Abstract
Background
Neuroferritinopathy is a rare inherited neurodegenerative disease with brain iron accumulation characterized by brain iron overload resulting in progressive movement disorders. No treatment is currently available.
Objective
We assessed conservative iron chelation with deferiprone at 30 mg/kg/day on the disease progression with controlled periods of discontinuation.
Methods
Four patients with confirmed molecular diagnosis of neuroferritinopathy were given deferiprone at different stages of disease progression and with clinical and biological monitoring to control benefit and risk.
Results
The four patients showed slight to high improvement. In one case, we managed to stabilize disease progression for more than 11 years. In another case, we were able to reverse symptoms after a few months of treatment. The earliest the treatment was started, the most efficient it was on disease progression.
Conclusions
Conservative iron chelation should be further assessed in neuroferritinopathy. © 2022 The Authors. Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Keywords: neuroferritinopathy, iron chelation, neuroprotection, Parkinson's disease, spinocerebellar ataxia
Neurodegeneration with brain iron accumulation (NBIA) is a group of rare inherited diseases with heterogeneous molecular entities. They are characterized by iron overload in various regions of the brain, predominantly in the basal ganglia and cerebellar nuclei, ultimately resulting in progressive childhood or young adult‐onset movement disorders and/or cerebellar signs. 1 The most common entity is pantothenate kinase‐associated neurodegeneration (PKAN).2, 3
Among other forms, neuroferritinopathy, also known as hereditary ferritinopathy or NBIA type 3, accounts for ~4% of patients with NBIA. 2 Neuroferritinopathy is cause by diverse mutations in the ferritin light chain gene (FTL) resulting in a frameshift leading to altered ability of ferritin to incorporate iron. 4 These autosomal dominant mutations with complete penetrance result in iron‐ferritin complex accumulation, most notably in the basal ganglia, thereby causing various clinical phenotypes including chorea, dystonia, parkinsonism, cerebellar signs, cognitive, and behavioral symptoms.5, 6, 7, 8
Currently, no treatment is available for neuroferritinopathy but following the example in PKAN, 9 iron chelation could theoretically limit iron‐related neurodegenerative progression.6, 7 Importantly, for a chelator to be of clinical value in disorders without systemic iron accumulation, it ought to be endowed with a requisite accessibility to the relevant sites. It allows this treatment to spare unaffected areas of the organism from scavenging iron, which is an essential element. 10 Deferiprone is exceptional among iron chelators in its ability to cross membranes, including the blood–brain barrier. Deferiprone has lower affinity for iron than transferrin and therefore, in conditions where transferrin is not fully saturated, it can transfer the excess labile iron to transferrin for normal use and avoid anemia. This is the concept of conservative iron chelation.10, 11
However, the ferritin depletion that characterizes neuroferritinopathy represents a classic contraindication to iron chelation because it suggests a depletion of iron storage. In neuroferritinopathy, ferritin depletion does not represent a depletion of iron but, on the contrary, a lack of secure storage of labile iron, which can be deposited in all tissues in excess and particularly the central nervous system that promotes iron‐related cell death.
Given that neuroferritinopathy is a very rare disease without therapeutic alternatives, we proposed a compassionate, open‐label use of conservative iron chelation by deferiprone 30 mg/kg/day to patients with neuroferritinopathy at different stages of the disease and different phenotypes to assess safety, feasibility, and the first parameters of efficacy. Clinical scores were performed by the same trained neurologist (D.D.) who had followed all four patients for many years in their usual care. Biological and radiological data were collected retrospectively from patient's medical records.
Cases
Clinical features and evolution with treatment are provided in Table 1.
TABLE 1.
Clinical and treatment features
| Case | Age at symptoms onset, y | Clinical features at disease onset | Clinical features at treatment onset | Duration of symptoms before treatment onset | Duration since treatment initiation | Treatment benefit | Treatment discontinuation |
|---|---|---|---|---|---|---|---|
| 1 | 26 | Foot dystonia | Dysphagia, dysarthria, oro‐facial dystonia, chorea of upper limb, mild cerebellar signs | 33 y | 62 m | Benefit: +, slight improvement of the last sign that appeared: apathy | Duration of 8 m with rapid degradation |
| 2 | 46 | Cerebellar syndrome | Cerebellar and extra‐pyramidal syndrome | 11 y | 141 m | Benefit: ++, mild improvement (UPDRS scores from 26 to 19) | 30 m with worsening of gait, balance, extrapyramidal syndrome, depression, apparition of dystonia |
| 3 | 28 | Spastic paraparesis | Spastic paraparesis | 3 y | 122 m | Benefit: +++, from 1 km to unlimited walking range | 41 m with worsening of spastic paraplegia |
| 4 | 48 | Extra‐pyramidal and cerebellar syndrome | Extra‐pyramidal and cerebellar syndrome | 4 y | 41 m | Benefit: ++++, improvement of cerebellar signs (SARA from 10 to 4) | No discontinuation |
y = years; m = month.
Case 1 (index)
A 44‐year‐old man with history of slowly progressing right foot dystonia since he was 26, presented with dysphagia, dysarthria, oro‐facial dystonia, distal chorea of the right upper limb, and mild dysdiadochokinesia. He also reported unusual anxiety and irritability. He then developed progressive akineto‐rigid syndrome (Unified Parkinson's disease rating scale [UPDRS]‐motor score: 11). A pathogenic variant c.458dup,p.(His153GInfs*28) in a heterozygous state in exon 4 of the FTL gene (NM_000146.3) was revealed. Akineto‐rigid syndrome worsened (UPDRS‐motor: 48), but with mild dopasensitivity of 25% (levodopa‐equivalent‐daily‐dose [LEDD]: 1165 mg/day). The patient further developed progressive cognitive subcorticofrontal impairment (Mattis: 128/144) with apathy (Lille Apathy Rating Scale: −8/36) and dysexecutive syndrome (Frontal Assessment Battery: 15/18). He was placed on deferiprone, 33 years after the onset of symptoms, with slight improvement of 23% on the UPDRS‐motor score (range, 48–37) and apathy reported by the family. Three months after treatment initiation, complete blood count revealed drug‐induced neutropenia and chelation therapy had to be discontinued. A trial with deferoxamine 30 mg/kg/day was carried out for 1 year but showed no real benefit with a rapid progression of handicap (UPDRS‐motor: 53). He died from aspiration pneumonia 5 years after initiating chelation therapy.
Case 2
Case 2 was the index‐case's sister. Past medical history was notable for bipolar disorder and depression since she was 40 years old, which were probable psychiatric manifestations of the disease. She presented at the age of 46 with cerebellar ataxia (scale for assessment and rating of ataxia [SARA]: 8.5). Ten years later, akineto‐rigid symptoms appeared (UPDRS‐motor: 26). Deferiprone 30 mg/kg/day was then proposed when diagnosis of neuroferritinopathy was confirmed, 11 years after the onset of cerebellar symptoms. Relative clinical stability was initially reported for 1 year (SARA: 8.5; UPDRS‐motor: 19), but gait disturbance slowly worsened with progressive upper motor neuron signs. Chelation therapy was discontinued for 2 years because hemoglobin was at the lower limit of normal and the patient complained of worsening fatigue. The patient then reported rapid worsening of parkinsonian signs, freezing of gait, falls and needing to use a cane to walk, and recurrence of depression. Clinical worsening was associated with increased R2* values on MRI in several regions of interest15 (Fig. 1). Despite reintroduction of deferiprone, the patient reported a slowing of handicap, which nevertheless progressively led to severe dysarthria with swallowing disorders requiring enteral tube feeding. The walking distance was limited to ~20 m indoors with human aid, and she lost the ability to walk outside 21 years after the disease onset.
FIG 1.
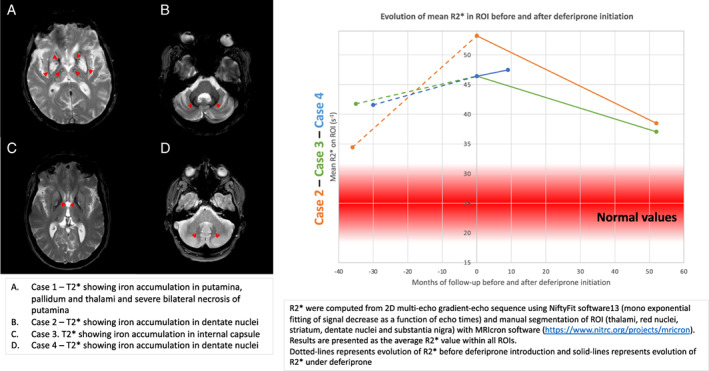
MRI pattern and evolution of mean R2* on thalami, red nuclei, striatum, dentate nuclei, and substantia nigra in cases 2, 3, and 4. (A) Case 1, T2* showing iron accumulation in putamina, pallidum, and thalami and severe bilateral necrosis of putamina. (B) Case 2, T2* showing iron accumulation in dentate nuclei. (C) Case 3, T2* showing iron accumulation in internal capsule. (D) Case 4, T2* showing iron accumulation in dentate nuclei. Estimation of brain iron overload in ROI using R2*. R2* were computed from 2D multi‐echo gradient‐echo sequence using NiftyFit software 12 (mono exponential fitting of signal decrease as a function of echo times) and manual segmentation of ROI (thalami, red nuclei, striatum, dentate nuclei, and substantia nigra) with MRIcron software (https://www.nitrc.org/projects/mricron). Results are presented as the average R2* value within all ROIs. Dotted lines represents evolution of R2* before deferiprone introduction and solid‐lines represents evolution of R2* under deferiprone. [Color figure can be viewed at wileyonlinelibrary.com]
Because of the rapid clinical worsening during the 2 years of deferiprone discontinuation, she chose to remain on deferiprone. R2* values decreased on MRI follow‐up (Fig. 1), and hemoglobin and ferritin counts remained at the lower limits of normal without anemia.
Case 3
Case 3 is the index‐case's niece and daughter of case 2. She presented at 28 years of age with fatigue and spastic paraparesis (maximal walking distance: 1 km) without affecting upper limbs and without cerebellar ataxia (SARA: 0). Deferiprone 30 mg/kg/day was initiated at the time of diagnosis, 3 years after symptom onset. Chelation therapy had to be discontinued before and during pregnancy, with worsening of gait and R2* values in the basal ganglia (Fig. 1). After giving birth and the reinitiating deferiprone, the feeling of aggravation disappeared and R2* values decreased (Fig. 1). Currently, the symptoms remain totally controlled, 11 years after treatment initiation with a SARA score of 0 and unlimited ambulation without assistance. She did not experience any side effects, new symptoms, or anemia.
Case 4
Case 4 is the index‐case's niece and cousin of case 3. She presented with a history of micrography and balance disturbance with onset at 48 years of age. Bilateral akineto‐rigid syndrome and cerebellar gait ataxia were identified (SARA: 10). Neuroferritinopathy was confirmed, and she was given deferiprone 4 years after symptom onset. Six months after initiating chelation therapy, she experienced subjective and objective improvement (SARA: 6). Sustained improvement was observed more than 3 years after treatment onset (SARA 4 at last visit). Magnetic resonance imaging (MRI) follow‐up showed relative stability of R2* values in the basal ganglia (Fig. 1). She did not experience any side effects, new symptoms, or anemia.
Discussion
Here, we observed that conservative iron chelation is feasible and safe in patients with neuroferritinopathy. Indeed, despite the low level of ferritin, deferiprone 30 mg/kg/day did not induce anemia even after more than 10 years of treatment. Deferiprone did not cause acute worsening of neurological symptoms on introduction or new neurological deficits under long‐term treatment. The main and well‐known complication of deferiprone is the risk of neutropenia, observed in 5.5% of treated patients 13 and here, in case 1, which needs to be monitored by weekly blood count for 6 months and then monthly for the duration of the treatment. This risk is mainly observed within the first months of treatment and should require discontinuation in case of agranulocytosis.
The most striking observation was that early treatment initiation resulted in better clinical response. In case 1, conservative iron chelation was introduced late in the disease course and the patient only experienced a slight benefit. We hypothesize that the lack of efficacy of deferoxamine in case 1 could be attributed to the fact that it is a large molecule that does not have the characteristics of deferiprone to easily cross membranes. 14 Case 2, who started treatment earlier, but still after 11 years of disease onset and suffering already from a high level of disability, experienced quantifiable benefit with slower worsening by comparing the period with and without deferiprone, but without recovery of the neurological state. Strikingly, in case 3, deferiprone, which was introduced 3 years after symptoms onset, when only slight symptoms of paraparesis were present, seemed to prevent disease progression for more than 11 years of follow‐up. Moreover, a significantly improvement in gait was reported, with no limit in terms of walking distance compared to the 1 km limit before treatment. Treatment was also started in case 4 at an early stage, 4 years after symptoms onset, when only slight symptoms of cerebellar ataxia were present, and resulted in the reversal of symptoms.
As expected, MRI data highlight increasing R2* values in each case when patients were not treated, indicating worsening of iron overload especially in the basal ganglia. We show a mild decrease in R2* values in cases 2 and 3 and an inflexion of the curve of iron accumulation in case 4 under deferiprone, suggesting that chelation therapy was effective in stalling iron overload. MRI measures the stored iron under the form of ferritin/hemosiderin and not the labile iron, which is the main chelated iron, and thereby could explain the small changes in MRI R2* in patients.
These results are consistent with a recent randomized control trial in PKAN. 9 Even though the trial failed to reach significance in improving dystonia at 18 months for all patients, subgroup analysis on classic and atypical PKAN suggests that the stage of the disease is a determining factor in response to treatment. Indeed, atypical PKAN is characterized by later onset, less severe impairment, slower disease evolution than in classic PKAN 15 and a better response to chelation therapy. 9 Patients with early progression are more prone to benefit from the treatment than those with advanced iron‐induced neurodegeneration. 9 We report data suggesting that early deferiprone initiation can induce a real improvement or stabilization of symptoms, whereas late treatment onset only affords mild slowing in progression of the disease or no benefit.
In conclusion, these first results of feasibility, safety, and clinical benefits are promising and support further validation on larger cohort to confirm efficiency of conservative iron chelation in neuroferritinopathy, either with a placebo group or n‐of‐1 design.
Author Roles
(A) Study concept and design; B: acquisition of data; (C) analysis and interpretation; (D) critical revision of the manuscript for important intellectual content; (E) study supervision.
F.M.: A, B, C
C.M.: A, C, D
G.K.: B, C, D
V.H.: B, C, D
L.D.: D
D.D.: A, B, C, D, E
The indicated authors take responsibility for data collection and analysis and the principal investigator (D.D), who had full access to all the study data, takes full responsibility for submitting the final work for publication.
Financial Disclosure
F.M., G.K., and V.H. have nothing to declare.
C.M. has received grants from the France Parkinson and Vaincre Parkinson charities, French Agence Nationale de la Recherche (ANR). She has received various honoraria from pharmaceutical companies for consultancy and lectures on Parkinson's disease at symposia, including Orkyn, Elivie, AbbVie, and Boston Scientific.
L.D. served on the Scientific Advisory Board for AbbVie and has received honoraria from pharmaceutical companies for consultancy and lectures including AbbVie, Novartis, Merz, Orkyn, and UCB.
D.D. has led four investigator‐driven studies funding by academic grants from France from the French Ministry of Health and research and European Commission Horizon 2020 PHC13 2014–2015 (No 633,190), involving deferiprone provided for free of charge by ApoPharma and Chiesi Canada Corp. (FAIRPARK‐I, FAIRPARK‐II, and SAFE‐FAIR ALS‐I, FAIR‐ALS‐II). He has served on advisory boards, served as a consultant and given lectures for pharmaceutical companies such as Orkyn, AbbVie, Medtronic, and Boston Scientific.
Acknowledgment
We thank the patients who participated, Fernando Tricta and Caroline Fradette (Chiesi Canada Corp.) for critical review and advice, and Sean Freeman for English editing.
Relevant conflicts of interest/financial disclosures: The authors have no financial disclosures to make or potential conflicts of interest to report in relation to this academic case report.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1. Schipper HM. Neurodegeneration with brain iron accumulation ‐ clinical syndromes and neuroimaging. Biochim Biophys Acta 2012;1822(3):350–360. [DOI] [PubMed] [Google Scholar]
- 2. Karin I, Büchner B, Gauzy F, Klucken A, Klopstock T. Treat iron‐related childhood‐onset neurodegeneration (TIRCON)‐an international network on care and research for patients with neurodegeneration with brain iron accumulation (NBIA). Front Neurol 2021;12:642228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hayflick SJ, Kurian MA, Hogarth P. Neurodegeneration with brain iron accumulation. Handb Clin Neurol 2018;147:293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Keogh MJ, Morris CM, Chinnery PF. Neuroferritinopathy. Int Rev Neurobiol 2013;110:91–123. [DOI] [PubMed] [Google Scholar]
- 5. Burn J, Chinnery PF. Neuroferritinopathy. Semin Pediatr Neurol 2006;13(3):176–181. [DOI] [PubMed] [Google Scholar]
- 6. Chinnery PF, Crompton DE, Birchall D, Jackson MJ, Coulthard A, Lombès A, et al. Clinical features and natural history of neuroferritinopathy caused by the FTL1 460InsA mutation. Brain 2007;130(Pt 1):110–119. [DOI] [PubMed] [Google Scholar]
- 7. Devos D, Tchofo PJ, Vuillaume I, Destée A, Batey S, Burn J, et al. Clinical features and natural history of neuroferritinopathy caused by the 458dupA FTL mutation. Brain 2009;132(Pt 6):e109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Batey S, Vuillaume I, Devos D, Destée A, Curtis AJ, Lombes A, et al. A novel FTL insertion causing neuroferritinopathy. J Med Genet 2010;47(1):71–72. [DOI] [PubMed] [Google Scholar]
- 9. Klopstock T, Tricta F, Neumayr L, Karin I, Zorzi G, Fradette C, et al. Safety and efficacy of deferiprone for pantothenate kinase‐associated neurodegeneration: a randomised, double‐blind, controlled trial and an open‐label extension study. The Lancet Neurology 2019;18(7):631–642. [DOI] [PubMed] [Google Scholar]
- 10. Cabantchik ZI, Munnich A, Youdim MB, Devos D. Regional siderosis: a new challenge for iron chelation therapy. Front Pharmacol 2013;4:167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Devos D, Moreau C, Devedjian JC, Kluza J, Petrault M, Laloux C, et al. Targeting chelatable iron as a therapeutic modality in Parkinson's disease. Antioxid Redox Signal 2014;21(2):195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Melbourne A, Toussaint N, Owen D, Simpson I, Anthopoulos T, De Vita E, et al. NiftyFit: a software package for multi‐parametric model‐fitting of 4D magnetic resonance imaging data. Neuroinform 2016;14(3):319–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tricta F, Uetrecht J, Galanello R, Connelly J, Rozova A, Spino M, et al. Deferiprone‐induced agranulocytosis: 20 years of clinical observations. Am J Hematol 2016;91(10):1026–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fredenburg AM, Sethi RK, Allen DD, Yokel RA. The pharmacokinetics and blood‐brain barrier permeation of the chelators 1,2 dimethly‐, 1,2 diethyl‐, and 1‐[Ethan‐1'ol]‐2‐methyl‐3‐hydroxypyridin‐4‐one in the rat. Toxicology 1996;108(3):191–199. [DOI] [PubMed] [Google Scholar]
- 15. Tomić A, Petrović I, Svetel M, Dobričić V, Dragašević Mišković N, Kostić VS. Pattern of disease progression in atypical form of pantothenate‐kinase‐associated neurodegeneration (PKAN) ‐ prospective study. Parkinsonism Relat Disord 2015;21(5):521–524. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


