Abstract
Astrocytes are not only the most populous cell type in the human brain, but they also have the most extensive and diverse sets of connections, across synapses, axons, blood vessels, as well as having their own internal network. Unsurprisingly, they are associated with many brain functions; from the synaptic transmission to energy metabolism and fluid homeostasis, and from cerebral blood flow and blood-brain barrier maintenance to neuroprotection, memory, immune defenses and detoxification, sleep, and early development. And yet, notwithstanding these key roles, so many current therapeutic approaches to a range of brain disorders have largely neglected their potential involvement. In this review, we consider the role of astrocytes in three brain therapies; two are emerging treatments (photobiomodulation and ultrasound), while the other is well-established (deep brain stimulation). In essence, we explore the issue of whether external sources, such as light, sound, or electricity, can influence the function of astrocytes, as they do neurons. We find that, when taken all together, each of these external sources can influence many, if not, all of the functions associated with astrocytes. These include influencing neuronal activity, prompting neuroprotection, reducing inflammation (astrogliosis) and potentially increasing cerebral blood flow and stimulating the glymphatic system. We suggest that astrocytes, just like neurons, can respond positively to each of these external applications and that their activation could each impart many beneficial outcomes on brain function; they are likely to be key players underpinning the mechanisms behind many therapeutic strategies.
Key Words: deep brain stimulation, high frequency, infrared, mitochondria, red, ultrasound
Introduction
Over the decades, an enormous number of different types of therapeutic - both medical and surgical - approaches have been developed for a range of neurological disorders, from Parkinson’s disease to epilepsy and from depression to schizophrenia. Most, if not all of these therapies were developed to influence the function and survival of the neuronal population of the brain. Whether or not any of them were effective on the glial cells, the other major cell type of the brain was really not given much attention. This “glial neglect” becomes all the more relevant when one considers that the precise mechanisms behind the bulk of the therapies for many neurological disorders are not clear.
In this review, we examine the effect of three different types of therapeutic approaches on the resident glial cells of the brain. Two of these are emerging treatments receiving considerable attention across the clinical, scientific, and wider communities (photobiomodulation and ultrasound), while the other is well-established (deep brain stimulation). In essence, we explore whether external sources, such as light, sound, or electricity, can influence the function of glial cells, as they do neurons. To this end, we focus on the astrocytes, the most populous of the glial cells and the ones most intimately associated with mechanisms of brain function and repair.
In the sections that follow, we will consider first the broad range of functions associated with astrocytes. Next, we outline the evidence that these cells are influenced by light, sound, and electricity; further, we suggest that the beneficial outcomes induced by these applications on brain function can potentially involve astrocytes as key players. In essence, we further the cause that the impact of any therapeutic neurological treatment should consider, not only the function of the neurons, but also of the astrocytes (Sofroniew and Vinters, 2010; Cicchetti and Barker, 2014; Bellesi et al., 2015; Finsterwald et al., 2015; Petit and Magistretti, 2016; Haydon, 2017; Magistretti and Allaman, 2018; Kim et al., 2019; Siracusa et al., 2019; Bozic et al., 2021; Vaidyanathan et al., 2021; Beard et al., 2022).
Search Strategy
Electronic searches of the PubMed database for literature with the key words “astrocytes and photobiomodulation”, “astrocytes and ultrasound”, “astrocytes and deep brain stimulation” were performed. The results were further screened by title and abstract to include articles on animal models and patients that were directly relevant to these key search items. This included publications up to January 2023. The articles that were not specific for the key words nor directly relevant to the issues considered in this narrative review, were not included.
Astrocytes and Their General Organization in the Nervous System
Astrocytes are the stars of the brain. Although many of them might look like stars (Figure 1A), hence their name, they have not always been considered the “stars”, at least in terms of being main players in brain function. Originally, they - and all glial cells - were thought to just form the framework within which neurons work within; they were the static scaffolds that keep the brain together structurally. Much has changed since that original view. Nowadays, astrocytes are known as the complete “busy-bodies” of the brain, involving themselves in practically every type of neural function; indeed, if you think of anything that the brain does, then astrocytes are invariably involved at some point along the way. One gains a clue that astrocytes are multi-functional if one considers their anatomy. Their processes, for example, extend all across the brain, reaching into every neuronal synapse - and there are billions and billions of them - they wrap around each one of the numerous cerebral vessels and they intermingle with the many axons (at nodes of Ranvier) of the white matter. They have an extensive communication system between themselves through rather distinct structures called gap junctions, formed by connexins. With this internal communication system in place, astrocytes can shuttle information over long distances (Sofroniew and Vinters, 2010; Clasadonte and Prevot, 2018).
Figure 1.
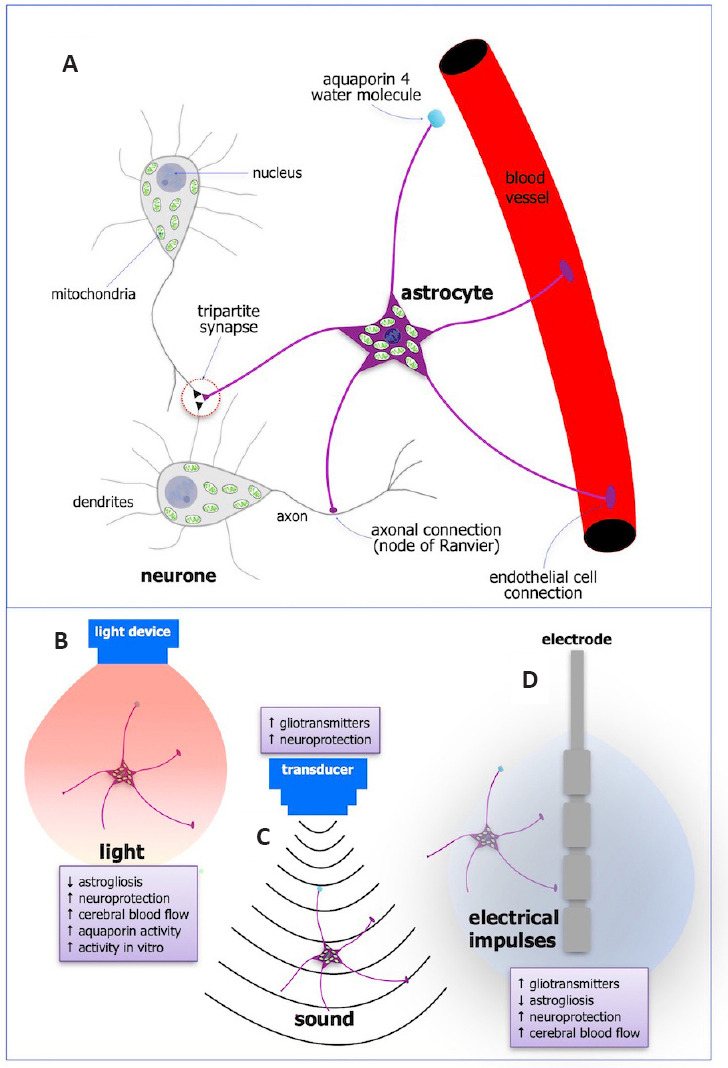
Schematic diagrams of the anatomy and functions of astrocytes (A) and the major effects on their function after photobiomodulation (B), ultrasound (C), and deep brain stimulation (D).
Astrocytes have a cell body with a nucleus and many mitochondria, and processes that extend to every neuronal synapse (tripartite synapse), to axons at the nodes of Ranvier, and to endothelial cells lining blood vessels. They also have aquaporin 4 water molecules on their end-feet (A). Taken all together, astrocytes are in a strategic position to influence many aspects of brain function. Their activity is influenced greatly after external treatment of light (photobiomodulation; B), sound (ultrasound; C), and electricity (deep brain stimulation; D), and these are listed in the small purple boxes next to each treatment. Created with Apple Keynote.
Through this extensive network of relationships - from synapses to blood vessels and to axons - astrocytes are in the unique position to monitor any extracellular changes and to offer a response to these changes and influence their immediate environment (Sofroniew and Vinters, 2010; Cicchetti and Barker, 2014; Bellesi et al., 2015; Finsterwald et al., 2015; Petit and Magistretti, 2016; Haydon, 2017; Magistretti and Allaman, 2018; Kim et al., 2019; Siracusa et al., 2019; Bozic et al., 2021; Vaidyanathan et al., 2021; Beard et al., 2022). They are by far and away the most populous cell in the brain and their higher numbers have been associated with higher-order brain function; humans generally have more glial cells than rodents (Sofroniew and Vinters, 2010; Herculano-Houzel, 2011) and even among humans, some of the smarter ones - like Albert Einstein - have been reported to have more of these cells than normal (Diamond et al., 1985). In the section below, we will consider first, the function of astrocytes under first, normal physiological conditions and second, pathological conditions.
Physiological conditions
General anatomy and physiology
Astrocytes have a cell body, complete with the nucleus and many organelles, such as the energy-producing mitochondria, which are, in fact, found in greater numbers in astrocytes than they are in neurons (Figure 1A). From each cell body, there are a number of fine, radiating processes. Astrocytes come in a range of different sizes and shapes and these, for the most part, are found in different regions of the brain. The major astrocyte types include; complex, star-shaped cells in the white matter (fibrous astrocytes), more basic-shaped cells in the grey matter (protoplasmic astrocytes); cells that have close links to the ependymal cells lining the ventricles (ependymal astrocytes); cells that relate to the migration of neurons during early development (radial glia) and cells whose end-feet processes relate closely to blood vessels (Claycomb et al., 2013). Together with their diverse anatomy, astrocytes show considerable molecular diversity. They can, for example, express a number of key proteins including glial fibrillary acidic protein (GFAP), glutamate transporter, glutamine synthetase, S100b calcium-binding protein B, N-Myc downstream-regulated gene 2 and aquaporin 4 (Zhang et al., 2019). Astrocytes have been shown to express potassium and sodium channels and can have evoked inward currents; unlike neurons, however, they do not generate action potentials but do show increases in intracellular calcium concentrations that can be viewed as a form of state of excitability (Sofroniew and Vinters, 2010). These patterns of excitability among astrocytes appear important in their communication not only between each other through the gap junctions but with neurons as well (Sofroniew and Vinters, 2010; Clasadonte and Prevot, 2018). Further, unlike neurons, astrocytes are not terminally differentiated cells and they retain their ability to divide and undergo mitosis into adulthood (Bozic et al., 2021).
Synaptic transmission
The astrocyte processes that involve themselves with synapses, particularly the glutamatergic ones, wrap around the synaptic cleft, and communicate with both the pre- and post-synaptic neurons; this is the so-called tripartite synapse (Figure 1A; Magistretti and Allaman, 2018; Beard et al., 2022). The astrocytes communicate with the neurons at the synapse via a release of various molecules including glutamate, adenosine triphosphate (ATP), γ-aminobutyric acid, and D-serine. These substances are referred to as “gliotransmitters” and are released in response to changes in the synaptic activity of the neurons; their release arises after an increase in the excitability (calcium influx) of the astrocytes (Sofroniew and Vinters, 2010).
Synaptic plasticity
Astrocytes have been shown to be involved in synaptic plasticity - that is, the formation and reorganization of synapses - in key structures associated with memory, such as the hippocampus. To regulate synaptic plasticity, astrocytes have been reported to send synaptogenic signals to neurons to rearrange and influence the functions of synapses, particularly excitatory ones. The extracellular matrix proteins thrombospondin 1 and 2 are secreted by astrocytes and these are important for synaptogenesis in the surrounding hippocampal neurons. Astrocytes have also been shown to release several cytokines, for example, tumor necrosis factor-alpha, that influence neuronal function and regulate their synaptic formations (Wang et al., 2022).
Fluid homeostasis
Astrocytes are in a key position to maintain extracellular fluid homeostasis and this is central for normal synaptic function. For example, astrocytes express the aquaporin 4 water channel (Figure 1A) that helps clear fluid from the brain and prevents vasogenic and/or cytotoxic edema; they also express transporters for the uptake of potassium, which act as buffers to limit the build-up of potassium in the extracellular space after neurons fire action potentials (Sofroniew and Vinters, 2010; Kim et al., 2019). At the synapse, astrocyte processes have been shown to express a range of neurotransmitter transporters, including those for glutamate, γ-aminobutyric acid, and glycine, that helps clear any excess from the synaptic cleft. The excess neurotransmitters are taken up by astrocytes and converted by enzymes (e.g., glutamine synthetase) into precursors (e.g., glutamine) that can be recycled back to the synapses as active neurotransmitters. In the case of glutamate, its clearance by astrocytes helps limit excitotoxicity and hence prevents neuronal damage (Sofroniew and Vinters, 2010).
Energy metabolism
Astrocytes, with such far-reaching processes extending to blood vessels, axons (at nodes of Ranvier), and synaptic clefts (Figure 1A; see above), are in a strategic position to take up glucose from blood vessels and distribute the resultant energy metabolites across the brain, in particular to the neurons that need them most. In addition, astrocytes are the principal sites of glycogen storage in the brain. Although glucose and glycogen are the main sources of energy used in the brain, other sources can be used under particular conditions. For instance, ketone bodies are used during early development and fasting, while lactate can be used during periods of intense physical activity and hypoglycemia (Magistretti and Allaman, 2018; Beard et al., 2022). It should be noted that astrocytes can produce lactate, from glucose or glycogen, under normal physiological conditions. The lactate is released into the extracellular space for uptake by neurons and can be used for induction of synaptic plasticity and during periods of high synaptic activity through a process known as the Astrocyte Neuron Lactate Shuttle (Sofroniew and Vinters, 2010; Suzuki et al., 2011; Magistretti and Allaman 2018). In general, lactate appears to maintain neuronal homeostasis by delivering an adequate energy supply to neurons, for normal synaptic function, plasticity, and memory (Suzuki et al., 2011).
Neurotrophic factors
Astrocytes have also been shown to produce and release a number of neurotrophic factors, for example, fibroblast growth factor 2 (Gonzalez et al., 1995) and brain-derived neurotrophic factor (BDNF; Jean et al., 2008). These neurotrophic factors have key roles in influencing not only normal synaptic transmission but also synaptic plasticity, in the formation and elimination of synapses across the brain during development and into adulthood. They are also beneficial in helping the survival of neurons in distress; that is, they are neuroprotective (Levine et al., 1995; Zechel et al., 2010; Kim et al., 2019).
Development
Astrocytes not only have a key role in the function of the adult brain, but they also play critical roles in the building of the brain during early development. From very early development, radial glia provide a set of tracks or guides for the migration of developing neurons from the ventricular zone to their final destination. Later, astrocytes establish molecular boundaries that help guide growing axons to their final targets and in the formation of developing synapses. Further, astrocytes, with their communications through gap junctions, help in the process of myelination of axons as the brain develops (Sofroniew and Vinters, 2010).
Blood flow and blood-brain barrier
Astrocytes form a key link between blood vessels and neurons. The end feet of astrocytes are intimate with the endothelium of blood vessels (Figure 1A) and they can influence blood flow, as well as the transport of substances in and out of the brain, including glucose (see above; Magistretti and Allaman, 2018). Astrocytes produce and release various molecules - for example, prostaglandins and nitric oxide - that can change the diameters of blood vessels and hence the flow of blood (Sofroniew and Vinters, 2010; MacVicar and Newman, 2015); such astrocyte-induced vascular changes are considered reflective of changes in the synaptic activity of the local neurons (Sofroniew and Vinters, 2010). With regard to the blood-brain barrier, a key interface between the vascular system and neural tissue, astrocytes appear essential for its formation during early development and potentially for its ongoing maintenance (Sofroniew and Vinters, 2010; Cabezas et al., 2014).
Sleep
Most, if not, all of the astrocytic functions that we have considered above have been when the brain is in a state of wakefulness. There is considerable evidence that astrocytes also have a key role in brain function during the state of sleep. In fact, it was Ramón y Cajal that first proposed this idea, suggesting that astrocytes extend processes into synapses during sleep and then retract them during wakefulness; he reasoned that this phenomenon would block and unblock synaptic activity during the different brain states (Haydon, 2017). Although Cajal’s reasoning was not entirely correct, his proposal was right, that astrocytes do play a role in the sleep process. It has been shown recently that astrocytic processes actually move closer to the synapse during wakefulness and away from the synapse during sleep. The astrocyte process retraction during sleep has been suggested to favor a glutamate spillover at the synapse, thereby promoting neuronal synchronization characteristic of non-rapid eye movement sleep (Bellesi et al., 2015). Astrocytes have been shown to have many other roles in sleep. They have G-protein-coupled receptors that detect the change in neuronal wave patterns reflective of sleep and wakefulness and can change their activity just before the transition between these brain states; they are important in the regulation of non-rapid eye movement sleep, in particular (Vaidyanathan et al., 2021). Astrocytes also influence the process of sleep homeostasis through the release of adenosine that promotes sleep (Haydon, 2017). More recently, they have been shown to be key players in the glymphatic system, which is involved in the clearance of fluid and toxic waste products - such as amyloid-β - from the brain during sleep. Fluid and waste are cleared through the brain via discrete perivascular channels and the aquaporin 4 water molecule on the astrocytes (Figure 1A), through to the meningeal lymphatic vessels and down into the neck. This “cleaning of the house” so-to-speak, is most active during sleep and is triggered by the change in neuronal brain waves associated with non-rapid eye movement sleep (δ and θ waves; Nedergaard and Goldman 2020). Through this shuttling system, astrocytes can also regulate the extracellular levels of glucose, glutamate, and lactate during the state of sleep (Petit and Magistretti, 2016).
Pathological conditions
Astrocytes not only have this multitude of roles for maintaining normal brain function in health, but they also play important roles during disease and/or after injury. When neurons suffer damage - either by stroke, trauma, or neurodegenerative disease - or during the process of natural aging, astrocytes become reactive. Under these conditions, they increase in size and number, as well as undergo many functional and molecular changes, such as an up-regulation of the protein GFAP. Taken together, these changes - referred to commonly as astrogliosis - have long been associated with toxic effects on the surrounding neurons, by either inhibiting axonal regeneration by forming glial scars and/or by secreting pro-inflammatory cytokines. More recently, however, astrogliosis has also been linked with beneficial effects, in particular, with the release of neuroprotective agents, such as neurotrophic factors (e.g., BDNF). The relationship between the toxic and beneficial function of astrocytes appears complex, being dependent on an array of different factors and molecular signaling mechanisms, and may change with time after the injury (Sofroniew and Vinters, 2010; Siracusa et al., 2019; Bozic et al., 2021; Beard et al., 2022).
In summary, astrocytes are such busy little cells, involving themselves in so many aspects of brain function in both health and disease in adulthood, and during the building of the brain in early development. They are essential for normal synaptic transmission, energy metabolism, fluid balance, sleep/wakefulness cycle, synaptic plasticity, blood flow, and the blood-brain barrier, as well as neuronal and axonal guidance during early development. They also undergo key changes when the brain becomes dysfunctional, during disease and/or after injury; depending on the condition and circumstances of the dysfunction, astrocytes can provide environments that are either toxic or of benefit to the suffering neurons.
Evidence that Light Stimulates Astrocytes
Light is classified as an electromagnetic wave made up of an extensive range of wavelengths, from the ones that we can see as blue (~400–500 nm) and red (~600–750 nm) to the ones that we cannot as infrared (> 750 nm). Over the last seventy years or so, scientists and clinicians have used a small part of this light spectrum - across the red to infrared range (~λ = 600–1300 nm) - to impart many beneficial effects on body function in both health and disease. These wavelengths of light have been shown to travel through body tissues, such as skin, bone, brain, and muscle and their therapeutic application has been referred to as photobiomodulation (Hamblin, 2016).
Although the precise mechanisms that underpin photobiomodulation are not entirely clear, the mitochondria within the cells - whether they are neurons or glia - appear to be the central targets. Quite remarkably, mitochondria house several different types of receptors for light, including cytochrome c oxidase and interfacial nanowater. These molecules have not been viewed as “receptors” in the classical sense, but once activated - for example, similar to how a drug may activate specific receptors in the cell - this leads to a chemical and molecular change within the cell. In particular, after cytochrome c oxidase and/or interfacial nanowater absorb the light, they undergo a conformational change, prompting the production of ATP energy. In addition to this short-term gain of generating more energy to fuel intrinsic cell function, this process induces more long-term changes, with the expression of a number of stimulatory and/or protective genes in the nucleus. It should be noted that such light-induced effects are not heat-mediated, but have been shown by many studies to be chemically medicated (Hamblin, 2016; Hamblin and Liebert, 2022).
With these many beneficial photobiomodulation-induced cellular outcomes, a large number of studies have shown that photobiomodulation can increase the survival of neurons in a range of animal models of disease, from Parkinson’s disease to multiple sclerosis and from Alzheimer’s disease to traumatic brain injury (Hamblin, 2016).
In addition to this impact on neurons, a number of studies have reported that photobiomodulation can influence astrocyte activity in these animal models of disease or trauma (Figure 1B). In particular, the astrogliosis evident in each case is very much reduced after photobiomodulation. In toxin-induced mouse and monkey models of Parkinson’s disease, photobiomodulation results in fewer GFAP+ astrocytes in the basal ganglia (El Massri et al., 2016). Similar reductions have been noted in the retina of a transgenic mouse model of age-related macular degeneration (Begum et al., 2013), in the striatum of a mouse model of aging (El Massri et al., 2018), and in the cerebral cortex of several transgenic mouse models of Alzheimer’s disease (Lu et al., 2017; Blivet et al., 2018; Wu et al., 2021). The neuroprotective effects of photobiomodulation may well involve an astrocyte-induced release of neurotrophic growth factors, such as BDNF. In a mouse model of traumatic brain injury, photobiomodulation stimulates the release of BDNF and this has been suggested to increase the proliferation of neuroprogenitor cells and the formation of new synapses (Xuan et al., 2015). Photobiomodulation also generates an increase in cerebral blood flow (e.g., Hipskind et al., 2019; Baik et al., 2021), and this key feature may be mediated by astrocytes through a prostaglandin-induced vasodilation (see above). Finally, there are indications that astrocytes could be involved in the photobiomodulation-induced clearance of fluid and toxic waste from the brain during the state of sleep (Semyachkina-Glushkovskaya et al., 2020, 2021a, b; Salehpour et al., 2022); this clearance may be due, at least in part, by stimulation of the glymphatic system, in particular, a stimulation of the aquaporin 4 water channel on the astrocytes (Valverde et al., 2022).
From all these studies it is not clear however, whether the particular changes in astrogliosis, neurotrophic factors, cerebral blood flow, and glymphatic activity are due to photobiomodulation having a direct primary effect on the astrocytes, or whether the effect is secondary to other factors, such as the protection of the neurons. Astrocytes have many mitochondria, in fact, more so than neurons (see above), so there is every reason to believe that the effect is direct. Indeed, a recent in vitro study has shown that photobiomodulation can stimulate astrocyte activity directly by increasing astrocyte proliferation, as well as the expression of GFAP (Yoon et al., 2021). One would assume that astrocytes in the retina, those cells that are exposed continually to the whole spectrum of wavelengths of light during the day, use the red and infrared component of the range to either maintain homeostasis and/or for the repair of retinal tissue in cases of disease or trauma (see above).
It should be noted that many studies have used optogenetics, a methodology that involves the introduction of light-sensitive opsins into particular targetted cells. The cells are then stimulated by light, usually, the shorter ones in the blue range (~400–500 nm), and the introduced opsins undergo a conformational change. This change then drives electrical changes across the plasma membrane. There are a number of different types of opsins that have been identified, for example, channelrhodopsin and melanopsin, and these can generate very different types of cellular responses. For example, optogenetic stimulation of channelrhodopsin in astrocytes has been shown to induce changes in intracellular calcium levels, leading to changes in the activity of neurons. Further, such optogenetic stimulation in astrocytes also induces the release of ATP energy (Mederos et al., 2019; Gerasimov et al., 2021; McNeill et al., 2022). Although this is not strictly a natural form of astrocyte stimulation by light - as say photobiomodulation - it is an interesting technology based on light stimulation.
In summary, there is evidence that light - photobiomodulation - can reduce the astrogliosis associated with various disease models, as well as increase astrocyte activity and proliferation in cell culture. Photobiomodulation promotes neuroprotection, synaptic plasticity, and neurogenesis by stimulating the release of neurotrophic factors, increases cerebral blood flow, and helps the clearance of fluid and waste from the brain; these functions may well be mediated by an activation of astrocytes (Figure 1B). Indirectly, through optogenetics, light-activated astrocytes have been shown to influence greatly the activity of neurons.
Does Sound Stimulate Astrocytes?
Ultrasound is a mechanical wave with frequencies ranging from about 1 to 15 MHz, well above that of human hearing (< 20 kHz). As with light, these waves can travel through body tissues, namely skin, bone, brain, and muscle. The best-known use for ultrasound is diagnostic (ranging from ~1–15 MHz), for the medical exploration of body organs. A much lesser known use for ultrasound is therapeutic and - as with photobiomodulation - it is being considered as the “next generation”, non-invasive stimulation technology for treating the spectrum of neurodegenerative and psychiatric disorders (~1 MHz; Tufail et al., 2010; Yulug et al., 2017; Oh et al., 2019; Deng et al., 2021; Balbi et al., 2022). Ultrasound has been used on patients to create small lesions in the brain, through rapid temperature rises, similar to those created after radio-frequency application. For example, focused ultrasound can create a thalamotomy, leading to a reduction in the signs of Parkinson’s disease (Magara et al., 2014; Schlesinger et al., 2015).
A key, recent development in the use of ultrasound as therapy is that, in experimental animals, it has been shown to be neuroprotective (Wang et al., 2014; Fan et al., 2016; Balbi et al., 2022). It may offer this neuroprotection in several ways. First, ultrasound can stimulate the production of neurotrophic factors, such as BDNF, and these could then promote the survival of distressed neurons (Tufail et al., 2010; Leinenga et al., 2016; Yulug et al., 2017; Balbi et al., 2022). Second, many studies have indicated that ultrasound has the ability to open, temporarily and safely, the blood-brain barrier. The transient opening of the blood-brain barrier is facilitated by intravenously injected gaseous “microbubbles”. The sound waves cause the microbubbles to expand, leading to the opening of their tight junctions. This enables the passage of endogenous blood-borne factors, together with any exogenously applied drugs and/or neuroprotective agents into the brain, those that would otherwise be prevented by an intact blood-brain barrier (Tufail et al., 2010; Leinenga et al., 2016; Yulug et al., 2017; Balbi et al., 2022).
Ultrasound has been shown to have a clear effect on the activity of neurons (Blakemore et al., 2019; Sanguinetti et al., 2020; Yu et al., 2021; Chu et al., 2022). The precise mechanisms that underpin the effect of ultrasound on neuronal function are not clear, but several recent studies have reported that ultrasonic waves have an impact on the function of mechanosensitive ion channels (Chu et al., 2022). For example, ultrasound administration has been shown to excite cortical neurons in culture through a mechanical mechanism mediated by specific calcium-selective mechanosensitive ion channels. This activation generates an accumulation of calcium resulting in a burst-firing response (Yoo et al., 2022).
Ultrasound has also been shown to impact the activity of astrocytes, again acting primarily on mechanosensitive ion channels of these cells. Ultrasound waves can open the transient receptor potential channel A1 in astrocytes, resulting in an influx of calcium through the open channels (Oh et al., 2019). This leads to a release of gliotransmitters, including glutamate, that then activates the surrounding neurons (Figure 1C). In addition, ultrasound has been reported to stimulate the release of exosomes - which play a pivotal role in removing intracellular materials, such as amyloid-β - from astrocytes. In this way, ultrasound could induce a neuroprotective role in the astrocytes by prompting the clearance of toxic substances from the brain (Deng et al., 2021). In addition, astrocytes could further contribute to ultrasound-induced neuroprotection by releasing BDNF into the extracellular space (Tufail et al., 2010; Leinenga et al., 2016; Yulug et al., 2017; Balbi et al., 2022).
In summary, ultrasound can directly influence astrocyte activity through mechanosensitive ion channels that then leads to the release of gliotransmitters to influence neuronal activity. An ultrasound activation of astrocytes may also induce neuroprotection, by stimulating the clearance of toxic waste from the brain via exosomes and by improving neuronal survival via the release of neurotrophic factors (Figure 1C).
What Does Electrical Stimulation Do for Astrocytes?
Deep brain stimulation at high frequency (> 100 Hz) relies on the propagation of electrical impulses across the brain after surgical implantation of an electrode into a targetted nucleus or region of the brain. Once thought to generate a “functional inactivation or lesion”, there is now increasingly more evidence indicating that deep brain stimulation at high frequency can have both inhibitory and excitatory effects on neurons, generating an extensive range of functional effects. This suggests that the impact of deep brain stimulation can work not only at a local level but at a global, network level as well (Fenoy et al., 2004; Chiken and Nambu, 2016; Ashkan et al., 2017).
Deep brain stimulation has been used as an effective therapeutic strategy for a wide range of neurological disorders, from Parkinson’s disease to epilepsy and from depression to chronic pain (Vedam-Mai et al., 2012; Ashkan et al., 2017). It has alleviated the signs and symptoms of these, and of many other, disorders in many patients over extended time-frames (i.e., years). And yet, notwithstanding extensive research over thirty years since its introduction, the precise mechanisms behind deep brain stimulation are still not entirely clear. Several have been suggested, however, and most of these have focused on the impact on the function of the neurons. Briefly, some of the suggested mechanisms include; a generation of local inhibition by either a release of inhibitory neurotransmitters from interneurons and/or inhibitory afferents; a “jamming” or depolarisation block, with electrical impulses from the electrode blocking the firing of neurons; an excitation of some neural pathways that result in an overall network change in functional connectivity, and; a dissociation of the synaptic inputs and outputs of neurons, thereby disrupting the flow of information through the targetted nucleus (Chiken and Nambu, 2016; Ashkan et al., 2017).
In more recent times, a number of authors have considered the effect of deep brain stimulation on astrocytes, and whether these cells play a role in the underlying mechanism. Indeed, astrocytes have been shown to be activated by electrical stimulation leading subsequently to changes in neuronal activity, both locally and globally. Some of the major changes after electrical stimulation of astrocytes will be considered below (Figure 1D).
First, deep brain stimulation has been shown to prompt a release of gliotransmitters (i.e., ATP and glutamate) from astrocytes. The release of ATP may contribute to the local inhibitory effect of deep brain stimulation; this inhibition of synaptic transmission is through the action of adenosine, which is converted from ATP by astrocytes, on post- and pre-synaptic A1 receptors (Fenoy et al., 2004; Vedam-Mai et al., 2012). By contrast, the release of glutamate by the astrocytes can potentially lead to a local excitation of neurons (Tawfik et al., 2010; Vedam-Mai et al., 2012). Second, deep brain stimulation has been shown to reduce astrogliosis and pro-inflammatory cytokines across the brain in animal models of disease, contributing to the overall reduction of inflammation generated by the particular conditions (e.g., Campos et al., 2020). It should be noted that the mechanical implantation of the electrode itself into the brain generates astrogliosis around the implant site. In fact, the resultant reactive astrocytes have been shown to release gliotransmitters that, in turn, contribute to the overall effects of deep stimulation (see above; Tawfik et al., 2010; Vedam-Mai et al., 2012; Cicchetti and Barker, 2014). Third, deep brain stimulation prompts the release of neuroprotective agents by astrocytes. When astrocytes are stimulated in cell culture, there is a release of a number of extracellular matrix proteins, such as adenosine, that may help promote synaptic plasticity and neuroprotection to neurons in distress (Fenoy et al., 2004; Jang et al., 2019). Further, there is evidence that deep brain stimulation increases the expression of BDNF that promotes synaptic plasticity and neuroprotection, and this increase may be induced by an astrocyte activation (Fischer and Sortwell, 2019). There is also evidence that deep brain stimulation induces neurogenesis, by increasing the proliferation of neurogenic astrocytes, that then later differentiate into neurons (Encinas et al., 2011; Vedam-Mai et al., 2012). Finally, deep brain stimulation generates a change in cerebral blood flow, that may well be mediated by an astrocyte activation, after a release of prostaglandin causing vasodilation (Perlmutter et al., 2002; Vedam-Mai et al., 2012).
In summary, many of the functional outcomes of deep brain stimulation have been related to the activation of astrocytes. Astrocytes, once activated electrically, have been associated with; an inhibition or excitation of neurons (via gliotransmitters), a reduction in inflammation (e.g., astrogliosis), a release of extracellular proteins involved in synaptic plasticity and neuroprotection, and a stimulation of neurogenesis. Further, the increases in cerebral blood flow may well be mediated by an activation of astrocytes (Figure 1D).
Potential Clinical Applications
There have been many studies exploring the clinical applications of light (e.g., Hamblin, 2016), sound (e.g., Leinenga et al., 2016), and electrical stimulation (e.g., Ashkan et al., 2017), particularly for the latter method. Given the wide range of effects each of these therapies have on astrocyte function, it may be instructive to consider a combined clinical approach for different neurological conditions. For example, in Parkinson’s disease, deep brain stimulation of the subthalamic nucleus (to stimulate basal ganglia function) and an intracranial optical fiber implant into the midbrain or ventricles (to reach deep zones of pathology in the brainstem) may be used, offering a combined symptomatic and potentially neuroprotective effect. For patients with Alzheimer’s disease, ultrasound, and photobiomodulation may be administered transcranially (to reach superficial zones of pathology in the cerebral cortex), together with deep brain stimulation of the fornix or stria terminalis could be considered (to stimulate memory centers; Rios et al., 2022). Future endeavors could explore different combinations of therapies in different neurological conditions, those that improve astrocyte function and the overall environment for neurons to perform most effectively.
Conclusions
The three brain therapies considered here - photobiomodulation, ultrasound, and deep brain stimulation - have a clear impact on the activity of astrocytes. When taken all together, each of these external sources - light, sound, and electricity - can influence many, if not, all of the functions associated with astrocytes. These include; influencing neuronal activity (both locally and globally through the large-scale networks, through the release of gliotransmitters), increasing cerebral blood flow (e.g., through prostaglandin-induced vasodilation), prompting neuroprotection (and synaptic plasticity and neurogenesis; e.g., through the release of various molecules) and stimulating the glymphatic system (e.g., through aquaporin 4 water channel). We suggest that astrocytes, just like neurons, can respond positively to each of these external applications and that their activation could each impart many beneficial outcomes on brain function. It remains to be determined whether any one of these therapies influences particular astrocytic functions better than the others and whether a combination of therapies and indeed which ones, might yield the best outcomes.
Footnotes
Funding: This work was supported by Fonds de dotation Clinatec and COVEA France (to JM).
Conflicts of interest: The authors declare no conflicts of interest.
Data availability statement: Not applicable.
C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- 1.Ashkan K, Rogers P, Bergman H, Ughratdar I. Insights into the mechanisms of deep brain stimulation. Nat Rev Neurol. 2017;13:548–554. doi: 10.1038/nrneurol.2017.105. [DOI] [PubMed] [Google Scholar]
- 2.Baik JS, Lee TY, Kim NG, Pak K, Ko SH, Min JH, Shin YI. Effects of photobiomodulation on changes in cognitive function and regional cerebral blood flow in patients with mild cognitive impairment:a pilot uncontrolled trial. J Alzheimers Dis. 2021;83:1513–1519. doi: 10.3233/JAD-210386. [DOI] [PubMed] [Google Scholar]
- 3.Balbi M, Blackmore DG, Padmanabhan P, Götz J. Ultrasound-mediated bioeffects in senescent mice and Alzheimer's mouse models. Brain Sci. 2022;12:775. doi: 10.3390/brainsci12060775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beard E, Lengacher S, Dias S, Magistretti PJ, Finsterwald C. Astrocytes as key regulators of brain energy metabolism:new therapeutic perspectives. Front Physiol. 2022;12:825816. doi: 10.3389/fphys.2021.825816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Begum R, Powner MB, Hudson N, Hogg C, Jeffery G. Treatment with 670 nm light up regulates cytochrome C oxidase expression and reduces inflammation in an age-related macular degeneration model. PLoS One. 2013;8:e57828. doi: 10.1371/journal.pone.0057828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellesi M, de Vivo L, Tononi G, Cirelli C. Effects of sleep and wake on astrocytes:clues from molecular and ultrastructural studies. BMC Biol. 2015;13:66. doi: 10.1186/s12915-015-0176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blackmore J, Shrivastava S, Sallet J, Butler CR, Cleveland RO. Ultrasound neuromodulation:a review of results, mechanisms and safety. Ultrasound Med Biol. 2019;45:1509–1536. doi: 10.1016/j.ultrasmedbio.2018.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blivet G, Meunier J, Roman FJ, Touchon J. Neuroprotective effect of a new photobiomodulation technique against Aβ25–35 peptide–induced toxicity in mice:Novel hypothesis for therapeutic approach of Alzheimer's disease suggested. Alzheimers Dement. 2018;4:54–63. doi: 10.1016/j.trci.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bozic I, Savic D, Lavrnja I. Astrocyte phenotypes:emphasis on potential markers in neuroinflammation. Histol Histopathol. 2021;36:267–290. doi: 10.14670/HH-18-284. [DOI] [PubMed] [Google Scholar]
- 10.Cabezas R, Ávila M, Gonzalez J, El-Bachá RS, Báez E, García-Segura LM, Jurado Coronel JC, Capani F, Cardona-Gomez GP, Barreto GE. Astrocytic modulation of blood brain barrier:perspectives on Parkinson's disease. Front Cell Neurosci. 2014;8:211. doi: 10.3389/fncel.2014.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campos ACP, Kikuchi DS, Paschoa AFN, Kuroki MA, Fonoff ET, Hamani C, Pagano RL, Hernandes MS. Unraveling the role of astrocytes in subthalamic nucleus deep brain stimulation in a Parkinson's disease rat model. Cell Mol Neurobiol. 2020;40:939–954. doi: 10.1007/s10571-019-00784-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiken S, Nambu A. Mechanism of deep brain stimulation. Neuroscientist. 2016;22:313–322. doi: 10.1177/1073858415581986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu YC, Lim J, Chien A, Chen CC, Wang JL. Activation of mechanosensitive ion channels by ultrasound. Ultrasound Med Biol. 2022;48:1981–1994. doi: 10.1016/j.ultrasmedbio.2022.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Cicchetti F, Barker RA. The glial response to intracerebrally delivered therapies for neurodegenerative disorders:is this a critical issue? Front Pharmacol. 2014;5:139. doi: 10.3389/fphar.2014.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clasadonte J, Prevot V. The special relationship:glia–neuron interactions in the neuroendocrine hypothalamus. Nat Rev Endocrinol. 2018;14:25–44. doi: 10.1038/nrendo.2017.124. [DOI] [PubMed] [Google Scholar]
- 16.Claycomb KI, Johnson KM, Winokur PN, Sacino AV, Crocker SJ. Astrocyte regulation of CNS inflammation and remyelination. Brain Sci. 2013;3:1109–1127. doi: 10.3390/brainsci3031109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng Z, Wang J, Xiao Y, Li F, Niu L, Liu X, Meng L, Zheng H. Ultrasound-mediated augmented exosome release from astrocytes alleviates amyloid-β-induced neurotoxicity. Theranostics. 2021;11:4351–4362. doi: 10.7150/thno.52436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diamond MC, Scheibel AB, Murphy GM, Harvey T. On the brain of a scientist:Albert Einstein. Exp Neurol. 1985;88:198–204. doi: 10.1016/0014-4886(85)90123-2. [DOI] [PubMed] [Google Scholar]
- 19.El Massri N, Moro C, Torres N, Darlot F, Agay D, Chabrol C, Johnstone DM, Stone J, Benabid AL, Mitrofanis J. Near-infrared light treatment reduces astrogliosis in MPTP-treated monkeys. Exp Brain Res. 2016;234:3225–3232. doi: 10.1007/s00221-016-4720-7. [DOI] [PubMed] [Google Scholar]
- 20.El Massri N, Weinrich TW, Kam JH, Jeffery G, Mitrofanis J. Photobiomodulation reduces gliosis in the basal ganglia of aged mice. Neurobiol Aging. 2018;66:131–137. doi: 10.1016/j.neurobiolaging.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Encinas JM, Hamani C, Lozano AM, Enikolopov G. Neurogenic hippocampal targets of deep brain stimulation. J Comp Neurol. 2011;519:6–20. doi: 10.1002/cne.22503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan CH, Ting CY, Lin CY, Chan HL, Chang YC, Chen YY, Liu HL, Yeh CK. Noninvasive, targeted, and non-viral ultrasound-mediated GDNF-plasmid delivery for treatment of Parkinson's disease. Sci Rep. 2016;6:19579. doi: 10.1038/srep19579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fenoy AJ, Goetz L, Chabardès S, Xia Y. Deep brain stimulation:are astrocytes a key driver behind the scene?CNS Neurosci Ther. 2014;20:191–201. doi: 10.1111/cns.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finsterwald C, Magistretti PJ, Lengacher S. Astrocytes:new targets for the treatment of neurodegenerative diseases. Curr Pharm Des. 2015;21:3570–3581. doi: 10.2174/1381612821666150710144502. [DOI] [PubMed] [Google Scholar]
- 25.Fischer DL, Sortwell CE. BDNF provides many routes toward STN DBS-mediated disease modification. Mov Disord. 2019;34:22–34. doi: 10.1002/mds.27535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerasimov E, Erofeev A, Borodinova A, Bolshakova A, Balaban P, Bezprozvanny I, Vlasova OL. Optogenetic activation of astrocytes—effects on neuronal network function. Int J Mol Sci. 2021;22:9613. doi: 10.3390/ijms22179613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamblin MR. Shining light on the head:photobiomodulation for brain disorders. BBA Clin. 2016;6:113–124. doi: 10.1016/j.bbacli.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamblin MR, Liebert A. Photobiomodulation therapy mechanisms beyond cytochrome c oxidase. Photobiomodul Photomed Laser Surg. 2022;40:75–77. doi: 10.1089/photob.2021.0119. [DOI] [PubMed] [Google Scholar]
- 29.Haydon PG. Astrocytes and the modulation of sleep. Curr Opin Neurobiol. 2017;44:28–33. doi: 10.1016/j.conb.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herculano-Houzel S. Scaling of brain metabolism with a fixed energy budget per neuron:implications for neuronal activity, plasticity and evolution. PLoS One. 2011;6:e17514. doi: 10.1371/journal.pone.0017514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hipskind SG, Grover FLJ, Fort TR, Helffenstein D, Burke TJ, Quint SA, Bussiere G, Stone M, Hurtado T. Pulsed transcranial red/near-infrared light therapy using light-emitting diodes improves cerebral blood flow and cognitive function in veterans with chronic traumatic brain injury:a case series. Photobiomodul Photomed Laser Surg. 2019;37:77–84. doi: 10.1089/photob.2018.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jang JS, Choi CI, Yi J, Butters K, Kim I, Bhagwate A, Jen J, Chang SY. High frequency electrical stimulation promotes expression of extracellular matrix proteins from human astrocytes. Mol Biol Rep. 2019;46:4369–4375. doi: 10.1007/s11033-019-04890-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim Y, Park J, Choi YK. The role of astrocytes in the central nervous system focused on BK channel and heme oxygenase metabolites:a review. Antioxidants (Basel) 2019;8:121. doi: 10.3390/antiox8050121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leinenga G, Langton C, Nisbet R, Götz J. Ultrasound treatment of neurological diseases--current and emerging applications. Nat Rev Neurol. 2016;12:161–174. doi: 10.1038/nrneurol.2016.13. [DOI] [PubMed] [Google Scholar]
- 35.Levine ES, Dreyfus CF, Black IB, Plummer MR. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci U S A. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacVicar BA, Newman EA. Astrocyte regulation of blood flow in the brain. Cold Spring Harb Perspect Biol. 2015;7:a020388. doi: 10.1101/cshperspect.a020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Magara A, Bühler R, Moser D, Kowalski M, Pourtehrani P, Jeanmonod D. First experience with MR-guided focused ultrasound in the treatment of Parkinson's disease. J Ther Ultrasound. 2014;2:11. doi: 10.1186/2050-5736-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magistretti PJ, Allaman I. Lactate in the brain:from metabolic end-product to signalling molecule. Nat Rev Neurosci. 2018;19:235–249. doi: 10.1038/nrn.2018.19. [DOI] [PubMed] [Google Scholar]
- 39.McNeill J, Rudyk C, Hildebrand ME, Salmaso N. Ion channels and electrophysiological properties of astrocytes:implications for emergent stimulation technologies. Front Cell Neurosci. 2021;15:644126. doi: 10.3389/fncel.2021.644126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mederos S, Hernández-Vivanco A, Ramírez-Franco J, Martín-Fernández M, Navarrete M, Yang A, Boyden ES, Perea G. Melanopsin for precise optogenetic activation of astrocyte-neuron networks. Glia. 2019;67:915–934. doi: 10.1002/glia.23580. [DOI] [PubMed] [Google Scholar]
- 41.Nedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science. 2020;370:50–56. doi: 10.1126/science.abb8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oh SJ, Lee JM, Kim HB, Lee J, Han S, Bae JY, Hong GS, Koh W, Kwon J, Hwang ES, Woo DH, Youn I, Cho IJ, Bae YC, Lee S, Shim JW, Park JH, Lee CJ. Ultrasonic neuromodulation via astrocytic TRPA1. Curr Biol. 2019;29:3386–3401. doi: 10.1016/j.cub.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 43.Perlmutter JS, Mink JW, Bastian AJ, Zackowski K, Hershey T, Miyawaki E, Koller W, Videen TO. Blood flow responses to deep brain stimulation of thalamus. Neurology. 2002;58:1388–1394. doi: 10.1212/wnl.58.9.1388. [DOI] [PubMed] [Google Scholar]
- 44.Petit JM, Magistretti PJ. Regulation of neuron-astrocyte metabolic coupling across the sleep-wake cycle. Neuroscience. 2016;323:135–156. doi: 10.1016/j.neuroscience.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Ríos AS, Oxenford S, Neudorfer C, Butenko K, Li N, Rajamani N, Boutet A, Elias GJB, Germann J, Loh A, Deeb W, Wang F, Setsompop K, Salvato B, Almeida LB de, Foote KD, Amaral R, Rosenberg PB, Tang-Wai DF, Wolk DA, et al. Optimal deep brain stimulation sites and networks for stimulation of the fornix in Alzheimer's disease. Nat Commun. 2022;7707;13 doi: 10.1038/s41467-022-34510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salehpour F, Khademi M, Bragin DE, DiDuro JO. Photobiomodulation therapy and the glymphatic system:promising applications for augmenting the brain lymphatic drainage system. Int J Mol Sci. 2022;23:2975. doi: 10.3390/ijms23062975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanguinetti JL, Hameroff S, Smith EE, Sato T, Daft CMW, Tyler WJ, Allen JJB. Transcranial focused ultrasound to the right prefrontal cortex improves mood and alters functional connectivity in humans. Front Hum Neurosci. 2020;14:52. doi: 10.3389/fnhum.2020.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlesinger I, Eran A, Sinai A, Erikh I, Nassar M, Goldsher D, Zaaroor M. MRI guided focused ultrasound thalamotomy for moderate-to-severe tremor in Parkinson's disease. Parkinsons Dis. 2015;2015:219149. doi: 10.1155/2015/219149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Semyachkina-Glushkovskaya O, Abdurashitov A, Dubrovsky A, Klimova M, Agranovich I, Terskov A, Shirokov A, Vinnik V, Kuzmina A, Lezhnev N, Blokhina I, Shnitenkova A, Tuchin V, Rafailov E, Kurths J. Photobiomodulation of lymphatic drainage and clearance:perspective strategy for augmentation of meningeal lymphatic functions. Biomed Opt Express. 2020;11:725. doi: 10.1364/BOE.383390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Semyachkina-Glushkovskaya O, Klimova M, Iskra T, Bragin D, Abdurashitov A, Dubrovsky A, Khorovodov A, Terskov A, Blokhina I, Lezhnev N, Vinnik V, Agranovich I, Mamedova A, Shirokov A, Navolokin N, Khlebsov B, Tuchin V, Kurths J. Transcranial photobiomodulation of clearance of beta-amyloid from the mouse brain:effects on the meningeal lymphatic drainage and blood oxygen saturation of the brain. Adv Exp Med Biol. 2021a;1269:57–61. doi: 10.1007/978-3-030-48238-1_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Semyachkina-Glushkovskaya O, Penzel T, Blokhina I, Khorovodov A, Fedosov I, Yu T, Karandin G, Evsukova A, Elovenko D, Adushkina V, Shirokov A, Dubrovskii A, Terskov A, Navolokin N, Tzoy M, Ageev V, Agranovich I, Telnova V, Tsven A, Kurths J. Night photostimulation of clearance of beta-amyloid from mouse brain:new strategies in preventing Alzheimer's disease. Cells. 2021b;10:3289. doi: 10.3390/cells10123289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siracusa R, Fusco R, Cuzzocrea S. Astrocytes:role and functions in brain pathologies. Front Pharmacol. 2019;10:1114. doi: 10.3389/fphar.2019.01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sofroniew MV, Vinters HV. Astrocytes:biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tawfik VL, Chang SY, Hitti FL, Roberts DW, Leiter JC, Jovanovic S, Lee KH. Deep brain stimulation results in local glutamate and adenosine release:investigation into the role of astrocytes. Neurosurgery. 2010;67:367–375. doi: 10.1227/01.NEU.0000371988.73620.4C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tufail Y, Matyushov A, Baldwin N, Tauchmann ML, Georges J, Yoshihiro A, Tillery SIH, Tyler WJ. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron. 2010;66:681–694. doi: 10.1016/j.neuron.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 57.Vaidyanathan TV, Collard M, Yokoyama S, Reitman ME, Poskanzer KE. Cortical astrocytes independently regulate sleep depth and duration via separate GPCR pathways. eLife. 2021;10:e63329. doi: 10.7554/eLife.63329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Valverde A, Hamilton C, Moro C, Billeres M, Magistretti P, Mitrofanis J. Lights at night:does photobiomodulation improve sleep? Neural Regen Res. 2022;18:474–477. doi: 10.4103/1673-5374.350191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vedam-Mai V, van Battum EY, Kamphuis W, Feenstra MGP, Denys D, Reynolds BA, Okun MS, Hol EM. Deep brain stimulation and the role of astrocytes. Mol Psychiatry. 2012;17:124–131. doi: 10.1038/mp.2011.61. [DOI] [PubMed] [Google Scholar]
- 60.Wang X, Cui G, Yang X, Zhang Z, Shi H, Zu J, Hua F, Shen X. Intracerebral administration of ultrasound-induced dissolution of lipid-coated GDNF microbubbles provides neuroprotection in a rat model of Parkinson's disease. Brain Research Bulletin. 2014;103:60–65. doi: 10.1016/j.brainresbull.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y, Fu AKY, Ip NY. Instructive roles of astrocytes in hippocampal synaptic plasticity:neuronal activity-dependent regulatory mechanisms. FEBS J. 2022;289:2202–2218. doi: 10.1111/febs.15878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xuan W, Agrawal T, Huang L, Gupta GK, Hamblin MR. Low-level laser therapy for traumatic brain injury in mice increases brain derived neurotrophic factor (BDNF) and synaptogenesis. J Biophoton. 2015;8:502–511. doi: 10.1002/jbio.201400069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoo S, Mittelstein DR, Hurt RC, Lacroix J, Shapiro MG. Focused ultrasound excites cortical neurons via mechanosensitive calcium accumulation and ion channel amplification. Nat Commun. 2022;13:493. doi: 10.1038/s41467-022-28040-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoon SR, Hong N, Lee MY, Ahn JC. Photobiomodulation with a 660-nanometer light-emitting diode promotes cell proliferation in astrocyte culture. Cells. 2021;10:1664. doi: 10.3390/cells10071664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu K, Niu X, Krook-Magnuson E, He B. Intrinsic functional neuron-type selectivity of transcranial focused ultrasound neuromodulation. Nat Commun. 2021;12:2519. doi: 10.1038/s41467-021-22743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yulug B, Hanoglu L, Kilic E. The neuroprotective effect of focused ultrasound:New perspectives on an old tool. Brain Res Bull. 2017;131:199–206. doi: 10.1016/j.brainresbull.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 67.Zechel S, Werner S, Unsicker K, von Bohlen und Halbach O. Expression and functions of fibroblast growth factor 2 (FGF-2) in hippocampal formation. Neuroscientist. 2010;16:357–373. doi: 10.1177/1073858410371513. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Z, Ma Z, Zou W, Guo H, Liu M, Ma Y, Zhang L. The appropriate marker for astrocytes:comparing the distribution and expression of three astrocytic markers in different mouse cerebral regions. Biomed Res Int. 2019;2019:e9605265. doi: 10.1155/2019/9605265. [DOI] [PMC free article] [PubMed] [Google Scholar]


