Key Words: cortex, functional recovery, JAK1, LZ-3, macrophage, microglia, monocyte locomotion inhibitory factor, phagocytosis, polarization, post-stroke, STAT6
Abstract
We previously found that monocyte locomotion inhibitory factor has a neuroprotective effect on ischemic brain injury during the acute phase of stroke. Therefore, we modified the structure of an anti-inflammatory monocyte locomotion inhibitory factor peptide to construct an active cyclic peptide—Cyclo (MQCNS) (LZ-3)—and investigated its effects on ischemic stroke. In this study, we established a rat model of ischemic stroke by occluding the middle cerebral artery and then administered LZ-3 (2 or 4 mg/kg) via the tail vein for 7 consecutive days. Our results showed that LZ-3 (2 or 4 mg/kg) substantially decreased infarct volume, reduced cortical nerve cell death, improved neurological function, reduced cortical and hippocampal injury, and decreased the levels of inflammatory factors in the blood and brain tissues. In a well-differentiated, oxygen-glucose deprivation/reoxygenation-induced BV2 cell model of post-stroke, LZ-3 (100 μM) inhibited the JAK1-STAT6 signaling pathway. LZ-3 regulated microglia/macrophage polarization from the M1 to the M2 type and inhibited microglia/macrophage phagocytosis and migration via the JAK1/STAT6 signaling pathway. To conclude, LZ-3 regulates microglial activation by inhibiting the JAK1/STAT6 signaling pathway and improves functional recovery post-stroke.
Introduction
Stroke is a leading contributor to death and disability globally, particularly in low- and middle-income countries. The increasing burden of stroke has become a serious social problem. Plasticity after stroke often contributes to reducing neural lesions; however, this reduction is often incomplete, and the degree of recovery of neurological function varies among individuals. Following a stroke, a significant number of patients have permanent disabilities. Several restorative therapies are being developed with the aim of amplifying functional restitution of damaged or nearby ipsilesional regions by fostering neuroplastic changes, but they do not sufficiently meet patient needs (Cassidy and Cramer, 2017).
Little is known about the chronic phase of post-stroke inflammation, despite a growing body of literature on the acute effects of neuroinflammation. Tumor necrosis factor-α (TNF-α) serum levels were found to be elevated in patients 6 months post-stroke, and were positively correlated with proapoptotic markers, implying that neuroinflammation may lead to continued cell death following a stroke (Pascotini et al., 2015). Endothelial necroptosis mediated by microglia-derived TNF-α after ischemic stroke aggravates disruption of the blood-brain barrier (Chen et al., 2019). In another study performed in rats, despite a reduction in inflammation at the primary infarct site, an increase in inflammation was observed in the ipsilateral thalamus 7 months after stroke (Walberer et al., 2014). Therefore, the inflammatory response caused by ischemic injury to the brain may cause inflammatory injury in other parts of the brain, or even throughout the whole brain. Consequently, chronic neuroinflammation may contribute to the onset of chronic neurological complications following stroke, as well as to ongoing neurological degeneration.
Microglia are the main immune cells involved in the inflammatory response in ischemic brain tissue. In response to various stressors, microglia are rapidly activated and polarize into M1- or M2-type microglia that increase or decrease injury (Wang et al., 2018; Chen et al., 2021; Lang et al., 2021; Yu et al., 2022). When an ischemic stroke occurs, microglia are activated, and circulating immune cells invade the infarct and peri-infarct region. Post-stroke inflammatory responses are orchestrated by resident and infiltrating immune cells, which communicate with each other and ischemic neurons using soluble and membrane-bound signaling molecules. After stroke, inflammation can have both negative and positive effects. In addition to contributing to infarct expansion, inflammation is responsible for infarction and remodeling (Lambertsen et al., 2019). Chronic inflammation is more likely to exacerbate injury than acute inflammation, which serves many protective functions. The Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway regulates cytokine signaling and is crucial to stroke pathogenesis and progression. JAK1 activation occurs after the binding of interleukin (IL)-4 to its receptor (Deszo et al., 2004), which forms an active receptor complex and phosphorylates JAK1. In response, activated JAKs transmit cytokine signals from membrane receptors, resulting in STAT phosphorylation (Stark et al., 1998). Phosphorylated STAT proteins dimerize, and the dimers translocate to the nucleus to regulate target gene transcription (Hou et al., 1994). In inflammatory diseases, IL-4 activates STAT6 to regulate M1/M2 polarization via a STAT6-dependent pathway (Koh et al., 2018). Chitinase-3-like protein 1 deletion accelerates stroke development by increasing neuroinflammation and decreasing STAT6-dependent M2 macrophage polarization (Im et al., 2020). Many drugs have neuroprotective effects by modulating microglial activation and polarization after ischemic stroke, according to accumulating evidence (Liu et al., 2019a; Jackson et al., 2020; Yang et al., 2020). Recent studies have also shown that inhibiting neuroinflammation can improve cerebral function after stroke (Zhang et al., 2021a, b; Liu et al., 2022). Therefore, microglial activation and polarization are the key factors that affect the inflammatory response after cerebral ischemia, as well as post-stroke recovery.
Cyclo (MQCNS) (LZ-3) is a cyclic bioactive peptide formed by dehydration and condensation of monocyte locomotion inhibitory factor methionine amino and serine carboxyl groups. Recently, research from our lab and others has suggested that monocyte locomotion inhibitory factor has neuroprotective effects against ischemic injury during the acute phase of stroke (Zhang et al., 2012; Zhu et al., 2016; Wang et al., 2017). However, whether LZ-3 improves nerve recovery and regulates microglial activation and polarization to reduce neuroinflammation post-stroke remains unknown.
Neuronal survival is dependent on microglia, and neuronal inflammation is caused by microglial activation after cerebral ischemia (Franco et al., 2021). Local microglia exhibit the anti-inflammatory M2 phenotype at the early stages of ischemic stroke but gradually transform into the proinflammatory M1 phenotype in peri-infarct regions (Hu et al., 2012). In this study, post-stroke recovery was evaluated using a middle cerebral artery occlusion (MCAO) rat model of stroke. We conducted a series of experiments to determine whether LZ-3 promotes behavioral and histological recovery in rats following stroke. In addition, we observed and evaluated the effects of LZ-3 on microglial polarization, phagocytosis, and migration. The molecular mechanism by which LZ-3 modulates microglial activation was also investigated.
Methods
Drug preparation
For the in vivo experiments, LZ-3 (Top-peptide, Shanghai, China; Figure 1A) was dissolved in 0.9% normal saline to concentrations of 1, 2, and 4 mg/ml before injection. For the in vitro experiments, LZ-3 and upadacitinib (a JAK1 inhibitor; Ark Pharm, Chicago, IL, USA) were dissolved in dimethyl sulfoxide to a concentration of 100 mM and stored at 4°C, and the experiments were performed by 1000-fold dilution of the drug to 100 μM in the cell growth medium. The final dimethyl sulfoxide concentration added to the culture medium was 0.1% (v/v), which had no significant effect on cell proliferation.
Figure 1.

LZ-3 reduces infarct volume and neuronal damage in rats post-stroke.
(A) Chemical structure of LZ-3. (B) Timeline of the in vivo study. Rats were injected with LZ-3, edaravone, or normal saline via the tail vein 3 days every 24 hours after reperfusion. TTC staining and immunofluorescence staining were performed on rat brain tissue 3 days after reperfusion. (C) TTC staining of coronal sections. Edaravone and LZ-3 (2 and 4 mg/kg) treatment reduced infarct volume compared with saline. (D) Infarct volumes in each group (n = 6). (E) Representative images of immunofluorescence staining for NeuN (red, Alexa Fluor® 647) in the cortex. Edaravone and LZ-3 (2 and 4 mg/kg) treatment also increased the number of live neurons compared with saline. Scale bar: 50 μm. (F) Number of NeuN-positive cells seen in E (n = 3). Data are expressed as mean ± SD. ###P < 0.001, vs. sham group; *P < 0.05, **P < 0.01, vs. model group (one-way analysis of variance followed by Tukey’s post hoc test). DAPI: 4′,6-Diamidino-2-phenylindole; ELISA: enzyme-linked immunosorbent assay; HE: hematoxylin-eosin; i.v: intravenous injection; MCAO: middle cerebral artery occlusion; Mod: model group; NeuN: neuronal nuclear protein; TTC: 2,3,5-triphenyte-trazoliumchloride; TUNEL: TdT-mediated dUTP nick-end labeling.
Animals
In this study, 200 specific pathogen-free adult male Sprague-Dawley rats (aged approximately 6–7 weeks, weighing 240–280 g) obtained from Slack Laboratory Animals Co., Ltd. (Shanghai, China; license No. SCXK (Hu) 2018-0006) were used. The rats were provided with food and water and housed at a constant temperature (24 ± 2°C) with controlled lighting conditions (12 hours of daylight). All rat experiments were approved by Shanghai University’s Animal Care Committee (approval No. ECSHU 2022-101, approved on March 4, 2022). All experiments were designed and reported according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines (Percie du Sert et al., 2020). The rats were randomly divided into six groups (sham, model, edaravone [6 mg/kg], and LZ-3 [1, 2, and 4 mg/kg] groups).
MCAO model and drug administration
In this study, we used Longa’s MCAO method to established the rat model of stroke (Longa et al., 1989). Rats were injected intraperitoneally with 2% sodium pentobarbital (50 mg/kg; Merck, Darmstadt, Germany), and cervical midline incisions were made. The rats were placed on a constant-temperature operating table, and the left cervical artery was carefully exposed. A sterile suture was used to close the exposed left external carotid artery, and vessel forceps were used to clamp the internal carotid arteries. Then, the proximal common carotid artery was tightly tied, and an incision was made in the common carotid artery. Suture silk (0.26 mm; Cinontech, Beijing, China) was inserted into the incision until it blocked blood flow to the middle cerebral artery (n = 180 rats). Blood flow in the left middle cerebral artery was detected with a Laser Doppler Flowmeter (OxyLab LDF; B&E TEKSYSTEMS LTD., Shanghai, China), and successful surgery was demonstrated by a reduction in cerebral blood flow to less than 20% of its starting value (Jin et al., 2018). The suture was removed after 2 hours to allow reperfusion. In the sham group (n = 20 rats), the operation was performed as described above, except blood flow to the middle cerebral artery was not blocked (only the left cervical artery was exposed, and no suture was inserted). After the full or sham surgery, the wounds were sutured. The rats were placed under a heat lamp to recover from the anesthesia and kept at an anal temperature of no less than 36°C.
Neurological scores were determined 24 hours after reperfusion. Rats with a neurological score of 0 (no neurological deficits or abnormal activity) were excluded. Rats with neurological scores between 1 and 4 were randomly assigned to the different treatment groups (n = 30 rats per group); the mean neurological scores of each group were similar. The sham group was injected with normal saline (3 mL/kg), the edaravone group was injected with edaravone (a brain protectant (free radical scavenger); Sinopharm, Beijing, China) at a dose of 6 mg/kg, and the LZ-3 groups were injected with LZ-3 (1, 2, or 4 mg/kg). The treatments were administered by tail vein injection every 24 hours from day 1 to day 7. The in vivo experimental design is shown in Figure 1B.
Cell culture and transfection
A mixture of 90% high-glucose Dulbecco’s modified Eagle’s medium and 10% fetal calf serum was used to culture BV2 cells (Biobw, Shanghai, China, Cat# Bio-73434, RRID: CVCL_0182). Cells were cultured for 7–10 days prior to the experiment at 37°C in humidified 5% CO2/95% air. The growth medium was changed daily. Cells in the exponential phase were used for each experiment. BV2 cells (2 × 105) were transfected with the pEX-3-JAK1 plasmid (2.4 μg, Genepharma, Shanghai, China) using Lipofectamine 3000 transfection reagent (Thermo Fisher Scientific, Waltham, MA, USA).
Oxygen-glucose deprivation/reoxygenation experiment and drug treatment
When the BV2 cells reached 80–90% confluency, they were washed three times in phosphate-buffered saline (PBS), seeded in glycoprival media (Hyclone, Logan, UT, USA), placed in an oxygen-deficient chamber (Billups Rothenberg, San Diego, CA, USA) filled with a 95% N2/5% CO2 gas mixture, and incubated at 37°C for 4 hours. The control cells were cultured in an incubator containing a standard oxygen-replete gas mixture, without oxygen-glucose deprivation/reoxygenation (OGD/R). The cells were then treated with dimethylsulfoxide, LZ-3 (100 μM), or upadacitinib (100 μM) diluted in Dulbecco’s modified Eagle medium for an additional 24 hours under normoxia (Additional Figure 1 (390.1KB, tif) ).
Neurological evaluation
Neurological scores for the rats in each group were compared from day 1 to day 7 following surgery using the Zea-Longa criteria (Longa et al., 1989). The Zea-Longa neurological deficit scores were as follows: 0, no neurological deficits or abnormal activity; 1, inverted tails, contralateral forepaws unable to stretch; 2, when crawling, turn to the opposite side; 3, walking with the body inclined to the opposite side; 4, loss of consciousness, unable to walk; 5, death.
Measurement of cerebral infarct area
The rats were sacrificed by intraperitoneal injection of 2% sodium pentobarbital (50 mg/kg) on day 3, and the brains were harvested. After rinsing with normal saline, the brains were immediately placed in the freezer for 15 minutes to remove excess water. The forebrains were dissected into six coronal sections (2 mm thick) (Chiang et al., 2011). Following 20 minutes of incubation at room temperature (18–25°C) in 2% 2,3,5-triphenyte-trazoliumchloride (Sigma Aldrich, St. Louis, MO, USA), the slices were fixed in 4% formaldehyde for 24 hours at 4°C. Afterwards, the brain slices were photographed and analyzed using ImageJ analysis software (v6.0; National Institutes of Health, Bethesda, MD, USA) (Schneider et al., 2012). The infarct volume (%) was calculated as left infarct volume/whole brain volume ×100 by counting the number of pixels within the infarct site.
Morris water maze test
From day 21 to day 26, spatial learning and memory were tested with the Morris water maze (Shanghai Ji Liang Software Technology Company, Shanghai, China). The maze consisted of a black circular pool 160 cm in diameter and 45 cm deep equipped with a camera system to monitor rat movements; parameters such as escape latency and time spent in the target quadrant were calculated using software. A platform was installed 1–2 cm below the surface of the water, and the pool was filled with water at 23 ± 1°C. There were two phases to the test: the navigation test, which was conducted for 5 consecutive days, followed by the spatial probe test, which was performed on day 6. At the beginning, the rats were placed in different quadrants of the pool on different days, and the swimming path was recorded. If the rat did not find the submerged platform within 60 seconds, it was guided to the platform and allowed to stay there for 10 seconds, and the escape latency was recorded as 60 seconds. The platform was removed for the spatial probe test. The rats were placed in water diagonally across from the original platform location, and swam to search for the platform. Both the number of times that they crossed the original platform location and the time spent in the target quadrant were recorded.
Enzyme-linked immunosorbent assay
After sacrifice by injection with 2% sodium pentobarbital (50 mg/kg) on day 3, rat blood and cortical tissue were harvested. To isolate serum, the blood samples were allowed to coagulate for 2 hours at room temperature and then centrifuged for 5 minutes at 4000 × g, after which the serum-containing supernatant was collected for testing. After homogenizing the ischemic cortical tissue in PBS, the homogenate was centrifuged at 12,000 × g for 15 minutes at 4°C, and the cortical supernatant was collected for testing. On the basis of the manufacturer’s instructions, enzyme-linked immunosorbent assay (ELISA) kits (R&D, Minneapolis, MN, USA) were used to detect IL-1β, IL-6, and TNF-α levels in the serum and cortical supernatant. The results were detected using a microplate reader (Multiskan MK3; Thermo Fisher Scientific).
Histological analysis
After the Morris water maze test was performed on day 26, rats were anesthetized with 2% sodium pentobarbital (50 mg/kg) and transcardially perfused with normal saline and 4% paraformaldehyde. Next, the brains were fixed in 4% formaldehyde, embedded in paraffin, and sliced into 5 µm-thick coronal sections. The slices were stained with hematoxylin (Beyotime, Shanghai, China) for 3–8 minutes, washed in tap water, fractionated in 1% hydrochloric acid for seconds, and then dehydrated with ethanol. The slices were then rinsed with tap water, treated with 0.6% ammonia until a blue color appeared, rinsed with distilled water, and stained with eosin for 1–3 minutes. A light microscope (Nikon, E100, Tokyo, Japan) was used to observe areas of interest.
A Nissl solution (Beyotime) was added to paraffin-embedded hippocampal sections before they were dewaxed using xylene (Sinopharm). The brain tissue was then treated with 95% ethanol to remove the stain from all areas except the Nissl bodies, which were stained dark blue. Hippocampal Nissl bodies in the CA1 region were observed and counted.
Next, TdT-mediated dUTP nick-end labeling (TUNEL) staining was performed using a kit (Roche, Shanghai, China) according to the manufacturer’s instructions to detect apoptotic cells. After dewaxing, the cortical sections were incubated with proteinase K at 37°C for 30 minutes, followed by incubation in film-breaking solution for 30 minutes at room temperature. Next, the sections were incubated at 37°C for 1 hour, followed by washing with TUNEL reagent. Following a 15-minute incubation in 3% hydrogen peroxide solution at room temperature, converter peroxidase was added, and the sections were incubated for 30 minutes at 37°C, after which color development was performed drop by drop with 3,3′-diaminobenzidine. Under a fluorescence microscope (Olympus, Tokyo, Japan), apoptotic neurons in the cortex were detected and counted.
Immunofluorescence staining
After sacrifice by 2% sodium pentobarbital (50 mg/kg) injection on day 3, the rats were transcardially perfused with normal saline and 4% paraformaldehyde. Brains were fixed with 4% formaldehyde then paraffin-embedded and sliced into 5 mm-thick sections. Antigen retrieval was performed at 100°C for 30 minutes using a 0.1 M sodium citrate buffer solution (pH 6.0). Slices were lysed with 0.1% Triton X-100 at room temperature for 5 minutes and incubated with 1% bovine serum albumin (BSA) for 1 hour. To label the neurons in the cortex, the slices were incubated with a rabbit anti-NeuN (1:250, Cell Signaling Technology, Boston, MA, USA, Cat# 24307, RRID: AB_2651140) primary antibody at 4°C overnight. After three 5-minute washes with PBS, the slices were incubated with the following secondary antibody: Alexa Fluor® 647 Donkey Anti-Rabbit IgG (1:200, Abcam, London, UK, Cat# ab150075, RRID: AB_2752244). Furthermore, nuclei were counterstained with 4′,6-diamidino-2-phenylindole (Beyotime) before the slices were mounted for microscopy.
To assess microglia/macrophage polarization and phagocytosis, co-immunofluorescence staining of Iba-1 with inducible nitric oxide synthase (iNOS), CD206, or NeuN was performed. Slices were treated with the following primary antibodies at 4°C overnight: mouse anti-Iba-1 (1:250, Abcam, Cat# ab15690, RRID: AB_2224403), rabbit anti-iNOS (1:250, Abcam, Cat# ab178945, RRID: AB_2861417), rabbit anti-CD206 (1:250, Cell Signaling Technology, Cat# 24595, RRID: AB_2892682), and rabbit anti-NeuN (1:250, Cell Signaling Technology, Cat# 24307, RRID: AB_2651140). After three 5-minute washes with PBS, the slices were incubated for 2 hours in the dark at room temperature with the following secondary antibodies: Alexa Fluor® 488 Goat Anti-Mouse IgG (1:200, Abcam, Cat# ab150113, RRID: AB_2576208) and Alexa Fluor® 647 Donkey Anti-Rabbit IgG (1:200, Abcam, Cat# ab150075, RRID: AB_2752244). Additionally, the cell nucleus was stained with 4′,6-diamidino-2-phenylindole for 10 minutes. Finally, a laser confocal microscope (Leica TCS SP5, Weztlar, Germany) was used to observe the cortical slices. To assess microglia/macrophage polarization, the number of Iba1-, iNOS-, or CD206-positive cells in three random fields (30×) from each cortical section was determined, and the average was taken as the final value. To assess microglia/macrophage phagocytosis, the number of Iba1- or NeuN-positive cells was counted in randomly selected fields (30×) in each cortical section.
Quantitative polymerase chain reaction
BV2 microglia were collected after OGD/R. Total RNA was isolated by treating the cells with 500 mL of RNAiso or TRIzol (Takara, Tokyo, Japan) and 100 mL of trichloromethane (Sinopharm) for 5 minutes and then centrifuging the solution for 15 minutes. The supernatant was diluted in an equal volume of isopropanol, mixed for 10 minutes, and centrifuged for 10 minutes. Afterward, 1 mL of 75% ethanol (Sinopharm) was added to the mixture, which was then centrifuged for 5 minutes. The pellet was then resuspended in RNase-free H2O (Takara), and the mixture was centrifuged at 12,000 × g, 4°C. Next, 1000 ng of purified total RNA was subjected to reverse transcription at 37°C for 15 minutes, then 5 seconds at 85°C with PrimeScript RT Master Mix (Takara). SYBR Premix Ex Taq (Takara) was used for quantitative polymerase chain reaction (qPCR) in a 20 μL reaction, using the reverse-transcribed cDNA as a template. The primer sequences are shown in Additional Table 1. A qPCR system (AB7500, Milwaukee, WI, USA) was used to perform the reaction at 95°C for 30 seconds followed by 45 cycles of 95°C for 15 seconds, 55°C for 5 seconds, and 72°C for 5 seconds. On the basis of the 2–ΔΔCt method (Livak and Schmittgen, 2001), the relative expression of mRNA was calculated.
AdditionalTable 1.
The primer sequences for quantitative polymerase chain reaction
| Gene | Forward | Reverse |
|---|---|---|
| GAPDH | 5′-CTTCACCACCATGGAGAAGGC-3′ | 5′-GGCATGGACTGTGGTCATGAG-3′ |
| CD11b | 5′-CCAAGACGATCTCAGCATCA-3′ | 5′-TTCTGGCTTGCTGAATCCTT-3′ |
| CD32 | 5′-AATCCTGCCGTTCCTACTGATC-3′ | 5′-GTGTCACCGTGTCTTCCTTGAG-3′ |
| iNOS | 5′-GGCAGCCTGTGAGACCTTTG-3′ | 5′-GCATTGGAAGTGAAGCGTTTC-3′ |
| Arg-1 | 5′-GAACACGGCAGTGGCTTTAAC-3′ | 5′-TGCTTAGCTCTGTCTGCTTTGC-3′ |
| CD206 | 5′-TTCGGTGGACTGTGGACGAGCA-3′ | 5′-ATAAGCCACCTGCCACTCCGGT-3′ |
| IL-10 | 5′-TAACTGCACCCACTTCCCAG-3′ | 5′-AGGCTTGGCAACCCAAGTAA-3′ |
Arg-1: Arginase 1; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; IL-10: interleukin-10; iNOS: inducible nitric oxide synthase.
Western blot analysis
Rats were sacrificed on day 3 after reperfusion. Tissues from the ipsilateral cortical ischemic penumbra were selected for western blotting. Brain tissues were rapidly removed, dissected, and homogenized. Total cellular protein was extracted from brain tissues or BV2 cells by incubating them in cell lysis buffer (Cat# 89900, Thermo Fisher Scientific) containing protease inhibitors (Merck, Burlington, MA, USA) and phosphatase inhibitors (Merck) on ice for 5 minutes, followed by centrifugation at 12,000 × g for 10 minutes at 4°C. A BCA protein quantitation kit (Thermo Fisher Scientific) was used to measure the protein levels in the supernatants. Next, the proteins were separated on a 10% sodium dodecyl sulfate polyacrylamide gel and transferred to a polyvinylidene fluoride membrane at 75 V. The membrane was incubated in the following primary antibodies overnight at 4°C: rabbit anti-JAK1 (1:1000, Cell Signaling Technology, Cat# 3344, RRID: AB_2265054), rabbit anti-phospho-JAK1 (p-JAK1, 1:1000, Cell Signaling Technology, Cat# 74129, RRID: AB_2799851), rabbit anti-signal transducer and activator of transcription (STAT6, 1:1000, Cell Signaling Technology, Cat# 5397, RRID: AB_11220421), and rabbit anti-phospho-signal transducer and activator of transcription (p-STAT6, 1:1000, Cell Signaling Technology, Cat# 56554, RRID: AB_2799514). Rabbit anti-glyceraldehyde-3-phosphate dehydrogenase (1:1000, Cell Signaling Technology, Cat# 5174, RRID: AB_10622025) was used to confirm equal loading. After three 5-minute washes with Tris-buffered saline/0.5% Tween, the membranes were incubated with the Alexa FluorTM 790 Goat anti-Rabbit IgG (1:10000, Thermo Fisher Scientific, Cat# A27041, RRID: AB_2536102) secondary antibody for 1.5 hours at room temperature. Odyssey Imager software (Li-Cor Biosciences, Lincoln, NE, USA) was used to image the membranes, and protein bands were quantified using ImageJ software based on grayscale values.
Cell migration assay
BV2 cells were cultured in monolayers in six-well plates. After 4 hours of OGD, artificial wounds were gently created with a 10-μL pipette tip. Then, the cells were washed with PBS to remove detached cells, and Dulbecco’s modified Eagle’s medium containing LZ-3 or upadacitinib was added. Micrographs were taken at 0, 12, and 24 hours after OGD to observe the cell migration distances. The amount of the scratched area covered by new cell growth was measured by ImageJ to calculate the migration rate.
Statistical analysis
No statistical methods were used to predetermine sample sizes, and no animals or data points were excluded from the analysis. The data were analyzed using SPSS software (Version 26.0, IBM Corp, Armonk, NY, USA). The data are expressed as means and standard deviations (SDs). One-way analysis of variance followed by Tukey’s post hoc test was used to compare groups. Longa scores were evaluated by non-parametric Kruskal-Wallis rank sum test. Statistical significance was defined as P < 0.05.
Results
LZ-3 reduces infarct volume and the neuronal damage caused by ischemia
The 2,3,5-triphenyte-trazoliumchloride staining results showed edaravone and LZ-3 (2 and 4 mg/kg) decreased infarct volume compared with the model group on day 3 (Figure 1C and D). To confirm the neuroprotective effect of LZ-3, we preformed immunofluorescence staining of neurons in the rat cortex in the early phase after stroke (Figure 1E) and found that LZ-3 (2 and 4 mg/kg) and edaravone (6 mg/kg) significantly increased the number of NeuN+ neurons on day 3 after stroke compared with no treatment (Figure 1F). Collectively, these results indicate that LZ-3 can reduce the damage to the brain cortex caused by stroke.
LZ-3 improves behavioral and cognitive functions in rats post-stroke
After 2 hours of MCAO-induced ischemia and 24 hours of reperfusion, rats were injected with LZ-3, edaravone, or saline every 24 hours for 7 days, and the Zea-Longa behavioral score was determined daily after the injection was administered. No significant differences in Zea-Longa behavioral scores were found among the five groups from day 1 to day 8 after cerebral ischemia, indicating that rat brain tissue underwent repair after cerebral ischemia/reperfusion injury (Figure 2A). On days 3 and 4, treatment with LZ-3 (2 and 4 mg/kg) significantly reduced the Zea-Longa score compared with the model group (P < 0.05, Figure 2B and C). These results suggest that LZ-3 can accelerate the recovery of behavioral function in rats after cerebral ischemia. On day 21 post-stroke, the Morris water maze test was performed to assess cognitive ability. For 5 consecutive days, we measured escape latency in the navigation test (Figure 2D). Interestingly, LZ-3 reduced the escape latency on day 25 compared with the model group (Figure 2E and F). In the spatial probe test performed on day 26, LZ-3 increased the time spent in the target quadrant and the number of platform crossings compared with the model group (P < 0.05; Figure 2G and H). These results indicated that LZ-3 treatment accelerates the recovery of neural function and improves cognitive function in rats post-stroke.
Figure 2.

LZ-3 improves behavioral and cognitive functions in rats on post-stroke.
(A–C) The neurological deficit score was evaluated from day 1 to day 8. (D–F) Escape latency in the Morris water maze navigation test. The Morris water maze test was performed from day 21 to day 26. (G and H) Time spent in the target quadrant and the number of platform crossings in the Morris water maze spatial probe test. Both values were determined for a 60-second period for each rat. Data are expressed as mean ± SD (n = 8). ##P < 0.01, vs. sham group; *P < 0.05, vs. model group. The neurological deficit scores were analyzed by non-parametric Kruskal-Wallis rank sum test, and the Morris water maze test results were analyzed by one-way analysis of variance followed by Tukey’s post hoc test. MCAO: Middle cerebral artery occlusion; Mod: model group.
LZ-3 ameliorates cortical and hippocampal injury in rats post-stroke
After the final Morris water maze test was completed, the rats were sacrificed, and the brain tissue was evaluated by hematoxylin-eosin staining. As shown in Figure 3A, severe liquefaction necrosis was observed in the cortex in the model group, and the structure of nerve cells in the cortical area was destroyed, while LZ-3 and edaravone reduced cortical voids and improved the structural integrity of cortical nerve cells. Moreover, to evaluate the effect of LZ-3 on cortical nerve cell apoptosis after cerebral ischemia, apoptotic bodies were observed by TUNEL staining (Figure 3B). We did not observe any apoptotic bodies in the cortex of sham-operated rats, and LZ-3 and edaravone significantly reduced the number of TUNEL+ cells in the cortex post-stroke compared to the model group (Figure 3C). Then, we evaluated the effect of LZ-3 on the structural integrity of the hippocampal CA1 region of rats post-stroke by Nissl staining (Figure 3D). In comparison to the model group, LZ-3 increased the number of hippocampal neurons and hippocampal structure integrity post-stroke (Figure 3E). These results indicate that LZ-3 treatment promotes cortical and hippocampal functional recovery in rats with ischemic brain injury.
Figure 3.
Effect of LZ-3 on brain histopathology in rats on day 26 after cerebral ischemia.
(A) Representative images of hematoxylin-eosin staining of the cortex show that LZ-3 reduced damage to the cortex on day 26 after reperfusion. LZ-3 (2 or 4 mg/kg) or edaravone treatment reduced the cortical damage caused by cerebral ischemia. Arrows indicate nerve cells in the cortex. Scale bars: 2 mm (low-magnification images) and 200 μm (enlarged images). (B, C) LZ-3 reduced the number of TUNEL+ cells in the rat cortex post-stroke. LZ-3 (2 or 4 mg/kg) or edaravone treatment reduced the number of apoptotic cells in the cortex after stroke. Arrows indicate apoptotic nerve cells in the cortex. Scale bar: 100 μm. (D, E) LZ-3 reduced hippocampal structure disruption post-stroke. LZ-3 and edaravone treatment increased the number of nerve cells in the hippocampal CA1 region compared with saline. Arrows indicate nerve cells in the hippocampal CA1 region. Scale bar: 100 μm. Data are expressed as mean ± SD (n = 3). ##P < 0.01, ###P < 0.001, vs. sham group; *P < 0.05, **P < 0.01, vs. model group (one-way analysis of variance followed by Tukey’s post hoc test). Mod: Model group; TUNEL: TdT-mediated dUTP nick-end labeling.
LZ-3 reduces serum and brain levels of inflammation markers in rats post-stroke
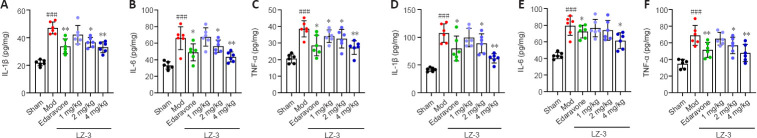
To determine whether LZ-3 reduces inflammation after ischemia in rats, on day 3 after LZ-3 injection we measured IL-1β, IL-6, and TNF-α levels in the cortex and serum by ELISA. As shown in Figure 4A–C, LZ-3 reduced serum levels of IL-1β, IL-6, and TNF-α compared with model group. Furthermore, LZ-3 reduced the levels of these inflammatory cytokines in the cortex after stroke (Figure 4D–F). These findings indicate that LZ-3 reduces brain damage following stroke by reducing the inflammatory response.
Figure 4.
LZ-3 reduces inflammatory factor levels in the serum and brain tissue of rats post-stroke.
Enzyme-linked immunosorbent assay was performed on serum and cerebral cortex tissue samples taken on day 3 post-stroke. (A–C) IL-1β, IL-6, and TNF-α serum levels. (D–F) IL-1β, IL-6, and TNF-α levels in the cerebral cortex. Data are expressed as mean ± SD (n = 6). ###P < 0.001, vs. sham group; *P < 0.05, **P < 0.01, vs. model group (one-way analysis of variance followed by Tukey’s post hoc test). IL: Interleukin; Mod: model group; TNF-α: tumor necrosis factor-α.
LZ-3 inhibits ischemia- or hypoxia-activated JAK1-STAT6 signaling
To dissect the mechanism by which LZ-3 improves recovery after stroke, we analyzed the brain levels of activated JAK1 and STAT6 to investigate inflammation-related signaling (Figure 5A). As shown in Figure 5B and C, LZ-3 reduced the levels of p-JAK1 and p-STAT6 proteins in rat brains affected by ischemia. Next, we analyzed the levels of p-JAK1 and p-STAT6 proteins in BV2 cells subjected to OGD/R. LZ-3 and upadacitinib both reduced p-JAK1 and p-STAT6 levels in BV2 cells (Figure 5D–F). To confirm that LZ-3 inhibits the JAK1-STAT6 signaling pathway, we transfected BV2 cells with the plasmid pEX-3-JAK1 to overexpress the JAK1 protein (Figure 5G). LZ-3 and upadacitinib significantly reduced hypoxia-induced p-JAK1 and p-STAT6 protein expression in cells overexpressing JAK1 (Figure 5H and I). Taken together, these findings suggest that LZ-3 improves recovery after cerebral ischemia in rats by inhibiting the JAK1-STAT6 signaling pathway.
Figure 5.
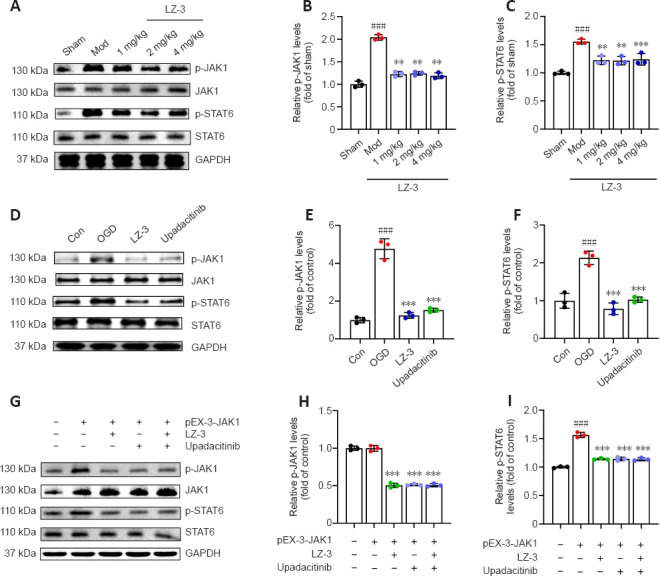
LZ-3 inhibits ischemia- or hypoxia-induced JAK1-STAT6 signaling.
(A) Representative western blot showing p-JAK1 and p-STAT6 expression in the cortex on day 3 after reperfusion. (B, C) Quantification of p-JAK1 and p-STAT6 expression based on western blotting (n = 3/group). ###P < 0.001, vs. sham group; **P < 0.01, ***P < 0.001, vs. model group. (D) Representative western blot showing p-JAK1 and p-STAT6 expression in BV2 cells treated with LZ-3 or upadacitinib after 24 hours of re-oxygenation after OGD. (E, F) Quantification of p-JAK1 and p-STAT6 expression based on western blotting (n = 3). (G) Representative western blot showing p-JAK1 and p-STAT6 expression in BV2 cells overexpressing JAK1 and treated with LZ-3 or upadacitinib after 24 hours of re-oxygenation after OGD. (H, I) Quantification of p-JAK1 and p-STAT6 expression based on western blotting (n = 3). ###P < 0.001, vs. control group; ***P < 0.001, vs. OGD group. Data are expressed as mean ± SD and were analyzed by one-way analysis of variance followed by Tukey’s post hoc test. Con: Control group; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; JAK1: Janus kinase 1; Mod: model group; OGD: oxygen-glucose deprivation; pEX-3-JAK1: peroxisomal biogenesis factor 3-Janus kinase 1; p-JAK1: phospho-Janus kinase 1; p-STAT6: phospho-signal transducer and activator of transcription; STAT6: signal transducer and activator of transcription.
LZ-3 regulates microglia/macrophage polarization by inhibiting JAK1
Next, immunofluorescence staining was performed to observe microglia/macrophage polarization in the rat cortex. The results showed that, compared with model group, treatment with LZ-3 or upadacitinib reduced the number of ischemia-activated iNOS+ and Iba-1+ microglia/macrophage cells (Figure 6A and B) and increased the number of CD206+ and Iba-1+ microglia/macrophage cells (Figure 6C and D). To analyze whether LZ-3 regulates microglia/macrophage polarization in vitro and in vivo, we detected polarization-related levels of marker mRNAs in BV2 cells by qPCR. As shown in Figure 6E and F, LZ-3 and upadacitinib reduced the mRNA levels of M1 markers (CD11b, CD32, and iNOS) compared with the model group. In addition, LZ-3 promoted microglia polarization toward the M2 phenotype, as indicated by increased mRNA levels of M2 markers (Arg-1, IL-10, and CD206). These findings show that LZ-3 affects M1- and M2-type polarization, and that the mechanism of this effect may be related to inhibition of JAK1.
Figure 6.

Effect of LZ-3 on microglia polarization in vivo and in vitro.
(A) Representative images of immunofluorescence staining for Iba-1 (green, Alexa Fluor® 488) and iNOS (red, Alexa Fluor® 647) in microglia after LZ-3 treatment. LZ-3 reduced the number of Iba-1- and iNOS-positive cells compared with Mod. Arrows indicate double-stained microglia. (B) The average number of Iba-1+ and iNOS+ cells detected as shown in A (n = 3). (C) Representative images of immunofluorescence staining for Iba-1 (green, Alexa Fluor® 488) and CD206 (red, Alexa Fluor® 647) in microglia after LZ-3 treatment. LZ-3 increased the number of Iba-1- and CD206-positive cells compared with Mod. Arrows indicate double-stained microglia. Scale bars: 100 μm. (D) The average number of Iba-1+ and CD206+ cells detected as shown in C (n = 3). ##P < 0.01, ###P < 0.001, vs. sham group; **P < 0.01, vs. model group. (E, F) LZ-3, upadacitinib, or PBS was added to the BV2 cell culture medium after 24 hours of re-oxygenation after OGD. Relative mRNA levels of CD11b, CD32, and iNOS as detected by qPCR (E), and relative mRNA levels of Arg-1, IL-10, and CD206 (F) (n = 3). ##P < 0.01, vs. control group; *P < 0.05, **P < 0.01, vs. OGD group. Data are expressed as mean ± SD and were analyzed by one-way analysis of variance followed by Tukey’s post hoc test. Arg-1: Arginase 1; Con: control group; DAPI: 4’,6-diamidino-2-phenylindole; IL-10: interleukin-10; iNOS: inducible nitric oxide synthase; Mod: model group; OGD: oxygen-glucose deprivation.
LZ-3 regulates hypoxia-induced changes in microglial function
To determine whether LZ-3 regulates microglial functions such as phagocytosis and migration after ischemia, the ability of microglia to phagocytose neurons in the cortex was observed by immunofluorescence staining (Figure 7A). We found that LZ-3 treatment reduced the microglial phagocytosis induced by ischemia, as well as the number of neurons (NeuN-positive cells) phagocytosed by microglia, compared with no treatment (Figure 7B). In addition, microglial migration was assessed by cell migration assay, which showed that treatment with LZ-3 or upadacitinib reduced the BV2 cell migration rate compared with the OGD group (Figure 7C and D). These findings demonstrated that LZ-3 inhibits ischemia-induced phagocytosis and microglia migration.
Figure 7.

LZ-3 decreases microglia phagocytosis and migration after ischemia or hypoxia.
(A) Representative images of immunofluorescence staining for NeuN (red, Alexa Fluor® 647) and Iba-1 (green, Alexa Fluor® 488) in the brain cortex. LZ-3 reduced the number of Iba-1- and NeuN-positive cells compared with Mod. Merged images show microglia phagocytosing neurons. Scale bars: 100 μm (left) and 10 μm (right). (B) The number of NeuN+ neurons phagocytosed by Iba-1+ microglia (30×) (n = 3). ##P < 0.01, vs. sham group; *P < 0.05, vs. model group. (C) Representative images of the cell migration assay performed after 4 hours of OGD. BV2 cells gradually migrated over the scratched area, and LZ-3 and upadacitinib were found to reduce the migration at 12 hours compared with the OGD group. Scale bar: 200 μm. (D) The migration rate of BV2 cells as shown in C (n = 3). ##P < 0.01, vs. control group; *P < 0.05, vs. OGD group. Data are expressed as mean ± SD and were analyzed by one-way analysis of variance followed by Tukey’s post hoc test. Con: Control group; DAPI: 4′,6-diamidino-2-phenylindole; Iba-1: ionized calcium binding adapter molecule-1; Mod: model group; NeuN: neuronal nuclear protein; OGD: oxygen-glucose deprivation.
Discussion
As acute stroke care improves and fewer patients die from stroke, the demand for post-stroke care will likely increase (Gagnon et al., 2020). After stroke, whether in the acute stage or in the recovery stage, the injured brain exhibits plasticity that, using a variety of mechanisms, may lead to recovery of motor function (Lindvall and Kokaia, 2015; Alia et al., 2016). Therefore, improving brain repair after stroke is crucial. However, most studies focus on acute cerebral ischemia, and the mechanisms of post-stroke injury and repair should be further understood to develop corresponding neuroprotective drugs (Dimyan and Cohen, 2011). Therefore, in this study, we focused on investigating the protective effects and specific mechanisms of LC-3 in the recovery period after cerebral ischemia. In this study, a rat model of MCAO was first established, the protective effects of LC-3 in the recovery period were investigated by assessing the area of cerebral infarction, histopathology, neurological conditions, and cognitive impairment, and the specific mechanism of action of LC-3 was explored to provide a basis for clinical use.
The inflammatory response is critical in the recovery period after cerebral ischemia (Mo et al., 2020). Inflammatory cytokines and the regulation of pro- or anti-inflammatory processes have gradually emerged as areas of intense research interest (Wang and He, 2018). Several drugs targeting the key proinflammatory cytokines TNF, IL-1β, and IL-6 and the anti-inflammatory factor IL-10 have been used to treat nonneurological diseases, such as rheumatoid arthritis, inflammatory bowel disease, and psoriasis (Steed et al., 2003; Wang and He, 2018; Kim et al., 2019). In recent years, many studies have reported that ischemia stimulates an anti-inflammatory response that prevents cell death in the injured tissue (Naderi et al., 2017; de Carvalho et al., 2019; Liu et al., 2019b). Therefore, in our study, we measured the levels of these inflammatory factors post-stroke. LC-3, a cyclic bioactive peptide, is derived from MLIF. Our group has demonstrated that MLIF has a protective effect against inflammatory damage during acute cerebral ischemia (Zhu et al., 2016), and thus we hypothesized that its cerebral ischemic protective effect is related to its anti-inflammatory effect. In this study, we found that LZ-3 treatment significantly reduced the levels of the inflammatory cytokines IL-1β, IL-6, and TNF-α in the serum and brain tissue of rats post-stroke compared with no treatment.
Several members of the STAT family are cytoplasmic proteins that play an important role in regulating the inflammatory response. Particularly, STAT6 signaling appears to induce macrophages to adopt an anti-inflammatory phenotype (Brunn et al., 2014). In MCAO-induced and bilateral common carotid artery stenosis-induced ischemic stroke models, STAT3 was also found to regulate M1 microglial polarization (Qin et al., 2017; Li et al., 2019). Inhibition of JAK2/STAT3 signaling promotes the polarization of quiescent microglia to an M2 phenotype and can reduce inflammation (Ding et al., 2019). Another study showed that AG490 (tyrosine kinase inhibitor of JAK2) decreased the expression of M1 markers such as iNOS, IL-1β, TNF-α, CD16, and CD32, as well as the phosphorylation and expression of IκBα in M1 microglia induced by soluble tumor necrosis factor ligand superfamily member 6 (Fas ligand)-activated translocation of p50/P65 (Meng et al., 2016). To better understand the mechanism by which LZ-3 exerts its protective effects post-stroke, western blotting was performed to determine p-JAK1 and p-STAT6 expression levels. The results showed that the expression levels of these proteins were significantly decreased by LZ-3 treatment. The protective effect of LC-3 was further demonstrated by overexpression of JAK1 in BV2 cells, which demonstrated that the protective effect of LC-3 was associated with JAK-STAT6 signaling. Taken together, our findings suggest that the inflammatory JAK1-STAT6 signaling pathway is activated in response to ischemia or hypoxia.
Microglia are the predominant immune cells involved in the inflammatory response in cerebral tissue after ischemia (Hou et al., 2021). Microglia are activated and undergo M1 or M2 polarization post-stroke, which can affect stroke recovery and is accompanied by changes in the expression levels of cell biomarkers (Xiong et al., 2016). The number of ischemia-induced M1 microglia increased over time during the first 2 weeks post-stroke, whereas M2 microglia numbers peaked on day 5 and then decreased sharply during the second week. Prolonged activation of M1 microglia can lead to secondary neuronal damage in the subsequent months (Taylor and Sansing, 2013). Our results are consistent with these findings and further show that LC-3 reverses the M2 to M1 transition by inhibiting JAK1-STAT6 signaling. In addition, we explored the effect of LC-3 on other processes in microglia. Microglia are highly motile phagocytic cells that infiltrate and settle in the developing brain, where they are thought to exert surveillance and scavenging functions (Paolicelli et al., 2011). After stroke, activated microglia migrate to the injured site, multiply, engulf surrounding cells, and release a variety of harmful or beneficial substances into surrounding tissues (Wolf et al., 2017; Prinz et al., 2019). We showed that LZ-3 reduced the number of neurons phagocytosed by microglia/macrophages compared with the model group. We found that BV2 cells treated with LZ-3 exhibited less migration than untreated cells after hypoxia in an in vitro cell migration assay. These findings suggest that LZ-3 can regulate microglial polarization from the proinflammatory M1 phenotype to the anti-inflammatory M2 phenotype, as well as microglial migration and phagocytosis, by inhibiting ischemia- or hypoxia-induced JAK1-STAT6 signaling.
Our study had several limitations. We used Iba-1 as a marker for microglia, although it is not strictly specific for microglia, because this marker is also expressed by macrophages (Nakamura et al., 2013). Considering that both androgens and estrogens can affect the pathophysiology of ischemic stroke (Ayala et al., 2011; Koellhoffer and McCullough, 2013), we only used male rats in this study; the effects of the drug on female rats will be explored in a future study. Perioperative pain management was not applied during surgery, and adequate post-surgical pain management should have been provided in the form of buprenorphine or similar drugs. Additionally, further mechanistic studies are required to identify whether microglia polarization is related to microglia phagocytosis and migration. In addition, we speculate that the effect of LZ-3 on microglial phagocytosis may be related to the efferosome, which will also be explored in further studies. On the basis of these in vitro and in vivo findings, we conclude that the neuroprotective effect of LZ-3 post-stroke is partially mediated by inhibition of microglia/macrophage activation, and that this effect could be attributable to inhibition of JAK1-STAT6 signaling pathway-mediated neuroinflammation.
Additional files:
Additional Table 1: The primer sequences for quantitative polymerase chain reaction.
Additional Figure 1 (390.1KB, tif) : Experimental design, grouping and timeline in in vitro experiment.
Experimental design, grouping and timeline in in vitro experiment.
Con: Control group; OGD/R: oxygen-glucose deprivation/reoxygenation; OGD: oxygen-glucose deprivation; pEx-3-JAK1: peroxisomal biogenesis factor 3-Janus kinase1; qPCR: quantitative polymerase chain reaction; WB: western blot.
Additional file 1: Open peer review reports 1 (78.5KB, pdf) –3 (89.9KB, pdf) .
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest.
Data availability statement: All data relevant to the study are included in the article or uploaded as Additional files.
Open peer reviewers: Anjali Chauhan, University of Texas Health Science Center at Houston, USA; Johannes Boltze, University of Warwick, UK; Dmitriy N. Atochin, Cardiovascular Research Center, USA.
P-Reviewers: Chauhan A, Boltze J, Atochin DN; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Crow E, Yu J, Song LP; T-Editor: Jia Y
References
- 1.Alia C, Spalletti C, Lai S, Panarese A, Micera S, Caleo M. Reducing GABA(A)-mediated inhibition improves forelimb motor function after focal cortical stroke in mice. Sci Rep. 2016;6:37823. doi: 10.1038/srep37823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayala P, Uchida M, Akiyoshi K, Cheng J, Hashimoto J, Jia T, Ronnekleiv OK, Murphy SJ, Wiren KM, Hurn PD. Androgen receptor overexpression is neuroprotective in experimental stroke. Transl Stroke Res. 2011;2:346–357. doi: 10.1007/s12975-011-0079-z. [DOI] [PubMed] [Google Scholar]
- 3.Brunn A, Mihelcic M, Carstov M, Hummel L, Geier F, Schmidt A, Saupe L, Utermöhlen O, Deckert M. IL-10, IL-4, and STAT6 promote an M2 milieu required for termination of P0(106-125)-induced murine experimental autoimmune neuritis. Am J Pathol. 2014;184:2627–2640. doi: 10.1016/j.ajpath.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Cassidy JM, Cramer SC. Spontaneous and therapeutic-induced mechanisms of functional recovery after stroke. Transl Stroke Res. 2017;8:33–46. doi: 10.1007/s12975-016-0467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen AQ, Fang Z, Chen XL, Yang S, Zhou YF, Mao L, Xia YP, Jin HJ, Li YN, You MF, Wang XX, Lei H, He QW, Hu B. Microglia-derived TNF-αmediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke. Cell Death Dis. 2019;10:487. doi: 10.1038/s41419-019-1716-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Chen YQ, Shi YJ, Ding SQ, Shen L, Wang R, Wang QY, Zha C, Ding H, Hu JG, Lü HZ. VX-765 reduces neuroinflammation after spinal cord injury in mice. Neural Regen Res. 2021;16:1836–1847. doi: 10.4103/1673-5374.306096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang T, Messing RO, Chou WH. Mouse model of middle cerebral artery occlusion. J Vis Exp. 2011:2761. doi: 10.3791/2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Carvalho TS, Sanchez-Mendoza EH, Nascentes LM, Schultz Moreira AR, Sardari M, Dzyubenko E, Kleinschnitz C, Hermann DM. Moderate protein restriction protects against focal cerebral ischemia in mice by mechanisms involving anti-inflammatory and anti-oxidant responses. Mol Neurobiol. 2019;56:8477–8488. doi: 10.1007/s12035-019-01679-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deszo EL, Brake DK, Kelley KW, Freund GG. IL-4-dependent CD86 expression requires JAK/STAT6 activation and is negatively regulated by PKCdelta. Cell Signal. 2004;16:271–280. doi: 10.1016/s0898-6568(03)00137-2. [DOI] [PubMed] [Google Scholar]
- 10.Dimyan MA, Cohen LG. Neuroplasticity in the context of motor rehabilitation after stroke. Nat Rev Neurol. 2011;7:76–85. doi: 10.1038/nrneurol.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding Y, Qian J, Li H, Shen H, Li X, Kong Y, Xu Z, Chen G. Effects of SC99 on cerebral ischemia-perfusion injury in rats:Selective modulation of microglia polarization to M2 phenotype via inhibiting JAK2-STAT3 pathway. Neurosci Res. 2019;142:58–68. doi: 10.1016/j.neures.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Franco R, Lillo A, Rivas-Santisteban R, Reyes-Resina I, Navarro G. Microglial adenosine receptors:from preconditioning to modulating the M1/M2 balance in activated cells. Cells. 2021;10:1124. doi: 10.3390/cells10051124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gagnon DJ, Leclerc AM, Riker RR, Brown CS, May T, Nocella K, Cote J, Eldridge A, Seder DB. Amantadine and modafinil as neurostimulants during post-stroke care:a systematic review. Neurocrit Care. 2020;33:283–297. doi: 10.1007/s12028-020-00977-5. [DOI] [PubMed] [Google Scholar]
- 14.Hou J, Schindler U, Henzel WJ, Ho TC, Brasseur M, McKnight SL. An interleukin-4-induced transcription factor:IL-4 Stat. Science. 1994;265:1701–1706. doi: 10.1126/science.8085155. [DOI] [PubMed] [Google Scholar]
- 15.Hou K, Li G, Yu J, Xu K, Wu W. Receptors, channel proteins, and enzymes involved in microglia-mediated neuroinflammation and treatments by targeting microglia in ischemic stroke. Neuroscience. 2021;460:167–180. doi: 10.1016/j.neuroscience.2021.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Gao Y, Chen J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- 17.Im JH, Yeo IJ, Park PH, Choi DY, Han SB, Yun J, Hong JT. Deletion of Chitinase-3-like 1 accelerates stroke development through enhancement of Neuroinflammation by STAT6-dependent M2 microglial inactivation in Chitinase-3-like 1 knockout mice. Exp Neurol. 2020;323:113082. doi: 10.1016/j.expneurol.2019.113082. [DOI] [PubMed] [Google Scholar]
- 18.Jackson L, Dong G, Althomali W, Sayed MA, Eldahshan W, Baban B, Johnson MH, Filosa J, Fagan SC, Ergul A. Delayed administration of angiotensin II type 2 receptor (AT2R) agonist compound 21 prevents the development of post-stroke cognitive impairment in diabetes through the modulation of microglia polarization. Transl Stroke Res. 2020;11:762–775. doi: 10.1007/s12975-019-00752-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin S, Kang M, Cho H. Cerebral blood perfusion deficits using dynamic susceptibility contrast MRI with gadolinium chelates in rats with post-ischemic reperfusion without significant dynamic contrast-enhanced MRI-derived vessel permeabilities:A cautionary note. PLoS One. 2018;13:e0201076. doi: 10.1371/journal.pone.0201076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HG, Yang WS, Hong YH, Kweon DH, Lee J, Kim S, Cho JY. Anti-inflammatory functions of the CDC25 phosphatase inhibitor BN82002 via targeting AKT2. Biochem Pharmacol. 2019;164:216–227. doi: 10.1016/j.bcp.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Koellhoffer EC, McCullough LD. The effects of estrogen in ischemic stroke. Transl Stroke Res. 2013;4:390–401. doi: 10.1007/s12975-012-0230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koh YC, Yang G, Lai CS, Weerawatanakorn M, Pan MH. Chemopreventive effects of phytochemicals and medicines on M1/M2 polarized macrophage role in inflammation-related diseases. Int J Mol Sci. 2018;19:2208. doi: 10.3390/ijms19082208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambertsen KL, Finsen B, Clausen BH. Post-stroke inflammation-target or tool for therapy? Acta Neuropathol. 2019;137:693–714. doi: 10.1007/s00401-018-1930-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang GP, Li C, Han YY. Rutin pretreatment promotes microglial M1 to M2 phenotype polarization. Neural Regen Res. 2021;16:2499–2504. doi: 10.4103/1673-5374.313050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Wang C, Jiang Y, Zhang X, Xian Y, Liu L, Zhao X, Gu H, Meng X, Li H, Wang Y, Wang Y. Rationale and design of patient-centered retrospective observation of guideline-recommended execution for stroke sufferers in China:China PROGRESS. Stroke Vasc Neurol. 2019;4:165–170. doi: 10.1136/svn-2019-000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindvall O, Kokaia Z. Neurogenesis following stroke affecting the adult brain. Cold Spring Harb Perspect Biol. 2015;7:a019034. doi: 10.1101/cshperspect.a019034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Nolte K, Brook G, Liebenstund L, Weinandy A, Höllig A, Veldeman M, Willuweit A, Langen KJ, Rossaint R, Coburn M. Post-stroke treatment with argon attenuated brain injury, reduced brain inflammation and enhanced M2 microglia/macrophage polarization:a randomized controlled animal study. Crit Care. 2019a;23:198. doi: 10.1186/s13054-019-2493-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Xiao G, Wang Y, Shang T, Li Z, Wang H, Pu L, He S, Shao R, Orgah JO, Zhu Y. Qishen Yiqi Dropping Pill facilitates post-stroke recovery of motion and memory loss by modulating ICAM-1-mediated neuroinflammation. Biomed Pharmacother. 2022;153:113325. doi: 10.1016/j.biopha.2022.113325. [DOI] [PubMed] [Google Scholar]
- 29.Liu ZJ, Ran YY, Qie SY, Gong WJ, Gao FH, Ding ZT, Xi JN. Melatonin protects against ischemic stroke by modulating microglia/macrophage polarization toward anti-inflammatory phenotype through STAT3 pathway. CNS Neurosci Ther. 2019b;25:1353–1362. doi: 10.1111/cns.13261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 32.Meng HL, Li XX, Chen YT, Yu LJ, Zhang H, Lao JM, Zhang X, Xu Y. Neuronal soluble fas ligand drives M1-microglia polarization after cerebral ischemia. CNS Neurosci Ther. 2016;22:771–781. doi: 10.1111/cns.12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mo Y, Sun YY, Liu KY. Autophagy and inflammation in ischemic stroke. Neural Regen Res. 2020;15:1388–1396. doi: 10.4103/1673-5374.274331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naderi Y, Sabetkasaei M, Parvardeh S, Zanjani TM. Neuroprotective effect of minocycline on cognitive impairments induced by transient cerebral ischemia/reperfusion through its anti-inflammatory and anti-oxidant properties in male rat. Brain Res Bull. 2017;131:207–213. doi: 10.1016/j.brainresbull.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura R, Nishimura T, Ochiai T, Nakada S, Nagatani M, Ogasawara H. Availability of a microglia and macrophage marker, iba-1, for differential diagnosis of spontaneous malignant reticuloses from astrocytomas in rats. J Toxicol Pathol. 2013;26:55–60. doi: 10.1293/tox.26.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 37.Pascotini ET, Flores AE, Kegler A, Gabbi P, Bochi GV, Algarve TD, Prado AL, Duarte MM, da Cruz IB, Moresco RN, Royes LF, Fighera MR. Apoptotic markers and DNA damage are related to late phase of stroke:Involvement of dyslipidemia and inflammation. Physiol Behav. 2015;151:369–378. doi: 10.1016/j.physbeh.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, et al. The ARRIVE guidelines 2.0:Updated guidelines for reporting animal research. PLoS Biol. 2020;18:e3000410. doi: 10.1371/journal.pbio.3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prinz M, Jung S, Priller J. Microglia biology:one century of evolving concepts. Cell. 2019;179:292–311. doi: 10.1016/j.cell.2019.08.053. [DOI] [PubMed] [Google Scholar]
- 40.Qin C, Fan WH, Liu Q, Shang K, Murugan M, Wu LJ, Wang W, Tian DS. Fingolimod protects against ischemic white matter damage by modulating microglia toward M2 polarization via STAT3 pathway. Stroke. 2017;48:3336–3346. doi: 10.1161/STROKEAHA.117.018505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ:25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 43.Steed PM, Tansey MG, Zalevsky J, Zhukovsky EA, Desjarlais JR, Szymkowski DE, Abbott C, Carmichael D, Chan C, Cherry L, Cheung P, Chirino AJ, Chung HH, Doberstein SK, Eivazi A, Filikov AV, Gao SX, Hubert RS, Hwang M, Hyun L, et al. Inactivation of TNF signaling by rationally designed dominant-negative TNF variants. Science. 2003;301:1895–1898. doi: 10.1126/science.1081297. [DOI] [PubMed] [Google Scholar]
- 44.Taylor RA, Sansing LH. Microglial responses after ischemic stroke and intracerebral hemorrhage. Clin Dev Immunol. 2013;2013:746068. doi: 10.1155/2013/746068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walberer M, Jantzen SU, Backes H, Rueger MA, Keuters MH, Neumaier B, Hoehn M, Fink GR, Graf R, Schroeter M. In-vivo detection of inflammation and neurodegeneration in the chronic phase after permanent embolic stroke in rats. Brain Res. 2014;1581:80–88. doi: 10.1016/j.brainres.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Xing H, Wan L, Jiang X, Wang C, Wu Y. Treatment targets for M2 microglia polarization in ischemic stroke. Biomed Pharmacother. 2018;105:518–525. doi: 10.1016/j.biopha.2018.05.143. [DOI] [PubMed] [Google Scholar]
- 47.Wang T, He C. Pro-inflammatory cytokines:The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38–50. doi: 10.1016/j.cytogfr.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Wang C, Yang Y, Ni J. New monocyte locomotion inhibitory factor analogs protect against cerebral ischemia-reperfusion injury in rats. Bosn J Basic Med Sci. 2017;17:221–227. doi: 10.17305/bjbms.2017.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolf SA, Boddeke HW, Kettenmann H. Microglia in physiology and disease. Annu Rev Physiol. 2017;79:619–643. doi: 10.1146/annurev-physiol-022516-034406. [DOI] [PubMed] [Google Scholar]
- 50.Xiong XY, Liu L, Yang QW. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog Neurobiol. 2016;142:23–44. doi: 10.1016/j.pneurobio.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 51.Yang HC, Zhang M, Wu R, Zheng HQ, Zhang LY, Luo J, Li LL, Hu XQ. C-C chemokine receptor type 2-overexpressing exosomes alleviated experimental post-stroke cognitive impairment by enhancing microglia/macrophage M2 polarization. World J Stem Cells. 2020;12:152–167. doi: 10.4252/wjsc.v12.i2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu SS, Li ZY, Xu XZ, Yao F, Luo Y, Liu YC, Cheng L, Zheng MG, Jing JH. M1-type microglia can induce astrocytes to deposit chondroitin sulfate proteoglycan after spinal cord injury. Neural Regen Res. 2022;17:1072–1079. doi: 10.4103/1673-5374.324858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L, Zhang L, Sui R. Ganoderic acid A-mediated modulation of microglial polarization is involved in depressive-like behaviors and neuroinflammation in a rat model of post-stroke depression. Neuropsychiatr Dis Treat. 2021a;17:2671–2681. doi: 10.2147/NDT.S317207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang X, Yuan M, Yang S, Chen X, Wu J, Wen M, Yan K, Bi X. Enriched environment improves post-stroke cognitive impairment and inhibits neuroinflammation and oxidative stress by activating Nrf2-ARE pathway. Int J Neurosci. 2021b;131:641–649. doi: 10.1080/00207454.2020.1797722. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, Chen J, Li F, Li D, Xiong Q, Lin Y, Zhang D, Wang XF, Yang P, Rui YC. A pentapeptide monocyte locomotion inhibitory factor protects brain ischemia injury by targeting the eEF1A1/endothelial nitric oxide synthase pathway. Stroke. 2012;43:2764–2773. doi: 10.1161/STROKEAHA.112.657908. [DOI] [PubMed] [Google Scholar]
- 56.Zhu Q, Zhang Y, Liu Y, Cheng H, Wang J, Zhang Y, Rui Y, Li T. MLIF alleviates SH-SY5Y neuroblastoma injury induced by oxygen-glucose deprivation by targeting eukaryotic translation elongation factor 1A2. PLoS One. 2016;11:e0149965. doi: 10.1371/journal.pone.0149965. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental design, grouping and timeline in in vitro experiment.
Con: Control group; OGD/R: oxygen-glucose deprivation/reoxygenation; OGD: oxygen-glucose deprivation; pEx-3-JAK1: peroxisomal biogenesis factor 3-Janus kinase1; qPCR: quantitative polymerase chain reaction; WB: western blot.