Abstract
Immune severe aplastic anemia (SAA) is characterized by pancytopenia and immune-mediated bone marrow destruction. SAA may be treated with hematopoietic stem cell transplantation (HSCT) or immunosuppressive therapy (IST). However, 30% of patients treated with IST relapse. We previously reported a clinical trial of alemtuzumab in which more than half of 25 relapsed SAA patients 56% responded hematologically. Here, we present long-term results of a total of 42 patients. Participants with SAA who had previously completed antithymocyte globulin (ATG)-based IST, but had relapsed, were enrolled on this study. Alemtuzumab was administered intravenously (IV) (n=28) or subcutaneously (SC) (n=14). The primary endpoint was hematologic response at 6 months. Secondary endpoints included relapse, clonal evolution, and survival. This trial was registered at clinicaltrials.gov (NCT00195624). Patients were enrolled over 9 years, with median follow-up of 6 years. Median age was 32 years, with 57% being female. At 6 months, 18 patients (43%) achieved response; 15 (54%) of those who received IV compared with 3 (21%) who received SC therapy. Seven patients (17%) had durable long-term response without need for subsequent AA-directed therapy or HSCT at 5 years post alemtuzumab. Nine patients had clonal evolution, with high-risk evolution occurring in 6. Overall survival was 67% at median follow-up of 6 years. Prolonged iatrogenic immunosuppression was observed as long as 2 years after alemtuzumab administration. Alemtuzumab induces responses in relapsed SAA, some of which are durable long-term. However, immunosuppression can persist for years, requiring long-term monitoring.
Keywords: aplastic anemia, bone marrow failure, alemtuzumab
Introduction
Immune aplastic anemia (AA) is characterized by pancytopenia and hypocellular bone marrow (BM). Although the exact etiology is not known, an immune-mediated mechanism is inferred from laboratory studies and clinical evidence of hematologic recovery following immunosuppression1. Hematopoietic stem cell transplant (HSCT) is curative, but immunosuppressive therapy (IST) is a robust alternative first-line therapy with long term responses in many patients. Standard front-line IST is usually horse antithymocyte globulin (hATG) and cyclosporine (CSA), as well as eltrombopag (EPAG)2, a thrombopoietin agonist. This triple-therapy regimen produces hematologic responses in over 80% of patients, but 20% are refractory, and 30-40% relapse after their initial response3,4. Persistent or recurrent pancytopenia can lead to life-threatening complications including risk of serious infection or bleeding. Second-line non-HSCT therapies for patients include repeat IST with rabbit ATG (rATG), thrombopoietin agonists, mycophenolate mofetil, and androgens, with varying rates of response, relapse, clonal evolution, and survival5.
Alemtuzumab is a humanized IgG1 monoclonal antibody that binds to CD52, an antigen which is highly expressed in T and B lymphocytes. Immunosuppression with alemtuzumab can result in prolonged lymphocyte depletion, with lesser effects on monocytes, natural killer cells, dendritic cells, and neutrophils6. We hypothesized that alemtuzumab might be effective in severe AA (SAA), due to its more potent and targeted immunosuppressive effect compared with hATG. We investigated alemtuzumab as monotherapy from 2005 to 2014 in three clinical trials in treatment-naïve (NCT00260689), relapsed (NCT00195624), and refractory (NCT00065260) SAA. Treatment-naïve patients treated with alemtuzumab had a low response (19%) compared to hATG and rATG, and the alemtuzumab arm was discontinued early. However, alemtuzumab was effective in patients with refractory or relapsed disease. At 6 months, there were improved hematologic response and 3-year survival (37% and 83%, respectively) in patients with refractory SAA treated with alemtuzumab, compared with rATG (33% and 60%, respectively).
In the relapsed SAA setting, in an initial cohort of 25 patients, alemtuzumab led to a hematologic response of 56% at 6 months and 86% overall survival (OS) at 3 years7. Here, we report long-term results of this clinical trial of alemtuzumab monotherapy in relapsed SAA, including a total of 42 patients, an additional 17 from the initial 25 reported. We evaluated hematologic response annually for five years, robustness of response, further relapse following initial response to alemtuzumab, long-term transfusion dependence, need for further immunosuppression or HSCT, clonal evolution to myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML), and survival. This trial is registered at clinicaltrials.gov (NCT00195624).
Methods
Patients
Enrollment was described in the initial report of this trial7. Briefly, patients were enrolled from November 2005 to August 2014 at the Warren Grant Magnuson Clinical Center of the National Institutes of Health. All adult patients or parents (or legal guardians) of children < 18 years of age signed informed consent following the Declaration of Helsinki, according to protocols approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute (NHLBI). SAA diagnosis was based on modified Camitta criteria defined as BM cellularity < 30% and severe pancytopenia with at least 2 of the following peripheral blood count criteria: absolute neutrophil count (ANC) < 0.5×109/L, absolute reticulocyte count (ARC) < 60×109/L, or platelet (PLT) count < 20×109/L8. Very SAA (VSAA) was defined as ANC ≤ 0.2×109/L. Patients enrolled on this study had a known diagnosis of SAA based on the above definition but had relapsed after an initial hematologic response to a course of hATG- or rATG-based IST and were aged ≥ 2 years old with weight > 12 kg. Relapse for inclusion in this study was defined as a drop in blood counts requiring further AA-directed therapy. Exclusion criteria were creatinine > 2.5 mg/dL, underlying carcinoma, a diagnosis of Fanconi anemia, prior history of alemtuzumab, human immunodeficiency virus seropositivity, evidence of a clonal disorder on BM cytogenetics, pregnancy, inability to understand the investigational nature of the study, or significant comorbidities such that imminent death was likely.
BM biopsy and aspirate for morphology and cytogenetics were performed prior to enrollment, 6 and 12 months after therapy, and then yearly. Children and young adults (< 40 years of age) had chromosome breakage studies to exclude Fanconi anemia. All patients were tested for paroxysmal nocturnal hemoglobinuria (PNH) by flow cytometry9; the presence of a clone was defined as the absence of glycosylphosphatidylinositol-anchored surface proteins in > 1% of neutrophils or RBCs.
Study Design
In relapsed SAA patients, alemtuzumab was administered in a non-randomized, single-arm study. If relapse reoccurred after successful treatment with alemtuzumab, CSA could be instituted on protocol (Table S1). Patients deemed non-responders at 6 months, and those with high-risk evolution, or who relapsed and required AA directed therapy other than CSA were taken off study. Responders remained on study up to 5 years.
Immunosuppressive Regimens
Alemtuzumab (Campath; Genzyme) was administered after a 1 mg test dose, at 10 mg daily for 10 days as an intravenous (IV) or subcutaneous (SC) infusion over 2 hours. Children < 50 kg received alemtuzumab 0.2 mg/kg/d for 10 days (not to exceed 10 mg/day). Alemtuzumab was administered IV from 2005 to 2011 in 28 patients and subcutaneously (SC) from 2012 to 2014 in 14 patients. CSA was not co-administered with alemtuzumab.
As prophylaxis for Pneumocystis jiroveci (PJP) pneumonia, all patients received aerosolized pentamidine monthly for at least 6 months. Prophylaxis for PJP was continued until CD4+ T cells were > 0.2×109/L. Trimethoprim/ sulfamethoxazole, dapsone or another prophylactic regimen against PJP was allowed as substitution. Ciprofloxacin 500 mg twice daily was administered as bacterial prophylaxis if ANC was < 0.2×109/L. Daily valacyclovir 500 mg daily for at least 8 weeks was used as Herpes simplex virus prophylaxis. Neither G-CSF nor prophylactic antifungal therapy were routinely administered. Because of reports of cardiotoxicity in alemtuzumab-treated patients, a 2D echocardiogram, 24-hour Holter monitoring, and troponin levels were performed before and after alemtuzumab infusion.
Study End Points
Hematologic response was defined as no longer meeting criteria for SAA8, and a robust response was defined as PLT or ARC of > 50×109/L at 6 months10. Relapse after alemtuzumab was defined as a drop in blood counts requiring further AA-directed therapy, which included re-institution of CSA, administration of another course of IST, or HSCT. As some enrolled patients’ baseline blood counts did not meet full modified Camitta criteria (ANC < 0.5×109/L, ARC < 60×109/L, PLT < 20×109/L) for SAA at time of alemtuzumab administration, in these cases, hematologic response was defined by protocol-prespecified improvements in blood counts. If baseline was ANC > 0.5×109/L, any increase in ANC of ≥ 0.5×109/L was deemed a response, while if baseline was ANC < 0.5×109/L, any increase in ANC of ≥ 0.3×109/L was deemed a response; if baseline PLT was < 70×109/L but > 50×109/L, an increase in PLT of > 30×109/L was deemed a response, while if baseline PLT < 50×109/L but >20×109/L, an increase in PLT of > 20×109/L was deemed a response; if baseline was red cell transfusion independence then an increase in hemoglobin (Hb) of 1.5g/dL was deemed a response, while if the patient was transfusion dependent then an increase in ARC to > 60×109/L was deemed a response (Full protocol details in Table S1).
The primary end point was hematologic response at 6 months. Secondary end points included hematologic response at 3 months and then at annual landmark visits for 3 years, robustness of hematologic response, relapse, clonal evolution including high-risk evolution (diagnosis of MDS by WHO definition for dysplastic morphology, or AML, or high-risk karyotype defined as chromosome 7 abnormality or complex karyotype) or low-risk evolution (isolated abnormal karyotype other than chromosome 7 or complex karyotype), and overall survival. For other long term outcomes, we also assessed need for alternative AA-directed therapy due to lack of response or relapse after alemtuzumab, and transfusion-independence, defined as the lack of requirement of transfusion at least 8 weeks following last red cell or platelet transfusion, in all patients with available clinical data.
Statistics
Patient baseline characteristics are reported as median and interquartile range for continuous variables, and count and proportion for categorical variables. Univariate logistic regression was used to assess predictors of 6-month response. Overall survival probabilities were evaluated using Kaplan-Meier estimates, as well as univariate and multivariate Cox proportional hazards models. Classification and regression tree analysis11 was performed on survival time as a method of feature selection. The Fine-Grey competing risk model12 was used for times to relapse and clonal evolution with death taken as a competing risk; cumulative incidence curves were used to display their probabilities over time. A p-value of <0.05 was considered statistically significant. All analyses were performed using R (version 4.0.3). Figures were generated using the software GraphPad Prism (version 9), R (version 4.0.3), and Microsoft Excel (version 16.0).
Results
In total, 42 patients received alemtuzumab after SAA relapse. Median age was 32 years, and 57% were female. Patients were enrolled over 9 years with median follow-up time 6 years (2180 days). Patient characteristics are described in Table 1. There were no differences in sex, age, disease severity, number of prior IST, size of PNH clones, or pre-treatment lab values in patients treated with IV or SC alemtuzumab.
Table 1.
Baseline Characteristics
| All Patients (n = 42) |
IV Administration (n = 28) |
SC Administration (n = 14) |
P-value | |
|---|---|---|---|---|
| Female Sex, n (%) | 24 (57) | 15 (54) | 9 (64) | 0.51 |
| Age (y), median (IQR) | 32.5 (22.3, 62.3) | 29.5 (22.0, 60.0) | 37.5 (23.8, 64.8) | 0.85 |
| Disease severity, n (%) | ||||
| SAA | 38 (90) | 25 (89) | 13 (93) | 1.0 |
| VSAA | 4 (10) | 3 (11) | 1 (7) | |
| Number of Prior Immunosuppressive Treatments, n (%) | ||||
| One | 31 (74) | 23 (82) | 8 (57) | 0.75 |
| Two | 9 (21) | 6 (21) | 3 (21) | |
| Three | 1 (2) | 1 (4) | 0 | |
| Four | 0 | 0 | 0 | |
| Five | 1 (2) | 0 | 1 (7) | |
| Laboratory Values, median (IQR) | ||||
| Hemoglobin (g/dL) | 8.8 (8.4, 9.2) | 8.8 (8.4, 9.2) | 8.8 (7.7, 9.2) | 0.93 |
| Platelets (x109/L) | 28.0 (17.3, 43.0) | 31.5 (19.5, 42.3) | 23.0 (10.8, 44.5) | 0.45 |
| Absolute Neutrophil Count (x109/L) | 0.8 (0.4, 1.3) | 0.7 (0.4, 1.2) | 0.8 (0.4, 1.5) | 0.62 |
| Absolute Lymphocyte Count (x109/L) | 0.2 (0.1, 0.4) | 0.2 (0.1, 0.5) | 0.3 (0.1, 0.4) | 1 |
| Absolute Reticulocyte Count (x109/L) | 29.9 (14.0, 46.8) | 30.6 (15.9, 43.7) | 21.8 (12.0, 46.8) | 0.87 |
| PNH Clone, n (%) | 19 (45) | 10 (36) | 9 (64) | 0.18 |
Abbreviations: IQR = interquartile range; IV = intravenous; PNH = paroxysmal nocturnal hemoglobinuria; SAA = severe aplastic anemia; SC = subcutaneous; VSAA = very severe aplastic anemia
Hematologic Response
The primary endpoint, hematologic response at 6 months, was achieved in 43% (n=18/42) of patients, with 67% (n=12/18) of responders demonstrating robust response (Table 2); response was achieved in 54% (n=15/28) of those who received alemtuzumab IV, compared to 21% (n=3/14) who received SC. Of the 3 patients who were previously treated with rATG, none were responders. At the 6-month assessment, 4 patients were off study and were deemed non-responders: 2 had died, and 2 had required subsequent therapy before 6-month evaluation.
Table 2.
Responses at 6 Months
| All Patients (n = 42) |
IV Administration (n = 28) |
SC Administration (n = 14) |
P-value† | |
|---|---|---|---|---|
| Overall Response, n (%) | 18 (43) | 15 (54) | 3 (21) | 0.06 |
| CR, n (%) | 1 (2) | 1 (4) | 0 | 0.1 |
| PR, n (%) | 17 (40) | 14 (50) | 3 (21) | |
| NR§, n (%) | 24 (57) | 13 (46) | 11 (79) | |
| Robust Response | 12 (67) | 10 (36) | 2 (14) | 0.3 |
ITT no response includes patients off study and dead
Fisher’s Exact Test p-value comparing IV and S/C
Abbreviations: CR = complete response; ITT = intention-to-treat; IV = intravenous; NR = no response; PR = partial response; SC = subcutaneous
Early response at 3 months occurred in 29% (n=12/42), 8 having received IV and 4 SC therapy. By 1 year, 14 of 19 patients who remained on study were responders, including 2 who responded late after 6 months. Of these, 79% (n=11/14) had received IV and 21% (n=3/14) SC treatment. By 5 years, 7 of 12 patients remaining on study were long-term responders. Detailed response data are available in Tables S2 and S3 as well as Figure S1.
Higher baseline ARC was predictive of response at 6 months, while Hb, platelet count, ANC, and presence of PNH clone >1% in neutrophils were not predictive (Table S4).
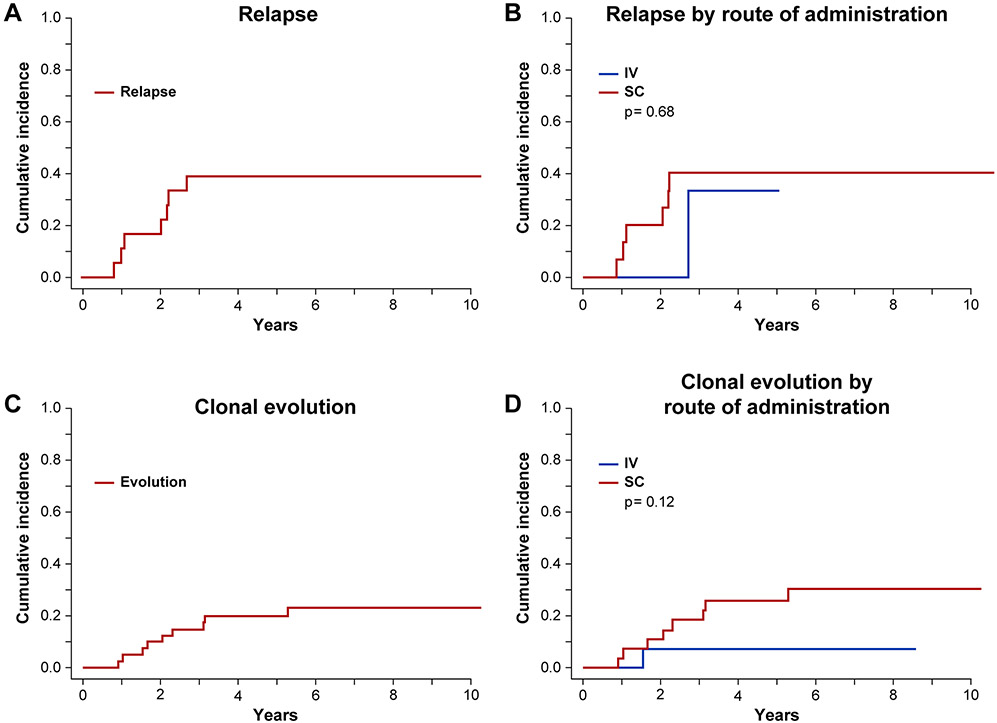
Relapse
Given low absolute numbers of responders, relapse was assessed for both IV and SC groups combined. The cumulative incidence of SAA relapse following alemtuzumab at median time of follow-up of 6 years (2180 days), was 39% (Figures 1A and 1B). Most relapses occurred within the first 2-3 years after treatment. Patients who were refractory to (n=24) or relapsed (n=8) after alemtuzumab proceeded to HSCT (n=4), other medical therapies (n=11), or both (n=10). Medical therapies included EPAG (n=13), r-ATG (n=8) CSA (n=6), androgens (n=3) or other (n=3), and were clinically determined. All patients who relapsed again after initial response to alemtuzumab received subsequent medical therapy and 38% (n=3/8) responded, while 15 patients refractory to alemtuzumab received subsequent medical therapy of whom 33% (n=5/15) responded. Subsequent therapies are detailed in Table S5.
Figure 1. Relapse and Clonal Evolution after Alemtuzumab.
Cumulative incidence curves were used to estimate the probability of relapse and clonal evolution after alemtuzumab, with death as a competing risk. Relapse and clonal evolution for the overall cohort (A, C) and divided by route of administration (B, D) are shown. The cumulative incidence of SAA relapse following alemtuzumab at median time of follow-up of 6 years (2180 days), was 39%. Clonal evolution occurred in 9 patients, with cumulative incidence of 23% at the median follow-up of 6 years (2180 days). Evolvers had a median time to evolution of over 2 years (755 days) following alemtuzumab administration. There was no significant difference seen in relapse or clonal evolution by route of administration.
Abbreviations: IV = intravenous; SC = subcutaneous
Clonal Evolution
Clonal evolution occurred in 9 patients, with cumulative incidence of 23% at the median follow-up of 6 years (2180 days). Patients with clonal evolution had a median time to evolution of over 2 years (755 days) following alemtuzumab administration (Figures 1C and 1D). Five of the patients who developed clonal evolution were non-responders at the primary endpoint of 6 months. Of the 9 evolved patients, 6 had high-risk evolution, defined as MDS, AML, or a high risk karyotype (chromosome 7 abnormality or complex karyotype). High-risk evolution events included myelodysplastic syndrome (MDS) with deletion of chromosome 7p (n=1), MDS with monosomy 7 (n=1), MDS-RAEB-1 with complex karyotype (n=1), MDS-RAEB-2 with trisomy 8 (n=1), and AML (n=1). Low-risk evolution events included isolated deletion 13q (n=2) and trisomy 21 (n=1). Six of the patients with clonal evolution underwent HSCT, including 4 with high-risk and 2 with low-risk evolution. Of the 3 who did not undergo HSCT, 2 died of disease progression, one patient 1 month after diagnosis of AML, and the other patient 6 months after diagnosis of MDS-RAEB-1 with complex karyotype. The third patient had low-risk clonal evolution and was alive at last follow-up.
PNH Clones
Presence of PNH clone >1% in neutrophils did not predict for either response or survival in alemtuzumab-treated patients (Table S4 and S6). There was no discernable pattern noted in PNH clonal expansion detected after alemtuzumab administration with some patients experiencing an increase and some a decrease in GPI-protein-negative neutrophils.
Long-term outcomes and survival
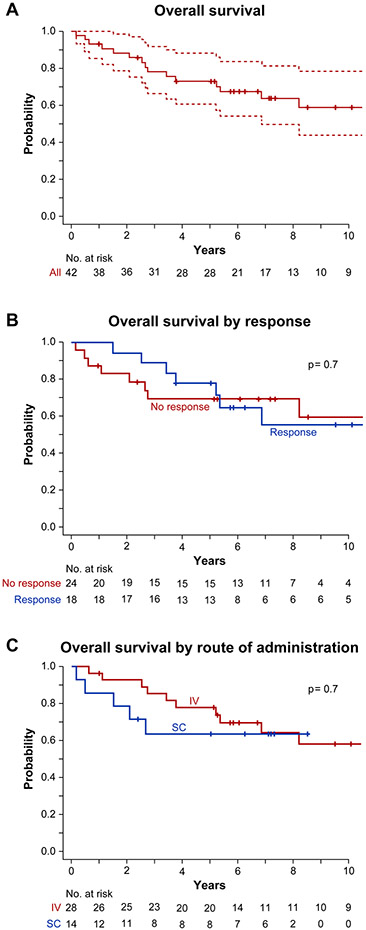
Overall survival was 67% at the median follow-up time of 6 years (2180 days) (Figure 2A), 69% in the IV group and 64% in the SC group (Figure 2C). There was no difference in survival between responders and non-responders (Figure 2B). Sixteen patients died, with median survival time of 989 days. Nine of the deaths were in non-responders, with a median survival time of 764 days. The remaining seven were responders, with a median survival time of 1378 days. Causes of death included sepsis with and without multi-organ failure in 4 patients, malignancy in 4 patients (2 with AML, 1 with MDS-RAEB-1 with complex karyotype, and 1 with DLBCL), pythiosis insidiosum in 1 patient, and an unknown cause of death for 7 patients. Predictors for survival using univariate analysis showed that baseline ARC > 36.9×109/L was favorable with a hazard ratio of 0.29 (Tables S6 and S7), while multivariate analysis showed that baseline ARC, ALC, PLT, and Hb were predictive of survival (Table S8).
Figure 2. Overall Survival.
Kaplan-Meier estimates were used to calculate overall survival for the cohort as a whole (A), divided by whether response was achieved at 6 months (B), and divided by route of administration (C). Overall survival was 67% at the median follow-up time of 6 years (2180 days). There was no significant difference seen in OS by route of administration. Overall survival was similar whether or not patients achieved response (p=0.7) and when administered through IV or SC routes of administration (p=0.7). Dotted lines in panel A indicate 95% confidence intervals.
Abbreviations: IV = intravenous; SC = subcutaneous
Twenty-six patients were alive at last follow-up; 6 responders and 4 non-responders to alemtuzumab at 6 months. Of these 26 patients, 11 patients (42%) were post-HSCT, 10 (38%) were transfusion independent, on no AA medical therapy, and without need for HSCT, 3 (12%) had stable blood counts on AA medical therapy, and for 2 patients status was unknown. Of the 10 patients who were both transfusion- and AA medication-independent at last follow-up, 4 had required subsequent AA therapy after alemtuzumab (eltrombopag n=3; CSA n=1). Therefore, a total 14% (6/42) of patients achieved a long-term response to alemtuzumab as 2nd line IST. All patients’ long-term clinical courses are depicted in Tables S2 and S3 as well as Figure S1.
Safety
Alemtuzumab was well tolerated and toxicity was similar to our prior report5 (Table S9). The most common serious adverse event was bacterial infection. Other rarer SAEs included infusion reactions, bleeding, cytopenias, and stroke. One patient who developed progressive multifocal leukoencephalopathy (PML; neurologic dysfunction with CSF demonstrating elevated JC virus) 14 months after alemtuzumab treatment was treated with cidofovir and was alive and well at last follow-up, 12 years after diagnosis, representing an unusual case in timing and disease course. No thyroid dysfunction or cardiac toxicity ≥ grade 3 or that required hospitalization were seen. Patients experienced prolonged immunosuppression after alemtuzumab. CD4+ count of < 0.2×109/L was observed in 21 (81%) of 26 patients with CD4+ data available at 6 months, 14 (67%) of 21 patients with data available at 1 year, and 7 (37%) of 19 patients with data available at 2 years (Figure S2). Stratified by route of administration, SC alemtuzumab resulted in 73% (n=8/11) of patients with CD4+ count <0.2×109/L at 6 months, 73% (n=8/11) at 1 year, and 38% (n=3/8) at 2 years. Among patients who received IV alemtuzumab, 93% (n=13/14) had CD4+ count <0.2×109/L at 6 months, 60% (n=6/10) at 1 year, and 45% (n=5/11) at 2 years.
Discussion
Alemtuzumab monotherapy is effective at inducing hematologic response in patients with relapsed SAA who previously have received at least one round of ATG-based IST. Response was achieved in 43% of patients in this larger cohort of 42 patients compared to the 56% 6-month response we reported in our initial study of 25 patients. Our results are comparable to previous studies of alemtuzumab in SAA showing 57% response in a cohort of 14 AA patients13, and 37% response in a cohort of 17 AA patients14. Alemtuzumab produced a slightly lower response rate compared to our prior study of rATG in relapsed SAA which reported a 6-month response rate of 68 in patients with similar baseline demographic characteristics15. Response rates of 53%16 and 61%17 have been reported in other studies evaluating treatment with either hATG or rATG for SAA relapse after ATG-based IST. However, survival in this context is comparable between both alemtuzumab and ATG. As monotherapy, the efficacy of alemtuzumab without requiring concurrent CSA makes it a good option for patients who are susceptible to complications from CSA such as renal impairment. Additionally, the adverse effect profile of alemtuzumab differs from ATG; in the case of ATG, serum sickness and hepatic or cardiac complications predominate, while in alemtuzumab most concerning effect is prolonged immunosuppression and risk of infection18. In the absence of trials that directly compare alemtuzumab and rATG in relapsed SAA, comparisons between the two regimens should be interpreted with caution, and an individualized patient approach for choice of immunosuppressive agent seems prudent.
Alemtuzumab was well tolerated by most patients. Infections and allergic reactions appeared to be more common in the IV treated patients. One patient developed PML more than a year after completing the alemtuzumab regimen. A recent review summarizes cases of PML in patients treated with alemtuzumab and reveals mostly poor outcomes. One 31-year-old patient developed PML 8 years after her multiple sclerosis diagnosis, 14 months after initiating alemtuzumab, and the clinical outcome was improvement. Three patients with chronic lymphocytic leukemia (CLL) developed PML: clinical data were available in two, who were 79 and 69 years of age, and developed PML 7 and 15 years after their CLL diagnoses, corresponding to 36 and 10 months respectively after initiating alemtuzumab; all three patients died19. While our patient had a favorable outcome from this rare side-effect, PML is potentially deadly, and patients must be appropriately counselled prior to alemtuzumab administration. We found that more than one-third of patients still had marked lymphopenia, low CD4+ counts, two years after treatment, consistent with prior reports20. Patients treated with alemtuzumab thus remain vulnerable to opportunistic infections, and clinical management should aim to prevent, detect, and treat complications, along with regular monitoring of lymphocyte subsets to guide PJP and viral prophylaxis. Appropriate prophylaxis mitigates the risk of acquiring these infections, as observed in our cohort. Per the alemtuzumab package insert, PJP and antiviral prophylaxis should be continued for 2 months after completion of alemtuzumab therapy or until the CD4+ count is ≥0.2×109/L, whichever occurs later21.
Alemtuzumab is effective when administered through IV or SC routes, but we observed more responses with the IV route (p=0.06). While others have reported that SC alemtuzumab produced similar response rates to IV in studies in BM failure22, comparison of their efficacy in various contexts of chronic lymphocytic leukemia has yielded contrasting results, with some reporting no difference between the routes23,24 and others premature termination of SC administration due to inferior efficacy25. In the present study, no difference was observed in baseline patient characteristics between IV and SC groups that would account for disparity in response. One explanation could be our specific SC administration regimen. In a trial evaluating the efficacy of alemtuzumab in patients with marrow failure of single or multiple lineages, SC dose-escalating administration of alemtuzumab over 4-5 days, with a total dose of 103 mg in SAA patients, produced a 58% response rate;22 some patients were also given long-term maintenance “boosters” of alemtuzumab every 2-8 weeks26. In patients with other hematologic disorders, both IV and SC alemtuzumab have been used interchangeably; MDS (64% response using same dosing regimen as our study)27, hemophagocytic lymphohistiocytosis (64% response)28, and chronic lymphocytic leukemia (54-87% response)23,29. In most cases, patients received a subsequent or maintenance dose of alemtuzumab. Therefore, while SC alemtuzumab has been shown efficacious both in SAA and other hematologic disorders, its use as a 10-day consecutive regimen in SAA may not be optimal and patients may require maintenance dosing when this route of administration is used.
Our study represents the largest relapsed SAA cohort treated with alemtuzumab in a prospective clinical trial. Some patients achieved durable long-term responses, with similar rates of clonal evolution, and survival to second-line therapy as reported with rATG15, with a favorable toxicity profile. The majority of responding patients ultimately required further AA-directed therapies or HSCT.
Alemtuzumab does not require concurrent CSA administration and may be advantageous in patients with CSA-related toxicity or renal impairment, but prolonged immunosuppression is a complication that necessitates patient education, long-term clinical monitoring, and antimicrobial prophylaxis.
Supplementary Material
Acknowledgements
This research was supported by intramural funding from the National Heart, Lung, and Blood Institute. Medical Illustrator Credit to Alan Hoofring, NIH Medical Arts Department.
Author N. Aggarwal: This research was made possible through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and contributions to the NIH from the Doris Duke Charitable Foundation (DDCF Grant #2014194), the American Association for Dental Research, the Colgate-Palmolive Company, Genentech, Elsevier, and other private donors.
Footnotes
The data used in this manuscript includes personally identifiable information and thus is stored securely on the NIH server. De-identified data will be available upon request.
Funding for this study was provided by the intramural research program of the National Heart, Lung, and Blood Institute. Patient consent was obtained prior to enrollment in the clinical trial, and the trial was approved by the IRB of the National Heart, Lung, and Blood Institute. The clinical trial is registered on clinicaltrials.gov with identifier NCT00195624.
This study was presented as a poster at the European Hematology Association 2022 Congress.
The authors do not have conflicts of interest to disclose.
References
- 1.Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006. Oct 15;108(8):2509–19. doi: 10.1182/blood-2006-03-010777. Epub 2006 Jun 15. https://ashpublications.org/blood/article/108/8/2509/22551/Current-concepts-in-the-pathophysiology-and [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Townsley DM, Scheinberg P, Winkler T, Desmond R, Dumitriu B, Rios O, Weinstein B, Valdez J, Lotter J, Feng X, Desierto M, Leuva H, Bevans M, Wu C, Larochelle A, Calvo KR, Dunbar CE, Young NS. Eltrombopag Added to Standard Immunosuppression for Aplastic Anemia. N Engl J Med. 2017. Apr 20;376(16):1540–1550. doi: 10.1056/NEJMoa1613878. https://www.nejm.org/doi/full/10.1056/NEJMoa1613878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young NS. Aplastic Anemia. N Engl J Med. 2018. Oct 25;379(17):1643–1656. doi: 10.1056/NEJMra1413485. https://pubmed.ncbi.nlm.nih.gov/30354958/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young NS. Current concepts in the pathophysiology and treatment of aplastic anemia. Hematology Am Soc Hematol Educ Program. 2013;2013(1):76–81. doi: 10.1182/asheducation-2013.1.76. https://pubmed.ncbi.nlm.nih.gov/24319166/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012. Aug 9;120(6):1185–96. doi: 10.1182/blood-2011-12-274019. Epub 2012 Apr 19. https://ashpublications.org/blood/article/120/6/1185/30513/How-I-treat-acquired-aplastic-anemia [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kashani N, Kelland EE, Vajdi B, Anderson LM, Gilmore W, Lund BT. Immune Regulatory Cell Bias Following Alemtuzumab Treatment in Relapsing-Remitting Multiple Sclerosis. Front Immunol. 2021. Oct 28;12:706278. doi: 10.3389/fimmu.2021.706278. https://www.frontiersin.org/articles/10.3389/fimmu.2021.706278/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheinberg P, Nunez O, Weinstein B, Scheinberg P, Wu CO, Young NS. Activity of alemtuzumab monotherapy in treatment-naive, relapsed, and refractory severe acquired aplastic anemia. Blood. 2012. Jan 12;119(2):345–54. doi: 10.1182/blood-2011-05-352328. Epub 2011 Nov 8. https://ashpublications.org/blood/article/119/2/345/105467/Activity-of-alemtuzumab-monotherapy-in-treatment [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenfeld SJ, Kimball J, Vining D, Young NS. Intensive immunosuppression with antithymocyte globulin and cyclosporine as treatment for severe acquired aplastic anemia. Blood. 1995. Jun 1;85(11):3058–65. https://ashpublications.org/blood/article/85/11/3058/123110/Intensive-immunosuppression-with-antithymocyte [PubMed] [Google Scholar]
- 9.Scheinberg P, Marte M, Nunez O, Young NS. Paroxysmal nocturnal hemoglobinuria clones in severe aplastic anemia patients treated with horse anti-thymocyte globulin plus cyclosporine. Haematologica. 2010. Jul;95(7):1075–80. doi: 10.3324/haematol.2009.017889. https://pubmed.ncbi.nlm.nih.gov/20595102/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003. Mar 5;289(9):1130–5. doi: 10.1001/jama.289.9.1130. https://pubmed.ncbi.nlm.nih.gov/12622583/ [DOI] [PubMed] [Google Scholar]
- 11.Calhoun P, Su X, Nunn M, Fan J. Constructing Multivariate Survival Trees: The MST Package for R. 2018. 2018;83(12):21. [Google Scholar]
- 12.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 13.Gómez-Almaguer D, Jaime-Pérez JC, Garza-Rodríguez V, Chapa-Rodríguez A, Tarín-Arzaga L, Herrera-Garza JL, Ruiz-Argüelles GJ, López-Otero A, González-Llano O, Rodríguez-Romo L. Subcutaneous alemtuzumab plus cyclosporine for the treatment of aplastic anemia. Ann Hematol. 2010. Mar;89(3):299–303. doi: 10.1007/s00277-009-0816-5. Epub 2009 Aug 25. https://pubmed.ncbi.nlm.nih.gov/19705116/ [DOI] [PubMed] [Google Scholar]
- 14.Kim H, Min YJ, Baek JH, Shin SJ, Lee EH, Noh EK, Kim MY, Park JH. A pilot dose-escalating study of alemtuzumab plus cyclosporine for patients with bone marrow failure syndrome. Leuk Res. 2009. Feb;33(2):222–31. doi: 10.1016/j.leukres.2008.08.004. Epub 2008 Sep 14. https://pubmed.ncbi.nlm.nih.gov/18790532/ [DOI] [PubMed] [Google Scholar]
- 15.Scheinberg P, Nunez O, Young NS. Retreatment with rabbit anti-thymocyte globulin and ciclosporin for patients with relapsed or refractory severe aplastic anaemia. Br J Haematol. 2006. Jun;133(6):622–7. doi: 10.1111/j.1365-2141.2006.06098.x. https://pubmed.ncbi.nlm.nih.gov/16704436/ [DOI] [PubMed] [Google Scholar]
- 16.Schrezenmeier H, Marin P, Raghavachar A, McCann S, Hows J, Gluckman E, Nissen C, van't Veer-Korthof ET, Ljungman P, Hinterberger W, et al. Relapse of aplastic anaemia after immunosuppressive treatment: a report from the European Bone Marrow Transplantation Group SAA Working Party. Br J Haematol. 1993. Oct;85(2):371–7. doi: 10.1111/j.1365-2141.1993.tb03181.x. https://pubmed.ncbi.nlm.nih.gov/8280610/ [DOI] [PubMed] [Google Scholar]
- 17.Tichelli A, Passweg J, Nissen C, Bargetzi M, Hoffmann T, Wodnar-Filipowicz A, Signer E, Speck B, Gratwohl A. Repeated treatment with horse antilymphocyte globulin for severe aplastic anaemia. Br J Haematol. 1998. Feb;100(2):393–400. doi: 10.1046/j.1365-2141.1998.00578.x. https://pubmed.ncbi.nlm.nih.gov/9488634/ [DOI] [PubMed] [Google Scholar]
- 18.Molldrem JJ, Leifer E, Bahceci E, Saunthararajah Y, Rivera M, Dunbar C, Liu J, Nakamura R, Young NS, Barrett AJ. Antithymocyte globulin for treatment of the bone marrow failure associated with myelodysplastic syndromes. Ann Intern Med. 2002. Aug 6;137(3):156–63. doi: 10.7326/0003-4819-137-3-200208060-00007. https://pubmed.ncbi.nlm.nih.gov/12160363/ [DOI] [PubMed] [Google Scholar]
- 19.Diamantopoulos PT, Kalopisis K, Tsatsou A, Efthymiou A, Giannakopoulou N, Hatzidavid S, Viniou NA. Progressive multifocal leukoencephalopathy in the context of newer therapies in hematology and review of new treatment strategies. Eur J Haematol. 2022. May;108(5):359–368. doi: 10.1111/ejh.13751. Epub 2022 Feb 6. [DOI] [PubMed] [Google Scholar]
- 20.Hill-Cawthorne GA, Button T, Tuohy O, Jones JL, May K, Somerfield J, Green A, Giovannoni G, Compston DA, Fahey MT, Coles AJ. Long term lymphocyte reconstitution after alemtuzumab treatment of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2012. Mar;83(3):298–304. doi: 10.1136/jnnp-2011-300826. Epub 2011 Nov 5. https://jnnp.bmj.com/content/jnnp/83/3/298.full.pdf [DOI] [PubMed] [Google Scholar]
- 21.Genzyme Corporation. Campath (alemtuzumab) [package insert]. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2001/alemmil050701LB.htm. Revised September 2003. [Google Scholar]
- 22.Risitano AM, Selleri C, Serio B, Torelli GF, Kulagin A, Maury S, Halter J, Gupta V, Bacigalupo A, Sociè G, Tichelli A, Schrezenmeier H, Marsh J, Passweg J, Rotoli B; Working Party Severe Aplastic Anaemia (WPSAA) of the European Group for Blood and Marrow Transplantation (EBMT). Alemtuzumab is safe and effective as immunosuppressive treatment for aplastic anaemia and single-lineage marrow failure: a pilot study and a survey from the EBMT WPSAA. Br J Haematol. 2010. Mar;148(5):791–6. doi: 10.1111/j.1365-2141.2009.08027.x. Epub 2009 Dec 7. https://pubmed.ncbi.nlm.nih.gov/19995389/ [DOI] [PubMed] [Google Scholar]
- 23.Lundin J, Kimby E, Björkholm M, Broliden PA, Celsing F, Hjalmar V, Möllgård L, Rebello P, Hale G, Waldmann H, Mellstedt H, Osterborg A. Phase II trial of subcutaneous anti-CD52 monoclonal antibody alemtuzumab (Campath-1H) as first-line treatment for patients with B-cell chronic lymphocytic leukemia (B-CLL). Blood. 2002. Aug 1;100(3):768–73. doi: 10.1182/blood-2002-01-0159. https://pubmed.ncbi.nlm.nih.gov/12130484/ [DOI] [PubMed] [Google Scholar]
- 24.Osterborg A, Foà R, Bezares RF, Dearden C, Dyer MJ, Geisler C, Lin TS, Montillo M, van Oers MH, Wendtner CM, Rai KR. Management guidelines for the use of alemtuzumab in chronic lymphocytic leukemia. Leukemia. 2009. Nov;23(11):1980–8. doi: 10.1038/leu.2009.146. Epub 2009 Jul 23. https://pubmed.ncbi.nlm.nih.gov/19626051/ [DOI] [PubMed] [Google Scholar]
- 25.Dearden CE, Khot A, Else M, Hamblin M, Grand E, Roy A, Hewamana S, Matutes E, Catovsky D. Alemtuzumab therapy in T-cell prolymphocytic leukemia: comparing efficacy in a series treated intravenously and a study piloting the subcutaneous route. Blood. 2011. Nov 24;118(22):5799–802. doi: 10.1182/blood-2011-08-372854. Epub 2011 Sep 26. https://pubmed.ncbi.nlm.nih.gov/21948296/ [DOI] [PubMed] [Google Scholar]
- 26.Thota S, Patel BJ, Sadaps M, Balasubramanian S, Sanikommu S, Hirsch C, Marotta S, Sekeres MA, Risitano AM, Maciejewski JP. Therapeutic outcomes using subcutaneous low dose alemtuzumab for acquired bone marrow failure conditions. Br J Haematol. 2018. Oct;183(1):133–136. doi: 10.1111/bjh.14907. Epub 2017 Sep 14. https://pubmed.ncbi.nlm.nih.gov/28905372/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranpura Vishal N., Wu Colin O., Olnes Matthew J., Parikh Ankur R., Shenoy Aarthi, Weinstein Barbara, Scheinberg Phillip, Barrett A. John, Neal S. Young, Hourigan Christopher S., Desmond Ronan. Alemtuzumab Is Safe and Associated With High Response Rates In Selected Patients With Myelodysplastic Syndrome. Blood. Volume 122, Issue 21. 2013, Page 593. ISSN 0006-4971. 10.1182/blood.V122.21.593.593 https://www.sciencedirect.com/science/article/pii/S0006497119638760 [DOI] [Google Scholar]
- 28.Marsh RA, Allen CE, McClain KL, Weinstein JL, Kanter J, Skiles J, Lee ND, Khan SP, Lawrence J, Mo JQ, Bleesing JJ, Filipovich AH, Jordan MB. Salvage therapy of refractory hemophagocytic lymphohistiocytosis with alemtuzumab. Pediatr Blood Cancer. 2013. Jan;60(1):101–9. doi: 10.1002/pbc.24188. Epub 2012 Apr 22. https://pubmed.ncbi.nlm.nih.gov/22522603/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faderl S, Ferrajoli A, Wierda W, O'Brien S, Lerner S, Keating MJ. Alemtuzumab by continuous intravenous infusion followed by subcutaneous injection plus rituximab in the treatment of patients with chronic lymphocytic leukemia recurrence. Cancer. 2010. May 15;116(10):2360–5. doi: 10.1002/cncr.24958. Erratum in: Cancer. 2010 Aug 15;116(16):3982. Dosage error in article text. https://pubmed.ncbi.nlm.nih.gov/20225334/ [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.