Abstract

We report that surrounding coordination of neutral six-membered arene rings affords molecularly well-defined organotransition metal nanoclusters. With the use of [2.2]paracyclophane as the face-capping arene ligand, we have isolated two polyarene palladium nanoclusters, one consisting of a hexakis-arene ligand shell and a hexagonal close-packed Pd13 anticuboctahedron trichloride core, and the other consisting of an octakis-arene ligand shell and a non-close-packed Pd17 square gyrobicupola dichloride core, both with Pd–Pd direct bonding. The μ4-facial coordination mode of arene was discovered through the structural characterization of the Pd13 cluster. Their Pd13 and Pd17 cores, which are distinct from the previously identified face-centered-cubic Pd13 core surrounded by seven-membered cycloheptatrienyl, are explained by stereochemical and theoretical analyses.
The size and packing-selective construction of molecular metal nanoclusters has been a longstanding challenge in inorganic chemistry, organometallic chemistry, catalysis, and materials science.1−4 For example, the 13-atom clusters have been thought to provide good models for studying metal nanoclusters because they possibly adopt three compact and symmetrical structures composed of 12 surface atoms and one interstitial atom, where each corresponds to face-centered-cubic (fcc) close-packed cuboctahedron, hexagonal close-packed (hcp) anticuboctahedron, and non-close-packed icosahedron.5−8 However, it is usually difficult to deduce the 13-atom metal core structure from its ligand shell due to the presence of many ligands for covering the surface sites.9−11 Recently, our group found that the use of C7H7 (Tr), a seven-membered cyclic unsaturated hydrocarbon (CUH), as the face-capping ligands provides an fcc-close-packed cuboctahedral 13-atom cluster [Pd13Tr6]2+ selectively.12 In the Pd13 cluster, only six Tr ligands surround the 13-atom metal core, giving a simple octahedral ligand geometry lying within the stereochemical regime developed for 6-coordinate mononuclear complexes. In order to verify the usefulness of this face-capping strategy using CUH ligands, it is necessary to understand whether and how the coordination of face-capping ligands affect the size and packing pattern of the metal core.13,14 Here we isolated and characterized transition metal nanoclusters surrounded by neutral six-membered arene rings (Figure 1). The distinct molecular structures of the polyarene nanoclusters, a 13-atom hcp-anticuboctahedral Pd cluster surrounded by six arene and three chloride ligands and a 17-atom non-close-packed square gyrobicupola Pd cluster surrounded by eight arene and two chloride ligands, were elucidated and characterized based on the experimental and theoretical analyses.
Figure 1.

Facial coordination of six-membered ring C6H4R2 to square faces gives hexagonal close-packed 13-atom anticuboctahedron, and that to triangular faces gives non-close-packed 17-atom square gyrobicupola, where each is further capped by chloride ligands (blue balls).
Although π-coordination of neutral six-membered arene rings has been widely used in organometallic chemistry,15,16 the arene π-coordination has not led to molecularly well-defined metal nanoclusters which contain more than 10 metal atoms. For example, the self-assembly of half-sandwich η6-arene mononuclear metal moieties has only provided small metal clusters containing up to four metal atoms.17 Although μ3-face-capping coordination of arenes has previously been identified in small Mm clusters (m ≤ 6),18−20 it is challenging to surround a metal nanocluster core by using multiple μ3-arene ligands to construct polyarene metal nanoclusters. Moreover, the fcc- and hcp-M13 cores have tetranuclear faces in addition to trinuclear faces, but it is not clear whether a six-membered arene ring has the ability to face-cap a tetranuclear face through μ4-coordination. The possible existence of polyarene metal nanoclusters containing up to 10 metal atoms was discussed previously on the basis of the mass spectrometric detection of Mm(benzene)n species (M = Fe, Co, Ni; m > n, m ≈ 10, n ≈ 6) in gas-phase reactions of metal vapor with benzene.21 However, such compounds have not been isolated and their atomic structures remain unexplained.
We selected [2.2]paracyclophane (PCP) as the arene ligand in our study because it shows a greater coordination ability than benzene due, in part, to transannular electronic interactions between the two parallel arene rings.22,23 Our group previously reported that the bis-μ3-arene trinuclear sandwich complex [Pd3(μ3-PCP)2(CH3CN)3][B(ArF)4]2 (1) [ArF = 3,5-(F3C)2C6H3] is isolable although the corresponding bis-benzene Pd3 complexes could not be obtained.24,25 Interestingly, 1H NMR monitoring of a solution of 1 in 1,2-dichloroethane-d4 (DCE-d4) at 70 °C showed a gradual consumption of 1, accompanied by the generation of two products exhibiting a large downfield shift for one of the two methylene proton resonances (δ = 20.00; δ = 9.74) and a large upfield shift for one of the two phenylene resonances (δ = −6.26; δ = −6.28), characteristic of paramagnetic compounds. Heating 1 in DCE for 1 day gave a 46% yield of a mixture of two products in a molar ratio of 56:44. Separation of the products by precipitation from DCE–Et2O gave a hexakis-PCP Pd13 cluster, [Pd13(μ4-PCP)6(μ-Cl)3][B(ArF)4]2 (2), in 15% yield, whereas an octakis-PCP Pd17 cluster, [Pd17(μ3-PCP)8(μ4-Cl)2][B(ArF)4]3 (3), was obtained in 8% yield by crystallization from the black supernatant (Figure 2a). It is reasonable to assume that B(ArF)4– behaves as a mild reducing reagent in the production of 2 and 3 in solution,26 as the formation of biaryl27 and CH3CN–B(ArF)328 were detected during the synthesis.29 The Cl ligands in 2 and 3 might be abstracted from the DCE solvent.
Figure 2.

(a) Synthesis of [Pd13(μ4-PCP)6(μ-Cl)3][B(ArF)4]2 (2) and [Pd17(μ3-PCP)8(μ4-Cl)2][B(ArF)4]3 (3). (b) Ball-and-stick representation of the X-ray structure of 2, a drawing of the anticuboctahedral Pd13 core of 2, and a drawing highlighting the μ4-coordination mode of the PCP ligands in 2. (c) Ball-and-stick representation of the X-ray structure of 3, a drawing of the square gyrobicupola Pd17 core of 3, and a drawing highlighting the μ3-coordination mode of the PCP ligands in 3. For (b) and (c), orange = Pd; green = Cl; dark gray = C; the counteranions and solvent molecules were omitted for clarity.
The structures of 2 and 3 were determined by single-crystal X-ray diffraction analysis. Nanocluster 2 contains an hcp-Pd13 core with an anticuboctahedral Pd12 surface arrangement (Johnson solid J27) and one interstitial Pd atom (Figure 2b). Each PCP ligand facially caps a Pd4 face of the Pd13 core through μ4-bridging coordination, which is an unprecedented coordination mode for six-membered arene rings. The observation of this coordination mode in 2 may result from the unique properties of the nanosized metal cluster core that are lacking in smaller Mn complexes (e.g., n = 4). Coverage of the six square Pd4 faces of the anticuboctahedral Pd13 core results in a triangular prismatic arrangement of the PCP ligands, providing additional spaces at the equatorial edges to accommodate three μ-Cl ligands. As a result, six PCP and three Cl ligands form a tricapped triangular prismatic ligand geometry with a symmetric (pseudo-D3h) structure. The Pd–Pd bond lengths in the Pd13 core of 2 [2.5392(9)–2.7837(6) Å] are in the normal range for Pd–Pd bond lengths (cf. 2.75 Å in bulk Pd)30 except for the elongated chloro-bridged Pd–Pd bonds [3.0167(6), 3.0146(8), and 3.0146(8) Å]. Although the anticuboctahedral M13 core is one of the basic close-packed geometries for metal nanoclusters, it has been rarely obtained except for the rhodium hydride carbonyl clusters [Rh13H5-n(CO)24]n−.9 Notably, formation of the hcp-anticuboctahedral Pd13 core with the use of arenes as the face-capping ligands is in sharp contrast to that of fcc-cuboctahedral Pd13 core in [Pd13Tr6]2+ where C7H7 ligands are employed as the face-capping ligands.12
The Pd17 core in 3 has a non-close-packed, square gyrobicupola geometry (Johnson solid J29) with one interstitial Pd atom (Figure 2c). The eight PCP ligands surround the Pd17 core in a square antiprismatic geometry, and each PCP ligand facially caps a triangular Pd3 face of the square gyrobicupola through μ3-bridging coordination, despite the presence of eight square Pd4 faces at the surface. The eight PCP and two Cl ligands adopt a bicapped square antiprismatic geometry with a pseudo-D4d symmetry. The Pd–Pd bond lengths [2.5649(4)–2.8937(5) Å] are in the normal range for Pd–Pd bonds,30 and the interstitial Pd atom is bonded to the eight Pd atoms at the square faces capped by Cl ligands but not to the Pd atoms forming the eight-membered equatorial ring (Pd···Pd ≥ 3.59 Å). The 17-atom square gyrobicupola nanocluster is unprecedented. It is noticeable that the icosahedral core, a more common non-close-packed M13 structure for group 11 metals,3,4 was not observed in the present case.
The different Pd packing in the 13-atom clusters 2 and [Pd13Tr6]2+ can be understood qualitatively by considering coordination numbers, valence electron numbers, and geometrical adaptation between the metal core and the ligand shell, where each is dependent on the ring-size of the facial CUH ligands. The difference of the coordination numbers between 2 and [Pd13Tr6]2+ results from the presence or absence of Cl ligands. According to the empirical or semiempirical approach,1 the M13 cluster in either cuboctahedron or anticuboctahedron would prefer 170 valence electrons. Although [Pd13Tr6]2+ is exactly the 170e compound and thus coordinatively saturated, [Pd13(μ4-arene)6]2+ which lacks Cl ligands might be highly electron-deficient (164e) because the number of electrons donated by a ligand is reduced by one when changing the ligands from seven-membered C7H7 to six-membered arene rings. Therefore, the acceptance of three μ-Cl ligands might well mitigate the electron-deficient state of the cluster.
From a stereochemical viewpoint, an hcp-anticuboctahedron has three uncoordinated edges after μ4-facial coordination of six CUH ligands, providing suitable sites for accommodation of μ-Cl ligands (Figure 3). On the other hand, it is unlikely that the fcc-cuboctahedron metal core possessing six face-capping CUH ligands accommodates additional Cl ligands, because all M–M edges are involved in the facial coordination of CUH ligands. It is noted that the observed metal core geometry correlates with the ideal ligand geometry expected from the ligand number. For 2, the square face augmentation31 of the anticuboctahedral core leads to the trigonal prismatic geometry of six face-capping ligands, and additional edge augmentation at the three uncoordinated equatorial edges gives a tricapped trigonal prism geometry, which is an ideal ligand geometry for 9-coordinate complexes (Figure S20).32 The case of [Pd13Tr6]2+ is simpler; square face augmentation of cuboctahedron produces octahedron, an ideal 6-coordinate ligand geometry.
Figure 3.

Summary of the four metal core structures: cuboctahedron, anticuboctahedron, icosahedron, and square gyrobicupola. The square faces or the triangular faces used for face-capping coordination of CUH ligands are shown in sky blue or orange. The edges which are not involved in the facial coordination of CUHs are shown in red.
Such consideration also explains the fact that the non-close-packed square gyrobicupola Pd17 cluster 3 is favorably formed, while the non-close-packed icosahedron Pd13 cluster is not. The number of valence electrons of 3 (225e, where μ4-Cl is considered as a five-electron-donor ligand) is close to that for an M17 cluster (226e) which is predicted by an empirical approach (Figure 3), where the accommodation of two μ4-Cl ligands mitigates the electron-deficiency of [Pd17(μ3-arene)8]3+ (215e). The μ4-coordination of arenes is not compatible with the μ4-Cl capping because the top and bottom Pd4 squares are used for the arene binding. A bicapped square antiprism geometry is known as an ideal ligand geometry for 10-coordinate metal complexes,32 to which the observed square gyrobicupola Pd17 core is geometrically adaptable through the triangular face augmentation with eight arenes and additional square-face augmentation with two Cl ligands (Figure S20). The non-close-packed icosahedral metal core would be unlikely to be formed when using face-capping CUH ligands, because the ligand geometry generated by face augmentation from icosahedron seems not to match any ideal coordination geometry of ligands.
In order to gain further insights into the present polyarene metal clusters, we carried out density functional theory (DFT) calculations on [Pd13(μ4-PCP)6(μ-Cl)3]2+ (22+), [Pd13(μ4-PCP)6(μ-Cl)3]+ (2+), [Pd17(μ3-PCP)8(μ4-Cl)2]3+ (33+), and [Pd17(μ3-PCP)8(μ4-Cl)2]4+ (34+). As shown in Figure 4a, the HOMO of 2+ mainly involves 4dσ–4dσ antibonding interactions in the top and bottom Pd3 moieties (these Pd3 moieties are defined in Figure 2b). Three MOs mainly composed of Pd 4d orbitals are found in unoccupied level (LUMO to LUMO+2), whereas a [Pd13]4+ core would have two MOs mainly composed of Pd 4d orbitals in the unoccupied level. This feature is not unreasonable at all because the 5s orbital at the interstitial Pd atom becomes occupied through Pd 4d–5s hybridization (Figure S19) and instead one MO mainly composed of Pd 4d becomes unoccupied (the LUMO). This LUMO involves the 4dπ–3pπ antibonding interaction between the equatorial Pd atoms and μ-Cl ligands, that is, the origin of the relatively short Pd–Pd bonds (2.5392(9) Å, 2.5477(6) Å) at the unbridged (no μ-Cl) equatorial positions. The degenerated LUMO+1 and LUMO+2 are featured by the antibonding interaction between the arene π-orbitals and surface Pd 4d orbitals (Figure 4b, left-hand side). These MOs are indeed reminiscent of the e″ orbitals of the 6-coordinate trigonal prismatic mononuclear complexes,33 and their vacancy suggests that donation occurs from the CUH ligands to the surface Pd atoms. In 34+, the LUMO and LUMO+1 are mainly featured by the σ-type antibonding interactions between the top and bottom Pd4 squares and equatorial Pd8 ring and also by the antibonding interaction between the arene π-orbitals and the surface Pd d-orbitals (Figure 4b, right-hand side). The HOMO involves the arene–Pd antibonding interaction, whereas the 4dσ–4dσ antibonding character is rather small. The 4d–5s hybridization occurs at the interstitial Pd atom as found in 2+. The Hirshfeld charges of the metal cores in 22+ and 33+ are similar; +1.507e and +1.579 e, respectively, despite the different numbers of Pd atoms, PCP ligands, and Cl ligands in addition to the different total positive charge (Table S6). DFT calculations also indicated that the accommodation of three Cl ligands in 22+ or two Cl ligands in 33+ from DCE is thermodynamically favorable (Table S7).
Figure 4.

(a) Frontier molecular orbitals of [Pd13(μ4-PCP)6(μ-Cl)3]+ (2+). (b) Antibonding interaction between the arene π-orbitals and Pd3 or Pd4 d-orbitals.
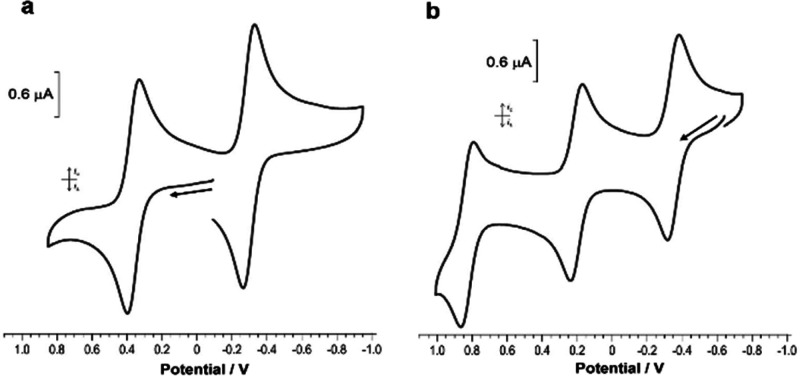
Cyclic voltammetry (CV) analyses of 2 and 3 indicated that there are several readily addressable oxidation states in which the cluster frameworks are retained: + 0.36 V (ΔEp = 68 mV) and −0.30 V (ΔEp = 66 mV) for 2; +0.83 V (ΔEp = 71 mV), +0.20 V (ΔEp = 68 mV), and −0.35 V (ΔEp = 65 mV) for 3 (Figure 5). A one-electron reduction product [Pd13(μ4-PCP)6(μ-Cl)3][B(ArF)4] (2′) from 2 and a one-electron oxidation product [Pd17(μ3-PCP)8(μ4-Cl)2][B(ArF)4]4 (3′) from 3 were generated in CD2Cl2 by addition of Et3N or AgPF6, respectively; each product showed 1H NMR resonances in the normal chemical-shift region for diamagnetic compounds. A single set of four resonances for the PCP ligands was observed in the 1H and 13C NMR spectra of 2′ and 3′ at 20 °C in CD2Cl2, and no apparent changes in their resonance patterns were observed, even at −90 °C (although a broadening of the resonance for the coordinated arene protons was observed), indicating that each PCP ligand rotates fluxionally on the NMR time scale in solution. The molecular structure of 2′ was determined by X-ray diffraction analysis, and its structure was found to be quite similar to that of 2 (Figure S15).
Figure 5.
(a) Cyclic voltammogram of 2. (b) Cyclic voltammogram of 3. For (a) and (b), a solution of the cluster (0.5 mM) in CH2Cl2 with [n-Bu4N][B(ArF)4] (0.1 M) was analyzed at a scan rate of 100 mV/s. The reference potential is that of the Fe(C5H5)2/[Fe(C5H5)2]+ redox couple.
In conclusion, we synthesized and structurally characterized the polyarene metal nanoclusters. It has been proven that the face-capping coordination of neutral six-membered arene rings well stabilizes symmetrical metal nanocluster cores. The μ4-coordination of arene was also discovered in metal nanoclusters. The analysis of the present organotransition metal nanoclusters suggested that the ring-size of facially coordinated CUH ligands has a pronounced impact on the structures of metal nanoclusters through steric and electronic perturbations.
Acknowledgments
We thank Yuta Takahira for his contribution to the characterization of the 13-atom clusters. This work was supported by JST-CREST (Grant JPMJCR20B6 to T.M. and S.S.), JSPS Grant-in-Aid for Scientific Research (Grants JP20H04805 and JP22H02093 to T.M., and JP19J23557 to T.S.), and Mitsubishi Foundation (to T.M.).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.3c02849.
Full experimental details, including synthetic procedures and characterization details, cyclic voltammetry, crystallographic and computational details (PDF)
Author Contributions
§ A.H. and T.S. contributed equally. The manuscript was written through contributions of all authors.
The authors declare no competing financial interest.
Supplementary Material
References
- The Chemistry of Metal Cluster Complexes; Shriver D. F., Kaesz H. D., Adams R. D., Eds.; VCH, 1990. [Google Scholar]
- Dyson P. J.; McIndoe J. S.. Transition Metal Carbonyl Cluster Chemistry; Gordon and Breach Science Publishers, 2000. [Google Scholar]
- Jin R.; Zeng C.; Zhou M.; Chen Y. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016, 116, 10346–10413. 10.1021/acs.chemrev.5b00703. [DOI] [PubMed] [Google Scholar]
- Chakraborty I.; Pradeep T. Atomically Precise Clusters of Noble Metals: Emerging Link between Atoms and Nanoparticles. Chem. Rev. 2017, 117, 8208–8271. 10.1021/acs.chemrev.6b00769. [DOI] [PubMed] [Google Scholar]
- Köster A. M.; Calaminici P.; Orgaz E.; Roy D. R.; Reveles J. U.; Khanna S. N. On the Ground State of Pd13. J. Am. Chem. Soc. 2011, 133, 12192–12196. 10.1021/ja203889r. [DOI] [PubMed] [Google Scholar]
- Pelzer A. W.; Jellinek J.; Jackson K. A. H2 Reactions on Palladium Clusters. J. Phys. Chem. A 2013, 117, 10407–10415. 10.1021/jp403089x. [DOI] [PubMed] [Google Scholar]
- Granja-DelRío A.; Abdulhussein H. A.; Johnston R. L. DFT-Based Global Optimization of Sub-nanometer Ni−Pd Clusters. J. Phys. Chem. C 2019, 123, 26583–26596. 10.1021/acs.jpcc.9b05970. [DOI] [Google Scholar]
- Alonso J. A.; López M. J. Palladium Clusters, Free and Supported on Surfaces, and Their Applications in Hydrogen Storage. Phys. Chem. Chem. Phys. 2022, 24, 2729–2751. 10.1039/D1CP03524J. [DOI] [PubMed] [Google Scholar]
- A rare example of the hcp-anticuboctahedral 13-atom cluster [Rh13H5–n(CO)24]n− was reported with employing CO and hydride ligands, but it is difficult to deduce the anticuboctahedral metal core structure from its complicated ligand shell containing more than 20 CO ligands.; a Albano V. G.; Ceriotti A.; Chini P.; Ciani G.; Martinengo S.; Anker W. M. Hexagonal Close Packing of Metal Atoms in the New Polynuclear Anions [Rh13(CO)24H5-n]n− (n = 2 or 3); X-ray Structure of [(Ph3P)2N]2[Rh13(CO)24H3]. J. Chem. Soc., Chem. Commun. 1975, 859–860. 10.1039/C39750000859. [DOI] [Google Scholar]; b Bau R.; Drabnis M. H.; Garlaschelli L.; Klooster W. T.; Xie Z.; Koetzle T. F.; Martinengo S. Five-Coordinate Hydrogen: Neutron Diffraction Analysis of the Hydrido Cluster Complex [H2Rh13(CO)24]3–. Science 1997, 275, 1099–1102. 10.1126/science.275.5303.1099. [DOI] [PubMed] [Google Scholar]
- Kharas K. C. C.; Dahl L. F. Ligand-Stabilized Metal Clusters: Structure, Bonding, Fluxionality, and the Metallic State. Adv. Chem. Phys. 1988, 70, 1–43. 10.1002/9780470122693.ch1. [DOI] [Google Scholar]
- Johnson B. F. G.; Roberts Y. V. The Ligand Polyhedral Model and Its Application to the Binary Carbonyls. Polyhedron 1993, 12, 977–990. 10.1016/S0277-5387(00)87174-0. [DOI] [Google Scholar]
- Teramoto M.; Iwata K.; Yamaura H.; Kurashima K.; Miyazawa K.; Kurashige Y.; Yamamoto K.; Murahashi T. Three-Dimensional Sandwich Nanocubes Composed of 13-Atom Palladium Core and Hexakis-Carbocycle Shell. J. Am. Chem. Soc. 2018, 140, 12682–12686. 10.1021/jacs.8b07430. [DOI] [PubMed] [Google Scholar]
- For the synthesis of Pd3 sheet sandwich cluster using the C7H7 ligands:; Murahashi T.; Fujimoto M.; Oka M.; Hashimoto Y.; Uemura T.; Tatsumi Y.; Nakao Y.; Ikeda A.; Sakaki S.; Kurosawa H. Discrete Sandwich Compounds of Monolayer Palladium Sheets. Science 2006, 313, 1104–1107. 10.1126/science.1125245. [DOI] [PubMed] [Google Scholar]
- For the synthesis of a rare 13-atom cuboctahedral Cu13 cluster having face-capping μ4-dithiocarbamate and μ3-alkynyl ligands:; Chakrahari K. K.; Liao J.-H.; Kahlal S.; Liu Y.-C.; Chiang M.-H.; Saillard J.-Y.; Liu C. W. [Cu13{S2CNnBu2}6(acetylide)4]+: A Two-Electron Superatom. Angew. Chem., Int. Ed. 2016, 55, 14704–14708. 10.1002/anie.201608609. [DOI] [PubMed] [Google Scholar]
- Fischer E. O.; Hafner W. Di-benzol-chrom Über Aromatenkomplex von Metallen I. Z. Naturforsch. B 1955, 10, 665–668. 10.1515/znb-1955-1201. [DOI] [Google Scholar]
- Elschenbroich C.Organometallics, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- a Olson W. L.; Dahl L. F. Stereochemistry and Bonding of the Tris(benzene)tricobalt Dicarbonyl monocation, [Co3(η6-C6H6)3(μ3-CO)2]+: A comparative Analysis of Its 48-Electron Co3(CO)2 Core with the 46-Electorn Co3(CO)2 Core of Co3(η5-C5Me5)3(μ3-CO)2 and Resulting Electronic Implications. J. Am. Chem. Soc. 1986, 108, 7657–7663. 10.1021/ja00284a033. [DOI] [PubMed] [Google Scholar]; b Meister G.; Rheinwald G.; Stoeckli-Evans H.; Süss-Fink G. Hydrogen Activation by Arene Ruthenium Complexes in Aqueous Solution. Part 2. Build-up of Cationic Tri- and Tetra-Nuclear Ruthenium Clusters with Hydrido Ligands. J. Chem. Soc., Dalton Trans. 1994, 3215–3223. 10.1039/DT9940003215. [DOI] [Google Scholar]
- Gomez-Sal M. P.; Johnson B. F. G.; Lewis J.; Raithby P. R.; Wright A. H. Benzene in a New Face-capping Bonding Mode: Molecular Structures of [Ru6C(CO)11(μ3-η2:η2:η2-C6H6)(η6-C6H6)] and [Os3(CO)9(μ3-η2:η2:η2-C6H6)]. J. Chem. Soc., Chem. Commun. 1985, 1682–1684. 10.1039/C39850001682. [DOI] [Google Scholar]
- Dyson P. J.; Johnson B. F. G.; Lewis J.; Martinelli M.; Braga D.; Grepioni F. Stepwise Formation of the Bis(benzene)hexaruthenium Carbido Carbonyl Cluster Ru6C(CO)11(η6-C6H6)(μ3-η2:η2:η2-C6H6) from Ru6C(CO)17. J. Am. Chem. Soc. 1993, 115, 9062–9068. 10.1021/ja00073a023. [DOI] [Google Scholar]
- Wadepohl H. Benzene and Its Derivatives as Bridging Ligands in Transition-Metal Complexes. Angew. Chem., Int. Ed. 1992, 31, 247–262. 10.1002/anie.199202473. [DOI] [Google Scholar]
- Kurikawa T.; Takeda H.; Hirano M.; Judai K.; Arita T.; Nagao S.; Nakajima A.; Kaya K. Electronic Properties of Organometallic Metal−Benzene Complexes [Mn(benzene)m (M = Sc−Cu)]. Organometallics 1999, 18, 1430–1438. 10.1021/om9807349. [DOI] [Google Scholar]
- Dyson P. J.; Johnson B. F G.; Martin C. M. Ruthenium Cluster-[2.2]Paracylophane Complexes. Coord. Chem. Rev. 1998, 175, 59–89. 10.1016/S0010-8545(98)00172-6. [DOI] [Google Scholar]
- Papoyan G. A.; Butin K. P.; Hoffmann R.; Rozenberg V. I. Theoretical Investigation of [2.2]Paracyclophane as a Donor toward a Cr(CO)3 Group. Russ. Chem. Bull. 1998, 47, 153–159. 10.1007/BF02495523. [DOI] [Google Scholar]
- Murahashi T.; Fujimoto M.; Kawabata Y.; Inoue R.; Ogoshi S.; Kurosawa H. Discrete Triangular Tripalladium Sandwich Complexes of Arenes. Angew. Chem., Int. Ed. 2007, 46, 5440–5443. 10.1002/anie.200701665. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y.; Kimura S.; Takase K.; Yamamoto K.; Kurashige Y.; Yanai T.; Murahashi T. Modulation of Benzene or Naphthalene Binding to Palladium Cluster Sites by the Backside-Ligand Effect. Angew. Chem., Int. Ed. 2015, 54, 2482–2486. 10.1002/anie.201409499. [DOI] [PubMed] [Google Scholar]
- Murahashi T.; Takase K.; Oka M.; Ogoshi S. Oxidative Dinuclear Addition of a PdI-PdI Moiety to Arenes: Generation of μ-η3:η3-Arene-PdII2 Species. J. Am. Chem. Soc. 2011, 133, 14908–14911. 10.1021/ja206076u. [DOI] [PubMed] [Google Scholar]
- Budiman Y. P.; Jayaraman A.; Friedrich A.; Kerner F.; Radius U.; Marder T. B. Palladium-Catalyzed Homocoupling of Highly Fluorinated Arylboronates: Studies of the Influcence of Strongly vs Weakly Coordinating Solvents on the Reductive Elimination Process. J. Am. Chem. Soc. 2020, 142, 6036–6050. 10.1021/jacs.9b11871. [DOI] [PubMed] [Google Scholar]
- a Bourke S. C.; MacLachlan M. J.; Lough A. J.; Manners I. Ring-Opening Protonolysis of Sila[1]ferrocenophanes as a Route to Stabilized Silylium Ions. Chem.—Eur. J. 2005, 11, 1989–2000. 10.1002/chem.200400859. [DOI] [PubMed] [Google Scholar]; b Herrington T. J.; Thom A. J. W.; White A. J. P.; Ashley A. E. Novel H2 Activation by a Tris[3,5-bis(trifluoromethyl)phenyl]borane Frustrated Lewis Pair. Dalton Trans. 2012, 41, 9019–9022. 10.1039/c2dt30384a. [DOI] [PubMed] [Google Scholar]
- Presumably, the transfer of ArF groups to the Pd3 core in 1 from the B(ArF)4 anions and subsequent reductive elimination of biaryl could generate reactive Pd0 species that could induce the Pd agglomeration. We did not obtain polyarene nanoclusters when the PF6– salt [Pd3(μ3-PCP)2(PhCN)3][PF6]2 (1′) was used as the starting complex.
- Murahashi T.; Kurosawa H. Organopalladium Complexes Containing Palladium−Palladium Bonds. Coord. Chem. Rev. 2002, 231, 207–228. 10.1016/S0010-8545(02)00121-2. [DOI] [Google Scholar]
- Alvarez S. Polyhedra in (Inorganic) Chemistry. Dalton Trans. 2005, 2209–2233. 10.1039/b503582c. [DOI] [PubMed] [Google Scholar]
- Muetterties E. L.; Wright C. M. Molecular Polyhedra of High Co-ordination Number. Q. Rev. Chem. Soc. 1967, 21, 109–194. 10.1039/qr9672100109. [DOI] [Google Scholar]
- a Hoffmann R.; Howell J. M.; Rossi A. R. Bicapped Tetrahedral, Trigonal Prismatic, and Octahedral Alternatives in Main and Transition Group Six-Coordination. J. Am. Chem. Soc. 1976, 98, 2484–2492. 10.1021/ja00425a016. [DOI] [Google Scholar]; b Cremades E.; Echeverría J.; Alvarez S. The Trigonal Prism in Coordination Chemistry. Chem.—Eur. J. 2010, 16, 10380–10396. 10.1002/chem.200903032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.