Abstract
Purpose
Despite the fact that childhood and young adult cancer survivors are at increased risk for chronic health problems as a result of their cancer treatment, many use tobacco, thereby increasing their risks. Perceptions of risk related to tobacco use can be targeted for interventions aimed at improving health behaviors for childhood, adolescent, and young adult cancer survivors. Understanding the covariates of perceptions of health risks among young adult survivors who smoke will help to determine targets for intervention.
Method
Three hundred seventy-four participants who were diagnosed with cancer prior to age 35, currently between 18 and 55 years of age, and current smokers were recruited as part of a larger smoking cessation study, Partnership for Health-2 (PFH-2). Data were collected by telephone survey.
Results
Overall, women had the highest perception of risk for serious health problems, a second cancer, and heart problems. Additionally, those participants who were dependent on nicotine endorsed that they were at higher risk of serious health problems and second cancers, but not heart problems. Finally, Hodgkin lymphoma survivors reported that they were at increased risk for second cancers and heart problems compared to their “healthy” peers.
Conclusion
Young adult cancer survivors who smoke correctly perceived some of their increased health risks. Additional motivation and education is needed for those young adult cancer survivors who perceive their increased health risks yet continue to smoke. Further education is needed for young survivors so they have a fully appropriate sense of risk, especially as it relates to their tobacco use.
Keywords: Childhood, Young adult, Cancer survivor, Risk perception
Over 80 % of childhood cancer survivors treated today will become long-term survivors [1]. In fact, one in every 570 young adults in the USA is a childhood cancer survivor [2]. Despite high survival rates, these survivors are at increased risk of second malignant tumors, pulmonary problems, and cardiovascular problems, in addition to a variety of other health risks, as a result of their cancer and related treatment [3]. By 30 years following their cancer diagnosis, 73 % of survivors of childhood cancers will have a chronic health condition and 42 % will have a severe, disabling, or life-threatening condition or have died from a chronic condition [3].
It is well known that, among healthy individuals, smoking harms nearly every organ of the body and is the most harmful behavior associated with preventable causes of death and diminished quality of life [4]. Among childhood cancer survivors, smoking potentiates the organ damage associated with many different treatment exposures, including irradiation to the head, neck, chest, abdomen, or pelvis and chemotherapy with pulmonary toxic agents (e.g., bleomycin, carmustine, and lomustine). Thus, the single most important risky health behavior to address among childhood cancer survivors is tobacco use [5]. Despite this, survivors continue to use tobacco. Although some studies have found that childhood cancer survivors smoke at somewhat lower smoking rates than the general population and age-matched controls [6–12], others have found similar rates of smoking [11–14]. Regardless of the relative smoking rates, 17 to 30 % of these survivors smoke, despite their increased health risks [8,14–16].
One explanation for this may be that survivors are often unfamiliar with the therapy that they received and are generally unaware of their risks [17]. The Institute of Medicine’s (IOM) 2003 and 2006 seminal reports recommend providing patients with a survivorship care plan written by their cancer treatment team [18,19] which is intended to assist survivors and their health care providers by providing information on health risks and posttreatment coordination of care [20,21]. However, only 15 % of childhood and adolescent cancer survivors report having received a treatment summary or survivorship care plan [22,23] and are therefore uneducated about their long-term health risks.
There is a small body of prior literature investigating childhood cancer survivors’ perceptions of risk which has reported mixed findings, with some studies indicating heightened concerns about health vulnerability [11,12,24,25], while others demonstrate fewer health concerns among survivors [26]. This variability in outcomes has also been found in the literature focused on healthy adolescents who smoke, with some studies finding higher perceived vulnerability among smokers and others finding lower sense of risk associated with smoking [27,28].
This study aims to assess perceived risk and related covariates among a fairly large cohort of survivors of childhood and young adult cancers (c/ya) who smoke, using multiple risk assessment methods. Specifically, we were interested in examining whether survivors of c/ya cancers who smoke accurately see themselves at increased risk for health problems. We also sought to examine whether having contact with the health care system and/or having a treatment summary was related to whether survivors accurately perceived themselves at risk.
Methods
Eligibility criteria and sample recruitment
Participants were recruited as part of a larger smoking cessation study, Partnership for Health-2 (PFH-2), which has been described in detail elsewhere [29]. Briefly, PFH-2 was conducted in collaboration with five pediatric cancer centers in the USA and Canada: St. Jude Children’s Research Hospital, Memorial Sloan-Kettering, Princess Margaret Hospital, Hospital for Sick Children, and Dana-Farber Cancer Institute. The study was also advertised on web sites designed for and about childhood and young adult cancer survivors and survivorship (e.g., American Cancer Society, Planet Cancer, The Doug Ulman Fund), and survivors could proactively contact the study team, be screened for eligibility, and provide consent.
To be eligible for PFH-2, survivors had to be diagnosed with cancer before age 35, currently be between ages 18 and 55, out of cancer treatment for at least 2 years, mentally able to provide informed consent, reachable by telephone, able to speak English, and be a current smoker (defined as having smoked at least one puff in the last 30 days). The institutional review boards at all participating institutions approved this study.
An introductory letter was sent to 4,397 cancer survivors and 4,312 did not opt out of further contact. In addition, 46 people contacted the study directly from postings on websites. Of the 3,258 survivors who were contacted, 22 % (n=719) were alive and eligible (ineligibility largely due to smoking status), and 51 % (n=374) of eligible survivors were enrolled in the study (Fig. 1).
Fig. 1.
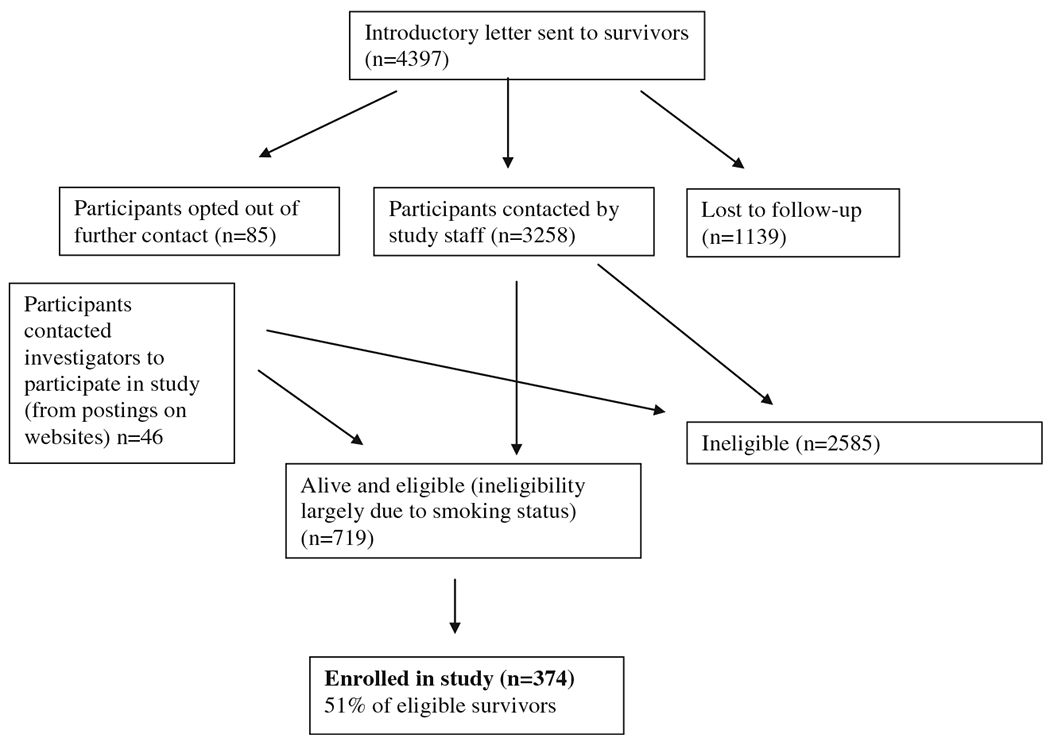
Recruitment flow chart
Measures
Perceptions of risk and related covariates were collected at baseline and all survey data was collected by telephone.
Sociodemographic characteristics and medical history
The following demographic data were collected: age, gender, race, ethnicity, marital status, education, and employment status. In addition, data were also collected on participants’ cancer and subsequent treatment.
Perceived risk
We assessed two types of perceived risk: absolute risk and comparative risk (relative risk compared to peers). Absolute risk was assessed with one question asking survivors to “rate the chance that they thought they would experience any serious health problem in the future” (six-point scale; no chance to certain to happen, which was collapsed into three categories for analysis: no chance/very unlikely, moderate chance/likely, and very likely/certain). Two comparative risk questions asked survivors their “chances, compared to individuals the same age and gender, that they would be diagnosed with cancer; or have heart problems in the future” (five-point scale, much less to much more likely). The peer comparison group was not specified and it is likely that participants compared themselves to a noncancer cohort of same age/gender peers. These questions have been used widely in the perceived risk literature [30–34].
Knowledge of smoking risks
Survivors were asked to what extent they agreed with the statement “Smoking may increase my chance of developing health problems” (four-point scale, completely agree to completely disagree).
Contact with the health care system
Participants were asked if they had a regular health care provider and whether their primary care physician or their oncologist had seen them in the past year. They were also asked if they had ever been given a written treatment summary and whether they could easily find this summary.
Smoking behavior
Smoking rate
Participants reported the number of cigarettes they smoked per day.
Nicotine dependence
Participants reported the number of minutes after waking that they smoked their first cigarette [35]; responses were dichotomized as <30 min (nicotine dependent) and ≥30 min (not nicotine dependent). This single item has been shown to have the greatest predictive validity of the larger measure and has been described as a good single-item measure of nicotine dependence [35].
Quit attempts
Participants reported the number of times in the previous 12 months that they tried to quit smoking and abstained from cigarettes for at least 24 h.
Data analysis
Descriptive analyses were used to characterize the study population. Bivariate analyses were conducted examining our perceived risk outcomes with sociodemographic and medical covariates. All outcome analyses were conducted using multiple imputation methods (in SAS MI) to account for missing data [36]. Bootstrapping methods of model development were used to create three multivariable models predicting perceived risk of any serious health problem and perceived risk of a second cancer/recurrence [37]. Multivariable models were constructed using all potential variables referenced in the bivariate models (Tables 2 and 3). Using bootstrap methods for predictive models, we finalized on the model that included variables that were included in 60 % of the bootstrapped models. After development and choice of this model, we explored whether other variables should have been included due to significant p values, better goodness of fit, or a change in significant variable coefficients and found none of the remaining variables fit any of these categories for consideration to be included. Smoking was treated as a continuous variable in each model. All models controlled for the cancer site from which participants were recruited.
Table 2.
Proportional odds of increase in the chance of experiencing serious health problems in the future, controlling for site
| Odds | 95 % CI | p value | |
|---|---|---|---|
| Demographics | |||
| Age | 1.04 | (1.02–1.07) | 0.0020 |
| Gender | 0.0023 | ||
| Male | Ref | ||
| Female | 1.89 | (1.25–2.86) | |
| Race | 0.0664 | ||
| White | 1.70 | (0.97–3.00) | |
| Non-White | Ref | ||
| Employment | 0.8755 | ||
| Yes | 0.96 | (0.59–1.58) | |
| No | Ref | ||
| Education | 0.8023 | ||
| Did not complete HS or GED | 1.06 | (0.48–2.35) | |
| Completed HS or GED | 0.88 | (0.51–1.51) | |
| Some college or training after college | 0.80 | (0.47–1.34) | |
| College graduate | Ref | ||
| Marital status | 0.3442 | ||
| Married | 0.55 | (0.26–1.17) | |
| Living with partner | 0.54 | (0.24–1.23) | |
| Never been married and not living with a partner | 0.51 | (0.25–1.06) | |
| Divorced or no longer living with partner | Ref | ||
| Medical characteristics | |||
| Diagnosis | 0.4272 | ||
| Leukemia | 0.91 | (0.38–2.18) | |
| Hodgkin’s disease | 1.53 | (0.64–3.70) | |
| CNS malignancy | 1.11 | (0.40–3.10) | |
| Non-Hodgkin’s lymphoma | 2.32 | (0.79–6.86) | |
| Bone cancer | 1.21 | (0.44–3.32) | |
| Other | 1.11 | (0.47–2.60) | |
| Germ cell | Ref | ||
| Cancer treatment | 0.3603 | ||
| Radiation | 0.78 | (0.47–1.31) | |
| Chemo | 0.62 | (0.36–1.08) | |
| Surgery | 0.95 | (0.49–1.85) | |
| All 3 | Ref | ||
| Smoking | |||
| Smoke at least a pack/day | 0.2662 | ||
| No | 0.77 | (0.48–1.22) | |
| Yes | Ref | ||
| Nicotine dependence | 0.0142 | ||
| No (more than 30 min after waking) | 0.57 | (0.36–0.89) | |
| Yes (less than 30 min after waking) | Ref | ||
| Quit attempts | 1.01 | (0.99–1.02) | 0.2438 |
| Health care system | |||
| For your non-emergency care, do you have a primary care physician or health care professional (other than your oncologist) whom you go to for medical care | 0.2748 | ||
| Yes | 1.32 | (0.80–2.18) | |
| No | Ref | ||
| Have you had a routine check-up from any primary care physician in the past year? | 0.5745 | ||
| Yes | 1.13 | (0.74–1.73) | |
| No | Ref |
Table 3.
Odds of increase in risk of cancer (slightly or much more versus about the same or slightly or much less), controlling for site
| Odds | 95 % CI | p value | |
|---|---|---|---|
| Demographics | |||
| Age | 0.99 | (0.96–1.02) | 0.3878 |
| Gender | 0.0125 | ||
| Male | Ref | ||
| Female | 1.72 | (1.12–2.63) | |
| Race | 0.0030 | ||
| White | 2.46 | (1.36–4.45) | |
| Non-White | Ref | ||
| Employment | 0.2873 | ||
| Yes | 1.32 | (0.79–2.21) | |
| No | Ref | ||
| Education | 0.0114 | ||
| Did not complete HS or GED | 0.34 | (0.15–0.79) | |
| Completed HS or GED | 0.43 | (0.24–0.78) | |
| Some college or training after college | 0.46 | (0.26–0.80) | |
| College graduate | Ref | ||
| Marital status | 0.9357 | ||
| Married | 1.03 | (0.47–2.24) | |
| Living with partner | 1.18 | (0.50–2.79) | |
| Never been married and not living with a partner | 0.96 | (0.45–2.06) | |
| Divorced or no longer living with partner | Ref | ||
| Medical characteristics | |||
| Diagnosis | 0.5402 | ||
| Leukemia | 0.71 | (0.28–1.79) | |
| Hodgkin’s disease | 1.31 | (0.51–3.35) | |
| CNS malignancy | 0.72 | (0.25–2.10) | |
| Non-Hodgkin’s lymphoma | 1.12 | (0.35–3.51) | |
| Bone cancer | 0.84 | (0.29–2.44) | |
| Other | 0.70 | (0.29–1.72) | |
| Germ cell | Ref | ||
| Cancer treatment | 0.2981 | ||
| Radiation | 0.62 | (0.36–1.06) | |
| Chemo | 0.92 | (0.52–1.64) | |
| Surgery | 0.68 | (0.34–1.36) | |
| All 3 | Ref | ||
| Smoking | |||
| Smoke at least a pack/day | 0.2786 | ||
| No | 0.77 | (0.47–1.24) | |
| Yes | Ref | ||
| Nicotine dependence | 0.0512 | ||
| No | 0.62 | (0.39–1.00) | |
| Yes | Ref | ||
| Quit attempts | 1.00 | (0.99–1.02) | 0.8581 |
| Health care system | |||
| For your non-emergency care, do you have a primary care physician or health care professional (other than your oncologist) whom you go to for medical care | 0.0017 | ||
| Yes | 2.30 | (1.37–3.86) | |
| No | Ref | ||
| Have you had a routine check-up from any primary care physician in the past year? |
0.0370 | ||
| Yes | 1.60 | (1.03–2.48) | |
| No | Ref |
Results
Participant characteristics
The mean age at enrollment was 32.4 years (σ=7.94). The study sample was 51 % male, 86 % White, and 30 % had at least a college degree. In addition, 48% were married or living with a partner, and 79.4% were employed in the last year. The mean years since cancer diagnosis was 20 (σ=9.61), and leukemia was the most common cancer diagnosis (22.7 %) reported by study participants. Almost half of the participants (45 %) reported combined modality cancer treatment. Over 92 % of the participants smoked in the last 7 days and 89 % smoked less than one pack of cigarettes during this time period. Forty-seven percent of the sample was dependent on nicotine. Sixty-six percent of the participants reported that their regular doctor, oncologist, nurse, or other health care provider had encouraged them to quit smoking during the past 15 months (Table 1).
Table 1.
Participant characteristics and smoking behavior (N=374)
| Numbera | Percentageb | |
|---|---|---|
| Sociodemographic characteristics | ||
| Age (range= 19–56), M (SD) | 32.4 (7.9) | |
| Education | ||
| ≥College | 111 | 29.7 |
| Some college/vocational school | 128 | 34.2 |
| High school or GED | 103 | 27.5 |
| <High school | 32 | 8.6 |
| Did you work in the past year | ||
| Yes | 297 | 79.4 |
| No | 77 | 20.6 |
| Gender | ||
| Male | 192 | 51.3 |
| Female | 182 | 48.7 |
| Race/ethnicity | ||
| White | 320 | 85.6 |
| Non-White | 54 | 14.4 |
| Marital status | ||
| Married or partnered | 176 | 47.2 |
| Unmarried or not partnered | 197 | 52.8 |
| Clinical characteristics | ||
| Childhood cancer diagnosis | ||
| Leukemia | 85 | 22.7 |
| Hodgkin’s disease | 73 | 19.5 |
| CNS malignancy | 35 | 9.4 |
| Non-Hodgkin’s lymphoma | 25 | 6.7 |
| Bone cancer | 29 | 7.8 |
| Other | 127 | 34.0 |
| Age at diagnosis, in years (range=0–31), M (SD) | 12.4 (8.1) | |
| Cancer treatment received: surgery | ||
| Yes | 265 | 72.6 |
| No | 100 | 27.4 |
| Cancer treatment received: radiation | ||
| Yes | 227 | 61.4 |
| No | 143 | 38.6 |
| Cancer treatment received: chemotherapy | ||
| Yes | 285 | 77.2 |
| No | 84 | 22.8 |
| Self-reported health status | ||
| Excellent or very good | 122 | 32.6 |
| Good | 153 | 40.9 |
| Fair or poor | 99 | 26.5 |
| Risk perceptions | ||
| Smoking may increase my chance of developing health problems | ||
| Disagree | 0 | 0 |
| Somewhat agree | 76 | 20.3 |
| Completely agree | 298 | 79.7 |
| Chances of diagnosis of cancer in the future | ||
| Much less/slightly less | 42 | 11.3 |
| Same | 109 | 29.4 |
| Slightly more | 146 | 39.4 |
| Much more | 74 | 19.9 |
| Chance of having heart problems | ||
| Much less/slightly less | 52 | 13.8 |
| About the same | 231 | 35.5 |
| Slightly more | 127 | 34.4 |
| Much more | 60 | 16.3 |
| Have a regular health care provider | ||
| Yes | 294 | 78.6 |
| No | 80 | 21.4 |
| Had a PCP or oncology exam in the past year | ||
| Yes | 284 | 75.9 |
| No | 90 | 24.1 |
| Smoking rate (avg # cigarettes/day), M (SD) | 12.6 (10.0) | |
| During the past 7 days, on average, # cigarettes/day | ||
| <1 | 7 | 2.0 |
| 1–10 | 185 | 53.3 |
| 11–20 | 116 | 33.4 |
| >20 | 39 | 11.3 |
| Number of minutes after waking until first cigarette | ||
| <30 min | 126 | 34.2 |
| 30+min | 242 | 65.8 |
Sample sizes may differ slightly due to missing data
Percentages may not sum to 100 due to rounding
Contact with the health care system
Over two-thirds (79 %) of the participants reported having a primary care physician (PCP) or health care professional to go to for their medical care. Sixty-six percent reported having had a routine check-up from a PCP in the past year, yet only 37 % reported seeing their oncologist or other cancer specialist in the past year. Taken together, 76 % reported having either a physical examination from their PCP or oncologist in the past year. Most participants (83 %) had not been hospitalized for any medical problem in the past year.
Only 37 % reported having ever been given a written summary of their cancer treatment. Of this subgroup of survivors, only 56 % of those could easily find that written summary (only 20 % of total sample) and 59 % (22 % of total sample) reported that their PCP had a copy of their treatment summary.
Knowledge of smoking risk
Eighty percent of the participants reported that they completely agreed that smoking may increase their chances of developing health problems in the future. Another 20 % endorsed somewhat agreeing with this statement.
Perception of absolute risk
Participants were asked the chances they would experience any serious health problem in the future. Twenty-one percent reported that there was no chance or it was unlikely that this would happen. Fifty-eight percent reported that there was a moderate or likely chance that they would have a serious health problem in the future, and 21 % reported that this was very likely or certain to happen in their future.
Perception of comparative risk
With regard to their comparative risk of specific health problems, 60 % of the participants endorsed that their risk of having a diagnosis of cancer in the future was more than others similar in age and gender. Slightly less reported being at higher risk for having heart problems (51 %) as compared to others of the same age and gender.
Bivariate analyses
In bivariate analyses, perceived risk of experiencing health problems in the future (Table 2) was found to be significantly related to survivors’ older (current) age (OR=1.04, CI=1.02–1.07), gender (females OR=1.89, CI=1.25–2.86), and nicotine dependence (not nicotine dependent OR=0.57, CI=0.36–0.89).
Bivariate analyses examining the comparative risk of being diagnosed with cancer again (recurrence or second malignant neoplasm) (Table 3) was significantly related to gender (male OR=0.58, CI=0.38–0.89), race (White OR=2.46, CI=1.36– 4.45), dependence on nicotine (no dependence OR=0.62, CI=0.39–1.0), having a health care provider for routine care (OR=2.3, CI=1.37–3.86), and having had a routine check-up in the past year (OR=1.60, CI=1.03–2.48). Risk of heart problems was significantly related to gender (female OR= 1.72, CI=1.12–2.63, p=0.0119), race (White OR=2.28, CI=1.24–4.19, p=0.0079), having a health care provider for routine care (OR=1.97, CI=1.16–3.33, p=0.012), and having a health care provider offer to help the survivor quit smoking (OR=1.89, CI=1.21–2.96, p=0.005).
Multivariate analysis
In our multivariate model to predict higher absolute perceived risk of having any serious health problems in the future, we found that gender and nicotine dependence were associated with higher perceived risk of future health problems. Overall, female gender (OR=2.06, CI=1.33–3.18, p=0.0012) and being dependent on nicotine (OR=1.71, CI=1.08–2.69, p=0.02) were significantly associated with higher absolute perceived risk (Table 4).
Table 4.
Proportional odds of increase in the chance of experiencing serious health problems in the future, controlling for site
| OR | CI | p value | |
|---|---|---|---|
| Independent variables | |||
| Gender | 0.0012 | ||
| Female | 2.06 | 1.33, 3.18 | |
| Male | Ref | ||
| Race | 0.0875 | ||
| White | 1.67 | 0.93, 3.02 | |
| Non-White | Ref | ||
| Nicotine dependency (1st cig. <30 min from waking) | 0.0210 | ||
| Yes | 1.71 | 1.08, 2.69 | |
| No | Ref | ||
| Controlling variable | |||
| Study site | 0.91 |
For this model, the proportion odds assumption p value=0.0795, the goodness of fit test p value is 0.0035 and the c-index is 0.62
In the model to predict comparative risk of having a diagnosis of cancer in the future, we found that education level, being dependent on nicotine, diagnosis, race, and gender were associated with higher perceived risk. More specifically, we found that non-Hispanic White ethnicity (OR=2.41, CI=1.25–4.66), females (OR=2.09, CI=1.26–3.46), those who were nicotine dependent (OR=1.67, CI=1.00–2.79), had been diagnosed with Hodgkin’s lymphoma (OR=2.55, CI=1.22–5.34), and had higher levels of education (OR=2.76, CI=1.08–7.1) perceived themselves to be at higher risk for another cancer, compared to individuals in the referent groups: Non-White, male, non-nicotine dependent, other cancer, and lower levels of education (Table 5).
Table 5.
Comparative risk (odds) of being diagnosed with cancer, controlling for site
| OR | CI | p value | |
|---|---|---|---|
| Independent variables | |||
| Education | 0.0918 | ||
| Did not complete HS or GED | Ref | ||
| Completed HS or GED | 1.33 | 0.55, 3.19 | |
| Some college or training after college | 1.78 | 0.74, 4.32 | |
| College graduate | 2.76 | 1.08, 7.10 | |
| Diagnosis | 0.1870 | ||
| Leukemia | 1.54 | 0.77, 3.05 | |
| Hodgkin’s disease | 2.55 | 1.22, 5.34 | |
| CNS malignancy | 1.19 | 0.47, 3.01 | |
| Non-Hodgkin’s lymphoma | 2.46 | 0.86, 7.01 | |
| Bone cancer | 1.35 | 0.50, 3.67 | |
| Germ cell | 2.89 | 1.02, 8.23 | |
| Other | Ref | ||
| Nicotine dependency (1st cig. <30 min from waking) | 0.0501 | ||
| Yes | 1.67 | 1.00, 2.79 | |
| No | Ref | ||
| Gender | 0.0043 | ||
| Female | 2.09 | 1.26, 3.46 | |
| Male | Ref | ||
| Race | 0.0086 | ||
| White | 2.41 | 1.25, 4.66 | |
| Non-White | Ref | ||
| Controlling variable | |||
| Study site | 0.36 |
For this model, the goodness of fit test p value is 0.0012 and the c-index is 0.70
In the model to predict comparative perceived risk of having heart problems in the future, we found that gender (OR=1.83, CI=1.17–2.86, p=0.0079) and cancer diagnosis (p=0.048) were associated with higher perceived risk. Specifically, women and Hodgkin lymphoma survivors had the highest perceived risk of heart problems (Table 6).
Table 6.
Comparative risk (odds of heart problems), controlling for site
| OR | CI | p value | |
|---|---|---|---|
| Independent variables | |||
| Diagnosis | 0.0488 | ||
| Leukemia | 1.17 | 0.63, 2.17 | |
| Hodgkin’s disease | 2.18 | 1.12, 4.22 | |
| CNS malignancy | 0.64 | 0.28, 1.48 | |
| Non-Hodgkin’s lymphoma | 2.00 | 0.78, 5.14 | |
| Bone cancer | 2.05 | 0.83, 5.04 | |
| Germ cell | 0.84 | 0.33, 2.17 | |
| Other | Ref | ||
| Gender | 0.0079 | ||
| Female | 1.83 | 1.17, 2.86 | |
| Male | Ref | ||
| Check-up from cancer specialist in past year | 0.6679 | ||
| Yes | Ref | ||
| No | 1.11 | 0.70, 1.75 | |
| Controlling variable | |||
| Study site | 0.57 |
Discussion
Absolute and comparative risk perceptions were assessed among a sample of childhood and young adult cancer survivors who reported smoking in the past 30 days. Over half (59 %) of the survivors thought their chances of having a diagnosis of cancer and/or any health problem in the future were higher compared to their same age/gender peers. Only about one-fifth of our sample (21 %) thought that the possibility of having a serious health problem in the future was unlikely. Despite having a higher perceived risk of medical problems in the future, 20 % did not completely agree that smoking may increase their chance of developing health problems in the future—a figure that may perhaps serve as a rationalization for continuing to smoke among a portion of this high-risk group. Despite the fact that a majority of our survivors agreed that smoking may increase their chance of health problems in the future, they continued to smoke— highlighting the importance of targeting other motivational factors for cessation for this group of cancer survivors.
While a majority of survivors had an identified health care professional and got routine checkups, only a small minority of these saw someone for follow-up or specialized survivorship care. Even more concerning was that less than 40 % reported ever being given a written treatment summary and only 22 % reported that their PCP had a copy, thereby diminishing the possibility that they would obtain risk-based follow-up care from their health care provider [22], especially since most survivors are followed by a PCP in the community years after their cancer treatment has commenced. Risk-based care refers to follow-up care dedicated to the screening, prevention, and treatment of late effects throughout the life span. This percentage is higher than what has been previously published in the literature [22,23] but is nevertheless striking and problematic. With so few PCPs having a copy of survivors’ cancer treatment summaries, it is unlikely that recommended surveillance is being recommended and completed. In post hoc analyses, we examined factors related to having a treatment summary and found that it was unrelated to sociodemographics or cancer diagnosis.
In previous studies, it has been demonstrated that most survivors are followed in the community and that these PCPs caring for them have limited knowledge regarding their health risks and their recommended long-term health care [22,23,38]. Despite the Children’s Oncology Group published guidelines for the care of childhood and adolescent cancer survivors, many adult survivors of c/ya cancer do not receive appropriate care focused on the risks arising from their prior cancer therapy [39,40]. The implication of this is that a discussion of general health behaviors, such as tobacco use, and its impact on cancer survivors is likely missed.
Even at National Cancer Institute (NCI) comprehensive cancer centers, where one might assume that tobacco use is addressed at higher rates than among community physicians, the provision of tobacco use treatment is not considered a core service at most cancer centers [41]. In fact, a recent survey of NCI cancer centers demonstrated that although 60% of cancer centers offered some form of tobacco use treatment, such services were often confined to one disease subpopulation (e.g., lung cancer) [41]. Additionally, fewer than half of the cancer centers surveyed had designated personnel to offer tobacco use treatment. Even when examining tobacco-related cancers and physician practices, there are significant gaps in the routine offer of tobacco assistance. In one recent study, thoracic oncologists reported recognizing the importance of tobacco cessation; however, most did not provide assistance to their patients as a routine part of cancer care [42]. Therefore, even at NCI comprehensive cancer centers, it is likely that we are falling short with regard to appropriately offering cessation programs and treatment to c/ya survivors who smoke.
In our multivariate analysis examining perceptions of risk, we found that women consistently perceived themselves at higher risk of health problems or a second cancer than men, thereby suggesting that our risk interventions may need to be targeted toward male survivors in order to motivate behavior change and communicate risks of smoking within the context of their cancer history. It also suggests that for women, we may need to think creatively about other targets of intervention for women besides increasing their perceptions of risk, which are already elevated. Additionally, heavier smokers perceived that they were at greater risk of a serious health problem; however, they did not perceive themselves to be a greater risk for a second cancer or recurrence, which should be targeted for intervention.
The literature suggests that survivors with lower risk perception may be less motivated to change their smoking behavior than smokers with a greater perceived risk of future health problems [43], which is consistent with the above finding for cancer recurrence. It is possible that survivors who feel relatively certain that they will experience a future health event demonstrate fatalistic beliefs about their future health potential, which are associated with an increased likelihood of smoking [44].
Finally, another interesting finding in our multivariable analyses was among older survivors in our sample who perceived themselves to be at higher risk for a serious health problem in the future but not at higher risk of a second cancer or recurrence. This may reflect an optimistic bias that if one hasn’t gotten a second cancer yet, perhaps they are “out of the woods”; however, fairly recent data suggests that does not reflect this and still indicates that risk of second cancers among childhood survivors continues to increase across the lifespan [3]. Additionally, the recent Surgeon General’s Report definitively concluded that smoking increases the risk of dying from cancer and other diseases in cancer patients and survivors [45], further highlighting the importance of tobacco cessation for childhood cancer survivors.
Limitations
This study had several limitations. First, we were unable to contact 24% of survivors who were identified by participating sites and did not opt out, and we do not know whether these individuals were eligible for the study. In many cases, the participating sites did not have accurate addresses on file. We made a concerted effort to locate these individuals by using internet searches and other telephone and mail-based tracing approaches. Second, 48% of known eligible survivors declined to participate, which introduces selection bias into the study; however, a 52 % recruitment rate among a sample of smokers who have not sought out smoking cessation treatment is reasonable. Third, our sample was predominately White, which limits our ability to generalize to other racial and ethnic groups, although it reflects the racial/ethnic characteristics of the participating treatment sites.
Strengths
This study also had several important strengths. First, study participants were recruited from five pediatric cancer treatment facilities in the USA and Canada. Thus, our results have higher external validity than if recruited from a single site. Second, we collected a fairly wide breadth of data including those on perceptions of risk, smoking behavior, and many other medical and psychosocial variables. This enabled us to look at multiple covariates of perceptions of risk among a group of young adult cancer survivors who are smokers. Additionally, this is one of the larger studies looking at perceived risk in cancer survivors, especially among a high-risk group of survivors.
Conclusions
The results of this study suggest that despite the fact that many of our cancer survivors perceived themselves to be at significantly increased risk of health problems, including a second cancer, they continued to smoke—indicating a need to identify alternative targets of intervention in addition to increasing perceived risk. Additionally, despite having a health care practitioner, few of our survivors were in risk-based care or had the treatment information needed to be able to obtain recommended risk-based care. Not having a survivorship care plan or treatment summary may be a significant contributing factor to survivors’ underestimates of their risk. Being engaged in risk-based care and having a care plan may both serve as a catalyst and teachable moment for health behavior change, including smoking cessation.
Perceptions of risk can be targeted for interventions aimed at improving health behaviors for childhood and young adult cancer survivors. It is likely that several interventions would need to be developed, one for young adult smokers (18–24 years old) and those for adult smokers (aged 25 and older) as they likely have different tobacco behaviors and attitudes. Therefore, understanding survivors’ risk perceptions and the related covariates are important when considering future health behavior interventions for this group. This is even more essential for those survivors who engage in tobacco use, as they are putting themselves at even greater increased health risks over time than that conferred by their cancer and its treatment.
Footnotes
Conflict of interest The authors declare they have no conflicts of interest.
Contributor Information
Jennifer S. Ford, Department of Psychiatry and Behavioral Sciences, Memorial Sloan-Kettering Cancer Center, 641 Lexington Avenue, 7th Floor, New York, NY 10022, USA
Elaine Puleo, University of Massachusetts, Boston, USA.
Kim Sprunck-Harrild, Dana-Farber Cancer Institute and Harvard School of Public Health, Boston, USA.
Janet deMoor, National Cancer Institute, Bethesda, MD, USA.
Karen M. Emmons, Dana-Farber Cancer Institute and Harvard School of Public Health, Boston, USA
References
- 1.Ries LAG et al. SEER cancer statistics review, 1975–2005, in NIH publication, SEER Program (National Cancer Institute (U.S.)), et al. , Editors. 2008, U.S. Dept. of Health and Human Services, Public Health Service, National Institutes of Health, National Cancer Institute: Bethesda, Md. p. v [Google Scholar]
- 2.National Cancer Institute (NCI) Office of Cancer Survivorship. U.S. National Institutes of Health. [cited 2013 April 22]; Available from: http://dccps.nci.nih.gov/ocs/
- 3.Oeffinger KC et al. (2006) Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 355(15):1572–1582 [DOI] [PubMed] [Google Scholar]
- 4.(CDC), C.f.D.C.a.P (2004) Surgeon General’s report—the health consequences of smoking. 2004: Atlanta, GA [Google Scholar]
- 5.Nathan PC et al. (2009) Health behaviors, medical care, and interventions to promote healthy living in the Childhood Cancer Survivor Study cohort. J Clin Oncol 27(14):2363–2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauld C et al. (2005) Health-risk behaviours among adolescent survivors of childhood cancer. Pediatr Blood Cancer 45(5):706–715 [DOI] [PubMed] [Google Scholar]
- 7.Clarke SA, Eiser C (2007) Health behaviours in childhood cancer survivors: a systematic review. Eur J Cancer 43(9):1373–1384 [DOI] [PubMed] [Google Scholar]
- 8.Frobisher C et al. (2008) Extent of smoking and age at initiation of smoking among adult survivors of childhood cancer in Britain. J Natl Cancer Inst 100(15):1068–1081 [DOI] [PubMed] [Google Scholar]
- 9.Mulhern RK et al. (1995) Health-related behaviors of survivors of childhood cancer. Med Pediatr Oncol 25(3):159–165 [DOI] [PubMed] [Google Scholar]
- 10.Tyc VL, Hudson MM, Hinds P (1999) Health promotion interventions for adolescent cancer survivors. Cogn Behav Pract 6(2):128–136 [Google Scholar]
- 11.Tyc VL, Hadley W, Crockett G (2001) Prediction of health behaviors in pediatric cancer survivors. Med Pediatr Oncol 37(1):42–46 [DOI] [PubMed] [Google Scholar]
- 12.Tyc VL, Hadley W, Crockett G (2001) Predictors of intentions to use tobacco among adolescent survivors of cancer. J Pediatr Psychol 26(2):117–121 [DOI] [PubMed] [Google Scholar]
- 13.Verrill JR et al. (2000) Aggression, antisocial behavior, and substance abuse in survivors of pediatric cancer: possible protective effects of cancer and its treatment. J Pediatr Psychol 25(7):493–502 [DOI] [PubMed] [Google Scholar]
- 14.Emmons K et al. (2002) Predictors of smoking initiation and cessation among childhood cancer survivors: a report from the childhood cancer survivor study. J Clin Oncol 20(6):1608–1616 [DOI] [PubMed] [Google Scholar]
- 15.Tao ML et al. (1998) Smoking in adult survivors of childhood acute lymphoblastic leukemia. J Natl Cancer Inst 90(3):219–225 [DOI] [PubMed] [Google Scholar]
- 16.Larcombe I, Mott M, Hunt L (2002) Lifestyle behaviours of young adult survivors of childhood cancer. Br J Cancer 87(11):1204–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadan-Lottick NS et al. (2002) Childhood cancer survivors’ knowledge about their past diagnosis and treatment—childhood cancer survivor study. JAMA J Am Med Assoc 287(14):1832–1839 [DOI] [PubMed] [Google Scholar]
- 18.Hewitt ME et al. (2006) From cancer patient to cancer survivor: lost in transition. National Academies Press, Washington, D.C., p 506, xxv [Google Scholar]
- 19.Hewitt M, Weiner S, and Simone J (2003) Childhood cancer survivorship: improving care and quality of life. Washington, D.C.: National Academies Press; [PubMed] [Google Scholar]
- 20.Landier W et al. (2004) Development of risk-based guidelines for pediatric cancer survivors: the Children’s Oncology Group Long-Term Follow-Up Guidelines from the Children’s Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol 22(24):4979–4990 [DOI] [PubMed] [Google Scholar]
- 21.Saslow D et al. (2007) American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 57(2):75–89 [DOI] [PubMed] [Google Scholar]
- 22.Nathan PC et al. (2008) Medical care in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol 26(27):4401–4409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oeffinger KC et al. (2004) Health care of young adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Ann Fam Med 2(1):61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zebrack BJ, Chesler M (2001) Health-related worries, self-image, and life outlooks of long-term survivors of childhood cancer. Health Soc Work 26(4):245–256 [DOI] [PubMed] [Google Scholar]
- 25.Zebrack BJ, Chesler MA (2001) A psychometric analysis of the Quality of Life-Cancer Survivors (QOL-CS) in survivors of childhood cancer. Qual Life Res 10(4):319–329 [DOI] [PubMed] [Google Scholar]
- 26.Tercyak KP et al. (2004) Brief report: health beliefs among survivors of childhood cancer. J Pediatr Psychol 29(5):397–402 [DOI] [PubMed] [Google Scholar]
- 27.Milam JE et al. (2000) Perceived invulnerability and cigarette smoking among adolescents. Addict Behav 25(1):71–80 [DOI] [PubMed] [Google Scholar]
- 28.Tyc VL et al. (2004) Predictors of smoking intentions and smoking status among nonsmoking and smoking adolescents. Addict Behav 29(6):1143–1147 [DOI] [PubMed] [Google Scholar]
- 29.de Moor JS et al. (2011) Disseminating a smoking cessation intervention to childhood and young adult cancer survivors: baseline characteristics and study design of the Partnership for Health-2 study. BMC Cancer 11:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lipkus IM et al. (1996) Colorectal screening patterns and perceptions of risk among African-American users of a community health center. J Community Health 21(6):409–427 [DOI] [PubMed] [Google Scholar]
- 31.Lipkus IM, Rimer BK, Strigo TS (1996) Relationships among objective and subjective risk for breast cancer and mammography stages of change. Cancer Epidemiol Biomarkers Prev 5(12):1005–1011 [PubMed] [Google Scholar]
- 32.Vernon SW et al. (2001) Factors associated with perceived risk in automotive employees at increased risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev 10(1):35–43 [PubMed] [Google Scholar]
- 33.Weinstein ND (1987) Unrealistic optimism about susceptibility to health problems: conclusions from a community-wide sample. J Behav Med 10(5):481–500 [DOI] [PubMed] [Google Scholar]
- 34.Weinstein ND, Klein WM (1995) Resistance of personal risk perceptions to debiasing interventions. Health Psychol 14(2):132–140 [DOI] [PubMed] [Google Scholar]
- 35.Baker TB et al. (2007) Time to first cigarette in the morning as an index of ability to quit smoking: implications for nicotine dependence. Nicotine Tob Res 9(Suppl 4):S555–S570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Y (2011) Multiple imputation using SAS software. J Stat Softw 45(6):1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Austin P, Tu J (2004) Boostrap methods for developing predictive models. Am Stat 58(2):131–137 [Google Scholar]
- 38.Henderson TO et al. (2010) Physician preference and knowledge gaps regarding the care of childhood cancer survivors: a mailed survey of pediatric oncologists. J Clin Oncol 28(5):878–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nathan PC et al. (2013) Family physician preferences and knowledge gaps regarding the care of adolescent and young adult survivors of childhood cancer. J Cancer Surviv 7:275–282 [DOI] [PubMed] [Google Scholar]
- 40.Nathan PC et al. (2010) Screening and surveillance for second malignant neoplasms in adult survivors of childhood cancer: a report from the childhood cancer survivor study. Ann Intern Med 153(7):442.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morgan G et al. (2011) National Cancer Institute conference on treatment of tobacco dependence at cancer centers. J Oncol Pract 7(3):178–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warren GW et al. (2013) Practice patterns and perceptions of thoracic oncology providers on tobacco use and cessation in cancer patients. J Thorac Oncol 8:543–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brewer NT et al. (2004) Risk perceptions and their relation to risk behavior. Ann Behav Med 27(2):125–130 [DOI] [PubMed] [Google Scholar]
- 44.Schnoll RA et al. (2002) Correlates of tobacco use among smokers and recent quitters diagnosed with cancer. Patient Educ Couns 46(2):137–145 [DOI] [PubMed] [Google Scholar]
- 45.U.S. Department of Health and Human Services. The health consequences of smoking—50 years of progress: a report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014 [Google Scholar]


