Abstract
Background
Inflammation is a risk factor for cardiovascular disease (CVD), and particular inflammatory parameters can be used to predict the incidence of CVD. The aim of this study was to assess the association between fibrinogen (FIB), interleukin-6 (IL-6), C-reactive protein (CRP) and galectin-3 (Gal-3) and the risk of cardiovascular disease using meta-analysis.
Methods
PubMed, Embase, Scopus, and Web of Science databases were searched with the appropriate strategies to identify observational studies relevant to this meta-analysis. A random-effects model was used to combine inflammation factor-associated outcomes and cardiovascular disease outcomes, except in the case of galectin-3, where a fixed-effects model was used because of less heterogeneity. Location, age, type of cardiovascular disease, and sample size factors were used to explore heterogeneity in stratification and metaregression for subgroup analysis. A case-by-case literature exclusion approach was used for sensitivity analysis. The funnel plot and Begg's test were combined to assess publication bias.
Results
Thirty-three papers out of 11,456 were screened for inclusion in the analysis. Four inflammation biomarkers were significantly associated with the development of CVD: FIB (OR: 1.21, 95% CI: 1.15–1.27, P < 0.001; HR: 1.04, 95% CI: 1.00–1.07, P < 0.05), IL-6 (HR: 1.16, 95% CI: 1.10–1.22, P < 0.001), CRP (OR: 1.25, 95% CI: 1.15–1.35, P < 0.001; HR: 1.20, 95% CI: 1.14–1.25, P < 0.001) and Gal-3 (HR: 1.09, 95% CI: 1.05–1.14, P < 0.001). Location factors help explain the source of heterogeneity, and there is publication bias in the Gal-3 related literature.
Conclusion
Taken together, the current research evidence suggests that high levels of fibrinogen, interleukin-6, C-reactive protein and galectin-3 are risk factors for cardiovascular disease and can be used as biomarkers to predict the development of cardiovascular disease to some extent.
Systematic Review Registration
https://www.crd.york.ac.uk/PROSPERO, identifier: CRD42023391844.
Keywords: fibrinogen, interleukin-6, C-reactive protein, galectin-3, cardiovascular disease, meta-analysis
Introduction
Cardiovascular disease (CVD) is the leading cause of death and disability worldwide (1). Prevalent cases of total CVD in 204 countries and territories worldwide nearly doubled from 271 million [95% uncertainty interval (UI): 257–285 million] in 1990 to 523 million (95% UI: 497–550 million) in 2019 and are an important contributor to the heavy disease burden in the world (2). In terms of developmental mechanisms, inflammation is not only the basis for the pathogenesis of atherosclerosis but also a common cause of cardiovascular disease (3); thus, certain inflammatory factors may act as biomarkers to predict the risk of cardiovascular disease.
Epidemiological studies have revealed an independent positive link between elevated plasma fibrinogen (FIB) levels and CVD, as well as a positive relationship with several established CVD risk factors (4, 5). In addition, many other factors are associated with subclinical atherosclerotic heart disease (6). Galectin-3 (Gal-3), a potential biomarker of cardiovascular inflammation, promotes the secretion of other proinflammatory factors, such as interleukin-6 (IL-6), in a dose-dependent manner by activating macrophages and is significantly and rapidly expressed in a variety of diseases, including cancer, diabetes, and heart disease (7, 8). IL-6 plays a key role as an upstream cytokine in the propagation of the inflammatory response downstream of atherosclerosis, and the high production of IL-6 stimulates hepatocytes to generate C-reactive protein (CRP), which further amplifies the inflammatory response (9). Inflammation and atherosclerosis are essential features of the pre-CVD condition. Although the results of most studies suggest that inflammatory factors appear to predict cardiovascular events, there are still investigations that yield nonsignificant or even negative correlations for specific subtypes of CVD (10–18). In addition, prevention of CVD is a key component, yet current meta-analyses have focused on clinical prognosis and mortality in CVD patients (19–22), lacking an integrated discussion of the predictive value of inflammatory factors in the development of CVD, and even fewer articles have included consideration of multiple biomarkers simultaneously for direct comparison. Therefore, this study aimed to assess the relationship between these four inflammation biomarkers (FIB, IL-6, CRP, and Gal-3) and the risk of first occurrence of CVD events by performing a meta-analysis of previously published observational studies and, thereby, providing better estimates of the odds ratios.
Methods
Search strategies
This meta-analysis is registered on PROSPERO (https://www.crd.york.ac.uk/PROSPERO) with the registration ID CRD42023391844. In databases (EMBASE) (all fields), PubMed (all fields), Web of Science (topic), and Scopus (article title, abstract and keywords), the search strategy “((FIB) OR (fibrinogen) OR (IL-6) OR (interleukin-6) OR (CRP) OR (c reactive protein) OR (Gal-3) OR (galectin-3)) AND (incidence rate) AND (cardiovascular disease)” was used to search for all publications included up to 30 May 2023. The search does not use any filters or restrictions. References of related articles were also manually indexed to ensure maximum comprehensiveness for literature inclusion. All citations were imported into NoteExpress 3.7.0 to remove duplicates.
Study selection criteria
This meta-analysis involved articles investigating the association between four inflammation markers (FIB, IL-6, CRP, and Gal-3) and the incidence of common CVD (including heart failure, myocardial infarction, coronary artery disease, stroke, coronary heart disease, atrial fibrillation.) selected according to the following specific inclusion and exclusion criteria.
The inclusion criteria were (1) observational studies (including prospective cohort, retrospective cohort, and case‒control); (2) Inflammation factors investigated included one or more of FIB, IL-6, CRP, Gal-3, and an endpoint of first cardiovascular disease; and (3) study data contained hazard ratio (HR)/relative risk (RR)/odds ratio (OR) with 95% confidence intervals (CI) per standard deviation (SD) increase in log-transformed biomarker level.
The exclusion criteria were (1) non-English works of literature; (2) generally nonhealthy adult population (based on history of certain diseases and current diseases or population treated with interventions such as surgery); (3) sample size <500; and (4) when multiple studies assessed identical factors and endpoints across the same group of participants, only the latest was included.
Data extraction and quality evaluation
The extraction of crucial data and the quality assessment of the study were performed independently by two investigators to ensure that data collection was as accurate and objective as possible. Any disagreements were resolved through further deliberation and intervention efforts involving a third investigator. The results of the maximum adjustment model were used for the selection of the ending indicator. The details and characteristics derived and recorded among all eligible full texts are listed below: (1) study location; (2) participant characteristics (age, sex, sample size); (3) study period; (4) study cohort; (5) type of cardiovascular disease; (6) OR/RR/HR with 95% CI; (7) covariates in the maximum-adjusted model. The Newcastle Ottawa Scale (NOS), developed for case‒control and cohort studies, was used to evaluate quality; the scale ranges from 0 to 9, with 7 and above indicating very good quality, 4–6 indicating fairly good quality, and 4 and below indicating poor quality (23, 24).
Statistical analysis
The correlation between biomarkers and the risk of CVD was commonly reported as OR/RR/HR values for multiple comparison forms. Studies in which the values were reported by comparing linear biomarker doubling to incident CVD or the 4th tertile of biomarkers compared to the bottom tertile were not considered, and only articles with a 1-SD log-biomarker increase were included. Thus, although total surveys of the same type were not enrolled, comparability of outcome indicators was ensured while introduction of errors by data estimation transformation was avoided. In this meta-analysis, we analyzed studies reporting HR separately, and studies reporting RR and OR were directly combined and transformed into OR for analysis (25, 26). Statistical heterogeneity was assessed with the I2 statistic and the Cochran Q test, where a P-value of <0.05 was considered significant for heterogeneity. Pooled results were calculated using random-effects models if I2 > 50%, which indicated a substantial degree of heterogeneity across studies, or using a fixed-effects model otherwise (27). We performed subgroup analysis and meta-regression to assess the impact of variables such as region, age, CVD type, and sample size on the pooled effects and heterogeneity. Sensitivity analysis was used to explore whether the presence of certain studies altered the pooled results significantly by removing each study one at a time. Potential publication bias was evaluated collectively with the visual inspection funnel plot and Begg's test (28, 29). Stata (Version 17; MP-64) software was used for the meta-analysis and statistical analysis.
Results
Literature search
The literature search and eligible study selection process is shown in Figure 1. A total of 11,456 papers from the Web of Science, PubMed, Embase, and Scopus databases were retrieved by the targeted search strategy, and 33 were finally included in this meta-analysis after removal of duplicates, browsing of abstracts, and refinement of the full text. We estimated that for each inflammation biomarker, there were 7–19 articles that included an evaluation of the biomarker's capacity to predict the risk of cardiovascular disease.
Figure 1.
Flow-chart of literature search for meta-analysis.
Study characteristics and quality evaluation
Table 1 summarizes the major information and quality assessment of the included observational studies. The research sites covered European countries such as England, France and Germany (11, 13, 16, 18, 30–38), as well as the United States (10, 12, 15, 39–52), Canada (17) and Japan (53). The research sample size ranged from 564 to 40,656 and was generally adjusted for multiple covariates common to CVD, such as sex, age, blood pressure, smoking, diabetes, BMI, and cholesterol. NOS values of 6–9 in the observational study indicate good quality.
Table 1.
Study characteristics and quality evaluation.
| Reference | Study location | Sample size | Age (years) | Male (%) | Study period | Study cohort | Biomarker | Outcome | Maximum adjusted model RR/HR/OR (95% CI) | Covariates in the maximum-adjusted model | Quality of study (Score 0–9) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Smith 1997 | United Kingdom | 1,592 | 64.4 ± 0.2 | 47.4 | 1988–1993 | Edinburgh Artery Study | FIB | MI | RR: 1.04 (0.89–1.22) | Age, sex, systolic blood pressure, LDL-cholesterol, cigarette smoking, and baseline disease | 7 |
| Stroke | RR: 1.52 (1.17–1.98) | ||||||||||
| CVD | RR: 1.15 (1.02–1.29) | ||||||||||
| Scarabin 1998 | France and Northern Ireland | 10,500 | 55.0 ± 2.0 | 100.0 | 1991–1993 | Prospective Epidemiological Study of Myocardial Infarction (PRIME Study) | FIB | CVD | OR: 1.26 (1.17–1.36) | Field center, age, smoking, BMI, waist-to-hip,hypertension, HDL cholesterol LDL-cbolesterol triglycerides and diabetes | 7 |
| Koenig 1999 | Germany | 936 | 45–64 | 100.0 | 1984–1992 | Monitoring Trends and Determinants in Cardiovascular Disease Augsburg Cohort Study (MONICA) | CRP | CHD | HR: 1.50 (1.14–1.97) | Age, smoking | 8 |
| Scarabin 2003 | France and Northern Ireland | 10,500 | 55.0 ± 2.0 | 100.0 | 1991–1993 | PRIME Study (French participants) | FIB | CHD | RR: 1.26 (1.03–1.60) | Age, BMI, systolic blood pressure, TC, HDL, diabetes, smoking status | 6 |
| PRIME Study (Northern Ireland participants) | RR: 1.42 (1.06–2.16) | ||||||||||
| Cesari 2003 | USA | 2,225 | 74.0 ± 2.8 | 44.6 | 1997–2004 | Health, Aging, and Body Composition (Health ABC study) | CRP | CHD | RR: 1.11 (0.96–1.29) | Age, gender, race, smoking, diabetes, hypertension, BMI, HDL cholesterol, triglyceride, and albumin | 9 |
| Stroke | RR: 1.18 (0.91–1.53) | ||||||||||
| HF | RR: 1.48 (1.23–1.78) | ||||||||||
| IL-6 | CHD | RR: 1.27 (1.10–1.48) | |||||||||
| Stroke | RR: 1.45 (1.12–1.86) | ||||||||||
| HF | RR: 1.72 (1.40–2.12) | ||||||||||
| Vasan 2003 | USA | 732 | 78.4 ± 4.5 | 33.3 | 1992–1994 | Framingham Heart Study (FHS) | IL-6 | HF | HR: 1.36 (1.06–1.74) | Age, sex, diabetes, systolic blood pressure, hypertension treatment, smoking status, BMI, total cholesterol/HDL, valve disease, prevalent atrial fibrillation, prevalent cardiovascular disease, and ECG-LVH | 9 |
| Engström 2006 | Sweden | 6,075 | 46.8 ± 3.7 | 100.0 | 1974–1984 | A screening programme is approved and funded by Malmö's health services authority | FIB | Stroke | RR: 1.13 (0.99–1.29) | Age, BMI, diabetes, systolic blood pressure, physical activity, antihypertensive medication, current smoking, tobacco consumption, cholesterol, high alcohol consumption, log triglycerides, angina,occupation, marital status | 7 |
| Jeppesen 2008 | Denmark | 2,357 | 41–71 | 49.5 | 1982–1991 | Monitoring trends and determinants in cardiovascular disease project (MONICA) | CRP | CVD | HR: 1.33 (1.14–1.55) | Age, sex, smoking habit and total cholestero,waist circumference,levels of triglycerides, high-density lipoprotein-cholesterol, systolic and diastolic blood pressures, and level of physical activity | 7 |
| Davidson 2009 | Canada | 1,794 | 46.3 ± 18.3 | 49.9 | 1995–1999 | The Canadian Nova Scotia Health Survey (NSHS95) | CRP | CHD | HR: 1.28 (1.04–1.57) | Sex and framingham risk score | 6 |
| IL-6 | 1.07 (0.90–1.27) | ||||||||||
| Schnabel 2009 | USA | 2,863 | 61 ± 9 | 45 | 1998–2001 | The Framingham Offspring cohort | FIB | AF | HR: 0.94 (0.79–1.12) | Age, sex, smoking, systolic blood pressure, hypertension treatment, body mass index, diabetes, alcohol consumption, electrocardiographic left ventricular hypertrophy, auscultatory valvular heart disease, myocardial infarction, and heart failure | 6 |
| CRP | HR: 1.05 (0.87–1.26) | ||||||||||
| IL-6 | 1.08 (0.91–1.29) | ||||||||||
| Smith 2010 | Sweden | 5,187 | 57.6 ± 5.9 | 41.0 | 1991–1994 | MDCS (Malmö Diet and Cancer Study)Cardiovascular Cohort (MDCCC) | CRP | HF | HR: 1.57 (1.28–1.94) | Age, sex, systolic blood pressure, diastolic blood pressure, use of antihypertensive treatment, body mass index, low-density lipoprotein, high-density lipoprotein, current smoking,history of diabetes mellitus, and history of myocardial infarction | 8 |
| AF | HR: 1.18 (1.03–1.34) | ||||||||||
| Chei 2011 | Japan | 13,314 | 40–85 | 51.0 | 1985–2000 | Circulatory Risk in Communities Study (CIRCS) | CRP | Stroke | OR: 1.17 (1.01–1.35) | Systolic blood pressure, antihypertensive medication use, body mass index, alcohol intake category, cigarette smoking status, serum total cholesterol levels, log-transformed tryglyceride levels, and serum glucose category as well as matching for sex, age, community, year of serum stored, and fasting status | 7 |
| Schnabel 2013 | USA | 3,035 | 61.0 ± 9.0 | 47.0 | N/A | Framingham Heart Study (FHS) | CRP | CVD | HR: 1.21 (1.06–1.39) | Age, sex, smoking, systolic blood pressure, hypertension treatment, total/HDL cholesterol, body mass index, and diabetes | 7 |
| FIB | HR: 1.18 (1.03–1.36) | ||||||||||
| IL-6 | HR: 1.16 (1.01–1.32) | ||||||||||
| Daniels 2014 | USA | 1,397 | 70.0 ± 11.0 | 35.8 | 1992–2009 | Rancho Bernardo Study | Gal-3 | CHD | HR: 1.09 (0.92–1.30) | Age, sex, diabetes, hypertension, current smoking, systolic blood pressure, total cholesterol, HDL, estimated GFR, BMI, log10NT-proBNP | 6 |
| Yin 2014 | USA | 942 | 65.0 ± 9.0 | 69.0 | 1991–2008 | Framingham Heart Study (FHS) | CRP | MI | OR: 1.87 (1.09–3.19) | age, sex, current smoking status, statin use, systolic blood pressure, hypertension treatment status, total cholesterol, high-density lipoprotein–cholesterol, diabetes mellitus status, and body mass index | 7 |
| CVD | OR: 1.38 (1.13–1.69) | ||||||||||
| Ho 2014 | USA | 3,306 | 58.0 ± 9.0 | 46.0 | 1995–1998 | The Framingham Offspring cohort | Gal-3 | AF | HR: 1.13 (0.95–1.36) | Age, sex, clinical risk factors, alcohol, eGFR, BNP, CRP, echocardiographic parameters | 7 |
| Jagodzinski 2015 | Finland | 8,444 | 25–74 | 50.8 | 1997–2012 | FINRISK97 Study | Gal-3 | MI | HR: 1.06 (0.94–1.20) | Region of Finland, HDL and total cholesterol, systolic blood pressure, antihypertensive medication, smoking, prevalent diabetes, prevalent valvular heart disease, eGFR, galectin-3, NT-proBNP | 6 |
| Stroke | HR: 1.06 (0.94–1.18) | ||||||||||
| HF | HR: 1.09 (0.99–1.19) | ||||||||||
| Seven 2015 | Denmark | 6,502 | 45.9 ± 7.9 | 48.1 | N/A | Inter99 Study | CRP | Stroke | HR: 1.08 (0.88–1.33) | Sex, age, intervention group,total cholesterol, HDL-cholesterol, smoking status, systolic blood pressure, treatment for hypertension, baseline diabetes,BMI, HOMA-IR, eGFR, adiponectin, leptin | 6 |
| IHD | HR: 1.15 (1.00–1.32) | ||||||||||
| Appiah 2015 | USA | 10,601 | 59.6 ± 5.6 | 43.0 | 1993–2012 | Atherosclerosis Risk in Communities study (ARIC) | FIB | CHD | HR: 1.00 (0.94–1.06) | Age, sex, race, ARIC center,education, smoking, alcohol intake, sports index, systolic blood pressure, body mass index, use of antihypertensive medications, diabetes mellitus, cholesterol medication, high-density cholesterol, and total cholesterol,total fibrinogen,high sensitivity C-reactive protein | 7 |
| Stroke | HR: 0.97 (0.87–1.07) | ||||||||||
| HF | HR: 1.05 (0.99–1.12) | ||||||||||
| AbouEzzeddine 2016 | USA | 1,614 | 54–71 | 47.0 | 1997–2009 | Epidemiologic research in Olmsted County | Gal-3 | HF | HR: 1.20 (1.03–1.41) | Age, sex, BMI, eGFR, hypertension, systolic blood pressure, CAD, diabetes mellitus, total cholesterol, HDL and smoking history | 9 |
| Dawood 2016 | USA | 25,841 | 64.5 ± 9.5 | 45.3 | 2003–2007 | REasons for Geographic And Racial Differences in Stroke Study (REGARDS) | CRP | Stroke | HR: 1.06 (1.01–1.12) | Age, sex, race and socioeconomic status, hypertension, diabetes mellitus, congestive heart failure and warfarin/aspirin use | 6 |
| Appiah 2016 | USA | 5,888 | ≥65 | 90.0 | 1992–2013 | CHS population-based cohort | FIB | CHD | HR: 1.02 (0.95–1.10) | Age, sex, race, years of education and CHS center, smoking status, alcohol intake, physical activity, systolic blood pressure, BMI, antihypertensive medications use, diabetes, cholesterol medication use, HDL cholesterol and total cholestero, total fibrinogen | 7 |
| Stroke | HR: 0.88 (0.77–1.00) | ||||||||||
| HF | HR: 1.00 (0.92–1.08) | ||||||||||
| Silverman 2016 | USA | 6,781 | 45–84 | 50.0 | 2000–2007 | Multi-Ethnic Study of Atherosclerosis (MESA) | CRP | HF | HR: 1.17 (0.93–1.46) | Gender, race/ethnicity, socioeconomic status, MESA site | 6 |
| IL-6 | HR: 1.32 (0.91–1.93) | ||||||||||
| Tunstall 2017 | United Kingdom | 15,737 | 49.0 ± 8.3 | 52.0 | 1995–2009 | Scottish Heart Health Extended Cohort (SHHEC) | CRP | CHD | HR: 1.13 (1.06–1.19) | Use the Best and Extended ASSIGN Models | 7 |
| Ghorbani 2018 | USA | 2,477 | 57.0 ± 9.0 | 45.0 | 1995–2018 | Framingham Heart Study (FHS) | Gal-3 | CVD | HR: 1.20 (1.02–1.41) | Baseline galectin-3 levels, age, sex, systolic blood pressure, antihypertensive treatment, diabetes, body mass index, smoking, left ventricular hypertrophy, HDL to cholesterol ratio, estimated glomerular filtration rate, prevalent cardiovascular disease | 9 |
| HF | HR: 1.26 (1.00–1.59) | ||||||||||
| de Boer 2018 | USA | 22,756 | 60.0 ± 13.0 | 56.9 | 1989–2002 | Cardiovascular Health Study (CHS); Framingham Heart Study (FHS); Multi-Ethnic Study of Atherosclerosis (MESA); Prevention of Renal and Vascular End-stage Disease (PREVEND) | FIB | HF | HR: 1.12 (1.03–1.22) | Age, sex, race/ethnicity, systolic blood pressure, hypertension treatment, body mass index, diabetes, smoking, presence of left ventricular hypertrophy or left bundle branch block, and previous myocardial infarction | 9 |
| IL-6 | HR: 1.10 (0.99–1.22) | ||||||||||
| CRP | HR: 1.04 (0.95–1.14) | ||||||||||
| Gal-3 | HR: 1.02 (0.93–1.12) | ||||||||||
| Subirana 2018 | Spain | 5,404 | 35–74 | 49.4 | 1995–2005 | REGICOR population-cohorts | CRP | CAD | HR: 1.00 (0.76, 1.33) | Systolic blood pressure, diastolic blood pressure, high-density lipoprotein-cholesterol, total cholesterol, diabetes, smoking | 6 |
| IL-6 | HR: 1.34 (1.03, 1.74) | ||||||||||
| Ho 2018 | USA | 3,523 | 62.0 ± 8.0 | 47.0 | 1998–2018 | Framingham Heart Study (FHS) | CRP | HF | HR: 1.38 (1.19–1.60) | Age, sex, systolic blood pressure, hypertension treatment, diabetes mellitus, body mass index, smoking, total and HDL cholesterol, and history of atrial fibrillation, prevalent myocardial infarction | 7 |
| Leening 2018 | USA | 3,285 | 50–79 | 0.0 | 1994–2005 | Women's Health Initiative Observational Study (WHI OS) | CRP | CVD | HR: 1.20 (1.11–1.30) | Age, race/ethnicity, treated and untreated systolic blood pressure, total and HDL cholesterol levels, diabetes mellitus, and smoking status | 7 |
| CHD | HR: 1.21 (1.09–1.34) | ||||||||||
| Stroke | HR: 1.23 (1.11–1.35) | ||||||||||
| Magnussen 2019 | Europe | 40,656 | 21–99 | 48.3 | N/A | DanMONICA, FINRISK, Moli-sani, Northern Sweden | CRP | HF | Men: HR: 1.31 (1.23–1.41) | Body mass index, systolic blood pressure, antihypertensive medication, total cholesterol, diabetes, daily smoking | 8 |
| Women: HR: 1.10 (1.00–1.20) | |||||||||||
| Aguilar 2020 | USA | 6,538 | 62.5 ± 5.6 | 58.1 | 2011–2013 | Atherosclerosis Risk in Communities study (ARIC) visit 5 | Gal-3 | CHD | HR: 1.33 (0.68–2.60) | Age, sex, race, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, antihypertensive medication, current smoking, and diabetes mellitus status except for heart failure, estimated glomerular filtration rate,log N-terminal pro-B-type natriuretic peptide and log high-sensitivity cardiac troponin | 7 |
| Stroke | HR: 1.02 (0.43–2.43) | ||||||||||
| HF | HR: 1.93 (1.15–3.24) | ||||||||||
| Fernandez 2020 | Sweden | 4,469 | 57.2 ± 5.9 | 39.5 | 1991–2016 | Malmö Diet and Cancer-Cardiovascular Cohort (MDC-CC) | IL-6 | HF | HR: 1.22 (1.10–1.37) | Age, sex,GDF-15, IL-6, ST2, uPAR | 6 |
| Dykun 2022 | N/A | 8,563 | 64.6 ± 9.3 | 78.0 | N/A | Assessment of Clinical Effects of Cholesteryl Ester Transfer Protein Inhibitor with Evacetra pib in Patients with High-Risk for Vascular Outcomes (ACCELERATE) | CRP | Stroke | HR: 1.32 (1.08–1.62) | Age, race, sex, region, smoking status, body mass index, diabetes, baseline high-sensitivity C-reactive protein, baseline low-density lipoprotein cholesterol, baseline high-density lipoprotein cholesterol, baseline systolic blood pressure, baseline statin use, and treatment group | 6 |
| MI | HR: 1.28 (1.12–1.46) |
IHD, ischemic heart disease; MI, myocardial infarction; CVD, cardiovascular disease; CHD, coronary heart disease; HF, heart failure; CAD, coronary artery disease; AF, atrial fibrillation.
Association of inflammation markers with the risk of CVD
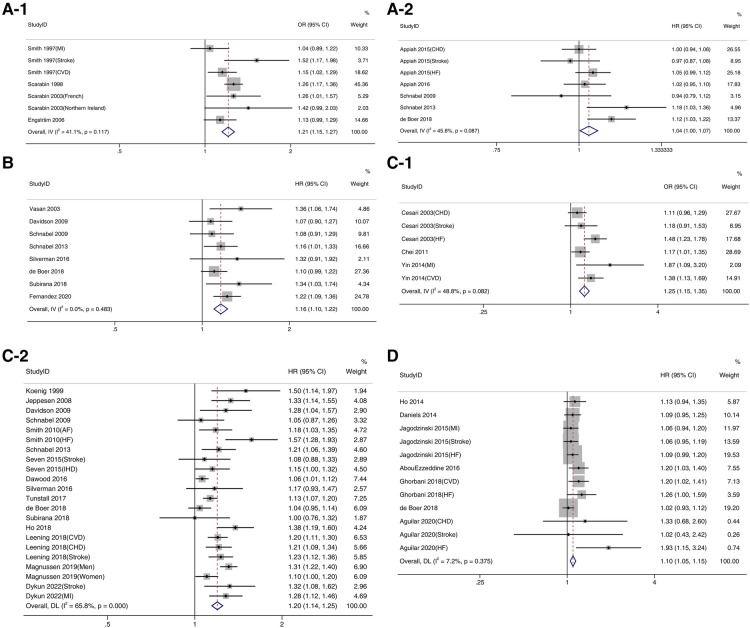
The results of the meta-analysis are shown in Figure 2 and Table 2, where one-standard deviation increases in the levels of all listed inflammation markers.
Figure 2.
Forest plot of inflammatory factors and incidence of cardiovascular disease.
Table 2.
Meta-analysis of inflammation markers and risk of CVD.
| Biomarker | Pooled results (95% CI) | Signifificance test (P-value) | No. of studies | No. of effffect estimates | P-value for Heterogeneity | I 2 |
|---|---|---|---|---|---|---|
| FIB | OR: 1.21 (1.15–1.27) | <0.001 | 4 | 7 | 0.117 | 41.1% |
| HR: 1.04 (1.00–1.07) | 0.029 | 5 | 7 | 0.087 | 45.8% | |
| IL-6 | HR: 1.16 (1.10–1.22) | <0.001 | 8 | 8 | 0.483 | 0.0% |
| CRP | OR: 1.25 (1.15–1.35) | <0.001 | 3 | 6 | 0.082 | 48.8% |
| HR: 1.20 (1.14–1.25) | <0.001 | 16 | 28 | <0.001 | 65.8% | |
| Gal-3 | HR: 1.09 (1.05–1.14) | <0.001 | 7 | 12 | 0.375 | 7.2% |
FIB (OR: 1.21, 95% CI: 1.15–1.27, P < 0.001, Figure 2A-1; HR: 1.04, 95% CI: 1.00–1.07, P < 0.05, Figure 2A-2), IL-6 (HR: 1.16, 95% CI: 1.10–1.22, P < 0.001, Figure 2B), CRP (OR: 1.25, 95% CI: 1.15–1.35, P < 0.001, Figure 2C-1; HR: 1.20, 95% CI: 1.14–1.25, P < 0.001, Figure 2C-2) and Gal-3 (HR: 1.09, 95% CI: 1.0–1.14, P < 0.001, Figure 2D) were significantly associated with the risk of developing CVD. There was high heterogeneity in the pooled analysis with CRP reported as HR (I2 = 65.8% ≥50.0%, P < 0.05).
Subgroup analysis and metaregression
We performed subgroup analysis based on predefined classification criteria, and the results are shown in Table 3. In most subgroups, high concentrations of FIB, IL-6, CRP, and Gal-3 were associated with an increased risk of CVD, with significantly lower intragroup heterogeneity in more than half of the subgroups. We used metaregression to detect the source of heterogeneity, and the results (Table 4) revealed that the covariate of study location (P = 0.004) could help explain heterogeneity in investigations of the biomarker FIB.
Table 3.
Subgroup analysis of inflammation markers and risk of CVD.
| Biomarker | Grouping criteria | Subgroup | Pooled results (95% CI) | Signifificance test (P-value) | No. of studies | No. of effffect estimates | P-value for Heterogeneity | I 2 |
|---|---|---|---|---|---|---|---|---|
| FIB | Location | EUR | 1.21 (1.15–1.27) | <0.001 | 3 | 4 | 0.117 | 41.1% |
| Age | <60 | 1.23 (1.16–1.31) | <0.001 | 1 | 3 | 0.453 | 0.0% | |
| ≥60 | 1.15 (1.05–1.26) | 0.002 | 5 | 7 | 0.053 | 66.0% | ||
| CVD type | CVD | 1.23 (1.15–1.31) | <0.001 | 2 | 2 | 0.199 | 39.3% | |
| MI | 1.04 (0.89–1.22) | 0.626 | 1 | 1 | – | – | ||
| Stroke | 1.20 (1.07–1.35) | 0.003 | 2 | 2 | 0.048 | 74.3% | ||
| CHD | 1.30 (1.08–1.57) | 0.006 | 1 | 2 | 0.576 | 0.0% | ||
| Sample Size | <5,000 | 1.15 (1.05–1.26) | 0.002 | 1 | 3 | 0.053 | 66.0% | |
| ≥5,000 | 1.23 (1.16–1.31) | <0.001 | 3 | 4 | <0.453 | 0.0% | ||
| IL-6 | Location | EUR | 1.24 (1.12–1.37) | <0.001 | 2 | 2 | 0.518 | 0.0% |
| USA | 1.14 (1.06–1.22) | <0.001 | 5 | 5 | 0.491 | 0.0% | ||
| CAN | 1.07 (0.90–1.27) | 0.441 | 1 | 1 | – | – | ||
| Age | <60 | 1.19 (1.09–1.30) | <0.001 | 3 | 3 | 0.294 | 18.3% | |
| ≥60 | 1.14 (1.06–1.22) | <0.001 | 5 | 5 | 0.491 | 0.0% | ||
| CVD type | CVD | 1.16 (1.01–1.32) | 0.030 | 1 | 1 | – | – | |
| CAD | 1.34 (1.03–1.74) | 0.029 | 1 | 1 | – | – | ||
| Stroke | 1.45 (1.12–1.86) | 0.004 | 1 | 1 | – | – | ||
| CHD | 1.07 (0.90–1.27) | 0.441 | 1 | 1 | – | – | ||
| HF | 1.18 (1.10–1.26) | <0.001 | 4 | 4 | 0.297 | 18.6% | ||
| AF | 1.08 (0.91–1.29) | 0.387 | 1 | 1 | – | – | ||
| Sample Size | <5,000 | 1.17 (1.09–1.25) | <0.001 | 5 | 5 | 0.429 | 0.0% | |
| ≥5,000 | 1.14 (1.04–1.25) | 0.006 | 3 | 3 | 0.287 | 19.8% | ||
| CRP | Location | EUR | 1.21 (1.13–1.30) | <0.001 | 7 | 10 | 0.001 | 67.5% |
| USA | 1.16 (1.09–1.24) | <0.001 | 7 | 9 | 0.002 | 67.4% | ||
| CAN | 1.28 (1.04–1.57) | 0.019 | 1 | 1 | – | – | ||
| Age | <60 | 1.22 (1.13–1.32) | <0.001 | 7 | 9 | 0.026 | 54.2% | |
| ≥60 | 1.19 (1.12–1.25) | <0.001 | 9 | 13 | <0.001 | 72.6% | ||
| CVD type | CVD | 1.22 (1.15–1.30) | <0.001 | 3 | 3 | 0.499 | 0.0% | |
| MI | 1.28 (1.12–1.46) | <0.001 | 1 | 1 | – | – | ||
| Stroke | 1.15 (1.03–1.29) | 0.010 | 4 | 4 | 0.018 | 70.3% | ||
| CHD | 1.20 (1.20–1.31) | <0.001 | 4 | 4 | 0.129 | 47.1% | ||
| HF | 1.23 (1.10–1.38) | <0.001 | 5 | 6 | <0.001 | 82.8% | ||
| IHD | 1.15 (1.00–1.32) | 0.048 | 1 | 1 | – | – | ||
| CAD | 1.00 (0.76–1.33) | >0.999 | 1 | 1 | – | – | ||
| AF | 1.13 (1.02–1.26) | 0.022 | 2 | 2 | 0.314 | 1.4% | ||
| Sample Size | <5,000 | 1.23 (1.18–1.29) | <0.001 | 7 | 9 | 0.359 | 9.2% | |
| ≥5,000 | 1.17 (1.10–1.24) | <0.001 | 9 | 13 | <0.001 | 72.5% | ||
| Gal-3 | Location | EUR | 1.07 (1.01–1.14) | 0.024 | 1 | 3 | 0.907 | 0.0% |
| USA | 1.11 (1.05–1.18) | <0.001 | 6 | 9 | 0.204 | 27.1% | ||
| Age | <60 | 1.10 (1.04–1.16) | <0.001 | 3 | 6 | 0.652 | 0.0% | |
| ≥60 | 1.08 (1.01–1.16) | 0.020 | 4 | 6 | 0.135 | 40.5% | ||
| CVD type | CVD | 1.20 (1.02–1.41) | 0.027 | 1 | 1 | – | – | |
| MI | 1.06 (0.94–1.20) | 0.350 | 1 | 1 | – | – | ||
| Stroke | 1.06 (0.95–1.99) | 0.316 | 2 | 2 | 0.931 | 0.0% | ||
| CHD | 1.10 (0.96–1.25) | 0.161 | 2 | 2 | 0.568 | 0.0% | ||
| HF | 1.09 (1.03–1.16) | 0.002 | 5 | 5 | 0.049 | 58.2% | ||
| AF | 1.13 (0.94–1.35) | 0.182 | 1 | 1 | – | – | ||
| Sample Size | <5,000 | 1.16 (1.08–1.25) | <0.001 | 6 | 5 | 0.782 | 0.0% | |
| ≥5,000 | 1.06 (1.01–1.12) | 0.017 | 3 | 7 | 0.362 | 8.8% |
Table 4.
Meta-regression analysis by potential modifier res % residual variation due to heterogeneity.
| Biomarker | Covariate | Exp (b) | P-value | I2 res |
|---|---|---|---|---|
| FIB | Location | 1.20 (1.09–1.32) | 0.004 | 41.08% |
| Age | 0.94 (0.76–1.17) | 0.525 | 41.24% | |
| CVD type | 0.98 (0.91–1.06) | 0.555 | 73.16% | |
| Sample Size | 1.06 (0.85–1.32) | 0.525 | 41.24% | |
| IL-6 | Location | 1.00 (0.90–1.11) | 0.936 | 7.30% |
| Age | 0.96 (0.83–1.10) | 0.457 | 0.00% | |
| CVD type | 0.99 (0.95–1.03) | 0.516 | 0.24% | |
| Sample Size | 0.98 (0.84–1.13) | 0.705 | 5.16% | |
| CRP | Location | 0.98 (0.93–1.03) | 0.338 | 65.61% |
| Age | 0.97 (0.88–1.08) | 0.591 | 67.35% | |
| CVD type | 0.99 (0.97–1.01) | 0.405 | 65.11% | |
| Sample Size | 0.94 (0.86–1.00) | 0.143 | 61.82% | |
| Gal-3 | Location | 1.05 (0.94–1.16) | 0.379 | 10.41% |
| Age | 0.98 (0.89–1.09) | 0.747 | 14.66% | |
| CVD type | 1.00 (0.96–1.03) | 0.766 | 14.81% | |
| Sample Size | 0.92 (0.89–1.02) | 0.090 | 0.00% |
Sensitivity analysis and publication bias
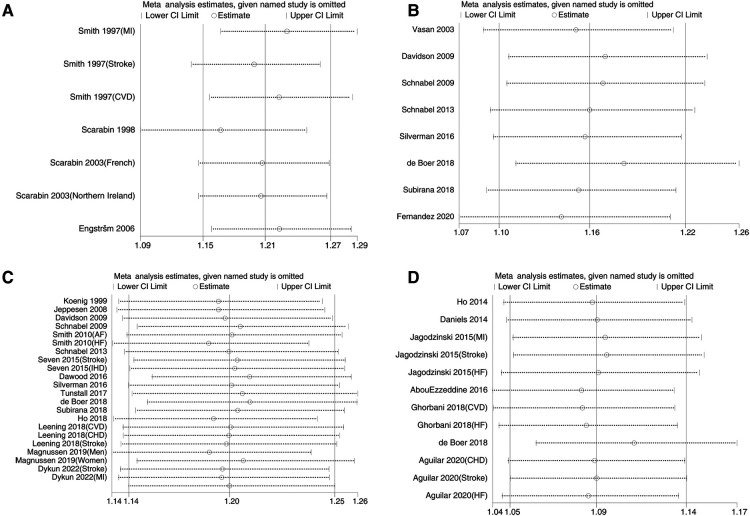
Figure 3 shows the sensitivity analysis of the pooled outcomes of the other studies after omitting one study at a time. The combined values of any remaining studies support the conclusion that these inflammation biomarkers are a risk for CVD occurrence, and the small difference between this value and the original effect size suggests that the combined results are not dominated by any one study and are relatively stable and reliable. Figure 4 shows mild asymmetry in the funnel plots of the Gal-3 studies. The results of Begg's test in Table 5 confirm that the Gal-3 related articles were associated with significant publication bias (P < 0.05).
Figure 3.
Sensitivity analysis of inflammatory factors and incidence of cardiovascular disease.
Figure 4.
Funnel plots of inflammatory factors and incidence of cardiovascular disease.
Table 5.
Results of publication bias analysis.
| Biomarker | Funnel plot symmetry | Begg's test (P-value) |
|---|---|---|
| FIB-OR | YES | 0.764 |
| FIB-HR | YES | 0.764 |
| IL-6 | YES | 0.266 |
| CRP-OR | YES | 0.452 |
| CRP-HR | YES | 0.259 |
| Gal-3 | NO | 0.047 |
Discussion
The pooled results of this meta-analysis showed that FIB, IL-6, CRP, and Gal-3 are risk factors for the development of CVD. A juxtaposition of the results showed that the pooled results of the four inflammatory factors were similar and that IL-6 had the best homogeneity of relevant studies. Location helps explain the heterogeneity of the included studies. Sensitivity analysis showed relatively stable combined results.
A number of biomarkers have become a major focus for improving methods of scoring CVD risk. The use of these biomarkers is attractive because they integrate signals from multiple pathophysiologic pathways and have some combined predictive utility (54). The results of the meta-analysis are consistent with previous studies, such as epidemiological investigations showing that for CVD-related diseases, circulating concentrations of FIB are associated with stroke and that their elevated levels predict the incidence of HF (55–57). As the accepted “gold standard,” CRP has been confirmed in numerous studies to be a marker of future CVD risk (58–63). Similar to CRP, levels of IL-6 in populations that appear to be healthy may also be used to predict future vascular risk. This finding was first established in men in 2000 (64), then validated in women (65), and then duplicated in more than 25 prospective epidemiological cohorts throughout the world (66). The findings of this investigation were consistent with those of a meta-analysis by the Emerging Risk Factors Collaborative, which established that a 1 SD rise in log IL-6 was related to a 25% increase in the risk of future vascular events (66). The relationship between the cascade response of IL-6 and CRP may help explain the similar ability of the two to predict the occurrence of CVD in the results of this study (67). Gal-3, as an emerging marker, has attracted increasing interest in recent years. Since 2014, the US Food and Drug Administration has included Gal-3 on the list of validated cardiovascular biomarkers (68). In a large population of 5,805 older adults, low Gal-3 levels were a good negative risk marker regarding the incidence of CVD events observed during short-term follow-up (69). Other factors not included have the potential to be explored as predictive markers, such as the soluble lectin-like oxidized low-density lipoprotein receptor-1 (sLOX-1) that is implicated in atherosclerotic cardiovascular disease (ASCVD) pathogenesis (70). It showed good discrimination in predicting plaque progression in patients with acute coronary syndromes (ACS) (70). sLOX-1 was further confirmed as an early diagnostic marker for ACS (71). Growth differentiation factor-15 (GDF-15) is cytokine involved in the regulation of multiple systems (72). Circulating levels of GDF-15 may be a biomarker of subclinical atherosclerosis in patients with psoriasis (73). In the general population, exploration of relevant markers remains to be further investigated.
A variety of factors can influence the predictive role of inflammation biomarkers of CVD development, including but not limited to those addressed in the exploration of heterogeneity above. Regarding age factors, observational studies on people aged 85 years indicate that a few of the classical risk factors, such as age, sex, systolic blood pressure, total lipoprotein cholesterol, diabetes, and smoking, become blurred or even play an inverse role in predicting cardiovascular disease under the influence of advanced age factors (74, 75). Markers such as C-reactive protein, interleukin 6, fibrinogen, and may help predict CVD status in the elderly population (76, 77). The magnitude of the predictive effect between different inflammation factors on specific CVD subtypes also varies. Any heterogeneity between CVD subtypes implies a search for increasingly specific etiological indicators (78). In a community-based cohort study, elevated plasma Gal-3 levels measured in a middle-aged population were associated not only with the onset of heart failure but also with coronary heart disease, ischemic stroke, and overall mortality (10). A previously published meta-analysis involved examining the relationship between Gal-3 and the incidence of HF, and the subgroup results of the present study were highly consistent in that context (79). Our findings show that IL-6 is one of the strongest inflammation biomarkers for predicting stroke. Indeed IL-6 has been one of the most studied biomarkers of inflammation in stroke patients, especially as a prognostic marker, and as a predictor of stroke risk (80). Similarly, in the case of stroke, CRP levels are rapidly elevated (81), and this acute phase response occurs in the context of widespread inflammation, reflecting the low specificity of CRP elevation. In population-based studies, elevated CRP levels not only increase the risk of stroke (82) but are also associated with poor functional outcome and mortality after stroke (83), and the time points of CRP elevation may reflect different phenomena, with early elevations associated with stroke severity and late elevations associated with post-stroke infection (84). In addition, IL-6 and CRP may be associated with diagnosis, risk stratification and prognosis in patients with acute myocardial infarction (85), and both are also significantly upregulated in acute coronary syndromes (86). There was an association between fibrinogen concentration and CVD, including CAD and stroke incidence, and an increased risk of future myocardial infarction, independent of other CVD and atherosclerotic factors (87, 88). The results of the present study also support this view at the CVD level, and the insignificant results of the subgroup analysis may be due to a bias caused by the small number of studies with the corresponding subgroups. The data in this paper are not grouped by gender, but studies show that women develop CVD later, experience more complications and have worse outcomes than men (89–91). Inflammatory biomarkers such as interleukins and tumor necrosis factor play a role in predisposing CVD risk, and gender-specific differences in CVD risk occurrence are associated with these biomarkers (92). In the Framingham Heart Study, cardiac biomarkers, including CRP, were observed to be substantially higher in premenopausal women than in men, and this difference was attenuated in postmenopausal women who did not receive hormone replacement therapy (93). The more widely supported view regarding the cause of this phenomenon of increased CVD and altered levels of inflammatory factors in women after menopause is that estrogen has an anti-inflammatory effect (94), and therefore, the protective effect of estrogen may be one of the reasons for the differences in biomarker prediction between men and women. A cohort study revealed that the association of some biomarkers with future cardiovascular events was influenced by ethnicity (95); data on CRP, IL-6 and FIB showed that CRP was predictive only in Caucasians, IL-6 was predictive only in African Americans, FIB was predictive in Caucasians, African Americans and Hispanics, and none of the biomarkers predicted CVD in the Chinese population. Despite the fact that Prof. Gu Dongfeng's team has developed a predictive model for assessing CVD in the Chinese population that takes into account differences in the disease spectrum and CVD risk factors compared to other ethnic groups (96), we encourage the continued exploration of the usefulness of novel biomarkers such as Gal-3 in predicting the effect of CVD.
Given modern developments, biomarkers are economical and convenient to measure, so the exploration of biomarkers has strong practical importance. As mentioned earlier, these predictive inflammation biomarkers help identify people at risk for CVD and assess the magnitude of an individual's future CVD risk. Appropriate use can be effective for CVD prevention in the general population. In populations already suffering from CVD, these factors can assist in diagnosis and predict disease prognosis. Nowadays, with the continuous development of anti-inflammatory drugs, certain factors are even indicators for the evaluation of anti-inflammatory effects and targets for treatment. For example, IL-6 has been rapidly gaining attention as a key targeted by the atherosclerosis research community (97). The anti-inflammatory drug ziltivekimab was effective in reducing IL-6 and subsequent hsCRP levels in patients with chronic kidney disease (98). Anti-inflammatory treatment with canakinumab targeting the interleukin-1β (IL-1β) innate immune pathway resulted in lower hsCRP levels and a significantly lower recurrence rate of cardiovascular events in the canakinumab group compared to the placebo group (99). Unlike canakinumab, which selectively inhibits IL-1β, the anti-inflammatory properties of colchicine involve multiple cellular and molecular mechanisms, and the use of small doses of colchicine reduces the composite risk of CV death, spontaneous myocardial infarction, ischemic stroke, or ischemia-driven coronary revascularization in patients with CAD (100).
This study involved pooling evidence from high-quality studies with long durations, large sample sizes, and cardiovascular disease occurrence as an endpoint; furthermore, most of the included studies adjusting for important confounders, including age, sex, BMI, smoking and alcohol consumption, and cholesterol. Multiple inflammatory factors were investigated in this study, and the use of these inflammatory markers in conjunction with each other or in combination with other risk factors may be a way forward in future CVD prediction. For example, in one of the studies, patients in the highest tertile with respect to a specific set of 3 inflammatory markers exhibited an extremely high risk of CVD (39). However, whether the multiple biomarker strategy is superior to the single biomarker strategy still needs further clarification (101). Despite these strengths, it is undeniable that our study has certain limitations. As described in the statistical analysis section, we were unable to include all studies of the same type to ensure reliable data, which inevitably led to some loss and waste of information. Similar to many meta-analyses, the large sample size of this study may have statistically inflated the estimate of heterogeneity (102, 103). In addition, most of the studies conducted in this area were in Europe and the United States, so the lack of findings from Asia, Africa, and Latin America in our included studies suggests a need for further research in the future.
Conclusion
In conclusion, FIB, IL-6, CRP, and Gal-3 are positively associated with the development of CVD in the general adult population and are risk factors for CVD. Combined consideration of the levels of these inflammatory factor can help in the prediction of future cardiovascular disease.
Funding Statement
This study is supported by Key Research and Development Projects of Ningxia Hui Autonomous Region (grant no. 2021BEG02030).
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Author contributions
YL, ZL and SG: constructed the design of the meta-analysis. YL: was involved in data collection, analysis and drafting of the manuscript with the assistance of SG, HX, NZ and MH. SG and ZL: critically reviewed the manuscript. ZL and YL: had primary responsibility for final content. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Collins DR, Tompson AC, Onakpoya IJ, Roberts N, Ward AM, Heneghan CJ. Global cardiovascular risk assessment in the primary prevention of cardiovascular disease in adults: systematic review of systematic reviews. BMJ Open. (2017) 7:e13650. 10.1136/bmjopen-2016-013650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the gbd 2019 study. J Am Coll Cardiol. (2020) 76:2982–3021. 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naghavi M, Abajobir AA, Abbafati C, Abbas KM, Abd-Allah F, Abera SF, et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the global burden of disease study 2016. Lancet. (2017) 390:1151–210. 10.1016/S0140-6736(17)32152-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stec JJ, Silbershatz H, Tofler GH, Matheney TH, Sutherland P, Lipinska I, et al. Association of fibrinogen with cardiovascular risk factors and cardiovascular disease in the framingham offspring population. Circulation. (2000) 102:1634–8. 10.1161/01.cir.102.14.1634 [DOI] [PubMed] [Google Scholar]
- 5.Folsom AR, Rosamond WD, Shahar E, Cooper LS, Aleksic N, Nieto FJ, et al. Prospective study of markers of hemostatic function with risk of ischemic stroke. The atherosclerosis risk in communities (aric) study investigators. Circulation. (1999) 100:736–42. 10.1161/01.cir.100.7.736 [DOI] [PubMed] [Google Scholar]
- 6.Schrage B, Geelhoed B, Niiranen TJ, Gianfagna F, Vishram-Nielsen J, Costanzo S, et al. Comparison of cardiovascular risk factors in European population cohorts for predicting atrial fibrillation and heart failure, their subsequent onset, and death. J Am Heart Assoc. (2020) 9:e15218. 10.1161/JAHA.119.015218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao Z, Liu Z, Wang R, Zheng Y, Li H, Yang L. Galectin-3 is a potential mediator for atherosclerosis. J Immunol Res. (2020) 2020:5284728. 10.1155/2020/5284728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papaspyridonos M, McNeill E, de Bono JP, Smith A, Burnand KG, Channon KM, et al. Galectin-3 is an amplifier of inflammation in atherosclerotic plaque progression through macrophage activation and monocyte chemoattraction. Arterioscler Thromb Vasc Biol. (2008) 28:433–40. 10.1161/ATVBAHA.107.159160 [DOI] [PubMed] [Google Scholar]
- 9.Ridkero PM. C-reactive protein: eighty years from discovery to emergence as a major risk marker for cardiovascular disease. Clin Chem. (2009) 55:209–15. 10.1373/clinchem.2008.119214 [DOI] [PubMed] [Google Scholar]
- 10.Aguilar D, Sun C, Hoogeveen RC, Nambi V, Selvin E, Matsushita K, et al. Levels and change in galectin-3 and association with cardiovascular events: the aric study. J Am Heart Assoc. (2020) 9:e15405. 10.1161/JAHA.119.015405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subirana I, Fitó M, Diaz O, Vila J, Francés A, Delpon E, et al. Prediction of coronary disease incidence by biomarkers of inflammation, oxidation, and metabolism. Sci Rep. (2018) 8:3191. 10.1038/s41598-018-21482-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silverman MG, Patel B, Blankstein R, Lima JAC, Blumenthal RS, Nasir K, et al. Impact of race, ethnicity, and multimodality biomarkers on the incidence of new-onset heart failure with preserved ejection fraction (from the multi-ethnic study of atherosclerosis). Am J Cardiol. (2016) 117:1474–81. 10.1016/j.amjcard.2016.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seven E, Husemoen LL, Sehested TS, Ibsen H, Wachtell K, Linneberg A, et al. Adipocytokines, c-reactive protein, and cardiovascular disease: a population-based prospective study. PLoS One. (2015) 10:e128987. 10.1371/journal.pone.0128987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Everett BM, Ridker PM, Cook NR, Pradhan AD. Usefulness of b-type natriuretic peptides to predict cardiovascular events in women (from the women’s health study). Am J Cardiol. (2015) 116:532–7. 10.1016/j.amjcard.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Appiah D, Schreiner PJ, MacLehose RF, Folsom AR. Association of plasma gamma ‘fibrinogen with incident cardiovascular disease the atherosclerosis risk in communities (aric) study. Arterioscler Thromb Vasc Biol. (2015) 35:2700–6. 10.1161/ATVBAHA.115.306284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jagodzinski A, Havulinna AS, Appelbaum S, Zeller T, Jousilahti P, Skytte-Johanssen S, et al. Predictive value of galectin-3 for incident cardiovascular disease and heart failure in the population-based finrisk 1997 cohort. Int J Cardiol. (2015) 192:33–9. 10.1016/j.ijcard.2015.05.040 [DOI] [PubMed] [Google Scholar]
- 17.Davidson KW, Schwartz JE, Kirkland SA, Mostofsky E, Fink D, Guernsey D, et al. Relation of inflammation to depression and incident coronary heart disease [from the Canadian Nova Scotia health survey (nshs95) prospective population study]. Am J Cardiol. (2009) 103:755–61. 10.1016/j.amjcard.2008.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith FB, Lee AJ, Fowkes FG, Price JF, Rumley A, Lowe GD. Hemostatic factors as predictors of ischemic heart disease and stroke in the Edinburgh artery study. Arterioscler Thromb Vasc Biol. (1997) 17:3321–5. 10.1161/01.atv.17.11.3321 [DOI] [PubMed] [Google Scholar]
- 19.Danesh J, Lewington S, Thompson SG, Lowe GD, Collins R, Kostis JB, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. (2005) 294:1799–809. 10.1001/jama.294.14.1799 [DOI] [PubMed] [Google Scholar]
- 20.Shivappa N, Godos J, Hebert JR, Wirth MD, Piuri G, Speciani AF, et al. Dietary inflammatory index and cardiovascular risk and mortality-a meta-analysis. Nutrients. (2018) 10(2):200. 10.3390/nu10020200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji M, Hong X, Chen M, Chen T, Wang J, Zhang N. Dietary inflammatory index and cardiovascular risk and mortality: a meta-analysis of cohort studies. Medicine. (2020) 99:e20303. 10.1097/MD.0000000000020303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonopoulos AS, Angelopoulos A, Papanikolaou P, Simantiris S, Oikonomou EK, Vamvakaris K, et al. Biomarkers of vascular inflammation for cardiovascular risk prognostication: a meta-analysis. JACC Cardiovasc Imaging. (2022) 15:460–71. 10.1016/j.jcmg.2021.09.014 [DOI] [PubMed] [Google Scholar]
- 23.Cai X, Zheng S, Liu Y, Zhang Y, Lu J, Huang Y. Nonalcoholic fatty liver disease is associated with increased risk of atrial fibrillation. Liver Int. (2020) 40:1594–600. 10.1111/liv.14461 [DOI] [PubMed] [Google Scholar]
- 24.Cai X, Sun L, Liu X, Zhu H, Zhang Y, Zheng S, et al. Non-alcoholic fatty liver disease is associated with increased risk of chronic kidney disease. Ther Adv Chronic Dis. (2021) 12:364072937. 10.1177/20406223211024361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jatho A, Cambia JM, Myung SK. Consumption of artificially sweetened soft drinks and risk of gastrointestinal cancer: a meta-analysis of observational studies. Public Health Nutr. (2021) 24:6122–36. 10.1017/S136898002100104X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JH, Chai YJ, Yi KH. Effect of cigarette smoking on thyroid cancer: meta-analysis. Endocrinol Metab. (2021) 36:590–8. 10.3803/EnM.2021.954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. (2003) 327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. PMID: 7786990. [PubMed] [Google Scholar]
- 29.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. (1997) 315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scarabin PY, Aillaud MF, Amouyel P, Evans A, Luc G, Ferrières J, et al. Associations of fibrinogen, factor vii and pai-1 with baseline findings among 10,500 male participants in a prospective study of myocardial infarction–the prime study. Prospective epidemiological study of myocardial infarction. Thromb Haemost. (1998) 80:749–56. PMID: 9843166. [PubMed] [Google Scholar]
- 31.Koenig W, Sund M, Fröhlich M, Fischer HG, Löwel H, Döring A, et al. C-reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the monica (monitoring trends and determinants in cardiovascular disease) augsburg cohort study, 1984–1992. Circulation. (1999) 99:237–42. 10.1161/01.CIR.99.2.237 [DOI] [PubMed] [Google Scholar]
- 32.Scarabin PY, Arveiler D, Amouyel P, Dos SC, Evans A, Luc G, et al. Plasma fibrinogen explains much of the difference in risk of coronary heart disease between France and northern Ireland. The prime study. Atherosclerosis. (2003) 166:103–9. 10.1016/s0021-9150(02)00309-x [DOI] [PubMed] [Google Scholar]
- 33.Engström G, Hedblad B, Rosvall M, Janzon L, Lindgärde F. Occupation, marital status, and low-grade inflammation: mutual confounding or independent cardiovascular risk factors? Arterioscler Thromb Vasc Biol. (2006) 26:643–8. 10.1161/01.ATV.0000200100.14612.bb [DOI] [PubMed] [Google Scholar]
- 34.Jeppesen J, Hansen TW, Olsen MH, Rasmussen S, Ibsen H, Torp-Pedersen C, et al. C-reactive protein, insulin resistance and risk of cardiovascular disease: a population-based study. Eur J Cardiovasc Prev Rehabil. (2008) 15:594–8. 10.1097/HJR.0b013e328308bb8b [DOI] [PubMed] [Google Scholar]
- 35.Smith JG, Newton-Cheh C, Almgren P, Struck J, Morgenthaler NG, Bergmann A, et al. Assessment of conventional cardiovascular risk factors and multiple biomarkers for the prediction of incident heart failure and atrial fibrillation. J Am Coll Cardiol. (2010) 56:1712–9. 10.1016/j.jacc.2010.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tunstall-Pedoe H, Peters S, Woodward M, Struthers AD, Belch J. Twenty-year predictors of peripheral arterial disease compared with coronary heart disease in the scottish heart health extended cohort (SHHEC) [published correction appears in J Am Heart Assoc. 2017 Dec 23;6(12):e004215]. J Am Heart Assoc. (2017) 6(9):e005967. 10.1161/JAHA.117.005967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Magnussen C, Niiranen TJ, Ojeda FM, Gianfagna F, Blankenberg S, Vartiainen E, et al. Sex-specific epidemiology of heart failure risk and mortality in Europe results from the biomarcare consortium. Jacc Heart Fail. (2019) 7:204–13. 10.1016/j.jchf.2018.08.008 [DOI] [PubMed] [Google Scholar]
- 38.Fernandez C, Rysä J, Ström K, Nilsson J, Engström G, Orho-Melander M, et al. Circulating protein biomarkers predict incident hypertensive heart failure independently of n-terminal pro-b-type natriuretic peptide levels. Esc Heart Fail. (2020) 7:1891–9. 10.1002/ehf2.12757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, et al. Inflammatory markers and onset of cardiovascular events: results from the health abc study. Circulation. (2003) 108:2317–22. 10.1161/01.CIR.0000097109.90783.FC [DOI] [PubMed] [Google Scholar]
- 40.Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, et al. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the framingham heart study. Circulation. (2003) 107:1486–91. 10.1161/01.cir.0000057810.48709.f6 [DOI] [PubMed] [Google Scholar]
- 41.Schnabel RB, Larson MG, Yamamoto JF, Kathiresan S, Rong J, Levy D, et al. Relation of multiple inflammatory biomarkers to incident atrial fibrillation. Am J Cardiol. (2009) 104:92–6. 10.1016/j.amjcard.2009.02.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schnabel RB, Yin X, Larson MG, Yamamoto JF, Fontes JD, Kathiresan S, et al. Multiple inflammatory biomarkers in relation to cardiovascular events and mortality in the community. Arterioscler Thromb Vasc Biol. (2013) 33:1728–33. 10.1161/ATVBAHA.112.301174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ho JE, Yin X, Levy D, Vasan RS, Magnani JW, Ellinor PT, et al. Galectin 3 and incident atrial fibrillation in the community. Am Heart J. (2014) 167:121–729. 10.1016/j.ahj.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yin X, Subramanian S, Hwang SJ, O'Donnell CJ, Fox CS, Courchesne P, et al. Protein biomarkers of new-onset cardiovascular disease: prospective study from the systems approach to biomarker research in cardiovascular disease initiative. Arterioscler Thromb Vasc Biol. (2014) 34:939–45. 10.1161/ATVBAHA.113.302918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daniels LB, Clopton P, Laughlin GA, Maisel AS, Barrett-Connor E. Galectin-3 is independently associated with cardiovascular mortality in community-dwelling older adults without known cardiovascular disease: the rancho bernardo study. Am Heart J. (2014) 167:674–82. 10.1016/j.ahj.2013.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.AbouEzzeddine OF, McKie PM, Scott CG, Rodeheffer RJ, Chen HH, Michael FG, et al. Biomarker-based risk prediction in the community. Eur J Heart Fail. (2016) 18:1342–50. 10.1002/ejhf.663 [DOI] [PubMed] [Google Scholar]
- 47.Dawood FZ, Judd S, Howard VJ, Limdi NA, Meschia JF, Cushman M, et al. High-sensitivity c-reactive protein and risk of stroke in atrial fibrillation (from the reasons for geographic and racial differences in stroke study). Am J Cardiol. (2016) 118:1826–30. 10.1016/j.amjcard.2016.08.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Appiah D, Heckbert SR, Cushman M, Psaty BM, Folsom AR. Lack of association of plasma gamma prime (γ’) fibrinogen with incident cardiovascular disease. Thromb Res. (2016) 143:50–2. 10.1016/j.thromres.2016.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Boer RA, Nayor M, DeFilippi CR, Enserro D, Bhambhani V, Kizer JR, et al. Association of cardiovascular biomarkers with incident heart failure with preserved and reduced ejection fraction. JAMA Cardiol. (2018) 3:215–24. 10.1001/jamacardio.2017.4987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghorbani A, Bhambhani V, Christenson RH, Meijers WC, de Boer RA, Levy D, et al. Longitudinal change in galectin-3 and incident cardiovascular outcomes. J Am Coll Cardiol. (2018) 72:3246–54. 10.1016/j.jacc.2018.09.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ho JE, Lyass A, Courchesne P, Chen G, Liu C, Yin X, et al. Protein biomarkers of cardiovascular disease and mortality in the community. J Am Heart Assoc. (2018) 7(14):e008108. 10.1161/JAHA.117.008108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leening MJG, Cook NR, Franco OH, Manson JE, Lakshminarayan K, LaMonte MJ, et al. Comparison of cardiovascular risk factors for coronary heart disease and stroke type in women. J Am Heart Assoc. (2018) 7(19):e007514. 10.1161/JAHA.117.007514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chei CL, Yamagishi K, Kitamura A, Kiyama M, Imano H, Ohira T, et al. C-reactive protein levels and risk of stroke and its subtype in Japanese: the circulatory risk in communities study (circs). Atherosclerosis. (2011) 217:187–93. 10.1016/j.atherosclerosis.2011.03.001 [DOI] [PubMed] [Google Scholar]
- 54.Welsh P, Hart C, Papacosta O, Preiss D, McConnachie A, Murray H, et al. Prediction of cardiovascular disease risk by cardiac biomarkers in 2 United Kingdom cohort studies does utility depend on risk thresholds for treatment? Hypertension. (2016) 67:309–15. 10.1161/HYPERTENSIONAHA.115.06501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Engström G, Hedblad B, Tydén P, Lindgärde F. Inflammation-sensitive plasma proteins are associated with increased incidence of heart failure: a population-based cohort study. Atherosclerosis. (2009) 202:617–22. 10.1016/j.atherosclerosis.2008.05.038 [DOI] [PubMed] [Google Scholar]
- 56.Sato S, Iso H, Noda H, Kitamura A, Imano H, Kiyama M, et al. Plasma fibrinogen concentrations and risk of stroke and its subtypes among Japanese men and women. Stroke. (2006) 37:2488–92. 10.1161/01.STR.0000242473.13884.8e [DOI] [PubMed] [Google Scholar]
- 57.Wattanakit K, Folsom AR, Chambless LE, Nieto FJ. Risk factors for cardiovascular event recurrence in the atherosclerosis risk in communities (aric) study. Am Heart J. (2005) 149:606–12. 10.1016/j.ahj.2004.07.019 [DOI] [PubMed] [Google Scholar]
- 58.Nafari A, Mohammadifard N, Haghighatdoost F, Nasirian S, Najafian J, Sadeghi M, et al. High-sensitivity c-reactive protein and low-density lipoprotein cholesterol association with incident of cardiovascular events: Isfahan cohort study. BMC Cardiovasc Disord. (2022) 22:241. 10.1186/s12872-022-02663-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee W, Yim HW, Lee Y. Cohort study of long working hours and increase in blood high-sensitivity c-reactive protein (hscrp) concentration: mechanisms of overwork and cardiovascular disease. J Occup Health. (2022) 64:e12359. 10.1002/1348-9585.12359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohen AJ, Teramoto K, Claggett B, Buckley LEO, Solomon S, Ballantyne C, et al. Mid- to late-life inflammation and risk of cardiac dysfunction, hfpef and hfref in late life. J Card Fail. (2021) 27:1382–92. 10.1016/j.cardfail.2021.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koosha P, Roohafza H, Sarrafzadegan N, Vakhshoori M, Talaei M, Sheikhbahaei E, et al. High sensitivity c-reactive protein predictive value for cardiovascular disease: a nested case control from Isfahan cohort study (ics). Glob Heart. (2020) 15:3. 10.5334/gh.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mo J, Chen Z, Xu J, Wang A, Meng X, Zhao X, et al. The impact of the cumulative burden of ldl-c and hs-crp on cardiovascular risk: a prospective, population-based study. Aging (Albany NY). (2020) 12:11990–2001. 10.18632/aging.103365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quispe R, Michos ED, Martin SS, Puri R, Toth PP, Al SJ, et al. High-sensitivity c-reactive protein discordance with atherogenic lipid measures and incidence of atherosclerotic cardiovascular disease in primary prevention: the aric study. J Am Heart Assoc. (2020) 9:e13600. 10.1161/JAHA.119.013600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. (2000) 101:1767–72. 10.1161/01.cir.101.15.1767 [DOI] [PubMed] [Google Scholar]
- 65.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. (2000) 342:836–43. 10.1056/NEJM200003233421202 [DOI] [PubMed] [Google Scholar]
- 66.Kaptoge S, Seshasai SR, Gao P, Freitag DF, Butterworth AS, Borglykke A, et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J. (2014) 35:578–89. 10.1093/eurheartj/eht367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ridker PM. From c-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ Res. (2016) 118:145–56. 10.1161/CIRCRESAHA.115.306656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun RR, Lu L, Liu M, Cao Y, Li XC, Liu H, et al. Biomarkers and heart disease. Eur Rev Med Pharmacol Sci. (2014) 18:2927–35. PMID: 25339488. [PubMed] [Google Scholar]
- 69.Mortensen MB, Fuster V, Muntendam P, Mehran R, Baber U, Sartori S, et al. Negative risk markers for cardiovascular events in the elderly. J Am Coll Cardiol. (2019) 74:1–11. 10.1016/j.jacc.2019.04.049 [DOI] [PubMed] [Google Scholar]
- 70.Kraler S, Wenzl FA, Georgiopoulos G, Obeid S, Liberale L, von Eckardstein A, et al. Soluble lectin-like oxidized low-density lipoprotein receptor-1 predicts premature death in acute coronary syndromes. Eur Heart J. (2022) 43:1849–60. 10.1093/eurheartj/ehac143 [DOI] [PubMed] [Google Scholar]
- 71.Pirillo A, Catapano AL. Soluble lectin-like oxidized low density lipoprotein receptor-1 as a biochemical marker for atherosclerosis-related diseases. Dis Markers. (2013) 35:413–8. 10.1155/2013/716325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eddy AC, Trask AJ. Growth differentiation factor-15 and its role in diabetes and cardiovascular disease. Cytokine Growth Factor Rev. (2021) 57:11–8. 10.1016/j.cytogfr.2020.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaiser H, Wang X, Kvist-Hansen A, Krakauer M, Gortz PM, McCauley BD, et al. Biomarkers of subclinical atherosclerosis in patients with psoriasis. Sci Rep. (2021) 11:21438. 10.1038/s41598-021-00999-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Bemmel T, Gussekloo J, Westendorp RG, Blauw GJ. In a population-based prospective study, no association between high blood pressure and mortality after age 85 years. J Hypertens. (2006) 24:287–92. 10.1097/01.hjh.0000200513.48441.8e [DOI] [PubMed] [Google Scholar]
- 75.Kannel WB. Coronary heart disease risk factors in the elderly. Am J Geriatr Cardiol. (2002) 11:101–7. 10.1111/j.1076-7460.2002.00995.x [DOI] [PubMed] [Google Scholar]
- 76.de Ruijter W, Westendorp RG, Assendelft WJ, den Elzen WP, de Craen AJ, le Cessie S, et al. Use of framingham risk score and new biomarkers to predict cardiovascular mortality in older people: population based observational cohort study. Br Med J. (2009) 338:a3083. 10.1136/bmj.a3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kritchevsky SB, Cesari M, Pahor M. Inflammatory markers and cardiovascular health in older adults. Cardiovasc Res. (2005) 66:265–75. 10.1016/j.cardiores.2004.12.026 [DOI] [PubMed] [Google Scholar]
- 78.Gaye B, Tafflet M, Arveiler D, Montaye M, Wagner A, Ruidavets JB, et al. Ideal cardiovascular health and incident cardiovascular disease: heterogeneity across event subtypes and mediating effect of blood biomarkers: the PRIME study [published correction appears in J Am Heart Assoc. 2017 Dec 23;6(12):e004201]. J Am Heart Assoc. (2017) 6(10):e006389. 10.1161/JAHA.117.006389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baccouche BM, Mahmoud MA, Nief C, Patel K, Natterson-Horowitz B. Galectin-3 is associated with heart failure incidence: a meta-analysis. Curr Cardiol Rev. (2023) 19(3):50–62. 10.2174/1573403X19666221117122012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bustamante A, Simats A, Vilar-Bergua A, Garcia-Berrocoso T, Montaner J. Blood/brain biomarkers of inflammation after stroke and their association with outcome: from c-reactive protein to damage-associated molecular patterns. Neurotherapeutics. (2016) 13:671–84. 10.1007/s13311-016-0470-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Emsley HC, Smith CJ, Gavin CM, Georgiou RF, Vail A, Barberan EM, et al. An early and sustained peripheral inflammatory response in acute ischaemic stroke: relationships with infection and atherosclerosis. J Neuroimmunol. (2003) 139:93–101. 10.1016/s0165-5728(03)00134-6 [DOI] [PubMed] [Google Scholar]
- 82.Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Chambless LE, et al. Lipoprotein-associated phospholipase a2, high-sensitivity c-reactive protein, and risk for incident ischemic stroke in middle-aged men and women in the atherosclerosis risk in communities (aric) study. Arch Intern Med. (2005) 165:2479–84. 10.1001/archinte.165.21.2479 [DOI] [PubMed] [Google Scholar]
- 83.Elkind MS, Tai W, Coates K, Paik MC, Sacco RL. High-sensitivity c-reactive protein, lipoprotein-associated phospholipase a2, and outcome after ischemic stroke. Arch Intern Med. (2006) 166:2073–80. 10.1001/archinte.166.19.2073 [DOI] [PubMed] [Google Scholar]
- 84.Montaner J, Fernandez-Cadenas I, Molina CA, Ribo M, Huertas R, Rosell A, et al. Poststroke c-reactive protein is a powerful prognostic tool among candidates for thrombolysis. Stroke. (2006) 37:1205–10. 10.1161/01.STR.0000217744.89208.4e [DOI] [PubMed] [Google Scholar]
- 85.Wang XY, Zhang F, Zhang C, Zheng LR, Yang J. The biomarkers for acute myocardial infarction and heart failure. Biomed Res Int. (2020) 2020:2018035. 10.1155/2020/2018035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaminska J, Koper OM, Siedlecka-Czykier E, Matowicka-Karna J, Bychowski J, Kemona H. The utility of inflammation and platelet biomarkers in patients with acute coronary syndromes. Saudi J Biol Sci. (2018) 25:1263–71. 10.1016/j.sjbs.2016.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kannel WB, Wolf PA, Castelli WP, D'Agostino RB. Fibrinogen and risk of cardiovascular disease. The framingham study. JAMA. (1987) 258:1183–6. PMID: 3626001. [PubMed] [Google Scholar]
- 88.Ma J, Hennekens CH, Ridker PM, Stampfer MJ. A prospective study of fibrinogen and risk of myocardial infarction in the physicians’ health study. J Am Coll Cardiol. (1999) 33:1347–52. 10.1016/s0735-1097(99)00007-8 [DOI] [PubMed] [Google Scholar]
- 89.Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, et al. Acute myocardial infarction in women: a scientific statement from the American heart association. Circulation. (2016) 133:916–47. 10.1161/CIR.0000000000000351 [DOI] [PubMed] [Google Scholar]
- 90.Dreyer RP, Beltrame JF, Tavella R, Air T, Hoffmann B, Pati PK, et al. Evaluation of gender differences in door-to-balloon time in st-elevation myocardial infarction. Heart Lung Circ. (2013) 22:861–9. 10.1016/j.hlc.2013.03.078 [DOI] [PubMed] [Google Scholar]
- 91.Shaw LJ, Bairey MC, Pepine CJ, Reis SE, Bittner V, Kelsey SF, et al. Insights from the nhlbi-sponsored women’s ischemia syndrome evaluation (wise) study: part i: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol. (2006) 47:S4–20. 10.1016/j.jacc.2005.01.072 [DOI] [PubMed] [Google Scholar]
- 92.Kottilil S, Mathur P. The influence of inflammation on cardiovascular disease in women. Front Glob Womens Health. (2022) 3:979708. 10.3389/fgwh.2022.979708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lau ES, Paniagua SM, Guseh JS, Bhambhani V, Zanni MV, Courchesne P, et al. Sex differences in circulating biomarkers of cardiovascular disease. J Am Coll Cardiol. (2019) 74:1543–53. 10.1016/j.jacc.2019.06.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Giordano S, Hage FG, Xing D, Chen YF, Allon S, Chen C, et al. Estrogen and cardiovascular disease: is timing everything? Am J Med Sci. (2015) 350:27–35. 10.1097/MAJ.0000000000000512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Veeranna V, Zalawadiya SK, Niraj A, Kumar A, Ference B, Afonso L. Association of novel biomarkers with future cardiovascular events is influenced by ethnicity: results from a multi-ethnic cohort. Int J Cardiol. (2013) 166:487–93. 10.1016/j.ijcard.2011.11.034 [DOI] [PubMed] [Google Scholar]
- 96.Yang X, Li J, Hu D, Chen J, Li Y, Huang J, et al. Predicting the 10-year risks of atherosclerotic cardiovascular disease in Chinese population: the China-par project (prediction for ascvd risk in China). Circulation. (2016) 134:1430–40. 10.1161/CIRCULATIONAHA.116.022367 [DOI] [PubMed] [Google Scholar]
- 97.Ridker PM, Rane M. Interleukin-6 signaling and anti-interleukin-6 therapeutics in cardiovascular disease. Circ Res. (2021) 128:1728–46. 10.1161/CIRCRESAHA.121.319077 [DOI] [PubMed] [Google Scholar]
- 98.Ridker PM. From rescue to zeus: will interleukin-6 inhibition with ziltivekimab prove effective for cardiovascular event reduction? Cardiovasc Res. (2021) 117:e138–40. 10.1093/cvr/cvab231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. (2017) 377:1119–31. 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 100.Kraler S, Wenzl FA, Luscher TF. Repurposing colchicine to combat residual cardiovascular risk: the lodoco2 trial. Eur J Clin Invest. (2020) 50:e13424. 10.1111/eci.13424 [DOI] [PubMed] [Google Scholar]
- 101.Pareek M, Bhatt DL, Vaduganathan M, Biering-Sorensen T, Qamar A, Diederichsen AC, et al. Single and multiple cardiovascular biomarkers in subjects without a previous cardiovascular event. Eur J Prev Cardiol. (2017) 24:1648–59. 10.1177/2047487317717065 [DOI] [PubMed] [Google Scholar]
- 102.Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta-analysis: i(2) is not an absolute measure of heterogeneity. Res Synth Methods. (2017) 8:5–18. 10.1002/jrsm.1230 [DOI] [PubMed] [Google Scholar]
- 103.Rucker G, Schwarzer G, Carpenter JR, Schumacher M. Undue reliance on i(2) in assessing heterogeneity may mislead. BMC Med Res Methodol. (2008) 8:79. 10.1186/1471-2288-8-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.