Abstract
Objective
To investigate the comparative vaccine effectiveness of heterologous booster schedules (ie, three vaccine doses) compared with primary schedules (two vaccine doses) and with homologous mRNA vaccine booster schedules (three vaccine doses) during a period of omicron predominance.
Design
Population based cohort analyses.
Setting
Denmark, Finland, Norway, and Sweden, 27 December 2020 to 31 December 2022.
Participants
All adults aged ≥18 years who had received at least a primary vaccination schedule of AZD1222 (Oxford-AstraZeneca) or monovalent SARS-CoV-2 wild type (ancestral) strain based mRNA vaccines BNT162b2 (Pfizer-BioNTech) or mRNA-1273 (Moderna), in any combination.
Main outcome measures
The main outcome measure was country combined risks of covid-19 related hospital admission and death with covid-19 and additional outcomes of covid-19 related admission to an intensive care unit and SARS-CoV-2 infection. During a period of omicron predominance, these outcomes were compared in those who received a heterologous booster versus primary schedule (matched analyses) and versus those who received a homologous mRNA vaccine booster (weighted analyses). Follow-up was for 75 days from day 14 after the booster dose; comparative vaccine effectiveness was calculated as 1–risk ratio.
Results
Across the four Nordic countries, 1 086 418 participants had received a heterologous booster schedule of AZD1222+BNT162b2 or mRNA-1273 and 2 505 093 had received a heterologous booster schedule of BNT162b2+mRNA-1273. Compared with the primary schedule only (two doses), the vaccine effectiveness of heterologous booster schedules comprising AZD1222+BNT162b2 or mRNA-1273 and BNT162b2+mRNA-1273 was 82.7% (95% confidence interval 77.1% to 88.2%) and 81.5% (78.9% to 84.2%) for covid-19 related hospital admission and 95.9% (91.6% to 100.0%) and 87.5% (82.5% to 92.6%) for death with covid-19, respectively. Homologous mRNA booster schedules were similarly associated with increased protection against covid-19 related hospital admission (≥76.5%) and death with covid-19 (≥84.1%) compared with previous primary course vaccination only. When a heterologous booster schedule was compared with the homologous booster schedule, vaccine effectiveness was 27.2% (3.7% to 50.6%) for AZD1222+BNT162b2 or mRNA-1273 and 23.3% (15.8% to 30.8%) for BNT162b2+mRNA-1273 schedules against covid-19 related hospital admission and 21.7% (−8.3% to 51.7%) and 18.4% (−15.7% to 52.5%) against death with covid-19, respectively.
Conclusion
Heterologous booster schedules are associated with increased protection against severe, omicron related covid-19 outcomes compared with primary course schedules and homologous booster schedules.
Introduction
Vaccination against covid-19 is currently the most invaluable way to control the pandemic globally, particularly as policy makers no longer consider non-drug interventions. Booster doses are recommended to prevent severe and fatal covid-19, and observational studies have provided evidence of the effectiveness of such doses when the vaccine used for the booster (third dose) matches that of the primary vaccination schedule (homologous schedules).1 2 3 Additionally, clinical data have shown that heterologous vaccination schedules (where covid-19 vaccines differ in the primary vaccination schedule or the booster) are at least as immunogenic as homologous schedules.4 5 6 7 8 How these immunological findings translate into effectiveness is less certain.9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 Additionally, the use of heterologous booster strategies facilitates a more flexible approach for vaccination roll-out, and simplifies logistics, but such practice should be supported by evidence of effectiveness. This evidence is currently lacking—in particular for effectiveness against severe covid-19 outcomes associated with the omicron variants.19 21 22 23 24 25
In our population based cohort analyses using data from Denmark, Finland, Norway, and Sweden, we estimated the comparative vaccine effectiveness of heterologous booster schedules comprising adenovirus vector based AZD1222 (Vaxzeria; Oxford-AstraZeneca) as well as monovalent BNT162b2 (Comirnaty; Pfizer-BioNTech) and mRNA-1273 (Spikevax; Moderna) SARS-CoV-2 wild type (ancestral) strain based mRNA vaccines against severe covid-19 outcomes during omicron predominance in the Nordic countries. We compared heterologous booster schedules with primary course vaccination schedules and heterologous booster schedules with homologous booster schedules.
Methods
Study design and cohort
We used nationwide demography and healthcare registers to obtain individual level information on vaccination status, covid-19 related outcomes, and relevant covariates (eg, age, sex, and comorbidity). Supplementary tables S1 and S2 provide details on the registers and variables. We constructed country specific cohorts including all adults aged 18 years or older at first vaccination with known residency within the specific country who had received at least a primary vaccination schedule with AZD1222, BNT162b2, mRNA-1273, or mixed, ascertained during the period 27 December 2020 to 31 December 2022. The uptake of covid-19 vaccination in the Nordic countries has been high (>90% in those aged ≥50 years).26 We classified participants according to vaccine schedule received and defined heterologous booster schedules as those using a mix of vaccine types (eg, AZD1222 as the priming dose, followed by BNT162b2 as second and booster (third) dose, or mRNA-1273 as both priming and second dose and BNT162b2 as booster (third) dose). The mRNA vaccines BNT162b2 and mRNA-1273 administered as part of the primary schedule or as a third dose were the original monovalent mRNA vaccines containing spike glycoprotein sequences from the wild type (ancestral) SARS-CoV-2 strain in all four countries. We defined two main heterologous booster groups consisting of schedules comprising AZD1222 (one or two doses) followed by BNT162b2 or mRNA-1273, and schedules containing a mix of mRNA vaccines only, but we also considered the individual heterologous schedule subtypes (see supplementary table S3 for included comparisons). A homologous booster schedule was defined as three doses of BNT162b2 or mRNA-1273. We excluded individuals with a previous documented SARS-CoV-2 infection (ie, any history of a positive SARS-CoV-2 polymerase chain reaction (PCR) test result) from the primary analyses. Heterologous booster schedules using all three vaccines were rare and therefore not analysed.
The study was conducted according to ethical and legal requirements of each country (see supplementary table S4). Supplementary text S1 provides an overview of our literature search strategy and synthesis of evidence before this study.
Booster versus primary schedule
A matched design was used to assess the effectiveness of a heterologous booster schedule compared with a primary schedule (see supplementary figure S1).2 We matched participants on the day they received a booster dose with reference participants being those who had received the same primary schedule but had not yet received a booster dose (ie, contributing to reference period up until this date). The day the booster dose was administered served as the index date for each matched pair. We additionally matched participants on the calendar month they had received their second vaccine dose, year of birth (in five year bins), and a propensity score (by logistic regression) that included sex, region of residence, vaccination priority group, and selected comorbidities as predictors for receiving the respective studied heterologous booster schedule (supplementary table S2). Matched reference participants who received a booster dose later than the assigned index date were allowed to re-enter in additional matched pairs on that given date as participants who had received the booster.
Heterologous versus homologous booster schedules
We assessed the comparative effectiveness of heterologous and homologous booster schedules using stabilised inverse probability of treatment weights that included calendar month of the index date, year of birth (in five year bins), sex, region of residence, vaccination priority group, and selected comorbidities (supplementary table S2). The index date was defined as the day the booster dose was administered (supplementary figure S2). To ensure that participants were of similar age in the comparison groups and had received booster vaccines within the same calendar period, we used the interval from the 2.5% to the 97.5% centiles of the age and vaccination date distribution in the heterologous group as eligibility criteria. In other words, participants who had received the heterologous or homologous schedule had to not be younger or older than these limits and had to have received their booster dose within this calendar period.
Outcomes
We considered four covid-19 outcomes of interest. The two primary outcomes were covid-19 related hospital admission (defined as a covid-19 related diagnosis and a positive PCR test result for SARS-CoV-2 within 14 days before to two days after the day of admission) and death with covid-19 (defined as death within 30 days of a positive PCR test result for SARS-CoV-2). Covid-19 related admission to an intensive care unit (ICU) and documented SARS-CoV-2 infection (ie, positive PCR test result) were included as additional outcomes (supplementary table S2). Follow-up was done separately for each outcome.
Statistical analysis
Follow-up began on day 14 after the index date (to ensure full immunisation) and ended on the day of the outcome, day 75 after the start of follow-up (about three months from the index date), death, emigration, or end of study, whichever occurred first. Specifically for the analyses comparing matched booster schedules with primary schedules, we also censored matched pairs if the reference participant received a booster dose during follow-up.2 For the analyses of covid-19 related hospital and ICU admissions, we censored participants with a positive SARS-CoV-2 PCR test result on day 14 (ie, if no hospital admission had occurred within 14 days of the test, participants could no longer be classified as having a covid-19 related hospital admission and were no longer followed up) and similarly on day 30 for death with covid-19. The start of the outcome ascertainment period was defined according to when the omicron variant (sublineages BA.1 and BA.2) accounted for more than 90% of all cases (based on national surveillance data): 28 December 2021 in Denmark and Norway, 1 January 2022 in Finland, and 3 January 2022 in Sweden (and ended on 31 December 2022). Participants with follow-up time after these respective calendar start dates contributed to the estimation of the cumulative incidences, which we obtained using the Kaplan-Meier estimator according to schedule in the matched analysis or the weighted analysis. Specifically, for the outcome analyses of documented SARS-CoV-2 infection, we ended follow-up on 28 February 2022 (instead of 31 December 2022) owing to important changes in national testing strategies and covid-19 surveillance efforts by the end of February 2022. Relative (ie, comparative vaccine effectiveness, calculated as 1–risk ratio) and absolute risk differences were estimated using the cumulative incidences at day 75. We calculated the corresponding 95% confidence intervals using the delta method and truncated the upper 95% confidence bound of the comparative vaccine effectiveness if it exceeded 100% (a computational consequence in some comparisons owed to a large standard deviation when the number of compared individuals and events were small). Country specific estimates were pooled into country combined comparative vaccine effectiveness and risk differences estimates by random effects meta-analyses using the mixmeta package in R. We considered an association to be statistically significant if the 95% confidence interval did not include zero. We calculated the stabilised inverse probability of treatment weights as ((1−p0)/(1−pc))/(p0/pc); where p0 is equal to the crude probability of the specific schedule and pc is equal to the probability of the specific schedule given the covariates; these probabilities were computed using logistic regressions.
Additional analyses included extending follow-up with estimation of vaccine effectiveness at days 120, 180, 270, and 365 from start of follow-up; comparing homologous BNT162b2 and mRNA-1273 booster schedules as well as homologous booster versus primary schedules; and examining the risk of documented SARS-CoV-2 infection with stratification according to previous infection (not possible for the severe covid-19 outcomes because of too few events; ie, not applying the exclusion criterion of previous SARS-CoV-2 infection). Moreover, in sensitivity analyses, we started follow-up on the day of the booster dose among Danish participants who had received a homologous mRNA booster schedule only for the primary outcomes and we adjusted for calendar week of the index date (instead of month; to assess potential residual confounding of calendar time; results not presented) for the outcome of documented SARS-CoV-2 infection.
Patient and public involvement
No patients or members of the public were formally involved in defining the research question, study design, or outcome measures, or the conduct of the study. Involvement was not possible because of privacy constraints, funding restrictions, and the short timeline during which the study was conducted.
Results
Population
A combined total of 1 799 287 participants who had received a heterologous booster (ie, third dose; 201 151 received AZD1222+BNT162b2 or mRNA-1273 and 1 598 676 received BNT162b2+mRNA-1273) were included for the comparisons with primary vaccination schedules, and 3 591 511 (1 086 418 received AZD1222+BNT162b2 or mRNA-1273 and 2 505 093 received BNT162b2+mRNA-1273) for the comparisons with homologous booster schedule (table 1 and supplementary figures S3 and S4). Overall, Sweden contributed the largest proportion of participants who had been vaccinated with a heterologous AZD1222+BNT162b2 or mRNA-1273 booster schedule (a total of 574 046, 52.8%), followed by Finland (289 158, 26.6%), Denmark (112 097, 10.3%), and Norway (111 117, 10.2%); 1 110 524 (44.3%) of participants who had received a heterologous BNT162b2+mRNA-1273 booster schedule were from Sweden, followed by Norway (814 570, 32.5%), Finland (577 164, 23.0%), and Denmark (2835, 0.1%). The majority of booster doses were administered from December 2021 to end of February 2022.
Table 1.
Baseline characteristics of vaccinated participants according to vaccine schedules in the Nordic countries. Values are number (percentage) unless stated otherwise
| Heterologous booster v primary course schedules | Heterologous v homologous booster schedules | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| AZD1222+BNT162b2 or mRNA-1273 | Corresponding primary course to AZD1222+BNT162b2 or mRNA-1273 | BNT162b2+mRNA-1273 | Corresponding primary course to BNT162b2+mRNA-1273 | AZD1222+BNT162b2 or mRNA-1273 | Corresponding homologous mRNA schedule to AZD1222+BNT162b2 or mRNA-1273* | BNT162b2+mRNA-1273 | Corresponding homologous mRNA schedule to BNT162b2+mRNA-1273* | ||
| No of participants overall | 201 151 | 170 173 | 1 598 676 | 1 578 322 | 1 086 418 | 5 431 461 | 2 505 093 | 8 345 491 | |
| No of participants by country: | |||||||||
| Denmark | 10 230 | 10 215 | 1869 | 1860 | 112 097 | 1 560 287 | 2835 | 2 907 378 | |
| Finland | 92 172 | 61 429 | 200 029 | 188 826 | 289 158 | 1 143 451 | 577 164 | 1 543 397 | |
| Norway | NE | NE | 602 520 | 598 891 | 111 117 | 927 767 | 814 570 | 1 566 553 | |
| Sweden | 98 749 | 98 529 | 794 258 | 788 745 | 574 046 | 1 799 956 | 1 110 524 | 2 328 163 | |
| Mean (SD) age (years): | |||||||||
| Denmark | 37.8 (12) | 37.6 (11.9) | 50.4 (19.9) | 50.4 (19.9) | 45.4 (11.9) | 47.5 (11.3) | 52.5 (19.9) | 56.1 (17) | |
| Finland | 63.4 (10.3) | 63.6 (11) | 48.6 (16.2) | 47.8 (15.9) | 64.6 (7.1) | 58.1 (11.8) | 52.4 (14.4) | 55.9 (16.4) | |
| Norway | NE | NE | 42.1 (14.3) | 42 (14.3) | 44.7 (12.6) | 47.3 (12.5) | 44.3 (13.8) | 53.8 (15.9) | |
| Sweden | 57.2 (18.4) | 57.1 (18.4) | 48.7 (14.8) | 48.6 (14.8) | 67 (11.7) | 58.2 (14) | 52.3 (14.8) | 57.1 (17.2) | |
| Female sex (%): | |||||||||
| Denmark | 80 | 76.7 | 52.5 | 46.9 | 80.3 | 49.4 | 52.1 | 50.6 | |
| Finland | 51 | 51.9 | 53.2 | 47.1 | 50.6 | 52.8 | 52.1 | 52.6 | |
| Norway | NE | NE | 48.5 | 45.9 | 77.2 | 51 | 48.2 | 50.7 | |
| Sweden | 62.7 | 59.2 | 48.4 | 46.3 | 55.7 | 51.3 | 48.7 | 51.9 | |
| Calendar period†: | |||||||||
| Denmark | 18/10/21-31/12/22 | 18/10/21-31/12/22 | 17/10/21-31/12/22 | 17/10/21-31/12/22 | 8/10/21-10/1/22 | 8/10/21-10/1/22 | 1/10/21-5/12/22 | 1/10/21-5/12/22 | |
| Finland | 17/10/21-31/12/22 | 17/10/21-31/12/22 | 17/10/21-31/12/22 | 17/10/21-31/12/22 | 3/11/21-16/2/22 | 3/11/21-16/2/22 | 25/11/21-27/4/22 | 25/11/21-27/4/22 | |
| Norway | NE | NE | 14/10/21-31/12/22 | 14/10/21-31/12/22 | 16/11/21-25/1/22 | 16/11/21-25/1/22 | 08/11/21-2/3/22 | 08/11/21-2/3/22 | |
| Sweden | 21/10/21-31/12/22 | 21/10/21-31/12/22 | 21/10/21-31/12/22 | 21/10/21-31/12/22 | 11/11/21-14/2/22 | 11/11/21-14/2/22 | 22/10/21-19/5/22 | 22/10/21-19/5/22 | |
| Vaccination priority groups | |||||||||
| Vulnerable individuals: | |||||||||
| Denmark | 8 (0.1) | 15 (0.1) | 80 (4.3) | 86 (4.6) | 49 (0.0) | 23 671 (1.5) | 123 (4.3) | 106 484 (3.7) | |
| Finland | 176 (0.2) | 146 (0.2) | 858 (0.4) | 813 (0.4) | 360 (0.1) | 4557 (0.4) | 1300 (0.2) | 6826 (0.4) | |
| Norway | NE | NE | 323 (0.1) | 301 (0.1) | 8 (0.0) | 363 (0.0) | 217 (0.0) | 2498 (0.2) | |
| Sweden | 111 (0.1) | 120 (0.1) | 1563 (0.2) | 1793 (0.2) | 326 (0.1) | 1685 (0.1) | 1976 (0.2) | 6144 (0.3) | |
| Healthcare workers: | |||||||||
| Denmark | 8728 (85.3) | 8569 (83.9) | 66 (3.5) | 89 (4.8) | 99 474 (88.7) | 140 098 (9.0) | 109 (3.8) | 173 949 (6.0) | |
| Finland | 12 710 (13.8) | 7898 (12.9) | 20 726 (10.4) | 15 527 (8.2) | 36 992 (12.8) | 146 669 (12.8) | 60 930 (10.6) | 182 871 (11.8) | |
| Norway | NE | NE | 46 064 (7.6) | 46 091 (7.7) | 86 997 (78.3) | 125 452 (13.5) | 63 037 (7.7) | 159 046 (10.2) | |
| Sweden | 30 872 (31.3) | 28 013 (28.4) | 43 559 (5.5) | 44 650 (5.7) | 103 347 (18.0) | 159 059 (8.8) | 61 692 (5.6) | 194 693 (8.4) | |
| Others: | |||||||||
| Denmark | 1494 (14.6) | 1631 (16.0) | 1723 (92.2) | 1685 (90.6) | 12 574 (11.2) | 1 396 518 (89.5)‡ | 2603 (91.8) | 2 626 945 (90.4)‡ | |
| Finland | 78 973 (85.7) | 52 170 (84.9) | 178 418 (89.2) | 171 698 (90.9) | 251 806 (87.1) | 992 225 (86.8) | 514 934 (89.2) | 1 353 700 (87.7) | |
| Norway | NE | NE | 556 133 (92.3) | 552 499 (92.3) | 24 112 (21.7) | 801 952 (86.4) | 751 316 (92.2) | 1 405 009 (89.7) | |
| Sweden | 67 766 (68.6) | 70 396 (71.4) | 749 136 (94.3) | 742 302 (94.1) | 470 373 (81.9) | 1 639 212 (91.1) | 1 046 856 (94.3) | 2 127 326 (91.4) | |
| Comorbidities | |||||||||
| Autoimmune disorder: | |||||||||
| Denmark | 301 (2.9) | 232 (2.3) | 47 (2.5) | 50 (2.7) | 3182 (2.8) | 51 681 (3.3) | 79 (2.8) | 102 921 (3.5) | |
| Finland | 5621 (6.1) | 2356 (3.8) | 4693 (2.3) | 4037 (2.1) | 13 298 (4.6) | 37 951 (3.3) | 14 570 (2.5) | 47 985 (3.1) | |
| Norway | NE | NE | 7773 (1.3) | 8296 (1.4) | 1825 (1.6) | 21 886 (2.4) | 10 107 (1.2) | 37 697 (2.4) | |
| Sweden | 4665 (4.7) | 4294 (4.4) | 29 833 (3.8) | 28 724 (3.6) | 30 958 (5.4) | 89 779 (5.0) | 44 823 (4.0) | 112 456 (4.8) | |
| Cancer: | |||||||||
| Denmark | 88 (0.9) | 84 (0.8) | 60 (3.2) | 40 (2.2) | 1650 (1.5) | 26 727 (1.7) | 103 (3.6) | 98 386 (3.4) | |
| Finland | 9642 (10.5) | 5120 (8.3) | 6942 (3.5) | 5791 (3.1) | 27 022 (9.3) | 66 286 (5.8) | 23 575 (4.1) | 90 071 (5.8) | |
| Norway | NE | NE | 4300 (0.7) | 3893 (0.7) | 751 (0.7) | 12 265 (1.3) | 6026 (0.7) | 33 109 (2.1) | |
| Sweden | 6343 (6.4) | 5652 (5.7) | 24 837 (3.1) | 21 927 (2.8) | 49 784 (8.7) | 103 450 (5.7) | 43 169 (3.9) | 139 078 (6.0) | |
| Chronic pulmonary disease: | |||||||||
| Denmark | 119 (1.2) | 135 (1.3) | 44 (2.4) | 40 (2.2) | 1513 (1.3) | 27 706 (1.8) | 75 (2.6) | 77 499 (2.7) | |
| Finland | 2505 (2.7) | 1555 (2.5) | 1194 (0.6) | 1190 (0.6) | 7671 (2.7) | 11 782 (1.0) | 4112 (0.7) | 16 326 (1.1) | |
| Norway | NE | NE | 28 947 (4.8) | 26 247 (4.4) | 6712 (6.0) | 62 089 (6.7) | 36 409 (4.5) | 124 993 (8.0) | |
| Sweden | 3887 (3.9) | 4308 (4.4) | 21 810 (2.7) | 21 735 (2.8) | 25 884 (4.5) | 68 375 (3.8) | 32 956 (3.0) | 94 479 (4.1) | |
| CVD or diabetes mellitus: | |||||||||
| Denmark | 138 (1.3) | 131 (1.3) | 99 (5.3) | 111 (6.0) | 2300 (2.1) | 53 909 (3.5) | 187 (6.6) | 191 124 (6.6) | |
| Finland | 34 772 (37.7) | 19 054 (31.0) | 20 089 (10.0) | 17 335 (9.2) | 109 125 (37.7) | 176 792 (15.5) | 68 049 (11.8) | 243 013 (15.7) | |
| Norway | NE | NE | 35 788 (5.9) | 33 479 (5.6) | 8710 (7.8) | 94 426 (10.2) | 46 797 (5.7) | 240 990 (15.4) | |
| Sweden | 16 484 (16.7) | 17 542 (17.8) | 74 239 (9.3) | 76 534 (9.7) | 130 745 (22.8) | 297 673 (16.5) | 126 125 (11.4) | 397 302 (17.1) | |
| Chronic kidney disease: | |||||||||
| Denmark | 14 (0.1) | 16 (0.2) | 19 (1.0) | 17 (0.9) | 251 (0.2) | 6437 (0.4) | 28 (1.0) | 25 450 (0.9) | |
| Finland | 1946 (2.1) | 853 (1.4) | 948 (0.5) | 789 (0.4) | 4006 (1.4) | 6799 (0.6) | 2748 (0.5) | 10 416 (0.7) | |
| Norway | NE | NE | 637 (0.1) | 825 (0.1) | 76 (0.1) | 1949 (0.2) | 909 (0.1) | 6076 (0.4) | |
| Sweden | 1208 (1.2) | 1640 (1.7) | 6781 (0.9) | 7488 (0.9) | 9184 (1.6) | 22 400 (1.2) | 11 326 (1.0) | 34 689 (1.5) | |
CVD=cardiovascular disease; NE=not estimable owing to no events among matched individuals who received heterologous or primary course vaccination schedule.
The adenovirus vector based AZD1222 vaccine is produced by Oxford-AstraZeneca and the monovalent SARS-CoV-2 wild type (ancestral) strain based mRNA vaccines BNT162b2 and mRNA-1273 are produced by Pfizer-BioNTech and Moderna, respectively.
Homologous booster schedules comprised BNT162b2 or mRNA-1273.
Three doses of the same mRNA vaccine used in the heterologous schedule.
Calendar period (minimum and maximum dates) for index date (ie, day of booster vaccination); see supplementary figures S3 and S4 for density plots of the distributions.
Includes 14 and 31 individuals categorised as close contacts, respectively; see supplementary table S2 for details on definitions of covariates within each country.
Booster versus primary schedule
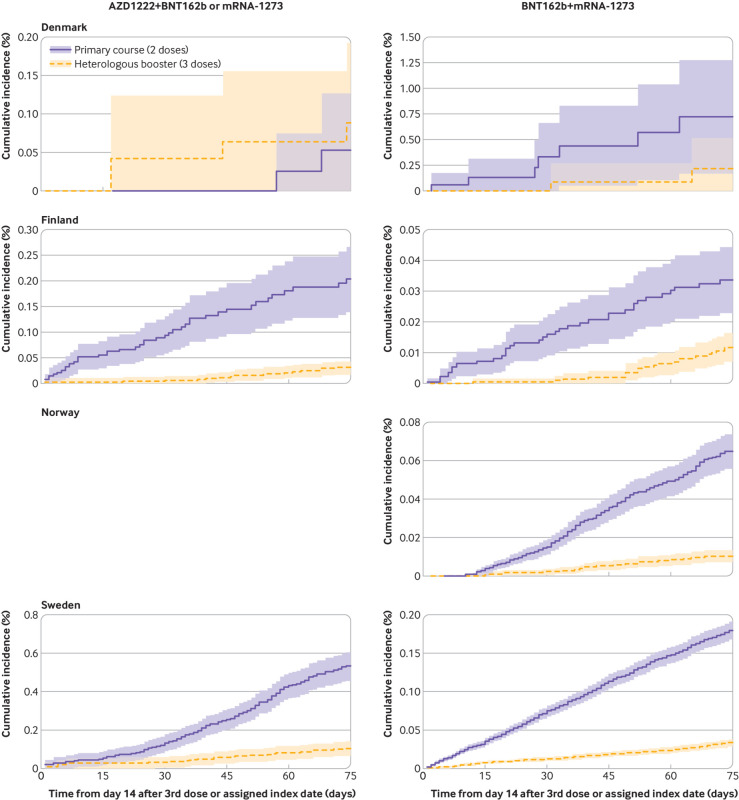
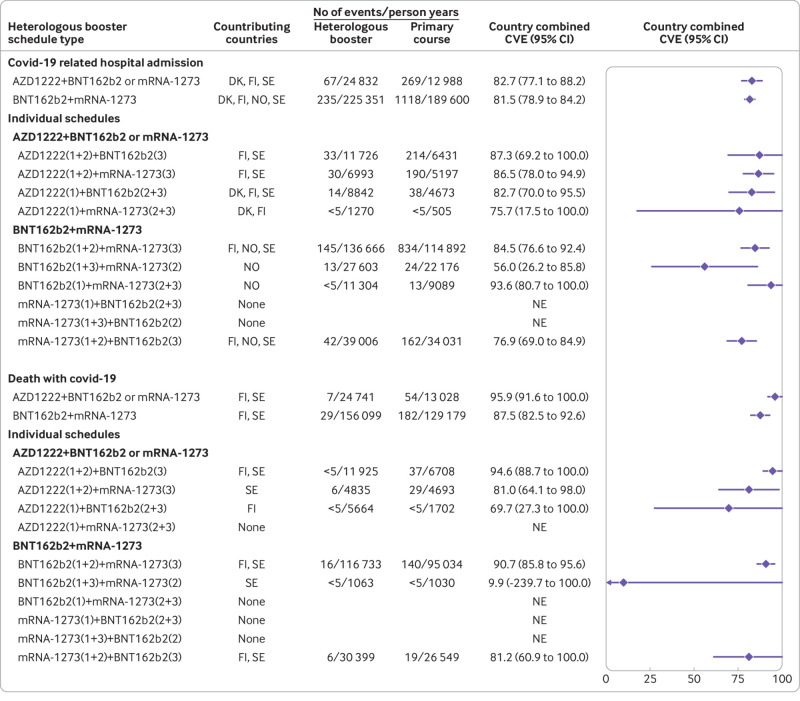
The cumulative incidences of covid-19 related hospital admission and death with covid-19 within 75 days of follow-up (ie, from day 14 after the booster dose) were low when participants who had received a heterologous booster schedule were compared with those who had received a primary schedule (fig 1 and fig 2). Compared with a primary schedule, a heterologous AZD1222+BNT162b2 or mRNA-1273 booster schedule was associated with a country combined vaccine effectiveness of 82.7% (95% confidence interval 77.1% to 88.2%) against covid-19 related hospital admission and 95.9% (91.6% to 100.0%) against death with covid-19 (fig 3; see supplementary table S5 for risk differences). Heterologous BNT162b2+mRNA-1273 booster schedules were associated with comparably high comparative vaccine effectiveness of 81.5% (78.9% to 84.2%) against covid-19 related hospital admission and 87.5% (82.5% to 92.6%) against death with covid-19. The comparative vaccine effectiveness for the individual subtypes of heterologous AZD1222+BNT162b2 or mMRNA-1273 and BNT162b2+mRNA-1273 booster schedules were overall compatible with those of the two main heterologous booster groups.
Fig 1.
Cumulative incidence curves of covid-19 related hospital admission comparing heterologous AZD1222 (Oxford-AstraZeneca), BNT162b2 (Pfizer-BioNTech), and mRNA-1273 (Moderna) booster schedules with primary schedules in Denmark, Finland, Norway, and Sweden. A panel for Norway is blank as cumulative incidence curves could not be generated for this specific country (row) comparison (column)
Fig 2.
Cumulative incidence curves of death with covid-19 comparing heterologous AZD1222 (Oxford-AstraZeneca), BNT162b2 (Pfizer-BioNTech), and mRNA-1273 (Moderna) booster schedules with primary schedules in Finland and Sweden. Analysis was not possible in Denmark and Norway because of too few events
Fig 3.
Comparative vaccine effectiveness for risk of covid-19 related hospital admission and death with covid-19 comparing heterologous AZD1222 (Oxford-AstraZeneca), BNT162b2 (Pfizer-BioNTech), and mRNA-1273 (Moderna) booster schedules with primary schedules in the Nordic countries. Estimates are adjusted for calendar month of the second vaccine dose, year of birth (five year bins), sex, region of residence, vaccination priority group, and selected comorbidities through a matched design. Supplementary table S5 presents risk difference estimates. Numbers in brackets represent 1st, 2nd, and 3rd vaccine doses. CVE=comparative vaccine effectiveness; DK=Denmark; FI=Finland, NE=not estimable; NO=Norway, SE=Sweden
For both heterologous AZD1222+BNT162b2 or mRNA-1273 and BNT162b2+mRNA-1273 booster schedules, we observed high comparative vaccine effectiveness against covid-19 related ICU admission (89.6% (95% confidence interval 75.7% to 100%) and 86.4% (74.0% to 98.9%), respectively), although these estimates were based on few events (supplementary table S6). The observed protection afforded by the two heterologous booster schedules was lower against documented SARS-CoV-2 infection (comparative vaccine effectiveness 40.5% (95% confidence interval 17.6% to 63.4%) and 50.0% (33.5% to 66.5%), respectively) than for the severe covid-19 outcomes (supplementary table S6 and supplementary figure S5).
Compared with only a previous primary vaccination schedule, homologous BNT162b2 and mRNA-1273 booster schedules were also associated with high protection—vaccine effectiveness of 76.5% (67.1% to 86.0%) and 80.7% (67.1% to 94.3%) against covid-19 related hospital admission and 84.1% (75.3% to 93.0%) and 90.0% (79.1% to 100.0%) against death with covid-19, respectively (supplementary figure S6 and supplementary table S7).
Heterologous versus homologous booster schedules
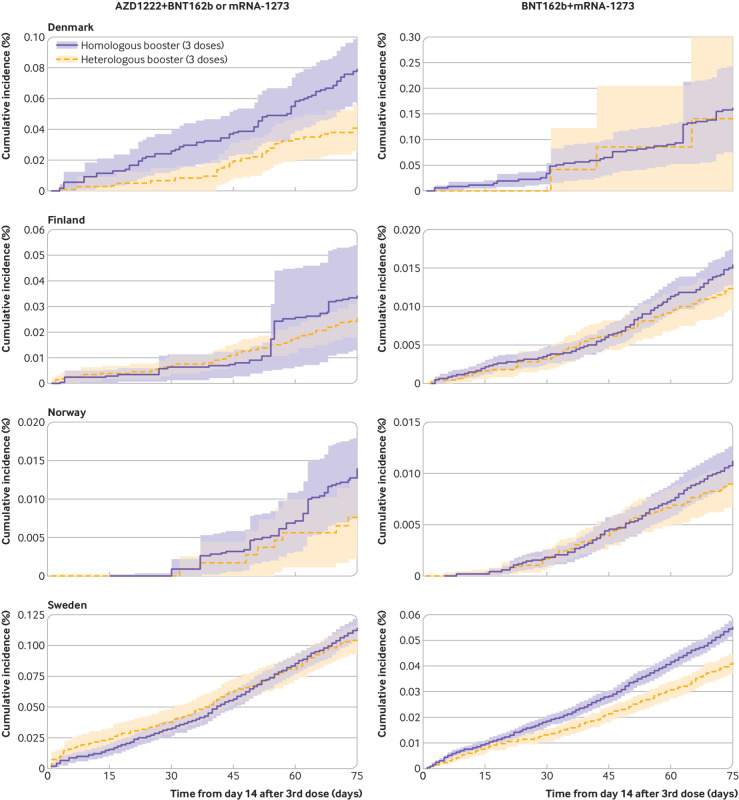
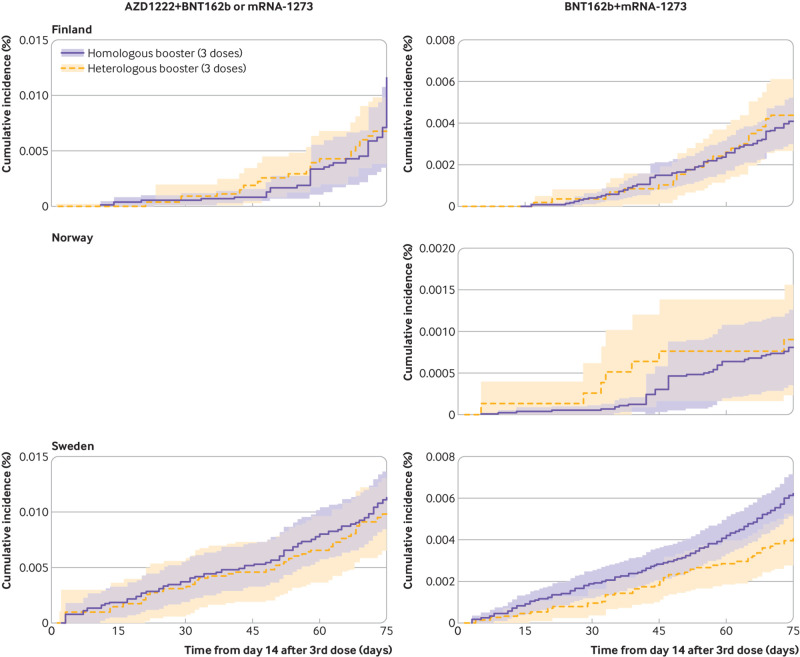
Compared with the homologous booster schedules, vaccine effectiveness of the heterologous AZD1222+BNT162b2 or mRNA-1273 booster schedules was 27.2% (3.7% to 50.6%) and 21.7% (−8.3% to 51.7%) for covid-19 related hospital admission and death with covid-19, respectively, whereas vaccine effectiveness of the heterologous BNT162b2+mRNA-1273 booster schedule was 23.3% (15.8% to 30.8%) and 18.4% (−15.7% to 52.5%), respectively (fig 4, fig 5, and fig 6; see supplementary table S8 for risk differences). The corresponding risk differences for the outcome of covid-19 related hospital admission were −10.1 (95% confidence interval −19.4 to −0.9) per 100 000 individuals and −6.0 (−12.8 to 0.9) per 100 000 individuals. When compared with homologous booster schedules for risk of covid-19 related ICU admission and documented SARS-CoV-2 infection, vaccine effectiveness of the AZD1222+BNT162b2 or mRNA-1273 booster schedules was 57.8% (−16.8% to 100.0%) and −11.2% (−24.4% to 2.0%), respectively, and of BNT162b2+mRNA-1273 was 4.4% (−73.8% to 82.7%) and 12.4% (−4.8% to 29.6%), respectively (supplementary table S9 and supplementary figure S7).
Fig 4.
Cumulative incidence curves of covid-19 related hospital admission comparing heterologous AZD1222 (Oxford-AstraZeneca), BNT162b2 (Pfizer-BioNTech), and mRNA-1273 (Moderna) booster schedules with homologous booster schedules in Denmark, Finland, Norway, and Sweden
Fig 5.
Cumulative incidence curves of death with covid-19 comparing heterologous AZD1222 (Oxford-AstraZeneca), BNT162b2 (Pfizer-BioNTech), and mRNA-1273 (Moderna) booster schedules with homologous booster schedules in Finland, Norway, and Sweden. A panel for Norway is blank as cumulative incidence curves could not be generated for this specific country (row) comparison (column). Analysis was not possible in Denmark because of too few events
Fig 6.
Comparative vaccine effectiveness for risk of covid-19 related hospital admission and death with covid-19 comparing heterologous AZD1222 (Oxford-AstraZeneca), BNT162b2 (Pfizer-BioNTech), and mRNA-1273 (Moderna) booster schedules with homologous booster schedules in the Nordic countries. Estimates are adjusted for calendar month the booster dose was received, year of birth (five year bins), sex, region of residence, vaccination priority group, and selected comorbidities using stabilised inverse probability of treatment weights. See supplementary table S8 for risk difference estimates. Bracketed numbers 1 to 3 refer to 1st, 2nd, and 3rd vaccine doses. CVE=comparative vaccine effectiveness, DK=Denmark; FI=Finland; NE=not estimable; NO=Norway; SE=Sweden
Compared with the homologous BNT162b2 booster schedule, the homologous mRNA-1273 booster schedule was associated with a vaccine effectiveness of 32.4% (12.8% to 52.0%) against covid-19 related hospital admission, 29.7% (−12.0% to 71.4%) against death with covid-19, 37.2% (-5.6% to 79.9%) against covid-19 related ICU admission, and 10.2% (3.1% to 17.3%) against documented SARS-CoV-2 infection (supplementary figure S8 and supplementary table S10).
Additional analyses
When we extended follow-up up to day 365, waning of vaccine effectiveness was observed (table 2; see supplementary figures S9-S11 and supplementary tables S11 and S12). When booster schedules were compared with primary course schedules for the outcome of covid-19 related hospital admission, the vaccine effectiveness of the heterologous AZD1222+BNT162b2 or mRNA-1273 and BNT162b2+mRNA-1273 booster schedules (which were 82.7% and 81.5% at day 75) was 62.6% (45.6% to 79.7%) and 67.7% (47.2% to 88.2%) at day 365 of follow-up, respectively. Similarly, modest waning was observed for death with covid-19 as well as for homologous booster schedules. When heterologous booster schedules were compared with homologous booster schedules, we observed a partial attenuation of the vaccine effectiveness estimates when increasing follow-up to day 365 for the outcomes of covid-19 related hospital admission and death with covid-19.
Table 2.
Comparative waning vaccine effectiveness against covid-19 related hospital admission and covid-19 related death in relation to heterologous booster schedules in the Nordic countries, with up to one year of follow-up
| Follow-up by schedule and outcome | Contributing countries | No of events/person years | Country combined CVE (%) (95% CI)) | |
|---|---|---|---|---|
| Heterologous booster schedules | Primary course/homologous booster schedules | |||
| Heterologous booster v primary course schedules | ||||
| Hospital admission: AZD122+BNT162b2 or mRNA-1273: | ||||
| Day 120 | DK, FI, SE | 125/39 972.02 | 318/18 434.35 | 75.1 (69.0 to 81.3) |
| Day 180 | DK, FI, SE | 167/56 654.72 | 348/24 598.56 | 69.6 (62.8 to 76.4) |
| Day 270 | DK, FI, SE | 209/74 606.74 | 404/32 594.11 | 64.9 (51.8 to 78.1) |
| Day 365 | DK, FI, SE | 230/82 964.96 | 444/39 392.43 | 62.6 (45.6 to 79.7) |
| Hospital admission: BNT162b2+mRNA-1273: | ||||
| Day 120 | DK, FI, NO, SE | 359/342 109.09 | 1440/278 529.01 | 71.5 (55.6 to 87.4) |
| Day 180 | DK, FI, NO, SE | 469/485 387.6 | 1588/389 533.01 | 68.1 (52.0 to 84.2) |
| Day 270 | DK, FI, NO, SE | 569/678 548.02 | 1860/545 228.98 | 66.1 (47.7 to 84.4) |
| Day 365 | DK, FI, NO, SE | 636/792 918.96 | 2121/642 158.93 | 67.7 (47.2 to 88.2) |
| Death: AZD1222+BNT162b2 or mRNA-1273: | ||||
| Day 120 | FI, SE | 21/39 939.53 | 74/18 412.68 | 91.1 (81.3 to 100.0) |
| Day 180 | FI, SE | 33/56 719.22 | 81/24 504.77 | 83.1 (75.4 to 90.9) |
| Day 270 | FI, SE | 44/74 679.79 | 93/32 356.35 | 81.8 (73.9 to 89.8) |
| Day 365 | FI, SE | 51/82 939.5 | 107/38 967.08 | 77.0 (66.6 to 87.5) |
| Death: BNT162b2+mRNA-1273: | ||||
| Day 120 | FI, SE | 75/235 482.05 | 305/189 554.22 | 77.1 (61.4 to 92.8) |
| Day 180 | FI, SE | 111/329 199.34 | 345/263 271.58 | 69.2 (43.7 to 94.6) |
| Day 270 | FI, SE | 136/447 455.03 | 397/363 477.25 | 67.0 (37.6 to 96.5) |
| Day 365 | FI, SE | 150/507 055.72 | 442/421 092.05 | 60.0 (2.2 to 100.0) |
| Heterologous v homologous booster schedules | ||||
| Hospital admission: AZD122+BNT162b2 or mRNA-1273: | ||||
| Day 120 | DK, FI, NO, SE | 948/295 951.81 | 3425/1 519 992.2 | 33.5 (11.2 to 55.9) |
| Day 180 | DK, FI, NO, SE | 1164/405 426.54 | 4111/2174 540.91 | 25.0 (2.0 to 48.0) |
| Day 270 | DK, FI, NO, SE | 1420/523 702.56 | 5128/3030 777.54 | 25.6 (4.2 to 46.9) |
| Day 365 | DK, FI, NO, SE | 1617/599 055.81 | 5894/3 602 580.34 | 24.6 (3.1 to 46.1) |
| Hospital admission: BNT162b2+mRNA-1273: | ||||
| Day 120 | DK, FI, NO, SE | 971/764 170.72 | 8493/2 312 781.97 | 22.9 (17.2 to 28.5) |
| Day 180 | DK, FI, NO, SE | 1225/1 118 938.46 | 11061/3 339 756.51 | 22.2 (16.6 to 27.8) |
| Day 270 | DK, FI, NO, SE | 1580/1 601 337.27 | 14083/4 710 036.8 | 15.6 (5.7 to 25.6) |
| Day 365 | DK, FI, NO, SE | 1857/1902086.04 | 16372/5587530.51 | 16.5 (8.4 to 24.6) |
| Death: AZD1222+BNT162b2 or mRNA-1273: | ||||
| Day 120 | FI, NO, SE | 127/276 888.29 | 357/1 164 326.68 | 30.2 (−0.5 to 60.8) |
| Day 180 | DK, FI, NO, SE | 183/411 680.65 | 557/2 220 376.13 | 14.8 (−5.8 to 35.4) |
| Day 270 | DK, FI, NO, SE | 224/530 572.56 | 704/3 080 606.66 | 27.6 (10.8 to 44.4) |
| Day 365 | DK, FI, NO, SE | 268/606 278.36 | 853/3 654 644.84 | 24.6 (6.8 to 42.3) |
| Death: BNT162b2+mRNA-1273: | ||||
| Day 120 | FI, NO, SE | 148/771 257.64 | 806/1 617 555.92 | 23.5 (−1.9 to 48.9) |
| Day 180 | DK, FI, NO, SE | 213/1 127 526.66 | 2580/3 404 773.44 | 18.4 (−5.4 to 42.2) |
| Day 270 | DK, FI, NO, SE | 272/1 611 025.4 | 3146/4 781 199.1 | 17.6 (−3.5 to 38.6) |
| Day 365 | DK, FI, NO, SE | 315/1 912 472.61 | 3666/5 662 053.88 | 16.1 (−7.7 to 39.9) |
CVE=comparative vaccine effectiveness; DK=Denmark; FI=Finland; NO=Norway; SE=Sweden.
The adenovirus vector based AZD1222 vaccine is produced by Oxford-AstraZeneca and the monovalent SARS-CoV-2 wild type (ancestral) strain based mRNA vaccines BNT162b2 and mRNA-1273 are produced by Pfizer-BioNTech and Moderna, respectively.
In the additional analyses of the risk of documented SARS-CoV-2 infection in stratified subgroups by previous SARS-CoV-2 infection, vaccine effectiveness did not differ materially between previously infected and non-infected participants, when comparing those who had received the booster schedule with those who had received the primary course schedule as well as those who received the heterologous booster schedule compared with those who received the homologous booster schedule (supplementary figures S12 and S13 and supplementary tables S13 and S14). For example, the vaccine effectiveness of the heterologous BNT162b2+mRNA-1273 booster schedule versus primary schedule in participants was 48.2% (32.9% to 63.5%) among those with no previous SARS-CoV-2 infection and 54.4% (25.9% to 83.0%) among those with previous infection; similarly, the vaccine effectiveness of heterologous AZD1222+BNT162b2 or mRNA-1273 versus homologous booster schedules was −10.7% (−23.1% to 1.8%) and −15.5% (−33.6% to 2.5%), respectively. Overall, the cumulative incidence of documented infection was lowest for those groups of participants with previous SARS-CoV-2 infection who received a booster dose.
Lastly, in sensitivity analyses the results did not change when we started follow-up on the day of the booster dose (see supplementary table S15) or adjusted for calendar weeks instead of months (data not shown).
Discussion
We estimated the vaccine effectiveness of heterologous booster schedules with AZD1222 and the monovalent SARS-CoV-2 wild type strain based BNT162b2 and mRNA-1273 vaccines during a period of omicron predominance in the Nordic countries. Overall, we found that both heterologous booster and homologous mRNA booster schedules were associated with improved protection (vaccine effectiveness ≥81.5% and ≥76.5%, respectively) against severe covid-19 compared with primary course vaccination only. We also found that heterologous booster schedules comprising AZD1222+BNT162b2 or mRNA-1273 and BNT162b2+mRNA-1273 both conferred modestly greater protection against covid-19 related hospital admission than homologous mRNA vaccine booster schedules. Moreover, for all comparisons, we observed a partial attenuation of the effect estimates when extending follow-up to one year after the day of booster vaccination.
Comparison with other studies
Studies reporting vaccine effectiveness estimates against severe covid-19 outcomes for heterologous booster schedules of AZD1222 as well as monovalent SARS-CoV-2 wild type (ancestral) strain based BNT162b2 or mRNA-1273 vaccines, or both, are few and mostly describe the effectiveness in periods before the emergence of omicron.20 21 22 23 24 25 In comparisons with unvaccinated individuals, previous studies have shown that heterologous AZD1222+BNT162b2 or mRNA-1273 and BNT162b2+mRNA-1273 booster schedules provide high vaccine effectiveness against severe covid-19 outcomes (vaccine effectiveness 74-99%), which overall appear to be similar to or modestly higher than the vaccine effectiveness of homologous mRNA or AZD1222 booster schedules when indirectly compared.20 21 22 23 Comparisons with unvaccinated people, however, are at risk of healthy vaccinee bias, particularly in populations with high vaccine uptake where unvaccinated people are poor representatives of the targeted general population for booster vaccination, and a cautious interpretation of indirect comparisons of different schedules is warranted. A UK study compared individuals who had received an AZD1222 primary vaccination course followed by a BNT162b2 booster with primary course vaccination during the delta period, and reported a comparative vaccine effectiveness of 80.6% (95% confidence interval 78.3% to 82.6%) against covid-19 related hospital admission and 86.6% (81.3% to 90.4%) against death with covid-19 at day 70 after booster vaccination.24 Similar results were found for homologous BNT162b2 booster schedules, with a comparative vaccine effectiveness of 79.3% (76.1% to 82.1%) for covid-19 related hospital admissions and 90.5% (85.6% to 93.7%) for death with covid-19.24 Our estimates for heterologous AZD1222+BNT162b2 or mRNA-1273 booster schedules (comparative vaccine effectiveness 82.7% (77.1% to 88.2%) and 95.9% (91.6% to 100.0%) for the two outcomes), obtained during the omicron period, are highly comparable to these previous findings during the delta period. Notably, as most of the booster doses in our study were administered from December 2021 to end of February 2022, estimates primarily reflect the effectiveness against covid-19 outcomes due to the omicron sublineages BA.1 and BA.2; BA.5 (and to a lesser extent BA.4) became the predominating variants only from June/July 2022. We observed a modestly lower risk of covid-19 related hospital admissions associated with both AZD1222+BNT162b2 or mRNA-1273 and BNT162b2+mRNA-1273 heterologous booster schedules compared with homologous booster schedules, but this was not statistically significant for death with covid-19. We were unable to identify any previous studies that directly compared heterologous AZD1222+BNT162b2 or mRNA-1273 with homologous booster schedules for the risk of severe covid-19 outcomes. However, although conducted during a combined delta and omicron predominance periods, a recent study produced comparable estimates for the outcome of covid-19 related hospital admissions, favouring a heterologous BNT162b2+mRNA-1273 booster schedule (defined as two BNT162b2 doses followed by a mRNA-1273 dose) over homologous BNT162b2 booster schedules, with a hazard ratio of 0.86 (95% confidence interval 0.75 to 1.00) at 20 weeks of follow-up.25 In addition, the study found a lower risk of covid-19 related hospital admission in association with homologous mRNA-1273 compared with homologous BNT162b2 booster schedules (0.89, 0.82 to 0.95), but the risk of death with covid-19 was not statistically significantly different (0.83, 0.58 to 1.19) (risk of death with covid-19 was not assessed for the heterologous BNT162b2+mRNA-1273 booster schedule). Although in our main 75 day follow-up (from day 14 after booster vaccination) period we observed similar risk estimates within the homologous mRNA-1273 versus BNT162b2 booster schedule comparison, the statistically significant difference in risk of covid-19 related hospital admission had waned at days 180, 270, and 365 (as was the case for all our comparisons). Waning vaccine effectiveness of homologous booster schedules has been described previously,27 28 but we are unaware of any studies reporting on waning of effectiveness estimates for heterologous booster schedules with the AZD1222, BNT162b2, and mRNA-1273 vaccines against severe covid-19 outcomes.
Despite the previous studies varying widely in study design, our results overall are in agreement with previous works that examined the effectiveness of other types of heterologous booster schedules that included the adenovirus vector based Ad26.COV2-S (Janssen/Johnson & Johnson) and inactivated virus based CoronaVac (Sinovac) vaccines and found that heterologous schedules were associated with improved protection against severe covid-19 outcomes that was comparable or slightly greater than that observed for homologous booster schedules.9 10 29 30 31 Similarly, our findings also expand on studies that found greater immunogenicity of heterologous booster schedules than of homologous booster schedules involving AZD1222, BNT162b2, and mRNA-1273.4 7 32
Our vaccine effectiveness estimates for booster schedules against documented SARS-CoV-2 infection ranged from 34% to 50% across booster schedules compared with primary course vaccination, which are similar to previously reported vaccine effectiveness estimates against omicron infection.13 14 15 33 34 35 36 37 Little data exist, however, for effectiveness of heterologous AZD1222+BNT162b2 or mRNA-1273 and BNT162b2+mRNA-1273 booster schedules for the risk of documented omicron infection.14 15 16 17 18 Similar to our results, the results of a study that compared 6436 healthcare workers who received a homologous mRNA booster schedule with 1160 healthcare workers who received a heterologous AZD1222+BNT162b2 or mRNA-1273 booster schedule did not observe a significant difference in the risk of omicron infection within 100 days of the booster dose (hazard ratio 0.86, 95% confidence interval 0.72 to 1.02).17 Although our comparison of a heterologous BNT162b2+mRNA-1273 booster schedule with homologous booster schedules for the risk of documented infection was not statistically significant (comparative vaccine effectiveness 12.4%, 95% confidence interval −4.8% to 29.6%), two other studies found lower rates of omicron infection among those who had received a heterologous BNT162b2+mRNA-1273 vaccine schedule.15 16 However, comparative vaccine effectiveness estimates against infection are inherently prone to vary across studies as the captured infection rates within studies are greatly influenced by factors such as the background population infection rate during a particular study period together with temporal changes in national covid-19 policies (including testing, restriction, and vaccination strategies) and in individual level testing as well as risk behaviour. In line with recent work,38 we found that those with a history of SARS-CoV-2 infection had better protection against later infection compared with those without such a history. This tendency was consistent regardless of whether a booster dose had been received. Furthermore, hybrid immunity from a booster dose along with previous infection conferred the greatest protection. Of additional relevance to vaccination strategies, we observed that the protection afforded by booster vaccination seemed robust irrespective of a history of infection.
Limitations of this study
Our study should be evaluated in light of potential limitations. Firstly, in our outcome ascertainment we potentially may have captured individuals with an outcome not directly related to covid-19 but where covid-19 was a contributing factor or co-occurred at the time of hospital admission or death. Our definition of covid-19 related hospital admission mitigated this by only including inpatient hospital admission events that were considered to be due to covid-19, but our covid-19 related death definition was most likely subject to a larger extent of case misclassification. Importantly, any potential outcome misclassifications are most likely to be non-differential between vaccinated comparator groups. Secondly, individuals within each country could potentially have been differentially selected between compared schedule groups (eg, individuals at higher risk of severe covid-19 being prioritised for earlier vaccination), but any potential selection bias would most likely have been moderated by the aggregation of comparative estimates across schedules and countries. Thirdly, although we matched or weighted on covariates considered to be potential confounders, owing to the observational nature of our study we cannot fully exclude residual confounding. Fourthly, as per the utilised matching and weighting confounder control approaches, our results reflect average treatment effect estimates on treated, and thus the individual comparisons should primarily be interpreted separately. Fifthly, this study did not assess the safety of heterologous booster vaccination. Some studies have found greater short term reactogenicity associated with heterologous vaccine schedules than with homologous schedules, but other studies have not been able to confirm this finding.7 In the clinical setting, the risk of severe adverse events does not seem to differ between heterologous and homologous booster schedules.39
Lastly, our analyses for the two main types of heterologous AZD1222+BNT162b2 or mRNA-1273 and BNT162b2+mRNA-1273 booster schedule composite groups were generally well powered throughout all comparisons, but we also presented estimates for each individual subtype of heterologous booster schedules to inform patients, clinicians, and regulatory authorities on the booster schedule specific effectiveness observed in the Nordic countries. Statistical precision was, however, lower for some comparisons because of the overall rarity of severe events, in part owed to the effectiveness of the covid-19 vaccination strategies.
Conclusion
In this study, we utilised the healthcare registers of Denmark, Finland, Norway, and Sweden to estimate the comparative effectiveness of heterologous booster schedules including AZD1222 as well as monovalent BNT162b2 and mRNA-1273 SARS-CoV-2 wild type (ancestral) strain based vaccines in the Nordic countries during predominance of the omicron variant. Both heterologous and homologous booster schedules were associated with high protection against severe covid-19 outcomes compared with primary course vaccination only. Additionally, heterologous booster schedules were associated with a modestly lower risk of covid-19 related hospital admission than homologous mRNA vaccine booster schedules when directly compared, but they did not differ in risk of death with covid-19, although statistical precision was limited. All comparative vaccine effectiveness estimates became attenuated over time.
What is already known on this topic
Booster doses against covid-19 are recommended to prevent severe and fatal covid-19
Changes in vaccination policies owing to specific safety concerns and logistical and supply issues have led to use of heterologous booster schedules in many countries
Implementation of heterologous booster strategies should be supported by evidence of effectiveness, including protection against severe covid-19 outcomes due to the omicron variant; however, current evidence is scarce
What this study adds
Compared with primary vaccination schedules, both heterologous booster schedules containing AZD1222 as well as monovalent BNT162b2 and mRNA-1273 SARS-CoV-2 ancestral strain based vaccines and homologous mRNA vaccine booster schedules were associated with significantly increased protection against severe covid-19 outcomes during a period of omicron predominance
Heterologous booster schedules were associated with modestly greater effectiveness against covid-19 related hospital admission than homologous mRNA vaccine booster schedules
This study adds evidence to support current and future covid-19 vaccination strategies that will increasingly rely on heterologous schedules
Web extra.
Extra material supplied by authors
Supplementary information: Additional tables S1-S15, figures S1-S13, and references
Contributors: NA, ET, and AH conceptualised the study. NA drafted the manuscript. ET, UB, JS, and NP carried out the statistical analyses. All authors interpreted the results and critically reviewed the manuscript. AH supervised the study. NA and AH are the guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. This document expresses the opinion of the authors of the paper and may not be understood or quoted as being made on behalf of, or reflecting the position of, the European Medicines Agency or one of its committees or working parties.
Funding: This research was supported by the European Medicines Agency. The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interest: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the European Medicines Agency; EP reports receiving a grant from The Finnish Medical Foundation; LL reports participation in research projects funded by Menarini Pharmaceuticals and LEO Pharma with funds paid to his institution and receiving personal fees for teaching epidemiological methods related to covid-19 research from Atrium, the Danish association of the pharmaceutical industry; all outside this submitted work; MAn reports previous participation in research projects funded by Pfizer, Janssen, AstraZeneca, H Lundbeck, and Mertz, and Novartis with grants paid to the institution (Karolinska Institutet; no personal fees); MAn reports having received personal fees for teaching from Atrium and his institution, Pharmacovigilance Research Centre, has been supported by a grant from the Novo Nordisk Foundation (NNF15SA0018404) to the University of Copenhagen; all outside this submitted work. JH reports participation in regulator mandated phase 4 studies funded by Alcon, Almirall, Astellas, Astra-Zeneca, Boehringer-Ingelheim, Novo Nordisk, Servier, and LEO Pharma with funds paid to the institution; all outside this submitted work. RL reports receiving grants from Sanofi Aventis paid to his institution and receiving personal fees from Pfizer; all outside the submitted work; no other financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The lead authors (NA and AH) affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: Studied participants were anonymised in the utilised data sources; and therefore, direct dissemination to study participants is not possible. The study results will be disseminated to the public and health professionals by a press release written using layman’s terms.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Not required according to Danish, Finnish, Norwegian, and Swedish law.
Data availability statement
No additional data available. Owing to data privacy regulations in each country, the raw data cannot be shared.
References
- 1. Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N Engl J Med 2021;385:1393-400. 10.1056/NEJMoa2114255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barda N, Dagan N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet 2021;398:2093-100. 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA Vaccine Boosters against SARS-CoV-2 Omicron Infection in Qatar. N Engl J Med 2022;386:1804-16. 10.1056/NEJMoa2200797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Munro APS, Janani L, Cornelius V, et al. COV-BOOST study group . Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 2021;398:2258-76. 10.1016/S0140-6736(21)02717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stuart ASV, Shaw RH, Liu X, et al. Com-COV2 Study Group . Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial. Lancet 2022;399:36-49. 10.1016/S0140-6736(21)02718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Borobia AM, Carcas AJ, Pérez-Olmeda M, et al. CombiVacS Study Group . Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet 2021;398:121-30. 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parker EPK, Desai S, Marti M, et al. Emerging evidence on heterologous COVID-19 vaccine schedules-To mix or not to mix? Lancet Infect Dis 2022;22:438-40. 10.1016/S1473-3099(22)00178-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tan CS, Collier AY, Yu J, et al. Durability of Heterologous and Homologous COVID-19 Vaccine Boosts. JAMA Netw Open 2022;5:e2226335. 10.1001/jamanetworkopen.2022.26335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jara A, Undurraga EA, Zubizarreta JR, et al. Effectiveness of homologous and heterologous booster doses for an inactivated SARS-CoV-2 vaccine: a large-scale prospective cohort study. Lancet Glob Health 2022;10:e798-806. 10.1016/S2214-109X(22)00112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mayr FB, Talisa VB, Shaikh O, Yende S, Butt AA. Effectiveness of Homologous or Heterologous Covid-19 Boosters in Veterans. N Engl J Med 2022;386:1375-7. 10.1056/NEJMc2200415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marra AR, Miraglia JL, Malheiros DT, et al. Effectiveness of Heterologous Coronavirus Disease 2019 (COVID-19) Vaccine Booster Dosing in Brazilian Healthcare Workers, 2021. Clin Infect Dis 2023;76:e360-6. 10.1093/cid/ciac430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Menni C, May A, Polidori L, et al. COVID-19 vaccine waning and effectiveness and side-effects of boosters: a prospective community study from the ZOE COVID Study. Lancet Infect Dis 2022;22:1002-10. 10.1016/S1473-3099(22)00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Accorsi EK, Britton A, Fleming-Dutra KE, et al. Association Between 3 Doses of mRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants. JAMA 2022;327:639-51. 10.1001/jama.2022.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Andrews N, Stowe J, Kirsebom F, et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N Engl J Med 2022;386:1532-46. 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Monge S, Rojas-Benedicto A, Olmedo C, et al. IBERCovid . Effectiveness of mRNA vaccine boosters against infection with the SARS-CoV-2 omicron (B.1.1.529) variant in Spain: a nationwide cohort study. Lancet Infect Dis 2022;22:1313-20. 10.1016/S1473-3099(22)00292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ono S, Michihata N, Yamana H, et al. Comparative Effectiveness of BNT162b2 and mRNA-1273 Booster Dose After BNT162b2 Primary Vaccination Against the Omicron Variants: A Retrospective Cohort Study Using Large-Scale Population-Based Registries in Japan. Clin Infect Dis 2023;76:18-24. 10.1093/cid/ciac763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guibert N, Trepat K, Pozzetto B, et al. A third vaccine dose equalizes the levels of effectiveness and immunogenicity of heterologous or homologous COVID-19 vaccine regimens. medRxiv 2023:2023.02.13.23285853. 10.1101/2023.02.13.23285853 [DOI] [PMC free article] [PubMed]
- 18. Suah JL, Tng BH, Tok PSK, et al. Real-world effectiveness of homologous and heterologous BNT162b2, CoronaVac, and AZD1222 booster vaccination against Delta and Omicron SARS-CoV-2 infection. Emerg Microbes Infect 2022;11:1343-5. 10.1080/22221751.2022.2072773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tan SHX, Pung R, Wang L-F, et al. Association of Homologous and Heterologous Vaccine Boosters With COVID-19 Incidence and Severity in Singapore. JAMA 2022;327:1181-2. 10.1001/jama.2022.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kirsebom FCM, Andrews N, Sachdeva R, Stowe J, Ramsay M, Lopez Bernal J. Effectiveness of ChAdOx1-S COVID-19 booster vaccination against the Omicron and Delta variants in England. Nat Commun 2022;13:7688. 10.1038/s41467-022-35168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Starrfelt J, Danielsen AS, Buanes EA, et al. Age and product dependent vaccine effectiveness against SARS-CoV-2 infection and hospitalisation among adults in Norway: a national cohort study, July-November 2021. BMC Med 2022;20:278. 10.1186/s12916-022-02480-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Andrews N, Stowe J, Kirsebom F, et al. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med 2022;28:831-7. 10.1038/s41591-022-01699-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baum U, Poukka E, Leino T, Kilpi T, Nohynek H, Palmu AA. High vaccine effectiveness against severe COVID-19 in the elderly in Finland before and after the emergence of Omicron. BMC Infect Dis 2022;22:816. 10.1186/s12879-022-07814-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hulme WJ, Williamson EJ, Horne E, et al. Effectiveness of BNT162b2 booster doses in England: an observational study in OpenSAFELY-TPP. medRxiv. 2022:2022.06.06.22276026 10.1101/2022.06.06.22276026. [DOI]
- 25. Hulme WJ, Horne EMF, Parker EPK, et al. Comparative effectiveness of BNT162b2 versus mRNA-1273 covid-19 vaccine boosting in England: matched cohort study in OpenSAFELY-TPP. BMJ 2023;380:e072808. 10.1136/bmj-2022-072808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathieu E, Ritchie H, Rodés-Guirao L, et al. Coronavirus Pandemic (COVID-19). Our World in Data Published Online First: 5 March 2020. https://ourworldindata.org/coronavirus-testing (accessed 20 Mar 2023).
- 27. Patalon T, Saciuk Y, Peretz A, et al. Waning effectiveness of the third dose of the BNT162b2 mRNA COVID-19 vaccine. Nat Commun 2022;13:3203. 10.1038/s41467-022-30884-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferdinands JM, Rao S, Dixon BE, et al. Waning of vaccine effectiveness against moderate and severe covid-19 among adults in the US from the VISION network: test negative, case-control study. BMJ 2022;379:e072141. 10.1136/bmj-2022-072141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Deng J, Ma Y, Liu Q, Du M, Liu M, Liu J. Comparison of the Effectiveness and Safety of Heterologous Booster Doses with Homologous Booster Doses for SARS-CoV-2 Vaccines: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health 2022;19:10752. 10.3390/ijerph191710752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ranzani OT, Hitchings MDT, de Melo RL, et al. Effectiveness of an inactivated Covid-19 vaccine with homologous and heterologous boosters against Omicron in Brazil. Nat Commun 2022;13:5536. 10.1038/s41467-022-33169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Natarajan K, Prasad N, Dascomb K, et al. Effectiveness of Homologous and Heterologous COVID-19 Booster Doses Following 1 Ad.26.COV2.S (Janssen [Johnson & Johnson]) Vaccine Dose Against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults - VISION Network, 10 States, December 2021-March 2022. MMWR Morb Mortal Wkly Rep 2022;71:495-502. 10.15585/mmwr.mm7113e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu X, Munro APS, Feng S, et al. COV-BOOST study group . Persistence of immunogenicity after seven COVID-19 vaccines given as third dose boosters following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK: Three month analyses of the COV-BOOST trial. J Infect 2022;84:795-813. 10.1016/j.jinf.2022.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tseng HF, Ackerson BK, Luo Y, et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat Med 2022;28:1063-71. 10.1038/s41591-022-01753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sheikh A, Kerr S, Woolhouse M, McMenamin J, Robertson C, EAVE II Collaborators . Severity of omicron variant of concern and effectiveness of vaccine boosters against symptomatic disease in Scotland (EAVE II): a national cohort study with nested test-negative design. Lancet Infect Dis 2022;22:959-66. 10.1016/S1473-3099(22)00141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ioannou GN, Bohnert ASB, O’Hare AM, et al. COVID-19 Observational Research Collaboratory (CORC) . Effectiveness of mRNA COVID-19 Vaccine Boosters Against Infection, Hospitalization, and Death: A Target Trial Emulation in the Omicron (B.1.1.529) Variant Era. Ann Intern Med 2022;175:1693-706. 10.7326/M22-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Buchan SA, Chung H, Brown KA, et al. Estimated Effectiveness of COVID-19 Vaccines Against Omicron or Delta Symptomatic Infection and Severe Outcomes. JAMA Netw Open 2022;5:e2232760. 10.1001/jamanetworkopen.2022.32760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lauring AS, Tenforde MW, Chappell JD, et al. Influenza and Other Viruses in the Acutely Ill (IVY) Network . Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ 2022;376:e069761. 10.1136/bmj-2021-069761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Altarawneh HN, Chemaitelly H, Ayoub HH, et al. Effects of Previous Infection and Vaccination on Symptomatic Omicron Infections. N Engl J Med 2022;387:21-34. 10.1056/NEJMoa2203965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Andersson NW, Thiesson EM, Laursen MV, Mogensen SH, Kjær J, Hviid A. Safety of heterologous primary and booster schedules with ChAdOx1-S and BNT162b2 or mRNA-1273 vaccines: nationwide cohort study. BMJ 2022;378:e070483. 10.1136/bmj-2022-070483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: Additional tables S1-S15, figures S1-S13, and references
Data Availability Statement
No additional data available. Owing to data privacy regulations in each country, the raw data cannot be shared.