Abstract
Administration of a single propofol bolus dose for anesthesia induction causes hypotension. We included 160 patients (74 males and 86 females; mean age, 42.4 ± 10.7 [range: 18–60] years) with the American Society of Anesthesiologists status I–II undergoing elective surgery under general anesthesia. Using simple randomization, the patients were divided into a conventional group (n = 80; received 2 mg/kg propofol at a rate of 250 mg/min) and titrated group (n = 80; received propofol at a rate of 1 mg/kg/min until the Observer's Assessment of Alertness/Sedation scale score reached 1 point). Fentanyl (4 µg/kg) and cisatracurium (0.2 mg/kg) were administered, as appropriate. Systolic blood pressure, diastolic blood pressure, mean blood pressure, and heart rate were recorded at different time points. Propofol consumption, hypotension, and other adverse events were recorded. All the patients were intubated without awareness. Compared with the conventional group, the titrated group showed more stable blood pressure (p < 0.05), as well as a lower decrease in systolic blood pressure, mean blood pressure at 1 and 3 min, and diastolic blood pressure at 1 min after propofol administration (p < 0.01). Moreover, compared with the conventional group, the titrated group showed a lower post-intubation hypotension incidence (9 vs. 19 cases; p = 0.04), as well as lower total propofol dosage and propofol dose per kilogram of body weight (93.57 ± 14.40 mg vs. 116.80 ± 22.37 mg and 1.73 ± 0.27 mg/kg vs. 2.02 ± 0.08 mg/kg, respectively, p < 0.01). Compared with conventional propofol usage, titrated propofol administration can reduce the incidence of hypotension and propofol consumption during anesthesia induction.
Keywords: Propofol, general anesthesia, rapid sequence induction and intubation, individuation, administration and dosage, hypotension
Introduction
Propofol is widely used for anesthesia induction given its rapid onset (approximately 40 s) and short elimination half-life. It can reduce the cardiovascular sympathetic response to intubation.1,2 The conventional single propofol dose (2–2.5 mg/kg) usually causes sharp hemodynamic fluctuations, with a significant decrease in blood pressure of ≥ 40%,3,4 especially in elderly individuals.5,6 Hypotension adversely affects patients with cerebrovascular disease, hypertension, and coronary heart disease due to the susceptibility to low perfusion in these patients.7–9 Studies have shown severe hypotension during the induction period is associated with prolonged postoperative residence time in the operation room or even death;10,11 moreover, it increases mortality in patients with serious cardiovascular disease. 12 Hemodynamic profile changes are influenced by both the rate and total dose of propofol administration.13,14 Recent guidelines recommend initiating propofol administration as a continuous infusion with gradual changes in the administration rate (>5-minute intervals) to minimize hypotension and avoid acute overdosage in intensive care unit patients’ sedation. However, propofol administration at 2–2.5 mg/kg with a rate of 40 mg/10 s is recommended for anesthesia induction. 15 Therefore, it is necessary to investigate the appropriate dose and its infusion speed of propofol during anesthesia induction.
Given the individual differences in constitution, airway status, and laryngoscopic grade, the anesthetic depth required for intubation and sensitivity to propofol vary across individuals.5,16 Previous studies have indicated that excessive and insufficient doses of propofol cause cardiovascular depression and intraoperative awareness, respectively.17–19 It is difficult to confirm the titration endpoint of propofol for anesthesia induction without a predetermined gold standard. Bispectral index (BIS) is a measure of cortical activity that reflects anesthesia depth. Although numerous studies have employed BIS to guide propofol administration,20–22 Avidan reported that BIS monitoring did not decrease the incidence of anesthesia awareness and anesthetic drug administration. 23 A recent study showed that BIS has a processing time delay (30.09 ± 18.73 s) between the predicted and real BIS. 24 As a clinical measure, the Observer's Assessment of Alertness/Sedation (OAA/S) scale 25 is more widely available and easily accessible for experienced anesthesiologists during the induction time and is well correlated with the BIS value.23,26 A recent study reported that BIS values are insufficiently sensitive for accurately reflecting the sedation depth during anesthesia induction. 27 Target-controlled infusion (TCI) system is theoretically ideal mode to administer propofol according to the predetermined effect site concentration or plasma concentration; however, the predicted concentration of TCI is inconsistent with the patient's organ actual concentration due to individual differences in pharmacokinetics.28,29 Consequently, the endpoint for titrated induction remains unclear. Therefore, there is a need to explore whether it is feasible to use an OAA/S score of 1 point as the induction endpoint during titrated propofol induction.
We hypothesized that titrated propofol administration at 1 mg/kg/min with an OAA/S score of 1 point as the induction endpoint may reduce the hypotension incidence and propofol consumption compared with the conventional propofol usage with a single bolus dose. This study aimed to test this hypothesis and investigate the factors influencing the dose requirement of propofol during induction.
Methods
Ethics
This study was approved by the Ethics Committee of the Sixth Affiliated Hospital of Sun Yat-Sen University (No. 2012017) and registered at ClinicalTrials.gov (NCT02199522, Date of registration: 24 July 2014). All the patients provided written informed consent.
Sample size
Based on the findings of the pilot study, we used the mean arterial pressure (MAP) of the two groups at four time points (before propofol administration, as well as 1, 3, and 5 min after propofol administration) to calculate the sample size using PASS software (NCSS, LLC, USA). We found that 80 participants would be required per group (assuming two-sided testing with a significance level of 0.05 and a power of 85%, as well as a 10% dropout rate).
Patient enrollment
We enrolled 160 patients (age range: 18–60 years) with the American Society of Anesthesiologists (ASA) status I–II undergoing elective surgery under general anesthesia. The exclusion criteria were as follows: hypertension; diabetes; organ dysfunction, including heart, pulmonary, brain, liver, and renal dysfunction; obesity (body mass index [BMI] > 30 kg/m2); predicted difficult intubation or known allergy to propofol and its fat emulsion; chronic alcohol abuse; and use of drugs or psychotropic agents.
Grouping
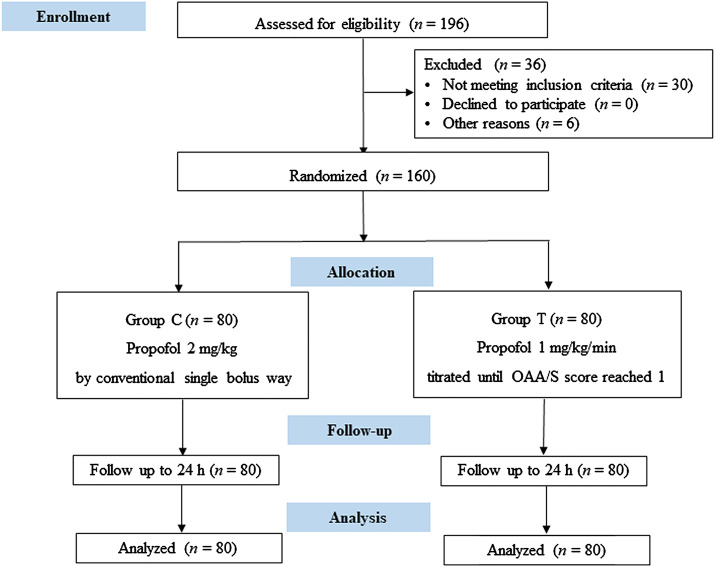
As shown in Consolidated Standards of Reporting Trials (CONSORT) flowchart (Figure 1), the patients were randomly assigned into two groups using SPSS random number generator as follows: the conventional group (Group C, who underwent conventional administration of propofol [KR093, AstraZeneca, United Kingdom]) and the titrated group (Group T, who underwent titrated administration of propofol). Patients in the conventional group received propofol at a bolus dose of 2 mg/kg using a Fresenius pump (Pilote Anesthesis 2/S, Fresenius, France) at a 250 mg/min rate following the drug manufacturer's instruction (approximately 40 mg per 10 s). For patients in the titrated group, a doctor not involved in sedation scale evaluation delivered propofol at a speed of 1 mg/kg/min by a Fresenius pump until the investigator evaluated the OAA/S score reached 1 point and told the drug administrator. Then propofol was administered at a speed of 0–1 mg/kg/h, which was adjusted to maintain the blood pressure stable and avoid the occurrence of hypertension and hypotension. We chose the titrated propofol infusion rate based on previous report. 17
Figure 1.
CONSORT diagram of the study. Group C: conventional group, propofol was conventionally administered as a single bolus dose calculated based on the body weight. Group T: titrated group, propofol was administered at a rate of 1 mg/kg/min until the OAA/S score reached 1 point. OAA/S: Observer’s Assessment of Alertness/Sedation.
Study design and anesthesia protocol
Before the operation, all the patients fasted for 8 h and drinking was not allowed for 4 h. Upon arrival in the operating room, a cannula was inserted into a forearm vein and 200 ml of Ringer's lactate solution was administered until completion of intubation. Routine monitoring of blood pressure, electrocardiogram, and pulse oxygen saturation were performed using a monitor (Datex Ohmeda S/5, Helsinki, Finland). Before induction, the patients underwent arterial cannulation at the left radial artery after local anesthesia with 2% lidocaine for continuous monitoring of arterial blood pressure.
This was a prospective, single-blind, randomized controlled trial. A doctor administered the drugs based on the group allocation. An investigator blinded to the group allocation assessed the OAA/S score at 20-second intervals during anesthesia induction and informed the drug administrator when the OAA/S score reached 1 point to change the drug administration regimen as per the study protocol.
Fentanyl (20140203, Enhua Pharmaceutical Company, Xuzhou, China) of 4 µg/kg was administered using another pump at a rate of 250 µg/min simultaneously with propofol administration. Cisatracurium (14062717, Hengrui Pharmaceutical Company, Jiangsu, China) of 0.2 mg/kg was intravenously injected when the patient fell asleep. Subsequently, the patient was intubated by an experienced anesthesiologist 4 min after muscle relaxant injection. Next, the patient was mechanically ventilated with sevoflurane 1% inhalation with the following respiratory parameters: tidal volume, 8 ml/kg and respiratory rate, 12 breaths per minute. Intraoperative anesthesia was maintained using sevoflurane, propofol, remifentanil, and cisatracurium.
Hypotension or hypertension and bradycardia
Baseline blood pressure was defined as the blood pressure before induction after a rest of at least 15 min after the completion of radial artery cannulation. Hypotension and hypertension were defined as a > 30% MAP decrease and increase, respectively, compared with baseline. If hypotension (MAP decreased > 30% of baseline) lasted 1 min or systolic blood pressure (SBP) was < 90 mmHg during anesthesia induction, 2 mg dopamine was administered,30,31 and it could be repeated if the blood pressure did not recover to the normal in 2 min. If the SBP > 160 mmHg or diastolic blood pressure (DBP) > 90 mmHg during endotracheal intubation, a single propofol bolus dose of 20 mg was administered, and it could be repeated if the blood pressure did not recover to the normal in 1 min. Atropine 0.5 mg was injected when the heart rate (HR) was < 45 bpm.
Observed variables
The primary endpoint was the hemodynamic changes in both groups during anesthesia induction. The secondary endpoint was propofol consumption during anesthesia induction.
SBP, DBP, MAP, HR recorded before induction (T0) after a rest of at least 15 min after the completion of radial artery cannulation were considered as the baseline data. Subsequently, SBP, DBP, MAP, and HR were recorded at different time points, including 1 (T1), 3 (T2), and 5 (T3) min after propofol administration, as well as immediately (T4), 1 (T5), 3 (T6), and 5 (T7) min after endotracheal intubation.
The total propofol dosage immediately before and after endotracheal intubation was recorded. Moreover, other data, including age; gender; body weight; predicted body weight, which was calculated according to the height; ASA status; Mallampati class; surgery types; doses of fentanyl and cisatracurium; incidence of hypotension and hypertension; dopamine administration; and propofol bolus administration due to hypertension, were recorded. All the patients were followed up to confirm whether they were aware during anesthesia induction on the next day.
Statistical analysis
The Shapiro–Wilk test was used to assess the normality of the distribution of continuous variables with p > 0.05 indicating normal distribution. Data are presented as mean ± standard deviation (SD), percentage, or number of cases. Continuous variables were compared using Student's t-test for independent samples. Between-group comparisons of hemodynamic effects were performed using repeated measures analysis of variance. Between-group comparisons of categorical data were performed using the chi-square test or Fisher's exact test, as appropriate. Ranked data were analyzed using the Mann–Whitney U test. Pearson's correlation analysis was used to assess the linear correlation between continuous variables related to total propofol consumption and per kilogram of body weight propofol consumption. The association between binary variables of factors related to total propofol consumption and propofol consumption per kilogram of body weight was analyzed using Pearson's chi-square independence test. All statistical analyses were performed using SPSS 16.0 software (SPSS Inc., USA). Statistical significance was set at a two-tailed p-value < 0.05.
Results
General characteristics
Tracheal intubation was successfully performed in all patients. Postoperative follow-up of the first day after operation showed that no intraoperative awareness occurred. Age, body weight, height, BMI, predicted body weight, body surface area, fentanyl dose, cisatracurium dose, and dose of propofol bolus were normally distributed (p>0.05, Shapiro–Wilk test). There were no between-group differences in any baseline characteristic (p>0.05, Table 1). Table 1 shows the cases of dopamine injection and additional propofol bolus administration due to hypertension, which showed no significant between-group differences (p>0.05, Table 1).
Table 1.
General characteristics of the patients.
| Group C (n = 80) | Group T (n = 80) | P | |
|---|---|---|---|
| Age (years) | 42.29 ± 10.97 | 42.45 ± 10.48 | 0.93 |
| Sex (male/female) | 32/48 | 42/38 | 0.08 |
| Body weight (kg) | 58.56 ± 10.48 | 56.62 ± 8.20 | 0.20 |
| Height (m) | 1.62 ± 0.80 | 1.63 ± 0.68 | 0.55 |
| BMI (kg/m2) | 22.19 ± 2.95 | 21.32 ± 2.54 | 0.06 |
| Predicted body weight (kg) | 57.95 ± 5.73 | 58.42 ± 4.84 | 0.58 |
| Body surface area (m2) | 1.59 ± 0.17 | 1.57 ± 0.13 | 0.39 |
| ASA status (I/II) | 32/48 | 24/56 | 0.25 |
| Mallampati class (I/II) | 48/32 | 56/24 | 0.19 |
| Dose of fentanyl (ug) | 234.6 ± 41.49 | 226.48 ± 32.78 | 0.17 |
| Dose of cisatracurium (mg) | 12.0 ± 1.99 | 11.79 ± 1.53 | 0.45 |
| Types of surgery | 0.26 | ||
| Gastrointestinal surgery | 49 (61%) | 56 (70%) | |
| Gynecologic surgery | 15 (19%) | 18 (22%) | |
| Orthopaedic surgery | 1 (1%) | 0 (0%) | |
| Otorhinolaryngologic surgery | 3 (4%) | 1 (1%) | |
| Thyroid breast surgery | 7 (9%) | 2 (3%) | |
| Urologic surgery | 1 (1%) | 2 (3%) | |
| Hepatobiliary surgery | 4 (5%) | 1 (1%) | |
| Bowel preparation | 55 (69%) | 59 (74%) | 0.49 |
| Dopamine bolus | 13 (16%) | 11 (13%) | 0.66 |
| Propofol bolus | 7 (9%) | 6 (8%) | 0.77 |
Data are presented as mean ± SD or number of patients (%). Group C: conventional group, propofol was conventionally administered as a single bolus dose calculated based on body weight. Group T: titrated group, propofol was administered at a rate of 1 mg/kg/min until the OAA/S score reached 1 point. ASA: American Society of Anesthesiologists; BMI: body mass index.
Blood pressure and HR
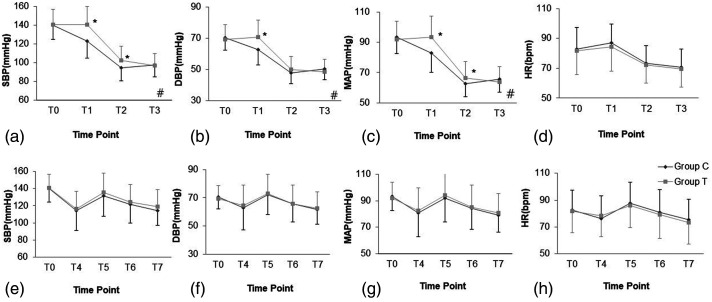
After propofol administration before intubation, there was a between-group difference in the changing trend of blood pressure (p < 0.05); specifically, compared with the conventional group, the titrated group showed a significantly lower decrease in SBP, MAP at 1 min and 3 min, and DBP at 1 min after propofol administration (p < 0.05, Figure 2a–2c). There was no between-group difference in the change in HR or incidence of hypotension (p > 0.05); moreover, incidence of hypotension was quite high in both groups (70%) (Figure 2d and Table 2). After tracheal intubation, there were no significant between-group differences in the changes in SBP, DBP, MAP, and HR (p>0.05, Figure 2e–2h); however, compared with the conventional group, the titrated group showed a lower incidence of hypotension (p < 0.05, Table 2).
Figure 2.
Changing trend of SBP, DBP, MAP, and HR before and after intubation. (a) Changing trend of SBP before intubation. (b) Changing trend of DBP before intubation. (c) Changing trend of MAP before intubation. (d) Changing trend of HR before intubation. (e) Changing trend of SBP after endotracheal intubation. (f) Changing trend of DBP after endotracheal intubation. (g) Changing trend of MAP after endotracheal intubation. (h) Changing trend of HR after endotracheal intubation. Group T and Group C: as shown in Figure 1. T0: baseline before propofol administration; T1, T2, and T3: 1 min, 3 min, and 5 min, respectively, after propofol administration; T4: immediately after endotracheal intubation; T5, T6, and T7: 1 min, 3 min, and 5 min, respectively, after endotracheal intubation. ANOVA for repeated measures. #p < 0.05 between two groups, *p < 0.01 is compared with the conventional group.
DBP: diastolic blood pressure; HR, heart rate; MAP: mean arterial pressure; SBP: systolic blood pressure.
Table 2.
Incidence of hypotension and hypertension during induction.
| Group C (n = 80) | Group T (n = 80) | P | |
|---|---|---|---|
| Hypotension before intubation | 56 (70%) | 55 (69%) | 0.87 |
| Hypotension after intubation | 19 (24%) | 9 (11%) * | 0.04 |
| Hypertension after intubation | 4 (5%) | 3 (4%) | 1.00 |
Data are presented as the number of patients (%). Group C: conventional group, propofol was conventionally administered as a single bolus dose calculated based on the body weight. Group T: titrated group, propofol was administered at a rate of 1 mg/kg/min until the OAA/S score reached 1 point. Hypotension and hypertension were defined as a decrease and increase in mean arterial blood pressure, respectively, by more than 30% from the baseline values. *P < 0.05 compared with the conventional group.
Propofol consumption
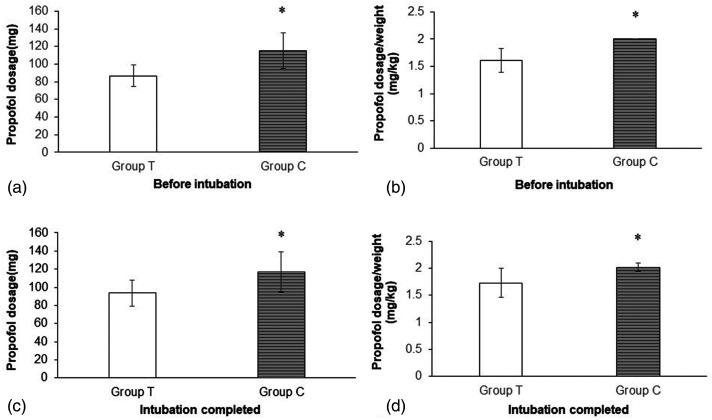
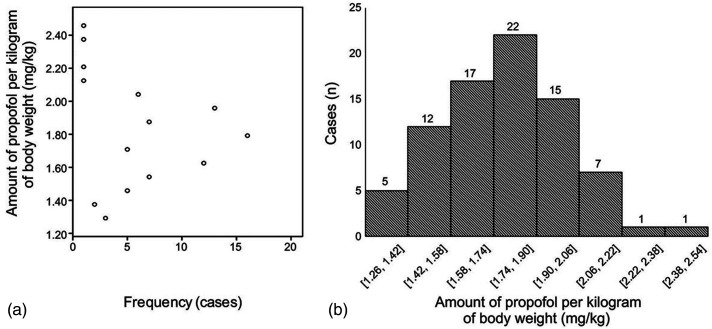
Both before and after endotracheal intubation, compared with the conventional group, the titrated group had a lower total propofol dose (86.84 ± 12.08 mg vs. 115.47 ± 20.44 mg, p < 0.01; 93.57 ± 14.40 mg vs. 116.80 ± 22.37 mg, p < 0.01, Figure 3a, 3c) and propofol dose per kilogram of body weight (1.61 ± 0.22 mg/kg vs. 2.0 mg/kg, p < 0.01; 1.73 ± 0.27 mg/kg vs. 2.02 ± 0.08 mg/kg, p < 0.01, Figure 3b, 3d).When the OAA/S score reached 1 point in the titrated group, the propofol dose per kilogram of body weight scattered within a large range (Figure 4a, 4b).
Figure 3.
Propofol consumption during induction before intubation and when intubation completed. Data are presented as mean ± SD. Group T and Group C: as shown in Figure 1. (a) Total propofol consumption before intubation. (b) Propofol consumption per kilogram of body weight before intubation. (c) Total propofol consumption at the completion of intubation. (d) Propofol consumption per kilogram of body weight at the completion of intubation. Student's t-test, *p < 0.01 is compared with the titrated group.
Figure 4.
Scattered profile of propofol consumption per kilogram of body weight before tracheal intubation in the titrated group. (a) Scattered plot of the frequency distribution of propofol dosage per kilogram of body weight. (b) Case distribution at different dose ranges per kilogram of body weight.
Predictive factors for appropriate propofol dosage for induction
We analyzed factors associated with propofol consumption in the titrated group during induction, including age, body weight, height, BMI, predicted body weight, body surface area, sex, ASA grade, and bowel preparation.
The propofol dose per kilogram of body weight was negatively correlated with BMI (r = −0.377, p < 0.01) and body weight (r = −0.230, p < 0.05); however, it was not correlated with age, height, predicted body weight, body surface area, sex, ASA grade, or bowel preparation (r = −0.193, 0.119, 0.12, −0.136, −0.137, 0.059, and 0.017, respectively; all p > 0.05).
The total propofol dosage was positively correlated with body surface area (r = 0.689, p < 0.001), body weight (r = 0.648, p < 0.001), predicted body weight (r = 0.573, p < 0.001), height (r = 0.572, p < 0.001), and BMI (r = 0.375, p < 0.01). Moreover, the total propofol dosage was negatively correlated with sex (r = −0.525, p < 0.001) and was not correlated with age, ASA grade, or bowel preparation (r = 0.097, 0.184, and 0.073, respectively; all p > 0.05). The aforementioned correlation coefficients were all < 0.7.
Discussion
In this study, compared with the conventional group, the titrated group showed significantly reduced propofol consumption and more stable blood pressure during induction. There is no exact factor for predicting the induction propofol dose.
A prospective multicenter observational study reported that hypotension occurrence during general anesthesia induction is dependent on age, preoperative degree of blood pressure decompensation, and presence of type 2 diabetes mellitus. 32 The present study included patients without hypertension and diabetes. There were no significant between-group differences in the baseline age or blood pressure; therefore, the occurrence of hypotension might be dependent on propofol, except for individual differences in the response to fentanyl. However, previous studies have reported that the appropriate dose of fentanyl and cisatracurium have an insignificant effect on hemodynamics.33,34 Propofol used for anesthesia induction can cause a dose-dependent decrease in arterial blood pressure and cardiac output.35,36 We found that the titrated group has a more stable hemodynamic profile than the conventional group. We performed real-time monitoring of the invasive blood pressure to facilitate prompt monitoring and correction of hypotension. Noninvasive blood pressure monitoring is widely used during induction, however, it cannot immediately detect hypotension. Given the sensitivity of invasive blood pressure, incidence of hypotension (almost 70% of the patients in both groups) before endotracheal intubation in our study (Table 2) was higher than that reported by previous studies (12.6% or 36.5%), which performed noninvasive blood pressure monitoring.4,35 Our findings suggest that hypotension during induction might be underestimated and needs to be given more attention. Moreover, a study using a lower rate of titrated propofol administration is warranted given the high incidence of hypotension in our study.
Appropriate anesthesia depth can help patients fall asleep quickly, eliminate intubation stress, and create favorable conditions for endotracheal intubation. Propofol is usually administered as a single bolus dose calculated based on the patients' body weight; however, there are individual differences in the tolerance to anesthetics. 37 Moreover, the bolus method might cause overdosage 38 or underdosage. 39 In our study, patients in the titrated group were successfully intubated without intraoperative awareness, which suggested that titrated propofol administration could allow an appropriate depth of anesthesia. Only seven and six patients in the conventional and titrated groups, respectively, required additional propofol administration due to hypertension, with no significant between-group difference. The conventional group had a higher incidence of hypotension after tracheal intubation, which suggested that conventional propofol usage could have resulted in propofol overdosage or underdosage in some patients. In the titrated group, some cases required additional propofol during intubation, which could be attributed to individual differences in intubation difficulty. In the titrated group, there was no factor associated with the total propofol dosage, which suggested that there were no predictive factors for propofol induction dose, including body weight and BMI. As shown in Figure 4, the propofol dose required for induction showed a large variance, indicating that the induction propofol dose should be individualized. This suggests that propofol should not be administered as a bolus dosage calculated according to body weight; rather, it should be administered using a slow infusion rate with an endpoint.
This study has several limitations. First, this study only concerned the propofol dosage and usage during induction, but how to titrate the whole process of general anesthesia was remained for future investigation. Second, we did not compare the BIS-targeted induction with the OAA/S-targeted induction, which should be considered in future studies. Third, we included relatively healthy adult patients and it would be more meaningful to use titration induction in older patients, patients with ASA status III–IV, and patients with obesity. Finally, there is a need for future large-scale studies to determine the more appropriate propofol infusion rate and the various titration endpoints for anesthesia induction.
Conclusion
In conclusion, titrated propofol administration guided by the OAA/S score reaching 1 point allows a stable hemodynamic profile, appropriate depth of anesthesia, and significant propofol dose reduction during anesthesia induction. There were no predictive factors for the induction propofol dose. The propofol dosage for anesthesia induction should be individualized using the titrated method with an endpoint.
Acknowledgments
The authors would like to thank Bo Li and Xinyang Li for their assistance in data collection.
Author biographies
Lihong Chen is an attending doctor of the Department of Anesthesiology, the Sixth Affiliated Hospital, Sun Yat-sen University. She mainly engaged in clinical research of propofol titration anesthesia; besides, she also participated in the clinical research on pressure-driven lidocaine spray on airway topical anesthesia for conscious sedation intubation.
Kun Lu is a resident doctor of the Department of Anesthesiology, the Sixth Affiliated Hospital, Sun Yat-sen University. He mainly engaged in clinical research of propofol titration anesthesia; besides, he also participated in the clinical research on safe duration of apnea and intubation time in face mask ventilation during induction of general anesthesia.
Tongfeng Luo is a resident doctor of the Department of Anesthesiology, the Sixth Affiliated Hospital, Sun Yat-sen University. She mainly engaged in clinical research of propofol titration anesthesia; besides, she also participated in the clinical research on a clinical prediction score for pathologically diagnosed aldosterone-producing adenoma.
Huiming Liang is an attending doctor of the Department of Anesthesiology, the Sixth Affiliated Hospital, Sun Yat-sen University. He mainly engaged in clinical research of propofol titration anesthesia; besides, he also participated in the clinical research on regional anesthesia and sedation on psychological stress and postoperative recovery.
Yuqin Gui is a resident doctor of the Department of Anesthesiology, the Sixth Affiliated Hospital, Sun Yat-sen University. She mainly participated in clinical research of propofol titration anesthesia.
Sanqing Jin is the chief and professor of the Department of Anesthesiology, the Sixth Affiliated Hospital, Sun Yat-sen University. He is the chairman of Anesthesiology Association of Cross-Straits Medicine Exchange. The main research area is organ protection of transient limb ischemia and propofol titration anesthesia.
Footnotes
Author contributions: Sanqing Jin designed the study and supervised the whole process. Lihong Chen and Kun Lu recruited the patients, performed the detailed experiment, and collected the data. Tongfeng Luo, Huiming Liang, and Yuqin Gui collected and checked the data. Sanqing Jin, Lihong Chen, and Tongfeng Luo carried out data analysis and wrote the manuscript. All the authors read and approved the final manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, author ship, and/or publication of this article: This study was supported by the Project for Science and Technology (No. 2010B080701073) and Guangdong Natural Science Foundation (No. 2016A030313303) from the Department of Science and Technology of Guangdong Province.
Ethics approval: Ethical approval to report this case series was obtained from Ethics Committee of the Sixth Affiliated Hospital of Sun Yat-Sen University (No.2012017).
Informed consent: Written informed consent was obtained from the patients for their anonymized information to be published in this article prior to study initiation.
ORCID iDs: Lihong Chen https://orcid.org/0000-0001-6883-8027
Sanqing Jin https://orcid.org/0000-0003-0919-8247
References
- 1.Chan VW, Chung FF. Propofol infusion for induction and maintenance of anesthesia in elderly patients: recovery and hemodynamic profiles. J Clin Anesth 1996; 8: 317–323. [DOI] [PubMed] [Google Scholar]
- 2.de Wit F, van Vliet AL, de Wilde RB, et al. The effect of propofol on haemodynamics: cardiac output, venous return, mean systemic filling pressure, and vascular resistances. Br J Anaesth 2016; 116: 784–789. [DOI] [PubMed] [Google Scholar]
- 3.Shah NK, Harris M, Govindugari K, et al. Effect of propofol titration v/s bolus during induction of anesthesia on hemodynamics and bispectral index. Middle East J Anaesthesiol 2011; 21: 275–281. [PubMed] [Google Scholar]
- 4.Reich DL, Hossain S, Krol M, et al. Predictors of hypotension after induction of general anesthesia. Anesth Analg 2005; 101: 622–628. [DOI] [PubMed] [Google Scholar]
- 5.Dundee JW, Robinson FP, McCollum JS, et al. Sensitivity to propofol in the elderly. Anaesthesia 1986; 41: 482–485. [DOI] [PubMed] [Google Scholar]
- 6.Kanonidou Z, Karystianou G. Anesthesia for the elderly. Hippokratia 2007; 11: 175–177. [PMC free article] [PubMed] [Google Scholar]
- 7.Duke T. A new intravenous anesthetic agent: propofol. Can Vet J 1995; 36: 181–183. [PMC free article] [PubMed] [Google Scholar]
- 8.Tsuchiya M, Yamada T, Asada A. Pleth variability index predicts hypotension during anesthesia induction. Acta Anaesthesiol Scand 2010; 54: 596–602. [DOI] [PubMed] [Google Scholar]
- 9.Liu Q, Kong AL, Chen R, et al. Propofol and arrhythmias: two sides of the coin. Acta Pharmacol Sin 2011; 32: 817–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sessler DI, Sigl JC, Kelley SD, et al. Hospital stay and mortality are increased in patients having a “triple low” of low blood pressure, low bispectral index, and low minimum alveolar concentration of volatile anesthesia. Anesthesiology 2012; 116: 1195–1203. [DOI] [PubMed] [Google Scholar]
- 11.Green RS, Butler MB. Postintubation hypotension in general anesthesia: a retrospective analysis. J Intensive Care Med 2016; 31: 667–675. [DOI] [PubMed] [Google Scholar]
- 12.Kirkbride DA, Parker JL, Williams GD, et al. Induction of anesthesia in the elderly ambulatory patient: a double-blinded comparison of propofol and sevoflurane. Anesth Analg 2001; 93: 1185–1187. [DOI] [PubMed] [Google Scholar]
- 13.Blum J, Kochs E, Forster N, et al. The influence of injection rate on the hypnotic effect of propofol during anesthesia: a randomized trial. PLoS Clin Trials 2006; 1: e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zahoor A, Ahmed N. The effects of duration of propofol injection on hemodynamics. Middle East J Anaesthesiol 2010; 20: 845–850. [PubMed] [Google Scholar]
- 15. U.S national library of medicine label : Propofol injection, emulsion https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fdb77e13-f5a7-4c4a-9f8e-338b702239c8&audience=consumer##. Accessed 20 August 2021.
- 16.McKinstry-Wu AR, Wasilczuk AZ, Harrison BA, et al. Analysis of stochastic fluctuations in responsiveness is a critical step toward personalized anesthesia. eLife 2019; 8:e50143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kazama T, Ikeda K, Morita K, et al. Relation between initial blood distribution volume and propofol induction dose requirement. Anesthesiology 2001; 94: 205–210. [DOI] [PubMed] [Google Scholar]
- 18.van Kralingen S, Diepstraten J, van de Garde EMW, et al. Comparative evaluation of propofol 350 and 200 mg for induction of anaesthesia in morbidly obese patients: a randomized double-blind pilot study. Eur J Anaesthesiol 2010; 27: 572–574. [DOI] [PubMed] [Google Scholar]
- 19.Goodchild CS, Serrao JM. Propofol-induced cardiovascular depression: science and art. Br J Anaesth 2015; 115: 641–642. [DOI] [PubMed] [Google Scholar]
- 20.Dussaussoy C, Peres M, Jaoul V, et al. Automated titration of propofol and remifentanil decreases the anesthesiologist's Workload during vascular or thoracic surgery: a randomized prospective study. J Clin Monit Comput 2014; 28: 35–40. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Li M, Yang D, et al. Closed-loop control better than open-loop control of profofol tci guided by bis: a randomized, controlled, multicenter clinical trial to evaluate the concert-cl closed-loop system. PLoS One 2015; 10: e0123862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orliaguet GA, Benabbes Lambert F, Chazot T, et al. Feasibility of closed-loop titration of propofol and remifentanil guided by the bispectral monitor in pediatric and adolescent patients. Anesthesiology 2015; 122: 759–767. [DOI] [PubMed] [Google Scholar]
- 23.Avidan MS, Zhang L, Burnside BA, et al. Anesthesia awareness and the bispectral index. N Engl J Med 2008; 358: 1097–1108. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira AL, Mendes JG, Nunes CS, et al. Evaluation of bispectral index time delay in response to anesthesia induction: an observational study. Braz J Anesthesiol 2019; 69: 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chernik DA, Gillings D, Laine H, et al. Validity and reliability of the observer's assessment of alertness/sedation scale: study with intravenous midazolam. J Clin Psychopharmacol 1990; 10: 244–251. [PubMed] [Google Scholar]
- 26.Kasuya Y, Govinda R, Rauch S, et al. The correlation between bispectral index and observational sedation scale in volunteers sedated with dexmedetomidine and propofol. Anesth Analg 2009; 109: 1811–1815. [DOI] [PubMed] [Google Scholar]
- 27.Lim TW, Choi YH, Kim JY, et al. Efficacy of the bispectral index and observer's Assessment of alertness/sedation scale in monitoring sedation during spinal anesthesia: a randomized clinical trial. J Int Med Res 2020; 48: 030006051989316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mu J, Jiang T, Xu XB, et al. Comparison of target-controlled infusion and manual infusion for propofol anaesthesia in children. Br J Anaesth 2018; 120: 1049–1055. [DOI] [PubMed] [Google Scholar]
- 29.Chen G, Buell O, Gruenewald M, et al. A comparison between target-controlled and manually controlled propofol infusions in patients undergoing routine surgical procedures. Eur J Anaesthesiol 2009; 26: 928–935. [DOI] [PubMed] [Google Scholar]
- 30.Kasaba T, Yamaga M, Iwasaki T, et al. Ephedrine, dopamine, or dobutamine to treat hypotension with propofol during epidural anesthesia. Can J Anaesth 2000; 47: 237–241. [DOI] [PubMed] [Google Scholar]
- 31.Gao WF, Zhou H, Chen C, et al. A comparison of effectiveness of three anesthetic agents in induction of general anesthesia in elderly surgical patients. Chinese Journal of General Practice 2015; 13 : 213–215(in Chinese). [Google Scholar]
- 32.Jor O, Maca J, Koutna J, et al. Hypotension after induction of general anesthesia: occurrence, risk factors, and therapy. A prospective multicentre observational study. J Anesth 2018; 32: 673–680. [DOI] [PubMed] [Google Scholar]
- 33.Vuyk J, Lim T, Engbers FHM, et al. The pharmacodynamic interaction of propofol and alfentanil during lower abdominal surgery in women. Anesthesiology 1995; 83: 8–22. [DOI] [PubMed] [Google Scholar]
- 34.Larsen R, Sonntag H, Schenk HD, et al. Haemodynamics, coronary blood flow and myocardial metabolism in man: effects of sufentanil and fentanyl. Anaesthesist 1980; 29: 277–279. [PubMed] [Google Scholar]
- 35.Saugel B, Bebert E-J, Briesenick L, et al. Mechanisms contributing to hypotension after anesthetic induction with sufentanil, propofol, and rocuronium: a prospective observational study. J Clin Monit Comput 2021. https://link.springer.com/article/10.1007/s10877-021-00653-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehandale S, Rajasekhar P. Perfusion index as a predictor of hypotension following propofol induction - a prospective observational study. Indian J Anaesth 2017; 61: 990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oliveira-Paula GH, Coeli-Lacchini FB, Ferezin LP, et al. Arginase ii polymorphisms modify the hypotensive responses to propofol by affecting nitric oxide bioavailability. Eur J Clin Pharmacol 2021; 77: 869–877. [DOI] [PubMed] [Google Scholar]
- 38.Purushothaman SS, Alex A, Kesavan R, et al. Ultrasound measurement of inferior vena cava collapsibility as a tool to predict propofol-induced hypotension. Anesth Essays Res 2020; 14: 199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uysal AI, Altiparmak B, Korkmaz TM, et al. The effect of preoperative anxiety level on mean platelet volume and propofol consumption. BMC Anesthesiol 2020; 20: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]