ABSTRACT
Aims/Introduction
To investigate the relationship between the metabolic score for insulin resistance (METS‐IR) index and major adverse cardiac events (MACEs) and to compare its ability to predict MACEs with other IR indices including homeostatic model assessment for IR (HOMA‐IR) and triglyceride glucose (TyG) index‐related parameters.
Materials and Methods
We conducted a cohort study enrolling 7,291 participants aged ≥40 years. Binary logistic regression and restricted cubic splines were performed to determine the association between METS‐IR and MACEs, and the receiver operating curve (ROC) was utilized to compare the predictive abilities of IR indices and to determine the optimal cut‐off points.
Results
There were 348 (4.8%) cases of MACEs during a median follow‐up of 3.8 years. Compared with participants with a METS‐IR in the lowest quartile, the multivariate‐adjusted RRs and 95% CIs for participants with a METS‐IR in the highest quartile were 1.47 (1.05–2.77) in all participants, 1.42 (1.18–2.54) for individuals without diabetes, and 1.75 (1.11–6.46) for individuals with diabetes. Significant interactions were found between the METS‐IR and the risk of MACEs by sex in all participants and by age and sex in individuals without diabetes (all P values for interaction < 0.05). In the ROC analysis, the METS‐IR had a higher AUC value than other indices for predicting MACEs in individuals with diabetes and had a comparable or higher AUC than other indices for individuals without diabetes.
Conclusions
The METS‐IR can be an effective clinical indicator for identifying MACEs, as it had superior predictive power when compared with other IR indices in individuals with diabetes.
Keywords: Major adverse cardiovascular events, Metabolic score for insulin resistance, Predictor
Elevated METS‐IR was independently associated with a greater risk of MACEs in individuals with and without diabetes. The METS‐IR can be a useful clinical indicator for identifying MACEs, as it had superior predictive power for MACEs when compared with other IR indices in individuals with diabetes.
INTRODUCTION
Cardiovascular disease (CVD) is currently the leading reason for death in the world, which has brought huge public health and economic burdens 1 . The epidemic of diabetes mellitus has further accelerated the alarming increase in the incidence of CVD. Insulin resistance (IR) refers to the decrease of insulin sensitivity in peripheral tissues, which is manifested by defective glucose uptake and oxidation. IR promotes a proinflammatory state and leads to dyslipidemia according to pathophysiological studies 2 , in addition, IR itself, as a metabolic risk factor, is closely related to the pathogenesis of diabetes and CVD, often occurring before the diagnosis of the disease 3 , 4 . Therefore, it is critical to detect and treat IR before the onset of clinical disease. Several methods have been developed to assess IR. The hyperinsulinemic–euglycemic clamp, despite being widely accepted as the gold standard and the reference method for determining IR, is hard to apply in clinical practice, especially in population‐based epidemiological studies, due to its high costs and complexity 5 . Thus, it is of great clinical significance to identify rapidly available and reliable IR markers. Homeostatic model assessment of IR (HOMA‐IR) is an insulin‐based index which is frequently applied presently. Besides, it has also been proposed that the triglyceride–glucose (TyG) index which is made up of the fasting plasma glucose (FPG) and triglycerides (TGs), has a high sensitivity for identifying IR and shows superior performance to the HOMA‐IR in assessing IR irrespective of the diabetes status 6 , 7 . Furthermore, several modified TyG indices which combine the TyG index with anthropometric parameters such as waist circumference (WC), waist to height ratio (WHtR), or body mass index (BMI), have been shown to enhance the predictability of IR considering the impact of obesity and visceral fat deposition on IR 8 .
The metabolic score for IR (METS‐IR) index is a newly developed alternative biomarker for IR, calculated from FPG, TGs, high density lipoprotein cholesterol (HDL‐C), and BMI. The index shows a strong correlation with the IR measured by the hyperinsulinemic–euglycemic clamp technique and is associated with fasting insulin, and accumulation of intrahepatic, intravisceral, and subcutaneous fat, which is a key mechanism in the development of IR 9 . The positive correlation between the METS‐IR index and metabolism‐related or inflammation‐related diseases, including diabetes 9 , metabolic syndrome 10 , hypertension 11 , 12 , and nonalcoholic fatty liver disease 13 has been found by previous research, each of which are recognized as significant risk factors for developing CVD. Several studies have shown the association of HOMA‐IR or TyG‐related indices with arterial stiffness 14 , 15 and CVD 16 , 17 . However, limited information is available on the longitudinal association between METS‐IR and incident MACEs in the general population and few studies have addressed the critical question of whether METS‐IR is a more effective predictor of MACEs than other IR indices in different diabetes status.
Accordingly, in this retrospective cohort study, we investigated the relationship between the METS‐IR index and the risk of clinical outcomes, including MACEs, in individuals with and without diabetes. At the same time, we further compared the predictive ability of the METS‐IR with other IR indices, including HOMA‐IR, TyG, TyG‐BMI, TyG‐WHtR, and TyG‐WC, for detecting MACEs. This may help to determine the most appropriate IR index for prediction of the risk of MACEs.
MATERIALS AND METHODS
Study population
The study was part of the China Cardiometabolic Disease and Cancer Cohort (4C) Study to which participants were recruited from Tianmen city, Hubei Province. The baseline data were collected from 2011 to 2012 and the follow‐up visit was conducted from 2014 to 2016, with an average of 3.8 years 18 , 19 , 20 , 21 . Of the 10,999 study participants aged over 40 years, 9,221 participants completed the follow‐up survey for a response rate of 83.8%. As shown in Figure S1, we excluded individuals who had previously been diagnosed with myocardial infarction (MI) or ischemic stroke (N = 182), who currently use anti‐hypertension medication, dyslipidemia medication, aspirin, or hypoglycemic medications (N = 934), had missing and incomplete clinical or demographic data (N = 651) or had missing outcome data (N = 163). Consequently, 7,291 individuals (2,499 males and 4,792 females) were included in the research.
Study outcomes
The primary outcome was the MACEs, which were defined as a composite of CVD death, MIs, and ischemic strokes 22 . The secondary outcomes included death from all causes, hospitalization for heart failure (HF), and diabetes mellitus which was diagnosed based on the World Health Organization criteria 23 . All clinical outcomes were confirmed by complete hospital records and death certificates.
Clinical and biochemical evaluation
Trained staff collected data using a standard questionnaire. Information on lifestyle risk factors (smoking, drinking habits), medical history, and sociodemographic characteristics were recorded. Smoking one or more cigarettes per day for at least 6 months was defined as current smoking, while drinking one or more drinks per week for at least 6 months was defined as current drinking. All participants were required to fast overnight before the blood collection and undergo standard anthropometric assessments. The glucose metabolism status of participants was evaluated by the oral glucose tolerance test (OGTT). The enzymatic hexokinase method was used to determine FPG and 2 h post‐load plasma glucose (2 h PG) concentrations. The high‐performance liquid chromatography method was used to measure glycated hemoglobin (HbA1c). Systolic and diastolic blood pressure were tested three times using an automated sphygmomanometer after the patients had been sitting for at least 5 min before measurement. Hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg and (or) diastolic blood pressure (DBP) ≥ 90 mmHg, or the use of anti‐hypertensive medication. Dyslipidemia was defined as serum total cholesterol (TC) level ≥ 6.22 mmol/L, TG ≥ 2.26 mmol/L, low density lipoprotein cholesterol (LDL‐C) ≥ 4.14 mmol/L, or HDL‐C < 1.04 mmol/L. The calculation of eGFR was based on the serum creatinine according to the Modification of Diet in Renal Disease for Chinese equation 24 . The IR indices were calculated as follows: METS‐IR = In (2 × FPG [mg/dL] + fasting TG [mg/dL]) × BMI [kg/m2]/ In (HDL‐C [mg/dL]) 9 , HOMA‐IR = (fasting insulin [mIU/L] × FPG [mmol/L])/22.5 25 , TyG = In (fasting TG [mg/dL] × FPG [mg/dL]/2) 26 , TyG‐BMI = TyG × BMI, TyG‐WC = TyG × WC, and TyG‐WHtR = TyG × WHtR 27 .
Statistical analysis
For continuous variables, means and standard deviations (SDs) were used to express normally distributed data, while medians (interquartile ranges, IQRs) were used to express asymmetrically distributed data. The categorical variables are expressed by numbers (proportions). Kolmogorov–Smirnov tests were used to determine the normal distribution of the data. The Kruskal‐Wallis test for continuous variables and the χ2 test for categorical variables were used to obtain P values. Risk ratios (RRs) and 95% confidence intervals (CIs) of clinical outcomes with the METS‐IR (continuous and quartiles) were calculated through the use of binary logistic regression analysis. The variance inflation factor (VIF) was calculated to test the multicollinearity between the variables. A variable was regarded as having high multicollinearity and was excluded from the multivariate regression analysis if its VIF value was more than 5. The METS‐IR index quartile was used as the ordinal variable to compute P values for trends. Restricted cubic spline analyses were conducted to investigate the possible nonlinear relationships between METS‐IR levels and the occurrence of MACEs 28 , 29 . The knots were located at the 25th, 50th, 75th, and 95th percentiles of the METS‐IR index. The likelihood ratio was used to test the nonlinear relationship. Stratified analyses were performed based on age, sex, hyperlipemia, hypertension, or eGFR at baseline, and the interactions of those variables were tested. The predictive values of IR indices for MACEs were estimated using receiver operating characteristic (ROC) curves. The performance of indices was assessed by the area under the curve (AUC) and the optimal cut‐off values of each index were determined using Youden's index. The difference between IR indices was judged by using the DeLong test. The incremental predictive value of the METS‐IR index for MACEs except for established risk factors was evaluated by the C‐index, continuous net reclassification improvement (NRI), and integrated discrimination index (IDI) 30 . Two‐tailed P < 0.05 was regarded as statistically significant. SPSS version 26.0 software (IBM, Chicago, IL, USA) and R version 4.2.0 software were used for all analyses.
RESULTS
Baseline characteristics
A total of 7,291 eligible participants with an average age of 60.37 (53.68–67.70) years were included in the present study. Table 1 presents the baseline characteristics of participants according to the quartiles of the METS‐IR. Compared with the lowest quartile of the METS‐IR, participants in the other groups were more likely to have higher levels of FPG, 2 h PG, HbA1c, BMI, TG, TC, and LDL, and lower levels of HDL and eGFR (all P values < 0.001). The prevalence of current smoking, current drinking, hypertension, hyperlipidemia, and diabetes was also elevated with higher quartiles of the METS‐IR (all P values < 0.001).
Table 1.
Baseline characteristics of the study population according to METS‐IR quartiles
| Characteristics | METS‐IR Quartiles | All | P Value | |||
|---|---|---|---|---|---|---|
| Q1 (<29.63) | Q2 (29.63–33.26) | Q3 (33.27–37.74) | Q4 (>37.74) | – | ||
| N | 1,823 | 1,823 | 1,822 | 1,823 | 7,291 | – |
| Age (years) | 59.12 (52.80–66.08) | 59.45 (53.31–66.63) | 60.62 (53.73–68.02) | 62.68 (54.89–70.30) | 60.37 (53.68–67.70) | <0.001 |
| Male (%) | 729 (39.99) | 647 (35.49) | 580 (31.83) | 543 (29.79) | 2,499 (34.28) | <0.001 |
| BMI (kg/m2) | 20.20 (19.23–21.17) | 22.60 (21.64–23.42) | 24.34 (23.29–25.31) | 27.10 (25.46–28.57) | 23.32 (21.37–25.44) | <0.001 |
| WHR | 0.84 (0.80–0.88) | 0.86 (0.82–0.89) | 0.87 (0.83–0.90) | 0.89 (0.86–0.92) | 0.87 (0.83–0.90) | <0.001 |
| WHtR | 0.46 (0.43–0.50) | 0.49 (0.46–0.52) | 0.52 (0.49–0.55) | 0.55 (0.52–0.59) | 0.51 (0.47–0.55) | <0.001 |
| SBP (mmHg) | 145.50 (129.50–163.00) | 147.50 (132–165.50) | 148.42 (133.00–166.67) | 153.00 (138.00–170.33) | 148.5 (133.00–166.50) | <0.001 |
| DBP (mmHg) | 78.50 (71.00–87.00) | 80.00 (72.50–88.00) | 81.00 (73.50–90.00) | 84.00 (76.50–92.33) | 80.67 (73.50–89.50) | <0.001 |
| HbA1c (%) | 5.60 (5.30–5.80) | 5.60 (5.30–5.90) | 5.70 (5.40–5.90) | 5.80 (5.50–6.10) | 5.70 (5.40–5.90) | <0.001 |
| FPG (mmol/L) | 5.15 (4.82–5.54) | 5.23 (4.89–5.62) | 5.29 (4.93–5.70) | 5.48 (5.09–6.10) | 5.28 (4.93–5.72) | <0.001 |
| 2 h PG (mmol/L) | 5.99 (5.06–7.18) | 6.08 (5.17–7.29) | 6.28 (5.34–7.55) | 6.75 (5.53–8.42) | 6.25 (5.27–7.60) | <0.001 |
| Fasting insulin (pmol/L) | 3.80 (2.70–5.10) | 4.70 (3.50–6.20) | 5.60 (4.30–7.20) | 6.70 (5.30–8.40) | 5.20 (3.70–6.90) | <0.001 |
| Fasting HDL (mmol/L) | 1.73 (1.51–1.96) | 1.53 (1.35–1.74) | 1.38 (1.22–1.57) | 1.23 (1.09–1.40) | 1.46 (1.25–1.71) | <0.001 |
| Fasting LDL (mmol/L) | 2.65 (2.18–3.13) | 2.81 (2.29–3.31) | 2.89 (2.41–3.46) | 2.90 (2.39–3.48) | 2.80 (2.31–3.34) | <0.001 |
| Fasting TG (mmol/L) | 0.96 (0.76–1.25) | 1.12 (0.85–1.49) | 1.38 (1.02–1.82) | 1.96 (1.40–2.72) | 1.27 (0.92–1.81) | <0.001 |
| Cholesterol (mmol/L) | 4.93 (4.38–5.54) | 4.92 (4.35–5.54) | 4.98 (4.35–5.67) | 5.13 (4.46–5.81) | 4.98 (4.38–5.63) | <0.001 |
| Currently smoking (%) | 309 (16.95) | 343 (18.82) | 390 (21.41) | 493 (27.04) | 1,535 (21.05) | <0.001 |
| Currently drinking (%) | 431 (23.64) | 449 (24.63) | 467 (25.63) | 572 (31.38) | 1,919 (26.32) | <0.001 |
| Creatinine (μmol/L) | 61.00 (55.90–67.20) | 61.50 (56.40–67.40) | 61.00 (56.18–67.50) | 62.30 (56.80–69.20) | 61.5 (56.3–67.80) | <0.001 |
| eGFR (mL/min/1.73m2) | 113.97 (102.52–126.31) | 113.07 (101.93–124.39) | 112.61 (101.01–123.53) | 109.43 (98.31–120.24) | 112.29 (100.88–123.71) | <0.001 |
| DM at baseline (%) | 97 (5.3) | 105 (5.8) | 119 (6.5) | 281 (15.4) | 602 (8.3) | <0.001 |
| Hypertension (%) | 1,082 (59.35) | 1,145 (62.81) | 1,187 (65.15) | 1,336 (73.29) | 4,750 (65.15) | <0.001 |
| Hyperlipidemia (%) | 169 (9.3) | 213 (11.7) | 371 (20.4) | 844 (46.3) | 1,597 (21.9) | <0.001 |
| HOMA‐IR | 0.88 (0.64–1.20) | 1.10 (0.81–1.47) | 1.34 (1.00–1.77) | 1.71 (1.29–2.24) | 1.23 (0.86–1.71) | <0.001 |
| TyG | 8.29 (8.04–8.58) | 8.46 (8.17–8.75) | 8.68 (8.36–8.98) | 9.11 (8.72–9.49) | 8.59 (8.24–8.97) | <0.001 |
| TyG‐BMI | 168.26 (159.83–176.36) | 191.41 (183.51–198.35) | 210.60 (202.52–219.55) | 242.92 (231.34–259.44) | 200.27 (179.86–224.79) | <0.001 |
| TyG‐WC | 612.91 (571.84–660.70) | 660.39 (616.32–705.94) | 710.28 (661.41–762.97) | 799.61 (735.94–860.46) | 688.43 (624.34–763.58) | <0.001 |
| TyG‐WHtR | 3.85 (3.58–4.16) | 4.15 (3.90–4.45) | 4.49 (4.18–4.81) | 5.02 (4.66–5.44) | 4.34 (3.94–4.83) | <0.001 |
| Outcomes | ||||||
| Primary outcome | ||||||
| MACEs (%) | 63 (3.5) | 77 (4.2) | 90 (4.9) | 118 (6.5) | 348 (4.8) | <0.001 |
| CVD death (%) | 30 (1.6) | 33 (1.8) | 40 (2.2) | 54 (3.0) | 157 (2.2) | 0.030 |
| MI (%) | 9 (0.5) | 10 (0.5) | 11 (0.6) | 20 (1.1) | 50 (0.7) | 0.102 |
| Stroke (%) | 24 (1.3) | 34 (1.9) | 39 (2.1) | 46 (2.5) | 143 (2.0) | 0.062 |
| Secondary outcomes | ||||||
| All‐cause death (%) | 75 (4.1) | 53 (2.9) | 74 (4.1) | 109 (6.0) | 311 (4.3) | <0.001 |
| HF (%) | 8 (0.4) | 13 (0.7) | 12 (0.7) | 16 (0.9) | 49 (0.7) | 0.442 |
| Newly diagnosed DM at visit (%) | 133 (7.3) | 115 (6.3) | 165 (9.1) | 263 (14.4) | 676 (9.3) | <0.001 |
Values are medians (interquartile ranges) for continuous variables with skewed distributions and numbers (proportions) for categorical variables. MACEs were defined as the composite of cardiovascular death, myocardial infarction, and ischemic stroke. Values in bold indicate statistical significance (P value < 0.05). BMI, body‐mass index; CVD, cardiovascular disease; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin A1c; HDL, high density lipoprotein; HF, hospitalization for heart failure; HOMA‐IR, homeostasis model assessment of insulin resistance; LDL, low density lipoprotein; MACEs, major adverse cardiovascular events; METS‐IR, metabolic score for insulin resistance; MI, myocardial infarction; SBP, systolic blood pressure; TG, triglycerides; TyG, triglyceride‐glucose index; WC, waist circumference; WHR, waist–hip ratio; WHtR, waist to height ratio; 2 h PG, 2 h plasma glucose concentration.
MACEs increased with METS‐IR
Of the 7,291 participants, 348 (4.8%) individuals developed MACEs, including 157 (2.2%) CVD deaths, 50 (0.7%) MIs, and 143 (2.0%) ischemic strokes during a mean follow‐up of 3.8 years (Table 1). Further analyses revealed that the incidence of MACEs increased with the METS‐IR quartile level in all subjects (P values < 0.05, Table 1) and in the subjects with or without diabetes (all P values < 0.05, Table S1). The incidences of CVD death (P values < 0.05, Table 1) were elevated with quartiles of METS‐IR in all subjects but were not found in individuals with or without diabetes (Table S1). In addition, the incidences of all‐cause mortality and diabetes (all P values < 0.05, Table S1) were elevated with quartiles of METS‐IR only in individuals without diabetes.
The relationship between METS‐IR levels and MACEs was determined using binary logistic regression analyses (Table 2). Elevated METS‐IR were significant independent predictive indicators of incident MACEs, no matter whether considered as a categorical or continuous variable. In all participants, the RRs (95% CIs) for MACEs were 1.47 (1.05–2.77) for the highest METS‐IR quartile when compared with the lowest quartile, and a 38% increased risk of MACEs was associated with each SD increase in METS‐IR levels after adjusting for several covariables. We further analyzed the association of METS‐IR levels with MACEs in individuals without and with diabetes. In the fully adjusted model (model 3), RRs (95% CIs) for MACEs were 1.42 (1.18–2.54) in the individuals without diabetes and 1.75 (1.11–6.46) in individuals with diabetes for the corresponding highest METS‐IR quartiles when compared with the lowest quartile, and each SD increase in METS‐IR levels was associated with a 36% increased risk of MACEs among individuals without diabetes and a 59% increased risk of MACEs among individuals with diabetes. The trend test for incident MACEs was significant with increasing METS‐IR quartiles in all participants and the participants stratified by diabetes status (all P for trend < 0.05).
Table 2.
Risk ratios (95% CI) for MACEs according to quartiles of METS‐IR
| METS‐IR index | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| RR (95% CI) | P Value | RR (95% CI) | P Value | RR (95% CI) | P Value | |
| All participants | ||||||
| Q1 (<29.63) | Reference | Reference | Reference | |||
| Q2 (29.63–33.26) | 1.23 (0.88–1.73) | 0.227 | 1.27 (0.90–1.78) | 0.174 | 1.18 (0.95–1.91) | 0.099 |
| Q3 (33.27–37.74) | 1.45 (1.04–2.02) | 0.026 | 1.53 (1.09–2.13) | 0.013 | 1.26 (1.12–2.27) | 0.010 |
| Q4 (>37.74) | 1.93 (1.41–2.64) | 0.000 | 2.08 (1.50–2.88) | 0.000 | 1.47 (1.05–2.77) | 0.000 |
| P for trend | 0.000 | 0.000 | 0.015 | |||
| Per SD increment | 1.44 (1.31–1.58) | 0.000 | 1.50 (1.36–1.66) | 0.000 | 1.38 (1.22–1.57) | 0.000 |
| Without diabetes | ||||||
| Q1 (<29.51) | Reference | Reference | Reference | |||
| Q2 (29.51–33.03) | 1.36 (0.95–1.93) | 0.091 | 1.39 (0.97–1.97) | 0.071 | 1.29 (1.03–2.14) | 0.035 |
| Q3 (33.04–37.28) | 1.53 (1.08–2.16) | 0.016 | 1.59 (1.12–2.25) | 0.009 | 1.34 (1.17–2.47) | 0.005 |
| Q4 (>37.28) | 1.68 (1.20–2.36) | 0.003 | 1.76 (1.25–2.48) | 0.001 | 1.42 (1.18–2.54) | 0.005 |
| P for trend | 0.002 | 0.000 | 0.001 | |||
| Per SD increment | 1.40 (1.26–1.56) | 0.000 | 1.44 (1.28–1.61) | 0.000 | 1.36 (1.19–1.56) | 0.000 |
| Diabetes | ||||||
| Q1 (<31.21) | Reference | Reference | Reference | |||
| Q2 (31.21–36.67) | 1.16 (0.41–3.28) | 0.781 | 1.41 (0.51–4.38) | 0.535 | 1.14 (0.44–4.46) | 0.570 |
| Q3 (36.68–43.46) | 1.31 (0.48–3.62) | 0.599 | 1.78 (0.64–5.29) | 0.299 | 1.23 (0.43–6.22) | 0.475 |
| Q4 (>43.46) | 2.61 (1.05–6.49) | 0.039 | 2.81 (1.38–6.85) | 0.010 | 1.75 (1.11–6.46) | 0.025 |
| P for trend | 0.027 | 0.030 | 0.004 | |||
| Per SD increment | 1.62 (1.22–2.15) | 0.001 | 1.83 (1.35–2.47) | 0.000 | 1.59 (1.11–2.89) | 0.018 |
Model 1 was unadjusted. Model 2 was adjusted for age, sex and WHR. Model 3 was adjusted for all variables in Model 2 plus HbA1c, fasting insulin, LDL, TC, hypertension, hyperlipidemia, current smoking, current drinking, and eGFR at baseline. Values in bold indicate statistical significance (P value < 0.05). CI, confidence interval; MACEs, major adverse cardiovascular events; METS‐IR, metabolic score for insulin resistance; RR, risk ratio; SD, standard deviation.
We also performed analyses for the relationship between the METS‐IR and each component of the MACEs (CVD death, MIs, and ischemic strokes) and each of the secondary clinical outcomes (all‐cause death, hospitalization for HF, and diabetes) in individuals with and without diabetes. Elevated METS‐IR levels were linked to an increasing risk of all‐cause death and diabetes among individuals without diabetes. As shown in Table S2, after adjusting for covariables, the participants in the highest quartile of the METS‐IR had a significantly higher risk of all‐cause death (RR 1.32, 95% CI 1.05–1.96; model 3) and diabetes (RR 2.25; 95% CI 1.72–2.93; model 3) than participants in the lowest quartile of the METS‐IR. However, no association was found between the METS‐IR and any single component of the MACEs or secondary clinical outcomes in individuals with diabetes.
As shown in Figure 1, multivariable‐adjusted restricted cubic spline analyses showed that METS‐IR levels were nonlinearly associated with the risk of MACEs in all participants (P for total < 0.001, P for nonlinear trend < 0.001; Figure 1a). After adjusting for covariables, a significant nonlinear relationship between METS‐IR levels and MACEs in individuals without diabetes (P for total < 0.001, P for nonlinear trend < 0.001; Figure 1b) and a linear relationship in individuals with diabetes (P for total <0.001, P for nonlinear trend = 0.892; Figure 1c) were indicated by further analysis.
Figure 1.
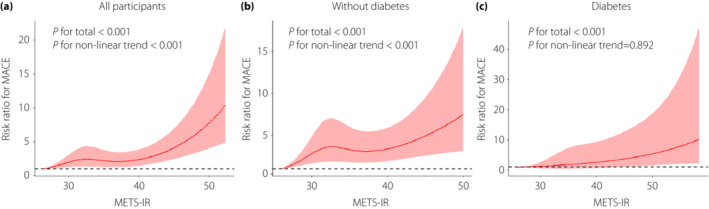
Multivariable‐adjusted risk ratios for MACEs in all participants (a), individuals without diabetes (b), and individuals with diabetes (c). The solid lines indicate multivariate‐adjusted risk ratios, and the shaded area indicates the 95% CIs derived from restricted cubic spline regression. Restricted cubic spline analysis has four knots at the 25th, 50th, 75th, and 95th percentiles for the METS‐IR index. The logistic regression was adjusted for age, sex, HbA1c, fasting insulin, LDL, TC, WHR, hypertension, hyperlipidemia, current smoking, current drinking, and eGFR at baseline.
Stratified analyses of MACEs
To explore the associations between METS‐IR and MACEs in more detail, we conducted stratified analyses based on age, sex, hyperlipemia, blood pressure, and eGFR at baseline (Figure 2). In all participants, we observed that the risk estimates for incident MACEs were mostly similar across subgroups except in subjects with eGFR <60 mL/min/1.73m2 (P value > 0.05). Significant interactions were found by sex (P for interaction = 0.005, Figure 2a) and the correlation seemed to be far more pronounced in females than in males. Further analyses showed that in individuals without diabetes, the association was diminished in individuals with an age ≤ 60 years or eGFR < 60 mL/min/1.73m2 (all P values > 0.05). The METS‐IR and age and sex had significant interactions (P for interaction < 0.05, Figure 2b). In individuals with diabetes, the association between METS‐IR levels and MACEs was diminished in males, in individuals with eGFR < 60 mL/min/1.73m2, and in individuals without hyperlipidemia or hypertension (all P values < 0.05, Figure 2c).
Figure 2.
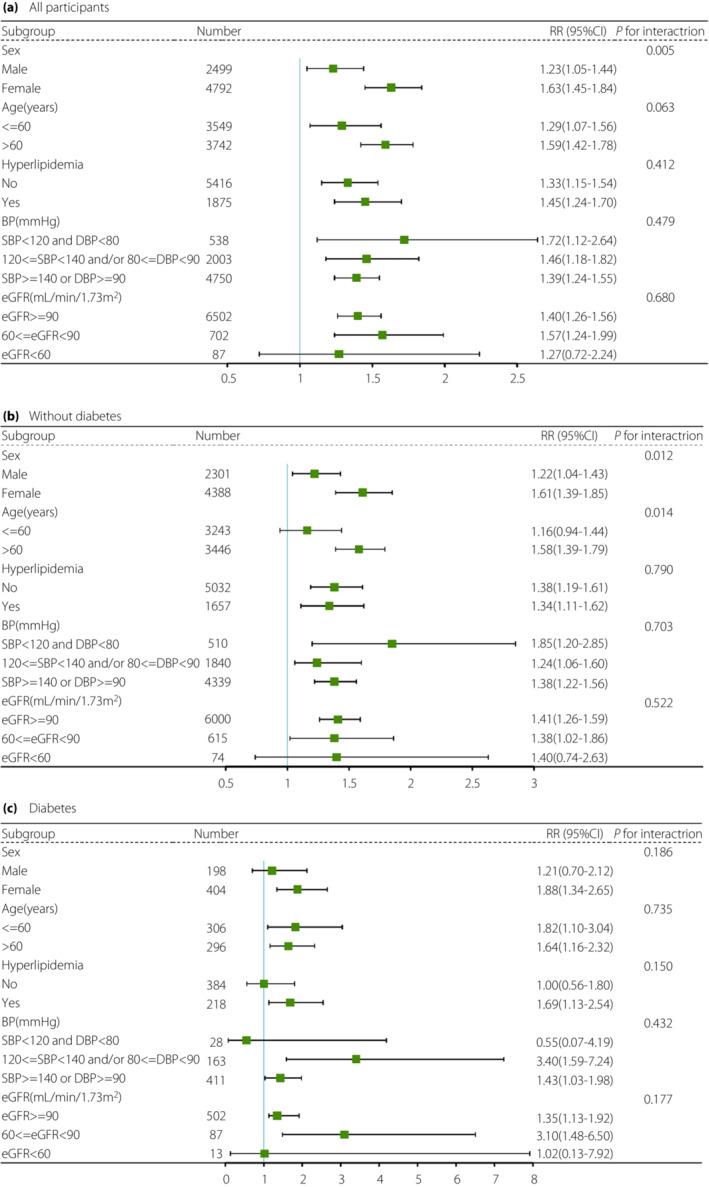
Stratified analyses of the RRs of MACEs according to per‐SD increments in METS‐IR in all participants (a), individuals without diabetes (b), and individuals with diabetes (c). The final model adjusted for age, sex, HbA1c, fasting insulin, LDL, TC, WHR, hypertension, hyperlipidemia, current smoking, current drinking, and eGFR at baseline, except for the strata variable. BP, blood pressure; eGFR, estimated glomerular filtration rate.
Comparison of the METS‐IR with other IR indices in predicting MACEs
The results of the comparison of ROC curves for predicting the incidence rate of MACEs by METS‐IR, HOMA‐IR, TyG, TyG‐BMI, TyG‐WHtR, and TyG‐WC are shown in Table 3. In all participants, the highest AUC was achieved by TyG [AUC (95% CI): 0.600 (0.589–0.612)], followed by HOMA‐IR [AUC (95% CI): 0.599 (0.588–0.611)] and METS‐IR [AUC (95% CI): 0.596 (0.585–0.607); P for comparison > 0.05]. The optimal cut‐offs of TyG, HOMA‐IR, and METS‐IR were 8.392, 1.128, and 28.529, respectively. Further analyses showed that in individuals without diabetes, the highest AUC was achieved by HOMA‐IR [AUC (95% CI): 0.597 (0.586–0.609)], followed by TyG [AUC (95% CI): 0.594 (0.582–0.605)] and METS‐IR [AUC (95% CI): 0.588 (0.576–0.599); P for comparison > 0.05]. However, in individuals with diabetes, METS‐IR [AUC (95% CI): 0.635 (0.595–0.674); P for comparison < 0.05] had the highest diagnostic value for MACEs among the IR indices, and, with a sensitivity of 63.4% and a specificity of 61.5%, a METS‐IR of 39.361 was determined to be the best cut‐off point for detecting MACEs.
Table 3.
Receiver operating characteristic analysis for the prediction of MACEs by IR indices
| Variables | AUC (95% CI) | Cut‐off value | Specificity | Sensitivity | P for comparison |
|---|---|---|---|---|---|
| All participants | |||||
| METS‐IR | 0.596 (0.585–0.607) | 28.529 | 0.546 | 0.627 | – |
| HOMA‐IR | 0.599 (0.588–0.611) | 1.128 | 0.503 | 0.673 | 0.835 |
| TyG | 0.600 (0.589–0.612) | 8.392 | 0.461 | 0.725 | 0.744 |
| TyG‐BMI | 0.571 (0.559–0.582) | 170.474 | 0.592 | 0.574 | 0.000 |
| TyG‐WHtR | 0.561 (0.550–0.573) | 4.236 | 0.490 | 0.667 | 0.014 |
| TyG‐WC | 0.547 (0.536–0.559) | 670.524 | 0.487 | 0.648 | 0.001 |
| Without diabetes | |||||
| METS‐IR | 0.588 (0.576–0.599) | 28.492 | 0.462 | 0.730 | – |
| HOMA‐IR | 0.597 (0.586–0.609) | 1.116 | 0.458 | 0.765 | 0.567 |
| TyG | 0.594 (0.582–0.605) | 8.219 | 0.483 | 0.728 | 0.678 |
| TyG‐BMI | 0.564 (0.552–0.576) | 168.304 | 0.657 | 0.514 | 0.000 |
| TyG‐WHtR | 0.553 (0.541–0.565) | 4.089 | 0.486 | 0.662 | 0.030 |
| TyG‐WC | 0.538 (0.526–0.550) | 690.675 | 0.619 | 0.521 | 0.004 |
| Diabetes | |||||
| METS‐IR | 0.635 (0.595–0.674) | 39.361 | 0.615 | 0.634 | – |
| HOMA‐IR | 0.598 (0.558–0.637) | 2.345 | 0.692 | 0.537 | 0.021 |
| TyG | 0.593 (0.553–0.633) | 9.163 | 0.582 | 0.629 | 0.019 |
| TyG‐BMI | 0.567 (0.526–0.607) | 198.591 | 0.453 | 0.734 | 0.000 |
| TyG‐WHtR | 0.587 (0.546–0.626) | 5.402 | 0.726 | 0.473 | 0.013 |
| TyG‐WC | 0.559 (0.519–0.599) | 739.920 | 0.459 | 0.692 | 0.018 |
P for significance of comparison between METS‐IR and other IR indices. Values in bold indicate statistical significance (P value < 0.05). AUC, area under the curve; CI, confidential interval; MACEs, major adverse cardiovascular events; METS‐IR, metabolic score for insulin resistance.
Additive effect of the METS‐IR index on established risk factors
We examined the C‐index, continuous NRI, and IDI to determine the incremental predictive value of the METS‐IR index for MACEs risk prediction (Table 4). In all participants, the addition of the METS‐IR index significantly improved the C‐index of the baseline risk model (from 0.707 to 0.716, P < 0.001). The risk reclassification and discrimination also significantly improved, with a continuous NRI of 0.173 (95% CI 0.063–0.292; P = 0.003) and an IDI of 0.010 (95% CI 0.006–0.014; P < 0.001). Similar results were observed in individuals with and without diabetes. These results suggested that the METS‐IR index has significant incremental predictive values beyond the established risk factors for incident MACEs.
Table 4.
Discrimination of each predictive model for MACEs using the C‐index, continuous NRI, and IDI
| C‐index (95% CI) | P Value | Continuous NRI (95% CI) | P Value | IDI (95% CI) | P Value | |
|---|---|---|---|---|---|---|
| All participants | ||||||
| Established risk factors | 0.707 (0.678–0.737) | Reference | Reference | |||
| Established risk factors + METS‐IR | 0.716 (0.686–0.746) | <0.001 | 0.173 (0.063–0.292) | 0.003 | 0.010 (0.006–0.014) | <0.001 |
| Without diabetes | ||||||
| Established risk factors | 0.698 (0.667–0.729) | Reference | Reference | |||
| Established risk factors + METS‐IR | 0.707 (0.675–0.738) | <0.001 | 0.155 (0.032–0.284) | 0.014 | 0.008 (0.004–0.011) | <0.001 |
| Diabetes | ||||||
| Established risk factors | 0.801 (0.725–0.876) | Reference | Reference | |||
| Established risk factors + METS‐IR | 0.817 (0.742–0.891) | <0.001 | 0.427 (0.037–0.833) | 0.043 | 0.023 (0.002–0.044) | 0.028 |
Established risk factors included age, sex, HbA1c, 2 h PG, fasting insulin, LDL, TC, WHR, hypertension, hyperlipidemia, current smoking, and current drinking. Values in bold indicate statistical significance (P value < 0.05). NRI, net reclassification index; IDI, integrated discrimination improvement; MACEs, major adverse cardiovascular event; METS‐IR, metabolic score for insulin resistance.
DISCUSSION
In this cohort study, we found that elevated levels of METS‐IR were positively associated with the risk of MACEs, and this association was independent of diabetes status. Moreover, the METS‐IR is a good indicator for MACEs compared with other IR indices, including HOMA‐IR, TyG, TyG‐BMI, TyG‐WHtR, and TyG‐WC. The METS‐IR showed better predictive ability for MACEs among individuals with diabetes and better or comparable predictive ability for MACEs in the total population and among individuals without diabetes. Adding the METS‐IR index to the model of established risk factors significantly improved the prediction value of MACEs.
Insulin resistance (IR) has been linked to an increased risk of cardiovascular disease as a component of metabolic syndrome 31 and its relationship with atherosclerosis has even been demonstrated in individuals without diabetes 32 . The METS‐IR index is a relatively new parameter for the assessment of IR and was first proposed in 2018 9 . They found that the use of the METS‐IR index was effective in predicting hypertension and arterial stiffness 33 . Recently, several studies have also shown similar findings 11 , 12 , 34 , 35 , 36 and therefore suggested a correlation between the METS‐IR index and CVD, as arterial stiffness and hypertension are the pathological basis and independent risk factors for CVD 37 , respectively. In our study, compared with the lowest quartile of METS‐IR, participants in the other groups had higher levels of FPG, 2 h PG, HbA1c, BMI, TG, TC, and LDL, and lower levels of HDL and eGFR. Additionally, the prevalence of current smoking, current drinking, hypertension, hyperlipidemia, and diabetes, all of which are classic risk factors for MACEs, was also elevated with an increase in METS‐IR. Our results that elevated levels of the METS‐IR index were independently associated with increased MACEs in individuals with and without diabetes suggested that the METS‐IR index might influence MACEs independently from diabetes. A previous study also reported the independent predictive value of the METS‐IR index among subjects without diabetes 38 . Intriguingly, our results further suggested that METS‐IR had stronger predictive power in individuals with diabetes than in those without diabetes since multivariable‐adjusted RRs for MACEs were 1.42 in the individuals without diabetes and 1.75 in individuals with diabetes for the corresponding fourth METS‐IR quartiles, and each SD increase in METS‐IR levels was associated with a 36% increased risk for MACEs in individuals without diabetes and a 59% increased risk for MACEs in individuals with diabetes. This may be because patients with diabetes likely have excess risk factors associated with MACEs 39 . Additionally, we found that the METS‐IR index was strongly correlated with the risk of all‐cause death and diabetes among individuals without diabetes, which is in agreement with previous studies 40 , 41 . A recent study 42 showed that METS‐IR was nonlinearly associated with all‐cause mortality and CVD‐related mortality in patients with diabetes, which is consistent with our findings because, in our study, the number of all‐cause and CVD deaths in the individuals with diabetes also increased with the elevation of METS‐IR, although this change was not statistically significant due to the lower event number. It is noteworthy that a linear relationship between METS‐IR levels and MACEs was observed in individuals with diabetes, whereas a nonlinear relationship was observed in those without diabetes. In individuals without diabetes, low‐grade IR may help to mobilize stored energy in response to infection or stress 43 and to maintain homeostasis in the internal environment, and therefore compensate for the adverse cardiovascular effects of hyperglycemia and dyslipidemia through enhanced cellular defense and anti‐oxidative stress 44 , 45 . This finding highlighted the importance of early clinical intervention to prevent cardiovascular events.
Additionally, we found that in most subgroups, the relationship between the METS‐IR index and MACEs was stable and persistent. It is worth noting that there was a statistically significant interaction between sex and METS‐IR for MACEs in all participants. The present research also revealed that sex and age should be essential considerations when referring to predicting MACEs in individuals without diabetes. Similarly, a previous study 46 reported that IR surrogate markers were linked to increased arterial stiffness and the relationship was stronger in females than in males. A cohort study in Tehran also reported that the elevated TyG index with an increased risk of CVD was only observed in females 47 . Moreover, recent findings demonstrate a sex‐specific interpretation of the connection between circulating cytokine levels and METS‐IR in females 48 . Sex differences in fat distribution and sex‐specific risk factors including menopause, reproductive endocrine disorders, and pregnancy complications may partially account for this phenomenon 49 . Collectively, understanding sex differences will aid in the development of individualized MACEs prevention and treatment interventions.
Our study directly compared the predictive values of METS‐IR with HOMA‐IR and TyG‐related indices in assessing MACEs risk. We identified that the METS‐IR had the best predictive power for MACEs among all indices for individuals with diabetes, as the METS‐IR had the highest AUC value in the ROC analysis. For individuals without diabetes, its performance was mediocre, as it had similar AUC values with HOMA‐IR and TyG. Similarly, METS‐IR has been shown to be superior to the TyG index in the diagnosis of diabetes 9 . In patients with NAFLD, Lee et al. found that METS‐IR had a higher predictive value for advanced liver fibrosis than the TyG index and HOMA‐IR 50 , which was consistent with the main finding from our study. Glucose and lipid metabolism disorders are key mechanisms leading to MACEs. METS‐IR incorporates FPG, blood lipids, and BMI, all of which represent general nutrition status, providing a more comprehensive reflection of metabolic dysfunction than HOMA‐IR and TyG. HOMA‐IR, despite including fasting insulin values, may not reflect the degree of IR well in individuals with diabetes as insulin levels decrease with islet β cell failure 51 . Moreover, compared with TyG‐BMI, TyG‐WHtR, and TyG‐WC, METS‐IR includes the HDL‐C parameter, an essential protective factor of CVD. HDL‐C has anti‐inflammatory, anti‐oxidative, and anti‐apoptotic effects, which make it an important contributor to overall cardiovascular health 52 . Moreover, our findings further suggested that the addition of METS‐IR index can predict the high risk of MACEs more accurately than models containing only established risk factors. As a simple measurement index, METS‐IR may have more applications in clinical practice. In terms of public health, the early identification of disease risk and timely intervention by calculating METS‐IR index may effectively reduce the incidence of MACEs in individuals.
There are several strengths to our research. First, this is the first large cohort study to explore the relationship between the METS‐IR and MACEs and to compare its performance with other IR indices in individuals with and without diabetes. Second, this is a community population‐based study with a relatively large sample size, and the results can be representative of the general population. Third, the use of a vigorous quality assurance program and standardized protocol for anthropometric measurements guarantee the accuracy of the study results. However, several limitations of the present study should be considered. First, the study was conducted among Chinese adults aged ≥40 years, which may limit the generalizability of our findings. Nevertheless, since cardiovascular events primarily occur in the elderly population, our results are practically applicable. Second, although we adjusted for confounding factors, the retrospective design may have introduced selection bias. However, the large sample size and complete follow‐up of this study partially mitigate this bias. Third, we only used baseline values of the METS‐IR index and other risk factors and did not account for changes in these parameters over the follow‐up period. Last, although our study had a large sample size, the limited number of events and short follow‐up duration may have reduced the statistical power. Therefore, to confirm our findings, further extensive research with longer follow‐up times is necessary.
In conclusion, our study confirmed that the METS‐IR index independently predicted incident MACEs in individuals with and without diabetes. Moreover, we found that the METS‐IR has a superior ability to predict MACEs than other indices (HOMA‐IR, TyG, TyG‐BMI, TyG‐WHtR, TyG‐WC), and its advantage was particularly pronounced for individuals with diabetes. These findings suggested that monitoring the METS‐IR index can assist in identifying high cardiovascular risk individuals and in providing active intervention.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: The medical ethics committee of Ruijin Hospital, Shanghai Jiao Tong University approved this study protocol.
Informed consent: Ethics approval and informed consent was obtained from each study participant.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Supporting information
Figure S1 Flow diagram for the study population selection.
Table S1 Outcomes according to quartile of METS‐IR level in individuals without and with diabetes
Table S2 Risk ratios for clinical outcomes according to quartiles of METS‐IR in individuals without and with diabetes
ACKNOWLEDGMENTS
We thank all the staff and participants of the 4C Study for making this study possible. The National Natural Science Foundation of China provided funding for this study (82270880, 81974109, 81570740).
Contributor Information
Yan Yang, Email: yangyan6910@163.com.
Xuefeng Yu, Email: xfyu188@163.com.
DATA AVAILABILITY STATEMENT
The data analyzed in the current study are available upon reasonable request from the corresponding author.
REFERENCES
- 1. Roth GA, Forouzanfar MH, Moran AE, et al. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med 2015; 372: 1333–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab 2011; 14: 575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reaven GM. Insulin resistance: the link between obesity and cardiovascular disease. Med Clin North Am 2011; 95: 875–892. [DOI] [PubMed] [Google Scholar]
- 4. DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard lecture 2009. Diabetologia 2010; 53: 1270–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979; 237: E214–E223. [DOI] [PubMed] [Google Scholar]
- 6. Guerrero‐Romero F, Simental‐Mendía LE, González‐Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic‐hyperinsulinemic clamp. J Clin Endocrinol Metab 2010; 95: 3347–3351. [DOI] [PubMed] [Google Scholar]
- 7. Vasques ACJ, Novaes FS, de Oliveira MS, et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract 2011; 93: e98–e100. [DOI] [PubMed] [Google Scholar]
- 8. Lim J, Kim J, Koo SH, et al. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: an analysis of the 2007‐2010 Korean National Health and nutrition examination survey. PLoS One 2019; 14: e0212963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bello‐Chavolla OY, Almeda‐Valdes P, Gomez‐Velasco D, et al. METS‐IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur J Endocrinol 2018; 178: 533–544. [DOI] [PubMed] [Google Scholar]
- 10. Mirr M, Skrypnik D, Bogdański P, et al. Newly proposed insulin resistance indexes called TyG‐NC and TyG‐NHtR show efficacy in diagnosing the metabolic syndrome. J Endocrinol Invest 2021; 44: 2831–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Han K‐Y, Gu J, Wang Z, et al. Association between METS‐IR and prehypertension or hypertension among Normoglycemia subjects in Japan: a retrospective study. Front Endocrinol 2022; 13: 851338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang X, Yu C, Ye R, et al. Correlation between non‐insulin‐based insulin resistance indexes and the risk of prehypertension: a cross‐sectional study. J Clin Hypertens (Greenwich) 2022; 24: 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cai X, Gao J, Hu J, et al. Dose‐response associations of metabolic score for insulin resistance index with nonalcoholic fatty liver disease among a nonobese Chinese population: retrospective evidence from a population‐based cohort study. Dis Markers 2022; 2022: 4930355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu S, Xu L, Wu M, et al. Association between triglyceride‐glucose index and risk of arterial stiffness: a cohort study. Cardiovasc Diabetol 2021; 20: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ho C‐T, Lin C‐C, Hsu H‐S, et al. Arterial stiffness is strongly associated with insulin resistance in Chinese—a population‐based study (Taichung Community Health Study, TCHS). J Atheroscler Thromb 2011; 18: 122–130. [DOI] [PubMed] [Google Scholar]
- 16. Yang K, Liu W. Triglyceride and glucose index and sex differences in relation to major adverse cardiovascular events in hypertensive patients without diabetes. Front Endocrinol 2021; 12: 761397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bonora E, Formentini G, Calcaterra F, et al. HOMA‐estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona diabetes complications study. Diabetes Care 2002; 25: 1135–1141. [DOI] [PubMed] [Google Scholar]
- 18. Ning G. Risk evaluation of cAncers in Chinese diabeTic individuals: a lONgitudinal (REACTION) study. J Diabetes 2012; 4: 172–173. [DOI] [PubMed] [Google Scholar]
- 19. Bi Y, Lu J, Wang W, et al. Cohort profile: risk evaluation of cancers in Chinese diabetic individuals: a longitudinal (REACTION) study. J Diabetes 2014; 6: 147–157. [DOI] [PubMed] [Google Scholar]
- 20. Ning G, Bloomgarden Z. Diabetes and cancer: findings from the REACTION study: REACTION. J Diabetes 2015; 7: 143–144. [DOI] [PubMed] [Google Scholar]
- 21. Lu J, Bi Y, Wang T, et al. The relationship between insulin‐sensitive obesity and cardiovascular diseases in a Chinese population: results of the REACTION study. Int J Cardiol 2014; 172: 388–394. [DOI] [PubMed] [Google Scholar]
- 22. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 23. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998; 15: 539–553. [DOI] [PubMed] [Google Scholar]
- 24. Ma Y‐C, Zuo L, Chen J‐H, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 2006; 17: 2937–2944. [DOI] [PubMed] [Google Scholar]
- 25. Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 26. Li H, Zuo Y, Qian F, et al. Triglyceride‐glucose index variability and incident cardiovascular disease: a prospective cohort study. Cardiovasc Diabetol 2022; 21: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Song K, Park G, Lee HS, et al. Comparison of the triglyceride glucose index and modified triglyceride glucose indices to predict nonalcoholic fatty liver disease in youths. J Pediatr 2022; 242: 79–85.e1. [DOI] [PubMed] [Google Scholar]
- 28. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989; 8: 551–561. [DOI] [PubMed] [Google Scholar]
- 29. Smith PL. Splines as a useful and convenient statistical tool. Am Stat 1979; 33: 57–62. [Google Scholar]
- 30. Pencina MJ, D'Agostino RB, Pencina KM, et al. Interpreting incremental value of markers added to risk prediction models. Am J Epidemiol 2012; 176: 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol 2014; 10: 293–302. [DOI] [PubMed] [Google Scholar]
- 32. Mehta R, Antonio‐Villa NE, Bello‐Chavolla OY, et al. Association between insulin resistance and arterial stiffness in Mexican patients without type 2 diabetes. Gac Med Mex 2021; 157: 522–530. [DOI] [PubMed] [Google Scholar]
- 33. Bello‐Chavolla OY, Antonio‐Villa NE, Vargas‐Vázquez A, et al. Prediction of incident hypertension and arterial stiffness using the non‐insulin‐based metabolic score for insulin resistance (METS‐IR) index. J Clin Hypertens (Greenwich) 2019; 21: 1063–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang X, Ye R, Yu C, et al. Correlation between non‐insulin‐based insulin resistance indices and increased arterial stiffness measured by the cardio‐ankle vascular index in non‐hypertensive Chinese subjects: a cross‐sectional study. Front Cardiovasc Med 2022; 9: 903307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu XZ, Fan J, Pan SJ. METS‐IR, a novel simple insulin resistance indexes, is associated with hypertension in normal‐weight Chinese adults. J Clin Hypertens (Greenwich) 2019; 21: 1075–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gu Q, Meng J, Hu X, et al. Isolated systolic hypertension and insulin resistance assessment tools in young and middle‐aged Chinese men with normal fasting glucose: a cross‐sectional study. Sci Rep 2022; 12: 758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hill MA, Yang Y, Zhang L, et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism 2021; 119: 154766. [DOI] [PubMed] [Google Scholar]
- 38. Yoon J, Jung D, Lee Y, et al. The metabolic score for insulin resistance (METS‐IR) as a predictor of incident ischemic heart disease: a longitudinal study among Korean without diabetes. J Pers Med 2021; 11: 742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stitziel NO, Kanter JE, Bornfeldt KE. Emerging targets for cardiovascular disease prevention in diabetes. Trends Mol Med 2020; 26: 744–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ausk KJ, Boyko EJ, Ioannou GN. Insulin resistance predicts mortality in nondiabetic individuals in the U.S. Diabetes Care 2010; 33: 1179–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cai X‐T, Zhu Q, Liu S‐S, et al. Associations between the metabolic score for insulin resistance index and the risk of type 2 diabetes mellitus among non‐obese adults: insights from a population‐based cohort study. Int J Gen Med 2021; 14: 7729–7740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang Z, Xie J, Wang J, et al. Association between a novel metabolic score for insulin resistance and mortality in people with diabetes. Front Cardiovasc Med 2022; 9: 895609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tsatsoulis A, Mantzaris MD, Bellou S. Insulin resistance: an adaptive mechanism becomes maladaptive in the current environment—an evolutionary perspective. Metabolism 2013; 62: 622–633. [DOI] [PubMed] [Google Scholar]
- 44. Hoehn KL, Salmon AB, Hohnen‐Behrens C, et al. Insulin resistance is a cellular antioxidant defense mechanism. Proc Natl Acad Sci USA 2009; 106: 17787–17792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nolan CJ, Ruderman NB, Kahn SE, et al. Insulin resistance as a physiological defense against metabolic stress: implications for the management of subsets of type 2 diabetes. Diabetes 2015; 64: 673–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nakagomi A, Sunami Y, Kawasaki Y, et al. Sex difference in the association between surrogate markers of insulin resistance and arterial stiffness. J Diabetes Complications 2020; 34: 107442. [DOI] [PubMed] [Google Scholar]
- 47. Barzegar N, Tohidi M, Hasheminia M, et al. The impact of triglyceride‐glucose index on incident cardiovascular events during 16 years of follow‐up: Tehran lipid and glucose study. Cardiovasc Diabetol 2020; 19: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Boccardi V, Mancinetti F, Baroni M, et al. Metabolic score for insulin resistance (METS‐IR) and circulating cytokines in older persons: the role of gender and body mass index. Nutrients 2022; 14: 3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gauci S, Cartledge S, Redfern J, et al. Biology, bias, or both? The contribution of sex and gender to the disparity in cardiovascular outcomes between women and men. Curr Atheroscler Rep 2022; 24: 701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee J‐H, Kwon Y‐J, Park K, et al. Metabolic score for insulin resistance is inversely related to incident advanced liver fibrosis in patients with non‐alcoholic fatty liver disease. Nutrients 2022; 14: 3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Katsuki A, Sumida Y, Urakawa H, et al. Neither homeostasis model assessment nor quantitative insulin sensitivity check index can predict insulin resistance in elderly patients with poorly controlled type 2 diabetes mellitus. J Clin Endocrinol Metab 2002; 87: 5332–5335. [DOI] [PubMed] [Google Scholar]
- 52. Groenen AG, Halmos B, Tall AR, et al. Cholesterol efflux pathways, inflammation, and atherosclerosis. Crit Rev Biochem Mol Biol 2021; 56: 426–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Flow diagram for the study population selection.
Table S1 Outcomes according to quartile of METS‐IR level in individuals without and with diabetes
Table S2 Risk ratios for clinical outcomes according to quartiles of METS‐IR in individuals without and with diabetes
Data Availability Statement
The data analyzed in the current study are available upon reasonable request from the corresponding author.


