Key Points
Question
What are the potential effects of earlier colorectal cancer (CRC) screening on disease incidence, mortality, and health care costs in Canada?
Findings
This modeling study followed simulated individuals representative of the Canadian population. Beginning screening by biennial fecal immunochemical test (FIT) at age 45 or 40, rather than 50 years, was associated with decreased CRC incidence, mortality, and overall CRC-related health care costs.
Meaning
Lowering the screening initiation age in Canada from the current start age of 50 years may be justified.
This economic evaluation computational study uses microsimulation modeling to estimate the association of a lowered initiation age for colorectal cancer screening by biennial fecal immunochemical test with colorectal cancer incidence, mortality, and health care system costs in Canada.
Abstract
Importance
Recent US guideline updates have advocated for colorectal cancer (CRC) screening to begin at age 45 years in average-risk adults, whereas Canadian screening programs continue to begin screening at age 50 years. Similarities in early-onset CRC rates in Canada and the US warrant discussion of earlier screening in Canada, but there is a lack of Canadian-specific modeling data to inform this.
Objective
To estimate the association of a lowered initiation age for CRC screening by biennial fecal immunochemical test (FIT) with CRC incidence, mortality, and health care system costs in Canada.
Design, Setting, and Participants/Exposures
This economic evaluation computational study used microsimulation modeling via the OncoSim platform.
Main Outcomes and Measures
Modeled rates of CRC incidence, mortality, and health care costs in Canadian dollars.
Results
This analysis included 4 birth cohorts (1973-1977, 1978-1982, 1983-1987, and 1988-1992) representative of the Canadian population accounting for previously documented effects of increasing CRC incidence in younger birth cohorts. Screening initiation at age 45 years resulted in a net 12 188 fewer CRC cases, 5261 fewer CRC deaths, and an added 92 112 quality-adjusted life-years (QALYs) to the cohort population over a 40-year period relative to screening from age 50 years. Screening initiation at age 40 years yielded 18 135 fewer CRC cases, 7988 fewer CRC deaths, and 150 373 QALYs. The cost per QALY decreased with younger birth cohorts to a cost of $762 per QALY when Canadians born in 1988 to 1992 began screening at age 45 years or $2622 per QALY with screening initiation at age 40 years. Although costs associated with screening and resulting therapeutic interventions increased with earlier screening, the overall health care system cost of managing CRC decreased.
Conclusions and Relevance
This economic evaluation study using microsimulation modeling found that earlier screening may reduce CRC disease burden and add life-years to the Canadian population at a modest cost. Guideline changes suggesting earlier CRC screening in Canada may be justified, but evaluation of the resulting effects on colonoscopy capacity is necessary.
Introduction
Organized colorectal cancer (CRC) screening programs exist or are planned in every Canadian province and territory, contributing to declining CRC incidence.1 As per 2016 Canadian Task Force on Preventive Health Care guidelines, screening currently targets individuals aged 50 to 74 years at average risk of developing CRC.2 In response to increasing CRC incidence in younger adults and updated decision-analysis modeling,3 several US organizations recommend initiating screening at age 45 rather than 50 years in average-risk Americans.4,5,6,7 Canadian data reveal similar rising early-onset CRC rates, suggesting a potential role for expansion of screening eligibility in Canada.2,8
Modeling studies have demonstrated incidence and mortality benefit in screening beginning at age 50 years,9,10 but there is a lack of Canadian literature assessing CRC screening before age 50 years. These economic data are essential in the context of the Canadian single-payer health care system. We used OncoSim, a publicly available microsimulation tool led by the Canadian Partnership Against Cancer (https://www.partnershipagainstcancer.ca), to model the effects of earlier screening on CRC incidence, mortality, and health care costs in Canada.
Methods
The OncoSim-CRC model is based on Canadian demographics, disease patterns, and screening/management practices.11 The model simulated individuals from birth to death to create a representative sample of the Canadian population. Details of the model and its validation have been described previously.11 We used OncoSim (version 3.4.0.3) to compare the current, guideline-recommended Canadian screening strategy (biennial fecal immunochemical test [FIT] from ages 50 to 74 years) with earlier screening (beginning at age 45 or 40 years, starting from the year 2022). To incorporate increased CRC incidence in recent birth cohorts, we adjusted the model using a similar approach to that by Peterse et al.3 Modeling of incidence data from the Canadian Cancer Registry was performed to estimate the cohort risk ratios (RRs) using the National Cancer Institute (NCI) Age Period Cohort Analysis Webtool.12 We used the 1968 to 1972 birth cohort as the reference because this was the latest cohort that would not be affected by lowering the screening age in 2022. This analysis was based on 4 birth cohorts: individuals born in 1973 to 1977, 1978 to 1982, 1983 to 1987, and 1988 to 1992, using a cohort RR of 1.17, 1.40, 1.91, and 2.29, respectively. These RRs were applied as the adenoma incidence multiplier for each cohort. Details of model parameters are outlined in eTables 1 and 2 in Supplement 1. Each scenario included 32 million simulated cases. All costs are expressed in 2019 Canadian dollars using a 0% discount rate.
Participation at first screening invitation was set to 43% based on published data.13,14 We carried out 2 sensitivity analyses: participation set to 60% and 43% participation with no adenoma incidence multiplier (eMethods in Supplement 1). OncoSim makes FIT screening available regardless of family history and offers screening by colonoscopy to individuals at above-average risk (eMethods in Supplement 1). Positive FIT results were followed by colonoscopy and surveillance as previously outlined.11
Results
Modeled Effect on CRC Incidence and Mortality
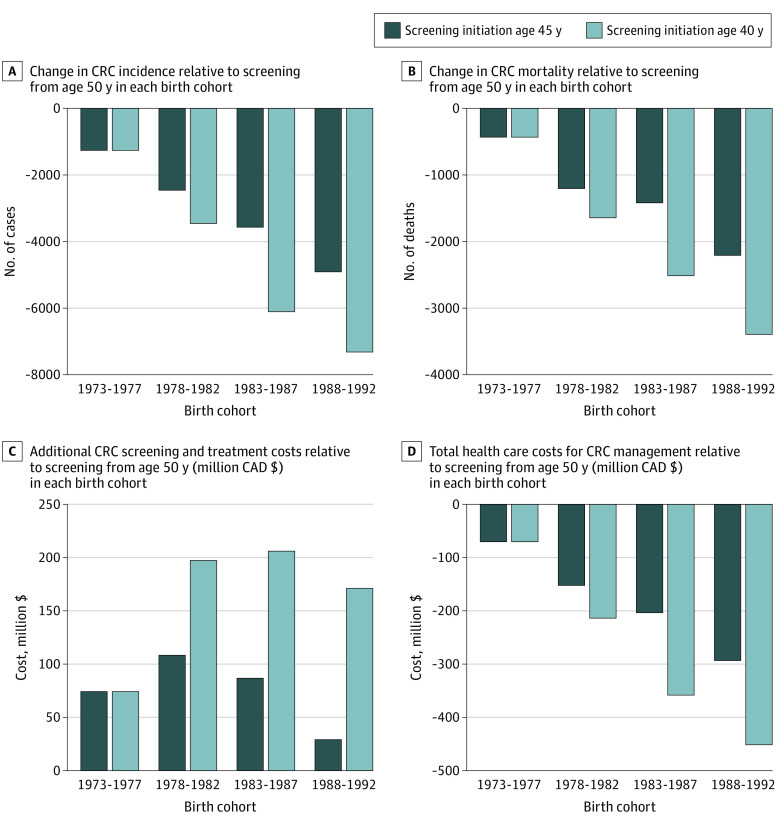
In this microsimulation, we observed decreased CRC incidence and mortality with earlier screening initiated at age 45 years (Figure). When results were expressed as cumulative values over 40 years (from the year a cohort’s oldest participants turn 40 years to the year where the youngest participants turn 75 years), screening beginning at age 45 years rather than 50 years yielded 12 188 fewer CRC cases and 5261 fewer CRC deaths in total among the 4 birth cohorts studied (Canadians born 1973-1992).
Figure. Yearly Cumulative Outcomes of Modeled Earlier Colorectal Cancer (CRC) Screening Initiation.
Cost of CRC management includes screening, diagnosis, treatment, management of recurrence, palliative and end-of-life care (for all CRC cases, diagnosed by screening or not). All costs are in 2019 Canadian dollars.
Modeled screening beginning at age 40 years resulted in 18 135 fewer CRC cases and 7988 fewer CRC deaths among the 4 birth cohorts over the 40-year interval compared with screening initiation at age 50 years. Both CRC incidence and mortality reductions were most pronounced in the younger birth cohorts observed (Table 1 and Figure).
Table 1. Cumulative Outcomes of Modeled Scenarios for CRC Screening Initiation at age 45 or 40 Yearsa,b.
| Measure | Birth cohort | Screening initiation age, y | ||
|---|---|---|---|---|
| ≥50 | 45 | 40 | ||
| Total cost, $ (billions) | 1973-1977 | 7.66 | 7.74 | 7.74 |
| 1978-1982 | 9.23 | 9.34 | 9.43 | |
| 1983-1987 | 12.34 | 12.43 | 12.55 | |
| 1988-1992 | 15.10 | 15.13 | 15.27 | |
| Cost of screening and diagnosis, $ (billions) | 1973-1977 | 2.70 | 2.84 | 2.84 |
| 1978-1982 | 3.04 | 3.30 | 3.46 | |
| 1983-1987 | 3.67 | 3.96 | 4.23 | |
| 1988-1992 | 4.23 | 4.55 | 4.85 | |
| Cost of CRC management, $ (billions)c | 1973-1977 | 4.97 | 4.89 | 4.89 |
| 1978-1982 | 6.19 | 6.03 | 5.97 | |
| 1983-1987 | 8.67 | 8.47 | 8.32 | |
| 1988-1992 | 10.87 | 10.58 | 10.42 | |
| CRC incidence | 1973-1977 | 87 732 | 86 472 | 86 472 |
| 1978-1982 | 109 472 | 107 014 | 106 015 | |
| 1983-1987 | 154 048 | 150 483 | 147 944 | |
| 1988-1992 | 192 302 | 187 396 | 184 987 | |
| CRC deaths | 1973-1977 | 30 331 | 29 900 | 29 900 |
| 1978-1982 | 37 880 | 36 676 | 36 235 | |
| 1983-1987 | 53 018 | 51 600 | 50 504 | |
| 1988-1992 | 66 568 | 64 361 | 63 171 | |
Abbreviation: CRC, colorectal cancer.
Values are cumulative over the 40-year period from the year the oldest participants of the cohort turn age 40 years to when the youngest participants turn age 75 years.
All costs are in 2019 Canadian dollars. Screening test results included screening done by fecal immunochemical test and colonoscopy (for eligible individuals with family medical history).
Cost of CRC management included screening, diagnosis, treatment, management of recurrence, palliative and end-of-life care (for all CRC cases, diagnosed by screening or not).
Modeled Effect on Costs
As expected, the microsimulation showed that earlier screening raised costs. Lowering the initiation age to 45 or 40 years cost an additional $298 million or $649 million, respectively, in screening and resultant treatment costs (cumulative total for all cohorts older than 40 years) (Figure). Conversely, we observed savings in the overall cost of CRC management (including costs associated with diagnosis, treatment, cancer recurrences, palliative, and end-of-life care for all CRC cases, diagnosed by screening or not) of $719 million and $1.1 billion for screening initiation at age 45 and 40 years, respectively, for all cohorts older than 40 years (Figure).
Quality-Adjusted Life-Years (QALYs)
OncoSim calculates QALYs by multiplying a simulated individual’s years of life by a utility value based on health status. Over the 40-year period, we observed a gain of 92 112 QALYs in the 4 cohorts with screening initiation at age 45 years and 150 373 additional QALYs with screening initiation at age 40 years (Table 2). The most benefit was observed in the youngest cohort (birth years 1988-1992), where screening initiation at age 45 yielded 38 268 additional QALYs at a cost of $762 per QALY. Screening beginning at age 40 years yielded 65 305 additional QALYs in this cohort at a cost of $2622 per QALY.
Table 2. Quality-Adjusted Life-Years (QALYs) Gained and Additional Cost With Modeled Earlier CRC Screening Relative to Screening Initiation at Age 50 Yearsa,b.
| Screening initiation age, y | Birth cohort | QALYs gained | Additional cost, $ (millions) | Cost per QALY, $ |
|---|---|---|---|---|
| 45 | 1973-1977 | 8342 | 74 | 8913 |
| 1978-1982 | 18 847 | 108 | 5745 | |
| 1983-1987 | 26 655 | 87 | 3251 | |
| 1988-1992 | 38 268 | 29 | 762 | |
| 40 | 1973-1977 | 8342 | 74 | 8913 |
| 1978-1982 | 28 192 | 198 | 7006 | |
| 1983-1987 | 48 534 | 206 | 4246 | |
| 1988-1992 | 65 305 | 171 | 2622 |
Abbreviation: CRC, colorectal cancer.
Values are cumulative over the 40-year period from the year the oldest participants of the cohort turn age 40 years to when the youngest participants turn age 75 years.
Cost is in 2019 Canadian dollars and includes costs associated with screening and treatment.
Sensitivity Analyses
When screening participation was 60%, outcomes followed a similar trend to the main analysis, notably with more QALYs at a similar cost to the main scenario (eTables 3 and 4 in Supplement 1). When no adenoma incidence rate multiplier was applied, we observed a more modest benefit from earlier screening but maintained cost-effectiveness (eResults in Supplement 1).
Discussion
In this economic evaluation computational study, OncoSim modeling showed a decrease in projected CRC incidence and mortality when screening was initiated at earlier ages, with increasing benefit in younger cohorts. Screening programs initiated at earlier ages add QALYs to this simulated population at a very modest cost per QALY compared with other life-prolonging interventions such as dialysis15 and decreasing cost in younger cohorts.
The main analysis (using a 43% participation rate and modified adenoma incidence by birth cohort) yielded a moderate estimate of benefits of earlier screening initiation. Sensitivity analysis using an aspirational estimate of 60% screening participation predicted increasing benefit to earlier screening if Canada is able to achieve this target (eTables 3 and 4 in Supplement 1). Sensitivity analysis using no adenoma rate multiplier predicted that earlier screening was still cost-effective even if rising CRC rates in younger generations have been overestimated (eTables 5 and 6 in Supplement 1).
Although earlier screening increased costs associated with screening and subsequent treatment, we observed overall health care costs related to CRC management to decrease. Earlier screening may identify more precancerous lesions and early-stage cancers, which are less costly to treat. However, effects on colonoscopy demand and quality, existing low rates of screening adherence, and other health system considerations need to be addressed before considering guideline changes. Although our economic data only apply directly to Canada, our findings may help justify earlier screening in other jurisdictions with similar public health care systems.
Strengths and Limitations
OncoSim is informed by historical sources of data derived from an older screening cohort because there are currently no randomized clinical trials exploring CRC screening in younger adults, to our knowledge. The rates of participation with initial FIT, repeat screening, and compliance with follow-up colonoscopy are not known in the younger population but we estimated it to match current Canadian participation in those aged 50 years or older. Of note, although we could not control for this, a major strength of this study is that we adjusted adenoma rates by birth cohort to more accurately reflect rising rates of malignant disease in younger individuals.
Conclusions
The findings of this economic evaluation computational modeling study suggest that lowering the screening initiation age to 45 or 40 years may reduce CRC disease burden and add life-years to the Canadian population at a cost comparable to other health care interventions.
eTable 1. OncoSim Input Parameters for FIT Screening Program
eTable 2. OncoSim Input Parameters for Colonoscopy Screening Program (for Individuals with at Least One 1st Degree Relative with CRC)
eMethods
eTable 3. Cumulative Outcomes of Modeled Scenarios for CRC Screening Initiation at age 45 or 40 When Screening Participation Set to 60%
eTable 4. Quality-Adjusted Life-Years Gained and Additional Cost with Earlier Screening Relative to Initiation at Age 50 and 60% Screening Participation
eTable 5. Cumulative Outcomes of Modeled Scenarios for CRC Screening Initiation at age 45 or 40 with Screening Participation at 43% and no Adenoma Incidence Multiplier
eTable 6. Quality-Adjusted Life-Years Gained and Additional Cost with Earlier Screening Relative to Initiation at Age 50 With Screening Participation at 43% and no Adenoma Incidence Multiplier
eResults. Sensitivity Analyses
Data Sharing Statement
References
- 1.Brenner DR, Poirier A, Woods RR, et al. ; Canadian Cancer Statistics Advisory Committee . Projected estimates of cancer in Canada in 2022. CMAJ. 2022;194(17):E601-E607. doi: 10.1503/cmaj.212097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalyta A, De Vera MA, Peacock S, et al. Canadian Colorectal Cancer Screening Guidelines: do they need an update given changing incidence and global practice patterns? Curr Oncol. 2021;28(3):1558-1570. doi: 10.3390/curroncol28030147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterse EFP, Meester RGS, Siegel RL, et al. The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening: microsimulation analysis I to inform the American Cancer Society colorectal cancer screening guideline. Cancer. 2018;124(14):2964-2973. doi: 10.1002/cncr.31543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68(4):250-281. doi: 10.3322/caac.21457 [DOI] [PubMed] [Google Scholar]
- 5.Davidson KW, Barry MJ, Mangione CM, et al. ; US Preventive Services Task Force . Screening for colorectal cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(19):1965-1977. doi: 10.1001/jama.2021.6238 [DOI] [PubMed] [Google Scholar]
- 6.Patel SG, May FP, Anderson JC, et al. Updates on age to start and stop colorectal cancer screening: recommendations from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2022;162(1):285-299. doi: 10.1053/j.gastro.2021.10.007 [DOI] [PubMed] [Google Scholar]
- 7.Ma W, Wang M, Wang K, et al. Age at initiation of lower gastrointestinal endoscopy and colorectal cancer risk among US women. JAMA Oncol. 2022;8(7):986-993. doi: 10.1001/jamaoncol.2022.0883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Sullivan DE, Ruan Y, Cheung WY, et al. Early-onset colorectal cancer incidence, staging, and mortality in Canada: implications for population-based screening. Am J Gastroenterol. 2022;117(9):1502-1507. doi: 10.14309/ajg.0000000000001884 [DOI] [PubMed] [Google Scholar]
- 9.Telford JJ, Levy AR, Sambrook JC, Zou D, Enns RA. The cost-effectiveness of screening for colorectal cancer. CMAJ. 2010;182(12):1307-1313. doi: 10.1503/cmaj.090845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heitman SJ, Hilsden RJ, Au F, Dowden S, Manns BJ. Colorectal cancer screening for average-risk North Americans: an economic evaluation. PLoS Med. 2010;7(11):e1000370. doi: 10.1371/journal.pmed.1000370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coldman AJ, Phillips N, Brisson J, et al. Using the Cancer Risk Management Model to evaluate colorectal cancer screening options for Canada. Curr Oncol. 2015;22(2):e41-e50. doi: 10.3747/co.22.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg PS, Check DP, Anderson WF. A web tool for age-period-cohort analysis of cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2296-2302. doi: 10.1158/1055-9965.EPI-14-0300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bretthauer M, Løberg M, Wieszczy P, et al. ; NordICC Study Group . Effect of colonoscopy screening on risks of colorectal cancer and related death. N Engl J Med. 2022;387(17):1547-1556. doi: 10.1056/NEJMoa2208375 [DOI] [PubMed] [Google Scholar]
- 14.Yong JHE, Mainprize JG, Yaffe MJ, et al. The impact of episodic screening interruption: COVID-19 and population-based cancer screening in Canada. J Med Screen. 2021;28(2):100-107. doi: 10.1177/0969141320974711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee CP, Chertow GM, Zenios SA. An empiric estimate of the value of life: updating the renal dialysis cost-effectiveness standard. Value Health. 2009;12(1):80-87. doi: 10.1111/j.1524-4733.2008.00401.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. OncoSim Input Parameters for FIT Screening Program
eTable 2. OncoSim Input Parameters for Colonoscopy Screening Program (for Individuals with at Least One 1st Degree Relative with CRC)
eMethods
eTable 3. Cumulative Outcomes of Modeled Scenarios for CRC Screening Initiation at age 45 or 40 When Screening Participation Set to 60%
eTable 4. Quality-Adjusted Life-Years Gained and Additional Cost with Earlier Screening Relative to Initiation at Age 50 and 60% Screening Participation
eTable 5. Cumulative Outcomes of Modeled Scenarios for CRC Screening Initiation at age 45 or 40 with Screening Participation at 43% and no Adenoma Incidence Multiplier
eTable 6. Quality-Adjusted Life-Years Gained and Additional Cost with Earlier Screening Relative to Initiation at Age 50 With Screening Participation at 43% and no Adenoma Incidence Multiplier
eResults. Sensitivity Analyses
Data Sharing Statement