Abstract
During growth on a glucose-tryptone medium, Bacillus subtilis 6051 (Marburg strain) exhibited three phases of isoprene (2-methyl-1,3-butadiene) formation, corresponding to (i) glucose catabolism and secretion of acetoin, (ii) catabolism of acetoin, and (iii) the early stages of sporulation. These results establish an experimental system for studying the biological role of isoprene formation.
Isoprene (2-methyl-1,3-butadiene) is a volatile hydrocarbon produced by a diverse group of organisms including bacteria, marine algae, animals, and a wide variety of plants (12, 13, 16, 23), sometimes in relatively large amounts. For example, global isoprene emission from green plants is estimated to be about 500 million tons per year (10), an amount which is comparable to global methane emissions of about 510 million tons per year (7). Surprisingly little is known about the biochemical rationale for isoprene production in any biological system (4, 14, 21).
Our discovery of isoprene formation in bacteria (12) has created the possibility of studying isoprene biosynthesis at the physiological, biochemical, and genetic levels in prokaryotic systems. A wide variety of bacterial species have been shown to produce isoprene, including both gram-positive and gram-negative strains (12, 23). Bacillus species were shown to be the most active isoprene producers on a variety of growth media (12). Here, we describe a more detailed exploration of Bacillus subtilis 6051 as an experimental system for studying isoprene biosynthesis and show that this wild-type strain exhibits a unique pattern of isoprene production during cellular growth and sporulation.
B. subtilis isoprene formation during aerobic growth.
With a stirred fermentor in which a variety of growth conditions (pH, aeration, and temperature) could be closely monitored and controlled, the production of isoprene was measured over the complete life cycle of B. subtilis. Wild-type B. subtilis 6051 (Marburg strain) was obtained from the American Type Culture Collection (Manassas, Va.) and maintained on AB3 plates (17.5 g of antibiotic medium 3 and 15 g of agar per liter; Difco). Each fermentor experiment consisted of growing a bacterial culture in 1 liter of F medium, a glucose-tryptone-salts medium (see legend to Fig. 1), with a BioFlo 2000 fermentation system (New Brunswick Scientific). Besides monitoring the pH and dissolved oxygen of the culture, we also measured the cell growth (optical density at 600 nm [OD600]), spore formation, isoprene production, and various extracellular metabolites in an attempt to determine how growth and differentiation affect isoprene formation. Heat-resistant spores were measured in aliquots removed from the fermentor and heated at 80°C for 20 min. Dilutions of these samples in sterile water were then plated on NB plates (8 g of nutrient broth and 15 g of agar per liter; Difco), and single colonies were counted following overnight incubation at 37°C. Isoprene in the exit gas from the fermentor was analyzed with a gas chromatography (GC) system which is highly sensitive to isoprene (8, 12). Isoprene production rates (nanomoles per liter of oxygen) were calculated by converting GC area units to nanomoles of isoprene via a standard isoprene concentration calibration curve. Glucose was measured in cell culture extracts with an assay kit (glucose oxidase-peroxidase; Sigma Chemical). Acetoin production was analyzed by high-performance liquid chromatography (HPLC) analysis of cell culture extracts which had been derivatized with 2,4-dinitrophenylhydrazine, as described in detail elsewhere (22).
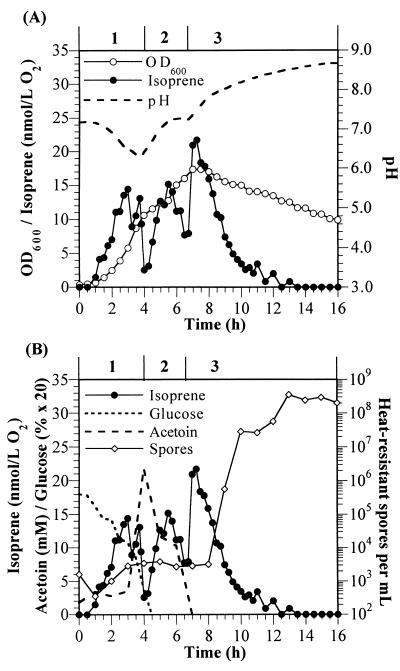
FIG. 1.
B. subtilis 6051 exhibits three phases of isoprene production during growth and sporulation. (A) Isoprene, growth, and pH profiles. (B) Isoprene, glucose, acetoin, and sporulation profiles. Cells were grown in 1 liter of F medium at 40°C with an oxygen flow of 1.5 liters min−1. F medium contained (per liter; pH 7.4) 10 g of glucose, 20 g of Bacto tryptone (Difco), 7 g of K2HPO4, 3 g of KH2PO4, 1 g of NH4Cl, 0.1 g of MgSO4 · 7H2O, 0.5 g of sodium citrate (dihydrate), 5.5 mg of CaCl2, 13.5 mg of FeCl2 · 6H2O, 1.0 mg of MnCl2 · 4H2O, 1.7 mg of ZnCl2, 0.4 mg of CuCl2 · 2H2O, 0.6 mg of CoCl2 · 6H2O, and 0.6 mg of Na2MoO4 · 2H2O. The dissolved oxygen was kept above a 15% minimum level by increasing agitation from 300 to 375 rpm. Phases 1, 2, and 3 of isoprene production are indicated at the top of each graph.
For these experiments, the inoculum was a 100-ml preculture grown for 22 to 24 h in F medium in a 37°C shaker. This large inoculum volume resulted in an initial OD600 value of approximately 0.4 for the 1-liter culture, the carryover of a small amount of spores (i.e., less than 0.01% heat-resistant spores as a fraction of total viable cells), and kept the subsequent elapsed time of a fermentor run as low as possible. Inoculation with a mid-exponential-phase culture significantly increased the lag at the beginning of the fermentor run but did not change the isoprene profile once cells began to grow.
Wild-type B. subtilis 6051 (Marburg strain) exhibited a unique isoprene production profile consisting of three distinct phases (Fig. 1). Isoprene was formed as soon as the cells began to grow, and during this first isoprene production phase, glucose was catabolized, the pH of the medium decreased, and the cells released large amounts of acetoin (3-hydroxy-2-butanone). Phase 1 isoprene production peaked at about the time that acetoin release began and then declined to a minimum coincident with exhaustion of glucose and a maximum in acetoin levels in the medium. Phase 2 isoprene production occurred during acetoin catabolism (Fig. 1B) as the pH increased (Fig. 1A), peaked when about half of the acetoin remained, and ended with depletion of acetoin in the medium (Fig. 1B). This decrease in isoprene release was similar to the trend in which phase 1 isoprene reached a minimum when glucose was exhausted. The third phase of isoprene production occurred when cell growth ceased, the pH of the medium had increased again, and the OD600 began to decrease as a result of spore formation; peak isoprene formation in phase 3 occurred immediately prior to the release of spores (Fig. 1).
The three phases of isoprene formation by strain 6051 in F medium were reproducible and were seen in numerous experiments. When we measured cellular growth and total isoprene production for each phase (isoprene release in the exit gas of the fermentor was integrated), we found that isoprene production and overall growth during individual phases differed by no more than 15% for cultures grown under identical conditions. When B. subtilis 6051 was grown on the standard F medium containing 1% glucose and 2% tryptone, the total isoprene formation during all three phases was approximately 9 μmol. While this represents only a very small fraction of glucose carbon provided in the medium (50 mmol), the high sensitivity of the GC system allowed us to accurately measure and easily detect changes in isoprene production levels.
B. subtilis phase 1 isoprene production.
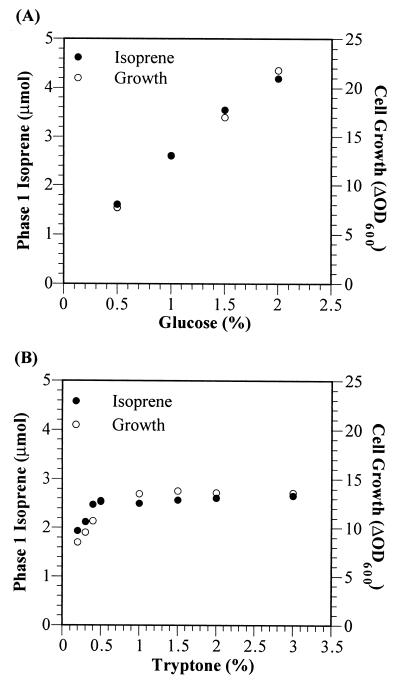
In an attempt to link phase 1 isoprene production directly to glucose catabolism, strain 6051 was grown in F medium containing various glucose concentrations while keeping the tryptone concentration constant at 2% (wt/vol). The total phase 1 isoprene production and cellular growth in each experiment were found to be directly proportional to the amount of glucose provided in the medium (Fig. 2A). This evidence is consistent with our observation that B. subtilis 6051 phase 1 isoprene becomes fully 13C labeled when cells are grown on uniformly labeled [13C]glucose (22).
FIG. 2.
The effects of varying glucose and tryptone on phase 1 isoprene formation and growth of B. subtilis 6051. (A) Total isoprene production and cellular growth during phase 1 for B. subtilis 6051 grown in F medium with 2% (wt/vol) tryptone and varying glucose concentrations. (B) Total isoprene production and cellular growth during phase 1 for B. subtilis 6051 grown in F medium with 1% (wt/vol) glucose and varying tryptone concentrations. ΔOD600, increase in OD600 during phase 1 growth. All data was collected from cultures grown as described in the legend to Fig. 1.
We also performed experiments in which the tryptone concentration was varied while the glucose concentration was kept constant at 1% (wt/vol). The tryptone level affected the phase 1 isoprene and growth yields only when the tryptone concentration was below 0.4% (Fig. 2B). In these cases, phase 1 isoprene production likely decreased because the cells had to use some of the glycolytic carbon for amino acid synthesis rather than simply utilizing amino acids from the medium. When minimal glucose medium (F medium with no tryptone) was used, a pronounced growth lag and poor growth occurred under the fermentation conditions described here; isoprene production was undetectable under these conditions.
It has previously been shown that B. subtilis secretes pyruvate, acetoin, acetate, and other metabolites when grown aerobically in glucose-based media; these metabolites can be used for subsequent growth when glucose is exhausted (9, 18). During the earliest stages of phase 1 growth on glucose, pyruvate accumulated to nearly 3 mM in the medium and then was rapidly assimilated immediately prior to the production of acetoin (data not shown). Acetoin production (Fig. 1B) rose to over 20 mM, as measured by HPLC methods, indicating that a sizable portion of initial glucose (about 50 mM) was converted to acetoin. Glucose represses the expression of acetoin catabolism genes (18), which explains why acetoin levels continually increased until glucose was exhausted at the end of phase 1.
Phases 2 and 3 of isoprene production.
As mentioned above, phase 2 of isoprene formation began as extracellular acetoin levels peaked and then began to decline. Since acetoin is not very volatile, this decline is consistent with cellular catabolism of acetoin. The pH of the medium increased during phase 2 (Fig. 1A), implying that acids secreted into the medium during phase 1 were also being metabolized (18). However, the levels of these acids were not measured. Unlike the first two phases of isoprene production, phase 3 of B. subtilis 6051 isoprene production occurred when the cells had stopped growing. The total isoprene production in phase 3 was greater than that in either of the first two isoprene phases, indicating that significant isoprene production can occur even in nongrowing cells. Preliminary experiments suggest that phase 3 isoprene production occurs before the IIii stage of sporulation (22).
Normalization of isoprene production.
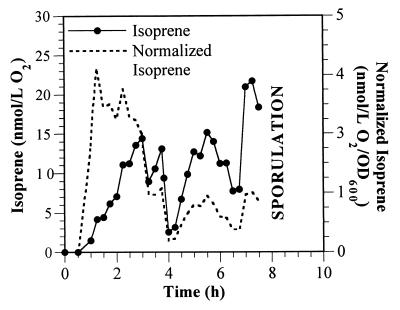
In analyzing the B. subtilis 6051 system, we also expressed isoprene production levels on a cellular basis. Isoprene concentrations were normalized to cell density (OD600) as shown in Fig. 3. The highest normalized isoprene production occurred during the first 2 to 3 h of exponential growth in phase 1 prior to acetoin formation. Normalized isoprene production in phase 2 was significantly lower. Once the cells started to sporulate during phase 3, the OD600 values gradually declined, and thus normalized isoprene levels are presented only for early phase 3 prior to this decline; at this early stage of spore development, isoprene formation on a cellular basis was similar to that in phase 2. These results suggest that while isoprene release from cells occurs under very different metabolic circumstances, including the initial stages of sporulation, maximal isoprene release per cell occurs in the initial stages of glycolysis. The lowest levels of normalized isoprene production occurred when primary carbon sources were exhausted (i.e., end of phases 1 and 2).
FIG. 3.
B. subtilis 6051 isoprene and normalized isoprene profiles. Data was taken from the experiment described in the legend to Fig. 1, and total isoprene production in the exit gas was calculated as the summation of the average isoprene concentrations (nanomoles per liter of O2) between successive time points with an oxygen flow rate of 1.5 liters min−1. Normalized isoprene formation on a cell basis was calculated by dividing the isoprene concentration by the cell density (OD600). Since cell growth ceased and sporulation began at about 7.5 to 8 h, normalized isoprene was not calculated beyond this point of the experiment.
What is the metabolic rationale for isoprene formation?
With B. subtilis 6051, we have established an experimental system in which isoprene formation is linked to three distinct processes during growth on a glucose-tryptone medium: glucose catabolism, acetoin catabolism, and sporulation. Since the underlying metabolic activities during these phases are seemingly very different, it is not immediately clear how to explain these releases of isoprene, and much more work is needed to do so. One possible mechanism under investigation is that isoprene is a metabolic overflow metabolite released when flow of carbon to higher isoprenoids is restricted. In B. subtilis, the isoprenoid building blocks isopentenyl diphosphate and dimethylallyl diphosphate (DMAPP) are used primarily for synthesis of the side chain of menaquinone, a component of the electron transport chain, and undecaprenyl phosphate, the glycosyl carrier lipid for cell wall synthesis (2, 20). Some DMAPP is also used for modifications of tRNATyr and tRNAPhe which contain 2-methylthio-N-6-isopentenyladenosine (19). Production of all of these isoprenoids is required for aerobic cellular growth. It seems likely that in Bacillus isoprene is a product of the deoxyxylulose phosphate pathway of isoprenoid biosynthesis (3, 21), arising from DMAPP as it does in plants (17). If this is the case, isoprene formation could result from DMAPP “overflow” from isoprenoid pathways. In this model, more isopentenyl diphosphate and DMAPP are synthesized than are needed for higher isoprenoid production, and isoprene formation acts as a safety valve freeing up cellular pyrophosphate for use in other cellular processes. Since isoprene is so volatile, it is easily released from the cells. This model might explain (a) why isoprene is released when cells are rapidly metabolizing available carbon sources, such as seen in phases 1 and 2, where carbon is shunted to the deoxyxylulose pathway for the synthesis of essential isoprenoids, and (b) why isoprene production declines during transitions in carbon assimilation pathways, when less carbon is available for isoprenoid synthesis. Metabolic overflow and metabolite secretion are well-known phenomena during aerobic bacterial growth on carbohydrates (15) and during transitions from glycolysis to citric acid cycle metabolism (6). For example, it has been found that when glucose is abundant in aerobic B. subtilis cultures, other components of the growth medium (i.e., phosphate and sulfate) may limit complete glucose oxidation, resulting in the excretion of metabolic intermediates such as acetate and pyruvate (15).
Why would production of isoprene continue when cell growth ceases and spore formation is initiated (i.e., phase 3 isoprene formation)? It may be relevant that both menaquinone production and tRNA modifications are enhanced during sporulation (1, 5). The overflow model would predict that during the onset of sporulation, carbon in excess of that needed for tRNA prenylation and menaquinone biosynthesis is shunted to the isoprenoid pathway, and this excess is released as isoprene.
This and other models for isoprene formation in B. subtilis will be more amenable to analysis when further information on the isoprene biosynthetic pathway is discovered. With genetic and molecular tools arising from the completion of the Bacillus genome project (11), it will be possible to relate isoprene formation during growth and sporulation to the expression of particular genes. With defined fermentation conditions, such as those described here, it will also be possible to relate isoprene formation in particular mutants to the metabolic status of the cell.
Acknowledgments
This research was supported by grant DE-FG03-97ER20274 from the U.S. Department of Energy, Office of Basic Energy Sciences, and the Colorado Institute for Research in Biotechnology (fellowship to W.P.W.).
We thank Jeff Heys for assistance in running fermentor experiments and Megan Shirk and Cindy Barnes for assistance with HPLC. We also thank Veronica Bierbaum and Robert Kuchta for valuable suggestions concerning data analysis.
REFERENCES
- 1.Bjork G, Ericson J, Gustafsson C, Hagervall T, Jonsson Y, Wikstrom P. Transfer RNA modifications. Annu Rev Biochem. 1987;56:263–287. doi: 10.1146/annurev.bi.56.070187.001403. [DOI] [PubMed] [Google Scholar]
- 2.Bugg T, Brandisch P. From peptidoglycan to glycoproteins: common features of lipid-linked oligosaccharide biosynthesis. FEMS Microbiol Lett. 1994;119:255–262. doi: 10.1111/j.1574-6968.1994.tb06898.x. [DOI] [PubMed] [Google Scholar]
- 3.Eisenreich W, Schwartz M, Cartayrade A, Arigoni D, Zenk M H, Bacher A. The deoxyxylulose pathway of terpenoid biosynthesis in plants and microorganisms. Chem Biol. 1998;5:R221–R233. doi: 10.1016/s1074-5521(98)90002-3. [DOI] [PubMed] [Google Scholar]
- 4.Fall R. Biogenic emissions of volatile organic compounds from higher plants. In: Hewitt C N, editor. Reactive hydrocarbons in the atmosphere. San Diego, Calif: Academic Press; 1999. pp. 41–96. [Google Scholar]
- 5.Farrand S K, Taber H W. Changes in menaquinone concentration during growth and early sporulation in Bacillus subtilis. J Bacteriol. 1974;117:324–326. doi: 10.1128/jb.117.1.324-326.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goel A, Domach M M, Hanley W, Lee J W, Ataai M M. Coordination of glycolysis and TCA cycle networks. Citrate-glucose cometabolism eliminates acids and reveals potential metabolic engineering strategies. Ann N Y Acad Sci. 1996;782:1–16. doi: 10.1111/j.1749-6632.1996.tb40542.x. [DOI] [PubMed] [Google Scholar]
- 7.Graedel T E, Crutzen P J. Atmospheric change. An earth system perspective. New York, N.Y: W. H. Freeman & Co.; 1993. [Google Scholar]
- 8.Greenberg J P, Zimmerman P R, Taylor B E, Silver G M, Fall R. Sub-parts per billion detection of isoprene using a reduction gas detector with a portable gas chromatograph. Atmos Environ. 1993;27A:2689–2692. [Google Scholar]
- 9.Grundy F J, Waters D A, Takova T Y, Henkin T M. Identification of genes involved in utilization of acetate and acetoin in Bacillus subtilis. Mol Microbiol. 1993;10:259–271. doi: 10.1111/j.1365-2958.1993.tb01952.x. [DOI] [PubMed] [Google Scholar]
- 10.Guenther A C, Hewitt C, Erickson D, Fall R, Geron C, Graedel T, Harley P, Klinger L, Lerdau M, McKay W, Pierce T, Scholes B, Steinbrecher R, Tallamraju R, Taylor J, Zimmerman P. A global model of natural volatile organic compound emissions. J Geophys Res. 1995;100:8873–8892. [Google Scholar]
- 11.Kunst F, et al. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 12.Kuzma J, Nemecek-Marshall M, Pollock W H, Fall R. Bacteria produce the volatile hydrocarbon isoprene. Curr Microbiol. 1995;30:97–103. doi: 10.1007/BF00294190. [DOI] [PubMed] [Google Scholar]
- 13.Lerdau M, Guenther A, Monson R. Plant production and emission of volatile organic compounds. BioScience. 1997;47:373–383. [Google Scholar]
- 14.Logan, B. A., and R. K. Monson. Lack of an enhancement of leaf thermotolerance during exposure of four isoprene-emitting species to exogenous isoprene. Plant Physiol., in press. [DOI] [PMC free article] [PubMed]
- 15.Neijssel O M, Tempest D W. The physiology of metabolite over-production. In: Bull A T, Ellwood D C, Ratledge C, editors. Microbial technology: current state, future prospects. Cambridge, United Kingdom: Cambridge University Press; 1979. pp. 53–82. [Google Scholar]
- 16.Sharkey T D. Isoprene synthesis by plants and animals. Endeavour. 1996;20:74–78. doi: 10.1016/0160-9327(96)10014-4. [DOI] [PubMed] [Google Scholar]
- 17.Silver G M, Fall R. Characterization of aspen isoprene synthase, an enzyme responsible for leaf isoprene emission to the atmosphere. J Biol Chem. 1995;270:13010–13016. doi: 10.1074/jbc.270.22.13010. [DOI] [PubMed] [Google Scholar]
- 18.Speck E L, Freese E. Control of metabolite secretion in Bacillus subtilis. J Gen Microbiol. 1973;78:261–275. doi: 10.1099/00221287-78-2-261. [DOI] [PubMed] [Google Scholar]
- 19.Sprinzl M, Moll J, Meissner F, Hartmann T. Compilation of tRNA sequences. Nucleic Acids Res. 1985;13:r1–r49. doi: 10.1093/nar/13.suppl.r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi I, Ogura K, Seto S. Heptaprenyl pyrophosphate synthetase from Bacillus subtilis. J Biol Chem. 1980;255:4539–4543. [PubMed] [Google Scholar]
- 21.Taucher J, Hansel A, Jordan A, Fall R, Futrell J H, Lindinger W. Detection of isoprene in expired air from human subjects using proton-transfer-reaction mass spectrometry. Rapid Commun Mass Spectrom. 1997;11:1230–1234. doi: 10.1002/(SICI)1097-0231(199707)11:11<1230::AID-RCM3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 22.Wagner W P. M.S. thesis. Boulder: University of Colorado; 1998. [Google Scholar]
- 23.Wilkins K. Volatile metabolites from Actinomycetes. Chemosphere. 1996;32:1427–1434. [Google Scholar]