INTRODUCTION
Early in the coronavirus disease 2019 (COVID-19) pandemic, the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network reported rates of asymptomatic (47%) and critical illness (4%) in pregnant patients in the United States. The frequency of cesarean delivery (adjusted relative risk [aRR] 1.57, 95% CI 1.30–1.90), preterm birth (aRR 3.52, 95% CI 2.42–5.14), and pregnancy-related hypertension (aRR 1.61, 95% CI 1.18–2.20) were increased in those with severe or critical illness.1 Before June 2021, the majority of infections sequenced at our institution were identified as the Alpha variant (B.1.1.7) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), but by mid-June, the Delta (B.1.617.2) variant predominated, with rates reaching 85% of cases by mid-July and 95% by early August. With the emergence of the Delta variant, we sought to report differences in perinatal outcomes after its emergence.
METHODS
We performed a retrospective cohort study of all pregnant patients with COVID-19 at the University of Alabama at Birmingham from March 22, 2020, to August 18, 2021. At our institution, we began universal inpatient testing of all obstetric patients on admission on April 15, 2020. This was briefly paused (June 21, 2021–August 1, 2021) for fully vaccinated, asymptomatic patients, but then resumed on August 1, 2021, given rising case numbers. During this pause, all unvaccinated patients and any symptomatic patients were still tested on admission. Based on the subsets of pregnant patients who underwent genotypic sequencing at our institution, patients were categorized as pre-Delta (March 22, 2020–May 31, 2021) or Delta (July 1, 2021–August 18, 2021; 95% of cases sequenced were Delta). We excluded patients diagnosed in June 2021 (given the multiple variants in sequencing data at our institution). Outcomes included admission rates and adverse maternal and neonatal outcomes. Outcomes were compared between the pre-Delta and Delta groups; log-binomial regression was used to estimate aRRs with 95% CIs. We performed a sensitivity analysis restricted to patients receiving prenatal care at our institution (compared with those transferred from another facility) to account for potential referral bias.
RESULTS
A total of 293 pregnant patients with COVID-19 were included: 224 in the pre-Delta period and 69 in the Delta period. Groups were similar, except there were more patients with asthma in the pre-Delta period. During the pre-Delta period, none of the patients who tested positive had been vaccinated, compared with 3% during the Delta period. The proportion of patients admitted to the intensive care unit (ICU) who had been transferred from other institutions was similar between groups (75% pre-Delta vs 70% Delta). August 2021 (only through the 18th) had the highest overall number of patients admitted per month to both the ICU and inpatient units (Fig. 1). Overall, the screen-positive rate (% positive/all patients tested on admission) increased on average from 3% in the pre-Delta period to 15% in the Delta period. Proportions of severe–critical disease (13% vs 36%, aRR 2.76, 95% CI 1.73–4.40) and ICU admissions (8% vs 29%, 3.42, 95% CI 1.91–6.11) were increased in the Delta cohort. Need for respiratory support, intubation, and pharmacologic treatment was also increased significantly (Table 1). Of the 91 (41%) patients in the pre-Delta period and 28 (41%) patients in the Delta period who delivered at our institution during their COVID-19 admission, the rates of cesarean delivery, preterm birth, and neonatal intensive care unit admission all increased in the Delta cohort (Table 1). Results were consistent when limited to patients who received prenatal care at our institution (n=237, relative risk [95% CI] for severe–critical disease 2.81 [1.33–5.94] and ICU admission 3.94 [1.33–11.67]).
Fig. 1.
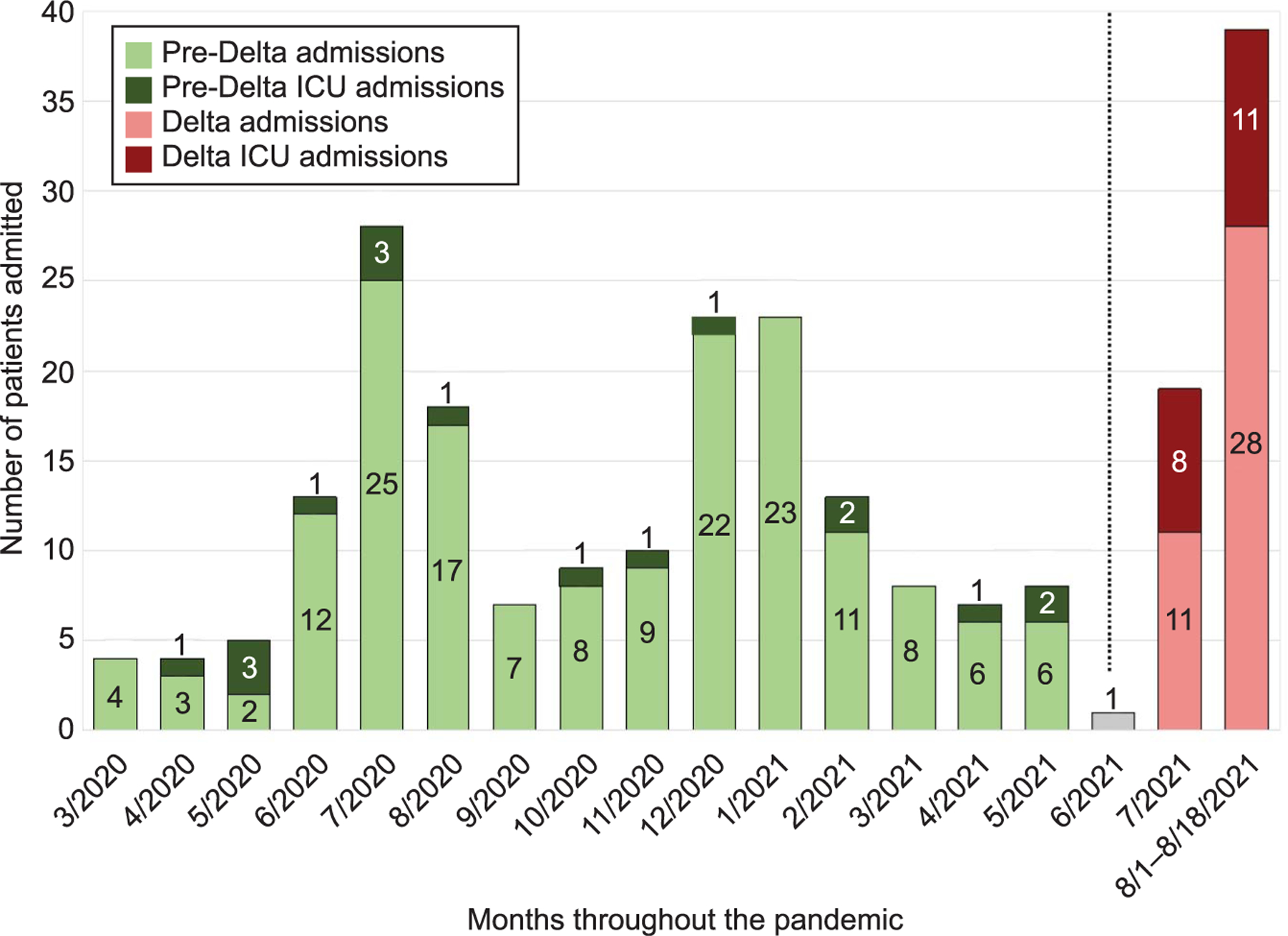
Admission trends in relationship to the rise in the Delta (B.1.617.2) variant among pregnant patients positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Dashed line represents transition from predominately Alpha to predominately Delta variant and denotes the period excluded from the analysis due to the multiple variants in the community at that time. ICU, intensive care unit.
Seasely. SARS-CoV-2 Delta Variant Outcomes in Pregnancy. Obstet Gynecol 2021.
Table 1.
Illness Severity and Perinatal Outcomes of Patients Who Required Delivery During Their Admission With Coronavirus Disease 2019 (COVID-19), According to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Variant Type
| Period |
P | RR (95% CI) | aRR (95% CI)* | ||
|---|---|---|---|---|---|
| Pre-Delta (n=224) |
Delta (n=69) |
||||
| Disease severity† | <.001 | ||||
| Asymptomatic | 104 (46) | 11 (16) | 0.34 (0.20–0.60) | 0.34 (0.19–0.59) | |
| Mild–moderate | 91 (41) | 33 (48) | 1.18 (0.88–1.58) | 1.22 (0.90–1.65) | |
| Severe–critical | 29 (13) | 25 (36) | 2.80 (1.76–4.44) | 2.76 (1.73–4.40) | |
| ICU admission | 18 (8) | 20 (29) | <.001 | 3.61 (2.03–6.42) | 3.42 (1.91–6.11) |
| Received pharmacologic treatment‡ | 35 (16) | 25 (36) | <.001 | 2.32 (1.50–3.59) | 2.31 (1.49–3.59) |
| Required respiratory support§ | 29 (13) | 25 (36) | <.001 | 2.80 (1.76–4.44) | 2.76 (1.73–4.40) |
| Intubation | 12 (5) | 16 (23) | <.001 | 4.33 (2.15–8.70) | 4.18 (2.06–8.48) |
| ECMO | 3 (1) | 3 (4) | .15 | 3.25 (0.67–15.72) | — |
| VTE | 5 (2) | 2 (3) | .67 | 1.30 (0.26–6.55) | — |
| Maternal death | 1 (1) | 1 (2) | .42 | 3.25 (0.21–51.22) | — |
| Patients who required delivery | n=91 | n=28 | |||
| Cesarean birth | 28 (31) | 17 (61) | .004 | 1.97 (1.29–3.03) | — |
| Indication, worsening maternal status | 4 (14) | 12 (71) | <.001 | 4.94 (1.90–12.88) | 4.94 (1.90–12.88) |
| Preterm birth at less than 37 wk | 30 (32) | 22 (73) | <.001║ | 2.37 (1.66–3.36) | 2.36 (1.68–3.32) |
| Gestational age at delivery (wk) | 35.6±5.6 | 33.7±4.8 | .14 | — | — |
| NICU admission | 37 (44) | 20 (74) | .008║ | 1.77 (1.23–2.53) | 1.77 (1.25–2.51) |
| Delivery complications | |||||
| Preeclampsia | 21 (23) | 5 (18) | .56 | 0.77 (0.32–1.86) | — |
| Abruption | 2 (2) | 1 (4) | .56¶ | 1.63 (0.15–17.26) | — |
| PPH | 10 (11) | 4 (14) | .74¶ | 1.30 (0.44–3.83) | — |
| Transfusion | 10 (11) | 4 (14) | .74¶ | 1.30 (0.44–3.83) | — |
| Stillbirth | 6 (6) | 1 (3) | .55║ | 0.53 (0.07–4.24) | — |
| Neonatal positive SARS-CoV-2 test result# | 0 (0) | 1 (4) | — | — | — |
RR, relative risk; aRR, adjusted relative risk; ICU, intensive care unit; ECMO, extracorporeal membrane oxygenation; VTE, venous thromboembolism; NICU, neonatal intensive care unit; PPH, postpartum hemorrhage; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Data are n (%) or mean±SD unless otherwise specified.
Adjusted relative risk (95% CI) with pre-Delta as reference, adjusted for asthma.
Disease severity based on the National Institutes of Health guidelines and categorized by the Metz et al study.1
Treatment includes azithromycin, cephalosporins, remdesivir, dexamethasone, or convalescent plasma.
Respiratory support included any form of supplemental oxygen (nasal cannula, high flow, bilevel positive airway pressure, and intubation).
Log-binomial regression models with repeated measures to account for correlation in data due to multiple gestations.
Fisher exact test.
Positive neonatal SARS-CoV-2 test result within 48 hours of delivery; placental studies in process to help determine whether this was a vertical transmission.
DISCUSSION
We present early findings of serious morbidity and adverse perinatal outcomes associated with the SARS-CoV-2 Delta variant in pregnancy. All cases of COVID-19 in pregnant patients admitted to the hospital occurred in unvaccinated patients. Two mild breakthrough cases in the Delta timeframe were diagnosed in fully vaccinated immunocompetent pregnant patients, both health care workers (vaccinated in January 2021). Neither required admission.
When considering the low (23%)2 vaccination rates in pregnant patients together with the overall low vaccination rate in Alabama (38%),3 this increase in COVID-19 morbidity is expected to have a negative influence on efforts to reduce maternal mortality. As such, we emphasize recommendations from the Society for Maternal-Fetal Medicine, the American College of Obstetricians and Gynecologists, and the Centers for Disease Control and Prevention to vaccinate all pregnant patients to mitigate severe perinatal morbidity and mortality.4,5
Footnotes
Each author has confirmed compliance with the journal’s requirements for authorship.
Financial Disclosure
Rachel G. Sinkey reports money was paid to her institution from GestVision and ProLab. Alan T. Tita reports money was paid to his institution from the NIH, CDC, and Pfizer for COVID-19 and RSV vaccine in pregnancy studies. Ashley N. Battarbee reports money was paid to her institution from the NIH and CDC for COVID-19 in pregnancy studies. Akila Subramaniam reports money was paid to her institution from the NIH. Jeff M. Szychowski reports money was paid to his institution from the NIH. Jodie Dionne-Odom currently receives grant funding from NIH/NICHD for an unrelated study on infection in pregnancy. Sixto M. Leal Jr. reports money was paid to his institution from the NIH, CDC, ADPH, CNINE, GenMark, mFluiDx, IMMY, SpeeDx, Abnova, Amplyx, IHMA, and JMI. Nitin Arora reports money was paid to his institution from the Congenital and Perinatal Infections Consortium, the UAB Sparkman Center for Global Health, and the Kaul Pediatric Research Institute Grant Program.
The other authors did not report any potential conflicts of interest.
PEER REVIEW HISTORY
Received August 31, 2021. Received in revised form September 16, 2021. Accepted September 23, 2021. Peer reviews are available at http://links.lww.com/AOG/C479.
REFERENCES
- 1.Metz TD, Clifton RG, Hughes BL, Sandoval G, Saade GR, Grobman WA, et al. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19). Obstet Gynecol 2021;137:571–80. doi: 10.1097/AOG.0000000000004339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Figure 3: percent of pregnant people aged 18–49 years receiving at least one dose of a COVID-19 vaccine during pregnancy overall, by race/ethnicity, and date reported to CDC – Vaccine Safety Datalink*, United States, December 14, 2020 – September 11, 2021 Accessed August 18, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccinations-pregnant-women
- 3.Centers for Disease Control and Prevention. COVID-19 vaccinations in the United States Accessed August 18, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total
- 4.American College of Obstetricians and Gynecologists. ACOG and SMFM recommend COVID-19 vaccination for pregnant individuals Accessed October 18, 2021. https://www.acog.org/news/news-releases/2021/07/acog-smfm-recommend-covid-19-vaccination-for-pregnant-individuals
- 5.Centers for Disease Control and Prevention. COVID-19 vaccines while pregnant or breastfeeding Accessed October 18, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html


