Abstract
Senescence is a natural phenomenon of growing old. It accelerates under certain conditions like diabetes mellitus resulting in early decline of bodily functions, which can be avoided by many claimed functional foods. The present study aims to investigate the anti-aging ability of Fenugreek seeds (Trigonellafoenum-graecum); a common ingredient of Indo-Pak cuisines. Briefly, the Fenugreek seeds extract (FgSE) in concentrationsof0.1, 0.5 and 1 mg/ml inhibited the formation of Advanced Glycation End products (AGEs) and fructosamine adducts in Bovine serum albumin (BSA)/fructose model in vitro. The BSA conformational analysis via Circular Dichorism and Congo red assays showed that it preserves secondary structure of BSA in aforementioned model. Although mechanistic studies revealed insignificant lysine blocking ability of Fenugreek by OPA assay, however carbonyl entrapping was found to be 24%, 34% and 42% at 0.1, 0.5 and 1 mg/ml, respectively. In vivo model of High Fructose diet (HFD) induced glycation, FgSE treatment in doses of 10, 25 & 50 mg/kg markedly improved Escape latency (p < 0.01) and preserved cognition in Morris Water Maze. Our data further exhibits significant decrease of CML (Nε-carboxymethyl lysine) levels in serum and hippocampus byFgSE treatment in comparison with HFD group. Therefore, we deduced that FgSE prevents glycation-induced memory decline via entrapping the reactive carbonyl intermediates, formed during production of AGEs. Hence, as a promising functional food it slows down the harmful process of glycation and aging associated morbidities.
Keywords: Carbonyl trapping, Glycation, Anti-aging functional foods, Trigonellafoenum-graecum
Highlights
-
•
Fenugreek seeds extract (FgSE) inhibited formation of advanced glycation end products.
-
•
Fenugreek seeds extract preserved the secondary structure of BSA in the presence of fructose.
-
•
Fenugreek seeds extract entrap the reactive carbonyl compounds required for the synthesis of AGEs.
-
•
Fenugreek seeds extract prevented the glycation-induced cognitive decline.
-
•
Fenugreek seeds extract reduced the CML levels in both hippocampus and serum of high fructose fed rats.
1. Introduction
Proteins play a vital role in maintaining structure and functions of human body. Their conformation in body is equally important as their concentration. A change in protein conformation has been reported to cause various neurodegenerative diseases like Alzheimer's &Parkinson's diseases [1]. One of the major underlying mechanisms that alter the structure of proteins is termed as glycation. It, also known as Milliard's reaction, has been receiving significant attention as a prime cause of loss of bodily functions attributed to aging [2]. It takes place in three steps. Initially binding of reducing sugars to free amino acids (lysine and arginine mainly) in proteins or lipids or DNA in absence of enzyme leads to formation of Schiff bases [3]. This first step, which may take few hours, is reversible and depending on concentration of sugars available. Schiff base undergoes rearrangement in chemical structures that leads to formation of relatively stable chemical structures known as Amadori products, ultimately leading to formation of irreversible advanced glycation end products within weeks to months [4]. This theory strongly suggested that reducing sugars act as a mediator of aging due to formation of Advanced glycation end products like N(Epsilon)- Carboxy Methyl Lysine (CML), N(Epsilon)- Carboxy Ethyl Lysine (CEL), Pyrraline, Methyl Glyoxyl Lysine Diamer (MOLD) and Glyoxyl Lysine Diamer (GOLD) [5,6]. It's most abundant concentration is found in old ages and strong scientific evidence supports that it has a role in decreasing life span [7]. Investigations in humans and rodents model revealed that calories restriction and low carbohydrate diet increases life span [8]. Confinement of dietary AGEs also leads to quick wound healing, decreased incidence of diabetes, obesity and cardiovascular diseases [9]. Enhanced glycemic index can lead to increase in glycative stress [10]. AGEs accumulation causes several pathologies and associated with faster memory decline and cognitive impairment [3]. It is also responsible for diabetic complications and other diseases like neurodegenerative diseases, arteriosclerosis, bone loss and cardiovascular diseases [11,12]. The detrimental effects of AGEs can occur either directly without receptors or via receptor for advanced glycation end products (RAGE) [13], which in turn activates transcription factor nuclear factor kappa B (NF-κB) thereby increasing the production of pro-inflammatory cytokines [14].
For last 160 years, life expectancy has exponentially expanded by a fourth of a year every year, an uncommon consistency of human accomplishment [15]. According to WHO, the world will have more individuals who live to see their 80s or 90s than any time in recent history. The quantity of individuals matured 80 years or more for instance, will have nearly quadrupled to 395 million in the vicinity of 2000 and 2050 [16]. This is not the complete picture of reality as quality of life is equally important as quantity. This also means that there is highest number of people living with morbidities of old age. Life longevity and aging research is gaining popularity. In recent years, the concept of preventive medicine for diseases gained much popularity and general population prefers not to take medicine unnecessarily before the onset of pathology. This reluctance towards needless medication for prevention of pathology introduced a newer concept of “functional foods” that emphasizes use of certain group of food products to prolong fitness [17]. This concept was tossed by the highest life expectancy countries like Japan and Australia where average life span is more than 80 years [18]. Functional foods are reported to have high impact on prevention of diseases particularly involving Glycation and Aging [19]. Many food substances are reported to be anti-aging by inhibiting non enzymatic glycosylation [20,21].
Fenugreek seeds (Trigonella foenum-graecum) have considerable health benefits. It is an esoteric Eastern herb frequently incorporated for decades in enhancing food color and taste. It iscommonly known as Meethi seedsand is incorporated in traditional practices for therapeutic purpose. It is long known for its ability to control diabetes and lipid profile of diabetic patients upon adding to the diet in powdered form [22]. It is also reported that fenugreek seeds contribute to memory preservation &neurogenesis in rats [23]. In Streptozotocin induced models of diabetes, it preserved memory and decreased the oxidative stress. It is of note that these results were similar to Metformin, which is a potential drug to control early diabetes and another recently known anti-aging drug [24,25]. Its benefits in short-term memory preservation is also reported [26]. Keeping into account aforementioned actions, the present study was designed to assess the anti-aging effect of Fenugreek seeds through interfering in the deleterious phenomenon of glycation. Limitations of previous studies are exhibited by the research gap in identifying exact mechanism in contribution against glycation and memory. Even the most recent work regarding Fenugreek seeds ability as skin anti-aging is quoted in terms of formulation. In certain in-vivo studies it is established to possess anti-aging effects associated with organs [27] however its association with attenuation of Advanced glycation end products like CML in rodents have not been demonstrated.
This research is an effort to highlight Fenugreek seed as functional food & its ability to be anti-aging as a whole without separating its components so general public could benefit. This approach of mechanistic studies highlights mechanism of action and its impact on cellular level. This approach is unique from the previous researches in our field as we studied phytochemical principals as a whole in multi system assessment. We have also incorporated protein conformational studies and impact of FgSE at molecular level of aggregation in BSA/fructose model.
2. Materials and methods
2.1. Animals
Male Wistar rats, aged 8–12 weeks weighing between 200 and 250 g were obtained from the Animal Resource Facility of ICCBS (International Center for Chemical and Biological Sciences), University of Karachi. All animals were kept under 12 h dark and light cycle and standard temperature conditions (25 ± 1 °C). All experiments were approved by Institutional Animal Care and Use Committee (ASP # 2017–0045).
2.2. Chemicals
The following chemicals were used in the study: 2,4,6-trinitrobenzenesulfonic acid (TNBSA), Acetic acid, d-Fructose, Formic acid, Nitroblue tetrazolium (NBT), O-Phenyldiamine, Phosphate buffer Saline, Sodium Azide, Sodium Carbonate, Sodium dihydrogen phosphate, Sodium hydrogen Phosphate & β-mercapethanol were obtained from Sigma Aldrich, (USA). Aminoguanidine (AG) andN-α-acetyl-l-lysine from Chemcruz, USA. Thioflavin T (ThT)from Santa Cruz Biotechnology, USA, Dimethyl sulfoxide (DMSO) and Chromium potassium sulfate from Merck, USA. Congo Red (CR) and Sodium bicarbonate from Bio Basic Inc.,Canada. Bovine Serum Albumin (BSA) from Imu med®, (USA).2-MethylQuinoxaline (2-MQ) and O-Phthaldialdehyde 98% (OPA) from Alfa Aesar (USA). Sodium dodecyl sulfate (SDS) from Kanto Chemical, (Japan). Sodium Thiopental from Abbott laboratories, (Pakistan). Ethanol and Methanol from Serva (Germany).
2.3. Preparation of hydro alcoholic extract
The Fenugreek seeds (1 Kg) were obtained from commercial source and submerged in Ethanol (70%). After maceration, the supernatant was collected for 5 days androtary evaporated (Bucchi rotavapor R-250, Tokyo, Japan)with vacuum (Bucchi vacuum pump V-700, Tokyo, Japan)to obtain thick concentrate. The extract (FgSE) was subsequently freeze-dried (model TR/FD/BT50 Trio Science Co. Tokyo, Japan)and storedat 4 °C until further use.
2.4. Phytochemical analysis by GC-MS
To identify the phytochemicals, present in Fenugreek seeds extract, GCMS-EI analysis of the hydroethanolic extract was conducted through Agilent Technologies 7000 A. Throughout the analysis the triple Quadrupole acquisition method was practiced. GCMS was available with a non-polar column i. e embedded with film that contain 95% Dimethylpolysiloxane and 5% phenyl (Agilent HP-5MS30 m length × 250 μm distance across × 0.25 μm film thickness). An electron ionization energy with 70eV was utilized for the detection of various compounds. As a carrier gas, ultra speckless helium gas (99.99%) was consumed (flow rate 1.5 ml/min) with 3 ml/min of septum purge flow. The infusion volume was 2 μL with 10:1 of split ratio. The pressure was 1.9952 psi and, the total run time was 71.429 min. Sample preparation was conducted by dissolving 1 g of extract in 20 ml of ethanol and then the solution was strained through Whatman filter paper No. 1 to remove stocky particles. Throughout the procedure analytical grade chemicals were used. The chromatograms were analyzed via Nist library.
2.5. AGEs formation assay
The reaction mixture consisted of FgSE (0.1, 0.5 or 1 mg/ml) mixed in a solution containing Bovine serum albumin (BSA, 10 mg/ml assuming it were 100% pure) and fructose (100 mM) in sodium phosphate buffer (0.2 M, pH 7.3) containing Sodium Azide (0.01%) as described earlier [28]. It was heated at 60 °C for 24 h followed by measurement of AGEs intrinsic fluorescence (excitation 360/40 nm and emission 460/40 nm) using Biotek Synergy HTX multimode reader. Aminoguanidine 2 mM was used as positive control. The percentage inhibition was calculated using following formula:
The reaction mixtures were further assessed in the following assays.
2.6. Estimation of fructosamine adducts
The Nitroblue Tetrazolium (NBT) assay was used to measure the formation fructosamine adducts [29]. Briefly, the reaction mixture (60 μl) was added to NBT (600 μl, 0.25 mM NBT was prepared in 10 ml of Bicarbonate buffer (pH 10.6). After incubation at 37 °C for 2 h, the absorbance was measured at 530 nm using Spectrophotometer (Biotek, Synergy HTX Multi-mode reader).
2.7. Estimation of lysine modification
Trinitro Benzene Sulfonic Acid (TNBSA)was used for estimation of lysine modification [29]. The 250 μl of TNBSA solution (0.1% w/v) was added to 500 μl of reaction mixture and incubated at 37 °C for 2 h. Absorbance was measured at 335 nm using spectrophotometer (Biotek, Synergy HTX Multi-mode reader).
2.8. Congo red assay
Congo red dye was used for the estimation of amyloid-like fibrils in the reaction mixture [30]. The reaction mixture (800 μl) was incubated with Congo red solution (100 μM, 200 μl) for 20 min. The absorbance was measured at 530 nm using Biotek, Synergy HTX Multi-mode reader.
2.9. Birefringence assay
In 80% ethanol prepared with deionized water, the saturating amount of NaCl was added to it. After filtration, the saturating amounts of Congo red is added and stain is stirred thoroughly and filtered [31]. This stain was used immediately after preparation. The reaction mixture (10 μl) was placed onto a glass slide and air dried. About 200-400μlof prepared stain was added to each slide and excess stain is blotted away. The slide was observed under microscope (Sundew Model MCX 1600)using polarized light for yellow/green birefringence.
2.10. Circular Dichorism
Circular dichorism (CD) technique was used to assess the secondary structure of BSA in reaction mixture using JASCO J810 Spectropolarimeter (Japan). All spectra were derived by scanning glycated protein samples at least four times in Far-UV amide region (190–250 nm) and UV-region (250–400 nm) at room temperature. CD spectral analysis was conducted to estimate secondary structural modification of protein by using online Dichro Web server [32].
2.11. Estimation of free lysine blockade
The OPA(o-phthaldialdehyde) assay was used to measure the interaction of fenugreek seeds extract with reactive lysine sites as described earlier [33]. The OPA reagent (100 ml) was prepared using 80 mg OPA dissolved in absolute ethanol (2 ml), 20%SDS (5 ml), β-Mercapethanol (200 μl) and Sodium Tetraborate buffer (50 ml, pH 10). This solution was stored in amber glass bottle. Lysine blockade estimation was done by taking 25 μg of protein sample (50 μl Liquid BSA), dissolved in de-ionized water along with FgSE and incubated for 15 min. Fluorometric analysis of available epsilon lysine amino group was estimated by checking the fluorescence immediately (Excitation at 360/40 nm and Emission at 460/40 nm) using Biotek, Synergy HTX Multi-mode reader. N-α-acetyl-lysine was used as standard dissolved in 0.1% formic acid at various concentrations for calibration curve from 50 μM to 100 μM.
2.12. Estimation of carbonyl trapping
Methyl glyoxal (MGO, 0.4 mg/ml) was prepared in 0.1 M phosphate buffer saline (pH 7.4). 100 μl was then dissolved in PBS (850 μl, 0.1 M, pH 7.4) and 50 μl of 5-MQ was added in this reaction mixture, which served as negative control. 5-MQ (1 mg/ml) in 50% methanol served as internal standard. 2-MQ was dissolved in 1 mg/ml of 50% methanol. Aminoguanidine was used as positive control in concentration of 1 m/ml. The carbonyl trapping ability of Fenugreek seeds extract was tested on three different doses (1 mg, 0.5 mg, and 0.1 mg) per ml. The 100 μl from each of this solution was incubated with 100 μl MGO, 50 μl 5-MQ and 750 μl of PBS. All the samples were thoroughly mixed and heated at 60 °C for 24 h. The OPD (10.8 mg/ml) is a derivatizing agent. After incubation, OPD solution (200 μl) was added to each reaction mixture. Again, incubate in dark for 30min to complete derivatization reaction. The quantification of MGO residue was done by amount of 2-MQ obtained in each sample [34]. For this purpose, HPLC analysis was done on Shimadzu Prominence HPLC system equipped with pump LC-20 A, autosampler SIL-20 A, and using SPD-M20A diode array UV detector. CBM-20 A connected Hardware to software LC solutions. Hibar® 250,4–6 Lichrospher® RP 18e (5 μM) preceded by guard column nucleosil 100-5 C 18 were used for chromatography separation. Mobile phase composition is 1:1 Methanol (HPLC grade) & DI water (filtered) with 5% glacial acetic acid (HPLC grade). Each sample was filtered with 0.22 μm syringe filter before transferring the samples into HPLC vial. Flow rate was kept at 1 ml/min with 20min runtime for complete quantification. Quantification of MGO was done by comparing 2-MQ with controls.
The percent decrease in amount of MGO is measured by this formula
| % Carbonyl trapping = [(MGO Control- Amount of MGO in test) / MGO control] x 100 |
2.13. In vivo study
The animal model of accelerated aging was developed by administering high amount of fructose as described earlier [28]. All animals were maintained on once daily (OD) dose of fructose in morning plus 10% fructose in drinking water (as libitum) for 40 days except for the saline group. The treatment arm was divided in three groups& treatment with FgSE (OD) was in the following fashion:
Group 1: FgSE (i.p. 10 mg/kg) + HFD.
Group 2: FgSE (i.p. 25 mg/kg) + HFD.
Group 3: FgSE (i.p. 50 mg/kg) + HFD.
High fructose diet (HFD) group:d- Fructose (i.p. 1000 mg/kg) plus 10% in drinking water for 40 days.
Normal Saline group: Received only 0.9% normal saline (i.p) for 40 days.
After 40 days, the cognitive function was evaluated using Morris's water maze. After probe trial animal was administered thiopental (50 mg/kg) for anesthesia and Blood was withdrawn by cardiac puncture later decapitation with rodent's guillotine was performed and brain and other organs were isolated all procedures were approved by Institutional Animal Care and Use Committee (ASP # 2017–0045).
2.14. Morris Water Maze
Spatial memory was assessed by Morris water maze as described previously [28]. It is a black water pool measuring 180 cm in diameter and 55 cm in height with a black color platform (10 cm Diameter, 20 cm Height) for escape. The platform position must be constant throughout the experiment [35]. The cues were fixed on the walls of the pool. The test was conducted over the period of five days after completion of 40 days dosing. Day 1 familiarization: In this session, platform was kept 1 cm above the water surface and rats were allowed to swim, if they were able to find the platform within 2 min, they were left on platform for 5 s. If they failed to reach the platform within 2 min, they were manually guided to platform and allowed to stay there for a period of 30 s. Upon entering the pool each time, we kept the face of rat towards the wall so it may remember the cue. During Day 2nd, 3rd& 4th (acquisition trail), the platform was kept 1 cm below the water surface and trails were conducted in the similar manner as that of day 1. On 5th day (Probe trial) platform was removed from the tank and rats were allowed to swim from the opposite quadrant containing the platform. It was allowed to swim for 120 min. Pool was drained and cleaned daily before starting experiment. A camera was fixed to monitor the behavior of rats in the paradigm. In order to assess the memory of rat, following parameters were noted i.e., number of crossings through platform position, time required to reach the platform position (escape latency), time spent in the target quadrant and time required to reach the target quadrant. The navigation maps were also prepared for comparison of spatial learning among groups.
2.15. Carboxymethyl-lysine measurement
After MWM, the Serum and hippocampus were obtained for the measurement of CML levels using ELISA kit (Glory Bioscience, UK) as described by the manufacturer protocol. Centrifuge, Spectrophotometer and Homogenizer were additionally required apart from this kit. This kit is based on Sandwich ELISA technique. Briefly, hippocampus from each group was homogenized in chilled PBS buffer (0.1 M pH 7.2) and centrifuged at 4250×g (3500 RPM in M2 rotor, Microcentrifuge; Labo-gene 1730 R ScanSpeed™, Denmark). The supernatant was collected and again centrifuged at 4 °C using 17000 g x g (14,000 RPM). The supernatant was stored (−20 °C) for CML measurement. In case of serum, after cardiac puncture, the blood was allowed to coagulate at room temperature for 10–20 min. Supernatant was picked and centrifuged at 4250×g (3500 RPM) for 20 min. The supernatant was collected and the remaining was discarded and stored at 4 °C for CML measurement. Standard Solution was prepared at the strengths of 400 ng/ml, 200 ng/ml, 100 ng/ml, 50 ng/ml and 25 ng/ml standard curve was constructed for calibration. The wells for the samples were set in the plate frame and 50 μl of the testing samples were added in each well. It was covered with the membrane and incubated at 37 °C for 30 min. Wells were washed with the wash buffer 3–4 times with multichannel pipette carefully without touching the walls or bottom of the well and allowed to dry. The HRP conjugate (supplied with the kit) was added (50 μl) in each well except blank and this plate was again incubated at 37 °C for 30 min. After second incubation, all the solution was discarded and the plate was washed (3–4 times) with wash buffer carefully. The wells were dried by gentle patting and first Chromogen Solution A (50 μl) was added and then Chromogen Solution B (50 μl) was added to each well. After firmly placing the membrane sealer, plate was incubated for 15 min at 37 °C. Afterwards, stop solution was added which changes the blue color to yellow. Reading was taken immediately after adding stop solution at 450 nm using UV spectrophotometer (Biotek Synergy HTX Multi-mode reader). The protein estimation (Bradford assay) was also performed to harmonize the results.
2.16. Statistical analysis
The data is presented as mean ± SEM of n = 6 animals per group. Difference among various means was computed using One-way ANOVA followed by LSD (Least significant difference) using statistical package IBM SPSS 21.0 software, USA. The p < 0.05 was considered minimum levels of significance throughout the experiments.
3. Results
3.1. GC-MS analysis of phytochemical principles
Analysis of Fenugreek phytochemicals revealed 25 compounds in total. Most abundant were Glucopyranoside52% and carbohydrate o-methyl D-glucose39.2%. Few gamma butyrolactones like Pantonolactone which is a precursor of Vit B5 Pantothenic Acid is also reported in this analysis 0.27%. Niacin commonly known as vitamin B3 is also found in 0.68%. Various other phytochemicals like Pyranones, Butanolides, Alkaloids, Phytosterols, Unsaturated fatty acids, Fatty amides, Ethyl esters, Ketones, Flavonoids, Aryl-aldehydes, Benzofurans, Furanones, Glucopyranoside and aziridine were found in this extract. Details of the Compounds, their molecular weights, Formulas, Structures and percentages are added in Appendix 1.
3.2. Anti-glycation assay
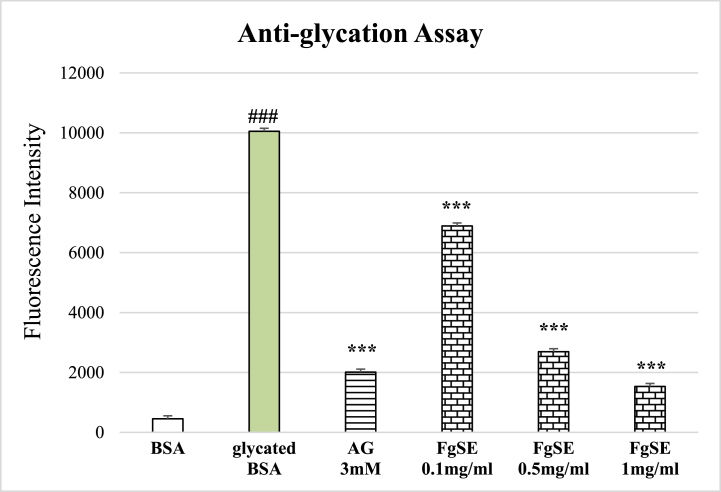
The glycated BSA showed significant (p < 0.005) increase in the intrinsic AGEs fluorescence as compared to native BSA (Fig. 1). In terms of similarity, with standard Aminoguanidine (3 mM), FgSE (0.1, 0.5 and 1 mg/ml) exhibited dose dependent decline in the fluorescence, when compared with glycated BSA.
Fig. 1.
Effect of Fenugreek Seeds extract (FgSE)on the intrinsic AGEs fluorescence in anti-glycation assay.
The Figure shows mean ± SEM of intrinsic fluorescence of AGEs in the presence of various treatment. The Figure shows significant increase in the intrinsic AGEs fluorescence in glycated samples of BSA as compared to native BSA. The Aminoguanidine (AG 3 mM) as well as FgSE (0.1, 0.5 and 1 mg/ml) has significantly reduced the formation of AGEs as compared to glycated BSA. Hash sign (###) represents statistical difference (p < 0.005) as compared to native BSA, while asterisks (***) indicate statistical difference (p < 0.005) as compared to glycated BSA.
3.3. Estimation of fructosamine adducts
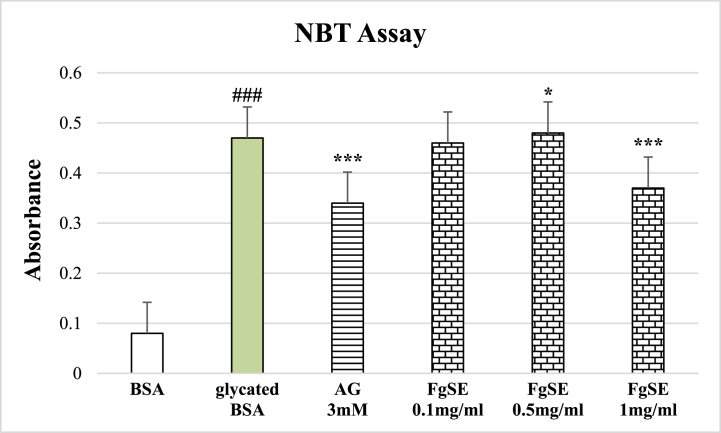
In comparison with native BSA, the glycated BSA showed significant (p < 0.005) increase in fructosamine adducts, which was prevented by both Aminoguanidine as well the FgSE in dose dependent fashion (Fig. 2).
Fig. 2.
Effects of fenugreek Seeds extract (FgSE) on Formation of intermediary Fructosamine adducts.
The Figure shows mean ± SEM of fructosamine adducts absorbance. The glycated BSA showed significant increase in the fructosamine adducts formation as compared to native BSA. The Aminoguanidine (AG 3 mM) as well as FgSE (0.5 and 1 mg/ml) has significantly reduced the formation of intermediate adducts as compared to glycated BSA. Hash sign (###) represents statistical difference (p < 0.005) as compared to native BSA, while asterisks (* and ***) indicate statistical difference (p < 0.05 and p < 0.005) as compared to glycated BSA.
3.4. Estimation of lysine modification
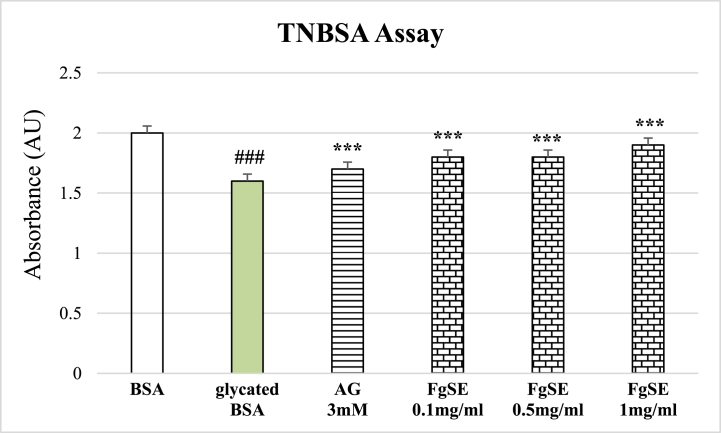
Significant lysine modification was observed in glycated samples as compared to the native BSA. However, the Aminoguanidine and FgSE treatment has significant preserved the lysine moiety in BSA (Figure-3).
Fig. 3.
Effects of Fenugreek seeds on lysine modification determined by TNBS assay.
This Figure shows mean ± SEMof lysine modification in the presence of various treatments. The data shows that glycated BSA has increased lysine modification and lesser available free lysine as compared to native BSA. The Aminoguanidine (AG 3 mM) as well as FgSE (1 mg/ml) has significantly reduced lysine modification as compared to glycated BSA. Hash sign (###) represents statistical difference (p < 0.005) as compared to native BSA, while asterisks (***) indicate statistical difference (p < 0.05) as compared to glycated BSA.
3.5. Congo red assay
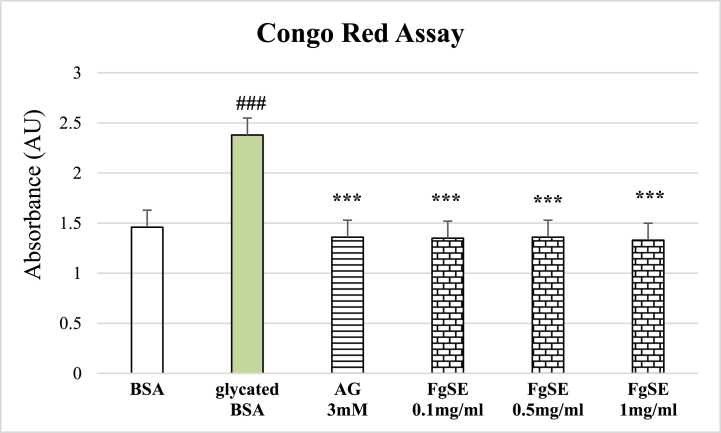
The Congo red analysis showed significantly raised amyloid-like structures in glycated samples as compared to native BSA. This raise was prevented by both Aminoguanidine and FgSE treatment (Fig. 4).
Fig. 4.
Effects of Fenugreek seeds extract (FgSE) on amyloid-like aggregation of BSA.
Fig. 4 shows mean ± SEM of amyloid-like aggregates formation in the presence of various treatments. The Figure shows significant increase in amyloid-like fibrils in glycated samples of BSA as compared to native BSA. Aminoguanidine (AG 3 mM) as well as FgSE (0.1, 0.5 and 1 mg/ml) has significantly reduced the formation amyloid firbrils as compared to glycated BSA. Hash sign (###) represents statistical difference (p < 0.005) as compared to native BSA, while asterisks (***) indicate statistical difference (p < 0.005) as compared to glycated BSA.
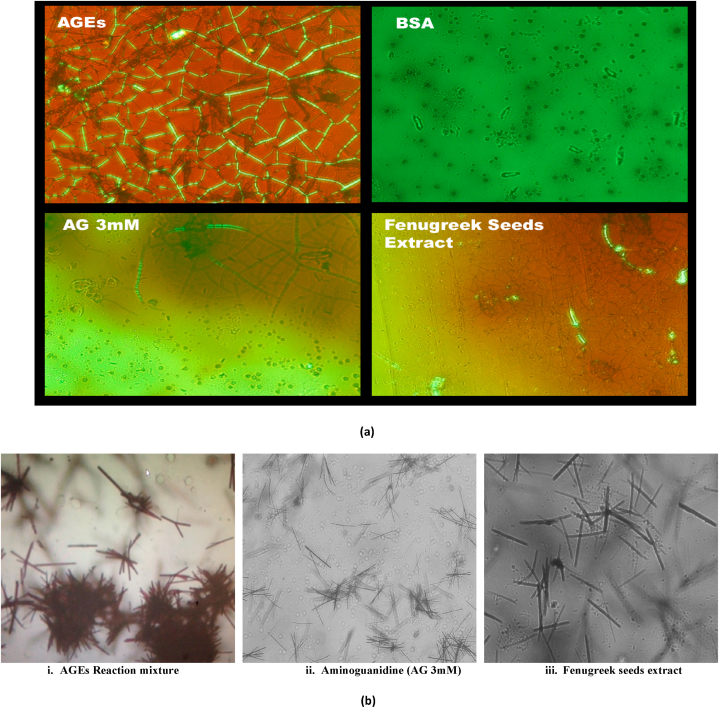
3.6. Congo red microscopy
Polarized light microscopy exhibited apple green and emerald green birefringence in AGEs reaction mixture (Fig. 5). In contrast treatment groups showed marked reduced birefringence and AG 3 μM showed native BSA like pattern (Fig. 5a). Congo red microscopy also revealed aggregation of BSA with FgSE and without treatment with FgSE extract (AGEs formation) and Aminoguanidine.
Fig. 5.
Effect of FgSE on glycation-induced change in the secondary structure of BSA. a Birefringence observed using Congo Red Staining. B Bright field microscopy of aggregates using Congo red staining
Fig. 5 (a and b) depicts the same finding under polarized light and bright field microscopy respectively following Congo red staining. Exhibition of birefringence in AGEs reaction mixture in the first image, Fig. 5a AGEs which glows apple green, upon exposure to polarized light which signifies presence of amyloid fibrils. Fig. 5 b also confirms this finding in bright field microscopy as clusters in image (i) AGEs reaction mixture. This birefringence is markedly reduced in reaction mixtures of Fenugreek seeds extract treated groups and positive control of Aminoguanidine 3 mM (Fig. 5a), which is also noticeable in images of bright field microscopy. Fig. 5b(i) Dense amyloid type aggregation can be seen with typical Congophilia. (ii) Aminoguanidine (AG 3 mM) which is a known anti-aging compound inhibits amyloid type aggregation and hence, decrease in extensive fibril formation. (iii) Fenugreek seeds extract exhibits AG like activity and a similar decrease can be seen in apple green birefringence in AG 3 mM and FgSE.
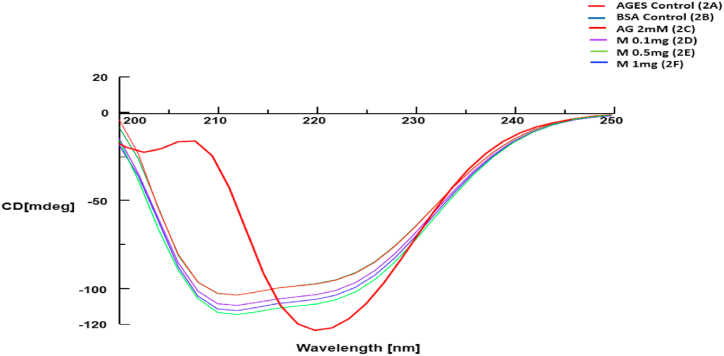
3.7. Circular Dichorism
The CD spectra showed the glycation-induced change in the secondary structure of BSA from alpha-helical to beta sheets (Fig. 6). The native structure was preserved by both Aminoguanidine and FgSE.
Fig. 6.
Effect of Fenugreek seeds extract on the conformation of BSA using Far-UV CD spectra.
Fig. 6 depicts that the glycation has significantly shifted the secondary structure of native BSA from alpha-helical to beta sheets. However, this change was prevented by Aminoguanidine and FgSE (0.1, 0.5, 1 mg/ml).
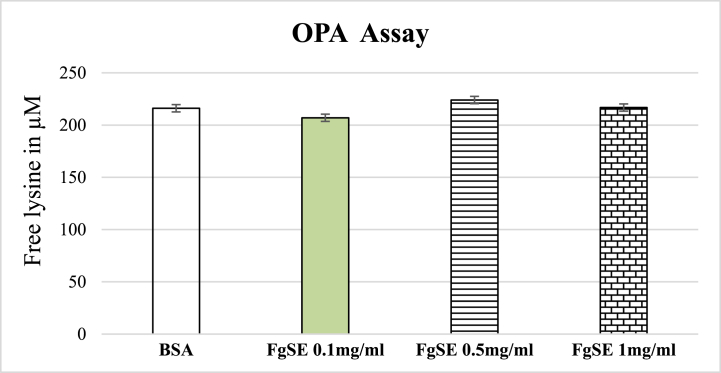
3.8. Estimation of free lysine blockade
No significant blocking of lysine was noted for FgSE (0.1, 0.5 and 1 mg/ml) and was similar to that of native BSA (Fig. 7).
Fig. 7.
Effects of Fenugreek seeds extract on lysine blockade by OPA Assay.
Fig. 7 depicts mean ± SEM of lysine blockade of BSA following various treatments. The data showed that FgSE did not have the ability to block free lysine residue in native BSA.
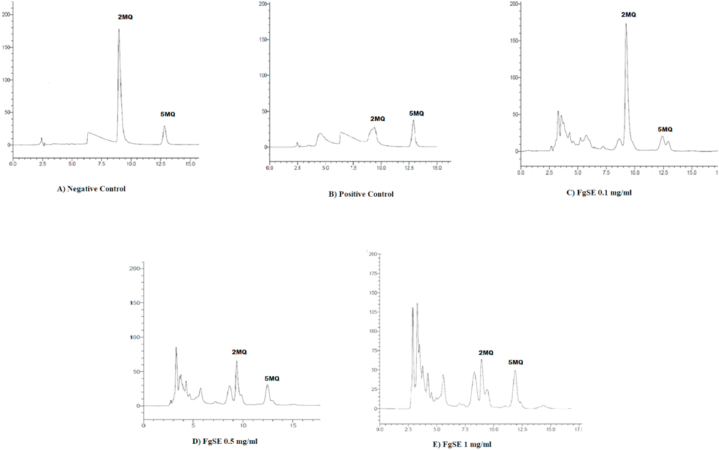
3.9. Estimation of carbonyl trapping
The aminoguanidine showed 86% carbonyl trapping, while FgSE has trapped 12%, 20% and 44% of the carbonyl moieties as the dose of 0.1, 0.5 and 1 mg/ml respectively (Fig. 8).
Fig. 8.
Effect of FgSEon Carbonyl entrapping using HPLC.
This Fig. 8 depicts the formation peaks of derivatized product 2MQ. Its AUC is markedly increased in failure of carbonyl entrapment which can be seen in Fig. 8-A however in presence of AG 3 mM and FgSE a dose dependent decline can be seen in AUC of 2MQ can be seen Fig. 8 B and whereas 5MQ is kept as internal standard.
The HPLC chromatograms showed that Aminoguanidine has entrapped 88% of carbonyl compounds, while FgSE has also shown 12%, 20% and 44% entrapments at respective doses of 0.1, 0.5 and 1 mg/ml.
3.10. Morris water maze
The HFD rats showed significant decline in the escape latency, time spent in target quadrant and number of crossings through platform position, while time to reach target quadrant was found to be significantly elevated as compared to control rats (Table-1). All of these impairments were significantly reversed by the FgSE treatment in dose depended manner.
Table 1.
Effect of FgSE treatment on learning & memory indicators in MWM, # (p < 0.05) and ## (p < 0.01) represent statistical comparison with NS, while ** (p < 0.01) and *** (p < 0.005) as compared to HFD group.
| Treatments | Learning & Memory Indicators in MWM |
|||
|---|---|---|---|---|
| Escape Latency (sec) | Time spent in target quadrant (sec) | Time to Reach Target Quadrant (sec) | Crossing through Platform Position | |
| Control | 4.8 ± 2 | 50 ± 5 | 3.5 ± 2 | 14 ± 1 |
| High fructose diet | 23 ± 2## | 30 ± 5### | 12.8 ± 2### | 6 ± 1### |
| FgSE (10 mg/kg) | 18.6 ± 2 | 46 ± 5** | 5.5 ± 2** | 8 ± 1 |
| FgSE (25 mg/kg) | 6.16 ± 2* | 66 ± 5*** | 3.5 ± 2*** | 12 ± 1*** |
| FgSE (50 mg/kg) | 6 ± 2** | 57 ± 5*** | 3.3 ± 2*** | 12 ± 1*** |
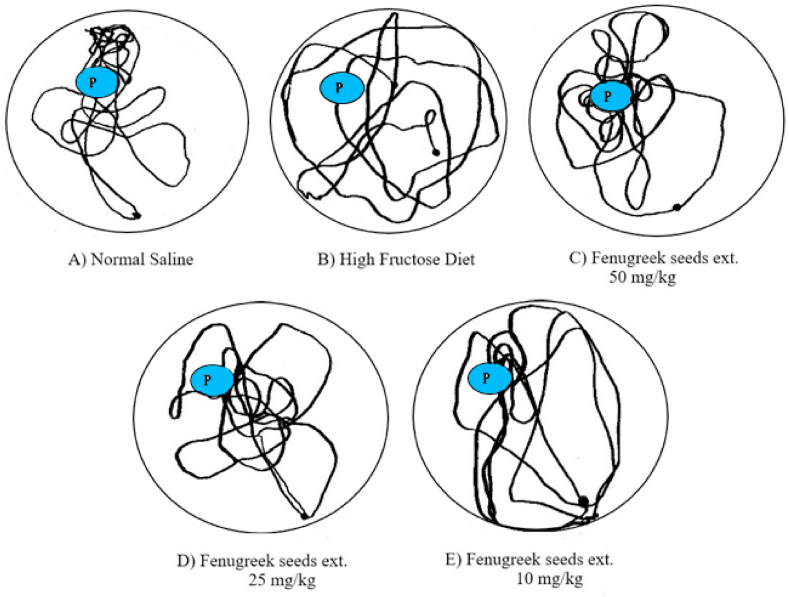
The navigation maps (Fig. 9 B) also exhibit that High fructose diet (HFD) group showed marked decline in finding Platform position, which was located in upper left quadrantrepresented in blue (Fig. 9). Normal saline group (NS) showed preserved memory as indicated by restricted movement in platform quadrant (Fig. 9A); the pattern also exhibited by FgSE treated group which became more obvious at highest tested dose of50 mg/kg (Fig. 9C).
Fig. 9.
Navigation Mapping inMorris water maze.
Fig. 9 depicts that HFD rats showed loss of memory in probe trail as indicated by random movement in the MWM pool. However, Aminoguanidine and FgSE treated rats showed more restricted movement in the platform quadrant, which is suggested of preserved training memory of rats during acquisition trials in MWM.
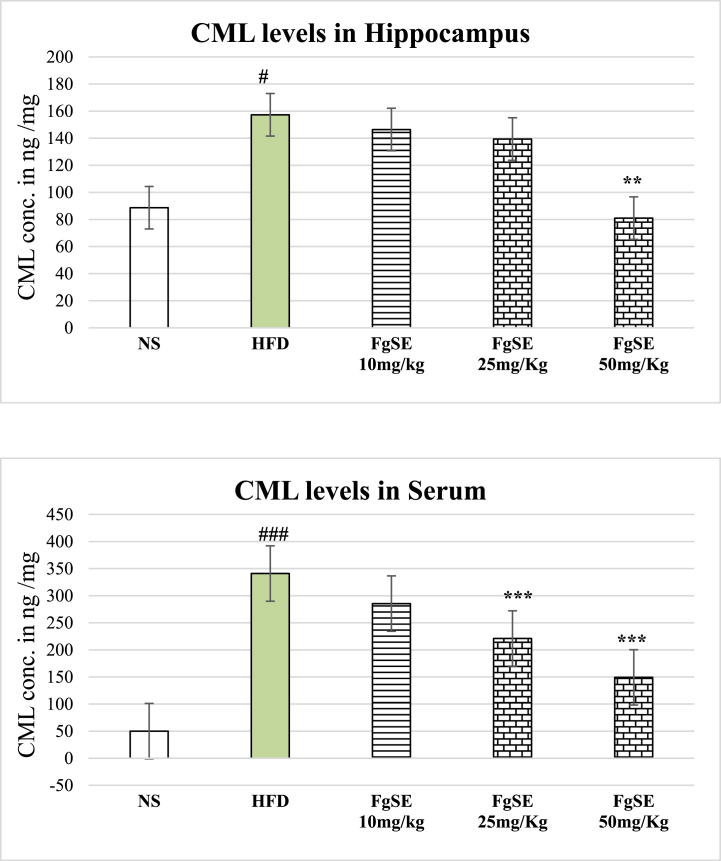
3.11. CML quantification
Our data showed significantly raise CML levels in both hippocampus and Serum of HFD rats (Fig. 10). However, the FgSE treatment has significantly reduced this burden.
Fig. 10.
Effects of Fenugreek Seeds extract on CML levels of Hippocampus and Serum of rats.
The data showed mean ± SEM of CML concentration. The HFD rats showed significant elevated levels of CML in their hippocampus and Serum. However, this CML load was found to be significantly reduced by FgSE treatment in dose dependent fashion. # (p < 0.05) and ## (p < 0.01) represent statistical comparison with NS, while ** (p < 0.01) and *** (p < 0.005) as compared to HFD group.
4. Discussion
Advanced glycation end products are known to be involved in the phenomenon of aging and associated ailments. It is associated with memory loss, cognitive decline and various other neurodegenerative disorders [36]. An easier approach is use of all those food substances that can nullify these effects of aging which are sometimes referred as functional foods [37]. Treatment with food is not a new concept. Ayurveda introduced the concept of “We are what We eat” [38]. Additionally, it's easier to convince large population to switch to healthier food rather consuming anti-aging medicine for lifetime. In today's post COVID world, there is incline towards natural source or remedies as more people are switching towards “organic foods and anti-oxidant supplements” [39]. Keeping this in view, the present study was designed to evaluate the ability of Fenugreek seeds in interfering with the harmful phenomenon of glycation.
Our data showed that FgSE inhibited the process of glycation in vitro (Figure-1), which is in line with the earlier report [40]. This is suggestive of the anti-aging potential of FgSE, Glycation is a complex multi steps phenomenon, which initially involved in the formation of fructosamine adducts. Our data (NBT Assay) showed that FgSE treated reaction mixtures demonstrated significantly lower load of these adducts as compared to glycated control (Figure-2). Furthermore, the glycation reaction proceeds with the interaction of fructose with the lysine amino acid in BSA [41]. The lysine 524 of BSA (equivalent to 525 in HSA) has been believed to be the primary residue involved in the process of glycation [42,43]. Hence, the TNBSA assay was performed to check the availability of free lysine in the BSA. Our data showed that significantly reduced lysine residues were found in glycated samples as compared to control (Figure-3). It is of note that FgSE treated samples exhibited enhanced free lysine availability as compared to glycated samples. This further strengthens the notion that FgSE is endowed with the ability to interfere in the process of glycation.
BSA is a 66 kDa protein, which is primarily composed of α-helical structure [44]. Glycation is known to induce the conformational changes in BSA i.e. it changes the secondary structure ordered amyloid-like or β enriched sheets [45]. In order see the effect of FgSE on this glycation mediated alteration in secondary structure, the CR assay and Circular dichroism was performed. Our CR data showed that glycated samples are rich in amyloid-like structures, while this transformation from α-helical to beta pleated sheets was significantly hampered by the presence of FgSE (Figure-4). It was further analyzed by Microscopy and Birefringence assay. All results affirmed the potential of fenugreek seeds extract as a potential AGE's inhibitor and prevention of protein mis-folding during the process of glycation (Fig. 5).
Circular dichorism is a robust method of determining secondary structures of protein. The native BSA with alpha helical rich structure gave distinct positive band at 192 nm and two negative bands around 208 and 222 nm. In case of glycated samples, the changes in protein conformation to beta sheet formation can easily be identified by the typical positive bands around 195 nm and a negative distinct band at 218 nm [46]. It is of note the Aminoguanidine and FgSE treatments has shown the CD spectra similar to that of native BSA, which is suggestive of preservation of secondary structure (Figure-6).
In order to explore the mechanism of anti-glycation action of FgSE, the OPA assay for lysine blockade and carbonyl entrapment assay was performed. The former OPA binds with non-conjugated epsilon lysine to produce highly fluorescent compound. In case of protection or blockade of lysine, the fluorescence intensity is decreased. Our data suggest that FgSE did not protect the lysine residue from the binding of fructose (Fig. 7). Hence, this is not the underlying mechanism of anti-glycation action of FgSE. Furthermore, the dicarbonyl intermediates, specifically MGO and GO are principal promoters of glycation process [47]. The entrapping of these detrimental compounds is also among of major pharmacological target for impeding the phenomenon of glycation. Our data showed that FgSE entrap MGO and decreased its availability for its derivatization to 2-MQ (Figure-8). The aminoguanidine showed 86% carbonyl trapping, while FgSE has trapped 12%, 20% and 44% of the carbonyl moieties as the dose of 0.1, 0.5 and 1 mg/ml respectively. This suggests that the FgSE inhibited the formation of AGEs through the mechanism of carbonyl entrapment.
As discussed earlier, the glycation leads of impairment in cognitive function as well. This conformational disorder leads to cognitive decline reciprocated in High fructose diet model in our studies using Morris Water Maze (Table-1). It is of note that all markers (escape latency, time to reach target quadrant, time spent in target quadrant and number of crossings through platform position) of cognitive impairment in MWM because of heavy fructose dosing were ameliorated by FgSE treatment in dose dependent fashion. The navigation maps in Fig. 9 also demonstrated that HFD group could not memorize and recall the platform position during the probe trial as indicated by random movement in the MWM pool (Fig. 9-B). On the contrary, the FgSE treated rats at highest dose showed more restricted movement in the platform quadrant much close to the normal rats with preserved memory (Fig. 9-A), which is suggested of preserved learning and memory function in these rats (Figure-9 C).
CML is one of the major advanced glycation end products (AGE) accumulates in pyramidal neurons in the hippocampus in an age‐dependent manner. This suggests a potential link between AGE‐accumulation and the aging process in neurons [48]. This Accumulation of AGEs is strongly related to risk factors for vascular dementia with aging [49]. Hence, following MWM study, CML levels were quantified in both hippocampus and Serum of rats. The data showed significantly elevated CML levels in HFD group as compared to control rats (Figure-10). It is of note that FgSE treated (10, 25 and 50 mg/kg) rats showed significantly lower load of CML. Hence, is can be deduced that the preservation of aforesaid cognitive function in FgSE group is most probably because its anti-glycation ability.
Search of literature revealed that the aforesaid outcome may be contributed by synergism of certain chemical constituents that are present in seeds and are already known anti-aging phytochemical principles [50] such as the trace amounts of Quercetin (0.009–0.012%)is known AGEs and Methylglyoxyl inhibitor [51]. However, according to our observation, the most neglected class of tannins is particularly undermined for their efficacy against glycation. These seeds have ample amount of tannins (2–5%) that are responsible for its antiaging effects via Modulation of NFkB signaling pathway and β-secretase inhibitor [52]. Gentianine of these seeds prevents glycative stress and diabetes complication [53]. Rhaponticin found in these seeds has retino-protective effects in oxidatives stress via NFkB signaling and is anti-glycating agent [54]. Vitexin & Isovitexin present in 3–12.8% are reported as α-glucosidase inhibitor and Isovitexin in a similar study from other food source is reported as AGEs inhibitor [55]. It is interesting to note that its aroma is due to volatile agentslike butanoic acid, isovaleric acid, 3-isopropyl-2-methoxypyrazine, caproic acid, eugenol, 3-Amino-4,5-dimethyl-3, linalool, (Z)-1,5-Octadiene-3-one, 4-dihydro-2(5H)-Furanone [56]. These are reported to be effective as polyphenols in Alzheimer's Disease [56]. Rutin and metabolites are novel AGEs inhibitor [57]. Upon analysis with GCMS we can safely state that this FgSE contains 25 compounds details of which are attached as Appendix 1. Particularly four of them “Maltol [58], Niacin [59], Linoleic acid [28]. & 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl [60]” are reported to be strong anti-aging and inhibit advanced glycation end products. Anti-aging studies & antiglycation effects are also reported for some derivatives of oleamide [61], 1, 2-cyclopentanedione [62], Pantothenic lactone [63]”. This may be termed as “Natural synergism”. Chemical composition may vary with seed type and variety but usual constituents remain same in fenugreek seeds. These anti-oxidant and anti-aging compounds are working together in this FgSE extract for natural synergism that explains observed in vivo and in vitro anti-aging ability in present study.
The major limitation involves the utilization of only one type of sugar for model development and single type of protein in vitro analysis in this study. Since it is edible, it should be tested in clinical settings against the patients of interest with diabetic complications and cognitive decline. There are numerous unexplored compounds with rich anti-oxidant properties like Coumaran should be investigated in future for its protentional anti-aging properties.
5. Conclusion
The present study highlights that Fenugreek seeds ethanolic extract is endowed with the ability to inhibit the deleterious phenomenon of glycation. This effect is most likely attribute to entrapment of carbonyl compounds. Hence, if efficiently incorporated in diet, the Fenugreek seeds can serve as a nutraceutical against aging and associated morbidities.
Author contribution statement
Laila Anwar: Performed the experiments; Wrote the paper.
Syed Abid Ali, Ghulam Abbas: Conceived and designed the experiments; Analyzed and interpreted the data.
Sana Khan, Mir Muhammad Uzairullah, Nazish Mustafa: Performed the experiments; Analyzed and interpreted the data.
Urooj Anwer Ali, Syed Jawad Rehmani: Analyzed and interpreted the data; Wrote the paper.
Faheema Siddiqui, Huma Aslam Bhatti: Contributed reagents, materials, analysis tools or data; Analyzed and interpreted the data.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e16866.
Appendix 1.
Phytochemical Analysis by GC-MS
| Compound Name | Molecular weight | Molecular formula | Retention time | ID number | % Peak area | Chemical structure | |
|---|---|---|---|---|---|---|---|
| 1 | 1,2- Cyclopentanedione | 98 | C5H6O2 | 6.959 | 60,722 | 0.83 | 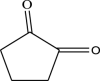 |
| 2 | Pantolactone Or Pantothenic lactone | 130 | C6H10O3 | 10.665 | 8012 | 0.27 | 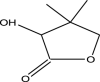 |
| 3 | Maltol | 126 | C6H6O3 | 11.303 | 17,332 | 0.25 | 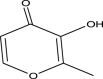 |
| 4 | 2(5H)-Furanone, 3-hydroxy-4,5-dimethyl | 128 | C6H8O3 | 11.93 | 45,390 | 0.07 | 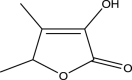 |
| 5 | 4H-Pyran-4-one,2,3-dihydro-3,5-dihydroxy-6-methyl | 144 | C6H8O4 | 12.885 | 1857 | 0.28 | 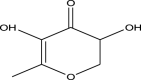 |
| 6 | Benzofuran,2,3-dihydro Or Coumaran | 120 | C8H8O | 14.635 | 16,156 | 1.02 | 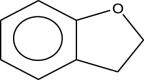 |
| 7 | 2-Furancarboxaldehyde,5-(hydroxymethyl)- | 126 | C6H6O3 | 14.817 | 60,271 | 0.51 |  |
| 8 | Niacin | 123 | C6H5NO2 | 15.209 | 16,856 | 0.68 | 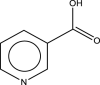 |
| 9 | Methyl 6-oxoheptanoate | 158 | C8H14O3 | 16.146 | 6770 | 0.35 | 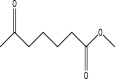 |
| 10 | 1-pyrrolid-2-one, N-carboxyhydrazide | 143 | C5H9N3O2 | 17.817 | 45,666 | 0.12 | 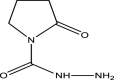 |
| 11 | N-[3-Hexylaminopropyl] aziridine | 184 | C11H24N2 | 18.248 | 14,285 | 0.47 | 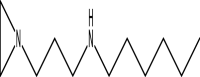 |
| 12 | α-[2-[N-Aziridyl]ethylamino] isobutyronitrile | 153 | C8H15N3 | 18.498 | 4530 | 0.21 | 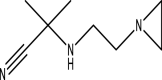 |
| 13 | 2-Carboxymethyl-3-methyl-cyclopentanecarboxylic acid | 186 | C9H14O4 | 20.742 | 43,577 | 0.09 | 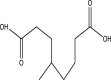 |
| 14 | Cyclohexane-propanoic acid,3,4-dihydroxy | 188 | C9H16O4 | 20.889 | 57,609 | 0.3 | 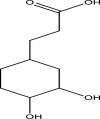 |
| 15 | α-d-Glucopyranoside,methyl | 194 | C7H14O6 | 22.935 | 27,200 | 52.53 | 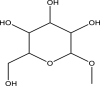 |
| 16 | 3-O-Methyl-d-glucose | 194 | C7H14O6 | 25.048 | 35,456 | 39.2 | 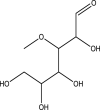 |
| 17 | n-Hexadecanoic acid | 256 | C16H32O2 | 27.204 | 8479 | 0.3 | 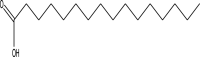 |
| 18 | Carbamic acid, [3-(triethoxysilyl)propyl]-, 5-methyl-2-(1-methylethyl)cyclohexylester | 403 | C20H41NO5Si | 29.235 | 129,958 | 0.73 | 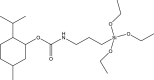 |
| 19 | 9,12-Octadecadienoic acid (z,z) Or Linoleic acid | 280 | C18H32O2 | 30.678 | 7212 | 0.55 | 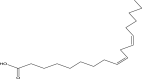 |
| 20 | Di-n-octyl phthalate | 390 | C24H38O4 | 49.537 | 19,967 | 0.32 |  |
| 21 | 9-Octadecenamide, (Z)- Or Oleamide | 281 | C18H35NO | 53.482 | 6574 | 0.07 |  |
| 22 | Methannone, bis[4-(dimethylamino)phenyl]- | 268 | C17H20N2O | 54.233 | 290,948 | 0.39 |  |
| 23 | 9,12-Octadecadienoic acid (z,z)-,2-hydroxy-1-(hydroxymethyl)ethyl ester | 354 | C21H38O4 | 55.68 | 28,833 | 0.12 |  |
| 24 | 9,12,15-Octadecadienoic acid, 2-[(trimethylsilyl)-1-[[(trimethylsilyl)oxy]methyl]ethyl ester, (Z,Z,Z) | 496 | C27H52O4Si2 | 59.757 | 3312 | 0.1 |  |
| 25 | Androst-7-ene-6,17-dione,2,3,14-trihydroxy-, (2β,3β,5α) Or Rubrosterone | 334 | C19H26O5 | 62.298 | 5584 | 0.24 | 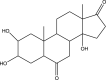 |
Appendix A. Supplementary data
The following are the Supplementary data to this article.
figs1.
References
- 1.Quist A., et al. Amyloid ion channels: a common structural link for protein-misfolding disease. Proc. Natl. Acad. Sci. U. S. A. 2005;102(30):10427–10432. doi: 10.1073/pnas.0502066102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross S.M. Sugar-induced aging: the deleterious effects of excess dietary sugar intake. Holist. Nurs. Pract. 2015;29(2):114–116. doi: 10.1097/HNP.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 3.Li J., et al. Advanced glycation end products and neurodegenerative diseases: mechanisms and perspective. J. Neurol. Sci. 2012;317(1–2):1–5. doi: 10.1016/j.jns.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed N. Advanced glycation endproducts—role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005;67(1):3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Luevano-Contreras C., Chapman-Novakofski K. Dietary advanced glycation end products and aging. Nutrients. 2010;2(12):1247–1265. doi: 10.3390/nu2121247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Münch G., et al. Advanced glycation endproducts in ageing and Alzheimer's disease. Brain Res. Rev. 1997;23(1–2):134–143. doi: 10.1016/s0165-0173(96)00016-1. [DOI] [PubMed] [Google Scholar]
- 7.Semba R.D., Nicklett E.J., Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J. Gerontol. Series A: Bio. Sci. Med. Sci. 2010;65(9):963–975. doi: 10.1093/gerona/glq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamada M., et al. Systemic amyloid deposition in old age and dementia of Alzheimer type: the relationship of brain amyloid to other amyloid. Acta Neuropathol. 1988;77(2):136–141. doi: 10.1007/BF00687423. [DOI] [PubMed] [Google Scholar]
- 9.Uribarri J., et al. Diet‐derived advanced glycation end products are major contributors to the body's AGE pool and induce inflammation in healthy subjects. Ann. N. Y. Acad. Sci. 2005;1043(1):461–466. doi: 10.1196/annals.1333.052. [DOI] [PubMed] [Google Scholar]
- 10.Farooqui A.A. Metabolic Syndrome. Springer; 2013. Glucose-and fructose-induced toxicity in the liver and brain; pp. 35–66. [Google Scholar]
- 11.Singh V.P., et al. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014;18(1):1–14. doi: 10.4196/kjpp.2014.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ott C., et al. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411–429. doi: 10.1016/j.redox.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheetz M.J., King G.L. Molecular understanding of hyperglycemia's adverse effects for diabetic complications. JAMA. 2002;288(20):2579–2588. doi: 10.1001/jama.288.20.2579. [DOI] [PubMed] [Google Scholar]
- 14.Lukic I.K., et al. The RAGE pathway. Ann. N. Y. Acad. Sci. 2008;1126(1):76–80. doi: 10.1196/annals.1433.059. [DOI] [PubMed] [Google Scholar]
- 15.Oeppen J., Vaupel J.W. Broken limits to life expectancy. Science. 2002;296(5570):1029–1031. doi: 10.1126/science.1069675. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization . World Health Organization; 2012. World Health Day 2012: Ageing and Health: Toolkit for Event Organizers. [Google Scholar]
- 17.Lobo V., et al. Free radicals, antioxidants and functional foods: impact on human health. Phcog. Rev. 2010;4(8):118. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathers C.D., et al. Healthy life expectancy in 191 countries, 1999. Lancet. 2001;357(9269):1685–1691. doi: 10.1016/S0140-6736(00)04824-8. [DOI] [PubMed] [Google Scholar]
- 19.Hasler C.M. vol. 52. Food Technology-Champaign Then Chicago; 1998. pp. 63–147. (Functional Foods: Their Role in Disease Prevention and Health Promotion). [Google Scholar]
- 20.Sarikhani M., et al. Anti-aging effects of peppermint (Mentha piperita L.) and Shirazi thyme (Zataria multiflora Boiss.) plant extracts. Food Biosci. 2021;41 [Google Scholar]
- 21.Justino A.B., et al. α-Glucosidase and non-enzymatic glycation inhibitory potential of Eugenia dysenterica fruit pulp extracts. Food Biosci. 2020;35 [Google Scholar]
- 22.Sharma R., Raghuram T., Rao N.S. Effect of fenugreek seeds on blood glucose and serum lipids in type I diabetes. Eur. J. Clin. Nutr. 1990;44(4):301–306. [PubMed] [Google Scholar]
- 23.Prema A., et al. Fenugreek seed powder nullified aluminium chloride induced memory loss, biochemical changes, Aβ burden and apoptosis via regulating Akt/GSK3β signaling pathway. PLoS One. 2016;11(11) doi: 10.1371/journal.pone.0165955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bafadam S., et al. Trigonella foenum-graceum seed (Fenugreek) hydroalcoholic extract improved the oxidative stress status in a rat model of diabetes-induced memory impairment. Horm. Mol. Biol. Clin. Invest. 2019;39(2) doi: 10.1515/hmbci-2018-0074. [DOI] [PubMed] [Google Scholar]
- 25.Anisimov V.N. Metformin: do we finally have an anti-aging drug? Cell Cycle. 2013;12(22):3483–3489. doi: 10.4161/cc.26928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Assad T., Khan R.A., Rajput M.A. Effect of Trigonella foenum-graecum Linn. seeds methanol extract on learning and memory. Metab. Brain Dis. 2018;33(4):1275–1280. doi: 10.1007/s11011-018-0235-1. [DOI] [PubMed] [Google Scholar]
- 27.Tewari D., et al. Fenugreek (Trigonella foenum-graecum L.) seeds dietary supplementation regulates liver antioxidant defense systems in aging mice. Nutrients. 2020;12(9):2552. doi: 10.3390/nu12092552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan S.A., et al. Gamma-linolenic acid ameliorated glycation-induced memory impairment in rats. Pharmaceut. Biol. 2017;55(1):1817–1823. doi: 10.1080/13880209.2017.1331363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cayot P., Tainturier G. The quantification of protein amino groups by the trinitrobenzenesulfonic acid method: a reexamination. Anal. Biochem. 1997;249(2):184–200. doi: 10.1006/abio.1997.2161. [DOI] [PubMed] [Google Scholar]
- 30.Buhimschi I.A., et al. Protein misfolding, congophilia, oligomerization, and defective amyloid processing in preeclampsia. Sci. Transl. Med. 2014;6(245):245ra92. doi: 10.1126/scitranslmed.3008808. [DOI] [PubMed] [Google Scholar]
- 31.Nilsson M.R. Techniques to study amyloid fibril formation in vitro. Methods. 2004;34(1):151–160. doi: 10.1016/j.ymeth.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Gil M.I., et al. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000;48(10):4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- 33.Goodno C.C., Swaisgood H.E., Catignani G.L. A fluorimetric assay for available lysine in proteins. Anal. Biochem. 1981;115(1):203–211. doi: 10.1016/0003-2697(81)90547-9. [DOI] [PubMed] [Google Scholar]
- 34.Kang J., et al. Chromatographic fingerprint analysis and characterization of furocoumarins in the roots of Angelica dahurica by HPLC/DAD/ESI-MS n technique. J. Pharmaceut. Biomed. Anal. 2008;47(4):778–785. doi: 10.1016/j.jpba.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Vorhees C.V., Williams M.T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006;1(2):848. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson J.L., et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain. 2018;141(7):2181–2193. doi: 10.1093/brain/awy146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nirmala C., Bisht M.S., Laishram M. Bioactive compounds in bamboo shoots: health benefits and prospects for developing functional foods. Int. J. Food Sci. Technol. 2014;49(6):1425–1431. [Google Scholar]
- 38.Morrison J. Simon and Schuster; 1995. The Book of Ayurveda. [Google Scholar]
- 39.Vecchio R., Cavallo C. Increasing healthy food choices through nudges: a systematic review. Food Qual. Prefer. 2019;78 [Google Scholar]
- 40.Abeysekera W. Anti-glycation and glycation reversing potential of fenugreek (Trigonella foenum-graecum) seed extract. Biomed. J. 2018;2:5. [Google Scholar]
- 41.Alam M.M., Ahmad I., Naseem I. Inhibitory effect of quercetin in the formation of advance glycation end products of human serum albumin: An in vitro and molecular interaction study. Int. J. Bio. Macromol. 2015;79:336–343. doi: 10.1016/j.ijbiomac.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 42.Garlick R.L., Mazer B.C. The principal site of nonenzymatic glycosylation of human serum albumin in vivo. J. Biol. Chem. 1983;258(10):6142–6146. [PubMed] [Google Scholar]
- 43.Arasteh A., et al. Glycated albumin: an overview of the in vitro models of an in vivo potential disease marker. J. Diabetes Metab. Disord. 2014;13(1):49. doi: 10.1186/2251-6581-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Babcock J.J., Brancaleon B.M. Bovine serum albumin oligomers in the E-and B-forms at low protein concentration and ionic strength. Int. J. Biol. Macromol. 2013;53:42–53. doi: 10.1016/j.ijbiomac.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iannuzzi C., Irace G., Sirangelo I. Differential effects of glycation on protein aggregation and amyloid formation. Front. Mol. Biosci. 2014;1:9. doi: 10.3389/fmolb.2014.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greenfield N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2006;1(6):2876–2890. doi: 10.1038/nprot.2006.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schalkwijk C.G., et al. Induction of 1, 2-dicarbonyl compounds, intermediates in the formation of advanced glycation end-products, during heat-sterilization of glucose-based peritoneal dialysis fluids. Perit. Dial. Int. 1999;19(4):325–333. [PubMed] [Google Scholar]
- 48.Jono T., et al. Accumulation of imidazolone, pentosidine and Nϵ‐(carboxymethyl) lysine in hippocampal CA4 pyramidal neurons of aged human brain. Pathol. Int. 2002;52(9):563–571. doi: 10.1046/j.1320-5463.2002.01390.x. [DOI] [PubMed] [Google Scholar]
- 49.Yanar K., et al. Biogerontology; 2020. Novel Biomarkers for the Evaluation of Aging-Induced Proteinopathies; pp. 1–18. [DOI] [PubMed] [Google Scholar]
- 50.Wani S.A., Kumar P. Fenugreek: a review on its nutraceutical properties and utilization in various food products. J. Saudi . Agric. Sci. 2018;17(2):97–106. [Google Scholar]
- 51.Li X., et al. Quercetin inhibits advanced glycation end product formation by trapping methylglyoxal and glyoxal. J. Agric. Food Chem. 2014;62(50):12152–12158. doi: 10.1021/jf504132x. [DOI] [PubMed] [Google Scholar]
- 52.Mori T., et al. Tannic acid is a natural β-secretase inhibitor that prevents cognitive impairment and mitigates Alzheimer-like pathology in transgenic mice. J. Biol. Chem. 2012;287(9):6912–6927. doi: 10.1074/jbc.M111.294025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qais F.A., et al. New Look to Phytomedicine. Elsevier; 2019. Understanding biochemical and molecular mechanism of complications of glycation and its management by herbal medicine; pp. 331–366. [Google Scholar]
- 54.Shi Q., et al. Effects of rhaponticin on retinal oxidative stress and inflammation in diabetes through NRF2/HO‐1/NF‐κB signalling. J. Biochem. Mol. Toxicol. 2020 doi: 10.1002/jbt.22568. [DOI] [PubMed] [Google Scholar]
- 55.Peng X., et al. Inhibitory effect of mung bean extract and its constituents vitexin and isovitexin on the formation of advanced glycation endproducts. Food Chem. 2008;106(2):475–481. [Google Scholar]
- 56.Reddy V.P., et al. Polyphenols in Alzheimer's disease and in the gut–brain Axis. Microorganisms. 2020;8(2):199. doi: 10.3390/microorganisms8020199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pashikanti S., et al. Rutin metabolites: novel inhibitors of nonoxidative advanced glycation end products. Free Radic. Biol. Med. 2010;48(5):656–663. doi: 10.1016/j.freeradbiomed.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 58.Sha J.y., et al. The p53/p21/p16 and PI3K/Akt signaling pathways are involved in the ameliorative effects of maltol on D‐galactose‐induced liver and kidney aging and injury. Phytother Res. 2021;35(8):4411–4424. doi: 10.1002/ptr.7142. [DOI] [PubMed] [Google Scholar]
- 59.Radicals, F., Nutritional and Botanical Approaches to Anti-aging.
- 60.Sothearith Y., et al. Evaluation of allelopathic potentials from medicinal plant species in Phnom Kulen national park, Cambodia by the sandwich method. Sustainability. 2020;13(1):264. [Google Scholar]
- 61.Bogdanowicz P., et al. Results from in vitro and ex vivo skin aging models assessing the antiglycation and anti-elastase MMP-12 potential of glycylglycine oleamide. Clin. Cosmet. Invest. Dermatol. 2016;9:143. doi: 10.2147/CCID.S98633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chung J.H., et al. 3-Methyl-1, 2-cyclopentanedione down-regulates age-related NF-κB signaling cascade. J. Agric. Food Chem. 2007;55(16):6787–6792. doi: 10.1021/jf070952p. [DOI] [PubMed] [Google Scholar]
- 63.Khayat Y. Anti-glycation supplements Part III (glycation: Part II of II) Perspectives on Health, Jan. 2015;26:1–52. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.