Abstract
Previous studies have demonstrated that Burkholderia pseudomallei secretes protease, lipase, and phospholipase C (PLC) into the extracellular milieu, but their mechanisms of secretion and roles in pathogenesis have not been elucidated. In this study, we isolated and characterized 29 transposon mutants unable to secrete protease, lipase, and PLC.
Melioidosis is an infection caused by the gram-negative bacillus Burkholderia pseudomallei (2, 15). In this report, we describe the isolation and characterization of B. pseudomallei 1026b transposon mutants deficient in secretion of protease, lipase, and phospholipase C (PLC). We also compared the relative virulence of 1026b and DD213, a representative secretion mutant, in the Syrian hamster model of melioidosis.
Isolation of secretion mutants of B. pseudomallei 1026b.
The bacterial strains and plasmids used in this study are listed in Table 1. The goal of this study was to identify mutants unable to secrete protease, lipase, and PLC in order to further characterize the genes involved in this process and examine the relative importance of these exoproducts in the pathogenesis of melioidosis. We screened approximately 15,000 Tn5-OT182 (3) mutants of B. pseudomallei 1026b for the inability to form a zone of clearing around isolated colonies on 3% skim milk agar (protease activity). Twenty-nine distinct mutants that did not demonstrate a zone of clearing around isolated colonies were identified. In fact, none of the 29 mutants demonstrated a zone of clearing on tributyrin agar (lipase activity) or formed a cloudy (opaque) zone around areas of bacterial growth on egg yolk agar (PLC activity). Taken together, these results demonstrate that all 29 mutants were unable to secrete protease, lipase, and PLC. This strongly suggests that this organism utilizes the same secretion pathway for these exoproducts.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Reference or source |
|---|---|---|
| Strains | ||
| Escherichia coli | ||
| SM10 | Mobilizing strain, transfer genes of RP4 integrated in chromosome; Kmr Sms | 14 |
| SM10λpir | SM10 with a λ prophage carrying the gene encoding the π protein | 8 |
| DH5α | High-efficiency transformation | Bethesda Research Laboratories |
| SURE | High-efficiency transformation; deficient in homologous recombination; Kmr Tcr | Stratagene |
| TOP10 | General cloning and blue/white screening | Invitrogen |
| B. pseudomallei | ||
| 1026b | Clinical isolate; Smr Tcs Tps | 3 |
| DD303-1 | 1026b::pDD303-1; Tcr | This study |
| PBS-1 | 1026b::pPBS-1; Tcr | 3 |
| DD301-1 | 1026b::pDD301-1; Tcr | This study |
| DD314-1 | 1026b::pDD314-1; Tcr | This study |
| DD315-1 | 1026b::pDD315-1; Tcr | This study |
| DD316-1 | 1026b::pDD316-1; Tcr | This study |
| Plasmids | ||
| pOT182 | pSUP102(Gm)::Tn5-OT182; Cmr Gmr Apr Tcr | 7 |
| pUCP28T | Broad-host-range vector; IncP OriT; pRO1600 ori; Tpr | 13 |
| pSKM11 | Positive selection cloning vector; IncP OriT; ColE1 ori; Apr Tcs | 9 |
| pC21Bg | 18.5-kb BglII fragment from C21 obtained by self-cloning; Apr Tcr | This study |
| pC21E | 16.0-kb EcoRI fragment from C21 obtained by self-cloning; Apr Tcr | This study |
| pDD226Nh | 31.0-kb NheI fragment from DD226 obtained by self-cloning; Apr Tcr | This study |
| pDD232E | 8.4-kb EcoRI fragment from DD232 obtained by self-cloning; Apr Tcr | This study |
| pDD235E | 11.6-kb EcoRI fragment from DD235 obtained by self-cloning; Apr Tcr | This study |
| pIR2 | pUCP28T containing a 670-bp PCR product encompassing gspC; Tpr | This study |
| pDD303-1 | pSKM11 containing 750-bp XhoI-BamHI fragment internal to gspE; Apr Tcr | This study |
| pPBS-1 | pSKM11 containing 3′ end of fliC and downstream DNA; Apr Tcr | 3 |
| pDD301-1 | pSKM11 containing 650-bp BamHI fragment internal to orfB; Apr Tcr | This study |
| pDD314-1 | pSKM11 containing a 390-bp PCR product internal to orfC; Apr Tcr | This study |
| pDD315-1 | pSKM11 containing a 480-bp PCR product internal to gspN; Apr Tcr | This study |
| pDD316-1 | pSKM11 containing 330-bp XhoI fragment internal to orfD; Apr Tcr | This study |
ori, origin of replication.
Protease and PLC are cell associated in B. pseudomallei DD213 and C21.
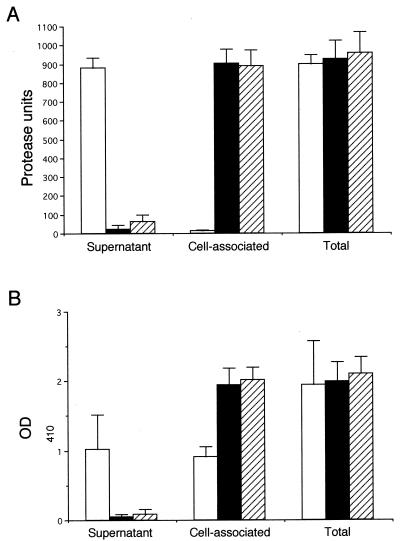
We performed quantitative protease and PLC assays on the culture supernatants and cell lysates of 1026b and two representative secretion mutants, DD213 and C21 (Fig. 1). More than 98% of the 1026b proteolytic activity was present in the culture supernatant, indicating that protease is efficiently secreted by B. pseudomallei (Fig. 1A). In contrast, only 2% of the DD213 proteolytic activity and 7% of the C21 proteolytic activity were present in the culture supernatant (Fig. 1A). The total amounts of proteolytic activity (culture supernatant plus cell lysate) produced by 1026b, DD213, and C21 were similar (Fig. 1A). Approximately 53% of the 1026b PLC activity was present in the culture supernatant, while the remaining 47% was cell associated (Fig. 1B). This result may indicate that PLC was inefficiently secreted under the assay conditions employed or that B. pseudomallei contains a cell-associated and a secreted PLC. DD213 and C21 culture supernatants contained only 2 and 3% of the total PLC activity, respectively (Fig. 1B). The total amounts of PLC activity produced by 1026b, DD213, and C21 were similar (Fig. 1B). These results clearly demonstrate that DD213 and C21 were secretion mutants; they produced normal levels of protease and PLC that accumulated intracellularly rather than being secreted into the extracellular milieu.
FIG. 1.
Quantitative enzymatic assay of secreted (supernatant), cell associated, and total proteolytic and PLC activities of B. pseudomallei 1026b (□), DD213 (■), and C21 (▨). Bacterial strains were grown in Luria-Bertani broth for 24 h at 37°C with shaking (250 rpm). (A) Protease assay (11). Numerical values are presented as protease units per milliliter of culture. One unit of protease is the amount of enzyme which yields an increase in absorbance (at 595 nm) of 0.25/h at 37°C. (B) PLC assay (5). Nitrophenylphosphorylcholine hydrolysis by PLC was monitored spectrophotometrically by the measurement of p-nitrophenol at 410 nm. Total enzymatic activity is the sum of the secreted and cell-associated activities. All numerical values are the means of three separate experiments performed in triplicate plus standard deviations (error bars).
Molecular characterization of the B. pseudomallei type II secretion pathway genes.
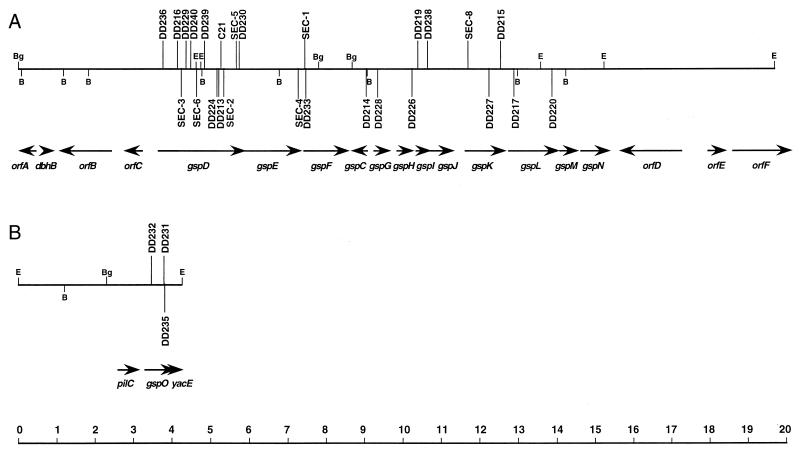
The 29 B. pseudomallei secretion mutants isolated in this study contain Tn5-OT182 integrations in two distinct genetic loci (Fig. 2). The 26 Tn5-OT182 integrations presented in Fig. 2A were found within a gene cluster that encodes products that are homologous to type II secretion pathway proteins in gram-negative bacteria (10, 12). The genes in this cluster were named gsp, for “general secretory pathway.” The three Tn5-OT182 integrations presented in Fig. 2B are found within a gene (gspO) that encodes a product that is homologous to type IV prepilin peptidases (6) (Fig. 2B).
FIG. 2.
Physical and genetic map of loci required for the secretion of protease, lipase, and PLC in B. pseudomallei. (A) Genetic locus containing 26 distinct Tn5-OT182 integrations in B. pseudomallei secretion mutants. (B) Genetic locus containing three distinct Tn5-OT182 integrations in B. pseudomallei secretion mutants. The horizontal lines represent B. pseudomallei chromosomal DNA, and the locations of relevant restriction endonuclease sites are shown (B, BamHI; Bg, BglII; E, EcoRI). The exact locations of Tn5-OT182 integrations in the secretion mutants are shown by vertical lines extending above and below the chromosomal DNA. The promoterless lacZ gene of Tn5-OT182 integrations is oriented from left to right (5′ to 3′) in the secretion mutants designated by vertical lines extending above the chromosomal DNA and from right to left (5′ to 3′) in the secretion mutants designated by vertical lines extending below the chromosomal DNA. The locations and directions of the transcription of the genes are represented by arrows, and the gene names are shown below them. A scale (in kilobases) is shown at the bottom.
Identification of the 5′ and 3′ termini of the type II secretion pathway gene cluster.
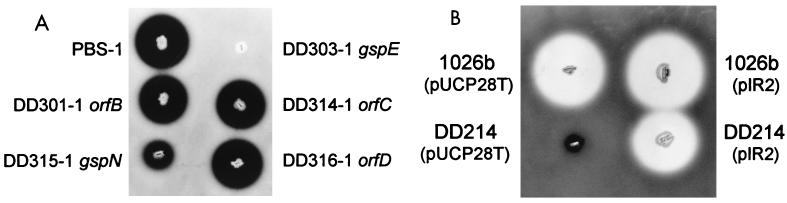
We constructed strains containing mutations in orfB, orfC, gspN, and orfD in order to investigate their roles in protein secretion (Fig. 2 and 3). The strains PBS-1 and DD303-1 were also constructed as positive and negative controls, respectively (Table 1). The zones of clearing produced by DD301-1 (orfB), DD314-1 (orfC), and DD316-1 (orfD) were indistinguishable from that produced by PBS-1 (Fig. 3A). Similar results were obtained with tributyrin agar and egg yolk agar (data not shown). The zone of clearing produced by DD315-1 (gspN) on 3% skim milk agar was consistently smaller than that produced by PBS-1 (Fig. 3A). Similar results were obtained with tributyrin agar and egg yolk agar (data not shown). The secretion phenotype of DD315-1 was probably not due to a polar effect because the gene immediately downstream of gspN (orfD) is not required for the secretion of exoproducts. These results demonstrate that gspN is required for efficient or maximal exoproduct secretion. The fact that we did not identify any Tn5-OT182 integrations in gspN supports the notion that mutations in this gene probably do not result in an absolute secretion-deficient phenotype. As orfC and orfD do not appear to be involved in the secretion of exoproteins by the type II pathway, we suggest that they define the 5′ and 3′ ends of the type II secretion pathway gene cluster, respectively (Fig. 2A).
FIG. 3.
Detection of protease secretion by B. pseudomallei strains on 3% skim milk agar. (A) Plasmid disruption of gspE and gspN resulted in strains that were completely or partially defective in protease secretion, respectively. The strains were grown on 3% skim milk agar plates containing tetracycline, and protease activity is indicated by a dark zone of clearing around the isolated colonies. (B) Complementation of the gspC::Tn5-OT182 mutation in DD214 by supplying gspC in trans on pIR2. The strains were grown on 3% skim milk agar plates containing trimethoprim, and protease activity is indicated by a light zone of clearing around the isolated colonies.
gspC is essential for secretion of exoproducts in B. pseudomallei.
The identification of a centrally located gene with reverse transcriptional polarity relative to the other type II secretion genes was quite surprising (Fig. 2). We attempted to complement the secretion-deficient phenotype of DD214 in order to determine if it was due to the disruption of gspC or to a polar effect by other gsp genes located upstream and/or downstream of gspC. We amplified by PCR a 670-bp product encompassing gspC and cloned it into the broad-host-range plasmid pUCP28T. The pUCP28T derivative pIR2 was able to complement the mutation in DD214 in trans (Fig. 3B). The zones of clearing around 1026b(pUCP28T), 1026b(pIR2), and DD214(pIR2) on 3% skim milk agar were similar. As expected, the negative control DD214(pUCP28T) did not produce a zone of clearing on 3% skim milk agar. Similar results were obtained with tributyrin agar and egg yolk agar (data not shown). These results clearly demonstrate that the mutation in DD214 did not have a polar effect on upstream or downstream genes and that gspC is essential for the secretion of exoproducts in B. pseudomallei.
Relative virulence of 1026b and DD213.
Syrian hamsters are highly susceptible to infection by B. pseudomallei (1, 4). The 50% lethal doses (LD50) of 1026b and DD213 in the hamster model of infection were <5 and 13, respectively. These represent increases in LD50 of 3- to 13-fold, depending on the actual LD50 for 1026b. DD213 was reisolated from the blood and livers of several infected animals and was found to be Tcr and deficient in exoproduct secretion, suggesting that the Tn5-OT182 integration in this strain was stable in the absence of selection. These results suggest that exoproducts secreted by the type II pathway probably play a minor role in B. pseudomallei pathogenesis.
Nucleotide sequence accession number.
The nucleotide sequences reported in this paper were deposited in the GenBank database under the accession no. AF110185 and AF110186.
REFERENCES
- 1.Brett P J, DeShazer D, Woods D E. Characterization of Burkholderia pseudomallei and Burkholderia pseudomallei-like strains. Epidemiol Infect. 1997;118:137–148. doi: 10.1017/s095026889600739x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dance D A B. Melioidosis. In: Cook G C, editor. Manson’s tropical diseases. 20th ed. London, England: W. B. Saunders Company Ltd.; 1996. pp. 925–930. [Google Scholar]
- 3.DeShazer D, Brett P J, Carlyon R, Woods D E. Mutagenesis of Burkholderia pseudomallei with Tn5-OT182: isolation of motility mutants and molecular characterization of the flagellin structural gene. J Bacteriol. 1997;179:2116–2125. doi: 10.1128/jb.179.7.2116-2125.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeShazer D, Woods D E. Animal models of melioidosis. In: Zak O, Sande M, editors. Handbook of animal models of infection. London, England: Academic Press Ltd.; 1999. pp. 199–203. [Google Scholar]
- 5.Kurioka S, Matsuda M. Phospholipase C assay using p-nitrophenylphosphorylcholine together with sorbitol and its application to studying the metal and detergent requirement of the enzyme. Anal Biochem. 1976;75:281–289. doi: 10.1016/0003-2697(76)90078-6. [DOI] [PubMed] [Google Scholar]
- 6.Lory S, Strom M S. Structure-function relationship of type-IV prepilin peptidase of Pseudomonas aeruginosa—a review. Gene. 1997;192:117–121. doi: 10.1016/s0378-1119(96)00830-x. [DOI] [PubMed] [Google Scholar]
- 7.Merriman T R, Lamont I L. Construction and use of a self-cloning promoter probe vector for gram-negative bacteria. Gene. 1993;126:17–23. doi: 10.1016/0378-1119(93)90585-q. [DOI] [PubMed] [Google Scholar]
- 8.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mongkolsuk S, Rabibhadana S, Vattanaviboon P, Loprasert S. Generalized and mobilizable positive-selection cloning vectors. Gene. 1994;143:145–146. doi: 10.1016/0378-1119(94)90620-3. [DOI] [PubMed] [Google Scholar]
- 10.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rinderknecht H, Geokas M C, Silverman P, Haverback B J. A new ultrasensitive method for the determination of proteolytic activity. Clin Chim Acta. 1968;21:197–203. doi: 10.1016/0009-8981(68)90127-7. [DOI] [PubMed] [Google Scholar]
- 12.Salmond G P C, Reeves P J. Membrane traffic wardens and protein secretion in Gram-negative bacteria. Trends Biochem Sci. 1993;18:7–12. doi: 10.1016/0968-0004(93)90080-7. [DOI] [PubMed] [Google Scholar]
- 13.Schweizer H P, Klassen T, Hoang T. Improved methods for gene analysis and expression in Pseudomonas spp. In: Nakazawa T, Furukawa K, Haas D, Silver S, editors. Molecular biology of pseudomonads. Washington, D.C: ASM Press; 1996. pp. 229–237. [Google Scholar]
- 14.Simon R, Priefer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 15.Woods D E, DeShazer D, Moore R A, Brett P J, Burtnick M N, Reckseidler S L, Senkiw M D. Current studies on the pathogenesis of melioidosis. Microbes Infect. 1999;1:157–162. doi: 10.1016/s1286-4579(99)80007-0. [DOI] [PubMed] [Google Scholar]