Abstract
The widespread clinical use of statins has contributed to significant reductions of cardiovascular morbidity and mortality. Increasing preclinical and epidemiological evidences have revealed that dyslipidemia is an important risk factor for carcinogenesis, invasion and metastasis, and that statins as powerful inhibitor of HMG-CoA reductase can exert prevention and intervention effects on cancers, and promote sensitivity to anti-cancer drugs. The anti-cancer mechanisms of statins include not only inhibition of cholesterol biosynthesis, but also their pleiotropic effects in modulating angiogenesis, apoptosis, autophagy, tumor metastasis, and tumor microenvironment. Moreover, recent clinical studies have provided growing insights into the therapeutic potentials of statins and the feasibility of combining statins with other anti-cancer agents. Here, we provide an updated review on the application potential of statins in cancer prevention and treatment and summarize the underneath mechanisms, with focuses on data from clinical studies.
Keywords: statins, cancer, cholesterol, angiogenesis, apoptosis, inflammation
1 Introduction
Cancer is the leading cause of death, although much effort has been directed at comprehending carcinogenesis with much progress achieved, effective drug treatment for most cancer types still lack. Dyslipidemia is an important risk factor for carcinogenesis, invasion, and metastasis (Liu et al., 2017a; Quan et al., 2020; Sun et al., 2020; Bian et al., 2021; Lim et al., 2021). Moreover, cancer cells are characterized with increased lipid biosynthesis that meets the metabolic needs of the fast-growing cells and provides cholesterol for membrane formation and stability. In this regard, the anti-cancer properties of lipid-lowering agents have attracted great interest (Matusewicz et al., 2015).
Statins are the most common lipid-lowering drugs, with an estimated 145.8 million users in 2018 (Blais et al., 2021). During recent decades, multiple studies on the anti-cancer effects of statins have been conducted, most of which indicate that statins reduce progression and prolong survival (Matusewicz et al., 2015; Wang et al., 2016; Mei et al., 2017; Chimento et al., 2018; Iarrobino et al., 2018). For examples, a retrospective study conducted on 146,326 women in the United States suggested that statins users had a significantly lower risk of cancer death [hazard ratio (HR), 0.78; 95% CI, 0.71–0.86] compared with never-users (Wang et al., 2016). Another 15-year large-scale observational study of a Danish subgroup including 13 cancers showed that all-cause mortality among patients with cancer who were taking statins was reduced by 15% (95% CI, 13–17) (Nielsen et al., 2012).
As a powerful inhibitor of 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase (HMGCR), statins blocks mevalonate pathway, inhibits de novo cholesterol synthesis, and also promotes serum low-density lipoprotein cholesterol (LDL-C) removal by upregulating LDL receptor (LDLR) expression in liver and peripheral tissues (Figure 1) (Stossel, 2008). Reduction of LDL-C hinders cancer progression mainly because rapidly dividing cells require more cholesterol for membrane synthesis (Nielsen et al., 2012; Gobel et al., 2020). Independent of cholesterol-lowering, statins also exhibit pleiotropic effects by downregulating other mevalonate pathway products and disrupting the prenylation of proteins to affect many signaling pathways (Ahmadi et al., 2020; Liu et al., 2020; Jiang et al., 2021; Yang et al., 2023). These cholesterol-independent actions also contribute to the statins’ impacts on growth, apoptosis, autophagy, angiogenesis, inflammation, and metastasis during cancer development (Figure 1) (Ahmadi et al., 2020; Jiang et al., 2021; Liu et al., 2022). Moreover, statins can modulate the tumor microenvironment (Wang et al., 2022a; Qiao et al., 2023) (Figure 1). Based on available pre-clinical and clinical studies, we comprehensively summarize the effects of statins in cancers and relevant mechanisms, and discuss the therapeutic potential and limitations of statin applications in cancer therapy.
FIGURE 1.
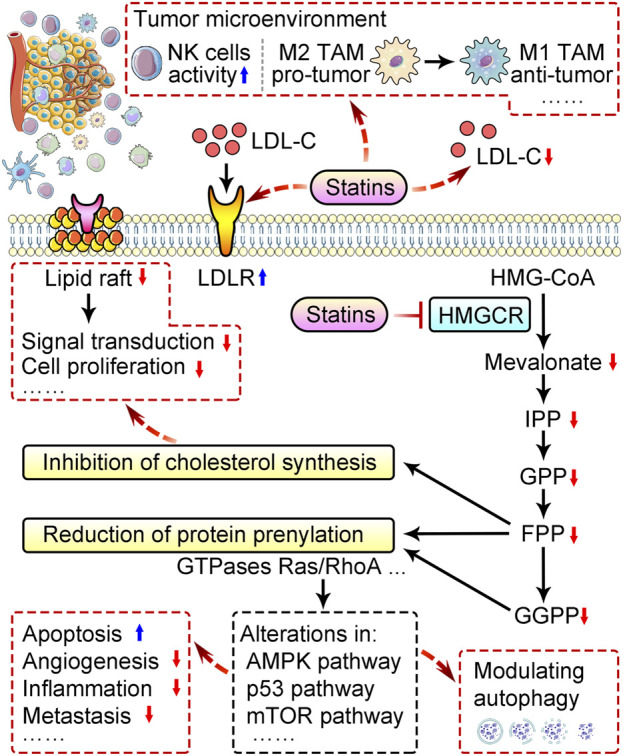
Schematic of mechanisms behind anti-cancer properties of statins. Statins remove serum low-density lipoprotein cholesterol (LDL-C) by upregulating LDL receptor (LDLR) expression in liver and peripheral tissues, and downregulates cholesterol biosynthesis by suppressing mevalonate pathway via inhibition of HMG-CoA reductase (HMGCR). Reduction of cholesterol disrupts the function of lipid rafts and suppresses cancer cell proliferation. Inhibition of the mevalonate pathway by statins also reduces prenylation of proteins like Ras and RhoA GTPases, and subsequently alter multiple pathways to modulate autophagy, promote apoptosis, and suppress angiogenesis, inflammation, metastasis, etc. Statins can also modulate tumor microenvironment via promoting the activity of natural killer (NK) cells, and M2-to-M1 switch, etc. TAM, tumor-associated macrophages; IPP, isopentenyl pyrophosphate; GPP, geranylgeranyl pyrophosphate; FPP, farnesyl pyrophosphate; GGPP, geranylgeranyl pyrophosphate.
2 Clinical data of statins in cancers
Despite of some inconsistent results possibly due to cohort diversity and differences in follow-up design, observational studies in the last decade have suggested overall positive impact of statins on clinical outcomes in an array of cancers, including but not limited to colorectal, gastric, breast, lung, liver and kidney cancers (Table 1) (Rosch and McCully, 2013; Wang et al., 2016; Mei et al., 2017; Liu et al., 2019; Tamburrino et al., 2020; Zeng et al., 2023). In a meta-analysis of over 1 million cancer patients, statins use reduces all-cause mortality and cancer-specific mortality by 30% and 40%, respectively (Mei et al., 2017). A recent meta-analysis involving 59,073 patients with hepatocellular carcinoma (HCC) shows that statins use is significantly associated with a reduced risk of HCC development (risk ratio, 0.54; 95% CI: 0.47–0.61) (Islam et al., 2020a). Large-scale observational studies also uncover significant correlation of statins with lower risks of several cancer types including prostate cancer and lymphoma (Table 1) (Graaf et al., 2004; Nielsen et al., 2012; Ren et al., 2021). These results warrant further randomized clinical trials to evaluate subtype-specific effects of statins in cancer prevention and treatment for certain cohorts.
TABLE 1.
Representative observational studies and interventional clinical studies regarding statins use in cancer.
| Large-scale observational studies | |||||||
|---|---|---|---|---|---|---|---|
| Statins | Cancer type | Study type/patient number | Findings | ||||
| Statins | Prostate cancer | Prospective cohort study/44,126 | Current statin use was associated with lower risk of PTEN-null and lethal prostate cancer (HR, 0.40; 95% CI, 0.19–0.87; and HR, 0.76; 95% CI, 0.60–0.96; respectively). Allott et al. (2020) | ||||
| Statins | Prostate cancer | Case-control study/42,480 | The use of statins was associated with a risk reduction of overall prostate cancer (adjusted OR, 0.94; 95% CI, 0.91–0.97) and specifically with advanced prostate cancer (adjusted OR, 0.90; 95% CI, 0.85–0.96). Jespersen et al. (2014) | ||||
| Statins (before cancer diagnosis) | Non-Hodgkin lymphoma | Case-control study/18,657 | Previous statin administration was associated with a reduced risk of subsequent non-Hodgkin lymphoma (adjusted OR, 0.52; 95% CI, 0.43–0.62). Cho et al. (2015) | ||||
| Statins | HCC | Meta-analysis/59,703 | Statin use was associated with a reduced risk of HCC development (risk ratio, 0.54; 95% CI, 0.47–0.61) compared with nonusers, supporting the beneficial inhibitory effect of statins on HCC incidence. Islam et al. (2020b) | ||||
| Statins | HCC | Meta-analysis/1,774,476 | Statin use was associated with reduced HCC risk (HR: 0.52; 95% CI, 0.37–0.72). Zeng et al. (2023) | ||||
| Statins (before cancer diagnosis) | Glioblastoma | Prospective cohort study/280,455 | Ever statin use (HR, 1.43, 95% CI, 1.10–1.86) was significantly associated with increased glioma risk. Cote et al. (2019) | ||||
| Statins (before cancer diagnosis) | Endometrial cancer | Case-control study/77,509 | The use of statins was not associated with the risk of endometrial cancer (OR, 1.03; 95% CI, 0.94–1.14). In addition, endometrial cancer risk did not vary substantially with duration or intensity of statin use. Sperling et al. (2017) | ||||
| Statins | Colorectal cancer | Meta-analysis/387,518 | The use of statins was significantly associated with a decrease in overall mortality (HR, 0.81; 95% CI, 0.76–0.86) and cancer-specific mortality (HR, 0.78; 95% CI, 0.72–0.85) of colorectal cancer. Li et al. (2021) | ||||
| Statins (simvastatin being the mostly prescribed lipophilic statin) | Breast cancer | Prospective cohort study/18,769 | Simvastatin was associated with a reduced risk of breast cancer recurrence among Danish women diagnosed with stage I–III breast carcinoma (adjusted 10-year risk difference = −0.10, 95% CI, −0.11 to −0.08) Ahern et al. (2011) | ||||
| Statins (after cancer diagnosis) | Breast cancer | Retrospective cohort study/17,880 | Statin use after a diagnosis of breast cancer reduced mortality due to breast cancer (adjusted HR, 0.84; 95% CI, 0.68–1.04). Cardwell et al. (2015) | ||||
| Statins | Gastric cancer | Retrospective cohort study/80,271 | Statin use was associated with a reduction of gastric cancer mortality in the general population but not with gastric cancer incidence. Cho et al. (2021) | ||||
| Atorvastatin, simvastatin, lovastatin, pravastatin, and rosuvastatin (both pre- and post- cancer diagnosis) | Lung cancer | Retrospective cohort study/19,974 | Overall baseline statin exposure was associated with a decrease in mortality risk for squamous-cell carcinoma patients (HR, 0.89; 95% CI, 0.82–0.96) and adenocarcinoma patients (HR, 0.87; 95% CI, 0.82–0.94), but not among those with SCLC. Post-diagnostic statin exposure was associated with prolonged survival in squamous-cell carcinoma patients (HR, 0.68; 95% CI, 0.59–0.79) and adenocarcinoma patients (HR, 0.78; 95% CI, 0.68–0.89). Baseline or post-diagnostic exposure to simvastatin and atorvastatin was associated with extended survival in NSCLC cancer subtypes. Ung et al. (2018) | ||||
| Statins | Kidney cancer | Meta-analysis/18,105 | Statin use was not significantly associated with PFS (pooled HR 0.92, 95% CI, 0.51–1.65); however, statin use was associated with marked improvements in cancer-specific survival (pooled HR 0.67, 95% CI, 0.47–0.94) and overall survival (pooled HR 0.74, 95% CI, 0.63–0.88) in patients with kidney cancer. Nayan et al. (2017) | ||||
| Statins (before cancer diagnosis) | 13 cancer types | Retrospective cohort study/295,925 | Statin use in patients with cancer was associated with reduced cancer-related mortality. Multivariable-adjusted HR for statin users, as compared with patients who had never used statins, were 0.85 (95% CI, 0.83–0.87) for death from any cause and 0.85 (95% CI, 0.82–0.87) for death from cancer. Nielsen et al. (2012) | ||||
| Statins | Not specified | Meta-analysis/1,111,407 | Statin use was significantly associated with decreased risk of all-cause mortality (HR, 0.70; 95% CI, 0.66–0.74) compared with nonusers. The observed pooled estimates were retained for cancer-specific mortality (HR, 0.60; 95% CI, 0.47–0.77), PFS (HR, 0.67; 95% CI, 0.56–0.81), recurrence-free survial (HR, 0.74; 95% CI, 0.65–0.83) and disease-free survival (HR, 0.53; 95% Cl, 0.40–0.72). Mei et al. (2017) | ||||
| Statins | Not specified | Prospective cohort study/146,326 | In a cohort of postmenopausal women, regular use of statins or other lipid-lowering medications was associated with decreased cancer death (HR, 0.78; 95% CI, 0.71–0.86), regardless of the type, duration, or potency of statin medications used. Wang et al. (2016) | ||||
| Statins | Not specified | Meta-analysis/175,000 | A median of 5 years of statin therapy had no effect on the incidence of, or mortality from, any type of cancer (or the aggregate of all cancer). Cholesterol Treatment Trialists et al. (2012) | ||||
| Interventional clinical studies | |||||||
| Intervention | Cancer type | Mechanisms | Study type/patient number | Findings | |||
| Lipophilic statins | |||||||
| Atorvastatin (80 mg/day before surgery) | Breast cancer | Inhibiting tumor cell growth by downregulation of cyclin D1 and p27 | Phase II, non-randomized, window of opportunity trial/50 | Atorvastatin treatment in patients with primary invasive breast cancer led to increased protein expression of the tumor suppressor p27, lower cyclin D1 expression, and a decrease in proliferation although not significantly (p = 0.08). (NCT00816244) Feldt et al. (2015) | |||
| Atorvastatin (10 mg/day) | HCC | NR | Phase IV, double-blind, randomized PC trial/recruiting | No results posted. The aims is to evaluate the effect of atorvastatin for preventing HCC recurrence after curative treatment. The primary endpoint is to compare the 3-year cumulative incidence of recurrent HCC between the intervention group and control counterpart. (NCT03024684) | |||
| Atorvastatin (20 mg/day) plus zoledronate | Kidney cancer | Bisphosphonates and statins target different steps in the mevalonate pathway, providing a synergistic effect | Phase II, single-arm, pilot trial/11 | The combination use of zoledronate and atorvastatin (or fluvastatin) were well tolerated, affected certain bone biomarkers and provided bone response. (NCT00490698) Manoukian et al. (2011) | |||
| Atorvastatin (Before surgery) plus metformin | Breast cancer | Inhibiting signaling pathways including PI3K/Akt/mTOR and AMPK | Phase I, window of opportunity trial/23 | No results posted. The aim is to assess whether tumor proliferation is reduced following ∼2 weeks of treatment with metformin plus atorvastatin in patients with newly diagnosed breast cancer. (NCT01980823) | |||
| Atorvastatin (40 mg/day) plus radiotherapy and temozolomide | Glioblastoma | NR | Phase II, single-arm trial/36 | 80% of patients discontinued because of disease progression. High LDL level was an important independent predictor of poor cancer-related outcome. (NCT02029573) Altwairgi FA et al. (2019) | |||
| Simvastatin (40 mg/day) | Ovarian cancer | NR | Phase I, single-arm, pilot trial/recruiting | No results posted. The aim is to evaluate the feasibility and effects of simvastatin to reduce cancer progression among patients with platinum-sensitive ovarian cancer treated with carboplatin and liposomal doxorubicin. (NCT04457089) | |||
| Simvastatin (40 mg/day) plus fluorouracil, adriamycin, and cyclophosphamide (FAC) | Locally advanced breast cancer | Inducing apoptosis and inhibiting tumor cell growth | Phase II, double-blind, randomized PC trial/70 | Simvastatin combined with FAC showed improvements in ORR and pathological response in patients with locally advanced breast cancer. Although no statistically significant difference was documented, there was a trend for better activity and tolerability. (NCT04418089) Yulian et al. (2021) | |||
| Simvastatin (80 mg/day) plus XELOX and bevacizumab | Metastatic colorectal cancer | Inducing tumor cell senescence and apoptosis, and showing anti-angiogenesis effect | Phase II, single-arm trial/60 | Addition of simvastatin to XELOX and bevacizumab showed comparable clinical efficacy in patients with metastatic colorectal cancer as first-line chemotherapy and did not increase toxicity. The median PFS was 10.4 months, the disease-control rate and overall RR was 88.3% and 58.3%. (NCT02026583) Kim et al. (2019) | |||
| Simvastatin (40 mg/day) plus gefitinib | NSCLC | Impairment of protein prenylation and interference with lipid rafts both affect the function of EGFR and EGFR signaling | Phase II, randomized trial/106 | The combination of simvastatin and gefitinib resulted in higher RR (40% vs. 0%, p = 0.043) and longer PFS (3.6 months vs. 1.7 months, p = 0.027) compared with gefitinib alone in subgroup of patients with wildtype EGFR non-adenocarcinomas. (NCT00452244) Han et al. (2011) | |||
| Simvastatin (40 mg/day) plus afatinib | NSCLC | Inhibition of RAS activation and downstream signaling cascades | Phase II, randomized trial/68 | Combination of simvastatin plus afatinib was well-tolerated, but did not improve RR and PFS compared with afatinib alone in patients with advanced non-adenocarcinomas who progressed after chemotherapy regimens. (NCT01156545) Lee et al. (2017) | |||
| Simvastatin (40 mg/day) plus capecitabine and cisplatin | Gastric cancer | Regulating modifications of Ras and RhoA, inducing apoptosis, and lowering VEGF serum levels | Phase III, double-blind, randomized PC trial/244 | Addition of simvastatin to capecitabine-cisplatin did not increase PFS in patients with previously untreated advanced gastric cancer, although it did not increase toxicity. (NCT01099085) Kim et al. (2014) | |||
| Simvastatin (40 mg/day) plus gemcitabine | Pancreatic cancer | Impairment of protein prenylation and interference with lipid rafts both affect the function of EGFR and EGFR signaling | Phase II, double-blind, randomized PC trial/114 | Addition of simvastatin to gemcitabine in advanced pancreatic cancer did not provide clinical benefit, although it did not result in increased toxicity. The median time to progression was not significantly different between the two arms (2.4 months vs. 3.6 months, p = 0.903). (NCT00944463) Hong et al. (2014) | |||
| Simvastatin (40 mg/day) plus FOLFIRI/XELIRI chemotherapy regimens | Metastatic colorectal cancer | Impairment of protein prenylation and intracellular signal transduction | Phase III, double-blind, randomized PC trial/269 | Addition of simvastatin to the regimens did not improve median PFS (5.9 months vs. 7.0 months, p = 0.826) in patients with previously treated metastatic colorectal cancer nor did it increase toxicity. (NCT01238094) Lim et al. (2015) | |||
| Simvastatin (40 mg/day) plus chemotherapy/radiation | Rectal cancer | NR | Phase II, double-blind, randomized PC trial/222 | No results posted. The primary objective is rates of favorable MRI-based tumor regression grading. Patients receive simvastatin or placebo daily for 90 days starting 1 week prior to standard preoperative chemoradiotherapy. (ACTRN 12617001087347) Jameson et al. (2019) | |||
| Simvastatin (80 mg/day) plus capecitabine | Locally advanced rectal cancer | NR | Phase II, single-arm trial/60 | No results posted. The aim is to investigate the synergistic effect of simvastatin combined with capecitabine and radiotherapy in locally advanced rectal cancer patients. The primary outcome is pathologic complete response rate. (NCT02161822) | |||
| Simvastatin (20 mg/day before surgery) plus metformin | Bladder cancer | Inhibiting signaling pathways including PI3K/Akt/mTOR and AMPK | Phase II, single-arm, window of opportunity trial/44 | No results posted. The aim is to evaluate the effect and feasibility of using a combination of metformin and simvastatin as a neoadjuvant treatment for patients with invasive bladder cancer who are to undergo cystectomy. (NCT02360618) | |||
| Lovastatin (0.5–2 mg/kg) plus thalidomide and dexamethasone | Multiple myeoloma | Anti-neoplasmatic property and inducing apoptosis | Randomized trial/91 | Lovastatin plus thalidomide–dexamethasone prolonged OS and PFS compared with thalidomide–dexamethasone alone in patients with relapsed or refractory multiple myeloma. Hus et al. (2011) | |||
| Fluvastatin (80 mg/day) | Localized prostate cancer | Inducing tumor cell apoptosis | Phase II, single-arm, window of opportunity, pilot trial/33 | A median 2.7-fold increase in cleaved Caspase-3 positivity (95% CI: 1.9–5.0, p = 0.007) was observed in post-fluvastatin RP tissues compared with matched pre-treatment biopsy controls. Fluvastatin was associated with promising effects on tumor cell apoptosis. Longo et al. (2020) | |||
| Fluvastatin (80 or 20 mg/day) | Breast cancer | Inducing apoptosis and suppressing tumor cell proliferation | Randomized, peri-operative window trial/40 | Administration of fluvastatin for 3–6 weeks before surgery decreased proliferation of high-grade tumors by a median of 7.2% (p = 0.008), and increased apoptosis in 60% of high-grade tumors; for low-grade tumors, these effects were less evident. Garwood et al. (2010) | |||
| Fluvastatin (80 mg/day) | Breast cancer | NR | Single-arm, non-randomized, biomarker modulation trial/30 | Lovastatin was technically feasible and generally well-tolerated in women at increased risk of developing breast cancer, but no significant biomarker modulation was observed. The results did not exclude a favorable effect on breast cancer risk. (NCT00285857) Vinayak et al., (2013) | |||
| Hydrophilic statins | |||||||
| Rosuvastatin (40 mg/day) plus chemoradiation therapy | Rectal cancer | Sensitizing cancer tissues and protects normal tissues to the effects of radiation | Phase II, single-arm trial/45 | No results posted. The aim is to evaluate whether the addition of rosuvastatin to standard chemoradiation therapy for the treatment of locally advanced rectal cancer may improve the pathological response rate and survival compared to standard chemoradiation therapy alone. Rosuvastatin treatment starts 2 weeks prior to the initiation of radiation at week 1 and stops 4 weeks after the completion of radiation. (NCT02569645) | |||
| Pravastatin (40 mg/day) combined with etoposide plus cisplatin or carboplatin | SCLC | NR | Phase III, double-blind, randomized PC trial/846 | Pravastatin plus standard chemotherapy did not offer additional benefit compared with chemotherapy alone. The median PFS was 7.7 months vs. 7.3 months. The median OS (pravastatin v placebo) was 14.6 months in both groups for limited disease and 9.1 months versus 8.8 months, respectively, for extensive disease. (NCT00433498) Seckl et al. (2017) | |||
| Pravastatin (40 mg/day) plus chemotherapy | Gastric carcinoma | NR | Phase II, randomized trial/30 | Addition of pravastatin to epirubicin/cisplatin/capecitabine did not improve progression-free rate at 6 months, RR, PFS and OS. Konings et al. (2010) | |||
| Pravastatin (40 mg/day) plus TAE and 5-FU | HCC | NR | Randomized trial/91 | Pravastatin prolonged the survival of patients with advanced HCC (median survival, pravastatin group vs. controls, 18 months vs. 9 months, p = 0.006). Kawata et al. (2001) | |||
| Pravastatin (40 mg/day) plus sorafenib | HCC | Inhibiting Raf-Ras -MAPK pathway. The anti-invasive and anti-metastatic action of pravastatin is a complement to the anti-angiogenic action of sorafenib | Phase III, randomized trial/312 | Addition of pravastatin to sorafenib did not improve survival in patients with advanced HCC, with no difference in median OS between sorafenib-pravastatin and sorafenib groups (10.7 months vs. 10.5 months; HR = 1.00; p = 0.975). (NCT01075555) Jouve et al. (2019) | |||
| Pravastatin (40 mg/day) | Esophageal cancer and stomach cancer | NR | Phase IV, randomized trial/recruiting | No results posted. The objective is to evaluate the efficacy of treatment (increase in survival and recurrence-free period of the disease) with pravastatin in patients with advanced esophageal cancer and stomach cancer. The experimental group will receive pravastatin orally daily for 2 years. (NCT01038154) | |||
Abbreviations: HCC, hepatocellular carcinoma; HR, hazard ratio; LDL, low density lipoprotein; NR, not reported; NSCLC, non-small cell lung cancer; OR, odds ratio; ORR, objective response rate; OS, overall survival; PC, placebo-controlled; PFS, progression-free survival; RR, response rate; SCLC, small cell lung cancer; TAE, transcatheter arterial embolization.
Various interventional clinical trials regarding anti-cancer ability of statins are ongoing, either given alone or in combination, with some already posted positive results (Table 1). A perioperative window trial in women with stage 0/1 breast cancer demonstrated that administration of fluvastatin for 3–6 weeks before surgery decreased proliferation of high-grade tumors by a median of 7.2% (p = 0.008), and increased apoptosis in 60% of high-grade tumors; while for low-grade tumors, these effects were less evident (Garwood et al., 2010). Similarly, 2-week atorvastatin treatment before surgery decreased tumor cell proliferation in patients with primary invasive breast cancer (Feldt et al., 2015). However, clinical trials applied statins in combination with other anti-cancer drugs gave less satisfactory results. A recent phase III trial found adding pravastatin to sorafenib did not improve survival in patients with advanced HCC, with no difference in median overall survival between sorafenib-pravastatin and sorafenib groups (10.7 months vs. 10.5 months; HR, 1.00; p = 0.975) (Jouve et al., 2019). Use of simvastatin in combination with chemotherapy drugs fail to benefit patients in most trials, except when combined with fluorouracil, adriamycin, and cyclophosphamide to treat patients with locally advanced rectal cancer (Table 1) (Yulian et al., 2021). How to take advantage of statins to promote current anti-cancer therapy remains a serious question awaiting in-depth mechanistic studies.
3 Mechanisms behind statins’ anti-cancer effects
The anti-cancer effect of statins are closely related to their inhibitory effect on HMG-CoA reductase and mevalonate pathway. Statins-mediated reduction of cholesterol leads to interruption of cell membrane structure and cholesterol-related biological function (Figure 1). Statins also downregulate non-cholesterol products of mevalonate pathway, including isopentenyl pyrophosphate (IPP), farnesyl pyrophosphate (FPP), and geranylgeranyl pyrophosphate (GPP), thereby suppress prenylation of proteins like small monomeric GTPases, primarily Ras and RhoA proteins, and consequently alter multiple cancer pathways (Figure 1) (Gobel et al., 2020; Jiang et al., 2021). Here, we introduce how statins exhibit tumor-suppressing activity by downregulating cholesterol, and how statins regulate multiple aspects including angiogenesis, apoptosis, autophagy, metastasis, tumor microenvironment and drug resistance in cancer.
3.1 Downregulating cholesterol
Cholesterol, the ubiquitous precursor to sterol hormones, is one of the basic building elements of cell membranes. Moreover, cholesterol regulate multiple signaling pathways involved in tumorigenesis and progression. Its endogenous synthesis is catalyzed by HMGCR (Mullen et al., 2016), while its uptake is regulated by LDLR. High intracellular cholesterol in normal cells blocks HMGCR mediated cholesterol biosynthesis and upregulates LXR α/β mediated cholesterol efflux transporter expression. In cancer cells, the presence of intracellular cholesterol does not affect cholesterol biosynthesis and uptake. The highly active cholesterol metabolism within cancer cells facilitates tumor progression and thus becomes a vulnerability that may be targeted (Mehta et al., 1998; Mullen et al., 2016; Zhou et al., 2018; Huang et al., 2020). Additionally, in the tumor microenvironment (TME), cholesterol metabolism is generally enhanced; thus targeting cholesterol synthesis can also modulate TME (Huang et al., 2020; Zhu et al., 2021). Indeed, in lung cancer cells, simvastatin remodels TME and reverses epithelial-mesenchymal transition (EMT) by re-polarizing tumor-associated macrophages (TAMs) from M2 to M1 via cholesterol-associated LXR/ABCA1 regulation (Jin et al., 2019).
Lipid raft, a specialized cholesterol-rich region of the cell membrane, facilitates membrane-initiated signaling events through compartmentalization of signaling pathways (Simons and Ikonen, 1997; Boudreau et al., 2010). Importantly, lipid raft is a key player in statin-mediated inhibition of tumor growth and migration (Simons and Ikonen, 1997; Boudreau et al., 2010; Yang et al., 2023). Simvastatin treatment reduced tumor cell growth, cellular cholesterol levels, cholesterol content in lipid rafts and membrane integrity (Zhuang et al., 2005; Menter et al., 2011). On the other hand, elevation of circulating cholesterol by cholesterol-enriched diet promoted tumor growth in a xenograft mouse model for prostate cancer (Zhuang et al., 2005). Disruption of lipid rafts by simvastatin also re-sensitized paclitaxel resistance in lung cancer cells by suppressing integrin-β3/FAK signaling pathway and focal adhesion formation (Jin et al., 2019). Moreover, in myeloproliferative neoplasms (MPN), aberrant JAK2 signaling plays a crucial tumor-promoting role, while JAK inhibitors did not induce patient remission; alternatively, simvastatin, lovastatin and atorvastatin inhibited mutated JAK2 localization to lipid rafts, consequently inhibited JAK2-V617-dependent growth and induced apoptosis in MPN cells, and suppressed primary erythroid colony formation of primary cells from MPN patients (Griner et al., 2013). These studies unequivocally suggest that statin-induced reduction on cholesterol alters signaling transduction to interfere with cell proliferation and metastasis, while the exact molecular alteration behind statin-induced changes of lipid raft remain not completely defined.
3.2 Inhibiting angiogenesis
Angiogenesis, formation of new blood vessels from pre-existing vessels, is an important event in cancer growth and hematogenous metastasis (Zhang et al., 2022; Xiong et al., 2023). Inhibition of angiogenesis with several FDA-approved inhibitors has been an established therapeutic strategy for many solid tumors (Chen et al., 2018; Li et al., 2019; Liu et al., 2019; Zhang et al., 2022). Anti-angiogenic effect of statins has attracted growing attention (Weis et al., 2002; Dulak and Jozkowicz, 2005; Zahedipour et al., 2022). The anti-angiogenic effect of cerivastatin is cholesterol-independently achieved by inhibiting the RhoA/focal adhesion kinase/AKT pathways (Vincent et al., 2001). Similarly, simvastatin interferes with angiogenesis by inhibiting RhoA geranylgeranylation (Park et al., 2002). Powerful anti-angiogenic effect of atorvastatin was evident in glioblastoma 3D spheroids by downregulating expression of VEGF and CD31 (Bayat et al., 2018), and reduction of angiogenesis by rosuvastatin was observed in tumor-bearing mice (Weis et al., 2002). Importantly, simvastatin potentiated the anti-angiogenic effects of bevacizumab on human colorectal cancer cells (Lee et al., 2014), and addition of simvastatin to XELOX and bevacizumab showed comparable clinical efficacy (disease-control rate, 88.3%) in patients with metastatic colorectal cancer with a favorable safety profile in a phase II study (Kim et al., 2019).
3.3 Inducing apoptosis
Statins induce cell apoptosis in different cancer types including lung, prostate, colorectal, and breast cancers (Zaleska et al., 2018; Ahmadi et al., 2020; Juarez and Fruman, 2021; Guo et al., 2022); with a tendency to induce greater-degree of apoptosis in malignant cells than in non-malignant ones (Wong et al., 2002; Wu et al., 2004). This is possibly due to enhanced dependency of malignant cells on signaling pathways including AMPK, AKT, mTOR, and p53 pathways, and autophagy pathway (Chou et al., 2019; Wang et al., 2022b). AMPK is a cellular energy sensor that inhibits cell proliferation and induces cancer cell apoptosis, and statins can activate AMPK pathway; moreover, statins-associated AMPK activation led to decreased lipid accumulation in liver which may decrease risk to liver cancer (Misirkic et al., 2012; Dehnavi et al., 2021). In glioma cell lines, simvastatin induced apoptosis by inhibiting AKT activation and mTOR pathways (Misirkic et al., 2012; Dehnavi et al., 2021). In lung adenocarcinoma, simvastatin enhanced caspase-dependent apoptotic progress by promoting mutant p53 protein degradation (Chou et al., 2019). Additionally, in small cell lung cancer, statins induced oxidative stress accumulation and apoptosis through suppressing the geranylgeranyl diphosphate (GGPP) synthase 1 (GGPS1)-RAB7A-autophagy axis, overcame both intrinsic and acquired chemoresistance in vivo across PDX models bearing high GGPS1 levels (Guo et al., 2022). The capacity of modulating apoptosis makes statins promising candidates for anti-cancer treatment.
3.4 Modulating autophagy
Autophagy plays a dual role in cancer, as either a promoter or a suppressor (Ashrafizadeh et al., 2020; Mengual et al., 2022). On one hand, statins can induce apoptosis via inhibiting autophagy (Chou et al., 2019; Guo et al., 2022); on the other hand, statins can suppress cancer cell viability via inducing autophagy in multiple cancers, such as ovarian cancer, lung adenocarcinoma, malignant pleural mesothelioma, melanoma, and pancreatic cancer (Ashrafizadeh et al., 2020; Mengual et al., 2022). Several signaling pathways have been implicated in the regulation of statin-mediated autophagy, including the mevalonate pathway, AMPK/mTOR pathway, and the nuclear accumulation of p53 (Yang and Chen, 2011; Zhang et al., 2012). For examples, fluvastatin reduced breast cancer cell viability by activating AMPK-mTOR dependent autophagy activation (Elimam et al., 2020), and prevented lung adenocarcinoma bone metastasis in nude mice via inducing autophagy that triggered by increased nuclear p53 expression (Yang et al., 2017). Moreover, the suppressive effect of lovastatin on primary tumors and metastasis in malignant mesothelioma was due to mTOR-independent induction of autophagic changes (Asakura et al., 2011). In lymphoma cells, fluvastatin treatment induced autophagy contributed to fluvastatin-induced apoptosis, which can be blocked by metabolic products of the HMG-CoA reductase reaction (Qi et al., 2013). However, in HCC and colorectal carcinoma cells, atorvastatin inhibited cell growth via inducing apoptosis, while promoted cell survival via inducing autophagy by activating AMPK/p21-dependent endoplasmic reticulum stress response (Yang et al., 2010). The mixed results in preclinical studies suggest that a refined classification needs to be considered when investigating the autophagy-related impacts of different statins in different cancer types. The combination treatment of statins and autophagic inhibitors in cancer therapy also warrants intensive investigation.
3.5 Reducing risks of metastasis
Metastasis is a major cause of cancer-related death. Take prostate cancer (PC) as an example, localized PC is frequently curable, while treatment for metastatic PC is challenging with limited therapeutic options and inevitable drug resistance (Scheinberg et al., 2023). Accumulating studies have suggested that circulating lipids were associated with PC aggressiveness and PC death, and that statin use was associated with reduced risks of metastatic PC and PC mortality (Raval et al., 2016; Van Rompay et al., 2019; Scheinberg et al., 2023). According to a large population-based cohort study with 25-year follow-up data, statins reduced the risk of aggressive PC (HR 0.52, 95% CI: 0.40–0.68), and statin users had a 49% lower risk of PC mortality (HR 0.51, 95% CI: 0.41–0.63) (Van Rompay et al., 2019). Similarly, a meta-analysis of 34 studies (including prospective randomized clinical trials and observational studies) showed that statins use was associated with over 20% reduction in the risks of both PC metastases (pooled HR 0.78, 95% CI: 0.68–0.87) and PC mortality (pooled HR 0.76, 95% CI: 0.63–0.91) (Raval et al., 2016). Moreover, in vivo studies found that simvastatin prevented the skeletal metastasis of breast cancer by inhibiting the expression of cancer stem cell marker CD44 and enhancing the expression of p53 (Mandal et al., 2011). Pravastatin reduced the lung metastasis of rat hepatocellular carcinoma by downregulating the expression and activity of liver matrix metalloproteinase-9 (Taras et al., 2007).
3.6 Modulating tumor microenvironment
Recent studies have demonstrated that tumor microenvironment (TME), which is characterized by metabolic reprogramming and hypoxia, play important roles in tumor progression (Chen et al., 2018; Liu et al., 2019). Cholesterol metabolism in TME is generally enhanced, as evidenced by increased cholesterol biosynthesis and uptake. In situations in which lipids and/or oxygen is limited, such as in the glioblastoma microenvironment, the master transcription factor SREBP2 and its downstream targets, including mevalonate-pathway enzymes are significantly upregulated in tumor (Lewis et al., 2015). Beyond SREBP2, another transcription factor, RORγ, which activates the cholesterol-biosynthesis program, is upregulated in triple-negative breast cancer and facilitates tumor progression (Cai et al., 2019). In addition to enhanced de novo cholesterol synthesis, increasing cholesterol uptake is observed in cancer cells. An extreme example is that some anaplastic large cell lymphoma cells express increased levels of LDLR and fully rely on cholesterol uptake to acquire exogenous cholesterol, thus supporting proliferation (Garcia-Bermudez et al., 2019). Moreover, another group of cholesterol metabolites, cholesteryl esters (CE) and oxysterols, are enriched in TME; accumulation of CE and oxysterols is another common signature in cancer (Li et al., 2016; Kloudova et al., 2017). Thus statins can regulate the metabolic TME due to its impact on multiple metabolic pathways (Chen et al., 2020; Huang et al., 2020; Liu et al., 2020; Zhu et al., 2021; Yang et al., 2023). For examples, simvastatin re-polarized TAMs, promoted M2-to-M1 phenotype switch, and suppressed epithelial-mesenchymal transition in lung cancer via cholesterol-associated LXR/ABCA1 regulation (Jin et al., 2019). Statins also downregulate the mevalonate-pathway product coenzyme Q (CoQ) and lead to severe oxidative stress, resulting in significant ROS production, which helps to improve the efficacy of chemotherapy (McGregor et al., 2020). Fatty acid synthesis increases along with the accumulation of H+, which contributes to the generation of acidic TME; while statins significantly reduced plasma free fatty acid concentrations (Sorrentino et al., 2014; Sahebkar et al., 2016; Chen et al., 2020; Liu et al., 2020; Yang et al., 2023). Pre-treatment of simvastatin reduces lactate content in head and neck tumors, and promotes tumor sensitivity to monocarboxylate transporter 1 (MCT1) inhibitors (Mehibel et al., 2018).
In addition, statins can alter the gene expression mediated by HIF-1α, a key regulator for hypoxia response, by stimulating HIF-1α ubiquitin/proteasome degradation (Hisada et al., 2012). In breast cancer, simvastatin-mediated activation of AMPK suppressed breast tumor angiogenesis by blocking HIF-1α (Fukamachi et al., 2013; Wang et al., 2018; Jin et al., 2019). Moreover, the anti-tumor effects of statins were associated with their effect on a variety of immune cells in TME other than TAMs, such as lymphocytes and natural killer cells (NK cells) (Wang et al., 2022a; Qiao et al., 2023). For examples, the combination of statins and difluoromethylornithine (DFMO) significantly suppressed colon cancer by increasing the activity of functional NK cells (Janakiram et al., 2016). Moreover, statins treatment induced MHC class I Chain-related protein A overexpression and sensitized tumor cells to lysis by NK cells (Pich et al., 2013). Whether these effects of statins can be adapted in improving anti-cancer immunotherapy awaits further experimental and clinical exploration.
3.7 Overcoming drug resistance
Cancer resistance, which is characterized by tumor relapse or spread, remains a major challenge in clinical oncology (Kartal-Yandim et al., 2016; Quan et al., 2020). A range of studies have reported the effects of statins on overcoming the resistance to various anti-cancer drugs (Tilija Pun and Jeong, 2021). For examples, simvastatin effectively improved doxorubicin cytotoxicity in human malignant mesothelioma cells (Riganti et al., 2006). In chronic lymphocytic leukemia, activation of RhoA/RhoA kinases, Ras/ERK1-2, Akt, HIF-1α, and P-glycoprotein protected cells from doxorubicin; while simvastatin inhibited these effects and sensitized cells to doxorubicin (Rigoni et al., 2015). Combined treatment of simvastatin 5-fluorouracil (5-FU) synergistically suppressed colon tumors in vivo by inhibiting inflammation, angiogenesis, and metastasis (Luput et al., 2020). In addition, chemo-resistant small cell lung cancer xenograft showed dependence on mevalonate-GGPP pathway, which can be suppressed by statins (Guo et al., 2022). Apart from chemotherapeutic drugs, statins also contributed to overcoming the resistance to targeted drugs including the widely applied EGFR tyrosine kinase inhibitor gefitinib. Addition of simvastatin to gefitinib enhanced apoptosis in gefitinib-resistant EGFR T790M mutant NSCLC cells by suppressing the activation of AKT and β-catenin/survivin (Hwang et al., 2014). Moreover, atorvastatin reversed KRAS-mediated gefitinib resistance in NSCLC cells by inhibiting HMG-CoA reductase-dependent disruption of Kras/Raf and Kras/PI3K complexes (Chen et al., 2013). There are ongoing trials of statins use combined with other anti-cancer agents in different cancers including NSCLC, SCLC, HCC, gastric cancer, locally advanced breast cancer, metastatic colorectal cancer, etc. (Table 1)
4 Conclusion and future perspectives
Accumulating pre-clinical and clinical trials of statins in different cancers suggested overall beneficial role of statins with a favorable safety profile in cancer treatment and prevention. The anti-cancer effects, as well as their well-tolerance, low cost, and much lower toxicity compared with the conventional chemotherapy drugs, attract increasing consideration of repurposing statins as a promising strategy for cancer treatments.
Beyond de novo cholesterol biosynthesis, most cells can acquire cholesterol via uptake extracellular cholesterol by various molecules including LDLR. Therefore, cancer cells may bypass their dependency on de novo cholesterol biosynthesis by relying on exogenous cholesterol, such as LDL/HDL, which limits the anti-cancer effect of statin treatment. Inhibition of cholesterol uptake has shown anti-cancer property in some cases, for examples, using shRNA for LDLR increases the efficacy of gemcitabine in pancreatic cancer (Guillaumond et al., 2015); an FDA approved cholesterol uptake blocker ezetimibe retards in vivo prostate cancer progression by inhibiting angiogenesis (Solomon et al., 2009). Therefore, combination of statins and cholesterol uptake blocker may provide enhanced anti-cancer effect, which warrants more in-depth studies. It is currently difficult to predict the type of cancers that particularly sensitive to statin therapy. However, encouraging results from some trials (Garwood et al., 2010; Bjarnadottir et al., 2013; Harshman et al., 2015) suggest that patients with hormone-dependent cancers, such as breast cancer and prostate cancer, may benefit from adding statins to their treatment. This may be partly because cholesterol is the precursor of hormones such as oestrogen and androgens, which have a major role in the development of these cancers (Finlay-Schultz and Sartorius, 2015). Clinical trials are required to further define the subset of cancers that are more statin-sensitive (Mullen et al., 2016).
The heterogeneous physiological effects of different types of statins in different cancer types need to be considered. Depending on chemical structure, statins are classified as either lipophilic or hydrophilic (Istvan and Deisenhofer, 2001; Sirtori, 2014). Some studies suggested stronger association of lipophilic statins than hydrophilic ones with lower cancer-specific mortality (Liu et al., 2017b; Majidi et al., 2021). A plausible reason is that compared with hydrophilic statins, lipophilic statins have higher pro-apoptotic activity, and a greater ability to penetrate cell membrane and enter cells through passive diffusion (Hamelin and Turgeon, 1998; Dulak and Jozkowicz, 2005; Kato et al., 2010; Menter et al., 2011), while further investigations are warranted.
Among many explanations of anti-cancer effects of statins, the cholesterol-dependent function has been comprehensively-characterized, while the cholesterol-independent impacts are relatively less studied. Many questions remain to be explored, such as determination of proper dosage of statins to avoid biphasic effects, whether statins can be applied in combination with anti-cancer drugs to improve therapy, etc. Improved understanding of relevant molecular mechanisms will help elucidating the anti-cancer properties of statins and guide future clinical trials.
Acknowledgments
We sincerely appreciate the investigators and authors who have contributed to this field and apologize that we could not discuss and cite all of them in this review due to space limitations.
Funding Statement
This work was supported by the National Key R&D Program of China (2022YFA0806101), the Natural Science Foundation of China (31971066 and 82273838), the China Postdoctoral Science Foundation (2021M700050), the Natural Science Foundation of Hubei Province (2021CFA004, 2021CFB250, and 2022CFB247), Wuhan Science and Technology Bureau Innovation Project (2022020801020526), and the Postdoctoral Innovation Research Program of Hubei Province.
Author contributions
All authors contributed to the conception and the main idea of the work. CL, HC, YC, and KH wrote the manuscript. CL, HC, BH, JS, YC, and KH analyzed the data and edited the manuscript. All authors have read and agreed to the final version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Ahern T. P., Pedersen L., Tarp M., Cronin-Fenton D. P., Garne J. P., Silliman R. A., et al. (2011). Statin prescriptions and breast cancer recurrence risk: A Danish nationwide prospective cohort study. J. Natl. Cancer Inst. 103 (19), 1461–1468. 10.1093/jnci/djr291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi M., Amiri S., Pecic S., Machaj F., Rosik J., Los M. J., et al. (2020). Pleiotropic effects of statins: A focus on cancer. Biochimica biophysica acta Mol. basis Dis. 1866 (12), 165968. 10.1016/j.bbadis.2020.165968 [DOI] [PubMed] [Google Scholar]
- Allott E. H., Ebot E. M., Stopsack K. H., Gonzalez-Feliciano A. G., Markt S. C., Wilson K. M., et al. (2020). Statin use is associated with lower risk of pten-null and lethal prostate cancer. Clin. cancer Res. official J. Am. Assoc. Cancer Res. 26 (5), 1086–1093. 10.1158/1078-0432.CCR-19-2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altwairgi Fa A. K., Alhussain H., Alsaeed E., Balbaid A., Aldandan S., Orz Y., et al. (2019). Phase ii study of atorvastatin in combination with radiotherapy and temozolomide in patients with glioblastoma (art): Final analysis report. Ann. Oncol. 30, ix20. 10.1093/annonc/mdz419 [DOI] [Google Scholar]
- Asakura K., Izumi Y., Yamamoto M., Yamauchi Y., Kawai K., Serizawa A., et al. (2011). The cytostatic effects of lovastatin on acc-meso-1 cells. J. Surg. Res. 170 (2), e197–e209. 10.1016/j.jss.2011.06.037 [DOI] [PubMed] [Google Scholar]
- Ashrafizadeh M., Ahmadi Z., Farkhondeh T., Samarghandian S. (2020). Modulatory effects of statins on the autophagy: A therapeutic perspective. J. Cell. physiology 235 (4), 3157–3168. 10.1002/jcp.29227 [DOI] [PubMed] [Google Scholar]
- Bayat N., Izadpanah R., Ebrahimi-Barough S., Norouzi Javidan A., Ai A., Mokhtari Ardakan M. M., et al. (2018). The anti-angiogenic effect of atorvastatin in glioblastoma spheroids tumor cultured in fibrin gel: In 3d in vitro model. Asian Pac. J. cancer Prev. APJCP 19 (9), 2553–2560. 10.22034/APJCP.2018.19.9.2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian X., Liu R., Meng Y., Xing D., Xu D., Lu Z. (2021). Lipid metabolism and cancer. J. Exp. Med. 218 (1), e20201606. 10.1084/jem.20201606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnadottir O., Romero Q., Bendahl P. O., Jirstrom K., Ryden L., Loman N., et al. (2013). Targeting hmg-coa reductase with statins in a window-of-opportunity breast cancer trial. Breast Cancer Res. Treat. 138 (2), 499–508. 10.1007/s10549-013-2473-6 [DOI] [PubMed] [Google Scholar]
- Blais J. E., Wei Y., Yap K. K. W., Alwafi H., Ma T. T., Brauer R., et al. (2021). Trends in lipid-modifying agent use in 83 countries. Atherosclerosis 328, 44–51. 10.1016/j.atherosclerosis.2021.05.016 [DOI] [PubMed] [Google Scholar]
- Boudreau D. M., Yu O., Johnson J. (2010). Statin use and cancer risk: A comprehensive review. Expert Opin. Drug Saf. 9 (4), 603–621. 10.1517/14740331003662620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D., Wang J., Gao B., Li J., Wu F., Zou J. X., et al. (2019). RORγ is a targetable master regulator of cholesterol biosynthesis in a cancer subtype. Nat. Commun. 10 (1), 4621. 10.1038/s41467-019-12529-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardwell C. R., Hicks B. M., Hughes C., Murray L. J. (2015). Statin use after diagnosis of breast cancer and survival: A population-based cohort study. Epidemiology 26 (1), 68–78. 10.1097/EDE.0000000000000189 [DOI] [PubMed] [Google Scholar]
- Chen J., Bi H., Hou J., Zhang X., Zhang C., Yue L., et al. (2013). Atorvastatin overcomes gefitinib resistance in Kras mutant human non-small cell lung carcinoma cells. Cell death Dis. 4 (9), e814. 10.1038/cddis.2013.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Liu X., Li Y., Quan C., Zheng L., Huang K. (2018). Lung cancer therapy targeting histone methylation: Opportunities and challenges. Comput. Struct. Biotechnol. J. 16, 211–223. 10.1016/j.csbj.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Yang D., Yang C., Zheng L., Huang K. (2020). Response to Comment on Chen et al. Clinical Characteristics and Outcomes of Patients With Diabetes and COVID-19 in Association With Glucose-Lowering Medication. Diabetes Care 2020;43:1399-1407. Diabetes Care 43 (10), e165–e166. 10.2337/dci20-0035 [DOI] [PubMed] [Google Scholar]
- Chimento A., Casaburi I., Avena P., Trotta F., De Luca A., Rago V., et al. (2018). Cholesterol and its metabolites in tumor growth: Therapeutic potential of statins in cancer treatment. Front. Endocrinol. 9, 807. 10.3389/fendo.2018.00807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M. H., Yoo T. G., Jeong S. M., Shin D. W. (2021). Association of aspirin, metformin, and statin use with gastric cancer incidence and mortality: A nationwide cohort study. Cancer Prev. Res. (Phila) 14 (1), 95–104. 10.1158/1940-6207.CAPR-20-0123 [DOI] [PubMed] [Google Scholar]
- Cho S. F., Yang Y. H., Liu Y. C., Hsiao H. H., Huang C. T., Wu C. H., et al. (2015). Previous exposure to statin may reduce the risk of subsequent non-hodgkin lymphoma: A nationwide population-based case-control study. PloS one 10 (10), e0139289. 10.1371/journal.pone.0139289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholesterol Treatment Trialists C., Emberson J. R., Kearney P. M., Blackwell L., Newman C., Reith C., et al. (2012). Lack of effect of lowering ldl cholesterol on cancer: Meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PloS one 7 (1), e29849. 10.1371/journal.pone.0029849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C. W., Lin C. H., Hsiao T. H., Lo C. C., Hsieh C. Y., Huang C. C., et al. (2019). Therapeutic effects of statins against lung adenocarcinoma via P53 mutant-mediated apoptosis. Sci. Rep. 9 (1), 20403. 10.1038/s41598-019-56532-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote D. J., Rosner B. A., Smith-Warner S. A., Egan K. M., Stampfer M. J. (2019). Statin use, hyperlipidemia, and risk of glioma. Eur. J. Epidemiol. 34 (11), 997–1011. 10.1007/s10654-019-00565-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehnavi S., Kiani A., Sadeghi M., Biregani A. F., Banach M., Atkin S. L., et al. (2021). Targeting ampk by statins: A potential therapeutic approach. Drugs 81 (8), 923–933. 10.1007/s40265-021-01510-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulak J., Jozkowicz A. (2005). Anti-angiogenic and anti-inflammatory effects of statins: Relevance to anti-cancer therapy. Curr. Cancer Drug Targets 5 (8), 579–594. 10.2174/156800905774932824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elimam H., El-Say K. M., Cybulsky A. V., Khalil H. (2020). Regulation of autophagy progress via lysosomal depletion by fluvastatin nanoparticle treatment in breast cancer cells. ACS Omega 5 (25), 15476–15486. 10.1021/acsomega.0c01618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldt M., Bjarnadottir O., Kimbung S., Jirstrom K., Bendahl P. O., Veerla S., et al. (2015). Statin-induced anti-proliferative effects via cyclin D1 and P27 in a window-of-opportunity breast cancer trial. J. Transl. Med. 13, 133. 10.1186/s12967-015-0486-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay-Schultz J., Sartorius C. A. (2015). Steroid hormones, steroid receptors, and breast cancer stem cells. J. Mammary Gland. Biol. Neoplasia 20 (1-2), 39–50. 10.1007/s10911-015-9340-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukamachi T., Wang X., Mochizuki Y., Maruyama C., Saito H., Kobayashi H. (2013). Acidic environments enhance the inhibitory effect of statins on proliferation of synovial cells. Int. Immunopharmacol. 17 (1), 148–153. 10.1016/j.intimp.2013.06.001 [DOI] [PubMed] [Google Scholar]
- Garcia-Bermudez J., Baudrier L., Bayraktar E. C., Shen Y., La K., Guarecuco R., et al. (2019). Squalene accumulation in cholesterol auxotrophic lymphomas prevents oxidative cell death. Nature 567 (7746), 118–122. 10.1038/s41586-019-0945-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwood E. R., Kumar A. S., Baehner F. L., Moore D. H., Au A., Hylton N., et al. (2010). Fluvastatin reduces proliferation and increases apoptosis in women with high grade breast cancer. Breast Cancer Res. Treat. 119 (1), 137–144. 10.1007/s10549-009-0507-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobel A., Rauner M., Hofbauer L. C., Rachner T. D. (2020). Cholesterol and beyond - the role of the mevalonate pathway in cancer biology. Biochimica biophysica acta Rev. cancer 1873 (2), 188351. 10.1016/j.bbcan.2020.188351 [DOI] [PubMed] [Google Scholar]
- Graaf M. R., Beiderbeck A. B., Egberts A. C., Richel D. J., Guchelaar H. J. (2004). The risk of cancer in users of statins. J. Clin. Oncol. 22 (12), 2388–2394. 10.1200/JCO.2004.02.027 [DOI] [PubMed] [Google Scholar]
- Griner L. N., McGraw K. L., Johnson J. O., List A. F., Reuther G. W. (2013). Jak2-V617f-Mediated signalling is dependent on lipid rafts and statins inhibit Jak2-V617f-dependent cell growth. Br. J. Haematol. 160 (2), 177–187. 10.1111/bjh.12103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaumond F., Bidaut G., Ouaissi M., Servais S., Gouirand V., Olivares O., et al. (2015). Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. Proc. Natl. Acad. Sci. U. S. A. 112 (8), 2473–2478. 10.1073/pnas.1421601112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C., Wan R., He Y., Lin S. H., Cao J., Qiu Y., et al. (2022). Therapeutic targeting of the mevalonate-geranylgeranyl diphosphate pathway with statins overcomes chemotherapy resistance in small cell lung cancer. Nat. Cancer 3 (5), 614–628. 10.1038/s43018-022-00358-1 [DOI] [PubMed] [Google Scholar]
- Hamelin B. A., Turgeon J. (1998). Hydrophilicity/Lipophilicity: Relevance for the Pharmacology and clinical effects of hmg-coa reductase inhibitors. Trends Pharmacol. Sci. 19 (1), 26–37. 10.1016/s0165-6147(97)01147-4 [DOI] [PubMed] [Google Scholar]
- Han J. Y., Lee S. H., Yoo N. J., Hyung L. S., Moon Y. J., Yun T., et al. (2011). A randomized phase ii study of gefitinib plus simvastatin versus gefitinib alone in previously treated patients with advanced non-small cell lung cancer. Clin. cancer Res. official J. Am. Assoc. Cancer Res. 17 (6), 1553–1560. 10.1158/1078-0432.CCR-10-2525 [DOI] [PubMed] [Google Scholar]
- Harshman L. C., Wang X., Nakabayashi M., Xie W., Valenca L., Werner L., et al. (2015). Statin use at the time of initiation of androgen deprivation therapy and time to progression in patients with hormone-sensitive prostate cancer. JAMA Oncol. 1 (4), 495–504. 10.1001/jamaoncol.2015.0829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisada T., Ayaori M., Ohrui N., Nakashima H., Nakaya K., Uto-Kondo H., et al. (2012). Statin inhibits hypoxia-induced endothelin-1 via accelerated degradation of HIF-1α in vascular smooth muscle cells. Cardiovasc Res. 95 (2), 251–259. 10.1093/cvr/cvs110 [DOI] [PubMed] [Google Scholar]
- Hong J. Y., Nam E. M., Lee J., Park J. O., Lee S. C., Song S. Y., et al. (2014). Randomized double-blinded, placebo-controlled phase ii trial of simvastatin and gemcitabine in advanced pancreatic cancer patients. Cancer Chemother. Pharmacol. 73 (1), 125–130. 10.1007/s00280-013-2328-1 [DOI] [PubMed] [Google Scholar]
- Huang B., Song B. L., Xu C. (2020). Cholesterol metabolism in cancer: Mechanisms and therapeutic opportunities. Nat. Metab. 2 (2), 132–141. 10.1038/s42255-020-0174-0 [DOI] [PubMed] [Google Scholar]
- Hus M., Grzasko N., Szostek M., Pluta A., Helbig G., Woszczyk D., et al. (2011). Thalidomide, dexamethasone and lovastatin with autologous stem cell transplantation as a salvage immunomodulatory therapy in patients with relapsed and refractory multiple myeloma. Ann. Hematol. 90 (10), 1161–1166. 10.1007/s00277-011-1276-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K. E., Kwon S. J., Kim Y. S., Park D. S., Kim B. R., Yoon K. H., et al. (2014). Effect of simvastatin on the resistance to egfr tyrosine kinase inhibitors in a non-small cell lung cancer with the T790m mutation of egfr. Exp. Cell Res. 323 (2), 288–296. 10.1016/j.yexcr.2014.02.026 [DOI] [PubMed] [Google Scholar]
- Iarrobino N. A., Gill B., Bernard M. E., Mishra M. V., Champ C. E. (2018). Targeting tumor metabolism with statins during treatment for advanced-stage pancreatic cancer. Am. J. Clin. Oncol. 41 (11), 1125–1131. 10.1097/COC.0000000000000433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M. M., Poly T. N., Walther B. A., Yang H. C., Jack Li Y. C. (2020a). Statin use and the risk of hepatocellular carcinoma: A meta-analysis of observational studies. Cancers (Basel) 12 (3), 671. 10.3390/cancers12030671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M. M., Poly T. N., Walther B. A., Yang H. C., Jack Li Y. C. (2020b). Statin use and the risk of hepatocellular carcinoma: A meta-analysis of observational studies. Cancers (Basel) 12 (3), 671. 10.3390/cancers12030671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Istvan E. S., Deisenhofer J. (2001). Structural mechanism for statin inhibition of hmg-coa reductase. Science 292 (5519), 1160–1164. 10.1126/science.1059344 [DOI] [PubMed] [Google Scholar]
- Jameson M. B., Gormly K., Espinoza D., Hague W., Asghari G., Jeffery G. M., et al. (2019). Spar - a randomised, placebo-controlled phase ii trial of simvastatin in addition to standard chemotherapy and radiation in preoperative treatment for rectal cancer: An agitg clinical trial. BMC Cancer 19 (1), 1229. 10.1186/s12885-019-6405-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janakiram N. B., Mohammed A., Bryant T., Zhang Y., Brewer M., Duff A., et al. (2016). Potentiating nk cell activity by combination of rosuvastatin and difluoromethylornithine for effective chemopreventive efficacy against colon cancer. Sci. Rep. 6, 37046. 10.1038/srep37046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen C. G., Norgaard M., Friis S., Skriver C., Borre M. (2014). Statin use and risk of prostate cancer: A Danish population-based case-control study, 1997-2010. Cancer Epidemiol. 38 (1), 42–47. 10.1016/j.canep.2013.10.010 [DOI] [PubMed] [Google Scholar]
- Jiang W., Hu J. W., He X. R., Jin W. L., He X. Y. (2021). Statins: A repurposed drug to fight cancer. J. Exp. Clin. Cancer Res. 40 (1), 241. 10.1186/s13046-021-02041-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H., He Y., Zhao P., Hu Y., Tao J., Chen J., et al. (2019). Targeting lipid metabolism to overcome EMT-associated drug resistance via integrin β3/FAK pathway and tumor-associated macrophage repolarization using legumain-activatable delivery. Theranostics 9 (1), 265–278. 10.7150/thno.27246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouve J. L., Lecomte T., Bouche O., Barbier E., Khemissa Akouz F., Riachi G., et al. (2019). Pravastatin combination with sorafenib does not improve survival in advanced hepatocellular carcinoma. J. hepatology 71 (3), 516–522. 10.1016/j.jhep.2019.04.021 [DOI] [PubMed] [Google Scholar]
- Juarez D., Fruman D. A. (2021). Targeting the mevalonate pathway in cancer. Trends Cancer 7 (6), 525–540. 10.1016/j.trecan.2020.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartal-Yandim M., Adan-Gokbulut A., Baran Y. (2016). Molecular mechanisms of drug resistance and its reversal in cancer. Crit. Rev. Biotechnol. 36 (4), 716–726. 10.3109/07388551.2015.1015957 [DOI] [PubMed] [Google Scholar]
- Kato S., Smalley S., Sadarangani A., Chen-Lin K., Oliva B., Branes J., et al. (2010). Lipophilic but not hydrophilic statins selectively induce cell death in gynaecological cancers expressing high levels of hmgcoa reductase. J. Cell. Mol. Med. 14 (5), 1180–1193. 10.1111/j.1582-4934.2009.00771.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawata S., Yamasaki E., Nagase T., Inui Y., Ito N., Matsuda Y., et al. (2001). Effect of pravastatin on survival in patients with advanced hepatocellular carcinoma. A randomized controlled trial. Br. J. Cancer 84 (7), 886–891. 10.1054/bjoc.2000.1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. T., Kang J. H., Lee J., Park S. H., Park J. O., Park Y. S., et al. (2014). Simvastatin plus capecitabine-cisplatin versus placebo plus capecitabine-cisplatin in patients with previously untreated advanced gastric cancer: A double-blind randomised phase 3 study. Eur. J. Cancer 50 (16), 2822–2830. 10.1016/j.ejca.2014.08.005 [DOI] [PubMed] [Google Scholar]
- Kim Y., Kim T. W., Han S. W., Ahn J. B., Kim S. T., Lee J., et al. (2019). A single arm, phase ii study of simvastatin plus xelox and bevacizumab as first-line chemotherapy in metastatic colorectal cancer patients. Cancer Res. Treat. 51 (3), 1128–1134. 10.4143/crt.2018.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloudova A., Guengerich F. P., Soucek P. (2017). The role of oxysterols in human cancer. Trends Endocrinol. metabolism TEM 28 (7), 485–496. 10.1016/j.tem.2017.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings I. R., van der Gaast A., van der Wijk L. J., de Jongh F. E., Eskens F. A., Sleijfer S. (2010). The addition of pravastatin to chemotherapy in advanced gastric carcinoma: A randomised phase ii trial. Eur. J. Cancer 46 (18), 3200–3204. 10.1016/j.ejca.2010.07.036 [DOI] [PubMed] [Google Scholar]
- Lee S. J., Lee I., Lee J., Park C., Kang W. K. (2014). Statins, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, potentiate the anti-angiogenic effects of bevacizumab by suppressing angiopoietin2, BiP, and Hsp90α in human colorectal cancer. Br. J. Cancer 111 (3), 497–505. 10.1038/bjc.2014.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Lee K. H., Lee G. K., Lee S. H., Lim K. Y., Joo J., et al. (2017). Randomized phase ii study of afatinib plus simvastatin versus afatinib alone in previously treated patients with advanced nonadenocarcinomatous non-small cell lung cancer. Cancer Res. Treat. 49 (4), 1001–1011. 10.4143/crt.2016.546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C. A., Brault C., Peck B., Bensaad K., Griffiths B., Mitter R., et al. (2015). Srebp maintains lipid biosynthesis and viability of cancer cells under lipid- and oxygen-deprived conditions and defines a gene signature associated with poor survival in glioblastoma multiforme. Oncogene 34 (40), 5128–5140. 10.1038/onc.2014.439 [DOI] [PubMed] [Google Scholar]
- Li J., Gu D., Lee S. S., Song B., Bandyopadhyay S., Chen S., et al. (2016). Abrogating cholesterol esterification suppresses growth and metastasis of pancreatic cancer. Oncogene 35 (50), 6378–6388. 10.1038/onc.2016.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Cui N., Hao T., Zou J., Jiao W., Yi K., et al. (2021). Statins use and the prognosis of colorectal cancer: A meta-analysis. Clin. Res. Hepatol. Gastroenterol. 45 (5), 101588. 10.1016/j.clinre.2020.101588 [DOI] [PubMed] [Google Scholar]
- Li S., Xu H. X., Wu C. T., Wang W. Q., Jin W., Gao H. L., et al. (2019). Angiogenesis in pancreatic cancer: Current research status and clinical implications. Angiogenesis 22 (1), 15–36. 10.1007/s10456-018-9645-2 [DOI] [PubMed] [Google Scholar]
- Lim S. A., Wei J., Nguyen T. M., Shi H., Su W., Palacios G., et al. (2021). Lipid signalling enforces functional specialization of T(reg) cells in tumours. Nature 591 (7849), 306–311. 10.1038/s41586-021-03235-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. H., Kim T. W., Hong Y. S., Han S. W., Lee K. H., Kang H. J., et al. (2015). A randomised, double-blind, placebo-controlled multi-centre phase iii trial of xeliri/folfiri plus simvastatin for patients with metastatic colorectal cancer. Br. J. Cancer 113 (10), 1421–1426. 10.1038/bjc.2015.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Yi Z., Guan X., Zeng Y. X., Ma F. (2017b). The relationship between statins and breast cancer prognosis varies by statin type and exposure time: A meta-analysis. Breast Cancer Res. Treat. 164 (1), 1–11. 10.1007/s10549-017-4246-0 [DOI] [PubMed] [Google Scholar]
- Liu C., Wang J., Wei Y., Zhang W., Geng M., Yuan Y., et al. (2020). Fat-specific knockout of Mecp2 upregulates slpi to reduce obesity by enhancing browning. Diabetes 69 (1), 35–47. 10.2337/db19-0502 [DOI] [PubMed] [Google Scholar]
- Liu C., Yan W., Shi J., Wang S., Peng A., Chen Y., et al. (2022). Biological actions, implications, and cautions of statins therapy in covid-19. Front. Nutr. 9, 927092. 10.3389/fnut.2022.927092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Sun Y., Jiang M., Li Y., Tian Y., Xue W., et al. (2017a). Glyceraldehyde-3-Phosphate dehydrogenase promotes liver tumorigenesis by modulating phosphoglycerate dehydrogenase. Hepatology 66, 631–645. 10.1002/hep.29202 [DOI] [PubMed] [Google Scholar]
- Liu X., Chen Y., Li Y., Petersen R. B., Huang K. (2019). Targeting mitosis exit: A brake for cancer cell proliferation. Biochimica biophysica acta Rev. cancer 1871 (1), 179–191. 10.1016/j.bbcan.2018.12.007 [DOI] [PubMed] [Google Scholar]
- Longo J., Hamilton R. J., Masoomian M., Khurram N., Branchard E., Mullen P. J., et al. (2020). A pilot window-of-opportunity study of preoperative fluvastatin in localized prostate cancer. Prostate Cancer Prostatic Dis. 23 (4), 630–637. 10.1038/s41391-020-0221-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luput L., Sesarman A., Porfire A., Achim M., Muntean D., Casian T., et al. (2020). Liposomal simvastatin sensitizes C26 murine colon carcinoma to the antitumor effects of liposomal 5-fluorouracil in vivo . Cancer Sci. 111 (4), 1344–1356. 10.1111/cas.14312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majidi A., Na R., Jordan S. J., De Fazio A., Webb P. M., Group O. S. (2021). Statin use and survival following a diagnosis of ovarian cancer: A prospective observational study. Int. J. cancer 148 (7), 1608–1615. 10.1002/ijc.33333 [DOI] [PubMed] [Google Scholar]
- Mandal C. C., Ghosh-Choudhury N., Yoneda T., Choudhury G. G., Ghosh-Choudhury N. (2011). Simvastatin prevents skeletal metastasis of breast cancer by an antagonistic interplay between P53 and Cd44. J. Biol. Chem. 286 (13), 11314–11327. 10.1074/jbc.M110.193714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoukian G. E., Tannir N. M., Jonasch E., Qiao W., Haygood T. M., Tu S. M. (2011). Pilot trial of bone-targeted therapy combining zoledronate with fluvastatin or atorvastatin for patients with metastatic renal cell carcinoma. Clin. Genitourin. Cancer 9 (2), 81–88. 10.1016/j.clgc.2011.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matusewicz L., Meissner J., Toporkiewicz M., Sikorski A. F. (2015). The effect of statins on cancer cells-review. Tumour Biol. 36 (7), 4889–4904. 10.1007/s13277-015-3551-7 [DOI] [PubMed] [Google Scholar]
- McGregor G. H., Campbell A. D., Fey S. K., Tumanov S., Sumpton D., Blanco G. R., et al. (2020). Targeting the metabolic response to statin-mediated oxidative stress produces a synergistic antitumor response. Cancer Res. 80 (2), 175–188. 10.1158/0008-5472.CAN-19-0644 [DOI] [PubMed] [Google Scholar]
- Mehibel M., Ortiz-Martinez F., Voelxen N., Boyers A., Chadwick A., Telfer B. A., et al. (2018). Statin-induced metabolic reprogramming in head and neck cancer: A biomarker for targeting monocarboxylate transporters. Sci. Rep. 8 (1), 16804. 10.1038/s41598-018-35103-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta N., Hordines J., Sykes D., Doerr R. J., Cohen S. A. (1998). Low density lipoproteins and lovastatin modulate the organ-specific transendothelial migration of primary and metastatic human colon adenocarcinoma cell lines in vitro . Clin. Exp. Metastasis 16 (7), 587–594. 10.1023/a:1006548902592 [DOI] [PubMed] [Google Scholar]
- Mei Z., Liang M., Li L., Zhang Y., Wang Q., Yang W. (2017). Effects of statins on cancer mortality and progression: A systematic review and meta-analysis of 95 cohorts including 1,111,407 individuals. Int. J. cancer 140 (5), 1068–1081. 10.1002/ijc.30526 [DOI] [PubMed] [Google Scholar]
- Mengual D., Medrano L. E., Villamizar-Villamizar W., Osorio-Llanes E., Mendoza-Torres E., Bolivar S. (2022). Novel effects of statins on cancer via autophagy. Pharm. (Basel) 15 (6), 648. 10.3390/ph15060648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menter D. G., Ramsauer V. P., Harirforoosh S., Chakraborty K., Yang P., Hsi L., et al. (2011). Differential effects of pravastatin and simvastatin on the growth of tumor cells from different organ sites. PloS one 6 (12), e28813. 10.1371/journal.pone.0028813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misirkic M., Janjetovic K., Vucicevic L., Tovilovic G., Ristic B., Vilimanovich U., et al. (2012). Inhibition of ampk-dependent autophagy enhances in vitro antiglioma effect of simvastatin. Pharmacol. Res. 65 (1), 111–119. 10.1016/j.phrs.2011.08.003 [DOI] [PubMed] [Google Scholar]
- Mullen P. J., Yu R., Longo J., Archer M. C., Penn L. Z. (2016). The interplay between cell signalling and the mevalonate pathway in cancer. Nat. Rev. Cancer 16 (11), 718–731. 10.1038/nrc.2016.76 [DOI] [PubMed] [Google Scholar]
- Nayan M., Punjani N., Juurlink D. N., Finelli A., Austin P. C., Kulkarni G. S., et al. (2017). Statin use and kidney cancer survival outcomes: A systematic review and meta-analysis. Cancer Treat. Rev. 52, 105–116. 10.1016/j.ctrv.2016.11.009 [DOI] [PubMed] [Google Scholar]
- Nielsen S. F., Nordestgaard B. G., Bojesen S. E. (2012). Statin use and reduced cancer-related mortality. N. Engl. J. Med. 367 (19), 1792–1802. 10.1056/NEJMoa1201735 [DOI] [PubMed] [Google Scholar]
- Park H. J., Kong D., Iruela-Arispe L., Begley U., Tang D., Galper J. B. (2002). 3-Hydroxy-3-Methylglutaryl coenzyme a reductase inhibitors interfere with angiogenesis by inhibiting the geranylgeranylation of rhoa. Circulation Res. 91 (2), 143–150. 10.1161/01.res.0000028149.15986.4c [DOI] [PubMed] [Google Scholar]
- Pich C., Teiti I., Rochaix P., Mariame B., Couderc B., Favre G., et al. (2013). Statins reduce melanoma development and metastasis through mica overexpression. Front. Immunol. 4, 62. 10.3389/fimmu.2013.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X. F., Kim D. H., Lee K. J., Kim C. S., Song S. B., Cai D. Q., et al. (2013). Autophagy contributes to apoptosis in A20 and El4 lymphoma cells treated with fluvastatin. Cancer Cell Int. 13 (1), 111. 10.1186/1475-2867-13-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao X., Hu Z., Xiong F., Yang Y., Peng C., Wang D., et al. (2023). Lipid metabolism reprogramming in tumor-associated macrophages and implications for therapy. Lipids health Dis. 22 (1), 45. 10.1186/s12944-023-01807-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan C., Chen Y., Wang X., Yang D., Wang Q., Huang Y., et al. (2020). Loss of histone lysine methyltransferase Ezh2 confers resistance to tyrosine kinase inhibitors in non-small cell lung cancer. Cancer Lett. 495, 41–52. 10.1016/j.canlet.2020.09.003 [DOI] [PubMed] [Google Scholar]
- Raval A. D., Thakker D., Negi H., Vyas A., Salkini M. W. (2016). Association between statins and clinical outcomes among men with prostate cancer: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 19 (2), 222. 10.1038/pcan.2016.3 [DOI] [PubMed] [Google Scholar]
- Ren Q. W., Yu S. Y., Teng T. K., Li X., Cheung K. S., Wu M. Z., et al. (2021). Statin associated lower cancer risk and related mortality in patients with heart failure. Eur. Heart J. 42 (32), 3049–3059. 10.1093/eurheartj/ehab325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riganti C., Orecchia S., Pescarmona G., Betta P. G., Ghigo D., Bosia A. (2006). Statins revert doxorubicin resistance via nitric oxide in malignant mesothelioma. Int. J. cancer 119 (1), 17–27. 10.1002/ijc.21832 [DOI] [PubMed] [Google Scholar]
- Rigoni M., Riganti C., Vitale C., Griggio V., Campia I., Robino M., et al. (2015). Simvastatin and downstream inhibitors circumvent constitutive and stromal cell-induced resistance to doxorubicin in ighv unmutated cll cells. Oncotarget 6 (30), 29833–29846. 10.18632/oncotarget.4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosch P. J., McCully K. (2013). Statin use and reduced cancer-related mortality. N. Engl. J. Med. 368 (6), 576. 10.1056/NEJMc1214827 [DOI] [PubMed] [Google Scholar]
- Sahebkar A., Simental-Mendia L. E., Pedone C., Ferretti G., Nachtigal P., Bo S., et al. (2016). Statin therapy and plasma free fatty acids: A systematic review and meta-analysis of controlled clinical trials. Br. J. Clin. Pharmacol. 81 (5), 807–818. 10.1111/bcp.12854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinberg T., Mak B., Butler L., Selth L., Horvath L. G. (2023). Targeting lipid metabolism in metastatic prostate cancer. Ther. Adv. Med. Oncol. 15, 17588359231152839. 10.1177/17588359231152839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckl M. J., Ottensmeier C. H., Cullen M., Schmid P., Ngai Y., Muthukumar D., et al. (2017). Multicenter, phase iii, randomized, double-blind, placebo-controlled trial of pravastatin added to first-line standard chemotherapy in small-cell lung cancer (lungstar). J. Clin. Oncol. 35 (14), 1506–1514. 10.1200/JCO.2016.69.7391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Ikonen E. (1997). Functional rafts in cell membranes. Nature 387 (6633), 569–572. 10.1038/42408 [DOI] [PubMed] [Google Scholar]
- Sirtori C. R. (2014). The Pharmacology of statins. Pharmacol. Res. 88, 3–11. 10.1016/j.phrs.2014.03.002 [DOI] [PubMed] [Google Scholar]
- Solomon K. R., Pelton K., Boucher K., Joo J., Tully C., Zurakowski D., et al. (2009). Ezetimibe is an inhibitor of tumor angiogenesis. Am. J. pathology 174 (3), 1017–1026. 10.2353/ajpath.2009.080551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino G., Ruggeri N., Specchia V., Cordenonsi M., Mano M., Dupont S., et al. (2014). Metabolic control of yap and taz by the mevalonate pathway. Nat. Cell Biol. 16 (4), 357–366. 10.1038/ncb2936 [DOI] [PubMed] [Google Scholar]
- Sperling C. D., Verdoodt F., Friis S., Dehlendorff C., Kjaer S. K. (2017). Statin use and risk of endometrial cancer: A nationwide registry-based case-control study. Acta Obstet. Gynecol. Scand. 96 (2), 144–149. 10.1111/aogs.13069 [DOI] [PubMed] [Google Scholar]
- Stossel T. P. (2008). The discovery of statins. Cell 134 (6), 903–905. 10.1016/j.cell.2008.09.008 [DOI] [PubMed] [Google Scholar]
- Sun Y., Wang Q., Zhang Y., Geng M., Wei Y., Liu Y., et al. (2020). Multigenerational maternal obesity increases the incidence of hcc in offspring via mir-27a-3p. J. hepatology 73 (3), 603–615. 10.1016/j.jhep.2020.03.050 [DOI] [PubMed] [Google Scholar]
- Tamburrino D., Crippa S., Partelli S., Archibugi L., Arcidiacono P. G., Falconi M., et al. (2020). Statin use improves survival in patients with pancreatic ductal adenocarcinoma: A meta-analysis. Dig. Liver Dis. 52 (4), 392–399. 10.1016/j.dld.2020.01.008 [DOI] [PubMed] [Google Scholar]
- Taras D., Blanc J. F., Rullier A., Dugot-Senant N., Laurendeau I., Vidaud M., et al. (2007). Pravastatin reduces lung metastasis of rat hepatocellular carcinoma via a coordinated decrease of mmp expression and activity. J. hepatology 46 (1), 69–76. 10.1016/j.jhep.2006.06.015 [DOI] [PubMed] [Google Scholar]
- Tilija Pun N., Jeong C. H. (2021). Statin as a potential chemotherapeutic agent: Current updates as a monotherapy, combination therapy, and treatment for anti-cancer drug resistance. Pharm. (Basel) 14 (5), 470. 10.3390/ph14050470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ung M. H., MacKenzie T. A., Onega T. L., Amos C. I., Cheng C. (2018). Statins associate with improved mortality among patients with certain histological subtypes of lung cancer. Lung Cancer 126, 89–96. 10.1016/j.lungcan.2018.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rompay M. I., Solomon K. R., Nickel J. C., Ranganathan G., Kantoff P. W., McKinlay J. B. (2019). Prostate cancer incidence and mortality among men using statins and non-statin lipid-lowering medications. Eur. J. Cancer 112, 118–126. 10.1016/j.ejca.2018.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinayak S., Schwartz E. J., Jensen K., Lipson J., Alli E., McPherson L., et al. (2013). A clinical trial of lovastatin for modification of biomarkers associated with breast cancer risk. Breast Cancer Res. Treat. 142 (2), 389–398. 10.1007/s10549-013-2739-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent L., Chen W., Hong L., Mirshahi F., Mishal Z., Mirshahi-Khorassani T., et al. (2001). Inhibition of endothelial cell migration by cerivastatin, an hmg-coa reductase inhibitor: Contribution to its anti-angiogenic effect. FEBS Lett. 495 (3), 159–166. 10.1016/s0014-5793(01)02337-7 [DOI] [PubMed] [Google Scholar]
- Wang A., Aragaki A. K., Tang J. Y., Kurian A. W., Manson J. E., Chlebowski R. T., et al. (2016). Statin use and all-cancer survival: Prospective results from the women's health initiative. Br. J. Cancer 115 (1), 129–135. 10.1038/bjc.2016.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C., Li X. X., Sun X., Li G. Y., Sun J. L., Ye Y. P., et al. (2018). Activation of AMPK by simvastatin inhibited breast tumor angiogenesis via impeding HIF-1α-induced pro-angiogenic factor. Cancer Sci. 109 (5), 1627–1637. 10.1111/cas.13570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. H., Liu C. H., Ding D. C. (2022a). Statins as repurposed drugs in gynecological cancer: A review. Int. J. Mol. Sci. 23 (22), 13937. 10.3390/ijms232213937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Chen Y., Xie Y., Yang D., Sun Y., Yuan Y., et al. (2022b). Histone H1.2 promotes hepatocarcinogenesis by regulating signal transducer and activator of transcription 3 signaling. Cancer Sci. 113 (5), 1679–1692. 10.1111/cas.15336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis M., Heeschen C., Glassford A. J., Cooke J. P. (2002). Statins have biphasic effects on angiogenesis. Circulation 105 (6), 739–745. 10.1161/hc0602.103393 [DOI] [PubMed] [Google Scholar]
- Wong W. W., Dimitroulakos J., Minden M. D., Penn L. Z. (2002). Hmg-coa reductase inhibitors and the malignant cell: The statin family of drugs as triggers of tumor-specific apoptosis. Leukemia 16 (4), 508–519. 10.1038/sj.leu.2402476 [DOI] [PubMed] [Google Scholar]
- Wu J., Wong W. W., Khosravi F., Minden M. D., Penn L. Z. (2004). Blocking the raf/mek/erk pathway sensitizes acute myelogenous leukemia cells to lovastatin-induced apoptosis. Cancer Res. 64 (18), 6461–6468. 10.1158/0008-5472.CAN-04-0866 [DOI] [PubMed] [Google Scholar]
- Xiong M., Chen H., Fan Y., Jin M., Yang D., Chen Y., et al. (2023). Tubular elabela-apj Axis attenuates ischemia-reperfusion induced acute kidney injury and the following aki-ckd transition by protecting renal microcirculation. Theranostics 13 (10), 3387–3401. 10.7150/thno.84308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Fan Y., Xiong M., Chen Y., Zhou Y., Liu X., et al. (2023). Loss of renal tubular G9a benefits acute kidney injury by lowering focal lipid accumulation via ces1. EMBO Rep. 24, e56128. 10.15252/embr.202256128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P. M., Chen C. C. (2011). Life or death? Autophagy in anticancer therapies with statins and histone deacetylase inhibitors. Autophagy 7 (1), 107–108. 10.4161/auto.7.1.13988 [DOI] [PubMed] [Google Scholar]
- Yang P. M., Liu Y. L., Lin Y. C., Shun C. T., Wu M. S., Chen C. C. (2010). Inhibition of autophagy enhances anticancer effects of atorvastatin in digestive malignancies. Cancer Res. 70 (19), 7699–7709. 10.1158/0008-5472.CAN-10-1626 [DOI] [PubMed] [Google Scholar]
- Yang Z., Su Z., DeWitt J. P., Xie L., Chen Y., Li X., et al. (2017). Fluvastatin prevents lung adenocarcinoma bone metastasis by triggering autophagy. EBioMedicine 19, 49–59. 10.1016/j.ebiom.2017.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yulian E. D., Siregar N. C., Bajuadji (2021). Combination of simvastatin and fac improves response to neoadjuvant chemotherapy in locally advanced breast cancer. Cancer Res. Treat. 53 (4), 1072–1083. 10.4143/crt.2020.1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahedipour F., Butler A. E., Rizzo M., Sahebkar A. (2022). Statins and angiogenesis in non-cardiovascular diseases. Drug Discov. Today 27 (10), 103320. 10.1016/j.drudis.2022.07.005 [DOI] [PubMed] [Google Scholar]
- Zaleska M., Mozenska O., Bil J. (2018). Statins use and cancer: An update. Future Oncol. 14 (15), 1497–1509. 10.2217/fon-2017-0543 [DOI] [PubMed] [Google Scholar]
- Zeng R. W., Yong J. N., Tan D. J. H., Fu C. E., Lim W. H., Xiao J., et al. (2023). Meta-Analysis: Chemoprevention of hepatocellular carcinoma with statins, aspirin and metformin. Aliment. Pharmacol. Ther. 57 (6), 600–609. 10.1111/apt.17371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Yang Y. J., Wang H., Dong Q. T., Wang T. J., Qian H. Y., et al. (2012). Autophagy activation: A novel mechanism of atorvastatin to protect mesenchymal stem cells from hypoxia and serum deprivation via amp-activated protein kinase/mammalian target of rapamycin pathway. Stem cells Dev. 21 (8), 1321–1332. 10.1089/scd.2011.0684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhou L., Xu Y., Zhou J., Jiang T., Wang J., et al. (2022). Targeting Smyd2 inhibits angiogenesis and increases the efficiency of apatinib by suppressing Egfl7 in colorectal cancer. Angiogenesis. 10.1007/s10456-022-09839-4 [DOI] [PubMed] [Google Scholar]
- Zhou M., Zheng J., Bi J., Wu X., Lyu J., Gao K. (2018). Synergistic inhibition of colon cancer cell growth by a combination of atorvastatin and phloretin. Oncol. Lett. 15 (2), 1985–1992. 10.3892/ol.2017.7480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P. F., Wang M. X., Chen Z. L., Yang L. (2021). Targeting the tumor microenvironment: A literature review of the novel anti-tumor mechanism of statins. Front. Oncol. 11, 761107. 10.3389/fonc.2021.761107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang L., Kim J., Adam R. M., Solomon K. R., Freeman M. R. (2005). Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J. Clin. investigation 115 (4), 959–968. 10.1172/JCI19935 [DOI] [PMC free article] [PubMed] [Google Scholar]


