Abstract
Background
Hepatic encephalopathy describes the spectrum of neuropsychiatric changes that may complicate the course of cirrhosis and detrimentally affect outcomes. Ammonia plays a key role in its development. Rifaximin is a non‐absorbable antibiotic that inhibits urease‐producing bacteria and reduces absorption of dietary and bacterial ammonia.
Objectives
To evaluate the beneficial and harmful effects of rifaximin versus placebo, no intervention, or non‐absorbable disaccharides for: (i) the prevention of hepatic encephalopathy, and (ii) the treatment of minimal and overt hepatic encephalopathy, in people with cirrhosis, both when used alone and when combined with a non‐absorbable disaccharide.
Search methods
We searched the Cochrane Hepato‐Biliary Group Clinical Trials Register, CENTRAL, MEDLINE, Embase, three other databases, the reference lists of identified papers, and relevant conference proceedings. We wrote to authors and pharmaceutical companies for information on other published, unpublished, or ongoing trials. Searches were performed to January 2023.
Selection criteria
We included randomised clinical trials assessing prevention or treatment of hepatic encephalopathy with rifaximin alone, or with a non‐absorbable disaccharide, versus placebo/no intervention, or a non‐absorbable disaccharide alone.
Data collection and analysis
Six authors independently searched for studies, extracted data, and validated findings. We assessed the design, bias risk, and participant/intervention characteristics of the included studies. We assessed mortality, serious adverse events, health‐related quality of life, hepatic encephalopathy, non‐serious adverse events, blood ammonia, Number Connection Test‐A, and length of hospital stay.
Main results
We included 41 trials involving 4545 people with, or at risk for, developing hepatic encephalopathy. We excluded 89 trials and identified 13 ongoing studies. Some trials involved participants with more than one type of hepatic encephalopathy or more than one treatment comparison. Hepatic encephalopathy was classed as acute (13 trials), chronic (7 trials), or minimal (8 trials), or else participants were considered at risk for its development (13 trials). The control groups received placebo (12 trials), no/standard treatment (1 trial), or a non‐absorbable disaccharide (14 trials). Eighteen trials assessed rifaximin plus a non‐absorbable disaccharide versus a non‐absorbable disaccharide alone. We classified 11 trials as at high risk of overall bias for mortality and 28 for non‐mortality outcomes, mainly due to lack of blinding, incomplete outcome data, and selective reporting.
Compared to placebo/no intervention, rifaximin likely has no overall effect on mortality (risk ratio (RR) 0.83, 95% confidence interval (CI) 0.50 to 1.38; P = 48, I2 = 0%; 13 trials, 1007 participants; moderate‐certainty evidence), and there may be no overall effect when compared to non‐absorbable disaccharides (RR 0.99, 95% CI 0.49 to 1.97; P = 0.97, I2 = 0%; 10 trials, 786 participants; low‐certainty evidence). However, there is likely a reduction in the overall risk of mortality when comparing rifaximin plus a non‐absorbable disaccharide to a non‐absorbable disaccharide alone (RR 0.69, 95% CI 0.55 to 0.86; number needed to treat for an additional beneficial outcome (NNTB) = 22; P = 0.001, I2 = 0%; 14 trials, 1946 participants; moderate‐certainty evidence).
There is likely no effect on the overall risk of serious adverse events when comparing rifaximin to placebo/no intervention (RR 1.05, 95% CI 0.83 to 1.32; P = 68, I2 = 0%; 9 trials, 801 participants; moderate‐certainty evidence) and there may be no overall effect when compared to non‐absorbable disaccharides (RR 0.97, 95% CI 0.66 to 1.40; P = 85, I2 = 0%; 8 trials, 681 participants; low‐certainty evidence). However, there was very low‐certainty evidence that use of rifaximin plus a non‐absorbable disaccharide may be associated with a lower risk of serious adverse events than use of a non‐absorbable disaccharide alone (RR 0.66, 95% CI 0.45 to 0.98; P = 0.04, I2 = 60%; 7 trials, 1076 participants).
Rifaximin likely results in an overall effect on health‐related quality of life when compared to placebo/no intervention (mean difference (MD) ‐1.43, 95% CI ‐2.87 to 0.02; P = 0.05, I2 = 81%; 4 trials, 214 participants; moderate‐certainty evidence), and may benefit health‐related quality of life in people with minimal hepatic encephalopathy (MD ‐2.07, 95% CI ‐2.79 to ‐1.35; P < 0.001, I2 = 0%; 3 trials, 176 participants). The overall effect on health‐related quality of life when comparing rifaximin to non‐absorbable disaccharides is very uncertain (MD ‐0.33, 95% CI ‐1.65 to 0.98; P = 0.62, I2 = 0%; 2 trials, 249 participants; very low‐certainty evidence). None of the combined rifaximin/non‐absorbable disaccharide trials reported on this outcome.
There is likely an overall beneficial effect on hepatic encephalopathy when comparing rifaximin to placebo/no intervention (RR 0.56, 95% CI 0.42 to 0.77; NNTB = 5; P < 0.001, I2 = 68%; 13 trials, 1009 participants; moderate‐certainty evidence). This effect may be more marked in people with minimal hepatic encephalopathy (RR 0.40, 95% CI 0.31 to 0.52; NNTB = 3; P < 0.001, I2 = 10%; 6 trials, 364 participants) and in prevention trials (RR 0.71, 95% CI 0.56 to 0.91; NNTB = 10; P = 0.007, I2 = 36%; 4 trials, 474 participants). There may be little overall effect on hepatic encephalopathy when comparing rifaximin to non‐absorbable disaccharides (RR 0.85, 95% CI 0.69 to 1.05; P = 0.13, I2 = 0%; 13 trials, 921 participants; low‐certainty evidence). However, there may be an overall beneficial effect on hepatic encephalopathy when comparing rifaximin plus a non‐absorbable disaccharide to a non‐absorbable disaccharide alone (RR 0.58, 95% CI 0.48 to 0.71; NNTB = 5; P < 0.001, I2 = 62%; 17 trials, 2332 participants; low‐certainty evidence).
Authors' conclusions
Compared to placebo/no intervention, rifaximin likely improves health‐related quality of life in people with minimal hepatic encephalopathy, and may improve hepatic encephalopathy, particularly in populations with minimal hepatic encephalopathy and when it is used for prevention. Rifaximin likely has no overall effect on mortality, serious adverse events, health‐related quality of life, or hepatic encephalopathy compared to non‐absorbable disaccharides. However, when used in combination with a non‐absorbable disaccharide, it likely reduces overall mortality risk, the risk of serious adverse events, improves hepatic encephalopathy, reduces the length of hospital stay, and prevents the occurrence/recurrence of hepatic encephalopathy. The certainty of evidence for these outcomes is very low to moderate; further high‐quality trials are needed.
Keywords: Humans, Ammonia, Disaccharides, Disaccharides/adverse effects, Hepatic Encephalopathy, Hepatic Encephalopathy/drug therapy, Hepatic Encephalopathy/prevention & control, Liver Cirrhosis, Liver Cirrhosis/complications, Quality of Life, Rifaximin, Rifaximin/therapeutic use
Plain language summary
Rifaximin for prevention and treatment of hepatic encephalopathy in people with cirrhosis
Key messages
The prevention and treatment of hepatic encephalopathy, in people with cirrhosis, largely depends on use of the compound lactulose. Rifaximin is not used to treat hepatic encephalopathy, at present, but it is used as an add‐on to lactulose to help prevent hepatic encephalopathy in people whose response to lactulose is inadequate.
We found that combining rifaximin with lactulose improved hepatic encephalopathy, reduced the risk of dying, and reduced the risk of developing side effects in addition to preventing future relapses.
Its wider use in the management of people with hepatic encephalopathy needs to be considered.
What are cirrhosis and hepatic encephalopathy?
Cirrhosis is a long‐term condition in which scar tissue (fibrosis) replaces normal liver tissue, often as a result of excess alcohol, being overweight, or having chronic hepatitis B/C infection. People with cirrhosis commonly develop a condition called hepatic encephalopathy which affects their mental function and their neurological function. This condition can have a negative effect on their survival. The exact reason why people with cirrhosis develop hepatic encephalopathy is unknown, but the toxin ammonia, which is produced mainly in the gut, is thought to play an important role. The severity of the symptoms of hepatic encephalopathy ranges from minor difficulties in mental function to obvious changes in movement, mental status, and consciousness. The minor changes in concentration, behaviour, and everyday function are classed as minimal hepatic encephalopathy. The more obvious abnormalities and changes in consciousness are classed as overt hepatic encephalopathy. The overt symptoms may occur in episodes or may be present at all times.
How is hepatic encephalopathy treated?
The non‐absorbable disaccharides (sugars), lactulose and lactitol, are the most commonly used treatment for hepatic encephalopathy. They reduce ammonia levels in the blood through multiple actions, mainly in the gut. Rifaximin is an antibiotic that is not absorbed into the blood stream but works solely in the gut, where it reduces the production of ammonia by the gut bacteria and ammonia absorption into the blood system. This effect may benefit people with hepatic encephalopathy.
What did we want to find out?
We wanted to find out if rifaximin could be used to prevent and treat hepatic encephalopathy in people with cirrhosis; whether it does this better than no drug treatment, a dummy pill (placebo), or non‐absorbable disaccharides; whether there may be additional benefit if rifaximin is used together with a non‐absorbable disaccharide; and whether there were any unwanted side effects.
What did we do?
We searched for studies that looked at rifaximin compared with no treatment, placebo, or non‐absorbable disaccharides in people with cirrhosis with, or at risk for developing, hepatic encephalopathy. We also searched for studies that used rifaximin plus non‐absorbable disaccharides compared with non‐absorbable disaccharides alone.
We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We identified 41 clinical studies involving 4545 people, who were randomly allocated to treatment groups. All participants had cirrhosis mainly due to excessive alcohol intake or chronic viral hepatitis. Participants were classed as having acute (13 studies), chronic (7 studies), or minimal (8 studies) hepatic encephalopathy, or were considered to be at risk for its development (13 studies). The studies compared rifaximin with a placebo (12 studies), no intervention (1 study), or lactulose/lactitol (14 studies). In 18 studies, rifaximin was given together with lactulose/lactitol and the results compared to the effect of giving lactulose/lactitol alone.
The analyses found that giving rifaximin alone may help improve health‐related quality of life and the performance of tests used to assess mental function in people with minimal hepatic encephalopathy. However, lactulose is probably as effective and is considerably cheaper. There were no differences in the benefits and side effects of rifaximin when directly compared with lactulose/lactitol. However, when rifaximin was given together with lactulose/lactitol, it reduced the risk of death (from 14.8% to 10.1%), reduced the risk of unwanted side effects (from 34.4% to 17.6%), and resulted in improvement in hepatic encephalopathy (from 86.9% to 33.8%) when compared to use of lactulose alone.
What are the limitations of the evidence?
We are uncertain about or have only moderate confidence in our findings, meaning we cannot make more certain conclusions about the effects of rifaximin. This was mainly because people in the studies might have been aware of which treatment they were getting and not all the studies provided data about the outcomes we were interested in. Also, many studies were too small for us to be certain about their results. More high‐quality studies are needed.
How up to date is this evidence?
The evidence is up to date to January 2023.
Summary of findings
Summary of findings 1. Summary of findings table ‐ Rifaximin compared to placebo/no intervention for prevention and treatment of hepatic encephalopathy in people with cirrhosis.
| Rifaximin compared to placebo/no intervention for prevention and treatment of hepatic encephalopathy in people with cirrhosis | ||||||
| Patient or population: prevention and treatment of hepatic encephalopathy in people with cirrhosis Setting: inpatient or outpatient Intervention: rifaximin Comparison: placebo/no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo/no intervention | Risk with rifaximin | |||||
| Mortality ‐ total number Follow‐up: mean 85.7 days | 58 per 1000 | 48 per 1000 (29 to 80) | RR 0.83 (0.50 to 1.38) | 1007 (13 RCTs) | ⊕⊕⊕⊝ Moderatea,b,c,d | Rifaximin likely results in little to no difference in mortality. |
| Serious adverse events ‐ total number of participants Follow‐up: mean 95.5 days | 184 per 1000 | 194 per 1000 (153 to 243) | RR 1.05 (0.83 to 1.32) | 801 (9 RCTs) | ⊕⊕⊕⊝ Moderateb,c,d,e | Rifaximin likely results in little to no difference in serious adverse events. |
| Health‐related quality of life ‐ assessed using: SIP score (3 trials) or EQ‐5D‐3L score (1 trial) Follow‐up: mean 64.5 days | The mean health‐related quality of life ‐ total ranged from 0 to 12 in SIP score or EQ‐5D‐3L score | MD 1.43 lower in SIP score or EQ‐5D‐3L score (2.87 lower to 0.02 higher) | ‐ | 214 (4 RCTs) | ⊕⊕⊕⊝ Moderatec,e,f,g | Rifaximin likely results in little to no difference in health‐related quality of life overall, although there is a suggestion of benefit in minimal hepatic encephalopathy. |
| Hepatic encephalopathy ‐ total number Follow‐up: mean 86 days | 479 per 1000 | 268 per 1000 (192 to 369) | RR 0.56 (0.42 to 0.77) | 1009 (13 RCTs) | ⊕⊕⊕⊝ Moderatec,e,h,i | Rifaximin likely improves hepatic encephalopathy overall and in minimal hepatic encephalopathy. |
| Non‐serious adverse events ‐ total number of particiapnts Follow‐up: mean 99.2 days | 312 per 1000 | 871 per 1000 (137 to 1000) | RR 2.79 (0.44 to 17.78) | 639 (6 RCTs) | ⊕⊝⊝⊝ Very lowc,e,j,k | The evidence is very uncertain about the effect of rifaximin on non‐serious adverse events overall. |
| Blood ammonia measured in μmol/L, μg/dL or mmol/L at trial end Follow‐up: mean 105 days | The mean blood ammonia ranged from 46 to 126.4 | MD 3.2 higher (7.74 lower to 14.14 higher) | ‐ | 381 (6 RCTs) | ⊕⊕⊝⊝ Lowc,e,g,h | Rifaximin may result in little to no difference in blood ammonia assessed at trial end. |
| Number Connection Test A assessed using: Z‐score (1 trial) or seconds (3 trials) assessed at trial end Follow‐up: mean 66.8 days | ‐ | SMD 0.31 SD lower (1.22 lower to 0.60 higher) | ‐ | 203 (4 RCTs) | ⊕⊕⊕⊝ Moderatec,e,l,m | Rifaximin likely results in little to no difference in Number Connection Test A performance at trial end. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised clinical trial; RR: risk ratio; SIP: sickness impact profile; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_429379845038926839. | ||||||
a Risk of bias: mortality outcomes unlikely to be affected by bias, not downgraded. b Inconsistency: I2 = 0 and all studies consistently show no net effect; not downgraded. c Indirectness: populations, interventions, outcomes, and comparisons are appropriate; not downgraded. d Imprecision: optimal information size not met, downgraded by 1 level. e Risk of bias: although trials were at a high risk of bias, sensitivity analyses did not change our findings; not downgraded. f Although the overall I2 statistic was 81, the inconsistencies could be explained by our subgroup analyses; not downgraded. g Imprecision: optimal information size met, but confidence interval includes both benefit and harm (overlaps 0); downgraded by 1 level. h Inconsistency: possible moderate heterogeneity within and between subgroups; downgraded by 1 level. i Imprecision: optimal information size met; not downgraded. j Inconsistency: possible substantial heterogeneity within and between subgroups; downgraded by 1 level. k Imprecision: optimal information size met; there were few events and the confidence intervals were wide, including both appreciable benefit and appreciable harm; downgraded by 2 levels. l Inconsistency: although heterogeneity exists overall and in subgroup analysis, there are few trials, of which all show no benefit, so are consistent; not downgraded. m Imprecision: standardised mean difference limits assessment; however, the small sample size increases imprecision; downgraded by 1 level.
Summary of findings 2. Summary of findings table ‐ Rifaximin compared to non‐absorbable disaccharide for prevention and treatment of hepatic encephalopathy in people with cirrhosis.
| Rifaximin compared to non‐absorbable disaccharide for prevention and treatment of hepatic encephalopathy in people with cirrhosis | ||||||
| Patient or population: prevention and treatment of hepatic encephalopathy in people with cirrhosis Setting: inpatient or outpatient Intervention: rifaximin Comparison: non‐absorbable disaccharide | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with non‐absorbable disaccharide | Risk with rifaximin | |||||
| Mortality ‐ total number Follow‐up: mean 62.2 days | 38 per 1000 | 38 per 1000 (19 to 75) | RR 0.99 (0.49 to 1.97) | 786 (10 RCTs) | ⊕⊕⊝⊝ Lowa,b,c,d | Rifaximin may result in little to no difference in mortality. |
| Serious adverse events ‐ total number of participants Follow‐up: mean 52.1 days | 121 per 1000 | 118 per 1000 (80 to 170) | RR 0.97 (0.66 to 1.40) | 681 (8 RCTs) | ⊕⊕⊝⊝ Lowb,c,e,f | Rifaximin may result in little to no difference in serious adverse events. |
| Health‐related quality of life assessed using: SF‐8 (1 trial) or SIP score (1 trial) at trial end Follow‐up: mean 144.5 days | The mean health‐related quality of life ‐ total ranged from 8.2 to 47.3 points | MD 0.33 lower (1.65 lower to 0.98 higher) | ‐ | 249 (2 RCTs) | ⊕⊝⊝⊝ Very lowb,c,g,h | The evidence is very uncertain about the effect of rifaximin on health‐related quality of life at trial end. |
| Hepatic encephalopathy ‐ total number Follow‐up: mean 57.9 days | 269 per 1000 | 229 per 1000 (186 to 283) | RR 0.85 (0.69 to 1.05) | 921 (13 RCTs) | ⊕⊕⊝⊝ Lowc,i,j,k | Rifaximin may result in little to no difference in hepatic encephalopathy. |
| Non‐serious adverse events ‐ total number of participants Follow‐up: mean 89.3 days | 161 per 1000 | 92 per 1000 (24 to 344) | RR 0.57 (0.15 to 2.13) | 396 (6 RCTs) | ⊕⊝⊝⊝ Very lowc,l,m,n | The evidence is very uncertain about the effect of rifaximin on non‐serious adverse events. |
| Blood ammonia measured in mmol/L, μg/dL, μmol/L, μg/100mL, or μg/mL at trial end Follow‐up: mean 27.4 days | The mean blood ammonia ranged from 47 to 128.3 | MD 6.78 lower (12.81 lower to 0.75 lower) | ‐ | 599 (10 RCTs) | ⊕⊝⊝⊝ Very lowc,f,m,o | The evidence is very uncertain about the effect of rifaximin on blood ammonia at trial end. |
| Number Connection Test A assessed using: grade (1 trial), seconds (4 trials), or Z‐score (2 trials) at trial end Follow‐up: mean 76.9 days | ‐ | SMD 0.18 SD lower (0.46 lower to 0.09 higher) | ‐ | 507 (7 RCTs) | ⊕⊝⊝⊝ Very lowc,p,q,r | The evidence is very uncertain about the effect of rifaximin on Number Connection Test A at trial end. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised clinical trial; RR: risk ratio; SIP: sickness impact profile; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_429380445436505482. | ||||||
a Risk of bias: mortality outcomes are unlikely to be affected by bias; not downgraded. b Inconsistency: I2 = 0 with all studies showing no effect; not downgraded. c Indirectness: populations, interventions, outcomes, and comparisons are appropriate. d Imprecision: optimal information size not met; there were few events and the confidence intervals were wide, including both appreciable benefit and appreciable harm; downgraded by 2 levels. e Risk of bias: even though sensitivity analyses did not affect our findings, only 2 trials remained from a total of 8, reducing certainty; downgraded by 1 level. f Imprecision: optimal information size met, but the confidence interval includes both benefit and harm (overlaps 1); downgraded by 1 level. g Risk of bias: excluding trials at high risk of bias leaves no trials ‐ we are therefore very uncertain about the evidence; downgraded by 2 levels. h Imprecision: insufficient studies at low risk of bias to calculate the optimal information size, few studies, and the confidence interval includes both benefit and harm (overlaps 0); downgraded by 2 levels. i Risk of bias: although sensitivity analyses did not change our findings, only 5 trials out of a total of 13 remained, reducing certainty of the evidence; downgraded by 1 level. j Heterogeneity: I2 = 0 for all but one subgroup analysis and overall, with all but one trial showing no effect; not downgraded. k Imprecision: optimal information size not met; downgraded by 1 level. l Risk of bias: sensitivity analysis for low‐risk trials shows a new benefit for minimal hepatic encephalopathy and a new harm for prevention trials with no change overall. Only one trial remains within each subgroup; the evidence is therefore very uncertain. m Heterogeneity: considerable heterogeneity may be present in multiple subgroup analyses in addition to the overall analysis; downgraded by 1 level. n Imprecision: optimal information size met; there were very few events and the confidence intervals were wide, including both appreciable benefit and appreciable harm; downgraded by 2 levels. o Risk of bias: sensitivity analysis for low‐risk trials shows a new subgroup‐level and overall benefit; some subgroup analyses have no data ‐ raising uncertainty; downgraded by 2 levels. p Risk of bias: sensitivity analysis for low‐risk trials differs from the main findings, with one or no trials within each subgroup ‐ the evidence is very uncertain; downgraded by 2 levels. q Inconsistency: I2 = 74% in acute hepatic encephalopathy trials with an overall statistic of 54%, and trials show inconsistent benefit between and within subgroups; downgraded by 1 level. r Imprecision: standardised mean difference used in analysis ‐ sample size was limited when selecting the most‐used measurement instrument, reducing certainty; downgraded by 1 level.
Summary of findings 3. Summary of findings table ‐ Rifaximin plus non‐absorbable disaccharides compared to non‐absorbable disaccharides alone for prevention and treatment of hepatic encephalopathy in people with cirrhosis.
| Rifaximin plus non‐absorbable disaccharides compared to non‐absorbable disaccharides alone for prevention and treatment of hepatic encephalopathy in people with cirrhosis | ||||||
| Patient or population: prevention and treatment of hepatic encephalopathy in people with cirrhosis Setting: inpatient or outpatient Intervention: rifaximin plus non‐absorbable disaccharides Comparison: non‐absorbable disaccharides alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with non‐absorbable disaccharides alone | Risk with rifaximin plus non‐absorbable disaccharides | |||||
| Mortality ‐ total number Follow‐up: mean 93 days | 148 per 1000 | 102 per 1000 (81 to 127) | RR 0.69 (0.55 to 0.86) | 1946 (14 RCTs) | ⊕⊕⊕⊝ Moderatea,b,c,d | Rifaximin plus non‐absorbable disaccharides likely reduces mortality slightly overall. |
| Serious adverse events ‐ total number of participants Follow‐up: mean 107.8 days | 256 per 1000 | 169 per 1000 (115 to 251) | RR 0.66 (0.45 to 0.98) | 1076 (7 RCTs) | ⊕⊝⊝⊝ Very lowc,d,e,f | The evidence is very uncertain about the effect of rifaximin plus non‐absorbable disaccharides on serious adverse events. |
| Hepatic encephalopathy ‐ total number Follow‐up: mean 82 days | 465 per 1000 | 270 per 1000 (223 to 330) | RR 0.58 (0.48 to 0.71) | 2332 (17 RCTs) | ⊕⊕⊝⊝ Lowc,g,h,i | Rifaximin plus non‐absorbable disaccharides may reduce hepatic encephalopathy overall. |
| Non‐serious adverse events ‐ total number of participants Follow‐up: mean 163.4 days | 592 per 1000 | 521 per 1000 (509 to 680) | RR 0.99 (0.86 to 1.15) | 384 (4 RCTs) | ⊕⊝⊝⊝ Very lowc,j,k,l | The evidence is very uncertain about the effect of rifaximin plus non‐absorbable disaccharides on non‐serious adverse events. |
| Blood ammonia measured in μg/mL at trial end Follow‐up: mean 143 days | The mean blood ammonia ranged from 88.6 to 109 | MD 6.88 lower (14.78 lower to 1.02 higher) | ‐ | 325 (2 RCTs) | ⊕⊝⊝⊝ Very lowc,m,n,o | The evidence is very uncertain about the effect of rifaximin plus non‐absorbable disaccharides on blood ammonia at trial end. |
| Number Connection Test A, assessed using: seconds (1 trial) or Z‐score (1 trial) at trial end Follow‐up: mean 68 days | ‐ | SMD 0.05 SD lower (1.28 lower to 1.17 higher) | ‐ | 76 (2 RCTs) | ⊕⊝⊝⊝ Very lowc,p,q,r | The evidence is very uncertain about the effect of rifaximin plus non‐absorbable disaccharides on Number Connection Test A at trial end. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised clinical trial; RR: risk ratio; SIP: sickness impact profile; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_429381317235175431. | ||||||
a Risk of bias: mortality outcome is unlikely to be affected by bias and sensitivity analysis did not change our findings; not downgraded. b Inconsistency: most studies showed no difference and I2 = 0% overall and for 2 subgroups, and 37% for acute hepatic encephalopathy; not downgraded. c Indirectness: populations, interventions, outcomes, and comparisons are appropriate; not downgraded. d Imprecision: optimal information size not met; downgraded by 1 level. e Risk of bias: with removal of high‐risk trials, sensitivity analysis leaves only 2 remaining studies with a new subgroup‐level benefit, limiting our certainty of the findings; downgraded by 2 levels. f Inconsistency: I2 = 71% in acute hepatic encephalopathy and 67% overall. Most trials show no difference but 3 favour rifaximin; downgraded by 1 level. g Risk of bias: only 2 trials remained in our sensitivity analysis from 17 trials. Although no change was observed, our certainty is therefore limited. Low‐risk trials did show similar direction of effect to high‐risk trials; downgraded by 1 level. h Inconsistency: I2 = 69% and 61% in 2 subgroup analyses, and 62% overall. Most trials show benefit, although there are some subgroup‐level outliers; downgraded by 1 level. i Imprecision: optimal information size met, with most studies showing a benefit; not downgraded. j Risk of bias: only 2 trials from 8 in total remained in our sensitivity analysis, which changed our findings, and therefore our certainty is very limited; downgraded by 2 levels. k Inconsistency: most trials show no difference, I2 = 0% in all analyses; not downgraded. l Some subgroups have few participants, causing wide confidence intervals; downgraded by 1 level. m Risk of bias: one of the two trials is at a high risk of bias. Although the sensitivity analysis does not change our findings, our certainty is therefore limited; downgraded by 1 level. n Inconsistency: both trials showed no effect, I2 = 0%; not downgraded. o Imprecision: optimal information size not met; there were few events and the confidence intervals were wide, including both appreciable benefit and appreciable harm; downgraded by 2 levels. p Risk of bias: only two trials were analysed, which are both at high risk. It is unclear whether low‐risk trials would show different findings and so our certainty is very limited; downgraded by 2 levels. q Inconsistency: despite the overall I2 = 84%, both trials showed no benefit; not downgraded. r Imprecision: only 72 participants in 2 trials were included, with the effect including both benefit and harm, severely limiting our certainty; downgraded by 2 levels.
Background
Description of the condition
Hepatic encephalopathy is the term used to describe the complex spectrum of neuropsychiatric change that can complicate the course of both acute and chronic liver disease. In this review, only the association with chronic liver disease will be considered. The joint guideline from the European and American Associations for the Study of the Liver defines hepatic encephalopathy as "brain dysfunction associated with liver insufficiency or portal systemic shunting" (EASL and AASLD guideline 2014).
Hepatic encephalopathy is broadly classified as: (i) overt, when there are manifest clinical abnormalities together with impairment of neurophysiological function and neuropsychometric performance; and (ii) minimal, when there is no clinical evidence of neuropsychiatric impairment but documented neurophysiological and neuropsychometric abnormalities (Morgan 2018; Weissenborn 2019). The term 'covert hepatic encephalopathy' has been introduced to encompass low‐grade overt hepatic encephalopathy (Grade 1) and minimal hepatic encephalopathy. However, the usefulness of this term is the subject of considerable debate (EASL Clinical Practice Guidelines 2022; Jalan 2022), particularly as there is evidence that people classified as having covert hepatic encephalopathy behave, when tested, as two relatively independent populations (Montagnese 2014; Zacharias 2017).
Clinically apparent or overt hepatic encephalopathy manifests as a neuropsychiatric syndrome encompassing a wide spectrum of mental and motor disorders (Patidar 2015; Weissenborn 2019). Based on its clinical course, overt hepatic encephalopathy is further classified as acute/episodic, recurrent, or chronic/persistent. Episodes of acute hepatic encephalopathy are often precipitated by events such as infection, dehydration, constipation, electrolyte disturbances, gastrointestinal bleeding, and drugs (Pantham 2017). In some instances, there may be more than one precipitant, while in others, no obvious precipitant is identified. These episodes can develop rapidly and without warning, and may recur (EASL and AASLD guideline 2014). Between episodes, people may return to their baseline neuropsychiatric state or show clinical evidence of ongoing impairment (Bajaj 2010a; Sharma 2010). Less frequently, the neuropsychiatric abnormalities become chronic, although they may still fluctuate in seriousness. The presence of persistent or chronic hepatic encephalopathy is often associated with extensive spontaneous portal‐systemic shunting or else surgically created or transjugular intrahepatic portosystemic shunts (Bai 2014; Zhuo 2019).
The changes in mental state associated with overt hepatic encephalopathy range from subtle alterations in personality, intellectual capacity, and cognitive function to more profound alterations in consciousness leading to deep coma with decerebrate posturing. The changes in motor function include rigidity, hypomimia, bradykinesia, ataxia, disorders of speech production, resting‐ and movement‐induced tremor, choreoathetoid movements, and transient focal abnormalities (Morgan 2018; Victor 1965; Weissenborn 2019). Asterixis (flapping tremor) is the best‐known of the motor abnormalities. Other abnormalities include impaired psychometric performance (Morgan 2018; Weissenborn 2019), disturbed neurophysiological function (Amodio 2015; Guérit 2009; Parsons‐Smith 1957), reductions in global and regional cerebral blood flow and metabolism (Bjerring 2018; O'Carroll 1991), and changes in cerebral fluid homeostasis (Cudalbu 2019). In general, the degree of impairment observed in these variables increases as the clinical condition worsens.
There is no gold standard for the diagnosis of hepatic encephalopathy (Morgan 2018). The initial assessment should include a careful and detailed neuropsychiatric history and examination (Montagnese 2004; Morgan 2018), with particular attention paid to changes in memory, concentration, cognition, and consciousness. The neurological examination should be comprehensive, looking particularly for evidence of subtle motor abnormalities. The West Haven Criteria can be used to grade mental status (Conn 1977), and the Glasgow Coma Score to grade the level of consciousness, if impaired (Teasdale 1974). The assessment should consider and exclude other potential causes of neuropsychiatric abnormalities such as concomitant neurological disorders, and metabolic abnormalities such as those associated with diabetes, renal failure, drugs, or alcohol intoxication. Thus, the history and clinical examination are useful for both the detection of overt hepatic encephalopathy and its exclusion.
Impaired psychometric performance is invariable in people with overt hepatic encephalopathy, and is one of the defining features of minimal hepatic encephalopathy (Amodio 2004; Montagnese 2004; Morgan 2016). Deficits in attention, visuospatial abilities, fine motor skills, and memory, together with relative preservation of other cognitive functions, are characteristic of minimal hepatic encephalopathy. Additional disturbances in psychomotor speed, executive function, and concentration are features of overt hepatic encephalopathy. A variety of single tests and test batteries are used to assess psychometric performance. The most frequently used single test is Number Connection Test‐A (Weissenborn 1998). The most frequently used, and best validated, test battery is the Psychometric Hepatic Encephalopathy Score (Schomerus 1998; Weissenborn 2001), which employs five paper and pencil tests to assess attention, visual perception, and visuo‐constructive abilities. Test scores are normalised to take account of factors such as age, sex, and educational level; normative data are available for populations in a number of countries world‐wide.
Neurophysiological abnormalities are common in people with hepatic encephalopathy (Guérit 2009). The electroencephalogram is the best known and most frequently used test system. Progressive slowing of the background activity of the electroencephalogram is seen in a high proportion of people with overt encephalopathy, and is one of the defining features of minimal hepatic encephalopathy (Amodio 2004; Montagnese 2004; Morgan 2016). Recent advances in electroencephalogram analysis should provide better quantifiable and more informative data (Morgan 2018). Other potential diagnostic techniques include the Critical Flicker Fusion Frequency (Kircheis 2002), and the Inhibitory Control Test (Bajaj 2008). Studies using structural and functional cerebral imaging techniques have helped to unravel the pathophysiology of hepatic encephalopathy, but offer little diagnostically (Berding 2009; Morgan 2018; Rose 2020).
Thus, the diagnosis of hepatic encephalopathy requires a detailed clinical assessment supported by neuropsychometric and neurophysiological testing. Specific guidelines are not available (EASL and AASLD guideline 2014; Ferenci 2002).
Hepatic encephalopathy is the commonest complication of cirrhosis. The overall incidence of hepatic encephalopathy in an older population of Americans with cirrhosis was 11.6 per 100 person‐years of follow‐up; the incidence was higher amongst those with alcohol‐related cirrhosis and portal hypertension (Tapper 2019).
The overall prevalence of overt hepatic encephalopathy, at the time of first diagnosis with cirrhosis, is 10% to 20% (D'Amico 1986; Jepsen 2010; Saunders 1981); the prevalence increases with the degree of hepatic decompensation. It is estimated that 30% to 40% of people with cirrhosis will develop hepatic encephalopathy during the course of their disease (D'Amico 1986; Jepsen 2010). The prevalence of minimal hepatic encephalopathy varies from 20% to 80%, depending on the population under study and the diagnostic test systems used (Groeneweg 1998; Schomerus 1998; Sharma 2007); it tends to exceed 50% in people with previous overt hepatic encephalopathy (Lauridsen 2011; Sharma 2010).
The risk of developing an episode of overt hepatic encephalopathy, within five years of presentation, varies from 5% to 25% depending on the presence or absence of other risk factors (Jepsen 2010). People with a previous episode of overt hepatic encephalopathy have a 42% risk of recurrence within one year (Sharma 2009), while those with recurrent overt hepatic encephalopathy have a 46% cumulative risk of a further recurrence within six months (Bass 2010; Sharma 2009). The presence of minimal hepatic encephalopathy significantly increases the risk of developing overt neuropsychiatric change; rates range from 20% to 30% (Das 2001; Romero‐Gómez 2001). The median cumulative one‐year incidence of overt hepatic encephalopathy, after insertion of a transjugular intrahepatic shunt, is significantly influenced by the criteria adopted for candidate selection, and so can range from 10% to 50% (Bai 2014; Fornio 2017; Nolte 1998; Riggio 2008; Zhu 2019).
Description of the intervention
Rifaximin is a virtually non‐absorbable, semisynthetic antibiotic with broad spectrum effects on both gram‐positive and gram‐negative bacteria (Calanni 2014; Scarpignato 2005). It is used to treat infectious diarrhoea and is currently licensed for use as an add‐on to lactulose for the prevention of recurrent hepatic encephalopathy. It is given orally. Common adverse events include nausea, flatulence, and diarrhoea. The risk of development of antibiotic resistance and of Clostridium difficile enteritis infection is low (Bass 2010). Combining rifaximin with lactulose does not result in any relevant changes to the antibiotic susceptibility profiles of the faecal microbiota nor in any clinically relevant antibiotic resistance (Frenette 2020a).
The non‐absorbable disaccharides, lactulose and lactitol, are used as osmotic laxatives for the treatment of constipation (Johanson 2007; Miller 2014). Lactulose was first used for the treatment of hepatic encephalopathy in 1966 (Bircher 1966). It is dispensed as a syrup, which is contaminated with other sugars; a pure crystalline preparation is available. Lactitol is a second‐generation non‐absorbable disaccharide that was first introduced into clinical practice in the early‐ to mid‐1980s (Bircher 1982). It is produced in pure crystalline form and is dispensed as a powder. Both are given orally. Common adverse events, mainly encountered with lactulose syrup and dose‐related, include nausea, abdominal discomfort, flatulence, and diarrhoea.
How the intervention might work
The exact pathogenesis of hepatic encephalopathy is unknown. Ammonia plays a key role (Morgan 2018; Rose 2020). The main sources of ammonia include nitrogenous products in the diet, bacterial metabolism of urea and proteins in the colon, deamination of glutamine in the small intestine, and release from the kidney (Levitt 2019). Thus, the gut microenvironment and the gut microbiota play an important role in ammoniagenesis (Acharya 2019; Iebba 2018; Levitt 2019). In consequence, most interventions for hepatic encephalopathy aim to reduce the production and/or increase the elimination of ammonia from the gut (EASL and AASLD guideline 2014; Morgan 2018; Rose 2020).
The exact mechanisms of action of rifaximin in hepatic encephalopathy are unknown, particularly in relation to ammonia homeostasis (Levitt 2019). Rifaximin may have an effect on the gut microbiome by changing its metabolic function rather than affecting relative bacterial abundance (Bajaj 2014; Bajaj 2016a; Bajaj 2021; Frenette 2020b). Rifaximin administered in combination with lactulose does not alter the bacterial composition or the richness of the stool microbiota (DuPont 2016; Schulz 2019). There are no identifiable differences in serum inflammatory markers when rifaximin is administered alone or in combination with a non‐absorbable disaccharide, suggesting that its action might be independent of systemic inflammatory processes (Bajaj 2020a).
The non‐absorbable disaccharides are not absorbed in the small intestine but are metabolised by colonic bacteria to volatile fatty acids and hydrogen. Their beneficial effects reflect their ability to reduce the intestinal production/absorption of ammonia, which is achieved in the following ways.
Catharsis: their colonic metabolism results in an increase in intraluminal gas formation; an increase in intraluminal osmolality; a reduction in intraluminal pH; and an overall decrease in transit time.
Bacterial uptake of ammonia: the intraluminal pH changes result in a leaching of ammonia from the circulation into the colon; the colonic bacteria use the released volatile fatty acids as substrate; they proliferate and use the trapped colonic ammonia as a nitrogen source for protein synthesis (Zhu 2016). The increase in bacterial numbers additionally ‘bulks’ the stool and contributes to the cathartic effect (Levitt 2019; Weber 1987).
Reduction in intestinal ammonia production: they inhibit glutaminase activity and interfere with the intestinal uptake of glutamine and its subsequent metabolism to ammonia (Levitt 2019; van Leeuwen 1988).
Beneficial effects on the gut microbiome: cirrhosis is associated with functional dysbiosis and changes to the colonic mucosal microbiome (Qin 2014); further changes may occur in hepatic encephalopathy (Bajaj 2012). Non‐absorbable disaccharides may have beneficial effects on the gut microbiota by modulating its composition and metabolic function (Riggio 1990; Schultz 2019).
A Cochrane Review demonstrated significant beneficial effects of the non‐absorbable disaccharides on both hepatic encephalopathy and survival (Gluud 2016). They are recommended as first‐line treatment for hepatic encephalopathy (EASL and AASLD guideline 2014; Gluud 2016; Morgan 2018; Rose 2020).
Rifaximin is licensed for the prevention of recurrent hepatic encephalopathy as an add‐on to lactulose. Studies have shown that it may have a beneficial effect in acute, chronic, and minimal hepatic encephalopathy, but study results are divergent and inconclusive. Several meta‐analyses of rifaximin in hepatic encephalopathy, against a variety of comparators, have been undertaken to date, but the number of included studies, the type of hepatic encephalopathy, and the outcomes vary considerably (Cheng 2021; Eltawil 2012; Fidel 2019; Fu 2022; Han 2021; Jiang 2008; Kimer 2014; Razzack 2021; Shukla 2011; Wu 2013; Zhuo 2019). No previous Cochrane Review of rifaximin for the prevention and treatment of hepatic encephalopathy has been undertaken.
Why it is important to do this review
The presence of hepatic encephalopathy, whether minimal or overt, has significant detrimental effects on outcomes in people with cirrhosis. It is associated with impairment in the performance of complex tasks, such as driving (Bajaj 2009; Kircheis 2009; Schomerus 1998); an increased risk of falls and injury (Roman 2011); and a significant detrimental effect on health‐related quality of life (Fabrellas 2020; Groeneweg 1998; Grønkjær 2018; Orr 2014). Hepatic encephalopathy also causes widespread distress, uncertainty, and anxiety for caregivers (Bajaj 2011a; Bajaj 2011b; Fabrellas 2020; Montagnese 2019; Shrestha 2020).
Hepatic encephalopathy has a significant negative effect on survival (Ampuero 2015; D'Amico 2006; Stewart 2007). The one‐ and five‐year mortality rates in people with hepatic encephalopathy at presentation are 64% and 85%, respectively (Jepsen 2010). Median survival times of 0.95 years have been reported for those aged over 65 and of 2.5 years in those who are younger (Tapper 2020). The in‐hospital mortality rate associated with an acute episode of hepatic encephalopathy is 15% (Stepanova 2012). The survival probability, after a first episode of hepatic encephalopathy, is 42% at one year and 23% at three years (Bustamante 1999). It follows that the development of an episode of overt hepatic encephalopathy identifies a population at high risk of short‐ and medium‐term mortality (Bustamante 1999). Liver transplant candidates with overt hepatic encephalopathy have a 90‐day mortality rate that is 66% higher than their unaffected counterparts with comparable Model of End‐Stage Liver Disease (MELD) scores (Wong 2014). Thus, hepatic encephalopathy is not just a symptom of liver failure, but may have independent pathophysiological and prognostic significance (Bohra 2020; Córdoba 2014).
The significant detrimental effects of hepatic encephalopathy on outcome results in frequent hospitalisation (Hirode 2019). The utilisation of healthcare resources associated with hepatic encephalopathy is greater than for any other complication of cirrhosis (Tapper 2016). Between 2010 and 2014, there was a 24.4% increase in the total number of hospitalisations with hepatic encephalopathy in the USA, with an associated 46% increase in total inpatient charges to USD 11.9 billion/annum (Hirode 2019). The readmission rates at 90 days were around 27%, adding another USD 200 million to the costs (Shaheen 2019). Comparable data are not available for Europe, although the annual admission costs are likely to be just as high (Di Pascoli 2017). There are no reliable estimates of the societal burden of hepatic encephalopathy, such as the costs associated with primary healthcare, disability and lost productivity, but they are likely to be substantial (Bajaj 2011b).
Hepatic encephalopathy can be prevented and treated. However, surveillance systems for diagnosis are poorly applied, and clear guidelines are lacking. Thus, many people, particularly those with minimal hepatic encephalopathy, escape detection and are denied the benefits of treatment. The non‐absorbable disaccharides are recommended as first line treatment for hepatic encephalopathy (EASL and AASLD guideline 2014), and there is good evidence for their efficacy and safety (Gluud 2016). However, information on long‐term compliance with treatment is lacking, but is assumed to be poor (Bajaj 2010b).
The results of individual trials show that rifaximin is superior to placebo/no intervention in minimal encephalopathy (Sidhu 2011), but not in chronic hepatic encephalopathy (Fera 1993), or for its prevention (Zeng 2021). Trials also show that rifaximin is comparable in effect to the non‐absorbable disaccharides in acute (Suzuki 2018), chronic (Massa 1993), and minimal (Pawar 2019) hepatic encephalopathy, and for its prevention (Higuera‐de‐la‐Tijera 2018). However, several trials have shown that combining rifaximin with a non‐absorbable disaccharide may be more beneficial than lactulose alone in acute hepatic encephalopathy (Ahmed 2018), and for preventing its recurrence (Bass 2010). A number of systematic reviews and meta‐analyses of the efficacy and safety of rifaximin in hepatic encephalopathy, against a variety of comparators, have been undertaken (Cheng 2021; Eltawil 2012; Fidel 2019; Fu 2022; Han 2021; Jiang 2008; Kimer 2014; Razzack 2021; Shukla 2011; Wang 2019a; Wu 2013; Zhuo 2019). They include varying numbers of studies, outcomes are generally limited to hepatic encephalopathy and mortality, and they are generally formulated to address specific aspects of the use of rifaximin in clinical practice.
Retrospective studies and decision analyses have addressed the cost‐efficiency of rifaximin compared to no intervention or to the non‐absorbable disaccharides, and have suggested that the expense of rifaximin may be counterbalanced by a decrease in hospitalisation rates, a reduction in readmission rates and healthcare costs (Courson 2016; Huang 2007; Leevy 2007; Neff 2013; Orr 2016; Tapper 2020).
Currently, rifaximin is only licensed for use combined with a non‐absorbable disaccharide for the prevention of recurrent hepatic encephalopathy (EASL and AASLD guideline 2014). It is unclear, from the current literature, whether it may also have treatment benefits when used alone or in combination with a non‐absorbable disaccharide in minimal, acute, and chronic hepatic encephalopathy. A systematic review and meta‐analysis of the efficacy and safety of rifaximin for both the treatment and prevention of hepatic encephalopathy, encompassing a wide variety of outcomes, is warranted.
Objectives
To evaluate the beneficial and harmful effects of rifaximin versus placebo, no intervention, or non‐absorbable disaccharides for: (i) the prevention of hepatic encephalopathy, and (ii) the treatment of minimal and overt hepatic encephalopathy, in people with cirrhosis, both when used alone and when combined with a non‐absorbable disaccharide.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised clinical trials irrespective of blinding, publication status, language, or outcomes reported in our primary analyses.
Types of participants
We included people with cirrhosis and hepatic encephalopathy from randomised clinical trials, irrespective of the aetiology and severity of their underlying liver disease. We included people with acute, chronic, or minimal hepatic encephalopathy, and people with recurrent episodes of hepatic encephalopathy. Participants were included regardless of sex, age, or the absence/presence of associated precipitating factors. If we identified trials including subsets of relevant participants with cirrhosis as well as participants without cirrhosis, we planned to exclude these trials in sensitivity analyses unless results were presented for the two groups separately or could be extrapolated from the data provided.
Types of interventions
The intervention comparisons were: i) rifaximin at any dose, duration, or mode of administration versus placebo or no intervention; ii) rifaximin at any dose, duration, or mode of administration versus non‐absorbable disaccharides (lactulose or lactitol); and iii) rifaximin at any dose, duration, or mode of administration co‐administered with a non‐absorbable disaccharide versus placebo, no intervention, or a non‐absorbable disaccharide. Co‐interventions and co‐medications administered equally to all allocation arms were allowed.
Types of outcome measures
We assessed all outcomes at the maximum duration of follow‐up.
Primary outcomes
All‐cause mortality
Serious adverse events, defined as any untoward medical occurrence, not necessarily having a causal relationship with the treatment, that at any dose resulted in death, were life‐threatening, required hospitalisation or prolongation of existing hospitalisation, resulted in persistent or significant disability or a congenital anomaly/birth defect, or any medical event that might have jeopardised the participant or required intervention to prevent one of the above outcomes (ICH GCP 2016). We analysed serious adverse events as a composite outcome (Peryer 2021). We excluded death events from the serious adverse events' outcome, and only reported the number of participants with adverse events rather than the total number of adverse events to prevent double counting.
Health‐related quality of life at the maximum point of follow‐up or as a change from baselines as per study authors' measurement
Hepatic encephalopathy, assessed by the number of participants without improved manifestations as per the study authors' assessment
Secondary outcomes
Non‐serious adverse events, defined as any untoward medical occurrence, not necessarily having a causal relationship with the treatment, that did not constitute a serious adverse event. We analysed non‐serious adverse events as a composite outcome by exploratory approach (Peryer 2021). We only reported the number of participants with adverse events rather than the total number of adverse events to avoid double counting.
Blood ammonia concentrations assessed at the maximum point of follow‐up and, where available, the difference between baseline and end of trial concentrations
Number Connection Test A (NCT‐A) time assessed at the maximum point of follow‐up and, where available, the difference between baseline and end of trial times
Length of hospital stay
Search methods for identification of studies
The last search update was January 2023.
Electronic searches
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register (searched through the Cochrane Library; 2022, Issue 5), Cochrane Central Register of Controlled Trials (2022, Issue 5) in the Cochrane Library, MEDLINE Ovid (1946 to 12 January 2023), Embase Ovid (1974 to 12 January 2023), Latin American and Caribbean Health Science Information database (LILACS) (Bireme; 1982 to 12 January 2023), Science Citation Index Expanded (1900 to 12 January 2023), and Conference Proceedings Citation Index – Science (1990 to 12 January 2023). The latter two were searched simultaneously through the Web of Science. All the searches were conducted without restrictions. The search strategies are presented in Appendix 1.
Searching other resources
We searched the conference proceedings from the American Gastroenterological Association (AGA), the American Association for the Study of Liver Diseases (AASLD), the European Association for the Study of the Liver (EASL), the Asian Pacific Association for the Study of the Liver (APASL), the International Society for Hepatic Encephalopathies and Nitrogen Metabolism (ISHEN), the British Society of Gastroenterology (BSG), the British Association for the Study of the Liver (BASL), and the United European Gastroenterology Week (UEGW). We searched the World Health Organization (WHO) online trial meta‐register (apps.who.int), the International Clinical Trials Registry Platforms (clinicaltrials.gov and www.clinicaltrialsregister.eu), the New Zealand Clinical Trials Register (NZCT, clinicaltrials.health.nz), and Google Scholar (scholar.google.com/) using the search terms cirrhosis AND rifaximin. Searches were performed up until 12 January 2023.
We searched the reference list of papers identified in the electronic searches and wrote to authors of the identified clinical trials and relevant pharmaceutical companies for additional information on completed randomised clinical trials, unpublished trials, and ongoing trials. We also sought and retrieved information from the Food and Drug Administration (www.fda.gov) and the European Medicines Agency Website (www.ema.europa.eu).
Data collection and analysis
Selection of studies
Six review authors (HDZ, FK, JT, NK, LG, and MYM), working independently, read the updated electronic searches, performed additional manual searches, and listed potentially eligible trials. All authors then read the potentially eligible trials and participated in the final selection of those to be included in the analyses. For trials reported in more than one publication, we selected the one reporting the longest duration of follow‐up as the primary reference. We included trials even if they did not report all of our selected outcomes. The final selection of trials was reached through discussion and agreed consensus between the authors.
If, during the selection of randomised clinical trials for inclusion in the review, we identified observational studies (i.e. quasi‐randomised studies, cohort studies, case‐series, or patient reports) that detailed adverse events caused by or associated with the interventions, then we reported this information in a review of adverse events additional to the main analyses. We did not specifically search for observational studies for inclusion in this review.
We completed a PRISMA flow diagram of the selection process (Page 2021a; Page 2021b; Figure 1). We listed details of included trials in a Characteristics of included studies table; listed all excluded trials with the reason for their exclusion in a Characteristics of excluded studies table; and listed details of relevant ongoing trials in a Characteristics of ongoing studies table.
1.
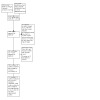
PRISMA flow diagram. Date of last search January 2023
Data extraction and management
All review authors participated in data extraction, and at least two review authors independently evaluated each clinical trial using a pilot Cochrane data extraction form. Key unpublished information that was missing from clinical trial reports was sought through correspondence with the primary investigators of the included randomised clinical trials. Where we were not able to gather sufficient data (number of events and participants) from the text and tables of the included trial reports or from correspondence with investigators, we attempted to extrapolate data, where possible, from graphs. We reported study characteristics based on PICOT (participants, interventions, comparisons, outcomes, and time) in order to explore and compare collected elements across trials. We gathered data on the following items.
Participants: mean age, proportion of men, proportion with cirrhosis, aetiology of cirrhosis, type of hepatic encephalopathy
Interventions: type, dose, duration of therapy, mode of administration, and concomitant therapies
Comparisons: type, dose, duration of therapy, mode of administration, and concomitant therapies
Outcomes: number and type assessed, criteria used in the assessment of hepatic encephalopathy
Trials and time: design (cross‐over or parallel); setting (hospital or outpatient; number of clinical sites), country of origin; inclusion period
We resolved any disagreements through discussion between review authors and consensus agreement.
Assessment of risk of bias in included studies
We assessed bias control using the domains described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017), and classified the risk of bias for separate domains as high, unclear, or low. We also included an overall assessment of bias control as described below.
Allocation sequence generation (concealment bias)
Low risk of bias: sequence generation achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, or throwing dice were adequate if performed by an independent person not otherwise involved in the trial.
Unclear risk of bias: the method of sequence generation was not specified.
High risk of bias: the sequence generation method was not random.
Allocation concealment (concealment bias)
Low risk of bias: allocation was controlled by a central and independent randomisation unit or similar adequate method (e.g. serially numbered opaque sealed envelopes) to ensure that the allocation sequence was unknown to the investigators (Savovic 2012).
Unclear risk of bias: the method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment.
High risk of bias: the allocation sequence was likely to be known to the investigators who assigned the participants.
Blinding of participants and personnel (performance bias)
Low risk of bias: blinding of participants and personnel performed adequately using a placebo, double‐dummy or similar. We defined lack of blinding (detection and performance bias) as not likely to affect the assessment of mortality.
Unclear risk of bias: insufficient information to assess blinding.
High risk of bias: no blinding or incomplete blinding.
Blinding of outcome assessors (detection bias)
Low risk of bias: blinding of outcome assessors performed adequately using a placebo. We defined lack of blinding as not likely to affect the outcome assessors' evaluation of mortality (Savovic 2012; Savovic 2018).
Unclear risk of bias: there was insufficient information to assess blinding.
High risk of bias: no blinding or incomplete blinding.
Incomplete outcome data (attrition bias)
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. The investigators used sufficient methods, such as intention‐to‐treat analyses with multiple imputations or carry‐forward analyses to handle missing data.
Unclear risk of bias: insufficient information to assess missing data.
High risk of bias: the results were likely to be biassed due to missing data.
Selective outcome reporting (reporting bias)
Low risk of bias: the trial reported clinically relevant outcomes (all‐cause mortality, hepatic encephalopathy, serious adverse events, health‐related quality of life (where feasible), non‐serious adverse events, blood ammonia (where feasible), length of hospital stay (where feasible), and NCT‐A (where feasible)). If we had access to the original trial protocol, the outcomes selected were those described in that protocol. If we obtained information from a trial registry (such as www.clinicaltrials.gov), we only used that information if the investigators registered the trial before the inclusion of the first participant. Due to the heterogenous nature of the condition and the variety of settings in which it is prevented or treated, outcomes are not always feasible to obtain or applicable to report (e.g. NCT‐A and health‐related quality of life in treatment trials in acute hepatic encephalopathy or blood ammonia in long‐term prevention trials); we assessed this on an individual trial basis.
Unclear risk of bias: predefined relevant outcomes were not reported fully, or the reporting was unclear.
High risk of bias: one or more predefined outcomes were not reported. The results have not been published in a full‐text paper, and/or the authors did not provide additional data.
Other bias
Low risk of bias: the trial appeared free of other biases including medicinal dosing problems or follow‐up (as defined below).
Unclear risk of bias: the trial may or may not have been free of other domains that could put it at risk of bias.
High risk of bias: there were other factors in the trial that could put it at risk of bias such as the administration of inappropriate treatments being given to the controls (e.g. an inappropriate dose) or follow‐up (e.g. the trial included different follow‐up schedules for participants in the allocation groups).
Overall bias assessment
Using the definitions described above, the overall bias assessment was classed as follows.
Low risk of bias: all domains were low risk of bias.
Some concerns: unclear risk of bias in at least one domain.
High risk of bias: one or more of the bias domains at high risk of bias or multiple domains were of unclear risk of bias.
We used the overall judgement per outcome (i.e. all‐cause mortality; serious adverse events; health‐related quality of life; hepatic encephalopathy; non‐serious adverse events; blood ammonia; length of hospital stay; and the NCT‐A time) to feed into the GRADE summary of findings tables.
Measures of treatment effect
We used risk ratios (RR) for dichotomous outcomes and the mean differences (MD) or standardised MDs for continuous outcomes, both with 95% confidence intervals (CI). For primary outcomes, we calculated the number needed to treat for an additional beneficial outcome (NNTB) as 1/risk difference (RD). We meta‐analysed our data using a random‐effects model.
Unit of analysis issues
Due to the fluctuating nature of hepatic encephalopathy and the nature of our primary outcomes, we included randomised clinical trials using a parallel group design and only utilised the first treatment period from trials with a cross‐over design (Higgins 2021a). We included separate pair‐wise comparisons of the interventions of interest from multi‐arm trials.
Dealing with missing data
We extracted data on all randomised participants, irrespective of compliance, protocol violations, or follow‐up, in order to allow intention‐to‐treat analyses. We planned to undertake analyses to evaluate the importance of missing data, including worst‐case scenario analysis (Higgins 2008), and extreme worst‐case and best‐case, and extreme‐best case scenario analyses (Deeks 2022).
Assessment of heterogeneity
We assessed heterogeneity through visual inspection of the forest plots and expressed heterogeneity as I2 values using the following thresholds: 0% to 40% (might not be important); 30% to 60% (may represent moderate heterogeneity); 50% to 90% (may represent substantial heterogeneity); and 75% to 100% (considerable heterogeneity) (Deeks 2022). Heterogeneity is included in Table 1; Table 2 and Table 3 (GRADEpro).
In the case of substantial or considerable heterogeneity, another author independently extracted the data to ensure they were concordant, and no errors had been made. If this did not resolve the issue, then we sought other potential causes of heterogeneity from the results of the additional subgroup analyses, by type of hepatic encephalopathy, and the results of the sensitivity analyses.
Assessment of reporting biases
We assessed reporting bias from missing outcomes in the included publications by comparing the available published data with those in trial registries and protocols, and by contacting study authors. We also assessed whether studies with limited outcome reporting were published as a peer‐reviewed full‐text article or solely in abstract form. If there was an unexplainable discrepancy between these various sources, leading to a suspicion of selective reporting, we categorised the bias assessment as 'high risk'.
For meta‐analyses reporting 10 or more trials, we drew up funnel plots to assess reporting bias from individual trials. These were made by plotting the risk ratio (RR) on a logarithmic scale against its standard error (Egger 1997; Page 2021). We examined the degree of asymmetry of the resulting funnel plots.
Data synthesis
We performed the analyses using Review Manager 5 and RevMan Web (Review Manager 2020; RevMan Web 2020). We used random‐effects model meta‐analyses for our main analyses but also performed fixed‐effect model meta‐analyses where indicated. The estimates of the random‐effects meta‐analysis might provide the most conservative estimate of intervention effects; however, the results of the fixed‐effect meta‐analyses are also reported, particularly if the overall results of the two models differed.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses to investigate heterogeneity based on the type of hepatic encephalopathy. We expressed differences between subgroups as P values (tests for subgroup differences).
Sensitivity analysis
We planned to perform sensitivity analyses including only randomised clinical trials at low risk of bias, only trials with no vested interests, and worse‐case, extreme worse case, best‐case, and extreme‐best case analyses.
Summary of findings and assessment of the certainty of the evidence
We used GRADEpro to generate Table 1, Table 2, and Table 3, with information about comparisons, outcomes, risk of bias, and results of the meta‐analyses (GRADEpro). We used the GRADEpro system to evaluate the certainty of the evidence for our primary outcomes in relation to the within‐study risk of bias (methodological quality); indirectness of evidence (population, intervention, control, outcomes); diversity (heterogeneity); imprecision of effect estimate (calculating optimal information size using trials at low risk of bias, (alpha 0.02, power 0.9) and identifying wide confidence intervals which may include no treatment effect); and risk of publication bias (GRADEpro; Jakobsen 2014). We assessed the aforementioned domains against each of our outcomes: mortality, serious adverse events, health‐related quality of life, hepatic encephalopathy, non‐serious adverse events, blood ammonia, and the NCT‐A time. We also assessed the risk of bias for length of hospital stay, but deemed this outcome to be less important to include in our summary of findings table than others as it can be influenced by factors other than need (e.g. bed availability).
Results
Description of studies
We included 41 randomised clinical trials in our quantitative and qualitative analyses (Ahmed 2018; Ali 2014; Babar 2017; Bajaj 2011; Bajaj 2019; Bass 2004; Bass 2010; Bucci 1993; Bureau 2021; Butt 2018; Fera 1993; Festi 1993; Gill 2014; Habib 2016; Hasan 2018; Higuera‐de‐la‐Tijera 2018; Kimer 2017; Loguercio 2003; Maharshi 2015; Majeed 2018; Manzhalii 2022; Mas 2003; Massa 1993; Moneim 2021; Muhammad 2016; Nawaz 2015; Paik 2005; Patel 2022; Pawar 2019; Poudyal 2019; Riggio 2005; Sharma 2013; Sharma 2014; Sidhu 2011; Sidhu 2016; Suzuki 2018; Tan 2022; Uthman 2020; Vyas 2017; Wahib 2014; Zeng 2021); a further 13 observational studies were included in an additional analysis of adverse events (Bohra 2020; Chang 2021; Jones 2020; Kang 2017; Mullen 2014; Oey 2019; Orr 2016; Salehi 2019; Suzuki 2019; Tatsumi 2021; Uchida 2020; Vlachogiannakos 2013; Walker 2020) (Characteristics of included studies; Figure 1).
We excluded 89 studies and have provided individual reasons for exclusion alongside details of their characteristics (Characteristics of excluded studies).
We identified 13 ongoing studies and have provided details of their characteristics (Characteristics of ongoing studies). There are no studies awaiting classification.
Results of the search
We identified 2019 potentially relevant references in the electronic database searches (Figure 1). Another 159 references were identified through manual searches from other sources. After excluding duplicates, 1447 records remained, which we assessed for eligibility. Of these, we excluded 1173 records that were reviews, cost‐effectiveness analyses, terminated or withdrawn trials, trials for cirrhosis but not evaluating rifaximin, and trials of rifaximin for indications other than cirrhosis. We identified 54 studies reported in 121 full‐text records which we included in our qualitative synthesis, and 41 randomised clinical trials reported in 108 full‐text references that fulfilled our inclusion criteria for our quantitative synthesis (meta‐analysis) (Ahmed 2018; Ali 2014; Babar 2017; Bajaj 2011; Bajaj 2019; Bass 2004; Bass 2010; Bucci 1993; Bureau 2021; Butt 2018; Fera 1993; Festi 1993; Gill 2014; Habib 2016; Hasan 2018; Higuera‐de‐la‐Tijera 2018; Kimer 2017; Loguercio 2003; Maharshi 2015; Majeed 2018; Manzhalii 2022; Mas 2003; Massa 1993; Moneim 2021; Muhammad 2016; Nawaz 2015; Paik 2005; Patel 2022; Pawar 2019; Poudyal 2019; Riggio 2005; Sharma 2013; Sharma 2014; Sidhu 2011; Sidhu 2016; Suzuki 2018; Tan 2022; Uthman 2020; Vyas 2017; Wahib 2014; Zeng 2021) (Characteristics of included studies).
We were able to retrieve additional information regarding trial design and outcome measures for two randomised clinical trials (Bass 2004; Bass 2010) from the FDA website (www.fda.gov), for six trials from Alfa Wassermann (Bucci 1993; Fera 1993; Festi 1993; Loguercio 2003; Mas 2003; Massa 1993), and for one trial from Salix Pharmaceuticals (Bajaj 2011). Information on randomisation methods and outcomes was also received from the authors of nine trials (Bajaj 2011; Gill 2014; Higuera‐de‐la‐Tijera 2018; Nawaz 2015; Patel 2022; Poudyal 2019; Riggio 2005; Sidhu 2011; Sidhu 2016). Additionally, we had access to individual participant data from seven trials (Bucci 1993; Fera 1993; Festi 1993; Kimer 2017; Loguercio 2003; Mas 2003; Massa 1993), including six‐month follow‐up data in one (Kimer 2017).
The countries where trials were undertaken included: Bangladesh (Hasan 2018), China (Tan 2022), Denmark (Kimer 2017), Egypt (Moneim 2021; Wahib 2014), France (Bureau 2021), India (Ali 2014; Maharshi 2015; Pawar 2019; Sharma 2013; Sharma 2014; Sidhu 2011; Sidhu 2016; Uthman 2020; Vyas 2017), Italy (Bucci 1993; Fera 1993; Festi 1993; Loguercio 2003; Massa 1993; Riggio 2005), Japan (Suzuki 2018), Mexico (Higuera‐de‐la‐Tijera 2018), Nepal (Poudyal 2019), Pakistan (Ahmed 2018; Babar 2017; Butt 2018; Gill 2014; Habib 2016; Majeed 2018; Muhammad 2016; Nawaz 2015), South Korea (Paik 2005), Spain (Mas 2003), Ukraine (Manzhalii 2022) the UK (Patel 2022), and the USA (Bajaj 2011). Some trials were conducted in several countries; namely, in Poland, Hungary, the USA, and the UK (Bass 2004); and Canada, Russia, and the USA (Bass 2010). The country of origin was not stated for one trial (Bajaj 2019).
Included studies
Participants
The 41 randomised clinical trials included 4545 participants with cirrhosis. The mean age ranged from 39 years to 65 years, and the proportion of men ranged from 37% to 91%. The proportion of participants with chronic hepatitis B/hepatitis C (HBV/HCV)‐related cirrhosis ranged from 10% to 100%, while the proportion with alcohol‐related cirrhosis ranged from 0% to 89%.
Twenty‐eight trials evaluated participants with hepatic encephalopathy (Ahmed 2018; Bajaj 2011; Bass 2004; Bucci 1993; Butt 2018; Fera 1993; Festi 1993; Gill 2014; Habib 2016; Hasan 2018; Kimer 2017; Loguercio 2003; Manzhalii 2022; Mas 2003; Massa 1993; Paik 2005; Patel 2022; Pawar 2019; Poudyal 2019; Sharma 2013; Sharma 2014; Sidhu 2011; Sidhu 2016; Suzuki 2018; Tan 2022; Uthman 2020; Vyas 2017; Wahib 2014). Three evaluated primary prevention (Higuera‐de‐la‐Tijera 2018; Maharshi 2015; Riggio 2005); eight trials evaluated secondary prevention (Ali 2014; Babar 2017; Bajaj 2019; Bass 2010; Majeed 2018; Moneim 2021; Muhammad 2016; Nawaz 2015), while two evaluated both primary and secondary prevention (Bureau 2021; Zeng 2021). Investigators classified the type of hepatic encephalopathy as acute (n = 13) (Ahmed 2018; Butt 2018; Gill 2014; Habib 2016; Hasan 2018; Mas 2003; Paik 2005; Poudyal 2019; Sharma 2013; Suzuki 2018; Uthman 2020; Vyas 2017; Wahib 2014), chronic (n = 7) (Bass 2004; Bucci 1993; Fera 1993; Festi 1993; Loguercio 2003; Massa 1993; Patel 2022), or minimal (n = 8) (Bajaj 2011; Kimer 2017; Manzhalii 2022; Pawar 2019; Sharma 2014; Sidhu 2011; Sidhu 2016; Tan 2022). Participants in the prevention trials were considered to be at risk for developing an episode of acute hepatic encephalopathy (n = 13) (Ali 2014; Babar 2017; Bajaj 2019; Bass 2010; Bureau 2021; Higuera‐de‐la‐Tijera 2018; Maharshi 2015; Majeed 2018; Moneim 2021; Muhammad 2016; Nawaz 2015; Riggio 2005; Zeng 2021) (Table 4).
1. Summary of included randomised clinical trials.
| Study | Intervention trial | Prevention triala | Type of hepatic encephalopathy | Comparison | Co‐administration of non‐absorbable disaccharide |
| Ahmed 2018 | ✓ | X | Acute episode | Rifaximin + lactulose vs lactulose | ✓ |
| Ali 2014 | X | ✓ (secondary) |
Rifaximin + lactulose vs lactulose | ✓ | |
| Babar 2017 | X | ✓ (secondary) |
Rifaximin + lactulose vs lactulose | ✓ | |
| Bajaj 2011 | ✓ | X | Minimal | Rifaximin vs placebo | X |
| Bajaj 2019 | X | ✓ (secondary) |
Rifaximin + lactulose vs lactulose | ✓ | |
| Bass 2004 | ✓ | X | Chronic | Rifaximin vs placebo | X |
| Bass 2010 | X | ✓ (secondary) |
Rifaximin + lactulose vs lactulose | ✓ | |
| Bucci 1993 | ✓ | X | Chronic | Rifaximin vs lactulose | X |
| Bureau 2021 | X | ✓ (primary and secondary) |
Rifaximin vs placebo | X | |
| Butt 2018 | ✓ | X | Acute episode | Rifaximin + lactulose vs lactulose | ✓ |
| Fera 1993 | ✓ | X | Chronic | Rifaximin vs placebo | X |
| Festi 1993 | ✓ | X | Chronic | Rifaximin vs lactulose | X |
| Gill 2014 | ✓ | X | Acute episode | Rifaximin + lactulose vs lactulose | ✓ |
| Habib 2016 | ✓ | X | Acute episode | Rifaximin + lactulose vs lactulose | ✓ |
| Hasan 2018 | ✓ | X | Acute episode | Rifaximin + lactulose vs lactulose | ✓ |
| Higuera‐de‐la‐Tijera 2018 | X | ✓ (primaryb) |
Rifaximin vs placebo, rifaximin vs lactulose | X | |
| Kimer 2017 | ✓ | X | Minimal | Rifaximin vs placebo | X |
| Loguercio 2003 | ✓ | X | Chronic | Rifaximin vs lactitol, rifaximin + lactitol vs lactitol | X |
| Maharshi 2015 | X | ✓ (primaryb) |
Rifaximin vs lactulose | X | |
| Majeed 2018 | X | ✓ (secondary) |
Rifaximin + lactulose vs lactulose | ✓ | |
| Manzhalii 2022 | ✓ | X | Minimal | Rifaximin vs lactulose | X |
| Mas 2003 | ✓ | X | Acute episode | Rifaximin vs lactitol | X |
| Massa 1993 | ✓ | X | Chronic | Rifaximin vs lactulose | X |
| Moneim 2021 | X | ✓ (secondary) |
Rifaximin + lactulose vs lactulose | ✓ | |
| Muhammad 2016 | X | ✓ (secondary) |
Rifaximin + lactulose vs lactulose | ✓ | |
| Nawaz 2015 | X | ✓ (secondary) |
Rifaximin + lactulose vs lactulose | ✓ | |
| Paik 2005 | ✓ | X | Acute episode | Rifaximin vs lactulose | X |
| Patel 2022 | ✓ | X | Acute episode or chronic | Rifaximin vs placebo | X |
| Pawar 2019 | ✓ | X | Minimal | Rifaximin vs lactulose, rifaximin vs placebo | X |
| Poudyal 2019 | ✓ | X | Acute episode | Rifaximin + lactulose vs lactulose | ✓ |
| Riggio 2005 | X | ✓ (primary) |
Rifaximin vs lactitol, rifaximin vs placebo | X | |
| Sharma 2013 | ✓ | X | Acute episode | Rifaximin + lactulose vs lactulose | ✓ |
| Sharma 2014 | ✓ | X | Minimal | Rifaximin vs placebo | X |
| Sidhu 2011 | ✓ | X | Minimal | Rifaximin vs placebo | X |
| Sidhu 2016 | ✓ | X | Minimal | Rifaximin vs lactulose | X |
| Suzuki 2018 | ✓ | X | Acute episode | Rifaximin vs lactitol | X |
| Tan 2022 | ✓ | X | Minimal | Rifaximin vs placebo | X |
| Uthman 2020 | ✓ | X | Acute episode | Rifaximin + lactulose vs lactulose | ✓ |
| Vyas 2017 | ✓ | X | Acute episode | Rifaximin + lactulose vs lactulose | ✓ |
| Wahib 2014 | ✓ | X | Acute episode | Rifaximin vs lactulose | X |
| Zeng 2021 | X | ✓ (primary and secondary) |
Rifaximin vs standard care | X |
aParticipants in the prevention trials either had (i) no history of hepatic encephalopathy but were at risk of developing an acute episode following certain procedures e.g. transhepatic intrahepatic portosystemic shunt (TIPS) insertion (primary prophylaxis); (ii) had experienced a previous episode(s) of hepatic encephalopathy but had recovered and exhibited no or only low grade hepatic encephalopathy at baseline (secondary prevention).
bParticipants in two trials were free of hepatic encephalopathy at the time of their admission with an acute variceal bleed, but it is unclear whether they had a previous history of hepatic encephalopathy.
Interventions
The dosage of rifaximin and the duration of treatment varied by type of hepatic encephalopathy. Participants with acute hepatic encephalopathy received a median (range) rifaximin dose of 1200 (1100 to 1200) mg/day for 10 (3 to 30) days; those with chronic hepatic encephalopathy received 1200 (1100 to 1200) mg/day for 15 (14 to 90) days; those with minimal hepatic encephalopathy 1100 (1000 to 1200) mg/day for 8 (4 to 12) weeks; those in the primary prophylaxis trials received 1200 mg/day for 7 (5 to 30) days while those in the secondary prophylaxis trials received 1100 (800 to 1200) mg/day for 6 (3 to 6) months.
The control groups received placebo/no intervention in 13 trials (Bajaj 2011; Bass 2004; Bureau 2021; Fera 1993; Higuera‐de‐la‐Tijera 2018; Kimer 2017; Patel 2022; Pawar 2019; Riggio 2005; Sharma 2014; Sidhu 2011; Tan 2022; Zeng 2021); or a non‐absorbable disaccharide in 14 trials (Bucci 1993; Festi 1993; Higuera‐de‐la‐Tijera 2018; Loguercio 2003; Maharshi 2015; Manzhalii 2022; Mas 2003; Massa 1993; Paik 2005; Pawar 2019; Riggio 2005; Sidhu 2016; Suzuki 2018; Wahib 2014). Twenty‐four trials assessed rifaximin as monotherapy (Bajaj 2011; Bass 2004; Bucci 1993; Bureau 2021; Fera 1993; Festi 1993; Higuera‐de‐la‐Tijera 2018; Kimer 2017; Loguercio 2003; Maharshi 2015; Manzhalii 2022; Mas 2003; Massa 1993; Paik 2005; Patel 2022; Pawar 2019; Riggio 2005; Sharma 2014; Sidhu 2011; Sidhu 2016; Suzuki 2018; Tan 2022; Wahib 2014; Zeng 2021), while 18 trials assessed rifaximin plus lactulose versus lactulose (Ahmed 2018; Ali 2014; Babar 2017; Bajaj 2019; Bass 2010; Butt 2018; Gill 2014; Habib 2016; Hasan 2018; Loguercio 2003; Majeed 2018; Moneim 2021; Muhammad 2016; Nawaz 2015; Poudyal 2019; Sharma 2013; Uthman 2020; Vyas 2017). An additional three trials comparing rifaximin versus placebo/no intervention allowed the use of non‐absorbable disaccharides in both trial arms; non‐absorbable disaccharide usage was reported in 22% (Kimer 2017), 66.7% (Suzuki 2018), and 37% (Patel 2022) of the participants.
Outcomes
Investigators assessed hepatic encephalopathy using a variety of methods (Table 5). Thirty‐one randomised clinical trials used the West Haven Criteria or the Conn Score to assess mental status (Ahmed 2018; Ali 2014; Babar 2017; Bajaj 2019; Bass 2004; Bass 2010; Bucci 1993; Bureau 2021; Butt 2018; Fera 1993; Festi 1993; Gill 2014; Habib 2016; Hasan 2018; Higuera‐de‐la‐Tijera 2018; Loguercio 2003; Maharshi 2015; Majeed 2018; Manzhalii 2022; Mas 2003; Massa 1993; Moneim 2021; Paik 2005; Patel 2022; Poudyal 2019; Riggio 2005; Sharma 2013; Uthman 2020; Vyas 2017; Wahib 2014; Zeng 2021). Several neuropsychiatric tests/systems were employed (Table 6). Nine randomised clinical trials used the Portal Systemic Hepatic Encephalopathy Sum, Index, and/or Ratio (Bass 2004; Bucci 1993; Fera 1993; Festi 1993; Mas 2003; Massa 1993; Suzuki 2018; Uthman 2020; Wahib 2014). Eighteen randomised clinical trials utilised neurocognitive tests either in batteries, such as the Portosystemic Hepatic Encephalopathy Score, or single or combined testing with the A‐Cancellation Test, Critical Flicker Frequency, Block Design Test, Digit Symbol Test, Inhibitory Control Test, Stroop test, Number Connection Test, Picture Completion Test and/or the Trail‐making (Reitan) Test (Bajaj 2011; Bass 2004; Bucci 1993; Bureau 2021; Fera 1993; Festi 1993; Higuera‐de‐la‐Tijera 2018; Kimer 2017; Manzhalii 2022; Mas 2003; Massa 1993; Paik 2005; Patel 2022; Pawar 2019; Sharma 2014; Sidhu 2011; Suzuki 2018; Tan 2022). Data on the performance of the NCT‐A were reported in 14 trials at baseline (Bajaj 2011; Bass 2004; Bucci 1993; Loguercio 2003; Mas 2003; Paik 2005; Patel 2022; Pawar 2019; Riggio 2005; Sharma 2014; Sidhu 2011; Sidhu 2016; Suzuki 2018; Wahib 2014), while 13 trials reported NCT‐A performance at the maximum time of follow‐up (Bajaj 2011; Bass 2004; Bucci 1993; Loguercio 2003; Mas 2003; Paik 2005; Patel 2022; Pawar 2019; Riggio 2005; Sidhu 2011; Sidhu 2016; Suzuki 2018; Wahib 2014); all 13 trials measured NCT‐A performance at baseline and at the maximum period of follow‐up, but the paired data were not extractable for three trials (Bass 2004; Sidhu 2011; Wahib 2014). Seventeen trials measured blood ammonia at baseline (Bajaj 2011; Bucci 1993; Bureau 2021; Fera 1993; Festi 1993; Kimer 2017; Loguercio 2003; Manzhalii 2022; Mas 2003; Massa 1993; Paik 2005; Patel 2022; Pawar 2019; Riggio 2005; Sharma 2013; Suzuki 2018; Wahib 2014); while 18 trials measured blood ammonia at the maximum time of follow‐up (Bajaj 2011; Bass 2010; Bucci 1993; Fera 1993; Festi 1993; Kimer 2017; Loguercio 2003; Manzhalii 2022; Mas 2003; Massa 1993; Paik 2005; Patel 2022; Pawar 2019; Riggio 2005; Suzuki 2018; Vyas 2017; Wahib 2014; Zeng 2021); 15 trials measured paired blood ammonia levels at baseline and at the maximum period of follow‐up (Bajaj 2011; Bucci 1993; Fera 1993; Festi 1993; Kimer 2017; Loguercio 2003; Manzhalii 2022; Mas 2003; Massa 1993; Paik 2005; Patel 2022; Pawar 2019; Riggio 2005; Suzuki 2018; Wahib 2014). However, the paired data were not extractable from two trials (Fera 1993; Massa 1993).
2. Definition of improvement of hepatic encephalopathy.
| Study | Number of participants | Nomenclature of hepatic encephalopathy at inclusion | Previous episodes of overt hepatic encephalopathy | Tools for assessing hepatic encephalopathy | Definition of improvement, maintenance or worsening of hepatic encephalopathy |
| Ahmed 2018 | 120 | Hepatic encephalopathy of any grade | Unknown |
|
Recovery from grade IV or grade III to grade I or below after 3 days of treatment |
| Ali 2014 | 126 | No hepatic encephalopathy at inclusion | Yes, at least 2 episodes of hepatic encephalopathy within the last 6 months |
|
Development of hepatic encephalopathy based on Conn score |
| Babar 2017 | 96 | No hepatic encephalopathy at inclusion | Yes, at least 2 episodes of hepatic encephalopathy within the last 6 months |
|
Development of hepatic encephalopathy based on Conn score |
| Bajaj 2011 | 42 | Minimal hepatic encephalopathy | No |
|
Improvement in mean Z‐score of the total battery of tests |
| Bajaj 2019 | 381 | No hepatic encephalopathy at inclusion | Yes, within the previous 6 months |
|
Conn score of 2 or more |
| Bass 2004 | 96 | Chronic hepatic encephalopathy grade 1 or 2 | Chronic |
|
Response was defined as change in baseline mental grade after at least 10 days of treatment. |
| Bass 2010 | 299 | No hepatic encephalopathy at inclusion | Yes, at least 2 episodes within the last 6 months |
|
Development of overt hepatic encephalopathy |
| Bucci 1993 | 58 | Porto‐systemic encephalopathy | Unknown |
|
Improvement in hepatic encephalopathy by PSE Sum |
| Bureau 2021 | 186 | No hepatic encephalopathy, or below grade 2 (23 (12%)) | 25 (13%) people had a history of overt hepatic encephalopathy |
|
Development of overt hepatic encephalopathy (grade 2 or higher by the West Haven Modified Criteria) |
| Butt 2018 | 130 | Overt hepatic encephalopathy | Unknown |
|
Reversal of hepatic encephalopathy |
| Fera 1993 | 40 | Stage 1 portosystemic encephalopathy and hyperammonaemia | Unknown |
|
Improvement in Conn's grading evaluated by investigator. Improvement in PSE index |
| Festi 1993 | 136 | Grade 1 hepatic encephalopathy | Unknown |
|
Reduction of neurological signs of hepatic encephalopathy (asterixis, Reitan‐test, ammonia levels, EEG) |
| Gill 2014 | 200 | Hepatic encephalopathy | Unknown |
|
Reversal of hepatic encephalopathy based on Conn's grading assessed by investigators |
| Habib 2016 | 122 | Hepatic encephalopathy | Unknown |
|
Reversal of hepatic encephalopathy based on Conn's grading assessed by investigators |
| Hasan 2018 | 91 | Hepatic encephalopathy grades I to IV | Unknown |
|
Any reduction in Conn scale |
| Higuera‐de‐la‐Tijera 2018 | 88 | No hepatic encephalopathy at inclusion | No previous episodes of minimal or overt hepatic encephalopathy |
|
Development of overt hepatic encephalopathy |
| Kimer 2017 | 54 | Minimal hepatic encephalopathy | 11 people had previous episodes of hepatic encephalopathy |
|
Improvement in PHES score and continuous reaction time |
| Loguercio 2003 | 33 | Overt chronic hepatic encephalopathy | Chronic encephalopathy |
|
Improvement in mental state evaluated by investigators using Conn's grading |
| Maharshi 2015 | 120 | No hepatic encephalopathy at inclusion | Unknown |
|
Development of overt hepatic encephalopathy by West Haven Criteria |
| Majeed 2018 | 120 | Hepatic encephalopathy; grade unknown | At least 2 episodes of overt hepatic encephalopathy in the previous 6 months |
|
Breakthrough episodes of hepatic encephalopathy |
| Manzhalii 2022 | 42 | Minimal, or grades 1 to 2 hepatic encephalopathy | At least 2 episodes of overt hepatic encephalopathy in the previous 6 months, with at least 1 episode in the previous 3 months |
|
Improvement in EncephalApp Stroop Test |
| Mas 2003 | 103 | Acute hepatic encephalopathy grade 1 to 3 > 48 hours prior to inclusion | Unknown |
|
Improvement in PSE Index Improvement in hepatic encephalopathy according to Conn's grading. |
| Massa 1993 | 40 | Hepatic encephalopathy | Unknown |
|
Improvement in hepatic encephalopathy, assessed by investigator according to West Haven criteria. |
| Moneim 2021 | 100 | Grade 1 hepatic encephalopathy or less | At least 1 episode of overt hepatic encephalopathy |
|
Increase in Conn score to a score of 2 or more |
| Muhammad 2016 | 98 | Hepatic encephalopathy with a Conn score of 2 or higher | Unknown |
|
A Conn score < 2 after 3 months |
| Nawaz 2015 | 150 | No hepatic encephalopathy at inclusion | At least 2 episodes of acute overt hepatic encephalopathy within the last 6 months | Method of assessment not stated | Acute attack of hepatic encephalopathy, diagnostics methods not stated |
| Paik 2005 | 54 | Hepatic encephalopathy grade 1 to 3 | Episodic |
|
Improvement in hepatic encephalopathy assessed by investigators according to Conn's modifications of Parsons‐Smith classification. |
| Patel 2022 | 38 | Chronic hepatic encephalopathy | Yes, all had either persistent and overt (at least grade 1) or at least 2 episodes of overt encephalopathy in the previous 6 months. 24/38 (63.2%) had overt encephalopathy at baseline. |
|
Recurrence of overt hepatic encephalopathy Normalisation of grade of encephalopathy from grade I to 0 Improvement in PHES |
| Pawar 2019 | 108 | Minimal hepatic encephalopathy | No history of hepatic encephalopathy |
|
Reversal of encephalopathy by PHES score more than ‐5, or ICT lures less than 14 |
| Poudyal 2019 | 132 | Type C acute hepatic encephalopathy of any grade | Unknown |
|
Complete reversal of clinical symptoms on the basis of the West Haven Criteria |
| Riggio 2005 | 75 | No hepatic encephalopathy at inclusion | Yes, overt hepatic encephalopathy in 11 cases |
|
Effect evaluated as development of hepatic encephalopathy based on West Haven criteria, asterixis and trail‐making test. |
| Sharma 2013 | 120 | Overt hepatic encephalopathy | Unknown |
|
Improvement in hepatic encephalopathy evaluated by investigators according to West Haven criteria. |
| Sharma 2014 | 124 | Minimal hepatic encephalopathy | Unknown, but no overt hepatic encephalopathy 6 weeks prior to enrolment |
|
Improvement in critical flocker fusion |
| Sidhu 2011 | 94 | Minimal hepatic encephalopathy | Unknown |
|
Normalisation of the abnormal neuropsychiatric tests |
| Sidhu 2016 | 112 | Minimal hepatic encephalopathy | Not reported |
|
If there was improvement in 2 or more of five neuropsychiatric tests |
| Suzuki 2018 | 172 | Overt hepatic encephalopathy grade 1 to 2 | Unknown |
|
Improvement in PSE Index Improvement in asterixis, ammonia levels, number connection test and EEG Improvement in hepatic encephalopathy |
| Tan 2022 | 40 | Minimal hepatic encephalopathy | Unknown, but no overt hepatic encephalopathy 3 months prior to enrolment |
|
Normalisation of the Psychometric Hepatic Encephalopathy Score or Stroop test time |
| Uthman 2020 | 84 | Overt hepatic encephalopathy grade 1 to 3 | Unknown |
|
Improvement of PSE Index Improvement in West Haven Criteria |
| Vyas 2017 | 73 | Grade III/IV hepatic encephalopathy | Unknown |
|
Improvement of hepatic encephalopathy from grade III/IV to grade II/I/no encephalopathy |
| Wahib 2014 | 50 | Overt hepatic encephalopathy grade 1 to 3 | Unknown |
|
Improvement in grading of hepatic encephalopathy based on West Haven criteria, improvement in blood ammonia levels, and improvement in hepatic encephalopathy index. |
| Zeng 2021 | 195 | Decompensated liver cirrhosis, of which participants could have hepatic encephalopathy | 24 (12.0%) participants had a history of hepatic encephalopathy > 1 month before the screening visit |
|
Development of hepatic encephalopathy adjusted by history of encephalopathy |
CCF: critical flicker fusion; EEG: electroencephalogram; ICT: inhibitory control test; MSE: mini mental state examination; NCT‐A: Number Connection Test‐A; NCT‐B: Number connection Test‐B;
3. Tools used to assess hepatic encephalopathy by the included studies.
| Description | Advantages | Disadvantages | |
| Asterixis Severity Scale (Williams 2000) |
Format: clinical grading score Approximate time required: 1 minute Grades the severity of asterixis Grade 0: no tremor Grade 1: rare flapping motions Grade 2: occasional, irregular flaps Grade 3: frequent flaps Grade 4: almost continuous flapping motions |
|
|
| Blood ammonia |
Format: a blood test, taken either as an arterial or venous sample, to measure the level of ammonia Approximate time required: 5 minutes, although time to results can vary |
|
|
| Clinical Hepatic Encephalopathy Staging Scale (CHESS) (Ortiz 2007) |
Format: a set of 9 questions that the observer must answer. It is graded on a scale from 0 to 9, designed to reduce interobserver variability Approximate time required: 10 minutes Domains tested
|
|
|
| Critical Flicker Frequency (CFF) (Kircheis 2002) |
Format: participants look into a viewing chamber at a flashing light, the frequency of which steadily increases or decreases. The flicker frequency is the frequency at which the continuous‐appearing light begins to flicker as the frequency decreases. Approximate time required: 20 minutes |
|
|
| Electroencephalogram |
Format: a neurophysiological test, providing a record of the brain's electric activity by placing electrodes over the surface of the scalp Approximate time required: 30 minutes People with hepatic encephalopathy may elicit slowing of normal higher frequencies, with bursts of activity in the low‐frequency theta and delta ranges |
|
|
| Inhibitory Control Test (ICT) (Bajaj 2007) |
Format: a computer‐based programme, showing a series of random letters which participants should either respond to (targets), or not respond to (lures). Approximate time required: 20 minutes Domains tested
|
|
|
| Portal‐Systemic Encephalopathy Sum (PSE Sum) and Index (PSE Index) (Conn 1977) | Provides an index of the severity of hepatic encephalopathy by adding scores for the degree of abnormality, expressed on a 0 to 4+ scale, for:
Each component is arbitrarily weighted in proportion to its importance: mental state is weighted by a factor of 3, whilst other variables are assigned a factor of 1. The PSE Sum is the total of the weighted scores; its maximum possible value is 28. The PSE index is the ratio of the estimated PSE Sum to the maximum possible. Approximate time required: dependent on the time taken to obtain the results of the blood ammonia and EEG |
|
|
| Psychometric Hepatic Encephalopathy Score (PHES) (Weissenborn 2001) |
Format: a battery of five pencil‐and‐paper tests Approximate time required: 20 minutes Domains tested
Tests include the number connection tests A and B, digit‐symbol test, line tracing test and serial dotting test. Figure connection tests may be used in illiterate people. Some studies in this review have used specific tests from this battery, but not the entire battery. A normalised 'z‐score' can be calculated, and thresholds for the diagnosis of hepatic encephalopathy vary by country and population. |
|
|
| Reitan Test (Reitan 1955) |
Format: a pencil‐and‐paper trail making test, where participants must connect a series of circles in ascending order Approximate time required: 10 minutes Domains tested
|
|
|
| Stroop Test (EncephalApp) (Bajaj 2013) |
Format: a smartphone‐based test, where participants must respond to the matching colour of a stimulus, presented as either hash signs (###; 'stroop off'), or as a distractor word (for example, 'blue'; 'stroop on'). Approximate time required: 5 to 10 minutes Domains tested
|
|
|
| Wechsler Adult Intelligence Scale (WAIS) (Lawton 1939) |
Format: an IQ test, of varying iterations, formed of multiple tests, split into verbal IQ and performance IQ:
Approximate time required: 60 to 85 minutes Domains tested
|
|
|
| West‐Haven Criteria/Conn Score (Conn 1977) |
Format: clinical grading score Approximate time required: < 5 minutes Grades the severity of hepatic encephalopathy into 4 main categories Minimal: abnormal results on psychometric or neurophysiological testing without clinical manifestations Grade I: changes in behaviour, mild confusion, slurred speech, impaired sleep, shortened attention span Grade II: lethargy, moderate confusion, apathy, subtle personality change and inappropriate behaviour Grade III: marked confusion, incoherent speech, somnolence to semi‐stupor but remains responsive to verbal stimuli, gross disorientation Grade IV: coma |
|
|
IQ: intelligence quotient
Vested interests funding
Seventeen randomised clinical trials did not receive funding, or else reported no involvement with for‐profit organisations (Ahmed 2018; Babar 2017; Bajaj 2019; Butt 2018; Maharshi 2015; Manzhalii 2022; Moneim 2021; Nawaz 2015; Pawar 2019; Poudyal 2019; Riggio 2005; Sharma 2013; Sharma 2014; Tan 2022; Uthman 2020; Vyas 2017; Zeng 2021). We classed eight trials as having an unclear risk of vested interests bias, including one trial that did not report direct funding, although the trial drugs may have been supplied by pharmaceutical companies (Sidhu 2016); one study which was funded by a grant from the French Public Health Ministry, but the corresponding author had received personal fees from relevant pharmaceutical companies (Bureau 2021); six randomised clinical trials that did not report disclosures or for‐profit funding (Gill 2014; Habib 2016; Hasan 2018; Majeed 2018; Muhammad 2016; Wahib 2014). Sixteen trials received support from for‐profit or pharmaceutical organisations in the form of financial support, assisted trial design, conduct, and the supply of interventional drugs; we classified these as being at high risk of vested interests bias (Ali 2014; Bajaj 2011; Bass 2004; Bass 2010; Bucci 1993; Fera 1993; Festi 1993; Higuera‐de‐la‐Tijera 2018; Kimer 2017; Loguercio 2003; Mas 2003; Massa 1993; Paik 2005; Patel 2022; Sidhu 2011; Suzuki 2018).
Excluded studies
We excluded 89 clinical trials. Of these, 75 were published as full‐text articles or as abstracts (Abd‐Elsalam 2016; Ahire 2017; Ahluwalia 2014; Anon 2014a; Anon 2014b; Bajaj 2013; Bajaj 2016b; Bajaj 2020a; Bajaj 2020b; Block 2010; Bohra 2020; Chang 2021; Cobbold 2018; Crisafulli 2016; Danulescu 2013; De Marco 1984; Deshmukh 2016; Diana‐Maria 2019; Di Piazza 1991; Dupont 2016; Frenette 2020a; Frenette 2020b; Gangarapu 2015; Giacomo 1993; Gupta 2021; Habib 2020; Hammond 2017; Hotten 2003; Huang 2018; Jain 2022; Jiménez 2022; John 2018; Jones 2020; Kaji 2017; Kalambokis 2012a; Kalambokis 2012b; Kalambokis 2012c; Kalambokis 2012d; Kang 2017; Kawaratani 2022; Khokhar 2015; Kimer 2018; Kimer 2022; Kubota 2022; Lauridsen 2018; Lighthouse 2004; Miglio 1997; Mohamed 2018; Mostafa 2015; Mullen 2014; Oey 2019; Orr 2016; Parini 1992; Pedretti 1991; Ponziani 2016; Pose 2020; Praharaj 2022; Saboo 2021; Salehi 2019; Sama 2004; Sarwar 2019; Schulz 2019; Seifert 2021; Song 2021; Suzuki 2019; Tatsumi 2021; Testa 1985; Uchida 2020; Venturini 2005; Vittitow 2018; Vlachogiannakos 2013; Walker 2020; Williams 2000; Yang 2016; Zeng 2015), while 14 were identified in trial registries (CTRI/2019/05/018966; EUCTR2014‐001856‐51‐DK; NCT00364689; NCT00748904; NCT01676597; NCT01846806; NCT01897051; NCT01951209; NCT02011841; NCT02485106; NCT03712280; NCT04159870; UMIN000036998; UMIN000038487).
We excluded studies for a variety of reasons; in many instances, we identified more than one reason.
Amongst the 75 published trials, we excluded eight because the comparator was another antibiotic that has been used to treat hepatic encephalopathy, including paromomycin, neomycin, and metronidazole (Abd‐Elsalam 2016; De Marco 1984; Di Piazza 1991; Miglio 1997; Mohamed 2018; Parini 1992; Pedretti 1991; Testa 1985). These trials will be included in a separate review (Jeyaraj 2017).
We excluded 10 trials because rifaximin was included in both trial arms. Five trials assessed the effects of lactulose and rifaximin versus rifaximin alone on hepatic encephalopathy, serum inflammatory markers, microbial cross resistance to antibiotics, or microbial diversity and composition (Bajaj 2020a; Diana‐Maria 2019; Frenette 2020a; Frenette 2020b; Schulz 2019); one trial assessed the effects of L‐ornithine L‐aspartate, lactulose, and rifaximin on hepatic encephalopathy compared with placebo, lactulose, and rifaximin (Jain 2022); one trial assessed the effects of bovine immunoglobulin and rifaximin versus rifaximin monotherapy on hepatic encephalopathy (John 2018); one trial assessed the effects of symbiotics and rifaximin versus rifaximin alone on hepatic encephalopathy (Lighthouse 2004); and one trial assessed L‐carnitine and rifaximin against rifaximin on hepatic encephalopathy (Kubota 2022). One additional trial compared rifaximin in combination with two other potentially active drugs against placebo (Lauridsen 2018).
Five dose‐finding randomised trials assessed rifaximin without a control group (Crisafulli 2016; Habib 2020; Khokhar 2015; Sarwar 2019; Williams 2000). One trial assessed the effects of a new formulation of rifaximin with limited extractable information on all formulations and with no clear information on the proportion of participants with hepatic encephalopathy at baseline or how it was assessed (Bajaj 2016b).
We excluded 13 trials because they assessed the effect of rifaximin on outcomes other than hepatic encephalopathy and did not provide information on any of our primary or secondary outcomes. These included the assessment of intestinal bacterial composition and biomarkers (Dupont 2016; Hotten 2003; Huang 2018; Saboo 2021; Schulz 2019), circulating endotoxins, benzodiazepine‐like compounds, and thrombocytopaenia (Kalambokis 2012b; Kimer 2018, Venturini 2005; Zeng 2015), and the effect of combined use of simvastatin and rifaximin on liver and muscle toxicity (Pose 2020). Others investigated the role of rifaximin in the management of complications of cirrhosis other than hepatic encephalopathy, such as spontaneous bacterial peritonitis (Gupta 2021; Mostafa 2015; Praharaj 2022).
The participants in five trials had liver disease other than cirrhosis, including non‐alcoholic fatty liver disease, non‐alcoholic steatohepatitis (Cobbold 2018; Gangarapu 2015), and severe alcoholic hepatitis (Jiménez 2022; Kimer 2022; Song 2021). One pharmacokinetic study was undertaken in healthy volunteers (Vittitow 2018).
We excluded a total of 26 studies because they were either not randomised or were not controlled, including: two case‐control studies (Danulescu 2013; Vlachogiannakos 2013), 17 prospective cohort studies (Ahire 2017; Ahluwalia 2014; Bajaj 2013; Bohra 2020; Chang 2021; Kaji 2017; Kalambokis 2012a; Kalambokis 2012c; Kalambokis 2012d; Mullen 2014; Oey 2019; Orr 2016; Ponziani 2016; Sama 2004; Tatsumi 2021; Uchida 2020; Walker 2020), and seven retrospective cohort studies (Hammond 2017; Jones 2020; Kang 2017; Kawaratani 2022; Salehi 2019; Seifert 2021; Suzuki 2019).
In three trials, there was insufficient information regarding the trial and outcome data (Bajaj 2020b; Deshmukh 2016; Giacomo 1993), and three reports could not be retrieved (Anon 2014a; Anon 2014b; Block 2010).
Of the 14 studies identified in the trial registries, we excluded nine that had been terminated or else had been inactive for at least five years without posted results (NCT00364689; NCT00748904; NCT01676597; NCT01846806; NCT01897051; NCT01951209; NCT02011841; NCT02485106; UMIN000036998). We also excluded a randomised, double‐blind trial in acute hepatic encephalopathy because rifaximin will be used in both trial arms (CTRI/2019/05/018966); a randomised double‐blind, placebo‐controlled trial of the effect of rifaximin on hepatic fibrosis because the majority of participants will have pre‐cirrhotic liver injury (EUCTR2014‐001856‐51‐DK); a randomised, open‐label trial comparing rifaximin and norfloxacin for the primary prophylaxis of spontaneous bacterial peritonitis (NCT04159870); a randomised, open‐label trial to assess the pharmacodynamics, safety, and pharmacokinetics of ornithine phenylacetate versus rifaximin as the comparator was not one stipulated for this review (NCT03712280), and a randomised, open‐label trial comparing the effects of rifaximin and standard treatment for the prevention of hepatorenal syndrome in which neuropsychiatric status will not be assessed (UMIN000038487).
Thirteen of these otherwise excluded studies contained information on adverse events, which we used in an additional analysis of harms (Bohra 2020; Chang 2021; Jones 2020; Kang 2017; Mullen 2014; Oey 2019; Orr 2016; Salehi 2019; Suzuki 2019; Tatsumi 2021; Uchida 2020; Vlachogiannakos 2013; Walker 2020) (Table 7).
4. Harms observed in observational studies, retrieved with the searches for randomised trials.
| Outcome | Study | Participants n (%) |
| Ascites/oedema | Chang 2021 | Rifaximin + lactulose vs lactulose alone: 58.3% vs 67.7% (P = 0.307) |
| Mullen 2014 | Rifaximin: 147/392 (37.5%) | |
| Salehi 2019 | Rifaximin vs no rifaximin: reduced (P = 0.024) | |
| Infection and spontaneous bacterial peritonitis | Kang 2017 | Rifaximin + lactulose: 32/318 (10.1%) Lactulose: 323/726 (44.5%) |
| Mullen 2014 | Rifaximin: 22/392 (5.6%) | |
| Oey 2019 | Pre‐ vs post‐rifaximin: 25.5% vs 22.3% | |
| Salehi 2019 | Rifaximin vs no rifaximin: reduced (P = 0.016) | |
| Vlachogiannakos 2013 | Rifaximin: 1/23 (4.3%) Control: 10/46 (21.7%) |
|
| Variceal/gastrointestinal bleed | Chang 2021 | Rifaximin + lactulose vs lactulose alone: 50% vs 100% (P = 0.313) |
| Kang 2017 | Rifaximin + lactulose: 30/318 (9.4%) Lactulose: 139/726 (19.2%) |
|
| Mullen 2014 | Rifaximin: 58/392 (14.8%) | |
| Salehi 2019 | Rifaximin vs no rifaximin: reduced (P = 0.024) | |
| Suzuki 2019 | Rifaximin: 3/65 (4.6%) | |
| Vlachogiannakos 2013 | Rifaximin: 5/23 (21.7%) Control: 21/46 (45.7%) |
|
| Hepatorenal syndrome | Kang 2017 | Rifaximin + lactulose: 25/318 (7.9%) Lactulose: 97/726 (13.4%) |
| Mullen 2014 | Rifaximin: 74/392 (18.9%) | |
| Vlachogiannakos 2013 | Rifaximin: 9/23 (39.1%) Control: 8/46 (17.4%) |
|
| Electrolyte imbalance | Mullen 2014 | Rifaximin: 52/392 (13.3%) |
| Coagulopathy/thrombocytopenia | Mullen 2014 | Rifaximin: 33/392 (8.4%) |
| Clostridium difficile infection | Kang 2017 | Rifaximin + lactulose: 1/318 (0.3%) Lactulose: 7/726 (1%) |
| Mullen 2014 | Rifaximin: 6/392 (1.5%) | |
| Oey 2019 | Rifaximin: 0/127 (0%) | |
| Orr 2016 | Rifaximin: 0/326 (0%) | |
| Uchida 2020 | Rifaximin: 0/95 (0%) | |
| Nausea | Oey 2019 | Rifaximin: 1/127 (0.8%) |
| Orr 2016 | Rifaximin: 2/326 (0.6%) | |
| Suzuki 2019 | Rifaximin: 3/65 (4.6%) | |
| Vlachogiannakos 2013 | Rifaximin: 2/23 (8.7%) | |
| Diarrhoea/enteritis | Suzuki 2019 | Rifaximin: 6/65 (9.2%) |
| Tatsumi 2021 | Rifaximin: 3/37 (8.0%) | |
| Vlachogiannakos 2013 | Rifaximin: 1/23 (4.4%) | |
| Rash | Oey 2019 | Rifaximin: 1/127 (0.8%) |
| Vlachogiannakos 2013 | Rifaximin: 1/23 (4.4%) |
Individual excluded studies with their corresponding reason for exclusion can be found in the Characteristics of excluded studies table.
Ongoing studies
Our search identified 13 planned/ongoing clinical trials which have the potential to provide additional information on the efficacy and safety of rifaximin for the prevention and treatment of hepatic encephalopathy in our populations of interest. Of these, one trial may provide information on the efficacy of rifaximin versus placebo in minimal hepatic encephalopathy (NCT02439307), and one the efficacy of rifaximin versus lactulose in acute hepatic encephalopathy (TCTR20180509001). One trial will provide information on rifaximin versus placebo for primary prevention of hepatic encephalopathy in people with no previous history of hepatic encephalopathy (NCT05071716 (RNLC3131)), while two trials will look at primary prevention of hepatic encephalopathy following insertion of a transjugular intrahepatic shunt, one comparing rifaximin plus lactulose and a vegetable protein diet versus no specific intervention (NCT02931123 (Riggio 2016)), and one comparing rifaximin plus lactulose versus lactulose alone (NCT04073290 (PEARL Study)). One trial will provide information on secondary prophylaxis of hepatic encephalopathy, comparing rifaximin versus placebo (NCT01846663).
A further seven trials will look at the effects of rifaximin on other complications of cirrhosis but will also collect information on hepatic encephalopathy. Of these, three trials will investigate the effects of rifaximin versus placebo on portal haemodynamics, portal hypertension, or variceal bleeding (ChiCTR1800018070; EUCTR2014‐000102‐35‐IT; NCT02508623); two will investigate the primary prophylaxis of spontaneous bacterial peritonitis comparing rifaximin with placebo or standard care (NCT03069131; NCT04775329), and one will assess the effects of rifaximin versus placebo on infection (EUDRACT2016‐002628‐96).
Finally, one trial looking at the mechanistic effects of rifaximin versus placebo/no treatment on lipopolysaccharides, thrombin generation, platelets, endotoxin and inflammatory markers, and faecal flora may generate clinically relevant data (EUCTR2017‐000488‐34‐IT).
Details of the ongoing studies can be found in the Characteristics of ongoing studies table.
Risk of bias in included studies
The assessment of bias included information retrieved from published trial reports, reports published by the Food and Drug Administration (www.fda.gov), and through correspondence with trial authors and with the pharmaceutical companies Salix Pharmaceuticals Inc. and Alfa Wassermann (Figure 2 and Figure 3).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
We classed 35 trials as at low risk for allocation sequence bias (Ahmed 2018; Ali 2014; Babar 2017; Bajaj 2011; Bass 2004; Bass 2010; Bucci 1993; Bureau 2021; Butt 2018; Fera 1993; Festi 1993; Gill 2014; Habib 2016; Hasan 2018; Higuera‐de‐la‐Tijera 2018; Kimer 2017; Loguercio 2003; Maharshi 2015; Manzhalii 2022; Mas 2003; Massa 1993; Moneim 2021; Muhammad 2016; Nawaz 2015; Paik 2005; Patel 2022; Pawar 2019; Riggio 2005; Sharma 2013; Sharma 2014; Sidhu 2011; Sidhu 2016; Suzuki 2018; Tan 2022; Zeng 2021); five trials were at unclear risk for allocation sequence bias (Bajaj 2019; Majeed 2018; Poudyal 2019; Uthman 2020; Vyas 2017), while one was at high risk (Wahib 2014).
We classified 21 trials as at low risk for allocation concealment bias (Ali 2014; Bajaj 2011; Bass 2010; Bucci 1993; Bureau 2021; Fera 1993; Hasan 2018; Higuera‐de‐la‐Tijera 2018; Kimer 2017; Loguercio 2003; Mas 2003; Massa 1993; Moneim 2021; Nawaz 2015; Patel 2022; Pawar 2019; Riggio 2005; Sharma 2013; Sharma 2014; Sidhu 2011; Sidhu 2016); two trials were at high risk of allocation concealment bias (Vyas 2017; Wahib 2014), while the risk was unclear in the remaining 18 (Ahmed 2018; Babar 2017; Bajaj 2019; Bass 2004; Butt 2018; Festi 1993; Gill 2014; Habib 2016; Maharshi 2015; Majeed 2018; Manzhalii 2022; Muhammad 2016; Paik 2005; Poudyal 2019; Suzuki 2018; Tan 2022; Uthman 2020; Zeng 2021).
Blinding
Seventeen randomised clinical trials were double‐blind, with blinding of both participants and study personnel and outcome evaluators; hence we classified these as at low risk of performance and detection bias (Ali 2014; Bajaj 2011; Bass 2010; Bucci 1993; Bureau 2021; Fera 1993; Gill 2014; Hasan 2018; Higuera‐de‐la‐Tijera 2018; Kimer 2017; Loguercio 2003; Mas 2003; Massa 1993; Nawaz 2015; Patel 2022; Sharma 2013; Sidhu 2011). We classified 17 trials as at high risk for both performance and detection bias as they were open‐label without blinding of participants or investigators, or else they omitted to report blinding procedures, or the blinding was inadequate (Ahmed 2018; Bajaj 2019; Festi 1993; Habib 2016; Maharshi 2015; Majeed 2018; Manzhalii 2022; Moneim 2021; Muhammad 2016; Paik 2005; Poudyal 2019; Riggio 2005; Sharma 2014; Tan 2022; Vyas 2017; Wahib 2014; Zeng 2021). We classified one open‐label trial as high‐risk for performance bias but low‐risk for detection bias as the evaluators were blinded (Suzuki 2018). Six trials had an unclear risk of performance bias, detection bias, or both, including: four that were placebo‐controlled and conducted double‐blind, but the methods for blinding and outcome assessment were not clearly stipulated (Babar 2017; Bass 2004; Pawar 2019; Uthman 2020); one study classified as unblinded in the published paper but which the investigators allege was blinded (Sidhu 2016); and one trial which was conducted single blinded, but no further details were provided (Butt 2018).
Incomplete outcome data
We classed 27 randomised clinical trials as at low risk of attrition bias (Ahmed 2018; Ali 2014; Bajaj 2011; Bass 2010; Bucci 1993; Bureau 2021; Fera 1993; Higuera‐de‐la‐Tijera 2018; Kimer 2017; Maharshi 2015; Manzhalii 2022; Mas 2003; Massa 1993; Moneim 2021; Paik 2005; Pawar 2019; Poudyal 2019; Riggio 2005; Sharma 2013; Sharma 2014; Sidhu 2011; Sidhu 2016; Suzuki 2018; Tan 2022; Uthman 2020; Wahib 2014; Zeng 2021). We considered eight trials to have a high risk of attrition bias because they did not account for participants with missing outcomes; excluded participants from the analysis; did not account for withdrawn participants; or because there was a discrepancy between reported participant numbers and participant outcomes (Bass 2004; Festi 1993; Gill 2014; Habib 2016; Hasan 2018; Loguercio 2003; Nawaz 2015; Patel 2022). Six trials had an unclear risk of attrition bias because they reported no data on missing participants, attrition, adverse events, or mortality, or because of unclear timing of outcome assessment (Babar 2017; Bajaj 2019; Butt 2018; Majeed 2018; Muhammad 2016; Vyas 2017).
Selective reporting
Twenty‐six randomised clinical trials reported predefined outcomes (mortality, hepatic encephalopathy, or both). We classified these trials as being at low risk of selective reporting (Ahmed 2018; Babar 2017; Bajaj 2011; Bass 2010; Bucci 1993; Bureau 2021; Fera 1993; Festi 1993; Higuera‐de‐la‐Tijera 2018; Kimer 2017; Maharshi 2015; Mas 2003; Massa 1993; Moneim 2021; Paik 2005; Patel 2022; Pawar 2019; Poudyal 2019; Riggio 2005; Sharma 2013; Sharma 2014; Sidhu 2011; Sidhu 2016; Suzuki 2018; Tan 2022; Zeng 2021).
We classified 13 trials as at high risk of reporting bias. Of these, four trials were published as conference abstracts with only a few outcomes or incomplete outcomes reported (Bass 2004; Gill 2014; Nawaz 2015; Vyas 2017), even though some additional information on hepatic encephalopathy and mortality were retrieved for three of these publications from www.fda.gov, the corresponding authors, and a sponsoring pharmaceutical company, Alfa Wassermann (Bass 2004; Gill 2014; Nawaz 2015). Five trials did not report mortality data, adverse events, or both (Butt 2018; Habib 2016; Majeed 2018; Muhammad 2016; Wahib 2014). One trial did not provide data on remission from hepatic encephalopathy in an extractable form, and although some additional data were provided from the sponsoring pharmaceutical company, these too were incomplete (Loguercio 2003). One trial reported data that differed between participant numbers and reported outcomes (Hasan 2018). One trial provided no information on hepatic encephalopathy except for the number in whom it resolved completely, and it was not registered (Uthman 2020). One trial did not report on changes in mental status in the 60% of participants presenting with grade I‐II hepatic encephalopathy (Manzhalii 2022).
Two trials had an unclear risk of reporting bias: one trial provided textural information on the participants in whom hepatic encephalopathy resolved completely but the table with details of the participants in whom it did not resolve was missing from the publication, as was the table of adverse events (Ali 2014); one study reported the combined results of two randomised trials but protocols/trial registrations were not available for either (Bajaj 2019).
Other potential sources of bias
We found no other potential sources of bias for 37 randomised clinical trials, and therefore classed them as having a low risk of bias for this domain (Ahmed 2018; Ali 2014; Babar 2017; Bajaj 2011; Bajaj 2019; Bass 2010; Bucci 1993; Bureau 2021; Butt 2018; Fera 1993; Festi 1993; Gill 2014; Habib 2016; Hasan 2018; Higuera‐de‐la‐Tijera 2018; Kimer 2017; Loguercio 2003; Maharshi 2015; Majeed 2018; Mas 2003; Massa 1993; Moneim 2021; Nawaz 2015; Paik 2005; Patel 2022; Pawar 2019; Poudyal 2019; Riggio 2005; Sharma 2014; Sidhu 2011; Sidhu 2016; Suzuki 2018; Tan 2022; Uthman 2020; Vyas 2017; Wahib 2014; Zeng 2021). Of the remainder, we classed two as having a high risk of other bias; one trial did not fulfil its pre‐estimated sample size (Bass 2004), and one showed possible participant selection bias (Muhammad 2016). Two further trials had an unclear risk of other bias: in one, there was a discrepancy in the number of participants recovering from hepatic encephalopathy in two published reports (Sharma 2013), and one trial submitted its registry entry after trial completion (Manzhalii 2022).
Funnel plots for primary meta‐analyses with 10 or more trials are shown in Figure 4, Figure 5, Figure 6, Figure 7, and Figure 8. All plots are visually symmetrical except for one outlier in Figure 5 (Patel 2022) and one in Figure 6 (Wahib 2014); this may indicate a low risk of publication bias overall.
4.

Funnel plot for studies assessing the effects of rifaximin versus placebo or no intervention on mortality outcomes
5.

Funnel plot for studies assessing the effects of rifaximin versus placebo or no intervention on hepatic encephalopathy outcomes
6.

Funnel plot for studies assessing the effects of rifaximin versus non‐absorbable disaccharide on hepatic encephalopathy outcomes
7.

Funnel plot for studies assessing the effects of rifaximin plus non‐absorbable disaccharide versus non‐absorbable disaccharide alone on mortality outcomes
8.

Funnel plot for studies assessing the effects of rifaximin plus non‐absorbable disaccharide versus non‐absorbable disaccharide alone on hepatic encephalopathy outcomes
Overall bias risk
We classed 25 randomised clinical trials as being at low risk of bias in the overall assessment of mortality (Ahmed 2018; Babar 2017; Bajaj 2011; Bass 2010; Bucci 1993; Bureau 2021; Fera 1993; Higuera‐de‐la‐Tijera 2018; Kimer 2017; Maharshi 2015; Mas 2003; Massa 1993; Moneim 2021; Paik 2005; Pawar 2019; Poudyal 2019; Riggio 2005; Sharma 2013; Sharma 2014; Sidhu 2011; Sidhu 2016; Suzuki 2018; Tan 2022; Wahib 2014; Zeng 2021), while a further 11 trials were at high risk of bias (Bass 2004; Butt 2018; Festi 1993; Gill 2014; Habib 2016; Hasan 2018; Loguercio 2003; Muhammad 2016; Nawaz 2015; Patel 2022; Uthman 2020), and five had an unclear risk (Ali 2014; Bajaj 2019; Majeed 2018; Manzhalii 2022; Vyas 2017).
We classed 12 randomised clinical trials as having a low risk of bias in the overall assessment of non‐mortality outcomes (Bajaj 2011; Bass 2010; Bucci 1993; Bureau 2021; Fera 1993; Higuera‐de‐la‐Tijera 2018; Kimer 2017; Mas 2003; Massa 1993; Pawar 2019; Sharma 2013 ; Sidhu 2011). A further 28 were at high risk of bias (Ahmed 2018; Ali 2014; Bajaj 2019; Bass 2004; Butt 2018; Festi 1993; Gill 2014; Habib 2016; Hasan 2018; Loguercio 2003; Maharshi 2015; Majeed 2018; Manzhalii 2022; Moneim 2021; Muhammad 2016; Nawaz 2015; Paik 2005; Patel 2022; Poudyal 2019; Riggio 2005; Sharma 2014; Sidhu 2016; Suzuki 2018; Tan 2022; Uthman 2020; Vyas 2017; Wahib 2014; Zeng 2021), and one had an unclear risk (Babar 2017).
Effects of interventions
See: Table 1; Table 2; Table 3
A total of 41 randomised clinical trials were included in the analyses; some included more than one comparison. Thirteen randomised clinical trials compared rifaximin with placebo or no intervention (Bajaj 2011; Bass 2004; Bureau 2021; Fera 1993; Higuera‐de‐la‐Tijera 2018; Kimer 2017; Patel 2022; Pawar 2019; Riggio 2005; Sharma 2014; Sidhu 2011; Tan 2022; Zeng 2021). Fourteen trials compared rifaximin to non‐absorbable disaccharides (Bucci 1993; Festi 1993; Higuera‐de‐la‐Tijera 2018; Loguercio 2003; Maharshi 2015; Manzhalii 2022; Mas 2003; Massa 1993; Paik 2005; Pawar 2019; Riggio 2005; Sidhu 2016; Suzuki 2018; Wahib 2014). Eighteen trials assessed rifaximin plus lactulose versus lactulose alone (Ahmed 2018; Ali 2014; Babar 2017; Bajaj 2019; Bass 2010; Butt 2018; Gill 2014; Habib 2016; Hasan 2018; Loguercio 2003; Majeed 2018; Moneim 2021; Muhammad 2016; Nawaz 2015; Poudyal 2019; Sharma 2013; Uthman 2020; Vyas 2017).
Thus, we assessed the effects of rifaximin versus placebo or no intervention (Table 1); the effects of rifaximin versus non‐absorbable disaccharides (Table 2); and the effects of rifaximin co‐administered with lactulose versus lactulose alone (Table 3). Each comparison is reported separately. We have also provided a summary of the primary and sensitivity analyses for the four primary outcomes for all three of the comparative regimens (Table 8).
5. Summary of the results of the primary and sensitivity analyses for the four primary outcomes in the three sets of comparator studies.
| Rifaximin versus placebo/no intervention | |||||
| Variable | Random‐effects meta‐analysis | Fixed‐effects meta‐analysis | Sensitivity (low bias trials) | Sensitivity (no vested interest trials) | Worse/best & extreme worse/extreme best ‐ case scenarios |
| Mortality | No effect | No effect | No effect | No effect | No effect |
| Serious adverse events | No effect | No effect | No effect | No effect | No effect |
| HRQoL | Beneficial effect overall and in minimal HE | Beneficial effect overall and in minimal HE | Beneficial effect overall and in minimal HE | n/a | n/a |
| Hepatic encephalopathy | Beneficial effect overall and in minimal HE | Beneficial effect overall and in minimal HE and the prevention trials | Beneficial effect overall and in minimal HE and the prevention trials | n/a | All four analyses: beneficial effect overall and in minimal HE Extreme worse case: possible detrimental effect in chronic HE |
| Rifaximin versus non‐absorbable disaccharides | |||||
| Mortality | No effect | No effect | No effect | No effect | No effect |
| Serious adverse events | No effect | No effect | No effect | n/a | No effect |
| HRQoL | No effect | No effect | n/a | n/a | n/a |
| Hepatic encephalopathy | No effect | No effect | No effect | n/a | No effect |
| Rifaximin plus a non‐absorbable disaccharide versus a non‐absorbable disaccharide alone | |||||
| Mortality | Beneficial effect overall | Beneficial effect overall | Beneficial effect overall | Beneficial effect overall | All four analyses ‐beneficial effect overall |
| Serious adverse events | No effect | Beneficial effect overall, in acute HE, and in the prevention trials. | n/a | n/a | Worst‐case, best‐case, extreme best‐case: beneficial effect overall, but not in any subgroup Extreme worse‐case: No effect |
| HRQoL | n/a | n/a | n/a | n/a | n/a |
| Hepatic encephalopathy | Beneficial effect overall | Beneficial effect overall | Beneficial effect overall | n/a | All four analyses ‐ beneficial effect overall |
Abbreviations: HE, hepatic encephalopathy; HRQoL, health‐related quality of life; n/a: not available/applicable
Rifaximin versus placebo/no intervention
Primary outcomes
Mortality
Random‐effects meta‐analysis of trials comparing rifaximin versus placebo/no intervention showed no overall effect on mortality (RR 0.83, 95% CI 0.50 to 1.38; P = 0.48, I2 = 0%; 13 trials, 1007 participants; moderate‐certainty evidence; Analysis 1.1 ). There was no evidence of differences in effect between subgroups by type of hepatic encephalopathy (Chi2 = 0.42, df = 2 (P = 0.81), I2 = 0%; Analysis 1.1).
1.1. Analysis.

Comparison 1: Rifaximin versus placebo/no intervention, Outcome 1: Mortality
Fixed‐effect meta‐analysis also showed no overall effect on mortality (RR 0.84, 95% CI 0.51 to 1.38; P = 0.69, I2 = 0%; 13 trials, 1007 participants).
A sensitivity analysis including only trials at low risk of overall bias for mortality outcomes did not affect the finding of no overall effect (RR 0.82, 95% CI 0.48 to 1.41; P = 0.47, I2 = 0%; 11 trials, 876 participants). A further sensitivity analysis excluding trials with unclear or known vested interests bias also showed no overall effect on mortality (RR 0.72, 95% CI 0.27 to 1.93; P = 0.51, I2 = 0%; 5 trials, 409 participants).
The certainty of evidence was moderate due to imprecision because the optimal information size was not met.
Serious adverse events
Random‐effects meta‐analysis of trials comparing rifaximin versus placebo/no intervention showed no overall difference in the risk of serious adverse events between interventions (RR 1.05, 95% CI 0.83 to 1.32; P = 0.68, I2 = 0%; 9 trials, 801 participants; moderate‐certainty evidence; Analysis 1.2). There was no evidence of differences in effect between subgroups (Chi2 = 1.13, df = 2 (P = 0.57), I2 = 0%; Analysis 1.2).
1.2. Analysis.

Comparison 1: Rifaximin versus placebo/no intervention, Outcome 2: Serious adverse events
Fixed‐effect meta‐analysis also showed no overall difference in the risk of serious adverse events by intervention (RR 1.09, 95% CI 0.86 to 1.38; P = 0.49, I2 = 0%; 9 trials, 801 participants).
A sensitivity analysis, including only trials at low risk of overall bias for non‐mortality outcomes, did not affect the finding of no overall difference in the risks of serious adverse events (RR 1.09, 95% CI 0.85 to 1.39; P = 0.49, I2 = 0%; 4 trials, 385 participants). A further sensitivity analysis excluding trials with unclear or known vested interests bias was not possible as all the included trials were at risk for this type of bias.
The certainty of evidence was moderate due to imprecision because the optimal information size was not met.
Health‐related quality of life
Random‐effects meta‐analysis of trials comparing rifaximin versus placebo/no intervention showed an overall benefit on health‐related quality of life, assessed with Sickness Impact Profile (SIP) score (three trials) or EQ‐5D‐3L score (one trial) (MD ‐1.43, 95% CI ‐2.87 to 0.02; P = 0.05, I2 = 81%; 4 trials, 214 participants; moderate‐certainty evidence; Analysis 1.3). A fixed‐effect meta‐analysis also showed an overall beneficial effect of rifaximin on health‐related quality of life (MD ‐1.05, 95% CI ‐1.56 to ‐0.54; P < 0.001, I2 = 81%; 4 trials, 214 participants; Analysis 1.3).
1.3. Analysis.

Comparison 1: Rifaximin versus placebo/no intervention, Outcome 3: Health‐related quality of life
There was evidence of differences in effect by subgroup (Chi2 = 15.58, df = 1 (P < 0.001), I2 = 93.6%); benefit was seen in minimal hepatic encephalopathy (MD ‐2.07, 95% CI ‐2.79 to ‐1.35; P < 0.001, I2 = 0%; 3 trials, 176 participants), but not in chronic hepatic encephalopathy (MD 0.00, 95% CI ‐0.73 to 0.73; 1 trial, 38 participants). None of the prevention trials reported data on this outcome.
A sensitivity analysis including the two trials in minimal hepatic encephalopathy at low risk of overall bias for non‐mortality outcomes confirmed the beneficial effect on health‐related quality of life (MD ‐2.05, 95% CI ‐2.78 to ‐1.32; P < 0.001, I2 = 0%; 2 trials, 136 participants); both trials were at high risk of vested interests bias.
One randomised trial reported that resolution of hepatic encephalopathy with rifaximin did not translate into an improvement in health‐related quality of life during the trial period (Patel 2022).
The certainty of evidence was moderate due to imprecision because, although the optimal information size was met, the confidence intervals were wide and included both benefit and harm.
Hepatic encephalopathy
Random‐effects meta‐analysis of trials comparing rifaximin versus placebo/no intervention showed an overall beneficial effect on hepatic encephalopathy (RR 0.56, 95% CI 0.42 to 0.77; NNTB = 5; P < 0.001, I2 = 68%; 13 trials, 1009 participants; moderate‐certainty evidence; Analysis 1.4). There was evidence of a difference in effect by subgroup (Chi2 = 8.97, df = 2 (P = 0.01), I2 = 77.7%); benefit was seen in minimal hepatic encephalopathy (RR 0.40, 95% CI 0.31 to 0.52; NNTB = 3; P < 0.001, I2 = 10%; 6 trials, 364 participants; Analysis 1.4), but not in chronic hepatic encephalopathy or in the prevention of hepatic encephalopathy (Analysis 1.4).
1.4. Analysis.

Comparison 1: Rifaximin versus placebo/no intervention, Outcome 4: Hepatic encephalopathy
Fixed‐effect meta‐analysis also showed an overall effect of rifaximin on hepatic encephalopathy (RR 0.58, 95% CI 0.49 to 0.67; NNTB = 5; P < 0.001, I2 = 68%; 3 trials, 1009 participants) with evidence of differences in effect by subgroup (Chi2 = 19.74, df = 2 (P < 0.001), I2 = 89.9%): the benefit in minimal hepatic encephalopathy was confirmed (RR 0.38, 95% CI 0.29 to 0.49; NNTB = 3; P < 0.001, I2 = 10%; 6 trials, 364 participants), while benefit was also seen in the prevention trials (RR 0.71, 95% CI 0.56 to 0.91; NNTB = 10; P = 0.007, I2 = 36%; 4 trials, 474 participants); there was no benefit in chronic hepatic encephalopathy (RR 0.92; 95% CI 0.64 to 1.32; P = 0.65, I2 = 47%; 3 trials, 171 participants).
A sensitivity analysis including only trials at low risk of overall non‐mortality bias showed benefit both in the group as a whole (RR 0.46, 95% CI 0.37 to 0.58; NNTB = 4; P < 0.001, I2 = 39%; 7 trials, 532 participants), and also in minimal hepatic encephalopathy (RR 0.33, 95% CI 0.24 to 0.47; NNTB = 3; P < 0.001, I2 = 0%; 4 trials, 263 participants), and in the prevention trials (RR 0.61, 95% CI 0.45 to 0.84; NNTB = 5; P = 0.002, I2 = 0%; 2 trials, 229 participants), but not in chronic hepatic encephalopathy (RR 0.60, 95% CI 0.17 to 2.18; P = 0.44; 1 trial, 40 participants). Only one trial was at low risk of bias in relation to vested interests bias, precluding further sensitivity analyses.
The certainty of evidence was moderate due to inconsistency arising from heterogeneity which was not explained by subgroup analysis.
Two of the 13 trials of rifaximin versus placebo/standard care had a high risk of attrition bias. Worst‐case scenario analyses (missing outcome data counted as failures in both groups) showed that rifaximin did not have an effect on mortality (RR 0.95, 95% CI 0.62 to 1.45; P = 0.81, I2 = 0%; 13 trials, 1007 participants; Analysis 1.5), or on serious adverse events (RR 1.06, 95% CI 0.85 to 1.32; P = 0.59, I2 = 0%; 9 trials, 801 participants; Analysis 1.6). However, it had a significant beneficial effect on hepatic encephalopathy overall (RR 0.58, 95% CI 0.42 to 0.79; P < 0.001, I2 = 74%; 13 trials, 1009 participants; Analysis 1.7) and in the subgroup with minimal hepatic encephalopathy (RR 0.40, 95% CI 0.31 to 0.52; P < 0.001, I2 = 10%; 6 trials, 364 participants; Analysis 1.7). The extreme worst‐case scenario analyses (missing outcome data counted as failures in the rifaximin group and successes in the control group) produced similar findings (Analysis 1.8, Analysis 1.9, and Analysis 1.10), except for a possible detrimental effect of rifaximin on hepatic encephalopathy in the small subgroup of trials in chronic hepatic encephalopathy (Analysis 1.10).
1.5. Analysis.

Comparison 1: Rifaximin versus placebo/no intervention, Outcome 5: Mortality (worst‐case)
1.6. Analysis.

Comparison 1: Rifaximin versus placebo/no intervention, Outcome 6: Serious adverse events (worst‐case)
1.7. Analysis.

Comparison 1: Rifaximin versus placebo/no intervention, Outcome 7: Hepatic encephalopathy (worst‐case)
1.8. Analysis.

Comparison 1: Rifaximin versus placebo/no intervention, Outcome 8: Mortality (extreme worst‐case)
1.9. Analysis.

Comparison 1: Rifaximin versus placebo/no intervention, Outcome 9: Serious adverse events (extreme worst‐case)
1.10. Analysis.

Comparison 1: Rifaximin versus placebo/no intervention, Outcome 10: Hepatic encephalopathy (extreme worst‐case)
Best‐case scenario analyses (missing outcome data counted as successes in both groups) showed that rifaximin did not have a beneficial effect on mortality (RR 0.83, 95% CI 0.50 to 1.38; P = 0.48, I2 = 0%; 13 trials, 1007 participants; Analysis 1.11), or on serious adverse events (RR 1.05, 95% CI 0.83 to 1.32; P = 0.68, I2 = 0%; 9 trials, 801 participants; Analysis 1.12). However, it had a significant beneficial effect on hepatic encephalopathy overall (RR 0.56, 95% CI 0.42 to 0.77; P < 0.001, I2 = 68%; 13 trials, 1009 participants; Analysis 1.13) and in the subgroup with minimal hepatic encephalopathy (RR 0.40, 95% CI 0.31 to 0.52; P < 0.001, I2 = 10%; 6 trials, 364 participants; Analysis 1.13). The extreme best‐case scenario analyses (missing outcome data counted as successes in the rifaximin group and failures in the control group) showed that rifaximin did not have an effect on overall mortality (RR 0.67, 95% CI 0.41 to 1.11; P = 0.12, I2 = 0%; 13 trials, 1007 participants; Analysis 1.14) or on the overall risk of serious adverse events (RR 0.91, 95% CI 0.60 to 1.36; P = 0.63, I2 = 0%; 9 trials, 801 participants; Analysis 1.15). However, it had a significant beneficial effect on hepatic encephalopathy overall (RR 0.55, 95% CI 0.41 to 0.73; P < 0.001, I2 = 66%; 13 trials, 1009 participants; Analysis 1.16) and in the subgroup with minimal hepatic encephalopathy (RR 0.40, 95% CI 0.31 to 0.52; P < 0.001, I2 = 10%; 6 trials, 364 participants; Analysis 1.16).
1.11. Analysis.

Comparison 1: Rifaximin versus placebo/no intervention, Outcome 11: Mortality (best‐case)
1.12. Analysis.

Comparison 1: Rifaximin versus placebo/no intervention, Outcome 12: Serious adverse events (best‐case)
1.13. Analysis.

Comparison 1: Rifaximin versus placebo/no intervention, Outcome 13: Hepatic encephalopathy (best‐case)
1.14. Analysis.

Comparison 1: Rifaximin versus placebo/no intervention, Outcome 14: Mortality (extreme best‐case)
1.15. Analysis.

Comparison 1: Rifaximin versus placebo/no intervention, Outcome 15: Serious adverse events (extreme best‐case)
1.16. Analysis.

Comparison 1: Rifaximin versus placebo/no intervention, Outcome 16: Hepatic encephalopathy (extreme best‐case)
The results of the primary and sensitivity analyses for these four primary outcomes are summarised in Table 8.
Secondary outcomes
Non‐serious adverse events
Random‐effects meta‐analysis of trials comparing rifaximin versus placebo/no intervention showed no overall difference in the risks of non‐serious adverse events by intervention (RR 2.79, 95% CI 0.44 to 17.78; P = 0.28, I2 = 89%; 6 trials, 639 participants; very low‐certainty evidence; Analysis 1.17). There was no evidence of differences in effect between subgroups (Chi2 = 0.16, df = 1 (P = 0.69), I2 = 0%; Analysis 1.17).
1.17. Analysis.

Comparison 1: Rifaximin versus placebo/no intervention, Outcome 17: Non‐serious adverse events
Fixed‐effect meta‐analysis showed a possible increase in the risk of non‐serious adverse events with rifaximin (RR 1.18, 95% CI 1.05 to 1.33; number needed to treat for an additional harmful outcome (NNTH) = 28; P = 0.006, I2 = 89%; 6 trials, 639 participants), with no evidence of differences in effect by subgroup (Chi2 = 1.49, df = 1 (P = 0.22), I2 = 33.0%).
A sensitivity analysis including only trials at low risk of overall bias for non‐mortality outcomes showed no overall difference in the risk of non‐serious adverse events by intervention (RR 1.01, 95% CI 0.93 to 1.10; P = 0.87, I2 = 10%; 4 trials, 404 participants). When additionally excluding trials with unclear or known vested interests bias, only one trial with reported outcomes remained.
The certainty of evidence was very low due to inconsistency, with possible substantial heterogeneity within and between subgroups, and imprecision as, although the optimal information size was met, few studies reported outcomes, and the confidence intervals were wide enough to include both substantial benefit and harm.
Blood ammonia
Random‐effects meta‐analysis of trials comparing rifaximin versus placebo/no intervention, at maximum follow‐up, showed no meaningful effect on blood ammonia concentrations (MD 3.20, 95% CI ‐7.74 to 14.14; P = 0.57, I2 = 70%; 6 trials, 381 participants; low‐certainty evidence; Analysis 1.18). There was no evidence of differences in effect between subgroups (Chi² = 0.74, df = 2 (P = 0.69), I² = 0%; Analysis 1.18).
1.18. Analysis.

Comparison 1: Rifaximin versus placebo/no intervention, Outcome 18: Blood ammonia
The results of the fixed‐effect meta‐analysis were similar.
A sensitivity analysis including only trials at low risk of overall bias for non‐mortality outcomes showed no overall effect on blood ammonia concentrations (MD ‐6.78, 95% CI ‐15.05 to 1.50; P = 0.11, I2 = 0%; 3 trials, 136 participants). None of the trials was at low risk of vested interests bias, precluding further sensitivity analyses.
Random‐effects meta‐analysis of trials reporting paired changes in blood ammonia from baseline to maximum follow‐up showed no overall effect of rifaximin (MD ‐3.40, 95% CI ‐8.10 to 1.30; P = 0.16, I2 = 81%; 4 trials, 184 participants; Analysis 1.19). There was evidence of differences in effect by subgroups (Chi2 = 9.31, df = 2 (P = 0.010), I2 = 78.5%; Analysis 1.19), with a small effect seen in the one included trial in chronic hepatic encephalopathy (MD ‐7.00, 95% CI ‐13.16 to ‐0.84; P = 0.03; 1 trial, 38 participants; Analysis 1.19).
1.19. Analysis.

Comparison 1: Rifaximin versus placebo/no intervention, Outcome 19: Blood ammonia (paired)
The certainty of evidence was low due to (a) imprecision because, although the optimal information size was met, the confidence intervals were wide and included both benefit and harm; and (b) inconsistency because of possible moderate heterogeneity between and within subgroups.
Number Connection Test A
Random‐effects meta‐analysis of trials comparing rifaximin versus placebo/no intervention at maximum follow‐up showed no overall effect on the performance of NCT‐A (SMD ‐0.31, 95% CI ‐1.22 to 0.60; P = 0.51, I2 = 90%; 4 trials, 203 participants; moderate‐certainty evidence; Analysis 1.20). There was no evidence of differences in effect between subgroups (Chi2 = 2.09, df = 2 (P = 0.35), I2 = 4.5%; Analysis 1.20).
1.20. Analysis.

Comparison 1: Rifaximin versus placebo/no intervention, Outcome 20: Number Connection Test A
The fixed‐effect meta‐analysis also showed no effect on test performance overall, but there was evidence of differences in effect by subgroup, with benefit seen in minimal hepatic encephalopathy (SMD ‐0.60, 95% CI ‐0.99 to ‐0.21; P = 0.003, I2 = 90%; 2 trials, 115 participants).
A sensitivity analysis including only trials at low risk of overall bias for non‐mortality outcomes showed no overall benefit of rifaximin on the performance of NCT‐A in minimal hepatic encephalopathy (SMD ‐1.01, 95% CI ‐2.87 to 0.85; P = 0.29, I2 = 94%; 2 trials, 115 participants). Only one study was at low risk for vested interest bias, precluding further sensitivity analyses.
Random‐effects meta‐analysis of trials reporting paired changes in NCT‐A performance from baseline to maximum follow‐up showed no overall effect of rifaximin (SMD 0.27, 95% CI ‐0.71 to 1.25; P = 0.59, I2 = 91%; 4 trials, 203 participants; Analysis 1.21). There was no evidence of differences in effect between subgroups (Chi2 = 1.26, df = 2 (P = 0.53), I2 = 0%; Analysis 1.21).
1.21. Analysis.

Comparison 1: Rifaximin versus placebo/no intervention, Outcome 21: Number Connection Test A (paired)
The certainty of evidence was moderate due to imprecision because of the small sample size.
Length of hospital stay
No data were available for the length of hospital stay.
Rifaximin versus non‐absorbable disaccharides
Primary outcomes
Mortality
Random‐effects meta‐analysis of trials comparing rifaximin versus non‐absorbable disaccharides showed no overall effect on mortality (RR 0.99, 95% CI 0.49 to 1.97; P = 0.97, I2 = 0%; 10 trials, 786 participants; low‐certainty evidence; Analysis 2.1). There was no evidence of differences in effect between subgroups (Chi2 = 1.89, df = 3 (P = 0.59), I2 = 0%; Analysis 2.1).
2.1. Analysis.

Comparison 2: Rifaximin versus non‐absorbable disaccharides, Outcome 1: Mortality
Fixed‐effect analysis also showed no effect on mortality overall (RR 0.94, 95% CI 0.48 to 1.83; P = 0.85, I2 = 0%; 10 trials, 786 participants), or in the individual subgroups.
A sensitivity analysis including only trials at low risk of overall bias for mortality outcomes did not affect the finding of no overall effect on mortality (RR 0.93, 95% CI 0.46 to 1.90; P = 0.85, I2 = 0%; 8 trials, 729 participants). The exclusion of trials with unclear or known vested interests bias likewise did not affect the finding of no overall effect on mortality (RR 1.10, 95% CI 0.50 to 2.45; P = 0.81, I2 = 0%; 3 trials, 222 participants).
The certainty of evidence was low due to imprecision because the optimal information size was not met and the relative estimates of effect included both appreciable benefit and appreciable harm.
Serious adverse events
Random‐effect meta‐analysis of trials comparing rifaximin versus non‐absorbable disaccharides showed no overall difference in the risk of serious adverse events by intervention (RR 0.97, 95% CI 0.66 to 1.40; P = 0.85, I2 = 0%; 8 trials, 681 participants; low‐certainty evidence; Analysis 2.2). There was no evidence of differences in effect between subgroups (Chi2 = 3.50, df = 2 (P = 0.17), I2 = 42.9%; Analysis 2.2).
2.2. Analysis.

Comparison 2: Rifaximin versus non‐absorbable disaccharides, Outcome 2: Serious adverse events
Fixed‐effect meta‐analysis also showed no difference in the risk of serious adverse events overall (RR 1.02, 95% CI 0.70 to 1.48; P = 0.92, I2 = 0%; 8 trials, 681 participants). There was no evidence of differences in risk effects between subgroups.
A sensitivity analysis, including only trials at low risk of overall bias for non‐mortality outcomes, did not affect the finding of no overall effect on the risk of serious adverse events by intervention (RR 0.81, 95% CI 0.14 to 4.72; P = 0.81, I2 = 17%; 2 trials, 146 participants). None of the included trials were at low risk for vested interest bias, precluding further sensitivity analyses.
The certainty of evidence was low due to a probable risk of bias and imprecision, as although the optimal information size was met, the confidence interval includes both benefit and harm.
Health‐related quality of life
The evidence from the random‐effects meta‐analysis of trials comparing rifaximin versus non‐absorbable disaccharides was insufficient to determine any difference in the effects on health‐related quality of life (MD ‐0.33, 95% CI ‐1.65 to 0.98; P = 0.62, I2 = 0%; 2 trials, 249 participants; very low‐certainty evidence; Analysis 2.3). There was no evidence of differences in effect between subgroups (Chi2 = 0.51, df = 1 (P = 0.47), I2 = 0%; Analysis 2.3).
2.3. Analysis.

Comparison 2: Rifaximin versus non‐absorbable disaccharides, Outcome 3: Health‐related quality of life
The results of the fixed‐effect meta‐analysis did not differ.
There were insufficient trials at low risk of overall bias for non‐mortality outcomes and vested interest bias to undertake sensitivity analyses.
The certainty of evidence was very low due to a high risk of bias and an insufficient number of studies resulting in confidence intervals including both benefit and harm.
Hepatic encephalopathy
Random‐effects meta‐analysis of trials comparing rifaximin versus non‐absorbable disaccharides showed no overall difference in the effect on hepatic encephalopathy (RR 0.85, 95% CI 0.69 to 1.05; P = 0.13, I2 = 0%; 13 trials, 921 participants; low‐certainty evidence; Analysis 2.4). There was no evidence of differences in effect between subgroups (Chi2 = 0.76, df = 3 (P = 0.86), I2 = 0%; Analysis 2.4).
2.4. Analysis.

Comparison 2: Rifaximin versus non‐absorbable disaccharides, Outcome 4: Hepatic encephalopathy
Fixed‐effect meta‐analysis also showed no difference in the effect on hepatic encephalopathy overall (RR 0.82, 95% CI 0.66 to 1.01; P = 0.06, I2 = 0%; 13 trials, 921 participants), with no evidence of differences in effect between subgroups.
A sensitivity analysis including only trials at low risk of overall bias for non‐mortality outcomes did not affect the finding of no overall effect on hepatic encephalopathy (RR 1.01, 95% CI 0.74 to 1.38; P = 0.95, I2 = 0%; 5 trials, 316 participants). There was only one trial at low risk of vested interests bias, precluding further sensitivity analyses.
The certainty of evidence was low due to a probable risk of bias and imprecision as the optimal information size was not met.
Two of the 14 trials of rifaximin versus a non‐absorbable disaccharide had a high risk of attrition bias. Worst‐case, extreme worse‐case, best‐case, and extreme best‐case scenario analyses showed no beneficial effects of rifaximin on overall mortality (Analysis 2.5; Analysis 2.6; Analysis 2.7; Analysis 2.8), the risk of serious adverse events (Analysis 2.9; Analysis 2.10; Analysis 2.11; Analysis 2.12), or hepatic encephalopathy (Analysis 2.13; Analysis 2.14; Analysis 2.15; Analysis 2.16).
2.5. Analysis.

Comparison 2: Rifaximin versus non‐absorbable disaccharides, Outcome 5: Mortality (worst‐case)
2.6. Analysis.

Comparison 2: Rifaximin versus non‐absorbable disaccharides, Outcome 6: Mortality (extreme worst‐case)
2.7. Analysis.

Comparison 2: Rifaximin versus non‐absorbable disaccharides, Outcome 7: Mortality (best‐case)
2.8. Analysis.

Comparison 2: Rifaximin versus non‐absorbable disaccharides, Outcome 8: Mortality (extreme best‐case)
2.9. Analysis.

Comparison 2: Rifaximin versus non‐absorbable disaccharides, Outcome 9: Serious adverse events (extreme best‐case)
2.10. Analysis.

Comparison 2: Rifaximin versus non‐absorbable disaccharides, Outcome 10: Serious adverse events (best‐case)
2.11. Analysis.

Comparison 2: Rifaximin versus non‐absorbable disaccharides, Outcome 11: Serious adverse events (worst‐case)
2.12. Analysis.

Comparison 2: Rifaximin versus non‐absorbable disaccharides, Outcome 12: Serious adverse events (extreme worst‐case)
2.13. Analysis.

Comparison 2: Rifaximin versus non‐absorbable disaccharides, Outcome 13: Hepatic encephalopathy (worst‐case)
2.14. Analysis.

Comparison 2: Rifaximin versus non‐absorbable disaccharides, Outcome 14: Hepatic encephalopathy (extreme worst‐case)
2.15. Analysis.

Comparison 2: Rifaximin versus non‐absorbable disaccharides, Outcome 15: Hepatic encephalopathy (best‐case)
2.16. Analysis.

Comparison 2: Rifaximin versus non‐absorbable disaccharides, Outcome 16: Hepatic encephalopathy (extreme best‐case)
The results of the primary and sensitivity analyses for these four primary outcomes are summarised in Table 8.
Secondary outcomes
Non‐serious adverse events
Random‐effects meta‐analysis of trials comparing rifaximin versus non‐absorbable disaccharides showed no overall difference in the risk of non‐serious adverse events by intervention (RR 0.57, 95% CI 0.15 to 2.13; P = 0.40, I2 = 57%; 6 trials, 396 participants; very low‐certainty evidence; Analysis 2.17). There was no evidence of differences in effect between subgroups (Chi2 = 0.98, df = 1 (P = 0.32), I2 = 0%; Analysis 2.17).
2.17. Analysis.

Comparison 2: Rifaximin versus non‐absorbable disaccharides, Outcome 17: Non‐serious adverse events
Fixed‐effect meta‐analysis showed an overall beneficial effect of rifaximin versus non‐absorbable disaccharides on the risk of non‐serious adverse events (RR 0.54, 95% CI 0.31 to 0.92; NNTB = 13; P = 0.02, I2 = 57%; 6 trials, 396 participants). There was no evidence of differences in effect by subgroups (Chi2 = 1.55, df = 1 (P = 0.21), I2 = 35.3%).
A sensitivity analysis, including only trials at low risk of overall bias for non‐mortality outcomes, showed no overall effect of rifaximin on the risk of non‐serious adverse events (RR 0.25, 95% CI 0.00 to 19.93; P = 0.53, I2 = 86%; 2 trials, 175 participants). There was evidence of differences in effect by subgroups (Chi2 = 5.57, df = 1 (P = 0.02), I2 = 82.0%), with benefit seen in minimal hepatic encephalopathy (RR 0.03, 95% CI 0.00 to 0.49; NNTB = 3; P = 0.01; 1 trial, 72 participants), but not in acute hepatic encephalopathy (RR 1.59, 95% CI 0.28 to 9.12; P = 0.60; 1 trial, 103 participants). There was only one trial at low risk of bias in relation to vested interests, precluding further sensitivity analyses.
The certainty of evidence was very low due to a probable risk of bias, considerable heterogeneity both overall and within subgroups, and imprecision because – although the optimal information size was met – the relative estimates of effects encompassed both appreciable benefit and appreciable harm.
One trial evaluating rifaximin versus lactulose reported no adverse events in the rifaximin group, but some participants in the lactulose group reported mild abdominal cramps and nausea (Festi 1993).
Blood ammonia
Random‐effects meta‐analysis of trials comparing rifaximin versus non‐absorbable disaccharides at maximum follow‐up showed a small overall beneficial effect on blood ammonia (MD ‐6.78, 95% CI ‐12.81 to ‐0.75; P = 0.03, I2 = 78%; 10 trials, 599 participants; very low‐certainty evidence; Analysis 2.18). There was evidence of differences in effect between subgroups (Chi2 = 31.56, df = 3 (P < 0.001), I2 = 90.5%; Analysis 2.18), with benefit seen in chronic hepatic encephalopathy (MD ‐5.30, 95% CI ‐9.02 to ‐1.58; P = 0.005, I2 = 0%; 4 trials, 141 participants; Analysis 2.18), and in minimal hepatic encephalopathy (MD ‐15.00, 95% CI ‐16.62 to ‐13.38; P < 0.001; 1 trial, 30 participants; Analysis 2.18).
2.18. Analysis.

Comparison 2: Rifaximin versus non‐absorbable disaccharides, Outcome 18: Blood ammonia
Fixed‐effect meta‐analysis showed a beneficial effect on blood ammonia overall (MD ‐13.01, 95% CI ‐14.47 to ‐11.56; P < 0.001, I2 = 78%; 10 trials, 599 participants). There was evidence of differences in effect by subgroup (Chi2 = 32.39, df = 3 (P < 0.001), I2 = 90.7%), with benefit seen in chronic hepatic encephalopathy (MD ‐5.30, 95% CI ‐9.02 to ‐1.58; P = 0.005, I2 = 0%; 4 trials, 141 participants), and in minimal hepatic encephalopathy (MD ‐15.00, 95% CI ‐16.62 to ‐13.38; 1 trial, 30 participants).
A sensitivity analysis including only trials at low risk of overall bias for non‐mortality outcomes did not affect the finding of an overall beneficial effect on blood ammonia concentrations (MD ‐12.43, 95% CI ‐24.55 to ‐0.30; P = 0.04, I2 = 72%; 3 trials, 201 participants). There was maintained evidence of differences in effect by subgroup (Chi2 = 5.94, df = 1 (P = 0.01), I2 = 83.2%), with a likely beneficial effect on blood ammonia in acute hepatic encephalopathy (MD ‐40.30, 95% CI ‐67.23 to ‐13.37; P = 0.003; 1 trial, 103 participants) and a continued benefit in chronic hepatic encephalopathy (MD ‐6.28, 95% CI ‐11.15 to ‐1.41; P = 0.01, I2 = 12%; 2 trials, 98 participants). None of the trials in minimal hepatic encephalopathy or for prevention qualified for this analysis.
None of the trials was at low risk of vested interests bias, precluding further sensitivity analyses.
Random‐effects meta‐analysis of trials reporting paired changes in blood ammonia from baseline to maximum follow‐up showed no overall effect of rifaximin versus non‐absorbable disaccharides (MD ‐1.65, 95% CI ‐4.48 to ‐1.18; P = 0.25, I2 = 95%; 9 trials, 565 participants; Analysis 2.19). There was evidence of differences in effect by subgroup (Chi2 = 124.98, df = 3 (P < 0.001), I2 = 97.6%; Analysis 2.19), with a suggestion that rifaximin had a more beneficial effect on blood ammonia than lactulose in minimal hepatic encephalopathy (MD ‐13.00, 95% CI ‐15.18 to ‐10.82; P < 0.001; 1 trial, 30 participants; Analysis 2.19), while lactulose had more benefit on blood ammonia in one prevention trial (MD 4.50, 95% CI 2.17 to 6.83; P < 0.001; 1 trial, 50 participants; Analysis 2.19).
2.19. Analysis.

Comparison 2: Rifaximin versus non‐absorbable disaccharides, Outcome 19: Blood ammonia (paired)
The certainty of the evidence was very low due to: (a) many studies having a high risk of bias; (b) imprecision, as although the optimal information size was met, the confidence intervals include both benefit and harm; and (c) considerable heterogeneity in several subgroup analyses.
Number Connection Test A
Random‐effects meta‐analysis of NCT‐A performance in trials comparing rifaximin versus non‐absorbable disaccharides, at maximum follow‐up, showed no overall difference in effect on test performance (SMD ‐0.18, 95% CI ‐0.46 to 0.09; P = 0.19, I2 = 54%; 7 trials, 507 participants; very low‐certainty evidence; Analysis 2.20). There was evidence of differences in effect by subgroup (Chi2 = 8.87, df = 3 (P = 0.03), I2 = 66.2%; Analysis 2.20), with a beneficial effect seen in chronic hepatic encephalopathy (SMD ‐0.49, 95% CI ‐0.93 to ‐0.04; P = 0.03, I2 = 0%; 2 trials, 80 participants; Analysis 2.20).
2.20. Analysis.

Comparison 2: Rifaximin versus non‐absorbable disaccharides, Outcome 20: Number Connection Test A
Fixed‐effect meta‐analysis also showed no overall effect of rifaximin on test performance (SMD ‐0.17, 95% CI ‐0.34 to 0.01; P = 0.07, I2 = 54%; 7 trials, 507 participants), but there was again evidence of effect differences between subgroups (Chi2 = 9.25, df = 3 (P = 0.03), I2 = 67.6%), which confirmed the beneficial effect in chronic hepatic encephalopathy (SMD ‐0.49, 95% CI ‐0.93 to ‐0.04; P = 0.03, I2 = 0%; 2 trials, 80 participants) and additionally showed a beneficial effect in acute hepatic encephalopathy (SMD ‐0.29, 95% CI ‐0.56 to ‐0.02; P = 0.04, I2 = 74%; 2 trials, 218 participants).
A sensitivity analysis including only trials at low risk of bias for non‐mortality outcomes found no effect on test performance overall (SMD ‐0.25, 95% CI ‐0.71 to 0.21; P = 0.28, I2 = 42%; 2 trials, 130 participants). There was only one trial at low risk of vested interests bias, precluding further sensitivity analyses.
Random‐effects meta‐analysis of trials reporting paired changes in NCT‐A performance, from baseline to maximum follow‐up, showed no beneficial effect of rifaximin compared to non‐absorbable disaccharides overall (SMD 0.15, 95% CI ‐0.85 to 1.16; P = 0.76, I2 = 97%; 8 trials, 610 participants; Analysis 2.21). There was evidence of differences in effect by subgroup (Chi2 = 80.22, df = 3 (P < 0.001), I2 = 96.3%; Analysis 2.21), with lactulose having a more beneficial effect than rifaximin in the one prevention trial with data (SMD 6.82, 95% CI 5.32 to 8.32; P < 0.001; 1 trial, 50 participants; Analysis 2.21).
2.21. Analysis.

Comparison 2: Rifaximin versus non‐absorbable disaccharides, Outcome 21: Number Connection Test A (paired)
The certainty of evidence was very low due to a high risk of bias, inconsistency, and imprecision because of the limited number of studies available.
One study did not find an overall effect in the number connection test when comparing rifaximin to lactitol; we did not extract these data as we could not calculate a standard deviation for each group (Mas 2003).
Length of hospital stay
Data were only available from one trial comparing rifaximin versus non‐absorbable disaccharides for the prevention of hepatic encephalopathy (Maharshi 2015). We analysed the data separately for those participants who did and those who did not develop hepatic encephalopathy. No overall effect was observed in the length of hospital stay in random‐effects meta‐analysis (MD ‐0.66, 95% CI ‐1.33 to 0.01; P = 0.05, I2 = 0%; 2 trials, 120 participants; Analysis 2.22).
2.22. Analysis.

Comparison 2: Rifaximin versus non‐absorbable disaccharides, Outcome 22: Length of hospital stay
Rifaximin plus non‐absorbable disaccharides versus non‐absorbable disaccharides alone
Primary outcomes
Mortality
Random‐effects meta‐analysis of trials comparing rifaximin plus a non‐absorbable disaccharide versus a non‐absorbable disaccharide alone showed an overall beneficial effect on mortality (RR 0.69, 95% CI 0.55 to 0.86; NNTB = 22; P = 0.001, I2 = 0%; 14 trials, 1946 participants; moderate‐certainty evidence; Analysis 3.1). There was no evidence of differences in effect by subgroup (Chi2 = 1.32, df = 1 (P = 0.25), I2 = 24.5%; Analysis 3.1).
3.1. Analysis.

Comparison 3: Rifaximin plus non‐absorbable disaccharides versus non‐absorbable disaccharides alone, Outcome 1: Mortality
The results of the fixed‐effect meta‐analysis were comparable.
A sensitivity analysis including only trials at low risk of overall bias for mortality outcomes confirmed the beneficial effect on mortality overall (RR 0.63, 95% CI 0.44 to 0.91; NNTB = 23; P = 0.01, I2 = 0%; 6 trials, 815 participants); there was no evidence of differences in effect by subgroup (Chi2 = 2.60, df = 1 (P = 0.11), I2 = 61.5%).
When additionally excluding trials with unclear or known vested interests bias, the beneficial effect on mortality was maintained overall (RR 0.58, 95% CI 0.39 to 0.87; NNTB = 15; P = 0.008, I2 = 0%; 5 trials, 516 participants).
The certainty of evidence was moderate due to imprecision because the optimal information size was not met.
Serious adverse events
Random‐effects meta‐analysis of trials showed very low‐certainty evidence that use of rifaximin plus a non‐absorbable disaccharide was associated with a lower risk of serious adverse events than use of a non‐absorbable disaccharide alone (RR 0.66, 95% CI 0.45 to 0.98; NNTB = 10; P = 0.04, I2 = 60%; 7 trials, 1076 participants; very low‐certainty evidence; Analysis 3.2). There was no evidence of differences in effect by subgroup (Chi2 = 0.01, df = 1 (P = 0.93), I2 = 0%; Analysis 3.2).
3.2. Analysis.

Comparison 3: Rifaximin plus non‐absorbable disaccharides versus non‐absorbable disaccharides alone, Outcome 2: Serious adverse events
Fixed‐effect meta‐analysis showed an overall beneficial effect on the risk of serious adverse events with the combined treatment (RR 0.62, 95% CI 0.49 to 0.79; NNTB = 10; P < 0.001, I2 = 60%; 7 trials, 1076 participants). A beneficial effect was seen in acute hepatic encephalopathy (RR 0.61, 95% CI 0.45 to 0.81; NNTB = 7; P < 0.001, I2 = 71%; 3 trials, 393 participants), and in the prevention trials (RR 0.65, 95% CI 0.44 to 0.97; P = 0.03, I2 = 64%;3 trials, 657 participants); data were not estimable for the one trial in chronic hepatic encephalopathy.
There was only one trial at low risk of overall bias for non‐mortality outcomes, precluding sensitivity analyses.
The certainty of evidence was very low due to: (a) all but one study having a high risk of bias; (b) imprecision as the optimal information size was not met; and (c) inconsistency both overall and within subgroups.
Health‐related quality of life
No trials provided data on health‐related quality of life in an extractable form.
One trial reported a higher time‐weighted average for the overall Chronic Liver Disease Questionnaire (CLDQ) score in the rifaximin plus lactulose group versus lactulose alone (P value ranging from 0.0087 to 0.0436), but only reported these data in a figure as means without confidence intervals (Bass 2010).
Hepatic encephalopathy
Random‐effects meta‐analysis of trials comparing rifaximin plus a non‐absorbable disaccharide versus a non‐absorbable disaccharide alone showed an overall beneficial effect on hepatic encephalopathy (RR 0.58, 95% CI 0.48 to 0.71; NNTB = 5; P < 0.001, I2 = 62%; 17 trials, 2332 participants; low‐certainty evidence; Analysis 3.3). There was no evidence of differences in effect by subgroup (Chi2 = 0.82, df = 2 (P = 0.66), I2 = 0%; Analysis 3.3).
3.3. Analysis.

Comparison 3: Rifaximin plus non‐absorbable disaccharides versus non‐absorbable disaccharides alone, Outcome 3: Hepatic encephalopathy
Fixed‐effect meta‐analysis also showed a beneficial effect on hepatic encephalopathy overall (RR 0.55, 95% CI 0.49 to 0.62; NNTB = 5; P < 0.001, I2 = 62%; 17 trials, 2332 participants). Again, there was no evidence of differences in effect by subgroup (Chi2 = 1.88, df = 2 (P = 0.39), I2 = 0%).
A sensitivity analysis including only trials at low risk of overall bias for non‐mortality outcomes retained the overall beneficial effect on hepatic encephalopathy (RR 0.48, 95% CI 0.36 to 0.65; NNTB = 5; P < 0.001, I2 = 0%; 2 trials, 416 participants), with no evidence of differences in effect by subgroup (Chi2 = 0.00, df = 1 (P = 0.99), I2 = 0%). There was only one trial at low risk of vested interests bias, precluding further sensitivity analyses.
The certainty of evidence was low as the majority of studies had a high risk of bias for non‐mortality outcomes and inconsistency as there was substantial heterogeneity both overall and within subgroups.
Four of the 17 trials of rifaximin plus a non‐absorbable disaccharide versus a non‐absorbable disaccharide alone had a high risk of attrition bias. Worst‐case, extreme worse‐case, best‐case, and extreme best‐case scenario analyses showed an overall beneficial effect of rifaximin plus a non‐absorbable disaccharide on mortality (Analysis 3.4; Analysis 3.5; Analysis 3.6; Analysis 3.7). Worst‐case, best‐case, and extreme best‐case analyses showed a beneficial effect of rifaximin plus a non‐absorbable disaccharide on serious adverse events (Analysis 3.8; Analysis 3.10; Analysis 3.11), but this was not seen in the extreme worst‐case scenario analysis (Analysis 3.9). Worst‐case, extreme worse‐case, best‐case, and extreme best‐case scenario analyses showed a beneficial effect of rifaximin plus a non‐absorbable disaccharide on hepatic encephalopathy overall (Analysis 3.12; Analysis 3.13; Analysis 3.14; Analysis 3.15).
3.4. Analysis.

Comparison 3: Rifaximin plus non‐absorbable disaccharides versus non‐absorbable disaccharides alone, Outcome 4: Mortality (extreme best‐case)
3.5. Analysis.

Comparison 3: Rifaximin plus non‐absorbable disaccharides versus non‐absorbable disaccharides alone, Outcome 5: Mortality (best‐case)
3.6. Analysis.

Comparison 3: Rifaximin plus non‐absorbable disaccharides versus non‐absorbable disaccharides alone, Outcome 6: Mortality (worst‐case)
3.7. Analysis.

Comparison 3: Rifaximin plus non‐absorbable disaccharides versus non‐absorbable disaccharides alone, Outcome 7: Mortality (extreme worst‐case)
3.8. Analysis.

Comparison 3: Rifaximin plus non‐absorbable disaccharides versus non‐absorbable disaccharides alone, Outcome 8: Serious adverse events (worst‐case)
3.10. Analysis.

Comparison 3: Rifaximin plus non‐absorbable disaccharides versus non‐absorbable disaccharides alone, Outcome 10: Serious adverse events (best‐case)
3.11. Analysis.

Comparison 3: Rifaximin plus non‐absorbable disaccharides versus non‐absorbable disaccharides alone, Outcome 11: Serious adverse events (extreme best‐case)
3.9. Analysis.

Comparison 3: Rifaximin plus non‐absorbable disaccharides versus non‐absorbable disaccharides alone, Outcome 9: Serious adverse events (extreme worst‐case)
3.12. Analysis.

Comparison 3: Rifaximin plus non‐absorbable disaccharides versus non‐absorbable disaccharides alone, Outcome 12: Hepatic encephalopathy (worst‐case)
3.13. Analysis.

Comparison 3: Rifaximin plus non‐absorbable disaccharides versus non‐absorbable disaccharides alone, Outcome 13: Hepatic encephalopathy (extreme worst‐case)
3.14. Analysis.

Comparison 3: Rifaximin plus non‐absorbable disaccharides versus non‐absorbable disaccharides alone, Outcome 14: Hepatic encephalopathy (best‐case)
3.15. Analysis.

Comparison 3: Rifaximin plus non‐absorbable disaccharides versus non‐absorbable disaccharides alone, Outcome 15: Hepatic encephalopathy (extreme best‐case)
The results of the primary and sensitivity analyses for these four primary outcomes are summarised in Table 8.
Secondary outcomes
Non‐serious adverse events
Random‐effects meta‐analysis of trials comparing rifaximin plus a non‐absorbable disaccharide versus a non‐absorbable disaccharide alone showed no overall difference in the risk of non‐serious adverse events between interventions (RR 0.99, 95% CI 0.86 to 1.15; P = 0.90, I2 = 0%; 4 trials, 384 participants; very low‐certainty evidence, Analysis 3.16). There was no evidence of differences in effect by subgroup (Chi2 = 1.74, df = 1 (P = 0.19), I2 = 42.5%; Analysis 3.16).
3.16. Analysis.

Comparison 3: Rifaximin plus non‐absorbable disaccharides versus non‐absorbable disaccharides alone, Outcome 16: Non‐serious adverse events
Fixed‐effect meta‐analysis also showed no overall difference in the risk of non‐serious adverse events between interventions (RR 0.98, 95% CI 0.84 to 1.15; P = 0.82, I2 = 0%; 4 trials, 384 participants). There was no evidence of differences in effect by subgroup (Chi2 = 1.76, df = 1 (P = 0.18), I2 = 43.3%).
There was only one trial at low risk of overall bias for non‐mortality outcomes, precluding sensitivity analysis.
The certainty of evidence was very low as the majority of studies had a high risk of bias and there was inconsistency due to substantial heterogeneity.
One trial evaluating rifaximin plus lactulose versus lactulose alone reported that the incidence of adverse events was similar between the two groups; however, these data were not extractable from the trial report (Ali 2014).
Blood ammonia
Random‐effects meta‐analysis of trials comparing rifaximin plus a non‐absorbable disaccharide versus a non‐absorbable disaccharide alone showed no overall effect on blood ammonia (MD ‐6.88, 95% CI ‐14.78 to 1.02; P = 0.09, I2 = 0%; 2 trials, 325 participants; very low‐certainty evidence; Analysis 3.17). There was no evidence of differences in effect by subgroup (Chi2 = 0.42, df = 1 (P = 0.52), I2 = 0%; Analysis 3.17).
3.17. Analysis.

Comparison 3: Rifaximin plus non‐absorbable disaccharides versus non‐absorbable disaccharides alone, Outcome 17: Blood ammonia
The results of the fixed‐effect meta‐analysis were similar.
Only one trial was at low risk of overall bias for non‐mortality outcomes in the prevention of hepatic encephalopathy, and this showed no overall effect (MD ‐4.70, 95% CI ‐14.99 to 5.59; 1 trial, 299 participants). No trials remained when including only those without vested interests bias, precluding further sensitivity analyses.
The certainty of evidence was very low due to studies having a high risk of bias, and imprecision as the optimal information size was not met, resulting in wide confidence intervals around relative estimates of effect that include both appreciable benefit and harm.
Random‐effects meta‐analysis showed no overall effect of rifaximin plus a non‐absorbable disaccharide on the paired observations of blood ammonia in the one available trial in chronic hepatic encephalopathy (MD ‐2.00, 95% CI ‐11.50, 7.50; P = 0.68; 1 trial, 26 participants; Analysis 3.18).
3.18. Analysis.

Comparison 3: Rifaximin plus non‐absorbable disaccharides versus non‐absorbable disaccharides alone, Outcome 18: Blood ammonia (paired)
One trial reported this outcome as an attainment of blood ammonia of ≤ 70 mg/dL; this was achieved in 22.9% of participants taking rifaximin plus lactulose compared with 21.1% of participants taking lactulose alone (Vyas 2017).
Number Connection Test A
Random‐effects meta‐analysis showed no overall effect of rifaximin plus a non‐absorbable disaccharide versus a non‐absorbable disaccharide alone on NCT‐A performance at maximum follow‐up (SMD ‐0.05, 95% CI ‐1.28, 1.17; P = 0.93, I2 = 84%; 2 trials, 76 participants; very low‐certainty evidence; Analysis 3.19). There were too few studies for subgroup analyses.
3.19. Analysis.

Comparison 3: Rifaximin plus non‐absorbable disaccharides versus non‐absorbable disaccharides alone, Outcome 19: Number Connection Test A
There were insufficient trials to undertake sensitivity analyses for those at low risk of overall bias for non‐mortality outcomes and those without vested interest bias.
The certainty of evidence was very low due to: (a) many studies having a high risk of bias; and (b) imprecision as the optimal information size was not met, so the relative estimates of effect included both appreciable benefit and appreciable harm.
Only one trial undertaken in chronic hepatic encephalopathy reported paired changes in NCT‐A performance, from baseline to maximum follow‐up, and showed no overall effect of rifaximin plus non‐absorbable disaccharide on test performance (MD 0.10, 95% CI ‐5.25 to 5.45; 1 trial, 26 participants; Analysis 3.20).
3.20. Analysis.

Comparison 3: Rifaximin plus non‐absorbable disaccharides versus non‐absorbable disaccharides alone, Outcome 20: Number Connection Test A (paired)
Length of hospital stay
Random‐effects meta‐analysis of trials comparing rifaximin plus a non‐absorbable disaccharide versus a non‐absorbable disaccharide alone showed a beneficial effect on length of hospital stay (MD ‐2.86, 95% CI ‐3.46 to ‐2.26; P < 0.001, I2 = 0%; 3 trials, 408 participants; Analysis 3.21). All three included trials assessed acute hepatic encephalopathy.
3.21. Analysis.

Comparison 3: Rifaximin plus non‐absorbable disaccharides versus non‐absorbable disaccharides alone, Outcome 21: Length of hospital stay
Only one trial was at low risk of overall bias for non‐mortality outcomes in acute hepatic encephalopathy; this showed an overall beneficial effect on length of hospital stay (MD ‐2.40, 95% CI ‐3.86 to ‐0.94; P = 0.001; 1 trial, 120 participants). This trial also declared no vested interests.
Harms observed in observational studies, retrieved with the searches for randomised trials
The main identified harms reported in 13 observational studies retrieved during the literature searches for randomised trials are shown in Table 7.
Ascites or oedema was reported in three studies (Chang 2021; Mullen 2014; Salehi 2019). In a two‐year, open‐label maintenance study, 37.5% of participants receiving rifaximin developed ascites/oedema during the study period (Mullen 2014). The proportion of participants with ascites/oedema was lower in one study in those given rifaximin plus lactulose compared to lactulose alone (Chang 2021), whereas in another the proportion of participants with ascites/oedema was significantly lower in those given rifaximin compared to no treatment (Salehi 2019).
Infection/spontaneous bacterial peritonitis was reported in five studies (Kang 2017; Mullen 2014; Oey 2019; Salehi 2019; Vlachogiannakos 2013). In a two‐year, open‐label maintenance study, 5.6% of participants receiving rifaximin developed an infection during the study period (Mullen 2014). In one study, the infection rates did not change after the introduction of rifaximin (Oey 2019). However, infection rates were lower in participants treated with rifaximin compared to no treatment in two other studies (Salehi 2019; Vlachogiannakos 2013), and lower in participants treated with rifaximin and lactulose compared to lactulose alone in another study (Kang 2017).
Variceal/gastrointestinal bleeding was reported in five studies. Gastrointestinal bleeding was reported in two 'real‐world' studies of long‐term rifaximin (14.8% of participants in Mullen 2014; 4.6% in Suzuki 2019). Two studies reported a reduction in the number of gastrointestinal bleeds when comparing rifaximin with no treatment (Salehi 2019; Vlachogiannakos 2013); two further studies reported a reduction in gastrointestinal bleeds when comparing rifaximin plus lactulose versus lactulose alone (Chang 2021; Kang 2017).
Hepatorenal syndrome was reported in three studies. In a two‐year, open‐label maintenance study, 18.9% of participants receiving rifaximin developed hepatorenal syndrome during the study period (Mullen 2014). In one study, the incidence of hepatorenal syndrome was reduced in participants receiving rifaximin plus lactulose compared to lactulose alone (Kang 2017), whereas in another study, the incidence of hepatorenal syndrome increased in the rifaximin‐treated group (Vlachogiannakos 2013).
In a two‐year, open‐label maintenance study (Mullen 2014), 13.0% of participants receiving rifaximin developed electrolyte imbalance while 8.4% developed a coagulopathy/thrombocytopenia.
In a two‐year, open‐label maintenance study, 1.5% of participants receiving rifaximin developed an infection with Clostridiumdifficile during the study period (Mullen 2014). There were no instances of C difficile infection in rifaximin‐treated participants in three long‐term studies (Oey 2019; Orr 2016; Uchida 2020). In one study, the C difficile infection rate was lower in participants receiving rifaximin plus lactulose than in those receiving lactulose alone (Kang 2017).
Nausea was reported in association with rifaximin use in four studies (Oey 2019; Orr 2016; Suzuki 2019; Vlachogiannakos 2013); diarrhoea or enteritis in three studies (Suzuki 2019; Tatsumi 2021; Vlachogiannakos 2013), and a skin rash in two studies (Oey 2019; Vlachogiannakos 2013).
No clinically applicable adverse events were reported in two identified observational studies (Kaji 2017; Sama 2004).
Discussion
Summary of main results
This review includes descriptive and quantitative information from 41 randomised clinical trials involving 4545 participants. We classed 11 trials as having a high risk of bias for mortality outcomes, 25 as having a low risk, and five as having an unclear risk. We classed 28 trials as having a high risk of bias for non‐mortality outcomes, 12 as having a low risk, and one as having an unclear risk.
Primary outcomes
The use of rifaximin likely results in little to no difference in mortality when compared to placebo/no intervention, or non‐absorbable disaccharides. However, rifaximin when used together with a non‐absorbable disaccharide shows an overall beneficial effect on mortality (NNTB = 22) compared to use of a non‐absorbable disaccharide alone.
The use of rifaximin likely results in little to no difference in the risk of serious adverse events when compared to placebo/no intervention or to non‐absorbable disaccharides. However, when used in combination with a non‐absorbable disaccharide, it likely reduces the risk of serious adverse events, although the evidence is very uncertain.
Very few trials investigated health‐related quality of life; thus data for this outcome are limited. The use of rifaximin likely improves health‐related quality of life in minimal hepatic encephalopathy when compared to placebo, but not when compared to lactulose. Given the paucity of data, it is difficult to draw certain conclusions.
Rifaximin may improve hepatic encephalopathy overall when compared to placebo/no intervention (NNTB = 5), although the effect is likely confined to a subgroup of people with minimal hepatic encephalopathy and when used for prevention. Rifaximin had no effect on hepatic encephalopathy when compared to non‐absorbable disaccharides. However, combining use of rifaximin with a non‐absorbable disaccharide may have an overall beneficial effect on hepatic encephalopathy (NNTB = 5) compared to use of a non‐absorbable disaccharide alone.
Secondary outcomes
Rifaximin may make little or no overall difference to the risk of non‐serious adverse events when compared to placebo/no intervention or to non‐absorbable disaccharides. Likewise, rifaximin in combination with a non‐absorbable disaccharide likely has no effect on the risk of non‐serious adverse events compared to use of a non‐absorbable disaccharide alone.
Rifaximin may have no effect on blood ammonia at maximum follow‐up when compared to placebo/no intervention. It may have a small beneficial effect on blood ammonia in chronic and minimal hepatic encephalopathy when compared to non‐absorbable disaccharides, but the evidence is very uncertain. There was no effect on blood ammonia when comparing rifaximin plus a non‐absorbable disaccharide to a non‐absorbable disaccharide alone in the one trial in chronic hepatic encephalopathy and the one prevention trial in which it was measured. Rifaximin may have a small beneficial effect on blood ammonia, measured as a change from baseline to maximum follow‐up, in chronic hepatic encephalopathy compared to placebo, and a beneficial effect in minimal hepatic encephalopathy when compared to non‐absorbable disaccharides, but lactulose may be more beneficial in reducing blood ammonia when used for prevention; the evidence from these paired observations is very uncertain. We chose not to include the paired data in our summary of findings tables, as there were fewer studies, and the results did not substantially change the uncertainty of the evidence.
NCT‐A was the most frequently used psychometric test in the included trials, but the number reporting performance data was small. Rifaximin may not improve NCT‐A performance when compared to placebo/no intervention at maximum follow‐up, but may slightly improve test performance in chronic hepatic encephalopathy when compared to a non‐absorbable disaccharide; these data are very uncertain. No overall benefit was seen in test performance comparing rifaximin plus a non‐absorbable disaccharide with a non‐absorbable disaccharide alone, but the analysis only included two trials. When NCT‐A performance was measured as a change from baseline to maximum follow‐up, there was no apparent beneficial effect of rifaximin when compared to placebo, or to non‐absorbable disaccharides, but lactulose may be more beneficial in improving test performance when used for prevention. We chose not to include the paired data in our summary of findings tables as there were fewer studies, and because the results did not affect the uncertainty of the evidence.
There is a paucity of trial data on the effects of rifaximin on the length of hospital stay. Use of rifaximin was associated with a reduction in hospital in‐patient stay, when compared to lactulose, in the one prevention trial with available data, while the use of rifaximin in combination with lactulose was associated with a reduction in hospital in‐patient stay, when compared to lactulose alone, in the three available trials in acute hepatic encephalopathy.
In conclusion, when compared to placebo/no intervention, rifaximin improves health‐related quality of life in minimal hepatic encephalopathy and may also improve hepatic encephalopathy in this population subgroup, and when used for prevention; it may also slightly reduce blood ammonia in people with chronic hepatic encephalopathy.
There were no differences in the effects of rifaximin and non‐absorbable disaccharides on any of the primary outcomes, but use of rifaximin may be associated with lower blood ammonia concentrations in chronic and minimal hepatic encephalopathy, and a slight improvement in the performance of NCT‐A in chronic hepatic encephalopathy. However, the evidence for the effects on this secondary outcome is very uncertain. Thus, we consider the effects of rifaximin and non‐absorbable disaccharides to be broadly similar.
Combining use of rifaximin with a non‐absorbable disaccharide may have several beneficial effects compared to use of a non‐absorbable disaccharide alone, although the evidence is uncertain. The combination likely reduces mortality, reduces the risk of serious adverse events, may improve hepatic encephalopathy and reduce the length of hospital stay, and may also reduce the risk of recurrence in the prevention trials. It is very unclear whether use of combination therapy affects the risk of non‐serious adverse events.
The combined evidence suggests that rifaximin, used in combination with a non‐absorbable disaccharide, should be considered in the management of people with cirrhosis who have or are at risk of developing hepatic encephalopathy.
Overall completeness and applicability of evidence
This review included randomised clinical trials of rifaximin versus placebo, no intervention, or non‐absorbable disaccharides, and trials of the combination of rifaximin with a non‐absorbable disaccharide against use of a non‐absorbable disaccharide alone. As a result, it was possible, where data allowed, to investigate the use of rifaximin as a stand‐alone treatment, to compare its effects with those of non‐absorbable disaccharides, the currently accepted first‐line treatment for hepatic encephalopathy (EASL and AASLD guideline 2014), and its use as a possible adjuvant to current first‐line therapy. This approach strengthens the completeness of the evidence.
The included trials were conducted world‐wide. The countries of origin were: Bangladesh (Hasan 2018), China (Tan 2022), Denmark (Kimer 2017), Egypt (Moneim 2021; Wahib 2014), France (Bureau 2021), India (Ali 2014; Maharshi 2015; Pawar 2019; Sharma 2013; Sharma 2014; Sidhu 2011; Sidhu 2016; Uthman 2020; Vyas 2017), Italy (Bucci 1993; Fera 1993; Festi 1993; Loguercio 2003; Massa 1993; Riggio 2005), Japan (Suzuki 2018), Mexico (Higuera‐de‐la‐Tijera 2018), Nepal (Poudyal 2019), Pakistan (Ahmed 2018; Babar 2017; Butt 2018; Gill 2014; Habib 2016; Majeed 2018; Muhammad 2016; Nawaz 2015), South Korea (Paik 2005), Spain (Mas 2003), Ukraine (Manzhalii 2022), the UK (Patel 2022), and the USA (Bajaj 2011). Some trials were conducted in several countries; for example, in Poland, Hungary, the USA and the UK (Bass 2004); and Canada, Russia and the USA (Bass 2010). The country of origin was not stated for one trial (Bajaj 2019). Treatment effects on minimal hepatic encephalopathy were assessed in trials undertaken in China, Denmark, India, Ukraine, and the USA; trials in acute hepatic encephalopathy were undertaken in Bangladesh, Egypt, India, Japan, Nepal, Pakistan, South Korea, Spain, and the UK, while trials in chronic hepatic encephalopathy were undertaken in Italy, the USA, and the UK. The prevention of hepatic encephalopathy was assessed in trials undertaken in Denmark, Egypt, France, India, Italy, Mexico, Pakistan, the USA, and multinationally.
The most important outcomes for people with cirrhosis and hepatic encephalopathy are mortality, morbidity, serious adverse events, and health‐related quality of life (Bajaj 2011a). Our systematic review with meta‐analyses provides information on all of these outcomes. Some difficulties were encountered in the extraction of data on adverse events. Many of the included trials did not report on the number of participants who experienced adverse events by intervention, but instead reported the number of participants by adverse events. This is a major limitation because if a given participant experienced more than one adverse event, this could falsely inflate the overall prevalence of individual events and lead to inconsistency in comparisons. Thus, we chose to exclude two trials that reported data in this way from our analyses of serious adverse events (Bass 2010; Moneim 2021) and 11 trials from our analyses of non‐serious adverse events (Bajaj 2011; Bajaj 2019; Bass 2010; Bucci 1993; Higuera‐de‐la‐Tijera 2018; Kimer 2017; Maharshi 2015; Moneim 2021; Patel 2022; Sharma 2013; Suzuki 2018).
The diagnostic assessment and classification of hepatic encephalopathy involves use of a variety of different clinical, neuropsychometric, neurophysiological, and psychophysical techniques. However, the approach to the diagnosis and evaluation of hepatic encephalopathy has changed in recent years, and although there is some commonality in the published guidelines, there is no absolute consensus (EASL and AASLD guideline 2014). The trials included in this review were undertaken between 1993 and 2022, and their evaluation of manifestations of improved hepatic encephalopathy varied widely. Accordingly, the between‐study heterogeneity was considerable. We were unable to identify sources of heterogeneity in subgroup analyses and so decided to accept the individual investigators’ classifications of hepatic encephalopathy, their selected outcome criteria, and their thresholds for improvement since these were likely to have been clinically relevant when the trials were conducted (Table 5; Table 6).
Hepatic encephalopathy varies widely in its manifestations. We included trials in our review involving people with minimal hepatic encephalopathy, people with single or recurrent episodes of acute hepatic encephalopathy, people with chronic hepatic encephalopathy associated with advanced liver disease, and with naturally occurring or surgically created portal‐systemic shunts. We included all clinical trials with extractable data in our primary analyses, and where this was not possible, reported data qualitatively. We also conducted subgroup and sensitivity analyses to determine the differential effects of interventions in the different population subgroups. The fact that the included trials encompass all subtypes of hepatic encephalopathy and that this was taken into account in the analysis strengthens the completeness of the evidence.
In 28 of the 41 randomised clinical trials included in this review, rifaximin, either alone or in combination with a non‐absorbable disaccharide, was used to treat hepatic encephalopathy, whereas in the remaining 13 trials it was used to prevent the occurrence of hepatic encephalopathy (Table 4). In five of the prevention trials, rifaximin was used to prevent the development of hepatic encephalopathy in people previously free of this complication; that is, for primary prevention. Two of the trials involved people undergoing insertion of a transjugular intrahepatic portosystemic shunt to prevent recurrent ascites or variceal bleeding (Bureau 2021; Riggio 2005), while a further two included people presenting with an acute variceal haemorrhage (Higuera‐de‐la‐Tijera 2018; Maharshi 2015). The fifth trial involved people with decompensated cirrhosis who had not yet experienced an episode of hepatic encephalopathy (Zeng 2021). In eight of the prevention trials, rifaximin, either alone or in combination with a non‐absorbable disaccharide, was used as secondary prophylaxis to prevent the recurrence of hepatic encephalopathy after one or more previous hospitalisations; that is, secondary prevention. Thus, we included trials encompassing use of rifaximin as treatment for hepatic encephalopathy as well as for its primary and secondary prevention, which strengthens the completeness of the evidence.
From a statistical perspective, the number of events for many of our analyses was generally low, meaning the standard meta‐analytic models we have utilised in this review may not have enough power to provide reliable estimates. To this end, reporting odds ratios versus risk ratios could be preferable, but this would likely produce similar results and, given it is the hardest summary statistic to understand and apply in practice, its use may have made this review more inaccessible for clinicians. Furthermore, where odds ratios are used, calculating summary estimates using the Peto fixed‐effect method in addition to our random‐effects analyses could help account for analyses where intervention effects and the number of events are small, and where comparator group sizes are comparable. However, as these criteria are not always fulfilled, Peto's method is not normally recommended as a default approach to meta‐analysis, and so we opted not to use this approach (Deeks 2022).
Hepatic encephalopathy is precipitated, in a high proportion of people with cirrhosis, by events such as infection, dehydration, constipation, dietary indiscretion, electrolyte disturbances, gastrointestinal bleeding, and certain drugs (Pantham 2017). The individual precipitating factors may act in concert; some may contribute more than others. Early recognition and correction of precipitating factors is an important first step in the management of overt hepatic encephalopathy; avoidance of these factors may reduce the risk of developing hepatic encephalopathy in the longer term. Detailed information on possible precipitating events, and on the effects of interventions designed to ameliorate them, were not provided in the trials included in this review. Thus, it is not clear whether the use of rifaximin provides additional benefit in situations where hepatic encephalopathy is precipitated by a treatable event. Future trials should endeavour to capture this information.
Hepatic encephalopathy has a considerable impact on health‐related quality of life (Fabrellas 2020; Groeneweg 1998; Grønkjær 2018; Orr 2016) and we included this as one of our primary outcomes. However, data on this variable were only reported in six of the 41 identified trials, including three comparing rifaximin with placebo (Bajaj 2011; Patel 2022; Sidhu 2011; Tan 2022), and two comparing rifaximin with a non‐absorbable disaccharide (Sidhu 2016; Suzuki 2018). None of the other trials reported data on this outcome in an extractable form. Health‐related quality of life should be assessed using validated tools as self‐reported outcomes often correlate poorly with those determined using physiological and performance variables, clinician‐reported outcomes, and biomarkers (Johnston 2021). Our finding of a possible beneficial effect of rifaximin on health‐related quality of life when used to treat minimal hepatic encephalopathy and for prevention needs to be evaluated in future studies.
We included trials in the review in which rifaximin was used alone or in combination with non‐absorbable disaccharides. However, in 20 of the 41 included trials, 22% to 100% of the participants were taking a non‐absorbable disaccharide (Ahmed 2018; Ali 2014; Babar 2017; Bajaj 2019; Bass 2010; Butt 2018; Gill 2014; Habib 2016; Hasan 2018; Kimer 2017; Majeed 2018; Moneim 2021; Muhammad 2016; Nawaz 2015; Patel 2022; Poudyal 2019; Sharma 2013; Suzuki 2018; Uthman 2020; Vyas 2017). In 11 trials, the dose of the lactulose was adjusted according to stool frequency and consistency (Bajaj 2019; Gill 2014; Hasan 2018; Higuera‐de‐la‐Tijera 2018; Moneim 2021; Muhammad 2016; Pawar 2019; Poudyal 2019; Sharma 2013; Sidhu 2016; Suzuki 2018). In six trials, lactulose was permitted in both groups, but the dose and frequency of administration were not reported (Ali 2014; Babar 2017; Bass 2010; Majeed 2018; Patel 2022; Uthman 2020). In 14 trials, lactulose was administered in a fixed dose (Ahmed 2018; Bucci 1993; Butt 2018; Fera 1993; Festi 1993; Habib 2016; Loguercio 2003; Maharshi 2015; Mas 2003; Massa 1993; Paik 2005; Riggio 2005; Vyas 2017; Wahib 2014). It is possible that the different dosing regimes may have influenced the results. However, there is considerable inter‐subject variability in the amount of non‐absorbable disaccharide needed to produce the desired effects on stool frequency and consistency, and it is likely that in most trials the dose of the non‐absorbable disaccharides was adjusted accordingly, even though not specifically stipulated or when seemingly given in a fixed regime. Differences in doses of the non‐absorbable disaccharides then become much less of a concern.
Some early trials used other 'anti‐encephalopathy' interventions such as dietary protein restriction (Bucci 1993; Fera 1993; Festi 1993; Mas 2003; Massa 1993; Paik 2005; Wahib 2014), although this is no longer recommended (EASL and AASLD guideline 2014). Participants randomised to experimental or control groups in these trial were equally protein‐restricted. Protein‐sufficient diets were instituted in three trials (Sidhu 2011; Sidhu 2016; Suzuki 2018). Protein restriction and as‐required or daily enemas were used in three trials (Fera 1993; Hasan 2018; Wahib 2014). Antibiotics were administered concomitantly in four trials: ceftriaxone in all participants in one trial (Sharma 2013), cephalosporin or quinolone given when needed in one trial (Higuera‐de‐la‐Tijera 2018), and intravenous antibiotics of non‐stipulated type in two trials (Hasan 2018; Poudyal 2019). Concomitant treatment administered equally between trial groups may cause heterogeneity but is not likely to produce systematic differences between groups.
Non‐compliance with medication is a recognised problem in people with hepatic encephalopathy (Pantham 2017). However, measures are invariably taken in clinical trials to ensure adherence to prescribed regimens. In the trials included in our review, the reported compliance rates were 84% to 100% (Bureau 2021; Higuera‐de‐la‐Tijera 2018; Kimer 2017; Majeed 2018; Manzhalii 2022; Paik 2005; Suzuki 2018; Zeng 2021), suggesting that non‐adherence with medication is unlikely to have influenced the results.
The length of treatment varied considerably in the trials in minimal and chronic hepatic encephalopathy, and in the prevention trials. A recent systematic review of randomised clinical trials, retrospective chart reviews, and real‐world and clinical practice open‐label studies, assessed the long‐term (six months or more) efficacy and safety of lactulose, rifaximin, or both for the prevention of hepatic encephalopathy (Hudson 2019). Lactulose reduced the occurrence of overt hepatic encephalopathy and related hospitalisations over the longer term; the combination of rifaximin and lactulose significantly reduced the risk of these events, compared to lactulose alone, without compromising tolerability. These findings support the results of our more restricted analysis.
Transjugular intrahepatic portosystemic shunts are used in the long‐term control of variceal bleeding and resistant ascites, but the benefits are off‐set by the development of post‐insertion hepatic encephalopathy in 35% to 50% of recipients (Bai 2014; Fornio 2017; Nolte 1998; Riggio 2008; Zhu 2019). We included two trials that were undertaken to determine if rifaximin is effective in preventing the development of post‐shunt hepatic encephalopathy, compared to placebo/no treatment (Bureau 2021; Riggio 2005). However, no subgroup analysis of the prevention trials was undertaken as there was significant trial heterogeneity. A recent meta‐analysis of rifaximin for the prevention of post‐shunt hepatic encephalopathy, published in abstract form (Razzack 2021), included these same two trials but also included data from a prevention study of lactulose plus rifaximin compared with lactulose alone, in which 32 (10.7 %) of the 299 participants had a transjugular intrahepatic portosystemic shunt (Bass 2010); it is not known if the authors relied solely on the published study data or were able to acquire additional information. Their conclusion that rifaximin is superior to placebo in preventing post‐shunt hepatic encephalopathy ignores the fact that lactulose co‐medication was allowed in the largest of their included trials. A recent systematic review emphasised that careful selection of candidates for this procedure is of utmost importance in reducing the risk of post‐shunt hepatic encephalopathy (Gairing 2022), and explored the efficacy of current pharmacological approaches to the prevention of post‐shunt hepatic encephalopathy. The review drew attention to the ongoing multicentre, randomised, double‐blind trial comparing rifaximin plus lactulose to placebo plus lactulose administered three days prior to shunt insertion to three months post‐shunt insertion (NCT04073290 (PEARL Study)); the results of this trial will be included in future versions of this review.
Acute‐on‐chronic liver failure (ACLF) is a recently‐defined syndrome arising in hospitalised people with acutely decompensated cirrhosis. It is characterised by failure of single or multiple extra‐hepatic organs, systemic inflammation, and an extremely poor prognosis (Arroyo 2020). Approximately 60% of people with ACLF develop hepatic encephalopathy, but this has different clinical, pathophysiological, and prognostic feature to the hepatic encephalopathy associated with decompensated cirrhosis per se (Córdoba 2014). It is likely that some of our included trials enroled people with ACLF, but their data were not available for separate analysis and so no comments can be made about outcomes in this population subgroup. Future trials will need to investigate the best treatment options in the ACLF population; currently lactulose is the recommended treatment of choice (Rose 2020).
Hepatic encephalopathy imposes a significant burden on healthcare systems and the resource utilisation associated with the management of people with hepatic encephalopathy is increasing (Elsaid 2020; Poordad 2007). Several studies have evaluated the cost‐effectiveness of rifaximin compared to lactulose (Elsaid 2020; Huang 2007; Kabeshova 2016; Leevy 2007; Neff 2010; Neff 2018; Orr 2016). These studies have generally shown that the combined use of rifaximin and lactulose for the secondary prevention of overt hepatic encephalopathy is highly cost‐effective because of the considerable savings in total health care resource utilisation associated with fewer hospitalisations and shorter lengths of hospital stay (Elsaid 2020; Orr 2016). A recent systematic review of the pharmacoeconomics of rifaximin concluded that the economic data favour use of rifaximin, with or without lactulose, for reducing the risk of the recurrence of overt hepatic encephalopathy (Neff 2018; Siddiqui 2021). Most of these analyses have focused on inpatient costs; further studies taking into account the costs associated with primary care resources are clearly warranted. None of the trials included in our review undertook a cost analysis and few looked at the associated effects on length of inpatient stay. However, the reduction in the number of episodes of hepatic encephalopathy associated with use of rifaximin together with lactulose in the prevention trials will undoubtedly have resulted in cost savings. Currently, rifaximin is considerably more expensive than lactulose and some other active interventions (BNF 2021). However, at some stage, rifaximin will come off‐licence and cheaper generic products may become available; this could change the cost‐benefit/cost‐effectiveness landscape considerably.
Finally, there is increasing evidence that rifaximin may have several clinically relevant beneficial effects in people with cirrhosis, in addition to any beneficial effects it might have on hepatic encephalopathy (Bass 2010; Caraceni 2021; Flamm 2018; Kang 2017; Vlachogiannakos 2013); it is thought that these additional benefits are mediated via the gut microbiota (Bajaj 2016a; Trebicka 2021). Most interest has been shown in the role of rifaximin for the prevention of spontaneous bacterial peritonitis, and this has been subject to no less than nine separate systematic reviews and meta‐analyses (Facciorusso 2019; Faust 2020; Goel 2017; Kamal 2017; Komolafe 2020; Menshawy 2019; Pimentel 2021; Soni 2020; Wang 2019b). Three of these meta‐analyses reviewed the efficacy and safety of rifaximin for primary and secondary prevention of spontaneous peritonitis compared to placebo, no intervention, or the oral quinolones, norfloxacin and ciprofloxacin, the current antibiotic treatment of choice in this situation. Rifaximin was found to be superior to placebo/no intervention and the quinolones in two studies (Goel 2017; Kamal 2017), but equivalent to the norfloxacin in the other (Menshawy 2019). The remaining six reviews included all identified antibiotic trials and used network meta‐analyses to rank their performance for primary and secondary prophylaxis of spontaneous bacterial peritonitis. Three of the meta‐analyses provided evidence for the superior efficacy of rifaximin compared to other antibiotics (Faust 2020; Soni 2020; Wang 2019b); two provided evidence for the superiority of the quinolones (Facciorusso 2019; Pimentel 2021); while the final meta‐analysis, undertaken as a Cochrane Review, stated that there was considerable uncertainty about whether antibiotic prophylaxis was beneficial for the prevention of spontaneous bacterial peritonitis, and if beneficial, which antibiotic regime would be most efficacious (Komolafe 2020). Oral administration of poorly absorbed antibiotics, such as norfloxacin or rifaximin, results in 'selective' intestinal decontamination, which might reduce the inflammatory response induced by bacterial translocation. This could potentially improve portal hypertension. A recent systematic review and meta‐analysis showed that neither rifaximin nor norfloxacin significantly reduce the hepatic venous pressure gradient in patients with cirrhosis and portal hypertension (Mendoza 2020). However, the trials in which antibiotics were used in the longer term, together with a beta‐blocker, reported a significant decrease in the hepatic venous pressure gradient. More long‐term trials are clearly warranted.
Although we included variceal bleeding, ascites, spontaneous bacterial peritonitis, acute kidney injury, and hepatorenal syndrome in our assessment of serious adverse events, we did not consider the potentially beneficial effects of rifaximin on the risks of these complications. Future trials of rifaximin in people with cirrhosis should attempt to assess as many clinically relevant outcomes as possible.
Quality of the evidence
This is the first Cochrane Review of rifaximin for the prevention and treatment of hepatic encephalopathy in people with cirrhosis. We identified a moderate number of randomised clinical trials, and additional information from authors and companies helped us to identify essential aspects of bias control. As recommended, we combined the individual bias domains in an overall assessment (Higgins 2021b). We also included an assessment of individual domains, focusing on randomised clinical trials with a low risk of selection bias (Higgins 2017; Savovic 2012). Based on previous evidence (Savovic 2012), we defined mortality, but not serious adverse events, as an outcome that is robust to performance and detection bias. This decision can be questioned as lack of blinding is also unlikely to influence the assessment of events such as variceal bleeding and the development of ascites (Savovic 2018). We included 17 randomised clinical trials with a low risk of performance and detection bias due to clear double‐blinding, but cannot exclude the possibility that our analyses overestimate the effect of rifaximin on hepatic encephalopathy due to insufficient or unclear blinding in the other 24 trials.
Overall, we classified 16 of the 41 trials as having a high or unclear risk of bias for mortality outcomes, while 29 of the 41 trials had a high or unclear risk of bias for non‐mortality outcomes. This was mainly the result of the use of open‐label designs, incomplete outcome data, and selective reporting.
We performed sensitivity analyses on the trials at a low risk of overall bias. Overall, the results of these analyses did not differ substantially from those of the original analysis for the four primary outcomes (Table 8).
Only 17 of the 41 included trials were free of possible vested interest bias. We did not include this as a bias domain (Boutron 2021), but intended to undertake sensitivity analyses excluding trials where this may have been an issue. However, the number of trials free of these possible influences was low, precluding many of the planned analyses. Where we were able to perform them, the results did not differ from those of the primary analyses (Table 8).
Overall, 11 of the 41 trials had a high risk of attrition bias. To explore the effect of data attrition, we conducted worst‐case, extreme worst‐case, best‐case, and extreme best‐case scenario analyses (Table 8). These did not substantially differ from the results of the primary analyses for the comparisons rifaximin versus non‐absorbable disaccharides and rifaximin plus a non‐absorbable disaccharide versus a non‐absorbable disaccharide alone. In the comparison rifaximin versus placebo/standard care, the extreme best‐case analysis showed a beneficial effect on mortality and serious adverse events in the chronic hepatic encephalopathy subgroup while the extreme worse‐case analysis showed a detrimental effect on hepatic encephalopathy in the same group (Table 8).
Thus, the results of our primary analysis appear robust, with little divergence when subjected to sensitivity analyses designed to account for the overall risk of bias, attrition bias, and vested interest bias.
Based on the revised assessment of bias control, combined with the assessment of the directness of evidence, heterogeneity, precision of effect estimate, and risk of publication bias, we classified the certainty of the evidence for our primary outcomes in the range of low certainty to moderate certainty for mortality, very low certainty to moderate certainty for serious adverse events, very low certainty to moderate certainty for health‐related quality of life, and very low certainty to low certainty for hepatic encephalopathy outcomes. For our secondary outcomes, we classified the certainty of the evidence as very low certainty for non‐serious adverse events, very low certainty for blood ammonia, and very low to low certainty for the NCT‐A. We did not assess certainty of evidence for length of hospital stay in our summary of findings tables, in line with the restrictions stipulated in the Cochrane Handbook, but deemed this to be the least useful outcome to assess (Schünemann 2021). A limitation with the assessment of imprecision is that hepatic encephalopathy is a heterogenous condition, so calculating optimal information size for overall outcomes may overlook the true incidence of outcomes in the subgroups by type of hepatic encephalopathy.
Potential biases in the review process
We undertook the review based on Cochrane's guidance when the protocol was initially developed (Higgins 2017). We attempted to minimise possible publication bias by using a comprehensive search strategy (Page 2014). We combined extensive and comprehensive electronic searches of the literature with extensive manual searches of reference lists, journals, and conference proceedings; most of the identified conference abstracts were subsequently published as full papers. We consider it unlikely that we failed to identify any published trials. We included 41 trials in our final review with a comprehensive range of both primary and secondary outcomes to assess efficacy and safety. Six of the included trials have only been published in abstract form (Bajaj 2019; Bass 2004; Fera 1993; Gill 2014; Nawaz 2015; Vyas 2017). Trials that report positive or significant results are more likely to be published as full papers and outcomes that are statistically significant have higher odds of being fully reported (Dwan 2008). Thus, if data are only available in abstract form, this could be a potential source of publication/reporting bias. We were provided with individual participant data from seven of the included trials (Bucci 1993; Fera 1993; Festi 1993; Kimer 2017; Loguercio 2003; Mas 2003; Massa 1993), extensive trial data from one further trial (Bajaj 2011), and additional information regarding trial design and outcome measures for two included trials from www.fda.gov (Bass 2004; Bass 2010). Information on randomisation methods and outcomes were also received following correspondence with the authors of 11 trials (Bajaj 2011; Gill 2014; Hasan 2018; Higuera‐de‐la‐Tijera 2018; Maharshi 2015; Nawaz 2015; Patel 2022; Poudyal 2019; Riggio 2005; Sidhu 2011; Sidhu 2016). We classified 10 trials as being at high risk for reporting bias, although we were able to retrieve important additional data for four of these from other sources.
Retrieving unpublished information may have reduced the risk of publication and reporting bias. In addition, the availability of individual participant data allowed us to calculate and include information on outcomes for several trials not provided in the original publications. The additional information on trial design and bias control allowed us to provide some standardisation in the determining of outcomes which would not otherwise have been possible. Overall, we were able to retrieve data on our primary outcomes of mortality from 33 of the 41 included trials, serious adverse events from 22, and improvement in hepatic encephalopathy from 39 trials. Only six trials reported on our primary outcome of health‐related quality of life. The number of trials that reported secondary outcomes, such as non‐serious adverse events, blood ammonia levels, and length of hospital stay, was very small. Conclusions based on the analyses of the secondary outcomes must be viewed with caution.
Agreements and disagreements with other studies or reviews
In this, the first Cochrane Review of the efficacy and safety of rifaximin in hepatic encephalopathy, we included 41 randomised clinical trials, undertaken between 1993 and 2022, involving 4545 people with cirrhosis and hepatic encephalopathy. We evaluated the use of rifaximin against placebo/no intervention; rifaximin against non‐absorbable disaccharides, which are currently first‐line treatment for this condition (Gluud 2016); and the use of rifaximin in combination with a non‐absorbable disaccharide against a non‐absorbable disaccharide alone. We chose not to look at rifaximin in comparison with other antibiotics as this will be the subject of a separate review (Jeyaraj 2017). We also chose not to compare rifaximin with other potentially active agents – for example, L‐ornithine L‐aspartate – as this comparison was included in a previous review (Goh 2018). We also undertook specific subgroup analyses in order to assess the impact of rifaximin on outcomes in participants with minimal, acute, and chronic hepatic encephalopathy and when used for primary and secondary prevention. This is, in consequence, the largest and most comprehensive review of rifaximin undertaken to date.
A number of systematic reviews and meta‐analyses of the efficacy and safety of rifaximin in people with cirrhosis and hepatic encephalopathy were undertaken between 2008 and 2022; the majority were published as full papers (Cheng 2021; Eltawil 2012; Fu 2022; Han 2021; Jiang 2008; Kimer 2014; Wang 2019a; Wu 2013; Zhuo 2019), although three have only been published in abstract form, to date (Fidel 2019; Razzack 2021; Shukla 2011). These meta‐analyses include between three and 28 randomised clinical trials, involving between 264 and 2979 participants; one meta‐analysis also included observational studies (Wang 2019a). Five of the meta‐analyses compared rifaximin with non‐absorbable disaccharides in people with minimal and overt hepatic encephalopathy (Cheng 2021; Jiang 2008; Shukla 2011; Wu 2013; Zhuo 2019); one compared rifaximin with non‐absorbable disaccharides and other antibiotics in overt hepatic encephalopathy (Eltawil 2012); one compared rifaximin with placebo/no treatment, non‐absorbable disaccharides, and other antibiotics in minimal and overt hepatic encephalopathy, and for prevention (Kimer 2014); one compared rifaximin with placebo/no treatment, non‐absorbable disaccharides, and other active interventions, including antibiotics and L‐ornithine L‐ aspartate, in minimal and overt hepatic encephalopathy, and for prevention (Han 2021); three compared rifaximin plus lactulose to lactulose alone in overt hepatic encephalopathy (Fidel 2019; Fu 2022; Wang 2019a), and one specifically compared rifaximin to placebo in the prevention of hepatic encephalopathy following insertion of a transjugular intrahepatic portosystemic shunt (Razzack 2021). In the meta‐analyses which included trials with several comparators, these were grouped together to form a single control group (Eltawil 2012; Han 2021; Kimer 2014), although subgroup analyses were sometimes available. In most meta‐analyses, the main outcome variables were hepatic encephalopathy and adverse events; five included mortality as an outcome (Fidel 2019; Fu 2022; Han 2021; Kimer 2014; Wang 2019a), while two included length of hospital stay (Han 2021; Wu 2013). Some included surrogate markers of hepatic encephalopathy, such as blood ammonia (Cheng 2021; Eltawil 2012; Han 2021; Kimer 2014; Wu 2013; Zhuo 2019), and psychometric performance (Eltawil 2012; Han 2021; Kimer 2014; Wu 2013). However, not all the data were extractable for comparison with the current review, particularly from the two meta‐analyses which combined placebo with other active interventions into one control group (Han 2021; Kimer 2014).
Where we were able to access data, we found no major discrepancies between our findings and those of previous meta‐analyses taken together. Thus, we found that rifaximin resulted in little to no difference in mortality when compared to placebo, no intervention, or non‐absorbable disaccharides; comparable findings were reported in two previous meta‐analyses (Han 2021; Kimer 2014). However, we did find that combining rifaximin with a non‐absorbable disaccharide appears to reduce overall mortality in people with hepatic encephalopathy, compared to a non‐absorbable disaccharide alone. Comparable findings were reported in the three previous meta‐analyses that reported on this outcome (Fidel 2019; Fu 2022; Wang 2019a).
Our finding that rifaximin improves minimal hepatic encephalopathy when compared to placebo or no intervention is in line with the findings reported in previous meta‐analyses (Han 2021; Kimer 2014; Wang 2019a). Our finding that rifaximin did not have a beneficial effect on hepatic encephalopathy or its prevention when compared to non‐absorbable disaccharides was in concert with the findings in several previous meta‐analyses (Cheng 2021; Eltawil 2012; Jiang 2008; Wu 2013; Zhuo 2019), but rifaximin was reported to have a more beneficial effect on hepatic encephalopathy and its prevention than non‐absorbable disaccharides in two meta‐analyses where comparative groups were combined (Han 2021; Kimer 2014), and in an early meta‐analysis reported in abstract form only (Shukla 2011). We found that use of rifaximin with a non‐absorbable disaccharide appears to have a beneficial effect on hepatic encephalopathy, when compared to use of a non‐absorbable disaccharide alone. The results of the three meta‐analyses that looked specifically at the effects of the combined use of rifaximin and a non‐absorbable disaccharide also reported increased benefit in relation to the resolution of hepatic encephalopathy, but they did not look at prevention (Fidel 2019; Fu 2022; Wang 2019a).
None of the previous meta‐analyses distinguished clearly between serious and non‐serious adverse events; some extractable data on adverse events are available from six previous meta‐analyses (Cheng 2021; Han 2021; Jiang 2008; Wang 2019a; Wu 2013; Zhuo 2019). We found very little or no overall difference in the risk of serious adverse events comparing rifaximin to placebo/no intervention or to the non‐absorbable disaccharides; we found that use of rifaximin with a non‐absorbable disaccharide appeared to decrease the risk of serious adverse events, both overall and in acute hepatic encephalopathy when compared to use of a non‐absorbable disaccharide alone; there are no other meta‐analyses suitable for comparison. We found a possible reduction in the risk of non‐serious adverse events in chronic hepatic encephalopathy with rifaximin compared to placebo/no intervention; no difference in the risk was reported in a previous meta‐analysis (Han 2021). We also identified a lower risk of non‐serious adverse events with rifaximin compared to non‐absorbable disaccharides in both chronic and minimal hepatic encephalopathy. Two previous meta‐analyses reported no difference in the frequency of adverse events between rifaximin and non‐absorbable disaccharides (Cheng 2021; Zhuo 2019), while lower frequencies of abdominal pain (Jiang 2008), and of abdominal pain and diarrhoea (Wu 2013), were reported in others. We found that use of rifaximin with a non‐absorbable disaccharide appears to slightly increase the risk of non‐serious adverse events when compared to use of a non‐absorbable disaccharide alone, whereas no difference in the frequency of adverse events was reported in one previous meta‐analysis (Wang 2019a).
We found very little effect of rifaximin on blood ammonia, whether used alone or in combination with a non‐absorbable disaccharide in comparison to placebo/no intervention or a non‐absorbable disaccharide alone. A similar lack of benefit of rifaximin on blood ammonia was reported in five meta‐analyses with extractable data comparing rifaximin with a non‐absorbable disaccharide (Cheng 2021; Eltawil 2012; Han 2021; Jiang 2008; Zhuo 2019).
Two network analyses have been undertaken, designed to identify the best options for treating minimal hepatic encephalopathy and preventing overt hepatic encephalopathy (Dhiman 2020), and for treating overt hepatic encephalopathy (Zhu 2015). In the first of these studies, standard meta‐analyses, network analyses, and surface under accumulated ranking (SUCRA) were undertaken to identify optimal treatments for minimal hepatic encephalopathy and the prevention of hepatic encephalopathy against placebo or no intervention (Dhiman 2020). The top two agents found to be effective in reversing minimal hepatic encephalopathy were rifaximin (OR 7.5, 95% predictive interval (PrI) 4.5 to 12.7; SUCRA, 89.2%; moderate quality) and lactulose (OR 5.39, 95% PrI 3.60 to 8.0; SUCRA, 67.2%; moderate quality); rifaximin was not significantly superior to lactulose – in concert with our findings. A significant reduction in the risk of developing overt hepatic encephalopathy, compared to placebo or no treatment, was reported for lactulose (OR 0.22, 95% PrI 0.09 to 0.52; SUCRA, 73.9%; moderate quality) but not for rifaximin (OR 0.44, 95% PrI 0.09 to 2.11; low quality). However, the three rifaximin trials included in the analysis for the prevention of overt hepatic encephalopathy involved people with minimal hepatic encephalopathy at baseline (Bajaj 2011; Sharma 2014; Sidhu 2011), followed for a maximum of eight weeks with few, if any, events reported. Our approach to the selection of trials for the prevention of overt hepatic encephalopathy analyses differed substantially from this, so the results cannot be compared.
The second network analysis looked at the comparative effectiveness and safety of interventions used to treat overt hepatic encephalopathy (Zhu 2015). Exact details of the 20 randomised clinical trials included in the analyses are not given and are difficult to access from the reference list. However, it would appear that only four of the nine available trials comparing rifaximin to a non‐absorbable disaccharide were included, together with a published direct meta‐analysis of eight trials. As such, the reported data cannot be compared with the results of our direct meta‐analysis of all included trials.
A number of systematic and narrative reviews have dealt with aspects of the prevention and treatment of hepatic encephalopathy not specifically addressed in our review. Thus, although we included health‐related quality of life as one of our primary outcomes, very few trials addressed this measure. We did, however, show that rifaximin benefits health‐related quality of life in minimal hepatic encephalopathy compared to placebo, and has an effect on health‐related quality of life equivalent to that of lactulose in minimal and acute hepatic encephalopathy. A recent systematic review of randomised clinical trials and prospective cohort studies examined the effects of lactulose and rifaximin on health‐related quality of life and other patient‐reported outcomes in people with hepatic encephalopathy (Moon 2023). Both lactulose and rifaximin were consistently associated with improvements in health‐related quality of life, sleep, social activity, and emotional behaviour, with no discernable difference between their effects. This highlights the importance of identifying these domains as outcomes of importance in future trials.
Authors' conclusions
Implications for practice.
The evidence provided in this review suggests that rifaximin, particularly when used in combination with a non‐absorbable disaccharide, may have a place in the management of people with cirrhosis and hepatic encephalopathy. The certainty of the evidence is generally low, but some implications for practice can be deduced.
Minimal hepatic encephalopathy detrimentally affects the performance of complex tasks, compromises personal safety, significantly impairs health‐related quality of life, and is a major risk factor for the development of overt hepatic encephalopathy. Treatment with rifaximin confers benefit in minimal hepatic encephalopathy, but its effects are not superior to those of non‐absorbable disaccharides, except to lower blood ammonia. Considering the clinical course of the condition, it is likely that treatment, once instigated, would need to be continued in the long term. Current cost considerations may favour use of a non‐absorbable disaccharide for this indication.
Development of an episode of acute hepatic encephalopathy is associated with significant reductions in short‐ and medium‐term survival. Rifaximin and non‐absorbable disaccharides may have equivalent effects on mortality and clinical recovery when used for this indication. However, combining the use of rifaximin with a non‐absorbable disaccharide may have beneficial effects on both mortality and resolution of the hepatic encephalopathy compared to use of a non‐absorbable disaccharide alone, with likely no increase in the risk of adverse events. While the combined use of rifaximin and lactulose is not licensed for this indication, use of the combination could be considered in this situation, particularly if supported by further studies.
The risk of developing further episodes of hepatic encephalopathy following an index event is high. Long‐term use of rifaximin and a non‐absorbable disaccharide to prevent hepatic encephalopathy may be effective in reducing the risk of recurrence, when compared to a non‐absorbable disaccharide alone, but does not influence mortality. Rifaximin is licensed for use, in combination with lactulose, for secondary prevention of hepatic encephalopathy, and our review findings support its use for this indication.
Rifaximin, whether as monotherapy (three trials) or in combination with a non‐absorbable disaccharide (one trial), does not appear to benefit chronic hepatic encephalopathy, although the number of included studies in our review was very small, so the evidence is very uncertain. Chronic hepatic encephalopathy is often associated with the presence of large spontaneous portosystemic shunts and consideration should be given to non‐invasive obliteration of these, if present. Similarly, consideration should be given to reducing the size of any previously inserted transjugular intrahepatic shunts. The presence of chronic hepatic encephalopathy should be an indication for assessment for liver transplantation, but not for this indication alone.
No implications for practice can be made, based on this review, for the prevention or treatment of hepatic encephalopathy following the insertion of a transjugular intrahepatic portosystemic shunt. Careful selection of candidates for this procedure is the most effective way to reduce the risk of post‐shunt hepatic encephalopathy. However, the results of two ongoing studies may help provide guidance.
Likewise, no implications for practice can be made, based on this review, for the treatment of hepatic encephalopathy in acute‐on‐chronic liver failure.
Implications for research.
When selecting trials for inclusion in this review, we identified 13 ongoing trials which might provide data suitable for inclusion in future review updates. Seven of the 13 ongoing trials involve other complications of cirrhosis or are mechanistic, and we are uncertain whether clinically relevant information will be extractable from these when completed. However, the remaining six ongoing trials should provide clinically important data which may allow us to be more certain about the conclusions of our present review. Thus, two trials – one multicentre and one single centre – are comparing rifaximin, with or without adjuvant lactulose, against standard care or placebo for the prevention of encephalopathy following insertion of a transjugular, intrahepatic portosystemic shunt. One trial is investigating rifaximin against placebo for primary prevention of hepatic encephalopathy, while another will investigate rifaximin against placebo for secondary prevention. One trial is investigating rifaximin versus placebo for minimal hepatic encephalopathy, while a further trial is investigating rifaximin versus lactulose for acute hepatic encephalopathy. Although the quality of these trials cannot be assessed until completed and published, the fact that they are funded and are already underway does have implications for future research undertakings.
Fewer than half of the trials included in our review were free of potential bias, and the majority only provided outcome information on hepatic encephalopathy, and less frequently, adverse events and mortality. In addition, a variety of diagnostic and monitoring techniques were used, making comparisons difficult. All future trials should be conducted to rigorous standards; they should use validated diagnostic procedures to characterise the trial populations; they should be designed to avoid bias by use of robust randomisation methods and blinding, and avoid incomplete or selective reporting of data; outcome measures should be predefined and should be robust and clinically relevant; the trials should be adequately powered.
The use of rifaximin to prevent and treat complications of cirrhosis other than hepatic encephalopathy – for example, portal hypertension and spontaneous bacterial peritonitis – is also being explored. These trials could be an important source of additional data on hepatic encephalopathy, and researchers undertaking these trials should be urged to include assessments of mental status and cognitive function. Likewise, researchers undertaking trials of rifaximin in hepatic encephalopathy, especially the longer‐term prevention trials, should be encouraged to assess and monitor other potential complications of cirrhosis.
We used the EPICOT format in the definition of implications for research (Brown 2006):
Evidence (what is the current state of the evidence?): this review includes 41 randomised clinical trials; we classed 18 as being at high risk of bias in the overall assessment of mortality and non‐mortality and a further 11 as being at high risk for non‐mortality outcomes only. We found moderate‐certainty evidence for a beneficial effect of rifaximin in minimal hepatic encephalopathy, health‐related quality of life, and performance of Number Connection Test A (NCT‐A) when compared to placebo. We found very low‐certainty evidence for beneficial effects of rifaximin plus a non‐absorbable disaccharide on mortality and on hepatic encephalopathy compared to use of a non‐absorbable disaccharide alone.
Participants (what is the population of interest?): people with cirrhosis with minimal, acute, and chronic hepatic encephalopathy; people with cirrhosis who are at risk for developing encephalopathy, for example, after a gastrointestinal bleed or insertion of a transjugular intrahepatic portosystemic shunt (primary preventions); people who have experienced one or more previous episodes of encephalopathy (secondary prevention); people with acute‐on‐chronic liver failure with hepatic encephalopathy.
Interventions (what are the interventions of interest?): rifaximin as monotherapy or combined with a non‐absorbable disaccharide.
Comparisons (what are the comparisons of interest?): placebo‐controlled trials of rifaximin could be considered in minimal hepatic encephalopathy and for primary prevention; some primary prevention trials have compared rifaximin with placebo and with other active agents such as lactulose and L‐ornithine L‐aspartate. Non‐absorbable disaccharides are the treatment of choice for hepatic encephalopathy and there appears to be additional benefit in combining the use of rifaximin with lactulose; future trials in acute/chronic hepatic encephalopathy and secondary prevention should compare rifaximin plus lactulose against lactulose and a placebo preparation. Trials of new, potentially active drugs should include suitably‐blinded comparisons with rifaximin, lactulose, and rifaximin plus lactulose.
Outcomes (what are the outcomes of interest?): information on mortality, hepatic encephalopathy, and adverse events should be collected in all future trials; health‐related quality of life is an outcome of interest, except in trials in acute hepatic encephalopathy where data would be difficult to obtain; surrogate markers such as psychometric tests and biomarkers such as blood ammonia, collected at the beginning and the end of treatment periods, are of value, particularly in trials in minimal hepatic encephalopathy.
Time stamp (date of literature search): January 2023
History
Protocol first published: Issue 3, 2015
Acknowledgements
We are grateful to the authors of studies and associated pharmaceutical companies who willingly answered our questions regarding the conduct of their trials and, where available, provided us with additional data.
Cochrane Hepato‐Biliary Group supported the authors in the development of this review.
The following people from the Editorial Team office of the Hepato‐Biliary conducted the editorial process for this article.
Sign‐off Editor (editorial decision): Agostino Colli, Deputy Co‐ordinating Editor, Hepato‐Biliary Group, Denmark
Managing Editor (selected peer reviewers, provided comments, provided editorial guidance to authors, edited the article): Dimitrinka Nikolova, Hepato‐Biliary Group, Denmark
Information Specialist (developing search strategies and trial search): Sarah Louise Klingenberg, Hepato‐Biliary Group, Denmark
Peer reviewers (provided expert comments): Piero Amodio, University of Padova, Italy; Guglielmo Trovato, The European Medical Association, Brussles, Belgium; Lorenzo Ridola, Department of Translational and Precision Medicine, "Sapienza", University of Rome, Italy
The following people from the Cochrane Central Editorial Service supported the production of his review.
Sign‐off Editor (final editorial decision): Toby Lasserson, Cochrane Evidence Production and Methods Directorate
Managing Editor (co‐ordinated methods input, edited the article): Anne‐Marie Stephani, Cochrane Central Editorial Service
Peer‐reviewers (provided comments and recommended an editorial decision): Rachel Richardson, Nuala Livingstone, Cochrane Evidence Production and Methods Directorate (methods review)
Robin Featherstone, Cochrane Central Editorial Service (peer reviewer on the search review)
Copy Editor (copy‐editing and production): Andrea Takeda, Cochrane Central Production Service
Cochrane Review Group funding acknowledgement: the Danish State is the largest single funder of the Cochrane Hepato‐Biliary Group through its investment in the Copenhagen Trial Unit, Centre for Clinical Intervention Research, the Capital Region of Denmark, Rigshospitalet, Copenhagen, Denmark. Disclaimer: the views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the Danish State or the Copenhagen Trial Unit.
Appendices
Appendix 1. Search strategies
| Database | Time span | Search strategy |
| The Cochrane Hepato‐Biliary Controlled Trials Register | 4 May 2022 | (rifaximin* OR xifaxan* OR rcifax* OR rifagut*) AND (encephalopath* OR liver disease* OR cirrho*) |
| Cochrane Central Register of Controlled Trials | Latest issue (2022, Issue 5) | #1 rifaximin* or xifaxan* or rifagut* or Rcifax* #2 MeSH descriptor: [Hepatic Encephalopathy] explode all trees #3 MeSH descriptor: [Liver Diseases] explode all trees #4 MeSH descriptor: [Fibrosis] explode all trees #5 encephalopath* or liver disease* or cirrho* #6 #2 or #3 or #4 or #5 #7 #1 and #6 |
| MEDLINE Ovid | 1946 to 4 May 2022 | 1. (rifaximin* or xifaxan* or rifagut* or Rcifax*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonym 2. exp Hepatic Encephalopathy/ 3. exp Liver Diseases/ 4. exp Fibrosis/ 5. (encephalopath* or liver disease* or cirrho*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 6. 2 or 3 or 4 or 5 7. 1 and 6 8. (randomized controlled trial or controlled clinical trial).pt. or clinical trials as topic.sh. or trial.ti. 9. (random* or blind* or placebo* or meta‐analys*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 10. 7 and (8 or 9) |
| Embase Ovid | 1974 to 4 May 2022 | 1. exp rifaximin/ 2. (rifaximin* or xifaxan* or rifagut* or Rcifax*).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] 3. 1 or 2 4. exp hepatic encephalopathy/ 5. exp liver disease/ 6. exp fibrosis/ 7. (encephalopath* or liver disease* or cirrho*).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] 8. 4 or 5 or 6 or 7 9. 3 and 8 10. Randomized controlled trial/ or Controlled clinical study/ or trial.ti. 11. (random* or blind* or placebo* or meta‐analys*).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] 12. 9 and (10 or 11) |
| LILACS (Bireme) | 1982 to 4 May 2022 | (rifaximin$ or xifaxan$ or rifagut$ or Rcifax$) [Words] and (encephalopath$ or liver disease$ or cirrho$) [Words] |
| Science Citation Index Expanded | 1900 to 4 May 2022 | #5 #4 AND #3 #4 TI=(random* or blind* or placebo* or meta‐analys* or trial*) OR TS=(random* or blind* or placebo* or meta‐analys*) #3 #2 AND #1 #2 TS=(encephalopath* or liver disease* or cirrho*) #1 TS=(rifaximin* or xifaxan* or rifagut* or Rcifax*) |
| Conference Proceedings Citation Index – Science (Web of Science) | 1990 to 4 May 2022 | #5 #4 AND #3 #4 TI=(random* or blind* or placebo* or meta‐analys* or trial*) OR TS=(random* or blind* or placebo* or meta‐analys*) #3 #2 AND #1 #2 TS=(encephalopath* or liver disease* or cirrho*) #1 TS=(rifaximin* or xifaxan* or rifagut* or Rcifax*) |
Data and analyses
Comparison 1. Rifaximin versus placebo/no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Mortality | 13 | 1007 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.50, 1.38] |
| 1.1.1 Chronic hepatic encephalopathy | 3 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.21, 4.00] |
| 1.1.2 Minimal hepatic encephalopathy | 6 | 354 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.21, 1.78] |
| 1.1.3 Prevention of hepatic encephalopathy | 4 | 482 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.49, 1.70] |
| 1.2 Serious adverse events | 9 | 801 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.83, 1.32] |
| 1.2.1 Chronic hepatic encephalopathy | 2 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.39, 2.87] |
| 1.2.2 Minimal hepatic encephalopathy | 3 | 188 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [0.57, 7.86] |
| 1.2.3 Prevention of hepatic encephalopathy | 4 | 482 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.81, 1.30] |
| 1.3 Health‐related quality of life | 4 | 214 | Mean Difference (IV, Random, 95% CI) | ‐1.43 [‐2.87, 0.02] |
| 1.3.1 Chronic hepatic encephalopathy | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.73, 0.73] |
| 1.3.2 Minimal hepatic encephalopathy | 3 | 176 | Mean Difference (IV, Random, 95% CI) | ‐2.07 [‐2.79, ‐1.35] |
| 1.4 Hepatic encephalopathy | 13 | 1009 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.42, 0.77] |
| 1.4.1 Chronic hepatic encephalopathy | 3 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.29, 1.95] |
| 1.4.2 Minimal hepatic encephalopathy | 6 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.31, 0.52] |
| 1.4.3 Prevention of hepatic encephalopathy | 4 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.54, 1.03] |
| 1.5 Mortality (worst‐case) | 13 | 1007 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.62, 1.45] |
| 1.5.1 Chronic hepatic encephalopathy | 3 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.61, 2.30] |
| 1.5.2 Minimal hepatic encephalopathy | 6 | 354 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.21, 1.78] |
| 1.5.3 Prevention of hepatic encephalopathy | 4 | 482 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.49, 1.70] |
| 1.6 Serious adverse events (worst‐case) | 9 | 801 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.85, 1.32] |
| 1.6.1 Chronic hepatic encephalopathy | 2 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.66, 1.89] |
| 1.6.2 Minimal hepatic encephalopathy | 3 | 188 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [0.57, 7.86] |
| 1.6.3 Prevention of hepatic encephalopathy | 4 | 482 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.81, 1.30] |
| 1.7 Hepatic encephalopathy (worst‐case) | 13 | 1009 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.42, 0.79] |
| 1.7.1 Chronic hepatic encephalopathy | 3 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.52, 1.51] |
| 1.7.2 Minimal hepatic encephalopathy | 6 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.31, 0.52] |
| 1.7.3 Prevention of hepatic encephalopathy | 4 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.54, 1.03] |
| 1.8 Mortality (extreme worst‐case) | 13 | 1007 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.62, 1.77] |
| 1.8.1 Chronic hepatic encephalopathy | 3 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 4.43 [0.43, 45.34] |
| 1.8.2 Minimal hepatic encephalopathy | 6 | 354 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.21, 1.78] |
| 1.8.3 Prevention of hepatic encephalopathy | 4 | 482 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.49, 1.70] |
| 1.9 Serious adverse events (extreme worst‐case) | 9 | 801 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.87, 2.19] |
| 1.9.1 Chronic hepatic encephalopathy | 2 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 3.75 [0.62, 22.68] |
| 1.9.2 Minimal hepatic encephalopathy | 3 | 188 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [0.57, 7.86] |
| 1.9.3 Prevention of hepatic encephalopathy | 4 | 482 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.81, 1.30] |
| 1.10 Hepatic encephalopathy (extreme worst‐case) | 13 | 1009 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.44, 0.87] |
| 1.10.1 Chronic hepatic encephalopathy | 3 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.03, 1.90] |
| 1.10.2 Minimal hepatic encephalopathy | 6 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.31, 0.52] |
| 1.10.3 Prevention of hepatic encephalopathy | 4 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.54, 1.03] |
| 1.11 Mortality (best‐case) | 13 | 1007 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.50, 1.38] |
| 1.11.1 Chronic hepatic encephalopathy | 3 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.21, 4.00] |
| 1.11.2 Minimal hepatic encephalopathy | 6 | 354 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.21, 1.78] |
| 1.11.3 Prevention of hepatic encephalopathy | 4 | 482 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.49, 1.70] |
| 1.12 Serious adverse events (best‐case) | 9 | 801 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.83, 1.32] |
| 1.12.1 Chronic hepatic encephalopathy | 2 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.39, 2.87] |
| 1.12.2 Minimal hepatic encephalopathy | 3 | 188 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [0.57, 7.86] |
| 1.12.3 Prevention of hepatic encephalopathy | 4 | 482 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.81, 1.30] |
| 1.13 Hepatic encephalopathy (best‐case) | 13 | 1009 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.42, 0.77] |
| 1.13.1 Chronic hepatic encephalopathy | 3 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.29, 1.95] |
| 1.13.2 Minimal hepatic encephalopathy | 6 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.31, 0.52] |
| 1.13.3 Prevention of hepatic encephalopathy | 4 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.54, 1.03] |
| 1.14 Mortality (extreme best‐case) | 13 | 1007 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.41, 1.11] |
| 1.14.1 Chronic hepatic encephalopathy | 3 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.08, 0.86] |
| 1.14.2 Minimal hepatic encephalopathy | 6 | 354 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.21, 1.78] |
| 1.14.3 Prevention of hepatic encephalopathy | 4 | 482 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.49, 1.70] |
| 1.15 Serious adverse events (extreme best‐case) | 9 | 801 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.60, 1.36] |
| 1.15.1 Chronic hepatic encephalopathy | 2 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.18, 0.90] |
| 1.15.2 Minimal hepatic encephalopathy | 3 | 188 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [0.57, 7.86] |
| 1.15.3 Prevention of hepatic encephalopathy | 4 | 482 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.81, 1.30] |
| 1.16 Hepatic encephalopathy (extreme best‐case) | 13 | 1009 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.41, 0.73] |
| 1.16.1 Chronic hepatic encephalopathy | 3 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.13, 1.86] |
| 1.16.2 Minimal hepatic encephalopathy | 6 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.31, 0.52] |
| 1.16.3 Prevention of hepatic encephalopathy | 4 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.54, 1.03] |
| 1.17 Non‐serious adverse events | 6 | 639 | Risk Ratio (M‐H, Random, 95% CI) | 2.79 [0.44, 17.78] |
| 1.17.1 Minimal hepatic encephalopathy | 3 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 4.22 [0.52, 33.97] |
| 1.17.2 Prevention of hepatic encephalopathy | 3 | 432 | Risk Ratio (M‐H, Random, 95% CI) | 2.29 [0.25, 20.54] |
| 1.18 Blood ammonia | 6 | 381 | Mean Difference (IV, Random, 95% CI) | 3.20 [‐7.74, 14.14] |
| 1.18.1 Chronic hepatic encephalopathy | 2 | 78 | Mean Difference (IV, Random, 95% CI) | ‐3.29 [‐23.18, 16.61] |
| 1.18.2 Minimal hepatic encephalopathy | 2 | 96 | Mean Difference (IV, Random, 95% CI) | 1.59 [‐13.32, 16.50] |
| 1.18.3 Prevention of hepatic encephalopathy | 2 | 207 | Mean Difference (IV, Random, 95% CI) | 10.49 [‐14.00, 34.98] |
| 1.19 Blood ammonia (paired) | 4 | 184 | Mean Difference (IV, Random, 95% CI) | ‐3.40 [‐8.10, 1.30] |
| 1.19.1 Chronic hepatic encephalopathy | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐7.00 [‐13.16, ‐0.84] |
| 1.19.2 Minimal hepatic encephalopathy | 2 | 96 | Mean Difference (IV, Random, 95% CI) | ‐4.92 [‐11.40, 1.56] |
| 1.19.3 Prevention of hepatic encephalopathy | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 1.50 [‐0.48, 3.48] |
| 1.20 Number Connection Test A | 4 | 203 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐1.22, 0.60] |
| 1.20.1 Chronic hepatic encephalopathy | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.34, 0.94] |
| 1.20.2 Minimal hepatic encephalopathy | 2 | 115 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐2.87, 0.85] |
| 1.20.3 Prevention of hepatic encephalopathy | 1 | 50 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.14, 0.99] |
| 1.21 Number Connection Test A (paired) | 4 | 203 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.71, 1.25] |
| 1.21.1 Chronic hepatic encephalopathy | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.54, 0.73] |
| 1.21.2 Minimal hepatic encephalopathy | 2 | 115 | Std. Mean Difference (IV, Random, 95% CI) | 0.69 [‐1.77, 3.15] |
| 1.21.3 Prevention of hepatic encephalopathy | 1 | 50 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.86, 0.25] |
Comparison 2. Rifaximin versus non‐absorbable disaccharides.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Mortality | 10 | 786 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.49, 1.97] |
| 2.1.1 Acute hepatic encephalopathy | 2 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.05, 5.66] |
| 2.1.2 Chronic hepatic encephalopathy | 2 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 2.80 [0.12, 63.20] |
| 2.1.3 Minimal hepatic encephalopathy | 3 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 3.93] |
| 2.1.4 Prevention of hepatic encephalopathy | 3 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.51, 2.36] |
| 2.2 Serious adverse events | 8 | 681 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.66, 1.40] |
| 2.2.1 Acute hepatic encephalopathy | 3 | 328 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.31, 2.97] |
| 2.2.2 Minimal hepatic encephalopathy | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 3.86 [0.86, 17.38] |
| 2.2.3 Prevention of hepatic encephalopathy | 3 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.58, 1.31] |
| 2.3 Health‐related quality of life | 2 | 249 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐1.65, 0.98] |
| 2.3.1 Acute hepatic encephalopathy | 1 | 171 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐1.87, 2.47] |
| 2.3.2 Minimal hepatic encephalopathy | 1 | 78 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐2.36, 0.96] |
| 2.4 Hepatic encephalopathy | 13 | 921 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.69, 1.05] |
| 2.4.1 Acute hepatic encephalopathy | 4 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.30, 1.38] |
| 2.4.2 Chronic hepatic encephalopathy | 4 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.49, 1.24] |
| 2.4.3 Minimal hepatic encephalopathy | 2 | 184 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.58, 1.45] |
| 2.4.4 Prevention of hepatic encephalopathy | 3 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.54, 1.46] |
| 2.5 Mortality (worst‐case) | 10 | 786 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.55, 1.56] |
| 2.5.1 Acute hepatic encephalopathy | 2 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.42, 2.17] |
| 2.5.2 Chronic hepatic encephalopathy | 2 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.12, 3.13] |
| 2.5.3 Minimal hepatic encephalopathy | 3 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 3.93] |
| 2.5.4 Prevention of hepatic encephalopathy | 3 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.51, 2.36] |
| 2.6 Mortality (extreme worst‐case) | 10 | 786 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.51, 2.03] |
| 2.6.1 Acute hepatic encephalopathy | 2 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.05, 5.66] |
| 2.6.2 Chronic hepatic encephalopathy | 2 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 4.67 [0.24, 88.96] |
| 2.6.3 Minimal hepatic encephalopathy | 3 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 3.93] |
| 2.6.4 Prevention of hepatic encephalopathy | 3 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.51, 2.36] |
| 2.7 Mortality (best‐case) | 10 | 786 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.49, 1.97] |
| 2.7.1 Acute hepatic encephalopathy | 2 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.05, 5.66] |
| 2.7.2 Chronic hepatic encephalopathy | 2 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 2.80 [0.12, 63.20] |
| 2.7.3 Minimal hepatic encephalopathy | 3 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 3.93] |
| 2.7.4 Prevention of hepatic encephalopathy | 3 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.51, 2.36] |
| 2.8 Mortality (extreme best‐case) | 10 | 786 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.43, 1.64] |
| 2.8.1 Acute hepatic encephalopathy | 2 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.05, 5.66] |
| 2.8.2 Chronic hepatic encephalopathy | 2 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.04, 2.61] |
| 2.8.3 Minimal hepatic encephalopathy | 3 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 3.93] |
| 2.8.4 Prevention of hepatic encephalopathy | 3 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.51, 2.36] |
| 2.9 Serious adverse events (extreme best‐case) | 8 | 681 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.66, 1.40] |
| 2.9.1 Acute hepatic encephalopathy | 3 | 328 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.31, 2.97] |
| 2.9.2 Minimal hepatic encephalopathy | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 3.86 [0.86, 17.38] |
| 2.9.3 Prevention of hepatic encephalopathy | 3 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.58, 1.31] |
| 2.10 Serious adverse events (best‐case) | 8 | 681 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.64, 1.36] |
| 2.10.1 Acute hepatic encephalopathy | 3 | 328 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.22, 2.16] |
| 2.10.2 Minimal hepatic encephalopathy | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 3.86 [0.86, 17.38] |
| 2.10.3 Prevention of hepatic encephalopathy | 3 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.58, 1.31] |
| 2.11 Serious adverse events (worst‐case) | 8 | 681 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.64, 1.36] |
| 2.11.1 Acute hepatic encephalopathy | 3 | 328 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.22, 2.16] |
| 2.11.2 Minimal hepatic encephalopathy | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 3.86 [0.86, 17.38] |
| 2.11.3 Prevention of hepatic encephalopathy | 3 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.58, 1.31] |
| 2.12 Serious adverse events (extreme worst‐case) | 8 | 681 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.64, 1.36] |
| 2.12.1 Acute hepatic encephalopathy | 3 | 328 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.22, 2.16] |
| 2.12.2 Minimal hepatic encephalopathy | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 3.86 [0.86, 17.38] |
| 2.12.3 Prevention of hepatic encephalopathy | 3 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.58, 1.31] |
| 2.13 Hepatic encephalopathy (worst‐case) | 13 | 921 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.68, 1.01] |
| 2.13.1 Acute hepatic encephalopathy | 4 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.30, 1.38] |
| 2.13.2 Chronic hepatic encephalopathy | 4 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.55, 1.06] |
| 2.13.3 Minimal hepatic encephalopathy | 2 | 184 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.58, 1.45] |
| 2.13.4 Prevention of hepatic encephalopathy | 3 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.54, 1.46] |
| 2.14 Hepatic encephalopathy (extreme worst‐case) | 13 | 921 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.71, 1.09] |
| 2.14.1 Acute hepatic encephalopathy | 4 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.30, 1.38] |
| 2.14.2 Chronic hepatic encephalopathy | 4 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.61, 1.40] |
| 2.14.3 Minimal hepatic encephalopathy | 2 | 184 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.58, 1.45] |
| 2.14.4 Prevention of hepatic encephalopathy | 3 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.54, 1.46] |
| 2.15 Hepatic encephalopathy (best‐case) | 13 | 921 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.69, 1.05] |
| 2.15.1 Acute hepatic encephalopathy | 4 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.30, 1.38] |
| 2.15.2 Chronic hepatic encephalopathy | 4 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.49, 1.24] |
| 2.15.3 Minimal hepatic encephalopathy | 2 | 184 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.58, 1.45] |
| 2.15.4 Prevention of hepatic encephalopathy | 3 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.54, 1.46] |
| 2.16 Hepatic encephalopathy (extreme best‐case) | 13 | 921 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.64, 0.99] |
| 2.16.1 Acute hepatic encephalopathy | 4 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.30, 1.38] |
| 2.16.2 Chronic hepatic encephalopathy | 4 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.43, 0.97] |
| 2.16.3 Minimal hepatic encephalopathy | 2 | 184 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.58, 1.45] |
| 2.16.4 Prevention of hepatic encephalopathy | 3 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.54, 1.46] |
| 2.17 Non‐serious adverse events | 6 | 396 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.15, 2.13] |
| 2.17.1 Acute hepatic encephalopathy | 2 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.29, 5.41] |
| 2.17.2 Chronic hepatic encephalopathy | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.17.3 Minimal hepatic encephalopathy | 3 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.02, 3.88] |
| 2.17.4 Prevention of hepatic encephalopathy | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.18 Blood ammonia | 10 | 599 | Mean Difference (IV, Random, 95% CI) | ‐6.78 [‐12.81, ‐0.75] |
| 2.18.1 Acute hepatic encephalopathy | 4 | 378 | Mean Difference (IV, Random, 95% CI) | ‐8.74 [‐26.31, 8.83] |
| 2.18.2 Chronic hepatic encephalopathy | 4 | 141 | Mean Difference (IV, Random, 95% CI) | ‐5.30 [‐9.02, ‐1.58] |
| 2.18.3 Minimal hepatic encephalopathy | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐15.00 [‐16.62, ‐13.38] |
| 2.18.4 Prevention of hepatic encephalopathy | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 2.60 [‐7.56, 12.76] |
| 2.19 Blood ammonia (paired) | 9 | 565 | Mean Difference (IV, Random, 95% CI) | ‐1.65 [‐4.48, 1.18] |
| 2.19.1 Acute hepatic encephalopathy | 4 | 378 | Mean Difference (IV, Random, 95% CI) | ‐3.11 [‐7.21, 0.98] |
| 2.19.2 Chronic hepatic encephalopathy | 3 | 107 | Mean Difference (IV, Random, 95% CI) | 2.69 [‐1.89, 7.27] |
| 2.19.3 Minimal hepatic encephalopathy | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐13.00 [‐15.18, ‐10.82] |
| 2.19.4 Prevention of hepatic encephalopathy | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 4.50 [2.17, 6.83] |
| 2.20 Number Connection Test A | 7 | 507 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.46, 0.09] |
| 2.20.1 Acute hepatic encephalopathy | 2 | 218 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐1.05, 0.21] |
| 2.20.2 Chronic hepatic encephalopathy | 2 | 80 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.93, ‐0.04] |
| 2.20.3 Minimal hepatic encephalopathy | 2 | 159 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.37, 0.25] |
| 2.20.4 Prevention of hepatic encephalopathy | 1 | 50 | Std. Mean Difference (IV, Random, 95% CI) | 0.54 [‐0.02, 1.11] |
| 2.21 Number Connection Test A (paired) | 8 | 610 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.85, 1.16] |
| 2.21.1 Acute hepatic encephalopathy | 3 | 321 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐1.77, 1.08] |
| 2.21.2 Chronic hepatic encephalopathy | 2 | 80 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.61 [‐3.97, 0.75] |
| 2.21.3 Minimal hepatic encephalopathy | 2 | 159 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.36, 0.26] |
| 2.21.4 Prevention of hepatic encephalopathy | 1 | 50 | Std. Mean Difference (IV, Random, 95% CI) | 6.82 [5.32, 8.32] |
| 2.22 Length of hospital stay | 1 | 120 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.33, 0.01] |
| 2.22.1 Prevention of hepatic encephalopathy | 1 | 120 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.33, 0.01] |
Comparison 3. Rifaximin plus non‐absorbable disaccharides versus non‐absorbable disaccharides alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Mortality | 14 | 1946 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.55, 0.86] |
| 3.1.1 Acute hepatic encephalopathy | 7 | 776 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.46, 0.92] |
| 3.1.2 Chronic hepatic encephalopathy | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 3.1.3 Prevention of hepatic encephalopathy | 6 | 1144 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.58, 1.39] |
| 3.2 Serious adverse events | 7 | 1076 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.45, 0.98] |
| 3.2.1 Acute hepatic encephalopathy | 3 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.37, 1.14] |
| 3.2.2 Chronic hepatic encephalopathy | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 3.2.3 Prevention of hepatic encephalopathy | 3 | 657 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.33, 1.40] |
| 3.3 Hepatic encephalopathy | 17 | 2332 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.48, 0.71] |
| 3.3.1 Acute hepatic encephalopathy | 8 | 944 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.45, 0.87] |
| 3.3.2 Chronic hepatic encephalopathy | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.28, 2.32] |
| 3.3.3 Prevention of hepatic encephalopathy | 8 | 1362 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.42, 0.69] |
| 3.4 Mortality (extreme best‐case) | 14 | 1947 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.49, 0.76] |
| 3.4.1 Acute hepatic encephalopathy | 7 | 776 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.42, 0.70] |
| 3.4.2 Chronic hepatic encephalopathy | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 3.56] |
| 3.4.3 Prevention of hepatic encephalopathy | 6 | 1144 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.58, 1.39] |
| 3.5 Mortality (best‐case) | 14 | 1947 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.55, 0.86] |
| 3.5.1 Acute hepatic encephalopathy | 7 | 776 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.46, 0.92] |
| 3.5.2 Chronic hepatic encephalopathy | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 3.5.3 Prevention of hepatic encephalopathy | 6 | 1144 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.58, 1.39] |
| 3.6 Mortality (worst‐case) | 14 | 1947 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.51, 0.78] |
| 3.6.1 Acute hepatic encephalopathy | 7 | 776 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.44, 0.72] |
| 3.6.2 Chronic hepatic encephalopathy | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.15, 5.67] |
| 3.6.3 Prevention of hepatic encephalopathy | 6 | 1144 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.58, 1.39] |
| 3.7 Mortality (extreme worst‐case) | 14 | 1947 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.57, 0.93] |
| 3.7.1 Acute hepatic encephalopathy | 7 | 776 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.45, 1.02] |
| 3.7.2 Chronic hepatic encephalopathy | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 4.67 [0.24, 88.96] |
| 3.7.3 Prevention of hepatic encephalopathy | 6 | 1144 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.58, 1.39] |
| 3.8 Serious adverse events (worst‐case) | 7 | 1077 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.47, 0.97] |
| 3.8.1 Acute hepatic encephalopathy | 3 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.37, 1.14] |
| 3.8.2 Chronic hepatic encephalopathy | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.15, 5.67] |
| 3.8.3 Prevention of hepatic encephalopathy | 3 | 657 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.34, 1.39] |
| 3.9 Serious adverse events (extreme worst‐case) | 7 | 1077 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.47, 1.04] |
| 3.9.1 Acute hepatic encephalopathy | 3 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.37, 1.14] |
| 3.9.2 Chronic hepatic encephalopathy | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 4.67 [0.24, 88.96] |
| 3.9.3 Prevention of hepatic encephalopathy | 3 | 657 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.35, 1.46] |
| 3.10 Serious adverse events (best‐case) | 7 | 1077 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.45, 0.98] |
| 3.10.1 Acute hepatic encephalopathy | 3 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.37, 1.14] |
| 3.10.2 Chronic hepatic encephalopathy | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 3.10.3 Prevention of hepatic encephalopathy | 3 | 657 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.33, 1.40] |
| 3.11 Serious adverse events (extreme best‐case) | 7 | 1077 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.44, 0.94] |
| 3.11.1 Acute hepatic encephalopathy | 3 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.37, 1.14] |
| 3.11.2 Chronic hepatic encephalopathy | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 3.56] |
| 3.11.3 Prevention of hepatic encephalopathy | 3 | 657 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.32, 1.35] |
| 3.12 Hepatic encephalopathy (worst‐case) | 17 | 2333 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.48, 0.70] |
| 3.12.1 Acute hepatic encephalopathy | 8 | 944 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.45, 0.83] |
| 3.12.2 Chronic hepatic encephalopathy | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.36, 1.75] |
| 3.12.3 Prevention of hepatic encephalopathy | 8 | 1362 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.42, 0.70] |
| 3.13 Hepatic encephalopathy (extreme worst‐case) | 17 | 2333 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.49, 0.71] |
| 3.13.1 Acute hepatic encephalopathy | 8 | 944 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.45, 0.83] |
| 3.13.2 Chronic hepatic encephalopathy | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.45, 2.78] |
| 3.13.3 Prevention of hepatic encephalopathy | 8 | 1362 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.42, 0.70] |
| 3.14 Hepatic encephalopathy (best‐case) | 17 | 2333 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.48, 0.71] |
| 3.14.1 Acute hepatic encephalopathy | 8 | 944 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.45, 0.87] |
| 3.14.2 Chronic hepatic encephalopathy | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.25, 2.18] |
| 3.14.3 Prevention of hepatic encephalopathy | 8 | 1362 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.42, 0.69] |
| 3.15 Hepatic encephalopathy (extreme best‐case) | 17 | 2333 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.47, 0.68] |
| 3.15.1 Acute hepatic encephalopathy | 8 | 944 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.45, 0.81] |
| 3.15.2 Chronic hepatic encephalopathy | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.20, 1.40] |
| 3.15.3 Prevention of hepatic encephalopathy | 8 | 1362 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.42, 0.69] |
| 3.16 Non‐serious adverse events | 4 | 384 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.86, 1.15] |
| 3.16.1 Chronic hepatic encephalopathy | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 1.95] |
| 3.16.2 Prevention of hepatic encephalopathy | 3 | 358 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.86, 1.16] |
| 3.17 Blood ammonia | 2 | 325 | Mean Difference (IV, Random, 95% CI) | ‐6.88 [‐14.78, 1.02] |
| 3.17.1 Chronic hepatic encephalopathy | 1 | 26 | Mean Difference (IV, Random, 95% CI) | ‐10.00 [‐22.32, 2.32] |
| 3.17.2 Prevention of hepatic encephalopathy | 1 | 299 | Mean Difference (IV, Random, 95% CI) | ‐4.70 [‐14.99, 5.59] |
| 3.18 Blood ammonia (paired) | 1 | 26 | Mean Difference (IV, Random, 95% CI) | ‐2.00 [‐11.50, 7.50] |
| 3.18.1 Chronic hepatic encephalopathy | 1 | 26 | Mean Difference (IV, Random, 95% CI) | ‐2.00 [‐11.50, 7.50] |
| 3.19 Number Connection Test A | 2 | 76 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐1.28, 1.17] |
| 3.19.1 Chronic hepatic encephalopathy | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.51, 0.08] |
| 3.19.2 Prevention of hepatic encephalopathy | 1 | 50 | Std. Mean Difference (IV, Random, 95% CI) | 0.54 [‐0.02, 1.11] |
| 3.20 Number Connection Test A (paired) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.20.1 Chronic hepatic encephalopathy | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.21 Length of hospital stay | 3 | 408 | Mean Difference (IV, Random, 95% CI) | ‐2.86 [‐3.46, ‐2.26] |
| 3.21.1 Acute hepatic encephalopathy | 3 | 408 | Mean Difference (IV, Random, 95% CI) | ‐2.86 [‐3.46, ‐2.26] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmed 2018.
| Study characteristics | ||
| Methods | Randomised, open‐label clinical trial | |
| Participants | The trial assessed the effects of rifaximin plus lactulose versus lactulose alone in 120 people with decompensated cirrhosis who presented with an acute episode of hepatic encephalopathy, grades I‐IV
There were 60 participants in each of the groups. Age (mean ± SD) years
Proportion of men (%)
Aetiology of cirrhosis (n: %)
|
|
| Interventions | Intervention: rifaximin 550 mg twice daily plus lactulose 30 mL thrice daily Control intervention: lactulose 30 mL thrice daily Co‐interventions: none Duration of treatment: 3 days |
|
| Outcomes | Neuropsychiatric assessment Mental status: West Haven criteria |
|
| Inclusion period | January 2017 to August 2017 | |
| Outcomes included in meta‐analyses | Mortality, hepatic encephalopathy | |
| Country of origin | Single centre in Pakistan | |
| Notes |
Publication status: full paper Vested interests bias: none reported. Additional information: overall 76.7% of the participants had grade III/IV hepatic encephalopathy at inclusion. Efficacy was determined by the reversal of grade III/IV hepatic encephalopathy to grade 0/1. However, 23.2% of the participants had grade I/II hepatic encephalopathy at inclusion and no mention is made about how treatment efficacy was measured in these. Mortality deduced as all participant outcomes were accounted for. Authors contacted for further data on the aetiology of cirrhosis of participants, trial registry status, conflicts of interest, mortality outcomes, and adverse events. Request sent on 3 April 2021; still awaiting response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation via lottery method |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open trial with no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open trial with no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants are accounted for and included in the results |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported on |
| Other bias | Low risk | No other bias identified |
| Overall bias assessment (mortality) | Low risk | Mortality deduced as all participant outcomes were accounted for |
| Overall bias assessment (non‐mortality outcomes) | High risk | Open trial with no blinding |
Ali 2014.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled clinical trial | |
| Participants | This trial assessed the effects of rifaximin versus placebo for secondary prevention of hepatic encephalopathy in 126 people with cirrhosis and a history of at least 2 episodes of overt hepatic encephalopathy in the preceding 6 months but who were in remission at baseline. As all participants were allowed to take lactulose throughout the trial, the comparison made was effectively rifaximin plus lactulose versus lactulose alone. There were 63 participants in the both the rifaximin and placebo groups. Age (mean ± SD) years
Proportion of men (%)
Aetiology of cirrhosis (n: %) Rifaximin plus lactulose
Placebo plus lactulose
|
|
| Interventions | Intervention: rifaximin 550 mg twice daily plus lactulose of unknown dose Control intervention: identically presented and packaged placebo twice daily plus lactulose of unknown dose Co‐intervention: all participants received lactulose before randomisation and continued on lactulose throughout the trial. Duration of treatment: 6 months or until first breakthrough episode of hepatic encephalopathy, adverse event or death |
|
| Outcomes | Neuropsychiatric assessment Mental status: West Haven criteria Breakthrough episode of hepatic encephalopathy defined as West Haven score of 2 or more. |
|
| Inclusion period | October 2012 to April 2013 | |
| Outcomes included in meta‐analyses | Mortality, adverse events, blood ammonia | |
| Country of origin | Single centre in India | |
| Notes |
Publication status: full paper Vested interests bias: rifaximin and placebo preparation supplied by Brooke's Pharmaceuticals who also paid an honorarium to the Principal Investigator. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and personnel using placebo of the same size, shape and colour with similar packing (page 270, first column). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment using placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants are accounted for and included in the description of the results with carry forward of the last observed response (126 participants are randomised and the same number of participants are included in the analysis). |
| Selective reporting (reporting bias) | Unclear risk | Clinically relevant outcomes are reported. Tables 3 and 4 are referred to, but missing in the published report and the data were not otherwise retrievable from the trialists or publisher. |
| Other bias | Low risk | No other biases identified |
| Overall bias assessment (mortality) | Unclear risk | Data on deaths pre‐trial and during the trial are reported in the text despite the missing Tables |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of reporting bias |
Babar 2017.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo controlled clinical trial | |
| Participants | This trial assessed the effects of rifaximin versus placebo for secondary prevention of hepatic encephalopathy in 96 people with cirrhosis and a history of at least 2 episodes of overt hepatic encephalopathy in the preceding 6 months but who were in remission at baseline. As all participants were allowed to take lactulose throughout the trial, the comparison made was effectively rifaximin plus lactulose vs lactulose alone There were 45 participants in the rifaximin plus lactulose group and 43 in the placebo plus lactulose group Age (mean ± SD) years
Proportion of men (%)
Aetiology of cirrhosis (n: %) Rifaximin plus lactulose
Placebo plus lactulose
|
|
| Interventions | Intervention: rifaximin 550 mg twice daily plus lactulose of unknown dose Control intervention: placebo twice daily (no details provided) plus lactulose of unknown dose Co‐intervention: participants were allowed to take lactulose throughout the trial Duration of treatment: 6 months or until first breakthrough episode of hepatic encephalopathy |
|
| Outcomes | Neuropsychiatric assessment Mental status: West Haven criteria Breakthrough episode defined by a West Haven score of 2 or more |
|
| Inclusion period | January 2016 to June 2016 | |
| Outcomes included in meta‐analyses | Mortality, hepatic encephalopathy, adverse events | |
| Country of origin | Single centre in Pakistan | |
| Notes |
Publication status: full paper Vested interests bias : none declared Additional information: authors contacted for further information on trial registration status, allocation concealment methods, blinding methods, and whether they collected data on our secondary outcomes. Request sent on 3 April 2021; still awaiting response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors state that: 'participants [...] were randomized by simple lottery method 1:1', page 16. |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind trial but no details are provided of the placebo preparation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind trial but no details are provided of the placebo preparation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants accounted for in report; unclear if the participants lost to follow‐up were included in the analyses |
| Selective reporting (reporting bias) | Low risk | All data published in full paper report |
| Other bias | Low risk | No other bias detected |
| Overall bias assessment (mortality) | Low risk | Low overall risk; deaths reported |
| Overall bias assessment (non‐mortality outcomes) | Unclear risk | Unclear risk of blinding and attrition bias |
Bajaj 2011.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled clinical trial | |
| Participants | This trial assessed the effects of rifaximin versus placebo on driving performance and neuropsychiatric status in 42 people with cirrhosis and minimal hepatic encephalopathy who had never experienced an episode of overt hepatic encephalopathy; were not receiving prophylactic medication; and, were current car drivers. There were 21 participants in each group. Age (mean ± SD) years
Proportion of men (%)
Aetiology of cirrhosis (n: %) Rifaximin
Control
|
|
| Interventions | Intervention: rifaximin 550 mg twice a day Control intervention: placebo ‐ no details provided Co‐intervention: not reported, although participants were excluded if they were taking lactulose or other antibiotics on enrolment. Duration of treatment: 8 weeks |
|
| Outcomes | Neuropsychiatric assessment Driving performance assessed using a simulator; number connection tests A and B, digit symbol, and block design test; number of lures in the inhibitory control test; venous blood ammonia |
|
| Inclusion period | October 2007 to February 2010 | |
| Outcomes included in meta‐analyses | Mortality, hepatic encephalopathy (as a percentage of people with improvement of performance in the cognitive battery), adverse events, health‐related quality of life (Sickness Impact Profile), venous blood ammonia | |
| Country of origin | Two centres in USA | |
| Notes |
Publication status: full paper Vested interests bias: support from Valeant Pharmaceuticals Inc stated at clinicaltrials.gov. Dr Bajaj had received funding and had been on advisory boards and acted as a consultant for Salix Pharmaceuticals and Ocera Therapeutics. Dr Sanyal was a consultant and was an advisory board member for Salix Pharmaceuticals at the time of publication. Salix provided funding but was not involved in protocol design and implementation, data collection, analysis or interpretation of the study results. Additional information: authors contacted April 2015 and data on follow‐up and mortality was retrieved. Authors contacted again for data on participant characteristics, randomisation methods, allocation concealment methods, blinding methods, and non‐serious adverse events. Request sent on 3 April 2021; still awaiting response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised equally into placebo and active drug groups base on blocks of four provided directly to the investigational pharmacy. |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study was double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | An intention‐to‐treat analysis was planned with the “last measurement carried forward” for all patients who did not come for their last visit. One participant in the placebo group withdrew after randomisation but was still included in the analysis. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes reported, data on mortality supplemented by corresponding author. |
| Other bias | Low risk | No other biases detected |
| Overall bias assessment (mortality) | Low risk | All domains are at a low risk of bias |
| Overall bias assessment (non‐mortality outcomes) | Low risk | All domains are at a low risk of bias |
Bajaj 2019.
| Study characteristics | ||
| Methods | Two randomised trials, from which data were pooled. Trial 1: randomised, phase 3, double‐blind trial Trial 2: randomised, phase 4, open‐label trial |
|
| Participants | These trials assessed the effect of rifaximin (± lactulose) versus lactulose (permitted in trial 1, mandatory in trial 2) (+ placebo) for the secondary prevention of hepatic encephalopathy in people with cirrhosis who had experienced an episode of acute hepatic encephalopathy in the preceding 6 months but currently had a West Haven score of 1 or less Rifaximin plus lactulose (n = 236); lactulose (plus placebo) alone (n = 145) Age (mean) years
Proportion of men (n: %)
Aetiology of cirrhosis: not reported Participants in complete remission at baseline ( %)
|
|
| Interventions | Intervention: Trial 1: rifaximin 550 mg twice daily with or without concurrent lactulose, titrated to produce 2 to 3 semi‐soft stools per day Trial 2: rifaximin 550 mg twice daily with concurrent lactulose, titrated to produce 2 to 3 soft stools per day Control interventions: Trial 1: placebo with or without concurrent lactulose, titrated to produce 2 to 3 semi‐soft stools per day Trial 2: lactulose, titrated to produce 2 to 3 semi‐soft stools per day Co‐interventions: not reported Duration of therapy: not specifically reported ‐ up to 6 months |
|
| Outcomes | Neuropsychiatric assessment Mental status: West Haven criteria Breakthrough episode of hepatic encephalopathy defined as West Haven criteria score of 2 or more |
|
| Inclusion period | Not reported | |
| Outcomes included in meta‐analyses | Mortality, serious adverse events (hospitalisation), hepatic encephalopathy, adverse events | |
| Country of origin | Unknown | |
| Notes |
Publication status: abstract only Vested interests bias: no conflicts of interest were declared Additional information: authors contacted for further information on study centres, co‐interventions used, participant characteristics, trial registration status, country of origin, conflicts of interest, randomisation and blinding methods, complete outcome reporting, and whether the data from the two pooled studies could be split to allow further analyses. The request was sent on 3 April 2021; still awaiting response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors report randomisation, but have not stated how this was conducted. |
| Allocation concealment (selection bias) | Unclear risk | No information provided by the authors |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Trial 1 was double‐blind ‐ although the methods of blinding are not reported by the authors. Trial 2 was open‐label, which introduces bias. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Trial 1 was double‐blind ‐ although the methods of blinding are not reported by the authors. Trial 2 was open‐label, which introduces bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported by the authors |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to a protocol or trial registry to assess whether all outcomes were reported. |
| Other bias | Low risk | No other bias identified |
| Overall bias assessment (mortality) | Unclear risk | One or more domains was deemed 'unclear risk' of bias. |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of performance and detection bias |
Bass 2004.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled clinical trial | |
| Participants | This trial assessed the effects of rifaximin versus placebo in 96 people with cirrhosis and mild to moderate chronic hepatic encephalopathy, who were intolerant of lactulose or lactitol There were 48 participants in the rifaximin group and 45 in the placebo group. Age (mean) years
Proportion of men
Aetiology of cirrhosis
|
|
| Interventions | Intervention: rifaximin 400 mg three times daily Control group: placebo three times daily Co‐interventions: participants did not receive concomitant disaccharides during the study Duration of treatment: 14 days |
|
| Outcomes | Neuropsychiatric assessment Modified PSE Sum/Index: mental status (West Haven criteria), asterixis, NCT‐A, blood ammonia |
|
| Inclusion period | Not reported | |
| Outcomes included in meta‐analyses | Mortality, hepatic encephalopathy, adverse events | |
| Country of origin | Multiple centres in the USA, Poland, Hungary, and the UK | |
| Notes |
Publication status: abstract only Vested interests bias: co‐author an employee of Salix pharmaceuticals Additional information: information on safety and mortality was retrieved from FDA: www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/GastrointestinalDrugsAdvisoryCommittee/UCM201081.pdf Accessed 26 January 2015. Study reference RFHE9901 by the FDA and Salix Pharmaceuticals. A later published FDA summary of the Salix sponsored rifaximin studies states "RFHE9901 is too short in duration and targeted an inappropriate patient population (patients with active HE)." Planned enrolment was 112 subjects (56 per group); 79 participants completed the trial (39 in rifaximin group, 40 in placebo group). Thus, the study is significantly underpowered. Authors contacted for more data on the characteristics of the participants, blinding methods, the handling of incomplete outcomes, adverse events, quality of life outcomes, and specific data on blood ammonia concentrations. Information requested on 3 April 2021; response received on 11 April 2021 agreeing to attempt to assist; still awaiting response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised to treatment with rifaximin or placebo; no more details available. |
| Allocation concealment (selection bias) | Unclear risk | No information available on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Methods of blinding of participants and personnel is not stated in abstract or supplemental information from FDA. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported in abstract or additional information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Follow‐up not reported in abstract Possible dropouts not reported in abstract. Additional information states that 14 participants were withdrawn from the trial (15%). The trial planned enrolment of 120 people. 93 were included. 79 completed the trial. |
| Selective reporting (reporting bias) | High risk | Results are published as a journal abstract containing few data. The primary end point is not reported in published abstract. Information on mortality, safety and attrition was obtained through official documents from the trial sponsor Salix Pharmaceuticals on the FDA web site but is still incomplete. A report from FDA states that: "Note, that from the statistical standpoint, the analysis of the numerous secondary endpoints and their varied post‐hoc analyses cannot establish evidence of a positive effect." However, the title of the published abstract states that rifaximin is beneficial for mild HE. Source: www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022554Orig1s000MedR.pdf, accessed 26 January 2016 A further FDA document comments that the trial was "too short in duration and targeted an inappropriate patient population (patients with active HE)." |
| Other bias | High risk | Power calculation states that 120 participants are needed. 93 participants were randomised, so the trial is underpowered. |
| Overall bias assessment (mortality) | High risk | Data on mortality not published in abstract. High risk of reporting and other bias. |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of reporting and other bias. |
Bass 2010.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled clinical trial | |
| Participants | This trial assessed the effects of rifaximin, compared to placebo, for secondary prevention of hepatic encephalopathy in 299 people with cirrhosis and a history of at least 2 episodes of acute hepatic encephalopathy in the preceding 6 months but who were currently in remission; the majority were taking lactulose on entry into the trial and throughout its duration. Thus, the comparison made was effectively rifaximin plus lactulose vs lactulose alone. There were 140 participants in the rifaximin + lactulose group and 159 in the placebo plus lactulose group. Age (mean ± SD) years
Proportion of men (n: %)
Aetiology of cirrhosis (n: %) Rifaximin plus lactulose
Placebo plus lactulose
|
|
| Interventions | Intervention: rifaximin 550 mg twice daily Control: identically presented placebo twice daily Co‐intervention: 91% of participants in both groups were taking lactulose at baseline and throughout the trial Duration of treatment: 180 days (6 months) |
|
| Outcomes | Neuropsychiatric assessment Mental status: West Haven criteria; asterixis |
|
| Inclusion period | December 2005 to August 2008 | |
| Outcomes included in meta‐analyses | Mortality, health‐related quality of life (Sickness Impact Profile), hepatic encephalopathy, adverse events, blood ammonia | |
| Country of origin | A total of 70 investigatory sites in the USA, Canada and Russia | |
| Notes |
Publication status: full paper Vested interests bias: study was funded by Bausch Health Americas, Inc. and supported by Salix Pharmaceuticals Additional Information: information on ammonia concentrations, causes of death, and disease aetiology was retrieved from: www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/GastrointestinalDrugsAdvisoryCommittee/UCM201081.pdf Authors contacted for more data on the allocation concealment of participants, blinding of outcome assessment, adverse events and tabular data on quality of life outcomes. Request sent on 3 April 2021, reply received on 11 April 2021 agreeing to attempt to assist with access to these data; still awaiting response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible participants were randomly assigned, in a 1:1 ratio, to receive either 550 mg of rifaximin or placebo; no further detals provided. |
| Allocation concealment (selection bias) | Low risk | Investigators were unaware of the allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as blinded to both authors and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in journal article. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in journal article |
| Other bias | Low risk | Secondary outcome reported in journal article |
| Overall bias assessment (mortality) | Low risk | All domains were at a low risk of bias. |
| Overall bias assessment (non‐mortality outcomes) | Low risk | All domains were at a low risk of bias. |
Bucci 1993.
| Study characteristics | ||
| Methods | Randomised, double‐blind, double‐dummy clinical trial | |
| Participants | This trial assessed the effects of rifaximin versus lactulose in 58 participants with cirrhosis and chronic hepatic encephalopathy. There were 30 participants in the rifaximin group and 28 in the placebo group. Age (mean ± SD) years
Proportion of men (n: %)
Aetiology of cirrhosis (n: %) Rifaximin
Lactulose
|
|
| Interventions | Intervention: rifaximin 2 tablets of 200 mg plus 10 gram sachets of placebo (sorbitol), thrice daily Control group: lactulose 10 gram sachets plus 2 tablets of rifaximin placebo, thrice daily Co‐interventions: none Duration of treatment: 15 days |
|
| Outcomes | Neuropsychiatric assessment Modified PSE Sum/Index comprises scores for: mental status (West Haven criteria), asterixis, cancelling A test, NCT‐A, EEG mean frequency, fasting venous blood ammonia |
|
| Inclusion period | Not reported | |
| Outcomes included in meta‐analyses | Hepatic encephalopathy defined by improvement of modified PSE Sum, serious adverse events, blood ammonia concentrations | |
| Country of origin | Single centre in Italy | |
| Notes |
Publication status: full paper Vested interests bias: trial sponsored by Alfa Wassermann Additional information: individual participants data supplied by Alfa Wassermann |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information from Alfa Wassermann: "List, manually prepared by Alfa Wassermann Medical Department." Judgement: probably random generation |
| Allocation concealment (selection bias) | Low risk | Quote: "...a double‐dummy experimental design" p. 110 in published report. Judgement: participants received rifaximin + placebo or lactulose + placebo. No risk of selection bias detected. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial stated as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No other biases detected |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All primary end points reported. No participants unaccounted for. |
| Selective reporting (reporting bias) | Low risk | Additional information on primary end point achieved from Alfa Wassermann (January 2013). |
| Other bias | Low risk | No other bias detected |
| Overall bias assessment (mortality) | Low risk | Low risk of bias in all domains |
| Overall bias assessment (non‐mortality outcomes) | Low risk | Low risk of bias in all domains |
Bureau 2021.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled clinical trial | |
| Participants | This trial assessed the effects of rifaximin versus placebo for the prevention of hepatic encephalopathy in people with cirrhosis being considered for insertion of a transjugular intrahepatic portosystemic shunt (TIPS) to control resistant ascites (86%) or prevent variceal re‐bleeding (16%). A total of 197 participants were randomised, but 11 were either ineligible; did not receive a TIPS; did not receive the study drug; or withdrew consent. Data on the remaining 186 eligible participants were included in the efficacy analysis. There were 93 participants in both groups. Age (mean (SEM)) years
Proportion of men (n: %)
Aetiology of cirrhosis (n: %) Rifaximin group
Placebo group
|
|
| Interventions | Intervention: rifaximin capsules 600 mg twice daily Control: identically presented placebo capsules twice daily Co‐intervention: lactulose was co‐administered in the event of an episode of overt hepatic encephalopathy developing during follow‐up, at which point the participant was withdrawn from the study; lactulose was otherwise prohibited. Duration of treatment: 15 days before TIPS and for 6 months post‐TIPS; study medication was stopped in the event of 2 episodes of overt hepatic encephalopathy and open‐label rifaximin provided. Follow‐up period: 6 months post‐TIPS, at which point study medication was stopped; further follow‐up every 3 months for 1 year, death, or liver transplantation. |
|
| Outcomes | Neuropsychiatric assessment Mental status assessed using modified West Haven criteria and the presence of asterixis. Overt hepatic encephalopathy was defined as grade 2 or more change in mental status or isolated asterixis; psychometric hepatic encephalopathy score (PHES) measured in the absence of overt hepatic encephalopathy; minimal hepatic encephalopathy defined as a score of ‐4 or below. |
|
| Inclusion period | October 2013 to June 2016 | |
| Outcomes included in meta‐analyses | Mortality, hepatic encephalopathy post‐TIPS, adverse events | |
| Country of origin | A total of 12 investigatory centres in France | |
| Notes |
Publication status: full paper Vested interests bias: The study was funded by the French ministry; they played no role in the conduct of the study or its subsequent publication. Dr Bureau received support from Alfa Wasserman outside this study; risk of bias unclear. Additional information: Participants with diabetes: rifaximin 43%; placebo 34%. In 24 participants with a history of overt hepatic encephalopathy (rifaximin = 12; placebo = 12), the cumulative incidence of overt encephalopathy post‐TIPS was 33% with rifaximin versus 83% with placebo (P < 0.05). In 162 participants without a history of previous overt encephalopathy, the cumulative incidence of overt encephalopathy post‐TIPS was 35% with rifaximin versus 51% with placebo (P = 0.070). A total of 23 participants had minimal hepatic encephalopathy at baseline; post‐TIPS insertion 39% of participants with and 44% without minimal encephalopathy at baseline developed overt encephalopathy. Rifaximin did not reduce the incidence of minimal encephalopathy (27% versus 29% in the rifaximin and placebo groups, respectively P = 0.74). Authors contacted for further information on the aetiology of cirrhosis and data on our secondary outcomes. Request sent on 13 April 2021; still awaiting response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to treatment groups in a 1:1 ratio using computer‐generated randomisation with random block sizes. Participants were stratified according to Child‐Pugh classification and history of overt hepatic encephalopathy. |
| Allocation concealment (selection bias) | Low risk | Investigators used a website which immediately sent the investigator the participant’s unique number in the study, the treatment group allocated to the participant and the number of the treatment bottle. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomised, double‐blind study, with the study drugs similar in size, shape and colour |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from supplementary material: "Up until the end of the study, neither the investigating physicians, nor the patient will know the group to which the patient has been assigned by randomisation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 197 participants were randomised, but 186 were analysed as the other 11 did not receive a TIPS, did not receive the study drug, withdrew consent or had a wrong indication for TIPS. For the remaining participants who received a TIPS, an intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined were fulfilled in results. |
| Other bias | Low risk | No other bias identified |
| Overall bias assessment (mortality) | Low risk | Low risk of bias in all domains |
| Overall bias assessment (non‐mortality outcomes) | Low risk | Low risk of bias in all domains |
Butt 2018.
| Study characteristics | ||
| Methods | Randomised, single‐blind clinical trial | |
| Participants | This trial assessed the effects of rifaximin plus lactulose versus lactulose in 130 participants with cirrhosis and an acute episode of hepatic encephalopathy, grade II‐IV. There were 65 participants in each group. Age (mean ± SD) years
Proportion of men (%)
Aetiology of cirrhosis
|
|
| Interventions | Intervention: rifaximin 550 mg twice daily plus lactulose 30 ml thrice daily Control group: lactulose 30 ml thrice daily Co‐interventions: none Duration of treatment: 10 days |
|
| Outcomes | Neuropsychiatric assessment Mental status: West Haven criteria Reversal criteria not defined |
|
| Inclusion period | December 2014 to June 2015 | |
| Outcomes included in meta‐analyses | Hepatic encephalopathy | |
| Country of origin | Single centre in Pakistan | |
| Notes |
Publication status: full paper Vested interests bias: no declarations of conflict Additional information: authors contacted on 20 March 2018 for further information on mortality and adverse events. Authors contacted again on 4 April 2021 for further information on the characteristics of the participants, conflicts of interest, blinding status, concealment of allocation, loss to follow‐up, mortality and adverse event, and data regarding our secondary outcomes; still awaiting response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients were divided into two groups, by lottery method" p. 116. Probably done. |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The trial was conducted single‐blind, but the authors do not specify whether it was the participants or the study personnel who were blinded; most likely the study personnel but uncertain. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial was conducted single‐blind, but the authors do not specify whether it was the participants or the study personnel who were blinded; most likely the study personnel but uncertain. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only primary outcome reported. No missing data reported. No participants reported lost to follow‐up or withdrawn from trial. |
| Selective reporting (reporting bias) | High risk | Outcome data on hepatic encephalopathy provided but no information available on missing data adverse events or mortality. |
| Other bias | Low risk | No other bias detected |
| Overall bias assessment (mortality) | High risk | Mortality not reported |
| Overall bias assessment (non‐mortality outcomes) | High risk | No reports on adverse events or compliance to treatment |
Fera 1993.
| Study characteristics | ||
| Methods | Randomised, double‐blind, double‐dummy clinical trial | |
| Participants | This trial assessed the effects of rifaximin versus placebo in 40 people with cirrhosis and chronic hepatic encephalopathy. There were 20 participants in each group. Age (mean ± SD) years
Proportion of men (n: %)
Aetiology of cirrhosis
|
|
| Interventions | Intervention: 200 mg x 2 rifaximin plus 2 sachets of lactulose placebo (sorbitol) thrice daily for the first 14 days of each month for 90 days (n = 20) Control group: rifaximin placebo tablets x 2 and 2 sachets of lactulose (20 mg each) three times a day for the first 14 days of each month for 90 days (n = 20) Co‐interventions: magnesium sulphate (10 to 20 mg thrice daily) and evacuation enemas were performed if indicated. Duration of treatment: 3 times 14 days over a 90‐day period |
|
| Outcomes | Neuropsychiatric assessment PSE Sum/Index: mental state (West Haven Criteria); asterixis; cancelling A test; NCT‐A; EEG mean frequency; fasting blood ammonia |
|
| Inclusion period | Not reported | |
| Outcomes included in meta‐analyses | Mortality, hepatic encephalopathy, adverse events, blood ammonia | |
| Country of origin | Single centre in Italy | |
| Notes |
Publication status: full paper Vested interests bias: trial sponsored by Alpha‐Wassermann Additional information: individual participant data and details of randomisation methods were supplied by Alfa Wasserman. Authors contacted for further information on the aetiology of the participans' liver disease, confirmation of mortality outcomes, data on adverse events, and data regarding our secondary outcomes. Request sent on 5 April 2021; still awaiting response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "..with randomised assignment of treatment." p. 59 in published report. Judgement: probably done |
| Allocation concealment (selection bias) | Low risk | Additional information from Alfa Wassermann: randomisation with list manually prepared by AW medical department. Central allocation and hence concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy, double‐blind design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐dummy, double‐blind design |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were withdrawn from the trial. |
| Selective reporting (reporting bias) | Low risk | All end points described are accounted for in the published report. |
| Other bias | Low risk | No other bias detected |
| Overall bias assessment (mortality) | Low risk | All domains at low risk of bias |
| Overall bias assessment (non‐mortality outcomes) | Low risk | All domains at low risk of bias |
Festi 1993.
| Study characteristics | ||
| Methods | Randomised, open‐label clinical trial | |
| Participants | This trial assessed the effects of rifaximin versus lactulose in 201 participants with cirrhosis and chronic hepatic encephalopathy grade 1. A total of 136 participants were eventually included in the study. Three studies were undertaken:* 1) Open study of rifaximin (80 participants received rifaximin) 2) Randomised clinical trial of rifaximin (n = 20) versus neomycin (n = 15) 3) Randomised clinical trial of rifaximin (n = 9) versus lactulose (n = 12) *Only the comparison between rifaximin and lactulose was assessed Age (mean) years
Proportion of men (n: %)
Aetiology of cirrhosis (total group) (n: %)
|
|
| Interventions | Intervention: rifaximin 1200 mg daily Control intervention: lactulose 40 mg daily Co‐intervention: none Duration of treatment: 21 days |
|
| Outcomes | Neuropsychiatric assessment PSE Sum/Index: Mental status (West Haven criteria), asterixis, NCT‐A, EEG mean frequency; blood ammonia |
|
| Inclusion period | 1988 to 1991 | |
| Outcomes included in meta‐analyses | Mortality, hepatic encephalopathy, adverse events, blood ammonia | |
| Country of origin | A total of 10 investigatory centres in Italy | |
| Notes |
Publication status : full paper Vested interests bias: trial sponsored by Alpha ‐Wassermann Additional information: individual participant data and information on primary end points and randomisation provided by Alfa Wassermann Alfasigma contacted on 5 April 2021 for further information on blinding status, concealment of allocation, handling of missing data, mortality, adverse events for the randomised clinical trial arm, and data on secondary outcomes; still awaiting response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned to rifaximin or lactulose in a randomised fashion." p. 600 in published report. Additional information from Alfa Wassermann: "Open comparative study design. Randomisation was done as subsequent patients in the office (pair/unpair). The investigators allocated participants based on an open table of random numbers". |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No information on blinding of outcome assessors but highly unlikely |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No information on participants withdrawn from the trial. No information on the number of participants completing the trial. |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Other bias | Low risk | No other bias detected |
| Overall bias assessment (mortality) | High risk | High risk of selection bias, attrition bias and detection performance |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of selection, attrition bias and detection performance |
Gill 2014.
| Study characteristics | ||
| Methods | Randomised, double‐blind clinical trial | |
| Participants | This trial assessed the effects of rifaximin versus placebo in 200 people with cirrhosis and an acute episode of hepatic encephalopathy. As all the participants received lactulose this was essentially a comparison of rifaximin plus lactulose versus lactulose alone. There were 100 participants in each group. Age (mean) years
Proportion of men (n: %)
Aetiology of cirrhosis
|
|
| Interventions | Intervention: rifaximin 550 mg twice daily Control group: placebo tablets twice daily Co‐intervention: all participants received lactulose 30 to 60 ml 2 to 3 times daily Duration of treatment: 10 days |
|
| Outcomes | Neuropsychiatric status Mental status: modified version of West Haven criteria No information provided on the definition of encephalopathy reversal. |
|
| Inclusion period | Not reported | |
| Outcomes included in meta‐analyses | Mortality, hepatic encephalopathy, length of hospital stay | |
| Country of origin | Single centre in Pakistan | |
| Notes |
Publication status: abstract only Vested interests bias: no information provided Additional information: randomisation methods and blinding were retrieved from the corresponding author in May 2014 and March 2016 Authors contacted again on 5 April 2021 for additional information on study characteristics, conflicts of interest, method of randomisation, allocation concealments, blinding of outcome data and dealing with incomplete outcome data, and data on both primary and secondary outcomes after further planned analysis; response still awaited. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated to 1 of 2 groups: group A was given rifixamin and lactulose and group B was given lactulose plus placebo pills. Corresponding author states that trial was randomised but the method was not specified. |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Information from authors: "Patients and research medical authors were blinded to the study (it was double‐blinded)." Judgement: Probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Information from authors: "Patients and research medical authors were blinded to the study (it was double‐blinded)." Judgement: Probably done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No data on attrition or withdrawal from trial are reported. Corresponding author states that follow‐up and participants are still under review. |
| Selective reporting (reporting bias) | High risk | Data on mortality and hospital stay not reported in abstract. Corresponding author states that follow‐up and participants are still under review. |
| Other bias | Low risk | None identified |
| Overall bias assessment (mortality) | High risk | High risk of detection, attrition and reporting bias |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of detection, attrition and reporting bias |
Habib 2016.
| Study characteristics | ||
| Methods | Randomised, open‐label clinical trial | |
| Participants | This trial assessed the effects of rifaximin plus lactulose versus lactulose alone in 22 participants with cirrhosis and an acute episode of hepatic encephalopathy, grade II‐IV. There were 61 participants in each group. Age (mean ± SD) years
Proportion of men (%)
Aetiology of cirrhosis
|
|
| Interventions | Intervention: rifaximin tablet 550 mg twice daily plus lactulose 30 ml thrice daily Control: lactulose 30 ml thrice daily Co‐intervention: none Duration of treatment: likely 7 days |
|
| Outcomes | Neuropsychiatric assessment Mental status: West Haven criteria No information provided on the criteria used to determine treatment response |
|
| Inclusion period | August 2014 to February 2015 | |
| Outcomes included in meta‐analyses | Hepatic encephalopathy | |
| Country of origin | Single centre in Pakistan | |
| Notes |
Publication status : full paper Vested interests bias: no information provided Additional information: no contact details provided in the article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly allocated to one of the two groups on lottery basis". P 38 |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding as trial conducted open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Duration of follow‐up is not reported. No data on mortality, adverse events or attrition reported. |
| Selective reporting (reporting bias) | High risk | Duration of follow‐up is not reported. The predefined outcome is reported for all participants. |
| Other bias | Low risk | No other bias detected. |
| Overall bias assessment (mortality) | High risk | Mortality is not reported. |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of selective reporting, performance and detection bias |
Hasan 2018.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled clinical trial | |
| Participants | This trial assessed the effects of rifaximin plus lactulose versus lactulose plus placebo in 91 people with cirrhosis admitted to hospital with an acute episode of hepatic encephalopathy, grade I‐IV. There were 45 participants in the rifaximin plus lactulose group and 46 in the placebo plus lactulose group. Age (mean ± SD) years
Proportion of men (n: %)
Aetiology of cirrhosis (n: %) Rifaximin plus lactulose group
Placebo plus lactulose group
|
|
| Interventions | Intervention: rifaximin 400 mg 3 times daily with lactulose 15 ml 3 to 4 times daily titrated to produce 3 to 4 loose stools per day Control intervention: placebo tablets three times daily plus lactulose 15 ml 3 to 4 times daily titrated to produce 3 to 4 loose stools per day Co‐interventions: both groups received supportive measures such as intravenous antibiotics and enemata as indicated, but the number requiring these measures was not provided. Duration of therapy: until recovery of hepatic encephalopathy or for a maximum of 10 days |
|
| Outcomes | Neuropsychiatric assessment Mental status: West Haven criteria Improvement was defined as any reduction in encephalopathy grade and worsening as any increase in encephalopathy grade. |
|
| Inclusion period | Not stated | |
| Outcomes included in meta‐analyses | Mortality, hepatic encephalopathy | |
| Country of origin | Single centre in Bangladesh | |
| Notes |
Publication status: full paper Vested interests bias: no information provided Additional information: authors contacted for further information on the aetiology of cirrhosis in the participants, inclusion period, conflicts of interest, blinding status, handling of incomplete outcomes, and data on our secondary outcomes. The request was made on 9 April 2021, response received on 12 April 2021 directing us to the online study site (www.japi.org/n3n5o506k424r4/f3j5v50694j4y4/v2c4). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a 1:1 allocation ratio using block randomisation in variable block sizes. |
| Allocation concealment (selection bias) | Low risk | Interventions were allocated in a sealed, coded packet containing a bottle of rifaximin or placebo. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was conducted double‐blind; the participants received the interventions in a coded, sealed packet. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study was conducted double‐blind; the participants received the interventions in a coded, sealed packet. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The authors did not report how missing outcome data were dealt with. In the assessment of outcomes, the percentages reported do not match with the total number of participants allocated to each group; it seems as though 2 from the rifaximin group and 6 from the lactulose group were lost to follow‐up. |
| Selective reporting (reporting bias) | High risk | All outcomes stated in the methodology were reported; however there are discrepancies in the number of participants randomised and the number for whom results are provided; we could not identify a clinical trial registry entry in order to compare with the study protocol. |
| Other bias | Low risk | No other bias detected |
| Overall bias assessment (mortality) | High risk | Incomplete outcomes, attrition bias |
| Overall bias assessment (non‐mortality outcomes) | High risk | One or more domains were classified as 'high risk'. |
Higuera‐de‐la‐Tijera 2018.
| Study characteristics | ||
| Methods | Randomised, double‐blind, three‐arm clinical trial | |
| Participants | The trial assessed the effects of rifaximin versus: (i) lactulose; (ii) L‐ornithine L‐aspartate; and, (iii) placebo for the prevention of hepatic encephalopathy in 88 people with cirrhosis admitted to hospital with an acute variceal bleed who had no evidence of minimal or overt hepatic encephalopathy at the time of admission. The comparisons assessed were (i) rifaximin versus placebo; (ii) rifaximin versus lactulose. There were 21 participants in the rifaximin group and 22 participants in both the lactulose and placebo groups. Age (mean ± SD) years
Proportion of men (n: %)
Aetiology of cirrhosis (n: %) Rifaximin
Lactulose
Placebo
|
|
| Interventions | Intervention: rifaximin: 400 mg thrice daily plus placebo lactulose (dextrose solution) 30 ml thrice daily (n = 21) Control intervention: lactulose 30 ml thrice daily, adjusted to achieve 2 to 3 semi‐soft stools per day plus placebo rifaximin (identically presented dextrose tablets) 2 thrice daily (n = 22) Control intervention: placebo, 30 ml placebo lactulose (dextrose solution) thrice daily plus placebo rifaximin (dextrose tablets) 2 thrice daily (n = 22) Co‐medications: intravenous quinolones or cephalosporins were given for primary prophylaxis of infections for 7 days in all groups except the rifaximin group Duration of therapy: 7 days, follow‐up extended to 28 days |
|
| Outcomes | Neuropsychiatric assessment Mental status: West Haven Criteria; PHES psychometric test battery and critical flicker frequency |
|
| Inclusion period | June 2014 to August 2016 | |
| Outcomes included in meta‐analyses | Mortality, serious adverse events, hepatic encephalopathy, non‐serious adverse events | |
| Country of origin | Single centre in Mexico | |
| Notes |
Publication status: full paper Vested interests bias: the publication fee was supported by Alfasigma Inc. Additional information: authors contacted for further information on adverse events and our secondary outcomes. The request was made on 4 April 2021; still awaiting response. No information is available on whether participants had a history of hepatic encephalopathy prior to the trial. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated table of random numbers considering four groups of equal size. |
| Allocation concealment (selection bias) | Low risk | Allocating investigator did not have contact with participants, blinded to the drugs administered in each group (only lettered groups). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participant, caregiver, and outcome assessors were blinded. All participants received treatment corresponding to the complementary placebos to ensure blinding of both investigator and participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One person in the rifaximin group withdrew from the study before receiving the treatment; this person was not included in the analysis. No participants were lost to follow‐up, and no participants discontinued the intervention. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed on the trial registry have been included and reported in the full‐text paper. |
| Other bias | Low risk | No other bias detected. |
| Overall bias assessment (mortality) | Low risk | All domains were at a low risk of bias. |
| Overall bias assessment (non‐mortality outcomes) | Low risk | All domains were at a low risk of bias. |
Kimer 2017.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled clinical trial | |
| Participants | This trial assessed the effects of rifaximin versus placebo on haemodynamics, renal function, and vasoactive hormones in 54 participants with cirrhosis and ascites with no evidence of overt hepatic encephalopathy. Data were available on neuropsychiatric status following a post‐hoc analysis, which was published separately. There were 36 participants in the rifaximin group and 18 in the placebo group; 34 (63%) of the 54 participants had evidence of minimal hepatic encephalopathy. Age (mean (range)) years
Proportion of men (n: %)
Aetiology of cirrhosis (n: %) Rifaximin
Placebo
|
|
| Interventions | Intervention: rifaximin 550 mg twice a day Control intervention: placebo tablet identically presented and packaged twice a day Co‐interventions: none Duration of treatment: 28 days; duration of follow‐up 6 months |
|
| Outcomes | Neuropsychiatric assessment Participants screened for minimal hepatic encephalopathy with the continuous reaction time test, the psychometric hepatic encephalopathy score (PHES), and arterial blood ammonia |
|
| Inclusion period | February 2013 to December 2015 | |
| Outcomes included in meta‐analyses | Mortality, serious and non‐serious adverse events, hepatic encephalopathy, arterial blood ammonia | |
| Country of origin | Single centre in Copenhagen, Denmark | |
| Notes |
Publication status: full paper Vested interests bias: several co‐authors have received support from Norgine pharmaceutics. Additional information: primary end points published in a full paper. Individual participant data were retrieved from the authors in October 2016. Authors were contacted for further information on our secondary outcomes on 10 April 2021; still awaiting response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Investigators were not aware of the allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study medication was packed according to the randomisation list. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in original paper and analysed as intention‐to‐treat. |
| Selective reporting (reporting bias) | Low risk | Predefined outcomes reported |
| Other bias | Low risk | No other bias detected |
| Overall bias assessment (mortality) | Low risk | All domains were classified as low risk of bias. |
| Overall bias assessment (non‐mortality outcomes) | Low risk | All domains were classified as low risk of bias. |
Loguercio 2003.
| Study characteristics | ||
| Methods | Randomised, double‐blind, double‐dummy clinical trial | |
| Participants | This trial was a three‐way comparison of the effects of rifaximin monotherapy, lactitol monotherapy, and rifaximin plus lactitol in 40 people with cirrhosis and chronic hepatic encephalopathy, grade I‐II Data analysis was confined to 33 participants; 12 in the rifaximin group; 10 in the lactitol group and 11 in the rifaximin plus lactitol group. The comparisons assessed were (i) rifaximin versus lactitol; (ii) rifaximin plus lactitol versus lactitol alone. Age (mean ± SD) years
Proportion of men (data not available for allocated participants)
Aetiology of cirrhosis (n: %)
|
|
| Interventions | Intervention: rifaximin 400 mg + sorbitol 20 gram thrice daily Control intervention 1: lactitol 20 gram + (inert placebo) thrice daily Control intervention 2: rifaximin 400 mg + lactitol 20 gram thrice daily Co‐intervention: lactulose allowed during trial Duration of treatment: 15 days on and 15 days off treatment for 3 cycles |
|
| Outcomes | Neuropsychiatric assessment Modified PSE Sum/Index: mental status (West Haven criteria), asterixis, number connection test‐ A, arterial blood ammonia |
|
| Inclusion period | Not reported | |
| Outcomes included in meta‐analyses | Mortality, serious adverse events, hepatic encephalopathy, non‐serious adverse events, arterial blood ammonia | |
| Country of origin | Single centre in Italy | |
| Notes |
Publication status: full paper Vested interests bias: study sponsored by Alfa‐Wassermann Additional information: individual participant data provided by Alfa‐Wassermann Authors contacted for further information on the trial inclusion period, adverse events regardless of whether related to the study drug or not, and more data on our secondary outcomes. Request made on 10 April 2021; still awaiting response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were enrolled, following a double‐blind design, completely randomised, with parallel groups Additional information from Alfa Wassermann: "Randomisation list manually prepared." |
| Allocation concealment (selection bias) | Low risk | Randomisation undertaken centrally with allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy double‐blind study design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information on blinding of outcome assessors but likely they were blinded as this is a double‐blind, double dummy study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 40 participants initially enrolled 33 completed the trial; limited information is available on the 7 non‐completers; 1 died, 2 developed ascites and 4 were lost to follow‐up; their data were not included in the analyses. |
| Selective reporting (reporting bias) | High risk | Data on remission from hepatic encephalopathy supplemented from Alfa Wassermann (May 2014). |
| Other bias | Low risk | No other bias detected |
| Overall bias assessment (mortality) | High risk | All participants are accounted for but 7 non‐completers were not included in the analyses. |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of attrition and reporting bias |
Maharshi 2015.
| Study characteristics | ||
| Methods | Randomised, open‐label clinical trial | |
| Participants | This trial assessed the effects of rifaximin versus lactulose for prevention of hepatic encephalopathy in 120 people with cirrhosis and an acute variceal bleed who were free of hepatic encephalopathy at the time of presentation. There were 60 participants in each group. Age (mean ± SD) years
Proportion of men (n: %)
Aetiology of cirrhosis
|
|
| Interventions | Intervention: rifaximin 400 mg thrice daily Control intervention: lactulose 30 ml 4 times daily Co‐intervention: both trial arms received concomitant standard treatment for acute variceal bleeding as per Baveno V guidelines, including antibiotics. Duration of treatment: 5 days |
|
| Outcomes | Development of overt hepatic encephalopathy defined using West Haven criteria | |
| Inclusion period | Not reported | |
| Outcomes included in meta‐analyses | Mortality, serious adverse events, hepatic encephalopathy, length of hospital stay | |
| Country of origin | Single centre in India | |
| Notes |
Publication status: letter to journal Vested interests bias: no conflicts of interest declared Additional information: authors confirmed that the 53 participants reported in their published abstract, Maharshi 2014, were included in this report. No information is available on whether participants had a history of hepatic encephalopathy prior to the trial. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | As stated in correspondence with authors 19 July 2015: "Patients were randomized by computer generated numbers." Authors' judgement: probably done |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | As stated in correspondence with authors 19 July 2015: "No blinding, this was open labelled study." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes from trial registry reported in the letter |
| Other bias | Low risk | No other bias detected |
| Overall bias assessment (mortality) | Low risk | Mortality data reported; no participants unaccounted for |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of selection, performance, detection bias |
Majeed 2018.
| Study characteristics | ||
| Methods | Randomised, 'placebo'‐controlled clinical trial | |
| Participants | This trial assessed the effects of rifaximin versus placebo for the prevention of hepatic encephalopathy in 120 people with cirrhosis and a history of at least 2 episodes of overt hepatic encephalopathy in the 6 months preceding the trial who were presumably free of hepatic encephalopathy at enrolment. However, the nature of the placebo is not provided. As the majority of participants received lactulose during the trial, the comparison is essentially rifaximin plus lactulose versus lactulose alone. There were 60 participants in each group. Age (mean ± SD) years
Proportion of men (n: %)
Aetiology of cirrhosis
|
|
| Interventions | Intervention: rifaximin (dose unknown) Control: placebo (no information provided) Co‐intervention: of the participants receiving placebo 91.2% also received lactulose during the trial; likewise 91.4% of the participants receiving rifaximin also received lactulose. Duration of treatment: rifaximin (128 ± 45.0)days; placebo (110 ± 61.2) days |
|
| Outcomes | Neuropsychiatric assessment Development of an episode of hepatic encephalopathy based on West Haven criteria |
|
| Inclusion period | January 2017 to June 2017 | |
| Outcomes included in meta‐analyses | Hepatic encephalopathy, adverse events | |
| Country of origin | Single centre in Pakistan | |
| Notes |
Publication status: full paper Vested interests bias: no information provided Additional information: placebo‐controlled but details of blinding not provided Authors were contacted on 10 April 2021 for further information on the characteristics of the participants and interventions, conflicts of interest, blinding and randomisation status, concealment of allocation, handling of missing data, data on mortality and serious adverse events, and data on our secondary outcomes; still awaiting response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "The treatment allocation were applied randomly a bunch or patients were provided with rifaximin and others with Placebo". |
| Allocation concealment (selection bias) | Unclear risk | Quote "The treatment allocation were applied randomly a bunch or patients were provided with rifaximin and others with Placebo". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding; no mention of the nature of the placebo |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of blinding; no mention of the nature of the placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information about intention to treat analysis. Unclear outcome follow‐up time: trial stopped when participants experienced an episode of HE, but unclear follow‐up for those that did not. Average duration was 128 days for rifaximin + NAD and 110 for NAD alone. |
| Selective reporting (reporting bias) | High risk | All outcomes listed on the trial registry have been included but details of the incidences of adverse events are only reported as percentages whereas the effect on the length of hospital stay is only reported as "rifaximin treatment also reduced the stay or risk of hospitalization due to HE". |
| Other bias | Low risk | No other bias identified |
| Overall bias assessment (mortality) | Unclear risk | No deaths are reported |
| Overall bias assessment (non‐mortality outcomes) | High risk | Attrition bias and blinding methods are unclear |
Manzhalii 2022.
| Study characteristics | ||
| Methods | Randomised, open‐label, prospective trial | |
| Participants | This trial assessed the effects of E. coli Nissle 1917 (EcN) compared to lactulose and rifaximin in 45 people with cirrhosis and minimal or grade 1 to 2 hepatic encephalopathy. Only the comparison between rifaximin and lactulose was assessed. Age (mean, SD) years
Proportion of men (n: %)
Aetiology of cirrhosis (n: %) Rifaximin
Lactulose
|
|
| Interventions | Intervention: rifaximin 500 mg twice daily Control intervention: lactulose 30 to 60 mL in 2 or 3 divided doses to pass 2 to 3 semi‐soft stools per day Co‐intervention: none Duration of treatment: 1 month |
|
| Outcomes | Neuropsychiatric assessment Mental status (West Haven criteria), Stroop test Ammonia |
|
| Inclusion period | 2017 to 2020 | |
| Outcomes included in meta‐analyses | Mortality, adverse events, blood ammonia | |
| Country of origin | Ukraine | |
| Notes |
Publication status : full paper Vested interests bias: none declared Additional information: some data, such as blood ammonia, have been extracted from graphical representations. This trial included participants with either minimal hepatic encephalopathy or low‐grade overt hepatic encephalopathy at baseline; however, the results have been reported as a composite of these groups. We therefore classed this trial as assessing minimal hepatic encephalopathy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using a computer‐generated numeric sequence |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label design |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label design |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants are accounted for, |
| Selective reporting (reporting bias) | High risk | Approximately 60% of the participants had grade I or II HE; the remainder had minimal HE. No comment is made about participants' mental status following treatment, |
| Other bias | Unclear risk | The trial registry was submitted after the study primary completion date. |
| Overall bias assessment (mortality) | Unclear risk | Mortality was not a stated outcome. All participants were accounted for; no deaths were reported; high risk of selective reporting. |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of selection, performance, detection and other bias; no information reported on mental status. |
Mas 2003.
| Study characteristics | ||
| Methods | Randomised, double‐blind, double‐dummy clinical trial | |
| Participants | This trial assessed the effects of rifaximin versus lactitol in 103 people with cirrhosis and an acute episode of hepatic encephalopathy, grade I‐III. There were 50 participants in the rifaximin group and 53 in the placebo group. Age (mean ± SD) years
Proportion of men (n: %)
Aetiology of cirrhosis (n: %) Rifaximin
Lactitol
|
|
| Interventions | Intervention: rifaximin 2 x 200 mg and 20 gram placebo three times per day Control intervention: lactitol 20 gram and 2 tablets of placebo three times per day Co‐intervention: none Duration of treatment: 5 to 10 days |
|
| Outcomes | Neuropsychiatric assessment PSE Sum /Index: mental status (West Haven criteria), asterixis, NCT‐A, EEG mean frequency, blood ammonia |
|
| Inclusion period | November 1995 to December 1997 | |
| Outcomes included in meta‐analyses | Mortality, serious adverse effects, hepatic encephalopathy, non‐serious adverse effects, blood ammonia | |
| Country of origin | A total of 13 investigatory sites in Spain | |
| Notes |
Publication status: full paper Vested interests bias: trial sponsored by Alpha ‐Wassermann. Additional information: individual participant data provided by Alpha‐Wassermann. The authors were contacted for further information on our secondary outcomes on 10 April 2021; still awaiting response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out centrally using serially numbered, sealed, opaque envelopes stratified by the centre. Additional information from Alfa Wassermann: "Randomisation with computer‐generated list." |
| Allocation concealment (selection bias) | Low risk | All experimental material was divided into ‘patient‐units’ characterised by a label containing the previously assigned randomised number. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind double‐dummy trial design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The statistical evaluation was performed by Clever Instruments (Barcelona, Spain) using a statistical package." p. 53 in published report. Judgement: outcome assessment performed by third‐party. Likely to have been blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for. |
| Selective reporting (reporting bias) | Low risk | All predetermined outcomes reported in full. |
| Other bias | Low risk | No other bias detected. |
| Overall bias assessment (mortality) | Low risk | All bias categories are judged to be low risk. |
| Overall bias assessment (non‐mortality outcomes) | Low risk | All bias categories are judged to be low risk. |
Massa 1993.
| Study characteristics | ||
| Methods | Randomised, double‐blind, double‐dummy clinical trial | |
| Participants | This trial investigates the effects of rifaximin versus lactulose in 40 people with cirrhosis and chronic hepatic encephalopathy, grade I‐III. There were 20 participants in each group. Age (mean ± SD) years
Proportion of men (n: %)
Aetiology of cirrhosis (n: %) Rifaximin group
Lactulose group
|
|
| Interventions | Intervention: rifaximin 200 mg x 2 plus 2 sachets of sorbitol 3 times per day Control intervention: placebo tablets x 2 plus 2 sachets of lactulose 10 g, 3 times per day Co‐intervention: none Duration of treatment: 15 days |
|
| Outcomes | Neuropsychiatric assessment PSE Sum/Index: Mental status (West Haven Criteria), asterixis, cancelling A test, NCT‐A, blood ammonia, EEG mean frequency |
|
| Inclusion period | Not reported | |
| Outcomes included in meta‐analyses | Hepatic encephalopathy, adverse events, blood ammonia | |
| Country of origin | Single centre in Italy | |
| Notes |
Publication status: full paper Vested interests bias: trial sponsored by Alpha‐Wasserman. Additional information: individual participant data supplied by Alfa Wassermann. Authors contacted on 10 April 2021 for further information on the study inclusion period, blinding of outcome assessment, and data on both our primary and secondary analyses; still awaiting response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out centrally using serially numbered, sealed, opaque envelopes stratified by the centre. Additional information from Alfa Wassermann: "Patients were randomised following a computer‐generated list." |
| Allocation concealment (selection bias) | Low risk | All experimental material was divided into ‘patient‐units’ characterized by a label containing the previously assigned randomised number. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The patients received two tablets of placebo, externally indistinguishable from the rifaximin tablets administered to the previous group." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, double‐dummy trial. Participants received either rifaximin plus sachets of sorbitol, indistinguishable from the lactulose sachets given to the other group, or placebo tablets, indistinguishable from the rifaximin tablets, and sachets of lactulose. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in published report. |
| Selective reporting (reporting bias) | Low risk | All end points reported in journal article. |
| Other bias | Low risk | No other bias detected. |
| Overall bias assessment (mortality) | Low risk | All bias categories are judged to be low risk. |
| Overall bias assessment (non‐mortality outcomes) | Low risk | All bias categories are judged to be low risk. |
Moneim 2021.
| Study characteristics | ||
| Methods | Randomised, open‐label clinical trial | |
| Participants | The trial assessed the effects of rifaximin plus lactulose versus lactulose alone in preventing the development of hepatic encephalopathy in 100 people with hepatitis C‐related liver cirrhosis and a history of at least one previous episode of overt hepatic encephalopathy. All participants were classified as Grade I or less on the West‐Haven (Conn's) criteria at inclusion. There were 50 participants in the rifaximin plus lactulose group, and 50 in the lactulose alone group. Age (mean ± SD) years
Proportion of men (%)
Aetiology of cirrhosis: all participants had hepatitis C‐related liver cirrhosis. |
|
| Interventions | Intervention: rifaximin 400 mg thrice daily plus lactulose 30 to 45 ml thrice daily to produce 2 to 3 soft stools per day Control intervention: lactulose 30 to 45 ml thrice daily to produce 2 to 3 soft stools per day. Co‐interventions: none Duration of treatment: 6 months |
|
| Outcomes | Neuropsychiatric assessment Mental status: West Haven criteria |
|
| Inclusion period | January 2015 to December 2018 | |
| Outcomes included in meta‐analyses | Mortality, hepatic encephalopathy, adverse events | |
| Country of origin | Single centre in Egypt | |
| Notes |
Publication status: full paper Vested interests bias: none reported Additional information: overall, 22% of participants in the intervention group and 20% in the control group had grade I hepatic encephalopathy at baseline. Recurrence defined as the development of grade II change or more. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment using a computer random sequence generator in a 1:1 allocation ratio. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed, envelopes were used and kept by the hospital pharmacist. These were opened only once participant details were written on the envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants who received treatment were included in the safety and efficacy analyses. Microbial resistance data was missing for 56 of the 100 participants due to technical problems; this was analysed using intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All clinically relevant outcomes reported in the full‐text when compared to the trial registry. |
| Other bias | Low risk | No other bias detected |
| Overall bias assessment (mortality) | Low risk | Low risk of bias in all domains except for performance and detection bias ‐ unlikely to influence mortality |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of bias in performance and detection domains |
Muhammad 2016.
| Study characteristics | ||
| Methods | Randomised, cross‐sectional, open‐label clinical trial | |
| Participants | This trial assessed the effects of rifaximin plus lactulose versus lactulose for the prevention of hepatic encephalopathy, over a 3‐month period, in 98 people with cirrhosis previously admitted with an episode of overt hepatic encephalopathy who had little or no evidence of encephalopathy on discharge from hospital. Although one group is referred to as the 'placebo' group they did not receive a placebo preparation but conventional treatment, i.e. lactulose 30 to 60 ml in 2 to 3 divided doses per day. There were 49 participants in each group. Age (mean ± SD) years
Proportion of men (n: %)
Aetiology of cirrhosis
|
|
| Interventions | Intervention: rifaximin 550 mg twice daily plus lactulose 30 to 60 ml daily in 2 to 3 divided doses Control intervention: lactulose 30‐60 ml daily in 2 to 3 divided doses Co‐intervention: none Duration of treatment: 3 months |
|
| Outcomes | Neuropsychiatric assessment Mental status: West Haven criteria Recurrence defined as the development of grade II change or more |
|
| Inclusion period | June 2015 to May 2016 | |
| Outcomes included in meta‐analyses | Hepatic encephalopathy | |
| Country of origin | Single centre in Pakistan | |
| Notes |
Publication status: full paper Vested interests bias: no information provided Additional information: trial not registered in clinicaltrials.gov |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Report states that:"Patients randomly divided into two groups i.e. treatment and placebo groups using random numbers generated from random table". Although one group is referred to as the 'placebo' group, they did not receive a placebo preparation but conventional treatment i.e. lactulose. |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of evaluators is mentioned in the paper. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants appear to be accounted for but no details are provided on time to the end point, which was the recurrence of hepatic encephalopathy. |
| Selective reporting (reporting bias) | High risk | The only reported outcome was 'recurrence of hepatic encephalopathy'; no information provided on mortality or serious adverse events. |
| Other bias | High risk | The full paper report does not mention how participants were selected; no data are provided on the numbers of people eligible for inclusion or the numbers excluded and the reasons why. Thus, selection bias, prior to inclusion, cannot be excluded. |
| Overall bias assessment (mortality) | High risk | No information provided on mortality in this 3‐month follow‐up study. |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of selection and performance bias; unclear risk of reporting bias |
Nawaz 2015.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled clinical trial | |
| Participants | This trial assessed the effects of rifaximin versus placebo in the prevention of hepatic encephalopathy, over a 6‐month period, in 150 people with cirrhosis and a history of 2 or more episodes of overt hepatic encephalopathy in the preceding 6 months. As it is highly likely, but not stipulated, that these participants would be taking prophylactic lactulose, this comparison is effectively rifaximin plus lactulose versus lactulose alone. There were 75 participants in each group. Age (mean ± SD) years
Proportion of men (n: %)
Aetiology of cirrhosis
|
|
| Interventions | Intervention: rifaximin 550 mg twice daily Control intervention: placebo twice daily ‐ no details provided Co‐intervention: all participants were permitted to use lactulose as standard of care. Duration of treatment: 6 months or until the first episode of hepatic encephalopathy |
|
| Outcomes | Neuropsychiatric assessment No information provided on methods for assessing hepatic encephalopathy but likely to be West Haven criteria. |
|
| Inclusion period | 2014 | |
| Outcomes included in meta‐analyses | Hepatic encephalopathy | |
| Country of origin | Single centre in Pakistan | |
| Notes |
Publication status: abstract only Vested interests bias: no conflicts of interest declared by the authors. Additional information: planned publication of full paper in future Authors contacted for further information 12 April 2021, response received 14 August 2021. Further information received included characteristics of participants, date of the study, conflicts of interest, randomisation methods, concealment of allocation, methods of blinding, handling of incomplete outcome data, trial registration status, publication status and data on our primary and secondary outcomes. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | All participants randomised by simple lottery method in a 1:1 manner |
| Allocation concealment (selection bias) | Low risk | Allocation concealed: codes allocated to both the groups were concealed from both the researcher and the participants. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from author: "it was a double‐blind placebo‐controlled trial, both the participants and the researchers were blinded, it was done by allocating codes to the drug and placebo." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from author: "it was a double‐blind placebo‐controlled trial, both the participants and the researchers were blinded, it was done by allocating codes to the drug and placebo." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Participants lost to follow‐up were excluded from the analyses. No intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | High risk | Published as an abstract, in which only data on hepatic encephalopathy were reported. Although further information was received from the study authors on mortality and adverse events, its completeness is uncertain. |
| Other bias | Low risk | No other bias detected |
| Overall bias assessment (mortality) | High risk | High risk of attrition and reporting bias |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of attrition and reporting bias |
Paik 2005.
| Study characteristics | ||
| Methods | Randomised, open‐label clinical trial | |
| Participants | This trial assessed the effects of rifaximin versus lactulose in 54 people with cirrhosis and an acute episode of hepatic encephalopathy, grade I‐III. There were 32 participants in the rifaximin groups and 22 in the lactulose group. Age (mean ± SD) years
Proportion of men (n: %)
Aetiology of cirrhosis (n: %) Rifaximin
Lactulose
|
|
| Interventions | Intervention: rifaximin 400 mg, 3 times per day Control intervention: lactulose 90 ml per day in divided doses Co‐intervention: none Duration of treatment: 7 days |
|
| Outcomes | Neuropsychiatric assessment Modified PSE Sum/Index: mental status (West Haven criteria), asterixis, NCT‐A, blood ammonia |
|
| Inclusion period | January 1997 to December 1998 | |
| Outcomes included in meta‐analyses | Hepatic encephalopathy, adverse events, blood ammonia concentrations | |
| Country of origin | Single centre in South Korea | |
| Notes |
Publication status: full paper Vested interests bias: rifaximin provided by pharmaceutical company Additional information: authors contacted on 9 April 2021 for further information on aetiology of cirrhosis, participant numbers with overt hepatic encephalopathy, co‐interventions used, method of randomisation, blinding status, allocation concealment, trial registration status, and data on our primary and secondary outcomes; still awaiting response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a computer‐generated sequential 3:2 block randomisation list, patients were randomised." |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Although this trial has a limitation due to it being an open‐label study," p. 406 in published report. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "no patient was withdrawn from the trial due to an undue adverse effect." Judgement: all participants accounted for in published report. There is mention in the article that 64 participants started treatment, but 10 were discontinued due to meeting exclusion criteria. |
| Selective reporting (reporting bias) | Low risk | Published as journal article in international journal |
| Other bias | Low risk | No other bias detected |
| Overall bias assessment (mortality) | Low risk | High risk of selection, detection and performance bias, although this will unlikely affect mortality data |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of selection, detection and performance bias |
Patel 2022.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled clinical trial | |
| Participants | This trial assessed the effect of rifaximin versus placebo in 38 people with cirrhosis and either chronic persistent hepatic encephalopathy or ≥ 2 episodes of overt hepatic encephalopathy in the preceding 6 months. There were 19 participants in each group. Age (mean (range)) years
Proportion of men (n: %)
Aetiology of cirrhosis: not reported |
|
| Interventions | Treatment intervention: rifaximin 550 mg twice daily Control intervention: identically presented placebo tablets, twice daily Co‐interventions: 7 participants in the rifaximin group and 7 in the placebo group were receiving lactulose at baseline. Duration of treatment: 90 days |
|
| Outcomes | Neuropsychiatric assessment Mental status (West Haven criteria); psychometric hepatic encephalopathy score (PHES); venous blood ammonia |
|
| Inclusion period | January 2015 to June 2016 | |
| Outcomes included in meta‐analyses | Mortality, serious adverse events, health‐related quality of life (3‐level EQ‐5D), non‐serious adverse events, venous blood ammonia | |
| Country of origin | Single centre in UK | |
| Notes |
Publication status: full paper Vested interests bias: the research was funded by Norgine UK Ltd; several authors have delivered paid lectures for Norgine Pharmaceuticals Ltd; the senior author has participated in advisory boards for Norgine Pharmaceuticals Ltd. Additional information: the study was intended to include 50 participants with cirrhosis and chronic hepatic encephalopathy randomised to either rifaximin or matching placebo twice daily for 90 days. A total of 38 participants were randomised; the authors explained that recruitment was not completed because during the trial period use of rifaximin was approved for the prevention of recurrent overt HE and so potential participants would be eligible for treatment with rifaximin as standard of care. However, use of rifaximin was not approved for treatment of persistent hepatic encephalopathy, so these participants could still have been recruited. At baseline the majority of participants, 14 /19 (74%) in the rifaximin group and 10/19 (53%) in the placebo group had clinical evidence of overt hepatic encephalopathy. It was stated that the randomised study participants had either persistent hepatic encephalopathy or had experienced ≥2 episodes of encephalopathy in the preceding 6 months. However, the distribution of the participants in the rifaximin and placebo groups is unknown. The maximum ever West Haven grade attained in the population overall was III; the maximum grade at the outset of the trial was I. Statistically, the changes in mental status are extremely difficult to interpret. In addition, while the number who developed an episode of hepatic encephalopathy is statistically higher in the placebo group it could simply reflect the distribution of cases between the two groups i.e. more people with a history of recurrent as opposed to persistent hepatic encephalopathy in the placebo group. The PHES score and two PHES components ‐ Trail A and Line Tracing tests showed significant improvement in the rifaximin group over 90 days but the difference between rifaximin and placebo groups was not significant. Thus, the data on hepatic encephalopathy could not be interpreted with any certainty and so have been excluded from the analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised in a 1:1 ratio using a validated program in block randomisation of blocks of 10. |
| Allocation concealment (selection bias) | Low risk | Allocation was conducted centrally by way of sequentially numbered containers, known only to the Clinical Trial Unit and drug manufacturers/suppliers. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were blinded, and personnel were only unblinded once the data were locked. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Allocation was revealed to the trial statistician at the end once the electronic database was completed and locked. Investigators were blinded throughout the study period. Emergency un‐blinding would mean a participant was withdrawn from the trial. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 19 participants allocated to intervention and control, 6 participants in each group were excluded from the analyses; the authors censored data for participants lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were reported by the authors. |
| Other bias | Low risk | No other bias identified |
| Overall bias assessment (mortality) | High risk | Attrition bias was deemed 'high risk' |
| Overall bias assessment (non‐mortality outcomes) | High risk | Attrition bias was deemed 'high risk' |
Pawar 2019.
| Study characteristics | ||
| Methods | Randomised, three‐way, double‐blind clinical trial | |
| Participants | This trial assessed the effects of rifaximin versus placebo and rifaximin versus lactulose in 108 people with cirrhosis and minimal hepatic encephalopathy. There were 37 participants in the rifaximin and lactulose groups and 36 in the placebo group. Age (mean ± SD) years
Proportion of men (n: %)
Aetiology of cirrhosis ( n: %) Rifaximin
Lactulose
Placebo
|
|
| Interventions | Treatment 1: rifaximin 550 mg twice daily: unclear if a lactulose placebo was used Treatment 2: lactulose 30 to 60 ml/day titrated to produce 2 semi‐soft stools/day: unclear if a placebo rifaximin was used. Control: 'placebo' vitamin B‐complex tablets twice daily Treatment duration: 3 months |
|
| Outcomes | Neuropsychiatric assessment Minimal hepatic encephalopathy was diagnosed based on a Psychometric Hepatic Encephalopathy Score (PHES) of ≤ ‐5 and/or Inhibitory Control Test lures ≥ 14 Reversal of minimal hepatic encephalopathy was defined as Psychometric Hepatic Encephalopathy Score ≥ ‐5 and or Inhibitory Control Test lures ≤ 14 |
|
| Inclusion period | May 2015 to March 2017 | |
| Outcomes included in meta‐analyses | Rifaximin versus placebo and rifaximin versus placebo considered separately Hepatic encephalopathy, adverse events |
|
| Country of origin | Single centre in India | |
| Notes |
Publication status: full paper Vested interests bias: none declared Additional information: authors contacted for further information on 14 September 2021 regarding whether the abstract Pawar 2016 included the same participant cohort as Pawar 2019; still awaiting response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised by an independent observer using a computer‐generated list of random numbers. |
| Allocation concealment (selection bias) | Low risk | Investigators were unaware of the allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind trial but no mention is made of the use of placebo equivalents for rifaximin and lactulose |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind trial but no mention is made of the use of placebo equivalents for rifaximin and lactulose |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other bias identified |
| Overall bias assessment (mortality) | Low risk | No participants lost to follow‐up, and low overall risk of bias |
| Overall bias assessment (non‐mortality outcomes) | Low risk | Low overall risk of bias |
Poudyal 2019.
| Study characteristics | ||
| Methods | Randomised, three‐armed, open‐label clinical trial | |
| Participants | This trial assessed the effects of lactulose, lactulose plus L‐ornithine L‐aspartate and lactulose plus rifaximin in 132 people with cirrhosis and an acute episode of hepatic encephalopathy, grade I‐IV. Comparisons between the lactulose plus rifaximin versus lactulose alone arms were included in the analyses. There were 44 participants in both included groups. Age (mean ± SD) years
Proportion of men (n: %)
Aetiology of cirrhosis (n: %) Lactulose plus rifaximin
Lactulose alone
|
|
| Interventions | Group 1: rifaximin 550 mg capsule twice daily plus lactulose 30 to 60 ml, thrice daily, to ensure passage of 2 to 3 semi‐soft stools a day Group 2: lactulose 30 to 60 ml, thrice daily, to ensure passage of 2 to 3 semi‐soft stools in a day Co‐intervention: other standard treatments according to need, including antibiotics Duration of treatment: until discharge from hospital or death |
|
| Outcomes | Neuropsychiatric assessment Mental status: West Haven criteria The criteria used to determine outcome were not detailed but were classified as complete reversal or treatment failure. |
|
| Inclusion period | February 2017 to January 2018 | |
| Outcomes included in meta‐analyses | Mortality, hepatic encephalopathy, length of hospital stay | |
| Country of origin | Single centre in Nepal | |
| Notes |
Publication status: full paper Vested interests bias: none Additional information: authors contacted on 13 April 2021 for further information on treatment duration, randomisation methods, allocation concealment, blinding methods, handling of incomplete data, trial registration status, serious adverse events, and data on our secondary outcomes. Response received on 16 April 2021. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors did not report the methods of random sequence generation, although the trial was reported as randomised. Author's response: "randomisation was simple randomisation". |
| Allocation concealment (selection bias) | Unclear risk | There was no information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Author's response: "It was single blinded. Patient was blinded" However, one group received an IV preparation of LOLA while the other group received oral rifaximin. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Author's response: "It was single blinded. Patient was blinded" However, one group received an IV preparation while the other comparison group received tablets. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes accounted for in the results |
| Other bias | Low risk | No other bias identified |
| Overall bias assessment (mortality) | Low risk | Deaths are reported. |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of detection bias |
Riggio 2005.
| Study characteristics | ||
| Methods | Randomised, open‐label, three‐arm clinical trial | |
| Participants | This trial assessed the effects of rifaximin versus lactitol and rifaximin versus no intervention in preventing the development of hepatic encephalopathy in 75 people with cirrhosis, free of encephalopathy, who underwent placement of a transjugular intrahepatic portosystemic shunt (TIPS) . Two comparisons were assessed; (i) rifaximin versus lactitol; (ii) rifaximin versus placebo. Age (mean ± SD) years
Proportion of men (n: %)
Aetiology of cirrhosis (n: %) Rifaximin
Lactitol
No treatment group
|
|
| Interventions | Intervention: rifaximin 400 mg thrice daily Control intervention (i): lactitol 60 grams daily in 3 divided doses Control intervention (ii): no treatment Co‐intervention: 120 ml sorbitol enema administered in the rifaximin and no treatment groups, if required Duration of treatment: 30 days or until occurrence of hepatic encephalopathy |
|
| Outcomes | Neuropsychiatric assessment Modified PSE Sum/Index: mental status (West Haven Criteria), asterixis, NCT‐A, venous blood ammonia |
|
| Inclusion period | November 1998 to September 2003 | |
| Outcomes included in meta‐analyses | Mortality, hepatic encephalopathy, adverse events, venous blood ammonia | |
| Country of origin | Single centre in Italy | |
| Notes |
Publication status: full paper Vested interests bias: none Additional information: additional unpublished data requested from authors; response received directing to a published article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed in blocks of 12 by sealed opaque envelopes." P 675, column 2, l. 61. Authors judgement: probably done |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes were used, see above. Authors judgement: Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Blindness was not considered due to the modifications in the bowel habits induced by one of the treatments." P 675, column 2, l. 62 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Blindness was not considered due to the modifications in the bowel habits induced by one of the treatments." P 675, column 2, l. 62 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in journal article. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in journal article. |
| Other bias | Low risk | No other bias detected |
| Overall bias assessment (mortality) | Low risk | Low risk of bias for mortality outcomes |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of performance and detection bias |
Sharma 2013.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled clinical trial | |
| Participants | This trial assessed the effects of rifaximin plus lactulose versus placebo plus lactulose in 120 people with cirrhosis and an acute episode of hepatic encephalopathy, grade II‐IV. There were 63 participants in the rifaximin plus lactulose group and 57 in the placebo plus lactulose group. Age (mean ± SD) years
Proportion of men (n: %)
Aetiology of cirrhosis (n: %)
|
|
| Interventions | Intervention: rifaximin 400 mg thrice daily plus lactulose 30 to 60 ml thrice daily titrated to allow passage of 2 to 3 semi‐soft stools daily Control intervention: placebo capsule (sugar) thrice daily plus lactulose 30 to 60 ml thrice daily titrated to allow passage of 2 to 3 semi‐soft stools daily Co‐intervention: people also received standard treatment which in 70 (58%) including antibiotics. In case of treatment failure, participants in group B were given rifaximin and those in group A were given L‐ornithine L‐aspartate ‐ however, no participants had refractory encephalopathy. Duration of treatment: until recovery, or for a maximum of 10 days |
|
| Outcomes | Neuropsychiatric assessment Mental status: West Haven criteria End point was 'complete reversal of hepatic encephalopathy' but no criteria for reversal provided, and no allowance made for improvement in mental status short of complete reversal or for deterioration. |
|
| Inclusion period | October 2010 to September 2012 | |
| Outcomes included in meta‐analyses | Mortality, hepatic encephalopathy, adverse events, length of hospital stay | |
| Country of origin | Single centre in India | |
| Notes |
Publication status: full paper Vested interests bias: none reported Additional information: authors contacted on 11 April 2021 for data on adverse events, quality of life outcomes, and blood ammonia levels. We also enquired regarding a discrepancy in participants recovering from hepatic encephalopathy between 2 publications; still awaiting response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Treatment envelopes with the randomisation code were distributed to the treating nurse by the statistician who was aware of the allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, investigators, and study staff (nurse) were blinded to treatment assignments. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The statistician was unblinded to allocation ‐ the authors report this was to prevent mixing of rifaximin and placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enrolled participants have outcome data. |
| Selective reporting (reporting bias) | Low risk | All end points reported. |
| Other bias | Unclear risk | There is a discrepancy between the full‐text paper published in the American Journal of Gastroenterology in 2013 and the 2012 abstract in the Journal of Gastroenterology and Hepatology in the number of people recovering from hepatic encephalopathy (25 vs 29 in the lactulose group, respectively), but it is not unusual to find minor discrepancies between abstracts and published papers. |
| Overall bias assessment (mortality) | Low risk | All but one categories are judged to be low risk. |
| Overall bias assessment (non‐mortality outcomes) | Low risk | All but one categories are judged to be low risk. |
Sharma 2014.
| Study characteristics | ||
| Methods | Randomised, open‐label, four‐arm clinical trial | |
| Participants | This trial assessed the effects of rifaximin in comparison to L‐ornithine L‐aspartate, probiotics and placebo in 124 people with cirrhosis and minimal hepatic encephalopathy. The comparison assessed in this review was rifaximin versus placebo. There were 31 participants in the rifaximin group and 30 in the placebo group. Age (mean ± SD) years
Proportion of men (n: %)
Aetiology of cirrhosis (n: %) Rifaximin
Placebo
|
|
| Interventions | Intervention: rifaximin 400 mg thrice daily Control intervention: placebo, 2 capsules per day (no details provided) Co‐intervention: none reported Duration of treatment: 2 months ± 3 days or until the participants developed overt hepatic encephalopathy, died or were lost to follow‐up |
|
| Outcomes | Neuropsychiatric assessment Clinical Hepatic Encephalopathy Staging Scale (CHESS) to exclude clinically overt hepatic encephalopathy The presence of minimal hepatic encephalopathy was assessed using the number connection, figure connection and digit symbol tests and the Critical Flicker Frequency (CFF) and diagnosed if any 2 of the 3 psychometric tests were abnormal and/or the CFF was < 39 Hz. |
|
| Inclusion period | August 2009 to August 2010 | |
| Outcomes included in meta‐analyses | Mortality, hepatic encephalopathy | |
| Country of origin | Single centre in India | |
| Notes |
Publication status: full paper Vested interests bias: none reported Additional information: authors contacted on 11 April 21 for more information on study characteristics, data on adverse events, and data on our secondary outcomes; still awaiting response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "..the block randomisation method was utilized for random allocation of drugs. The sequence remained concealed from the investigator and the generator of the random blocks did not participate in screening, enrolment or drug delivery" p. 227 in journal article. Authors judgement: random allocation was probably done |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed from investigators and participants. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The study was not blinded" p. 227 in journal article. Judgement: no blinding of participants or personnel regarding study medication. No blinding of outcome assessors reported. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 people were reported as lost to follow‐up, 6 people deteriorated into overt hepatic encephalopathy, and 4 people expired. Intention‐to‐treat analyses were performed. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in journal article. |
| Other bias | Low risk | No source of support or conflicts of interest reported, no other bias detected. |
| Overall bias assessment (mortality) | Low risk | Low risk of bias for mortality outcomes |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of performance bias and unclear risk of detection bias |
Sidhu 2011.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled clinical trial | |
| Participants | This trial assessed the effects of rifaximin and placebo in 94 people with cirrhosis and minimal hepatic encephalopathy; 49 randomised to rifaximin and 45 to placebo. Age (mean (range)) years
Proportion of men (n: %)
Aetiology of cirrhosis (n: %) Rifaximin
Placebo
|
|
| Interventions | Intervention: rifaximin 200 mg, 2 tablets thrice daily Control intervention: placebo tablets, 2 tablets thrice daily Co‐intervention: none Duration of treatment: 8 weeks |
|
| Outcomes | Neuropsychiatric assessment Minimal hepatic encephalopathy was diagnosed if, in the absence of clinically obvious neuropsychiatric change, any 2 of 5 tests in a neuropsychometric battery, comprised of number and figure connection, picture completion, digit symbol, and block design, were beyond 2 SD of normal. |
|
| Inclusion period | December 2008 to November 2009 | |
| Outcomes included in meta‐analyses | Mortality, reversal of minimal hepatic encephalopathy; development of overt hepatic encephalopathy, adverse events, health‐related quality of life (Sickness Impact Profile) | |
| Country of origin | Single centre in India | |
| Notes |
Publication status: full paper Vested interests bias: study drugs (rifaximin and placebo) were provided by LUPIN limited, Laxmi towers, Bandra Kurla Complex, Mumbai‐ 400051, India. Additional information: Nil |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All patients diagnosed to have MHE were randomised into two groups (group A and B) using computer‐generated randomisation" p. 309 in journal article. Judgement: random sequence generation done |
| Allocation concealment (selection bias) | Low risk | Quote: "Sequentially numbered, opaque envelopes were used for treatment allocation by a coordinator, who was not investigator." p. 309 in journal article. Judgement: allocation concealment adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The participant, investigator, data‐entry operator, and statistician were blinded regarding the treatment drugs. The code was broken only after the study was complete and analysis of the results was carried out." p. 309 in journal article. Judgement: blinding adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The participant, investigator, data‐entry operator, and statistician were blinded regarding the treatment drugs. The code was broken only after the study was complete and analysis of the results was carried out." p. 309 in journal article. Judgement: blinding adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants lost to follow‐up, participant drop‐outs, and severe adverse events accounted for in journal article. Intention‐to‐treat analyses were performed. Outcome data adequate. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in journal article. |
| Other bias | Low risk | No other bias detected. |
| Overall bias assessment (mortality) | Low risk | Low risk of bias in all domains |
| Overall bias assessment (non‐mortality outcomes) | Low risk | Low risk of bias in all domains |
Sidhu 2016.
| Study characteristics | ||
| Methods | Randomised, open‐label, non‐inferiority clinical trial | |
| Participants | This trial assessed the effects of rifaximin versus lactulose in 112 people with cirrhosis and minimal hepatic encephalopathy. There were 57 participants in the rifaximin group and 55 in the lactitol group. Age (median (range)) years
Proportion of men (n: %)
Aetiology of cirrhosis (n: %) Rifaximin
Lactulose
|
|
| Interventions | Intervention: rifaximin 400 mg thrice daily Control intervention: lactulose 30 to 120 ml daily Dosage adjusted to ensure passage of 2 to 3 semi‐formed stools daily Co‐intervention: none Duration of treatment: 90 days Duration of follow‐up: 9 months after inclusion |
|
| Outcomes | Neuropsychiatric assessment Battery of 5 psychometric tests: number connection, figure connection, digit symbol, picture completion, block design. Participants were diagnosed as having minimal hepatic encephalopathy if two or more of the psychometric tests were abnormal. |
|
| Inclusion period | March 2011 to August 2013 | |
| Outcomes included in meta‐analyses | Mortality, reversal of minimal hepatic encephalopathy, development of overt hepatic encephalopathy, adverse events, health‐related quality of life (Sickness Impact Profile) | |
| Country of origin | Single centre in India | |
| Notes |
Publication status: full paper Vested interests bias: none Additional information: published initially as abstract. Additional information on randomisation methods, blinding and mortality was obtained from the corresponding author in May 2014. Published as full paper article in 2015, with a follow‐up in 2017. Further information on randomisation methods, blinding of statistician, adverse events, and whether data were collected for ammonia levels and length of hospital stay. We also clarified other identified references to include from this study group. This information was obtained from the corresponding author on 18 April 2021. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomised in to two groups (group A and B) using computer‐generated randomisation (1:1)." p. 2. Information from authors confirms this: "Patients were randomised using computer‐generated randomisation 1:1." This was done by the lead statistician, who was blinded at that point. Judgement: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "..concealed by using sealed envelopes.." p. 2. Information from corresponding author confirms this: "Sequentially numbered, sealed, opaque envelopes were used for treatment allocation by a coordinator who was not an investigator." Judgement: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This study is reported is the published paper as unblinded: "Another limitation was that our study was unblinded." We did however receive Information from the corresponding author to the effect that: "Investigators, data entry operator and statistician (up to the point of analysis) were blinded regarding treatment drugs" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | See above. Further information from the author: "The statistician was not strictly blinded during analysis. However, he preferred to be genuinely concerned about the blinding in this study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals and dropouts reported in information from the corresponding author, and on p. 3‐4 in paper |
| Selective reporting (reporting bias) | Low risk | Published as a full report |
| Other bias | Low risk | No other bias detected |
| Overall bias assessment (mortality) | Low risk | Low risk in all domains except detection bias |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of detection bias |
Suzuki 2018.
| Study characteristics | ||
| Methods | Randomised, evaluator blinded clinical trial | |
| Participants | This trial assessed the effects of rifaximin versus lactitol in 172 people with cirrhosis and an acute episode of hepatic encephalopathy, grade I or II. There were 84 participants in the rifaximin group and 87 in the lactitol group. Age (median (range)) years
Proportion of men (n: %)
Aetiology of cirrhosis (n: %) Rifaximin
Lactitol
|
|
| Interventions | Intervention: rifaximin tablets 400 mg thrice daily Control Intervention: lactitol 6‐12 g thrice daily Co‐intervention: none Duration of treatment: 14 days |
|
| Outcomes | Neuropsychiatric assessment PSE Sum/Index: mental status (West Haven criteria), asterixis, NCT‐A and B, and digit symbol test, blood ammonia, EEG mean frequency |
|
| Inclusion period | 2013 to 2015 | |
| Outcomes included in meta‐analyses | Mortality, adverse events, health‐related quality of life, hepatic encephalopathy, blood ammonia | |
| Country of origin | A total of 37 investigatory sites in Japan | |
| Notes |
Publication status: full paper Vested interests bias: trial conducted under the auspices of ASKA Pharmaceutical Co. Ltd; a company employee was a co‐author Additional information: the published report included data not only from the 14‐day randomised comparison of rifaximin versus lactitol but also from a 10 week roll‐over study of rifaximin treatment alone. Only the randomised trial was included in the analyses. Authors were contacted on 20 March 2018 for further information on improvement in the hepatic encephalopathy; they were further contacted on 11 April 2021 for data on non‐serious adverse events and length of hospital stay; still awaiting response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised in a 1:1 ratio using a web assignment system |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment stated in report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in report. All participants who had completed at least one dose of the investigational drug and underwent at least one efficacy evaluation were included in the statistical analysis. |
| Selective reporting (reporting bias) | Low risk | No reporting bias detected |
| Other bias | Low risk | No other bias identified |
| Overall bias assessment (mortality) | Low risk | Although the study was not double‐blind, this is unlikely to affect mortality outcomes. |
| Overall bias assessment (non‐mortality outcomes) | High risk | One or more domains classified as 'high' risk of bias |
Tan 2022.
| Study characteristics | ||
| Methods | Randomised, open‐label, prospective clinical trial | |
| Participants | This trial assessed the effects of high and low‐dose rifaximin versus placebo in 40 people with cirrhosis and 'covert' hepatic encephalopathy. There were 12 participants in the low‐dose rifaximin group, 14 in the high‐dose group, 14 in the control group. Age (median (range)) years
Proportion of men (n: %)
Aetiology of cirrhosis (n: %) Rifaximin low‐dose
Rifaximin high‐dose
Control
|
|
| Interventions | Intervention: low‐dose rifaximin 400 mg twice daily; high‐dose rifaximin 600 mg twice daily Control Intervention: placebo, unknown Co‐intervention: none Duration of treatment: 8 weeks |
|
| Outcomes | Neuropsychiatric assessment Psychometric Hepatic Encephalopathy Score, Stroop Test |
|
| Inclusion period | 2017 to 2020 | |
| Outcomes included in meta‐analyses | Mortality, adverse events, health‐related quality of life (Sickness Impact Profile), hepatic encephalopathy | |
| Country of origin | China | |
| Notes |
Publication status: full paper Vested interests bias: none declared Additional information: in the published paper the participants are referred to as having covert hepatic encephalopathy (a group comprising of minimal and grade I‐II overt encephalopathy) but none appears to have had any clinically defining features of overt hepatic encephalopathy; the diagnosis was made based on abnormalities of both the Stroop test and the PHES score and hence they are classified, for purposes of this review as having minimal hepatic encephalopathy. The published report includes high and low‐dose rifaximin as separate groups. In our analyses, we have combined the rifaximin doses as a single group as authors reported similar outcomes between low and high‐dose groups. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally‐generated random assignment table used |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis used for missing data |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes reported |
| Other bias | Low risk | None identified |
| Overall bias assessment (mortality) | Low risk | Low risk of bias for mortality outcomes |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of bias from non‐mortality outcomes due to open‐label design |
Uthman 2020.
| Study characteristics | ||
| Methods | Randomised, single‐centre, placebo‐controlled clinical trial | |
| Participants | This trial assessed the effects of rifaximin plus lactulose, compared to placebo plus lactulose, for the treatment of overt hepatic encephalopathy in 84 people with cirrhosis. There were 42 participants in the rifaximin + lactulose group and 42 in the placebo plus lactulose group. Age (reported as % participants < 60 years)
Proportion of men (n: %)
Aetiology of cirrhosis not stated |
|
| Interventions | Intervention: rifaximin 550 mg twice daily Control: placebo, frequency unknown Co‐intervention: none Duration of treatment: 15 days or until discharge |
|
| Outcomes | Neuropsychiatric assessment Mental status ( West Haven criteria); portosystemic encephalopathy index |
|
| Inclusion period | July 2019 to December 2019 | |
| Outcomes included in meta‐analyses | Mortality | |
| Country of origin | India | |
| Notes |
Publication status: full paper Vested interests bias: none Additional Information: although data on the number of participants in whom hepatic encephalopathy completely resolved is provided (rifaximin plus lactulose 34/42 (80.9%); placebo + lactulose alone 20/42 (47.6%); chi2 P = 0.003, no information is provided on the number of participants in whom there was no change or worsening of hepatic encephalopathy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study is classified as randomised but no details are provided on randomisation method ‐ participants were divided into 2 groups. |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment methods provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were randomised to rifaximin plus lactulose or placebo plus lactulose, but no information is provided on the placebo preparation or blinding, |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Participants were randomised to rifaximin plus lactulose or placebo plus lactulose, but no information is provided on the placebo preparation or blinding, |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in final analyses. |
| Selective reporting (reporting bias) | High risk | Mortality can be deduced from the data although were not specifically reported; the number of people in whom hepatic encephalopathy resolved completely is reported but the numbers in whom hepatic encephalopathy remained unchanged or worsened is not provided. Trial not available in registries. |
| Other bias | Low risk | No other bias identified |
| Overall bias assessment (mortality) | High risk | High risk of bias in more than one domain |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of bias in more than one domain |
Vyas 2017.
| Study characteristics | ||
| Methods | Randomised, open‐label clinical trial | |
| Participants | The trial assessed the effects of lactulose plus rifaximin or lactulose alone in participants with cirrhosis and acute‐on‐chronic liver failure who were admitted to hospital with grade III/IV hepatic encephalopathy. There were 38 participants in the rifaximin plus lactulose group and 35 in the lactulose alone group. Age
Proportion of men
Aetiology of cirrhosis
|
|
| Interventions | 86 participants initially received lactulose 100 ml followed by 30 ml hourly for 24 hours to ensure passage of 4 to 6 bowel movements. The 73 participants in whom blood ammonia concentration were still > 70 ug/dl following purgation were then randomised as follows. Intervention: rifaximin 400 mg every 8 hours plus enteral lactulose 30 ml every 6 hours Control group: lactulose 30 ml every 6 hours Co‐interventions: none Duration of treatment: 72 hours Follow‐up duration: 30 days |
|
| Outcomes | Neuropsychiatric assessment Mental status (most likely West Haven criteria); blood ammonia |
|
| Inclusion period | November 2014 to December 2015 | |
| Outcomes included in meta‐analyses | Mortality, hepatic encephalopathy, blood ammonia (primary end point attainment of a blood ammonia of 70 mg/dl; this was achieved in 21.1% of participants on lactulose and 22.9% on rifaximin plus lactulose, but data were not extractable for our meta‐analysis). | |
| Country of origin | Single centre in India | |
| Notes |
Publication status: abstract only Vested interests bias: none Additional information: authors contacted on 11 April 2021 for further information on participant and study characteristics, randomisation methods and concealment of allocation, handling of incomplete outcome data, clarification of data extracted regarding hepatic encephalopathy outcomes, and data on our secondary outcomes. We also requested access to their blood ammonia data by treatment groups rather than as a 'primary end point'; still awaiting response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Active arm was randomised but no details provided. |
| Allocation concealment (selection bias) | High risk | Participants had already been treated with lactulose, so unclear if the allocation to additional treatment was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was an open‐label study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | This was an open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Outcome data presented as percentages not as actual numbers. |
| Selective reporting (reporting bias) | High risk | Compared to the trial registry, outcomes of duration of hepatic encephalopathy post‐inclusion, and duration of intensive care unit stay, were not reported by the authors. |
| Other bias | Low risk | No other biases identified. |
| Overall bias assessment (mortality) | Unclear risk | Deaths reported for both arms and, within the total population, for those in whom blood ammonia did or did not improve, but as percentages rather than actual numbers. |
| Overall bias assessment (non‐mortality outcomes) | High risk | Selection, performance, detection bias selective reporting bias identified as high risk. |
Wahib 2014.
| Study characteristics | ||
| Methods | Randomised, open‐label clinical trial | |
| Participants | This trial assessed the effects of rifaximin versus lactulose in 50 people with cirrhosis and an acute episode of hepatic encephalopathy, grade I‐III. There were 25 participants in each group. Age
Proportion of men
Aetiology of cirrhosis
|
|
| Interventions | Rifaximin: rifaximin 400 mg thrice daily Control intervention: lactulose 30 ml thrice daily Co‐interventions: daily enemata in both groups Duration of treatment: 7 days |
|
| Outcomes | Neuropsychiatric assessment Modified PSE Sum/Index: mental state (West Haven Criteria), asterixis, NCT‐A, venous blood ammonia |
|
| Inclusion period | Not reported | |
| Outcomes included in meta‐analyses | Mortality, hepatic encephalopathy, blood ammonia | |
| Country of origin | Single centre in Egypt | |
| Notes |
Publication status: full paper Vested interests bias: no information provided Additional information: authors contacted on 11 April 2021 for further information on participant and study characteristics, conflicts of interest, randomisation methods, allocation concealment, blinding status, trial registry status, clarification on missing outcomes, and data on both our primary and secondary outcomes; still awaiting response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were classified into two groups: no mention of randomisation. |
| Allocation concealment (selection bias) | High risk | No mention of randomisation; open‐label trial. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding in journal article. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of blinding in journal article. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in journal article; no deaths reported. |
| Selective reporting (reporting bias) | High risk | No prespecified outcomes; no information provided on side effects; trial not registered in trial database. |
| Other bias | Low risk | No other bias detected. |
| Overall bias assessment (mortality) | Low risk | All participants alive at the end of the study. |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of selection, detection, performance, and reporting bias; no information on side effects of treatment. |
Zeng 2021.
| Study characteristics | ||
| Methods | Randomised, open‐label clinical trial | |
| Participants | This trial assessed the effects of long‐term administration of low‐dose rifaximin versus 'conventional therapy' in preventing complications and prolonging survival in 195 people with decompensated cirrhosis. All participants were free of hepatic encephalopathy at baseline; the effects of rifaximin versus standard treatment for preventing the development of overt hepatic encephalopathy were assessed for this review. There were 97 participants in the rifaximin group and 98 in the standard of care group. Age (mean ± SD) years
Proportion of men (n: %)
Aetiology of cirrhosis (n: %) Rifaximin
Standard care
|
|
| Interventions | Intervention: rifaximin 400 mg twice daily Control intervention: standard care Co‐interventions: none Duration of treatment: 6 months treatment, with 6 months follow‐up |
|
| Outcomes | Neuropsychiatric assessment Mental status ‐ presumably West Haven criteria |
|
| Inclusion period | September 2014 to November 2017 | |
| Outcomes included in meta‐analyses | Mortality, hepatic encephalopathy, adverse events, blood ammonia | |
| Country of origin | A total of 8 investigatory centres in China | |
| Notes |
Publication status: full paper Vested interests bias: none Additional information: there was a significant imbalance in the proportion of participants in the rifaximin and control groups who had a history of hepatic encephalopathy at baseline (20.6 vs 4.1%; P < 0.001). Hence, the comparison of the number of episodes of overt hepatic encephalopathy during the follow‐up period between the two groups was analysed by adjusted logistic regression. The authors were contacted on 11 April 2021 for further information on co‐interventions given, quality of life outcomes, and hospital length of stay: response still awaited. Trial identifier: NCT: clinicaltrials.gov/ct2/show/NCT02190357 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible individuals were randomly allocated into a rifaximin group and a control group with a randomised block digital table in a ratio of 1:1. |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outlined number of participants screened (n = 265), the number subsequently enrolled (n = 200) and the remaining participants after exclusion or withdrawal. All participants who discontinued the study drug were accounted for (intention‐to‐treat). |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | No other bias detected. |
| Overall bias assessment (mortality) | Low risk | Low risk of bias for mortality outcomes |
| Overall bias assessment (non‐mortality outcomes) | High risk | Blinding and allocation concealment have a high risk of bias. |
EEG: electroencephalogram; NAFLD/NASH: non‐alcoholic fatty liver disease/non‐alcoholic steatohepatitis; NCT‐A: Number Connection Test A; PHES: portosystemic hepatic encephalopathy score; PSE: portosystemic encephalopathy; SD: standard deviation; SEM: standard error of the mean; TIPS: transhepatic portalsystemic shunt
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abd‐Elsalam 2016 | Randomised clinical trial involving 262 participants with cirrhosis, ascites and a previous episode of spontaneous bacterial peritonitis assigned to receive either rifaximin or norfloxacin for 6 months. We excluded this study as the comparator was another antibiotic. |
| Ahire 2017 | A prospective observational study involving 60 participants with cirrhosis and hepatic encephalopathy allocated to treatment with rifaximin plus lactulose (n = 32) or lactulose alone (n = 28) by physicians decision. We excluded the study because the treatment allocation was not randomised. |
| Ahluwalia 2014 | Observational study involving 20 participants with cirrhosis and minimal hepatic encephalopathy who underwent cognitive testing before and after 8 week treatment with rifaximin. We excluded this study because it was uncontrolled. |
| Anon 2014a | Paper not retrievable. Springer Nature Group were contacted on 12 April 2021, but their response on 30 April 2021 was: "due to a technical error, it is unfortunately impossible to upload the missing pages". |
| Anon 2014b | Paper not retrievable. Springer Nature Group were contacted on 12 April 2021 but their response on 30 April 2021 was: "due to a technical error, it is unfortunately impossible to upload the missing pages". |
| Bajaj 2013 | Observational study involving the same 20 participants with cirrhosis and minimal hepatic encephalopathy as Ahluwalia 2014; participants underwent cognitive testing, serum endotoxin analysis, urine/serum metabolomics, and faecal microbiome assessment before and after 8 week treatment with rifaximin. We excluded this study because it was uncontrolled. |
| Bajaj 2016b | Randomised, double‐blind, placebo controlled dose‐finding study in 518 people with early decompensated cirrhosis allocated to rifaximin at varying dose levels (n = 422) or placebo (n = 94). We excluded this study as its primary aim was to test the efficacy of a new formulation of rifaximin. The amount of extractable information was limited. In particular, we could not identify information on the proportion of participants with hepatic encephalopathy at baseline or how it was assessed. |
| Bajaj 2020a | Post hoc analysis of an open‐label randomised control trial involving 221 participants assigned to rifaximin (n = 113) or rifaximin plus lactulose (n = 108). The study end point was the number of episodes of hepatic encephalopathy in participants in whom lactulose was withdrawn before randomisation against those who continued lactulose. The study was excluded as both groups received rifaximin. Full data from the trial can be accessed on ClinicalTrials.gov site. |
| Bajaj 2020b | Randomised, placebo‐controlled, double‐blind, multi‐arm, dose‐ranging, parallel assignment, phase 2 trial in 71 people with overt hepatic encephalopathy at baseline or grade ll‐lll hepatic encephalopathy following 8 to 12 hr of intravenous fluids and lactulose. Participants received rifaximin immediate‐release soluble solid dispersion in combination with lactulose, or placebo. We excluded this study as there were no extractable data in the abstract or press releases (below). Information from Bausch Health America Inc, received on 2 September 2020, confirmed that the study was ongoing and that recruitment was complete. Last study update posted March 2021 as completed but with no reported data. Further information from Bausch Health America Inc on 19 April 2021: "The study in question is currently under review with the Food and Drug Administration for a separate indication, and the sponsor is not able to share data beyond what has already been presented in the press release." A press release (available at https://ir.bauschhealth.com/news‐releases/2020/03‐31‐2020‐120048038) [last accessed 8 May 2021], stated: "In the double‐blinded, placebo‐controlled multi‐arm, dose‐ranging study, the treatment arm evaluating 40 mg BID of rifaximin SSD IR plus standard of care therapy met its primary endpoint of time to resolution of overt hepatic encephalopathy using the Hepatic Encephalopathy Grading Instrument (HEGI) scale. The 40 mg BID rifaximin SSD IR in combination with standard of care therapy treatment arm was statistically significantly superior to the placebo plus standard of care therapy treatment arm with median time to resolution being 21.1 hours versus 62.7 hours, respectively. The rates of adverse events were comparable across all treatment arms of the study" This information was insufficient to allow inclusion of this trial at this time. |
| Block 2010 | Unable to retrieve study. The publisher was contacted on 7 November 2020 and 13 April 2021; we still have not received a response. |
| Bohra 2020 | Observational study involving 188 participants with cirrhosis and hepatic encephalopathy who were treated with rifaximin. Outcomes were assessed up to 48 months following admission, and included 12‐month survival. We excluded this study as it is observational; however, we included the information on adverse events in our review of harms. |
| Chang 2021 | Retrospective cohort study involving 43 participants with previous hepatic encephalopathy allocated to rifaximin plus lactulose (n = 12) or lactulose alone (n = 31) for prevention of recurrence. We excluded this study as group allocation was not randomised, but we included information on adverse events in our review of harms. |
| Cobbold 2018 | An open‐label pilot study in participants with biopsy‐proven NASH and elevated serum ALT activity who received 6 weeks rifaximin 400 mg twice daily, followed by a 6‐week observation period. The primary end point was change in serum ALT after 6 weeks and changes in hepatic lipid content and insulin sensitivity. We excluded this study because it was not randomised, none of the patients had cirrhosis and the endpoints did not include hepatic encephalopathy. |
| Crisafulli 2016 | Randomised, dose‐comparison study in 77 participants with cirrhosis and acute hepatic encephalopathy allocated to high dose rifaximin plus lactulose (n = 39) or standard dose rifaximin plus lactulose (n = 38). We excluded the study as there was no control group. |
| CTRI/2019/05/018966 | Randomised, double‐blind, study comparing lactulose plus rifaximin versus lactulose, rifaximin and L‐ornithine L‐aspartate for the treatment of overt grade lll‐lV hepatic encephalopathy in 124 people with cirrhosis. We excluded this study as rifaximin will be given to participants in both study arms. The trial is not yet recruiting. |
| Danulescu 2013 | Non‐randomised, case‐control study involving 46 participants with severely decompensated cirrhosis and refractory ascites, followed over a period of 6 months. Of these 22 received rifaximin for the treatment of hepatic encephalopathy while 24 did not. The primary outcome was the development of spontaneous bacterial peritonitis. We excluded this trial because it was observational. |
| De Marco 1984 | Randomised study involving 32 participants with cirrhosis and varying degrees of hepatic encephalopathy allocated to treatment with rifaximin (n = 18) or paromomycin (n = 14) over a 6 to 15‐day period. We excluded this study as the comparison was with another antibiotic. |
| Deshmukh 2016 | Randomised, 6‐month open‐label study evaluating the efficacy and safety of rifaximin versus lactulose for the treatment of minimal/covert hepatic encephalopathy in an unspecified number of people with cirrhosis. The study is published in abstract form; quantitative and qualitative data were not provided. The study has not been published as a full paper and no response has been obtained from the trialists. |
| Di Piazza 1991 | Randomised, double‐blind, cross‐over study involved 14 participants with chronic persistent or recurrent hepatic encephalopathy allocated to rifaximin or neomycin for one week with a one‐week washout. Concomitant treatment with lactulose was allowed. We excluded this study because the comparator was another antibiotic. |
| Diana‐Maria 2019 | Randomised trial involving 66 participants with cirrhosis admitted with an episode of acute hepatic encephalopathy who were allocated equally to lactulose and rifaximin or lactulose plus rifaximin plus L‐ornithine L‐aspartate. We excluded this study as rifaximin was given in both treatment arms. |
| Dupont 2016 | Randomised, 6‐month, open‐label study of the effect of rifaximin, with or without lactulose, on stool microbiota and antimicrobial susceptibility in people with cirrhosis and recurrent overt hepatic encephalopathy in remission. We excluded this study because there were no extractable data on hepatic encephalopathy. |
| EUCTR2014‐001856‐51‐DK | A randomised double‐blind, placebo‐controlled, single‐centre trial involving participants with alcohol‐related liver injury and hepatic fibrosis classified, using the Ishak score, from F1‐F4, allocated to rifaximin or placebo. We excluded this study as the majority of the participants would not have established cirrhosis (F4) and because it is unclear whether outcomes of interest will be available as clinical data collection is not mentioned. |
| Frenette 2020a | Randomised, open‐label trial evaluating rifaximin versus rifaximin plus lactulose for 6 months in 64 participants with previous hepatic encephalopathy now in remission. The trial focused on colonic microbial cross‐resistance to other antibiotics in the people treated with rifaximin alone vs rifaximin plus lactulose. We excluded this trial as rifaximin was administered to both groups, and no data on hepatic encephalopathy were reported. |
| Frenette 2020b | Randomised, open‐label trial evaluating rifaximin versus rifaximin plus lactulose for 6 months in 66 participants with previous hepatic encephalopathy now in remission. The trial focused on the impact of rifaximin alone or rifaximin plus lactulose treatment on stool microbiota in people with a history of OHE. We excluded this trial as rifaximin was administered to both groups, and no data on hepatic encephalopathy were reported. |
| Gangarapu 2015 | A prospective, open‐label, observational cohort study in participants with biopsy‐proven NAFLD/NASH in whom circulating endotoxins and cytokines were measures before and after 28 days of rifaximin. We excluded this study because it was not randomised or controlled and the participants did not have cirrhosis or hepatic encephalopathy. |
| Giacomo 1993 | Randomised, double‐blind, double‐dummy clinical trial involving 40 participants with mild hepatic encephalopathy allocated to rifaximin (n = 20), or lactulose (n = 20). We have excluded this study as we were unable to extract quantitative data. We contacted Alfa Sigma for further information on 3 April 2021 but have b not received a response. |
| Gupta 2021 | A prospective, open‐label, randomised clinical trial comparing rifaximin versus rifaximin plus norfloxacin for secondary prophylaxis of spontaneous bacterial peritonitis in 62 participants with cirrhosis and hepatic encephalopathy. We excluded this study because both trial groups received rifaximin. |
| Habib 2020 | Randomised, dose‐comparison trial evaluating rifaximin 400 mg daily and rifaximin 1100 mg daily in 80 participants with cirrhosis and hepatic encephalopathy. We excluded this trial as there was no suitable comparator. |
| Hammond 2017 | Retrospective cohort study in which participants who had experienced an episode of hepatic encephalopathy in the previous 6 months were reviewed and outcomes in those who had received lactulose plus rifaximin (n = 169) were compared with those receiving lactulose alone (n = 437). We excluded this study because it was not randomised. |
| Hotten 2003 | Randomised trial involving 30 participants with compensated Child B cirrhosis allocated in equal numbers to receive 3‐week treatment with (i) lactitol; (ii) rifaximin; or (iii) the symbiotic SCM‐III. The primary objective was the effect of the three treatments on faecal organic acid excretion and gut flora changes. We excluded this study as none of the participants had hepatic encephalopathy at baseline and neuropsychiatric status was not monitored during the study. |
| Huang 2018 | This abstract reports the results of an analysis of gut microbiome and its metabolites in participants with cirrhosis who had a variceal haemorrhage, underwent endoscopic intervention for prophylaxis of re‐bleeding and received 8‐week rifaximin treatment based on a randomised open‐label trial of rifaximin versus no antibiotic treatment; this trial appears to have two registrations on clinical trials.gov‐‐ NCT02991612 and NCT02964195. The only results reported to date appear to be those pertaining to the gut microbiome. We excluded this study as hepatic encephalopathy was not included as a primary or secondary end point. |
| Jain 2022 | A double‐blind, randomized, placebo‐controlled trial, involving 140 participants with cirrhosis and acute hepatic encephalopathy who received a combination of L‐ornithine L‐aspartate, lactulose, and rifaximin or placebo, lactulose, and rifaximin. We excluded this study as both trials groups received rifaximin. |
| Jiménez 2022 | A multicentre, open, comparative pilot study exploring the use of rifaximin as an add‐on to standard therapy in a prospective cohort of 21 participants with severe alcoholic hepatitis; outcomes were compared with a matched historical cohort of 42 participants with severe alcoholic hepatitis from other countries; the primary outcome was the incidence of infection. We excluded this study because it was not randomised; the proportion of participants with underlying cirrhosis was not specified and hepatic encephalopathy, although monitored, was not one of the stated outcomes. |
| John 2018 | Randomised trial involving participants with cirrhosis and overt hepatic encephalopathy randomised in groups of 20 to (i) rifaximin plus branched chain amino acids (BCAA); (ii) rifaximin plus bovine immunoglobulin (IG) or (iii) rifaximin plus BCAA plus bovine IG. The study was excluded as a non‐rifaximin control group was not included. |
| Jones 2020 | Retrospective, data‐linked analysis of 4669 people with newly diagnosed hepatic encephalopathy treated with either rifaximin, lactulose or a combination of the two in primary care. Treatment was assumed to last for 28 days either side of the prescription date. We excluded this study from the main analyses as it was not randomised, but included survival data in our review of harms. |
| Kaji 2017 | Observational study examining the effects of 4‐weeks' treatment with rifaximin in 20 participants with decompensated cirrhosis. The study was excluded from the quantitative analyses as it was not controlled, but data on the adverse events were included in our review of harms. |
| Kalambokis 2012a | Open‐label, observational study to assess the effects of 4 weeks of treatment with rifaximin on systemic haemodynamics and renal function in 13 participants with alcohol‐related cirrhosis and ascites. The study was excluded because it was observational and uncontrolled. |
| Kalambokis 2012b | Open‐label, observational study to assess the effects of 4 weeks of treatment with rifaximin on platelet counts, plasma endotoxin and serum interleukin‐1 (IL‐1), interleukin‐6 (IL‐6) and tumour necrosis factor‐α (TNF‐α) in 25 participants with alcohol‐related cirrhosis and thrombocytopenia. Four participants had hepatic encephalopathy at baseline (two in each group); no follow‐up data were provided on neuropsychiatric status; no adverse events were reported. We excluded this study as it was observational and did not report on our end points of interest. |
| Kalambokis 2012c | Open‐label, observational study to assess the effects of either 4 weeks treatment with rifaximin (n = 9) or no treatment (n = 7) on the occurrence of spontaneous bacterial peritonitis (SBP) in participants with cirrhosis and ascites but no history of previous SBP. The study was observational and did not include an assessment of neuropsychiatric status; no side effects of treatment were recorded. |
| Kalambokis 2012d | Open‐label, observational study to assess the effects of 8 weeks treatment with rifaximin on endotoxaemia, liver function and disease severity in nine liver transplant candidates with alcohol‐related cirrhosis. Hepatic encephalopathy was assessed as none (n = 4) or suppressed by medication (n = 5) both before and after treatment with rifaximin; it was unclear whether the participants with suppressed hepatic encephalopathy were receiving other anti‐encephalopathy medication. The trial was excluded as it was observational and uncontrolled; no adverse events were reported. |
| Kang 2017 | Retrospective study comparing the effects of rifaximin plus lactulose versus lactulose alone in 1042 participants with a previous episode of hepatic encephalopathy. We excluded this trial as it was not randomised; however, we considered the data on adverse events in our assessment of harms. |
| Kawaratani 2022 | A multicenter, retrospective, observational, cohort study of 215 consecutive participants with cirrhosis and at least Grade 1 hepatic encephalopathy treated with rifaximin for > 12 months. The primary outcome was the effectiveness of long‐term rifaximin treatment; the secondary outcome was the safety of long‐term rifaximin treatment. We excluded this study because it was uncontrolled and because no serious adverse events were reported. |
| Khokhar 2015 | Randomised, 6‐month, dose‐comparison study involving 306 participants with cirrhosis with at least one previous episode of overt hepatic encephalopathy allocated to rifaximin 550 mg once daily (n = 128) or rifaximin 550 mg twice daily (n = 178). We excluded this study as there was no control arm. |
| Kimer 2018 | Randomised, placebo‐controlled, double‐blind clinical trial involving 54 participants with cirrhosis and ascites allocated to rifaximin (n = 36) or placebo (n = 18) for 28 days. No clinical data were reported in this publication but clinically relevant data were reported from the same dataset of participants in Kimer 2017. |
| Kimer 2022 | An open‐label, randomised, clinical trial involving 32 participants with severe alcoholic hepatitis who were allocated to standard medical therapy (SMT) or SMT plus rifaximin. We excluded this study because it was unclear whether some or all of the participants had cirrhosis and because although neurocognitive status was assessed at baseline it did not appear to have been monitored during the study. |
| Kubota 2022 | Randomised, open‐label study involving 83 people with cirrhosis and Grade I or II hepatic encephalopathy, refractory to non‐absorbable disaccharides, allocated to 12‐weeks treatment with rifaximin (n = 42) or rifaximin plus L‐carnitine (n = 41). Lactulose treatment was continued throughout. We excluded this study because there was no appropriate control group as rifaximin was given in both study arms. |
| Lauridsen 2018 | Randomised, double‐blind, study comparing the effects of 3 months treatment with lactulose, branched‐chain amino acids and rifaximin versus triple placebo in 44 participants with cirrhosis none of whom had evidence of overt hepatic encephalopathy although 22 manifest features of minimal hepatic encephalopathy on testing. We excluded this study as participants receive other potentially active anti‐encephalopathy medication in addition to rifaximin. |
| Lighthouse 2004 | Randomised open‐label trial comparing the effects of (i) rifaximin (2 weeks); (ii) SCM‐III (symbiotic) (2 weeks); and, (iii) rifaximin (1 week) followed by SCM‐III (5 weeks) on circulating benzodiazepine‐like substances, ammonia and endotoxin in 30 participants with viral‐related cirrhosis. All participants were taking low dose non‐absorbable disaccharide before the trial, but this was stopped 2 weeks before enrolment. We excluded this study as there was no appropriate control group and neuropsychiatric status was not assessed. |
| Miglio 1997 | Randomised trial involving 49 people with cirrhosis allocated to either rifaximin or neomycin for 14 consecutive days each month, for a period of six months. We excluded this study as it compared rifaximin with another antibiotic. |
| Mohamed 2018 | Randomised comparison of the effects of rifaximin (n = 60) versus metronidazole (n = 60) for the treatment of an acute episode of hepatic encephalopathy in participants with cirrhosis. We excluded this study as it compared rifaximin with another antibiotic. |
| Mostafa 2015 | Randomised single‐blind comparison of the effects of 6‐months treatment with rifaximin (n = 40) or norfloxacin (n = 30) for the prevention of spontaneous bacterial peritonitis in participants with cirrhosis and ascites. The study was excluded because it compared rifaximin with another antibiotic; neuropsychiatric status was not evaluated or monitored. |
| Mullen 2014 | A 24‐month, open label maintenance study of rifaximin in 392 participants with hepatic encephalopathy who had either participated in a prior randomised clinical trial or were newly enrolled. The primary outcome was safety, namely adverse events and clinical laboratory parameters. We excluded the trial as it was observational; however, we did include data on adverse events in our review of harms. |
| NCT00364689 | A single‐centre, randomised, controlled trial evaluating the efficacy and safety of rifaximin, given alone or in combination with lactulose, as compared to lactulose given alone, in people in remission from prior acute episodes of HE. We excluded this trial as it was terminated before completion because of recruitment difficulties. |
| NCT00748904 | A randomised, open label, single‐centre trial of rifaximin versus lactulose in hospital inparticipants with cirrhosis with progressive renal failure and stage 0 to 2 hepatic encephalopathy; the primary end points are progression to severe hepatic encephalopathy and severe adverse events (renal failure). Extractable data might have been available from this trial, once completed, but it has now been withdrawn. |
| NCT01676597 | Randomised, double‐blind trial to evaluate the effects of pentoxifylline in participants with clinical or subclinical hepatopulmonary syndrome; those not responding will continue treatment with pentoxifylline but will also be randomised to either rifaximin or placebo. We excluded this trials as neuropsychiatric status will not be monitored. The trial's current status is unknown. |
| NCT01846806 | Observational study examining the effects of 14 days treatment with rifaximin on bacterial overgrowth and delayed intestinal transit in 10 participants with cirrhosis. The trial was terminated but would have been excluded as it was observational and uncontrolled. |
| NCT01897051 | Randomised study comparing the effects of propranolol plus rifaximin versus propranolol plus placebo on the hepatic vein pressure gradient and the occurrence of upper gastrointestinal bleeding in 140 participants with cirrhosis and portal hypertension. The trial's current status is unknown. However, we excluded the trial as propranolol can have adverse effects on hepatic encephalopathy. |
| NCT01951209 | A pilot study of the effect of rifaximin on B‐cell dysregulation in cirrhosis. The study was terminated due to failed recruitment but would have been excluded as it did not appear that relevant clinical data would be collected. |
| NCT02011841 | Randomised study to compare the effects of rifaximin and ciprofloxacin for the secondary prevention of spontaneous bacterial peritonitis in participants with cirrhosis. This trial was terminated early because of poor recruitment but would have been excluded as it involved comparison with another antibiotic, and it was unclear if data on hepatic encephalopathy would be collected. |
| NCT02485106 | Randomised, open‐label study to evaluate the effect of rifaximin as an adjuvant to treatment with steroids or pentoxifylline in 170 participants with severe alcoholic hepatitis. The current status of this study is unknown, but it would be excluded as participants may or may not have coexisting cirrhosis and hepatic encephalopathy was not one of the stated outcomes. |
| NCT03712280 | A randomised, open‐label, completed comparator study to assess the pharmacodynamics, safety and pharmacokinetics of 3 different dose regimes of L‐ornithine phenylacetate versus rifaximin over a 5‐day period in 50 people with cirrhosis and a history of previous episodes of hepatic encephalopathy. The trial is completed, and the results posted on the clinicaltrials.gov website. We excluded this study as the comparator was not one of those stipulated for this review. |
| NCT04159870 | Randomised, open‐label trial comparing rifaximin versus norfloxacin for the primary prophylaxis of spontaneous bacterial peritonitis in 322 adults with cirrhosis and ascites. The study is active but not recruiting. We excluded this trial as the comparator was another antibiotic, and we were uncertain if relevant clinical data on hepatic encephalopathy would be collected. |
| Oey 2019 | Observational study involving 127 people with cirrhosis and overt hepatic encephalopathy allocated to rifaximin, with or without concomitant lactulose who were followed for at least 6 months or until death, liver transplantation, or permanent discontinuation of rifaximin. We excluded this study as it is observational; however, we included the reported adverse events in our review of harms. |
| Orr 2016 | Observational study involving 326 participants with cirrhosis receiving rifaximin for the secondary prevention of hepatic encephalopathy. We excluded this study as it was observational; however, we included the data on adverse events in out review of harms. |
| Parini 1992 | Randomised trial involving 30 adults with cirrhosis and an acute episode of hepatic encephalopathy allocated to rifaximin (n = 15) or paromomycin (n = 15) for 15 days. We excluded this trial as the comparator was another antibiotic. |
| Pedretti 1991 | Randomised, double‐blind study involving 30 participants with cirrhosis and an acute episode of hepatic encephalopathy allocated to rifaximin (n = 15) or neomycin (n = 15) for 21 days. We excluded this study as the comparator was another antibiotic. |
| Ponziani 2016 | An open‐label, observational study aimed at exploring the correlation between gut microbiota modulation and symptoms improvement in participants undergoing rifaximin treatment, including a small number with cirrhosis and hepatic encephalopathy (n = 4). We excluded this study as it was observational and did not provide quantifiable data. |
| Pose 2020 | Randomised, double‐blind, placebo‐controlled, dose‐safety study in 50 people with decompensated cirrhosis and moderate‐to‐severe liver failure assigned to simvastatin 20 mg/day plus rifaximin 1200 mg/day (n = 18), simvastatin 40 mg/day plus rifaximin 1200 mg/day (n = 16), or double placebo (n = 16). The primary end points were liver and muscle toxicity, estimated by changes in circulating enzyme activities. This study was excluded because no relevant clinical data were collected. This is the preliminary safety study for an efficacy study registered as ClinicalTrials.gov NCT03780673 which is currently recruiting. |
| Praharaj 2022 | Randomised, open‐label 6‐month study of rifaximin versus norfloxacin for the primary (n = 59) and secondary (n = 59) prevention of spontaneous bacterial peritonitis in participants with cirrhosis and ascites. Participants with prior overt or recurrent hepatic encephalopathy were excluded. We excluded this study as the comparator was another antibiotic and hepatic encephalopathy was not one of the primary outcomes. |
| Saboo 2021 | Cross‐sectional study of sex‐differences in gut microbial function and composition in participants with cirrhosis and hepatic encephalopathy receiving rifaximin plus lactulose (n = 170,) participants with cirrhosis and hepatic encephalopathy receiving lactulose alone (n = 130), participants with cirrhosis and no evidence of hepatic encephalopathy (n = 319) and healthy controls (n = 142). We excluded this study as it was not randomised and did not report clinically relevant outcomes. |
| Salehi 2019 | A retrospective analysis of 101 participants with at least two episodes of overt hepatic encephalopathy resulting in hospitalisation, or else encephalopathy at the time of assessment. Rifaximin‐treated or rifaximin‐naive participants were analysed. We excluded this study as it was not randomised; however, we included the data on adverse events in our review of harms. |
| Sama 2004 | Observational, open‐label study involving 26 participants with cirrhosis and hepatic encephalopathy previously intolerant or non‐responsive to treatment with lactulose who were treated with rifaximin for a 10‐day period. We excluded this trial as it was not controlled but considered the data on adverse events in our review of harms. |
| Sarwar 2019 | Randomised, double‐blind, quasi‐experimental trial involving 75 participants with decompensated cirrhosis but no history of hepatic encephalopathy allocated to rifaximin 400 mg (n = 34), or 1100 mg (n = 41) daily. We excluded this study as it was essentially dose ‐finding exercise with no control group. |
| Schulz 2019 | Observational study to characterise the active bacterial assemblages in the upper gut and stool samples in participants with cirrhosis and minimal hepatic encephalopathy before, during and after long‐term treatment with rifaximin. The participants comprised a subset of 5 of the 60 individuals recruited to the “RiMINI” trial; they were allocated to either 3‐months of rifaximin monotherapy (n = 1) or to 3‐months of rifaximin plus lactulose (n = 4). We excluded this trial as rifaximin was used in both study arms and no relevant outcomes were reported. |
| Seifert 2021 | A retrospective study looking at the prevention of hepatic encephalopathy post insertion of a transjugular intrahepatic portosystemic shunt; participants either received no treatment (n = 83) lactulose (n = 85), rifaximin (n = 6), rifaximin plus lactulose (n = 59) with or without L‐ornithine L‐aspartate. We excluded this study as it was a retrospective and treatment was not randomised. |
| Song 2021 | A pilot, multicentre, open‐label, randomised clinical trial in 42 participants with severe alcoholic hepatitis receiving corticosteroids or pentoxifylline; participants were randomized to rifaximin or control stratified by treatment for the underlying condition. We excluded this study because the participants did not have cirrhosis and hepatic encephalopathy was not an end point. |
| Suzuki 2019 | Retrospective 'real‐world' cohort study involving 65 participants with cirrhosis and hepatic encephalopathy on long‐term treatment with rifaximin. Participants were allowed to take other potentially active anti‐encephalopathy medications such as non‐absorbable disaccharides and branched‐chain amino acids. We excluded this study as it was not randomised or controlled; however, we considered the data on adverse events in our review of harms. |
| Tatsumi 2021 | Observational study involving 37 participants with cirrhosis and hepatic encephalopathy who were switched from treatment with kanamycin sulphate to rifaximin. We excluded this study as it was observational; however, we considered the reported adverse events in our review of harms. |
| Testa 1985 | Observational study involving 20 people with cirrhosis and minimal hepatic encephalopathy who were pretreated for one week with lactulose and were then randomly allocated to treatment with rifaximin or paromomycin for a further 5 days. We excluded this study as the comparator was another antibiotic. |
| Uchida 2020 | Retrospective, observational study involving 95 participants with decompensated cirrhosis. Those with unmanageable overt hepatic encephalopathy and/or hyperammonaemia received rifaximin, with or without a concomitant non‐absorbable disaccharides. We excluded this study as it was not randomised; however, we included data on adverse events in our review of harms. |
| UMIN000036998 | A randomised, open‐label controlled trial comparing zinc‐rifaximin combination therapy with zinc monotherapy in participants with hypozincaemia and minimal hepatic encephalopathy. This study has been terminated but would have been excluded because there was no inert control group. |
| UMIN000038487 | Randomised, open‐label trial comparing the effects of rifaximin for 12 weeks versus standard treatment for the prevention of hepatorenal syndrome in 94 participants with cirrhosis and ascites. The trial is ongoing but would be excluded as it does not appear that participants' neuropsychiatric status will not be assessed. |
| Venturini 2005 | Randomised study involving 18 participants with cirrhosis with no evidence of hepatic encephalopathy allocated, in groups of six, to either rifaximin, lactulose or placebo for 7 days to assess the effects on serum benzodiazepine‐like compounds. The study was excluded as none of the participants had hepatic encephalopathy and no information was provided on relevant outcomes. |
| Vittitow 2018 | Pharmacokinetic study of two formulations of rifaximin in healthy volunteers. |
| Vlachogiannakos 2013 | Assessment of the effects of long‐term rifaximin on outcomes in 23 participants with decompensated alcohol‐related cirrhosis who had shown improved liver haemodynamics while taking rifaximin and in 46 (pair matched) participants who did not receive rifaximin; both groups were followed for up to 5 years, death, or liver transplantation. We excluded this study as treatment allocation was not randomised, although we included the data on adverse events in our review of harms. |
| Walker 2020 | Observational study involving 389 adults with cirrhosis and persistent hepatic encephalopathy. Outcomes were assessed for a minimum of 12 months, and compared between those receiving rifaximin (n = 280) and those receiving standard care (n = 89). We excluded this study as it was not randomised; however, we included the data on adverse events in our review of harms. |
| Williams 2000 | Randomised, double‐blind, dose‐finding study involving 54 participants with cirrhosis and mild to moderate hepatic encephalopathy allocated to rifaximin 600 mg daily (n = 18), 1200 mg daily (n = 19) or 2400 mg daily (n = 17). We excluded this study as it lacked a control group. |
| Yang 2016 | Randomised, trial comparing the effects of (i) L‐ornithine L‐aspartate; (ii) rifaximin and (iii) L‐ornithine L‐aspartate plus rifaximin in 80 participants with cirrhosis and an acute episode of hepatic encephalopathy. We excluded this study as no suitable control group was included, and hepatic encephalopathy outcomes were not reported. |
| Zeng 2015 | Randomised open‐label study involving 43 participants with decompensated cirrhosis free of overt hepatic encephalopathy allocated, for a period of 2 weeks, to low‐dose rifaximin (800 mg daily; n = 14); high‐dose rifaximin (1200 mg/day; n = 14) or no treatment (n = 15). We excluded the trial from our main analyses as no information was provided on neuropsychiatric status during or following treatment, but we included data on adverse events in our review of harms. |
ALT: serum alanine aminotransferase; NAFLD/NASH: non‐alcoholic fatty liver disease/non‐alcoholic steatohepatitis; OHE: overt hepatic encephalopathy
Characteristics of ongoing studies [ordered by study ID]
ChiCTR1800018070.
| Study name | Clinical study of rehoci combined with long‐acting octreotide in reducing the risk of rebleeding in patients with cirrhosis and portal hypertension |
| Methods | Trial to determine if rifaximin (rehoci) has a beneficial effect on the risk of rebleeding in participants with cirrhosis and portal hypertension when used as monotherapy or when combined with octreotide. If data are reported on hepatic encephalopathy then we will be able to compare outcomes in the rifaximin monotherapy and placebo groups. Blinding not stated. |
| Participants | Planned recruitment sample size: 80 people. People aged 18 to 75 years of any gender with cirrhosis, portal hypertension, and confirmed oesophageal varices of any cause. Participants must be classified as Child‐Pugh grade B or C for over 1 month Participants will be excluded if suffering from moderate to severe hepatic encephalopathy "in recent months". |
| Interventions | Participants will be randomised to one of four groups of 20 participants each:
Dose and timings not stated |
| Outcomes | CT, MRI, ultrasonography, liver function, blood ammonia |
| Starting date | 1 January 2019 to 30 June 2022 |
| Contact information | Guan Jiao, tel: +86 18817821667; email: 0727guanjiao@163.com |
| Notes | Last update 28 August 2018, as 'not yet recruiting' This study might allow outcomes of interest to be compared between the rifaximin and placebo group. |
EUCTR2014‐000102‐35‐IT.
| Study name | Effect of administration "add on" of rifaximin on portal hypertension of patients with liver cirrhosis and oesophageal varices on standard therapy with propranolol |
| Methods | Randomised, double‐blind, inpatient prevention trial |
| Participants | Participants who meet the following criteria.
|
| Interventions | Intervention: rifaximin (unknown dose) Control intervention: placebo (details not stated) Duration of treatment: 60 days |
| Outcomes | Adverse events |
| Starting date | December 2014 |
| Contact information | Clinica Medica 5, Department of Medicine DIMED Padova, Italy 00390498212927 webmaster.medicinadimed@unipd.it |
| Notes | Country of origin: Italy Last update: 19 March 2018 Authorised‐recruitment may be ongoing or finished Monetary Support: Alfa Wassermann S.p.A. URL: www.clinicaltrialsregister.eu/ctr‐search/trial/2014‐000102‐35/IT This study will should allow access to data on outcomes of interest comparing rifaximin and placebo |
EUCTR2017‐000488‐34‐IT.
| Study name | Rifaximin blunted higher levels of endotoxin in cirrhosis patients: a randomized, double‐blind, short term interventional trial ‐ rifaximin in cirrhotic patient |
| Methods | Randomised, double‐blind clinical trial |
| Participants | Adults with decompensated cirrhosis (Child‐Pugh grade B or C), without overt hepatic encephalopathy at baseline |
| Interventions | Intervention: rifaximin 550 mg twice daily orally Control: film‐coated tablet placebo orally |
| Outcomes | Bacterial lipopolysaccharide concentration, thrombin generation, platelet activation indexes, adverse events, mortality, clinical outcomes Duration of intervention: 14 days Duration of follow‐up: 60 days after discontinuation of drug |
| Starting date | Unknown |
| Contact information | Stefania Basili (Sapienza‐Universita di Roma), stefania.basili@uniroma1.it, 3393452523 |
| Notes | Monetary support: Alfa Wassermann S.p.A Trial registered: 8 June 2021 Last update: 24 August 2021 This is a mechanistic trial which may provide data of relevance on hepatic encephalopathy. |
EUDRACT2016‐002628‐96.
| Study name | A multi‐centre, double‐blind, randomised, controlled clinical trial of rifaximin to reduce infection in patients admitted to hospital with decompensated cirrhosis |
| Methods | Randomised, double‐blinded, parallel assignment, multicentre, inpatient prevention trial |
| Participants | Inpatients with cirrhosis complications (e.g. alcoholic hepatitis, sepsis, variceal bleeding) and who are currently on antimicrobial therapy |
| Interventions | Treatment: rifaximin 550 mg twice daily Control: placebo 550 mg twice daily Treatment duration: 6 months |
| Outcomes |
|
| Starting date | April 2016 |
| Contact information | Dr Harry Antoniades / Dr Rooshi Nathwani ‐ see note below 10th Floor QEQM, St Mary's, South Wharf Rd London W2 1NY UK Tel (Dr H Antoniades): +442033126454 / fax: +442077249369 / mob (Dr R Nathwani): +44 7415 871928 c.antoniades@imperial.ac.uk / rooshi.nathwani08@imperial.ac.uk |
| Notes |
Principal investigator Harry Antoniades died 2 April 2018 Funded by Norgine Ltd. and Alfa Wasserman Registered as ISRCTN10994757 Intended to publish in January 2020. The outcome data collected in this study could be included in subsequent versions of this review, if the trial is completed or if sufficient data were collected before April 2018. |
NCT01846663.
| Study name | The efficacy, safety, and pharmacokinetics of rifaximin in subjects with severe hepatic impairment and hepatic encephalopathy. |
| Methods | Randomised, double‐blind (subject, caregiver, investigator, outcomes assessor), placebo controlled, parallel assignment, phase 4 trial |
| Participants | People in remission from overt hepatic encephalopathy and with MELD score of 19 or higher. |
| Interventions | Intervention: rifaximin (550 mg twice daily) Control intervention: placebo (orally administered) Duration of treatment: 6 months |
| Outcomes |
Duration of follow‐up: 6 months |
| Starting date | 2013 |
| Contact information | Erica Bullock erica.bullock@salix.com Tel: 919‐862‐1854 Salix Pharmaceuticals |
| Notes | Country of origin: USA URL: http://clinicaltrials.gov/show/NCT01846663 Information from Salix Pharmaceuticals regarding this trial: This study is ongoing. "The recruitment is difficult due to high MELD scores requirement" received on 7 November 2016 Last update posted 1 December 2021 with an estimated primary completion date of June 2022 Data from this study, when completed, could be included in future versions of this review |
NCT02439307.
| Study name | Effect of rifaximin on minimal hepatic encephalopathy and small intestinal bacterial overgrowth |
| Methods | Randomised, parallel assignment, double‐blind (participant, caregiver, investigator) trial |
| Participants | People with cirrhosis and diagnosed with minimal hepatic encephalopathy and small intestinal bacterial overgrowth |
| Interventions | Intervention: rifaximin (dose unknown) Control intervention: placebo (dose unknown) |
| Outcomes |
|
| Starting date | 2015 |
| Contact information | Coordinación de Investigación en Salud, Mexico |
| Notes | Country of origin: Mexico Update posted in July 2020 that the hospital had been converted to care solely for COVID‐19 patients; hence, the study was suspended. Response from the author 16 April 2021: "the clinical trial was discontinued because now the hospital accepts principa"lly patients with COVID. We don't have a date to reopen this clinical trial" URL: clinicaltrials.gov/show/NCT02439307 It is not known if the trial will resume as the pandemic wanes or if sufficient numbers of participants were recruited prior to closure to allow publication. Outcome data from this trial may still be available for inclusion in future versions of this review. |
NCT02508623.
| Study name | Effect of administration of rifaximin on the portal pressure of patients with liver cirrhosis and oesophageal varices (ERASE) |
| Methods | Randomised, parallel assignment, double‐blind (participant, caregiver, investigator, outcomes assessor), phase 3 trial |
| Participants | People with cirrhosis and presence of oesophageal varices at high risk of bleeding (60 participants) |
| Interventions | Intervention: rifaximin (550 mg 1 tablet twice daily for 60 days) Control intervention: placebo (Placebo 1 tablet twice daily for 60 days) |
| Outcomes | Change of hepatic venous pressure gradient, cognitive function, systemic inflammatory response and modification of faecal bacteria |
| Starting date | 2014 |
| Contact information | Piero Amodio ‐‐PI has retired Francesca Campagna his colleague remains in post Claudio Dario francescacampagna3@gmail.com Tel: +39 0498218675 /+39 0498212105 University of Padua, Italy |
| Notes | Outcome data from this study, if completed, could be included in future versions of this review |
NCT02931123 (Riggio 2016).
| Study name | A RCT comparing lactulose and rifaximin associated with a vegetable diet in the prevention of post‐TIPS overt hepatic encephalopathy |
| Methods | Randomised, open‐label, parallel assignment prevention study |
| Participants | People with cirrhosis undergoing TIPS placement |
| Interventions | Active: rifaximin and lactulose plus a predominantly vegetable protein diet Control: no intervention Duration: one month |
| Outcomes | Incidence of hepatic encephalopathy |
| Starting date | November 2015 |
| Contact information | Professor Oliviero Riggio Department of Clinical Medicine University of Roma La Sapienza Rome, RM, Italy, 00100 +390649972021: oliviero.riggio@uniroma1.it |
| Notes | Estimated enrolment n = 58 Current status: unknown; an update in October 2016 confirmed that the study was ongoing. Outcome data on the efficacy of rifaximin in combination with lactulose and a vegetable protein diet for primary prevention of post‐TIPS hepatic encephalopathy could be included in future versions of this review. |
NCT03069131.
| Study name | Two strategies of primary prophylaxis of spontaneous bacterial peritonitis in severe cirrhotic patients with ascites (ProPILARifax) |
| Methods | Randomised, double‐blind (participant, caregiver, investigator, outcomes assessor), parallel assignment, phase 3 trial |
| Participants | People with cirrhosis and grade 3 ascites or higher, and low ascitic protein (< 15 g/L) and renal or hepatic impairment. |
| Interventions | Intervention: rifaximin (550 mg 2 times daily, orally administered) Control: placebo (orally administered) Duration of treatment: unknown |
| Outcomes |
|
| Starting date | 2018 |
| Contact information | Thierry Thevenot tthevenot@chu‐besancon.fr Tel: +33381668594 Centre Hospitalier Universitaire de Besancon |
| Notes | Country of origin: France URL: clinicaltrials.gov/ct2/show/NCT03069131 This study is funded by Alfasigma S.p.A, LC2 Pharma Information from the author regarding this trial: "this trial is still ongoing" received 12 April 2021. Last update posted 17 July 2018 with an estimated study completion date of May 2021. Outcome data from this trial, when completed, could be added to future versions of this review. |
NCT04073290 (PEARL Study).
| Study name | Prevention of hepatic encephalopathy by administration of rifaximin and lactulose in patients with liver cirrhosis undergoing TIPS placement: a multi‐centre randomized, double‐blind, placebo‐controlled trial |
| Methods | Randomised, double‐blind (participant, care provider, investigator), parallel assignment, multicentre, phase 4 trial |
| Participants | People with cirrhosis and undergoing TIPS placement for refractory ascites or recurrent variceal bleeding |
| Interventions | Intervention: rifaximin (550 mg twice daily) Control intervention: placebo (twice daily) Co‐intervention: TIPS + 25mL twice daily lactulose (667 mg/ml, dose adjusted based on soft stool frequency) Duration: 72 hours before TIPS placement, until 3 months post‐TIPS |
| Outcomes | Overt hepatic encephalopathy (incidence at 3, 12 months and time to development of any episode), 90‐day mortality, PHES score, blood molecular composition, quality of life, cost‐effectiveness |
| Starting date | 21 January 2020 |
| Contact information | Koos de Wit, MD
0031‐20‐5668468 k.dewit1@amsterdamumc.nl |
| Notes | PEARL Study:
NCT04073290
EUCTR2018‐004323‐37
ZonMw848017009 Last updated on January 15, 2021 as 'recruiting'; author response 13 April 2021: "we can confirm that we are recruiting patients in this trial." Estimated primary completion date: 30 September 2022; Estimated study completion date: 30 September 2023 Trial protocol published in BMJ Open Gastroenterology: bmjopengastro.bmj.com/content/7/1/e000531 (last accessed 6 May 2021) Dr R B Takkenberg: r.b.takkenberg@amsterdamumc.nl Data from this trial will be included in future versions of this review. |
NCT04775329.
| Study name | Primary prophylaxis for spontaneous bacterial peritonitis in decompensated chronic liver disease with small bowel bacterial overgrowth: a randomised trial |
| Methods | A randomised, triple‐blind, placebo‐controlled study comparing rifaximin to standard of care for the prevention of spontaneous bacterial peritonitis in participants with decompensated liver disease and small bowel bacterial overgrowth. |
| Participants | Estimated enrolment number of 72 participants (recruiting as of 25 February 2021). |
| Interventions | Intervention: rifaximin 1000 mg once per day in the morning Control: standard of care Co‐interventions: unknown |
| Outcomes | Primary outcome: incidence of spontaneous bacterial peritonitis Secondary outcomes: hepatic encephalopathy, variceal bleeding, acute‐on‐chronic liver failure Follow‐up duration: 12 months |
| Starting date | 1 November 2020 |
| Contact information | Yu Jun Wong, Changi General Hospital, eugene.wong.y.j@singhealth.com.sg Prem Harichander Thurairajah, thurairajah.prem.harichander@singhealth.com.sg |
| Notes | Estimated completion date July 2023 The data on secondary outcomes from the completed study could be included in future versions of this review. |
NCT05071716 (RNLC3131).
| Study name | A randomized, double‐blind, placebo‐controlled, multicenter study to assess the efficacy and safety of rifaximin soluble solid dispersion (SSD) for the delay of encephalopathy decompensation in cirrhosis |
| Methods | Randomised, double‐blind, multicentre, phase 3 primary prevention trial |
| Participants | Inclusion criteria (target sample size = 466)
|
| Interventions | Intervention: rifaximin soluble solid dispersion immediate release 40 mg twice daily Control: placebo twice daily |
| Outcomes |
Assessment period: 72 weeks |
| Starting date | 7 April 2022 |
| Contact information | John Lahey (Bausch Health), john.lahey@bauschhealth.com, 908‐541‐8631 |
| Notes | Estimated primary completion date: January 2025 Estimated study completion date: January 2025 Data from this study, when completed, could be included in future versions of this review. |
TCTR20180509001.
| Study name | Comparison between rifaximin vs lactulose for treatment of hepatic encephalopathy. |
| Methods | Randomised, double‐blind, parallel‐assignment, efficacy, treatment phase 3 trial |
| Participants | Adults with cirrhosis and hepatic encephalopathy with Conn score ≥ 1. Those with spontaneous bacterial peritonitis or septicaemia were treated with antibiotics before recruitment; and people with gastrointestinal haemorrhage had bleeding controlled. Participants could receive lactulose less than a day before enrolment. |
| Interventions | Intervention: rifaximin, dose not specified Control intervention: lactulose, dose not specified Duration of treatment: unclear |
| Outcomes | Primary outcome: improvement of clinical syndrome of hepatic encephalopathy, by Conn score and asterixis grading Secondary outcomes: adverse events, including length of hospital stay and mortality Duration of follow‐up: unknown |
| Starting date | 22 December 2016; currently recruiting |
| Contact information | Contact for Scientific Queries: Watcharasak Chotiyaputta / Patchara Pannin 022549008 watcharasak.cho@mahidol.ac.th / patchara_p@bio‐nnova.com Bangkok, Thailand 10700 |
| Notes | Target sample size = 80. www.clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsearch&smenu=fulltext&task=search&task2=view1&id=3507 www.thaiclinicaltrials.org/TCTR20180509001 Sponsor: Bio‐innova and Synchron Co., Ltd Outcome data from this trial, once completed, could be included in future versions of this review |
CT: computerised tomography; MELD: model for end‐stage liver disease; MRI: magnetic resonance imaging; PHES: psychometric hepatic encephalopathy score; TIPS: transjugular intrahepatic portosystemic shunt
Differences between protocol and review
In the USA and in many European countries the only licensed indication for the use of rifaximin is as co‐medication with a non‐absorbable disaccharide for the prevention of episodes of hepatic encephalopathy following an index event. In consequence, the majority of recent trials have involved comparisons of rifaximin plus a non‐absorbable disaccharide against a non‐absorbable disaccharide alone. To better capture this we have changed the title of the review from 'Rifaximin for the treatment of hepatic encephalopathy in people with cirrhosis' to 'Rifaximin for prevention and treatment of hepatic encephalopathy in people with cirrhosis'. In addition, we have included 'prevention' as an outcome category in addition to the three categories, minimal hepatic encephalopathy, acute hepatic encephalopathy and chronic hepatic encephalopathy as specified in the original protocol.
Several of the medical interventions used to treat hepatic encephalopathy listed in the original protocol have either not been compared with rifaximin in randomised clinical trials, or else the number of trials available was too small for meaningful comparison. However, separate Cochrane Reviews evaluating these interventions have now been undertaken, including: probiotics (Dalal 2017), branched chain amino acids (Gluud 2017), L‐ornithine L‐aspartate (Goh 2018), and ammonia lowering agents (Zacharias 2019).
To avoid heterogeneity and multiple testing, and to establish analyses with clinically relevant outcomes, we also excluded trials comparing rifaximin to other antibiotics. A separate Cochrane Review will evaluate the use of aminoglycosides, vancomycin, and metronidazole versus no intervention, placebo, or other potentially beneficial pharmacological interventions for the prevention and treatment of hepatic encephalopathy in people with cirrhosis (Jeyaraj 2017).
We did not identify any trials which contained a mixture of participants with and without cirrhosis, and so we did not need to adjust the analyses to take account of this, as stipulated in the protocol.
We did not conduct trial sequential analysis for our outcomes, in light of new methodology recommendations.
We included the NCT‐A as a secondary outcome as this was commonly cited as an outcome in the included trials.
Contributions of authors
Harry Zacharias (HZ), Jaclyn Tan (JT), Fady Kamel (FK), Nina Kimer (NK), Lise Lotte Gluud (LG) and Marsha Morgan (MM) performed literature searches and listed studies for possible inclusion. All authors contributed to the selection of studies for inclusion and exclusion. HZ, JT, FK, NK, LG, and MM extracted data independently. HZ, JT, FK, NK and LG performed the statistical analyses. All authors participated in the interpretation of the analyses. All authors played a role in drafting the review. HZ and MM completeed the final draft of the review. All authors reviewed and approved the final manuscript.
NK and LG did not contribute to the overall study inclusion and exclusion criteria, and did not make study eligibility decisions about, extract data from, carry out the risk of bias assessment for, or perform GRADE assessments for the included study they authored (Kimer 2017).
Sources of support
Internal sources
-
No internal funding, Other
The authors received no internal funding
External sources
-
No external funding, Other
The authors received no external funding
Declarations of interest
HZ: has declared that they have no conflict of interest.
FK: has declared that they have no conflict of interest.
JT: has declared that they have no conflict of interest.
NK: reports being employed as a postdoctoral fellow in translational medicine by the University of Copenhagen, between 2016 and 2019 supported by the Novo Nordisk Foundation (grant no: NNF18SA0034956); she reports authorship of one of the trials included in this review (Kimer 2017) and of a previous systematic review and meta‐analysis of the effects of rifaximin in hepatic encephalopathy (Kimer 2014).
LG: reports receiving funding for research from Novo Nordisk, Alexion, Gilead, and Sobi International; she reports being a member of an advisory board for Novo Nordisk; she reports authorship of one of the trials included in this review (Kimer 2017) and of a previous systematic review and meta‐analysis of the effects of rifaximin in hepatic encephalopathy (Kimer 2014).
MM: has declared that they have no conflict of interest.
New
References
References to studies included in this review
Ahmed 2018 {published data only (unpublished sought but not used)}
- Ahmed S, Ahmad S, Khan SU. Lactulose alone versus lactulose + rifaximin for the management of hepatic encephalopathy. Pakistan Journal of Medical and Health Sciences 2018;12(3):1269-71. [Google Scholar]
Ali 2014 {published data only (unpublished sought but not used)}
- Ali B, Zaidi YA, Alam A, Anjum HS. Efficacy of rifaximin in prevention of recurrence of hepatic encephalopathy in patients with cirrhosis of liver. Journal of the College of Physicians and Surgeons Pakistan 2014;24(4):269-73. [PMID: ] [PubMed] [Google Scholar]
Babar 2017 {published data only (unpublished sought but not used)}
- Babar AN, Ali A, Khan MA, Tanveer A, ul Haq M. Rifaximin versus placebo preventing the recurrence of clinically overt hepatic encephalopathy in patients having cirrhosis of liver. Medical Forum 2017;28(12):15-8. [Google Scholar]
Bajaj 2011 {published data only}
- Bajaj JS, Heuman DM, Wade JM, Gibson DP, Saeidan K, Wegelin JA, et al. Rifaximin improves driving simulator performance in a randomized trial of patients with minimal hepatic encephalopathy. Gastroenterology 2011;140(2):478-87. [DOI: 10.1053/j.gastro.2010.08.061] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00533910. Rifaximin in minimal hepatic encephalopathy. clinicaltrials.gov/ct2/show/NCT00533910 (First posted 24 September 2007).
Bajaj 2019 {published data only (unpublished sought but not used)}
- Bajaj J, Heimanson Z, Israel R. Rifaximin and lactulose combination therapy versus lactulose alone for prevention of overt hepatic encephalopathy recurrence: a pooled analysis of two randomized trials. Gastroenterology 2019;156(6):S560. [DOI: 10.1016/S0016-5085(19)38289-7] [DOI] [Google Scholar]
- Bajaj J, Sanyal A, Sundaram V, Frenette C, Heimanson Z, Israel R, et al. Rifaximin plus lactulose is more efficacious than lactulose alone for risk reduction of overt hepatic encephalopathy (OHE) recurrence: a subgroup analysis by viral or alcohol cirrhosis etiology. American Journal of Gastroenterology 2021;116(Suppl 1):S570. [DOI: 10.14309/01.ajg.0000778480.00043.a7] [DOI] [Google Scholar]
Bass 2004 {published and unpublished data}
- Bass NM, Ahmed A, Johnson L, Gardner JD. Rifaximin treatment is beneficial for mild hepatic encephalopathy. Hepatology 2004;40(4 Suppl):646A. [Google Scholar]
- Dimick-Santos L. Meeting of The Gastrointestinal Drugs Advisory Committee (GIDAC) to evaluate rifaximin 550 mg tablets for the proposed indication of maintenance of remission of hepatic encephalopathy (HE) in patients 18 years old and older. NDA 22-554 clinical review. www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/GastrointestinalDrugsAdvisoryCommittee/UCM201079.pdf (Accessed February 2014).
- Gardner J, Bass N, Johnson L, Ahmed A. The number connection test can predict the likelihood of asterixis and abnormal mental state in patients with hepatic encephalopathy treated with rifaximin. Hepatology 2004;40(4 Suppl 1):636-7A. [DOI: 10.1002/hep.1840400507] [DOI] [Google Scholar]
- Salix Pharmaceuticals Inc. Xifaxan (rifaximin) tablets 550 mg for hepatic encephalopathy. Committee USFaDAGDA. www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/GastrointestinalDrugs AdvisoryCommittee/UCM203248.pdf ( Accessed February 2015).
- Xifaxan (rifaximin) tablets, 550 mg NDA 22-554. Briefing document for the gastrointestinal drugs advisory committee meeting 23 February 2010: FDA; 2010. www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/GastrointestinalDrugsAdvisoryCommittee/UCM201081.pdf ( Accessed February 2015).
Bass 2010 {published data only (unpublished sought but not used)}
- Alivisatos R. EC. 21-361 Xifaxan medical review part 6 access data FDA. Salix Pharmaceuticals, FDA. www.accessdata.fda.gov/drugsatfda_docs/nda/2004/21-361_Xifaxan_Medr_P6.pdf (Accessed February 2015).
- Bass N, Mullen K, Sigal S, Sanyal A, Poordad F, Merchant K, et al. Rifaximin is effective in maintaining remission in hepatic encephalopathy: results of a large, randomized, placebo-controlled trial. Journal of Hepatology 2009;50(Suppl 1):S39. [DOI: 10.1016/S0168-8278(09)60095-7] [DOI] [Google Scholar]
- Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, et al. Rifaximin treatment in hepatic encephalopathy. New England Journal of Medicine 2010;362(12):1071-81. [DOI: 10.1056/NEJMoa0907893] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Brown RS, Bass N, Sanyal AJ, Poordad F, Sheikh MY, Mullen KD. Rifaximin significantly improved critical flicker frequency, and time-weighted CFF correlated with overt hepatic encephalopathy as assessed by Conn score in a 6 month study. Hepatology 2009;50(Suppl 4):449-50A. [DOI: 10.1002/hep.23301] [DOI] [Google Scholar]
- Butterworth RF, Golden PL, Bortey E, Paterson C, Forbes WP. A critical flicker frequency value of 32 HZ predicts recurrence of overt hepatic encephalopathy in a double-blind, placebo controlled trial of rifaximin in patients with cirrhosis. Hepatology 2012;56(Suppl 1):916-7A. [Google Scholar]
- Flamm SL, Bajaj JS, Heimanson Z, Neff G. Efficacy and safety of rifaximin treatment for reducing the risk of overt hepatic encephalopathy by baseline hepatic impairment. Gastroenterology 2019;156(Suppl 6):S113. [DOI: 10.1016/S0016-5085(19)37075-1] [DOI] [Google Scholar]
- Flamm SL, Heimanson Z, Israel RJ, Jesudian AB. Precipitation factors of overt hepatic encephalopathy occurrence: an analysis of 3 rifaximin clinical trials. Hepatology 2020;72(Suppl 1):1079A. [DOI: 10.1002/hep.31579] [DOI] [Google Scholar]
- Flamm SL, Mullen KD, Heimanson Z, Sanyal AJ. Rifaximin has the potential to prevent complications of cirrhosis. Therapeutic Advances in Gastroenterology 2018;11(1):1-10. [DOI: 10.1177/1756284818800307] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanein T, Barakat F, Barrett AC, Bortey E, Paterson C, Forbes WP. Utility of the hepatic encephalopathy scoring algorithm (HESA) for diagnosing hepatic encephalopathy in a randomized, controlled trial of rifaximin vs. placebo. Gastroenterology 2013;144(5):S997-8. [DOI: 10.1016/S0016-5085(13)63704-X] [DOI] [Google Scholar]
- Mullen KD, Sanyal AJ, Bass NM, Poordad F, Huang S, Merchant K, et al. The long term efficacy and safety of rifaximin in the maintenance of remission from overt hepatic encephalopathy in cirrhotic patients. Gastroenterology 2012;142(5 Suppl 1):S41-2. [DOI: 10.1016/S0016-5085(12)60157-7] [DOI] [Google Scholar]
- Mullen KD, Sanyal AJ, Bass NM, Poordad FF, Sheikh MY, Frederick RT, et al. Rifaximin is safe and well tolerated for long-term maintenance of remission from overt hepatic encephalopathy. Clinical Gastroenterology and Hepatology 2014;12(8):1390-7. [DOI: 10.1016/j.cgh.2013.12.021] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT00298038. A 6-month efficacy, safety, and tolerability study of rifaximin in preventing hepatic encephalopathy. clinicaltrials.gov/ct2/show/NCT00298038 (First posted 1 March 2006).
- NCT00686920. Safety and tolerability study of rifaximin in participants with a history of hepatic encephalopathy. clinicaltrials.gov/ct2/show/NCT00686920 (First posted 30 May 2008).
- Neff G, Leevy CB, Frederick T, Merchant K, Huang S, Shaw AL, et al. Rifaximin reduces the risk of hospitalizations in patients with previous episodes of hepatic encephalopathy: results from a phase 3 placebo-controlled trial. Gastroenterology 2009;136:A11-2. [DOI: 10.1016/S0016-5085(09)60056-1] [DOI] [Google Scholar]
- Neff GW, Barrett AC, Bortey E, Paterson C, Forbes WP. Efficacy and tolerability of rifaximin in hepatitis C patients with recurrent hepatic encephalopathy. Gastroenterology 2013;144(5 Suppl 1):S451-2. [DOI: 10.1016/S0016-5085(13)61667-4] [DOI] [Google Scholar]
- Poordad F, Bass N, Sanyal AJ, Sheukh MY, Mullen KD, Sigal S. The protective effect of rifaximin (1100mg daily) from hepatic encephalopathy observed in a double-blind placebo controlled study is substantiated and durable over the long term. Hepatology 2009;50(Suppl 4):448-9A. [DOI: 10.1002/hep.23301] [DOI] [Google Scholar]
- Sanyal A, Bass N, Mullen K, Poordad F, Shaw A, Merchant K. Rifaximin treatment improved quality of life in patients with hepatic encephalopathy: results of a large, randomized, placebo-controlled trial. Journal of Hepatology 2010;52(Suppl 1):S7-8. [CLINICALTRIALS.GOV: NCT00298038] [Google Scholar]
- Sanyal A, Bass N, Teperman L, Sigal S, Hillebrand D, Merchant K, et al. Chronic administration of rifaximin for the maintenance of remission of hepatic encephalopathy: a subgroup analysis of a phase 3 trial. Journal of Hepatology 2009;50(Suppl 1):S90. [DOI: 10.1016/S0168-8278(09)60224-5] [DOI] [Google Scholar]
- Sanyal A, Mullen KD, Bass NM, Frederick T, Poordad F, Hee J, et al. Rates of commonly occuring infections in cirrhosis patients remain stable during long-term rifaximin treatment. Journal of Hepatology 2012;56(Suppl 2):S255-6. [DOI: 10.1016/S0168-8278(12)60658-8] [DOI] [Google Scholar]
- Sanyal A, Younossi ZM, Bass NM, Mullen KD, Poordad F, Brown RS, et al. Randomised clinical trial: rifaximin improves health-related quality of life in cirrhotic patients with hepatic encephalopathy - a double-blind placebo-controlled study. Alimentary Pharmacology & Therapeutics 2011;34:853-61. [DOI: 10.1111/j.1365-2036.2011.04808.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Story BT, Kowdley KV. ACP Journal Club: Rifaximin maintained remission from hepatic encephalopathy longer than placebo in patients with cirrhosis. ACP Journal Club 2010;153(4):JC2-8. [DOI: 10.7326/0003-4819-153-4-201008170-02008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bucci 1993 {published and unpublished data}
- Bucci L, Palmieri GC. Double-blind, double-dummy comparison between treatment with rifaximin and lactulose in patients with medium to severe degree hepatic encephalopathy. Current Medical Research Opinion 1993;13(2):109-18. [DOI: 10.1185/03007999309111539] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bureau 2021 {published data only (unpublished sought but not used)}
- Bureau C, Jezequel C, Archambault I, Thabut D, Dharancy S, Marc Perarnau J, et al. Rifaximin for the prevention of hepatic encephalopathy in patients treated by TIPS: a multicenter randomized placebo-controlled trial. Hepatology 2019;70(Suppl 1):10A. [Google Scholar]
- Bureau C, Thabut D, Jezequel C, Archambeaud I, D’Alteroche L, Dharancy S, et al. The use of rifaximin in the prevention of overt hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. A randomized controlled trial. Annals of Internal Medicine 2021;174(5):633-40. [DOI: 10.7326/M20-0202] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Koretz RL. In patients having transjugular intrahepatic portosystemic shunt, rifaximin prevented overt hepatic encephalopathy. Annals of Internal Medicine 2021;174(7):JC78. [DOI: 10.7326/ACPJ202107200-078] [DOI] [PubMed] [Google Scholar]
- NCT02016196. Double blind randomized study, comparing rifaximin vs placebo for the prevention of encephalopathy in patients treated by TIPS. clinicaltrials.gov/ct2/show/study/NCT02016196 (First posted 19 December 2013).
Butt 2018 {published data only (unpublished sought but not used)}
- Butt NI, Butt UI, Kakar AA, Malik T, Siddiqui AM. Is lactulose plus rIfaximin better then lactulose alone in the management of hepatic encephalopathy? Journal of the College of Physicians and Surgeons Pakistan 2018;28(2):115-7. [DOI: 10.29271/jcpsp.2018.02.115] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fera 1993 {published and unpublished data}
- Fera G, Agostinacchio F, Nigro M, Schiraldi O, Ferrieri A. Rifaximin in the treatment of hepatic encephalopathy. European Journal of Clinical Research 1993;4(Suppl 1):57-66. [Google Scholar]
Festi 1993 {published and unpublished data}
- Festi D, Mazella G, Orsini M, Sottili S, Sangermano A, Bassi L, et al. Rifaximin in the treatment of chronic hepatic encephalopathy; results of a multicenter study of efficacy and safety. Current Therapeutic Research 1993;54(5):598-609. [Google Scholar]
Gill 2014 {published and unpublished data}
- Gill ML, Niaz T, Aziz H, Khan S. Outcomes of rifaximin plus lactulose versus lactulose alone in treatment of overt hepatic encephalopathy. Journal of Hepatology April 2014;60(Suppl 1):S215. [DOI: 10.1016/S0168-8278(14)60602-4] [DOI] [Google Scholar]
Habib 2016 {published data only}
- Habib H, Nayab DE, Hayat Z, Jamil S, Khan H. Evaluation of efficacy of rifaximin in the treatment of hepatic encephalopathy in patients with cirrhosis. Gomal Journal of Medical Sciences 2016;14(1):37-40. [Google Scholar]
Hasan 2018 {published data only (unpublished sought but not used)}CTRI/2016/08/007215
- Hasan S, Datta S, Bhattacherjee S, Banik S, Saha S, Bandyopadhyay D. A randomized controlled trial comparing the efficacy of a combination of rifaximin and lactulose with lactulose only in the treatment of overt hepatic encephalopathy. Journal of the Association of Physicians of India 2018;66(1):32-6. [PMID: ] [PubMed] [Google Scholar]
Higuera‐de‐la‐Tijera 2018 {published data only (unpublished sought but not used)}
- NCT02158182. Lactulose, L-ornithine L-aspartate, or rifaximin versus placebo for preventing hepatic encephalopathy in variceal bleeding. clinicaltrials.gov/show/NCT02158182 (First posted 6 June 2014).
- La Tijera FH, Servín-Caamaño AI, Abdo-Francis JM, Salas-Gordillo F, Pérez-Hernández JL, Camacho-Aguilera J, et al. A randomized, double-blind, clinical trial comparing lactulose, l-ornithine l-aspartate, or rifaximin, versus placebo, as primary prophylaxis of hepatic encephalopathy in cirrhotic patients with variceal bleeding. Journal of Hepatology 2017;66(Supplement 1):S49. [DOI: 10.1016/S0168-8278(17)30362-8] [DOI] [Google Scholar]
- la Tijera FH, Servín-Caamaño AI, Salas-Gordillo F, Pérez-Hernández JL, Abdo-Francis JM, Camacho-Aguilera J, et al. Primary prophylaxis to prevent the development of hepatic encephalopathy in cirrhotic patients with acute variceal bleeding. Canadian Journal of Gastroenterology and Hepatology 2018;2018:1-10. [DOI: 10.1155/2018/3015891] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kimer 2017 {published and unpublished data}EUCTR2012‐002890‐71‐DK
- EudraCT 2012-002890-71. Intestinal decontamination with rifaximin. Effects on the inflammatory and circulatory state in patients with cirrhosis and ascites - a randomised controlled clinical study. www.clinicaltrialsregister.eu/ctr-search/search?query=2012-002890-71 2012.
- Kimer N, Gluud LL, Pedersen JS, Tavenier J, Møller S, Bendtsen F. The psychometric hepatic encephalopathy syndrome score does not correlate with blood ammonia, endotoxins or markers of inflammation in patients with cirrhosis. Translational Gastroenterology and Hepatology 2021;6(8):1-9. [DOI: 10.21037/tgh.2020.02.14] [PMCID: PMC7724176] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimer N, Pedersen JS, Busk TM, Gluud LL, Hobolth L, Krag A, et al. Rifaximin has no effect on hemodynamics in decompensated cirrhosis: a randomized, double-blind, placebo-controlled trial. Hepatology 2017;65(2):592-603. [DOI: 10.1002/hep.28898] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Kimer N, Pedersen JS, Tavenier J, Christensen JE, Busk TM, Hobolth L, et al. Rifaximin has limited effect on the inflammatory state and bacterial translocation in the patient with stable decompensated cirrhosis. Results from a randomized, placebo controlled trial. Hepatology 2016;64(Suppl 1):1041A. [DOI: 10.1002/hep.28799] [DOI] [Google Scholar]
- Kimer N, Pedersen JS, Tavenier J, Christensen JE, Busk TM, Hobolth L, et al. Rifaximin has minor effects on bacterial composition, inflammation and bacterial translocation in cirrhosis; A randomized trial. Journal of Gastroenterology and Hepatology 2018;33(1):307-14. [DOI: 10.1111/jgh.13852] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Kimer N, Pedersen JS, Busk TM, Hobolth L, Gluud LL, Krag A, et al. Rifaximin has no effect on hemodynamics in decompensated cirrhosis ‐ A randomized, double blind, placebo controlled trial. Hepatology 2016;64(Suppl 1):1041A. [DOI: 10.1002/hep.28800] [DOI] [PubMed] [Google Scholar]
- NCT01769040. Intestinal decontamination with rifaximin. The inflammatory and circulatory state in patients with cirrhosis. clinicaltrials.gov/ct2/show/NCT01769040 (First posted 16 January 2013).
Loguercio 2003 {published and unpublished data}
- Loguercio C, Federico A, De Girolamo V, Ferrieri A, Del Vecchio Blanco C. Cyclic treatment of chronic hepatic encephalopathy with rifaximin. Results of a double-blind clinical study. Minerva Dietologica e Gastroenterologica 2003;49:53-62. [PMID: ] [PubMed] [Google Scholar]
Maharshi 2015 {published and unpublished data}
- Maharshi S, Sharma BC, Srivastava S, Jindal A. Prophylaxis of hepatic encephalopathy in acute variceal bleed in patients with cirrhosis: an open label randomized controlled trial of lactulose versus rifaximin. Journal of Clinical and Experimental Hepatology 2014;4(Suppl 2):S52-3. [Google Scholar]
- Maharshi S, Sharma BC, Srivastava S, Jindal A. Randomised controlled trial of lactulose versus rifaximin for prophylaxis of hepatic encephalopathy in patients with acute variceal bleed. Gut 2015;64:1341-2. [DOI: 10.1136/gutjnl-2014-308521] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Maharshi S, Sharma BC, Srivastava S, Jindal A. Prophylaxis of hepatic encephalopathy in acute variceal bleed in patients with cirrhosis: An open label controlled trial of lactulose versus rifaximin. Journal of Hepatology 2014;60(Suppl):S59. [DOI: 10.1016/S0168-8278(14)60021-0] [DOI] [Google Scholar]
Majeed 2018 {published data only (unpublished sought but not used)}
- Majeed A, Butt MW, Aziz HA. A comparison of rifaximin with placebo for the treatment of hepatic encephalopathy. Journal of Medical and Health Sciences 2018;12(1):362-4. [Google Scholar]
Manzhalii 2022 {published data only}
- Manzhalii E, Moyseyenko V, Jondratiuk V, Molochek N, Falalyeyeva T, Kobyliak N. Effect of a specific Escherichia coli Nissle 1917 strain on minimal/mild hepatic encephalopathy treatment. World Journal of Hepatology 2022;14(3):634-46. [DOI: 10.4254/wjh.v14.i3.634] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04787276. Efficacy and Safety of E.Coli Nissle 1917 in Patients With Mild (Stage 1-2) or Minimal Hepatic Encephalopathy. https://clinicaltrials.gov/ct2/show/NCT04787276 (First posted 8 March 2021).
Mas 2003 {published and unpublished data}
- Mas A, Rodés J, Sunyer L, Rodrigo L, Planas R, Vargas V, et al. Comparison of rifaximin and lactitol in the treatment of hepatic encephalopathy: results of a randomized, double-blind, double-dummy, controlled clinical trial. Journal of Hepatology 2003;38:51-8. [DOI: 10.1016/s0168-8278(02)00350-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Mas A. Treatment of hepatic encephalopathy (HE) with rifaximin compared with lactitol. Multicenter randomized, double-blind, double dummy study [Tratamiento de la encefalopatia hepatica (EH) con rifaximina en comparacion con lactitol. Estudio multicentrico aleatorizado, doble ciego, doble simulacion.]. Gastroenterologia y Hepatologia 1999;22(Suppl 1):40. [Google Scholar]
Massa 1993 {published and unpublished data}
- Massa P, Vallerino P, Dodero M. Treatment of hepatic encephalopathy with rifaximin. The European Journal of Clinical Research 1993;4:7-18. [Google Scholar]
Moneim 2021 {published data only}
- Moneim MA, Abdelaziz DH, Nagy YI, Baki AA, Attia AS, Sabry N. Rifaximin microbial resistance and its efficacy and safety as secondary prophylaxis of hepatic encephalopathy in patients with hepatitis C virus-related cirrhosis. International Journal of Clinical Practice 2021;75(11):1-11. [DOI: 10.1111/ijcp.14807] [DOI] [PubMed] [Google Scholar]
Muhammad 2016 {published data only}
- Muhammad N, Khan MAR, Hussain A. Role of rifaximin in preventing the recurrence of hepatic encephalopathy in patients with chronic liver disease. Pakistan Journal of Medical and Health Sciences 2016;10(4):1417-20. [Google Scholar]
Nawaz 2015 {published and unpublished data}
- Nawaz A, Ghafoor A, Khan A, Ullah H. Rifaximin versus placebo in reducing the risk of clinically overt hepatic encephalopathy recurrence in patients having cirrhosis of liver. Hepatology International 2015;9(Suppl 1):S104-5. [Google Scholar]
Paik 2005 {published and unpublished data}
- Kim MH, Han KH, Lee KS, Paik YH, Song KH, Na HK. Efficacy and safety of short-term administration of rifaximin in the treatment of hepatic encephalopathy. Korean Journal of Hepatology 2001;7(1):55-60. [onlinelibrary.wiley.com/o/cochrane/clcentral/articles/272/CN-01045272/frame.html] [Google Scholar]
- Paik Y, Lee KS, Han K, Song KH, Kim MH, Moon BS, et al. Comparison of rifaximin and lactulose for the treatment of hepatic encephalopathy: a prospective randomized study. Yonsei Medical Journal 2005;46(3):399-407. [DOI: 10.3349/ymj.2005.46.3.399] [PMCID: PMC2815818] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song KH, Lee KS, Kim MH, Paik YH, Moon BS, Yoon SH, et al. The clinical efficacy of rifaximin in the treatment of hepatic encephalopathy (Comparison with lactulose). Hepatology 2000;32(4):407A. [Google Scholar]
Patel 2022 {published and unpublished data}2013‐004708‐20
- EUCTR2013-004708-20-GB. A trial to determine if rifaximin is more efficient than placebo in treating systemic inflammation and neutrophil (white blood cells) malfunction in patients with cirrhosis and chronic hepatic encephalopathy. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2013-004708-20 (Registered 3 March 2014).
- NCT02019784. RIFSYS: randomised controlled trial of mechanistic effects of rifaximin in cirrhosis and chronic hepatic encephalopathy. clinicaltrials.gov/show/NCT02019784 (First posted 24 December 2013).
- Patel V, Lee S, McPhail M, De Silva K, Guilly S, Zamalloa A, et al. Rifaximin reduces gut-derived inflammation and mucin degradation in cirrhosis and encephalopathy: RIFSYS randomised controlled trial. Journal of Hepatology 2022;76(2):332-42. [DOI: 10.1016/j.jhep.2021.09.010] [DOI] [PubMed] [Google Scholar]
- Patel V, McPhail MJ, Zamalloa A, Støy S, Vijay GM, Huang X, et al. Results of a placebo-controlled double blind randomised trial to investigate the efficacy of rifaximin-alpha versus placebo in improving systemic inflammation in patients with cirrhosis and chronic hepatic encephalopathy (RIFSYS Trial). Journal of Hepatology 2018;68(Suppl 1):S107-8. [DOI: 10.1016/S0168-8278(18)30432-X] [DOI] [Google Scholar]
- Patel V, Shawcross D, McPhail M. A placebo controlled single centre double blind randomised trial to investigate the efficacy of RIFaximin versus placebo in improving SYStemic inflammation and neutrophil malfunction in patients with cirrhosis and chronic hepatic encephalopathy. Scientific Report 2018:1-97.
- Patel VC, McPhail MJ, Zamalloa A, Stoy S, Vijay GK, Witherden E, et al. Improvement in hepatic encephalopathy with rifaximin is associated with distinct changes in the metagenomic species in the saliva and stool microbiome and a reduction in systemic inflammation – results of the RIFSYS randomized controlled trial. Hepatology 2018;68(6):1462A-3A. [DOI: 10.1002/hep.30353] [DOI] [Google Scholar]
Pawar 2019 {published data only (unpublished sought but not used)}
- Pawar V, Sonthalia N, Rathi P, Contractor Q, Surude R, Jain S, et al. Prospective randomized study of effect of lactulose or rifaximin or placebo in minimal hepatic encephalopathy. Indian Journal of Gastroenterology 2016;35(Suppl 1):A44. [DOI: 10.1007/s12664-016-0715-3] [DOI] [Google Scholar]
- Pawar VB, Surude RG, Sonthalia N, Zanwar V, Jain S, Contractor Q, et al. Minimal hepatic encephalopathy in Indians: psychometric hepatic encephalopathy score and inhibitory control test for diagnosis and rifaximin or lactulose for its reversal. Journal of Clinical & Translational Hepatology 2019;7(4):304-12. [DOI: 10.14218/JCTH.2017.00037] [PMCID: PMC6943207] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Poudyal 2019 {published and unpublished data}
- Poudyal N, Chaudhary S, Kc S, Paudel B, Basnet B, Mandal A, et al. Precipitating factors and treatment outcomes of hepatic encephalopathy in liver cirrhosis. Cureus 2019;11(4):e4363. [DOI: 10.7759/cureus.4363] [PMCID: PMC6550494] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silwal NP, Kc S, Chaudhary S, Sharma M. Treatment outcome of hepatic encephalopathy in liver cirrhosis. Journal of Clinical and Experimental Hepatology 2018;8(S1):S51. [DOI: 10.1016/j.jceh.2018.06.363] [DOI] [Google Scholar]
Riggio 2005 {published data only (unpublished sought but not used)}
- Masini A, Nicolao F, Efrati C, Merli M, Attili AF, Riggio O. Preliminary results of a randomised controlled trials in the prevention of early post-tips hepatic encephalopathy: a comparison between rifaximin and lactitol. Journal of Hepatology 2002;36(Suppl 1):205. [DOI: 10.1016/S0168-8278(02)80730-9] [DOI] [Google Scholar]
- Riggio O, Masini A, Efrati C, Nicolao F, Angeloni S, Salvatori F, et al. Pharmacological prophylaxis of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt: a randomized controlled study. Journal of Hepatology 2005;42(4):674-9. [DOI: 10.1016/j.jhep.2004.12.028] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sharma 2013 {published data only (unpublished sought but not used)}CTRI/2013/07/003845
- Lunia M, Sharma P, Sharma B, Sarin S. A double blind randomised controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of hepatic encephalopathy. Journal of Gastroenterology and Hepatology 2012;27(Suppl 5):52. [DOI: 10.1111/jgh.12005] [DOI] [PubMed] [Google Scholar]
- Lunia MK, Sharma BC, Sharma P, Sarin SK. A randomised double blind controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of hepatic encephalopathy. Journal of Clinical and Experimental Hepatology 2013;3(Suppl 1):S42. [DOI: 10.1016/j.jceh.2013.03.085] [DOI] [PubMed] [Google Scholar]
- NCT01218568. Rifaximin plus lactulose versus lactulose alone for the treatment of hepatic encephalopathy: a double blind randomized trial. clinicaltrials.gov/ct2/show/record/NCT01218568 (First posted 11 October 2010).
- Sharma BC, Sharma P, Lunia MK, Srivastava S, Goyal R, Sarin SK. A randomised double blind controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Journal of Clinical and Experimental Hepatology 2013;3(1S):S5-7. [DOI: 10.1016/j.jceh.2013.03.011] [DOI] [PubMed] [Google Scholar]
- Sharma BC, Sharma P, Lunia MK, Srivastava S, Goyal R, Sarin SK. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. American Journal of Gastroenterology 2013;108(9):1458-63. [DOI: 10.1038/ajg.2013.219] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sharma 2014 {published data only (unpublished sought but not used)}
- Sharma K, Pant S, Dwivedi M, Misra SP, Misra A, Narang S. Minimal hepatic encephalopathy: effect of rifaximin, probiotics, and L-ornithine L-aspartate. Journal of Gastroenterology and Hepatology 2012;27(Suppl 5):275-6. [DOI: 10.1111/jgh.12006] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, Pant S, Misra S, Dwiwedi M, Misra A, Narang S, et al. Effect of rifaximin, probiotics, and l-ornithine l-aspartate on minimal hepatic encephalopthy: a randomized controlled trial. Saudi Journal of Gastroenterology 2014;20(4):225-32. [DOI: 10.4103/1319-3767.136975] [PMCID: PMC4131305] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sidhu 2011 {published data only}CTRI/2009/091/000979
- Sidhu SS, Goyal O, Mishra BP, Sood A, Chhina RS, Soni RK. Rifaximin improves psychometric performance and health-related quality of life in patients with minimal hepatic encephalopathy (The RIME trial). American Journal of Gastroenterology 2011;106:307-16. [DOI: 10.1038/ajg.2010.455] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sidhu 2016 {published and unpublished data}CTRI/2010/091/003045
- Goyal O, Sidhu SS, Kishore H. Minimal hepatic encephalopathy in cirrhosis - how long to treat? Annals of Hepatology 2017;16(1):115-22. [DOI: 10.5604/16652681.1226822] [DOI] [PubMed] [Google Scholar]
- Goyal O, Sidhu SS, Parker RA, Prasad J, Kishore H, Sood A, et al. Rifaximin versus lactulose in the treatment of minimal hepatic encephalopathy in patients with cirrhosis: a prospective, randomised, active-comparator, non-inferiority trial. Journal of Hepatology 2014;60(Suppl 1):S526. [Google Scholar]
- Goyal O, Sidhu S, Kishore H. Relapse of minimal hepatic encephalopathy after treatment in patients with liver cirrhosis. Hepatology International 2017;11(Suppl 1):S188-9. [DOI: 10.1007/s12072-016-9783-9] [DOI] [Google Scholar]
- Sidhu SS, Goyal O, Parker RA, Prasad J, Kishore H, Sood A. Rifaximin vs. lactulose in treatment of minimal hepatic encephalopathy. Liver International 2016;36(3):378-85. [DOI: 10.1111/liv.12921] [DOI] [PubMed] [Google Scholar]
- Wander P, Goyal O, Sidhu SS, Kishore H. Minimal hepatic encephalopathy in cirrhosis: how long to treat? Does minimal hepatic encephalopathy relapse after treatment? American Journal of Gastroenterology 2016;111(Suppl 1):S371. [Google Scholar]
Suzuki 2018 {published data only (unpublished sought but not used)}JapicCTI‐132086JapicCTI‐132087
- Phase II/III clinical trial of L-105 in patients with hepatic encephalopathy. www.clinicaltrials.jp/cti-user/trial/ShowDirect.jsp?japicId=JapicCTI-132086 (First posted 19 March 2013).
- Phase III clinical trial of L-105 in patients with hepatic encephalopathy. www.clinicaltrials.jp/cti-user/trial/ShowDirect.jsp?japicId=JapicCTI-132087 (First posted 19 March 2013).
- Suzuki K, Endo R, Takikawa Y, Moriyasu F, Aoyagi Y, Moriwaki H, et al. Efficacy and safety of rifaximin in Japanese patients with hepatic encephalopathy: a phase II/III, multicenter,randomized, evaluator-blinded, active-controlled trial and a phase III, multicenter, open trial. Hepatology Research 2018;48(6):411-23. [DOI: doi: 10.1111/hepr.13045] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tan 2022 {published data only}
- NCT03077217. Low-dose Rifaximin in the Treatment of Covert Hepatic Encephalopathy. https://clinicaltrials.gov/ct2/show/NCT03077217 ( First posted 10 March 2017).
- Tan W, Wang J, Shi PM, Feng LM, Shi J, Ning BF, et al. Effects of low-dose and high-dose rifaximin in the treatment of covert hepatic encephalopathy. Journal of Clinical and Translational Hepatology 2022 ;10(6):1099-1106. [DOI: 10.14218/JCTH.2021.00457] [PMCID: PMC9634763] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Uthman 2020 {published data only}
- Uthman M, Mujtaba SWA, Qaisar AM. Examine the efficacy of rifaximin for hepatic encephalopathy patients with chronic liver disease. Medical Forum Monthly 2020;31(4):19-22. [Google Scholar]
Vyas 2017 {published data only (unpublished sought but not used)}
- NCT02321371. Effect of goal directed ammonia lowering therapy in acute on chronic liver failure patients with hepatic encephalopathy. clinicaltrials.gov/ct2/show/NCT02321371 (First posted 22 December 2014).
- Vyas T, Maiwall R, Jinadal A, Sarin S. Goal directed ammonia lowering therapy in acute on chronic liver failure (ACLF) with hepatic encephalopathy (HE): a randomized trial. Journal of Clinical and Experimental Hepatology 2017;7(Suppl 1):S1-21. [DOI: 10.1016/j.jceh.2017.01.026] [NCT: NCT02321371] [DOI] [Google Scholar]
- Vyas TS, Jindal A, Maiwall R, Sahney A, Kalal C, Premkumar M, et al. Goal directed ammonia lowering therapy in acute on chronic liver failure (ACLF) with hepatic encephalopathy (HE): a randomized trial. Hepatology 2016;64(Suppl 1):704-5A. [DOI: 10.1002/hep.28799] [DOI] [Google Scholar]
- Vyas TS, Sarin SK, Maiwall R. Effect of goal directed ammonia lowering therapy in acute-on-chronic liver failure patients with hepatic encephalopathy. Indian Journal of Gastroenterology 2016;35(Suppl):A67-8. [DOI: 10.1007/s12664-016-0715-3] [DOI] [Google Scholar]
Wahib 2014 {published data only (unpublished sought but not used)}
- Wahib AA, El-Deen Salem MN, Ahmed MA, El-Dessouky YM, El-Tiby DM, El-Mola K, et al. Evaluation of rifaximin in management of hepatic encephalopathy. Journal of the Egyptian Society of Parasitology 2014;44(3):677-85. [DOI: 10.12816/0007871] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zeng 2021 {published data only (unpublished sought but not used)}
- NCT02190357. Rifaximin reduces the complications of decompensated cirrhosis: a randomized controlled trial. www.clinicaltrials.gov/ct2/show/NCT02190357 (First posted 15 July 2014).
- Zeng X, Sheng X, Wang PQ, Xin HG, Guo YB, Lin Y, et al. Low-dose rifaximin prevents complications and improves survival in patients with decompensated liver cirrhosis. Hepatology International 2021;15(1):155-65. [DOI: 10.1007/s12072-020-10117-y] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abd‐Elsalam 2016 {published data only}
- Abd-Elsalam S, Ali LA, Soliman S, Ibrahim S, Elfert A. Randomized controlled trial of rifaximin versus norfloxacin for secondary prophylaxis of spontaneous bacterial peritonitis. Journal of Hepatology 2016;64(S2):S211. [CLINICALTRIALS.GOV: NCT02120196] [DOI: 10.1016/S0168-8278(16)00176-8] [DOI] [PubMed] [Google Scholar]
- Elfert A, Abo Ali L, Soliman S, Ibrahim S, Abd-Elsalam S. Randomized-controlled trial of rifaximin versus norfloxacin for secondary prophylaxis of spontaneous bacterial peritonitis. European Journal of Gastroenterology & Hepatology 2016;28(12):1450-4. [DOI: 10.1097/MEG.0000000000000724] [DOI] [PubMed] [Google Scholar]
- NCT02120196. Comparative study of rifaximin versus norfloxacin in the secondary prophylaxis of spontaneous bacterial peritonitis (SBP). clinicaltrials.gov/ct2/show/NCT02120196 (First posted 22 April 2014).
Ahire 2017 {published data only}
- Ahire K, Sonawale A. Comparison of rifaximin plus lactulose with the lactulose alone for the treatment of hepatic encephalopathy. Journal of the Association of Physicians of India 2017;65:42-6. [PubMed] [Google Scholar]
Ahluwalia 2014 {published data only}
- Ahluwalia V, Wade JB, Heuman DM, Hammeke TA, Sanyal AJ, Sterling RK, et al. Enhancement of functional connectivity, working memory and inhibitory control on multi-modal brain MR imaging with rifaximin in cirrhosis: implications for the gut-liver-brain axis. Metabolic Brain Disease 2014;29(4):1017-25. [DOI: 10.1007/s11011-014-9507-6] [PMCID: PMC4155029] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anon 2014a {published data only}
- Hepatic encephalopathy. Rifaximin opens new perspectives. MMW Fortschritte der Medizin 2014;156(13):78-9. [PubMed]
Anon 2014b {published data only}
- Intestine specific broad spectrum antibiotic. Rifaximin: therapy option in hepatic encephalopathy. MMW Fortschritte der Medizin 2014;156(3):64-5. [PubMed]
Bajaj 2013 {published data only}
- Bajaj JS, Heuman DM, Sanyal AJ, Hylemon PB, Sterling RK, Stravitz RT, et al. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLOS One 2013;8(4):e60042. [CLINICALTRIALS.GOV: NCT01069133] [DOI: 10.1371/journal.pone.0060042] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bajaj 2016b {published data only (unpublished sought but not used)}
- Bajaj J, Flamm SL, Chalassani NP, Diaz E, Kayali Z, Kang R, et al. Oral rifaximin soluble solid dispersion immediate release 40 mg prevents development of cirrhosis related complications: A phase 2, randomized, multicenter, double-blind, placebo-controlled trial. Hepatology 2016;64(Suppl 1):1027A. [Google Scholar]
- Kakiyama G, Pandak W, Heimanson Z, Zhou H, Thacker L, Zhao D, et al. Rifaximin solube solid dispersion tablets modify gut microbial function, as shown by increased faecal secondary bile acid levels compared with placebo, in patients with early decompensated cirrhosis. United European Gastroenterology Journal 2020;8(Suppl 8):611. [DOI: 10.1177/2050640620927345] [DOI] [Google Scholar]
- Kakiyama G, Pandak W, Heimanson Z, Zhou H, Thacker L, Zhao D, et al. Rifaximin soluble solid dispersion tablets modify gut microbial function, as shown by increased fecal secondary bile acid levels compared with placebo, in patients with early decompensated cirrhosis. Gastroenterology 2020;158(6 Suppl 1):S1456. [DOI: 10.1016/S0016-5085(20)34307-9] [DOI] [Google Scholar]
- NCT01904409. A randomized, double-blind, placebo-controlled, dose-ranging, multicenter study to assess the efficacy and safety of rifaximin soluble solid dispersion (SSD) tablets for the prevention of complications in subjects with early decompensated liver cirrhosis. clinicaltrials.gov/ct2/show/record/NCT01904409 (First 22 July 2013).
- Sanyal AJ, Chalassani N, Bajaj J, Diaz E, Kayali Z, Harmon M, et al. Impact of baseline chronic liver disease characteristics on the efficacy of oral rifaximin soluble solid dispersion tablets for the prevention of further decompensation or all-cause mortality in patients with cirrhosis and ascites. Hepatology 2016;64(Suppl 1):47A. [Google Scholar]
Bajaj 2020a {published data only}
- Bajaj J, Sundaram V, Frenette C, Heimanson Z, Israel R, Sanyal A. Lactulose withdrawal can potentiate breakthrough overt hepatic encephalopathy in patients controlled with rifaximin plus lactulose therapy: a post hoc analysis of a randomized controlled trial. Journal of Hepatology 2020;73(S1):S721. [CLINICALTRIALS.GOV: NCT01842581] [DOI: 10.1016/S0168-8278(20)31898-5] [DOI] [Google Scholar]
- Flamm S, Mullen K, Heimanson Z, Zeev P, Sanyal A. Assessment of potential impact of demographic and baseline disease characteristics on rifaximin monotherapy versus lactulose combination therapy for the prevention of overt hepatic encephalopathy (OHE) recurrence. American Journal of Gastroenterology 2017;112(S1):S494. [DOI: 10.1038/ajg.2017.305] [DOI] [Google Scholar]
- NCT01842581. The safety/efficacy of rifaximin with/without lactulose in participants with a history of recurrent hepatic encephalopathy. clinicaltrials.gov/ct2/show/NCT01842581 (First posted 29 April 2013).
- Sanyal A, Heimanson Z, Flamm S. SAT-110-Impact of rifaximin monotherapy or rifamixin plus lactulose on markers of inflammation in patients with cirrhosis and a history of hepatic encephalopathy. Journal of Hepatology 2019;70(Suppl 1):e677-8. [DOI: 10.1016/S0618-8278(19)31349-0] [DOI] [Google Scholar]
- Sanyal A, Heimanson Z, Israel R, Mullen K, Flamm S. Prevention of overt hepatic encephalopathy recurrence with rifaximin alone versus rifaximin plus lactulose therapy: impact on quality of life and caregiver burden in patients with cirrhosis. Journal of Hospital Medicine 2018;13(4):1. [Google Scholar]
- Sanyal AJ, Hassanein TI, Kayali Z, Kang R, Wolf RA, Mullen KD. Efficacy and safety of rifaximin monotherapy versus lactulose combination therapy for the prevention of overt hepatic encephalopathy (HE) recurrence. Hepatology (Baltimore, Md.) 2016;64(Suppl 1):130-1A. [DOI: 10.1002/hep.28796] [DOI] [Google Scholar]
- Sanyal AJ, Heimanson Z, Isreal R, Mullen KD, Flamm SL. Impact of rifaximin monotherapy versus rifaximin and lactulose combination therapy on quality of life in patients with a history of overt hepatic encephalopathy (OHE). Hepatology (Baltimore Md.) 2017;66(S1):268A. [Google Scholar]
Bajaj 2020b {published data only}
- Bajaj JS, Rahimi RS, Hassanein T, Pyrsopoulos NT, Heimanson Z, Israel RJ, et al. Investigational water-soluble rifaximin formulation significantly shortens time to recovery in hospitalized patients with overt hepatic encephalopathy (OHE): a phase 2, randomized, double-blind, placebo-controlled trial. Hepatology 2020;72(Suppl 1):57-8A. [DOI: 10.1002/hep.31578] [DOI] [Google Scholar]
- NCT03515044. Rifaximin Soluble Solid Dispersion (SSD) tablets plus lactulose for the treatment of overt hepatic encephalopathy (OHE). clinicaltrials.gov/ct2/show/study/NCT03515044 (First posted 2 May 2018).
Block 2010 {published data only}
- Block JP. Rifaximin improves the treatment of hepatic encephalopathy. Journal of Clinical Outcomes Management 2010;17(5):16-9. [Google Scholar]
Bohra 2020 {published data only}
- Bohra A, Worland T, Hui S, Terbah R, Farrell A, Robertson M. Prognostic significance of hepatic encephalopathy in patients with cirrhosis treated with current standards of care. World Journal of Gastroenterology 2020;26(18):2221-31. [DOI: 10.3748/wjg.v26.i18.2221] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2021 {published data only}
- Chang C, Huang CH, Tseng HJ, Yang FC, Chien RN. Real-world experience of the one-year efficacy of rifaximin add-on to lactulose is superior to lactulose alone in patients with cirrhosis complicated with recurrent hepatic encephalopathy in Taiwan. Journal of Personalized Medicine 2021;11(6):478. [PMID: 10.3390/jpm11060478] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cobbold 2018 {published and unpublished data}
- Cobbold JFL, Atkinson S, Marchesi JR, Smith A, Wai SN, Stove J, et al. Rifaximin in non-alcoholic steatohepatitis: An open-label pilot study. Hepatology Research 2018;48(1):69-77. [DOI: 10.1111/hepr.12904] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT01355575. Rifaximin in fatty liver disease (RiFL). clinicaltrials.gov/ct2/show/NCT01355575 First posted 18 May 2011.
Crisafulli 2016 {published data only}
- Crisafulli A, Demma S, Rigano G, Bertino G. Treatment with rifaximin high dose plus lactulose vs rifaximin standard dose plus lactulose for acute hepatic encephalopathy. Journal of Hepatology 2016;64(2):S258. [DOI: 10.1016/S0168-8278(16)00286-5] [DOI] [Google Scholar]
CTRI/2019/05/018966 {published data only}
- CTRI/2019/05/018966. A double blind randomised controlled trial comparing lactulose plus rifaximin with lactulose plus rifaximin plus L-ornithin L-aspartate in the treatment of overt hepatic encephalopathy (grade III-grade IV) in patients with cirrhosis. www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=32692&EncHid=&modid=&compid=%27,%2732692det%27 (Registered 7 May 2019).
Danulescu 2013 {published data only}
- Danulescu RM, Ciobica A, Stanciu C, Trifan A. The role of rifaximine in the prevention of the spontaneous peritonitis. Revista Medico-Chirurgicala Societatii de Medici si Naturalisti din Iasi 2013;117(2):315-20. [PMID: ] [PubMed] [Google Scholar]
De Marco 1984 {published data only}
- De Marco F, Santamaria Amato P, D'Arienzo A. Rifaximin in collateral treatment of portal systemic encephalopathy: a preliminary report. Current Therapeutic Research 1984;36(4):668-74. [Google Scholar]
Deshmukh 2016 {published data only (unpublished sought but not used)}
- Deshmukh PG, Konar A. An open label randomized study to evaluate the efficacy and safety of lactulose versus rifaximin in cirrhotics with covert hepatic encephalopathy. Indian Journal of Gastroenterology 2016;35(Suppl 1):LO-66. [Google Scholar]
Diana‐Maria 2019 {published data only}
- Diana-Maria S, Leahu A, Asaftei M, Garleanu I, Petrea O, Stanciu C, et al. Therapeutic approaches in patients with overt hepatic encephalopathy and liver cirrhosis. Journal of Gastrointestinal and Liver Diseases 2019;28(Suppl 1):68-9. [Google Scholar]
Di Piazza 1991 {published data only}
- Di Piazza S, Gabriella Filippazzo M, Valenze LM, Morello S. Rifaximin versus neomycine in the treatment of portosystemic encephalopathy. Italian Journal of Gastroenterology 1991;23(7):403-7. [PubMed] [Google Scholar]
Dupont 2016 {published data only}
- DuPont HL, Flamm SL, Wolf RA, Sanyal AJ. Changes in antimicrobial susceptibility of fecal bacteria during rifaximin treatment for the prevention of overt hepatic encephalopathy (HE) recurrence. Hepatology 2016;64(Suppl 1):1047A. [Google Scholar]
- DuPont HL, Hassanein TI, Sanyal AJ, Wolf RA, Mullen KD. Genomic characterization of stool microbiota with rifaximin monotherapy versus lactulose in combination therapy for prevention of overt hepatic encephalopathy (HE) recurrence. Hepatology 2016;64(Suppl 1):720A. [Google Scholar]
EUCTR2014‐001856‐51‐DK {published data only}
- Anti-fibrotic and molecular aspects of rifaximin in alcoholic liver disease: A randomized placebo controlled clinical trial. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2014-001856-51 (Date of registration 10 June 2014).
Frenette 2020a {published data only}
- Frenette C, Sundaram V, Heimanson Z, Israel R, Sanyal A. Lack of colonic microbial cross-resistance to other antibiotics in patients treated with rifaximin alone vs rifaximin plus lactulose for reducing the risk of overt hepatic encephalopathy (OHE) recurrence. American Journal of Gastroenterology 2020;115(Supplement):S517. [DOI: 10.14309/01.ajg.0000706100.58826.f6] [DOI] [Google Scholar]
Frenette 2020b {published data only}
- Frenette C, Vinay S, Heimanson Z, Israel R, Sanyal A. Lactulose on intestinal microbial diversity and composition in patients with cirrhosis. American Journal of Gastroenterology 2020;115(Supplement):S517-8. [DOI: 10.14309/01.ajg.0000706104.30097.6e] [DOI] [Google Scholar]
Gangarapu 2015 {published data only}
- Gangarapu V, Ince AT, Baysal B, Kayar Y, Kılıç U, Gök Ö, et al. Efficacy of rifaximin on circulating endotoxins and cytokines in patients with nonalcoholic fatty liver disease. European Journal of Gastroenterology and Hepatology 2015;27(7):840-5. [DOI: 10.1097/MEG.0000000000000348] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT02009592. Efficacy of rifaximin on hepatosteatosis and steatohepatitis patients.. clinicaltrials.gov/ct2/show/NCT02009592 First posted 12 December 2013.
Giacomo 1993 {published data only}
- Giacomo F, Francesco A, Michele N, Oronzo S, Antonella F. Rifaximin in the treatment of hepatic encephalopathy. European Journal of Clinical Research 1993;4(Suppl 1):57-66. [Google Scholar]
Gupta 2021 {published data only}
- CTRI/2021/09/036321. Comparative study of Rifaximin alone versus combination therapy with Norfloxacin for secondary prophylaxis of spontaneous bacterial peritonitis with hepatic encephalopathy. https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2021/09/036321 2021.
- Gupta T, Gaur V. Rifaximin alone versus combination with norfloxacin for secondary prophylaxis of spontaneous bacterial peritonitis with hepatic encephalopathy: a randomized-controlled trial. Hepatology (Baltimore, Md.) 2021;74(Suppl.1):1232A: 2073. [DOI: 10.1002/hep.32188] [DOI] [Google Scholar]
Habib 2020 {published data only}
- Habib N, Amjad M, Afzal M, Iqbal MZ, Shehzad MA. Compare the efficacy of low versus high dose of rifaximin for primary prophylaxis of protosystemic encephalopathy. Pakistan Journal of Medical & Health Sciences 2020;14(3):843-5. [Google Scholar]
Hammond 2017 {published data only}
- Hammond DA, Dayama N, Martin BC. Impact of rifaximin and lactulose versus lactulose alone on hospitalization for acute recurrent hepatic encephalopathy. Value in Health 2017;20(5):A181. [Google Scholar]
Hotten 2003 {published data only}
- Hotten P, Marotta F, Naito Y, Minelli E, Helmy A, Lighthouse J, et al. Effects of probiotics, lactitol and rifaximin on intestinal flora and fecal excretion of organic acids in cirrhotic patients. Chinese Journal of Digestive Diseases 2003;4(1):13-8. [DOI: 10.1046/j.1443-9611.2003.00110.x] [DOI] [Google Scholar]
Huang 2018 {published data only}
- Huang X, Chen S. Effect of rifaximin on gut microbiome in cirrhotic patients with variceal bleeding. Journal of Gastroenterology and Hepatology 2018;33(S4):53: OE-0898 (PD-0020). [DOI: 10.1111/jgh.14481] [DOI] [Google Scholar]
- NCT02964195. Efficacy of rifaximin in treatment of cirrhotic gastroesophageal variceal hemorrhage: A multi-center randomized controlled clinical trial .. clinicaltrials.gov/ct2/show/NCT02964195 First posted 10 November 2016.
- NCT02991612. Efficacy of rafiximin in patients with cirrhotic gastroesophageal variceal hemorrhage: A single-center pilot study. clinicaltrials.gov/ct2/show/NCT02991612 First posted December 13, 2016: Sudy completed February 28, 2018.
Jain 2022 {published data only}
- Jain A, Sharma BC, Mahajan B, Srivastava S, Kumar A, Sachdeva S, et al. L- ornithine L-aspartate in acute treatment of severe hepatic encephalopathy: A double-blind randomized controlled trial. Hepatology (Baltimore, Md) 2022;75(5):1194-1203. [DOI: 10.1002/hep.32255.] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jiménez 2022 {published data only}
- Jiménez C, Ventura-Cots M, Sala M, Calafat M, Garcia-Retortillo M, Cirera I, et al. Effect of rifaximin on infections, acute-on-chronic liver failure and mortality in alcoholic hepatitis: A pilot study (RIFA-AH). Liver International 2022;42(5):1109-20. [DOI: 10.1111/liv.15207] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
John 2018 {published data only}
- John N, Aloysius M, Zaman M. Bovine immunoglobulin orally with modified doses of rifaximin for advanced cirrhotics in overt hepatic encephalopathy: a randomized placebo control prospective clinical pilot trial (BRAIN trial). Hepatology International 2018;12(2):S298. [DOI: 10.1007/s12072-018-9852-3] [DOI] [Google Scholar]
Jones 2020 {published data only}
- Jones B, Berni E, Poole C, Jenkins-Jones S, Orr J, Amlani B, et al. Increased survival in patients with hepatic encephalopathy treated with rifaximin-alpha in combination with lactulose: an observational study from UK clinical practice. Journal of Hepatology 2020;73(Suppl 1):S37. [DOI: 10.1016/S0168-8278(20)30626-7] [DOI] [Google Scholar]
Kaji 2017 {published data only}UMIN000029127
- Kaji K, Takaya H, Saikawa S, Furukawa M, Sato S, Kawaratani H, et al. Rifaximin ameliorates hepatic encephalopathy and endotoxemia without affecting the gut microbiome diversity. World Journal of Gastroenterology 2017;23(47):8355-66. [DOI: 10.3748/wjg.v23.i47.8355] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaya H, Kaji K, Saikawa S, Furukawa M, Sato S, Sawada Y, et al. Rifaximin ameliorated hepatic encephalopathy and endotoxemia without affecting the gut microbiome diversity. Journal of Hepatology 2018;68(Suppl 1):S752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalambokis 2012a {published data only}
- Kalambokis GN, Mouzaki A, Rodi M, Pappas K, Fotopoulos A, Xourgia X, et al. Rifaximin improves systemic hemodynamics and renal function in patients with alcohol-related cirrhosis and ascites. Clinical Gastroenterology and Hepatology 2012;10(7):815-8. [DOI: 10.1016/j.cgh.2012.02.025] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kalambokis 2012b {published data only}
- Kalambokis G, Tsianos EV. Thrompocytopenia associated with chronic liver disease: effects of rifaximin on platelet count. American Journal of Gastroenterology December 2010;105:2705-7. [DOI] [PubMed] [Google Scholar]
- Kalambokis, Mouzaki A, Rodi M, Tsianos EV. Rifaximin improves thrombocytopenia in patients with alcoholic cirrhosis in association with reduction of endotoxiemia. Liver International 2012;32(3):467-75. [DOI] [PubMed] [Google Scholar]
Kalambokis 2012c {published data only}
- Kalambokis GN, Mouzaki A, Rodi M, Tsianos EV. Rifaximin for the prevention of spontaneous bacterial peritonitis. World Journal of Gastroenterology 2012;18(14):1700-2. [DOI: 10.3748/wjg.v18.i14.1700] [PMCID: PMC3325539] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalambokis 2012d {published data only}
- Kalambokis GN, Tsianos EV. Rifaximin reduces endotoxemia and improves liver function and disease severity in patients with decompensated cirrhosis. Hepatology 2012;55(2):655-6. [DOI] [PubMed] [Google Scholar]
Kang 2017 {published data only}
- Kang SH, Lee YB, Lee JH, Nam JY, Chang Y, Cho H, et al. Rifaximin treatment is associated with reduced risk of cirrhotic complications and prolonged overall survival in patients experiencing hepatic encephalopathy. Alimentary Pharmacology & Therapeutics 2017;46(9):845-5. [DOI: 10.1111/apt.14275] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Kang SH, Yoo JJ, Lee JH, Nam JY, Chang Y, Cho H, et al. Rifaximin reduces cirrhotic complications and prolongs overall survival in patients experiencing hepatic encephalopathy. Journal of Hepatology 2017;66(Suppl 1):S572-3. [DOI: 10.1016/S0168-8278(17)31566-0] [DOI] [PubMed] [Google Scholar]
Kawaratani 2022 {published data only}
- Kawaratani H, Kondo Y, Tatsumi R, Kawabe N, Tanabe N, Sakamaki A, et al. Long-term efficacy and safety of rifaximin in japanese patients with hepatic encephalopathy: a multicenter retrospective study. Journal of Clinical Medicine 2022;157110.3390/jcm11061571.(6):1571. [DOI: 10.3390/jcm11061571.] [PMCID: PMC8948903] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khokhar 2015 {published data only}
- Khokhar N, Qureshi MO, Ahmad S, Ahmad A, Khan HH, Shafqat F, et al. Comparison of once a day rifaximin to twice a day dosage in the prevention of recurrence of hepatic encephalopathy in patients with chronic liver disease. Journal of Gastroenterology and Hepatology 2015;30:1420-2. [DOI: 10.111/jgh.12970] [DOI] [PubMed] [Google Scholar]
- Qureshi MO, Shafqat F, Salih, M, Khokhar N. Daily single dose rifaximin for prevention of hepatic encephalopathy in patients with chronic liver disease. Hepatology International 2015;9(Suppl 1):S325. [DOI] [PubMed] [Google Scholar]
Kimer 2018 {published data only}
- Kimer N, Gudmann NS, Pedersen J, Møller S, Nielsen MJ, Leeming DJ, et al. No effect of rifaximin on soluble CD163, mannose receptor or type III and IV neoepitope collagen markers in decompensated cirrhosis: Results from a randomized, placebo controlled trial. PLOS One 2018;13(9):1-12. [DOI: 10.1371/journal.pone.0203200] [EUDRACT: 2012-002890-71] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kimer 2022 {published data only}
- EUCTR2014-002264-33-DK. Rifaximin (an antibiotic) treatment of acute alcoholic liver injury. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2014-002264-33-DK 2014.
- Kimer N, Meldgaard M, Hamberg O, Kronborg TM, Lund AM, Møller HJ, et al. The impact of rifaximin on inflammation and metabolism in alcoholic hepatitis: a randomized clinical trial. PLoS One 2022;17(3):e0264278. [DOI: 10.1371/journal.pone.0264278] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kubota 2022 {published data only}
- Kubota K, Uojima H, Shao X, Iwasaki S, Hidaka H, Wada N, et al. Additional L-carnitine reduced the risk of hospitalization in patients with overt hepatic encephalopathy on rifaximin. Digestive Diseases 2022;40(3):313-21. [DOI: 10.1159/000518067] [PMID: ] [DOI] [PubMed] [Google Scholar]
- UMIN000027511. Combination therapy with levocarnitine and rifaximin for hepatic encephalopathy in patients with cirrhosis: a randomized study. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000031535 Registered 7 June 2017.
Lauridsen 2018 {published data only}
- Lauridsen MM, Gram J, Vilstrup H, Schaffalitzky de Muckadell OB. The continuous reaction times test for minimal hepatic encephalopathy validated by a randomized controlled multi-modal intervention. Journal of Clinical and Experimental Hepatology 2017;7(Suppl 1):S12-3. [DOI: 10.1016/j.jceh.2017.01.017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauridsen MM, Gram J, Muckadell OB, Vilstrup H. The continuous reaction time test for minimal hepatic encephalopathy validated by a randomized controlled multimodal intervention. Journal of Hepatology 2018;68(Suppl 1):S740-1. [DOI: 10.1016/S0168-8278(18)31744-6] [DOI] [Google Scholar]
- Lauridsen MM, Mikkelsen S, Svensson T, Holm J, Klüver C, Gram J, et al. The continuous reaction time test for minimal hepatic encephalopathy validated by a randomized controlled multi-modal intervention - a pilot study. PLOS One 2017;12(10):e0185412. [DOI: 10.1371/journal.pone.0185412] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01773538. Diagnosis of covert/minimal hepatic encephalopathy by means of continuous reaction time measurement. clinicaltrials.gov/ct2/show/NCT01773538 (First posted 23 January 2013).
Lighthouse 2004 {published data only}
- Lighthouse J, Naito Y, Helmy A, Hotten P, Fuji H, Min CH, et al. Endotoxinemia and benzodiazepine-like substances in compensated cirrhotic patients: a randomized study comparing the effect of rifaximine alone and in association with a symbiotic preparation. Hepatology Research 2004;28(3):155-60. [DOI: 10.1016/j.hepres.2003.11.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Miglio 1997 {published data only}
- Miglio F, Valpiani D, Rossellini SR, Ferrieri A. Rifaximin, a non-absorbable rifamycin, for the treatment of hepatic encephalopathy. A double-blind, randomized trial. Current Medical Research and Opinion 1997;13(10):593-601. [DOI: 10.1185/03007999709113333] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mohamed 2018 {published data only}
- Mohamed AM, Ahmad RR, Marwa AG, Mohamed OAM, Yosef MS. Rifaximin versus metronidazole in management of acute episode of hepatic encephalopathy: An open labeled randomized clinical trial. Arab Journal of Gastroenterology 2018;19(2):76-9. [DOI: 10.1016/j.ajg.2018.06.001] [DOI] [PubMed] [Google Scholar]
Mostafa 2015 {published data only}
- Mostafa T, Badra G, Abdallah M. The efficacy and the immunomodulatory effect of rifaximin in prophylaxis of spontaneous bacterial peritonitis in cirrhotic Egyptian patients. Turkish Journal of Gastroenterology 2015;26(2):163-9. [DOI: 10.5152/tjg.2015.7782] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mullen 2014 {published data only}
- Mullen K, Sanyal A, Bass N, Poordad F, Huang S, Merchant K. The long term efficacy and safety of rifaximin in the maintenance of remission from overt hepatic encephalopathy in cirrhotic patients. Gastroenterology 2012;142(5 Suppl 1):S41-2. [DOI: 10.1016/S0016-5085(12)60157-7] [DOI] [Google Scholar]
- Mullen K, Sanyal A, Bass N, Poordad F, Sheikh M, Frederick RT, et al. Rifaximin is safe and well tolerated for long-term maintenance of remission from over hepatic encephalopathy. Clincial Gastroenterology and Hepatology 2014;12(8):1390-7. [DOI: 10.1016/j.cgh.2013.12.021] [DOI] [PubMed] [Google Scholar]
- Neff GW, Flamm SL, Mullen KD, Barrett AC, Bortey E. Improved outcomes in hepatic encephalopathy using rifaximin monotherapy compared to rifaximin and lactulose combination therapy. Gastroenterology 2013;144(Suppl 5):S-451. [DOI: 10.1016/S0016-5085(13)61665-0] [DOI] [Google Scholar]
- Poordad F, Bass N, Sanyal AJ, Sheikh MY, Mullen KD, Sigal S, et al. The protective effect of rifaximin (1100 mg daily) from hepatic encephalopathy observed in a double-blind placebo controlled study is substantiated and durable over the long term. Hepatology 2009;50(Suppl 4):448-9A. [DOI: 10.1002/hep.23301] [DOI] [Google Scholar]
- Sanyal A, Mullen KD, Bass NM, Frederick T, Poordad F, Hee J, et al. Rates of commonly occuring infections in cirrhosis patients remain stable during long-term rifaximin treatment. Journal of Hepatology 2012;56(Suppl 2):S255-6. [DOI: 10.1016/S0168-8278(12)60658-8] [DOI] [Google Scholar]
NCT00364689 {published data only}
- RICE Trial: Rifaximin In Chronic Hepatic Encephalopathy - A Randomized, Controlled Trial. https://clinicaltrials.gov/show/NCT00364689.
NCT00748904 {published data only}
- NCT00748904. A Randomized Comparison of Rifaximin Versus Lactulose in Hospitalized Cirrhotic Patients With Progressive Renal Failure. ClinicalTrials.gov: https://www.clinicaltrials.gov/ct2/show/NCT00748904 First posted 9 September 2008.
NCT01676597 {published data only}
- NCT01676597. Efficacy of pentoxifylline and rifaximin combination in pentoxifylline refractory clinical and subclinical hepatopulmonary syndrome. clinicaltrials.gov/ct2/show/NCT01676597 (First posted 31 August 2012).
NCT01846806 {published data only}
- NCT01846806. The role of bacterial overgrowth and delayed intestinal transit in hepatic encephalopathy. clinicaltrials.gov/ct2/show/NCT01846806 (First posted 3 May 2013).
NCT01897051 {unpublished data only}
- NCT01897051. Rifaximin and propranolol combination therapy versus propranolol monotherapy in cirrhotic patients (RECOVER) [Hemodynamic response of rifaximin and non-selective β-blocker combination therapy versus non-selective β-blocker monotherapy in cirrhotic patients with esophageal varices]. clinicaltrials.gov/show/nct01897051 (First posted 11 July 2013).
NCT01951209 {published data only}
- NCT01951209. Pilot study of the effect of rifaximin on B-cell dysregulation in cirrhosis. clinicaltrials.gov/ct2/show/NCT01951209 (First posted 26 September 2013).
NCT02011841 {published data only}
- NCT02011841. Rifaximin for the secondary prevention of spontaneous bacterial peritonitis recurrence in cirrhotic patients. clinicaltrials.gov/ct2/show/NCT02011841 (First posted 13 December 2013).
NCT02485106 {published data only}
- NCT02485106. Rifaximin use in severe alcoholic hepatitis. clinicaltrials.gov/ct2/show/NCT02485106 (First posted 30 June 2015).
NCT03712280 {published data only}
- NCT03712280. MNK6106 for liver disease (hepatic cirrhosis) that in the past has affected the brain (hepatic encephalopathy). clinicaltrials.gov/ct2/show/NCT03712280 (First posted 19 October 2018).
NCT04159870 {published data only}
- NCT04159870. Rifaximin versus norfloxacin in the primary prophylaxis of spontaneous bacterial peritonitis. clinicaltrials.gov/ct2/show/NCT04159870 (First posted 12 November 2019).
Oey 2019 {published data only}
- Oey RC, Buck LE, Erler NS, Burren HR, Man RA. The efficacy and safety of rifaximin-α: a 2-year observational study of overt hepatic encephalopathy. Therapeutic Advances in Gastroenterology 2019;12(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Orr 2016 {published data only}
- Orr J, Currie CJ, Berni E, Anurag B, Goel KJ, Moriarty A. The impact on hospital resource utilisation of treatment of hepatic encephalopathy with rifaximin-α. Liver International 2016;36(9):1295-303. [DOI: 10.1111/liv.13111] [DOI] [PubMed] [Google Scholar]
Parini 1992 {published data only}
- Parini P, Cipolla A, Ronchi M, Salzetta A, Mazella G, Roda E. Effect of rifaximin and paromomycin in the treatment of portal-systemic encephalopathy. Current Therapeutic Research 1992;52(1):34-9. [Google Scholar]
Pedretti 1991 {published data only}
- Pedretti G, Calzetti C, Missale G, Fiaccadori F. Rifaximin versus neomycin on hyper-ammoniemia in chronic portal systemic encephalopathy of cirrhotics. A double-blind, randomized trial. Italian Journal of Gastroenterology 1991;23:175-8. [PubMed] [Google Scholar]
Ponziani 2016 {published data only}
- Ponzani FR, Scaldaferri F, Pecere S, Sterbini F, Petito V, Lopetuso LR, et al. Increased abundance of beneficial bacteria is associated with clinical improvement after rifaximin treatment. Digestive and Liver Disease 2016;48(Suppl 2):E176-7. [DOI: 10.1016/S1590-8658(16)30264-X] [DOI] [Google Scholar]
- Ponziani FR, Scaldaferri F, Sterbini FP, Petito V, Pecere S, Lopetuso LR, et al. Increased abundance of beneficial bacteria is associated with clinical improvement in patients receiving rifaximin treatment. United European Gastroenterology Journal 2016;4(Suppl 5):A649-50. [DOI: 10.1177/2050640616663689] [DOI] [Google Scholar]
Pose 2020 {published data only}
- EUCTR2018-001698-25-GB. Efficacy of the combination of simvastatin plus rifaximin in patients with decompensated cirrhosis to prevent ACLF development: a multicenter, double-blind, placebo controlled randomized clinical trial. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2018-001698-25 (Date of registration 01 October 2018).
- NCT03780673. Efficacy of the combination of simvastatin plus rifaximin in patients with decompensated cirrhosis to prevent ACLF development. clinicaltrials.gov/ct2/show/NCT03780673 (First posted 19 December 2018).
- Pose E, Napoleone L, Amin A, Campion D, Jimenez C, Piano S, et al. Safety of two different doses of simvastatin plus rifaximin in decompensated cirrhosis (LIVERHOPE-SAFETY): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. Gastroenterology and Hepatology 2020;5(1):31-41. [DOI: 10.1016/S2468-1253(19)30320-6] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Pose E, Napoleone L, Amin A, Campion D, Jimenez C, Piano S, et al. Safety and tolerability of two doses of simvastatin associated with rifaximin in patients with decompensated cirrhosis: a double‐blind, randomized, placebo‐controlled trial. Hepatology 2018;68(51):176A. [DOI: 10.1002/hep.30256] [DOI] [Google Scholar]
Praharaj 2022 {published data only}
- NCT03695705. Rifaximin and norfloxacin for prevention of SBP in adults with decompensated cirrhosis. clinicaltrials.gov/ct2/show/NCT03695705 (First posted 4 October 2018).
- Praharaj D, Taneja S, Duseja A, Chawla YK, Dhiman RK. Randomized control trial of rifaximin and norfloxacin in primary and secondary prophylaxis of spontaneous bacterial peritonitis in cirrhotic patients. Indian Journal of Gastroenterology 2017;36(1):A57-8. [DOI: 10.1007/s12664-017-0798-5] [DOI] [Google Scholar]
- Praharaj D, Taneja S, Duseja A, Chawla YK, Dhiman RK. Randomized control trial of rifaximin and norfloxacin in primary and secondary prophylaxis of spontaneous bacterial peritonitis (SBP) in cirrhotic patients. Journal of Clinical and Experimental Hepatology 2017;7(S2):S71. [DOI: 10.1016/j.jceh.2017.05.133] [DOI] [Google Scholar]
- Praharaj D. Randomized control trial of rifaximin and norfloxacin in primary and secondary prophylaxis of spontaneous bacterial peritonitis (SBP) in cirrhotic patients. Hepatology International 2018;12(2):S553-4. [DOI: 10.1007/s12072-018-9852-3] [DOI] [Google Scholar]
- Praharaj DL, Premkumar M, Roy A, Verma N, Taneja S, Duseja A, Dhiman RK. Rifaximin vs. norfloxacin for spontaneous bacterial peritonitis prophylaxis: A randomized controlled trial. Journal of Clinical and Experimental Hepatology 2022;12(2):336-342. [DOI: 10.1016/j.jceh.2021.08.010] [PMCID: PMC9077172] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saboo 2021 {published data only}
- Saboo K, Shamsaddini A, Iyer M, Hu C, Fagan A, Gavis E. Sex is associated with differences in gut microbial composition and function in hepatic encephalopathy. Journal of Hepatology 2021;74(1):80-8. [DOI: 10.1016/j.jhep.2020.06.046] [PMCID: PMC7749850] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salehi 2019 {published data only}
- Salehi S, Lim S, Heneghan M, Aluvihare V, Heaton N, Patel V, et al. Rifaximin reduced the incidence of sepsis and all cause admissions whilst on the liver transplant waiting list. Journal of Hepatology 2018;68(Suppl 1):S119-20. [DOI: 10.1016/S0168-8278(18)30454-9] [DOI] [Google Scholar]
- Salehi S, Tranah T, Lim S, Heaton N, Heneghan M, Aluvihare V, et al. Rifaximin reduces the incidence of spontaneous bacterial peritonitis, variceal bleeding and all-cause admissions in patients on the liver transplant waiting list. Alimentary Pharmacology & Therapeutics 2019;50(4):435-41. [DOI: 10.1111/apt.15326] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sama 2004 {published data only}
- Sama C, Morselli-Labate AM, Pianta P, Lambertini L, Berardi S, Martini G. Clinical effects of rifaximin in patients with hepatic encephalopathy intolerant or nonresponsive to previous lactulose treatment: an open-label, pilot study. Current Therapeutic Research 2004;65(5):413-22. [DOI: 10.1016/j.curtheres.2004.10.002] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sarwar 2019 {published data only}
- Sarwar S, Muhyuddin B, Aleem A, Nadeem MA. Primary prophylaxis of hepatic encephalopathy in decompensated cirrhosis: low dose vs. full dose rifaximin. Pakistan Journal of Medical Sciences 2019;35(5):1446-50. [DOI: 10.12669/pjms.35.5.549] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 2019 {published data only}
- Schulz C, Schütte K, Vilchez-Vargas R, Vasapolli R, Malfertheiner P. Long-term effect of rifaximin with and without lactulose on the active bacterial assemblages in the proximal small bowel and faeces in patients with minimal hepatic encephalopathy. Digestive Diseases 2019;37(2):161-9. [DOI: 10.1159/000494216] [DOI] [PubMed] [Google Scholar]
- Schulz C, Schütte K, Kropf S, Schmitt FC, Vasapolli R, Kliegis LM, et al. RiMINI - the influence of rifaximin on minimal hepatic encephalopathy (MHE) and on the intestinal microbiome in patients with liver cirrhosis: study protocol for a randomized controlled trial. Trials 2016;17(1):111. [DOI: 10.1186/s13063-016-1205-8] [EUDRA-CT: 2013-004414-18] [PMCID: PMC4770677] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Seifert 2021 {published data only}
- Seifert LL, Schindler P, Schoster M, Weller JF, Wilms C, Schmidt HH. Recurrence of hepatic encephalopathy after TIPS: effective prophylaxis with combination of lactulose and rifaximin. Journal of Clinical Medicine 2021;10(20):1-12. [DOI: 10.3390/jcm10204763] [PMCID: PMC8537523] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 2021 {published data only}
- Song DS, Yang JM, Jung YK, Yim H-J, Kim HY, Kim CW, et al. Rifaximin treatment in patients with severe alcoholic hepatitis; A multicenter, open-label, pilot randomized controlled trial. Hepatology (Baltimore, Md.) 2021;74(Suppl. 1):250A: 390. [DOI: 10.1002/hep.32188] [DOI] [Google Scholar]
Suzuki 2019 {published data only}
- Suzuki H, Sezaki H, Suzuki F, Kasuya K, Sano T, Fujiyama S, et al. Real-world effects of long-term rifaximin treatment for Japanese patients with hepatic encephalopathy. Hepatology Research 2019;49(12):1406-13. [DOI: 10.1111/hepr.13415] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tatsumi 2021 {published data only}
- Tasumi R, Suii H, Yamaguchi M, Arakawa T, Nakajima T, Kuwata T, et al. Efficacy of switching from kanamycin sulfate to rifaximin in patients with hepatic cirrhosis. Internal Medicine 2021;60(1):1501-7. [DOI: 10.2169/internalmedicine.6039-20] [DOI] [PMC free article] [PubMed] [Google Scholar]
Testa 1985 {published data only}
- Eftimiadi C, Deleo C, Schito GC. Treatment of hepatic encephalopathy with L/105, a new non-absorbable rifamycin. Drugs under Experimental and Clinical Research 1984;10(10):691-6. [PubMed]
- Testa R, Eftimiadi C, Sukkar GS, De Leo C, Rovida S, Schito GC et al. A non-absorbable rifamycin for treatment of hepatic encephalopathy. Drugs Under Experimental and Clinical Research 1985;11(6):387-92. [PMID: ] [PubMed] [Google Scholar]
Uchida 2020 {published data only}
- Uchida Y, Tsuji S, Uemura H, Kouyama J, Naiki K, Sugawara K. Furosemide as a factor to deteriorate therapeutic efficacy of rifaximin in patients with decompensated cirrhosis. Hepatology Research 2020;50(11):1264-74. [DOI: 10.1111/hepr.13564] [DOI] [PubMed] [Google Scholar]
UMIN000036998 {published data only}
- Randomized controlled trial comparing zinc-rifaximin combination therapy with zinc monotherapy in liver cirrhosis patients with minimal hepatic encephalopathy. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000042151 (Registered 10 June 2019). 2019. [UMIN: 000036998]
UMIN000038487 {published data only}UMIN000038487
- UMIN000038487. Use of rifaximin therapy in primary prevention of hepatorenal syndrome in cirrhotic patients with ascites. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000043845 (Registered 3 November 2019).
Venturini 2005 {published data only}
- Venturini I, Ferrieri A, Farina F, Avallone R, Corsi L, Baraldi M, et al. Evaluation of rifaximin, placebo and lactulose in reducing the levels of benzodiazepine-like compounds in patients with liver cirrhosis: A pilot study. Drugs Under Experimental and Clinical Research 2005;31(4):161-8. [PMID: ] [PubMed] [Google Scholar]
Vittitow 2018 {published data only}
- Vittitow J, Heimanson Z, Israel R, Lowe E. Pharmacokinetics study of 2 investigational rifaximin soluble solid dispersion formulations (immediate release and sustained extended release) in healthy volunteers. Journal of Hepatology 2018;68(Suppl 1):S456. [DOI: 10.1016/S0168-8278(18)31175-9] [DOI] [Google Scholar]
Vlachogiannakos 2013 {published data only}
- Vlachogiannakos J, Viazis N, Vasianopoulou P, Vafiadis I, Karamanolis DG, Ladas SD. Long-term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. Journal of Gastroenterology and Hepatology 2013;28(3):450-5. [DOI: 10.1111/jgh.12070] [DOI] [PubMed] [Google Scholar]
Walker 2020 {published data only}
- Krag A, Schuchmann M, Sodatonou H, Pilot J, Whitehouse J, Strasser S, et al. Design of the Prospective Real-world Outcomes Study of hepatic encephalopathy Patients’ Experience on Rifaximin-α (PROSPER): an observational study among 550 patients. Hepatology, Medicine and Policy 2018;3(4):1-8. [DOI: 10.1186/s41124-017-0029-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard N, Pilot J, Holt A. PROSPER: Prospective Real World Outcomes Study of Hepatic Encephalopathy Patients' Experience on Rifaximin-a (TARGAXAN®/XIFAXAN®) 550 mg. clinicaltrials.gov/ct2/show/NCT02488993 (First posted 2 July 2015).
- Walker D. PROSPER study reveals reduction in bed days per hospitalisation and decreased risk of mortality for patients observed on rifaximin-alpha 550mg compared to a cohort on standard of care. Journal of Hepatology 2020;73(Suppl 1):S705. [DOI: 10.1016/S0168-8278(20)31873-0] [DOI] [Google Scholar]
Williams 2000 {published data only}
- Williams R, James OFW, Warnes TW, Morgan MY. Evaluation of the efficacy of hepatic encephalopathy: a double-blind, randomized, dose-finding multi-centre study. European Journal of Gastroenterology and Hepatology 2000;12(2):203-8. [DOI] [PubMed] [Google Scholar]
Yang 2016 {published data only}
- Yang J, Zhou SC, Zang JL, Yang YX. Clinical effects of ornithine aspartate combined with rifaximin in the treatment of acute hepatic encephalopathy. World Chinese Journal of Digestology 2016;24(3):415-9. [DOI: 10.11569/wcjd.v24.i3.415] [DOI] [Google Scholar]
Zeng 2015 {published data only (unpublished sought but not used)}
- NCT02074280. Rifaximin predicts the complications of decompensated cirrhosis: a randomized controlled trial. clinicaltrials.gov/ct2/show/NCT02074280 First posted 28 February 2014.
- Zeng X, Tang X, Sheng X, Ni W, Xin H, et al. Does low-dose rifaximin ameliorate endotoxiemia in patients with liver cirrhosis: a prospective study. Journal of Digestive Diseases 2015;16(11):665-74. [DOI: 10.1111/cdd.12294] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR1800018070 {published data only}
- ChiCTR1800018070. Clinical study of rehoci combined with long-acting octreotide in reducing the risk of rebleeding in patients with cirrhosis and portal hypertension. www.chictr.org.cn/showprojen.aspx?proj=30575 (Registered 29 August 2018).
EUCTR2014‐000102‐35‐IT {published data only}
- EUCTR2014-000102-35-IT. Effect of administration "add on" of Rifaximin on portal hypertension of patients with liver cirrhosis and esophageal varices in standard therapy with propranolol. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2014-000102-35 (Date of registration 24 October 2014).
EUCTR2017‐000488‐34‐IT {published data only}EUCTR2017‐000488‐34‐IT
- EUCTR2017-000488-34-IT. Rifaximin blunted higher levels of endotoxin in cirrhosis patients: a randomized, double blind, short term interventional trial - rifaximin in cirrhotic patient. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2017-000488-34 (Date of registration 8 June 2021).
EUDRACT2016‐002628‐96 {published data only}
- EUDRACT2016-002628-96. A multi-centre, double-blind, randomised, controlled clinical trial of Rifaximin to reduce infection in patients admitted to hospital with decompensated cirrhosis. www.clinicaltrialsregister.eu/ctr-search/search?query=2016-002628-96 (Date of registration September 2016).
- ISRCTN10994757. A multi-centre, double-blind, randomised, controlled clinical trial of Rifaximin to reduce infection in patients admitted to hospital with decompensated cirrhosis. www.isrctn.com/ISRCTN10994757 (Trial start date 15 April 2016).
NCT01846663 {published data only}
- NCT01846663. The efficacy, safety, and pharmacokinetics of rifaximin In subjects with severe hepatic impairment and hepatic encephalopathy. clinicaltrials.gov/ct2/show/NCT01846663 (First posted 3 May 2013).
NCT02439307 {published data only}
- NCT02439307. Effect of rifaximin on minimal hepatic encephalopathy and small intestinal bacterial overgrowth. clinicaltrials.gov/show/NCT02439307 (First posted 8 May 2015).
NCT02508623 {published data only}NCT02508623
- NCT02508623. Effect of administration of rifaximin on the portal pressure of patients with liver cirrhosis and esophageal varices (ERASE). clinicaltrials.gov/show/NCT02508623 (First posted 27 July 2015).
NCT02931123 (Riggio 2016) {published data only}
- NCT02931123. A RCT comparing lactulose and rifaximin associated with a vegetable diet in the preventions of post-TIPS overt hepatic encephalopathy. clinicaltrials.gov/show/NCT02931123 (First posted 12 October 2016).
NCT03069131 {published data only}
- NCT03069131. Two strategies of primary prophylaxis of spontaneous bacterial peritonitis in severe cirrhotic patients with ascites (ProPILARifax). clinicaltrials.gov/ct2/show/NCT03069131 (First posted 3 March 2017).
NCT04073290 (PEARL Study) {published data only}EUCTR2018‐004323‐37
- EUCTR2018-004323-37-NL. Prevention of hepatic encephalopathy by administration of rifaximin and lactulose in patients with liver cirrhosis undergoing placement of a transjugular intrahepatic portosystemic shunt: a multi-centre randomized, double blind, placebo controlled trial. The PEARL trial. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2018-004323-37 (Date of registration 07 January 2019). [DOI] [PMC free article] [PubMed]
- NCT04073290. Prevention of post-TIPS hepatic encephalopathy by administration of rifaximin and lactulose (PEARL). clinicaltrials.gov/ct2/show/NCT04073290 (First posted 29 August 2019).
- Wit K, Schaapman JJ, Nevens F, Verbeek J, Coenen S, Cuperus FJ, et al. Prevention of hepatic encephalopathy by administration of rifaximin and lactulose in patients with liver cirrhosis undergoing placement of a transjugular intrahepatic portosystemic shunt (TIPS): a multicentre randomised, double blind, placebo controlled trial (PEARL trial). BMJ Open Gastroenterology 2020;7(1):e000531. [DOI: 10.1136/bmjgast-2020-000531] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT04775329 {published data only}
- NCT04775329. Primary prophylaxis for spontaneous bacterial peritonitis (SIBOC). clinicaltrials.gov/ct2/show/NCT04775329 (First posted 1 March 2021).
NCT05071716 (RNLC3131) {published data only}
- NCT05071716. A randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of rifaximin soluble solid dispersion (SSD) for the delay of encephalopathy decompensation in cirrhosis. clinicaltrials.gov/ct2/show/NCT05071716 (First posted 8 October 2021).
TCTR20180509001 {published data only}
- TCTR20180509001. Comparison between rifaximin vs lactulose for treatment of hepatic encephalopathy. www.thaiclinicaltrials.org/show/TCTR20180509001 (Date of registration 09 May 2018).
Additional references
Acharya 2019
- Acharya C, Bajaj JS. Altered microbiome in patients with cirrhosis and complications. Clinical Gastroenterology and Hepatology 2019;17(2):307-21. [DOI: 10.1016/j.cgh.2018.08.008] [PMCID: PMC6314917DO] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Amodio 2004
- Amodio P, Montagnese S, Gatta A, Morgan MY. Characteristics of minimal hepatic encephalopathy. Metabolic Brain Disease 2004;19(3-4):253-67. [DOI: 10.1023/b:mebr.0000043975.01841.de] [PMID: ] [DOI] [PubMed] [Google Scholar]
Amodio 2015
- Amodio P, Montagnese S. Clinical neurophysiology of hepatic encephalopathy. Journal of Clinical and Experimental Hepatology 2015;5(Suppl 1):S60-8. [DOI: 10.1016/j.jceh.2014.06.007] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ampuero 2015
- Ampuero J, Simon M, Montoliu C, Jover R, Serra MA, Córdoba J, et al. Minimal hepatic encephalopathy and critical flicker frequency are associated with survival of patients with cirrhosis. Gastroenterology 2015;149(6):1483-9. [DOI: 10.1053/j.gastro.2015.07.067] [PMID: ] [DOI] [PubMed] [Google Scholar]
Arroyo 2020
- Arroyo V, Moreau R, Jalan R. Acute-on-chronic liver failure. New England Journal of Medicine 2020;382(22):2137-45. [DOI: 10.1056/NEJMra1914900] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bai 2014
- Bai M, Qi X-S, Yang Z-P, Yang M, Fan D-M, Han G-H, et al. TIPS improves liver transplantation free survival in cirrhotic patients with refractory ascites: an updated meta-analysis. World Journal of Gastroenterology 2014;20(10):2704-14. [DOI: 10.3748/wjg.v20.i10.2704] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bajaj 2007
- Bajaj JS, Saeian K, Verber MD, Hischke D, Hoffmann RG, Franco J, et al. Inhibitory control test is a simple method to diagnose minimal hepatic encephalopathy and predict development of overt hepatic encephalopathy. American Journal of Gastroenterology 2007;102(4):754-60. [DOI: 10.1111/j.1572-0241.2007.01048.x] [DOI] [PubMed] [Google Scholar]
Bajaj 2008
- Bajaj JS. Minimal hepatic encephalopathy matters in daily life. World Journal of Gastroenterology 2008;14(23):3609-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bajaj 2009
- Bajaj J, Saeian K, Schubert C, Hafeezullah M, Franco J, Varma R, et al. Minimal hepatic encephalopathy is associated with motor vehicle crashes: the reality beyond the driving test. Hepatology 2009;50(4):1175-83. [DOI: 10.1002/hep.23128] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bajaj 2010a
- Bajaj JS, Schubert CM, Heuman DM, Wade JB, Gibson DP, Topaz A, et al. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology 2010;138(7):2332-40. [DOI: 10.1053/j.gastro.2010.02.015] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bajaj 2010b
- Bajaj JS, Sanyal AJ, Bell D, Gilles H, Heuman DM. Predictors of the recurrence of hepatic encephalopathy in lactulose-treated patients. Alimentary Pharmacology & Therapeutics 2010;31(9):1012-7. [DOI: 10.1111/j.1365-2036.2010.04257.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bajaj 2011a
- Bajaj JS, Cordoba J, Mullen KD, Amodio P, Shawcross DL, Butterworth RF, et al. Review article: the design of clinical trials in hepatic encephalopathy - an International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) consensus statement. Alimentary Pharmacology & Therapeutics 2011;33(7):739-47. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bajaj 2011b
- Bajaj JS, Wade JB, Gibson DP, Heuman DM, Thacker LR, Sterling RK, et al. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. American Journal of Gastroenterology 2011;106(9):1646-53. [DOI: 10.1038/ajg.2011.157] [PMCID: PMC3989143] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bajaj 2012
- Bajaj JS, Gillevet PM, Patel NR, Ahluwalia V, Ridlon JM, Kettenmann B, et al. A longitudinal systems biology analysis of lactulose withdrawal in hepatic encephalopathy. Metabolic Brain Disease 2012;27:205-15. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bajaj 2014
- Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. Journal of Hepatology 2014;60(5):940-7. [DOI: 10.1016/j.jhep.2013.12.019] [PMCID: PMC3995845] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bajaj 2016a
- Bajaj JS. Review article: potential mechanisms of action of rifaximin in the management of hepatic encephalopathy and other complications of cirrhosis. Alimentary Pharmacology & Therapeutics 2016;43(S1):11-26. [DOI: 10.1111/apt.13435] [DOI] [PubMed] [Google Scholar]
Bajaj 2021
- Bajaj JS, Shamsaddini A, Fagan A, Sterling R, Acharya C, Lee H, et al. Rifaximin and fecal microbiota transplant beneficially modulate gut metagenomic virulence factors in cirrhosis: analysis of two trials. Journal of Hepatology 2021;75(Suppl 2):S403-4. [DOI: 10.1016/S0168-8278(21)01843-2] [DOI] [Google Scholar]
Berding 2009
- Berding G, Banati RB, Buchert R, Chierichetti F, Grover VP, Kato A, et al. Radiotracer imaging studies in hepatic encephalopathy: ISHEN practice guidelines. Liver International 2009;29:621-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bircher 1966
- Bircher J, Müller J, Guggenheim P, Haemmerli UP. Treatment of chronic portal-systemic encephalopathy with lactulose [in German]. Lancet 1966;1(7443):890-2. [DOI: 10.1016/s0140-6736(66)91573-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bircher 1982
- Bircher J, Bührer M, Franz K, Velthuijsen JA. 1st use of lactitol in the treatment of porto-systemic encephalopathy. Schweizerische Medizinische Wochenschrift 1982;112(38):1306-7. [PMID: ] [PubMed] [Google Scholar]
Bjerring 2018
- Bjerring PN, Gluud LL, Larsen FS. Cerebral Blood Flow and Metabolism in Hepatic Encephalopathy—A Meta-Analysis. Journal of Clinical Experimental Hetatology 2018;8(3):286-93. [DOI: 10.1016/j.jceh.2018.06.002] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
BNF 2021
- BMJ Group and the Royal Pharmaceutical Society of Great Britain. Rifaximin. In: British National Formulary. BMJ Group and the Royal Pharmaceutical Society of Great Britain, 2019. [ISBN: 978 0 85711 369 6] [Google Scholar]
Boutron 2021
- Boutron I, Page MJ, Higgins JP, Altman DG, Lundh A, Hróbjartsson A. Chapter 7: Considering bias and conflicts of interest among the included studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Brown 2006
- Brown P, Brunnhuber K, Chalkidou K, Chalmers I, Clarke M, Fenton M, et al. How to formulate research recommendations. BMJ (Clinical Research Ed.) 2006;333:804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bustamante 1999
- Bustamante J, Rimola A, Ventura PJ, Navasa M, Cirera I, Reggiardo V, et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. Journal of Hepatology 1999;30:890-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Calanni 2014
- Calanni F, Renzulli C, Barbanti M, Viscomi GC. Rifaximin: beyond the traditional antibiotic activity. Journal of Antibiotics 2014;67:667 – 70. [DOI: 10.1038/ja.2014.106] [PMID: ] [DOI] [PubMed] [Google Scholar]
Caraceni 2021
- Caraceni P, Vargas V, Solà E, Alessandria C, Wit K, Trebicka J, et al. The use of rifaximin in patients with cirrhosis. Hepatology 2021;74(3):1600-73. [DOI: 10.1002/hep.31708] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheng 2021
- Cheng J, Chen Y, Cao W, Zuo G. Is rifaximin better than nonabsorbable disaccharides in hepatic encephalopathy?: A meta-analysis. Medicine 2021;100(51):e28232. [DOI: 10.1097/MD.0000000000028232] [DOI] [PMC free article] [PubMed] [Google Scholar]
Conn 1977
- Conn HO, Leevy CM, Vlahcevic ZR, Rodgers JB, Maddrey WC, Seeff L, et al. Comparison of lactulose and neomycin in the treatment of chronic portal-systemic encephalopathy. A double blind controlled trial. Gastroenterology 1977;72:573-83. [PMID: ] [PubMed] [Google Scholar]
Courson 2016
- Courson A, Jones GM, Twilla JD. Treatment of acute hepatic encephalopathy: comparing the effects of adding rifaximin to lactulose on patient outcomes. Journal of Pharmacy Practice 2016;29(3):212-7. [DOI] [PubMed] [Google Scholar]
Cudalbu 2019
- Cudalbu C, Taylor-Robinson SD. Brain edema in chronic hepatic encephalopathy. Journal of Clinical and Experimental Hepatology 2019;9(3):362-82. [DOI: 10.1016/j.jceh.2019.02.003] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Córdoba 2014
- Córdoba J, Ventura-Cots M, Simón-Talero M, Amorós À, Pavesi M, Vilstrup H, et al. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute-on-chronic liver failure (ACLF). Journal of Hepatology 2014;60(2):275-81. [DOI: 10.1016/j.jhep.2013.10.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
D'Amico 1986
- D'Amico G, Morabito A, Pagliaro L, Marubini E. Survival and prognostic indicators in compensated and decompensated cirrhosis. Digestive Diseases and Sciences 1986;31:468-75. [DOI] [PubMed] [Google Scholar]
D'Amico 2006
- D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. Journal of Hepatology 2006;44(1):217-31. [DOI: 10.1016/j.jhep.2005.10.013] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dalal 2017
- Dalal R, McGee RG, Riordan SM, Webster AC. Probiotics for people with hepatic encephalopathy. Cochrane Database of Systematic Reviews 2017, Issue 2(2):CD008716. Art. No: CD008716. [DOI: 10.1002/14651858.CD008716.pub3] [PMCID: 6464663] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Das 2001
- Das A, Dhiman RK, Saraswat VA, Verma M, Nail SR. Prevalence and natural history of subclinical hepatic encephalopathy in cirrhosis. Journal of Gastroenterology and Hepatology 2001;16(5):531-5. [DOI: 10.1046/j.1440-1746.2001.02487.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Deeks 2022
- Deeks J, Higgins J, Altman D. Analysing data and undertaking meta-analyses. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. 6.3 edition. Cochrane, 2022. [Google Scholar]
Dhiman 2020
- Dhiman RK, Thumburu KK, Verma N, Chopra M, Rathi S, Dutta U, et al. Comparative efficacy of treatment option for minimal hepatic encephalopathy: a systematic review and network meta-analysis. Clinical Gastroenterology and Hepatology 2020;18(4):800-12. [DOI: 10.1016/j.cgh.2019.08.047] [DOI] [PubMed] [Google Scholar]
Di Pascoli 2017
- Di Pascoli M, Ceranto E, De Nardi P, Donato D, Gatta A, Angeli P, et al. Hospitalizations due to cirrhosis: clinical aspects in a large cohort of Italian patients and cost analysis report. Digestive Diseases 2017;35(5):433-8. [DOI: 10.1159/000458722] [PMID: ] [DOI] [PubMed] [Google Scholar]
DuPont 2016
- DuPont HL, Hassanein TI, Sanyal AJ, Wolf RA, Millen KD. Genomic characterization of stool microbiota with rifaximin monotherapy versus lactulose combination therapy for prevention of overt hepatic encephalopathy (HE) recurrence. Hepatology 2016;64(Suppl 1):720-1A. [DOI: 10.1002/hep.28799] [DOI] [Google Scholar]
Dwan 2008
- Dwan K, Altman DG, Arnaiz JA, Bloom J, Chan AW, Cronin E, et al. Systematic review of the empirical evidence of study publication bias and outcome reporting bias. PLOS ONE 2008;3:e3081. [CMR-12056] [DOI] [PMC free article] [PubMed] [Google Scholar]
EASL and AASLD guideline 2014
- Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014;60:715-35. [DOI: 10.1002/hep.27210] [PMID: ] [DOI] [PubMed] [Google Scholar]
EASL Clinical Practice Guidelines 2022
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of hepatic encephalopathy. Journal of Hepatology 2022;77(3):807-824. [DOI: 10.1016/j.jhep.2022.06.001] [PMID: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. British Medical Journal (Clinical Research Ed.) 1997;315(7121):1533-7. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elsaid 2020
- Elsaid M, John T, Li Y, Pentakota SR, Rustgi VK. The health care burden of hepatic encephalopathy. Clinics in Liver Disease 2020;24(2):263-75. [DOI: 10.1016/j.cld.2020.01.006] [DOI] [PubMed] [Google Scholar]
Eltawil 2012
- Eltawil KM, Laryea M, Peltekian K, Molinari M. Rifaximin vs. conventional oral therapy for hepatic encephalopathy: a meta-analysis. World Journal of Gastroenterology 2012;18:767-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fabrellas 2020
- Fabrellas N, Moreira R, Carol M, Cervera M, Prada G, Perez M, et al. Psychological burden of hepatic encephalopathy on patients and caregivers. Clinical and Translational Gastroenterology 2020;11(4):e00159. [DOI: 10.14309/ctg.0000000000000159] [PMCID: PMC7263656] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Facciorusso 2019
- Facciorusso A, Papagiouvanni I, Cela M, Buccino VR, Sacco R. Comparative efficacy of long-term antibiotic treatments in the primary prophylaxis of spontaneous bacterial peritonitis. Liver International 2019;39(8):1448-58. [DOI: 10.1111/liv.14109] [PMID: ] [DOI] [PubMed] [Google Scholar]
Faust 2020
- Faust N, Yamada A, Haider H, Komaki Y, Komaki F, Micic D, et al. Systemic review and network meta-analysis: Prophylactic antibiotic therapy for spontaneous bacterial peritonitis. World Journal of Hepatology 2020;12(5):239-52. [DOI: 10.4254/wjh.v12.i5.239] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferenci 2002
- Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002;35:716-21. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fidel 2019
- Fidel KP, Payawal DA. The efficacy of rifaximin plus lactulose versus lactulose alone in the treatment of hepatic encephalopathy among patients with decompensated chronic liver disease: a meta-analysis. Hepatology International 2019;13(Suppl 1):S238. [DOI: 10.1007/s12072-019-09936-5] [DOI] [Google Scholar]
Flamm 2018
- Flamm SL, Mullen KD, Heimanson Z, Sanyal AJ. Rifaximin has the potential to prevent complications of cirrhosis. Therapeutic Advances in Gastroenterology 2018;11:1756284818800307. [CLINICALTRIALS.GOV IDENTIFIER: NCT00298038] [DOI: 10.1177/1756284818800307] [PMCID: PMC6166307] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fornio 2017
- Fonio P, Discalzi A, Calandri M, Doriguzzi Breatta A, Bergamasco L, Martini S, et al. Incidence of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt (TIPS) according to its severity and temporal grading classification. La Radiologia medica 2017;122(9):713-21. [DOI: 10.1007/s11547-017-0770-6] [DOI] [PubMed] [Google Scholar]
Fu 2022
- Fu J, Gao Y, Shi L. Combination therapy with rifaximin and lactulose in hepatic encephalopathy: A systematic review and meta-analysis.. PLoS One 2022;17(4):e0267647. [DOI: 10.1371/journal.pone.0267647] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gairing 2022
- Gairing SJ, Müller L, Kloeckner R, Galle PR, Christian Labenz. Review article: post-TIPSS hepatic encephalopathy-current knowledge and future perspectives. Alimentary Pharmacology & Therapeutics 2022;55(10):1265-76. [DOI: 10.1111/apt.16825] [DOI] [PubMed] [Google Scholar]
Gluud 2016
- Gluud LL, Vilstrup H, Morgan MY. Non-absorbable disaccharides versus placebo/no intervention and lactulose versus lactitol for the prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database of Systematic Reviews 2016, Issue 5. Art. No: CD003044. [DOI: 10.1002/14651858.CD003044.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gluud 2017
- Gluud LL, Dam G, Les I, Marchesini G, Borre M, Aagaard NK, Vilstrup H. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database of Systematic Reviews 2017, Issue 5(5):CD001939. Art. No: CD001939. [DOI: 10.1002/14651858.CD001939.pub4] [PMCID: 6481897] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goel 2017
- Goel A, Rahim U, Nguyen LH, Stave C, Nguyen MH. Systematic review with meta-analysis: rifaximin for the prophylaxis of spontaneous bacterial peritonitis. Alimentary Pharmacology & Therapeutics 2017;46(11-12):1029-36. [DOI: 10.1111/apt.14361] [PMID: ] [DOI] [PubMed] [Google Scholar]
Goh 2018
- Goh ET, Stokes CS, Sidhu SS, Vilstrup H, Gluud LL, Morgan MY. L-ornithine L-aspartate for prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No: CD012410. [DOI: 10.1002/14651858.CD012410.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- GRADEpro. Brozek J, Oxman A, Schünemann H, Version 3.2 for Windows. Grade Working Group, 2008.
Groeneweg 1998
- Groeneweg M, Quero JC, De Bruijn I, Hartmann IJ, Essink-bot ML, Hop WC, et al. Subclinical hepatic encephalopathy impairs daily functioning. Hepatology 1998;28:45-9. [DOI: 10.1016/s0168-8278(00)80243-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Grønkjær 2018
- Grønkjær LL, Sehstedt TH, Norlyk A, Vilstrup H. Overt hepatic encephalopathy experienced by individuals with cirrhosis: a qualitative interview study. Gastroenterology Nursing 2018;41(6):468-76. [DOI: 10.1097/SGA.0000000000000286] [PMCID: PMC6259814] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guérit 2009
- Guérit JM, Amantini A, Fischer C, Kaplan PW, Mecarelli O, Schnitzler A, et al. Neurophysiological investigations of hepatic encephalopathy: ISHEN practice guidelines. Liver International 2009;29:789-96. [PMID: ] [DOI] [PubMed] [Google Scholar]
Han 2021
- Han X, Luo Z, Wang W, Zheng P, Li T, Mei Z, et al. Efficacy and safety of rifaximin versus placebo or other active drugs in critical ill patients with hepatic encephalopathy. Frontiers in Pharmacology 2021;12:1-12. [DOI: 10.3389/fphar.2021.696065] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, White IR, Wood AM. Imputation methods for missing outcome data in meta-analysis of clinical trials. Clinical Trials 2008;5:225-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2021a
- Higgins JP, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Higgins 2021b
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Hirode 2019
- Hirode G, Vittinghoff E, Wong RJ. Increasing burden of hepatic encephalopathy among hospitalized adults: an analysis of the 2010-2014 National Inpatient Sample. Digestive Diseases and Sciences 2019;64(6):1448-57. [DOI: 10.1007/s10620-019-05576-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Huang 2007
- Huang E, Esrailian E, Spiegel BMR. The cost-effectiveness and budget impact of competing therapies in hepatic encephalopathy - a decision analysis. Alimentary Pharmacology & Therapeutics 2007;26:1147-61. [DOI: 10.1111/j.1364-2036.2007.03464.x] [DOI] [PubMed] [Google Scholar]
Hudson 2019
- Hudson M, Schuchmann M. Long-term management of hepatic encephalopathy with lactulose and/or rifaximin: a review of the evidence. European Journal of Gastroenterology & Hepatology 2019;31(4):434-50. [DOI: 10.1097/MEG.0000000000001311] [DOI] [PMC free article] [PubMed] [Google Scholar]
ICH GCP 2016
- International Council for Harmonisation of technical requirements for pharmaceuticals for human use (ICH). ICH Harmonised Guideline. Integrated addendum to ICH E6(R1): guideline for good clinical practice E6(R2). database.ich.org/sites/default/files/E6_R2_Addendum.pdf (accessed 26 April 2022).
Iebba 2018
- Iebba V, Guerrieri F, Di Gregorio V, Levrero M, Gagliardi A, Santangelo F, et al. Combining amplicon sequencing and metabolomics in cirrhotic patients highlights distinctive microbiota features involved in bacterial translocation, systemic inflammation and hepatic encephalopathy. Scientific Reports 2018;8(1):8210. [DOI: 10.1038/s41598-018-26509-y] [PMCID: PMC5974022] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jakobsen 2014
- Jakobsen JC, Weterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Medical Research Methodology 2014;14:120. [DOI: 10.1186/1471-2288-14-120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jalan 2022
- Jalan R, Rose CF. Heretical thoughts into hepatic encephalopathy. Journal of Hepatology 2022;77(2):539-548. [DOI: 10.1016/j.jhep.2022.03.014] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jepsen 2010
- Jepsen P, Ott P, Andersen PK, Sørensen HT, Vilstrup H. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology 2010;51:1675-82. [DOI] [PubMed] [Google Scholar]
Jeyaraj 2017
- Jeyaraj R, Morgan MY, Gluud LL. Aminoglycosides and metronidazole for people with cirrhosis and hepatic encephalopathy. Cochrane Database of Systematic Reviews 2017, Issue 7. Art. No: CD012734. [DOI: 10.1002/14651858.CD012734] [DOI] [Google Scholar]
Jiang 2008
- Jiang Q, Jiang XH, Zheng MH, Jiang LM, Chen YP, Wang L. Rifaximin versus nonabsorbable disaccharides in the management of hepatic encephalopathy: a meta-analysis. European Journal of Gastroenterology and Hepatology 2008;20:1064-70. [DOI] [PubMed] [Google Scholar]
Johanson 2007
- Johanson JF. Review of the treatment options for chronic constipation. Medscape General Medicine 2007;9:25. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Johnston 2021
- Johnston BC, Patrick DL, Devji T, Maxwell LJ, Bingham III CO, Beaton D, et al. Chapter 18: Patient-reported outcomes. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Kabeshova 2016
- Kabeshova A, Ben Hariz S, Tsakeu E, Benamouzig R, Launois R. Cost-effectiveness analysis of rifaximin-α administration for the reduction of episodes of overt hepatic encephalopathy in recurrence compared with standard treatment in France. Therapeutic Advances in Gastroenterology 2016;9(4):473-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kamal 2017
- Kamal F, Khan MA, Khan Z, Cholankeril G, Hammad T, Lee WM, et al. Rifaximin for the prevention of spontaneous bacterial peritonitis and hepatorenal syndrome in cirrhosis: a systematic review and meta-analysis. European Journal of Gastroenterology & Hepatology 2017;29(10):1109-17. [DOI: 10.1097/MEG.0000000000000940] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kimer 2014
- Kimer N, Krag A, Moller S, Bendtsen F, Gluud LL. Systematic review with meta-analysis: the effects of rifaximin in hepatic encephalopathy. Alimentary Pharmacology & Therapeutics 2014;40(2):123-32. [DOI: 10.1111/apt.12803] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kircheis 2002
- Kircheis G, Wettstein M, Timmermann L, Schnitzler A, Haussinger D. Critical flicker frequency for quantification of low-grade hepatic encephalopathy. Hepatology 2002;35:357-66. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kircheis 2009
- Kircheis G, Knoche A, Hilger N, Manhart F, Schnitzler A, Schulze H, et al. Hepatic encephalopathy and fitness to drive. Gastroenterology 2009;137:1706-15. [PMID: ] [DOI] [PubMed] [Google Scholar]
Komolafe 2020
- Komolafe O, Roberts D, Freeman S, Wilson P, Sutton A, Cooper N, et al. Antibiotic prophylaxis to prevent spontaneous bacterial peritonitis in people with liver cirrhosis: a network meta-analysis. Cochrane Database of Systematic Reviews 2020, Issue 1. Art. No: CD013125. [DOI: 10.1002/14651858.CD013125.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lauridsen 2011
- Lauridsen MM, Jepsen P, Vilstrup H. Critical flicker frequency and continuous reaction times for the diagnosis of minimal hepatic encephalopathy: a comparative study of 154 patients with liver disease. Metabolic Brain Disease 2011;26:135-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lawton 1939
- Lawton G. The measurement of adult intelligence. Baltimore: the Williams and Wilkins Company, 1939. [Google Scholar]
Leevy 2007
- Leevy CB, Phillips JA. Hospitalizations during the use of rifaximin versus lactulose for the treatment of hepatic encephalopathy. Digestive Diseases and Sciences 2007 ;52(3):737-41. [DOI: 10.1007/s10620-006-9442-4] [DOI] [PubMed] [Google Scholar]
Levitt 2019
- Levitt MD, Levitt DG. Use of quantiative modelling to elucidate the roles of the liver, gut, kidney, and muscle in ammonia homeostasis and how lactulose and rifaximin alter this homeostasis. International Journal of General Medicine 2019;12:367-80. [DOI: 10.2147/IJGM.S218405] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mendoza 2020
- Mendoza YP, Rodrigues SG, Bosch J, Berzigotti A. Effect of poorly absorbable antibiotics on hepatic venous pressure gradient in cirrhosis: A systematic review and meta-analysis. Digestive and Liver Disease 2020;52(9):958-65. [DOI: 10.1016/j.dld.2020.06.048] [PMID: ] [DOI] [PubMed] [Google Scholar]
Menshawy 2019
- Menshawy A, Mattar O, Barssoum K, AboEl-Naga AM, Salim HM, Mohamed AM, et al. Safety and efficacy of rifaximin in prophylaxis of spontaneous bacterial peritonitis: A systematic review and meta-analysis. Current Drug Targets 2019;20(4):380-7. [DOI: 10.2174/1389450119666180924145156] [DOI] [PubMed] [Google Scholar]
Miller 2014
- Miller LE, Tennila J, Ouwehand AC. Efficacy and tolerance of lactitol supplementation for adult constipation: a systematic review and meta-analysis. Clinical and Experimental Gastroenterology 2014;7:241-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Montagnese 2004
- Montagnese S, Amodio P, Morgan MY. Methods for diagnosing hepatic encephalopathy in patients with cirrhosis: a multidimensional approach. Metabolic Brain Disease 2004;19:281-312. [DOI: 10.1023/b:mebr.0000043977.11113.2a] [PMID: ] [DOI] [PubMed] [Google Scholar]
Montagnese 2014
- Montagnese S, Balistreri E, Schiff S, De Rui M, Angeli P, Zanus G, et al. Covert hepatic encephalopathy: agreement and predictive validity of different indices. World Journal of Gastroenterology 2014;20(42):15756-62. [DOI: 10.3748/wjg.v20.i42.15756] [PMCID: 4229541] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Montagnese 2019
- Montagnese S, Bajaj JS. Impact of Hepatic Encephalopathy in Cirrhosis on Quality-of-Life Issues. Drugs 2019;79(Suppl 1):11-6. [DOI: 10.1007/s40265-018-1019-y] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moon 2023
- Moon AM, Kim HP, Jiang Y, Lupu G, Bissram JS, Barritt AS, Tapper EB. Systematic review and meta-analysis on the effects of lactulose and rifaximin on patient-reported outcomes in hepatic encephalopathy. American Journal of Gastroenterology 2023;118(2):284-293. [DOI: 10.14309/ajg.0000000000002008] [PMCID: PMC9904367] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morgan 2016
- Morgan MY, Amodio P, Cook NA, Jackson CD, Kircheis G, Lauridsen MM, et al. Qualifying and quantifying minimal hepatic encephalopathy. Metabolic Brain Disease 2016;31(6):1217-29. [DOI: 10.1007/s11011-015-9726-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Morgan 2018
- Morgan MY. Hepatic Encephalopathy in Patients with Cirrhosis . In: Dooley JS, Lok ASF, Garcia-Tsao G, Pinzani M, editors(s). Sherlock's Diseases of the Liver and Biliary System. 13 edition. Chichester, West Sussex: John Wiley & Sons Ltd, 2018:Chapter 10. [DOI: ] [Google Scholar]
Neff 2010
- Neff G. Pharmacoeconomics of hepatic encephalopathy. Pharmacotherapy 2010;30:28S-32S. [PMID: ] [DOI] [PubMed] [Google Scholar]
Neff 2013
- Neff G, Kemmer N, Duncan C, Alsina A. Update on the management of cirrhosis - focus on cost-effective preventative strategies. ClinicoEconomics and Outcomes Research 2013;5:143-52. [DOI: 10.2147/CEOR.S30675] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neff 2018
- Neff G, Zachry W. Systematic review of the economic burden of overt hepatic encephalopathy and pharmacoeconomic impact of rifaximin. Pharmacoeconomics 2018;36(7):809-22. [DOI: 10.1007/s40273-018-0641-6] [PMCID: PMC5999147] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nolte 1998
- Nolte W, Wiltfang J, Schindler C, Münke H, Unterberg K, Zumhasch U, et al. Portosystemic hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in patients with cirrhosis: clinical, laboratory, psychometric, and electroencephalographic investigations. Hepatology 1998;28(5):1215-25. [DOI: 10.1002/hep.510280508] [PMID: ] [DOI] [PubMed] [Google Scholar]
O'Carroll 1991
- O'Carroll RE, Hayes PC, Ebmeier KP, Dougall N, Murray C, Best JJ, et al. Regional cerebral blood flow and cognitive function in patients with chronic liver disease. Lancet 1991;337:1250-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Orr 2014
- Orr JG, Homer T, Ternent L, Newton J, McNeil CJ, Hudson M, et al. Health related quality of life in people with advanced chronic liver disease. Journal of Hepatology 2014;61(5):1158-65. [DOI: 10.1016/j.jhep.2014.06.034] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ortiz 2007
- Ortiz M, Córdoba J, Doval E, Jacas C, Pujadas F, Esteban R, et al. Development of a clinical hepatic encephalopathystaging scale. Alimentary Pharmacology& Therapeutics 2007;26(6):859-67. [DOI: ] [DOI] [PubMed] [Google Scholar]
Page 2014
- Page MJ, McKenzie JE, Kirkham J, Dwan K, Kramer S, Green S, et al. Bias due to selective inclusion and reporting of outcomes and analyses in systematic reviews of randomised trials of healthcare interventions. Cochrane Database of Systematic Reviews 2014, Issue 10. Art. No: MR000035. [DOI: 10.1002/14651858.MR000035.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2021
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Page 2021a
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Research Ed.) 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2021b
- Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ (Clinical Research Ed.) 2021;372:n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pantham 2017
- Pantham G, Post A, Venkat D, Einstadter D, Mullen KD. A new look at precipitants of overt hepatic encephalopathy in cirrhosis. Digestive Diseases and Sciences 2017;62(8):2166-73. [DOI: 10.1007/s10620-017-4630-y] [PMID: ] [DOI] [PubMed] [Google Scholar]
Parsons‐Smith 1957
- Parsons-Smith BG, Summerskill WH, Dawson AM, Sherlock S. The electroencephalograph in liver disease. Lancet 1957;273:867-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
Patidar 2015
- Patidar KR, Bajaj JS. Covert and overt hepatic encephalopathy: diagnosis and management. Clinical Gastroenterology and Hepatology 2015;13(12):2048-61. [DOI: 10.1016/j.cgh.2015.06.039] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peryer 2021
- Peryer G, Golder S, Junqueira D, Vohra S, Loke YK. Chapter 19: Adverse effects. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Pimentel 2021
- Pimentel R, Gregório C, Figueiredo P. Antibiotic prophylaxis for prevention of spontaneous bacterial peritonitis in liver cirrhosis: systematic review. Acta Gastro-enterologica Belgica 2021;84(2):333-42. [DOI: 10.51821/84.2.333] [DOI] [PubMed] [Google Scholar]
Poordad 2007
- Poordad FF. Review article: the burden of hepatic encephalopathy. Alimentary Pharmacology & Therapeutics 2007;25:3-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Qin 2014
- Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014;513(7516):59-64. [DOI: 10.1038/nature13568] [PMID: ] [DOI] [PubMed] [Google Scholar]
Razzack 2021
- Razzack AA, Hussain N, Hassan SA, Gulzar R, Karam A, Panday P, et al. Rifaximin for prophylaxis of post-TIPPS hepatic encephalopathy-a meta analysis. Journal of Hepatology 2021;75(Suppl 2):S383. [DOI: 10.1016/S0168-8278(21)01843-2] [DOI] [Google Scholar]
Reitan 1955
- Reitan RM. The relation of the trail making test to organic brain damage. Journal of Consulting Psychology 1955;19(5):393-4. [DOI: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager (RevMan). Version 5.4. The Cochrane Collaboration, 2014.
RevMan Web 2020 [Computer program]
- Review Manager Web (RevMan Web). Version 1.22.0. Cochrane Collaboration, 2020.
Riggio 1990
- Riggio O, Varriale M, Testore GP, Di Rosa R, Di Rosa E, Merli M, et al. Effect of lactitol and lactulose administration on the fecal flora in cirrhotic patients. Journal of Clinical Gastroenterology 1990;12:433-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Riggio 2008
- Riggio O, Angeloni S, Salvatori FM, De Santis A, Cerini F, Farcomeni A, et al. Incidence, natural history, and risk factors of hepatic encephalopathy aftertransjugular intrahepatic portosystemic shunt with polytetrafluoroethylenecoveredstent grafts. American Journal of Gastroenterology 2008;103(11):2738-46. [DOI: 10.1111/j.1572-0241.2008.02102.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Roman 2011
- Roman E, Córdoba J, Torrens M, Torras X, Villanueva C, Vargas V, et al. Minimal hepatic encephalopathy is associated with falls. American Journal of Gastroenterology 2011;106:476-82. [DOI] [PubMed] [Google Scholar]
Romero‐Gómez 2001
- Romero- Gómez M, Boza F, Garcia-Valdecasas MS, García E, Aguilar-Reina J. Subclinicalhepatic encephalopathy predicts the development of overt hepatic encephalopathy. American Journal of Gastroenterology 2001;96(9):2718-23. [DOI: 10.1111/j.1572-0241.2001.04130.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rose 2020
- Rose CF, Amodio P, Bajaj JS, Dhiman RK, Montagnese S, Taylor-Robinson SD, et al. Hepatic encephalopathy: Novel insights into classification, pathophysiology and therapy. Journal of Hepatology 2020;73(6):1526-47. [DOI: 10.1016/j.jhep.2020.07.013] [PMID: ] [DOI] [PubMed] [Google Scholar]
Saunders 1981
- Saunders JB, Walters JR, Davies AP, Paton AA. 20-year prospective study of cirrhosis. British Medical Journal 1981;282(6260):263-6. [DOI: 10.1136/bmj.282.6260.263] [PMCID: PMC1504019] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Savovic 2012
- Savovic J, Jones H, Altman D, Harris RJ, Juni P, Pildal J et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta-epidemiological studies. Health Technology Assessment 2012;16(35):1-82. [DOI] [PubMed] [Google Scholar]
Savovic 2018
- Savovic J, Turner RM, Mawdsley D, Jones HE, Beynon R, Higgins JPT, et al. Association between risk-of-bias assessments and results of randomized trials in Cochrane Reviews: The ROBES meta-epidemiologic study. American Journal of Epidemiology 2018;187(5):1113-22. [DOI: 10.1093/aje/kwx344] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scarpignato 2005
- Scarpignato C, Pelosini I. Rifaximin, a poorly absorbed antibiotic: pharmacology and clinical potential. Chemotherapy 2005;51(Suppl 1):36-66. [DOI: 10.1159/000081990.] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schomerus 1998
- Schomerus H, Hamster W. Neuropsychological aspects of portal-systemic encephalopathy. Metabolic Brain Disease 1998;13:361-77. [DOI: 10.1023/a:1011610427843] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schultz 2019
- Schulz C, Schütte K, Vilchez-Vargas R, Vasapolli R, Malfertheiner P. Long-term effect of rifaximin with and without lactulose on the active bacterial assemblages in the proximal small bowel and faeces in patients with minimal hepatic encephalopathy.. Digestive Diseases 2019;37(2):161-9. [DOI: 10.1159/000494216] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Shaheen 2019
- Shaheen A-A, Nguyen HH, Congly SE, Kaplan GG, Swain MG. Nationwide estimates and risk factors of hospital readmission in patients with cirrhosis in the United States. Liver International 2019;39(5):878-84. [DOI: 10.1111/liv.14054] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sharma 2007
- Sharma P, Sharma BC, Puri V, Sarin SK. Critical flicker frequency: diagnostic tool for minimal hepatic encephalopathy. Journal of Hepatology 2007;47(1):67-73. [DOI: 10.1016/j.jhep.2007.02.022] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sharma 2009
- Sharma BC, Sharma P, Agrawal A, Sarin SK. Secondary prophylaxis of hepatic encephalopathy: an open-label randomized controlled trial of lactulose versus placebo. Gastroenterology 2009;137(3):885-91. [DOI: 10.1053/j.gastro.2009.05.056] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sharma 2010
- Sharma P, Sharma BC, Sarin SK. Prevalence of abnormal psychometric tests and critical flicker frequency after clinical recovery of overt hepatic encephalopathy. Neurology 2010;58:220-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Shrestha 2020
- Shrestha D, Rathi S, Grover S, Taneja S, Duseja A, Chawla YK, et al. Factors affecting psychological burden on the informal caregiver of patients with cirrhosis: looking beyond the patient. Journal of Clinical and Experimental Hepatology 2020;10(1):9-16. [DOI: 10.1016/j.jceh.2019.06.002] [PMCID: PMC6995890] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shukla 2011
- Shukla S, Mehboob S, Guha S. Comparison of efficacy of rifaximin and non-absorbable disaccharides in management of hepatic encephalopathy: a meta-analysis. Gastroenterology 2011;140(5 Suppl 1):S955. [DOI: 10.1016/S0016-5085(11)63955-3] [DOI] [Google Scholar]
Siddiqui 2021
- Siddiqui K, Attria S, Olando M, Lelli F, Maida V, Thabut D. Cost-effectiveness of rifaximin/alpha versus lactulose in preventing recurrent episodes of overt hepatic encephalopathy: a systematic review and meta-analysis. Journal of Hepatology 2021;75(Suppl 2):S356-7. [Google Scholar]
Soni 2020
- Soni H, Kumar-M P, Sharma V, Bellam BL, Mishra S, Mahendru D, et al. Antibiotics for prophylaxis of spontaneous bacterial peritonitis: systematic review & Bayesian network meta-analysis. Hepatology International 2020;14(3):399-413. [DOI: 10.1007/s12072-020-10025-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stepanova 2012
- Stepanova M, Mishra A, Venkatesan C, Younossi ZM. In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clinical Gastroenterology and Hepatology 2012;10(9):1034-41. [DOI: 10.1016/j.cgh.2012.05.016] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stewart 2007
- Stewart CA, Malinchoc M, Kim WR, Kamath PS. Hepatic encephalopathy as a predictor of survival inpatients with end-stage liver disease. Liver Transplantation 2007;13(10):1366–71. [DOI: 10.1002/lt.21129] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tapper 2016
- Tapper EB, Finkelstein D, Mittleman MA, Piatkowski G, Chang M, Lai M. A quality improvement initiative reduces 30-day rate of readmission for patients with cirrhosis. Clinical Gastroenterology and Hepatology 2016;14(5):753-9. [DOI: 10.1016/j.cgh.2015.08.041] [PMCID: PMC5394424] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tapper 2019
- Tapper EB, Henderson JB, Parikh ND, Ioannou GN, Lok AS. Incidence of and risk factors for hepatic encephalopathy in a population-based cohort of Americans with cirrhosis. Hepatology Communications 2019;3(11):1510-9. [DOI: 10.1002/hep4.1425] [PMCID: PMC6824059] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tapper 2020
- Tapper EB, Aberasturi D, Zhao Z, Hsu CY, Parikh ND. Outcomes after hepatic encephalopathy in population-based cohorts of patients with cirrhosis. Alimetary Pharmacology & Therapeutics 2020;51(12):1397-405. [DOI: 10.1111/apt.15749] [DOI] [PMC free article] [PubMed] [Google Scholar]
Teasdale 1974
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;2:81-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Trebicka 2021
- Trebicka J, Macnaughtan J, Schnabl B, Shawcross DL, Bajaj JS. The microbiota in cirrhosis and its role in hepatic decompensation. Journal of Hepatology 2021;75(Suppl 1):S67-S81. [DOI: 10.1016/j.jhep.2020.11.013] [PMCID: PMC8973011] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Leeuwen 1988
- Leeuwen PA, Berlo CL, Soeters PB. New mode of action for lactulose. Lancet 1988;1(8575-6):55-6. [DOI: 10.1016/s0140-6736(88)91033-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Victor 1965
- Victor M, Adams RD, Cole M. The acquired (non-Wilsonian) type of chronic hepatocerebral degeneration. Medicine 1965;44:345-96. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wang 2019a
- Wang Z, Chu P, Wang W. Combination of rifaximin and lactulose improves clinical efficacy and mortality in patients with hepatic encephalopathy. Drug Design, Development and Therapy 2019;13:1-11. [DOI: 10.2147/DDDT.S172324] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2019b
- Wang W, Yang J, Liu C, Song P, Wang W, Xu H, et al. Norfloxacin, ciprofloxacin, trimethoprim-sulfamethoxazole, and rifaximin for the prevention of spontaneous bacterial peritonitis: a network meta-analysis. Journal of Gastroenterology and Hepatology 2019;31(8):905-10. [DOI: 10.1097/MEG.0000000000001446] [PMID: ] [DOI] [PubMed] [Google Scholar]
Weber 1987
- Weber FL Jr, Banwell JG, Fresard KM, Cummings JH. Nitrogen in fecal bacterial, fibre, and soluble fractions ofpatients with cirrhosis: effects of lactulose and lactuloseplus neomycin. Journal of Laboratory and Clinical Medicine 1987;110(3):259-63. [PMID: ] [PubMed] [Google Scholar]
Weissenborn 1998
- Weissenborn K, Rückert N, Hecker H, Manns MP. The number connection tests A and B: interindividual variability and use for the assessment of early hepatic encephalopathy. Journal of Hepatology 1998;28(4):646-53. [DOI: 10.1016/s0168-8278(98)80289-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Weissenborn 2001
- Weissenborn K, Ennen JC, Schomerus H, Ruckert N, Hecker H. Neuropsychological characterization of hepatic encephalopathy. Journal of Hepatology 2001;34:768-73. [PMID: ] [DOI] [PubMed] [Google Scholar]
Weissenborn 2019
- Weissenborn K. Hepatic encephalopathy: definition, clinical grading and diagnostic principles. Drugs 2019;79(Suppl 1):5-9. [DOI: 10.1007/s40265-018-1018-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2014
- Wong RJ, Gish RG, Ahmed A. Hepatic encephalopathyis associated with significantly increased mortalityamong patients awaiting liver transplantation. Liver Transplantation 2014;20(12):1454–61. [DOI: 10.1002/lt.23981] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wu 2013
- Wu D, Wu SM, Lu J, Zhou YQ, Xu L, Guo CY. Rifaximin versus non-absorbable disaccharides for the treatment of hepatic encephalopathy: a meta-analysis. Gastroenterology Research and Practice 2013;2013:1-9. [DOI: 10.1155/2013/236963] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zacharias 2017
- Zacharias HD, Jackson CD, Morgan MY, Olesen SS. Letter: stepwise diagnosis in covert hepatic encephalopathy - critical flicker frequency and MELD-score as a first-step approach. Replication and pitfalls. Alimentary Pharmacology and Therapeutics 21017;45(1):187-9. [DOI: 10.1111/apt.13830] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zacharias 2019
- Zacharias HD, Zacharias AP, Gluud LL, Morgan MY. Pharmacotherapies that specifically target ammonia for the prevention and treatment of hepatic encephalopathy in adults with cirrhosis. Cochrane Database of Systematic Reviews 2019, Issue 6(6) CD012334. Art. No: CD012334. [DOI: 10.1002/14651858.CD012334.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhu 2015
- Zhu GQ, Shi KQ, Huang S, Wang LR, Lin YQ, Huang GQ, et al. Systematic review with network meta-analysis: the comparative effectiveness and safety of interventions in patients with overt hepatic encephalopathy. Alimentary Pharmacology and Therapeutics 2015;41(7):624-35. [DOI: 10.1111/apt.13122] [DOI] [PubMed] [Google Scholar]
Zhu 2016
- Zhu ZY, Cui Di, Gai H, Dong FY, Liu XC, Liu F, et al. Efficient synthesis and activity of beneficial intestinal flora of two lactulose-derived oligosaccharides. European Journal of Medicinal Chemisttry 2016;114:8-13. [DOI: 10.1016/j.ejmech.2016.03.007] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhu 2019
- Zuo L, Iv Y, Wang Q, Yin Z, Wang Z, He C, et al. Early-recurrent overt hepatic encephalopathy is associated with reduced survival in cirrhotic patients after transjugular intrahepatic portosystemic shunt creation. Journal of Vascular and Interventional Radiology 2019;30(2):148-52.e2. [DOI: 10.1016/j.jvir.2018.08.023] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhuo 2019
- Zhuo GY, Xiang T, Zhang PY, Zhang XD, Luo L, Zhang JM, et al. Effects of rifaximin versus nonabsorbable disaccharides in hepatic encephalopathy. GastroHep 2019;1(1):22-32. [DOI: 10.1002/ygh2.207] [DOI] [Google Scholar]
References to other published versions of this review
Kimer 2015
- Kimer N, Krag A, Bendtsen F, Møller S, Gluud LL. Rifaximin for people with hepatic encephalopathy. Cochrane Database of Systematic Reviews 2015, Issue 3. Art. No: CD011585. [DOI: 10.1002/14651858.CD011585] [DOI] [Google Scholar]


