Abstract
The TPS1 gene from Hansenula polymorpha, which encodes trehalose-6-phosphate (Tre6P) synthase, has been isolated and characterized. The deletion of TPS1 rendered H. polymorpha cells incapable of trehalose synthesis under conditions where wild-type cells normally accumulate high levels of trehalose. Interestingly, the loss of Tre6P synthase did not cause any obvious growth defects on a glucose-containing medium, even at high temperatures, but seriously compromised the cells’ ability to acquire thermotolerance.
The accumulation of the nonreducing disaccharide trehalose is an element of the adaptive response of various microorganisms to stress conditions such as nutrient starvation and heat shock. Consistent with the suggestion that trehalose may, therefore, act as a stabilizer of cellular structures, in vitro studies have revealed the exceptional capability of trehalose in protecting biological membranes and enzymes from stress-induced damage (for reviews see references 5, 18, and 19). Moreover, genetic studies in yeast have shown that the loss of trehalose-6-phosphate (Tre6P) synthase activity severely compromises the cells’ ability to acquire tolerance towards heat stress (references 6 and 16 and references therein). In agreement with these findings, it was recently demonstrated that trehalose is important for both maintaining proteins in their native forms and suppressing the aggregation of denatured proteins during heat shock in living cells (17). In this context, we chose to study the trehalose metabolism in the thermophilic, methylotrophic budding yeast Hansenula polymorpha, because this yeast is particularly well adapted to growth at very high temperatures (up to 48°C) and may therefore provide further insight into the role of trehalose as a stress protectant.
Trehalose metabolism in H. polymorpha.
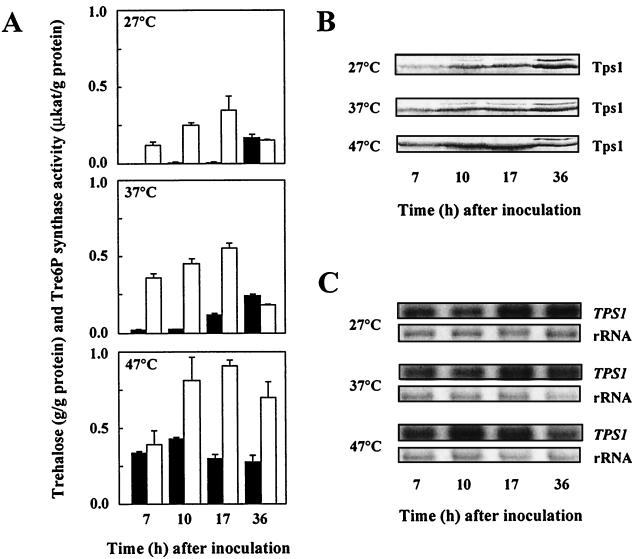
Trehalose accumulation in fungi has been reported to occur particularly during starvation conditions (e.g., entry into stationary phase) or during a mild heat shock (for reviews see references 13, 22, and 24). Consistent with these observations, cells of a thermophilic, homothallic H. polymorpha wild-type strain were found to have low trehalose levels during exponential growth and to accumulate trehalose when grown to stationary phase at 27 and 37°C (Fig. 1A). In parallel, the levels of Tre6P synthase activity (Fig. 1A), Tps1 protein (Fig. 1B), and TPS1 mRNA (Fig. 1C) also increased upon entry of cells into stationary phase when grown at 27 and 37°C. Interestingly, cells grown at 47°C were found to reach high levels of trehalose during exponential growth and to increase levels of Tre6P synthase activity (Fig. 1A), Tps1 protein (Fig. 1B), and TPS1 mRNA (Fig. 1C) during late exponential growth phase, just before entry into stationary phase. Taken together, these results show that trehalose accumulation in H. polymorpha, as in other fungi, is part of the carbon source starvation response and that trehalose synthesis is at least partially controlled at a transcriptional level under these conditions.
FIG. 1.
Levels of trehalose and Tre6P synthase activity (A), Tps1 protein (B), and TPS1 mRNA (C) of wild-type H. polymorpha cells grown to stationary phase at 27, 37, and 47°C. Cultures were pregrown overnight at the temperatures indicated and diluted (at time zero) with fresh YPD medium to an initial optical density at 600 nm of 0.2. Samples were taken in exponential phase (7 h), late exponential phase (10 h), early stationary phase (17 h), and late stationary phase (36 h). Glucose was consumed after 12 h (at 27°C), 11 h (at 37°C), or 12 h (at 47°C). (A) Shaded bars denote trehalose levels (determined as described in reference 7), and open bars denote Tre6P synthase activities (assayed as described in references 6 and 7). Error bars indicate standard deviations. (B) Immunoblot analysis of the Tps1 protein levels by using polyclonal rabbit antibodies raised against a peptide (Gly-Val-Asp-Arg-Leu-Asp-Tyr-Ile-Lys-Gly-Val-Pro-Gln-Lys) corresponding to a highly conserved amino acid sequence in Tps1 proteins of various fungi. Several proteins cross-reacted weakly with these antibodies, and the identification of the correct band was, therefore, verified in each case by the use of an extract from an H. polymorpha Δtps1 strain (data not shown). (C) Total RNAs were extracted (14), and equal amounts (10 μg) were probed with an internal 650-bp fragment of the H. polymorpha TPS1 gene after electrophoresis and blotting. The application and transfer of equal amounts of RNA were verified by ethidium bromide staining.
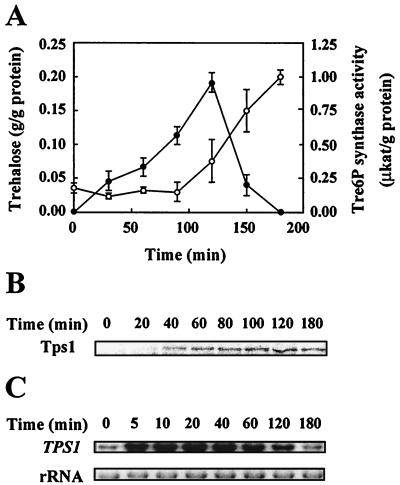
Similar to other fungi, H. polymorpha cells were also found to accumulate trehalose under heat shock conditions. However, as expected on the basis of the higher temperature optimum for growth (than that of other yeast species such as Schizosaccharomyces pombe and Saccharomyces cerevisiae), H. polymorpha cells accumulated large amounts of trehalose only when subjected to a temperature shift from 27 to 47°C (instead of 40°C). Accordingly, cells were found to accumulate large amounts of trehalose during a 2-h heat shock at 47°C (Fig. 2A). In parallel, the levels of Tre6P synthase activity (Fig. 2A), Tps1 protein (Fig. 2B), and TPS1 mRNA (Fig. 2C) also increased significantly under these conditions. Notably, while the levels of both Tre6P synthase activity (Fig. 2A) and Tps1 protein (Fig. 2B) increased with relatively slow kinetics, the level of TPS1 mRNA increased dramatically shortly after the temperature shift (5 min), reaching a maximum after 20 to 40 min and decreasing slightly after 2 h at 47°C (Fig. 2C). Thus, similar to the situation in other fungi (4, 25, 26), trehalose synthesis is an element of the heat shock response in H. polymorpha, which is at least partially controlled by transcriptional mechanisms. In accordance with this notion, the TPS1 mRNA levels (Fig. 2C) decreased to almost pre-heat shock levels (time zero) when cells were allowed to recover from the heat shock for 30 min at 27°C. Despite the fact that the level of Tps1 protein remained high and that even the in vitro-measured Tre6P synthase activity increased (Fig. 2A and B), trehalose was found to be completely and rapidly mobilized, possibly due to the activation of trehalases, when heat-shocked cells were allowed to recover for 30 min at 27°C (Fig. 1A).
FIG. 2.
Levels of trehalose and Tre6P synthase activity (A), Tps1 protein (B), and TPS1 mRNA (C) of wild-type H. polymorpha cells during heat shock and subsequent recovery. Cultures were grown at 27°C on YPD medium to early exponential phase, shifted to 47°C at time zero, and transferred back to 27°C after 120 min. (A) Black circles, trehalose levels; white circles, Tre6P synthase activities. Error bars indicate standard deviations. (B) Protein samples were extracted at the times indicated and subjected to immunoblot analysis of Tps1 protein levels (for further details see the legend for Fig. 1). (C) Total RNAs were extracted (14) at the times indicated, and equal amounts (10 μg) were probed as described in the legend for Fig. 1.
Isolation of the H. polymorpha TPS1 gene.
Based on a comparison of known TPS1 genes from S. cerevisiae, S. pombe, Kluyveromyces lactis, and Aspergillus niger, two degenerate oligonucleotides were designed which correspond to highly conserved regions in these genes. These two oligonucleotides were used as primers in a PCR with genomic DNA from H. polymorpha as a template. The resulting 650-bp fragment was subsequently used as a probe to screen an H. polymorpha genomic library (8) by colony filter hybridization. Two positive clones (pHRP20-1 and pHRP21-3) were found to contain the entire H. polymorpha TPS1 gene. A segment of 2,695 bp containing the entire H. polymorpha TPS1 gene, including flanking sequences, was sequenced on both strands by the cycle sequencing method with AmpliTaq DNA polymerase (FS), BigDye terminators, and the ABI Prism 310 capillary sequencer (Perkin-Elmer Applied Biosystems). Analysis of the H. polymorpha TPS1 sequence revealed an open reading frame of 1,428 bp coding for a putative protein of 476 amino acids (54.4 kDa). As expected, the predicted H. polymorpha Tps1 sequence exhibits similarity to Tps1 sequences from other organisms. Sequence identities range from 35% (with Rhizobium sp. Tps1) to 74% (with Candida albicans Tps1) over the full length of the shorter protein. Analysis of the DNA sequence (including 972 bp) immediately upstream of the putative H. polymorpha TPS1 start codon revealed the existence of two elements (TGAAGCCTCTTGAAA and TGAATATAAAGGAAA at positions −94 and −166, respectively) with high similarity to S. cerevisiae and Drosophila heat shock elements which are typically composed of multiple, contiguous, and possibly inverted 5-bp NGAAN repeats (9).
Complementation of S. cerevisiae and S. pombe tps1 mutants by H. polymorpha TPS1.
In order to test whether we had isolated a functional TPS1 gene, H. polymorpha TPS1 was analyzed for its ability to complement the phenotypes of S. cerevisiae and S. pombe tps1 mutants. To this end, a 2.6-kb HindIII/HindIII fragment containing the full-length H. polymorpha TPS1 gene, including 0.6 and 0.2 kb of the immediate up- and downstream flanking regions, respectively, was excised from plasmid pHRP21-3, isolated, and cloned at the HindIII sites of YEplac195 (10) and pUR19 (1), thus creating YEp-HpTPS1 and pUR-HpTPS1, respectively. Using these plasmids, we found that H. polymorpha TPS1 restored, albeit to a different extent, the ability to synthesize trehalose in S. cerevisiae and S. pombe tps1 mutants (Table 1). In a further experiment, the S. cerevisiae Δtps1 strain YSH 6.106.-1A (15) was transformed with either YEp-HpTPS1 or the corresponding control plasmid (YEplac195). As expected, S. cerevisiae Δtps1 cells containing the control plasmid were able to grow on a galactose-containing medium but not on a glucose-containing medium (data not shown). In contrast, S. cerevisiae Δtps1 cells containing YEp-HpTPS1 were able to grow on both galactose- and glucose-containing media. Accordingly, H. polymorpha TPS1 also complements the growth defect of an S. cerevisiae Δtps1 mutant on glucose. Together, these results show that H. polymorpha TPS1 codes for a functional Tre6P synthase.
TABLE 1.
Complementation of the trehalose synthesis defect of S. cerevisiae and S. pombe Δtps1 mutants by H. polymorpha TPS1a
| Strain | Relevant genotype | Mean amt of trehalose ± SE (g/g of protein)
|
|
|---|---|---|---|
| After heat shock | In stationary phase | ||
| S. cerevisiae | |||
| YSH 6.106.-3A | TPS1 | 0.150 ± 0.012 | 0.008 ± 0.001 |
| YSH 6.106.-1A | Δtps1::TRP1 | <0.001 | <0.001 |
| YSH 6.106.-1A/ YEp-HpTPS1 | Δtps1::TRP1/TPS1 | 0.015 ± 0.006 | 0.009 ± 0.004 |
| S. pombe | |||
| PB003 | tps1+ | 0.255 ± 0.015 | 0.225 ± 0.018 |
| PBL-17 | tps1::LEU2 | <0.001 | <0.001 |
| PBL-17/pUR-HpTPS1 | tps1::LEU2/TPS1 | 0.187 ± 0.018 | 0.050 ± 0.010 |
Cells were grown at 27°C to exponential phase (for heat shock experiments) or stationary phase (4 days) on synthetic defined media containing either 2% galactose (YSH 6.106.-1A [MATα leu2 ura3 trp1 his3 ade2 Δtps1::TRP1] and its isogenic wild type YSH 6.106.-3A [MATα leu2 ura3 trp1 his3 ade2]) (15) or 2% glucose (PBL-17 [h+ ade6-M216 leu1-32 ura4-D18 tps1::LEU2] and its isogenic wild type PB003 [h+ ade6-M216 leu1-32 ura4-D18]) (16) as the carbon source. For heat shock experiments, cells growing exponentially at 27°C were shifted to 40°C for 2 h. Results are the means ± standard errors from three independent experiments. The detection limit for trehalose levels was 0.001 g/g of protein.
Effects of the H. polymorpha TPS1 deletion.
To determine the consequences of the loss of Tps1, we replaced the complete H. polymorpha TPS1 coding region with the kanMX2 module coding for Geneticin resistance (23). To this end, two DNA fragments of 0.6 and 1.0 kb, containing sequences immediately up- and downstream of the TPS1 start and stop codons, respectively, were amplified by PCR. NotI-SpeI and SalI-EcoRI restriction sites were introduced with the PCR primers at the ends of the 5′ and 3′ fragments, respectively. The resulting fragments were purified, digested with NotI/SpeI or SalI/EcoRI, and coligated with an SpeI-SalI fragment containing the kanMX2 module to NotI/EcoRI-digested pBluescript KS(+) (Stratagene). The deletion cassette with flanking sequences of TPS1 separated by the kanMX2 module was then excised from the resulting plasmid with NotI and EcoRI and transformed into the H. polymorpha wild-type strain. Two independent Geneticin-resistant colonies were isolated, and the correct integration of the marker at the TPS1 locus was verified by PCR and Southern blot analysis (data not shown). On plates, the H. polymorpha Δtps1 strains had no obvious growth defect at 27°C on all carbon sources we tested, including glucose, fructose, sucrose, galactose, glycerol, and ethanol. In addition, we did not observe any growth defect on glucose-containing plates or liquid media for the H. polymorpha Δtps1 mutant at any temperature tested (i.e., 27, 37, and 47°C). Thus, the TPS1 gene is not essential for growth in H. polymorpha.
As expected, and in contrast to wild-type cells, H. polymorpha Δtps1 mutant cells grown on 1% yeast extract–2% peptone–2% glucose (YPD) medium had no in vitro-detectable Tre6P synthase activity during exponential growth, in stationary phase, or under heat shock conditions (detection limit, 0.001 μkat/g of protein). Consistent with these findings, H. polymorpha Δtps1 mutants were unable to synthesize detectable amounts of trehalose upon entry into stationary phase and during heat shock, while wild-type cells accumulated large amounts of trehalose under the same conditions (detection limit, 0.001 g/g of protein). Together, these findings indicate that the isolated H. polymorpha TPS1 gene encodes the only functional Tre6P synthase in H. polymorpha.
Previous genetic approaches to determine the specific role of trehalose for the heat-induced thermotolerance in S. cerevisiae have been hampered by the finding that the deletion of TPS1 causes a variety of pleiotropic effects, including the inability to grow on glucose-containing media (6). However, studies of an S. pombe tps1 mutant, which reportedly has no such growth defects, showed that trehalose synthesis is indeed important for the acquisition of thermotolerance (16). Since the loss of Tps1 causes no obvious growth defect in H. polymorpha on glucose-containing media, we could also assess the role of trehalose synthesis in this particularly thermophilic yeast species. We found that unconditioned H. polymorpha Δtps1 cells were as heat sensitive to a 40-min challenging heat shock at 56.5°C as wild-type cells. However, while a 1-h conditioning heat shock at 47°C increased the survival rate of wild-type cells towards the challenging heat shock (40 min at 56.5°C) more than 1,000-fold, H. polymorpha Δtps1 mutant cells remained similarly sensitive to the challenging heat shock with or without a prior conditioning heat shock (data not shown). These results clearly indicate that trehalose synthesis is an important factor for the acquisition of thermotolerance in H. polymorpha. Notably, in this context, while trehalose synthesis was found to be important for the acquisition of thermotolerance in H. polymorpha, it was completely dispensable for growth at high temperatures. Thus, the high levels of trehalose found in wild-type cells growing exponentially at 47°C are not essential for growth at these temperatures but rather may function as a guard against potentially harsher heat conditions.
Effect of Tre6P on the H. polymorpha glucose-phosphorylating activity.
The deletion of TPS1 in H. polymorpha, unlike deletions of the corresponding genes in S. cerevisiae and K. lactis but similar to the tps1+ deletion in S. pombe, did not result in any obvious growth defect on a glucose-containing medium. A somewhat intermediate situation has been reported for A. niger and C. albicans, in which the loss of Tre6P synthase(s) caused an apparent growth defect only at very high glucose concentrations and/or at elevated temperatures, respectively (26, 27). A model which may explain these different phenotypic consequences of the loss of Tre6P synthase suggests that they arise from the different susceptibilities of the corresponding hexokinases to inhibition by Tre6P, which in some Tre6P synthase mutants would lead to an unrestrained flux through hexokinases and consequently the inhibition of growth on glucose (4; for a review including further models see reference 20). Accordingly, while the major hexokinase activities of S. pombe, A. niger, and S. cerevisiae (and K. lactis) were found to be insensitive, weakly sensitive, and strongly sensitive, respectively, to Tre6P, the deletion of the Tre6P synthase-encoding genes in the corresponding fungi caused no defect, a weak defect (see above), and a strong defect, respectively, for growth on glucose-containing media (2, 3, 4, 11, 12, 21, 26). Surprisingly, and at variance with this model, the loss of Tre6P synthase in H. polymorpha did not impair growth on glucose even at elevated temperatures, despite the fact that we found glucose-phosphorylating activity from H. polymorpha to be rather strongly inhibited by 1 mM Tre6P (53.7 and 9.6% inhibition in the presence of 1 and 10 mM glucose, respectively; for a description of the assay see reference 3). These results may be reconciled with the above-presented model, however, if H. polymorpha did not rely exclusively on Tre6P synthase-dependent control mechanisms but also on Tre6P synthase-independent control mechanisms to restrict the initial steps of glycolysis. Possibly, since the demand for a high glycolytic flux is particularly strong during fermentative growth, the loss of the Tre6P synthase-mediated control mechanisms may pose a severe problem only for fungi with a high fermentative capacity (S. cerevisiae and K. lactis) and not for fungi with prevailingly respiratory metabolisms, such as A. niger, C. albicans, and H. polymorpha. Thus, the importance of Tre6P synthase for the control of glycolysis in fungi may be determined not only by the susceptibility of hexokinases to Tre6P-mediated inhibition but also by the general (fermentative or respirative) nature of the carbohydrate metabolism.
Nucleotide sequence accession number.
A segment of 2,695 bp was submitted as H. polymorpha TPS1 to the EMBL database under accession no. AJ010725.
Acknowledgments
Anke Reinders and Ivano Romano contributed equally to the manuscript.
We are grateful to P. Piper and R. Hilbrands for supplying the H. polymorpha strain and the genomic DNA library, respectively. We thank M. Ribeiro and P. Strickner for excellent technical assistance.
This work was supported by Swiss National Science Foundation grants 4235.94 (to A.W.) and 3100-052245.97 (to C.D.V.).
REFERENCES
- 1.Barbert N, Muriel W J, Carr A M. Versatile shuttle vectors and genomic libraries for use with Schizosaccharomyces pombe. Gene. 1992;114:59–66. doi: 10.1016/0378-1119(92)90707-v. [DOI] [PubMed] [Google Scholar]
- 2.Bell W, Klaassen P, Ohnacker M, Boller T, Herweijer M, Schoppink P, van der Zee P, Wiemken A. Characterization of the 56-kDa subunit of yeast trehalose-6-phosphate synthase and cloning of its gene reveal its identity with the product of CIF1, a regulator of carbon catabolite inactivation. Eur J Biochem. 1992;209:951–959. doi: 10.1111/j.1432-1033.1992.tb17368.x. [DOI] [PubMed] [Google Scholar]
- 3.Blázquez M A, Lagunas R, Gancedo C, Gancedo J M. Trehalose-6-phosphate, a new regulator of yeast glycolysis that inhibits hexokinases. FEBS Lett. 1993;329:51–54. doi: 10.1016/0014-5793(93)80191-v. [DOI] [PubMed] [Google Scholar]
- 4.Blázquez M A, Stucka R, Feldmann H, Gancedo C. Trehalose-6-P synthase is dispensable for growth on glucose but not for spore germination in Schizosaccharomyces pombe. J Bacteriol. 1994;176:3895–3902. doi: 10.1128/jb.176.13.3895-3902.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowe J H, Hoekstra F A, Crowe L M. Anhydrobiosis. Annu Rev Physiol. 1992;54:579–599. doi: 10.1146/annurev.ph.54.030192.003051. [DOI] [PubMed] [Google Scholar]
- 6.De Virgilio C, Hottiger T, Dominguez J, Boller T, Wiemken A. The role of trehalose synthesis for the acquisition of thermotolerance in yeast. I. Genetic evidence that trehalose is a thermoprotectant. Eur J Biochem. 1994;219:179–186. doi: 10.1111/j.1432-1033.1994.tb19928.x. [DOI] [PubMed] [Google Scholar]
- 7.De Virgilio C, Bürckert N, Bell W, Jenö P, Boller T, Wiemken A. Disruption of TPS2, the gene encoding the 100-kDa subunit of the trehalose-6-phosphate synthase/phosphatase complex in Saccharomyces cerevisiae, causes accumulation of trehalose-6-phosphate and loss of trehalose-6-phosphate phosphatase activity. Eur J Biochem. 1993;212:315–323. doi: 10.1111/j.1432-1033.1993.tb17664.x. [DOI] [PubMed] [Google Scholar]
- 8.Faber K N, Swaving G J, Faber F, Ab G, Harder W, Veenhuis M, Haima P. Chromosomal targeting of replicating plasmids in the yeast Hansenula polymorpha. J Gen Microbiol. 1992;138:2405–2416. doi: 10.1099/00221287-138-11-2405. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes M, Xiao H, Lis J T. Fine structure analyses of the Drosophila and Saccharomyces heat shock factor-heat shock element interactions. Nucleic Acids Res. 1994;22:167–173. doi: 10.1093/nar/22.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gietz R D, Sugino A. New yeast-Escherichia coli shuttle vectors with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- 11.Gonzáles M I, Stucka R, Blázquez M A, Feldmann H, Gancedo C. Molecular cloning of CIF1, a yeast gene necessary for growth on glucose. Yeast. 1992;8:183–192. doi: 10.1002/yea.320080304. [DOI] [PubMed] [Google Scholar]
- 12.Luyten K, de Koning W, Tesseur I, Ruiz M C, Ramos J, Cobbaert P, Thevelein J M, Hohmann S. Disruption of the Kluyveromyces lactis GGS1 gene causes inability to grow on glucose and fructose and is suppressed by mutations that reduce sugar uptake. Eur J Biochem. 1993;217:701–713. doi: 10.1111/j.1432-1033.1993.tb18296.x. [DOI] [PubMed] [Google Scholar]
- 13.Piper P W. Molecular events associated with acquisition of heat tolerance by the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev. 1993;11:339–356. doi: 10.1111/j.1574-6976.1993.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 14.Piper P W. Measurement of transcription. In: Johnston J R, editor. Molecular genetics of yeast. A practical approach. Oxford, England: IRL Press; 1994. pp. 147–159. [Google Scholar]
- 15.Reinders A, Bürckert N, Hohmann S, Thevelein J M, Boller T, Wiemken A, De Virgilio C. Structural analysis of the subunits of the trehalose-6-phosphate synthase/phosphatase complex in Saccharomyces cerevisiae and their function during heat shock. Mol Microbiol. 1997;24:687–695. doi: 10.1046/j.1365-2958.1997.3861749.x. [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro M J S, Reinders A, Boller T, Wiemken A, De Virgilio C. Trehalose synthesis is important for the acquisition of thermotolerance in Schizosaccharomyces pombe. Mol Microbiol. 1997;25:571–581. doi: 10.1046/j.1365-2958.1997.4961856.x. [DOI] [PubMed] [Google Scholar]
- 17.Singer M A, Lindquist S. Multiple effects of trehalose on protein folding in vitro and in vivo. Mol Cell. 1998;1:639–648. doi: 10.1016/s1097-2765(00)80064-7. [DOI] [PubMed] [Google Scholar]
- 18.Singer M A, Lindquist S. Thermotolerance in Saccharomyces cerevisiae: the Yin and Yang of trehalose. Trends Biotechnol. 1998;16:460–468. doi: 10.1016/s0167-7799(98)01251-7. [DOI] [PubMed] [Google Scholar]
- 19.Thevelein J M. Regulation of trehalose metabolism and its relevance to cell growth and function. In: Brambl R, Marzluff G A, editors. The mycota III. Biochemistry and molecular biology. Berlin, Germany: Springer-Verlag; 1996. pp. 395–420. [Google Scholar]
- 20.Thevelein J M, Hohmann S. Trehalose synthase: guard to the gate of glycolysis in yeast? Trends Biochem Sci. 1995;20:3–10. doi: 10.1016/s0968-0004(00)88938-0. [DOI] [PubMed] [Google Scholar]
- 21.Van Aelst L, Hohmann S, Bulaya B, de Koning W, Sierkstra L, Neves M J, Luyten K, Alijo R, Ramos J, Coccetti P, Martegani E, de Magalhães-Rocha N M, Brandão R L, Van Dijck P, Vanhalewyn M, Durnez P, Jans A W H, Thevelein J M. Molecular cloning of a gene involved in glucose sensing in the yeast Saccharomyces cerevisiae. Mol Microbiol. 1993;8:927–943. doi: 10.1111/j.1365-2958.1993.tb01638.x. [DOI] [PubMed] [Google Scholar]
- 22.Van Laere A. Trehalose, reserve and/or stress metabolite? FEMS Microbiol Rev. 1989;63:201–210. [Google Scholar]
- 23.Wach A, Brachat A, Pöhlmann R, Philippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- 24.Wiemken A. Trehalose in yeast, stress protectant rather than reserve carbohydrate. Antonie Leeuwenhoek. 1990;58:209–217. doi: 10.1007/BF00548935. [DOI] [PubMed] [Google Scholar]
- 25.Winderickx J, de Winde J H, Crauwels M, Hino A, Hohmann S, Van Dijck P, Thevelein J M. Regulation of genes encoding subunits of the trehalose synthase complex in Saccharomyces cerevisiae: novel variations of STRE-mediated transcription control? Mol Gen Genet. 1996;252:470–482. doi: 10.1007/BF02173013. [DOI] [PubMed] [Google Scholar]
- 26.Wolschek M F, Kubicek C P. The filamentous fungus Aspergillus niger contains two “differentially regulated” trehalose-6-phosphate synthase-encoding genes, tpsA and tpsB. J Biol Chem. 1997;272:2729–2735. doi: 10.1074/jbc.272.5.2729. [DOI] [PubMed] [Google Scholar]
- 27.Zaragoza O, Blázquez M A, Gancedo C. Disruption of the Candida albicans TPS1 gene encoding trehalose-6-phosphate synthase impairs formation of hyphae and decreases infectivity. J Bacteriol. 1998;180:3809–3815. doi: 10.1128/jb.180.15.3809-3815.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]