Abstract
The starter unit used in the biosynthesis of daunorubicin is propionyl coenzyme A (CoA) rather than acetyl-CoA, which is used in the production of most of the bacterial aromatic polyketides studied to date. In the daunorubicin biosynthesis gene cluster of Streptomyces peucetius, directly downstream of the genes encoding the β-ketoacyl:acyl carrier protein synthase subunits, are two genes, dpsC and dpsD, encoding proteins that are believed to function as the starter unit-specifying enzymes. Recombinant strains containing plasmids carrying dpsC and dpsD, in addition to other daunorubicin polyketide synthase (PKS) genes, incorporate the correct starter unit into polyketides made by these genes, suggesting that, contrary to earlier reports, the enzymes encoded by dpsC and dpsD play a crucial role in starter unit specification. Additionally, the results of a cell-free synthesis of 21-carbon polyketides from propionyl-CoA and malonyl-CoA that used the protein extracts of recombinant strains carrying other daunorubicin PKS genes to which purified DpsC was added suggest that this enzyme has the primary role in starter unit discrimination for daunorubicin biosynthesis.
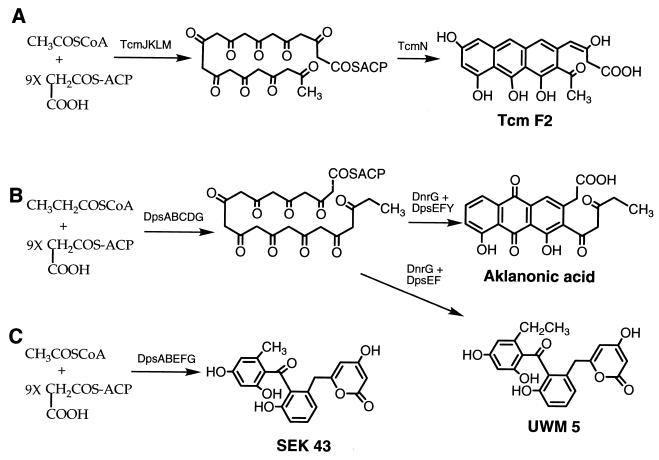
Daunorubicin (DNR) and its C-14-hydroxylated derivative doxorubicin are among the most important antitumor antibiotics in current use. Both antibiotics are produced by Streptomyces peucetius through a pathway involving a type II polyketide synthase (PKS), which executes the condensation of propionyl coenzyme A (CoA), as the starter unit, and nine malonyl-CoA extender units in the production of a 21-carbon decaketide (8, 11, 12). Subsequent intramolecular aldol condensations of the decaketide and C-12 oxidation form the 21-carbon tricyclic aromatic pigment aklanonic acid (AA) (8, 11), the first identifiable polyketide intermediate in the DNR pathway (Fig. 1B). When compared with other aromatic polyketides like tetracenomycin (Tcm) C, a 20-carbon polyketide produced by a type II PKS that uses acetyl-CoA as the starter unit (Fig. 1A), the DNR system stands out in terms of its use of a distinct starter unit and possibly also a PKS-dedicated malonyl-CoA:acyl carrier protein acyltransferase (MCAT) (8, 11).
FIG. 1.
(A) Biosynthesis of Tcm F2 from malonyl-ACP and starter unit acetyl-CoA by the Tcm PKS enzymes. (Acetyl-CoA may not be the actual substrate since formation of the starter unit by decarboxylation of malonyl-ACP has been demonstrated in vitro [1, 6].) (B) Biosynthesis of AA and UWM5 from malonyl-ACP and starter unit propionyl-CoA by the daunorubicin PKS proteins. (C) Production of the 20-carbon polyketide SEK43 from malonyl-ACP and acetyl-CoA by Dps PKS enzymes without DpsC, DpsD, and DpsY.
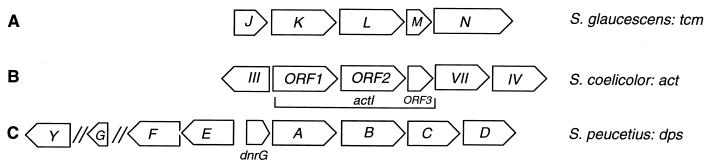
Compared with different sets of type II PKS genes, the cluster of DNR PKS (dps) genes contains several unique features (Fig. 2) (8, 24). Directly downstream of the genes encoding the β-ketoacyl:acyl carrier protein synthase (KS) subunits are two unique genes, dpsC and dpsD, rather than an acyl carrier protein (ACP) gene, which is found in all other PKS gene clusters (Fig. 2A and B). The ACP gene, dpsG, has an atypical position within the cluster, approximately 6.8 kb upstream of the genes encoding the KS subunits (8, 24) (Fig. 2C). In addition, sequence analysis has indicated that the dpsA and dpsB genes encode the KS subunits, dpsE encodes a ketoreductase, and dpsF and dpsY each encode a cyclase enzyme. Since the dpsC and dpsD genes are unique among PKS gene clusters, we are currently investigating the role of the gene products they encode in DNR biosynthesis. On the basis of a high sequence similarity, Grimm et al. (8) proposed that the function of DpsC was analogous to that of the Escherichia coli FabH enzyme, a β-ketoacyl:ACP synthase III (KS III) and a component of a type II fatty acid synthase. KS III catalyzes the condensation of acetyl-CoA with malonyl-ACP in the production of the first intermediate, acetoacetyl-ACP, in E. coli fatty acid biosynthesis. DpsD has a high similarity (48 to 50%) with the MCAT enzymes of bacterial fatty acid synthases and contains the expected active-site signature sequence (xGHSxGE) with the essential Ser residue, suggesting that it functions as an acyltransferase (8). Either or both of the DpsC and DpsD enzymes could be responsible for the starter unit specification in DNR polyketide biosynthesis (8). Using heterologous expression of two plasmids carrying the dnrI regulatory gene and dpsABCEF, dpsG, and other PKS genes in Streptomyces lividans, Grimm et al. (8) isolated and identified AA. Since the two plasmids did not carry dpsD, this result suggested that the enzyme encoded by dpsD had no specific purpose in AA biosynthesis or that a related enzyme supplanted its function in the heterologous system (8).
FIG. 2.
Physical map of Tcm, actinorhodin (ACT), and DNR PKS gene clusters. The actI-ORF1, actI-ORF2, tcmK, tcmL, dpsA, and dpsB genes encode the KS subunits; the actI-ORF3, tcmM, and dpsG genes encode the ACPs; the actVII, actIV, tcmJ, tcmN, dpsF, and dpsY genes encode the polyketide cyclases; and the actIII and dpsE genes encode ketoreductases. The dnrG gene specifies an anthraquinol oxygenase, and the dpsC and dpsD genes encode KS III-like and acyltransferase enzymes, respectively. ORF, open reading frame; //, indication that the genes are separated by several kilobases.
On the other hand, the fact that the active-site cysteine that is well conserved among KS III and thiolase enzymes is replaced with a serine in DpsC (8, 19, 24) has led to skepticism about the proposed starter unit specificity function (24). Metabolite studies by Rajgarhia and Strohl (19) and Gerlitz et al. (7), in which heterologous expression of minimal dps genes was found to result in apparent AA production in the absence of dpsC and dpsD, suggested that these genes were dispensable for the biosynthesis of AA. That is, the PKS consisting of the products of dnrG (which governs C-12 oxidation of the AA precursors [Fig. 1B]) and the dpsABEFG genes appeared to be responsible for the choice of starter unit and also the reduction, folding, and cyclization of the nascent 21-carbon decaketide to form AA (7, 19). Gerlitz et al. (7) noted that the strain also made the 20-carbon compound SEK43 (Fig. 1C). A later study by Strohl et al. (20) using a DNR-producing strain with disrupted dpsC and dpsD genes revealed that a 20-carbon anthracycline was the major metabolite produced. Taken together, these results imply that promiscuous starter unit selection can occur in the absence of dpsC and dpsD. We approached the question of starter unit specification by constructing different combinations of the DNR PKS genes in expression vectors and studied metabolite production by feeding the cultures with 14C-labeled precursors. In addition, a cell-free system was employed to synthesize 14C-labeled polyketides in vitro to study the function of dpsC and dpsD. The results of this research clearly demonstrate that dpsC is the primary genetic determinant of starter unit specificity in the biosynthesis of AA.
Construction of expression plasmids.
The plasmid pWHM75 (8) was used as the source of the dpsABCDEF genes. Plasmids pWHM346 (14) and pWHM555 (13) were used to obtain dpsY and dpsG, respectively. The plasmid pWHM1013, which is pGEM7zf (Promega, Madison, Wis.) containing the dpsBCDG genes, was created as described below and used as the starting point for constructing three other plasmids. A 1.35-kb PvuII fragment containing dpsD was subcloned into pGEM7zf. The SacII-PvuII fragment containing the 3′ end of dpsD was then removed from this clone and replaced with a synthetic oligonucleotide linker approximately 50 bp in length that recreated the end of dpsD and added an MroI site directly behind its stop codon. The resulting dpsD gene was excised as a 940-bp BamHI-MroI fragment and ligated into pGEM7zf along with a 2.4-kb BamHI-XhoI fragment containing dpsBC and a 540-bp PinAI-KpnI fragment containing dpsG, to yield pWHM1013.
Plasmid pWHM1010 (tcmJ dpsABCDG) was made by removing the 3.9-kb HindIII-XhoI fragment from pWHM1013 and ligating it into pWHM3 (22) along with a 2-kb EcoRI-XhoI fragment from pWHM885 (17) that contains the Streptomyces promoter ermE*p (4) and tcmJ (3, 17) (the tcmJ gene is incidental and was carried along solely for convenience). An 85-bp SphI-AatII linker was then inserted into pWHM1013 to recreate the 3′ end of dpsG and to add several cloning sites so that the 1.85-kb XhoI-AvrII dpsEF fragment could be added to the plasmid. The 3.8-kb NsiI-HindIII fragment containing dpsCDGEF was then cut out and substituted into pWHM1010 at the same sites to form pWHM1011 (tcmJ dpsABCDGEF).
Plasmid pWHM1012 (tcmJ dpsABCDGEFY) was created in the same manner as pWHM1011, except that a 1-kb XhoI-BglI fragment containing dpsY was also inserted into the linker behind dpsEF before replacing the NsiI-HindIII fragment in pWHM1010. In both cases, the dpsEF and dpsY fragments were first subcloned into pUC19 (23), pLitmus38 (New England Biolabs, Beverly, Mass.), or pGEM7zf to pick up appropriate restriction sites for easy insertion of these fragments into the linker. The Streptomyces expression plasmid containing dpsEF (pWHM1015) was constructed by treating the 1.85-kb XhoI-AvrII dpsEF fragment described above with the Klenow fragment (GIBCO BRL, Gaithersburg, Md.), to fill in the ends, and by ligating the resulting product into the HincII site of pUC19. The resulting fragment containing dpsEF was cut out of pUC19 with XbaI and HindIII and ligated into the same restriction sites of pWHM1250 (15) to form plasmid pWHM1015, in which the dpsEF genes are expressed from ermE*p.
For the construction of the dpsCD expression plasmid, a NdeI site was introduced at the translational start codon of dpsC and a HindIII site was introduced downstream of the translational stop codon of dpsD. The primers used for PCR were 5′-GGGAATTCCATATGAGCGTGCCGCAGGGGG-3′ and 5′-GGGTATTAAGCTTATCGACGTGCCCGTCC-3′. (Italics indicate the NdeI and HindIII restriction sites, respectively.) PCR was carried out with 2.5 U of Pwo polymerase (Boehringer Mannheim, Indianapolis, Ind.), 0.4 μg of each primer, 1 μg of pWHM75 (8) DNA as the template, EasyStart PCR mix in a tube (Molecular Bioproducts, San Diego, Calif.), and water to a total volume of 100 μl. Amplification was achieved with 30 cycles of denaturation at 94°C for 30 s, annealing at 45°C for 30 s, and extension at 70°C for 2 min. The 2.1-kb PCR product was recovered by 0.8% agarose gel electrophoresis, digested with the NdeI-HindIII fragment, and ligated into the T7 expression plasmid pT7SC (5). A 2.2-kb XbaI-HindIII fragment from the resulting plasmid was excised and ligated into pWHM1250 at the same restriction sites to yield pWHM1014, containing the dpsC and dpsD genes expressed from ermE*p.
Metabolites isolated from cultures of recombinant strains.
Recombinant S. lividans 1326 (10) strains each containing one of the plasmids pWHM80, pWHM1010, pWHM1011, or pWHM1012 (Table 1) were prepared by standard methods (10) and grown in 5 ml of R2YE medium (10) containing thiostrepton (40 μg/ml) in 25-ml tubes at 30°C and 280 rpm for 48 h. This seed culture was used to inoculate 50 ml of R2YE cultures in 250 baffled flasks with thiostrepton (20 μg/ml) that were grown at 30°C and 280 rpm for 20 h. To each flask, 1 μCi of [1-14C]propionic acid or [1-14C]acetate (Sigma, St. Louis, Mo.), not diluted with unlabeled carrier, was added and the cultures were grown for another 10 h. Before extraction with ethyl acetate (twice with 40 ml each time), acetic acid (0.1 ml) was added to each flask to acidify the culture. The ethyl acetate extract was dried under vacuum, and the metabolites were dissolved in methanol for further analysis. High-performance liquid chromatography (HPLC) on a C18 reverse-phase HPLC column (Novapak, 4 μm in diameter, 8 by 100 mm; Waters, Midford, Mass.) was used with a gradient of acetonitrile-water-acetic acid (20:80:0.1 [vol/vol] for 2 min to 100:0:0.1 in 12 min) at a flow rate of 2 ml/min to separate the metabolites. The purified metabolites were detected with a Waters 484 variable wavelength absorbance detector and a Radiomatic Flo-One/Beta A-515 radiochromatography detector (Packard, Downers Grove, Ill.). Based on the total radioactivity recorded in the peak corresponding to the 14C-labeled product, a total incorporation of 50% into SEK43 or UWM5 was calculated. The identity of each to known compounds was verified by comparison HPLC and confirmed by liquid chromatography-mass spectrometry analysis.
TABLE 1.
Metabolite production by strains carrying plasmids used in this work
| Plasmid | Genes | Metabolite from [1-14C]acetic acid | Metabolite from [1-14C]propionic acid |
|---|---|---|---|
| pWHM80 | dnrG dpsABEFG | SEK43 | None |
| pWHM1011 | tcmJ dpsABCDGEF | UWM5 | UWM5 |
| pWHM1010 | tcmJ dpsABCDG | —a | — |
| pWHM1012 | tcmJ dpsABCDGEFY | AA | AA |
—, a small amount of unidentified material was produced.
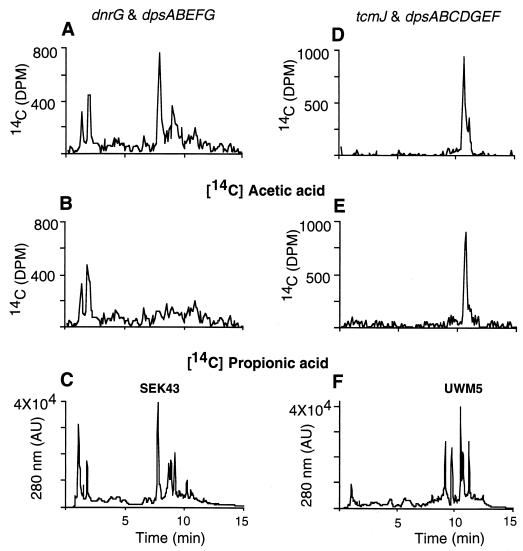
In the extract from S. lividans cultures with the plasmid carrying the dnrG and dpsABEFG genes (pWHM80), the 20-carbon polyketide SEK43, formed by aberrant cyclization of the 20-carbon decaketide (Fig. 1C), was the major product in the extract (Fig. 3C; Table 1). When [1-14C]propionic acid was fed to this culture, no apparent labeled products were detected, although SEK43 remained the primary product of this fermentation (Fig. 3B and C). When [1-14C]acetic acid was added, SEK43 again was the major 14C-labeled product (Fig. 3A and C). Significantly, no 14C-labeled AA was identified in the cultures to which either [1-14C]propionic acid or [1-14C]acetic acid had been added (Fig. 3A to C) (AA is eluted from the column in 11.5 min under the specified condition). Therefore, the dnrG and dpsABEFG genes in pWHM80 do not allow for the incorporation of propionic acid into the polyketide, and they do not produce AA (contrary to a previous report [7], presumably due to inadequate chemical characterization of the product and to the absence of the dpsY gene). An earlier investigation revealed that enzymes expressed from the dpsAB tcmMN genes in S. lividans resulted in the synthesis of a 20-carbon polyketide, Tcm F2 (17). This work, together with our present results, reveals that the DpsA and DpsB KS subunits have the same function as their tcm counterparts, TcmK and TcmL, and that they do not contain the information necessary to direct the starter unit specificity for DNR biosynthesis.
FIG. 3.
HPLC analysis of metabolites produced by S. lividans with plasmids pWHM80 (A, B, and C) and pWHM1011 (D, E, and F). Cultures were fed with [1-14C]acetic acid (A and D), [1-14C]propionic acid (B and E), or nothing (C and F). AU, absorbancy units.
The behavior of the strain with pWHM1011, which contains the dpsC and dpsD genes in addition to the tcmJ and dpsABEFG genes, was dramatically different in terms of the compounds produced from that of the strain containing pWHM80. In these cultures, a 21-carbon polyketide shunt product (UWM5) (Fig. 1B) was the major compound identified (Fig. 3F). When [1-14C]propionic or [1-14C]acetic acid was added to this culture, UWM5 was the dominant product labeled (Fig. 3D and E), indicating that both labeled precursors are incorporated into UWM5. Since without dpsC and dpsD a 20-carbon polyketide is formed, these results show that dpsC and/or dpsD is the primary genetic factor dictating starter unit specificity of DNR biosynthesis.
In strains harboring pWHM1010, which contains only the tcmJ and dpsABCDG genes, some unidentified products were made and were 14C labeled with both of the aforementioned labeled fatty acid precursors (Table 1). The structures of these products have not been fully elucidated, but the preliminary data show that they resemble typical aromatic shunt products formed by type II PKSs. The fact that S. lividans(pWHM1010) was able to incorporate the correct starter unit, propionyl-CoA, into these polyketides supports the belief that the dpsC and/or dpsD gene plays a role in the specification of the starter unit.
Previous work by Lomovskaya et al. (14) had shown that disruption of the dpsY gene in S. peucetius resulted in the production of UWM5 (Fig. 1B), an aberrantly cyclized 21-carbon compound, which led to the suggestion that dpsY maintains a role in the cyclization of the nascent polyketide backbone. We tested this idea by adding the dpsY gene to pWHM1011 to make pWHM1012, which contains the tcmJ and dpsABCDGEFY genes. The S. lividans strain carrying this plasmid produced large quantities of the DNR biosynthetic intermediate AA, which was labeled with both [1-14C]propionic and [1-14C]acetic acid (Table 1). (The host strain apparently supplies a protein with the function normally belonging to the dnrG product in S. peucetius.) These results confirm earlier reports that the function of dpsY is that of a polyketide cyclase in AA biosynthesis.
Analysis of metabolites produced in vitro.
Cell-free synthesis of polyketides has been successfully used to study the functions of PKS enzymes in the biosynthesis of tetracenomycin (1) and actinorhodin (6), two well-characterized bacterial aromatic polyketides. Accordingly, we employed a cell-free system to study DNR biosynthesis, in hopes of illustrating the function of the PKS enzymes involved in this biosynthetic pathway. Cultures of S. lividans strains with plasmid pWHM80, pWHM1010, pWHM1011, pWHM1012, pWHM1014, or pWHM1015 were treated as described above without the addition of 14C-labeled precursors. Cells were harvested, washed, and sonicated, and protein extracts were prepared as described earlier (1). Ammonium sulfate (504 g/liter) was added to the protein extract, and the precipitate was collected by centrifugation (25,240 × g, 20 min). The resulting pellet was dissolved in 100 mM sodium phosphate buffer (pH 7.2) with 2 mM dithiothreitol and 10% glycerol. A PD-10 column (Pharmacia) was used to desalt the solution into the same buffer at a protein concentration of 2.5 mg/ml. The complete assay solution (250 μl) contained 50 μM propionyl-CoA, 150 μM [2-14C]malonyl-CoA, 2 mM dithiothreitol, and 50 μl of protein extract in 0.1 M phosphate buffer (pH 7.5). The assay solution was incubated at 30°C for 100 min, and the reaction was terminated by the addition of 150 mg of NaH2PO4. The products were extracted with ethyl acetate (0.3 ml, three times), and the samples for HPLC analysis were prepared as described earlier (1). HPLC analysis of the products was done as described above.
The protein extract from cultures harboring pWHM80 made small quantities of SEK43 and Tcm F2, both of which are 20-carbon polyketides, from malonyl-CoA (Table 2). The addition of TcmN to the cell-free reaction mixture resulted in the production of Tcm F2 only (Fig. 4A and B), indicating that the DpsF cyclase does not function properly with an unreduced 20-carbon polyketide backbone, as noted previously (18). As anticipated, incorporation of radioactivity from labeled propionyl-CoA was not observed in the absence of DpsC and DpsD. The addition of a protein extract containing DpsC and DpsD to the reaction mixtures containing the DpsABEFG enzymes resulted in the predominant formation of UWM5 (Table 2), which substantiates the role of DpsC and/or DpsD in starter unit specification. Although the DpsABCDG proteins failed to make any identifiable products (Table 2), the addition of a protein extract containing DpsE and DpsF (prepared as described above) to the reaction mixture resulted in the production of UWM5 (Table 2), indicating that the enzymes encoded by the dpsEF genes also play a role in the production of UWM5. As predicted, enzymes from strains containing construct pWHM1011 with the dpsABCDGEF genes made UWM5 (Fig. 4B).
TABLE 2.
Cell-free synthesis of polyketides
| Plasmids | Proteins present | Metabolite from [1-14C]malonyl CoA |
|---|---|---|
| pWHM80 | DnrG and DpsABEFG | —a |
| pWHM80 | DnrG and DpsABEFG + purified TcmN | Tcm F2 |
| pWHM1011 | TcmJ and DpsABCDGEF | UWM5 |
| pWHM1010 + pWHM1015 | TcmJ and DpsABCDG + DpsEF | UWM5 |
| pWHM1080 + pWHM1014 | DnrG and DpsABEFG + DpsCD | UWM5 |
| pWHM80 | DnrG and DpsABEFG + purified DpsC | UWM5 |
—, small amount of SEK43 was produced.
FIG. 4.
HPLC analysis with UV absorbance and 14C radioactivity detection of metabolites produced from malonyl-CoA by DNR PKS enzymes. (A) DnrG and DpsABEFG plus TcmN; (B) TcmJ and DpsABCDGEF. AU, absorbance units.
The results of the above-described experiments have recently been extended by demonstrating cell-free synthesis of UWM5 by the protein extract of the strain containing pWHM80, to which purified DpsC was added (Table 2) (2). At this time, it appears that DpsC is solely responsible for the choice of starter unit for AA and DNR biosynthesis. DpsC thus plays a role comparable to that of FabH in type II fatty acid biosynthesis in Streptomyces glaucescens (9, 21) and E. coli (16). On the basis of in vitro experiments conducted with different starter units (acetyl-CoA, butyryl-CoA, or isobutyryl-CoA), Han et al. (9) found that the S. glaucescens FabH functions as a KS III to catalyze the first condensation step and also appears to specify the starter units for biosynthesis of both straight- and branched-chain fatty acids in S. glaucescens. A similar type of activity has been demonstrated for DpsC (2), which may function as a KS III with DpsG and DpsD or with DpsG, DpsD, and DpsAB, to synthesize the first five-carbon unit of AA. DpsC was found to maintain a very high specific activity for propionyl-CoA in that work (2). Earlier reports by Grimm et al. (8) showed that dpsD mutants retained the ability to choose the correct starter unit in the formation of AA. However, these strains undoubtedly harbored enzymes with a nonspecific MCAT activity that may substitute for the DpsD MCAT (21).
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health (CA35381).
REFERENCES
- 1.Bao W, Wendt-Pienkowski E, Hutchinson C R. Reconstitution of the interactive type II polyketide synthase for tetracenomycin F2 biosynthesis. Biochemistry. 1998;37:8132–8138. doi: 10.1021/bi980466i. [DOI] [PubMed] [Google Scholar]
- 2.Bao, W., P. J. Sheldon, and C. R. Hutchinson. Biochemistry, in press.
- 3.Bibb M J, Biro S, Montamedi H, Collin J, Hutchinson C R. Analysis of the nucleotide sequence of the Streptomyces glaucescens tcmI genes provided key information about the enzymology of polyketide tetracenomycin C antibiotic biosynthesis. EMBO J. 1989;8:2727–2736. doi: 10.1002/j.1460-2075.1989.tb08414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bibb M J, White J, Ward J M, Janssen G R. The mRNA for the 23S rRNA methylase encoded by the ermE gene of Saccharopolyspora erythraea is translated in the absence of a conventional ribosome-binding site. Mol Microbiol. 1994;14:533–545. doi: 10.1111/j.1365-2958.1994.tb02187.x. [DOI] [PubMed] [Google Scholar]
- 5.Brown W C, Campbell J L. A new cloning vector and expression strategy for genes encoding proteins toxic to Escherichia coli. Gene. 1993;127:99–103. doi: 10.1016/0378-1119(93)90622-a. [DOI] [PubMed] [Google Scholar]
- 6.Carreras C W, Khosla C. Purification and in vitro reconstitution of the essential protein components of an aromatic polyketide synthase. Biochemistry. 1998;37:2084–2088. doi: 10.1021/bi972919+. [DOI] [PubMed] [Google Scholar]
- 7.Gerlitz M, Meurer G, Wendt-Pienkowski E, Madduri K, Hutchinson C R. Effect of the daunorubicin dpsH gene on the choice of starter unit and cyclization pattern reveals that type II polyketide synthase can be unfaithful yet intriguing. J Am Chem Soc. 1997;119:7392–7393. [Google Scholar]
- 8.Grimm A, Madduri K, Ali A, Hutchinson C R. Characterization of the Streptomyces peucetius ATCC 29050 genes encoding doxorubicin polyketide synthase. Gene. 1994;151:1–10. doi: 10.1016/0378-1119(94)90625-4. [DOI] [PubMed] [Google Scholar]
- 9.Han L, Lobo S, Reynolds K. Characterization of β-ketoacyl-acyl carrier protein synthase III from Streptomyces glaucescens and its role in initiation of fatty acid biosynthesis. J Bacteriol. 1998;180:4481–4486. doi: 10.1128/jb.180.17.4481-4486.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hopwood D A, Bibb M J, Chater K F, Bruton C J, Kieser H M, Lydiate D J, Smith C P, Ward J M, Schrempf H. Genetic manipulation of streptomyces, a laboratory manual. Norwich, United Kingdom: The John Innes Foundation; 1985. [Google Scholar]
- 11.Hutchinson C R. Biosynthetic studies of daunorubicin and tetracenomycin C. Chem Rev. 1997;97:2525–2535. doi: 10.1021/cr960022x. [DOI] [PubMed] [Google Scholar]
- 12.Hutchinson C R. Antibiotics from genetically engineered microorganisms. In: Strohl W R, editor. Biotechnology of antibiotics. 2nd ed. New York, N.Y: Marcel Dekker; 1997. pp. 683–702. [Google Scholar]
- 13.Lomovskaya N, Otten S L, Doi-Katayama Y, Fonstein L, Liu X-C, Takatsu T, Inventi-Solari A, Filippini S, Torti F, Colombo A L, Hutchinson C R. Doxorubicin overproduction in Streptomyces peucetius: cloning and characterization of the dnrU ketoreductase and dnrV genes and the doxA cytochrome P-450 hydroxylase gene. J Bacteriol. 1999;181:305–318. doi: 10.1128/jb.181.1.305-318.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lomovskaya N, Doi-Katayama Y, Filippini S, Nastro C, Fonstein L, Gallo M, Colombo A L, Hutchinson C R. The Streptomyces peucetius dpsY and dnrX genes govern early and late steps of daunorubicin and doxorubicin biosynthesis. J Bacteriol. 1998;180:2379–2386. doi: 10.1128/jb.180.9.2379-2386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madduri K, Kennedy J, Rivola G, Inventi-Solari A, Filippini S, Zanuso G, Colombo A L, Gerwain K M, Occi J L, MacNeil D J, Hutchinson C R. Production of the antitumor drug epirubicin (4′-epidoxorubicin) and its precursor by a genetically engineered strain of Streptomyces peucetius. Nat Biotechnol. 1998;16:69–74. doi: 10.1038/nbt0198-69. [DOI] [PubMed] [Google Scholar]
- 16.Magnuson K, Jackowski S, Rock C O, Cronan J E., Jr Regulation of fatty acid biosynthesis in Escherichia coli. Microbiol Rev. 1993;57:522–542. doi: 10.1128/mr.57.3.522-542.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meurer G, Hutchinson C R. Daunorubicin type II polyketide synthase enzymes DpsA and DpsB determine neither the choice of starter unit nor the cyclization pattern of aromatic polyketide. J Am Chem Soc. 1995;117:5899–5900. [Google Scholar]
- 18.Meurer G, Gerlitz M, Wendt-Pienkowski E, Vining L C, Rohr J, Hutchinson C R. Iterative, type II polyketide synthases, cyclases and ketoreductases exhibit context dependent behavior in the biosynthesis of linear and angular decapolyketides. Chem Biol. 1997;4:433–443. doi: 10.1016/s1074-5521(97)90195-2. [DOI] [PubMed] [Google Scholar]
- 19.Rajgarhia V B, Strohl W R. Minimal Streptomyces sp. strain C5 daunorubicin polyketide biosynthesis genes required for aklanonic acid biosynthesis. J Bacteriol. 1997;179:2690–2696. doi: 10.1128/jb.179.8.2690-2696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strohl W R, Rajgarhia V B, Priesley N D. Proceedings of the International Interdisciplinary Conference on Polyketides. II—Chemistry, biochemistry and molecular genetics. Bristol, United Kingdom: University of Bristol; 1998. Streptomyces sp. strain C5 mutant disrupted in daunorubicin-specific polyketide synthase genes, dpsC and dpsD, produce novel anthracyclines suggesting promiscuous starter unit selection. [Google Scholar]
- 21.Summers R G, Ali A, Shen B, Wessel W A, Hutchinson C R. Malonyl coenzyme A:acyl carrier protein acetyltransferase of Streptomyces: a possible link between fatty acid and polyketide biosynthesis. Biochemistry. 1995;34:9389–9402. doi: 10.1021/bi00029a015. [DOI] [PubMed] [Google Scholar]
- 22.Vara J, Lewandowska-Sharbeck M, Wang Y-G, Donadio S, Hutchinson C R. Cloning of genes governing the deoxysugar portion of the erythromycin biosynthesis pathway in Saccharopolyspora erythraea (Streptomyces erythreus) J Bacteriol. 1989;171:5872–5881. doi: 10.1128/jb.171.11.5872-5881.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 24.Ye J, Dickens M L, Plater R, Li Y, Lawrence J, Strohl W R. Isolation and sequence analysis of polyketide synthase genes from the daunomycin-producing Streptomyces sp. strain C5. J Bacteriol. 1994;176:6270–6280. doi: 10.1128/jb.176.20.6270-6280.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]