Abstract
Purpose: To investigate the clinical characteristics, pathological features, and outcomes of patients with antineutrophil cytoplasmic antibody (ANCA)-positive systemic lupus erythematosus (SLE) in northwest China.Methods: This retrospective study included 491 patients with SLE tested for ANCA antibodies and 171 patients with ANCA-associated vasculitis (AAV) as controls. Subgroup analysis limited to those with renal involvement, and by ANCA antibody subtype (PR3 vs MPO). To compare the proteinuria remission rates between ANCA-positive and ANCA-negative lupus nephritis (LN) groups, a logistic regression model was used for propensity score matching based on age, hemoglobin, and baseline estimated glomerular filtration rate (eGFR).Results: Compared to ANCA-negative SLE (n = 442), ANCA-positive SLE (n = 46) occur in older patients; however, these patients were younger than those with AAV (n = 167). The eGFR of patients with ANCA-positive LN (n = 25) was higher than that of patients having AAV with renal involvement (n = 56) but lower than that of patients with ANCA-negative LN (n = 163). Patients with SLE who had MPO-ANCA (n = 16) had higher levels of serum creatinine compared to those with PR3-ANCA (n = 30) (156.5 µmol/L vs. 45.5 µmol/L, p = 0.005). During the follow-up period, the remission rate of proteinuria in patients with ANCA-positive LN was lower than that of patients with ANCA-negative LN (50% vs. 75%, p = 0.008).Conclusion: Patients with ANCA-positive LN may have worse baseline renal function and lower protein remission rates compared to patients with ANCA-negative LN. ANCA titers should be regularly monitored throughout the follow-up period in patients with SLE, especially in cases of renal involvement.
Keywords: Anti-neutrophil cytoplasmic antibodies, clinicopathological characteristics, lupus nephritis, systemic lupus erythematosus
1. Introduction
Systemic lupus erythematosus (SLE) is a common, complex, multisystem autoimmune disease that affects multiple organs. Lupus nephritis (LN) is one of the most frequent and serious complications of SLE that affects 30%–60% of patients with SLE [1]. LN is characterized by a series of inflammatory responses triggered by the deposition of immune complexes in the glomeruli.
Antineutrophil cytoplasmic autoantibodies (ANCAs) are unique autoantibodies that target cytoplasmic components of human neutrophils, including protease (PR3) and myeloperoxidase (MPO) [2], and are primarily associated with small-vessel vasculitis [3]. ANCAs are detectable in 15%–20% of patients with SLE [4], especially those with LN [5]. Several studies have suggested that ANCAs are associated with diffuse proliferative LN, especially class IV segment-type LN [6]. However, the role of ANCAs in SLE, especially LN, and their association with the disease activity, histological features, and prognosis of SLE remain controversial [7–9].
In our study, we analyzed the clinical characteristics of patients with SLE with co-existing ANCA positivity and compared these characteristics with those of patients with ANCA-negative SLE and AAV. Additionally, we conducted subgroup analyses based on renal involvement and ANCA serum types to clarify the role of ANCA in SLE.
2. Methods
2.1. Patients
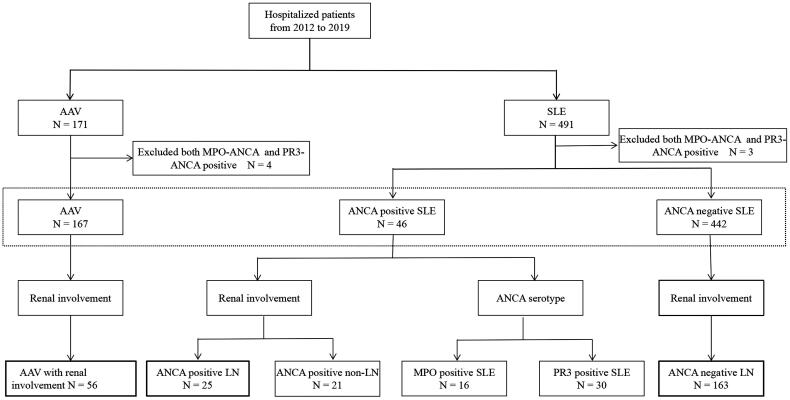
For this study, we recruited a total of 491 patients diagnosed with SLE who had their ANCA serology assessed between December 2012 and October 2019. Additionally, we selected 171 patients diagnosed with AAV as the control group. Patient data were obtained through the electronic medical record database of the First Affiliated Hospital of Xi’an Jiaotong University (Figure 1).
Figure 1.
Flowchart of study participants note: ANCA: antineutrophil cytoplasmic antibody; SLE: systemic lupus erythematosus; AAV: ANCA-associated vasculitis; MPO: myeloperoxidase; PR3: proteinase 3.
The inclusion criteria for SLE, with or without ANCA positivity, were as follows: (1) meeting the SLE grading criteria established by the American College of Rheumatology [10] and (2) positive anti-MPO and anti-PR3 titers by ELISA, defining ANCA positivity [11]. Patients with incomplete medical records were excluded from the study. None of the selected patients were using medications known to cause ANCA positivity, such as propylthiouracil, isoniazid, or hydralazine. After excluding three patients who were MPO-positive and PR3-positive in the selected group of patients with SLE, the remaining patients were divided into two groups based on their ANCA serology: ANCA-positive SLE (n = 46) and ANCA-negative SLE (n = 442). Within the ANCA-positive SLE group, patients were further divided into those with renal involvement (n = 25) and those without renal involvement (n = 21), based on the 2021 Kidney Disease Improving Global Outcomes guidelines [12]. The renal involvement group consisted of patients with ≥2+ proteinuria determined by dipstick protein, a spot urine protein to creatinine ratio >500 mg/g, or those confirmed as having renal involvement by renal biopsy. Furthermore, within the renal involvement group, patients were divided into the following subgroups based on elevated titers of anti-PR3 or anti-MPO antibodies: MPO-positive (n = 16) and PR3-positive (n = 30). In the ANCA-negative SLE group, patients were further divided into ANCA-negative LN (n = 163) and non-LN groups based on the presence or absence of renal involvement.
Patients with AAV met the criteria established by the Chapel Hill Consensus Conference for the definition of AAV [13]. Patients with secondary vasculitis or those with anti-glomerular basement membrane antibodies were excluded. From the AAV group, 56 patients who had undergone renal biopsies were chosen as the subset control group after excluding four patients who tested positive for both MPO and PR3 antibodies.
2.2. Clinical and laboratory data
Demographic and clinical data, including age, sex, medical history, and duration of follow-up were retrospectively analyzed. Laboratory data collected included hemoglobin (HGB), white blood cell (WBC) count, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), serum albumin and serum creatinine (CREA) levels, 24-h urine protein, serum immunoglobulin G (IgG), serum immunoglobulin A (IgA), serum immunoglobulin M (IgM), complement C3 and complement C4 levels, serum antinuclear antibody (ANA), serum anti-dsDNA antibody and its titer, and ANCA serotype. ANCA titers, dsDNA titers, and creatinine levels were collected from patients with ANCA-positive SLE who had undergone multiple tests during their hospital visits. The estimated glomerular filtration rate (eGFR) was determined using the Chronic Kidney Disease Epidemiology Partnership study equation [14]. The duration of follow-up was determined from the first hospitalization due to ANCA-positive SLE to the date of the last follow-up (up to August 2, 2021). The follow-up period for patients with ANCA-negative LN started at the time of renal biopsy during hospitalization and continued until the same cutoff date as for ANCA-positive SLE. The follow-up procedures for ANCA-positive SLE and ANCA-negative SLE were consistent. The primary endpoint of the study was death, and the secondary endpoint was defined as end-stage renal disease (ESRD). ESRD was defined as the requirement of continuous dialysis or kidney transplantation, or an eGFR ≤15 mL/min/1.73 m2. Death and ESRD were considered composite endpoints. Urinary protein remission rate was defined as the remission of proteinuria by greater than 50% from the baseline value or a final proteinuria level of less than 0.5 g/24 h [15]. If a patient was lost to follow-up, the duration of follow-up was based on the data available in the last medical record.
2.3. Renal histology
Renal tissue samples were obtained using ultrasound-guided percutaneous renal biopsy. Under a light microscope, paraffin sections of the samples were stained with hematoxylin and eosin, Masson’s trichrome stain, periodic acid-Schiff, and periodic acid silver methenamine. These stains enabled the visualization of different tissue aspects such as glomerular hyperplasia, necrosis and exudative lesions, the basement membrane, glomerular thrombosis, and tubulointerstitial inflammation. The renal cortex was examined by electron microscopy to verify the location of the deposits. LN was classified according to the criteria established by the International Society of Nephrology and the Society of Renal Pathology. The disease activity of LN and chronic index (CI) were also assessed. Standard immunofluorescence microscopy was employed to stain immunoglobulin (Ig) G, IgA, IgM, C3, and C1q antigens. The staining intensity was semi-quantitatively scored on a scale ranging from 0 to 3 + [16].
2.4. Statistical analysis
Statistical analyses were conducted using SPSS version 26.0 (IBM Corp, Armonk, NY, USA) and GraphPad Prism 8 (version 8.0.2; GraphPad Software, La Jolla, CA, USA). Continuous variables were expressed as mean ± standard deviation or median (interquartile range, IQR), depending on data distribution. Categorical variables were described in terms of frequency and percentage. The normality of data distribution was assessed using the single-sample Kolmogorov–Smirnov test. For comparison among three groups with variables that conformed to a normal distribution, a one-way analysis of variance (ANOVA) was used, and least significance difference (LSD) or Tamhane T2 test was used for post hoc comparison if the results were statistically significant. The Kruskal-Wallis test was used to compare the three groups of skewed distribution variables, then variations of statistical significance were further subjected to post hoc pairwise analysis by applying the Bonferroni’s correction. To compare the normal distribution or skew distribution variables between the two groups of independent samples, the t-test or the Mann–Whitney U test were used, respectively. The Chi-square test or Fisher’s exact test was used to compare categorical variables, where appropriate. Correlation analysis between ANCA titers and clinical characteristics was performed using Spearman’s rank correlation coefficient.
Kaplan–Meier survival analysis was used to compare patient survival between the various SLE subgroups (ANCA-positive LN vs. ANCA-negative LN, MPO-positive SLE vs. PR3-positive SLE). To compare proteinuria remission rates between ANCA-positive and ANCA-negative LN groups, a propensity score-matched analysis was conducted to reduce the effects of bias, using a logistic regression model based on variables such as age, HGB, and baseline eGFR. Pairs of ANCA-positive and ANCA-negative LN groups were created using greedy nearest neighbor matching with a propensity score caliper of 0.02 [17]. A P-value < 0.05 indicated statistical significance.
3. Results
3.1. Comparison of clinical characteristics between patients with ANCA-positive SLE, ANCA-negative SLE, and AAV
The clinical and laboratory features of the patients in the ANCA-positive SLE, ANCA-negative SLE, and AAV groups are shown in Table 1. Overall, significant differences were observed among the three groups in terms of age, male gender, WBC count, HGB, serum albumin, CRP, ESR, CREA, eGFR, IgG, IgA, and C3 and C4 levels (p < 0.05). Patients in the AAV group had a higher mean age (64.41 ± 12.05 years) compared to those in the ANCA-positive SLE (43.76 ± 14.23 years) and ANCA-negative SLE (39.18 ± 14.21 years) groups, and this difference was statistically significant. On conducting multiple comparisons, we observed that the ANCA-positive SLE group had significantly lower levels of HGB (96.28 ± 19.79 g/L) compared to the ANCA-negative SLE group (103.36 ± 22.15 g/L). Moreover, the ANCA-positive SLE group had higher levels of IgG (20.24 ± 10.12 g/L) compared to the ANCA-negative SLE group (14.87 ± 7.04 g/L). Furthermore, the ANCA-positive SLE group had lower eGFR compared to the ANCA-negative SLE group (107.72 [IQR, 43.13–131.27] mL/min/1.73 m2 vs. 112.59 [IQR, 79.50–128.00] mL/min/1.73 m2), although the difference was not statistically significant. The positivity rates of anti-MPO and anti-PR3 antibodies were lower in the ANCA-positive SLE group (34.8% and 65.2%, respectively) compared to the AAV group (76.1% and 9.0%, respectively), and these differences were statistically significant (p = 0.000). The levels of anti-MPO and anti-PR3 antibodies were significantly lower in the ANCA-positive SLE group (68.16 [IQR, 48.58–106.30] RU/mL and 56.70 [IQR, 32.96–83.27] RU/mL, respectively) than in the AAV group (148.54 [IQR, 65.95–211.22] RU/mL and 290.52 [IQR, 130.39–396.43] RU/mL, respectively; p = 0.003 and p = 0.000, respectively).
Table 1.
Comparison of clinical characteristics between patients with ANCA-positive SLE, ANCA-negative SLE, and AAV.
| Variable | ANCA-positive SLE N = 46 | ANCA-negative SLE N = 442 | AAV N = 167 | P-value |
|---|---|---|---|---|
| Age (years) | 43.76 ± 14.23a | 39.18 ± 14.21b | 64.41 ± 12.05c | 0.000 |
| Male, n (%) | 2 (4.1)ab | 56 (12.7)b | 97 (56.7)c | 0.000 |
| WBC (× 109/L) | 4.25 (2.74,5.56)ab | 4.76 (3.35, 7.23)b | 7.50 (5.88, 10.47)c | 0.000 |
| HGB (g/L) | 96.28 ± 19.79ac | 103.36 ± 22.15b | 98.44 ± 23.21c | 0.011 |
| Serum albumin (g/L) | 37.66 ± 11.76a | 33.34 ± 10.36bc | 34.36 ± 7.74c | 0.015 |
| CRP (mg/L) | 3.30 (3.30, 18.25)ab | 9.90 (3.30, 15.20)b | 36.75 (11.10, 81.78)c | 0.000 |
| ESR (mm/h) | 54.75 ± 33.12abc | 42.52 ± 32.85b | 64.47 ± 35.24c | 0.000 |
| CREA (µmol/L) | 52.00 (41.50, 131.50)ab | 57.00 (46.00, 80.00)b | 185.00 (73.00, 438.50)c | 0.000 |
| eGFR (mL/min/1.73m2) | 107.72 (43.13, 131.27)ab | 112.59 (79.50, 128.00)b | 28.06 (10.05, 80.00)c | 0.000 |
| 24-h urine protein (g) | 0.425 (0.10, 1.46) | 1.13 (0.14, 2.50) | 0.91 (0.34, 1.90) | 0.179 |
| IgG, g/L | 20.24 ± 10.12a | 14.87 ± 7.04bc | 14.82 ± 5.13c | 0.000 |
| IgM, g/L | 1.11 (0.80, 1.59) | 0.96 (0.64, 1.41) | 0.91 (0.65, 1.40) | 0.339 |
| IgA, g/L | 3.15 ± 1.48ac | 2.64 ± 1.27bc | 2.76 ± 1.23c | 0.039 |
| C3, g/L | 0.64 ± 0.29ab | 0.60 ± 0.30b | 1.02 ± 0.23c | 0.000 |
| C4, g/L | 0.13 ± 0.08ab | 0.12 ± 0.09b | 0.27 ± 0.12c | 0.000 |
| Anti-dsDNA, n (%) | 21 (55.3) | 99 (41.9) | – | 0.125 |
| Anti-dsDNA titer (IU/mL) | 5.96 (1.17, 71.24.00) | 9.79 (1.88, 51.87) | – | 0.703 |
| Anti-SmD1, n (%) | 20 (54.1) | 64 (27.1) | – | 0.001 |
| ANA, n (%) | 29 (63.0)ab | 220 (56.0)b | 11 (7.1)c | 0.000 |
| Anti-MPO, n (%) | 16 (34.8) | – | 118 (76.1) | 0.000 |
| Anti-PR3, n (%) | 30 (65.2) | – | 14 (9.0) | 0.000 |
| Anti-MPO titer (RU/mL) | 68.16 (48.58, 106.30) | – | 148.54 (65.95, 211.22) | 0.003 |
| Anti-PR3 titer (RU/mL) | 56.70 (32.96, 83.27) | – | 290.52 (130.39, 396.43) | 0.000 |
NOTE: a, b, and c represent the results of multiple comparisons between groups.
ANCA: antineutrophil cytoplasmic antibody; SLE: systemic lupus erythematosus; AAV: ANCA-associated vasculitis; WBC: white blood cell; HGB: hemoglobin; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; CREA: serum creatinine; eGFR: estimated glomerular filtration rate; IgG: serum immunoglobulin G, normal values: 7–16 g/L; IgA: serum immunoglobulin A, normal values: 0.7–3.8 g/L; C3: complement 3, normal values: 0.8–1.85 g/L; C4: complement 4; normal values: 0.1–0.4 g/L; ds-DNA: double-stranded DNA antibodies; normal values: 0–7I U/mL; SmD1: Smith antigen D1; ANA: antinuclear antibody; MPO: myeloperoxidase; PR3: proteinase 3; anti-MPO titer: normal value: < 20 RU/mL; anti-PR3 titer: normal value: < 20 RU/mL.
3.2. Comparison of clinical characteristics between patients with ANCA-positive LN, ANCA-negative LN, and those having AAV with renal involvement
The clinical characteristics of patients with ANCA-positive LN, ANCA-negative LN, and those having AAV with renal involvement are shown in Table 2. Statistically significant differences were observed among the three groups in terms of age, male gender, WBC count, HGB, CRP, ESR, CREA, eGFR, 24-h urine protein, IgG, and complement C3 and C4 levels (p < 0.05). The ANCA-positive LN group had significantly lower eGFR (54.48 [IQR, 14.81–114.15 mL/min/1.73 m2] compared to the ANCA-negative LN group (105.64 [IQR, 54.95–124.70] mL/min/1.73 m2), whereas it was higher than the eGFR observed in the AAV with renal involvement group (17.89 [IQR, 8.17–34.20] mL/min/1.73 m2).
Table 2.
Comparison of clinical characteristics between patients with ANCA-positive LN, ANCA-negative LN, and AAV with renal involvement.
| Variable | ANCA-positive LN N = 25 |
ANCA-negative LN N = 163 |
AAV with renal involvement N = 56 |
P-value |
|---|---|---|---|---|
| Age (years) | 45.73 ± 14.17a | 35.19 ± 12.60b | 61.54 ± 10.15c | 0.000 |
| Male, n (%) | 1 (3.8)ab | 25 (15.3)b | 35 (62.5)c | 0.001 |
| White blood cell (× 109/L) | 3.97 (2.76, 5.55)ab | 4.53 (3.48, 7.02)b | 7.06 (5.50, 9.79)c | 0.000 |
| HGB (g/L) | 91.54 ± 22.70a | 101.24 ± 21.49b | 89.22 ± 18.44ac | 0.001 |
| Serum albumin (g/L) | 36.21 ± 13.03 | 33.90 ± 12.96 | 37.57 ± 9.22 | 0.138 |
| CRP (mg/L) | 3.00 (3.00, 19.15)ab | 9.90 (9.90, 9.90)b | 45.80 (19.70, 79.60)c | 0.000 |
| ESR (mm/h) | 60.25 ± 36.88ab | 46.92 ± 30.84b | 86.33 ± 26.46c | 0.000 |
| CREA (µmol/L) | 107.95 (49.75, 298.25) ab | 66.00 (48.00, 114.00)b | 266.50 (174.25, 534.75)c | 0.000 |
| eGFR (mL/min/1.73m2) | 54.48 (14.81, 114.15)a | 105.64 (54.95, 124.70)b | 17.89 (8.17, 34.20)c | 0.000 |
| 24h urine protein (g) | 1.36(0.66, 3.39)abc | 2.24 (1.23, 3.75)b | 1.37 (0.79, 2.39)c | 0.001 |
| IgG, g/L | 18.42 ± 9.79ac | 12.41 ± 5.62b | 15.01 ± 5.43c | 0.000 |
| IgM, g/L | 0.85 (0.44, 1.47) | 0.97 (0.61, 1.43) | 0.91 (0.65, 1.33) | 0.901 |
| IgA, g/L | 2.99 ± 1.36 | 2.53 ± 1.20 | 2.67 ± 0.82 | 0.159 |
| C3, g/L | 0.54 ± 0.27ab | 0.49 ± 0.27b | 1.02 ± 0.21c | 0.000 |
| C4, g/L | 0.14 ± 0.09ab | 0.10 ± 0.07b | 0.29 ± 0.08c | 0.000 |
| Anti-dsDNA, n (%) | 12 (50.0) | 25 (53.2) | – | 0.799 |
| Anti-dsDNA titer (IU/mL) | 6.63 (2.29, 100.00) | 63.62 (9.33, 100.00) | – | 0.201 |
| Anti-SmD1, n (%) | 11 (47.8) | 17 (36.2) | – | 0.350 |
| ANA, n (%) | 15 (57.7)ab | 39 (33.6)b | 1 (2.3) | 0.001 |
| Anti-MPO, n (%) | 11 (44.0) | – | 33 (76.7) | 0.006 |
| Anti-PR3, n (%) | 14 (56.0) | – | 8 (18.6) | 0.001 |
| Anti-MPO titer (RU/mL) | 73.63 (63.84, 105.82) | – | 159.42 (106.28, 212.09) | 0.006 |
| Anti-PR3 titer (RU/mL) | 55.20 (31.86, 72.55) | – | 298.78 (138.05, 390.77) | 0.000 |
NOTE: a, b, and c represent the results of multiple comparisons between groups.
ANCA: antineutrophil cytoplasmic antibody; SLE: systemic lupus erythematosus; AAV: ANCA-associated vasculitis; WBC: white blood cell; HGB: hemoglobin; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; CREA: serum creatinine; eGFR: estimated glomerular filtration rate; IgG: serum immunoglobulin G, normal values: 7–16 g/L; IgA: serum immunoglobulin A, normal values: 0.7–3.8 g/L; C3: complement 3, normal values: 0.8–1.85 g/L; C4: complement 4; normal values: 0.1–0.4 g/L; ds-DNA: double-stranded DNA antibodies; normal values: 0–7 IU/mL; SmD1: Smith antigen D1; ANA: antinuclear antibody; MPO: myeloperoxidase; PR3: proteinase 3; anti-MPO titer: normal value: <20 RU/mL; anti-PR3 titer: normal value: <20 RU/mL.
Additionally, the positivity rate for anti-PR3 antibodies was significantly higher in the ANCA-positive LN group than in the AVV group (56.0% vs. 18.6%, p = 0.001). However, the positivity rate for anti-MPO antibodies was lower in the ANCA-positive LN group than in the AVV group (44.0% vs. 76.7%, p = 0.006). The levels of anti-MPO and anti-PR3 antibodies were significantly lower in the ANCA-positive LN group than in the AVV group (73.63 [IQR, 63.84–105.82] vs. 159.42 [IQR, 106.28–212.09] RU/mL; p = 0.006; 55.20 [IQR, 31.86–72.55] vs. 298.78 [IQR, 138.05–390.77] RU/mL; p = 0.000).
3.3. Comparison of clinical characteristics between patients with ANCA-positive LN and ANCA-positive SLE without renal involvement
The clinical and laboratory features of the patients in the ANCA-positive LN and ANCA-positive non-LN groups are shown in Supplementary Table S1. The ANCA-positive LN group had significantly higher levels of CREA (107.95 [IQR, 49.75–298.25] vs. 43.00 [IQR, 37.51–53.00] µmol/L; p = 0.001), higher levels of 24-h urine protein (1.36 [IQR, 0.66–3.39] vs 0.10 [IQR, 0.05–0.25] g/L; p = 0.000), lower eGFR (54.48 [IQR, 14.81–114.15] vs. 123.42 [IQR, 107.44–137.16] mL/min/1.73 m2; p = 0.001), lower levels of IgM (0.85 [IQR, 0.44–1.47] vs. 1.39 [IQR, 1.10–1.91] g/L; p = 0.007), and lower levels of complement C3 (0.54 ± 0.27 vs. 0.75 ± 0.26 g/L; p = 0.011) compared to the ANCA-positive non-LN group.
3.4. Clinical characteristics of patients with ANCA-positive SLE based on ANCA serotypes
Serum CREA levels were significantly higher in the MPO-ANCA group than in the PR3-ANCA group (156.5 [IQR, 45.75–350.75] vs. 45.50 [IQR, 37.76–58.60] µmol/L; p = 0.005), whereas eGFR and IgG levels in the MPO-ANCA group were lower than those in the PR3-ANCA group (36.15 [IQR, 12.43–126.40] vs. 114.11 [IQR, 100.35–131.54] mL/min/1.73 m2; p = 0.015 and 13.80 [IQR, 7.70–23.70] vs. 22.50 [IQR, 14.30–29.10] µmol/L; p = 0.034, respectively). The levels of complement C4 were significantly higher in the MPO-ANCA group than in the PR3-ANCA group (0.17 ± 0.08 vs. 0.12 ± 0.08] g/L; p = 0.037; Table 3).
Table 3.
Comparison of clinical characteristics between patients with MPO-ANCA-positive and PR3-ANCA-positive SLE.
| MPO-ANCA positivity N = 16 | PR3-ANCA positivity N = 30 | P | |
|---|---|---|---|
| Age (years) | 47.88 ± 17.23 | 41.57 ± 12.09 | 0.154 |
| Male, n (%) | 2 (12.5) | 0 (0.0) | 0.116* |
| diagnosis of SLE to ANCA positive (months) | 13.50 (1.75, 114.00) | 12 (4.50, 72.00) | 0.917 |
| WBC (× 109/L) | 3.72 (2.81, 5.69) | 4.32 (2.73, 5.80) | 0.517 |
| HGB (g/L) | 92.29 ± 23.23 | 98.21 ± 18.02 | 0.364 |
| Serum albumin (g/L) | 39.39 ± 14.90 | 36.74 ± 9.86 | 0.529 |
| CRP (mg/L) | 14.20 (9.90, 22.75) | 9.90 (9.90, 14.95) | 0.127 |
| ESR (mm/h) | 57.33 ± 37.47 | 53.74 ± 32.11 | 0.788 |
| CREA (µmol/L) | 156.50 (45.75, 350.75) | 45.50 (37.76, 58.60) | 0.005 |
| eGFR (mL/min/1.73m2) | 36.15 (12.43, 126.40) | 114.11 (100.35, 131,54) | 0.015 |
| 24-h urine protein (g) | 0.96 (0.12, 1.48) | 0.40 (0.08, 1.87) | 0.340 |
| IgG, g/L | 13.80 (7.70, 23.70) | 22.50 (14.30, 29.10) | 0.034 |
| IgM, g/L | 0.89 (0.41, 1.27) | 1.27 (0.85, 1.70) | 0.053 |
| IgA, g/L | 2.56 ± 1.16 | 3.44 ± 1.54 | 0.058 |
| C3, g/L | 0.64 ± 0.25 | 0.64 ± 0.31 | 0.977 |
| C4, g/L | 0.17 ± 0.08 | 0.12 ± 0.08 | 0.037 |
| Anti-dsDNA, n (%) | 8 (57.1) | 13 (54.2) | 0.859 |
| Anti-dsDNA titer (IU/mL) | 2.76 (0.79, 52.98) | 10.92 (1.49, 85.62) | 0.604 |
| Anti-SmD1, n (%) | 5 (35.7) | 15 (65.2) | 0.081 |
| ANA, n (%) | 8 (50.0) | 21 (70.0) | 0.181 |
| Anti-RNP, n (%) | 4 (57.1) | 10 (45.5) | 0.458* |
| Anti-MPO titer (RU/mL) | 83.94 ± 50.14 | – | – |
| Anti-PR3 titer (RU/mL) | – | 56.70 (32.95, 83.27) | – |
Fisher’s exact test.
ANCA: antineutrophil cytoplasmic antibody; SLE: systemic lupus erythematosus; AAV: ANCA-associated vasculitis; WBC: white blood cell; HGB: hemoglobin; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; CREA: serum creatinine; eGFR: estimated glomerular filtration rate; IgG: serum immunoglobulin G, normal values: 7–16 g/L; IgA: serum immunoglobulin A, normal values: 0.7–3.8 g/L; C3: complement 3, normal values: 0.8–1.85 g/L; C4: complement 4; normal values: 0.1–0.4 g/L; ds-DNA: double-stranded DNA antibodies; normal values: 0–7 IU/mL; SmD1: Smith antigen D1; ANA: antinuclear antibody; MPO: myeloperoxidase; PR3: proteinase 3; anti-MPO titer: normal value: <20 RU/mL; anti-PR3 titer: normal value: <20 RU/mL.
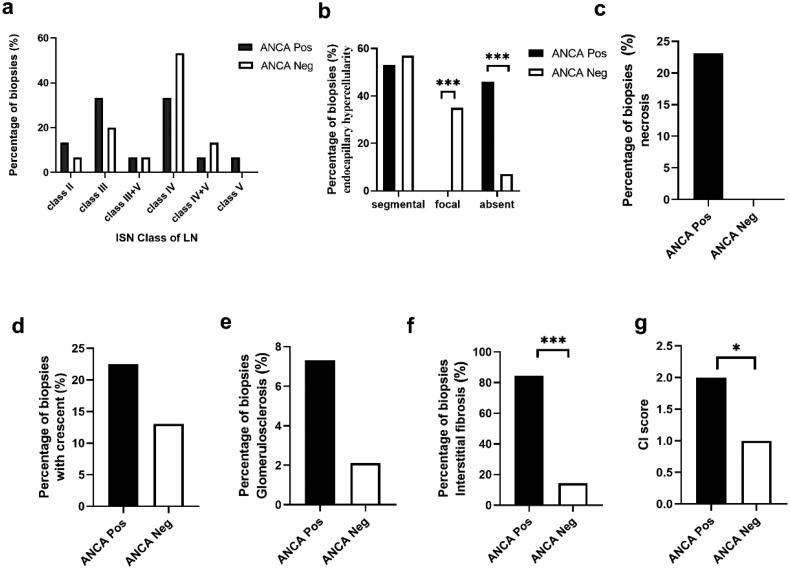
3.5. Evaluation of renal pathology in LN
The characteristics of renal histopathology of LN in patients with and without ANCA are shown in Figure 2. No significant differences were observed in the overall histopathological features of LN between the ANCA-positive and ANCA-negative groups (Figure 2a). However, on comparing biopsy specimens with proliferative LN (class III and class IV), focal endocapillary hypercellularity was not observed in the ANCA-positive LNs, whereas it was observed in 35.7% of ANCA-negative LNs (p < 0.01, Figure 2b). The proportion of tubulointerstitial fibrosis was higher in the ANCA-positive group than in the ANCA-negative group (84.6% vs. 14.3%, p < 0.001, Figure 2f). Although not statistically significant, a higher proportion of biopsy specimens in the ANCA-positive group showed necrosis (23.0% vs. 0%), crescent formation (22.5% vs. 13.0%), and glomerulosclerosis (7.3% vs. 2.1%) compared to the ANCA-negative LN group (Figure 2c–e). Moreover, patients with ANCA-positive LN had higher median CI scores compared to those with ANCA-negative LN (2.1 vs. 0.9, p = 0.017, Figure 2g).
Figure 2.
Histopathological features of lupus nephritis (LN). (a) Percentage of biopsy specimens for each type of LN in relation to antineutrophil cytoplasmic antibody (ANCA) positivity and negativity. (b–e) Image shows only Class III and IV LN (i.e. proliferative LN). Comparison of the patterns of (b) endocapillary hypercellularity, (c) necrosis, (d) crescents, (e) glomerulosclerosis, and (f) interstitial fibrosis. (g) Shows a comparison of the chronic index scores. *P < 0.05; ***P < 0.01
Immunofluorescence staining revealed that the prevalence of immune complex deposition was lower in the ANCA-positive LN group than in the ANCA-negative LN group. Furthermore, the ANCA-positive LN group exhibited a lower average intensity of IgG (p = 0.013) on renal biopsy specimens compared to the ANCA-negative LN group (Supplementary Table S2).
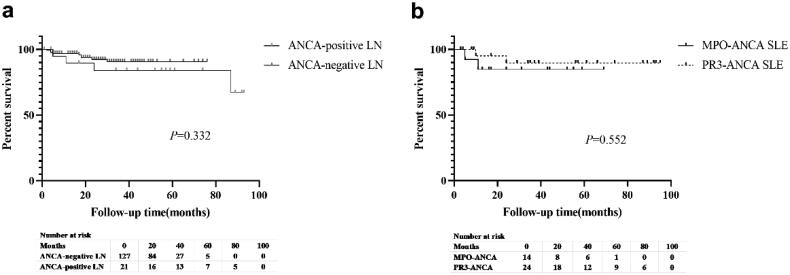
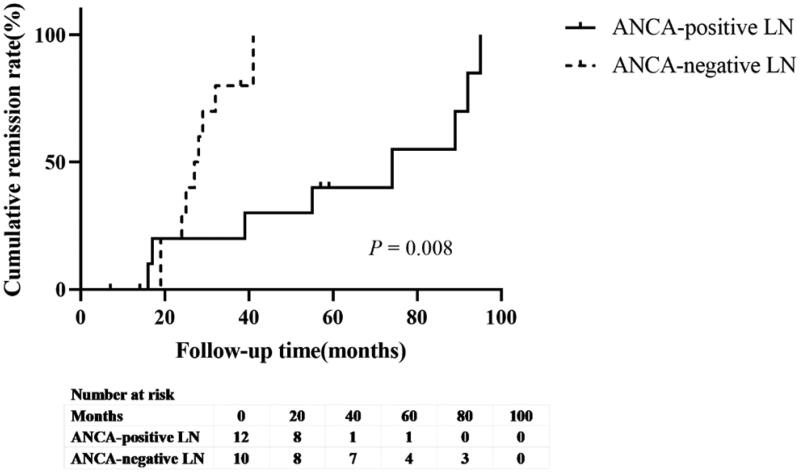
3.6. Long-term outcomes
In the ANCA-positive group, 7 patients were lost to follow-up and 39 patients were followed-up for a duration ranging from 3 to 95 (mean 42.42 ± 29.32) months. At the end of the study, four patients died and three patients underwent maintenance hemodialysis. The difference in the time to reach composite endpoints between the ANCA-positive and ANCA-negative LN groups was not statistically significant (p = 0.332; Figure 3a). Similarly, no significant difference was observed in the long-term outcomes between the MPO-ANCA and PR3-ANCA groups (p = 0.552; Figure 3b). To compare the outcomes of patients with ANCA-positive LN and those with ANCA-negative LN who had a similar risk of poor outcomes, the patients were matched based on age, HGB, and baseline eGFR using propensity scoring at a 1:1 ratio of patients with ANCA-negative to those with ANCA-positive LN. The proteinuria remission rate in patients with ANCA-positive LN was lower than that in patients with ANCA-negative LN patients (50% vs. 75%, p = 0.008, Figure 4).
Figure 3.
Kaplan–Meier survival curves comparing survival between the (a) antineutrophil cytoplasmic antibody (ANCA)-positive lupus nephritis (LN) group (25 patients) and the ANCA-negative LN group (163 patients) and (b) myeloperoxidase ANCA (16 patients) and proteinase 3-ANCA (30 patients) groups among patients with systemic lupus erythematosus.
Note: Survival refers to both kidney and patient survival.
Figure 4.
Kaplan–Meier survival curve of proteinuria remission rate between the antineutrophil cytoplasmic antibody (ANCA)-positive lupus nephritis (LN) group and the ANCA-negative LN group.
4. Discussion
In this study, the prevalence of AAV was found to be higher in elderly individuals, and patients with ANCA-positive SLE tended to be older than those with ANCA-negative SLE. On assessing the renal function (eGFR) among the three patient groups, we found that the AAV group had the most impaired renal function, followed by the ANCA-positive and ANCA-negative SLE groups. However, no statistically significant difference in renal function was observed between the ANCA-positive and ANCA-negative SLE groups. With regard to renal involvement, the AAV group with renal involvement had the most impaired renal function, whereas the ANCA-positive LN group showed worse baseline renal function and a lower proteinuria remission rate compared to the ANCA-negative LN group. Furthermore, the ANCA-positive SLE group had worse renal function compared to the ANCA-positive non-LN group. However, ANCA positivity itself was not associated with poor outcomes in patients with LN.
Activated neutrophils effectively capture and eliminate pathogens by releasing neutrophil extracellular traps (NETs), a cell capture net composed of decondensed chromatin and intracellular granule proteins. Current evidence suggests that neutrophils, NETosis, and complement activation contribute to tissue damage in SLE. Patients with SLE exhibit significantly abnormal neutrophil phenotype and function, and their neutrophils are more prone to undergo apoptosis and NETosis [18]. ANCA has been shown to induce endothelial cell injury by triggering the release of granules from neutrophils and monocytes; it is also involved in the acceleration of neutrophil apoptosis [4]. These findings suggest a potential pathogenic role of ANCA in SLE. Numerous clinical retrospective studies have explored the role of ANCA in SLE. Some studies have found associations between ANCA titers, disease activity, and the presence of anti-dsDNA antibodies in patients with newly diagnosed SLE [9]. ANCAs can be detected in the serum of patients with SLE, and some ANCA subtypes have been associated with particular clinical manifestations [19]. Patients with both active and inactive SLE have been found to have high defensin and cathepsin G-ANCA levels [20]. However, most of these studies have concentrated on the relationship between ANCA detected in the serum and disease activity in patients with SLE.
There have been limited studies comparing renal function between ANCA-positive and ANCA-negative SLE. In our study, we observed that patients with ANCA-positive SLE tended to have poorer renal function, although statistical significance was not reached. However, we noted that patients with ANCA-positive LN had worse renal function compared to patients with ANCA-positive non-LN. Based on these findings, we concluded that the presence of ANCAs in patients with SLE may indicate the possibility of LN. Therefore, we considered whether ANCAs could be used as a supplementary indicator to distinguish LN from SLE without nephritis. Recently, to diagnose specific clinical SLE phenotypes, Mirjana et al. assessed the level, avidity, and specificity of ANCA in SLE and found a correlation between renal manifestations and higher levels of lactoferrin (LF)-ANCAs (p < 0.01) and avidity (p < 0.05) in patients with LF-ANCA positivity [21].
An analysis of the subgroup of patients with ANCA-positive SLE with renal involvement revealed that the age of onset of ANCA-positive LN was higher than that of ANCA-negative LN but lower than the age of onset of AAV. This finding is consistent with previous studies that have shown older people to be more susceptible to AAV; however, the difference in the age of onset between patients with ANCA-positive and ANCA-negative LN has not been consistently reported [7,22–24]. Patients with ANCA-positive LN are more likely to have worse baseline renal function (lower eGFR) compared to those with ANCA-negative LN, which is consistent with previous studies [7,25]. However, the association of ANCA positivity in LN with poor prognosis remains controversial [6,22–24]. Our survival analysis did not reveal a significant difference in the time to reach the endpoint between patients with ANCA-positive and ANCA-negative LN, although a lower proteinuria remission rate was observed in patients with ANCA-positive LN than in those with ANCA-negative LN. Another study found that patients with both ANCA-positive and ANCA-negative LN exhibited significant improvement in proteinuria during the first six months after biopsy [25]. Moreover, the follow-up data in our study showed that ANCA titers seemed to decrease concomitantly with improvement in renal function (Supplementary Figure S1). Therefore, we hypothesized that ANCA can be used as an indicator of disease activity in LN, although further research is required to confirm this. Nevertheless, we recommend that all patients with SLE, especially those with LN, should be regularly monitored for ANCA levels during treatment follow-up.
ANCA mediates acute injury and induces a chronic response to injury [26]. Our results showed that patients with ANCA-positive LN are more likely to have severe interstitial fibrosis and show higher CI scores on renal biopsy specimens compared to patients with ANCA-negative LN. These findings are consistent with previous studies [7,24] and suggest increased chronic inflammation in ANCA-positive LN due to ANCAs. Other studies have reported that patients with ANCA-positive LN are more likely to have segmental endocapillary hypercellularity [23,25]. However, in our study, LN was more likely to result in segmental endocapillary hypercellularity in patients with ANCA positivity than in those without ANCA positivity, although the difference between the groups was not statistically significant.
In our study, we analyzed 16 (34.8%) cases of MPO-ANCA and 30 (65.2%) cases of PR3-ANCA among patients with ANCA-positive SLE. Previous epidemiological investigations have shown that MPO-ANCA-associated vasculitis and PR3-ANCA-associated vasculitis have different geographic distributions [26]. In China, the incidence of MPO-ANCA-positive AAV ranges from 80% to 95% [27]. Our study results were inconsistent with those of previous studies in several aspects [19,28]. First, Harper and Savage found that serum MPO-ANCA was more prevalent in older patients with ANCAs than in younger patients [29]. Findings from a study conducted on the Chinese population were consistent with the results of this study [27]. In our study, patients with ANCA-positive SLE were younger (43.27 ± 13.98 years) than the patients in previous studies. Second, the majority of patients in our study were treated with glucocorticoids and immunosuppressants for SLE, and ANCA serology was assessed at the time of kidney biopsy. Recent reports indicate that patients with MPO-ANCA-positive LN show poor renal function and high activity on renal histological examination [30,31]. Although the ratio of MPO-positive cases to PR3-positive cases was lower in our study, patients with MPO-ANCA-positive SLE were more likely to have worse baseline renal function. A follow-up analysis of the survival outcomes in both groups revealed that the survival outcome of patients with MPO-ANCA SLE was potentially worse than that of patients with PR3-ANCA SLE, although the difference did not reach statistical significance. Although one cohort study on patients with LN in China indicated that patients with MPO-positive LN had a poorer prognosis compared to those with PR3-positive LN, research on the prognosis of patients with these two types of SLE remains limited [28]. To better understand the prognosis of patients with SLE who had MPO and PR3 positivity, further larger-scale studies with larger sample sizes and homogenized baseline values are required.
This study has several limitations that should be considered. First, it was a single-center study, which may limit the generalizability of the findings. In addition, the number of patients was not large enough to conduct a multivariable analysis. Second, although we followed up on the changes in ANCA titers during the treatment period to determine whether the dynamic evolution of ANCA is related to LN activity, the continuity of variables was insufficient owing to the limitations of the retrospective nature of the study. Third, a previous study reported that elevated serum anti-dsDNA antibody levels may lead to false positive results in the MPO-ANCA test [32,33]. We investigated potential cross-reactivity between ANCA and autoantibodies by analyzing the correlation between ANCA levels and autoantibody seropositivity and titer (Supplementary Figure S2). We found no significant correlation between the ANCA titer and antibody seropositivity in the ANCA-positive SLE group and observed that elevated ds-DNA levels were not associated with elevated ANCA titers. Therefore, we speculated that there was no ANCA cross-reactivity in this study; however, further experimental verification is warranted to confirm our findings.
In conclusion, our study found that ANCAs are associated with poor baseline renal function in LN and delayed remission of urinary proteins. However, prospective studies are needed to confirm whether ANCAs have a pathogenic effect on SLE and to determine the specific mechanisms involved, to guide the development of targeted therapies. In addition, regular monitoring of ANCA titers in patients with ANCA-positive SLE, especially those with renal involvement, is recommended.
Supplementary Material
Acknowledgments
We thank the Research Square platform for accepting our following article as a preprint: Wang, Y., Yu, X.Y, Xie, X.F, et al. Clinical features and outcomes of patients with antineutrophil cytoplasmic antibody-positive systemic lupus erythematosus; 09 May 2022, PREPRINT (Version 1) available at Research Square [https://doi.org/10.21203/rs.3.rs-1624967/v1]. We also thank Bullet Edits for the linguistic editing and proofreading of this manuscript.
Funding Statement
This study was supported by the Clinical Research Award of the First Affiliated Hospital of Xi’an Jiaotong University, China (XJTU1AF-CRF-2017-018) and the Key Research and Development Program of Shaanxi Province, China (2019 KW-040).
Authors’ contributions
Xinfang Xie and Wanhong Lu designed the study, Ying Wang and Xiaoyang Yu wrote the initial draft of the paper, Wei Yang and Yu Liang collected the data, and Huixian Li and Ying Wang conducted the statistical analysis.
Ethical approval
This study adhered to the Declaration of Helsinki and was approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University (XJTU1AF2021LSK-214). Owing to the retrospective nature of the study, the requirement for obtaining informed consent from the participants was waived by the Research Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University, Shaanxi, China.
Disclosure statement
All authors declare no competing interests.
References
- 1.Davidson A. What is damaging the kidney in lupus nephritis? Nat Rev Rheumatol. 2016;12(3):1–10. doi: 10.1038/nrrheum.2015.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jennette JC, Wilkman AS, Falk RJ.. Anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and vasculitis. Am J Pathol. 1989;135(5):921–930. [PMC free article] [PubMed] [Google Scholar]
- 3.Windpessl M, Bettac EL, Gauckler P, et al. ANCA status or clinical phenotype - What counts more? Curr Rheumatol Rep. 2021;23(6):37. doi: 10.1007/s11926-021-01002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sen D, Isenberg DA.. Antineutrophil cytoplasmic autoantibodies in systemic lupus erythematosus. Lupus. 2003;12(9):651–658. doi: 10.1191/0961203303lu456rr. [DOI] [PubMed] [Google Scholar]
- 5.Chin HJ, Ahn C, Lim CS, et al. Clinical implications of antineutrophil cytoplasmic antibody test in lupus nephritis. Am J Nephrol. 2000;20(1):57–63. doi: 10.1159/000013557. [DOI] [PubMed] [Google Scholar]
- 6.Hill GS, Delahousse M, Nochy D, et al. Class IV-S versus class IV-G lupus nephritis: clinical and morphologic differences suggesting different pathogenesis. Kidney Int. 2005;68(5):2288–2297. doi: 10.1111/j.1523-1755.2005.00688.x. [DOI] [PubMed] [Google Scholar]
- 7.Li C, Zhou M-L, Liang D-D, et al. Treatment and clinicopathological characteristics of lupus nephritis with anti-neutrophil cytoplasmic antibody positivity: a case-control study. BMJ Open. 2017;7(7):e015668. doi: 10.1136/bmjopen-2016-015668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nasr SH, D’Agati VD, Park H-R, et al. Necrotizing and crescentic lupus nephritis with antineutrophil cytoplasmic antibody seropositivity. Clin J Am Soc Nephrol. 2008;3(3):682–690. doi: 10.2215/cjn.04391007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan H-F, Fang X-H, Wu G-C, et al. Anti-neutrophil cytoplasmic antibodies in new-onset systemic lupus erythematosus and lupus nephritis. Inflammation. 2008;31(4):260–265. doi: 10.1007/s10753-008-9073-3. [DOI] [PubMed] [Google Scholar]
- 10.Hochberg MC. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 11.Bossuyt X, Cohen Tervaert J-W, Arimura Y, et al. Position paper: revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis. Nat Rev Rheumatol. 2017;13(11):683–692. doi: 10.1038/nrrheum.2017.140. [DOI] [PubMed] [Google Scholar]
- 12.Kidney Disease: Improving Global Outcomes Glomerular Diseases Work Group . KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4S):S1–S276. doi: 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 13.Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37(2):187–192. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korbet SM, Lewis EJ, Schwartz MM, et al. Factors predictive of outcome in severe lupus nephritis. Lupus nephritis collaborative study group. Am J Kidney Dis. 2000;35(5):904–914. doi: 10.1016/s0272-6386(00)70262-9. [DOI] [PubMed] [Google Scholar]
- 16.Sethi S, Haas M, Markowitz GS, et al. Mayo clinic/renal pathology society consensus report on pathologic classification, diagnosis, and reporting of GN. J Am Soc Nephrol. 2016;27(5):1278–1287. doi: 10.1681/ASN.2015060612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan MJ. Neutrophils in the pathogenesis and manifestations of SLE. Nat Rev Rheumatol. 2011;7(12):691–699. doi: 10.1038/nrrheum.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galeazzi M, et al. Anti-neutrophil cytoplasmic antibodies in 566 european patients with systemic lupus erythematosus: prevalence, clinical associations and correlation with other autoantibodies. European concerted action on the immunogenetics of SLE. Clin Exp Rheumatol. 1998;16(5):541–546. [PubMed] [Google Scholar]
- 20.Tamiya H, Tani K, Miyata J, et al. Defensins- and cathepsin G-ANCA in systemic lupus erythematosus. Rheumatol Int. 2006;27(2):147–152. doi: 10.1007/s00296-006-0173-9. [DOI] [PubMed] [Google Scholar]
- 21.Gajic-Veljic M, Lekic B, Nikolic M, et al. Level and avidity of antineutrophil cytoplasmic antibodies specific to lactoferrin are useful biomarkers in systemic lupus erythematosus. Clin Rheumatol. 2022;41(3):709–720. doi: 10.1007/s10067-021-05926-x. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Huang X, Cai J, et al. Clinicopathologic characteristics and outcomes of lupus nephritis with antineutrophil cytoplasmic antibody: a retrospective study. Medicine (Baltimore). 2016;95(4):e2580. doi: 10.1097/MD.0000000000002580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S, Shang J, Xiao J, et al. Clinicopathologic characteristics and outcomes of lupus nephritis with positive antineutrophil cytoplasmic antibody. Ren Fail. 2020;42(1):244–254. doi: 10.1080/0886022X.2020.1735416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pyo JY, Jung SM, Song JJ, et al. ANCA positivity at the time of renal biopsy is associated with chronicity index of lupus nephritis. Rheumatol Int. 2019;39(5):879–884. doi: 10.1007/s00296-019-04263-2. [DOI] [PubMed] [Google Scholar]
- 25.Turner-Stokes T, Wilson HR, Morreale M, et al. Positive antineutrophil cytoplasmic antibody serology in patients with lupus nephritis is associated with distinct histopathologic features on renal biopsy. Kidney Int. 2017;92(5):1223–1231. doi: 10.1016/j.kint.2017.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jennette JC, Xiao H, Falk RJ.. Pathogenesis of vascular inflammation by anti-neutrophil cytoplasmic antibodies. J Am Soc Nephrol. 2006;17(5):1235–1242. doi: 10.1681/ASN.2005101048. [DOI] [PubMed] [Google Scholar]
- 27.Yates M, Watts R.. ANCA-associated vasculitis. Clin Med (Lond). 2017;17(1):60–64. doi: 10.7861/clinmedicine.17-1-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen M, Yu F, Zhang Y, et al. Antineutrophil cytoplasmic autoantibody-associated vasculitis in older patients. Medicine (Baltimore). 2008;87(4):203–209. doi: 10.1097/MD.0b013e31817c744b. [DOI] [PubMed] [Google Scholar]
- 29.Li C, Wang J-J, Zhou M-L, et al. Differences in clinico-pathological characteristics and outcomes between proteinase 3-ANCA positivity and myeloperoxidase-ANCA positivity in lupus nephritis. Lupus. 2019;28(9):1111–1119. doi: 10.1177/0961203319861680. [DOI] [PubMed] [Google Scholar]
- 30.Harper L, Savage CO.. ANCA-associated renal vasculitis at the end of the twentieth century–a disease of older patients. Rheumatology (Oxford). 2005;44(4):495–501. doi: 10.1093/rheumatology/keh522. [DOI] [PubMed] [Google Scholar]
- 31.Yang J, Liang D, Zhang H, et al. Long-term renal outcomes in a cohort of 1814 chinese patients with biopsy-proven lupus nephritis. Lupus. 2015;24(14):1468–1478. doi: 10.1177/0961203315593166. [DOI] [PubMed] [Google Scholar]
- 32.Korbet SM, Schwartz MM, Evans J, et al. Severe lupus nephritis: racial differences in presentation and outcome. J Am Soc Nephrol. 2007;18(1):244–254. doi: 10.1681/asn.2006090992. [DOI] [PubMed] [Google Scholar]
- 33.Fussner LA, Hummel AM, Schroeder DR, et al. Factors determining the clinical utility of serial measurements of antineutrophil cytoplasmic antibodies targeting proteinase 3. Arthritis Rheumatol. 2016;68(7):1700–1710. doi: 10.1002/art.39637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.