Abstract
A set of Saccharomyces cerevisiae strains with variable expression of only the high-affinity Hxt7 glucose transporter was constructed by partial deletion of the HXT7 promoter in vitro and integration of the gene at various copy numbers into the genome of an hxt1-7 gal2 deletion strain. The glucose transport capacity increased in strains with higher levels of HXT7 expression. The consequences for various physiological properties of varying the glucose transport capacity were examined. The control coefficient of glucose transport with respect to growth rate was 0.54. At high extracellular glucose concentrations, both invertase activity and the rate of oxidative glucose metabolism increased manyfold with decreasing glucose transport capacity, which is indicative of release from glucose repression. These results suggest that the intracellular glucose concentration produces the signal for glucose repression.
Metabolism of glucose in Saccharomyces cerevisiae proceeds via sugar transport across the plasma membrane and oxidation to pyruvate via the common glycolytic pathway. The flux through these steps determines the rates of fermentation and respiration. The contribution of the individual enzymatic steps of glycolysis to flux through the pathway has been examined in S. cerevisiae, with the surprising conclusion that the enzymes can be overexpressed up to 10-fold without significant effects on growth or ethanol production (26, 27). These observations lend support to the proposal (11) that glucose transport limits the rate of glycolysis. Control of flux by the transport step may be more pronounced in cells growing at low glucose concentrations and expressing high-affinity glucose transporters (28).
Evaluation of the degree to which glycolysis in S. cerevisiae is limited by glucose transport is complicated by the large number of transporters expressed by that yeast (16). Metabolic control analysis offers both a theoretical basis and a set of experimental approaches for that evaluation. Metabolic control analysis describes the control of flux through a metabolic pathway in terms of the control coefficient of each step. In principle, every step in a pathway shares the control of that pathway; the sum of the control coefficients in a pathway is 1 (7, 15). In order to estimate the control coefficient of an individual step under defined conditions, its activity should be varied by small amounts and the magnitude of the effect of each variation on flux should be measured.
Glucose plays a regulatory role in yeast in addition to its importance as a nutrient. When glucose is available, it is used preferentially to other carbon sources. This is achieved, in part, by transcriptional repression of genes that are required for respiratory metabolism and utilization of other carbon sources (12). The molecular mechanisms of this signal transduction pathway have been described in considerable detail (2, 14). However, the nature of the signal that the cell perceives from glucose in its environment is still unknown.
The promoter of the HXT7 gene in plasmid p21 (encoding the high-affinity glucose transporter Hxt7; reference 25) was progressively deleted by exonuclease III deletion (13). Selected deletion mutants were integrated at the URA3 locus of S. cerevisiae KY73 (MATα hxt1Δ::HIS3::Δhxt4 hxt5::LEU2 hxt2Δ::HIS3 hxt3Δ::LEU2::Δhxt6 hxt7Δ::HIS3 gal2Δ::DR ura3-52 MAL2 SUC2 MEL; reference 17). The endpoints of the deletions were determined by DNA sequence analysis, and the locations and copy numbers of the integrated HXT7 genes were determined by Southern blotting with an HXT7-specific oligonucleotide probe (4) and a DNA probe from the URA3 gene. Isolates with various HXT7 promoter lengths and copy numbers were selected for further study based on their growth characteristics on solid glucose medium (unpublished data).
Cells of four selected HXT7 integrant strains and isogenic strains MC996A (wild type; MATα ura3-52 his3-11,15 leu2-3,112 MAL2 SUC2 GAL MEL; reference 25) and RE607B (HXT7 only; MATα hxt1Δ::HIS3::Δhxt4 hxt5::LEU2 hxt2Δ::HIS3 hxt3Δ::LEU2::Δhxt6 ura3-52 MAL2 SUC2 GAL MEL; reference 25) were grown in liquid medium containing 1% yeast extract, 2% peptone, and 1% (approximately 55 mM) glucose. Growth was monitored by measurement of the optical density at 600 nm (OD600) at various time points. The residual glucose in the medium at each time point was determined enzymatically (1). Cells were harvested at a residual glucose concentration of approximately 40 mM for the following analyses. Transport of glucose was measured with the 5-s [14C]glucose uptake assay described by Walsh et al. (30). For strains containing only HXT7, the glucose concentrations in the assay were 1 and 10 mM; for wild-type strain MC996A, transport was assayed at 10 glucose concentrations ranging from 0.25 to 250 mM. Invertase activity was measured as described by Walsh et al. (29). The rate of oxygen consumption by the cultures was measured in an Oxygraph equipped with a Clark oxygen electrode. Hxt7 protein abundance was estimated by Western blotting of 10-μg samples of whole-cell extracts with anti-Hxt7 antibody (kind gift of E. Boles) as previously described (17) and densitometric scanning of the resulting chemiluminograms. Total cell protein was estimated by the method of Lowry et al. (18) using bovine serum albumin as the standard. The results of these analyses are shown in Table 1.
TABLE 1.
Effect of glucose transport on growth rate and glucose repression of wild-type and Hxt7-only S. cerevisiae strainsa
| Strain | HXT7 promoter length (bp) | HXT7 copy no. | Growth rate (h−1) | Hxt7 expression level (arbitrary units) | Glucose transport capacityb (nmol · min−1 · mg of protein−1) | Invertase activity (nmol · min−1 · mg of protein−1) | Oxygen consumption rate (nmol · min−1 · mg of protein−1) | Glucose flux ratec (nmol · min−1 · mg of protein−1) |
|---|---|---|---|---|---|---|---|---|
| MC996A | 1,148 | 1 | 0.39 ± 0.02 (100) | NDd | 364 ± 26 (100) | 183 ± 80 (100) | 41 ± 1 (100) | 494 ± 56 (100) |
| LYY4 | 729 | 12 | 0.32 ± 0.03 (82) | 100 ± 1 | 245 ± 22 (67) | 229 ± 111 (125) | 53 ± 2 (129) | 340 ± 1 (69) |
| LYY0 | 1,148 | 2 | 0.31 ± 0.03 (79) | 93 ± 3 | 217 ± 14 (60) | 429 ± 105 (234) | 59 ± 4 (144) | 309 ± 35 (63) |
| RE607B | 1,148 | 1 | 0.30 ± 0.03 (77) | 69 ± 3 | 201 ± 22 (55) | 430 ± 108 (235) | 61 ± 8 (149) | 328 ± 4 (66) |
| LYY8 | 507 | 1 | 0.27 ± 0.01 (69) | 50 ± 1 | 195 ± 9 (54) | 973 ± 2 (532) | 75 ± 6 (184) | 234 ± 21 (47) |
| LYY16 | 179 | 8 | 0.11 ± 0.02 (28) | ND | 27 ± 1 (7) | 1406 ± 163 (768) | 144 ± 13 (351) | 47 ± 2 (10) |
Values are means ± standard deviations. Values represent at least two independent experiments as described in the text. The glucose concentrations of these cultures varied from 30 to 47 mM at the time of harvest. Values in parentheses are percentages of the wild-type value.
The transport capacity of the strains is the maximal velocity determined by fitting the zero-trans influx rates to the Michaelis-Menten equation, with a Km of 16 mM for the wild-type strain and a Km of 2.2 mM for the HXT7-only strains.
Estimation of the glucose flux rate was based on the total consumption of extracellular glucose (millimolar concentration of glucose per unit of OD600) and the rate of growth at the time of harvest. The mean protein concentration was 0.3 (range, 0.25 to 0.35) mg · ml−1 · OD600U−1.
ND, not detectable.
Glucose transport exerts a high level of control over growth.
Wild-type strain MC996A grew faster than all other strains. At this stage of growth, Hxt7 protein was not detected in the wild-type strain; HXT7 expression is low in wild-type S. cerevisiae at glucose concentrations of >20 mM (data not shown; see also reference 4). For the HXT7-only strains, the growth rate correlated well with the level of Hxt7 protein expressed.
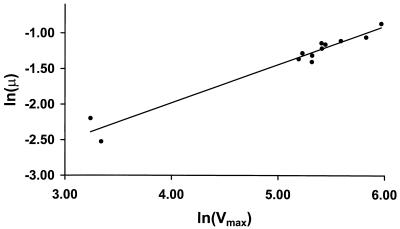
The glucose transport capacity of these strains was also correlated with the growth rate. When the logarithm of the growth rate is plotted against the logarithm of the Vmax for glucose transport (Fig. 1), the data fall on a straight line with a slope of 0.54 ± 0.04 (R2 = 0.96). According to metabolic control analysis theory, the slope of the line produced by plotting a flux versus a catalytic activity in double-logarithmic space is equal to the control coefficient of that activity over that flux (8).
FIG. 1.
Control of growth rate by glucose transport. The rate of growth of the wild-type MC996A strain and strains expressing only HXT7 to various levels is plotted as a function of the maximal velocity of glucose transport (logarithmic scales). Cells were harvested during exponential phase, 8 h after inoculation (OD600, 0.4 to 1.1; residual glucose, 30 to 47 mM).
These results are consistent with previous reports that glucose transport exerts a high level of control over growth and glycolytic flux by Saccharomyces. When maltose was used to inhibit glucose transport in wild-type S. bayanus, it was found that the control coefficient ranged from 0.5 to 1, depending on the extracellular glucose concentration (5). In another study, the coefficients of glucose transport control over glycolytic flux in nongrowing S. cerevisiae cell suspensions were 0.64 at pH 5.5 and 0.83 at pH 4.5; under the same conditions, the control coefficients for phosphofructokinase were 0.10 and 0.12, respectively (9). The control coefficient for phosphofructokinase (often considered to be “the rate-limiting step of glycolysis” [11]) has been calculated by other investigators to be 0.3 (3, 8).
Glucose transport affects glucose repression.
The status of glucose repression in these cultures was determined by measuring their invertase activity and oxygen consumption rate. The strains with reduced glucose transport capacity expressed higher levels of invertase activity (Table 1). Similarly, the specific oxygen consumption rate was inversely correlated with transport capacity. The invertase assay used here measures the total cellular activity of this enzyme. Using standard culture conditions for repression and derepression of secreted invertase (23), we found that the repressed level of total invertase in the wild-type MC996A strain was 361 nmol · min−1 · mg of protein−1, and the derepressed level is 3,897 nmol · min−1 · mg of protein−1. By comparison with Table 1, these values demonstrate that invertase was fully repressed at the highest glucose uptake capacities and was significantly derepressed at the lowest uptake capacity.
Lower levels of glucose transport activity in yeast have previously been found to diminish glucose repression. In Kluyveromyces lactis strains containing two low-affinity glucose transporter genes, endogenous β-galactosidase activity was fully repressed during growth on glucose. Null mutations of either gene resulted in partial derepression of β-galactosidase, and in a double null mutant strain, the activity was completely derepressed (31). In S. cerevisiae, the dominant mutations HTR1-23 and DGT1-1 resulted in decreased levels of HXT gene expression and glucose transport activity. Both mutations alleviated glucose repression of enzymes such as invertase, maltase, malate dehydrogenase, glutamate dehydrogenase, and cytochrome c oxidase (10, 22). However, it was not resolved whether the reduced repression levels were consequences of the mutations or of the reduced glucose transport activities. In an S. cerevisiae strain with null mutations in HXT1 to HXT7, glucose repression of maltase was completely relieved. In related strains with single HXT genes, the extent of glucose repression was strongly correlated with the glucose consumption rate of the strain. In particular, increasing the copy number of HXT1 stepwise from 1 to 3 in this hxt null strain increased the glucose consumption rate and decreased the maltase activity (24).
In contrast to these results that suggest that the flux of glucose into the cell determines the degree of glucose repression, Meijer et al. (20) found that repression of the SUC2 gene was dependent on the external glucose concentration and was fully derepressed at glucose concentrations of <14 mM. In contrast, the level of SUC2 expression was independent of the glucose flux.
Mutations of HXK2, encoding hexokinase II, also lead to relief of glucose repression (6, 19, 21). It has been pointed out that intracellular glucose is the metabolite that links glucose transport and hexokinase and that the intracellular glucose concentration is a likely signal for the glucose repression pathway (28).
We observed that at lower rates of transport, a higher fraction of glucose was oxidized via the respiratory pathway (Table 1). Therefore, the effect on the growth rate of the decrease in glucose uptake was partly compensated for by a difference in glucose metabolism, with relatively more glucose being metabolized by oxidative phosphorylation (which generates more ATP per mole of glucose) at low rates of glucose uptake. These results demonstrate that glucose transport plays important roles in determining the relative activities of the fermentative and respiratory pathways of glucose metabolism, both by delivering glucose across the plasma membrane to the glycolytic pathway and by influencing the glucose repression status of various metabolic activities.
Acknowledgments
This work was financially supported by the Association of Biotechnology Centers in the Netherlands (ABON).
We are grateful to Marco de Groot for constructing plasmid pBCY7.
REFERENCES
- 1.Bergmeyer H U. Methods of enzymatic analysis. Weinheim, Germany: Verlag Chemie GmbH; 1974. [Google Scholar]
- 2.Carlson M. Regulation of glucose utilization in yeast. Curr Opin Genet Dev. 1998;8:560–564. doi: 10.1016/s0959-437x(98)80011-7. [DOI] [PubMed] [Google Scholar]
- 3.Davies S E, Brindle K M. Effects of overexpression of phosphofructokinase on glycolysis in the yeast Saccharomyces cerevisiae. Biochemistry. 1992;31:4729–4735. doi: 10.1021/bi00134a028. [DOI] [PubMed] [Google Scholar]
- 4.Diderich J A, Schepper M, van Hoek P, Luttik M A H, van Dijken J P, Pronk J T, Klaassen P, Boelens H F M, Teixeira de Mattos M J, van Dam K, Kruckeberg A L. Glucose uptake kinetics and transcription of HXT genes in chemostat cultures of Saccharomyces cerevisiae. J Biol Chem. 1999;274:15350–15359. doi: 10.1074/jbc.274.22.15350. [DOI] [PubMed] [Google Scholar]
- 5.Diderich, J. A., B. Teusink, J. Valkier, J. Anjos, I. Spencer-Martins, K. van Dam, and M. C. Walsh. Inhibition characteristics of glucose transport and strategies to determine the control of glucose transport on glycolysis in yeast. Submitted for publication.
- 6.Entian K D. Genetic and biochemical evidence for hexokinase PII as a key enzyme involved in carbon catabolite repression in yeast. Mol Gen Genet. 1980;178:633–637. doi: 10.1007/BF00337871. [DOI] [PubMed] [Google Scholar]
- 7.Fell D A. Metabolic control analysis: a survey of its theoretical and experimental development. Biochem J. 1992;286:313–330. doi: 10.1042/bj2860313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fell D A. Understanding the control of metabolism. 1st ed. London, England: Portland Press; 1997. [Google Scholar]
- 9.Galazzo J L, Bailey J E. Fermentation pathway kinetics and metabolic flux control in suspended and immobilized Saccharomyces cerevisiae. Enzyme Microb Technol. 1990;12:162–172. [Google Scholar]
- 10.Gamo F J, Lafuente M J, Gancedo C. The mutation DGT1-1 decreases glucose transport and alleviates carbon catabolite repression in Saccharomyces cerevisiae. J Bacteriol. 1994;176:7423–7429. doi: 10.1128/jb.176.24.7423-7429.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gancedo C, Serrano R. Energy-yielding metabolism. In: Rose A H, Harrison J S, editors. The yeasts. 2nd ed. Vol. 3. London, England: Academic Press; 1989. pp. 205–259. [Google Scholar]
- 12.Gancedo J M. Yeast carbon catabolite repression. Microbiol Mol Biol Rev. 1998;62:334–361. doi: 10.1128/mmbr.62.2.334-361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984;28:351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- 14.Johnston M. Feasting, fasting and fermenting: glucose sensing in yeast and other cells. Trends Genet. 1999;15:29–33. doi: 10.1016/s0168-9525(98)01637-0. [DOI] [PubMed] [Google Scholar]
- 15.Kacser H, Burns J A. The molecular basis of dominance. Genetics. 1981;97:639–666. doi: 10.1093/genetics/97.3-4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kruckeberg A L. The hexose transporter family of Saccharomyces cerevisiae. Arch Microbiol. 1996;166:283–292. doi: 10.1007/s002030050385. [DOI] [PubMed] [Google Scholar]
- 17.Kruckeberg A L, Ye L, Berden J A, van Dam K. Functional expression, quantitation and cellular localization of the Hxt2 hexose transporter of Saccharomyces cerevisiae tagged with the green fluorescent protein. Biochem J. 1999;339:299–307. [PMC free article] [PubMed] [Google Scholar]
- 18.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 19.Ma H, Bloom L M, Walsh C T, Botstein D. The residual enzymatic phosphorylation activity of hexokinase II mutants is correlated with glucose repression in Saccharomyces cerevisiae. Mol Cell Biol. 1989;9:5643–5649. doi: 10.1128/mcb.9.12.5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meijer M M, Boonstra J, Verkleij A J, Verrips C T. Glucose repression in Saccharomyces cerevisiae is related to the glucose concentration rather than the glucose flux. J Biol Chem. 1998;273:24102–24107. doi: 10.1074/jbc.273.37.24102. [DOI] [PubMed] [Google Scholar]
- 21.Michels C A, Hahnenberger K M, Sylvestre Y. Pleiotropic mutations regulating resistance to glucose repression in Saccharomyces carlsbergensis are allelic to the structural gene for hexokinase B. J Bacteriol. 1983;153:574–578. doi: 10.1128/jb.153.1.574-578.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Özcan S, Freidel K, Leuker A, Ciriacy M. Glucose uptake and catabolite repression in dominant HTR1 mutants of Saccharomyces cerevisiae. J Bacteriol. 1993;175:5520–5528. doi: 10.1128/jb.175.17.5520-5528.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Özcan S, Vallier L G, Flick J S, Carlson M, Johnston M. Expression of the SUC2 gene of Saccharomyces cerevisiae is induced by low levels of glucose. Yeast. 1997;13:127–137. doi: 10.1002/(SICI)1097-0061(199702)13:2<127::AID-YEA68>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 24.Reifenberger E, Boles E, Ciriacy M. Kinetic characterization of individual hexose transporters of Saccharomyces cerevisiae and their relation to the triggering mechanisms of glucose repression. Eur J Biochem. 1997;245:324–333. doi: 10.1111/j.1432-1033.1997.00324.x. [DOI] [PubMed] [Google Scholar]
- 25.Reifenberger E, Freidel K, Ciriacy M. Identification of novel HXT genes in Saccharomyces cerevisiae reveals the impact of individual hexose transporters on glycolytic flux. Mol Microbiol. 1995;16:157–167. doi: 10.1111/j.1365-2958.1995.tb02400.x. [DOI] [PubMed] [Google Scholar]
- 26.Rosenzweig R F. Regulation of fitness in yeast overexpressing glycolytic enzymes: parameters of growth and viability. Genet Res. 1992;59:35–48. doi: 10.1017/s0016672300030159. [DOI] [PubMed] [Google Scholar]
- 27.Schaaff I, Heinisch J, Zimmermann F K. Overproduction of glycolytic enzymes in yeast. Yeast. 1989;5:285–290. doi: 10.1002/yea.320050408. [DOI] [PubMed] [Google Scholar]
- 28.Teusink B, Diderich J A, Westerhoff H V, van Dam K, Walsh M C. Intracellular glucose concentration in derepressed yeast cells consuming glucose is high enough to reduce the glucose transport rate by 50% J Bacteriol. 1998;180:556–562. doi: 10.1128/jb.180.3.556-562.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walsh M C, Scholte M, Valkier J, Smits H P, van Dam K. Glucose sensing and signalling properties in Saccharomyces cerevisiae require the presence of at least two members of the glucose transporter family. J Bacteriol. 1996;178:2593–2597. doi: 10.1128/jb.178.9.2593-2597.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walsh M C, Smits H P, Scholte M, van Dam K. Affinity of glucose transport in Saccharomyces cerevisiae is modulated during growth on glucose. J Bacteriol. 1994;176:953–958. doi: 10.1128/jb.176.4.953-958.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weirich J, Goffrini P, Kuger P, Ferrero I, Breunig K D. Influence of mutations in hexose-transporter genes on glucose repression in Kluyveromyces lactis. Eur J Biochem. 1997;249:248–257. doi: 10.1111/j.1432-1033.1997.t01-1-00248.x. [DOI] [PubMed] [Google Scholar]