Abstract
MarR negatively regulates expression of the multiple antibiotic resistance operon (marRAB) in Escherichia coli. In this study, it was demonstrated that sodium salicylate, plumbagin, 2,4-dinitrophenol, and menadione–inducers of the marRAB operon in whole cells–all interfered with the repressor activity of MarR in vitro. It is proposed that these compounds can interact directly with MarR to affect its repressor activity.
The multiple antibiotic resistance locus (mar) of Escherichia coli controls intrinsic susceptibility to multiple antibiotics, organic solvents, oxidative stress agents, and household disinfectants (3, 19). In E. coli and Salmonella typhimurium the mar locus is organized into two divergently positioned transcriptional units, marC and marRAB, whose expression is under the control of a centrally located promoter and operator region, marO (5, 28). In the absence of an appropriate stimulus, MarR negatively regulates expression of the marRAB operon (5) by binding to two regions, sites I and II (15), within marO. MarR repression is alleviated following exposure to a variety of diverse compounds (4, 6, 8, 15, 25).
Previous experiments in vitro demonstrated that radiolabeled salicylic acid bound MarR with a Kd of 0.5 mM, and sodium salicylate inhibited the formation of MarR-marO complexes as judged by a gel retardation assay (15). Although some evidence for tetracycline binding to MarR (Kd > 10 mM) was also demonstrated, these earlier studies did not detect any effect of tetracycline, chloramphenicol, or other structurally different chemicals on MarR function (15).
MarR is a member of a newly recognized family of regulatory proteins, many of which may interact with phenolic compounds (29). Two MarR homologs, Ec17kd and MprA (EmrR), when expressed from plasmids, negatively regulated expression of a marOR-lacZ fusion (29), and the repressor function of both proteins in whole cells was antagonized by salicylate (12, 29). CinR, the MarR homolog from Butyrivibrio fibrisolvens E14, is antagonized in vitro by two compounds that contain ferrulic acid, a cinnamic acid (salicylate-like compound in plants) derivative (7).
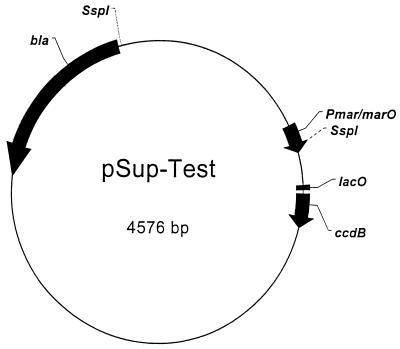
In this study, a restriction enzyme site protection assay was used to test the abilities of different chemicals to interfere with a MarR-marO interaction in vitro. The basis of the assay was plasmid pSup-Test, which contains two SspI sites: one within marO at 913 bp and the other elsewhere on the plasmid at 4,385 bp (Fig. 1). Wild-type MarR was specified by pMarR-WT, a medium-copy-number high-level wild-type MarR expression vector (2) constructed in pET13a (27), a kanamycin-resistant version of pET11a (Novagen, Madison, Wis.).
FIG. 1.
Map of plasmid pSup-Test showing the positions of the SspI sites within the plasmid.
MarR was purified from E. coli BL21(DE3) (Novagen) containing pMarR-WT essentially as described previously (2). Frozen cell pellets (2 g) were lysed in 8 ml of buffer P (100 mM sodium phosphate [pH 7.4] containing 0.5 ml of a protease inhibitor cocktail [Sigma, St. Louis, Mo.]), ion-exchange chromatography on sulfopropyl-Sepharose HiTrap columns (Pharmacia Biotech, Piscataway, N.J.) was performed in 10 mM sodium phosphate (pH 7.4), and the purified protein was dialyzed against 333 volumes of a solution containing 50 mM Tris-HCl (pH 7.4), 100 mM NaCl, 10% glycerol, and 1 mM phenylmethylsulfonyl fluoride (serine protease inhibitor) overnight at 4°C. Samples of the purified MarR, judged to be >99% pure on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis Coomassie blue-stained gel, were stored at −70°C until further use.
Analysis of MarR function in vitro.
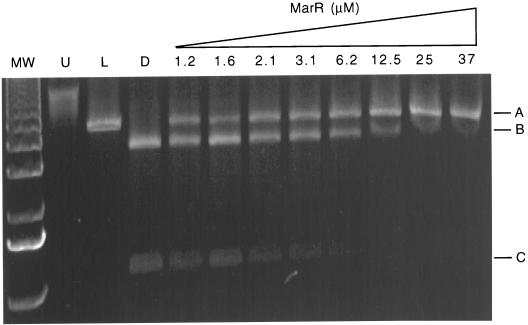
Reaction mixtures were prepared as previously described (2). A single linearly cut plasmid (4,576 bp) indicated that MarR protected the SspI site within site I of marO and that the second SspI site at 4,385 bp in pSup-Test was accessible to digestion (Fig. 2, lane L, fragment A). The production of two smaller fragments, of 3,472 and 1,104 bp, indicated that the SspI recognition sequence within site I of marO was no longer protected (Fig. 2, lane D, fragments B and C).
FIG. 2.
Binding of MarR to marO assayed by a restriction enzyme site protection assay. In the absence of MarR, the SspI recognition sequence in marO is not protected and the plasmid is cut into two pieces of 3,472 bp (fragment B) and 1,104 bp (fragment C) in length. A single cut in the non-marO SspI restriction site results in fragment A (4,576 bp) and indicates protection of the SspI site in marO. Serial increases in the concentration of MarR led to increased protection, as indicated by the conversion of the two smaller bands, fragments B and C, to a single 4,756-bp fragment. MW, molecular weight standards; U, uncut pSup-Test from the original plasmid preparation; L, pSup-Test digested with BamHI (linear form); D, pSup-Test digested with SspI (double-cut form).
Previous studies demonstrated that the MarR-marO interaction was highly specific (Kd ≈ 1 to 5 nM) (15, 25). We estimated the affinity of MarR for marO by determining the point of 50% protection, judged by the visual inspection of ethidium bromide-stained gels (9, 18, 26). Consistently, average ∼Kds of 2.1 and 0.95 μM (assuming the monomeric and dimeric forms of MarR, respectively) were obtained (Fig. 2). However, these values do not represent true Kd values for many reasons. Both competition between MarR and the restriction endonuclease and the continual depletion of the amount of target DNA (marO) due to the restriction enzyme contribute to an underestimation of the true Kd. Although nonspecific protein-nucleic acid interactions have been observed for other bacterial transcription factors (9), nonspecific binding for MarR was not evident, since the nonoperator SspI recognition sequence was accessible at the highest protein concentrations tested (Fig. 2, last two lanes). In these experiments, the off-rate for the MarR-marO interaction must be sufficiently low in order to protect marO from cleavage. This assay measures the presence of MarR on site I only (see reference 3 for a review regarding DNA-protein interactions at marO). However, since sites I and II are separated by a very short distance, it is anticipated, as observed for other prokaryotic transcription factors, that protein-protein communication among repressors at these sites would exist. The major advantage of the restriction enzyme site protection assay over a standard gel shift assay is that it is performed at equilibrium.
Effects of many chemicals on MarR repressor activity.
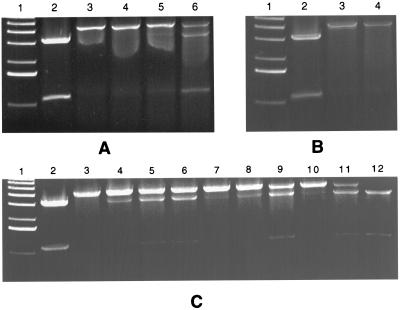
The restriction enzyme site protection assays were performed in the presence of various inducers to determine if any of these chemicals could antagonize repressor function directly in vitro. SspI digestion of pSup-Test in the presence of the various marRAB operon inducers and control compounds showed no effect on the activity of the restriction endonuclease (data not shown). These chemicals were then tested for their effect on the DNA binding activity of MarR in vitro (Fig. 3A, B, and C). Sodium salicylate, at a concentration of 2 mM, interfered with the DNA binding activity of MarR (Fig. 3A, lane 5), and this effect was more pronounced at higher concentrations (Fig. 3A, lane 6).
FIG. 3.
Restriction enzyme site protection assays in the presence of structurally dissimilar compounds. In all panels, lane 1 shows molecular weight standards, lane 2 shows the vector (pSup-Test [3.4 nM]) alone digested with SspI, and lane 3 shows pSup-Test in the presence of MarR (2.92 to 3.6 μg or 9.1 to 11.2 μM [assuming the monomeric form of MarR]) digested with SspI. (A) The concentrations of sodium salicylate in lanes 4 to 6 were 0.8, 2, and 5 mM. (B) Lane 4, paraquat at a concentration of 5 mM. (C) Plumbagin (lanes 4 to 6) and 2,4-dinitrophenol (lanes 7 to 9) were tested at concentrations of 0.25, 0.5, and 1 mM; menadione (lanes 10 to 12) was tested at concentrations of 0.8, 2, and 5 mM.
Paraquat, an oxidative stress agent, did not show any effect at the highest concentration tested, 5 mM (Fig. 3B, lane 4). Since 50 μM paraquat induced the expression of an inaA-lacZ fusion (inaA is part of the Mar regulon, but its function is unknown [3]) equally in a mar+ or Δmar background in E. coli, this induction appeared to be independent of the mar locus (24). Another group found that paraquat at a much higher concentration (1.3 mM) induced the expression of a marO-lacZ fusion 2.7-fold in a wild-type host and 0.98-fold in a Δmar background (25). Thus, at high amounts paraquat affected mar expression (25). The results found in vitro suggest that paraquat at high concentrations induces expression of the marRAB operon by an indirect mechanism.
Plumbagin, an oxidative stress agent, and 2,4-dinitrophenol, an uncoupler, were the most effective compounds tested with visible deprotection at 250 μM (Fig. 3C, lanes 4 and 7). Menadione caused deprotection at 800 μM (Fig. 3C, lane 10).
In the in vitro assays, ampicillin at a concentration of 5 mM appeared to antagonize the DNA binding activity of MarR (data not shown). However, unlike that of other active agents, this effect was not observed at lower concentrations, i.e., 1 or 2.5 mM (data not shown). Ampicillin does not induce marRAB expression in whole cells (8). Since this finding may have resulted from its being kept out of the cell by the AcrAB multidrug efflux system (21, 22), we tested its ability to induce mar in E. coli AG100A (22), which has AcrAB deleted. No MarA expression was detected (with MarA polyclonal antibodies [16]) despite exposure to 2 mg of ampicillin per ml (data not shown). These results suggest that ampicillin’s effect at high concentrations in vitro is nonspecific.
No effect on MarR repressor activity was detected with chloramphenicol and norfloxacin at 5 mM, but a slight deprotection was observed when the chloramphenicol concentration was increased to ∼10 mM (data not shown). In previous experiments, only a marginal level of binding (Kd > 10 mM) of MarR to tetracycline was seen, but tetracycline had no effect on nucleoprotein complexes (15). It is therefore probable that the induction of marRAB expression in whole cells by chloramphenicol and tetracycline (8) occurs indirectly. An unidentified cellular product generated upon exposure to either of these compounds may function as the inducer (3). Alternatively, both antibiotics may simply increase mRNA stability (13).
The relatively high inducer concentrations in these assays correlate with their activities in whole cells (8). A specificity is evident from the lack of activity by other compounds but leaves open the possibility that an intrinsic cell-mediated inducer exists, which has not been identified (3). Still, the variety of structures that cause induction in vitro suggest that MarR has a broadly specific, low-affinity substrate binding site.
Experiments in which MarR was added to pSup-Test before the inducer produced results identical to those described above (data not shown). These findings suggest that compounds which induce marRAB expression can interact with MarR whether it is bound to DNA or free. In both instances this interaction altered the DNA binding activity of the repressor.
The low background level of ethidium bromide staining seen with some samples is attributed to two factors: the intrinsic fluorescence of the inducers under UV light and the formation of unique nucleoprotein complexes. Purified MarR forms multimers (15, 25) which are seen on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels containing 8 M urea (data not shown). The background staining seen in samples containing MarR, but not with plasmid alone, may represent different multimeric forms of MarR complexed with DNA.
Conclusions.
The findings in this report provide evidence that multiple structurally unrelated chemicals (inducers) interfere directly with MarR function in vitro. The multidrug binding profiles of efflux proteins have been demonstrated (11, 20, 23). Fewer examples of multidrug binding to cytoplasmic proteins have been reported. BmrR, the positive regulator of the Bacillus subtilis Bmr multidrug transporter, binds rhodamine 6G (Kd ≈ 1 μM [14]) and tetraphenylphosphonium (Kd ≈ 100 μM [14]), which are also substrates of the pump (1, 30). The gene product of fabI in E. coli, encoding enoyl reductase, binds natural fatty acid substrates with high affinity and interacts with at least two different chemicals, triclosan and diazaborine, that inhibit the function of the protein (10, 17). By responding with different affinities to many unrelated chemicals, MarR is well adapted to control the cell’s rapid response to multiple environmental hazards.
Acknowledgments
We are grateful to Laura McMurry, Michael Malamy, Bruce Demple, and the excellent reviewers for their thoughtful and helpful comments on the manuscript.
This work was supported by NIH grant GM 51661.
REFERENCES
- 1.Ahmed M, Borsch C M, Taylor S S, Vazquez-Laslop N, Neyfakh A A. A protein that activates expression of a multidrug efflux transporter upon binding the transporter substrates. J Biol Chem. 1994;269:28506–28513. [PubMed] [Google Scholar]
- 2.Alekshun M N, Levy S B. Characterization of MarR superrepressor mutants. J Bacteriol. 1999;181:3303–3306. doi: 10.1128/jb.181.10.3303-3306.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alekshun M N, Levy S B. Regulation of chromosomally mediated multiple antibiotic resistance: the mar regulon. Antimicrob Agents Chemother. 1997;41:2067–2075. doi: 10.1128/aac.41.10.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ariza R R, Cohen S P, Bachhawat N, Levy S B, Demple B. Repressor mutations in the marRAB operon that activate oxidative stress genes and multiple antibiotic resistance in Escherichia coli. J Bacteriol. 1994;176:143–148. doi: 10.1128/jb.176.1.143-148.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen S P, Hächler H, Levy S B. Genetic and functional analysis of the multiple antibiotic resistance (mar) locus in Escherichia coli. J Bacteriol. 1993;175:1484–1492. doi: 10.1128/jb.175.5.1484-1492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen S P, Levy S B, Foulds J, Rosner J L. Salicylate induction of antibiotic resistance in Escherichia coli: activation of the mar operon and a mar-independent pathway. J Bacteriol. 1993;175:7856–7862. doi: 10.1128/jb.175.24.7856-7862.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalrymple B P, Swadling Y. Expression of a Butyrivibrio fibrisolvens E14 gene (cinB) encoding an enzyme with cinnamoyl ester hydrolase activity is negatively regulated by the product of an adjacent gene (cinR) Microbiology. 1997;143:1203–1210. doi: 10.1099/00221287-143-4-1203. [DOI] [PubMed] [Google Scholar]
- 8.Hächler H, Cohen S P, Levy S B. marA, a regulated locus which controls expression of chromosomal multiple antibiotic resistance in Escherichia coli. J Bacteriol. 1991;173:5532–5538. doi: 10.1128/jb.173.17.5532-5538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joachimiak A, Kelley R L, Gunsalus R P, Yanofsky C, Sigler P B. Purification and characterization of trp aporepressor. Proc Natl Acad Sci USA. 1983;80:668–672. doi: 10.1073/pnas.80.3.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy C W, Roujeinikova A, Sedelnikova S, Baker P J, Stuitje A R, Slabas A R, Rice D W, Rafferty J B. Molecular basis of triclosan activity. Nature. 1999;398:383–384. doi: 10.1038/18803. [DOI] [PubMed] [Google Scholar]
- 11.Lewis K. Multidrug resistance pumps in bacteria: variations on a theme. Trends Biochem Sci. 1994;19:119–123. doi: 10.1016/0968-0004(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 12.Lomovskaya O, Lewis K, Matin A. EmrR is a negative regulator of the Escherichia coli multidrug resistance pump EmrAB. J Bacteriol. 1995;177:2328–2334. doi: 10.1128/jb.177.9.2328-2334.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez P J, Marchand I, Yarchuk O, Dreyfus M. Translation inhibitors stabilize Escherichia coli mRNAs independently of ribosome protection. Proc Natl Acad Sci USA. 1998;95:6067–6072. doi: 10.1073/pnas.95.11.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Markham P N, LoGuidice J, Neyfakh A. Broad ligand specificity of the transcriptional regulator of the Bacillus subtilis multidrug transporter Bmr. Biochem Biophys Res Commun. 1997;239:269–272. doi: 10.1006/bbrc.1997.7467. [DOI] [PubMed] [Google Scholar]
- 15.Martin R G, Rosner J L. Binding of purified multiple antibiotic-resistance repressor protein (MarR) to mar operator sequences. Proc Natl Acad Sci USA. 1995;92:5456–5460. doi: 10.1073/pnas.92.12.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDermott P F, White D G, Podglajen I, Alekshun M N, Levy S B. Multidrug resistance following expression of the Escherichia coli marA gene in Mycobacterium smegmatis. J Bacteriol. 1998;180:2995–2998. doi: 10.1128/jb.180.11.2995-2998.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMurry L M, Oethinger M, Levy S B. Triclosan targets lipid synthesis. Nature. 1998;394:531–532. doi: 10.1038/28970. [DOI] [PubMed] [Google Scholar]
- 18.Melville S B, Gunsalus R P. Isolation of an oxygen-sensitive FNR protein of Escherichia coli: interaction at activator and repressor sites of FNR-controlled genes. Proc Natl Acad Sci USA. 1996;93:1226–1231. doi: 10.1073/pnas.93.3.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moken M C, McMurry L M, Levy S B. Selection of multiple-antibiotic-resistant (Mar) mutants of Escherichia coli by using the disinfectant pine oil: roles of the mar and acrAB loci. Antimicrob Agents Chemother. 1997;41:2770–2772. doi: 10.1128/aac.41.12.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikaido H. Multidrug efflux pumps of gram-negative bacteria. J Bacteriol. 1996;178:5853–5859. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikaido H, Basina M, Nguyen V, Rosenberg E Y. Multidrug efflux pump AcrAB of Salmonella typhimurium excretes only those β-lactam antibiotics containing lipophilic side chains. J Bacteriol. 1998;180:4686–4692. doi: 10.1128/jb.180.17.4686-4692.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okusu H, Ma D, Nikaido H. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J Bacteriol. 1996;178:306–308. doi: 10.1128/jb.178.1.306-308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paulsen I T, Brown M H, Skurray R A. Proton-dependent multidrug efflux systems. Microbiol Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosner J L, Slonczewski J L. Dual regulation of inaA by the multiple antibiotic resistance (mar) and superoxide (soxRS) stress response systems of Escherichia coli. J Bacteriol. 1994;176:6262–6269. doi: 10.1128/jb.176.20.6262-6269.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seoane A S, Levy S B. Characterization of MarR, the repressor of the multiple antibiotic resistance (mar) operon of Escherichia coli. J Bacteriol. 1995;177:3414–3419. doi: 10.1128/jb.177.12.3414-3419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith H Q, Somerville R L. The tpl promoter of Citrobacter freundii is activated by the TyrR protein. J Bacteriol. 1997;179:5914–5921. doi: 10.1128/jb.179.18.5914-5921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 28.Sulavik M C, Dazer M, Miller P F. The Salmonella typhimurium mar locus: molecular and genetic analyses and assessment of its requirement for virulence. J Bacteriol. 1997;179:1857–1866. doi: 10.1128/jb.179.6.1857-1866.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sulavik M C, Gambino L F, Miller P F. The MarR repressor of the multiple antibiotic resistance (mar) operon in Escherichia coli: prototypic member of a family of bacterial regulatory proteins involved in sensing phenolic compounds. Mol Med. 1995;1:436–446. [PMC free article] [PubMed] [Google Scholar]
- 30.Zheleznova E E, Markham P N, Neyfakh A A, Brennan R G. Structural basis of multidrug recognition by BmrR, a transcription activator of a multidrug transporter. Cell. 1999;96:353–362. doi: 10.1016/s0092-8674(00)80548-6. [DOI] [PubMed] [Google Scholar]