Abstract
Studies have shown that as many as 1 in 10 adults with chronic kidney disease has a monogenic form of disease. However, genetic services in adult nephrology are limited. An adult Kidney Genetics Clinic was established within the nephrology division at a large urban academic medical center to increase access to genetic services and testing in adults with kidney disease. Between June 2019 and December 2021, a total of 363 patients were referred to the adult Kidney Genetics Clinic. Of those who completed genetic testing, a positive diagnostic finding was identified in 27.1%, a candidate diagnostic finding was identified in 6.7% of patients, and a nondiagnostic positive finding was identified in an additional 8.6% of patients, resulting in an overall yield of 42.4% for clinically relevant genetic findings in tested patients. A genetic diagnosis had implications for medical management, family member testing, and eligibility for clinical trials. With the utilization of telemedicine, genetic services reached a diverse geographic and patient population. Genetic education efforts were integral to the clinic’s success, as they increased visibility and helped providers identify appropriate referrals. Ongoing access to genomic services will remain a fundamental component of patient care in adults with kidney disease.
Keywords: genetics clinic, genetic testing, kidney genetics, nephrology, renal genetics
1 |. INTRODUCTION
Chronic kidney disease (CKD) is a debilitating disorder associated with significant morbidity and mortality and affects approximately 15% of adults in the United States (CDC, 2021). A diagnosis of CKD is often based on overlapping, nonspecific clinical features, and histology findings, and in adults, the underlying etiology remains unknown in many cases. While the underlying genetic contribution to pediatric kidney disease is well established, the role of genetics in adult-onset kidney disease has been overlooked until recently.
Recent studies have shown that 1 in 10 adults with CKD have a monogenic form of disease (Devuyst et al., 2014; Groopman et al., 2019; Mallett et al., 2014). This study, along with several others (Connaughton et al., 2019; Vivante & Hildebrandt, 2016), identified monogenic etiologies of kidney disease across the spectrum of clinical diagnoses in kidney disease. Notably, a diagnostic yield of ~20% was reported in those with nephropathy of unknown origin (Groopman et al., 2019; Hays, Groopman, & Gharavi, 2020). Similarly, a high yield of genetic diagnoses has been found in individuals with cystic kidney disease, congenital anomalies of the kidney and urinary tract (CAKUT), and glomerular disorders (Bullich et al., 2018; Groopman et al., 2019; Westland, Renkema, & Knoers, 2020). Genetic causes of other types of kidney disease, such as tubulopathies and complement disorders, are also common, especially when accompanied by other risk factors for a genetic etiology, such as a family history of kidney disease, young age of onset, or extra-renal features (Cocchi, Nestor, & Gharavi, 2020). Thus, genetic testing is an emerging tool to aid in identifying the underlying etiology and diagnosis of CKD.
Establishing a genetic diagnosis in a patient with kidney disease can clarify the diagnosis, identify extra-renal manifestations for which the patient may be at risk, and determine the genetic risk to other family members. In addition, a genetic diagnosis can also aid in the identification of eligible donors for kidney transplantation (Garg, Levey, Kasiske, Cheung, & Lentine, 2020; Mann et al., 2019; Niaudet, 2010), prevent the use of unnecessary immunosuppression (Preston, Stuart, & Lennon, 2019), and increase patient eligibility for a growing number of clinical trials based on genetic disease status (Milo Rasouly & Marasa, 2018). As such, clinical genetic testing is increasingly being recommended as a tool for the diagnosis and management of kidney disease (Knoers et al., 2022).
With these emerging data, the nephrology medical specialty is increasingly utilizing genomic medicine in clinical practice, similar to other specialties, such as oncology (Hampel, Bennett, Buchanan, Pearlman, & Wiesner, 2015) or cardiology (Hershberger et al., 2018; Mital et al., 2016), where genetics clinics have become well integrated into clinical practices. Establishing these clinics has been associated with positive patient outcomes, such as increased knowledge, positive health behaviors, and decreased anxiety and decisional conflict (Madlensky et al., 2017). Newly formed renal genetic clinics have reported their early experiences with genetic testing, citing impressively high diagnostic rates in their clinics ranging from 33% to 60% (Alkanderi, Yates, Johnson, & Sayer, 2017; Amlie-Wolf et al., 2021; Elhassan et al., 2022; Lundquist et al., 2020; Mallett, Fowles, McGaughran, Healy, & Patel, 2016; Pode-Shakked et al., 2022; Thomas et al., 2020). These clinics utilized the expertise of clinical geneticists, genetic counselors, and nephrologists to guide clinical assessment and interpretation of genetic findings. Other reported benefits of their clinics included increasing access to genetic testing and improving diagnosis and management of those found to have a genetic etiology of kidney disease. Previously established renal genetics clinics have implemented a diverse array of workflows for how to see patients, including novel collaborations between genetic counselors and pediatric nephrologists (Amlie-Wolf et al., 2021), collaborations between departments of nephrology and genetics (Mallett et al., 2016), and even seeing entire families together for consults (Alkanderi et al., 2017). The integration of genetic counselors or geneticists in nephrology aims to address nephrologists’ lack of training and low confidence in interpreting genetic results (Berns, 2010; Jayasinghe et al., 2020).
However, the utilization of genetic services remains limited in the adult nephrology population. These recent renal genetics clinics have reported diverse initial experiences, differing in their clinical setting, team members’ roles, and populations served. Many of the previously established renal genetics clinics report a low patient volume or describe only their initial experiences in maintaining the clinic. Additionally, many clinics primarily see pediatric or young adult patient populations. While Lundquist et al. identified criteria they felt were necessary for a successful renal genetics clinic, including affordability and administrative support (Lundquist et al., 2020), few other groups report on the aspects of their experiences that facilitated the establishment of their renal genetics clinic. In addition, the demographics of the patient population served by these new clinics have not been well described in the literature; thus, it remains unknown which patient populations are served by these renal genetics clinics.
Therefore, the experience of establishing and maintaining a high-volume kidney genetics clinic within an adult nephrology division at a large, urban academic medical center is presented here. The variables that contributed to the successful implementation and integration of the Kidney Genetics Clinic and patient demographics are reported and show that this novel kidney genetics clinic serves a diverse patient population and can overcome several barriers to the successful uptake of genetic testing in this patient population.
2 |. METHODS
2.1 |. Laying the foundation
Prior to establishing the Kidney Genetics Clinic, several key research initiatives were implemented within the Division of Nephrology at Columbia University Irving Medical Center (CUIMC), which were central to the success of the clinic. Following a study on 2,187 patients enrolled at CUIMC that reported that 1 in 10 adults with CKD has a monogenic form of kidney disease (Groopman et al., 2019), a pilot study was carried out to return those research genetic results to participants (Nestor et al., 2020). In a continuation of these studies, a monthly genetic variant sign-out meeting and educational series were established. At the sign-out meetings, clinical and genetic information on research cases from the ongoing research studies were discussed among nephrologists, geneticists, genetic counselors, and genetics researchers. The group then agreed upon which variants would be clinically confirmed and returned to the participants. The referring clinical nephrologists were involved throughout this process. The educational series included an interactive, biweekly renal genetics case series to familiarize and engage with the faculty and staff within the Division of Nephrology on renal genetics. Topics from these cases highlighted themes such as: when to suspect a genetic kidney condition, genetic test selection considerations, aspects and interpretation of genetic results, key management implications of the genetic diagnosis, and cascade testing of at-risk family members. Essential genetic topics and vocabulary, such as the types of inheritance patterns, penetrance, and variable expressivity, as well as their clinical implications, were introduced and discussed. Familiarizing nephrologists with the research workflow and educating them on genetic topics commonly encountered in the clinic facilitated the establishment and utilization of the Kidney Genetics Clinic.
2.2 |. Setting and clinic structure
In June 2019, an adult Kidney Genetics Clinic was created within the Division of Nephrology in the Department of Medicine at CUIMC. CUIMC is a large academic and clinical medical institution located in the Washington Heights neighborhood of Manhattan, NY.
Within the Kidney Genetics Clinic, two visit types were established for new patients: Full Genetic Consults (staffed by a genetic counselor [GC] and nephrologist) and Genetic Counseling Visits (staffed by a GC only). Full Genetic Consults involved obtaining a complete medical and family history, performing a personalized genetic risk assessment, and, when applicable, obtaining informed consent and sample coordination for clinical genetic testing. Genetic Counseling Visits typically involved obtaining informed consent and sample collection for a specific genetic test recommended by the patient’s treating nephrologist, obtaining consent for cascade testing for a known familial variant, or counseling on genetic test results. To return genetic results and follow-up on genetic testing previously ordered by the Kidney Genetics Clinic, the clinic offered Return Patient Visits (staffed by a GC only). The full workflow for the Kidney Genetics Clinic, from referral to return of results, can be found in Figure 1.
FIGURE 1.

Current workflow for referrals received in the Kidney Genetics Clinic through return of genetic results at Columbia University Irving Medical Center
The clinic was held weekly with a maximum of two concurrent visits and six visits a day (six 1-hour appointment slots for Full Genetic Consults and six 1-hour Genetic Counseling Visits or Return Patient Visits). Each clinic was staffed by two genetic counselors and one of four rotating nephrologists with specialized training or interest in genetics and precision medicine. Administrative help was also provided for scheduling and insurance support.
Both Full Genetic Consults and Genetic Counseling Visits occurred in-person at the Washington Heights medical campus location prior to March 2020, at which point the clinic briefly halted operations until May 2020 due to the COVID-19 pandemic, then transitioned almost exclusively to telemedicine (virtual video appointments). Thereafter, virtual video appointments became the default method for all new patient genetic consults, although in-person appointments remained available based on patient preference. Return Patient Visits for return of genetic results occurred in-person, by phone, or video prior to the pandemic and transitioned exclusively to telemedicine format in March 2020.
2.3 |. Referrals
Clinical providers were given many options regarding how to refer their patients, including messaging a clinic-specific email, online referral form, phone call, or email to a clinic staff’s personal email. The phone number, email, and referral form were made accessible online (Figure S1). In February 2020, the institution transitioned to EPIC as their Electronic Medical Record (EMR), and direct referrals to the Kidney Genetics Clinic through the EMR became available to providers. Referrals were accepted from both internal CUIMC and external providers, as well as directly from patients. The administrative staff made two contact attempts to schedule each referred patient, and all outcomes were documented. All referrals and accompanying clinical information were stored in a Columbia University REDCap project.
2.4 |. Genetic testing
All diagnostic genetic tests ordered through the Kidney Genetics Clinic were sent to CLIA (Clinical Laboratory Improvement Amendments)-certified laboratories, and variants were classified according to the laboratory protocols. A positive diagnostic finding was considered a pathogenic or likely pathogenic variant(s) in a gene that fully or partially explains the patient’s features. A positive nondiagnostic finding was defined as either a common risk factor (such as a high-risk APOL1 genotype) or a pathogenic or likely pathogenic variant that does not explain the patient’s kidney disease. A candidate diagnostic finding was defined as a variant or variants of uncertain significance identified in a gene that is associated with a condition that has a significant clinical overlap with the patient’s presentation or when there is preliminary evidence that the variant may be related to the condition, but not enough to meet criteria for classification as likely pathogenic or pathogenic (Richards et al., 2015).
When genetic testing was ordered on affected patients, several factors were considered, including the clinical diagnosis, the presence of extrarenal features, family history of disease and consanguinity, patient motivation, cost of testing and insurance coverage, methodology, and diagnostic yield. If needed, several tests were ordered concurrently or sequentially, with diagnostic genetic testing focusing on known gene-disease associations and research testing focusing on novel gene discovery. During the consenting process for all patients, the risks, limitations, and benefits of each test were discussed, including, but not limited to, the types of results that can be expected, privacy concerns, research and sharing of data, and the Genetic Information Nondiscrimination Act (GINA).
When genetic testing was ordered on healthy family members or potential kidney donors, additional factors, such as the presence of a known familial variant, turn-around time, and potential for identifying secondary/nondiagnostic findings were also considered during the test selection process. If an unaffected family member was referred for genetic testing, including those being evaluated as a kidney transplant donor, it was recommended to refer and test the affected family member, or transplant recipient, first. This testing strategy was intended t establish a genetic diagnosis in the family, thus allowing for targeted testing in other family members, and identifying true-negative test results. However, if time was a factor, concurrent genetic testing in both the affected and unaffected family member, or recipient and donor, was recommended to prevent delays in treatment and management.
2.5 |. Data analysis
A retrospective chart review was performed on all patients referred to the Kidney Genetics Clinic from June 2019 to December 2021. Demographic information, clinical features, family history, referral indications, and contact outcomes were collected from referral documents and data. For patients scheduled in the Kidney Genetics Clinic, demographic and referral information, detailed clinical and family history, genetic testing results, management implications, and subsequent referrals were collected from the EMR, testing documents, and directly from the patients. All data collection was documented in the REDCap database and performed in accordance with the Genetic Studies of Constitutional Disorders protocol (Institutional Review Board Protocol Number AAAS7948), approved by the Columbia University Institutional Review Board.
3 |. RESULTS
3.1 |. Referral indications and methodologies
Between June 2019 and December 2021, a total of 363 patients were referred to the Kidney Genetics Clinic from multiple sources, including internal CUIMC providers, external providers, and self-referrals (Figure 2). Referrals from CUIMC providers (internal referrals) represented 73% of referrals and spanned eight different divisions and departments, including Nephrology, Cardiology, Cancer Genetics, Reprogenetics/OBGYN, Ophthalmology, Internal Medicine, and Gastroenterology. Eleven percent of referrals were self-referrals, and 16% of referrals came from over 30 different external institutions (Table S1). 76.3% of referrals were received directly through the Kidney Genetics Clinic email, 6.8% through the EMR, 4.1% through the clinic online referral form, and 13.0% through other mechanisms, such as a phone call or personal correspondence (Table S1). In self-referred patients, phone or email was the preferred referral method, while the online referral form was mostly utilized for referrals from external institutions (Table S1).
FIGURE 2.
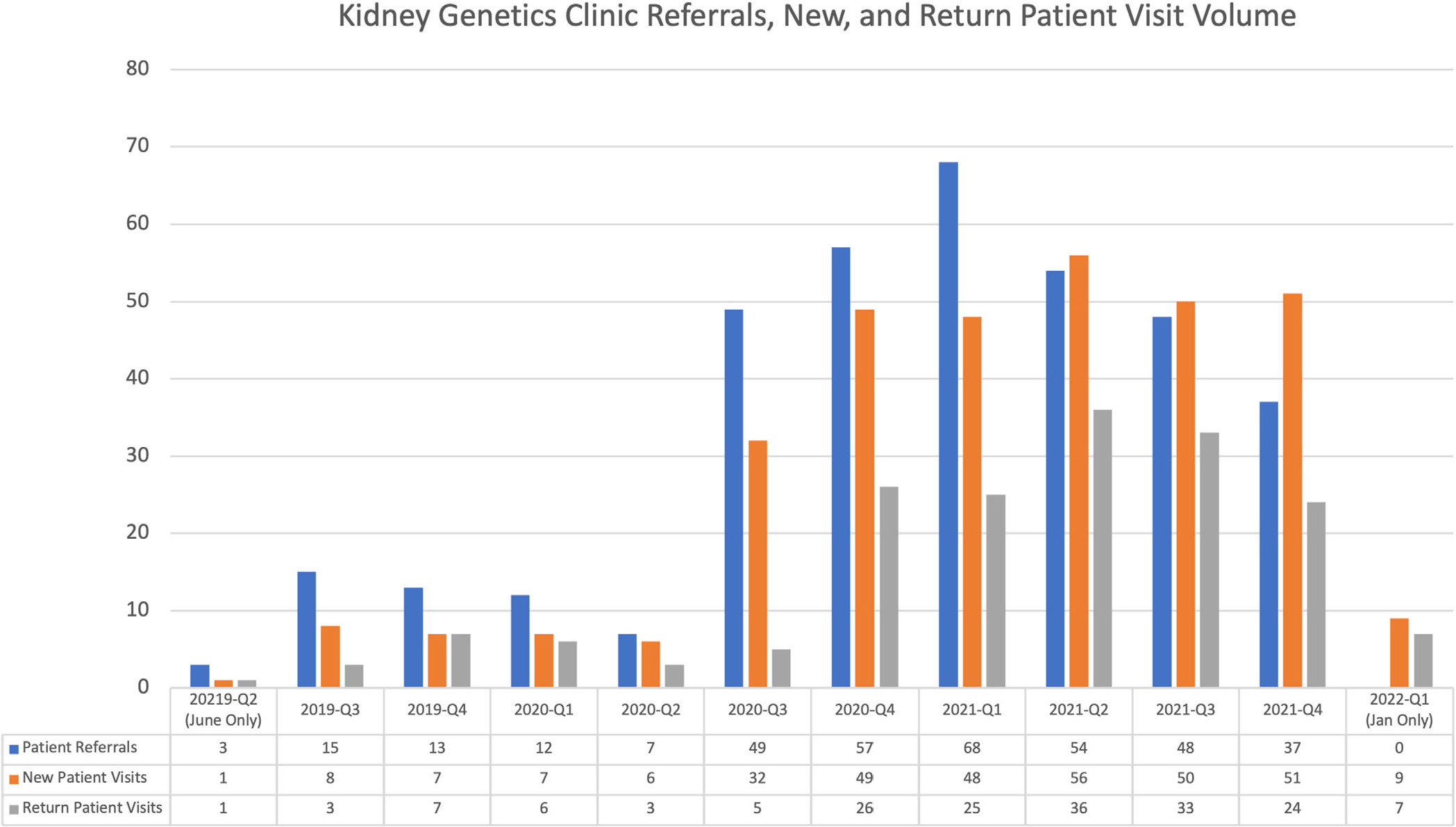
Kidney Genetics Clinic volume of referrals, new patient consults, and return patient visits from June 2019 to January 2022
Of all patients referred, 304 patients (84%) were scheduled for a new patient genetic consult. The average wait time between an initial referral request and a scheduled appointment was 37 days. For patients referred for donor evaluation, the average wait time from referral to appointment was 5 days. There was no significant difference in scheduling rates between internal, external, or self-referrals (Table S1). Among the referrals where a new patient genetic consult was not scheduled, no contact with the patient (n = 33) was the most cited barrier, followed by patient declined or lack of interest (n = 15). Access limitations (n = 7), including lack of professional state licensure, technology issues, and cost, were rare but reported barriers to scheduling new patient genetic consults. Four patients were referred to the Kidney Genetics Clinic, but their referral was determined to be not clinically relevant for the Kidney Genetics Clinic, and they were referred to other genetics clinics at CUIMC.
Overall, 324 new patient genetic consults were scheduled in the Kidney Genetics Clinic at CUIMC. This included 20 rescheduled patient visits where the patient did not present for their originally scheduled appointment. Ultimately, 279 patient visits were completed, resulting in an overall no-show rate of 13.9% (Figure 2). In addition to new patient genetic consults, 176 follow-up visits occurred from June 2019 to January 2022. Overall, most scheduled visits were telemedicine appointments via virtual video visits (61%) or phone appointments (33%, Table 1).
TABLE 1.
Scheduled appointments by visit type and modality in the Kidney Genetics Clinic
| Visit type | In-person | Phone | Video | Total visits |
|---|---|---|---|---|
| Complete genetics consult | 28 | 3 | 233 | 264 |
| Genetic counseling visit | 2 | 20 | 38 | 60 |
| Return patient visit | 1 | 143 | 32 | 176 |
| Total | 31 (6.2%) | 166 (33.2%) | 303 (60.6%) | 500 |
Most patients seen in the Kidney Genetics Clinic were referred to clarify a clinical diagnosis (n = 186), but referral indications also included clarification of biopsy findings (n = 20), transplant donor and recipient evaluation (n = 53), and cascade and family member genetic testing (n = 39). Patients had a variety of kidney-related clinical indications and diagnoses, many of which were overlapping. These included: suspected or clinical diagnosis of Alport spectrum disorder (n = 49), hematuria and/or proteinuria (n = 45), focal segmental glomerulosclerosis (FSGS) (n = 37), cystic kidney disease (including PKD) (n = 35), tubulopathy or electrolyte disorder (n = 28), tubulointerstitial disease (n = 26), complement dysregulation (n = 12), CAKUT (n = 8), tumor or cancer (n = 5), and CKD of unknown etiology (n = 29). In addition, 32 individuals were healthy or unaffected, referred for pre-symptomatic testing, segregation analysis, or donor selection.
3.2 |. Patient demographics
The Kidney Genetics Clinic patient population was relatively diverse, with 49% who self-identified as white, 21% Hispanic/Latinx, 15% Black/African American, 7% Asian, 1% Native Hawaiian/Pacific Islander, and 7% other or preferred not to specify (Table 2a–e). Most consultations were in English, but 5.7% were held in Spanish. The majority of patients were female (57%), and of those seen in the Kidney Genetics Clinic, most patients (59%) had private insurance, while 28% had government insurance (Medicare or Medicaid), and insurance type was unknown for 13%. The average age of the patient population was 44 years old and ranged from 18 to 87 years. A family history of kidney disease was reported in 104 patients (37%), and 69 patients (25%) had a personal or family history of a known genetic diagnosis at the time of their appointment (Tables 3–5). The Kidney Genetics Clinic patient population primarily resided in the NY-CT-NJ tri-state area (87%), but 13% resided in 10 additional states, three other countries, and one US territory.
TABLE 2.
Demographic details of the Kidney Genetics Clinic patient population
| (a) Self-reported race and ethnicity | ||
|---|---|---|
|
| ||
| n | % | |
| Asian | 20 | 7.2 |
| Black/African American | 41 | 14.7 |
| Hispanic/Latinx | 60 | 21.5 |
| Native Hawaiian/Pacific Islander | 4 | 1.4 |
| Other | 6 | 2.2 |
| Unknown/not specified | 13 | 4.7 |
| White | 138 | 49.5 |
| (b) Self-reported gender | ||
|
| ||
| n | % | |
| Male | 120 | 43 |
| Female | 159 | 57 |
| (c) Patient insurance | ||
|
| ||
| n | % | |
| Medicaid (NY) | 41 | 14.7 |
| Medicaid (other) | 7 | 2.5 |
| Medicare | 31 | 11.1 |
| No insurance/self-pay | 10 | 3.6 |
| Private insurance | 166 | 59.5 |
| Unknown | 24 | 8.6 |
| (d) Patient state of residence | ||
|
| ||
| n | % | |
| Californiaa | 3 | 1.1 |
| Connecticuta | 9 | 3.2 |
| Delawarea | 1 | <0.5 |
| Florida | 5 | 1.8 |
| Illinoisa | 1 | <0.5 |
| Indianaa | 1 | <0.5 |
| International | 7 | 2.5 |
| Maryland | 1 | <0.5 |
| Massachusettsa | 2 | 0.7 |
| New Jerseya | 57 | 20.4 |
| New York | 179 | 64.2 |
| North Carolina | 1 | <0.5 |
| Pennsylvaniaa | 5 | 1.8 |
| Puerto Rico | 1 | <0.5 |
| Virginiaa | 6 | 2.2 |
| (e) Preferred language | ||
|
| ||
| n | % | |
| Arabic | 3 | 1.1 |
| English | 258 | 92.5 |
| Korean | 1 | <0.5 |
| Mandarin | 1 | <0.5 |
| Spanish | 16 | 5.7 |
States with genetic counseling licensure.
TABLE 3.
Referral and clinical information of the kidney genetics clinic patient population
| Clinical indication and diagnostic yield | |||
|---|---|---|---|
|
| |||
| n | % | Diagnostic yield (%) | |
| CAKUT | 8 | 2.9 | 25.0% |
| Tubulointerstitial disease | 26 | 9.3 | 15.4% |
| Alport spectrum disorder | 49 | 17.6 | 38.8% |
| Focal segmental glomerulosclerosis (FSGS) | 37 | 13.3 | 10.8% |
| Tubulopathy/electrolyte disorder | 28 | 10.0 | 3.6% |
| Cystic kidney disease | 35 | 12.5 | 42.9% |
| Hematuria | 14 | 5.0 | 35.7% |
| Proteinuria/nephrotic syndrome | 31 | 11.1 | 19.4% |
| Complement dysregulation | 12 | 4.3 | 0.0% |
| Tumor/cancer | 5 | 1.8 | 0.0% |
| CKD of unknown etiology | 29 | 10.4 | 10.3% |
| Healthy relative | 32 | 11.5 | 12.5% |
| Other (Hypertension, diabetes) | 12 | 4.3 | 8.3% |
Note: Patients with a clinical indication and percentage they make up of the total cohort. Diagnostic yield in patients with that clinical indication. Patients can have multiple clinical indications.
TABLE 5.
Personal and family history and diagnostic yield
| n | % | Diagnostic yield (%) | |
|---|---|---|---|
| Personal history of genetic diagnosis | 26 | 9.3 | n/a |
| FH of kidney disease | 104 | 37.3 | 26.0% |
| FH of genetic diagnosis | 43 | 15.4 | 27.9% |
Note: Patients with a personal or family history noted at the time of referral and percentage they make up of the total cohort, as well as the diagnostic yield in patients with a noted family history of kidney disease or genetic diagnosis.
3.3 |. Genetic test ordering and results
During the new patient genetic consults, several CLIA-certified genetic tests were ordered, including small (<100 genes) and large (>100 genes) multi-gene panels, exome sequencing, chromosome microarrays, single-gene sequencing, and targeted variant testing (Table 3a, Table S2). A total of 249 clinical genetic tests were ordered on 231 patients (82.8%), with 18 patients having concurrent or reflex genetic testing ordered. Nineteen tests were canceled, as the patients never submitted samples for testing, and results for five tests were still pending at the time of publication. In patients for whom genetic testing was not ordered, testing was not clinically indicated in six patients, 15 patients declined testing or did not provide consent, genetic testing results already were available for 24 patients, and financial concerns were cited for three patients.
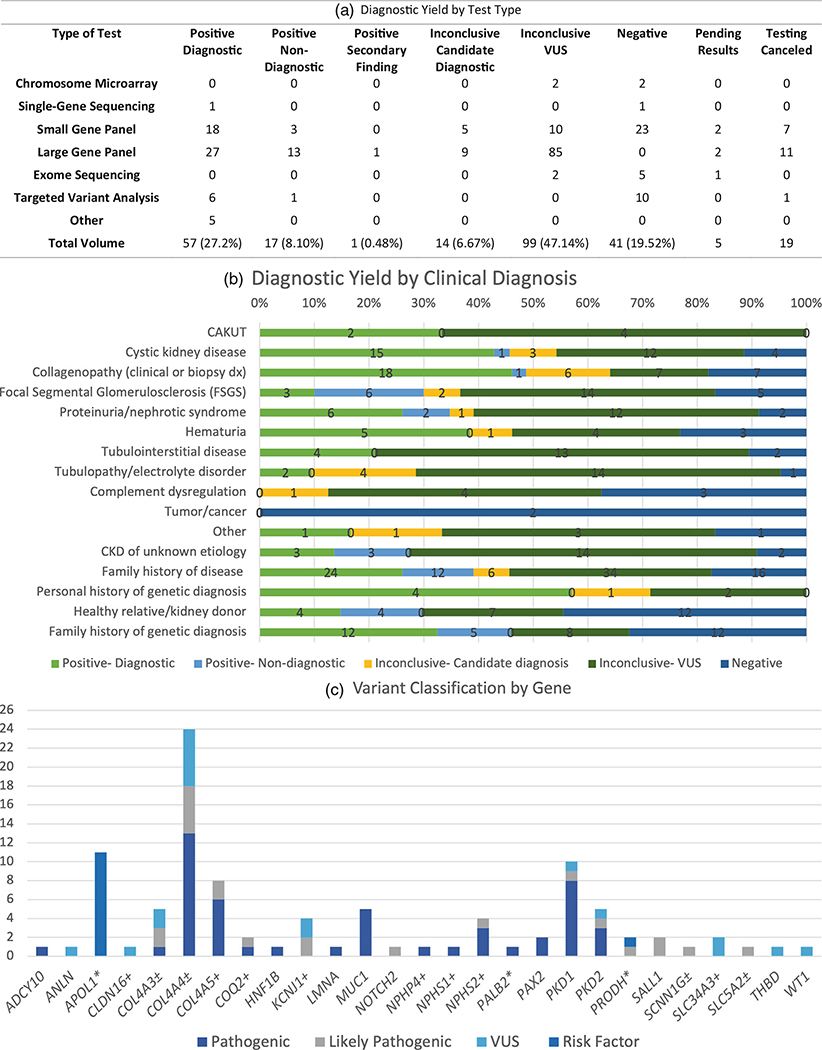
Presently, 225 genetic testing results were complete and available for 210 patients seen in the Kidney Genetics Clinic. Among these patients, a diagnostic finding was identified in 57 (27.14%) patients, and a candidate diagnostic finding was identified in 14 (6.7%) patients, resulting in a genetic finding potentially explaining the patient’s kidney disease in 33.8% of the Kidney Genetics Clinic patient population. A nondiagnostic positive finding was identified in an additional 18 (8.6%) patients. Nondiagnostic findings included secondary findings (n = 1), carrier status in phase testing (n = 5), and risk factors, such as an APOL1 high-risk genotype (n = 12). The age range for patients diagnosed with a monogenic disorder was 22–87, with the average age of 41 years old.
For results considered positive (diagnostic or nondiagnostic) or candidate diagnostic findings, 99 variants in 27 unique genes were identified (Figure 3c, Table S3). One patient was found to have dual diagnostic findings, one patient had a secondary finding, and four patients had both a diagnostic or candidate diagnostic finding and a nondiagnostic finding. The original results were classified as candidate diagnoses for two patients, but since the initial report was issued, the results were upgraded to diagnostic findings by the laboratory. The clinic facilitated this process by providing clinical and segregation data to the laboratories. The diagnostic yield was highest among patients with either cystic kidney disease or clinical suspicion of Alport syndrome or Alport-spectrum phenotype based on clinical features or kidney biopsy results (Figure 3b, Table 3).
FIGURE 3.
Genetic test results. (a) Diagnostic yield by test type. (b) Diagnostic yield by clinical indication. The highest diagnostic yield was found among patients with either cystic kidney disease or clinical suspicion of Alport spectrum disorder based on either clinical features or kidney biopsy results. (c) Variant classification by gene
3.4 |. Impact on management
Based on their genetic results, a referral to at least one specialist outside of nephrology was made for 40 patients (including ENT, ophthalmology, cardiology, hepatology, etc.). Of the 53 patients referred for transplant evaluation, 15 were found to have a genetic finding that could impact donor selection. Testing of family members for segregation analysis was recommended for 12 patients to assist in VUS resolution. Two patients with a clinical diagnosis of ADPKD were eligible for initiating tolvaptan based on clinical features, but insurance denied coverage of tolvaptan because there was a lack of family history of PKD. In these cases, a molecular diagnosis of ADPKD was established, which was successfully used to appeal the insurance company’s denial of coverage. In addition, 28 patients with at least one pathogenic or likely pathogenic in COL4A3, COL4A4, or COL4A5, and 34 patients with a PKD1 or PKD2 pathogenic or likely pathogenic variant were potentially eligible for participation in ongoing clinical trials for the treatment of Alport syndrome and ADPKD, respectively. Patients were referred to the trial if they met the inclusion criteria for enrolling.
In patients with nondiagnostic findings, additional genetic testing options were discussed during their return of results appointment. Based on the clinical suspicion of a genetic disorder, reflex to exome sequencing or referrals for specific genetic tests (e.g., targeted MUC1 testing) were made. When additional testing was not an option due to cost or patient interest, patients were referred to ongoing genetics and clinical research studies at CUIMC to further elucidate the genetics of kidney disease via novel gene discovery.
4 |. DISCUSSION
Genomic medicine has entered mainstream medicine and is increasingly available in medical specialties. However, the integration of genomic medicine in nephrology has been limited, in part due to nephrologists’ lack of confidence and knowledge of genetic information (Berns, 2010; Jayasinghe et al., 2020). To address these deficiencies, nephrologists and genetic counselors have collaborated to create a comprehensive Kidney Genetics Clinic within the Division of Nephrology at CUIMC. This clinic provides complete genetic care for adult patients with kidney and associated diseases.
4.1 |. Successes
The structure of the Kidney Genetics Clinic was created to allow for flexibility, offering multiple appointment types and points of entry into the clinic workflow. This workflow was specifically designed to cater to and appeal to the diverse level of providers’ genomic literacy. For example, a patient could be referred for a Full Genetic Consult by their treating nephrologist if they suspected an underlying genetic condition, but a specific gene or condition was not highly suspected, or if the patient had a complex clinical presentation. This visit type also could be utilized by non-nephrology providers with little experience with kidney-specific disorders and genetics. However, if the referring provider already suspected a specific genetic condition and testing, the patient could be referred for a Genetic Counseling Visit. During this visit, the patient would have a targeted counseling session and consent for the most appropriate genetic test. In addition, the genetic counseling visit also allowed for nephrologists and other referring providers to order genetic testing directly and refer for genetic counseling services based on the patient results and the providers’ knowledge and comfort with the resulting genetic information. As such, the Genetic Counseling Visits provide access to selected genomic services while integrating care with patients’ treating doctors, while the full genetic consult offers comprehensive genetic care, regardless of the referring providers’ knowledge of renal disease or genetics.
Several factors likely contributed to the kidney genetics clinic’s success and recognition across the institution, as illustrated by the 22 referrals from seven additional non-nephrology departments and divisions within CUIMC. Visibility among other specialties was likely due to interdepartmental collaborations when caring for patients seen in the Kidney Genetics Clinic. Different divisions and departments were often consulted on the management of patients with complex and syndromic features seen in the clinic, and referrals to other specialties were made when extra-renal manifestations were identified in clinic patients. This interdepartmental collaboration also facilitated cross-referrals between specialties and led to the identification of patients that could benefit from a referral to the Kidney Genetics Clinic within other specialty areas. Additionally, the Kidney Genetics Clinic providers, including the genetic counselors, collaborated with clinicians and genetic counselors in other divisions on interdepartmental educational initiatives, including seminars, case series, and other educational opportunities. These efforts laid the foundation for interdivisional and departmental collaborations, which allowed the clinic to gain broad recognition across the institution.
4.2 |. Access
The efficiency of the Kidney Genetics Clinic is highlighted by the short wait time for new patient visits, with the average time from referral to appointment being 37 calendar days and 5 days for patients referred for donor evaluation. This wait time is likely an overestimate of the typical patient’s wait time for an appointment, as this includes patients who were contacted multiple times over the course of a few weeks, as well as patients who were never able to be contacted and were referred again at a later date. Comparatively, the average wait time for a new patient visit in a traditional genetics clinic is 3–4 months (Maiese, Keehn, Lyon, Flannery, & Watson, 2019). In the nephrology setting, especially in transplantation, there can be an urgency associated with genetic results, and the timeline becomes critical. This model has the capacity to reduce barriers to genetic testing and counseling, such as long wait times for general genetics clinics.
While data is not available on the financial burden patients incur from genetic testing or counseling, the high uptake of genetic services and low cancelation rates due to financial constraints suggest that cost is not a significant barrier to accessing genomic services in nephrology. During the genetic consults, patients are informed about the genetic laboratory’s billing practices, and consent for testing is obtained. When cost is identified as a concern for patients, either during the visit or after, alternative testing options, such as industry-sponsored tests or research programs, are investigated as a cost-saving alternative. While the most common reason for test cancelation was due to patients not carrying out the testing (i.e., not sending in a sample or completing test consents), these rates were similar regardless of insurance status or type. This suggests that, although financial concerns cannot be ruled out as a consideration, it does not disproportionally affect a particular demographic in the Kidney Genetics Clinic.
4.3 |. Telemedicine utilization
The Kidney Genetics Clinic was established in June 2019, prior to the COVID-19 Pandemic, and operations were significantly impacted in response to the global pandemic. In mid-March 2020, the clinic stopped all activities in order to divert clinical resources to the pandemic. The clinic slowly began to see new patients again in May 2020 as it switched to a telemedicine model, and all patient visits at this time occurred via video visit or phone call, depending on the needs of the patient. During this time, the relaxed medical professional licensure rules greatly increased nephrologists’ and genetic counselors’ ability to provide virtual healthcare to patients across the country, significantly expanding access to genomic services in nephrology.
As such, the Kidney Genetics Clinic experienced a significant increase in referrals during the summer of 2020 (Figure 2), and the number of monthly referrals has since remained stable. Since then, telemedicine models have become more prevalent across healthcare specialties, driven by increased access to care (Garfan et al., 2021). There were no differences in conversion rate from referral to attending scheduled visits based on visit type, service model, referral source, or patient demographics, suggesting this model was successful in bringing genomic medicine to a diverse group of adults with kidney disease. By continuing to utilize a combination of in-person appointments and telemedicine, the Kidney Genetics Clinic will help genetic services reach a broad geographic region and diverse patient population.
Given the success of the implementation of telemedicine in this clinic, the Kidney Genetics Clinic continued primarily with a telemedicine model even after routine in-person visits began to resume in the nephrology division. Telemedicine remains a preferred method of visit for many patients because of the convenience, as well as the high proportion of nephrology patients who are immunocompromised. However, if a patient requested an in-person visit or if an in-person visit was deemed more accessible by the patient and provider based on technological issues or language barriers, in-person visits were, and continue to be, available and accommodated on a case-by-case basis. This dual appointment model further increases the access to genomic services in this patient population.
While telemedicine has been reported to reduce barriers to accessing genomic services, there are still challenges with this service model. A smartphone and reliable internet access are required for telemedicine visits, and a certain level of technology literacy is necessary to access the visits remotely. In addition, certain aspects of the traditional clinical genetics visit must be modified to accommodate telehealth visits. This is most obvious with the physical exam, which is utilized to identify dysmorphic and extra-renal features in the patient. Also adding to the difficulties of the telemedicine model, there is additional complexity when scheduling and conducting visits in other languages. These added challenges can further exacerbate existing barriers to access for specific patient populations.
To accommodate and address these barriers, several steps were taken by the clinical team to address the shortcomings of the telemedicine model. When patients had trouble utilizing the technology for video visits, appointments were conducted over the phone via conference call. The built-in telemedicine capability in the EMR also facilitated patient communication. During the patient visit, to address the absence of the physical exam, key extra-renal features (such as ear pits and tags, polydactyly, observation of hearing aids, etc.) were directly assessed through a targeted medical history and direct observation whenever possible. Lastly, conducting visits in the patient’s native language through translation services helped overcome some of the communication barriers. In the Kidney Genetics Clinic, these additional considerations were utilized routinely to increase access to genomic services and provide patient care in the nephrology specialty.
4.4 |. Implications of genetic diagnoses
The identification of genetic diagnoses in this patient population has several clinical implications, including changes in management, eligibility for genetically stratified clinical trials, and treatment implications. The diagnosis in patients impacted several areas of clinical care, including referrals to specialists, kidney donor selection, clinical trial eligibility (for example, in patients with a genetic diagnosis of Alport Syndrome), and increased access to medications (such as tolvaptan in patients with PKD1 variants). When diagnostic findings were identified in genes associated with non-kidney-related phenotypes, referrals to appropriate specialists were made.
Patients with candidate diagnoses presented a unique challenge in the clinic. Often, variants in this category were classified as having uncertain significance but were associated with conditions with a high degree of clinical overlap with the patient’s clinical presentation. In these situations, changes to clinical management were not routinely made, as these findings are not considered diagnostic, but attempts to resolve the classification were made. This involved familial testing and segregation analysis in affected and unaffected family members, phase testing, and additional clinical workup, when applicable. In two patients, this additional workup led to candidate variants being reclassified from variants of uncertain significance to likely pathogenic and thus became diagnostic for these patients. It is important to note, however, that each case was individually reviewed in the context of their clinical presentation and genetic results, and as such, nephrologists retained the right to exercise clinical judgment as necessary.
The nondiagnostic findings were typically the APOL1-high risk genotype, and patients were counseled depending on their CKD status, transplant plans, and family history. Those with nondiagnostic results were referred to ongoing genetics and clinical research studies at the medical center to elucidate the genetics of kidney disease further. Similar to positive cases, negative cases were also reviewed in the context of their clinical features, and additional genetic testing was discussed as needed. When there was high suspicion for a genetic diagnosis, for example, in patients with a severe phenotype and young age of onset, strong family history of CKD, or multisystem involvement, and negative genetic testing, additional genetic testing was considered. This is highlighted by patients with MUC1-associated autosomal dominant tubulointerstitial kidney disease (ADTKD). All patients evaluated in the clinic with positive MUC1 findings initially had a negative large gene panel analysis. However, upon review, these patients were all determined to be high-risk for a genetic condition and thus referred for additional testing. This subsequent genetic testing was instrumental in identifying a genetic diagnosis in this patient population.
4.5 |. Case series
To demonstrate an example of the specific implications of a genetic diagnosis in the setting of kidney disease, a case is presented below.
A 34-year-old female was referred to the clinic with a clinical diagnosis of autosomal dominant polycystic kidney disease (ADPKD). ADPKD was incidentally diagnosed on abdominal imaging in her 20s after a car accident. Imaging showed bilaterally enlarged kidneys with innumerable cysts. She had a history of hypertension since her 20s, as well as preeclampsia and proteinuria during pregnancy. She was also previously found to have mitral valve prolapse. She had no history of kidney stones or cysts in other organs. Based on the diagnosis of ADPKD and the associated risk for cerebral aneurysms, she had a past brain MRI, which was unremarkable. Notably, she had no family history of polycystic kidney disease or any features that would suggest that any family members were also affected. Her three siblings all had abdominal imaging based on her diagnosis and had no renal cysts. Based on her clinical presentation, kidney volume and progression, the patient was deemed to be eligible for tolvaptan, a medication used to slow the decline of kidney function in individuals with ADPKD. However, the patient’s health insurance cited the lack of family history of ADPKD as a justification to deny coverage of the medication. The patient underwent genetic testing for ADPKD in our clinic and was found to have a pathogenic PKD1 variant, which explained her personal history. She was counseled on the risk to her family members, including the risk to her children. While other family members did not complete genetic testing to our knowledge, it is likely that the variant was de novo, which is the case in ~15% of individuals with ADPKD. Most importantly for her health, the genetic diagnosis was used to successfully appeal the insurance company’s denial of coverage, and she was initiated on tolvaptan shortly after.
4.6 |. Cost considerations
The Kidney Genetics Clinic was implemented in a large academic medical center, with several financial resources at its disposal. The clinic utilized a billing model that many clinical genetics departments routinely use. In this model, only initial appointments and the physician’s time is billed. Historically, there is low reimbursement from health insurance companies for genetic counseling services, and thus, visits where only a genetic counselor was present, such as genetic counseling only and routine return of results visits, were not billed.
At CUIMC, this lost revenue was absorbed by the Division of Nephrology; however, over time, this can lead to a significant financial strain and burden for departments and institutions. Therefore, alternate payment models, such as a self-pay model for genetic counseling services, are being explored. Additionally, CUIMC, as well as other institutions, have created interdepartmental genetic counselors, where multiple departments collaborate to hire genetic counselors that would work across different departments; in this way, the cost is shared by multiple departments. Despite the shortcomings, the billing model utilized by the Kidney Genetics Clinic ensures continued access to genetic services without a significant financial burden on the patients. We note that these financial considerations apply to genetics clinics across all subspecialties. It is expected that the introduction of legislation that will enable billing for genetics counseling services will help address some of the financial challenges associated with establishing genetics clinics (Nowlen & Flores, 2021).
The COVID-19 pandemic forced many healthcare providers into a telemedicine model, which has been adopted permanently by many institutions and clinics. In response to this shift, reimbursement for telehealth services also changed dramatically, including broad coverage of telehealth services by private insurance companies and Medicare and Medicaid. Similarly, many states issued a temporary emergency waiver of licensure requirements. During this time, the Kidney Genetics Clinic used this opportunity to provide care to those outside of New York, as illustrated by patients residing in 12 additional states. However, most of those waivers have now expired, and physician and genetic counselor licensure must be considered when seeing patients who are physically located in other states. Efforts in this area have involved obtaining physician and genetic counselor licensure in the tri-state area (NY, NJ, CT), as well as in other states where the clinic receives a substantial number of referrals. However, the resource-intensive and time-consuming nature of applying for additional state licensure cannot be underestimated. Therefore, as the rules and regulations for telehealth continue to evolve, including licensure and reimbursement, it will be essential to monitor any changes and their potential impact on the clinic.
4.7 |. Future considerations
The Kidney Genetics Clinic continues to increase access to genetic testing and counseling while reducing barriers that might contribute to health inequities. While educating nephrologists on the impact and importance of genetic counseling and testing contributed to the success of the clinic, there are still areas where additional education can improve referral recognition and rates. Regular seminars and educational initiatives aimed at nephrologists and other advanced care practitioners in nephrology will continue to be integral. In addition, while the focus of educational initiatives has been on nephrologists, expanding the scope of these programs to include other specialties and genetic counselors can further increase the identification and appropriate referrals to genetics for this patient population. Finally, although patients can self-refer to the Kidney Genetics Clinic, few patients utilize this resource. Additional educational initiatives targeting the general kidney disease patient population can raise patient awareness and increase self-referrals. Ultimately, the Kidney Genetics Clinic will continue to facilitate conversations about genetics among and between patients and providers to improve access to genetic services in this patient population.
Supplementary Material
TABLE 4.
Referral reason
| n | % | |
|---|---|---|
| Clarification of clinical diagnosis | 186 | 66.7 |
| Clarification of biopsy finding | 20 | 7.2 |
| Counseling of genetic diagnosis | 32 | 11.5 |
| Cascade/family member testing | 39 | 14.0 |
| Transplant recipient evaluation | 40 | 14.3 |
| Transplant donor evaluation | 13 | 4.7 |
| Other (medication, clinical trial) | 2 | 0.7 |
Note: Patients with their referral indication and percentage they make up of the total cohort.
Footnotes
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Alkanderi S, Yates LM, Johnson SA, & Sayer JA (2017). Lessons learned from a multidisciplinary renal genetics clinic. QJM: An International Journal of Medicine, 110(7), 453–457. 10.1093/qjmed/hcx030 [DOI] [PubMed] [Google Scholar]
- Amlie-Wolf L, Baker L, Hiddemen O, Thomas M, Burke C, Gluck C, … Gripp KW (2021). Novel genetic testing model: A collaboration between genetic counselors and nephrology. American Journal of Medical Genetics Part A, 185(4), 1142–1150. 10.1002/ajmg.a.62088 [DOI] [PubMed] [Google Scholar]
- Berns JS (2010). A survey-based evaluation of self-perceived competency after nephrology fellowship training. Clinical Journal of the American Society of Nephrology, 5(3), 490–496. 10.2215/CJN.08461109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullich G, Domingo-Gallego A, Vargas I, Ruiz P, Lorente-Grandoso L, Furlano M, … Ars E (2018). A kidney-disease gene panel allows a comprehensive genetic diagnosis of cystic and glomerular inherited kidney diseases. Kidney International, 94(2), 363–371. 10.1016/j.kint.2018.02.027 [DOI] [PubMed] [Google Scholar]
- CDC. (2021). Chronic kidney disease in the United States, 2021. Retrieved from https://www.cdc.gov/kidneydisease/publications-resources/ckd-national-facts.html
- Cocchi E, Nestor JG, & Gharavi AG (2020). Clinical genetic screening in adult patients with kidney disease. Clinical Journal of the American Society of Nephrology, 5(10), 1497–1510. 10.2215/CJN.15141219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connaughton DM, Kennedy C, Shril S, Mann N, Murray SL, Williams PA, … Hildebrandt F (2019). Monogenic causes of chronic kidney disease in adults. Kidney International, 95(4), 914–928. 10.1016/j.kint.2018.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devuyst O, Knoers NV, Remuzzi G, Schaefer F, & Board of the Working Group for Inherited Kidney Diseases of the European Renal Association and European Dialysis and Transplant Association. (2014). Rare inherited kidney diseases: Challenges, opportunities, and perspectives. Lancet, 383(9931), 1844–1859. 10.1016/S0140-6736(14)60659-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhassan EAE, Murray SL, Connaughton DM, Kennedy C, Cormican S, Cowhig C, … Conlon PJ (2022). The utility of a genetic kidney disease clinic employing a broad range of genomic testing platforms: Experience of the Irish Kidney Gene Project. Journal of Nephrology, 35, 1655–1665. 10.1007/s40620-021-01236-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfan S, Alamoodi AH, Zaidan BB, Al-Zobbi M, Hamid RA, Alwan JK, … Momani F (2021). Telehealth utilization during the Covid-19 pandemic: A systematic review. Computers in Biology and Medicine, 138, 104878. 10.1016/j.compbiomed.2021.104878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg AX, Levey AS, Kasiske BL, Cheung M, & Lentine KL (2020). Application of the 2017 KDIGO guideline for the evaluation and care of living kidney donors to clinical practice. Clinical Journal of the American Society of Nephrology, 15(6), 896–905. 10.2215/CJN.12141019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groopman EE, Marasa M, Cameron-Christie S, Petrovski S, Aggarwal VS, Milo-Rasouly H, … Gharavi AG (2019). Diagnostic utility of exome sequencing for kidney disease. The New England Journal of Medicine, 380(2), 142–151. 10.1056/NEJMoa1806891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H, Bennett RL, Buchanan A, Pearlman R, & Wiesner GL (2015). A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: Referral indications for cancer predisposition assessment. Genetics in Medicine, 17(1), 70–87. 10.1038/gim.2014.147 [DOI] [PubMed] [Google Scholar]
- Hays T, Groopman EE, & Gharavi AG (2020). Genetic testing for kidney disease of unknown etiology. Kidney International, 98(3), 590–600. 10.1016/j.kint.2020.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershberger RE, Givertz MM, Ho CY, Judge DP, Kantor PF, McBride KL, … Ware SM (2018). Genetic evaluation of cardiomyopathy—A Heart Failure Society of America Practice Guideline. Journal of Cardiac Failure, 24(5), 281–302. 10.1016/j.cardfail.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasinghe K, Quinlan C, Mallett AJ, Kerr PG, McClaren B, Nisselle A, … Stark Z (2020). Attitudes and practices of Australian nephrologists toward implementation of clinical genomics. Kidney International Reports, 6(2), 272–283. 10.1016/j.ekir.2020.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoers N, Antignac C, Bergmann C, Dahan K, Giglio S, Heidet L, … Schaefer F (2022). Genetic testing in the diagnosis of chronic kidney disease: Recommendations for clinical practice. Nephrology, Dialysis, Transplantation, 37(2), 239–254. 10.1093/ndt/gfab218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist AL, Pelletier RC, Leonard CE, Williams WW, Armstrong KA, Rehm HL, & Rhee EP (2020). From theory to reality: Establishing a successful Kidney Genetics Clinic in the outpatient setting. Kidney360, 1(10), 1099–1106. 10.34067/KID.0004262020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madlensky L, Trepanier AM, Cragun D, Lerner B, Shannon KM, & Zierhut H (2017). A rapid systematic review of outcomes studies in genetic counseling. Journal of Genetic Counseling, 26(3), 361–378. 10.1007/s10897-017-0067-x [DOI] [PubMed] [Google Scholar]
- Maiese DR, Keehn A, Lyon M, Flannery D, & Watson M (2019). Current conditions in medical genetics practice. Genetics in Medicine, 21(8), 1874–1877. 10.1038/s41436-018-0417-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallett A, Fowles LF, McGaughran J, Healy H, & Patel C (2016). A multidisciplinary renal genetics clinic improves patient diagnosis. The Medical Journal of Australia, 204(2), 58–59. 10.5694/mja15.01157 [DOI] [PubMed] [Google Scholar]
- Mallett A, Patel C, Salisbury A, Wang Z, Healy H, & Hoy W (2014). The prevalence and epidemiology of genetic renal disease amongst adults with chronic kidney disease in Australia. Orphanet Journal of Rare Diseases, 9, 98. 10.1186/1750-1172-9-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann N, Braun DA, Amann K, Tan W, Shril S, Connaughton DM, … Hildebrandt F (2019). Whole-exome sequencing enables a precision medicine approach for kidney transplant recipients. Journal of the American Society of Nephrology, 30(2), 201–215. 10.1681/ASN.2018060575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milo Rasouly H, & Marasa M (2018). Pitfalls and challenges of consenting to genetic research studies. Kidney International Reports, 3(6), 1245–1248. 10.1016/j.ekir.2018.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mital S, Musunuru K, Garg V, Russell MW, Lanfear DE, Gupta RM, … Ware S (2016). Enhancing literacy in cardiovascular genetics: A scientific statement from the American Heart Association. Circulation. Cardiovascular Genetics, 9(5), 448–467. 10.1161/HCG.0000000000000031 [DOI] [PubMed] [Google Scholar]
- Nestor JG, Marasa M, Milo-Rasouly H, Groopman EE, Husain SA, Mohan S, … Gharavi AG (2020). Pilot study of return of genetic results to patients in adult nephrology. Clinical Journal of the American Society of Nephrology, 15(5), 651–664. 10.2215/CJN.12481019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaudet P (2010). Living donor kidney transplantation in patients with hereditary nephropathies. Nature Reviews. Nephrology, 6(12), 736–743. 10.1038/nrneph.2010.122 [DOI] [PubMed] [Google Scholar]
- Nowlen C, & Flores K (2021). Impact of the access to genetic counselor services act. Delaware Journal of Public Health, 7(5), 40–41. 10.32481/djph.2021.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pode-Shakked B, Ben-Moshe Y, Barel O, Regev LC, Kagan M, Eliyahu A, … Vivante A (2022). A multidisciplinary nephrogenetic referral clinic for children and adults-diagnostic achievements and insights. Pediatric Nephrology, 37(7), 1623–1646. 10.1007/s00467-021-05374-4 [DOI] [PubMed] [Google Scholar]
- Preston R, Stuart HM, & Lennon R (2019). Genetic testing in steroid-resistant nephrotic syndrome: Why, who, when and how? Pediatric Nephrology, 34(2), 195–210. 10.1007/s00467-017-3838-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, … Rehm HL (2015). Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics in Medicine, 17(5), 405–424. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CP, Freese ME, Ounda A, Jetton JG, Holida M, Noureddine L, & Smith RJ (2020). Initial experience from a renal genetics clinic demonstrates a distinct role in patient management. Genetics in Medicine, 22(6), 1025–1035. 10.1038/s41436-020-0772-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivante A, & Hildebrandt F (2016). Exploring the genetic basis of early-onset chronic kidney disease. Nature Reviews. Nephrology, 2(3), 133–146. 10.1038/nrneph.2015.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westland R, Renkema KY, & Knoers NVAM (2020). Clinical integration of genome diagnostics for congenital anomalies of the kidney and urinary tract. Clinical Journal of the American Society of Nephrology, 16(1), 128–137. 10.2215/CJN.14661119 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.