Abstract
Paraneoplastic neurological syndrome refers to certain malignant tumors that have affected the distant nervous system and caused corresponding dysfunction in the absence of tumor metastasis. Patients with this syndrome produce multiple antibodies, each targeting a different antigen and causing different symptoms and signs. The CV2/collapsin response mediator protein 5 (CRMP5) antibody is a major antibody of this type. It damages the nervous system, which often manifests as limbic encephalitis, chorea, ocular manifestation, cerebellar ataxia, myelopathy, and peripheral neuropathy. Detecting CV2/CRMP5 antibody is crucial for the clinical diagnosis of paraneoplastic neurological syndrome, and anti-tumor and immunological therapies can help to alleviate symptoms and improve prognosis. However, because of the low incidence of this disease, few reports and no reviews have been published about it so far. This article intends to review the research on CV2/CRMP5 antibody-associated paraneoplastic neurological syndrome and summarize its clinical features to help clinicians comprehensively understand the disease. Additionally, this review discusses the current challenges that this disease poses, and the application prospects of new detection and diagnostic techniques in the field of paraneoplastic neurological syndrome, including CV2/CRMP5-associated paraneoplastic neurological syndrome, in recent years.
Key Words: autoimmunity, CRMP5, CV2, CV2/CRMP5 antibody, paraneoplastic neurological syndromes, paraneoplastic syndromes, tumor
Introduction
CV2/collapsin response mediator protein 5 (CRMP5) antibody-associated paraneoplastic neurological syndrome (PNS) is a relatively common type of PNS. It is not a direct invasion of tissues or organs by the tumor but a distant effect of the tumor. Its pathogenesis is immune-mediated, and specific neuronal antibodies can often be detected in patients (Graus et al., 2021). The CV2/CRMP5 antibody is a kind of neuronal intracellular antigen antibody and a well-characterized onconeural antibody (Graus et al., 2004; Grativvol et al., 2018). According to the 2004 PNS diagnostic criteria, the presence of this antibody can lead to a PNS diagnosis, regardless of the presence of a tumor (Graus et al., 2004), and the 2021 update of the PNS diagnostic criteria reclassified it as a high-risk antibody (Graus et al., 2021). It is currently considered that the CV2/CRMP5 antibody is not pathogenic, and the pathogenic mechanism of CV2/CRMP5 antibody-associated PNS is mainly progressive, irreversible neuronal damage or apoptosis mediated by cytotoxic T lymphocytes (Grativvol et al., 2018). This disease has diverse clinical manifestations and is poorly responsive to immunotherapy. The detection of CV2/CRMP5 antibodies in serum and/or cerebrospinal fluid (CSF) is useful for the clinical diagnosis of CV2/CRMP5 antibody-associated PNS. This type of PNS is rare, and there are few related reports. There has been no review published about this disease until now. In this paper, we review the pathogenesis, clinical manifestations, auxiliary examination, diagnosis and differential diagnosis, treatment, and prognosis of CV2/CRMP5 antibody-associated PNS to provide a reference for the clinical treatment of this disease.
Retrieval Strategy
We used “CV2/CRMP5”, “CV2/CRMP-5”, “CV2”, “CRMP5”, “CRMP-5”, “antibody”, “paraneoplastic syndromes”, and “paraneoplastic neurological syndromes” as MeSH terms or keywords and searched the related literature published up to October 2022 through PubMed and Web of Science databases. Then, we analyzed and summarized the pathogenesis, clinical manifestations, auxiliary examination, diagnosis and differential diagnosis, treatment, and prognosis of CV2/CRMP5 antibody-associated PNS from the included literature.
Pathogenesis
The CV2 antibody was originally identified in a breast cancer patient presenting with cerebellar ataxia, uveitis, and peripheral neuropathy (Antoine et al., 1993); it reacted with a subpopulation of oligodendrocytes in the adult brain (Honnorat et al., 1996). The antibody was “rediscovered” as the CRMP5 antibody in 2001 (Yu et al., 2001). After an initial confrontation with the laboratory that characterized CV2 antibodies, everybody now agrees that the CV2 and CRMP5 antibodies are the same. One study also found that CV2 antibodies reacted with at least four of the five known isoforms of the CRMPs, with 100% of CV2 antibodies recognizing CRMP5, 40% recognizing CRMP3, 6% recognizing CRMP2, and 33% recognizing CRMP1 (Honnorat et al., 2001).
CRMP5 is a protein of 564 amino acids (molecular weight: 62 kDa) encoded by the human chromosome 2 (GenBank AC01347.3). It belongs to the CRMP family and shares 49–50% amino acid identity with the four other known CRMPs (1–4) (Fukada et al., 2000; Yu et al., 2001). In mouse embryos, CRMP5 binds to itself, CRMP2–4 (not CRMP1), and dihydropyrimidinase, forming hetero-multimeric structures (Fukada et al., 2000). The N-terminal sequences of CRMP isoforms are very similar and carry consensus sequences for phosphorylation sites, including tyrosine kinase, protein kinase A, protein kinase C, and casein kinase II phosphorylation sites (Fukada et al., 2000). A study showed that serum immunoglobulin G (IgG) from CRMP5 antibody-positive patients bound to the N-terminal epitope of recombinant CRMP5, but not to human-derived CRMP2/CRMP3, suggesting that the N-terminal epitope of CRMP5 is unique (Yu et al., 2001). The C-terminal region of human-derived CRMP5 is more conserved than the N-terminal region, suggesting that the C-terminal region has a specific role (Fukada et al., 2000). Besides, CRMP5 plays an important role in neuronal migration, axon guidance, dendrite growth, and synapse formation by interacting with neuronal cytoskeleton-associated proteins MAP2 and β III-microtubulin (Jeanne et al., 2021). CRMP5 is located only in the cytoplasm under physiological conditions; it is highly expressed in the nervous system during developmental stages and significantly less in adulthood (Bretin et al., 2005). CRMP5 is widely distributed in various regions, including the developing cerebral cortex, hippocampus, cerebellum, thalamus, retina, optic nerve, olfactory epithelium (Yu et al., 2001; Cross et al., 2003) as well as the spinal cord (Fukada et al., 2000), sensory neurons, axons of peripheral nerves, Schwann cells, and some tumors (Camdessanché et al., 2012; Brot et al., 2013). CRMP5 mRNA expression can also be detected in neurons of the cortex, amygdala, brainstem, and cerebellum (Bretin et al., 2005). The wide distribution of CRMP5 at multiple sites may be the anatomical and physiological basis for the diversity of clinical manifestations of CV2/CRMP5 antibody-associated PNS.
The CV2/CRMP5 antibody is a neuronal intracellular antigen antibody. Most researchers believe that this type of antibody is not directly pathogenic, in essence. Rather, they participate in the production of antigen-specific CD8+ T lymphocytes which cause neuronal damage and rapid and widespread cell death (Berzero and Psimaras, 2018). The pathogenic process may be as follows (Figure 1): the tumor cells undergo apoptosis after being attacked by the immune system, resulting in antigen exposure; antigen-presenting cells present antigens, and then naive lymphocytes are activated into cytotoxic CD8+ T cells and CD4+ T helper cells respectively; B lymphocytes differentiate into plasma cells and produce antibodies against the exposed antigens; plasma cells, CD4+ T helper cells, and cytotoxic CD8+ T cells cross the blood-brain barrier, and CD8+ T cells attack neurons that express the same antigen as the tumor cells, causing a broad immune response (Darnell and Posner, 2003). This is a hypothetical mechanism for CV2/CRMP5 antibody associated PNS, and some studies have also observed T lymphocyte infiltration in patients with positive CV2/CRMP5 antibody (Cross et al., 2003; Muehlschlegel et al., 2005).
Figure 1.
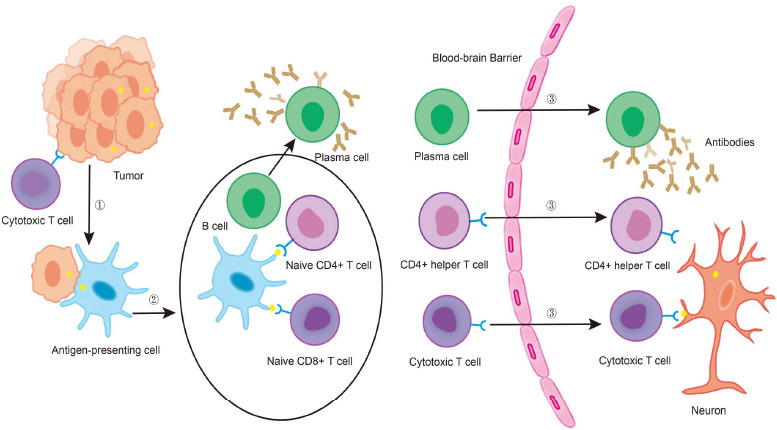
The pathogenesis of CV2/CRMP5 antibody-associated paraneoplastic neurological syndrome.
(1) The immune system attacks tumor cells, inducing apoptosis and resulting in antigen exposure; (2) antigen-presenting cells swallow the antigen and travel to local lymph nodes, naive lymphocytes are activated into cytotoxic T cells and CD4+ helper T cells, respectively, and B lymphocytes differentiate into plasma cells to produce CV2/CRMP5 antibodies; (3) plasma cells, CD4+ helper T cells and cytotoxic T cells cross the blood-brain barrier and cytotoxic T cells attack neurons that express the same antigen as the tumor cells. CRMP5: collapsin response mediator protein 5.
Clinical Manifestations
CV2/CRMP5 antibody-associated PNS is rare. For example, a study identified 28 PNS patients in Olmsted County (Minnesota, USA) from January 1, 1987, to December 31, 2018. The associated incidence rate was 0.6/100000 person-years, and only 2 of the 28 PNS patients were positive for the CV2/CRMP5 antibody (Shah et al., 2022). A previous review showed that the age of onset was 60–70 years and identified no gender difference (Sechi and Flanagan, 2021). Another study found that the median age was 69 years (44–88 years), and 54% of patients were male (Dubey et al., 2018). The clinical manifestations are varied and include limbic encephalitis (LE), chorea, ocular manifestation, cerebellar ataxia (CA), myelopathy, and peripheral neuropathy (Honnorat et al., 2009; Dubey et al., 2018; Sechi and Flanagan, 2021). These symptoms can coexist, and CV2/CRMP5 antibody-associated PNS commonly causes symptoms involving multiple areas of the nervous system (paraneoplastic encephalomyelitis). No CV2/CRMP5 antibody-associated PNS cases with isolated neurological syndromes have been reported. Patients with CV2/CRMP5 antibody-associated PNS have a longer course of disease, higher modified Rankin score, and longer median survival time than patients with Hu/ANNA-1 antibody-associated PNS (Honnorat et al., 2009).
Limbic encephalitis
Autoimmune LE is a group of inflammatory lesions of limbic structures that cause medial temporal lobe symptoms. It classically presents with the subacute onset of short-term memory deficits, seizures, or psychiatric symptoms (Budhram et al., 2019). CV2/CRMP5 antibody-associated LE is a relatively rare disease (Ibrahim Ismail et al., 2020) that often manifests as dementia, subacute progressive near-memory loss, sleep disturbance (insomnia/somnolence), mental and behavioral abnormalities (depression, paranoia, irritability, restlessness, upset, attention-deficit/hyperactivity disorder) (Li et al., 2018; Ibrahim Ismail et al., 2020; Tolkovsky et al., 2021), autonomic dysfunction (dysuria, difficulty defecating, profuse sweating) (Yan et al., 2020; Tolkovsky et al., 2021), and atrial tachycardia (Jia et al., 2020). Some patients experience seizures (Dubey et al., 2018), and a study described a patient without seizures having bitemporal epileptiform discharges appearing on an electroencephalogram (Ibrahim Ismail et al., 2020), which should be distinguished from other types of autoimmune epilepsy. Compared with Hu/ANNA-1 antibody-associated LE, CV2/CRMP5 antibody-associated LE is less likely to develop into isolated or typical LE, and its brain magnetic resonance imaging (MRI) features are rarely confined to the medial temporal lobe (Tüzün and Dalmau, 2007).
Chorea
In some patients with immune-related chorea (a rare form of hyperkinetic movement disorder), onconeural antibodies can be detected. Immune-mediated chorea subtypes associated with cancer are called paraneoplastic chorea and are possibly caused by the distal effect of an underlying tumor on the basal ganglia (Ha et al., 2019). They account for approximately 1.2% of PNS cases, and 64% of patients with paraneoplastic chorea detect CV2/CRMP5 antibodies (Vigliani et al., 2011). One study found that patients with CV2/CRMP5 antibody-related chorea had a mean age of onset of 69 years (Vernino et al., 2002), and typically had subacute generalized chorea, manifesting as involuntary, irregular, and symmetrical choreiform movements of the limbs or head and face. This disease can also manifest as orolingual facial dyskinesia, dystonia, and stereotyped movements, more obvious in the hands and feet. When it involves the neck, face, and perioral area, it leads to hyperkinetic dysarthria. Chorea can be bilateral or unilateral, appearing during the day and disappearing during sleep (Vigliani et al., 2011; Graus and Dalmau, 2012; Lim, 2017; Vaswani et al., 2020). However, the cause of extrapyramidal symptoms in patients with CV2/CRMP5 antibody-associated PNS is unclear. Compared with non-paraneoplastic chorea, paraneoplastic chorea is more common in males, has an older age of onset, has more severe chorea-like symptoms, may coexist with other central and peripheral nervous system symptoms, and may be associated with weight loss, often without oculomotor abnormalities (O’Toole et al., 2013; Lim, 2017). The most common antibody in paraneoplastic chorea is the CV2/CRMP5 antibody, although the Hu/ANNA-1, LGI1, CASPR2, GAD65, and NMDAR antibodies can also be detected. Around 35% of patients with IgLON5 antibody disease manifested with chorea, and the two (CV2/CRMP5 antibody-related chorea and IgLON5 antibody disease) need to be differentiated (Kyle et al., 2022; Sturchio et al., 2022).
Ocular manifestations
CV2/CRMP5 antibodies can be detected in some patients with optic neuritis, retinitis, and uveitis of unknown origin (Bataller and Dalmau, 2004). One study showed that 7% of CV2/CRMP5 antibody-associated PNS cases manifested only as ocular manifestations in the initial stages of the disease, without other neurological manifestations (Yu et al., 2001). Optic neuritis cases present with sudden bilateral visual loss, optic disc edema, and visual field defects, usually associated with encephalomyelitis. Retinitis symptoms include painless visual loss, photosensitivity, peripheral scotomas, and ring-like scotomas (Bataller and Dalmau, 2004). Uveitis may manifest as loss of vision, blurred vision, and flashing sensation. Scholars found that 69% of CV2/CRMP5 antibody-associated optic neuritis cases were associated with retinitis and vitreous inflammation, with an age of onset of 52–74 years, and often presented with subacute painless vision loss in both eyes, optic disc edema, defect of visual field (enlarged blind spots, arcuate and altitudinal defects, paracentral scotomata, and constriction of the visual field) (Cross et al., 2003), oculomotor disturbances (central nystagmus and diplopia), uveitis and iritis (Cohen et al., 2020), and also opsoclonus (Yu et al., 2001). MRI examination does not reveal optic nerve enhancement, and optic disc edema can be observed by fundoscopy, but rarely alone (Cohen et al., 2020). Immunotherapy improves visual function in 50% of patients and a minority of patients without immunotherapy developed optic nerve atrophy during the follow-up (Cohen et al., 2020). Improvement in visual acuity may be associated with the regression of optic disc swelling (Pulido et al., 2008). Besides CV2/CRMP5 antibodies, optic neuritis is more commonly associated with myelin oligodendrocyte glycoprotein and aquaporin 4 antibodies. Therefore, the detection of various autoimmune antibodies such as CV2/CRMP5 is recommended for unexplained subacute painless vision loss, optic disc edema, retinitis, and vitreous inflammation (Cross et al., 2003; Cohen et al., 2020).
Cerebellar ataxia
CV2/CRMP5 antibody-associated CA accounts for 2% of paraneoplastic CA cases (Rogemond and Honnorat, 2000), and about 26% of CV2/CRMP5 antibody-positive patients present with CA (Narayan et al., 2020). CA mainly manifests as vertigo, limb and gait ataxia, dysarthria, and nystagmus (Saloustros et al., 2010; Aliprandi et al., 2015)—of which 25–50% cases are gaze-evoked nystagmus or down beating nystagmus—and rarely presents as isolated vertigo (Narayan et al., 2020). In most cases, ataxia precedes the underlying tumor, and the brain MRI examination is initially normal or shows mild meningeal or cortical enhancement, and may show diffuse cerebellar atrophy later, which may indicate a poor response to immunotherapy (Narayan et al., 2020) .
Myelopathy
Since the 1980s, onconeural antibodies associated myelopathy has been considered to be associated with paraneoplastic disease, also known as paraneoplastic myelopathy (Babikian et al., 1985). Note-worthily, CV2/CRMP5 antibodies are detectable in 16–19% of patients with this disease (Yu et al., 2001; Flanagan et al., 2011; Shah et al., 2021). This rare and rapidly progressing, disabling disease can exist alone or in conjunction with encephalopathy, cranial neuropathy, or CA (Flabeau et al., 2022). It is most commonly seen in women, with a median age of 62 years (37–79 years). It is associated with breast and lung carcinomas, and usually manifests as transverse myelitis (Flanagan and Keegan, 2013), which is characterized by subacute/occult progressive weakness and paresthesia, sensory ataxia, bladder dysfunction, and orthostatic intolerance, as well as gastrointestinal dysfunction (Dubey et al., 2018). Physical examination findings include hyporeflexia or hyperreflexia of the distal reflexes, positive pathological reflexes, impaired vibration, and proprioception (Shah et al., 2021). Additionally, most patients may become wheelchair dependent as the disease progresses (Flanagan and Keegan, 2013). Longitudinal extensive damage has been reported in 25% of CV2/CRMP5 antibody-associated myelopathies (Dericioglu et al., 2018). Several cases of CV2/CRMP5 antibody-related symptoms caused by the use of immune checkpoint inhibitors (ICIs) have been reported (Gill et al., 2019; Kunchok et al., 2020; Wang et al., 2021). Moreover, a patient with small cell lung cancer (SCLC) received programmed cell death ligand 1 inhibitors and developed CRMP5-IgG-associated myelopathy, which manifested as bilateral lower limb weakness, Lhermitte’s sign, and urinary retention (Kunchok et al., 2020). Another patient with extensive-stage SCLC developed CV2/CRMP5 antibody-positive acute myelitis after undergoing durvalumab plus etoposid-platinum chemotherapy. This manifested as numbness of the lower limbs, which gradually developed to the proximal thigh root and hands, bladder dysfunction, and even inability to walk unaided (Wang et al., 2021). Due to the rarity of the disease and the lack of large-scale studies, its treatment outcome and prognosis rely on expert experience (Flanagan et al., 2011).
Peripheral neuropathy
Approximately one-third of PNS cases present with peripheral neuropathy (Giometto et al., 2010). Additionally, CV2/CRMP5, Hu/ANNA-1, and other onconeural antibodies can be detected in about one-third of PNS-associated peripheral neuropathy cases (Voltz, 2002). Peripheral neuropathies associated with CV2/CRMP5 antibodies are mostly mixed axonal and demyelinating peripheral neuropathies (Antoine et al., 2001; Honnorat et al., 2009). Besides, 54% of CV2/CRMP5 antibody-associated neuropathy cases present with painful axonal asymmetric polyradiculoneuropathy (Dubey et al., 2018), with predominant sensory or sensorimotor involvement (less commonly pure motor involvement, which mainly affects the distal extremities), sensory disturbance involving all the modalities of sensation, and depressed or absent tendon reflexes (Antoine et al., 2001). When pure sensory involvement is present, it is subacute and progresses rapidly, commonly in the upper limbs, with early loss of nociception followed by moderate to severe pain requiring pain relief medication, namely opioid analgesics in 39% of cases (Dubey et al., 2018). When mixed sensorimotor involvement is present, sensory symptoms are predominant, lower limbs are more commonly and severely involved than upper limbs, and pain is relatively uncommon (Antoine et al., 2001). The CV2/CRMP5 antibody can coexist with the VGCC, PCA-2/MAP1B, amphiphysin, and SOX1/AGNA antibodies in this group of patients, and the coexisting antibodies do not affect the location of peripheral neuropathy or the development of pain (Dubey et al., 2018). In addition, peripheral neuropathy associated with the CV2/CRMP5 antibody can involve cranial nerves other than the optic nerve and manifest as facial numbness (Maramattom, 2013), loss of taste (Wu et al., 2019), and loss of olfaction (Maramattom, 2013).
Other symptoms
The other symptoms of CV2/CRMP5 antibody-associated PNS are mostly derived from case reports and include: (1) Myasthenic syndrome, such as the Lambert-Eaton myasthenic syndrome (LEMS), which may be accompanied by other antibodies. It manifests as progressive symmetrical fluctuating proximal muscle weakness (Li et al., 2018). In a few patients, CV2/CRMP5 antibodies are just an immunological accompaniment reflecting the presence of cancer, whereas the LEMS is caused by concurrent pathogenic antibodies. Another example is myasthenia gravis, a generalized or oculomotor pattern combined with thymoma (Monstad et al., 2009). (2) Opsoclonus-myoclonus ataxia syndrome, causing ocular myoclonus, myoclonus, and ataxia (Nakajima et al., 2018; Popławska-Domaszewicz et al., 2018); (3) Parkinsonism, which often manifests as slow movement, festination, masked face, increased muscle tone, postural balance disorders, involuntary limb tremors, static tremor (Tada et al., 2016; Wu et al., 2019), and lead-pipe rigidity (Vakrakou et al., 2020). When combined with rapidly progressive autonomic dysfunction, it can easily be misdiagnosed as multiple system atrophy (Song et al., 2021). (4) Brainstem encephalitis, which causes salivation, somnolence, dysphagia, bilateral vocal cord paralysis (Tolkovsky et al., 2021), dysarthria (Tada et al., 2016; Nakajima et al., 2018), non-fluent speech (Wu et al., 2019), and central hypoventilation. (5) Motor neuron disease, which provokes signs and symptoms of upper and lower motor neuron injury and visible muscle atrophy without sensory abnormalities, and is easily misdiagnosed as amyotrophic lateral sclerosis (Tolkovsky et al., 2021). (6) Devic syndrome, a myelopathy accompanied by optic neuritis (Cross et al., 2003; Ducray et al., 2007). (7) Chronic intestinal pseudo-obstruction, which is characterized by symptoms of mechanical intestinal obstruction rather than mechanical bowel obstruction and is associated with autonomic neuropathy (Yan et al., 2020).
Oncological associations
The CV2/CRMP5 antibody is now known as a high-risk antibody, and its presence indicates a potential tumor (Graus et al., 2021). About 90% of CV2/CRMP5 antibody-associated PNS cases are accompanied by tumors (Yu et al., 2001; Budhram et al., 2019), with SCLC and thymoma being the most common (Honnorat et al., 2009). A study on Hu/ANNA-1 and CV2/CRMP5 antibody-associated PNS found that malignant thymoma was only found in CV2/CRMP5 antibody-positive patients, and patients with thymoma were younger and more likely to develop myasthenia gravis (Honnorat et al., 2009) and to have higher antibody titers (Ibrahim Ismail et al., 2020) than those with SCLC. CV2/CRMP5 antibody-associated PNS can also accompany breast cancer (Antoine et al., 1993; Wu et al., 2019), lymphoma (Jia et al., 2020), renal carcinoma (Dubey et al., 2018), colon cancer (Popławska-Domaszewicz et al., 2018), testicular tumor, uterine sarcoma (Antoine et al., 2001), prostate cancer (Aliprandi et al., 2015), undifferentiated carcinoma (Antoine et al., 1993), thyroid cancer (Cross et al., 2003), squamous cell carcinoma of tongue (Saloustros et al., 2010), and adenocarcinoma of the lung (Yap et al., 2021). Some patients have both breast and lung cancer (Jarius et al., 2012). A rare case reported that biopsies revealed a solitary lymph node involvement associated with SCLC in a patient who was both CV2/CRMP5 antibody-positive and Hu/ANNA-1 antibody-positive (Khan and Warriach, 2020). Although some studies have also found that 22% of patients with CV2/CRMP5 antibody-associated PNS do not have tumors (Rogemond and Honnorat, 2000), the possibility of occult malignancy cannot be ruled out as the majority of these patients have the neurological symptoms before the tumor is detected (Dubey et al., 2018). Moreover, some tumors can be eliminated by the immune response during the disease, preventing their detection (Graus et al., 2004).
Auxiliary Examinations
CSF and serological tests
Positive detection of CV2/CRMP5 antibodies in the CSF and/or serum is a specific indicator for the diagnosis of the disease. Detection methods include immunofluorescence, western blotting (Aliprandi et al., 2015; Figure 2), immunohistochemistry (Graus et al., 2004; Sabater et al., 2016), and line blotting (Ruiz-García et al., 2020). A study on the detection of CV2/CRMP5 antibodies found that immunoblotting missed 7.5% of the serum samples tested positive by immunohistochemistry (Sabater et al., 2016). Line blotting is more sensitive to low CV2/CRMP5 antibody levels, but can yield false positives (Ruiz-García et al., 2020). A recent study reported a cell-based assay for the detection of CRMP5 antibodies (CRMP5-CBA) that was more sensitive than immunohistochemistry (Totland et al., 2021). Overall, CV2/CRMP5 antibody detection methods still require optimization. Only half of the patients with high serum antibody titers had CV2/CRMP5 antibodies in the CSF (Rogemond and Honnorat, 2000); 37% of patients had CSF CRMP5 IgG titers significantly higher or equal to serum titers (Yu et al., 2001). In general, CSF antibody testing has a higher clinical specificity than serum testing. Therefore, for patients with suspected PNS and atypical clinical manifestations, simultaneous serum and CSF antibody testing are recommended to maximize specificity (Berzero and Psimaras, 2018; Graus et al., 2021).
Figure 2.
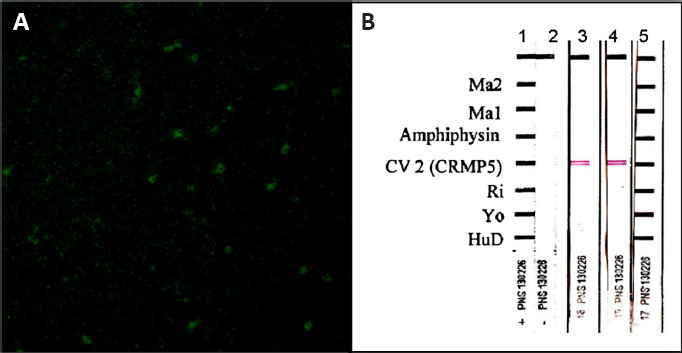
Patient’s serum antibody reactivity.
(A) A pattern suggesting anti-CV2 positivity (cytoplasmic staining with nuclear sparing) obtained by indirect immunofluorescence performed with our patient’s serum on primate cerebellum (Inova Diagnostics, San Diego); the estimated titer was 1:500. (B) Subsequent immunoblot verification (Ravo Diagnostika) of anti-CV2 isolated presence in serum obtained from the proband in two separate occasions (lanes 3 and 4); serum dilution 1:2000. Reproduced with permission from Aliprandi et al. (2015).
A study observed CV2/CRMP5 antibody-positivity in 5% of patients with SCLC, 12% of patients with myasthenia gravis and thymoma, and 0.6% of healthy individuals (Monstad et al., 2008). In another autoimmune antibody test in 116 SCLC patients, only 6 patients were CV2/CRMP5 antibody-positive, which is a positivity rate of 5.17% (Zekeridou et al., 2019; Totland et al., 2021). Besides, CV2/CRMP5 was expressed in 98.6% of highly differentiated neuroendocrine lung cancer specimens (Dalmau and Rosenfeld, 2008). Qvale et al. (2014) tested the sera of 379 smokers with chronic obstructive pulmonary disease, and only one (0.3%) was CV2/CRMP5 antibody-positive. Meanwhile, 4010 patients with neuropsychiatric symptoms of unknown etiology were tested for autoimmune encephalitis (AE) and PNS antibodies, and 72 of them were found positive. Among the 44 cases with complete clinical data, 13.6% were CV2/CRMP5 antibody-positive (Seluk et al., 2019). Although PNS can manifest as polyneuropathies, a study of sera from 283 patients with polyneuropathies showed no antibody positivity. Therefore, routine screening for onconeural antibodies is not necessary for patients with unknown etiology and possibly idiopathic polyneuropathies (Guillain-Barré syndrome and chronic inflammatory demyelinating polyradiculoneuropathy) (Berger et al., 2020).
The majority of patients with CV2/CRMP5 antibody-associated PNS have abnormalities in the CSF, often manifesting as pleocytosis and increased protein levels (Yu et al., 2001; Cohen et al., 2020). Some have elevated IgG index and/or elevated IgG synthesis rates (Yu et al., 2001), and a few have positive oligoclonal bands. More than two-third of CV2/CRMP5 antibody-positive patients were positive to other antibodies (Cross et al., 2003; Cohen et al., 2020), including Yo/PCA-1 antibody (Vakrakou et al., 2020), AMPAR antibody (Jia et al., 2020), GABAB receptor antibody (Li et al., 2018), VGCC antibody (Vernino et al., 2002), NMDAR antibody (Qiao et al., 2022), LGI1 antibody, CASPR2 antibody, amphiphysin antibody, PCA-2/MAP1B antibody, Ri/ANNA-2 antibody (Dubey et al., 2018), Ma2/Ta antibody (Hu et al., 2015), ZIC4 antibody (Cross et al., 2003), and acetylcholine receptors (AChR) antibody (Monstad et al., 2009), in addition to the most common Hu/ANNA-1 antibody (Antoine et al., 2001) and SOX1/AGNA antibody (Shibata et al., 2021). CV2/CRMP5 antibodies can be accompanied by the Hu/ANNA-1 and SOX1/AGNA antibodies (Hean et al., 2020), Hu/ANNA-1 and VGCC antibodies (Nakajima et al., 2018), Hu/ANNA-1 and amphiphysin antibodies (Carette et al., 2021). Besides, a patient with immunomyelopathy was positive to CV2/CRMP5, SOX1/AGNA, ZIC4, and MOG antibodies after immunosuppressive therapy (Wang et al., 2021). Overall, coexisting antibodies have no significant impact on the clinical phenotype (Dubey et al., 2018), but patients positive to multiple antibodies may have a worse prognosis than patients positive to the CV2/CRMP5 antibody alone.
Table 1 displays the combined antibodies classification according to the updated PNS diagnostic criteria of 2021. The ZIC4 antibody (neural intracellular antigen antibody) is partially characterized as an onconeural antibody (Graus et al., 2004). The AChR antibody (neural surface antigen antibody) is associated with PNS (Grativvol et al., 2018).
Table 1.
Classification of combined antibodies
| Antigen Localization | Risk Classification | Antibody |
|---|---|---|
| Neural intracellular antigen antibody | High-risk antibodies | CV2/CRMP5 antibody, Hu/ANNA-1 antibody, SOX1/AGNA antibody, Ma2/Ta antibody, Yo/PCA-1 antibody, Ri/ANNA-2 antibody, amphiphysin antibody, PCA-2/MAP1B antibody |
| Neural surface antigen antibody | Intermediate-risk antibodies | AMPAR antibody, GABAB receptor antibody, VGCC antibody, NMDAR antibody, CASPR2 antibodya |
| Low-risk antibodies | LGI1 antibody, CASPR2 antibody, MOG antibody |
CASPR2 antibodya, only in the case of manifested as morvan syndrome CASPR2 antibody is considered as intermediate-risk antibody. AGNA: Anti-glial nuclear antibody; AMPAR:α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor; ANNA: antineuronal nuclear antibody; CASPR2: contactin-associated protein-like 2; CRMP5: collapsin response mediator protein 5; GABAB: gamma-aminobutyric acid B; LGI1: leucine-rich glioma-inactivated 1; MAP1B: microtubule associated protein 1B; MOG: myelin oligodendrocyte glycoprotein; NMDAR: N-methyl-D-aspartate receptor; PCA: Purkinje cell antibody; VGCC: voltage -gated calcium channel.
Neuroimaging studies
MRI can find various abnormalities in the brain of patients with CV2/CRMP5 antibody-associated PNS, providing more valuable information for the diagnosis. 18F-fluoro-2-deoxy-d-glucose positron emission tomography (18F-FDG PET) is more sensitive to potential abnormalities, which may contribute to the early diagnosis of the disease.
MRI
MRI results of patients with CV2/CRMP5 antibody-associated PNS can be normal or show multiple foci, mainly involving the basal ganglia, medial temporal lobe (Maramattom, 2013; Figure 3), but also extensive white matter (Vigliani et al., 2011), hippocampus (Ibrahim Ismail et al., 2020), the cerebellum, insula, optic nerve, thalamus, and frontal lobe. T2-weighted image/fluid attenuated inversion recovery (T2WI/FLAIR) sequences will often show increased signals in the affected areas (Wu et al., 2019). The involvement of the frontostriatal circuit, which connects frontal regions to the basal ganglia (striatum), may be associated with personality changes, obsessive behavior, and cognitive deficits (Muehlschlegel et al., 2005; Tüzün and Dalmau, 2007). Myelopathy usually presents with extensive symmetric T2WI high signal and symmetric enhancement involving more than three segments confined to the spinal tract (lateral/dorsal) or gray matter (Flanagan et al., 2011; Graus and Dalmau, 2012).
Figure 3.
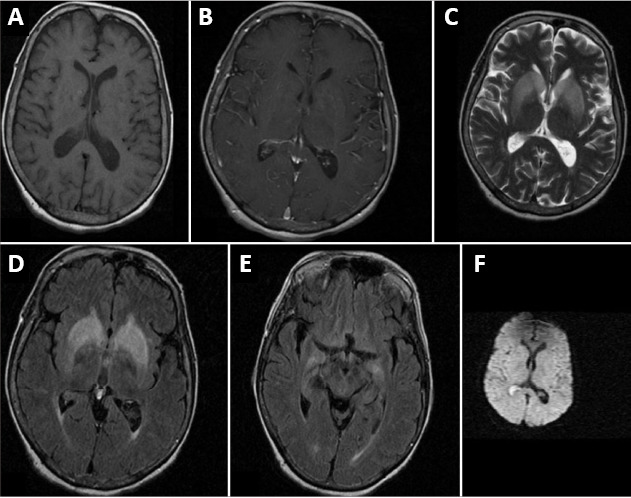
MRI of a patient with CV2/CRMP5 antibody-associated paraneoplastic neurological syndrome.
(A, B) T1-weighted plain and contrast MRI results are normal. (C, D) T2-weighted and FLAIR images show bilateral symmetrical basal ganglia hyperintensities. (E) FLAIR image shows bilateral mesial temporal hyperintensities. (F) Diffusion weighted image is normal. Reproduced with permission from Maramattom (2013). CRPM5: Collapsin response mediator protein 5; MRI: magnetic resonance imaging.
PET/computed tomography
There are case reports of patients with CV2/CRMP5 antibody-related chorea that showed bilateral caudate nucleus hyperintensity on T2WI and hypometabolism in the corresponding region on 18F-FDG PET/computed tomography (CT) (Vernino et al., 2002; Vigliani et al., 2011; Dericioglu et al., 2018). Another CV2/CRMP5 antibody-positive patient showed autonomic dysfunction and Parkinson’s disease without brain stem or cerebellar atrophy on brain MRI and symmetrical hypometabolism in the bilateral frontoparietal lobes on 18F-FDG PET/CT scans (Song et al., 2021). Overall, in patients with PNS, 18F-FDG PET/CT is more sensitive to potential abnormalities and has a higher abnormality detection rate than MRI in the early course of the disease (Masangkay et al., 2014). Moreover, 18F-FDG PET/CT scans are important for the early diagnosis of malignancy and can show hypermetabolism at sites where CT or MRI are inefficient (Grativvol et al., 2018; Opalińska et al., 2022). In patients with a high suspicion of paraneoplastic neurological disorder, occult cancer can be detected before clinical manifestations (McKeon et al., 2010; Sechi and Flanagan, 2021). If the first examination is negative, it should be repeated in the next 3–6 months and then every 6 months for up to 4 years (Vedeler et al., 2006).
Neuropathological studies
Regarding the pathology of CV2/CRMP5 antibody-associated PNS, perivascular and parenchymal T lymphocyte infiltration, neuronal loss, gliosis (Muehlschlegel et al., 2005; Figure 4), microglial activation, and rare microglial nodules formed by focal microglial hyperplasia (Cross et al., 2003) are seen in the brain and spinal cord. Cerebellar cortex atrophy and Purkinje cell decrease can be seen in the lesions that are restricted to the cerebellum (Vernino et al., 2002). One case study showed that the lymphocytic infiltrate within the parenchyma consisted exclusively of CD8+ T lymphocytes, whereas the perivascular infiltrate contained both B and T lymphocytes (CD4- and CD8-reactive; Muehlschlegel et al., 2005).
Figure 4.
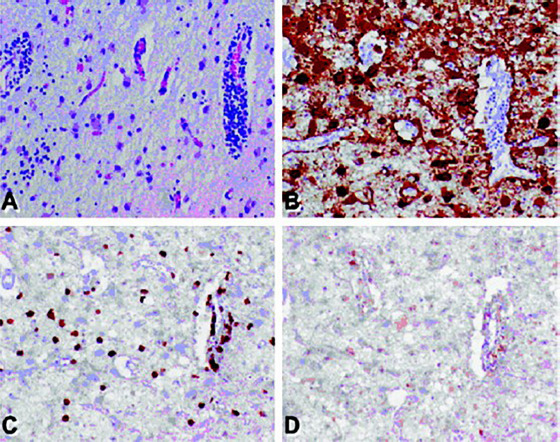
Pathological findings in patients with CV2/CRMP5 antibody-associated paraneoplastic neurological syndrome.
(A) Hematoxylin and eosin-stained section from the caudate nucleus showing perivascular and parenchymal infiltrates of mature lymphocytes, diffuse neuronal loss, and reactive (hypertrophic) gliosis. (B) Glial fibrillary acidic protein-immunostained section showing reactive gliosis. (C) CD8-immunostained section showing strong reactivity, consistent with cytotoxic/suppressor T lymphocytes. (D) CD4 (T-helper cell)-immunostained section showing only rare positivity. Original magnification 500×. Reproduced with permission from Muehlschlegel et al. (2005). CRMP5: Collapsin response mediator protein 5.
The pathological findings of the optic nerve are similar to those of the brain and spinal cord. The autopsy of a CV2/CRMP5 antibody-associated optic neuritis patient showed T lymphocyte infiltration (mainly CD8+ T lymphocytes), axonal and myelin loss, and increased pleomorphic cells and reactive lymphocytes in the vitreous humor (Cross et al., 2003). Cervical cord biopsies of CV2/CRMP5 antibody-positive patients reported by Ducray et al. (2007) showed reactive gliosis, edema, and necrosis with massive macrophage and perivascular lymphocyte infiltration. The extensive T lymphocyte infiltration supports the hypothesis of irreversible neuronal damage caused by cytotoxic T lymphocytes (Shah et al., 2021). Additionally, pathological biopsies of the peroneal nerve showed fiber loss, Wallerian degeneration, demyelination, and onion bulb formation (Antoine et al., 2001).
Other examinations
Fundoscopy, electroencephalography (EEG), and electromyography have unique advantages in the diagnosis of CV2/CRMP5 antibody-associated PNS.
Fundoscopy
Cross et al. (2003) found that all but one of 15 patients with documented fundoscopy showed optic disc edema, commonly combined with hemorrhage of the nerve fiber layer, some with fan-shaped or diffuse atrophy of the optic disc. Fluorescence fundus angiography showed enhanced penetration and leakage of the optic papilla, and some cases had subretinal fluid and dye leakage from vessels distant from the optic disc, suggesting vascular insufficiency (Cross et al., 2003; Figure 5). Another study found that all 12 patients with ocular symptoms had optic disc edema in the fundus (Cohen et al., 2020).
Figure 5.
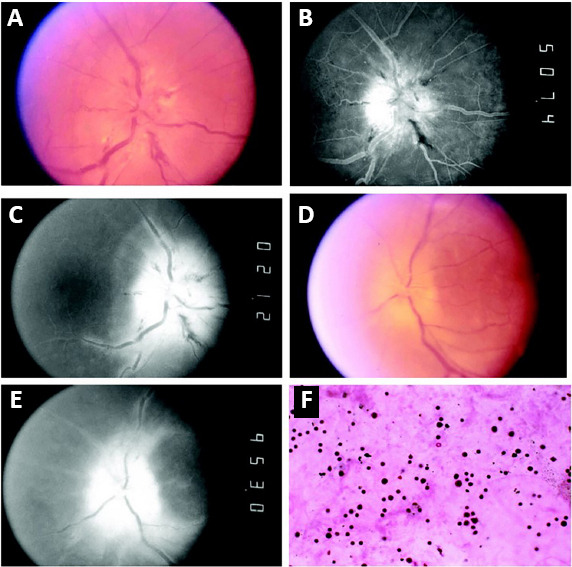
Optic disc photographs, fluorescein images, and vitreous biopsy.
(A) Right eye shows severe acute optic disc swelling with tortuous vessels, cotton wool spots, and nerve fiber layer hemorrhages. (B) Late arterial fluorescein image shows dye leakage on disc surface, and (C) late phase shows dye persistence at the disc and perivenous fluorescence indicates vascular incompetence. (D, E) Left eye shows chronic disc swelling, (D) optic nerve atrophy with dye leakage and (E) vascular incompetence remote from the disc. (F) Pleomorphic cellularity consistent with reactive lymphocytosis in the vitreous humor of the right eye (hematoxylin and eosin, original magnification 400×). Reproduced with permission from Cross et al. (2003).
EEG
EEG is non-specific and may reveal epileptic waves involving the temporal lobe, with focal or global activity (Ibrahim Ismail et al., 2020). Besides, persistent spikes and slow waves may occur during sleep (Hu et al., 2015).
Electromyography
Patients with peripheral neuropathy often have severe axonal damage, mild demyelination, and slowed nerve conduction velocity. Attention should be paid to the presence of LEMS when the amplitudes of the compound motor action potential decrease (Antoine et al., 2001; Honnorat et al., 2009).
Diagnosis and Differential Diagnosis
The diagnostic criteria for PNS were established in 2004 and updated in 2021. Therefore, the diagnosis of PNS is well documented. Antibody detection is of great value in the diagnosis of PNS. Moreover, different antibodies correspond to different types of PNS, which have different clinical characteristics. Summarizing their unique clinical characteristics can help identify and diagnose antibody-negative PNS, especially when multiple antibodies coexist, and help discern whether one of them is a pathogenic antibody or only an immune concomitant antibody reflecting the presence of cancer. In conclusion, documenting the unique clinical characteristics corresponding to different types of antibodies is crucial to improving the accuracy of clinical diagnosis and precision of treatment. Based on this, we summarized and condensed the clinical features of CV2/CRMP5 antibody-associated PNS based on the available literature as follows:
1. Clinical manifestations:
(1) Common symptoms
• LE: dementia, memory impairment, sleep abnormalities, mental behavior abnormalities, and autonomic dysfunction
• Chorea: involuntary, irregular, and symmetrical choreiform movements of the limbs or head and face
• Ocular manifestation: subacute painless vision loss in both eyes, optic disc edema, and defect of the visual field
• CA: vertigo, limb and gait ataxia, dysarthria, and nystagmus
• Myelopathy: transverse myelitis
• Peripheral neuropathy: sensory or sensorimotor involvement mainly in the distal extremities, sensory loss involving all the modalities of sensation, and decreased or absent tendon reflexes
(2) Other symptoms
• Myasthenic syndrome: LEMS: progressive symmetrical fluctuating proximal muscle weakness; myasthenia gravis:, muscle weakness and fatigue
• Opsoclonus-myoclonus ataxia syndrome: ocular myoclonus, myoclonus, and ataxia
• Parkinsonism: slow movements, increased muscle tone, involuntary limb tremors, and static tremor
• Brainstem encephalitis: salivation, somnolence, and dysphagia
• Motor neuron disease: signs and symptoms of upper and lower motor neuron injury
• Devic syndrome: myelopathy and optic neuritis
• Chronic intestinal pseudo-obstruction: symptoms of mechanical intestinal obstruction
(3) Oncological associations: SCLC, thymoma, breast cancer, and other tumors
2. Auxiliary examination
• Antibody: CV2/CRMP5 antibody-positive serum and/or CSF
• CSF: often manifesting as pleocytosis; increased protein levels, sometimes elevated IgG index and/or elevated IgG synthesis rates, and rarely positive oligoclonal bands
• MRI: The brain/spinal cord/optic nerve may appear normal or show multiple lesions, with hyperintensity on T2WI/FLAIR sequences at lesions
• 18F-FDG PET/CT: hypometabolic changes in the corresponding region
• Fundoscopy: optic disc edema, atrophy, nerve fiber layer hemorrhages, and subretinal fluid
• EEG: non-specific changes with epileptic waves
• Electromyography: severe axonal damage, mild demyelination, slow conduction velocity, and reduced compound motor action potential amplitude
• Pathology: perivascular and parenchymal T lymphocyte infiltration, microglial activation, gliosis, neuronal loss, and rare microglial nodules
• Imaging/pathological findings that indicate or confirm the presence of a tumor
The diagnosis of CV2/CRMP5 antibody-associated PNS is based on: (1) the presence of a clinical syndrome as described above, (2) the presence of CV2/CRMP5 antibodies in the serum or CSF, and (3) reasonable exclusion of alternative causes, including those causing CV2/CRPM5 antibody production (i.e., LEMS). According to the diagnostic criteria for PNS updated in 2021, CV2/CRMP5 antibody-positive patients can be diagnosed with definite/probable/possible PNS based on the PNS-Care score. The diagnostic level of “probable” or “possible” may change with the follow-up time (> or < 2 years, found tumor/not found) (Graus et al., 2021). Overall, the different manifestations of CV2/CRMP5 antibody-associated PNS need to be differentiated from other diseases (Table 2).
Table 2.
Differential diagnosis of CV2/CRMP5 antibody-associated PNS
| Manifestations | Differential diagnosis | References |
|---|---|---|
| LE | Gliomas, herpes virus, status epilepticus, anti-LGI1 LE | – |
| Chorea | Anti-IgLON5 disease, Huntington’s disease, atypical manifestations of degenerative and hereditary neurological disorders, toxic and metabolic causes | Kyle et al., 2022; Sturchio et al., 2022 |
| Ocular manifestations | Ischemic causes, infectious inflammatory diseases caused by viruses, bacteria, or spirochetes, nodular disease, lymphoma, multiple sclerosis, and neuromyelitis optica | Cross et al., 2003 |
| CA | Infection encephalopathy, heredity encephalopathy, vasculitis encephalopathy, poisoning encephalopathy, metabolism encephalopathy, cerebellar form of Creutzfeldt-Jacob disease, multiple system atrophy, and GAD antibody-associated CA | Narayan et al., 2020 |
| Myelopathy | Neuromyelitis optica, ischemic myelopathy, multiple sclerosis, and vitamin B12 deficiency | Keegan et al., 2008; Flanagan et al., 2011 |
| Peripheral neuropathy | Diabetes, hypothyroidism/hyperthyroidism, vitamin B12/folic acid deficiency, rheumatoid arthritis, infectious causes, subacute onset peripheral neuropathies, chronic inflammatory demyelinating polyneuropathy, vasculitis, and other PNS affecting peripheral nerves | Castelli et al., 2020; Zoccarato et al., 2021 |
CA: Cerebellar ataxia; GAD: glutamic acid decarboxylase; IgLON5: immunoglobulin-like cell adhesion molecule 5; LE: limbic encephalitis; LGI1: leucine-rich glioma-inactivated 1; PNS: paraneoplastic neurological syndrome.
Treatment
The treatments of CV2/CRMP5 antibody-associated PNS include immunotherapy, detection and treatment of tumors, and symptomatic treatment. Immunotherapy agents include glucocorticoids, intravenous immunoglobulin, plasma exchange, cyclophosphamide, and rituximab (Grativvol et al., 2018). Patients with myelopathy receiving ICIs should stop ICIs and be treated with corticosteroids (Kunchok et al., 2020; Wang et al., 2021). Although most available evidence suggests that intracellular antibody-associated PNS, including CV2/CRMP5 antibody-associated PNS, respond poorly to immunotherapy and that immunotherapy may not improve neurological symptoms, early and aggressive immunotherapy is still recommended (Rosenfeld and Dalmau, 2013). CV2/CRMP5 antibody-associated PNS is highly associated with tumors, and early detection and removal of the tumor is the best option by far. In some cases, neurological deficits improve with anti-tumor therapy (Vernino et al., 2002; Grativvol et al., 2018). In addition, symptomatic and supportive treatment can be given depending on the symptoms of each patient.
Prognosis
The prognosis of CV2/CRMP5 antibody-associated PNS depends on multiple factors, such as the severity of neurological damage at the time of diagnosis, whether the tumor is detected, the stage of the tumor, whether the tumor treatment is effective, and whether early immunotherapy is administered. Overall, intracellular antibody-associated PNS, including CV2/CRMP5 antibody-associated PNS, poorly responds to immunotherapy and anti-tumor therapy, leading to a poor prognosis (Dericioglu et al., 2018). The majority (71%) of CV2/CRMP5 antibody-associated PNS patients exhibited worsening neuropathy impairment scores during treatment (Dubey et al., 2018). However, patients with CV2/CRMP5 antibody-associated PNS had a longer mean survival time and better prognosis than patients with Hu/ANNA-1 antibody-associated PNS, independent of tumor type (Honnorat et al., 2009). In patients with identified malignancies, rapid and aggressive tumor treatment may improve outcomes (Narayan et al., 2020). Finally, neurological adverse effects resulting from the application of ICIs may lead to irreversible or fatal outcomes if not treated in a timely and appropriate manner (Wang et al., 2018).
Limitations
Although it has been nearly 30 years since the discovery of the CV2 antibody, in clinical practice, it is easy to miss CV2/CRMP5 antibody-associated PNS diagnosis because of the low incidence rate and the low awareness of clinicians. During the literature search, we found relatively few studies to include in our study, which brought some difficulties to the writing of the review. Moreover, some strongly relevant articles were not recent, but we still cited them for comprehensiveness; this may affect the review to a certain extent.
Conclusion
CV2/CRMP5 antibody-associated PNS is closely related to tumors, with a long course of disease, diverse clinical manifestations, and poor prognosis. The presence of the CV2/CRMP5 antibody in the CSF and/or serum is a specific indicator for the diagnosis of this disease. Currently, the CV2/CRMP5 antibody is not considered pathogenic, and the most likely underlying mechanism is progressive, irreversible neuron damage and apoptosis mediated by cytotoxic T lymphocytes.
Existing Challenges and Prospects
During the literature collection and summary, we found that some reported cases of CV2/CRMP5 antibody-associated encephalitis actually meet the diagnostic criteria for PNS, and similar cases have frequently been diagnosed as CV2/CRMP5 antibody-associated PNS cases in other reports. Our long-term study also found that the clinical manifestations of antibody-associated PNS and antibody-associated AE overlap and intersect, and the intrinsic association between them needs to be explored and clarified by further studies.
Due to the low incidence of CV2/CRMP5 antibody-associated PNS, the scarcity of case reports, and a lack of long-term systematic research on this disease, no summary of its characteristic clinical manifestations exists. The diagnosis of these cases highly depends on the positive detection of CV2/CRMP5 antibodies, which is restricted by various conditions, and current methods can yield false negative and false positive results. Summarizing its characteristic clinical manifestations will not only help to systematically recognize the disease, but also help to identify antibody-negative CV2/CRMP5 antibody-associated PNS and avoid clinical underdiagnosis or misdiagnosis. When multiple antibodies are present, the characteristic clinical manifestations can be used to identify CV2/CRMP5 antibodies as pathogenic or as concomitant antibodies reflecting the presence of cancer. A comprehensive summary and systematic understanding of the disease will help to promote accurate diagnosis and early treatment of the disease and ultimately improve the prognosis.
Regarding the diagnostic criteria for CV2/CRMP5 antibody-associated PNS, besides continuously enriching the understanding of the characteristic clinical manifestations of the disease, the rapid development of imaging technology and laboratory technology will help to achieve breakthroughs. Note-worthily, 18F-FDG PET/CT has a higher abnormality dection rate than MRI and other techniques in the early stage of the disease. It is also more sensitive to potential abnormalities in AE, and its use is recommended for the brain examination of patients with suspected AE, especially those with normal or non-specific changes on MRI examinations (Probasco et al., 2017; Solnes et al., 2017; Bordonne et al., 2021). Applying it to patients with suspected CV2/CRMP5 antibody-associated PNS would undoubtedly improve diagnosis accuracy. Another method that could improve CV2/CRMP5 antibody-associated PNS diagnosis is high-field MRI, which has been widely used in the study of cerebrovascular diseases and other neurological diseases.
CV2/CRMP5 antibody detection methods include immunofluorescence, western blotting, immunohistochemistry, and CRMP5-CBA. The sensitivity of these methods varies, which may lead to missed diagnoses. It is recommended to use a combination of two methods to increase the positivity rate of antibody testing. Theoretically, CSF antibody testing has higher clinical specificity than serum antibody testing. Therefore, for patients with suspected CV2/CRMP5 antibody-associated PNS and atypical clinical manifestations, simultaneous serum and CSF antibody testing are recommended to maximize specificity. In recent years, immunoadsorption has been used to treat autoimmune diseases. Performing antibody detection in immunoadsorption eluent would help diagnose of CV2/CRMP5 antibody-associated PNS. In some antibody-associated autoimmune diseases, antibody titers are associated with disease severity (Zhang et al., 2018; Fang et al., 2021), poor prognosis (Gresa-Arribas et al., 2014; Wang et al., 2020), and relapse (Zhong et al., 2022). Whether a similar quantitative-effect relationship exists for CV2/CRMP5 antibody-associated PNS needs to be determined. Quantifying CV2/CRMP5 antibodies and exploring the relationship between quantity and disease onset, progression, treatment efficacy, and prognosis may improve the diagnosis and treatment precision of CV2/CRMP5 antibody-associated PNS.
Immune repertoire analysis is an emerging method in immunology research. Combining it with single-cell sequencing technology to study CV2/CRMP5 antibody-associated PNS will help uncover the molecular mechanism of immune abnormalities in this disease and find new therapeutic targets.
In conclusion, there is a paucity of detection and diagnosis methods for CV2/CRMP5 antibody-associated PNS, and effective detection and treatment tools are lacking. However, with the introduction of a large number of new highly sensitive and specific detection technologies, as well as new research methods and technologies, we believe that the diagnosis and treatment of CV2/CRMP5 antibody-associated PNS will soon greatly improve, which will ultimately help improve the prognosis of the disease and bring good news to patients.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China, No. U1604181, Henan Province Key R&D and Promotion Special Project (Science and Technology Tackle), No. 212102310834, Henan Medical Education Research Project, No. Wjlx2020531, and the Joint project of Medical Science and Technology Research Program of Henan Province, No. LHGJ20190078 (all to JW).
Conflicts of interest: The authors report no conflicts of interest in this work.
Data availability statement: Not applicable.
Editor’s evaluation: The authors review the current state of knowledge and research regarding CV2/CRMP5 antibody-associated paraneoplastic neurological syndromes. Specifically, clinical manifestations- including chorea, ocular manifestations, myelopathy, neuropathy, cerebellar ataxia, limbic encephalitis, and other symptoms. They also discuss the natural history of the disease, diagnostic evaluation, treatment, and prognosis. The authors assert that they are the first group to compile a review on this disease.
C-Editor: Zhao M; S-Editor: Li CH; L-Editors: Li CH, Song LP; T-Editor: Jia Y
References
- 1.Aliprandi A, Terruzzi A, Rigamonti A, Bazzigaluppi E, Tremolizzo L, Ferrarese C, Salmaggi A. Paraneoplastic cerebellar degeneration with anti-CV2/CRMP5 antibodies and prostate adenocarcinoma. Neurol Sci. 2015;36:1501–1503. doi: 10.1007/s10072-015-2113-5. [DOI] [PubMed] [Google Scholar]
- 2.Antoine JC, Honnorat J, Vocanson C, Koenig F, Aguera M, Belin MF, Michel D. Posterior uveitis, paraneoplastic encephalomyelitis and auto-antibodies reacting with developmental protein of brain and retina. J Neurol Sci. 1993;117:215–223. doi: 10.1016/0022-510x(93)90176-y. [DOI] [PubMed] [Google Scholar]
- 3.Antoine JC, Honnorat J, Camdessanché JP, Magistris M, Absi L, Mosnier JF, Petiot P, Kopp N, Michel D. Paraneoplastic anti-CV2 antibodies react with peripheral nerve and are associated with a mixed axonal and demyelinating peripheral neuropathy. Ann Neurol. 2001;49:214–221. doi: 10.1002/1531-8249(20010201)49:2<214::aid-ana41>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 4.Babikian VL, Stefansson K, Dieperink ME, Arnason BG, Marton LS, Levy BE. Paraneoplastic myelopathy:antibodies against protein in normal spinal cord and underlying neoplasm. Lancet. 1985;2:49–50. doi: 10.1016/s0140-6736(85)90107-2. [DOI] [PubMed] [Google Scholar]
- 5.Bataller L, Dalmau J. Neuro-ophthalmology and paraneoplastic syndromes. Curr Opin Neurol. 2004;17:3–8. doi: 10.1097/00019052-200402000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Berger B, Klingler AJ, Rauer S, Stich O. Absent well-characterized onconeural antibodies in 283 patients with various polyneuropathies:A retrospective monocenter study. J Neurol Sci. 2020;413:116804. doi: 10.1016/j.jns.2020.116804. [DOI] [PubMed] [Google Scholar]
- 7.Berzero G, Psimaras D. Neurological paraneoplastic syndromes:an update. Curr Opin Oncol. 2018;30:359–367. doi: 10.1097/CCO.0000000000000479. [DOI] [PubMed] [Google Scholar]
- 8.Bordonne M, Chawki MB, Doyen M, Kas A, Guedj E, Tyvaert L, Verger A. Brain (18)F-FDG PET for the diagnosis of autoimmune encephalitis:a systematic review and a meta-analysis. European journal of nuclear medicine and molecular imaging. 2021;48:3847–3858. doi: 10.1007/s00259-021-05299-y. [DOI] [PubMed] [Google Scholar]
- 9.Bretin S, Reibel S, Charrier E, Maus-Moatti M, Auvergnon N, Thevenoux A, Glowinski J, Rogemond V, Prémont J, Honnorat J, Gauchy C. Differential expression of CRMP1, CRMP2A, CRMP2B, and CRMP5 in axons or dendrites of distinct neurons in the mouse brain. J Comp Neurol. 2005;486:1–17. doi: 10.1002/cne.20465. [DOI] [PubMed] [Google Scholar]
- 10.Brot S, Malleval C, Benetollo C, Auger C, Meyronet D, Rogemond V, Honnorat J, Moradi-Améli M. Identification of a new CRMP5 isoform present in the nucleus of cancer cells and enhancing their proliferation. Exp Cell Res. 2013;319:588–599. doi: 10.1016/j.yexcr.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Budhram A, Leung A, Nicolle MW, Burneo JG. Diagnosing autoimmune limbic encephalitis. CMAJ. 2019;191:E529–534. doi: 10.1503/cmaj.181548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camdessanché JP, Ferraud K, Boutahar N, Lassablière F, Mutter M, Touret M, Kolattukudy P, Honnorat J, Antoine JC. The collapsin response mediator protein 5 onconeural protein is expressed in Schwann cells under axonal signals and regulates axon-Schwann cell interactions. J Neuropathol Exp Neurol. 2012;71:298–311. doi: 10.1097/NEN.0b013e31824d1df2. [DOI] [PubMed] [Google Scholar]
- 13.Carette T, Mulquin N, van Pesch V, London F. Simultaneous bilateral optic neuropathy and myelitis revealing paraneoplastic neurological syndrome associated with multiple onconeuronal antibodies. Mult Scler Relat Disord. 2021;49:102789. doi: 10.1016/j.msard.2021.102789. [DOI] [PubMed] [Google Scholar]
- 14.Castelli G, Desai KM, Cantone RE. Peripheral neuropathy:evaluation and differential diagnosis. Am Fam Physician. 2020;102:732–739. [PubMed] [Google Scholar]
- 15.Cohen DA, Bhatti MT, Pulido JS, Lennon VA, Dubey D, Flanagan EP, Pittock SJ, Klein CJ, Chen JJ. Collapsin response-mediator protein 5-associated retinitis, vitritis, and optic disc edema. Ophthalmology. 2020;127:221–229. doi: 10.1016/j.ophtha.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Cross SA, Salomao DR, Parisi JE, Kryzer TJ, Bradley EA, Mines JA, Lam BL, Lennon VA. Paraneoplastic autoimmune optic neuritis with retinitis defined by CRMP-5-IgG. Ann Neurol. 2003;54:38–50. doi: 10.1002/ana.10587. [DOI] [PubMed] [Google Scholar]
- 17.Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol. 2008;7:327–340. doi: 10.1016/S1474-4422(08)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349:1543–1554. doi: 10.1056/NEJMra023009. [DOI] [PubMed] [Google Scholar]
- 19.Dericioglu N, Gocmen R, Tan E. Paraneoplastic striatal encephalitis and myelitis associated with anti-CV2/CRMP-5 antibodies in a patient with small cell lung cancer. Clin Neurol Neurosurg. 2018;170:117–119. doi: 10.1016/j.clineuro.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Dubey D, Lennon VA, Gadoth A, Pittock SJ, Flanagan EP, Schmeling JE, McKeon A, Klein CJ. Autoimmune CRMP5 neuropathy phenotype and outcome defined from 105 cases. Neurology. 2018;90:e103–110. doi: 10.1212/WNL.0000000000004803. [DOI] [PubMed] [Google Scholar]
- 21.Ducray F, Roos-Weil R, Garcia PY, Slesari J, Heinzlef O, Chatelain D, Toussaint P, Roullet E, Honnorat J. Devic's syndrome-like phenotype associated with thymoma and anti-CV2/CRMP5 antibodies. J Neurol Neurosurg Psychiatry. 2007;78:325–327. doi: 10.1136/jnnp.2006.097972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang H, Hu W, Jiang Z, Yang H, Liao H, Yang L, Wu L. Autoimmune glial fibrillary acidic protein astrocytopathy in children:a retrospective analysis of 35 cases. Front Immunol. 2021;12:761354. doi: 10.3389/fimmu.2021.761354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flabeau O, Laurent C, Schneider S, Honnorat J, Ellie E. Spinal cord tractopathy in paraneoplastic anti-CV2/CRMP5 myelitis responsive to plasma exchange. Rev Neurol (Paris) 2022;178:280–282. doi: 10.1016/j.neurol.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Flanagan EP, McKeon A, Lennon VA, Kearns J, Weinshenker BG, Krecke KN, Matiello M, Keegan BM, Mokri B, Aksamit AJ, Pittock SJ. Paraneoplastic isolated myelopathy:clinical course and neuroimaging clues. Neurology. 2011;76:2089–2095. doi: 10.1212/WNL.0b013e31821f468f. [DOI] [PubMed] [Google Scholar]
- 25.Flanagan EP, Keegan BM. Paraneoplastic myelopathy. Neurol Clin. 2013;31:307–318. doi: 10.1016/j.ncl.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Fukada M, Watakabe I, Yuasa-Kawada J, Kawachi H, Kuroiwa A, Matsuda Y, Noda M. Molecular characterization of CRMP5, a novel member of the collapsin response mediator protein family. J Biol Chem. 2000;275:37957–37965. doi: 10.1074/jbc.M003277200. [DOI] [PubMed] [Google Scholar]
- 27.Gill A, Perez MA, Perrone CM, Bae CJ, Pruitt AA, Lancaster E. A case series of PD-1 inhibitor-associated paraneoplastic neurologic syndromes. J Neuroimmunol. 2019;334:576980. doi: 10.1016/j.jneuroim.2019.576980. [DOI] [PubMed] [Google Scholar]
- 28.Giometto B, Grisold W, Vitaliani R, Graus F, Honnorat J, Bertolini G. Paraneoplastic neurologic syndrome in the PNS Euronetwork database:a European study from 20 centers. Arch Neurol. 2010;67:330–335. doi: 10.1001/archneurol.2009.341. [DOI] [PubMed] [Google Scholar]
- 29.Grativvol RS, Cavalcante WCP, Castro LHM, Nitrini R, Simabukuro MM. Updates in the diagnosis and treatment of paraneoplastic neurologic syndromes. Curr Oncol Rep. 2018;20:92. doi: 10.1007/s11912-018-0721-y. [DOI] [PubMed] [Google Scholar]
- 30.Graus F, Delattre JY, Antoine JC, Dalmau J, Giometto B, Grisold W, Honnorat J, Smitt PS, Vedeler C, Verschuuren JJ, Vincent A, Voltz R. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004;75:1135–1140. doi: 10.1136/jnnp.2003.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graus F, Dalmau J. Paraneoplastic neurological syndromes. Curr Opin Neurol. 2012;25:795–801. doi: 10.1097/WCO.0b013e328359da15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graus F, Vogrig A, Muñiz-Castrillo S, Antoine JG, Desestret V, Dubey D, Giometto B, Irani SR, Joubert B, Leypoldt F, McKeon A, Prüss H, Psimaras D, Thomas L, Titulaer MJ, Vedeler CA, Verschuuren JJ, Dalmau J, Honnorat J. Updated diagnostic criteria for paraneoplastic neurologic syndromes. Neurol Neuroimmunol Neuroinflamm. 2021;8:e1014. doi: 10.1212/NXI.0000000000001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gresa-Arribas N, Titulaer MJ, Torrents A, Aguilar E, McCracken L, Leypoldt F, Gleichman AJ, Balice-Gordon R, Rosenfeld MR, Lynch D, Graus F, Dalmau J. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis:a retrospective study. Lancet Neurol. 2014;13:167–177. doi: 10.1016/S1474-4422(13)70282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ha J, Na BS, Ahn JH, Kim M, Kim JW, Lee JH, Cho JW, Kim JS, Youn J. Anti-CV2/CRMP5 paraneoplastic chorea effectively managed with intravenous amantadine. Tremor Other Hyperkinet Mov (N Y) 2019 doi: 10.7916/tohm.v0.701. doi:10.7916/tohm.v0.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hean V, Camdessanché JP, Cathébras P, Killian M. Paraneoplastic subacute sensory neuropathy with triple positive antineuronal antibodies associated with small-cell lung cancer. BMJ Case Rep. 2020;13:e235668. doi: 10.1136/bcr-2020-235668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Honnorat J, Antoine JC, Belin MF. Are the “newly discovered”paraneoplastic anticollapsin response-mediator protein 5 antibodies simply anti-CV2 antibodies? Ann Neurol. 2001;50:688–691. doi: 10.1002/ana.1270. [DOI] [PubMed] [Google Scholar]
- 37.Honnorat J, Antoine JC, Derrington E, Aguera M, Belin MF. Antibodies to a subpopulation of glial cells and a 66 kDa developmental protein in patients with paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 1996;61:270–278. doi: 10.1136/jnnp.61.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Honnorat J, Cartalat-Carel S, Ricard D, Camdessanche JP, Carpentier AF, Rogemond V, Chapuis F, Aguera M, Decullier E, Duchemin AM, Graus F, Antoine JC. Onco-neural antibodies and tumour type determine survival and neurological symptoms in paraneoplastic neurological syndromes with Hu or CV2/CRMP5 antibodies. J Neurol Neurosurg Psychiatry. 2009;80:412–416. doi: 10.1136/jnnp.2007.138016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu LY, Shi XY, Feng C, Wang JW, Yang G, Lammers SH, Yang XF, Ebrahimi-Fakhari D, Zou LP. An 8-year old boy with continuous spikes and waves during slow sleep presenting with positive onconeuronal antibodies. Eur J Paediatr Neurol. 2015;19:257–261. doi: 10.1016/j.ejpn.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 40.Ibrahim Ismail I, J KJ, Ibrahim M, Al-Hashel JY. Paraneoplastic limbic encephalitis associated with anti-CV2/CRMP5 antibodies secondary to thymoma in an adolescent. Case Rep Neurol. 2020;12:50–55. doi: 10.1159/000505232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jarius S, Wandinger KP, Borowski K, Stoecker W, Wildemann B. Antibodies to CV2/CRMP5 in neuromyelitis optica-like disease:case report and review of the literature. Clin Neurol Neurosurg. 2012;114:331–335. doi: 10.1016/j.clineuro.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 42.Jeanne M, Demory H, Moutal A, Vuillaume ML, Blesson S, Thépault RA, Marouillat S, Halewa J, Maas SM, Motazacker MM, Mancini GMS, van Slegtenhorst MA, Andreou A, Cox H, Vogt J, Laufman J, Kostandyan N, Babikyan D, Hancarova M, Bendova S, et al. Missense variants in DPYSL5 cause a neurodevelopmental disorder with corpus callosum agenesis and cerebellar abnormalities. Am J Hum Genet. 2021;108:951–961. doi: 10.1016/j.ajhg.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jia Y, Wang J, Xue L, Hou Y. Limbic encephalitis associated with AMPA receptor and CRMP5 antibodies:A case report and literature review. Brain Behav. 2020;10:e01528. doi: 10.1002/brb3.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keegan BM, Pittock SJ, Lennon VA. Autoimmune myelopathy associated with collapsin response-mediator protein-5 immunoglobulin G. Ann Neurol. 2008;63:531–534. doi: 10.1002/ana.21324. [DOI] [PubMed] [Google Scholar]
- 45.Khan U, Warriach SA. Rare case: paraneoplastic syndrome affecting peripheral nerves, associated with anti-collapsin-response mediator protein-5 (anti-CRMP5) antibodies, as early manifestation of small cell lung cancer confined to a solitary lymph node without evidence of lung mass on routine CT thorax. BMJ Case Rep. 2020;13:e232656. doi: 10.1136/bcr-2019-232656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kunchok A, Zekeridou A, Pittock S. CRMP5-IgG-associated paraneoplastic myelopathy with PD-L1 inhibitor therapy. JAMA Neurol. 2020;77:255–256. doi: 10.1001/jamaneurol.2019.4379. [DOI] [PubMed] [Google Scholar]
- 47.Kyle K, Bordelon Y, Venna N, Linnoila J. Autoimmune and paraneoplastic chorea:a review of the literature. Front Neurol. 2022;13:829076. doi: 10.3389/fneur.2022.829076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H, Zhang A, Hao Y, Guan H, Lv Z. Coexistence of Lambert-Eaton myasthenic syndrome and autoimmune encephalitis with anti-CRMP5/CV2 and anti-GABAB receptor antibodies in small cell lung cancer:A case report. Medicine (Baltimore) 2018;97:e0696. doi: 10.1097/MD.0000000000010696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lim TT. Paraneoplastic autoimmune movement disorders. Parkinsonism Relat Disord. 2017;44:106–109. doi: 10.1016/j.parkreldis.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 50.Maramattom BV. Paraneoplastic CRMP-5 basal ganglionitis and limbic encephalitis in an elderly Indian lady. Neurol India. 2013;61:534–535. doi: 10.4103/0028-3886.121941. [DOI] [PubMed] [Google Scholar]
- 51.Masangkay N, Basu S, Moghbel M, Kwee T, Alavi A. Brain 18F-FDG-PET characteristics in patients with paraneoplastic neurological syndrome and its correlation with clinical and MRI findings. Nucl Med Commun. 2014;35:1038–1046. doi: 10.1097/MNM.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 52.McKeon A, Apiwattanakul M, Lachance DH, Lennon VA, Mandrekar JN, Boeve BF, Mullan B, Mokri B, Britton JW, Drubach DA, Pittock SJ. Positron emission tomography-computed tomography in paraneoplastic neurologic disorders:systematic analysis and review. Arch Neurol. 2010;67:322–329. doi: 10.1001/archneurol.2009.336. [DOI] [PubMed] [Google Scholar]
- 53.Monstad SE, Nøstbakken JK, Vedeler CA. CRMP5 antibodies found in a patient with limbic encephalitis and myasthenia gravis. J Neurol Neurosurg Psychiatry. 2009;80:241–242. doi: 10.1136/jnnp.2008.149336. [DOI] [PubMed] [Google Scholar]
- 54.Monstad SE, Drivsholm L, Skeie GO, Aarseth JH, Vedeler CA. CRMP5 antibodies in patients with small-cell lung cancer or thymoma. Cancer Immunol Immunother. 2008;57:227–232. doi: 10.1007/s00262-007-0369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muehlschlegel S, Okun MS, Foote KD, Coco D, Yachnis AT, Fernandez HH. Paraneoplastic chorea with leukoencephalopathy presenting with obsessive-compulsive and behavioral disorder. Mov Disord. 2005;20:1523–1527. doi: 10.1002/mds.20570. [DOI] [PubMed] [Google Scholar]
- 56.Nakajima M, Uchibori A, Ogawa Y, Miyazaki T, Ichikawa Y, Kaneko K, Takahashi T, Nakashima I, Shiraishi H, Motomura M, Chiba A. CV2/CRMP5-antibody-related paraneoplastic optic neuropathy associated with small-cell lung cancer. Intern Med. 2018;57:1645–1649. doi: 10.2169/internalmedicine.9736-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Narayan RN, McKeon A, Fife TD. Autoimmune vestibulocerebellar syndromes. Semin Neurol. 2020;40:97–115. doi: 10.1055/s-0039-3402061. [DOI] [PubMed] [Google Scholar]
- 58.O'Toole O, Lennon VA, Ahlskog JE, Matsumoto JY, Pittock SJ, Bower J, Fealey R, Lachance DH, McKeon A. Autoimmune chorea in adults. Neurology. 2013;80:1133–1144. doi: 10.1212/WNL.0b013e3182886991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Opalińska M, Sowa-Staszczak A, Wężyk K, Jagiełła J, Słowik A, Hubalewska-Dydejczyk A. Additional value of [18F]FDG PET/CT in detection of suspected malignancy in patients with paraneoplastic neurological syndromes having negative results of conventional radiological imaging. J Clin Med. 2022;11:1537. doi: 10.3390/jcm11061537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Popławska-Domaszewicz K, Florczak-Wyspiańska J, Kozubski W, Michalak S. Paraneoplastic movement disorders. Rev Neurosci. 2018;29:745–755. doi: 10.1515/revneuro-2017-0081. [DOI] [PubMed] [Google Scholar]
- 61.Probasco JC, Solnes L, Nalluri A, Cohen J, Jones KM, Zan E, Javadi MS, Venkatesan A. Abnormal brain metabolism on FDG-PET/CT is a common early finding in autoimmune encephalitis. Neurol Neuroimmunol Neuroinflamm. 2017;4:e352. doi: 10.1212/NXI.0000000000000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pulido J, Cross SA, Lennon VA, Pulido J, Swanson D, Muench M, Lachance DH. Bilateral autoimmune optic neuritis and vitreitis related to CRMP-5-IgG:intravitreal triamcinolone acetonide therapy of four eyes. Eye (Lond) 2008;22:1191–1193. doi: 10.1038/sj.eye.6702959. [DOI] [PubMed] [Google Scholar]
- 63.Qiao S, Zhang SC, Wang ZH, Wang L, Zhang RR, Li HY, Jin Y, Liu LL, Wang ML, Wang AH, Liu XW. Coexistence of multiple anti-neuronal antibodies in autoimmune encephalitis in China:A multi-center study. Front Immunol. 2022;13:858766. doi: 10.3389/fimmu.2022.858766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qvale TH, Storstein A, Mazengia K, Eagan TM, Bakke PS, Vedeler CA. Paraneoplastic Hu and CRMP5 antibodies are present in smokers without cancer or neurological disease. Respirology. 2014;19:730–734. doi: 10.1111/resp.12292. [DOI] [PubMed] [Google Scholar]
- 65.Rogemond V, Honnorat J. Anti-CV2 autoantibodies and paraneoplastic neurological syndromes. Clin Rev Allergy Immunol. 2000;19:51–59. doi: 10.1385/CRIAI:19:1:51. [DOI] [PubMed] [Google Scholar]
- 66.Rosenfeld MR, Dalmau J. Diagnosis and management of paraneoplastic neurologic disorders. Curr Treat Options Oncol. 2013;14:528–538. doi: 10.1007/s11864-013-0249-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ruiz-García R, Martínez-Hernández E, Saiz A, Dalmau J, Graus F. The diagnostic value of onconeural antibodies depends on how they are tested. Front Immunol. 2020;11:1482. doi: 10.3389/fimmu.2020.01482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sabater L, Saiz A, Dalmau J, Graus F. Pitfalls in the detection of CV2 (CRMP5) antibodies. J Neuroimmunol. 2016;290:80–83. doi: 10.1016/j.jneuroim.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 69.Saloustros E, Zaganas I, Mavridis M, Vamvakas L, Plaitakis A, Georgoulias V, Mavroudis D. Anti-CV2 associated cerebellar degeneration after complete response to chemoradiation of head and neck carcinoma. J Neurooncol. 2010;97:291–294. doi: 10.1007/s11060-009-0022-2. [DOI] [PubMed] [Google Scholar]
- 70.Sechi E, Flanagan EP. Antibody-mediated autoimmune diseases of the CNS:challenges and approaches to diagnosis and management. Front Neurol. 2021;12:673339. doi: 10.3389/fneur.2021.673339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seluk L, Taliansky A, Yonath H, Gilburd B, Amital H, Shoenfeld Y, Kivity S. A large screen for paraneoplastic neurological autoantibodies;diagnosis and predictive values. Clin Immunol. 2019;199:29–36. doi: 10.1016/j.clim.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 72.Shah S, Vazquez Do Campo R, Kumar N, McKeon A, Flanagan EP, Klein C, Pittock SJ, Dubey D. Paraneoplastic myeloneuropathies:clinical, oncologic, and serologic accompaniments. Neurology. 2021;96:e632–639. doi: 10.1212/WNL.0000000000011218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shah S, Flanagan EP, Paul P, Smith CY, Bryant SC, Devine MF, Lennon VA, McKeon A, Pittock SJ, Dubey D. Population-based epidemiology study of paraneoplastic neurologic syndromes. Neurol Neuroimmunol Neuroinflamm. 2022;9:e1124. doi: 10.1212/NXI.0000000000001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shibata K, Nishimura Y, Sakura H. Spontaneous regression of small cell lung carcinoma and associated hemichorea. Intern Med. 2021;60:3817–3821. doi: 10.2169/internalmedicine.7190-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Solnes LB, Jones KM, Rowe SP, Pattanayak P, Nalluri A, Venkatesan A, Probasco JC, Javadi MS. Diagnostic value of (18)F-FDG PET/CT versus MRI in the setting of antibody-specific autoimmune encephalitis. J Nucl Med. 2017;58:1307–1313. doi: 10.2967/jnumed.116.184333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Song J, Zhang Y, Lang Y, Wang YH, Shao J, Cui L. Parkinsonism and dysautonomia with anti-CV2/CRMP5 associated paraneoplastic neurological syndromes mimicking multiple system atrophy:a case report. BMC Neurol. 2021;21:408. doi: 10.1186/s12883-021-02448-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sturchio A, Dwivedi AK, Gastaldi M, Grimberg MB, Businaro P, Duque KR, Vizcarra JA, Abdelghany E, Balint B, Marsili L, Espay AJ. Movement disorders associated with neuronal antibodies:a data-driven approach. J Neurol. 2022;269:3511–3521. doi: 10.1007/s00415-021-10934-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tada S, Furuta M, Fukada K, Hirozawa D, Matsui M, Aoike F, Okuno T, Sawada J, Mochizuki H, Hazama T. Severe parkinsonism associated with anti-CRMP5 antibody-positive paraneoplastic neurological syndrome and abnormal signal intensity in the bilateral basal ganglia. J Neurol Neurosurg Psychiatry. 2016;87:907–910. doi: 10.1136/jnnp-2015-311569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tolkovsky A, Kipervasser S, Fainmesser Y, Alcalay Y, Gadoth A. A paraneoplastic syndrome misdiagnosed as ALS:What are the red flags?A case report and review of the literature. J Neuroimmunol. 2021;358:577635. doi: 10.1016/j.jneuroim.2021.577635. [DOI] [PubMed] [Google Scholar]
- 80.Totland C, Haugen M, Vedeler C. CRMP5 antibodies-diagnostic challenges. Front Neurol. 2021;12:729075. doi: 10.3389/fneur.2021.729075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tüzün E, Dalmau J. Limbic encephalitis and variants:classification, diagnosis and treatment. Neurologist. 2007;13:261–271. doi: 10.1097/NRL.0b013e31813e34a5. [DOI] [PubMed] [Google Scholar]
- 82.Vakrakou A, Constantinides VC, Velonakis G, Tzartos JS, Stefanis L, Kapaki E, Paraskevas GP. Paraneoplastic basal ganglia encephalitis associated with anti-CV2/CRMP-5 and anti-Yo antibodies in a patient with non-small-cell lung cancer. Neurol Sci. 2020;41:2649–2651. doi: 10.1007/s10072-020-04399-1. [DOI] [PubMed] [Google Scholar]
- 83.Vaswani PA, Kimchi EY, Hung AY. CRMP-5-IgG associated paraneoplastic chorea. Mov Disord Clin Pract. 2020;7:713–715. doi: 10.1002/mdc3.13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vedeler CA, Antoine JC, Giometto B, Graus F, Grisold W, Hart IK, Honnorat J, Sillevis Smitt PA, Verschuuren JJ, Voltz R Paraneoplastic Neurological Syndrome Euronetwork. Management of paraneoplastic neurological syndromes:report of an EFNS Task Force. Eur J Neurol. 2006;13:682–690. doi: 10.1111/j.1468-1331.2006.01266.x. [DOI] [PubMed] [Google Scholar]
- 85.Vernino S, Tuite P, Adler CH, Meschia JF, Boeve BF, Boasberg P, Parisi JE, Lennon VA. Paraneoplastic chorea associated with CRMP-5 neuronal antibody and lung carcinoma. Ann Neurol. 2002;51:625–630. doi: 10.1002/ana.10178. [DOI] [PubMed] [Google Scholar]
- 86.Vigliani MC, Honnorat J, Antoine JC, Vitaliani R, Giometto B, Psimaras D, Franchino F, Rossi C, Graus F. Chorea and related movement disorders of paraneoplastic origin:the PNS EuroNetwork experience. J Neurol. 2011;258:2058–2068. doi: 10.1007/s00415-011-6074-1. [DOI] [PubMed] [Google Scholar]
- 87.Voltz R. Paraneoplastic neurological syndromes:an update on diagnosis, pathogenesis, and therapy. Lancet Neurol. 2002;1:294–305. doi: 10.1016/s1474-4422(02)00135-7. [DOI] [PubMed] [Google Scholar]
- 88.Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, Rathmell WK, Ancell KK, Balko JM, Bowman C, Davis EJ, Chism DD, Horn L, Long GV, Carlino MS, Lebrun-Vignes B, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 4:1721–1728. doi: 10.1001/jamaoncol.2018.3923. Erratum in: JAMA Oncol. 2018;4:1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang L, Lou H, Li B, Li J, Yang YM. Paraneoplastic myelitis associated with durvalumab treatment for extensive-stage small cell lung cancer. Invest New Drugs. 2021;40:151–156. doi: 10.1007/s10637-021-01154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Y, Miao A, Shi Y, Ge J, Wang L, Yu C, Xu H, Yu Y, Huang S, Li Y, Wang X. Influencing electroclinical features and prognostic factors in patients with anti-NMDAR encephalitis:a cohort follow-up study in Chinese patients. Sci Rep. 2020;10:10753. doi: 10.1038/s41598-020-67485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu X, Wang H, Xu G, Lin Y. Anti-CV2 autoimmune encephalitis with parkinson-like symptoms and bilateral leukoencephalopathy-A case report. Front Neurol. 2019;10:1064. doi: 10.3389/fneur.2019.01064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yan J, Chen Z, Liang Y, Yang H, Cao L, Zhou Y, Zhao Y, Zhang Y. Anti-CV2/CRMP5 antibody-positive paraneoplastic neurological syndromes with chronic intestinal pseudo-obstruction in a small-cell lung cancer patient:a case report and literature review. J Int Med Res. 2020;48:300060520974466. doi: 10.1177/0300060520974466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yap JY, Wan HItam WH, Abdul Halim S, Masnon NA. Paraneoplastic optic neuropathy secondary to adenocarcinoma of the lung. BMJ Case Rep. 2021;14:e242082. doi: 10.1136/bcr-2021-242082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu Z, Kryzer TJ, Griesmann GE, Kim K, Benarroch EE, Lennon VA. CRMP-5 neuronal autoantibody:marker of lung cancer and thymoma-related autoimmunity. Ann Neurol. 2001;49:146–154. [PubMed] [Google Scholar]
- 95.Zekeridou A, Majed M, Heliopoulos I, Lennon VA. Paraneoplastic autoimmunity and small-cell lung cancer:Neurological and serological accompaniments. Thorac Cancer. 2019;10:1001–1004. doi: 10.1111/1759-7714.13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang Y, Liu G, Jiang M, Chen W, He Y, Su Y. Clinical characteristics and prognosis of severe anti-N-methyl-D-aspartate receptor encephalitis patients. Neurocrit Care. 2018;29:264–272. doi: 10.1007/s12028-018-0536-6. [DOI] [PubMed] [Google Scholar]
- 97.Zhong R, Chen Q, Zhang X, Zhang H, Lin W. Relapses of anti-NMDAR, anti-GABABR and anti-LGI1 encephalitis:a retrospective cohort study. Front Immunol. 2022;13:918396. doi: 10.3389/fimmu.2022.918396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zoccarato M, Grisold W, Grisold A, Poretto V, Boso F, Giometto B. Paraneoplastic neuropathies:what's new since the 2004 recommended diagnostic criteria. Front Neurol. 2021;12:706169. doi: 10.3389/fneur.2021.706169. [DOI] [PMC free article] [PubMed] [Google Scholar]


