Abstract
Aims
To investigate the prospective associations of the loneliness and social isolation scales with cardiovascular disease (CVD) risk in diabetes patients and compare the relative importance of loneliness and social isolation with traditional risk factors. Also, the interactions of loneliness or isolation with the degree of risk factor control in relation to CVD risk were evaluated.
Methods and results
A total of 18 509 participants diagnosed with diabetes from the UK Biobank were included. A two-item scale and a three-item scale were used to assess loneliness and isolation levels, respectively. The degree of risk factor control was defined as numbers of glycated hemoglobin (HbA1c), blood pressure (BP), low-density lipoprotein cholesterol (LDL-C), smoking, and kidney condition controlled within the target range. During a mean follow-up of 10.7 years, 3247 total CVD incidents were documented, including 2771 coronary heart disease and 701 strokes. In the fully adjusted model, compared with participants with the lowest loneliness score (zero), hazard ratios (95% confidence interval) for CVD were 1.11 (1.02 and 1.20) and 1.26 (1.11 and 1.42) for participants with a loneliness scale of 1 and 2, respectively (P-trend < 0.001). No significant associations were observed for social isolation. Loneliness ranked higher in relative strength for predicting CVD than the lifestyle risk factors in diabetes patients. A significant additive interaction between loneliness and the degree of risk factor control on the risk of CVD was observed (P for additive interaction = 0.005).
Conclusion
Among diabetes patients, loneliness, but not social isolation scale, is associated with a higher risk of CVD and shows an additive interaction with the degree of risk factor control.
Keywords: Loneliness, Social isolation, Diabetes patients, Cardiovascular disease
Structured Graphical Abstract
Structured Graphical Abstract.
Loneliness, social isolation and traditional risk factors for cardiovascular disease in diabetes patients. BMI, body mass index; GFR, glomerular filtration rate; LDL, low-density lipoprotein.
See the editorial comment for this article ‘A lonely heart is a broken heart: it is time for a biopsychosocial cardiovascular disease model', by K. G. Kahl et al ., https://doi.org/10.1093/eurheartj/ehad310.
Introduction
Individuals with diabetes have a two- to three-fold higher risk of cardiovascular disease (CVD) than individuals without diabetes.1,2 Recently, the American Heart Association issued a statement calling for attention to the importance of the social determinants of health (SDOH) in diabetes patients in addition to traditional risk factors.3 However, the relevant evidence is still lacking.
Loneliness and social isolation are two important components of SDOH, reflecting different aspects of social contact.4,5 Loneliness usually refers to the emotional feelings related to the quality of social relations, while isolation refers to the quantity of social relations in behavior.6,7 Although the concepts of loneliness and social isolation are often discussed and compared to each other, they are quite different in nature and have different health consequences.6–9 Several previous studies in the general population have found that loneliness and social isolation are both significantly related to a higher risk of CVD.6,10,11 Notably, growing evidence shows that individuals with diabetes experience significantly higher levels of loneliness and isolation than individuals without diabetes,12,13 whereas little is known about the association of loneliness or social isolation with CVD risk in diabetes patients.
Moreover, previous studies showed that controlling for multiple traditional risk factors, such as high glycemia, high blood pressure (BP), dyslipidemia, smoking, and a poor kidney condition, might lower but not entirely eliminate the diabetes-related excess risk of CVD.14,15 It is unclear whether the association between traditional risk factor control and the risk of CVD differs by the status of loneliness or isolation. Therefore, in this study, we investigated the prospective associations of the loneliness and social isolation scales with CVD risk in diabetes patients and particularly compared the relative importance of loneliness and social isolation to traditional risk factors (e.g. lifestyle factors and metabolic risk factors) in predicting CVD risk. Moreover, we also evaluated the interactions of loneliness or isolation with the degree of risk factor control in relation to CVD risk by applying multiplicative interaction and additive interaction more relevant to the public health measures.
Methods
Study design and population
The UK Biobank Study is a population-based cohort study; the detailed study design and methods have been described previously.16 Briefly, UK Biobank recruited more than 0.5 million participants, aged 37 to 73, at 22 assessment centers throughout England, Wales, and Scotland from 2006 to 2010. All participants provided written informed consent, and the study was approved by the North West Multi-Centre Research Ethics Committee and the Tulane University Biomedical Committee Institutional Review Board.
In this study, our analyses were restricted to participants who had diabetes at baseline, which was defined based on the onset time of diabetes (before or equal to the date of attending the assessment center) or a self-reported history of diabetes diagnosed by a doctor (n = 26 863) (see Supplementary material online, Table S1). A total of 18 509 participants were included in the main analysis after excluding 6219 participants with prevalent coronary heart disease (CHD), stroke, or heart failure and 2135 participants with missing values on information about loneliness or isolation.
Definition of loneliness and isolation scales
In the UK Biobank, the scales of loneliness and social isolation were constructed based on several previous studies with a self-reported questionnaire.7–9,11 The loneliness scale was assessed through two questions, “Do you often feel lonely?” and “How often are you able to confide in someone close to you?” High-risk loneliness factors were defined as feeling lonely and being able to confide less than once a month. The social isolation scale was measured with three questions: (i) “Including yourself, how many people are living together in your household?”; (ii) “How often do you visit friends or family or have them visit you?”; (iii) “Which of the following leisure/social activities do you engage in once a week or more often?”. High-risk isolation factors were defined as living alone, having friends and family visit less than once a month, and no participating in social activity at least once per week. Participants with high-risk factors were coded as 1, and those with low-risk factors were coded as 0. The scale of loneliness or social isolation was calculated by summing the individual scores of the two or three corresponding factors, respectively, with a range of 0–2 or 0–3. Higher scores indicate higher levels of loneliness or social isolation. We further classified the loneliness status as loneliness (loneliness scale =2) and non-loneliness (loneliness scale <2).7–9,11 Social isolation status was classified as isolation (isolation scale ≥2) and non-isolation (isolation scale <2).7–9,11
Definition of traditional risk factor control
According to the previous studies and guidelines,14,15,17,18 five risk factors were used to define diabetes patients with different numbers of traditional risk factor control, including glycated hemoglobin (HbA1c), BP, low-density lipoprotein (LDL) cholesterol, smoking, and kidney condition. We defined glycemic control as HbA1c level <53 mmol/mol (7.0%), lipid control as LDL cholesterol level <100 mg/dL, BP control as mean systolic BP and diastolic BP level <140/90 mm Hg, non-current smoking as a combination of never and past smoking, and control of kidney condition as a combination of an albumin-to-creatinine ratio (ACR) <10 mg/g and an estimated glomerular filtration rate (eGFR) ≥90 mL/min/1.73 m2.14,15,19 This cut point for ACR was chosen according to the previous evidence that diabetes patients with an ACR value between 10 and 29 mg/g already had a significant higher CVD risk compared with patients with ACR <10 mg/g19,20 Moreover, a more relaxed cut point was used to define optimal control of the kidney condition (ACR <30 mg/g and eGFR ≥90 mL/min/1.73 m2) in sensitivity analysis.21 Also, because it is difficult and often impossible to make eGFR better even though the rate of eGFR decline can be changed, we performed a sensitivity analysis by excluding eGFR from the risk factor control to provide a point of view on clinical application. The degree of traditional risk factor control was classified as low (0–1 risk factor control), medium (2–3 risk factor control), and high (4–5 risk factor control).
Outcomes
Baseline participants were linked to hospital inpatient records to obtain data on admissions, diagnoses, and deaths. The primary outcomes of the present study were incident CVD and its two major component endpoints: CHD and stroke. We defined outcomes according to the International Classification of Diseases, 10th revision (ICD-10) codes: I20–I25 for CHD and I60–I64 for stroke. The follow-up time was calculated from the date of the baseline to the diagnosis of the outcome, death, or the censoring date (23 May 2021), whichever occurred first. Detailed information on the ascertainment of outcomes is available online at https://biobank.ctsu.ox.ac.uk/showcase/label.cgi? id=2000.
Covariates
Self-reported information was collected by a touch-screen questionnaire, including age, sex, race, the Townsend deprivation index, physical activity (minutes of moderate or vigorous physical activity or an equivalent combination per week22) moderate drinking (alcohol intake >0 g and ≤14 g/day for women and alcohol intake >0 g and ≤28 g/day for men23), healthy diet score, depression score, family history of heart disease or stroke (among first-degree relatives including father, mother, and siblings), diabetes medication (insulin use, oral antidiabetic drugs only, and neither), antihypertensive medication, and statin or other lipid lowering medication. The Townsend deprivation index is a composite measure of deprivation based on unemployment, non-car ownership, non-home ownership, and household overcrowding.24 A healthy diet score was calculated based on vegetable intake (≥median), fruit intake (≥median), fish intake (≥median), red meat intake (<median), and processed red meat intake (<median); one point was given for each favorable diet factor, and the total diet score ranges from 0 to 5.25 Depressive symptoms were measured with the two questions from the Patient Health Questionnaire-2, including the frequency of depressed mood and disinterest or absence of enthusiasm in the previous 2 weeks. For each item, if participants answered, “not at all,” “several days,” “more than half the days,” or “nearly every day,” they were coded as 0, 1, 2, and 3, respectively. The total depression score ranged from 0 to 6 by adding the scores for the two items.26 Information on diabetes medication was extracted from both self-reported data and the nurse’s verbal interview.27
Height and weight were measured during the assessment visit, and body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m²). Albumin-to-creatinine ratio was calculated by the concentration of urine albumin and creatinine.21 The eGFR was calculated by serum creatinine and age according to race and sex.28 Information on diabetes duration, diabetes complications (diabetes-related amputation, neuropathy, cataract, retinopathy, and arthropathy), and diabetes types was defined through the inpatient health record. We coded missing covariates as a missing indicator category for categorical variables and with mean values for continuous variables. In sensitivity analysis, we impute missing covariate values with multivariate imputation by chained equations and repeat the analyses. Further detailed descriptions of these measurements are provided in the Supplementary material and can be found at the UK Biobank website (https://biobank.ctsu.ox.ac.uk/showcase).
Statistical analysis
Analyses of covariance (generalized linear models) and chi-square were used for comparison of continuous and categorical variables, respectively, between diabetes patients who were lonely and those who were not lonely and between those who were isolated and those who were not isolated. Cox proportional hazards models were used to evaluate the association between the loneliness or isolation scale and the risk of CVD separately, and follow-up years were used as the underlying time metric. The proportional hazards assumption was tested based on Schoenfeld residuals, and all analyses were satisfied. In the multivariable adjusted model, we controlled for age, sex, race, the Townsend deprivation index, diabetes duration, diabetes complications, types of diabetes, family history of CVD, CHD or stroke (in the corresponding model), BMI, the use of diabetes medication, cholesterol-lowering medication, and antihypertensive medication. Next, we further included lifestyle factors (physical activity, healthy diet score, and moderate drinking), depression score, and the control of traditional risk factors into the model, separately and simultaneously. A directed acyclic graph explaining the association between the exposures, the outcome, and the covariates is available in Supplementary material online, Figure S1. To estimate whether the association of loneliness or social isolation with incident CVD was independent of each other, a sensitive analysis was performed to mutually adjust. Also, another sensitive analysis was performed to take the competing risk of death into account by using Fine and Gray’s proportional sub-hazards model.
To estimate how important loneliness or isolation is in predicting CVDs, we analyzed the relative importance of loneliness or isolation and other traditional risk factors by calculating the R2 values of the Cox models.14,29 The explainable log-likelihood attributed to each risk factor was also calculated to test the consistency of our results.14
In addition, we classified participants according to the joint categories of loneliness or isolation and the degree of risk factor control. To investigate whether the association of loneliness or isolation with CVD is modified by the degree of risk factor control, we performed both multiplicative and additive interaction analyses. The multiplicative interaction was accessed by adding the product terms to the original Cox models. To assess the additive interaction, we coded variables by assigning the stratum with the lowest risk as the reference (non-loneliness and five risk factors controlled) and considered them as continuous variables.30 The relative excess risk due to interaction (RERI) and the attributable proportion (AP) were assessed as described in our previous studies.25,31
Statistical analyses were performed with SAS version 9.4 (SAS Institute Inc. Cary, NC, USA) and R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). All P-values were two-sided, and P < 0.05 was considered statistically significant.
Results
Study population
The baseline information of diabetes patients without prevalent CVD according to the loneliness scale or social isolation scale is presented in Table 1 and Supplementary material online, Table S2, respectively. Among 18 509 diabetes patients, 61.1%, 29.6%, and 9.3% participants were defined as having a loneliness scale of 0, 1, or 2, respectively. The corresponding percentage was 44.9%, 41.9%, and 13.2% for participants with isolation scale of 0, 1, or ≥2. Patients who had a higher loneliness or isolation scale were younger, had higher Townsend deprivation index, were less likely to be non-current smoker, drink in moderation, and eat a healthy diet, as compared with their counterparts who had a lower loneliness or social isolation scale. Patients with a higher loneliness or isolation scale tended to have higher BMI, HbA1c, depression score, and a lower degree of risk factor control and were more likely to use insulin and to have diabetes complications.
Table 1.
Baseline characteristics of participants with diabetes by loneliness score
| Characteristics | Loneliness score | ||
|---|---|---|---|
| 0 | 1 | 2 | |
| No. of participants (%) | 11303 (61.1) | 5481 (29.6) | 1725 (9.3) |
| Age, years | 59.6 ± 7.1 | 59.1 ± 7.2 | 57.5 ± 7.4 |
| Female sex, n (%) | 4543 (40.2) | 2269 (41.4) | 730 (42.3) |
| White ethnicity, n (%) | 10250 (91.0) | 4677 (85.8) | 1509 (87.9) |
| Townsend deprivation index | −1.0 ± 3.0 | −0.2 ± 3.5 | 0.3 ± 3.6 |
| Diabetes duration, years | 8.7 ± 10.4 | 9.2 ± 10.8 | 9.0 ± 10.3 |
| Type 1 diabetes, n (%) | 1009 (8.9) | 468 (8.5) | 173 (10.0) |
| Diabetes complications, n (%) | 285 (2.5) | 191 (3.5) | 61 (3.5) |
| Non-current smoker, n (%) | 10218 (90.4) | 4780 (87.2) | 1449 (84.0) |
| Moderate drinker, n (%) | 4980 (44.1) | 2137 (39.0) | 636 (36.9) |
| Physical activity, minutesa | 369.9 ± 639.0 | 337.3 ± 639.8 | 359.7 ± 1044.1 |
| Healthy diet score | 2.9 ± 1.2 | 2.7 ± 1.3 | 2.6 ± 1.3 |
| Depression score | 0.4 ± 0.9 | 1.1 ± 1.5 | 2.1 ± 1.8 |
| Body mass index, kg/m2 | 30.9 ± 5.7 | 31.6 ± 6.2 | 32.2 ± 6.4 |
| Estimated GFR, mL/min/1.73 m2 | 89.7 ± 15.5 | 89.7 ± 16.6 | 91.9 ± 16.7 |
| Albumin-to-creatinine ratio, mg/g | 37.4 ± 182.1 | 51.0 ± 287.5 | 49.3 ± 217.3 |
| Family history, n (%) | |||
| Heart disease | 5120 (45.3) | 2452 (44.7) | 770 (44.6) |
| Stroke | 3272 (29.0) | 1562 (28.5) | 500 (29.0) |
| Glycated hemoglobin | |||
| Millimoles per mole | 51.9 ± 13.0 | 53.3 ± 14.7 | 54.5 ± 15.2 |
| Percent | 6.9 ± 1.2 | 7.0 ± 1.3 | 7.1 ± 1.4 |
| LDL cholesterol | |||
| Millimoles per liter | 2.7 ± 0.8 | 2.7 ± 0.8 | 2.8 ± 0.8 |
| Milligrams per deciliter | 105.2 ± 29.7 | 106.0 ± 30.4 | 107.3 ± 31.5 |
| Blood pressure, mm Hg | |||
| Systolic | 142.2 ± 16.9 | 141.3 ± 17.3 | 138.9 ± 16.9 |
| Diastolic | 82.1 ± 9.5 | 82.1 ± 9.6 | 82.2 ± 9.8 |
| Antihypertensive medication, n (%) | 6703 (59.3) | 3266 (59.6) | 995 (57.7) |
| Cholesterol-lowering medication, n (%) | 8444 (74.7) | 4097 (74.8) | 1280 (74.2) |
| Diabetes medication, n (%) | |||
| Oral antidiabetic drugs only | 5557 (49.2) | 2758 (50.3) | 846 (49.0) |
| Insulin | 2156 (19.1) | 1104 (20.1) | 416 (24.1) |
| Neither | 3590 (31.8) | 1619 (29.5) | 463 (26.8) |
| Number of risk factor control | 2.9 ± 1.1 | 2.8 ± 1.1 | 2.7 ± 1.1 |
Data are present as mean ± SD or n (%).
Physical activity was calculated as minutes of moderate or vigorous physical activity or an equivalent combination per week. The body mass index is the weight in kilograms divided by the square of the height in meters; the GFR was estimated with the use of the Modification of Diet in Renal Disease equation. To convert values for cholesterol to milligrams per deciliter, divide by 0.02586; to convert values for glycated hemoglobin to percent, divide by 10.929 and plus 2.15. LDL, low-density lipoprotein; GFR, glomerular filtration rate.
Loneliness or isolation scale with risk of cardiovascular disease among diabetic patients
During a mean follow-up of 10.7 years, a total of 3247 incident CVD events were observed, including 2771 incident CHD events and 701 incident stroke events. We found that the loneliness scale, but not the isolation scale, was significantly associated with higher risks of total CVD and CHD among diabetes patients (Table 2 and Supplementary material online, Table S3). In the multivariable adjusted model, compared with participants with the lowest loneliness score (0), hazard ratios (HRs) [95% confidence interval (CI)] for total CVD were 1.15 (1.07, 1.25) and 1.38 (1.23, 1.54) for participants with a loneliness scale of 1 and 2, respectively (P-trend < 0.001) (Table 2). The results were not appreciated changed if we further adjusted for lifestyle factors (physical activity, moderate drinking, and healthy diet score), depression score, or traditional risk factor control (HbA1c control, BP control, LDL cholesterol control, no current smoking, and control of kidney function) in the model. The association was slightly attenuated but still significant if all these covariates were included in the model, with HRs (95% CI) of 1.11 (1.02, 1.20) and 1.26 (1.11, 1.42) for participants with a loneliness scale of 1 and 2 (P-trend <0.001). Similar results were observed for CHD. For stroke, in the model that included all the covariates, the loneliness scale was not significantly associated with stroke. We did not find a significant association between the social isolation scale and CVD, CHD, or stroke, if we included all the covariates in the models (see Supplementary material online, Table S3). Similar associations were observed when we adjusted for loneliness and isolation mutually in one model in sensitive analysis (see Supplementary material online, Table S4). Additionally, the results did not appreciably change when the competing risk of death was considered in the analysis or when multiple imputation was used to impute the missing covariates (see Supplementary material online, Tables S5 and S6). Furthermore, we observed that the association between loneliness and CVD did not appear to be modified by covariates (see Supplementary material online, Table S7).
Table 2.
Multivariable-adjusted HRs (95% CIs) of loneliness scale for cardiovascular diseases among diabetes patients
| Loneliness scale | P-trend | |||
|---|---|---|---|---|
| 0 | 1 | 2 | ||
| Cardiovascular disease | ||||
| No. of cases/total | 1852/11303 | 1034/5481 | 361/1725 | |
| Multivariable adjusteda | 1 (Reference) | 1.15 (1.07, 1.25) | 1.38 (1.23, 1.54) | <0.001 |
| Multivariable adjusteda +lifestyle factors | 1 (Reference) | 1.15 (1.06, 1.24) | 1.36 (1.21, 1.52) | <0.001 |
| Multivariable adjusteda +depression score | 1 (Reference) | 1.12 (1.04, 1.22) | 1.28 (1.14, 1.45) | <0.001 |
| Multivariable adjusteda +traditional risk factor control | 1 (Reference) | 1.14 (1.06, 1.23) | 1.36 (1.21, 1.53) | <0.001 |
| Model included all covariates | 1 (Reference) | 1.11 (1.02, 1.20) | 1.26 (1.11, 1.42) | <0.001 |
| Coronary heart disease | ||||
| No. of cases/total | 1566/11303 | 895/5481 | 310/1725 | |
| Multivariable adjusteda | 1 (Reference) | 1.18 (1.08, 1.28) | 1.38 (1.22, 1.56) | <0.001 |
| Multivariable adjusteda +lifestyle factors | 1 (Reference) | 1.17 (1.07, 1.27) | 1.36 (1.20, 1.54) | <0.001 |
| Multivariable adjusteda +depression score | 1 (Reference) | 1.14 (1.05, 1.24) | 1.28 (1.12, 1.46) | <0.001 |
| Multivariable adjusteda +traditional risk factor control | 1 (Reference) | 1.17 (1.07, 1.27) | 1.37 (1.21, 1.55) | <0.001 |
| Model included all covariates | 1 (Reference) | 1.13 (1.04, 1.23) | 1.26 (1.10, 1.44) | <0.001 |
| Stroke | ||||
| No. of cases/total | 401/11303 | 225/5481 | 75/1725 | |
| Multivariable adjusteda | 1 (Reference) | 1.15 (0.98, 1.36) | 1.30 (1.01, 1.67) | 0.016 |
| Multivariable adjusteda +lifestyle factors | 1 (Reference) | 1.15 (0.97, 1.35) | 1.29 (1.01, 1.66) | 0.021 |
| Multivariable adjusteda +depression score | 1 (Reference) | 1.14 (0.97, 1.35) | 1.27 (0.97, 1.65) | 0.039 |
| Multivariable adjusteda +traditional risk factor control | 1 (Reference) | 1.12 (0.95, 1.33) | 1.25 (0.97, 1.61) | 0.045 |
| Model included all covariates | 1 (Reference) | 1.11 (0.94, 1.32) | 1.22 (0.94, 1.59) | 0.091 |
Adjusted for sex, age, race, Townsend deprivation index, diabetes duration, diabetes complications, type of diabetes, family history of cardiovascular disease, coronary heart disease or stroke (in the corresponding model), body mass index, the use of diabetes medication, cholesterol-lowering medication, and antihypertensive medication; lifestyle factors including physical activity, healthy diet score, and moderate drinking; traditional risk factor control including glycated hemoglobin control, blood pressure control, low-density-lipoprotein cholesterol control, no current smoking, and control of kidney condition; HR, hazard ratio; CI, confidence interval.
The relative importance of loneliness and isolation comparing with traditional risk factors in predicting cardiovascular disease among diabetic patients
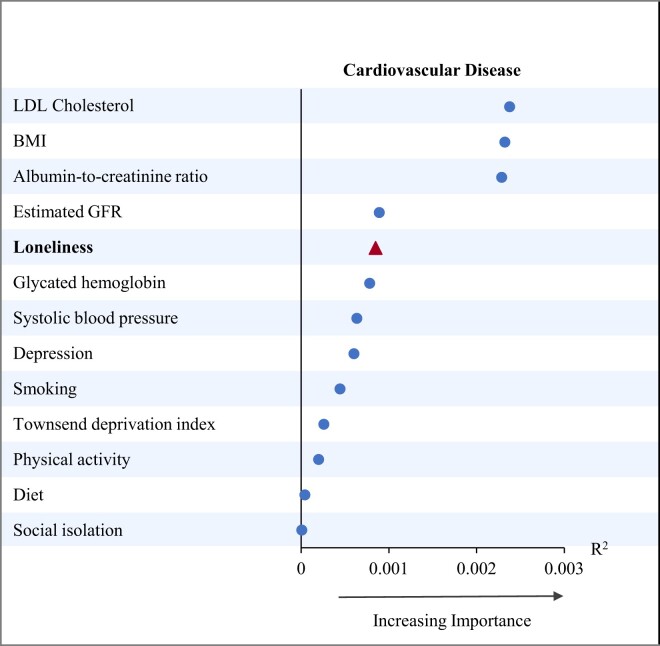
We assessed the relative importance of loneliness and isolation in predicting CVD among diabetes patients in comparison to other traditional risk factors (Figure 1). We observed that, among a few selected factors, loneliness was in fifth place for CVD risk in diabetes patients. Loneliness ranked lower in relative strength for predicting CVD than LDL cholesterol, BMI, and ACR, similar to eGFR, HbA1c, and systolic BP levels, but higher than depression score and lifestyle risk factors such as smoking, physical activity, and diet. To validate our findings, we also examined the relative strengths of these risk factors for CVDs using explained log-likelihood (see Supplementary material online, Figure S2) and found that the ranking of loneliness was consistent with that observed using explained relative risk (R2) model.
Figure 1.
Relative importance of risk factors for predicting cardiovascular disease among patients with diabetes. Analysis was restricted to 15 456 participants who had complete data on loneliness and risk-factor control. BMI, body mass index; GFR, glomerular filtration rate; LDL, low-density lipoprotein.
Joint association of loneliness and risk factor control with cardiovascular disease among diabetic patients
We further assessed the joint association between the scale of loneliness and the degree of risk factor control with the risk of CVD (Table 3). The baseline characteristics of diabetes patients according to the degree of risk factor control are presented in Supplementary material online, Table S8. Compared with individuals (reference group) who did not feel lonely and had a high degree of risk factor control (controlling 4–5 risk factors), the HR was 1.50 (95% CI,1.30–1.73) for individuals who did not feel lonely and had a low degree of risk factor control (0–1 factor), whereas the HR further increased to 1.93 (95% CI, 1.47–2.54) in individuals who felt lonely and had a low degree of risk factor control (0–1 factor). We found a significant additive interaction between loneliness and the degree of risk factor control on the risk of CVD (Table 4). The RERI is 0.11 (0.03 to 0.18), and the AP to the additive interaction was 8.5% (95% CI, 4.2%–12.8%), with a P-value for the additive interaction of 0.005. We did not find a significant multiplicative interaction (P = 0.098) between loneliness and the degree of risk factor control on the risk of CVD (Table 3). Similar patterns of interaction were observed in sensitive analysis if a more relaxed cut point (ACR <30 mg/g and eGFR ≥90 mL/min/1.73 m2) was used to define optimal control of kidney condition (see Supplementary material online, Table S9, P for additive interaction = 0.035; P for multiplicative interaction = 0.232), or if eGFR was excluded from the definition of risk factor control (see Supplementary material online, Table S10, P for additive interaction = 0.002; P for multiplicative interaction = 0.071).
Table 3.
Joint association of loneliness and the degree of risk factor control on the risk of cardiovascular diseases among diabetes patients
| Loneliness Status | The degree of risk factor control | |||||
|---|---|---|---|---|---|---|
| High (4–5 factors) | Medium (2–3 factors) | Low (0–1 factor) | ||||
| Cases/total | HR (95% CI) | Cases/total | HR (95% CI) | Cases/total | HR (95% CI) | |
| Not lonely | 370/2761 | 1 (Reference) | 1675/9500 | 1.26 (1.12, 1.41) | 383/1779 | 1.50 (1.30, 1.73) |
| Loneliness | 31/268 | 0.82 (0.57, 1.18) | 204/914 | 1.58 (1.32, 1.89) | 62/234 | 1.93 (1.47, 2.54) |
| P for multiplicative interaction = 0.098 | ||||||
| P for additive interaction = 0.005 | ||||||
Analysis was restricted to 15 456 participants who had complete data on loneliness and risk-factor control. Five risk factor control including glycated hemoglobin control, blood pressure control, low-density-lipoprotein cholesterol control, non-current smoking and control of kidney function (range from 0 to 5); models are adjusted for sex, age, race, Townsend deprivation index, diabetes duration, diabetes complications, type of diabetes, family history of cardiovascular disease, body mass index, the use of diabetes medication, cholesterol-lowering medication, antihypertensive medication, physical activity, healthy diet score, moderate drinking, and depression score.
Table 4.
Attributing effects to additive interaction between loneliness and low degree of risk factor control on cardiovascular risk
| Cardiovascular disease | |
|---|---|
| Main effects | |
| Per addition decreases in risk factor control | 1.14 (1.09, 1.19) |
| Loneliness | 0.97 (0.69, 1.35) |
| Joint effect | 1.26 (1.02, 1.50) |
| Relative excess risk due to interaction | 0.11 (0.03, 0.18) |
| P-value | 0.005 |
| Attributable proportion, % | 8.48 (4.20, 12.77) |
Models are adjusted for sex, age, race, Townsend deprivation index, diabetes duration, diabetes complications, type of diabetes, family history of cardiovascular disease, body mass index, the use of diabetes medication, cholesterol-lowering medication, antihypertensive medication, physical activity, healthy diet score, moderate drinking, and depression score.
Discussion
This study generated two key findings. First, we found that a higher loneliness scale, but not social isolation scale, was significantly associated with a higher risk of CVD in diabetes patients, and loneliness ranked higher in relative strength for predicting CVD than the lifestyle risk factors (Structured Graphical Abstract). Second, we observed that the risk of CVD associated with a combination of loneliness and a low degree of traditional risk factor control was greater than the addition of the risk associated with each of these factors, indicating a significant interaction on an additive scale.
Our study, for the first time, showed that loneliness was significantly related to a higher risk of CVD in diabetic patients. Our findings were supported by the results from several previous studies studying the general population, in which loneliness was found to be associated with higher risks of CVD events.32,33 A cohort study from England showed that a one-point increase in the loneliness scale was associated with a 5%–8% increase in the hazard of CVD in the general population.32 Another study from the UK showed that loneliness was significantly associated with a higher risk of acute myocardial infarction in the general population when separately adjusting for biological factors, health behaviors, depressive symptoms, or socioeconomic factors, whereas such an association was no longer significant when these factors were fully adjusted in the model (HR 1.06, 95% CI, 0.96–1.17).11 In our study, the associations between the loneliness scale and the risks of CVD and CHD among diabetes patients remained significant after adjusting for similar covariates. Our findings could be potentially explained by the evidence that diabetic patients not only have a higher risk of CVD but also experience higher levels of loneliness than individuals without diabetes.13 Moreover, we compared the relative importance of loneliness with traditional risk factors in predicting CVD among diabetes patients and found that the relative strength of loneliness was greater than the lifestyle risk factors, including smoking, physical activity, and diet.
In this study, we did not find a significant association between social isolation and CVDs among diabetes patients. Consistent with our findings, a prospective study from the Women’s Health Initiative also did not find a significant association between social network size (constructed by married or in an intimate relationship, times attending activities, and number of relatives) and CHD risk among women with diabetes.34 Our findings are also supported by the results of intervention studies examining the impact of social support on outcomes in diabetes patients,35–40 which found that behavior-related interventions had no effect on changes in cardiometabolic biomarkers such as HbA1c and BP,36,37 whereas emotion-related interventions such as peer support consistently resulted in greater improvement in these biomarkers.38–40 Different from loneliness, which refers to a negative emotional feeling toward social contacts and relationships, social isolation refers to the scarcity of social contacts and relationships in behavior.6,41,42 Our findings suggest that the quality of social context (emotional) may play a more important role than quantity (behavioral) in determining CVD among diabetes patients.
We observed that among diabetes patients, the CVD risk associated with a combination of loneliness and a low degree of risk factor control was greater than the addition of the risks associated with each of these factors, indicating a significant interaction on an additive scale. Specifically, if both loneliness and a low degree of risk factor control were present, this would result in an additional 8.5% of cases of CVD. The observed effect size of additive interaction in this study is modest in magnitude. However, given the high prevalence of diabetes and the increasing trend worldwide, such a percentage increase is considerable regarding the number of patients affected by CVD complications and therefore has important public health implications. The additive interactions could distinguish whether the effect of the risk factor on a certain disease differed across subgroups; thus, they are more relevant to public health as compared with multiplicative interactions. Our findings indicate that the public health consequence of a low degree of risk factor control would be greater in diabetes patients with loneliness.
The major strengths of this study include the prospective study design, the large sample size of diabetes patients with available data on loneliness and isolation, and the comprehensive and detailed information on covariates in diabetes patients. We also acknowledge several potential limitations of this study. Firstly, due to the nature of an observational study, we cannot draw any conclusions about the causality between the loneliness scale and the risk of CVD. Second, the loneliness and social isolation scales were constructed based on simple questions, which may not be able to adequately assess the complex phenomenon of social networking and interaction. However, these scales have been widely used in several previous studies from different cohorts,7–9,11,43 suggesting such scales are effective in population studies. Third, despite the large sample size of the study and long follow-up period, the incidence of stroke was still low; thus, our results on stroke should be interpreted as exploratory. Fourth, we did not adjust for cognitive function in this study because only a limited number of UK Biobank participants completed the cognitive test at baseline. Future studies considering these factors are warranted. Fifth, only ∼12% of our study participants were non-White European; whether our findings could be generalized to other race/ethnic groups would need to be further tested. Sixth, the participants from the UK Biobank are more likely to have healthier behaviors and may not be a representation of the general UK population.16 However, a valid assessment of exposure–disease relationships may not require a representative population.44
Conclusion
Our findings indicate that a higher loneliness scale, but not isolation scale, was significantly associated with a higher risk of CVD in diabetic patients, and loneliness ranked higher in relative strength for predicting CVD than the lifestyle risk factors. The effect of loneliness and a low degree of risk factor control on the risk of CVD is greater than additive among diabetic patients. These results highlight the importance of loneliness in the prediction of CVDs among diabetic patients.
Supplementary data
Supplementary data is available at European Heart Journal online.
Supplementary Material
Contributor Information
Xuan Wang, Department of Epidemiology, Tulane University School of Public Health and Tropical Medicine, 1440 Canal Street, New Orleans, LA 70112, USA.
Hao Ma, Department of Epidemiology, Tulane University School of Public Health and Tropical Medicine, 1440 Canal Street, New Orleans, LA 70112, USA.
Xiang Li, Department of Epidemiology, Tulane University School of Public Health and Tropical Medicine, 1440 Canal Street, New Orleans, LA 70112, USA.
Yoriko Heianza, Department of Epidemiology, Tulane University School of Public Health and Tropical Medicine, 1440 Canal Street, New Orleans, LA 70112, USA.
Vivian Fonseca, Section of Endocrinology and Metabolism, Tulane University School of Medicine, New Orleans, LA, USA; Southeast Louisiana Veterans Health Care System, New Orleans, LA, USA.
Lu Qi, Department of Epidemiology, Tulane University School of Public Health and Tropical Medicine, 1440 Canal Street, New Orleans, LA 70112, USA; Department of Nutrition, Harvard T.H. Chan School of Public Health, 677 Huntington Ave, Boston, MA 02115, USA.
Data availability
This study has been conducted using the UK Biobank Resource, approved project number 29256. The UK Biobank will make the source data available to all bona fide researchers for all types of health-related research that is in the public interest, without preferential or exclusive access for any persons. All researchers will be subject to the same application process and approval criteria as specified by UK Biobank. For more details on the access procedure, see the UK Biobank website: http://www.ukbiobank.ac.uk/register-apply.
Funding
The study was supported by grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, HL126024) and the National Institute of Diabetes and Digestive and Kidney Diseases (DK115679, DK091718, DK100383, DK078616).
Role of the Funder/Sponsor: The funding sources and sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author contributions
L.Q. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: L.Q., X.W., and H.M.
Acquisition, analysis, or interpretation of data: L.Q., X.W., and H.M.
Critical revision of the manuscript for important intellectual content: All authors.
Drafting of the manuscript: L.Q., X.W., and H.M.
Statistical analysis: X.W., and H.M.
Transparency statement: L.Q. affirms that the manuscript is an honest, accurate, and transparent account of the study being reported, that no important aspects of the study have been omitted, and that any discrepancies from the study as planned have been explained.
References
- 1. Rawshani A, Rawshani A, Franzén S, Eliasson B, Svensson A, Miftaraj M, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med 2017;376:1407–1418. 10.1056/NEJMoa1608664 [DOI] [PubMed] [Google Scholar]
- 2. Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol 2015;3:105–113. 10.1016/S2213-8587(14)70219-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Joseph JJ, Deedwania P, Acharya T, Aguilar D, Bhatt DL, Chyun DA, et al. Comprehensive management of cardiovascular risk factors for adults with type 2 diabetes: a scientific statement from the American Heart Association. Circulation 2022;145:e722–722e759. 10.1161/CIR.0000000000001040 [DOI] [PubMed] [Google Scholar]
- 4. Powell-Wiley TM, Baumer Y, Baah FO, Baez AS, Farmer N, Mahlobo CT, et al. Social determinants of cardiovascular disease. Circ Res 2022;130:782–799. 10.1161/CIRCRESAHA.121.319811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hill-Briggs F, Adler NE, Berkowitz SA, Chin MH, Gary-Webb TL, Navas-Acien A, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care 2020;44:258–279. 10.2337/dci20-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Christiansen J, Lund R, Qualter P, Andersen CM, Pedersen SS, Lasgaard M. Loneliness, social isolation, and chronic disease outcomes. Ann Behav Med 2021;55:203–215. 10.1093/abm/kaaa044 [DOI] [PubMed] [Google Scholar]
- 7. Elovainio M, Hakulinen C, Pulkki-Råback L, Virtanen M, Josefsson K, Jokela M, et al. Contribution of risk factors to excess mortality in isolated and lonely individuals: an analysis of data from the UK Biobank cohort study. Lancet Public Health 2017;2:e260–e266. 10.1016/S2468-2667(17)30075-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salinas J, Beiser AS, Samra JK, O'Donnell A, DeCarli CS, Gonzales MM, et al. Association of loneliness with 10-year dementia risk and early markers of vulnerability for neurocognitive decline. Neurology 2022;98:e1337–e1348. 10.1212/WNL.0000000000200039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elovainio M, Komulainen K, Sipilä PN, Pulkki-Råback L, Alonso LC, Pentti J, et al. Association of social isolation and loneliness with risk of incident hospital-treated infections: an analysis of data from the UK Biobank and Finnish Health and Social Support studies. Lancet Public Health 2023;8:e109–e118. 10.1016/S2468-2667(22)00253-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Valtorta NK, Kanaan M, Gilbody S, Ronzi S, Hanratty B. Loneliness and social isolation as risk factors for coronary heart disease and stroke: systematic review and meta-analysis of longitudinal observational studies. Heart 2016;102:1009–1016. 10.1136/heartjnl-2015-308790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hakulinen C, Pulkki-Råback L, Virtanen M, Jokela M, Kivimäki M, Elovainio M. Social isolation and loneliness as risk factors for myocardial infarction, stroke and mortality: UK Biobank cohort study of 479 054 men and women. Heart 2018;104:1536–1542. 10.1136/heartjnl-2017-312663 [DOI] [PubMed] [Google Scholar]
- 12. Brinkhues S, Dukers-Muijrers NH, Hoebe CJ, Van Der Kallen CJH, Dagnelie PC, Koster A, et al. Socially isolated individuals are more prone to have newly diagnosed and prevalent type 2 diabetes mellitus-the Maastricht study. BMC Public Health 2017;17:955. 10.1186/s12889-017-4948-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hackett RA, Hudson JL, Chilcot J. Loneliness and type 2 diabetes incidence: findings from the English Longitudinal Study of Ageing. Diabetologia 2020;63:2329–2338. 10.1007/s00125-020-05258-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rawshani A, Rawshani A, Franzén S, Sattar N, Eliasson B, Svensson A, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2018;379:633–644. 10.1056/NEJMoa1800256 [DOI] [PubMed] [Google Scholar]
- 15. Wright AK, Suarez-Ortegon MF, Read SH, Kontopantelis E, Buchan I, Emsley R, et al. Risk factor control and cardiovascular event risk in people with type 2 diabetes in primary and secondary prevention settings. Circulation 2020;142:1925–1936. 10.1161/CIRCULATIONAHA.120.046783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Association AD . 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021;44:S125–S150. 10.2337/dc21-S010 [DOI] [PubMed] [Google Scholar]
- 18. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice: developed by the task force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European Association of Preventive Cardiology (EAPC). Eur Heart J 2021;42:3227–3337.34458905 [Google Scholar]
- 19. Scirica BM, Mosenzon O, Bhatt DL, Jacob AU, Steg PG, McGuire DK, et al. Cardiovascular outcomes according to urinary albumin and kidney disease in patients with type 2 diabetes at high cardiovascular risk: observations from the SAVOR-TIMI 53 trial. JAMA Cardiol 2018;3:155–163. 10.1001/jamacardio.2017.4228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fox CS, Matsushita K, Woodward M, Bilo HJG, Chalmers J, Heerspink HJL, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet 2012;380:1662–1673. 10.1016/S0140-6736(12)61350-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levey AS, Eckardt K, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005;67:2089–2100. 10.1111/j.1523-1755.2005.00365.x [DOI] [PubMed] [Google Scholar]
- 22. Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, et al. The physical activity guidelines for Americans. JAMA 2018;320:2020–2028. 10.1001/jama.2018.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th Edition. 2015, 1–122. http://health.gov/dietaryguidelines/2015/guidelines/ (1 November 2022, date last accessed).
- 24. Yousaf S, Bonsall A. UK Townsend Deprivation Scores from 2011 Census Data. Colchester, UK: UK Data Service; 2017. [Google Scholar]
- 25. Ma H, Zhou T, Li X, Maraganore D, Heianza Y, Qi L. Early-life educational attainment, APOE ε4 alleles, and incident dementia risk in late life. Geroscience 2022;44:1479–1488. 10.1007/s11357-022-00545-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dregan A, Rayner L, Davis KAS, Bakolis I, De La Torre JA, Das-Munshi J, et al. Associations between depression, arterial stiffness, and metabolic syndrome among adults in the UK Biobank population study: a mediation analysis. JAMA Psychiatry 2020;77:598–606. 10.1001/jamapsychiatry.2019.4712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eastwood SV, Mathur R, Atkinson M, Brophy S, Sudlow C, Flaig R, et al. Algorithms for the capture and adjudication of prevalent and incident diabetes in UK Biobank. PLoS One 2016;11:e0162388. 10.1371/journal.pone.0162388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heller G. A measure of explained risk in the proportional hazards model. Biostatistics 2012;13:315–325. 10.1093/biostatistics/kxr047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Knol MJ, VanderWeele TJ, Groenwold RHH, Klungel OH, Rovers MM, Grobbee DE. Estimating measures of interaction on an additive scale for preventive exposures. Eur J Epidemiol 2011;26:433–438. 10.1007/s10654-011-9554-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Song Q, Sun D, Zhou T, Li X, Ma H, Liang Z, et al. Perinatal exposure to maternal smoking and adulthood smoking behaviors in predicting cardiovascular diseases: a prospective cohort study. Atherosclerosis 2021;328:52–59. 10.1016/j.atherosclerosis.2021.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bu F, Zaninotto P, Fancourt D. Longitudinal associations between loneliness, social isolation and cardiovascular events. Heart 2020;106:1394–1399. 10.1136/heartjnl-2020-316614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Valtorta NK, Kanaan M, Gilbody S, Hanratty B. Loneliness, social isolation and risk of cardiovascular disease in the English Longitudinal Study of Ageing. Eur J Prev Cardiol 2018;25:1387–1396. 10.1177/2047487318792696 [DOI] [PubMed] [Google Scholar]
- 34. Miao Jonasson J, Hendryx M, Shadyab AH, Kelley E, Johnson KC, Kroenke CH, et al. Social support, social network size, social strain, stressful life events, and coronary heart disease in women with type 2 diabetes: a cohort study based on the women’s health initiative. Diabetes Care 2020;43:1759–1766. 10.2337/dc19-2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Strom JL, Egede LE. The impact of social support on outcomes in adult patients with type 2 diabetes: a systematic review. Curr Diab Rep 2012;12:769–781. 10.1007/s11892-012-0317-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Frosch DL, Uy V, Ochoa S, Mangione CM. Evaluation of a behavior support intervention for patients with poorly controlled diabetes. Arch Intern Med 2011;171:2011–2017. 10.1001/archinternmed.2011.497 [DOI] [PubMed] [Google Scholar]
- 37. McEwen MM, Pasvogel A, Gallegos G, Barrera L. Type 2 diabetes self-management social support intervention at the U.S.-Mexico border. Public Health Nurs 2010;27:310–319. 10.1111/j.1525-1446.2010.00860.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Murrock CJ, Higgins PA, Killion C. Dance and peer support to improve diabetes outcomes in African American women. Diabetes Educ 2009;35:995–1003. 10.1177/0145721709343322 [DOI] [PubMed] [Google Scholar]
- 39. Heisler M, Vijan S, Makki F, Piette JD. Diabetes control with reciprocal peer support versus nurse care management: a randomized trial. Ann Intern Med 2010;153:507–515. 10.7326/0003-4819-153-8-201010190-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Long JA, Jahnle EC, Richardson DM, Loewenstein G, Volpp KG. Peer mentoring and financial incentives to improve glucose control in African American veterans: a randomized trial. Ann Intern Med 2012;156:416–424. 10.7326/0003-4819-156-6-201203200-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de Jong Gierveld J, Van Tilburg T, Dykstra PA. Loneliness and Social Isolation: Cambridge handbook of personal relationships; 2006: 485. [Google Scholar]
- 42. Perlman D. Loneliness: A Sourcebook of Current Theory, Research and Therapy: John Wiley & Sons Incorporated; 1982: 36. [Google Scholar]
- 43. Smith RW, Barnes I, Green J, Reeves GK, Beral V, Floud S. Social isolation and risk of heart disease and stroke: analysis of two large UK prospective studies. Lancet Public Health 2021;6:e232–e239. 10.1016/S2468-2667(20)30291-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol 2017;186:1026–1034. 10.1093/aje/kwx246 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study has been conducted using the UK Biobank Resource, approved project number 29256. The UK Biobank will make the source data available to all bona fide researchers for all types of health-related research that is in the public interest, without preferential or exclusive access for any persons. All researchers will be subject to the same application process and approval criteria as specified by UK Biobank. For more details on the access procedure, see the UK Biobank website: http://www.ukbiobank.ac.uk/register-apply.