Abstract
Background:
Remarkable progress has been made in expanding access to services addressing the pediatric HIV epidemic, including programs to prevent mother-to-child transmission, early diagnosis, and treatment for children living with HIV. Few long-term data are available from rural sub-Saharan Africa to assess implementation and impact of national guidelines.
Methods:
Results from three cross-sectional studies and one cohort study conducted at Macha Hospital in Southern Province, Zambia from 2007-2019 were summarized. For infant diagnosis, maternal antiretroviral treatment, infant test results, and turnaround times for results were evaluated by year. For pediatric HIV care, the number and age of children initiating care and treatment, and treatment outcomes within 12 months were evaluated by year.
Results:
Receipt of maternal combination antiretroviral treatment increased from 51.6% in 2010-2012 to 93.4% in 2019, and the proportion of infants testing positive decreased from 12.4% to 4.0%. Turnaround times for results returning to clinic varied but were shorter when labs consistently used a text messaging system. The proportion of mothers receiving results was higher when a text message intervention was piloted. The number of children living with HIV enrolled into care and the proportion initiating treatment with severe immunosuppression and dying within 12 months decreased over time.
Conclusions:
These studies demonstrate the long-term beneficial impact of implementing a strong HIV prevention and treatment program. While expansion and decentralization brought challenges, the program succeeded in decreasing the rate of mother-to-child transmission and ensuring that children living with HIV benefit from access to life-saving treatment.
Keywords: HIV, pediatrics, early infant diagnosis, antiretroviral therapy, sub-Saharan Africa
Introduction
Sub-Saharan Africa is the epicenter of the pediatric human immunodeficiency virus (HIV) epidemic, with approximately 90% of new perinatal HIV infections occurring in the region.1 Over the past two decades, significant progress was made in expanding access to HIV services, including programs to prevent mother-to-child transmission (PMTCT) for women living with HIV, early testing and diagnosis for HIV-exposed infants, and treatment for children living with HIV.2 This effort was driven by a global plan to eliminate pediatric HIV infection, implemented in 2011, with a goal to reduce new pediatric HIV infections by 90% by reducing the rate of mother-to-child transmission below 5% and providing at least 90% of pregnant women with antiretroviral drugs for PMTCT.3 For children living with HIV, goals were set to reduce AIDS-related deaths by >50% and provide treatment for all children. Twenty-two priority countries were identified that were home to almost 90% of pregnant women living with HIV. The list consisted of India and 21 countries in sub-Saharan Africa, including Zambia. At a national level, Zambia has had success in reaching these goals. From 2010 to 2021, the estimated number of new pediatric infections decreased from 8800 to 3800,4,5 and the proportion of pregnant women living with HIV accessing antiretroviral drugs increased from 71% to 97%.4,5 Fewer data are available at a local level but would be useful to assess implementation of national guidelines and policies, particularly in rural areas.
Macha Research Trust, in collaboration with Johns Hopkins University, has conducted pediatric studies in Southern Province, Zambia since 2007. These studies provide the unique opportunity to understand the evolution of the pediatric HIV epidemic over more than a decade in a rural setting. The objective of this analysis was to evaluate use of antiretroviral drugs during pregnancy among women living with HIV, the rate of mother-to-child HIV transmission, turnaround times for infant testing, and pediatric care and treatment from 2007 to 2019 in rural Zambia.
Materials and Methods
Study setting
The studies in this analysis were conducted in the catchment area of Macha Hospital, a district-level hospital located in rural Choma District, Southern Province, Zambia (see Figure; Supplemental Digital Content 1). HIV prevalence in Southern Province decreased from 14.5% among adults 15-49 years in 20076 to 12.4% in 2018.7 Antiretroviral therapy (ART) became available for treatment and PMTCT at the HIV clinic associated with Macha Hospital in 2005 (see Figure; Supplemental Digital Content 2). In 2010, mobile outreach clinics were established at surrounding rural health centers with ART provided by the Macha HIV clinic.8 In 2017, these rural health centers became independent HIV clinics. Pediatric guidelines for starting ART expanded from treating only children with severe immunosuppression and disease progression in 20079 to treating all children <15 years in 2013.10 Guidelines for PMTCT also expanded from use of single dose nevirapine and short-course ART to implementation of Option B+ in 2013, in which all pregnant women living with HIV receive lifelong ART.11 In 2016, all individuals were recommended to receive ART after diagnosis regardless of clinical or immunologic stage.12 Infant diagnosis of HIV, which requires a nucleic acid amplification test (NAAT) for diagnosis prior to 18 months of age, became available in 2008 with recommended testing starting at 6 weeks of age, and then starting at birth in 2016.12
Studies evaluating infant diagnosis
Three studies were conducted to evaluate infant diagnosis of HIV infection. The first study, the Dried Blood Spot (DBS) study, was conducted at the Macha HIV clinic from August 1, 2010 to March 1, 2012 to measure turnaround times for infant diagnosis.13 All HIV NAATs performed were abstracted from the laboratory logbook. Study staff documented when results returned from the designated laboratory in Lusaka to the clinic, the test result, and maternal receipt of results.
The second study, the Early Infant Diagnosis (EID) study, was a cross-sectional study conducted at the Macha HIV clinic from April 1, 2013 to October 31, 2015.14 The objectives were to validate a locally performed p24 antigen assay for diagnosis and evaluate a mobile phone intervention to decrease the turnaround time for mothers to receive test results. All infants brought to the clinic for infant diagnosis were eligible for enrollment and could be enrolled multiple times if brought for additional testing. After enrollment, a questionnaire was administered, a blood spot card was prepared, and the p24 antigen assay was performed. A DBS card was also sent to the designated laboratory in Lusaka or Livingstone for an HIV NAAT as part of routine care. Study staff documented when and how (short message service [SMS] or hard copy) the routine test results returned to the clinic and the test result. As part of the study, mothers were contacted by phone or through the local community health worker when routine test results were available
The third study, the Novel Screening for Exposed Babies (NSEBA) study, was a prospective cohort study conducted at several health facilities in the Macha Hospital catchment area from February 1, 2016 to March 31, 2020.15,16 The objective was to evaluate strategies for implementing point-of-care technologies for infant diagnosis in Zambia. All HIV-exposed infants attending the study sites for infant diagnosis were eligible for enrollment. Infants were enrolled from birth or their first infant diagnosis visit and followed until their post-weaning visit or December 31, 2019. After enrollment, a questionnaire was administered, a blood spot card was prepared, and a point-of-care test was performed. Beginning in September 2018, the point-of-care test performed was the GeneXpert HIV-1 Qual assay (Cepheid, Sunnyvale, CA) and the results were provided to the parents.16 A DBS card was also sent to the designated laboratory in Livingstone or Choma for an HIV NAAT as part of routine clinical care. Study staff were alerted when the routine test result was returned to the clinic by SMS or hard copy. As the NSEBA study was conducted in several sites, data for this analysis included only participants enrolled at Macha Hospital after birth for comparison with the prior studies.
Study evaluating pediatric care and treatment
One cohort study, the Pediatric Antiretroviral Therapy (PART) study, was conducted at the Macha HIV clinic to evaluate pediatric HIV care and treatment. The PART study is an ongoing open cohort study that began in September 2007.17,18 All children 0-15 years of age registered for care were eligible for enrollment; more than 95% of eligible children are estimated to have been enrolled. Study visits occurred every three months, at which time a questionnaire was administered, height and weight were measured, and clinical and laboratory data were abstracted from the medical record. Participants missing at least two consecutive study visits were followed up in their homes to ascertain vital status.
Statistical analysis
For all studies, trends over time were summarized and depicted graphically. For infant diagnosis, outcomes included the proportion of women receiving any ART regimen, ART regimens received, and the proportion of infants testing positive by ART regimen. ART regimens included single-dose nevirapine during labor, short-course ART (use of one or two antiretroviral drugs during pregnancy and post-partum), and combination ART (use of at least three antiretroviral drugs during pregnancy and post-partum). Outcomes were summarized by year, although outcomes between 2010 and 2012 were aggregated due to the timing of the DBS study. For these analyses, the last available valid HIV NAAT result was used for each participant. Results by study were compared using a chi-square test. Turnaround times for results and the proportion of mothers receiving results were also evaluated by year. Turnaround times were summarized as median, interquartile range, and range. For these analyses, all available HIV NAAT results were used for each participant. Results by study or year were compared using a Wilcoxon rank-sum test for turnaround times and a chi-square test for the proportion of results returned.
For HIV care, outcomes included the number and age distribution of children initiating HIV services, enrolling into the study, and initiating HIV treatment by year, and the distribution of immunosuppression at ART initiation. Level of immunosuppression was defined based on age according to the World Health Organization (WHO) guidelines.19 In addition, treatment outcomes after 6 and 12 months were evaluated among children initiating treatment after study enrollment. Children not known to have died or transferred to another clinic were considered still in care if their last follow-up visit occurred within 3 months of the date of administrative censoring (6 or 12 months after treatment initiation).
Results
Infant diagnosis
From 2010 to 2019, the proportion of women receiving any ART during pregnancy increased from 79.6% to 93.4% (p<0.0001; Figure 1A). Among women receiving ART during pregnancy, the proportion receiving combination ART increased from 64.9% to 100% (p<0.0001). During the study period, the designated testing laboratory changed as the number of reference laboratories in the country expanded. The median turnaround time for returning results to the clinic more than doubled, from 36 to 133 days, when the reference laboratory changed from Lusaka to Livingstone in 2015 (Figure 2). In 2018, the reference laboratory changed again to Choma and median turnaround times decreased to 31 days in 2019, but remained higher than the maximum of 28 days recommended by the WHO to return results to the mother.20 In 2013, a national SMS system was implemented to decrease the turnaround time for returning laboratory results to the clinic. With increasing use of this system by the laboratory in Lusaka, the median turnaround time decreased from 54 days in 2010-2012 to 36 days in 2014 (p<0.0001; Figure 2), but again remained higher than the recommended maximum of 28 days. The SMS system was rarely used from 2015-2019 at the other laboratories. The proportion of mothers receiving results was similar in 2010-2012 and 2017-2019 at approximately 75-85% but higher in 2013-2015 (95-97%; p<0.0001) when a phone text messaging intervention was piloted to contact mothers when results were available.14
Figure 1.
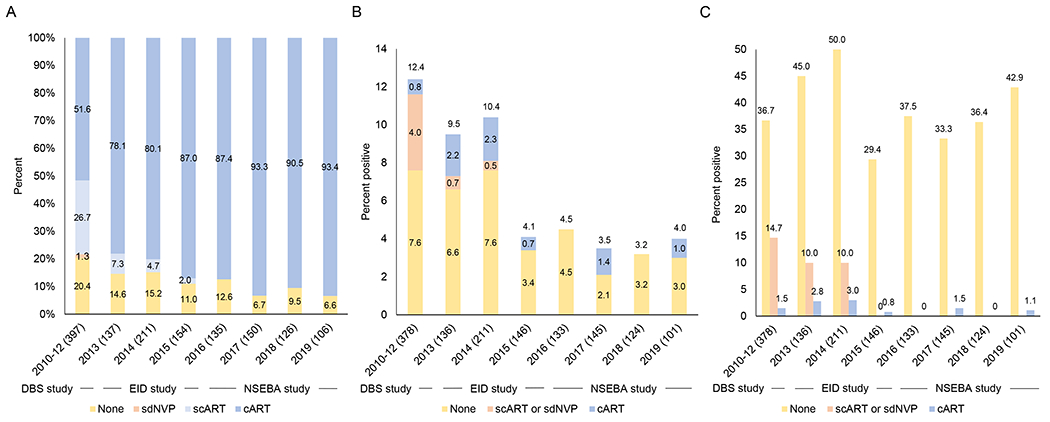
A) Percent of women living with HIV receiving antiretroviral therapy during pregnancy by year; B) Percent of infants who tested HIV positive by year, showing maternal antiretroviral regimen; C) Percent of infants who tested HIV positive by year and maternal antiretroviral regimen during pregnancy
ART: antiretroviral therapy; scART: short-course ART; cART: combination ART; sdNVP: single dose nevirapine
Note: Sample sizes are provided in parentheses after the year. The sample sizes in 1A and 1B/C do not match as some infants did not have valid test results available.
Figure 2.
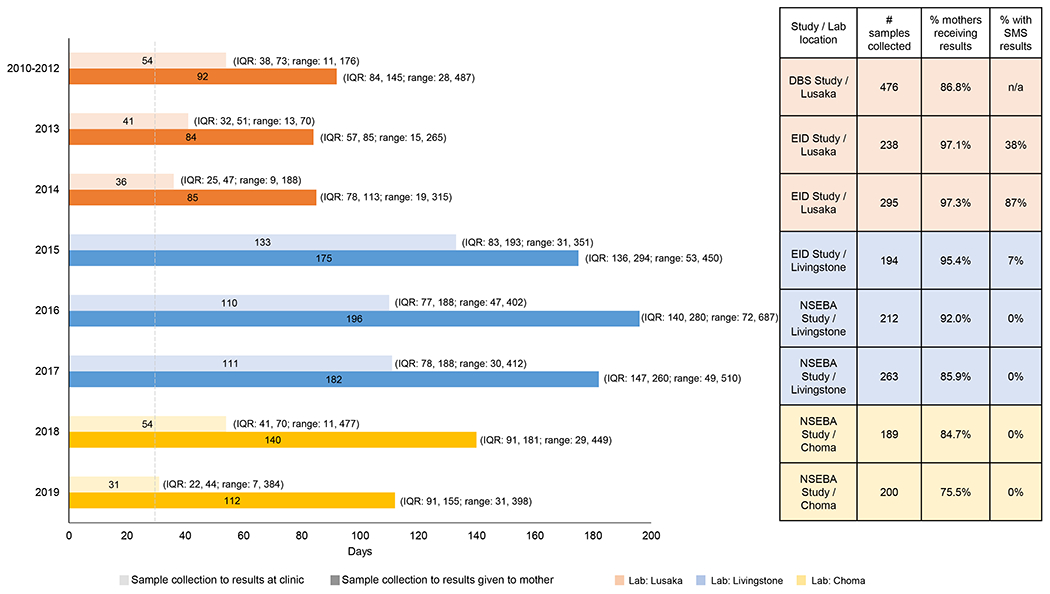
Turnaround time for test results from infant diagnosis of HIV by year
Note: Grey dashed line at 28 days represents the WHO recommended time to return results to the mother/caregiver.20
The proportion of infants testing positive decreased significantly from 12.4% in 2010-2012 to 4.1% in 2015 (p=0.04) and then remained stable through 2019 (p=0.98; Figure 1B). The proportion of infants testing positive differed significantly by maternal ART status (p<0.0001 in each study; Figure 1C). As the proportion of women receiving combination ART during pregnancy increased, their contribution to the proportion of infants testing positive also increased, from 6.5% (0.8%/12.4%) in 2010-2012 to 15.8% (0.6%/3.8%) in 2016-2019 (Figure 1B).
Pediatric HIV care and treatment
The number of children enrolling into the PART Study increased through 2010 and then decreased each subsequent year, to a low of 9 in 2019 (Figure 3A). A similar trend was observed by year of enrollment into HIV care (Figure 3B). The number of children initiating ART increased, particularly after infant diagnosis became available in 2008 and treatment guidelines expanded to all children 0-15 years of age in 2013 (Figure 3C).
Figure 3.
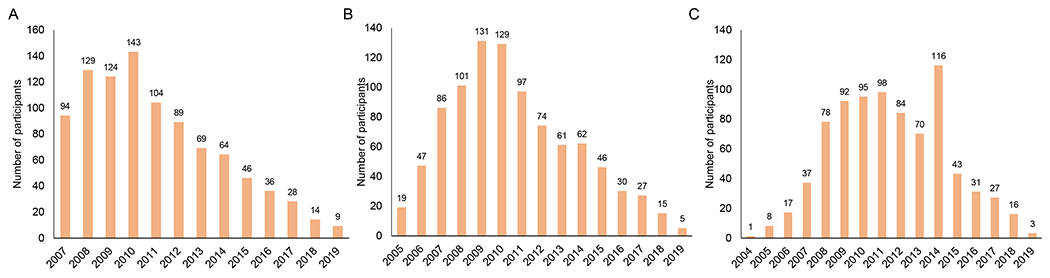
A) Enrollment into the Pediatric Antiretroviral Therapy (PART) Study by year; B) Enrollment into care at the Macha HIV clinic by year; and C) Treatment initiation by year
Note: Panel A shows the number of participants enrolling in the study by calendar year starting in 2007 when the study began and ending in 2019 (end of analysis). Panel B shows the number of study participants enrolling into care by calendar year. As participants enrolling in the study may have previously enrolled into care, particularly in the early years of the study, the time period starts in 2005 when the HIV clinic opened and ends in 2019. A small number of participants (n=48 out of 949; range: 0-7 per year) transferred into HIV care after having initiated treatment at another clinic. Panel C shows the number of study participants initiating treatment by calendar year. As some participants in the study previously initiated treatment either at the Macha HIV clinic or at another clinic, the time period starts in 2004 and ends in 2019.
The age distribution of children enrolling in the HIV clinic changed over time (Figure 4A), with more children 0-2 years of age enrolling after infant diagnosis became available in 2008. Thereafter, the proportion of children 5-15 years of age increased as the overall number of children diagnosed and registering for care decreased. A similar change was observed for the age of children initiating treatment (Figure 4B), particularly after treatment guidelines expanded in 2013. The proportion of children initiating ART with severe immunosuppression decreased over time (Figure 4C).
Figure 4.
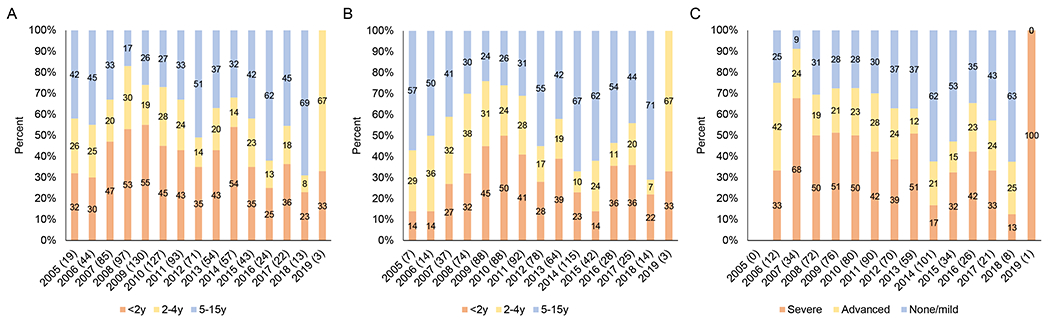
Distribution among study participants of A) age at registration for care at the Macha HIV clinic by year; B) age at treatment initiation by year; C) level of immune suppression at treatment initiation by year
Note: Sample sizes are provided in parentheses after the year. Children who transferred to the Macha HIV clinic already receiving treatment were excluded. Immune suppression defined according to WHO guidelines based on age and CD4+ T-cell percentage or count (0-11 months: none/mild - ≥30%, advanced – 25-29%, severe - <25%; 12-35 months: none/mild - ≥25%, advanced – 20-24%, severe - <20%; 36\59 months: ≥20%, advanced – 15\19%, severe - <15%; ≥5 years: none/mild - ≥350, advanced – 200\349, severe - <200 or <15%).19
Trends in treatment outcomes were evaluated (Figure 5). The proportion of children dying after treatment initiation decreased from 29% and 43% at 6 and 12 months, respectively, in 2007 to none from 2017 to 2019 (p<0.0001). The proportion of children transferring to other clinics was consistently low until 2017 when HIV services were decentralized. Loss to follow-up was low through 2018; only two children were enrolled in 2019 and one was lost to follow-up.
Figure 5.
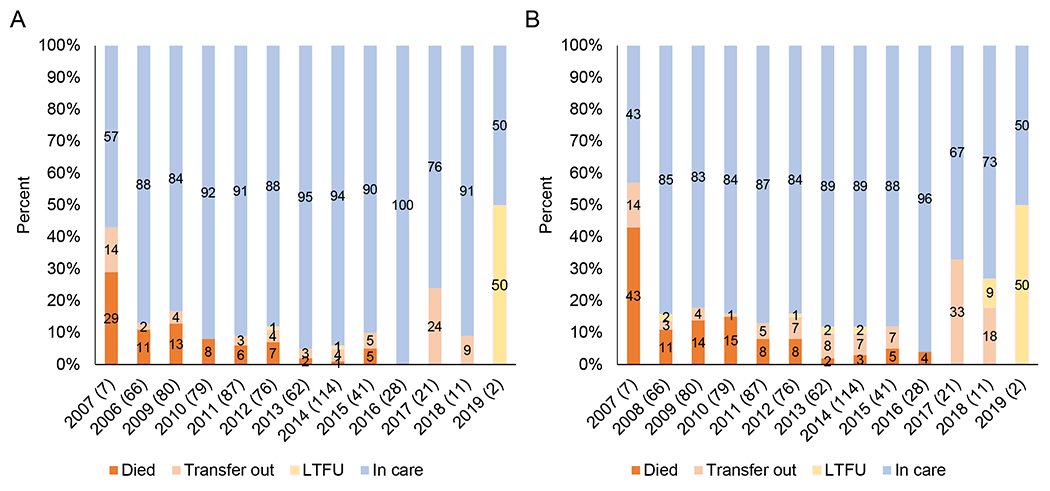
Distribution of outcomes among study participants after A) 6 months and B) 12 months of treatment by year of initiation.
Note: Sample sizes are provided in parentheses after the year. The analysis was limited to children who initiated treatment after enrolling into the study. Children who had not died or transferred to another clinic were considered to still be in care if their last follow-up visit occurred within 3 months of the date of administrative censoring (6 or 12 months after treatment initiation).
Discussion
In this series of studies, infant diagnosis of HIV and pediatric HIV care were evaluated over more than a decade in Macha, Zambia. To our knowledge, this is the only set of studies with data consistently available at a single site in rural sub-Saharan Africa. As per national guidelines, maternal use of combination ART during pregnancy increased over time and coincided with a decreasing proportion of infants testing positive. The turnaround times for results varied, but were shorter when mobile technologies were used to return results to the clinic and notify mothers of availability of results. Consistent with declining mother-to-child transmission, the number of children enrolling into HIV care decreased over time. With expanding treatment guidelines, the number of children receiving ART increased and the proportion initiating ART with severe immunosuppression decreased over time. Taken together, these findings suggest successful and sustained implementation of the HIV program in this area that has benefited women and children living with HIV.
The proportion of women receiving combination ART during pregnancy increased over time, with larger increases observed in 2013 and 2017, suggesting rapid implementation of the 2013 and 2016 national guidelines recommending Option B+ and a ‘test-and-treat all’ approach, respectively.10,12 From 2017-2019, over 90% of women received combination ART during pregnancy. While the studies only included women bringing their children for infant diagnosis which may overestimate coverage, they are consistent with Zambia’s reported high ART coverage (>95%) among pregnant women living with HIV in 2018.4,21 Similar to other studies, the rate of mother-to-child transmission was significantly lower with maternal use of combination ART,22–24 and therefore increased maternal use of combination ART coincided with a decrease in the proportion of children testing positive. While it is encouraging that improvements in use of combination ART and mother-to-child transmission were sustained over time, no additional gains were observed from 2017-2019, suggesting that identifying and reaching the remaining 5-10% of women in need will be difficult. A large study of pregnant women living with HIV in Southern Province found that women who did not receive ART were younger, more likely to be diagnosed with HIV during pregnancy, and less likely to have disclosed their status to their partner or received antenatal care.25 Increased efforts to ensure repeated testing during pregnancy and counseling for women newly diagnosed with HIV during pregnancy, as well as potential inclusion of pre-exposure prophylaxis in services offered to pregnant women, may be needed to further reduce new pediatric infections.
Turnaround times for returning test results to the clinic and caregivers varied by year and laboratory but were consistently longer than the WHO recommended 28 days,20 similar with other reports from the region.26–28 These studies took place over a decade during which Zambia expanded capacity for infant diagnosis outside a few centralized laboratories in Lusaka and Ndola. In Southern Province, laboratories in Livingstone and Choma became the designated testing centers for Macha in 2015 and 2018, respectively. However, decentralization of testing did not necessarily result in shorter turnaround times. While Livingstone is closer to Macha (~215 km) than Lusaka (~300 km), turnaround times were longer due to reagent stockouts and changes in laboratory capacity during the study period.29 The laboratory in Choma is approximately 70 km from Macha and had turnaround times comparable to the laboratory in Lusaka. Even with shorter distances, transporting samples to the laboratory and results back to the clinic remains challenging, making it difficult for clinics outside urban centers where reference laboratories are located to achieve the desired turnaround time. A significant improvement in turnaround times was observed with implementation of a national SMS system at the laboratories in 2013, but improvements were not sustained with inconsistent use of the system. A pilot text message intervention targeting turnaround times from the clinic to the caregiver and implemented from 2013-2015 increased the proportion of caregivers receiving test results,14 an improvement that also was not sustained when the pilot program ended. Text-based programs have been effective in reducing turnaround times, delivering results to caregivers, and improving retention for infant diagnosis in other settings,30–32 but require investments in human resources and technology and a sustained commitment for implementation to be effective. These challenges highlight the potential utility of point-of-care technologies for providing same-day results to mothers and improving linkage to care for children living with HIV.33 Despite requiring infrastructure and technology investments, point-of-care testing has been found to be cost-effective,34–36 and is recommended for use in high-burden settings.33
Consistent with an expanding program for infant diagnosis, the number and proportion of infants and young children enrolling in the pediatric HIV cohort increased from 2008 to 2010. With decreasing rates of mother-to-child-transmission of HIV, the number of children enrolling then steadily decreased to a low of 9 in 2019. The large increase in the number of children and proportion of older children initiating treatment in 2014 suggests rapid implementation of guidelines for expanded access to treatment. In addition, the decreasing proportion of children initiating treatment with severe immunosuppression and dying within 6-12 months support a benefit of earlier treatment initiation.37,38 These changes are consistent with observations from other cohorts in the region.39
This analysis had several limitations. First, while the goal was to evaluate the rate of mother-to-child transmission, results of HIV testing post-weaning were not available from all studies. Therefore, the proportion of children testing positive over time, using their last available NAAT during the study period, was used as a proxy. Second, for treatment initiation, a relevant metric would have been the proportion of eligible children initiating ART; however, evolving guidelines made such an analysis challenging. Instead, the number of children initiating each year was summarized. Lastly, these results were generated within the context of research studies conducted at one health facility over a decade. These findings may not represent the experience of all facilities in rural areas but they highlight the successes and challenges common to many programs in the region.
In conclusion, these studies conducted in a rural area of southern Zambia over the past decade demonstrate at a local level the impact of implementing a strong HIV prevention and treatment program. Expansion and decentralization brought challenges, particularly around testing, that will continue to need to be addressed. However, with sustained effort and rapid adoption of national guidelines, the program succeeded in dramatically decreasing the rate of mother-to-child transmission of HIV and ensuring that children living with HIV could benefit from access to life-saving treatment.
Supplementary Material
Acknowledgements
We thank the children and their parents for participating in the studies, and the staff at the Macha HIV clinic and Macha Research Trust for assisting with the studies.
Conflicts of interest and source of funding:
The authors have no conflicts of interest to declare. The DBS, EID and PART studies were supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through Cooperative Agreements (U62/CCU322428 to WJM and 5U2GPS001930 to PET) from the Department of Health and Human Services (DHHS)/Centers for Disease Control and Prevention (CDC), Global AIDS Program. The NSEBA study was supported by a grant from the National Institutes of Allergy and Infectious Disease (R01AI116324 to WJM). The findings and conclusions included in its content are solely the responsibility of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.UNAIDS. Fact Sheet - Global HIV Statistics. 2020. https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (accessed September 11 2020).
- 2.UNAIDS. Global AIDS Update 2020: Seizing the moment. Tackling entrenched inequalities to end epidemics. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS), 2021. [Google Scholar]
- 3.UNAIDS. Global Plan Towards the Elimination of new HIV Infections Among Children by 2015 and Keeping Their Mothers Alive. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS, 2011. [Google Scholar]
- 4.UNAIDS. UNAIDS data 2019. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS), 2019. [Google Scholar]
- 5.UNAIDS. Country Factsheets Zambia 2021. https://www.unaids.org/en/regionscountries/countries/zambia (accessed November 13 2022).
- 6.Central Statistical Office (CSO), Ministry of Health (MOH), Tropical Diseases Research Centre (TDRC), University of Zambia, Macro International Inc. Zambia Demographic and Health Survey 2007. Calverton, Maryland, USA: CSO and Macro International Inc., 2009. [Google Scholar]
- 7.Zambia Statistics Agency, Ministry of Health (MOH), ICF. Zambia Demographic and Health Survey 2018. Lusaka, Zambia and Rockville, Maryland, USA: Zambia Statistics Agency, Ministry of Health, and ICF, 2019. [Google Scholar]
- 8.van Dijk JH, Moss WJ, Hamangaba F, Munsanje B, Sutcliffe CG. Scaling-up access to antiretroviral therapy for children: A cohort study evaluating care and treatment at mobile and hospital-affiliated HIV clinics in rural Zambia. PLoS One 2014; 9(8): e104884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kankasa C Guidelines for Antiretroviral Therapy of HIV infection in Infants and Children: Towards universal access: Zambia Ministry of Health and UNICEF Zambia, 2007. [Google Scholar]
- 10.Ministry of Health Republic of Zambia. Zambia Consolidated Guidelines for Treatment and Prevention of HIV Infection. Lusaka, Zambia, 2013. [Google Scholar]
- 11.Ministry of Health Republic of Zambia. Lifelong Antiretroviral Drugs (ARV’s) for all HIV positive Pregnant Women in Zambia. Policy Guidelines for Health Facilities in Zambia. January 2013. Lusaka, Zambia: Government of the Republic of Zambia, Ministry of Health, 2013. [Google Scholar]
- 12.Ministry of Health (MOH). Zambia consolidated guidelines for treatment & prevention of HIV infection. Lusaka, Zambia: Republic of Zambia Ministry of Health, 2016. [Google Scholar]
- 13.Sutcliffe CG, van Dijk JH, Hamangaba F, Mayani F, Moss WJ. Turnaround time for early infant HIV diagnosis in rural Zambia: a chart review. PLoS One 2014; 9(1): e87028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutcliffe CG, Thuma PE, van Dijk JH, et al. Use of mobile phones and text messaging to decrease the turnaround time for early infant HIV diagnosis and notification in rural Zambia: an observational study. BMC pediatrics 2017; 17(1): 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutcliffe CG, Mutanga J, Moyo N, et al. Point-of-care p24 antigen detection for early infant diagnosis of HIV infection: cross-sectional and longitudinal studies in Zambia. BMC Infect Dis 2021; 21(1): 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutcliffe CG, Moyo N, Schue JL, et al. The NSEBA Demonstration Project: implementation of a point-of-care platform for early infant diagnosis of HIV in rural Zambia. Trop Med Int Health 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Dijk JH, Sutcliffe CG, Munsanje B, Hamangaba F, Thuma PE, Moss WJ. Barriers to the care of HIV-infected children in rural Zambia: a cross-sectional analysis. BMC Infect Dis 2009; 9: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schue JL, van Dijk JH, Hamangaba F, et al. Treatment outcomes among children younger than five years living with HIV in rural Zambia, 2008-2018: a cohort study. BMC pediatrics 2021; 21(1): 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: towards universal access, 2006. [Google Scholar]
- 20.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. Second Edition 2016. Gevena, Switzerland: World Health Organization, 2016. [PubMed] [Google Scholar]
- 21.Ministry of Health Zambia. Zambia Population-based HIV Impact Assessment (ZAMPHIA) 2016: Final Report. Lusaka, Zambia: Ministry of Health, February 2019. [Google Scholar]
- 22.Mutanga JN, Mutembo S, Ezeamama AE, et al. Tracking progress toward elimination of mother to child transmission of HIV in Zambia: Findings from the early infant diagnosis of HIV program (2009-2017). J Trop Pediatr 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muyunda B, Musonda P, Mee P, Todd J, Michelo C. Effectiveness of lifelong ART (Option B+) in the prevention of mother-to-child transmission of HIV programme in Zambia: Observations based on routinely collected health data. Front Public Health 2019; 7: 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gumede-Moyo S, Filteau S, Munthali T, Todd J, Musonda P. Implementation effectiveness of revised (post-2010) World Health Organization guidelines on prevention of mother-to-child transmission of HIV using routinely collected data in sub-Saharan Africa: A systematic literature review. Medicine (Baltimore) 2017; 96(40): e8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamahuwa M, Phiri Y, Manyepa P, et al. Factors associated with PMTCT utilization among HIV-infected women in Southern Province, Zambia. American Society of Tropical Medicine and Hygiene; 2018 October 28 - November 1; New Orleans, LA; 2018. [Google Scholar]
- 26.Gill MM, Hoffman HJ, Mokone M, et al. Assessing very early infant diagnosis turnaround times: Findings from a birth testing pilot in Lesotho. AIDS Res Treat 2017; 2017: 2572594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bwana VM, Mfinanga SG, Simulundu E, Mboera LEG, Michelo C. Accessibility of early infant diagnostic services by under-5 years and HIV exposed children in Muheza District, North-East Tanzania. Front Public Health 2018; 6: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ali H, Minchella P, Chipungu G, et al. Infant HIV diagnosis and turn-around time for testing in Malawi, 2015. Afr J Lab Med 2020; 9(1): 904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutcliffe CG, Mutanga JN, Moyo N, et al. Acceptability and feasibility of testing for HIV infection at birth and linkage to care in rural and urban Zambia: a cross-sectional study. BMC Infect Dis 2020; 20(1): 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finocchario-Kessler S, Gautney BJ, Khamadi S, et al. If you text them, they will come: using the HIV infant tracking system to improve early infant diagnosis quality and retention in Kenya. AIDS 2014; 28 Suppl 3: S313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finocchario-Kessler S, Gautney B, Cheng A, et al. Evaluation of the HIV Infant Tracking System (HITSystem) to optimise quality and efficiency of early infant diagnosis: a cluster-randomised trial in Kenya. Lancet HIV 2018; 5(12): e696–e705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deo S, Crea L, Quevedo J, et al. Implementation and operational research: Expedited results delivery systems using GPRS technology significantly reduce early infant diagnosis test turnaround times. J Acquir Immune Defic Syndr 2015; 70(1): e1–4. [DOI] [PubMed] [Google Scholar]
- 33.WHO. Updated recommendations on HIV prevention, infant diagnosis, antiretroviral initiation and monitoring: March 2021. Geneva, Switzerland: World Health Organization, 2021. [PubMed] [Google Scholar]
- 34.De Broucker G, Salvatore PP, Mutembo S, et al. The cost-effectiveness of scaling-up rapid point-of-care testing for early infant diagnosis of HIV in southern Zambia. PLoS One 2021; 16(3): e0248217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salvatore PP, de Broucker G, Vojnov L, Moss WJ, Dowdy DW, Sutcliffe CG. Modeling the cost-effectiveness of point-of-care platforms for infant diagnosis of HIV in sub-Saharan African countries. AIDS 2021; 35(2): 287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frank SC, Cohn J, Dunning L, et al. Clinical effect and cost-effectiveness of incorporation of point-of-care assays into early infant HIV diagnosis programmes in Zimbabwe: a modelling study. Lancet HIV 2019; 6(3): e182–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 2008; 359(21): 2233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azzoni L, Barbour R, Papasavvas E, et al. Early ART results in greater immune reconstitution benefits in HIV-infected infants: Working with data missingness in a longitudinal dataset. PLoS One 2015; 10(12): e0145320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iyun V, Technau KG, Vinikoor M, et al. Variations in the characteristics and outcomes of children living with HIV following universal ART in sub-Saharan Africa (2006–17): a retrospective cohort study. Lancet HIV 2021; 8(6): e353–e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


