed using a SEM coupled with an EDS detector. High-resolution images showed that the structure of cystine stones was not affected by L-Erg treatment, neither on the stone surface, where the hexagonal prismatic crystals were preserved and no signs of corrosion were found, nor in the core of the stone, where the same stone cleavage pattern was observed (Fig. 3e). Also, control and treated cystine stones showed the same elemental composition spectra (Fig. 3e).
Fig. 3.
Long-term L-Erg treatment increases cystine solubility without affecting physiological parameters and cystine crystal structure. (A) Urinary excretion normalized by body surface area in control and L-Erg treated mice. (B) Urinary pH in control and L-Erg treated mice. (C) Urinary cystine concentration in μM in WT, control and L-Erg treated mice. (D)l-cystine precipitate relative to control mice after adding a supersaturated l-cystine solution (4 mM) to a urine pool from control and L-Erg treated mice. (E) Representative SEM images of the surface (I) and core (II) of a cystine stone from control mice and of the surface (III) and core (IV) of a cystine stone from treated mice, and their corresponding EDS spectra. Each dot represents an individual mouse, and the bars indicate the mean ± SEM. p-value is shown at the top if significant after Mann-Whitney-Wilcoxon test (*p < 0.05). C = Carbon, N = Nitrogen, O = Oxygen, Al = Aluminum, S =Sulphur.
3.4. High-dose L-Erg treatment prevents cystine lithiasis
To determine whether the effect observed after L-Erg treatment could be improved, a high-dose L-Erg treatment was performed from weaning and up to 4-month-old (Fig. 4a). The dose selected was 200 mg/kg/day, corresponding to a Human Equivalent Dose (HED) of 1 g/day for a 60 kg adult [46]. During the 4-month treatment, no effect of L-Erg on mice weight, water intake and urinary cystine concentration was observed (Supplementary Figs. S3–S4). However, the treated group showed a 70% decrease in the rate of stone former mice (relative risk = 0.283), a delay in the stone onset and a lower post-mortem stone weight (Fig. 4b–d). A slight increase in effectivity was observed compared to the previous dose, but in the treated group still two mice developed stones. Then, urinary levels of L-Erg and S-Met-L-Erg were assessed in control, low and high-dose treated mice. Regardless of treatment, all stone-former mice had the lowest urinary S-Met/L-Erg ratio of each condition due to a lower amount of S-Met-L-Erg (Fig. 4e–g), suggesting a poorer internal metabolism of L-Erg in stone-former mice and revealing the importance of L-Erg metabolism rather than the L-Erg levels in urine.
Fig. 4.
High-dose L-Erg treatment reduces and delays stone formation in theSlc7a9−/−mouse model. (A) Timeline of preventive L-Erg (200 mg/kg/day) treatment from weaning to 4 months of age in Slc7a9−/− mice. (B) Evolution of the rate of stone-former mice in control and high-dose-treated groups (N = 55 per group). (C) Comparison of the monthly stone weight evolution in control and high-dose-treated stone-former mice. The stone weight follow-up was estimated by X-ray images. (D) Post-mortem stone weight in control and high-dose-treated stone-former mice after L-Erg treatment. Urinary levels of (E) L-Erg, (F) S-Met-L-Erg and (G) their ratio in control, low-dose and high-dose-treated non-stone-former (NSF) and stone-former (SF) mice grouped by sex. Control and treated mice are plotted in different graphs for visualization purposes. Each dot represents an individual mouse, and the bars indicate the mean ± SEM. p-value is shown at the top if significant after Mann-Whitney-Wilcoxon test (*p < 0.05, **p < 0.01). M = Month of age, XR = X-ray, M = male, F = Female, Cntrl = Control, LD = Low-dose (16 mg/kg/day), HD = High-dose (200 mg/kg/day), NSF = Non-stone-former, SF = Stone-former.
3.5. L-Erg metabolism is essential for treatment effectiveness
As the above results suggested that L-Erg internal metabolism was necessary to prevent the stone formation process, 16 mg/kg/day of L-Erg was administered to the Slc7a9−/−Slc22a4−/− mice (cystinuria model lacking L-Erg transporter). Preventive treatment from weaning to 4 months of age did not show significant differences in stone onset, growth, or post-mortem weight compared to control group (Fig. 5a–c). Results evidence that L-Erg internalization and metabolization are essential for treatment effectiveness. To explore whether S-Met-L-Erg was the metabolite responsible for the increased solubility of cystine in the urine, a cystine precipitation assay was performed to compare the precipitate obtained in urines from both L-Erg treatments and urines supplemented in vitro with L-Erg or S-Met-L-Erg. While both treatments significantly reduced cystine precipitate by 50%, independently of the urine's pH (Supplementary Fig. S5), no effect was observed in the in vitro supplemented ones (Fig. 5d).
Fig. 5.
L-Erg metabolism is essential for treatment effectiveness. (A) Evolution of the rate of stone-former mice percentage in control and treated dKO mice (N = 22 per group). (B) Comparison of the monthly stone weight evolution in control and treated dKO stone-former mice. The stone weight follow-up was estimated by X-ray images. (C) Post-mortem stone weight in control and treated dKO stone-former mice after L-Erg treatment. (D) l-cystine precipitate relative to control mice after adding a supersaturated l-cystine solution (4 mM) to a urine pool from Slc7a9−/− control mice (Cntrl), Slc7a9−/− mice treated with a low-dose of L-Erg (16 mg/kg/day) (Treated LD), Slc7a9−/− mice treated with a high dose of L-Erg (200 mg/kg/day) (Treated HD), Slc7a9−/− control mice but in vitro supplemented with L-Erg to obtain a final urine concentration of 0.5 mM (Cntrl + L-Erg) and Slc7a9−/− control mice but in vitro supplemented with S-Met-L-Erg to obtain a final urine concentration of 0.5 mM (Cntrl + S-Met). Each dot represents an individual mouse, and the bars indicate the mean ± SEM. p-value is shown at the top if significant after Mann-Whitney-Wilcoxon test (*p < 0.05). dKO = Slc7a9−/−Slc22a4−/− mice, Cntrl = Control, LD = Low dose (16 mg/kg/day), HD = High dose (200 mg/kg/day).
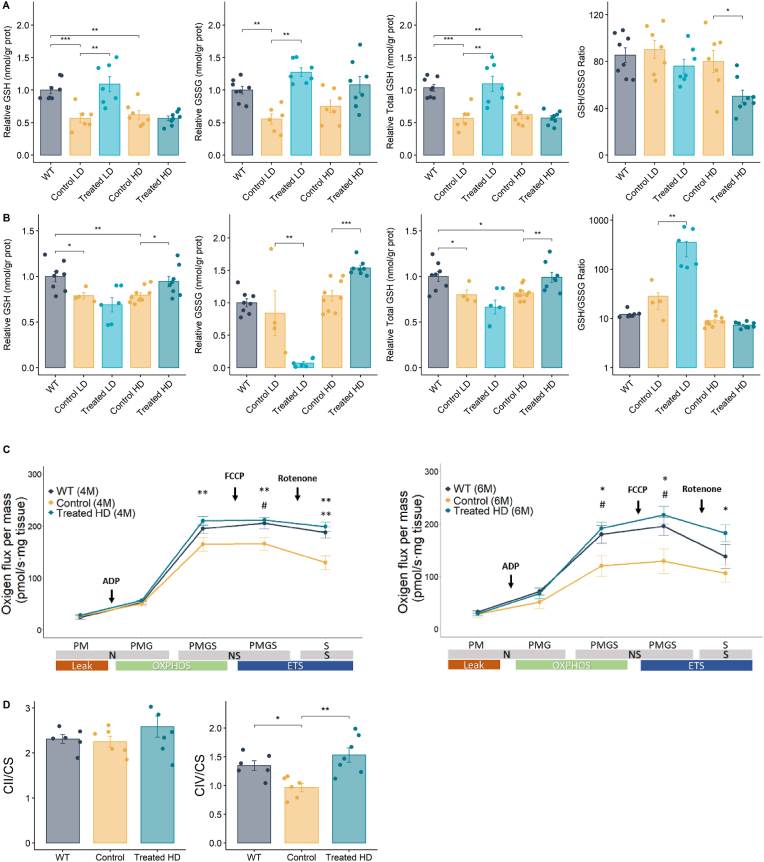
3.6. Long-term L-Erg treatment ameliorates oxidative damage in cystinuric mice
As L-Erg properties described so far are related to its condition as an antioxidant molecule and cysteine is the limiting precursor of GSH, the oxidative status of WT, control and treated mice was studied. Intracellular GSH and GSSG pools in the kidneys of Slc7a9−/− mice were about 40% lower than those of WT mice, revealing a decrease in total GSH content in cystinuric mice, although GSH/GSSG ratio was preserved (Fig. 6a). Low-dose L-Erg treatment significantly increased GSH and GSSG content recovering total kidney GSH, but such effect was not observed in high-dose treated mice (Fig. 6a). Similar results were obtained in the liver, where total GSH content was decreased by 25% in cystinuric mice compared to WT mice, while no differences in GSH/GSSG ratio were detected (Fig. 6b). Low- and high-dose L-Erg treatment also showed disparities, as low-dose treatment significantly increased GSH/GSSG ratio due to lower GSSG levels, while high-dose treatment restored total liver GSH content without changing GSH/GSSG ratio (Fig. 6b).
Fig. 6.
Long-term L-Erg treatment ameliorates oxidative damage and mitochondrial function in Slc7a9−/− mice. (A) Kidney and (B) liver reduced (GSH), oxidized (GSSG) and total (GSH + GSSG) GSH content, and GSH/GSSG ratio of WT, low-dose (LD) and high-dose (HD) L-Erg treated mice, and their respective control mice. Levels were normalized by kidney or liver total protein content (grams of proteins) and plotted normalized to WT levels. (C) Ex vivo mitochondrial respiration of fresh saponin permeabilized kidney biopsies of WT, control and high-dose L-Erg treated mice at 4 (left graph) and 6 (right graph) months of age. N-Leak respiration was measured adding pyruvate and malate (PM), N-OXPHOS capacity adding ADP and glutamate (PMG), NS-OXPHOS capacity adding succinate (PMGS), NS-ETS capacity adding FCCP and S-ETS capacity adding rotenone. Oxygen flux was normalized by mg of kidney tissue. The upper p-value corresponds to the comparison between control and treated mice, and the lower p-value, to WT and control comparison. N = 5–7. (D) Kidney mitochondrial succinate dehydrogenase (CII) and cytochrome c oxidase (CIV) activity in control and high-dose L-Erg treated mice at 4 months of age. Activity levels were calculated in nmol/min per mg of protein and normalized to citrate synthase (CS) activity. Each dot represents an individual mouse, and the bars indicate the mean ± SEM. p-value is shown at the top after Mann-Whitney-Wilcoxon test. #p < 0.1, *p < 0.05, **p < 0.01, ***p < 0.001. Cntrl = Control, LD = Low-dose (16 mg/kg/day), HD = High-dose (200 mg/kg/day), N = NADH linked pathway, NS = NADH and succinate linked pathways, S = succinate linked pathway, OXPHOS = oxidative phosphorylation, ETS = Electron transport system, FCCP = Carbonyl cyanide 4-(trifluoromethoxy)-phenylhydrazone, CII = Complex II, CIV = Complex IV, CS = Citrate Synthase.
Moreover, mitochondria function was assessed in the kidneys of cystinuric mice by high-resolution respirometry. At 4 months of age, cystinuric mice showed lower maximal mitochondrial respiration capacity after adding the uncoupling agent FCCP and, when complex I was inhibited with rotenone, lower respiration was also detected, suggesting dependence on complex I to maintain mitochondrial respiration in cystinuria (Fig. 6c). At 6 months of age, differences between WT and cystinuric mice increased, indicating an age-related decrease in mitochondrial respiration in our model (Fig. 6c). Nevertheless, high-dose L-Erg treatment restored mitochondrial respiration at both 4 and 6 months of age (Fig. 6c). The enzymatic activity of complex II and IV was also evaluated at 4 months of age and, while non-significant differences were observed in complex II, a decrease in complex IV activity was observed in cystinuric mice compared to WT mice, defect that was restored by L-Erg treatment (Fig. 6d).
4. Discussion
Current therapeutical approaches for cystinuria aim to prevent the precipitation of cystine crystals and the consequent stone formation. However, many patients continue to develop cystine stones every few years [47]. Here, we have demonstrated the therapeutical effect of L-Erg preventing cystine lithiasis in the Slc7a9−/− mouse model by increasing urinary cystine solubility and improving some parameters related to the oxidative stress status in the kidney. L-Erg is an antioxidant and cytoprotective molecule which safety has been deeply studied and approved by regulatory agencies as FDA [48] and EFSA [49].
As cystinuria is a lifelong disease with an early onset, mice were treated from weaning and for 6 months. L-Erg (16 mg/kg/day) treatment decreased the rate of stone formation and delayed the lithiasis onset in those treated mice that developed stones. No effect was observed regarding mice weight and development, water intake, urinary excretion levels, or urinary pH, allowing to ensure the safety of L-Erg and discarding a diuretic or urine alkalinizing mechanism of action. Furthermore, although L-Erg is a thiol-thione molecule, we demonstrated by two different approaches that L-Erg does not chelate cysteine in urines, and thus does not act as a thiol-binding drug. Supporting this finding, L-Erg-cysteine dimerization has been only observed in strongly acidic solutions [50].
The HED of L-Erg 16 mg/kg/day treatment is 77.8 mg/day for a 60 kg adult [46] and, as the L-Erg NOAEL (No Observed Adverse Effect Level) established for adults, pregnant and infants is 800 mg/kg/day [48], we increase the dose administered. High-dose (200 mg/kg/day) L-Erg treatment confirmed the preventive effect by decreasing the rate of stone formation and delaying the stone onset. However, although the administered dose was 10-fold higher, the additional benefits observed were slight. By analyzing the urinary levels of L-Erg and its metabolite S-Met-L-Erg, we found that all stone-former mice, including the ones in the treated group, had the lowest S-Met/L-Erg ratio of their condition due to lower levels of S-Met-L-Erg. These results revealed, in addition to confirming that the ratio of S-Met-L-Erg to L-Erg is a robust marker of cystine lithiasis [26], that the internal metabolism of L-Erg is involved in the prevention of cystine lithiasis. This hypothesis was confirmed when Slc7a9−/−Slc22a4−/− mice were treated with L-Erg (16 mg/kg/day), as no differences were observed in stone formation rate or onset between control and treated mice. This could explain the slight additional benefits observed in the high-dose treatment, as a pharmacokinetic study of L-Erg in mouse tissues showed no differences in the accumulation of L-Erg and its metabolites in the kidney when comparing two different doses: 35 and 70 mg/kg/day. Therefore, a dose lower than 35 mg/kg/day may saturate the intracellular accumulation and metabolism of L-Erg [51].
Although no differences in urinary cystine concentration were detected between control and treated mice at the end of the experiments, a significant increase in cystine solubility was observed in treated urines, regardless of urinary pH, cysteine chelation, or induction of hydration or diuresis. Consistent with stone formation rate results, no differences in cystine solubility were observed between urines from low- and high-dose L-Erg treatments: both decreased cystine precipitate by 50%. But, when L-Erg or S-Met-L-Erg were added in vitro to control urines, no changes in cystine solubility were detected, suggesting that they are not the direct metabolites responsible for preventing cystine precipitation. Remarkably, the antioxidant α-lipoic acid also increases cystine solubility in the urine of cystinuric mice through its derived metabolism, but the specific metabolite(s) involved have not yet been identified [23]. Similarly, Salvianolic acid B, reduced cystine crystal formation when administered to mice, but its mechanism of action has not been elucidated beyond relating it to its antioxidant and cytoprotective capacity [24].
Disruptions in oxidative stress have been observed in cystinuria. In patients, decreased GSH levels in leukocytes [52], accumulation of lipid peroxidation and variations in superoxide dismutase (SOD), glutathione peroxidase (GPx) and inducible nitric oxide synthase (iNOS) antioxidant enzyme activities in blood have been described [24,53]. Likewise, decreased GSH levels in livers of the Slc3a1−/− mouse model [54] and alterations in lipid peroxidation and SOD and GPx enzyme activities in kidneys of the Slc7a9−/− mouse model have been observed [24]. In this work, GSH levels were determined in kidneys and livers of the Slc7a9−/− mouse model and, in both tissues, a lower GSH content was observed compared to WT mice. These findings strengthen the evidence for a compromised antioxidant capacity in cystinuria that could be improved with antioxidant treatments.
Low-dose L-Erg treatment restored total GSH levels in the kidneys and increased GSH/GSSH ratio in the livers of cystinuric mice. However, high-dose L-Erg treatment had no effect on the kidneys but restored total GSH content in the livers. These differences in kidney between low and high-dose treatment could be explained as it has been described that after the neutralization of reactive species by L-Erg, L-Erg can be regenerated by consuming GSH [55]. Thus, the increase in GSH consumption could counteract the effect observed in the low-dose treatment and lead to a decrease in the GSH/GSSG ratio. As the liver is the organ with the highest GSH levels and it is responsible for whole organism GSH homeostasis, this could explain why is need a high dose of L-Erg to restore its GSH levels. Previous L-Erg treatments in rat models of type 2 diabetes and cisplatin-evoked nephrotoxicity, in addition to restoring kidney GSH concentration, increased antioxidant defenses such as SOD and GPx, and decreased lipid peroxidation [33,56]. Thus, L-Erg treatment might be able to recover the antioxidant defect described in cystinuria [24,[52], [53], [54]].
Here, we have also described an OXPHOS-related impairment of mitochondrial activity in our cystinuria model at 4 and 6 months of age that could be restored with L-Erg treatment. It has been described that complex IV is the rate-limiting enzyme of the mitochondrial respiratory chain [57,58]. Thus, the lower activity of complex IV observed in cystinuric mice could explain the decrease in their maximal respiratory capacity, which plays a crucial role in organs under chronic disease-related stress [[59], [60], [61]]. Although only in vitro studies have described the presence of the L-Erg transporter OCTN1 in the mitochondrial membrane [[62], [63], [64]], L-Erg detection in mitochondria fractions [38] demonstrated that targets this organelle. In addition, L-Erg administration has shown to prevent ROS production both in vitro [[65], [66], [67]] and in kidneys [68].
Oxidative stress and mitochondrial dysfunction have been linked to the pathophysiology of other urolithiasis [[69], [70], [71]]. The interaction of crystals with renal epithelial cells promotes cytotoxicity, which leads to membrane disruption, ROS production and mitochondrial damage [[72], [73], [74]]. Antioxidants that target mitochondrial defects showed to decrease crystal depositions in mouse models of kidney stone diseases by lowering ROS and lipid peroxidation, and recovering antioxidant defenses and OXPHOS activity [[74], [75], [76], [77], [78], [79]]. In vitro studies demonstrated cytotoxicity of cyst(e)ine on renal epithelial cells, leading to lipid peroxidation and necrosis induction [[80], [81], [82]]. Interestingly, a recent proteomic analysis of cystine stones from mice revealed that the proteins overrepresented in the initial stage of cystine stone formation, apart from ribosomal proteins, were OXPHOS and the TCA cycle elements [83], closely related to mitochondrial metabolism. Similarly, mitochondrial proteins have been found in stone matrices of calcium oxalate stones being related to crystal nucleation [84]. Thus, high cystine concentrations in the renal tubules that induce cell death and lipid peroxidation, together with low GSH levels and mitochondrial dysfunction that induce apoptotic cell death, could lead to the release of cellular debris and cell organelles into the lumen favoring the nucleation and aggregation of cystine crystals.
A weakness of the study could be the method of drug delivery chosen. Other methods were considered to better control the dose administered, but oral administration via drinking water has been applied in studies to test cystinuria treatments [85] and ensures continuous drug delivery while minimizing handling and stress to the mice, essential in long-term treatments. Also, although it saturates the L-Erg transporter, high-dose treatment was unable to totally prevent cystine stone formation probably due to differences in L-Erg metabolism. In humans, studies addressing the saturation point of L-Erg transporter have not yet been conducted, but there are SLC22A4 polymorphisms related to an increase [86] or impaired L-Erg transport [87]. More evidence is needed to understand the role of SLC22A4 gene in cystinuria patients and to establish the appropriate dose of L-Erg to prevent cystine lithiasis.
To date, there is only one study that addresses the administration of L-Erg in humans [88], although different clinical trials are underway to determine the potential benefit of this molecule in different pathological conditions and to understand its pharmacokinetics better. From the published results, we can extrapolate that by administering a dose of 25 mg/kg/day of L-Erg for 7 days to humans (mouse equivalent dose of 307.5 mg/kg/day), both L-Erg and S-Met-L-Erg levels increase proportionally in urine [88]. This suggests that L-Erg metabolism in humans is saturated at higher doses than in mice. Moreover, patients who have taken this dose have reported no side effects. However, to evaluate the effective dose and the optimal schedule of L-Erg administration, recurrent cystine precipitation assays of the urine of treated patients should be performed to assess treatment response.
Cytoprotective properties [24,89], induction of GSH synthesis [89,90] and improvement of mitochondrial function [91,92] have also been reported for α-Lipoic Acid and Salvianolic acid B. These common pathways with L-Erg could explain their effect on cystine lithiasis. However, the HED of the doses tested in cystinuria animal models are 2700 [23] and 326 [24] mg/day for α-Lipoic Acid and Salvianolic acid B, respectively, which are higher than the doses tested that cause side effects in humans. Regarding α-Lipoic Acid, in humans, doses of 600, 1200 and 1800 mg/day reported a 27%, 43% and 54% of side effects [93]. Most of them, were gastrointestinal: nausea, vomiting and dizziness, which can lead to treatment discontinuation. Furthermore, the effect of α-Lipoic Acid on cystine lithiasis has already been reported to be dose-dependent in mice, being half as effective at half the dose of 2700 mg/day [23]. As for Salvianolic acid B, at the dose tested in mice, mild renal and hepatic damage was observed in humans [94]. We therefore believe that L-Erg, for which no side effects have been reported in humans at doses higher than those administered in the mouse model [48] is a molecule with high potential for the treatment of cystine lithiasis.
In conclusion, L-Erg treatment prevented and delayed cystine lithiasis in a cystinuria mouse model by increasing urinary cystine solubility and restored kidney GSH content and OXPHOS function. Further evidence from clinical trials in cystinuria patients is needed to evaluate L-Erg effect in combination with conservative therapies as a preventive treatment for cystine lithiasis.
Funding
This work has been funded by the Instituto de Salud Carlos III through the projects PI16/00267-R-FEDER and PI20/00200 to VN (Co-funded by European Regional Development Fund. ERDF, a way to build Europe), and by La Marató de TV3 through the project 202025-30 to VN and 202025-32 to FVP. Generalitat de Catalunya Grant SGR2017-191 to VN. We also thank CERCA Programme/Generalitat de Catalunya for institutional support.
Authors’ contribution
Secure Funding: VN; treatments experimental design: CM-V, CV, MLdH and VN; L-Erg and S-Met-L-Erg analysis: CM-V, MLdH, LM, CS and AL; treatment follow-up and analyses: LG, EP, CM-V, MLdH and VN, cystine analyses: CM-V, MC and RA, GSH and GSSG analyses: MAA, CM-V, FVP; Mitochondrial analyses: CM-V, PMG-R, MEG and GG, manuscript drafts and figure versions: CM-V and MLdH, with the supervision of VN. All authors revised the manuscript and approved the final version.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Virginia Nunes’ group received support funded by AVEROA SAS.
Acknowledgements
We acknowledge B. García and M. Vecino for technical support; M. Fernández-Vaquero and L. Lucena for support in cystine lithiasis quantification; E. Tobias for her guidance in the measurement of respiratory chain enzymatic activities; Dr. O. Jauregui for her guidance in LC-MS/MS analysis; Dr. Y. Kato for the mouse model Slc22a4−/−; JC Yadan for providing l-Ergothioneine and S-Met-L-Erg; RA thanks to Turrons Vicens and Carmen de Torres grant (Institut de recerca Sant Joan de Déu) for the support to his laboratory work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2023.102801.
Contributor Information
Clara Mayayo-Vallverdú, Email: cmayayo@idibell.cat.
Virginia Nunes, Email: vnunes@idibell.cat.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Modersitzki F., Pizzi L., Grasso M., Goldfarb D.S. Health-related quality of life (HRQoL) in cystine compared with non-cystine stone formers. Urolithiasis. 2014;42(1):53–60. doi: 10.1007/s00240-013-0621-4.Health-related. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Streeper N.M., Wertheim M.L., Nakada S.Y., Penniston K.L. Cystine stone formers have impaired health-related quality of life compared with noncystine stone formers: a case-referent study piloting the Wisconsin stone quality of life questionnaire among patients with cystine stones. J. Endourol. 2022;31(1):48–53. doi: 10.1089/end.2016.0564. [DOI] [PubMed] [Google Scholar]
- 3.Feliubadaló L., Font M., Purroy J., et al. Non-type I cystinuria caused by mutations in SLC7A9, encoding a subunit (bo,+AT) of rBAT. Nat. Genet. 1999;23(1):52–57. doi: 10.1038/12652. [DOI] [PubMed] [Google Scholar]
- 4.Pras E., Sood R., Raben N., Aksentijevich I., Chen X., Kastner D.L. Genomic organization of SLC3A1, a transporter gene mutated in cystinuria. Genomics. 1996;36(1):163–167. doi: 10.1006/geno.1996.0437. [DOI] [PubMed] [Google Scholar]
- 5.Pfeiffer R., Loffing J., Rossier G., et al. Luminal heterodimeric amino acid transporter defective in cystinuria. Mol. Biol. Cell. 1999;10:4135–4147. doi: 10.1091/mbc.10.12.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morin C.L., Thompson M.W., Jackson S.H., Sass-Kortsak A. Biochemical and genetic studies in cystinuria: observations on double heterozygotes of genotype I-II. J. Clin. Invest. 1971;50(9):1961–1976. doi: 10.1172/JCI106688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dent C., Rose G. Amino acid metabolism in cystinuria. J. Med. 1951;20:205–219. doi: 10.3181/00379727-78-19189. [DOI] [PubMed] [Google Scholar]
- 8.Stein W.H. Excretion of amino acids in cystinuria. Proc. Soc. Exp. Biol. Med. 1951;78(3):705–708. doi: 10.3181/00379727-78-19189. [DOI] [PubMed] [Google Scholar]
- 9.Königsberger E., Wang Z., Königsberger L.-C. Solubility of L-cystine in NaCl and artifcial urine solutions. Chem. Mon. 2000;131:39–45. doi: 10.1007/s007060050004. [DOI] [Google Scholar]
- 10.Leusmann D.B., Blaschke R., Schmandt W. Results of 5035 stone analyses: a contribution to epidemiology of urinary stone disease. Scand. J. Urol. Nephrol. 1990;24:205–210. doi: 10.3109/00365599009180859. [DOI] [PubMed] [Google Scholar]
- 11.Erbagci A., Ergbagci A., Yilmaz M., et al. Pediatric urolithiasis. Scand. J. Urol. Nephrol. 2003;37:129–133. doi: 10.1080/00365590310008866. [DOI] [PubMed] [Google Scholar]
- 12.Eggermann T., Venghaus A., Zerres K. Cystinuria : an inborn cause of urolithiasis. Orphanet J. Rare Dis. 2012;7(19):1–11. doi: 10.1186/1750-1172-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knoll T., Zöllner A., Maurice G.W., Michel S. Cystinuria in childhood and adolescence : recommendations for diagnosis , treatment , and follow-up. Pediatr. Nephrol. 2005;20:19–24. doi: 10.1007/s00467-004-1663-1. [DOI] [PubMed] [Google Scholar]
- 14.Thomas K., Wong K., Withington J., Bultitude M., Doherty A. Cystinuria — a urologist ’ s perspective. Nat. Rev. Urol. 2014;11:270–277. doi: 10.1038/nrurol.2014.51. [DOI] [PubMed] [Google Scholar]
- 15.Kum F., Wong K., Game D., Bultitude M., Thomas K. Hypertension and renal impairment in patients with cystinuria : findings from a specialist cystinuria centre. Urolithiasis. 2019;47(4):357–363. doi: 10.1007/s00240-019-01110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Servais A., Thomas K., Dello Strologo L., et al. Cystinuria: clinical practice recommendation. Kidney Int. 2020;99(1):48–58. doi: 10.1016/j.kint.2020.06.035. [DOI] [PubMed] [Google Scholar]
- 17.Deberardinis R.J., Coughlin C.R., Kaplan P. Penicillamine therapy in pediatric cystinuria: experience from a cohort of American children. J. Urol. 2008;180(6):2620–2623. doi: 10.1016/j.juro.2008.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prot-Bertoye C., Lebbah S., Daudon M., et al. Adverse events associated with currently used medical treatments for cystinuria and treatment goals: results from a series of 442 patients in France. BJU Int. 2019;124(5):849–861. doi: 10.1111/bju.14721. [DOI] [PubMed] [Google Scholar]
- 19.Bai Y., Tang Y., Wang J., et al. Tolvaptan treatment of cystine urolithiasis in a mouse model of cystinuria. World J. Urol. 2021;39(1):263–269. doi: 10.1007/s00345-020-03166-3. [DOI] [PubMed] [Google Scholar]
- 20.Lee M.H., Sahota A., Ward M.D., Goldfarb D.S. Cystine growth inhibition through molecular mimicry: a new paradigm for the prevention of crystal diseases. Curr. Rheumatol. Rep. 2015;17(5):1–6. doi: 10.1007/s11926-015-0510-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu L., Yang Y., Sloysius H., et al. L-cystine diamides as L-cystine crystallization inhibitors for cystinuria. Physiol. Behav. 2016;176(1):139–148. doi: 10.1021/acs.jmedchem.6b00647.L-Cystine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y., Albanyan H., Lee S., et al. Bioorganic & Medicinal Chemistry Letters Design , synthesis , and evaluation of L -cystine diamides as L -cystine crystallization inhibitors for cystinuria. Bioorg. Med. Chem. Lett. 2018;28:1303–1308. doi: 10.1016/j.bmcl.2018.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zee T., Bose N., Zee J., et al. α-Lipoic acid treatment prevents cystine urolithiasis in a mouse model of cystinuria. Nat. Med. 2017;23(3):288–290. doi: 10.1038/nm.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yifan Z., Luwei X., Kai L., Liuhua Z., Yuzheng G., Ruipeng J. Protective effect of salvianolic acid B against oxidative injury associated with cystine stone formation. Urolithiasis. 2019;47(6):503–510. doi: 10.1007/s00240-019-01114-4. [DOI] [PubMed] [Google Scholar]
- 25.Mohammadi M., Shohani A., Khorami H., et al. The effect of selenium supplementation on cystine crystal volume in patients with cystinuria. Biomed. 2018;8(4):28–32. doi: 10.1051/bmdcn/2018080426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez de Heredia M., Muñoz L., Carru C., et al. S-Methyl-L-Ergothioneine to L-ergothioneine ratio in urine is a marker of cystine lithiasis in a cystinuria mouse model. Antioxidants. 2021;10(9):1424. doi: 10.3390/antiox10091424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang N.C., Lin H.-C., Wu J.-H., et al. Ergothioneine protects against neuronal injury induced by b-amyloid in mice. Food Chem. Toxicol. 2012;50(11):3902–3911. doi: 10.1016/j.fct.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 28.Cheah I.K., Tang R., Ye P., Yew T.S.Z., Lim K.H.S., Halliwell B. Liver ergothioneine accumulation in a Guinea pig model of non-alcoholic fatty liver disease. A possible mechanism of defence? Free Radic. Res. 2016;50(1):14–25. doi: 10.3109/10715762.2015.1099642. [DOI] [PubMed] [Google Scholar]
- 29.Smith E., Ottosson F., Hellstrand S., et al. Ergothioneine is associated with reduced mortality and decreased risk of cardiovascular disease | Enhanced Reader. Heart. 2020;106(9):691–697. doi: 10.1136/heartjnl-2019-315485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morillon A.-C., Williamson R.D., Baker P.N., et al. Effect of L-Ergothioneine on the metabolic plasma profile of the RUPP rat model of pre-eclampsia. PLoS One. 2020;15(3) doi: 10.1371/journal.pone.0230977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makiishi S., Furuichi K., Yamamura Y., et al. Carnitine/organic cation transporter 1 precipitates the progression of interstitial fibrosis through oxidative stress in diabetic nephropathy in mice. Sci. Rep. 2021;11:9093. doi: 10.1038/s41598-021-88724-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shinozaki Y., Furuichi K., Toyama T., et al. Impairment of the carnitine/organic cation transporter 1–ergothioneine axis is mediated by intestinal transporter dysfunction in chronic kidney disease. Kidney Int. 2017;92(6):1356–1369. doi: 10.1016/j.kint.2017.04.032. [DOI] [PubMed] [Google Scholar]
- 33.Dare A., Channa M.L., Nadar A. L-ergothioneine and its combination with metformin attenuates renal dysfunction in type-2 diabetic rat model by activating Nrf2 antioxidant pathway. Biomed. Pharmacother. 2021;141 doi: 10.1016/j.biopha.2021.111921. [DOI] [PubMed] [Google Scholar]
- 34.Pfeiffer C., Bauer T., Surek B., Schömig E., Gründemann D. Cyanobacteria produce high levels of ergothioneine. Food Chem. 2011;129(4):1766–1769. doi: 10.1016/j.foodchem.2011.06.047. [DOI] [Google Scholar]
- 35.Genghof D.S. Biosynthesis of ergothioneine and hercynine by mycobacteria. J. Bacteriol. 1964;103(2):475–478. doi: 10.1128/jb.103.2.475-478.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kornberg H.L., Krebs H.A. Biosynthesis of ergothioneine. Gr Nat. Publ. 1957;180:756–757. [Google Scholar]
- 37.Kato Y., Kubo Y., Iwata D., et al. Gene knockout and metabolome analysis of carnitine/organic cation transporter OCTN1. Pharm. Res. (N. Y.) 2010;27(5):832–840. doi: 10.1007/s11095-010-0076-z. [DOI] [PubMed] [Google Scholar]
- 38.Kawano H., Otani M., Takeyama K., Kawai Y., Mayumi T., Hama T. Studies on ergothioneine. Distribution and fluctuations of ergothioneine in rats. Chem. Pharm. Bull. 1982;30(5):1760–1765. doi: 10.1248/cpb.30.1760. [DOI] [PubMed] [Google Scholar]
- 39.Halliwell B., Cheah I.K., Drum C.L. Ergothioneine, an adaptive antioxidant for the protection of injured tissues? A hypothesis. Biochem. Biophys. Res. Commun. 2016;470(2):245–250. doi: 10.1016/j.bbrc.2015.12.124. [DOI] [PubMed] [Google Scholar]
- 40.Feliubadaló L., Arbonés M., Mañas S., et al. Slc7a9-deficient mice develop cystinuria non-l and cystine urolithiasis. Hum. Mol. Genet. 2003;12(17):2097–2108. doi: 10.1093/hmg/ddg228. [DOI] [PubMed] [Google Scholar]
- 41.Casado M., Sierra C., Batllori M., Artuch R., Ormazabal A. A targeted metabolomic procedure for amino acid analysis in different biological specimens by ultra-high-performance liquid chromatography–tandem mass spectrometry. Metabolomics. 2018;14(6):1–12. doi: 10.1007/s11306-018-1374-4. [DOI] [PubMed] [Google Scholar]
- 42.Escobar J., Sánchez-illana Á., Kuligowski J., et al. Analysis Development of a reliable method based on ultra-performance liquid chromatography coupled to tandem mass spectrometry to measure thiol-associated oxidative stress in whole blood samples. J. Pharm. Biomed. Anal. 2016;123:104–112. doi: 10.1016/j.jpba.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Cantó C., Garcia-Roves P.M. High-resolution respirometry for mitochondrial characterization of ex vivo mouse tissues. Curr. Protoc. Mol. Biol. 2015;5(2):135–153. doi: 10.1002/9780470942390.mo140061. [DOI] [PubMed] [Google Scholar]
- 44.Doerrier C., Garcia-Souza L., Krumschnabel G., Wohlfater Y., Meszaros A., Gnaiger E. High-resolution FluoRespirometry and OXPHOS protocols for human cells, permeabilized fibers from small biopsies of muscle, and isolated mitochondria. Methods Mol. Biol. 2018;1782:31–70. doi: 10.1007/978-1-4939-7831-1_3. [DOI] [PubMed] [Google Scholar]
- 45.Spinazzi M., Casarin A., Pertegato V., Salviati L., Angelini C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat. Protoc. 2012;7(6):1235–1246. doi: 10.1038/nprot.2012.058. [DOI] [PubMed] [Google Scholar]
- 46.Nair A.B., Jacob S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016;7(2):27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore S.L., Cook P., de Coninck V., et al. Outcomes and long-term follow-up of patients with cystine stones: a systematic review. Curr. Urol. Rep. 2019;20(6):1–7. doi: 10.1007/s11934-019-0891-7. [DOI] [PubMed] [Google Scholar]
- 48.FDA U. 2017. GRAS Notification of Ergothioneine. Published online. [Google Scholar]
- 49.Turck D., Bresson J., Burlingame B., et al. Statement on the safety of synthetic l‐ergothioneine as a novel food – supplementary dietary exposure and safety assessment for infants and young children, pregnant and breastfeeding women. EFSA J. 2017;15(11) doi: 10.2903/j.efsa.2017.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heath H., Toennies G. The preparation and properties of ergothioneine disulphide. Biochem. J. 1958;68:204–210. doi: 10.1042/bj0680204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang R., Cheah I., Yew T., Halliwell B. Distribution and accumulation of dietary ergothioneine and its metabolites in mouse tissues. Sci. Rep. 2018;8(1):1–15. doi: 10.1038/s41598-018-20021-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mårtensson J., Denneberg T., Lindell Å., Textorius O. Sulfur amino acid metabolism in cystinuria: a biochemical and clinical study of patients. Kidney Int. 1990;37(1):143–149. doi: 10.1038/ki.1990.20. [DOI] [PubMed] [Google Scholar]
- 53.Al-Shehabat M.A., Hani I.B., Jaradat S., Hunaiti A.A. Evaluation and comparison of a set of oxidative and antioxidative biomarkers in cystinuric patients with age- and sex-matched healthy subjects. Comp. Clin. Pathol. 2017;26(2):411–416. doi: 10.1007/s00580-016-2393-z. [DOI] [Google Scholar]
- 54.Woodard L.E., Welch R.C., Veach R.A., et al. Metabolic consequences of cystinuria. BMC Nephrol. 2019;20(1):1–9. doi: 10.1186/s12882-019-1417-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stoffels C., Oumari M., Perrou A., et al. Ergothioneine stands out from hercynine in the reaction with singlet oxygen: resistance to glutathione and TRIS in the generation of specific products indicates high reactivity. Free Radic. Biol. Med. 2017;113(July):385–394. doi: 10.1016/j.freeradbiomed.2017.10.372. [DOI] [PubMed] [Google Scholar]
- 56.Salama S.A., Abd-Allah G.M., Mohamadin A.M., Elshafey M.M., Gad H.S. Ergothioneine mitigates cisplatin-evoked nephrotoxicity via targeting Nrf2, NF-κB, and apoptotic signaling and inhibiting γ-glutamyl transpeptidase. Life Sci. 2021;278(March) doi: 10.1016/j.lfs.2021.119572. [DOI] [PubMed] [Google Scholar]
- 57.Li Y., Park J.S., Deng J.H., Bai Y. Cytochrome c oxidase subunit IV is essential for assembly and respiratory function of the enzyme complex. J. Bioenerg. Biomembr. 2006;38(5–6):283–291. doi: 10.1007/s10863-006-9052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elena Dalmonte M., Forte E., Luisa Genova M., Giuffrè A., Sarti P., Lenaz G. Control of respiration by cytochrome c oxidase in intact cells. J. Biol. Chem. 2009;284(47):32331–32335. doi: 10.1074/jbc.M109.050146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bell S.M., De Marco M., Barnes K., et al. Deficits in mitochondrial spare respiratory capacity contribute to the neuropsychological changes of Alzheimer's disease. J. Personalized Med. 2020;10(2) doi: 10.3390/jpm10020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mali V.R., Pan G., Deshpande M., et al. Cardiac mitochondrial respiratory dysfunction and tissue damage in chronic hyperglycemia correlate with reduced aldehyde dehydrogenase-2 activity. PLoS One. 2016;11(10) doi: 10.1371/journal.pone.0163158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Czajka A., Malik A.N. Hyperglycemia induced damage to mitochondrial respiration in renal mesangial and tubular cells: implications for diabetic nephropathy. Redox Biol. 2016;10(September:100–107. doi: 10.1016/j.redox.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lamhonwah A.-M., Tein I. Novel localization of OCTN1, an organic cation/carnitine transporter, to mammalian mitochondria q. Biochem. Biophys. Res. Commun. 2006;345:1315–1325. doi: 10.1016/j.bbrc.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 63.Lamhonwah A.-M., Tein I. Expression of the organic cation/carnitine transporter family (Octn1,-2 and-3) in mdx muscle and heart: implications for early carnitine therapy in Duchenne muscular dystrophy to improve cellular carnitine homeostasis. Clin. Chim. Acta. 2020;505:92–97. doi: 10.1016/j.cca.2020.02.015. [DOI] [PubMed] [Google Scholar]
- 64.Shitara Y., Nakamichi N., Norioka M., Shima H., Kato Y., Horie T. Role of organic cation/carnitine transporter 1 in uptake of phenformin and inhibitory effect on complex I respiration in mitochondria. Toxicol. Sci. 2012;132(1):32–42. doi: 10.1093/toxsci/kfs330. [DOI] [PubMed] [Google Scholar]
- 65.D’onofrio N., Servillo L., Giovane A., et al. Ergothioneine oxidation in the protection against high-glucose induced endothelial senescence: involvement of SIRT1 and SIRT6. Free Radic. Biol. Med. 2016;96:211–222. doi: 10.1016/j.freeradbiomed.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 66.Markova N.G., Karaman-Jurukovska N., Dong K.K., Damaghi N., Smiles K.A., Yarosh D.B. Skin cells and tissue are capable of using l-ergothioneine as an integral component of their antioxidant defense system. Free Radic. Biol. Med. 2009;46(8):1168–1176. doi: 10.1016/j.freeradbiomed.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 67.Obayashi K., Kurihara K., Okano Y., Masaki H., Yarosh D. L-Ergothioneine scavenges superoxide and singlet oxygen and suppresses TNF-a and MMP-1 expression in UV-irradiated human dermal fibroblasts. Int. J. Cosmet. Sci. 2005;27:191–192. doi: 10.1046/j.1467-2494.2001.00096.x. [DOI] [PubMed] [Google Scholar]
- 68.Williamson R.D., McCarthy F.P., Manna S., et al. L-(+)-Ergothioneine significantly improves the clinical characteristics of preeclampsia in the reduced uterine perfusion pressure rat model. Hypertension. 2020;75(2):561–568. doi: 10.1161/HYPERTENSIONAHA.119.13929. [DOI] [PubMed] [Google Scholar]
- 69.Cao L.C., Honeyman T.W., Cooney R., Kennington L., Scheid C.R., Jonassen J.A. Mitochondrial dysfunction is a primary event in renal cell oxalate toxicity. Kidney Int. 2004;66(5):1890–1900. doi: 10.1111/j.1523-1755.2004.00963.x. [DOI] [PubMed] [Google Scholar]
- 70.Farooq S.M., Boppana N.B., Asokan D., Sekaran S.D., Shankar E.M. C-phycocyanin confers protection against oxalate-mediated oxidative stress and mitochondrial dysfunctions in MDCK cells. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0093056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Niimi K., Yasui T., Hirose M., et al. Contribution Mitochondrial permeability transition pore opening induces the initial process of renal calcium crystallization. Free Radic. Biol. Med. 2012;52:1207–1217. doi: 10.1016/j.freeradbiomed.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 72.Sun X.Y., Ouyang J.M., Gan Q.Z., Liu A.J. Renal epithelial cell injury induced by calcium oxalate monohydrate depends on their structural features: size, surface, and crystalline structure. J. Biomed. Nanotechnol. 2016;12(11):2001–2014. doi: 10.1166/jbn.2016.2289. [DOI] [PubMed] [Google Scholar]
- 73.Peerapen P., Chaiyarit S., Thongboonkerd V. Protein network analysis and functional studies of calcium oxalate crystal-induced cytotoxicity in renal tubular epithelial cells. Proteomics. 2018;18(8):1–23. doi: 10.1002/pmic.201800008. [DOI] [PubMed] [Google Scholar]
- 74.Hirose M., Yasui T., Okada A., et al. Renal tubular epithelial cell injury and oxidative stress induce calcium oxalate crystal formation in mouse kidney. Int. J. Urol. 2010;17(1):83–92. doi: 10.1111/j.1442-2042.2009.02410.x. [DOI] [PubMed] [Google Scholar]
- 75.Li C.Y., Deng Y.L., Sun B.H. Taurine protected kidney from oxidative injury through mitochondrial-linked pathway in a rat model of nephrolithiasis. Urol. Res. 2009;37(4):211–220. doi: 10.1007/s00240-009-0197-1. [DOI] [PubMed] [Google Scholar]
- 76.Marhoume F.Z., Aboufatima R., Zaid Y., et al. Antioxidant and polyphenol-rich ethanolic extract of Rubia Tinctorum L prevents urolithiasis in an ethylene glycol experimental model in rats. Molecules. 2021;26(4):1–17. doi: 10.3390/molecules26041005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Niimi K., Yasui T., Okada A., et al. Novel effect of the inhibitor of mitochondrial cyclophilinD activation, N-methyl-4-isoleucine cyclosporin, on renal calcium crystallization. Int. J. Urol. 2014;21(7):707–713. doi: 10.1111/iju.12425. [DOI] [PubMed] [Google Scholar]
- 78.Sharma M., Kaur T., Singla S.K. Protective effects of N-acetylcysteine against hyperoxaluria induced mitochondrial dysfunction in male wistar rats. Mol. Cell. Biochem. 2015;405(1–2):105–114. doi: 10.1007/s11010-015-2402-6. [DOI] [PubMed] [Google Scholar]
- 79.Sharma M., Kaur T., Singla S.K. Role of mitochondria and NADPH oxidase derived reactive oxygen species in hyperoxaluria induced nephrolithiasis: therapeutic intervention with combinatorial therapy of N-acetyl cysteine and Apocynin. Mitochondrion. 2016;27:15–24. doi: 10.1016/j.mito.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 80.Nakanishi T., Akabane E.R., Nanami M., et al. Comparison of cytotoxicity of cysteine and homocysteine for renal epithelial cells. Nephron Exp. Nephrol. 2005;100(1):11–21. doi: 10.1159/000084108. [DOI] [PubMed] [Google Scholar]
- 81.Mulay S.R., Desai J., Kumar S.V., et al. Cytotoxicity of crystals involves RIPK3-MLKL-mediated necroptosis. Nat. Commun. 2016;7 doi: 10.1038/ncomms10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nishiuch Y., Sasaki M., Nakayasu M., Oikawa A. Cytotoxicity of cysteine in culture media. In Vitro. 1976;12(9):635–638. doi: 10.1007/BF02797462. [DOI] [PubMed] [Google Scholar]
- 83.Rose J., Basisty N., Zee T., et al. Comprehensive proteomic quantification of bladder stone progression in a cystinuric mouse model using data-independent acquisitions. PLoS One. 2022;17(6) doi: 10.1371/journal.pone.0250137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Govindaraj A., Selvam R. Increased calcium oxalate crystal nucleation and aggregation by peroxidized protein of human kidney stone matrix and renal cells. Urol. Res. 2001;29(3):194–198. doi: 10.1007/s002400100177. [DOI] [PubMed] [Google Scholar]
- 85.Font-Llitjós M., Feliubadaló L., Espino M., et al. Slc7a9 knockout mouse is a good cystinuria model for antilithiasic pharmacological studies. Am. J. Physiol. Ren. Physiol. 2007;293(3):732–740. doi: 10.1152/ajprenal.00121.2007. [DOI] [PubMed] [Google Scholar]
- 86.Taubert D., Grimberg G., Jung N., Rubbert A., Schömig E. Functional role of the 503F variant of the organic cation transporter OCTN1 in Crohn's disease. Gut. 2005;54(10):1505–1506. doi: 10.1136/gut.2005.075820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Toh D.S.L., Cheung F.S.G., Murray M., Pern T.K., Lee E.J.D., Zhou F. Functional analysis of novel variants in the organic cation/ergothioneine transporter 1 identified in Singapore populations. Mol. Pharm. 2013;10(7):2509–2516. doi: 10.1021/mp400193r. [DOI] [PubMed] [Google Scholar]
- 88.Cheah I., Tang R., Yew T., Li K., Halliwell B. Administration of pure ergothioneine to healthy human subjects: uptake, metabolism, and effects on biomarkers of oxidative damage and inflammation. Antioxidants Redox Signal. 2017;26(5):193–206. doi: 10.1089/ars.2016.6778. [DOI] [PubMed] [Google Scholar]
- 89.Skibska B., Goraca A., Skibska A., Stanczak A. Effect of alpha-lipoic acid on rat ventricles and atria under LPS-induced oxidative stress. Antioxidants. 2022;11(4) doi: 10.3390/antiox11040734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cao L., Yin G., Du J., et al. Salvianolic acid B regulates oxidative stress , autophagy and apoptosi against cyclophosphamide-induced hepatic injury in nile Tilapia. Animals. 2023;13:341–358. doi: 10.3390/ani13030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dieter F., Esselun C., Eckert G.P. Redox active α-lipoic acid differentially improves mitochondrial dysfunction in a cellular model of alzheimer and its control cells. Int. J. Mol. Sci. 2022;23(16) doi: 10.3390/ijms23169186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pan Y., Zhao W., Zhao D., et al. Salvianolic Acid B improves mitochondrial function in 3T3-L1 adipocytes through a pathway involving PPARγ coactivator-1α (PGC-1α) Front. Pharmacol. 2018;9(JUL):1–9. doi: 10.3389/fphar.2018.00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ziegler D., Ametov A., Barinov A., et al. Oral treatment with α-lipoic acid improves symptomatic diabetic polyneuropathy. Diabetes Care. 2006;29(11):2365–2370. doi: 10.2337/dc06-1216. [DOI] [PubMed] [Google Scholar]
- 94.Cheng J., Long J., Zhang J., et al. Safety, tolerance, and pharmacokinetics of salvianolic acid B in healthy Chinese volunteers: a randomized, double-blind, placebo-controlled phase 1 clinical trial. Front. Pharmacol. 2023;14(April:1–9. doi: 10.3389/fphar.2023.1146309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.