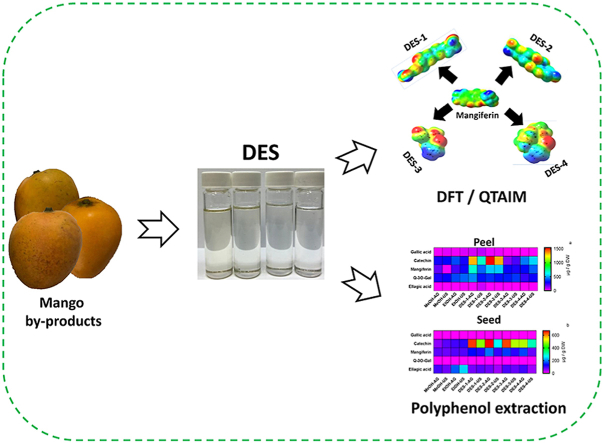
Abstract
Deep eutectic solvents (DESs) potential for the extraction of polyphenolic compounds (PC) from mango by-products (peel and seed) was evaluated. Ultrasound (US) and agitation were applied to evaluate the effects of solvent and extraction methodology. The extracts were characterized with antioxidant capacity and HPLC-DAD profile. A theoretical study was performed using density functional theory and the QTAIM approach. β-alanine and choline chloride based DESs were effective to extract PC from peel and seed. Some DES increased PC extraction up to three times for peel (23.05 ± 1.22 mg/g DW) and up to five time for seeds (60.01 ± 1.40 mg/g DW). The PC profile varied with the solvent (DES vs EtOH/MeOH), procedure (US vs agitation) and material (peel or seed). Mangiferin extraction from peels was significantly increased with β-alanine based DES (676.08 ± 20.34 μg/gDW). The strength of H-bonds had a determining effect on the viscosity of DESs. The solute-solvent solvation energy was suitable to estimate the strength of H-bond interactions between DES and target compounds. This study demonstrates the remarkable capacity of DESs to extract PC from mango by-products and provides insights into the factors controlling extraction properties.
Keywords: Bioactive, Mango, Mangiferin, Eutectic solvent, DFT
Graphical abstract
1. Introduction
Mango (Mangifera indica L.) is grown in many parts of the world, particularly in tropical countries. Over 1000 mango varieties are available worldwide, of these, only a few are grown on commercial scales and traded [1]. Seed and peel represent 35–60% of the whole fruit in some cultivars; these by-products are usually discarded in mango industry representing a significant source of pollution [2,3].
Mango peels have aroused considerable attention since their high content of polyphenols, carotenoids, enzymes, vitamins E and C, among other bioactive compounds [4]. Mango seed has also attracted considerable interest due to its fatty acids, polyphenols and dietary fiber contents [5,6]. These mango by-products’ properties make the biomass of mango processing an important material for the extraction and recovery of bioactive compounds with potential applications; in addition to contributing to a circular economy based on zero waste [7]. Among the polyphenols present in mango by-products, mangiferin stands out due to its high antioxidant potential, which would be responsible for its beneficial properties for human health [8]. Flavonoids are also present in mango by-products and they are of particular interest due to their bioactive properties such as anti-inflammatory, anti-proliferative and anti-microbial among others [7,9].
Extraction of bioactive compounds is traditionally achieved by solid−liquid extractions; these procedures employ solvents, mostly organic, such as acetone and ethyl acetate, or alkyl alcohols like methanol (MeOH), ethanol (EtOH), propanol or their mixtures [5,10]. In order to reduce solvent utilization and contaminant emission, novel technologies have been applied, of these, ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE) and supercritical fluid extraction (SFE) have been used for the extraction of bioactive compounds from mango by-products [7,9,10].
A more in-depth approach to clean technologies is green chemistry; it aims to preserve the environment by using the minimum quantity of non-hazardous solvents and eco-friendly solubilization techniques. In this context, it has been paid great attention to the replacement of many of the current organic solvents by deep eutectic solvents (DESs), generally reported as green solvents [11,12]. DESs were first described by Abbott, Capper, Davies, Rasheed, & Tambyrajah, 2003 as solvents which were obtained by combining a hydrogen bond acceptor (HBA) such as quaternary ammoniums, and a hydrogen bond donor (HBD) as, for example, urea, carboxylic acids or amines [13,14]. These compounds are capable of self-association, often through hydrogen bond interactions, and the resulting eutectic mixture has a melting point that is lower than those of the individual components [15]. DESs have unique physicochemical properties, such as chemical and thermal stability, no flammability, low toxicity, high electrical conductivity, and a good solubilizing capacity for several organic compounds [[12], [13], [16]]. The high viscosity of some DESs hinders their efficiency as extraction solvents due to the low mass transport efficiency. In order to overcome this situation, water could be added (to a certain point) to reduce viscosity [13,14]. The combination with other extraction strategies (stirring, ultrasound, microwave, etc.) could also be applied to improve the extraction of bioactive compounds [1,11,17]. Recently, DESs alone or combined with different technologies were successfully used for the extraction of various groups of bioactive compounds from different plants and agro-industrial waste material, including mango peels [14,[18], [19], [20]]. DES combined with heat and UAE applied to mango peels increased the extraction of pectins and other polyphenols [21,22].
Several properties of DESs have been related to the molecular interactions among DES components, in particular the H-bond interactions [13,14]. In this regard, computational chemistry has become a widely used tool to study molecular interactions [23]. Density functional theory (DFT) provides a good description of the geometry and electronic structure of chemical systems, and Quantum Theory of Atoms in Molecules (QTAIM) allows the characterization of bonding interactions [24]. These approaches have been applied to the study of H-bond interactions in DESs, allowing to explain and predict some of their properties [[25], [26], [27], [28]]. Nowadays, as DESs are in the limelight due to their potential applications and sustainability, molecular-level understanding of their structure and intermolecular interaction acquires great importance. This information could be used to develop tailor-made DESs to extract molecules of interest based on the starting material and the extraction technique.
This study aimed to evaluate the ability of DESs combined with agitation or UAE for the extraction of phenolic compounds from mango ‘criollo’ by-products. The results were evaluated in terms of total phenolic compounds, flavonoid content, antioxidant capacity and HPLC profile of five major phenolic compounds. In order to gain insight into the interactions among the components of the DES and between mangiferin, as a model of target molecule, a theoretical study was performed using DFT and QTAIM approaches. This works reports for the first time a study involving the effect of extraction techniques and DESs on enhancing polyphenols extraction from mango ‘criollo’ peel and seed. Also, to the best of our knowledge, a theoretical approach that explains the results based on the interactions between mangiferin and DES is reported for the first time.
2. Experimental section
2.1. Reagents and standards
Choline chloride (ChCl, ≥98%), dl-malic acid (DL-MA, ≥98%), β-alanine (β-Ala, 99%), citric acid (CA, 99%), ethylene glycol (EGly, anhydrous, 99.8%), gallic acid (≥98%), catechin (≥98%), ellagic acid (≥98%), quercetin-3-O-galactoside (Q-3O-Gal, ≥98%) and mangiferin (≥98%) were supplied by from Sigma-Aldrich, St Lois, MO, USA. Glycerol (Gly, >95%) from Glentham Life Sciences (Corsham, UK), formic acid (98%) from Panreac Química (Barcelona, Spain). Methanol and ethanol HPLC grade were provided by Lab-Scan (Dublin, Ireland).
2.2. Mango by-products
Three kilograms (20 fruit) of fully ripe mango ‘criollo’ were harvested from trees in the city of Corrientes Argentina (-27.510501795690335, -58.82515496578836). The fruit were washed with water, drained over paper and the peel and seed were manually removed. Two products were obtained: mango ‘criollo’ peel (MCP) and mango ‘criollo’ seed (MCS). These products were freeze-dried during 72 h at −58 °C, 0.035 mBar using a lyophilizer Christ Alpha 1–4 LD (Osterode, Germany) and powdered with an industrial grinder Arcano FW 100 (Beijing, China) until obtaining a fine powder (d < 0.025 mm) that was stored in well-sealed black PVC containers without headspace, at −20 °C until experiment (<30 days).
2.3. DESs preparation
The DESs investigated in this study (Table 1) were prepared by mixing the desired molar proportions of HBA and HBD under constant agitation at 70 ± 2 °C [16,19]. Eutectic mixtures were prepared gravimetrically (±10−4 g) in closed glass vials and stirred until the formation of a homogeneous liquid. The stability of the mixtures was evaluated by the absence of macroscopic crystallization (Fig. S1a) after a period of 7 days at room temperature (25 ± 2 °C).
Table 1.
Details and characteristics of the DESs systems evaluated in this work.
| Starting components |
Characteristics |
||||||
|---|---|---|---|---|---|---|---|
| Code | HBA | HBD | Other | Molar ratio | Synthesis time (min) | Aspect | Viscosity (cP) |
| DES-1 | β-Ala | DL-MA | H2O | 1:1:3 | 85 | Clear light brown | 750 ± 30 |
| DES-2 | β-Ala | CA | H2O | 1:1:3 | 120 | Clear light brown | 2350 ± 25 |
| DES-3 | ChCl | EGly | – | 1:2 | 20 | Clear colorless | 150 ± 15 |
| DES-4 | ChCl | Gly | – | 1:2 | 25 | Clear colorless | 450 ± 20 |
2.4. DESs viscosity
The viscosity test was performed in a thermostatic block (25 ± 1 °C) using a Brookfield LVDVII+ viscometer (Middleboro, MA, USA) with LV spind probe n°4 and rotational speed of 6 and 12 rpm [16]. The determination was performed in triplicate for each rotational speed and the results were expressed in centipoises (cP) as an average of the six determinations.
2.5. Extraction of phenolic compounds
2.5.1. Conventional extraction with agitation
Extracts from MCP and MCS were prepared using EtOH and MeOH as solvents. Based on preliminary experiments; a solid: solvent ratio of 1:60 (g:mL) was adequate to obtain maximum values of total phenolic compounds (Fig. S1b). Extracts were prepared with 80% v/v EtOH or MeOH under magnetic stirring (800 rpm) for 30 min at 25 °C. The extracts were centrifuged at 20,000 g for 30 min and the supernatant concentrated at 40 °C by evaporation in a rotator evaporator. The extracts were named EtOH-AG and MeOH-AG and stored at −20 °C until analysis, extraction was performed in triplicate (n = 3).
2.5.2. Extraction with DESs and agitation
Maintaining the 1:60 ratio (g:mL), MCP and MCS powders were mixed with each DES and magnetically stirred (800 rpm) for 30 min at 25 °C. Samples were centrifuged at 20,000 g during 30 min; the supernatant was referred as DES-AG and stored at −20 °C until analysis. Extraction was performed in triplicate (n = 3).
2.5.3. Ultrasound-assisted extraction (UAE)
Ultrasound assisted extraction using EtOH, MeOH or DESs was performed for 5 min with a solid tip of 19 mm (60 μm max amplitude range) connected to an ultrasound generator equipment set at 60% of amplitude (Fisherbrand Model 505 Sonic Dismembrator - 500 W - 20 kHz - Fisher Scientific™). These experimental conditions were selected based on previous works [10,18]. The extracts prepared with EtOH or MeOH were concentrated in a rotary evaporator and referred as EtOH-US or MeOH-US. The extracts prepared with DESs were centrifuged at 20,000 g for 30 min and the supernatants were referred as DES-US. Extraction was performed in triplicate (n = 3). All extracts were stored at −20 °C until analysis.
2.6. Quantitative analysis
Before quantitative analysis, all the extracts were dissolved in a 1:4 ratio with distilled water and loaded into 1 g C18 SPE cartridges (Purple Series, Análisis Vínicos, Tomelloso, Spain). The cartridges were previously conditioned following manufacturer instructions, once samples were loaded; 4 mL of distilled water were flushed. Final elution was performed with 3 mL of methanol acidified with formic acid 0.1% v/v. This extract was used for the quantification of total phenolic and flavonoid content, antioxidant capacity and HPLC-DAD analysis.
2.6.1. Total phenolic content
Total phenolic compounds were determined using Folin Ciocalteu reagent and Na2CO3 and absorbance was determined at 760 nm in a microplate reader (BioTek Instruments Inc., Bad Friedrichshall, Germany) [10]. Three replicate analyses were performed, and results were expressed on a dry weight basis as mg gallic acid equivalents (GAE)/g dry weight (DW).
2.6.2. Total flavonoid content
Total flavonoid content was evaluated with the Na2NO2/AlCl3/NaOH technique determining the absorbance at 510 nm [9]. The results were expressed as mg of catechin equivalents (CE)/g dry weight (DW). Analysis was performed in triplicate (n = 3).
2.6.3. Antioxidant capacity
The antioxidant capacity was analyzed by evaluating the free radical-scavenging effects of the extracts on 2,2-diphenyl- 1-picrylhydrazyl (DPPH•) and 2,2′-azinobis(3-ethylbenzothiazoline)- 6-sulphonate (ABTS•+) radicals. Diluted extracts were mixed with freshly prepared DPPH• and ABTS•+ solutions and allowed to react for 30 min. Absorbance was determined at 517 nm and 734 nm for DPPH• and ABTS•+ assays, respectively [9,10]. Trolox equivalent antioxidant capacity (TEAC) values for both DPPH• and ABTS•+ radicals were obtained from standard curves built at different concentrations of Trolox. Results were expressed as μmol of TEAC/g dry weight (DW). Analysis was performed in triplicate (n = 3).
2.6.4. HPLC-DAD analysis
The separation and quantification of major phenolic compounds present in MCP and MCS were achieved using an Agilent 1200 series HPLC system (Agilent Technologies, Santa Clara, CA, USA) equipped with a quaternary pump, integrated degasser and a diode array detector (DAD). Separation was carried out on a Poroshell C18 column of 4.6 × 250 mm id., 4.0 μm particle size (Agilent Technologies, Santa Clara, CA, USA). The mobile phase consisted of a linear gradient of 0.1% formic acid in Milli-Q-water (A) and 0.1% formic acid in acetonitrile (B) as follows: 0 min, 95% A; 5 min, 95% A; 10 min, 85% A; 20 min, 75% A; 30 min, 70% A; 60 min, 60% A; 65 min, 95% A; 70 min, 95%A. The flow rate was fixed at 0.7 mL/min and the injection volume of 20 μL. Runs were monitored at 360 nm, 320 nm, 280 nm and 255 nm [5]. Commercial standards of gallic acid, catechin, mangiferin, quercetin-3-O-galactoside and ellagic acid were used to build calibration curves in the range of 0.05–50 μg/mL.
2.7. Computational methods
Several starting geometries for DES complexes were built considering the Electrostatic Potential surfaces (ESP) of each solvent molecule (Supporting Information). Starting geometries of DES-3 and DES-4 were generated from previously reported geometries [29]. Then, these structures were optimized using DFT calculation, at B3LYP/6-31G(d,p) level of theory [30,31]. DFT has been shown to be useful for describing intermolecular interactions and properties of the DES [25,26]. Frequency calculations were performed to verify the stationary points on the potential energy surface. The interaction energies (ΔE) were calculated as the difference between the total energy of the DES complexes and the sum of total energies of the isolated monomers. All calculations were carried out using the Gaussian 09 suite of programs [32]. To obtain more information about the nature of the hydrogen bonds in the systems under study, a topological analysis of the electron density distribution based on the quantum theory of atom in molecules (QTAIM) [24] was performed using the AIMAII program [33].
2.8. Statistical analysis
The results were statistically analyzed using analysis of variance (ANOVA) (α = 0.05). The differences among means were tested for statistical significance using a multiple-range least significant difference (LSD) and orthogonal contrasts tests with Info-Stat Statistical Software 2015 (Córdoba, Argentina).
3. Results and discussion
3.1. DESs characterization
The DESs were prepared by mixture and stirring of HBA and HDB in an appropriate molar ratio to form deep eutectic mixtures already mentioned in the literature [16,19]. The synthesis time varied from 20 to 120 min, depending on the starting components. DES-3 and DES-4 showed shorter synthesis time, while DES-1 and DES-2 had the longest formation time (Table 1). The visual appearance of the four DESs is presented in Fig. S1a. All DESs showed as clear liquids at room temperature, the stability was evaluated in terms of absence of macroscopic crystallization after seven days at room temperature, and all the DESs maintained as clear liquids.
The viscosity indicated that DES-3 was the least viscous while DES-2 had the highest viscosity value (Table 1). This property is highly dependent on the composition of the DES and is a result of the interactions between molecules and the intensity of these interactions. In general, as explained further on, the viscosity could be related to the hydrogen bonds between HBA and HBD [15].
3.2. Total phenolic content (TPC)
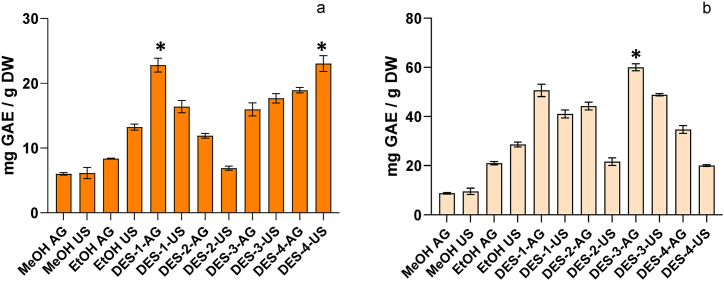
The extraction of phenolic compounds using conventional solvents (EtOH or MeOH) was influenced by the type of solvent and extraction procedure. Methanolic extraction was not improved with ultrasound (US), in average; MCP had a content of 6.00 ± 0.19 mg GAE/g DW, while MCS had an average content of 9.74 ± 0.32 mg GAE/g DW among both procedures (MeOH-AG and MeOH-US).
Ethanolic extraction achieved a higher extraction yield of TPC when US was applied; being 62% higher in MCP extracted with US and 40% higher for MCS when compared to EtOH-AG (Fig. 1a and b). Ethanol + US significantly improved the extraction of phenolic compounds; these results are in coincidence with previous works which indicated that aqueous ethanol was more effective than methanol to extract phenolic compounds from mango [7,10].
Fig. 1.
Total phenolic content of mango ‘criollo’ peel (a) and seed (b) extracts obtained with agitation or ultrasound using traditional solvents and DESs. *indicates significant differences (p < 0.05) among DES evaluated by orthogonal comparison test.
Extraction from MCP was significantly increased using DESs, the extracts DES-1-AG and DES-4-US presented the highest values for TPC and the extraction yield was improved by a factor of 3, being 40% higher than conventional extraction (Fig. 1a). The efficiency of the extraction could be related to the physicochemical properties of the DESs as well as the extraction procedure [11,12]. In some cases the combination of DES + US lead to higher values than those obtained by DES + agitation (DES-3-US > DES-3-AG and DES-4-US > DES-4-AG). Contrarily, the application of US with DES-1 and DES-2 had lower extraction yield compared to DES-1-AG and DES-2-AG. Independently of the extraction method, orthogonal contrast indicated that DES-1 and DES-4 were the eutectic solvents that significantly (p < 0.05) improved extraction of TPC (Fig. 1a). Given that US did not improve extraction, these results could be a direct consequence of the affinity of the DESs for phenolic compounds [34]. Moreover, the viscosity of the DESs, particularly DES-2, could also have a significant impact on the extraction efficiency and might explain the differences between agitation and UAE procedures [14,18]. Water addition in DES-1 and DES-2 could increase polarity hence improving polar phenolic compounds extraction [35,36]. Polarity of phenolic compounds also determine its extraction potential by DES, namely penta-O-galloyl glucose, which is more polar than gallic acid, is extracted in higher rate by polar DES [12,15].
The extraction of TPC from MCS was also increased when DESs were used, combined with agitation, DES-3 and DES-1 had the highest values for TPC, being 60.01 ± 1.40 mg GAE/g DW and 50.61 ± 2.51 mg GAE/g DW respectively. These values were 5 to 3 times higher than those obtained with EtOH-AG and MeOH-AG, which means 150%–200% higher extraction efficiency of DES, DES-2-AG and DES-4-AG also increased significantly (p < 0.05) the extraction of TPC, however, orthogonal contrast indicated that extraction with DES-3 was significantly higher than the other solvents (Fig. 1b). The application of DESs + US, although had significantly higher values than extraction with conventional solvents, was in general, less efficient than DES + agitation (Fig. 1b). These results could indicate that the combination of DESs + US was not effective to increase the extraction of TPC from MCS, which could be a consequence of other factors like matrix effect, viscosity, phenolic content and chemical diversity present in each section, etc. [7,20].
The differential results obtained for each section of the fruit highlights the importance of considering a wise selection of solvent and extraction procedure to obtain maximal extraction of phenolic compounds. Indeed, the use of DESs enhanced the extraction of phenolic compounds and represents an innovative technique for the recuperation of bioactive compounds from mango by-products.
3.3. Total flavonoid content (TFC)
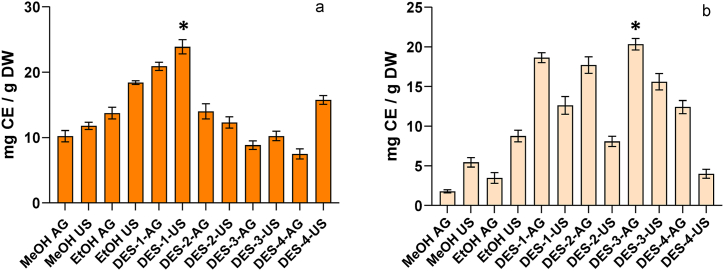
Flavonoids are a large group of polyphenolic compounds having a benzo-γ-pyrone structure. The chemical properties of flavonoids depend on their structural class, degree of hydroxylation, other substitutions and conjugations [1,4,9].
The use of conventional solvents to extract flavonoids indicated that EtOH was more effective than MeOH to extract this group of compounds from MCP; this result is in agreement with previous works [9,10]. The combination of EtOH + US increased in 38% the extraction of flavonoids when compared to other conventional procedures (Fig. 2a). Conventional extraction of flavonoids from MCS was also solvent and procedure-dependent, being EtOH-US the technique that achieved the highest value for TFC (8.76 ± 0.72 mg CE/g DW) using conventional solvents.
Fig. 2.
Total flavonoid content of mango ‘criollo’ peel (a) and seed (b) extracts obtained with agitation or ultrasound using traditional solvents and DESs. *indicates significant differences (p < 0.05) among DES evaluated by orthogonal comparison test.
Contrarily to the results obtained for TPC, the only DES that significantly increased flavonoid extraction from MCP was DES-1 and the combination of this solvent with ultrasound (DES-1-US) had higher extraction values than DES-1-AG (Fig. 2a). None of the other combinations of DES and procedures (agitation or ultrasound) achieved higher values than EtOH-AG and EtOH-US. These results could indicate that the chemical properties of DES-1 could have a significant impact in flavonoid extraction, indicating some degree of specificity. Other authors have indicated that DESs formed by β-alanine and dl-malic acid were promising systems for flavonoid extraction [19,37].
On the other hand, the extraction of flavonoids from MCS was significantly increased with the different DESs and extraction procedures evaluated. The highest values for TFC using agitation were obtained as follow DES-3-AG > DES-1-AG > DES-2-AG > DES-4-AG (Fig. 2b). The extraction increased by a factor of 4 for DES-3-AG and by a factor of 2 for DES-2-AG when compared to the conventional extraction that achieved the highest extraction (EtOH-US). Orthogonal contrast indicated DES3 as the most effective to achieve high TFC extraction (Fig. 2b). These results indicate that DESs did not only increase flavonoid extraction but also that some DES were more effective or selective. These findings are in agreement with the results observed for TPC in MCS and might help to elucidate the underlying mechanism for the specificity of DESs [12]. The application of US, although increased flavonoid extraction with DES-1 and DES-4 when compared to conventional extraction, was in general, significantly lower than the combination of DES and agitation (Fig. 2b). The relatively low extractive efficiency for TFC of DES with US might be a consequence of the high viscosity, particularly for DES-2, which might reduce ultracavitation phenomena [17,18].
The extraction of flavonoids was significantly affected by the type of DES and the extraction procedure. The results varied among the material, flavonoids extraction from MCP was mainly influenced by the chemical composition of DES. Meanwhile, flavonoid extraction from MCS was influenced not only by the chemical composition of the solvent but also by the extraction procedure.
Different mechanisms could affect flavonoid extraction; however, the chemical characteristics of the compounds might rule the process. The presence or absence of substitutions, particularly methoxyl and/or carbohydrates, has a significant impact in solubility and molecular properties [13,14,37]. Therefore, these results indicate that the differential extraction capability of DESs could be a consequence of several factors (fruit section, type of flavonoid, molecular interactions, etc.) which should be considered in order to obtain high extraction efficiency.
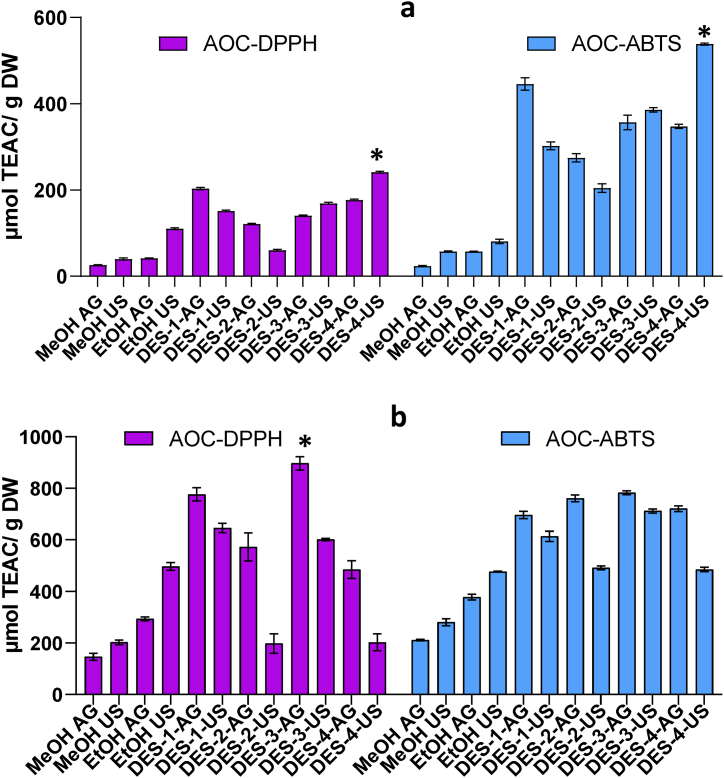
3.4. Antioxidant capacity (AOC)
The antioxidant capacity of mango fruit extracts is generally attributed to phenolic compounds, carotenoids and some organic acids [3,5].
For conventional extraction, the AOC of MCP and MCS yield the highest value with EtOH-US, as observed for TFC and TPC, extraction with MeOH had values significantly lower than EtOH (Fig. 3a and b).
Fig. 3.
Antioxidant capacity evaluated by DPPH• and ABTS•+ assays in mango ‘criollo’ peel (a) and seed (b) extracts obtained with agitation or ultrasound using traditional solvents and DESs. *indicates significant differences (p < 0.05) among DES evaluated by orthogonal comparison test.
The AOC of MCS was higher than MCP independently of the procedure; these results could have two interpretations, firstly the different content of antioxidants among MCP and MCS. Secondly, the differences could be attributed to the differential reactivity between DPPH• and ABTS•+ and antioxidant species [4,7,9].
The extraction of antioxidant compounds from MCP was significantly increased with DESs-mediated extraction. As observed in Fig. 3a, DES-1 and DES-4 achieved the highest AOC values with both techniques (DPPH• and ABTS•+). The highest values for AOC were obtained for DES-4-US (241.11 ± 2.01 μmol TEAC/g DW) followed by DES-1-AG (203.28 ± 2.56 μmol TEAC/g DW). Orthogonal contrast analysis indicated extracts obtained with DES-4 had the highest AOC-DPPH and AOC-ABTS (Fig. 3a). In view of these results, it can be seen that both, the chemical composition of the DES as well as the extraction procedure (US or agitation) influenced the AOC of the extracts. The other evaluated DESs achieved high rates of extraction for antioxidant compounds, being three to four times higher than the results obtained with conventional extraction. The AOC evaluated with DPPH• for DES-2-US was significantly (p < 0.05) lower than all the other DES-mediated extraction procedures; however, it was in the same order as EtOH-US (Fig. 3a), this particular result was consistent with the result obtained for TPC in the same extract.
The extraction of antioxidants species from MCS was also improved with some of the DESs evaluated. Extraction with DES-3-AG increased up to four times the AOC evaluated with DPPH• when compared to the highest value obtained with conventional solvent. DES-3 was also indicated as the most suitable to obtain high AOC-DPPH when orthogonal comparison was evaluated (Fig. 3b). In general, the combination of DES and agitation had higher values for AOC-DPPH than DESs + US, particularly DES-2-US and DES-4-US had low extraction yields. These results could be related to the viscosity of the DES, especially for DES-2 which could reduce the efficiency of ultrasound to extract antioxidant species [11,18]. On the other hand, the AOC of MCS extracts evaluated with ABTS•+ was significantly increased with all the eutectic solvents combined with agitation as extraction procedure. Orthogonal comparison indicated no statistically significant differences (p > 0.05), however, the AOC-ABTS obtained with DES-3-AG and DES-2-AG were 2.5 times higher than with conventional solvents (Fig. 3b). The combination of DESs + US significantly reduced the extraction of antioxidant compounds; however, DES-3-US and DES-1-US achieved significantly (p < 0.05) higher values for AOC-ABTS than conventional extraction with EtOH.
In view of these results, three major findings were analyzed: a) peel and seed extracts differ in their AOC, being the antioxidant potential of seed extracts higher than peel extracts. Also b) depending on the DES and fruit section, the AOC could be increased by agitation or US. Finally, c) the differential results for DPPH• and ABTS•+ could indicate that DESs have differential extractive properties [12,14]. Extraction of antioxidants is an important step in recovering bioactive compounds from fruit material. The stability of antioxidants is also determinant when DES are combined with other technology i.e., DESs combined with MAE are not satisfactory for the extraction of unstable substances because they may cause local overheating.
In this work, the effectiveness of extraction of antioxidant compounds by a given solvent was dependent on the chemical composition of the solvent as well as the extraction methodology.
3.5. Phenolic profile (HPLC-DAD)
Mango by-products have been extensively studied as an interesting source of polyphenolic compounds with antioxidant potential [1,3,38]. Phenolic compounds represented by gallic acid, gallates, gallotanins, ellagic acid and catechin were reported in peel, pulp and seed of Keitt, Ataulfo, Kent and other varieties [5,7,9]. Flavonoids represented by quercetin derivatives and xantones like mangiferin and its derivatives were also reported in mango by-products and are of particular interest because of their bioactive properties [3,4].
3.5.1. Gallic acid
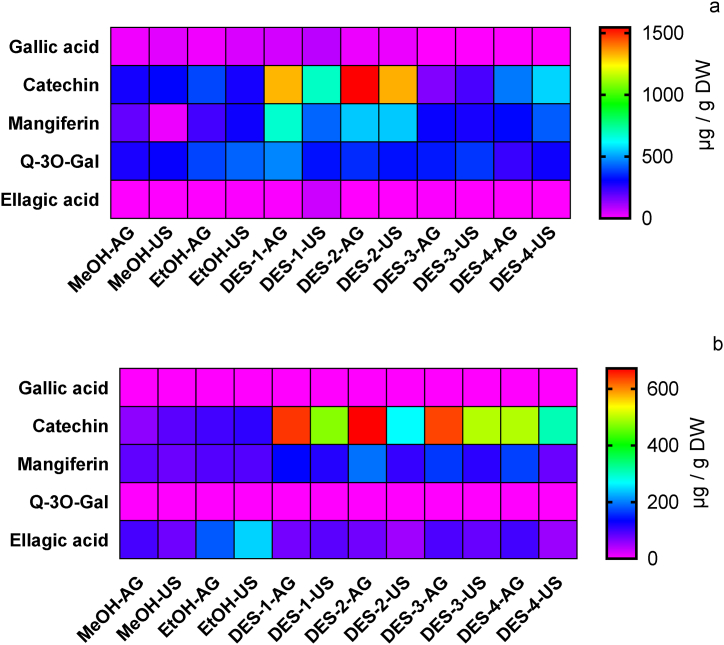
The extraction of gallic acid from MCP was significantly increased using DES-1-US (80.80 ± 3.42 μg/g DW), followed by DES-1 with agitation (53.11 ± 1.96 μg/g DW). Orthogonal comparison indicated DES-1 as the best solvent to recover gallic acid (p < 0.05) (Table S1). The use of conventional solvents with agitation or ultrasound had values that were significantly lower than those obtained by DES-1-US (Fig. 4a and Table S1). Given that DES-2 had a similar extraction potential for gallic acid to conventional solvents, and no gallic acid was detected in the extraction with DES-3 nor DES-4; it can be seen some selectivity in the extraction achieved by DES-1. These results indicate that the extraction of gallic acid from peel could be enhanced by ultrasound but also by the particular chemical properties of the eutectic solvent (Table S1) [11,12]. Although some authors have informed gallic acid present in mango seeds [2,5], under the evaluated conditions in this work, no free gallic acid was detected for any of the evaluated procedures in MCS. These results could be associated with cultivar characteristics or indicate that gallic acid might be combined with other compounds or strongly attached to matrix components.
Fig. 4.
Heat map representing the absolute content of major phenolic compounds in mango ‘criollo’ peel (a) and seed (b) extracts obtained with agitation or ultrasound using traditional solvents and DESs.
3.5.2. Catechin
Catechin was detected in MCP and MCS extracts; the use of DESs significantly increased the extraction of this compound (Fig. 4a and b). Catechin extraction from MCP using DES-2 and DES-1 increased up to six times when compared to EtOH or MeOH extraction (Fig. 4b). Both DESs were more effective combined with agitation than with US, orthogonal comparison indicated DES2 as the most effective (Table S1). These results could indicate that the extraction of catechin using DES would not be improved by the cavitation phenomena of ultrasound waves. This is an interesting result since it could give insights into the mechanisms involved in the extraction, moreover, the molecular interactions between catechin and DES-2 or DES-1 could be weakened by ultrasound waves [39]. Also considering the viscosity of these DESs higher amplitude or longer times could be required [11,18]. Extraction with DES-4 achieved similar values to extraction with conventional solvents (Table S1), while DES-3 did not improve the extraction of catechin (Fig. 4a). Organic-acid based eutectic solvents (DES-1 and DES-2) would be more suitable than polyol-based eutectic solvents (DES-3 and DES-4) for catechin recovery [15].
The results of catechin extraction from MCS were in part similar to those found for MCP. Extraction with DES-2-AG and DES-1-AG also achieved the highest extraction rates for catechin (Fig. 4b and Table S1). The orthogonal comparison indicated that extraction with DES-1 was significantly higher than DES-2. Surprisingly, extraction with DES-3 was improved up to six times (635.86 ± 17.03 μg/g DW), a result that could indicate a strong matrix effect in the extraction process since this solvent did not efficiently extract this molecule from peel. The use of DES-4 also increased catechin extraction in comparison to conventional method. Therefore, catechin extraction was in general improved by DESs, being agitation technique more efficient than US.
3.5.3. Mangiferin
Mangiferin is a unique polyphenol present in mango with notable antioxidant properties [3,4]. The extraction of this compound from MCP was significantly increased with DES-1, DES-2 and DES-4, while DES-3 extracted amounts similar to those obtained with EtOH (Fig. 4a). For DES-1, agitation was more effective than ultrasound, which could indicate that the structure of DES-1 is less effective in extracting mangiferin under US conditions. This could be a consequence of network destabilization due to ultrasound waves or lower efficiency due to viscosity [11,15,18]. Orthogonal comparison pointed DES-1 as the most effective solvent for mangiferin recovery (Table S1). Extraction with DES-2 was independent of the extraction methodology; this could indicate that for this solvent, the chemical composition might be relevant for mangiferin extraction rather than the extraction technique. To the best of our knowledge this is the first report concerning the use of β-alanine: citric acid: H2O DES for the recovery of mangiferin, however, this solvent has been reported for the extraction of flavonoids glycosides and aglycones [19,37].
The extraction of mangiferin from MCS was also significantly increased with all the DESs, being agitation technique more efficient than UAE. In decreasing order, the extraction of mangiferin was DES-2-AG > DES-4-AG > DES-3-AG > DES-1-AG (Table S1). The combination of DES + US increased 15–30% mangiferin extraction compared with conventional solvents; these values were lower than those obtained by DES-AG indicating that ultrasound methodology did not have a significant impact when combined with DES. These findings could be attributed to low mass transfer phenomena due to the viscosity of the DES or matrix particularities (fibre, fat and protein content) [14,18]. The chemical composition of the DESs seems to have a significant impact in mangiferin extraction, glycerol: sodium acetate DES was previously reported as an efficient solvent for mangiferin extraction [20], results that are in coincidence with the results obtained with DES-4 in this work.
3.5.4. Quercetin derivatives
Flavonoids in mango are mainly represented by quercetin derivatives, particularly; significant quantities of quercetin-3-O-galactoside (Q-3-O-Gal) were reported in mango by-products [5,7,9]. The extraction of Q-3-O-Gal from MCP using DES-1-AG was the only procedure that achieved values in the order of those obtained with EtOH (Fig. 4a). Therefore, the physicochemical properties of β-alanin: dl-malic acid: water (DES-1) might be in part responsible for the extraction rather than the methodology (agitation or ultrasound) [19,37]. Moreover, the extraction achieved by the other DESs evaluated was as follows DES-3 > DES-2 > DES-4; however, neither of them was able to extract Q-3-O-Gal at higher rates than EtOH-US or EtOH-AG. Although Q-3-O-Gal was reported for seed extracts of Keitt, Sensation and Gomera varieties [5], this compound was not detected in mango ‘criollo’ seed extracts.
3.5.5. Ellagic acid
The extraction of ellagic acid from MCP was significantly increased with DES-1-US (61.13 ± 0.23 μg/g DW); the yield was increased by a factor of 10 in comparison to EtOH-US (Table S1). The extraction of this compound with DES-1-AG (5.40 ± 0.14 μg/g DW) was in the order of EtOH-US (4.21 ± 0.05 μg/g DW). In view of the results, there might be a synergistic effect between the application of US and DES-1, since the combination of these factors produced a significant increase in ellagic acid extraction when compared to DES-1-AG and EtOH-US. It is remarkable the high extraction achieved by DES-1-US in mango peel, this finding becomes particularly relevant since after an exhaustive bibliographical search there were no previous reports concerning ellagic acid extraction with β-alanine: dl-malic acid: water DES. Further studies are needed to elucidate the possible synergistic effect aforementioned.
The extraction of ellagic acid from MCS had values that were significantly higher than those obtained in peel. However, neither of the DESs evaluated increased the extraction of ellagic acid compared to EtOH-US (Fig. 4b). These results exert the influence that could have fruit matrix on the extraction with DESs [12]. Since no significant increases were found in the extraction with DES-AG or DES-US, compared to conventional extraction, we could infer that there might be several intermolecular interactions or matrix-related effects that hinder the interaction between ellagic acid and the eutectic solvent. Nevertheless, all DESs achieved extraction yields similar to those obtained with MeOH; therefore, DES-mediated extraction still represents a more sustainable alternative.
The use of DESs in the extractions of polyphenols has yielded promising results, several works have reported success in isolation and extraction of target compounds, as well as competitive, if not superior, extraction rates compared with conventional solvents [11,37].
3.6. Computational chemistry calculations
3.6.1. Interactions among components of the DESs
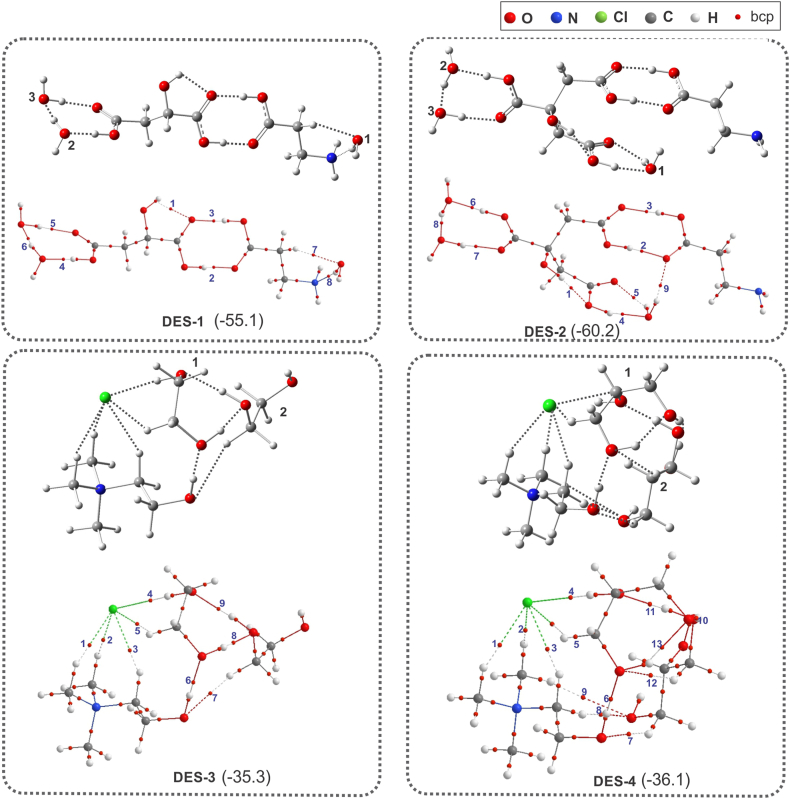
It is known that the properties of DESs and DESs are closely related to the interactions between HBA and HBD. Therefore, in order to obtain insight into the effect of H-bond interactions upon an important property of the DESs under study, namely their viscosity, a DFT and QTAIM analysis was performed (Table 1 and Fig. S2). The most stable optimized geometries of DESs along with their corresponding molecular graphs and interaction energies (ΔE) are shown in Fig. 5.
Fig. 5.
Optimized geometries (top) depicting H-bond interactions (dotted lines) and the molecular graphs (bottom) calculated for the most stable structures of the DESs under study. Interaction energy is given in parentheses (in kcal/mol). ΔE measures the total interaction energy in a molecular complex; and it is considered an estimation of the strength H-bonds in it.
The calculated absolute value of ΔE increases in the order DES-3 < DES-4 < DES-1 < DES-2, which is consistent with the increase in viscosity of DESs. As suggested for some choline chloride-based DESs, this result indicates that the H-bond strength exerts a dominant effect on the viscosity of DESs [15,40].
To further investigate the nature of H-bonds among components of the DESs, QTAIM analysis was carried out. Based on the QTAIM approach [41], the presence of a bond critical point (bcp) between an HBD group and an HBA group along with the bond path connecting two interacting atoms is considered to be a characteristic of H-bond interaction [13]. Several topological parameters evaluated at the bcp can be used to obtain information about the nature and features of the chemical bonds (properties at the bcp are labelled with the subscript “b”). The electron density (ρb) at the bcp reflects the strength of a bond and its Laplacian (∇2ρb) measures the local charge concentration (∇2ρb < 0) or local charge depletion (∇2ρb > 0). For H-bonds, it is crucial that ∇2ρb has a positive value. These two properties along with total energy density (Hb) are used to analyze the covalent character of an interaction [42]. As shown in Fig. 5 (topological parameters are shown in Table S2), there is a H-bonds network connecting the components of DESs.
In DES-1 and DES-2 a carboxylic group of malic acid and citric acid, respectively, interacts with the carboxylic group of the β-alanine forming a cyclic structure involving the H-bonds bcp 2 and 3. The other carboxylic acid group interacts with two water molecules forming also a cyclic structure. The strongest H-bond is the O–H⋯O H-bond in which malic acid (bcp 4) and citric acid (bcp 6), are acting as HBD and a water molecule as HBA. This result highlights the fundamental role of water molecules in the formation of this type of DES.
In DES-1 the H-bonds bcps 2–4, 6 and 8, and in DES-2 the H-bonds bcps 2–6, 8 and 9 are relatively strong with Hb < 0, which indicate that such interactions are partially covalent in nature. Additionally, most of the bcps of H-bond in DES-1 (bcp 2–4, 6 and 8) and in DES-2 (bcp 2–6, 8 and 9), the values of ρb are relatively high and ∇2ρb > 0 and Hb < 0, which indicate that such interactions are partially covalent in nature.
In DES-3 and DES-4, the geometry of ChCl remains generally unchanged upon interaction with the ethylene glycol and glycerol, respectively, and new H-bonds are formed between the Cl anion in the ChCl and the O–H (bcp 4) and C–H (bcp 5) bonds in the alcohols. These interactions showed to be the most relevant in the ChCl: ethylene glycol mixture at 1:1 M ratio [36].
Additionally, the hydroxyl group of ChCl forms a H-bond with an oxygen atom of OH groups of the alcohols. The interactions O–H⋯Cl and O–H⋯H (bcp 4 and 6), wherein the ChCl acts as HBA and HBD, respectively, are relatively strong and partially covalent (Hb < 0). Also, in these DESs, H-bonds of partially covalent nature are observed between the two molecules of ethylene glycol (bcp 8 and 9) and glycerol (bcp 10 and 11), which suggests that as the concentration of alcohol in the DES increases, the interactions between the molecules become more important in the stabilisation of the DESs. Also, in DES-4, the third hydroxyl group of glycerol allows the formation of additional intermolecular H-bonds, explaining its higher interaction energy with regard to DES-3.
Furthermore, it has been found that the sum of the ρb values at all intermolecular H-bond bcps (Σρb) in a complex is a measure of the H-bonds strength and it increases linearly with ΔE. Accordingly, a good linear correlation between Σρb and the ΔE (R2 = 0.988) is obtained for DES under study (Fig. S3).
3.6.2. Interactions between mangiferin and DESs
Based on prior research, the formation of complexes between mangiferin (Man) and DES can be explained by intermolecular interactions, particularly hydrogen bonding [13]. Therefore, our objective was to investigate the potential formation of H-bonds between DES and Man. For this purpose, Man molecule was placed in different spatial positions with regard to DESs structures, taking into account their electrostatic potentials surface maps. The same methodology for the DESs optimization was followed to obtain DES-Man complexes optimized structures. Then, the lowest energy structures were selected for further analysis using QTAIM approach. The interaction energies of DES-Man complexes were calculated using the following equation:
| ΔEDES-Man = EDES-Man − (EDES + EMan) |
where EDES-Man, EDES and EMan stand for the total energy of the DES-Man complex, DES, and the mangiferin molecule, in their optimized structure, respectively.
The molecular graphs of the most stable DES-Man complexes are shown in Fig. 6 along with the interaction energies. The topological parameters of the H-bond interactions between DESs and Man are shown in Table S3. The values of ΔE lie in the narrow range from −12.1 to −10.1 kcal/mol, suggesting that the formation of DES-Man complexes is favorable (negative sign of ΔE).
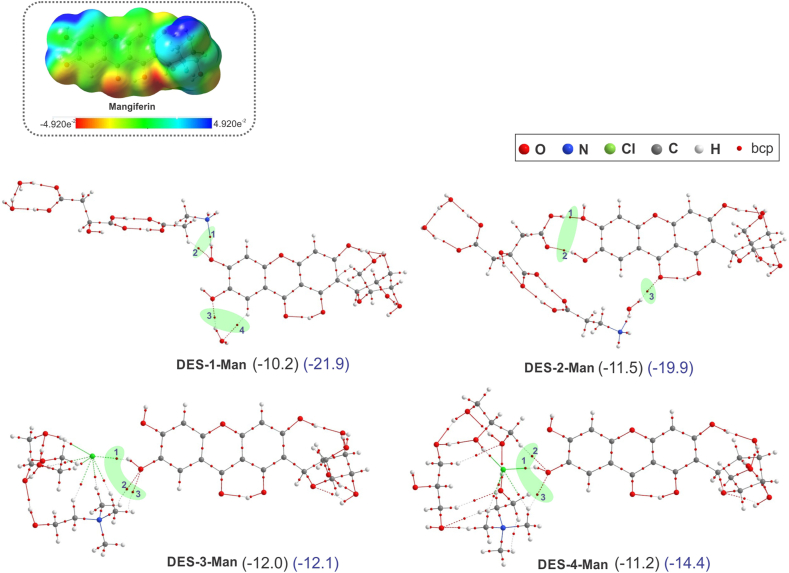
Fig. 6.
Electrostatic potential surface of mangiferin (top). Molecular graphs of the most stable DES-Man complexes in which the bcp corresponding to the H-bonds between DES and Man are highlighted in green. The interaction energy and solvation energy (blue) are given between parentheses and expressed in kcal/mol.
In DES-1-Man there is a strong H-bond (high value of ρb and ∇2ρb positive and the bcp 1) between N atom of β-alanine and O–H bond of mangiferin, which shows partially covalent property, (Hb < 0). The same O–H of mangiferin can act as an H-bond acceptor by interacting with a C–H bond of the β-alanine component of DES-1, forming a weak C–H⋯O H-bond. Also, one of the water molecules of DES-1 is acting as donor and acceptor of H-bond forming O⋯HO (bcp 3) and CH⋯O (bcp 4) H-bonds with the mangiferin.
In DES-2-Man complex, the presence of an extra carboxyl group in citric acid in DES-2 relative to malic acid in DES-1, allows it to interact with two adjacent OH groups of the Man, forming a cyclic structure linked by two moderate H-bonds (bcp 1 and 2). Also, a O–H bond of water molecule can contact with an oxygen atom of OH bond of Man via C–H⋯O H-bond (bcp 3), all these H-bonds show characteristics of pure electrostatic interaction.
In DES-3-Man and DES-4-Man complexes, the Cl anion of ChCl form an O–H⋯Cl H-bond (bcp 1) with an O–H bond of Man (OH⋯Cl), which is stronger in the second one. The same OH of Man also acts as HBA forming two weak H-bonds (bcp 2 and 3). All H-bonds between DES-3 and DES-4 and Man have characteristics of pure electrostatic interaction. Although these results suggest that Cl anion is very important in the formation of H-bonds, and therefore, in the extraction of compounds, the contribution of weaker H-bonds cannot be ruled out.
The H-bonds involved in the formation of DES-1-Man and DES-2-Man complexes are stronger than those in complexes of DES-3-Man and DES-4-Man, however, the strength of the H-bonds evaluated through the Σρb at all H-bonds bcps between DESs and Man (Σρb = 0.091, 0.086, 0.036 and 0.048 au. for DES-1,2,3,4 and Man complexes, respectively) does not reflect a direct correlation with the ΔEDES-Man. This could be due to the stability of a complex not only depends on its molecular interactions but also it is influenced by the deformation of the components upon formation of the complex.
Thus, to estimate the net interaction energy between the DES and Man we used the solute-solvent interaction energy defined for hydrated organic complex [43]. Based on this definition the DES-mangiferin interaction energy was defined as:
| ΔEDES∩Man = EDES-Man − (EcDES + EcMan) |
where EcDES, EcMan, indicate the DES and the Man energies, both of them calculated on the complex geometry.
The results show that ΔEDES∩Man increase in the order DES-1-Man > DES-2-Man > DES-4-Man > DES-3-Man which is in agreement with the extraction of mangiferin from MCP through agitation method, however, it differs from those extracted from MCS, which can be interpreted as a significant influence of the matrix.
Accordingly, a good linear correlation (R2 = 0.988) between the Σρb at all bcps of H-bonds between DES and Mangiferin and the ΔEDES∩Man was obtained (Fig. S4), demonstrating the relevance of H-bonds in the interaction between DESs and Man and therefore, in its recovery. These findings indicate that DESs under study form H-bonds with mangiferin molecule, acting both as HBD and HBA which is necessary to recover this kind of target molecule.
Although there might be other configurations of DESs-Man complexes, our computational study provides a valuable tool to assess the interaction between DES and a target molecule, which would be useful to predict the recovery of other polyphenolic compounds.
4. Conclusion
Deep eutectic solvents were used as an alternative to valorize mango by-products via the extraction of phenolic compounds. Agitation and UAE produced varying yields of total phenolic and flavonoid compounds for peel and seed extracts. β-alanine and choline chloride based DESs were effective for phenolic compounds extraction. Methodology and material affected phenolic profile and antioxidant capacity.
Theoretical studies showed that H-bond strength determined the viscosity of DESs. This study indicated that mangiferin and DESs form H-bonds of varying characteristics, which are determinant for effective compound recovery. Additionally, solvation energy between solute and solvent was identified as a reliable measure of DES-Mangiferin interaction strength.
These discoveries underline the significance of comprehending extraction mechanisms and the correlation with DESs physicochemical properties. This knowledge can significantly expand the application scope and benefits of these innovative solvents.
Author contribution statement
Gonzalo A. Ojeda: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Margarita M. Vallejos: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Sonia C. Sgroppo: Analyzed and interpreted the data; Wrote the paper.
Concepción Sánchez-Moreno, Begoña de Ancos: Contributed reagents, materials, analysis tools or datas; Analyzed and interpreted the data; Wrote the paper.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the grant PID2019-107980RB-I00 funded by MCIN/AEI/10.13039/501100011033. The authors are grateful to the graduate Lucía Gimenez for her technical support. Dr. Ojeda thanks the Carolina Foundation and the Ministry of Education of Argentina for the grant received. Dr. Sgroppo thanks the Argentinean Agency for the Scientific and Technological Promotion (ANPCyT) PICT 2019/3395 and SGCYT UNNE (PI 18F020).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e16912.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kaur B., Panesar P.S., Anal A.K., Ky S.C. Recent trends in the management of mango by-products. Food Rev. Int. 2022:1–21. doi: 10.1080/87559129.2021.2021935. [DOI] [Google Scholar]
- 2.Torres-León C., Rojas R., Contreras-Esquivel J.C., Serna-Cock L., Belmares-Cerda R.E., Aguilar C.N. Mango seed: functional and nutritional properties. Trends Food Sci. Tech. 2016;55:109–117. doi: 10.1016/j.tifs.2016.06.009. [DOI] [Google Scholar]
- 3.Serna-Cock L., García-Gonzales E., Torres-León C. Agro-industrial potential of the mango peel based on its nutritional and functional properties. Food Rev. Int. 2016;32:364–376. doi: 10.1080/87559129.2015.1094815. [DOI] [Google Scholar]
- 4.Jahurul M.H.A., Zaidul I.S.M., Ghafoor K., Al-Juhaimi F.Y., Nyam K.L., Norulaini N.A.N., Sahena F., Mohd Omar A.K. Mango (Mangifera indica L.) by-products and their valuable components: a review. Food Chem. 2015;183:173–180. doi: 10.1016/j.foodchem.2015.03.046. [DOI] [PubMed] [Google Scholar]
- 5.Dorta E., González M., Lobo M.G., Sánchez-Moreno C., de Ancos B. Screening of phenolic compounds in by-product extracts from mangoes (Mangifera indica L.) by HPLC-ESI-QTOF-MS and multivariate analysis for use as a food ingredient. Food Res. Int. 2014;57:51–60. doi: 10.1016/j.foodres.2014.01.012. [DOI] [Google Scholar]
- 6.Torres-León C., Ventura-Sobrevilla J., Serna-Cock L., Ascacio-Valdés J.A., Contreras-Esquivel J., Aguilar C.N. Pentagalloylglucose (PGG): a valuable phenolic compound with functional properties. J. Func. Foods. 2017;37:176–189. doi: 10.1016/j.jff.2017.07.045. [DOI] [Google Scholar]
- 7.Quintana S.E., Salas S., García-Zapateiro L.A. Bioactive compounds of mango (Mangifera indica): a review of extraction technologies and chemical constituents. J. Sci. Food Agric. 2021;101:6186–6192. doi: 10.1002/jsfa.11455. [DOI] [PubMed] [Google Scholar]
- 8.Jangra A., Arora M.K., Kisku A., Sharma S. The multifaceted role of mangiferin in health and diseases: a review. Adv. Trad. Med. 2021;21:619–643. doi: 10.1007/s13596-020-00471-5. [DOI] [Google Scholar]
- 9.Ballesteros-Vivas D., Álvarez-Rivera G., Morantes S.J., Sánchez-Camargo A. del P., Ibáñez E., Parada-Alfonso F., Cifuentes A. An integrated approach for the valorization of mango seed kernel: efficient extraction solvent selection, phytochemical profiling and antiproliferative activity assessment. Food Res. Int. 2019;126 doi: 10.1016/j.foodres.2019.108616. [DOI] [PubMed] [Google Scholar]
- 10.Martínez-Ramos T., Benedito-Fort J., Watson N.J., Ruiz-López I.I., Che-Galicia G., Corona-Jiménez E. Effect of solvent composition and its interaction with ultrasonic energy on the ultrasound-assisted extraction of phenolic compounds from Mango peels (Mangifera indica L.) Food Bioprod. Process. 2020;122:41–54. doi: 10.1016/j.fbp.2020.03.011. [DOI] [Google Scholar]
- 11.Balaraman H., Selvasembian R., Rangarajan V., Rathnasamy S. Sustainable and green engineering insights on deep eutectic solvents toward the extraction of nutraceuticals. ACS Sustain. Chem. Eng. 2021;9:11290–11313. doi: 10.1021/acssuschemeng.1c03034. [DOI] [Google Scholar]
- 12.Duan L., Dou L.L., Guo L., Li P., Liu E.H. Comprehensive evaluation of deep eutectic solvents in extraction of bioactive natural products. ACS Sustain. Chem. Eng. 2016;4:2405–2411. doi: 10.1021/acssuschemeng.6b00091. [DOI] [Google Scholar]
- 13.Dai Y., Witkamp G.J., Verpoorte R., Choi Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015;187:14–19. doi: 10.1016/j.foodchem.2015.03.123. [DOI] [PubMed] [Google Scholar]
- 14.Cao J., Cao J., Wang H., Chen L., Cao F., Su E. Solubility improvement of phytochemicals using (natural) deep eutectic solvents and their bioactivity evaluation. J. Mol. Liq. 2020;318 doi: 10.1016/j.molliq.2020.113997. [DOI] [Google Scholar]
- 15.Wang H., Liu S., Zhao Y., Wang J., Yu Z. Insights into the hydrogen bond interactions in deep eutectic solvents composed of choline chloride and polyols. ACS Sustain. Chem. Eng. 2019;7:7760–7767. doi: 10.1021/acssuschemeng.8b06676. [DOI] [Google Scholar]
- 16.Abbott A.P., Capper G., Davies D.L., Rasheed R.K., Tambyrajah V. Novel solvent properties of choline chloride/urea mixtures. Chem. Comm. 2003:70–71. doi: 10.1039/b210714g. [DOI] [PubMed] [Google Scholar]
- 17.Sui M., Feng S., Liu G., Chen B., Li Z., Shao P. Deep eutectic solvent on extraction of flavonoid glycosides from Dendrobium officinale and rapid identification with UPLC-Triple-TOF/MS. Food Chem. 2022 doi: 10.1016/j.foodchem.2022.134054. [DOI] [PubMed] [Google Scholar]
- 18.Patil S.S., Pathak A., Rathod V.K. Optimization and kinetic study of ultrasound assisted deep eutectic solvent based extraction: a greener route for extraction of curcuminoids from Curcuma longa. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oomen W.W., Begines P., Mustafa N.R., Wilson E.G., Verpoorte R., Choi Y.H. Natural deep eutectic solvent extraction of flavonoids of scutellaria baicalensis as a replacement for conventional organic solvents. Molecules. 2020;25:1–11. doi: 10.3390/molecules25030617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pal C.B.T., Jadeja G.C. Optimization and kinetics of polyphenol recovery from raw mango (Mangifera indica L.) peel using a glycerol-sodium acetate deep eutectic solvent system. Biomass Conv. Biorefinery. 2022 doi: 10.1007/s13399-022-02550-w. [DOI] [Google Scholar]
- 21.Chen S., Xiao L., Li S., Meng T., Wang L., Zhang W. The effect of sonication-synergistic natural deep eutectic solvents on extraction yield, structural and physicochemical properties of pectins extracted from mango peels. Ultrason. Sonochem. 2022;86 doi: 10.1016/j.ultsonch.2022.106045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanjekar K.J., Gokhale S., Rathod V.K. Utilization of waste mango peels for extraction of polyphenolic antioxidants by ultrasound-assisted natural deep eutectic solvent. Bioresour. Tech. Rep. 2022;18 doi: 10.1016/j.biteb.2022.101074. [DOI] [Google Scholar]
- 23.Tolmachev D., Lukasheva N., Ramazanov R., Nazarychev V., Borzdun N., Volgin I., Andreeva M., Glova A., Melnikova S., Dobrovskiy A., Silber S.A., Larin S., de Souza R.M., Ribeiro M.C.C., Lyulin S., Karttunen M. Computer simulations of deep eutectic solvents: challenges, solutions, and perspectives. Int. J. Molecular Sci. 2022;23 doi: 10.3390/ijms23020645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bader R.F.W. Clarendon Press; Oxford: 1990. Atoms in Molecules: A Quantum Theory. [Google Scholar]
- 25.García G., Atilhan M., Aparicio S. An approach for the rationalization of melting temperature for deep eutectic solvents from DFT. Chem. Phys. Lett. 2015;634:151–155. doi: 10.1016/j.cplett.2015.06.017. [DOI] [Google Scholar]
- 26.Zhu K., Wei Q., Li H., Ren X. Recovery of titanium from ilmenite HCl leachate using a hydrophobic deep eutectic solvent. ACS Sustain. Chem. Eng. 2022;10:2125–2135. doi: 10.1021/acssuschemeng.1c07204. [DOI] [Google Scholar]
- 27.Xia Q., Liu Y., Meng J., Cheng W., Chen W., Liu S., Liu Y., Li J., Yu H. Multiple hydrogen bond coordination in three-constituent deep eutectic solvents enhances lignin fractionation from biomass. Green Chem. 2018;20:2711–2721. doi: 10.1039/c8gc00900g. [DOI] [Google Scholar]
- 28.Jiang B., Zhang H., Zhang L., Zhang N., Huang Z., Chen Y., Sun Y., Tantai X. Novel deep eutectic solvents for highly efficient and reversible absorption of SO2 by preorganization strategy. ACS Sustain. Chem. Eng. 2019;7:8347–8357. doi: 10.1021/acssuschemeng.8b06822. [DOI] [Google Scholar]
- 29.Naseem Z., Shehzad R.A., Ihsan A., Iqbal J., Zahid M., Pervaiz A., Sarwari G. Theoretical investigation of supramolecular hydrogen-bonded choline chloride-based deep eutectic solvents using density functional theory. Chem. Phys. Lett. 2021;769 doi: 10.1016/j.cplett.2021.138427. [DOI] [Google Scholar]
- 30.Lee C., Yang W., Parr R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B. 1988;37:785–789. doi: 10.1103/PhysRevB.37.785. [DOI] [PubMed] [Google Scholar]
- 31.Parr R.G. In: Density Functional Theory of Atoms and Molecules BT - Horizons of Quantum Chemistry. Fukui K., Pullman B., editors. Springer Netherlands; Dordrecht: 1980. pp. 5–15. [Google Scholar]
- 32.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Petersson G.A., Nakatsuji H., Li X., Caricato M., Marenich A.V., Bloino J., Janesko B.G., Gomperts R., Mennucci B., Hratchian H.P., Ortiz J.V., Izmaylov A.F., Sonnenberg J.L., Williams, Ding F., Lipparini F., Egidi F., Goings J., Peng B., Petrone A., Henderson T., Ranasinghe D., Zakrzewski V.G., Gao J., Rega N., Zheng G., Liang W., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Throssell K., Montgomery J.A., Jr., Peralta J.E., Ogliaro F., Bearpark M.J., Heyd J.J., Brothers E.N., Kudin K.N., Staroverov V.N., Keith T.A., Kobayashi R., Normand J., Raghavachari K., Rendell A.P., Burant J.C., Iyengar S.S., Tomasi J., Cossi M., Millam J.M., Klene M., Adamo C., Cammi R., Ochterski J.W., Martin R.L., Morokuma K., Farkas O., Foresman J.B., Fox D.J. Gaussian, Inc.; Wallin: 2016. G16_C01, Gaussian 16, Revision C.01. [Google Scholar]
- 33.Keith T.A., Gristmill T.K. 2016. Software, AIMAII (Version 11.05.16) [Google Scholar]
- 34.Wang M., Wang J., Zhou Y., Zhang M., Xia Q., Bi W., Chen D.D.Y. Ecofriendly mechanochemical extraction of bioactive compounds from plants with deep eutectic solvents. ACS Sustain. Chem. Eng. 2017;5:6297–6303. doi: 10.1021/acssuschemeng.7b01378. [DOI] [Google Scholar]
- 35.Choi Y.H., van Spronsen J., Dai Y., Verberne M., Hollmann F., Arends I.W.C.E., Witkamp G.J., Verpoorte R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011;156:1701–1705. doi: 10.1104/pp.111.178426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alizadeh V., Malberg F., Pádua A.A.H., Kirchner B. Are there magic compositions in deep eutectic solvents? Effects of composition and water content in choline chloride/ethylene glycol from ab initio molecular dynamics. J. Phys. Chem. B. 2020;124:7433–7443. doi: 10.1021/acs.jpcb.0c04844. [DOI] [PubMed] [Google Scholar]
- 37.Skarpalezos D., Detsi A. Deep eutectic solvents as extraction media for valuable flavonoids from natural sources. Appl. Sci. (Switzerland) 2019;9 doi: 10.3390/app9194169. [DOI] [Google Scholar]
- 38.Dorta E., Lobo M.G., González M. Using drying treatments to stabilise mango peel and seed: effect on antioxidant activity. LWT - Food Sci. Tech. 2012;45:261–268. doi: 10.1016/j.lwt.2011.08.016. [DOI] [Google Scholar]
- 39.Venegas-Sanchez J.A., Tagaya M., Kobayashi T. Effect of ultrasound on the aqueous viscosity of several water-soluble polymers. Polym. J. 2013;45:1224–1233. doi: 10.1038/pj.2013.47. [DOI] [Google Scholar]
- 40.Florindo C., Oliveira F.S., Rebelo L.P.N., Fernandes A.M., Marrucho I.M. Insights into the synthesis and properties of deep eutectic solvents based on cholinium chloride and carboxylic acids. ACS Sustain. Chem. Eng. 2014;2:2416–2425. doi: 10.1021/sc500439w. [DOI] [Google Scholar]
- 41.Arunan E., Desiraju G.R., Klein R.A., Sadlej J., Scheiner S., Alkorta I., Clary D.C., Crabtree R.H., Dannenberg J.J., Hobza P., Kjaergaard H.G., Legon A.C., Mennucci B., Nesbitt D.J. Defining the hydrogen bond: an account (IUPAC technical report) Pure Appl. Chem. 2011;83:1619–1636. doi: 10.1351/PAC-REP-10-01-01. [DOI] [Google Scholar]
- 42.Cremer D., Kraka E. Chemical bonds without bonding electron density — does the difference electron-density analysis suffice for a description of the chemical bond? Angewandte Chem. Int. Ed. Eng. 1984;23:627–628. [Google Scholar]
- 43.Vallejos M.M., Peruchena N.M. Preferential formation of the different hydrogen bonds and their effects in tetrahydrofuran and tetrahydropyran microhydrated complexes. J. Phys. Chem. A. 2012;116:4199–4210. doi: 10.1021/jp301498n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.