Abstract
Aims
Cardiac troponin T and I can be measured using a number of high-sensitivity (hs) assays. This study aimed to characterize correlations between four such assays and test their comparative associations with mortality.
Methods and results
Among adults without cardiovascular disease in the 1999–2004 National Health and Nutrition Examination Survey, hs-troponin T was measured using one assay (Roche) and hs-troponin I using three assays (Abbott, Siemens, and Ortho). Cox regression was used to estimate associations with all-cause and cardiovascular mortality. Pearson’s correlation coefficients comparing concentrations from each assay ranged from 0.53 to 0.77. There were 2188 deaths (488 cardiovascular) among 9810 participants. Each hs-troponin assay [log-transformed, per 1 standard deviation (SD)] was independently associated with all-cause mortality: hazard ratio (HR) 1.20 [95% confidence interval (CI) 1.13–1.28] for Abbott hs-troponin I; HR 1.10 (95% CI 1.02–1.18) for Siemens hs-troponin I; HR 1.23 (95% CI 1.14–1.33) for Ortho hs-troponin I; and HR 1.31 (95% CI 1.21–1.42) for Roche hs-troponin T. Each hs-troponin assay was also independently associated with cardiovascular mortality (HR 1.44 to 1.65 per 1 SD). Associations of hs-troponin T and all-cause and cardiovascular mortality remained significant after adjusting for hs-troponin I. Furthermore, associations of hs-troponin I remained significant after mutually adjusting for hs-troponin I from the other individual assays: e.g. cardiovascular mortality HR 1.46 (95% CI 1.19–1.79) for Abbott after adjustment for the Siemens assay and HR 1.29 (95% CI 1.09–1.53) for Abbott after adjustment for the Ortho assay.
Conclusion
This study demonstrates only modest correlations between hs-troponin T and three hs-troponin I assays and that hs-troponin I assays can provide distinct risk information for mortality in the general population.
Keywords: High-sensitivity cardiac troponin, All-cause mortality, Cardiovascular mortality, NHANES
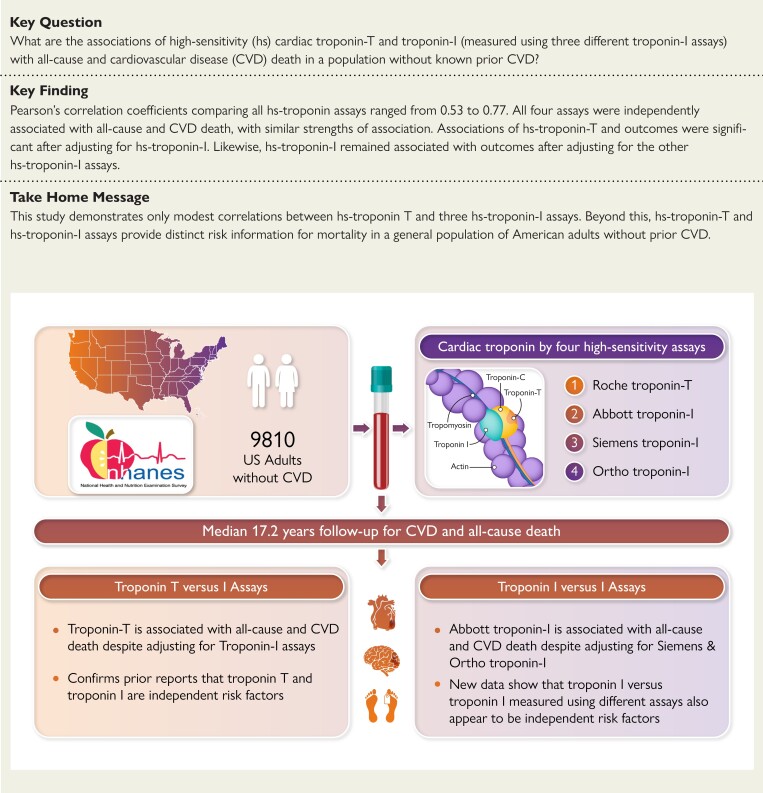
Structured Graphical Abstract
Structured Graphical Abstract.
CVD, cardiovascular disease; F/U, follow-up; NHANES, National Health and Nutrition Examination Survey; yr, year.
See the editorial comment for this article ‘Comparisons of multiple troponin assays for detecting chronic myocardial injury in the general population: redundant or complementary?', by J. A. de Lemos and J. D. Berry, https://doi.org/10.1093/eurheartj/ehad414.
Introduction
High-sensitivity cardiac troponin (hs-troponin) assays have transformed the practice of modern cardiology.1,2 For example, the heightened sensitivity of these assays compared to previous generation assays enables the earlier diagnosis of acute myocardial infarction (MI).3,4 Studies in selected cohorts of middle-aged and older adults have also demonstrated that elevated hs-troponin is an important independent risk factor for future cardiovascular disease (CVD) events, both among adults with a history of prior CVD (secondary prevention)5,6 and among asymptomatic ambulatory adults without a history of prior CVD (primary prevention).7–10
Few data are available in younger adults and associations of hs-troponin with all-cause and CVD mortality have not been reported in a cohort that was specifically designed to be representative of adults in the general population. In addition, while research indicates that the I and T subunits of hs-troponin may provide independent prognostic information in primary prevention,11,12 few large epidemiologic studies have conducted head-to-head comparisons of the various hs-troponin I assays available from different manufacturers.
We measured hs-troponin using three different hs-troponin I assays and one hs-troponin T assay in stored serum from adults aged 18 or older participating in the 1999–2004 National Health and Nutrition Examination Survey (NHANES). The aims of the present analysis were to (i) compare the prevalence of elevated hs-troponin concentrations using each of these four assays in a representative sample of adults from the general US population without CVD, (ii) evaluate correlations between the four assays, and (iii) characterize the associations of these four hs-troponin assays with all-cause and CVD mortality, overall and across age groups.
Methods
Study population
The NHANES is designed to be a nationally representative sample of the US population. Participants were selected from the US non-institutionalized, civilian population using a complex, stratified, multistage probability cluster sampling design.13 We included individuals with available stored blood in NHANES 1999–2004. There was a total of 13 252 adults aged 18 years or older in NHANES 1999–2004 with available specimen and data on mortality linkage. Of this group, we excluded participants with a self-reported a history of CVD (congestive heart failure, coronary heart disease, angina, heart attack, or stroke) diagnosed by a healthcare provider (n = 1370) or missing data on relevant covariates (n = 2072). Our final analytic sample included 9810 adults with available hs-troponin concentrations for all four assays.
The NHANES protocols and the measurement of hs-troponin in stored specimens were approved by the National Center for Health Statistics ethics review board. Written informed consent was obtained from all participants.
High-sensitivity troponin measurement
We measured hs-troponin concentrations in stored serum samples at the University of Maryland School of Medicine between 2018 and 2020. Prior to 2018, these samples had been stored by the Centers for Disease Control and Prevention (CDC). The majority (93%) of stored serum samples had never undergone a prior freeze–thaw cycle. High-sensitivity troponin T was measured with the Roche Cobas e601 using Elecsys reagents [Food and Drug Administration (FDA) cleared 18 January 2016]. The reported lower limit of detection (LoD) for this 5th generation assay is 3 ng/L. High-sensitivity troponin I (Abbott) was measured using ARCHITECT i2000SR (FDA cleared 25 September 2019). The LoD for this assay is 1.7 ng/L. High-sensitivity troponin I (Siemens) was measured using Centaur XPT (FDA cleared 12 July 2018). The LoD for this assay is 1.6 ng/L. Finally, hs-troponin I (Ortho) was measured using Vitros 3600, which is not currently FDA cleared. The LoD for this assay is 0.39 ng/L.
Other variables of interest
Self-reported information on demographics and lifestyle, including race/ethnicity, smoking, family history, and medication use, was collected during a computer-assisted personal interview.
Height and weight were measured at the mobile examination center and were used to calculate body mass index (BMI) in kg/m2. Hypertension was defined using a mean systolic blood pressure ≥140 mmHg, or mean diastolic blood pressure ≥90 mmHg, or anti-hypertensive medication use. We defined diabetes as a self-reported history of diagnosis by healthcare provider or current use of diabetes medication. Total cholesterol was measured using an enzymatic method. Hypercholesterolemia was defined as total cholesterol ≥240 mg/dL or self-reported lipid-lowering medication use. Glomerular filtration rate [estimated glomerular filtration ratio (eGFR)–CrCys in mL/min/1.73 m2] was estimated using the 2021 Chronic Kidney Disease Epidemiology Collaboration equation without race.14 Race/ethnicity was self-reported based on categories provided by NHANES investigators (non-Hispanic White, non-Hispanic Black, Mexican–American, or other).
Outcomes: all-cause and cardiovascular mortality
The vital status of participants was ascertained through a probabilistic match between NHANES personal identifiers and linkage to death certificates from the National Death Index through 31 December 2019. Cardiovascular disease mortality was ascertained according to the recorded cause of death using International Classification of Diseases (ICD) 10 codes (I00-I78).
Statistical analysis
We accounted for the complex survey design and used survey weights in all analyses to generate estimates generalizable to the 1999–2004 US adult population. Standard errors for all estimates were obtained using Taylor series linearization. We summarized the weighted prevalence of US adults (both as proportions and in millions of persons using 2003–04 US Census data) who had elevated hs-troponin concentrations by each of the four assays. Our definition of elevated hs-troponin was a concentration above the sex-specific 99th percentile upper reference limit (URL) for each assay reported by the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC).15 For females and males, respectively, these concentrations were 14 and 22 ng/L for Roche hs-troponin T, 17 and 35 ng/L for Abbott hs-troponin I, 39.6 and 58 ng/L for Siemens hs-troponin I, and 9 and 12 ng/L for Ortho hs-troponin I.15 Concentrations above these thresholds are used clinically to represent myocardial injury.16
After log-transformation to approximate a normal distribution, we generated scatterplots and evaluated the pairwise Pearson’s correlation coefficient between the four hs-troponin assays and the root mean squared error (RMSE) from linear regression models. We excluded participants with concentrations below the limit of the blank for each assay in these correlation calculations.
We cross-categorized participants according to quartiles of hs-troponin concentration for each assay and generated heat maps to compare incidence rates (per 1000 person-years) of all-cause and CVD mortality using Poisson regression. We also evaluated the cumulative incidence of all-cause and CVD mortality according to hs-troponin elevation status and for each assay. We used Cox regression to model the prospective associations of baseline hs-troponin with incident all-cause and CVD mortality. We modeled hs-troponin concentrations as continuous variables [per 1 standard deviation (SD) increase on the log scale] and in categories (quartiles). We also modeled hs-troponin using restricted cubic splines (knots at the 5th, 35th, 65th, and 95th percentiles) to flexibly evaluate the shape of associations between log(hs-troponin) and all-cause and CVD mortality. To put the different assays on equal footing and since hs-troponin concentrations below the LoD have been reported to contain prognostic information,17 we included concentrations below the LoD for each assay in all Cox models.18 Model 1 included age, sex, and race/ethnicity. Model 2 included all variables in Model 1 plus BMI, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, cigarette smoking status, diabetes mellitus, family history of CVD, use of blood pressure medications, use of cholesterol-lowering medications, and eGFR. The proportionality assumption was assessed visually. We also conducted stratified analyses and tested for multiplicative effect modification by age, sex, and race/ethnicity groups. To test whether each of the hs-troponin assays was independent of the others, we adjusted for the covariates in Model 2 and then further adjusted the model for one of the three other hs-troponin assays.
We conducted a number of sensitivity analyses. First, we stratified the Cox models by baseline hs-troponin above or below the 75th percentile. Second, we estimated the associations of each hs-troponin assay with non-CVD death. Third, we conducted analyses of CVD mortality using a competing risk approach (i.e. Fine–Gray models), with non-CVD death handled as a competing outcome. Fourth, we used C-statistics to compare model discrimination for all-cause and CVD mortality comparing the base model with only one hs-troponin assay to a model with an additional hs-troponin T or I assay.19
Stata version 17.0 (StataCorp, College Station, TX, USA) was used for all analyses and a two-sided P-value of <0.05 was considered statistically significant.
Results
Prevalence of US adults with elevated high-sensitivity troponin
Our analysis included 9810 study participants without a history of CVD [mean (SD) age 44.3 (16.0) years, 52.7% female]. The prevalence of elevated hs-troponin T value above the manufacturer-designated sex-specific 99th percentile reported by IFCC was 3.1%, corresponding to 6.6 million adults (Table 1 and Supplementary material online, Table S1). The corresponding sex-specific 99th percentile URL prevalence estimates (number of US adults) were 0.6% (1.3 million) for hs-troponin I (Abbott), 0.9% (2 million) for hs-troponin I (Siemens), and 0.7% (1.6 million) for hs-troponin I (Ortho) (Table 1 and Supplementary material online, Table S1). The prevalence of elevated hs-troponin was highest in older adults and among adults with hypertension, hypercholesterolemia, diabetes, or reduced eGFR. The prevalence of elevated hs-troponin was lower in current smokers across all assays.
Table 1.
Prevalence of elevated hs-troponina, overall and according to characteristics of US adults aged 18 or older without a history of CVD, NHANES 1999–2004
| Elevated (>99 percentile) hs-troponin T by Rochea | Elevated (>99 percentile) hs-troponin I by Abbotta | Elevated (>99 percentile) hs-troponin I by Siemensa | Elevated (>99 percentile) hs-troponin I by Orthoa | |
|---|---|---|---|---|
| Total, % | 3.11 | 0.60 | 0.93 | 0.75 |
| Gender, % | ||||
| Male | 2.54 | 0.52 | 1.02 | 0.66 |
| Female | 3.62 | 0.67 | 0.85 | 0.82 |
| Age categories, % | ||||
| 18–39 years | 0.33 | 0.36 | 0.41 | 0.32 |
| 40–59 years | 1.74 | 0.38 | 0.70 | 0.48 |
| 60 years or older | 12.6 | 1.64 | 2.64 | 2.32 |
| Race/ethnicity, % | ||||
| NH White | 3.34 | 0.55 | 0.81 | 0.74 |
| NH Black | 4.35 | 1.48 | 1.71 | 1.50 |
| Mexican–American | 1.19 | 0.43 | 0.88 | 0.22 |
| Other/other Hispanic | 1.66 | 0.16 | 1.00 | 0.41 |
| Hypertension, % | ||||
| No | 1.51 | 0.37 | 0.60 | 0.44 |
| Yes | 7.70 | 1.21 | 1.82 | 1.57 |
| Hypercholesterolemia, % | ||||
| No | 2.49 | 0.51 | 0.79 | 0.65 |
| Yes | 5.15 | 0.89 | 1.40 | 1.05 |
| eGFR, mL/min/1.73 m2, % | ||||
| >90 | 1.84 | 0.34 | 0.57 | 0.36 |
| 60–90 | 21.3 | 0.96 | 2.36 | 1.28 |
| <60 | 65.3 | 7.86 | 5.35 | 11.4 |
| Smoking status, % | ||||
| Current | 2.08 | 0.49 | 0.62 | 0.43 |
| Former | 4.75 | 0.77 | 1.38 | 1.37 |
| Never | 2.84 | 0.57 | 0.87 | 0.61 |
| Family history of CVD, % | ||||
| No | 3.32 | 0.64 | 1.02 | 0.79 |
| Yes | 1.83 | 0.36 | 0.36 | 0.50 |
| Diabetes mellitus, % | ||||
| No | 2.73 | 0.55 | 0.88 | 0.74 |
| Yes | 10.1 | 1.55 | 1.97 | 0.91 |
| BMI, kg/m2, % | ||||
| <25 | 3.36 | 0.63 | 0.98 | 0.72 |
| 25–30 | 2.87 | 0.53 | 0.99 | 0.85 |
| >30 | 3.10 | 0.64 | 0.81 | 0.65 |
BMI, body mass index; CVD, cardiovascular disease; eGFR, estimated glomerular filtration ratio using the creatinine cystatin equation; HDL, high-density lipoprotein; NH, non-Hispanic; SBP, systolic blood pressure; URL, upper reference limit.
Values above the manufacturer-designated assay- and sex-specific 99th percentile URL myocardial injury threshold were defined as elevated; troponin T 14(f)/22(m) ng/L; troponin I, Abbott 17(f)/35(m) ng/L; troponin I, Siemens 39.6(f)/58(m) ng/L; troponin I, Ortho 9(f) 12(m) ng/L.
High-sensitivity troponin and mortality outcomes
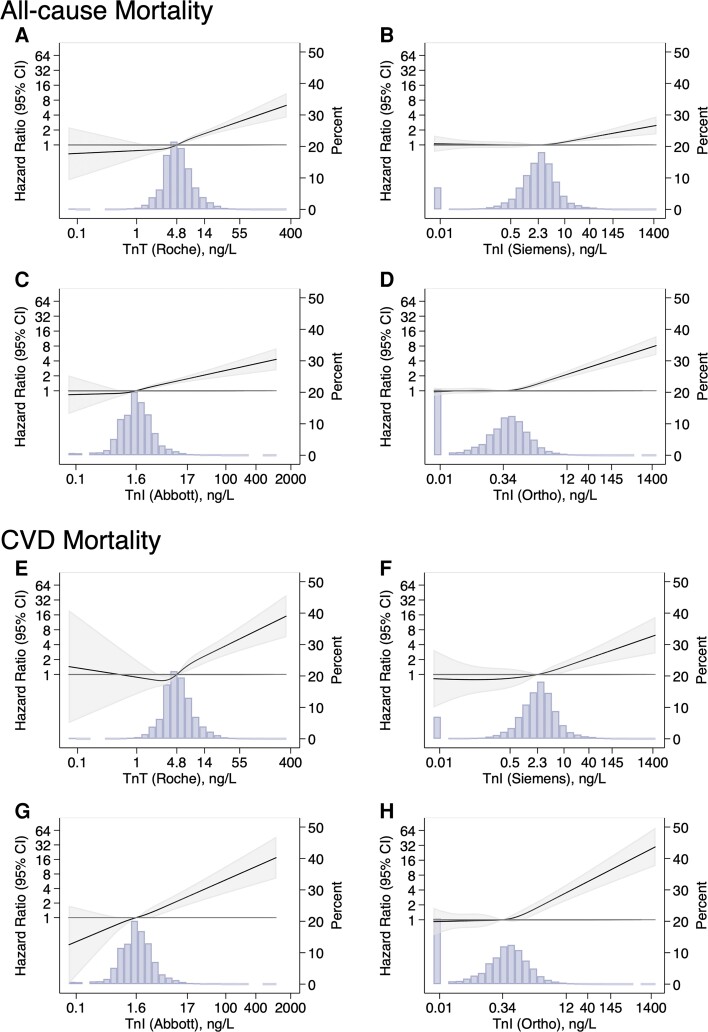
Over a median follow-up of 17.2 years, there were 2188 deaths (488 from cardiovascular causes). High-sensitivity troponin according to all four assays was robustly and independently associated with all-cause and CVD mortality (Table 2 and Supplementary material online, Table S2). The strength of association (slope) between each assay and both all-cause and CVD mortality was similar overall (Figure 1). Of note, while the point estimated of the hazard ratio for all-cause death was higher for hs-troponin T (per 1 SD increase) than for the hs-troponin I assays, the 95% confidence intervals were mostly overlapping (Table 2). The hazard ratio for CVD mortality was similar for all assays (per 1 SD increase, Table 2). The shape of associations between elevated log-transformed hs-troponin and both all-cause and CVD mortality was also roughly linear down to concentrations close to the assay LoD (Figure 1). Findings with hs-troponin modeled on the arithmetic scale are in Supplementary material online, eFigures S1 and S2.
Table 2.
Adjusteda HR (95% CI) of log-transformed hs-troponin (per 1 SD increase) with all-cause and cardiovascular mortality, overall and by subgroups of interest
| n/N | Roche hs-troponin T | Abbott hs-troponin I | Siemens hs-troponin I | Ortho hs-troponin I | |
|---|---|---|---|---|---|
| hazard ratio (95% CI) | hazard ratio (95% CI) | hazard ratio (95% CI) | hazard ratio (95% CI) | ||
| All-cause mortality | |||||
| Overall | 2188/9810 | 1.31 (1.21–1.42) | 1.20 (1.13–1.28) | 1.10 (1.02–1.18) | 1.23 (1.14–1.33) |
| Age group | |||||
| 18–39 years | 113/3902 | 1.02 (0.83–1.24) | 0.97 (0.78–1.22) | 0.92 (0.76–1.11) | 1.02 (0.79–1.31) |
| 40–59 years | 392/3022 | 1.30 (1.09–1.54) | 1.10 (0.96–1.24) | 0.97 (0.82–1.15) | 1.15 (0.98–1.34) |
| 60 years or older | 1683/2886 | 1.33 (1.21–1.45) | 1.30 (1.23–1.37) | 1.30 (1.19–1.42) | 1.36 (1.19–1.55) |
| P-interaction | <0.001 | 0.01 | <0.001 | 0.01 | |
| Sex | |||||
| Male | 1163/4579 | 1.18 (1.06–1.30) | 1.15 (1.05–1.26) | 1.03 (0.90–1.18) | 1.13 (0.99–1.29) |
| Female | 1025/5231 | 1.49 (1.32–1.67) | 1.26 (1.16–1.36) | 1.14 (1.03–1.26) | 1.31 (1.15–1.49) |
| P-interaction | 0.01 | 0.13 | 0.22 | 0.18 | |
| Race/ethnic group | |||||
| Non-Hispanic White | 1267/4966 | 1.30 (1.19–1.42) | 1.22 (1.12–1.33) | 1.12 (1.00–1.25) | 1.26 (1.13–1.41) |
| Non-Hispanic Black | 385/1730 | 1.39 (1.17–1.64) | 1.14 (1.03–1.27) | 1.11 (0.89–1.40) | 1.11 (0.95–1.30) |
| Mexican American | 413/2305 | 1.23 (0.97–1.57) | 1.22 (1.03–1.44) | 1.07 (0.78–1.48) | 1.33 (1.09–1.63) |
| Other | 123/809 | 1.39 (1.12–1.73) | 1.15 (0.89–1.49) | 0.98 (0.80–1.20) | 1.13 (0.78–1.64) |
| P-interaction | 0.004 | 0.009 | 0.209 | 0.004 | |
| CVD mortality | |||||
| Overall | 488/9810 | 1.49 (1.28–1.75) | 1.44 (1.26–1.66) | 1.45 (1.06–1.97) | 1.65 (1.23–2.22) |
| Age group | |||||
| 18–39 year | 16/3902 | 0.91 (0.38–2.19) | 0.92 (0.51–1.64) | 0.88 (0.33–2.35) | 0.70 (0.37–1.34) |
| 40–59 year | 75/3022 | 1.31 (0.95–1.80) | 1.16 (0.82–1.64) | 0.97 (0.60–1.58) | 1.32 (0.87–2.02) |
| 60 year or older | 397/2886 | 1.57 (1.28–1.91) | 1.63 (1.40–1.89) | 1.89 (1.50–2.39) | 2.04 (1.54–2.72) |
| P-interaction | 0.69 | 0.42 | 0.05 | 0.03 | |
| Sex | |||||
| Male | 265/4579 | 1.33 (1.09–1.62) | 1.38 (1.17–1.62) | 1.34 (0.96–1.88) | 1.64 (1.17–2.30) |
| Female | 223/5231 | 1.68 (1.33–2.11) | 1.50 (1.24–1.83) | 1.49 (1.01–2.19) | 1.62 (1.12–2.35) |
| P-interaction | 0.006 | 0.149 | 0.319 | 0.56 | |
| Race/ethnic group | |||||
| Non-Hispanic White | 296/4966 | 1.54 (1.28–1.85) | 1.52 (1.29–1.78) | 1.65 (1.18–2.32) | 1.89 (1.34–2.67) |
| Non-Hispanic Black | 94/1730 | 1.41 (0.97–2.05) | 1.16 (0.90–1.48) | 1.07 (0.59–1.92) | 1.38 (0.97–1.96) |
| Mexican American | 75/2305 | 1.40 (0.88–2.23) | 1.33 (0.76–2.32) | 1.28 (0.97–1.67) | 1.33 (0.80–2.22) |
| Other | 23/809 | 1.03 (0.60–1.77) | 1.05 (0.71–1.53) | 0.81 (0.36–1.82) | 0.74 (0.40–1.38) |
| P-interaction | 0.011 | 0.035 | 0.030 | 0.001 | |
CVD, cardiovascular disease; SD, standard deviation; eGFR, estimated glomerular filtration ratio.
hs-troponin modeled as a continuous (per 1 SD increase on the log scale) exposure and adjusted for age, sex, race/ethnicity, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, cigarette smoking status, diabetes mellitus, family history of CVD, body mass index, use of blood pressure medications, use of cholesterol-lowering medications, and eGFR.
Figure 1.
Hazard ratios (95% confidence intervals) for four high-sensitivity troponin assays with all-cause mortality (panel A, high-sensitivity troponin T, Roche; panel B, high-sensitivity troponin I, Siemens; panel C, high-sensitivity troponin I, Abbott; panel D, high-sensitivity troponin I, Ortho) and cardiovascular disease mortality (panel E, high-sensitivity troponin T, Roche; panel F, high-sensitivity troponin I, Siemens; panel G, high-sensitivity troponin I, Abbott; panel H, high-sensitivity troponin I, Ortho) among US adults without a history of cardiovascular disease, NHANES 1999–2004. High-sensitivity troponin was log-transformed and modeled as a restricted cubic spline (solid line). Knots were placed at 5th, 35th, 65th, 95th, percentiles. The shaded areas on each side of the regression line are the 95% confidence intervals. The background shaded area is the distribution (histogram) of each high-sensitivity troponin assay in the population. The models were adjusted for age, sex, race/ethnicity, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, cigarette smoking status, diabetes mellitus, family history of cardiovascular disease, body mass index, use of blood pressure medications, use of cholesterol-lowering medications, and estimated glomerular filtration ratio. CI, confidence interval; CVD, cardiovascular disease; Tn, troponin.
Associations of hs-troponin with all-cause and CVD mortality tended to be stronger among older adults (≥60 years) compared to younger adults (Table 2). When analyzing the associations between each of the four hs-troponin assays and both all-cause and CVD death, six out of eight tests for interaction by age were statistically significant (P-interaction < 0.05). Except for hs-troponin T, associations of hs-troponin with all-cause and CVD mortality were similar by sex. The hazard ratio point estimates for each hs-troponin assay were also similar when comparing non-Hispanic Whites, non-Hispanic Blacks, and Mexican–Americans. Nonetheless, interaction P-values by race/ethnicity were <0.05, a finding that appeared to be driven by more statistically significant associations in non-Hispanic Whites.
High-sensitivity troponin T was independently associated with all-cause and CVD mortality after individual adjustment for each of the hs-troponin I assays (Table 3). Except for the Siemens assay, hs-troponin I concentrations were independently associated with all-cause and CVD mortality after adjustment for hs-troponin T. The Abbott hs-troponin I assay remained significantly associated with all-cause and CVD mortality after adjustment for the Siemens hs-troponin I or the Ortho hs-troponin I assay. The Ortho hs-troponin I was also significantly associated with CVD and all-cause mortality after further adjustment for each of the other hs-troponin I assays. The Siemens hs-troponin I assay was no longer significantly associated with CVD or all-cause mortality after adjustment for the Abbott or Ortho hs-troponin I assays.
Table 3.
Adjusteda HR (95% CI) for log-transformed hs-troponin (per 1 SD) with all-cause mortality with and without mutual adjustment for the other hs-troponin assays
| Roche hs-troponin T | Abbott hs-troponin I | Siemens hs-troponin I | Ortho hs-troponin I | |
|---|---|---|---|---|
| hazard ratio (95% CI) | hazard ratio (95% CI) | hazard ratio (95% CI) | hazard ratio (95% CI) | |
| All-cause mortality | ||||
| Model 1 | 1.46 (1.35–1.58) | 1.29 (1.23–1.36) | 1.18 (1.10–1.27) | 1.31 (1.22–1.42) |
| Model 2 | 1.31 (1.21–1.42) | 1.20 (1.13–1.28) | 1.10 (1.02–1.18) | 1.23 (1.14–1.32) |
| Model 2 plus Roche hs-troponin Tb | - | 1.10 (1.01–1.19) | 0.99 (0.92–1.06) | 1.11 (1.02–1.20) |
| Model 2 plus Abbott hs-troponin Ib | 1.25 (1.14–1.38) | - | 0.94 (0.84–1.05) | 1.12 (1.02–1.22) |
| Model 2 plus Siemens hs-troponin Ib | 1.31 (1.21–1.42) | 1.24 (1.12–1.37) | - | 1.22 (1.12–1.32) |
| Model 2 plus Ortho hs-troponin Ib | 1.27 (1.16–1.38) | 1.15 (1.06–1.24) | 1.02 (0.94–1.10) | - |
| CVD mortality | ||||
| Model 1 | 1.77 (1.53–2.04) | 1.60 (1.41–1.81) | 1.66 (1.23–2.24) | 1.91 (1.48–2.47) |
| Model 2 | 1.49 (1.28–1.75) | 1.44 (1.26–1.66) | 1.45 (1.06–1.97) | 1.65 (1.23–2.22) |
| Model 2 plus Roche hs-troponin Tb | - | 1.28 (1.11–1.47) | 1.20 (0.91–1.58) | 1.39 (1.03–1.88) |
| Model 2 plus Abbott hs-troponin Ib | 1.30 (1.11–1.52) | - | 0.98 (0.71–1.36) | 1.29 (0.94–1.78) |
| Model 2 plus Siemens hs-troponin Ib | 1.41 (1.22–1.64) | 1.46 (1.19–1.79) | - | 1.52 (1.11–2.07) |
| Model 2 plus Ortho hs-troponin Ib | 1.35 (1.13–1.60) | 1.29 (1.09–1.53) | 1.19 (0.87–1.63) | - |
CVD, cardiovascular disease; SD, standard deviation; eGFR, estimated glomerular filtration ratio.
Model 1: association of each hs-troponin assay (Columns 2–5) with events after adjusting for age, sex, race/ethnicity. Model 2: adjusted for age, sex, race/ethnicity, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, cigarette smoking status, diabetes mellitus, family history of CVD, body mass index, use of blood pressure medications, use of cholesterol-lowering medications, and eGFR.
These models report the association of each hs-troponin exposure with mortality, after adjustment for the variables in Model 2 and further adjustment for concentrations from one other hs-troponin assay (i.e. these models contain two hs-troponin assays).
C-statistics demonstrated statistically significant improvements in model discrimination when hs-troponin T was added to base models that included each of hs-troponin I assays. For CVD mortality, the addition of either Abbott hs-troponin I or Ortho hs-troponin I increased the C-statistic of base models that included Siemens hs-troponin I (see Supplementary material online, Table S3).
In a sensitivity analysis, we repeated the continuous Cox models after first stratifying our sample into persons with baseline hs-troponin I (Abbott) concentrations above or below the 75th percentile. These analyses suggest that the independent prognostic value of the various hs-troponin assays was present at high and low concentrations (i.e. across the entire range of hs-troponin values) (see Supplementary material online, Tables S4 and S5). In sensitivity analyses for CVD death, using Fine–Gray models that accounted for non-CVD death as a competing outcome, results did not differ meaningfully from the main Cox regression analyses (see Supplementary material online, Table S6).
In analyses of the association of hs-troponin with non-CVD mortality, hs-troponin T, hs-troponin I (Abbott), and hs-troponin I (Ortho) were significantly associated with death in Model 2 (see Supplementary material online, Table S7). While hs-troponin T remained associated with non-CVD mortality even when adjusted for each of the three hs-troponin I assays, none of hs-troponin I assays remained significant predictors in models that adjusted for hs-troponin T.
Discordance between high-sensitivity troponin assay concentrations
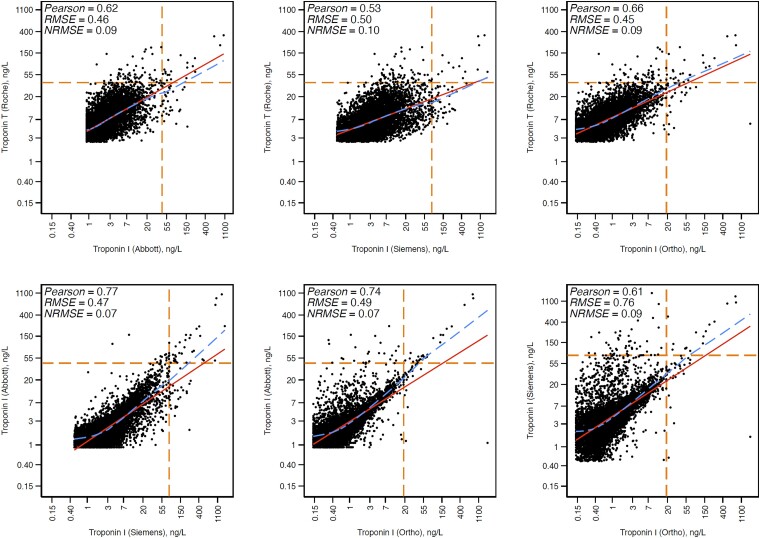
The pairwise Pearson’s correlations between assays were modest, ranging from r = 0.53 for hs-troponin T and Siemens hs-troponin I to r = 0.77 for Abbott and Siemens hs-troponin I (Figure 2). The typical deviation (RMSE) from the regression line ranged from 0.45 to 0.76.
Figure 2.
Unweighted pairwise correlations between log-transformed high-sensitivity troponin assays among primary prevention US adults aged 18 or older with concentrations above the assay limit of blank, NHANES 1999–2004. Unweighted Scatterplots. Non-linear dashed line, locally weighted scatterplot smoothing; straight unbroken line, linear regression; horizontal and vertical intersecting dashed lines, 99th percentile of troponin in the analytic sample. Participants with high-sensitivity troponin concentrations below the limit of blank for each assay were excluded from correlation analyses. RMSE, root mean square error; NRMSE, normalized root mean square error.
When participants were cross-categorized according to quartiles of hs-troponin concentration for two assays, absolute mortality incidence rates were highest when both assays had concentrations in the upper quartile (see Supplementary material online, eFigure S3). Mortality rates were lower in the presence of discordant concentrations of hs-troponin by the two assays under comparison or when both assays had concentrations in the lowest quartile. These findings were similar whether Abbott hs-troponin I was being compared to hs-troponin T or to another hs-troponin I assay. Qualitatively similar results were also obtained when the hs-troponin assays were modeled as binary exposures (elevated vs. non-elevated) (see Supplementary material online, eFigure S4).
Discussion
To our knowledge, this is the first prognostic study of multiple hs-troponin assays from a single cohort, comparing not just hs-troponin T vs. hs-troponin I but also comparing the various hs-troponin I assays available from manufacturers (i.e., hs-troponin I vs. hs-troponin I). Two major findings emerged from these comparisons. First, in this nationally representative sample of US adults, we found that 3.1% of all adults without a history of CVD had hs-troponin T concentrations above the manufacturer-reported threshold used to define myocardial injury. By contrast, fewer than 1% of the population had hs-troponin I concentrations above thresholds used to define myocardial injury. This finding highlights a lack of uniformity in the approach taken by manufacturers to derive 99th percentiles for each hs-troponin assay. Second, we demonstrated that correlations between the four assays tested were moderate. In keeping with these lower than anticipated correlations, not only was hs-troponin T independently associated with mortality outcomes despite adjustment for hs-troponin I (as previously described), but also associations of the Abbott and Ortho hs-troponin I assays with mortality outcomes remained significant even after adjustment for each other or for the Siemens hs-troponin I assay (Structured Graphical Abstract).
Our results call into some question the applicability of manufacturer-designated hs-troponin myocardial injury thresholds when applied to the full age range of the entire primary prevention adult population. The estimated overall 3.1% prevalence of elevated hs-troponin T in the entire US adult population without CVD might be considered high given the threshold for myocardial injury is based in theory on a 99th percentile concentration derived from a healthy reference sample (accordingly one would expect a prevalance of elevated hs-troponin T in the primary prevention population closer to 1%). Indeed, the prevalence of elevated hs-troponin T in our primary prevention sample was as high as 12.6% among adults aged 60 years or older. One explanation for this might be that the strict definition of health required in selecting the reference samples used to derive 99th percentile upper reference limits (URLs) means that hs-troponin T URLs do not generalize to the entire primary prevention population (including older adults and those with comorbidities). By contrast, the prevalence of myocardial injury using manufacturer-designated URL thresholds for the hs-troponin I assays in the entire primary prevention population appeared more consistent with a 99th percentile, with our analyses indicating that <1% of all primary prevention adults are above these thresholds. On the other hand, this <1% overall prevalence above manufacturer-designated 99th percentile URLs for the hs-troponin I assays could also suggest that these thresholds may be too high when applied to a strictly defined healthy representative subgroup that excludes all US adults with any CVD risk factors or other markers of ill-health. Irrespective, what is clear is that our results point to a lack of uniformity in the approach taken by manufacturers to derive 99th percentiles for the hs-troponin T vs. hs-troponin I assays.
Perhaps more importantly, our data also suggest that measurement of hs-troponin using at least two assays appears to provide additional independent information regarding mortality risk in the general adult population. The independent association of hs-troponin T with CVD and all-cause mortality after adjustment for the different hs-troponin I assays is consistent with other studies in older primary prevention populations11,12 and also in secondary prevention settings.20,21 These prior studies also suggest that hs-troponin T may be a stronger risk factor for non-CVD outcomes, which is consistent with our results. It has been speculated that these independent associations may relate, in part, to low correlation between the hs-troponin T and hs-troponin I assays due to factors like skeletal muscle disease.22,23 Another potential explanation for the independent associations of hs-troponin T with events may relate to differences in the relationship between renal dysfunction and concentrations of hs-troponin T vs. hs-troponin I.24
However, we are not aware of prior studies that have conducted head-to-head comparisons of the different hs-troponin I assays. The independent prognostic value of the Abbott and Ortho hs-troponin I assays even after adjusting for each other or the Siemens hs-troponin I assay suggests that hs-troponin I assays provide distinct information for risk stratification. The exact reasons for this merit further study. The clinical implications of our findings will also depend on the future uptake of hs-troponin testing in assessing cardiovascular risk among ambulatory primary prevention populations. While this practice is not yet commonplace, hs-troponin I (Abbott) does have an indication for risk assessment, and guidelines like the 2022 one from the American Diabetes Association25 are increasingly supporting the use of biomarker testing to assess risk and screen for subclinical disease. Whether the independent prognostic information carried by various hs-troponin assays warrants a strategy of dual vs. single-assay testing when estimating prognosis in ambulatory populations will need to be assessed.
Imperfect correlation between the various hs-troponin I assay concentrations has been previously noted in cohorts of adults without CVD.26 One explanation is that our results are from primary prevention adults mostly with hs-troponin concentrations in the low range (where the assays may be less reliable). However, modest concordance across hs-troponin assays has also been noted in acutely symptomatic patients with suspected MI and high hs-troponin concentration.27–29 High-sensitivity troponin assays are heterogeneously affected by heterophile antibodies, macro-troponin, and spuriously elevated results (so-called fliers).30–35 The hs-troponin I assays can also differ with respect to binding sites of capture and detection antibodies.15,36 These antigen differences may be relevant in part because troponin I is often fragmented in circulation.37–39 Indeed, when looking more closely at the correlation plots (Figure 2), the projected intercept of the regression line for all comparisons is highly unlikely to cross zero, suggesting there may be a fixed bias when comparing hs-troponin I concentrations across assays. This, along with the independent prognostic information in comparing the hs-troponin I assays, suggests that there are fundamental differences in what is being measured by these hs-troponin I assays. Before their deployment into clinical risk prediction, a better understanding of these differences and their mechanisms is needed.
Important limitations to consider in the interpretation of our results include the following: first, the ascertainment of all-cause and CVD death in NHANES was based exclusively on ICD codes and we did not have information on non-fatal cardiovascular events. Cardiovascular disease death is only available in NHANES as a composite outcome, and we were unable to study specific causes of death like coronary death. Second, hs-troponin measurements were obtained from long-term stored serum samples, though the inter-assay coefficients of variation for these hs-troponin assays were excellent and they have previously been shown to be accurate and reliable in long-term stored samples.15,40–43 Third, history of CVD was self-reported. Self-reported CVD is known to be specific but lacks sensitivity.44 We excluded persons with CVD from the current analysis and plan to study those individuals separately. However, we acknowledge that our sample may have included persons with atrial fibrillation or other forms of subclinical CVD. Fourth, our ability to examine mortality among some subgroups was limited, e.g. due to the low the number of deaths at younger ages. Fifth, we used manufacturer-designated 99th percentile URLs to report on prevalence of elevated hs-troponin or myocardial injury (which are provided by the IFCC15) though these manufacturer-reported thresholds have changed over time depending on assay iteration and equipment and other factors. Sixth, our results relate to current generation hs-troponin assays, and findings may differ for the ultra-hs assays in development.
The strengths of this study include the measurement of multiple hs-troponin assays in a large, diverse, and nationally representative population. Our analysis also benefited from rigorous and standardized ascertainment of CVD risk factors.
In conclusion, the prevalence of myocardial injury in the general US adult population is often >1%, particularly among older persons, and there are differences in myocardial injury prevalence between hs-troponin T and I assays. For cardiac troponin concentrations above the assay limit of detection, each of the four hs-troponin assays tested had a similar shape and strength of association with all-cause and CVD death. Compared to hs-troponin I, hs-troponin T was more robustly associated with non-CVD death. In US adults, we also found that the different hs-troponin assays provided independent prognostic information for mortality, even after mutual adjustment. These results should motivate research into understanding the modest correlations between hs-troponin assays and understanding the reasons why they provide independent prognostic information.
Supplementary Material
Acknowledgments
Reagents for hs-troponin assays were donated by the manufacturers.
Contributor Information
John W McEvoy, University of Galway School of Medicine and National Institute for Prevention and Cardiovascular Health, Moyola Lane, Newcastle, Galway H91-FF68, Connacht, Ireland; Johns Hopkins Hospital and Johns Hopkins University School of Medicine, 1800 Orleans Street, Baltimore, MD 21287, USA; Department of Epidemiology and Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins Bloomberg School of Public Health, 2024 E Monument Street, Baltimore, MD 21205, USA.
Natalie Daya, Department of Epidemiology and Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins Bloomberg School of Public Health, 2024 E Monument Street, Baltimore, MD 21205, USA.
Olive Tang, Johns Hopkins Hospital and Johns Hopkins University School of Medicine, 1800 Orleans Street, Baltimore, MD 21287, USA.
Michael Fang, Department of Epidemiology and Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins Bloomberg School of Public Health, 2024 E Monument Street, Baltimore, MD 21205, USA.
Chiadi E Ndumele, Johns Hopkins Hospital and Johns Hopkins University School of Medicine, 1800 Orleans Street, Baltimore, MD 21287, USA.
Josef Coresh, Department of Epidemiology and Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins Bloomberg School of Public Health, 2024 E Monument Street, Baltimore, MD 21205, USA.
Robert H Christenson, Department of Pathology, University of Maryland School of Medicine, Baltimore, MD, USA.
Elizabeth Selvin, Department of Epidemiology and Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins Bloomberg School of Public Health, 2024 E Monument Street, Baltimore, MD 21205, USA.
Supplementary data
Supplementary data is available at European Heart Journal online.
Pre-registered clinical trial number
None supplied.
Ethical approval
The NHANES protocols and the measurement of hs-troponin in stored specimens were approved by the National Center for Health Statistics ethics review board. Written informed consent was obtained from all participants.
Data availability
The NHANES data, including cardiac troponin, underlying this article are available in the Center for Disease Control website (https://wwwn.cdc.gov/nchs/nhanes/).
Funding
This work was funded by a grant from the Foundation for the National Institutes of Health Biomarkers Consortium to the Johns Hopkins Bloomberg School of Public Health (PI: E.S.). The Foundation for the National Institutes of Health received support for this project from Abbott Laboratories, AstraZeneca, Johnson & Johnson, the National Dairy Council, Ortho Clinical Diagnostics, Roche Diagnostics, and Siemens Healthcare Diagnostics. E.S. was also supported by NIH/NHLBI (National Institutes for Health/National Heart, lung, and Blood Institute) grant K24 HL152440.
References
- 1. Raber I, McCarthy CP, Januzzi JL Jr. A test in context: interpretation of high-sensitivity cardiac troponin assays in different clinical settings. J Am Coll Cardiol 2021;77:1357–1367. 10.1016/j.jacc.2021.01.011 [DOI] [PubMed] [Google Scholar]
- 2. Sandoval Y, Apple FS, Mahler SA, Body R, Collinson PO, Jaffe AS, et al. High-sensitivity cardiac troponin and the 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guidelines for the evaluation and diagnosis of acute chest pain. Circulation 2022;146:569–581. 10.1161/CIRCULATIONAHA.122.059678 [DOI] [PubMed] [Google Scholar]
- 3. Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009;361:858–867. 10.1056/NEJMoa0900428 [DOI] [PubMed] [Google Scholar]
- 4. Twerenbold R, Costabel JP, Nestelberger T, Campos R, Wussler D, Arbucci R, et al. Outcome of applying the ESC 0/1-hour algorithm in patients with suspected myocardial infarction. J Am Coll Cardiol 2019;74:483–494. 10.1016/j.jacc.2019.05.046 [DOI] [PubMed] [Google Scholar]
- 5. Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, et al. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med 2009;361:2538–2547. 10.1056/NEJMoa0805299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aimo A, Januzzi JL Jr, Vergaro G, Ripoli A, Latini R, Masson S, et al. Prognostic value of high-sensitivity troponin T in chronic heart failure: an individual patient data meta-analysis. Circulation 2018;137:286–297. 10.1161/CIRCULATIONAHA.117.031560 [DOI] [PubMed] [Google Scholar]
- 7. deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA 2010;304:2494–2502. 10.1001/jama.2010.1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA 2010;304:2503–2512. 10.1001/jama.2010.1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McEvoy JW, Chen Y, Ndumele CE, Solomon SD, Nambi V, Ballantyne CM, et al. Six-Year change in high-sensitivity cardiac troponin T and risk of subsequent coronary heart disease. Heart failure, and death. JAMA Cardiol 2016;1:519–528. 10.1001/jamacardio.2016.0765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Willeit P, Welsh P, Evans JDW, Tschiderer L, Boachie C, Jukema JW, et al. High-sensitivity cardiac troponin concentration and risk of first-ever cardiovascular outcomes in 154,052 participants. J Am Coll Cardiol 2017;70:558–568. 10.1016/j.jacc.2017.05.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Welsh P, Preiss D, Hayward C, Shah ASV, McAllister D, Briggs A, et al. Cardiac troponin T and troponin I in the general population. Circulation 2019;139:2754–2764. 10.1161/CIRCULATIONAHA.118.038529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jia X, Sun W, Hoogeveen RC, Nambi V, Matsushita K, Folsom AR, et al. High-sensitivity troponin I and incident coronary events, stroke, heart failure hospitalization, and mortality in the ARIC study. Circulation 2019;139:2642–2653. 10.1161/CIRCULATIONAHA.118.038772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, et al. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Stat 2 2013;161:1–24. [PubMed] [Google Scholar]
- 14. Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med 2021;385:1737–1749. 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. The International Federation of Clinical Chemistry and Laboratory Medicine. High-Sensitivity Cardiac Troponin I and T Assay Analytical Characteristics Designated by Manufacturer IFCC Committee on Clinical Applications of Cardiac Bio-markers (C-CB) v092021 . 2022. https://ifcc.web.insd.dk/media/479205/high-sensitivity-cardiac-troponin-i-and-t-assay-analytical-characteristics-designated-by-manufacturer-v092021-3.pdf. Accessed on 6 April 2023.
- 16. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol 2018;72:2231–2264. 10.1016/j.jacc.2018.08.1038 [DOI] [PubMed] [Google Scholar]
- 17. Parikh RH, Seliger SL, de Lemos J, Nambi V, Christenson R, Ayers C, et al. Prognostic significance of high-sensitivity cardiac troponin T concentrations between the limit of blank and limit of detection in community-dwelling adults: a metaanalysis. Clin Chem 2015;61:1524–1531. 10.1373/clinchem.2015.244160 [DOI] [PubMed] [Google Scholar]
- 18. National Healthand Nutrition Eamination Survey . 1999–2004 Data Documentation, Codebook, and Frequencies. https://wwwn.cdc.gov/Nchs/Nhanes/1999-2000/SSTROP_A.htm; accessed 28/9/2022.
- 19. Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation 2009;119:2408–2416. 10.1161/CIRCULATIONAHA.109.192278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tveit SH, Myhre PL, Hanssen TA, Forsdahl SH, Iqbal A, Omland T, et al. Cardiac troponin I and T for ruling out coronary artery disease in suspected chronic coronary syndrome. Sci Rep 2022;12:945. 10.1038/s41598-022-04850-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Omland T, Pfeffer MA, Solomon SD, de Lemos JA, Rosjo H, Saltyte Benth J, et al. Prognostic value of cardiac troponin I measured with a highly sensitive assay in patients with stable coronary artery disease. J Am Coll Cardiol 2013;61:1240–1249. 10.1016/j.jacc.2012.12.026 [DOI] [PubMed] [Google Scholar]
- 22. du Fay de Lavallaz J, Prepoudis A, Wendebourg MJ, Kesenheimer E, Kyburz D, Daikeler T, et al. Skeletal muscle disorders: a noncardiac source of cardiac troponin T. Circulation 2022;145:1764–1779. 10.1161/CIRCULATIONAHA.121.058489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmid J, Liesinger L, Birner-Gruenberger R, Stojakovic T, Scharnagl H, Dieplinger B, et al. Elevated cardiac troponin T in patients with skeletal myopathies. J Am Coll Cardiol 2018;71:1540–1549. 10.1016/j.jacc.2018.01.070 [DOI] [PubMed] [Google Scholar]
- 24. Needham DM, Shufelt KA, Tomlinson G, Scholey JW, Newton GE. Troponin I and T levels in renal failure patients without acute coronary syndrome: a systematic review of the literature. Can J Cardiol 2004;20:1212–1218. [PubMed] [Google Scholar]
- 25. Pop-Busui R, Januzzi JL, Bruemmer D, Butalia S, Green JB, Horton WB, et al. Heart failure: an underappreciated complication of diabetes. A consensus report of the American Diabetes Association. Diabetes Care 2022;45:1670–1690. 10.2337/dci22-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Christenson RH, Jacobs E, Uettwiller-Geiger D, Estey MP, Lewandrowski K, Koshy TI, et al. Comparison of 13 commercially available cardiac troponin assays in a multicenter North American study. J Appl Lab Med 2017;1:544–561. 10.1373/jalm.2016.022640 [DOI] [PubMed] [Google Scholar]
- 27. van der Linden N, Wildi K, Twerenbold R, Pickering JW, Than M, Cullen L, et al. Combining high-sensitivity cardiac troponin I and cardiac troponin T in the early diagnosis of acute myocardial infarction. Circulation 2018;138:989–999. 10.1161/CIRCULATIONAHA.117.032003 [DOI] [PubMed] [Google Scholar]
- 28. Karady J, Mayrhofer T, Ferencik M, Nagurney JT, Udelson JE, Kammerlander AA, et al. Discordance of high-sensitivity troponin assays in patients with suspected acute coronary syndromes. J Am Coll Cardiol 2021;77:1487–1499. 10.1016/j.jacc.2021.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arnadottir A, Pedersen S, Bo Hasselbalch R, Goetze JP, Friis-Hansen LJ, Bloch-Munster AM, et al. Temporal release of high-sensitivity cardiac troponin T and I and copeptin after brief induced coronary artery balloon occlusion in humans. Circulation 2021;143:1095–1104. 10.1161/CIRCULATIONAHA.120.046574 [DOI] [PubMed] [Google Scholar]
- 30. Warner JV, Marshall GA. High incidence of macrotroponin I with a high-sensitivity troponin I assay. Clin Chem Lab Med 2016;54:1821–1829. 10.1515/cclm-2015-1276 [DOI] [PubMed] [Google Scholar]
- 31. Lam L, Aspin L, Heron RC, Ha L, Kyle C. Discrepancy between cardiac troponin assays due to endogenous antibodies. Clin Chem 2020;66:445–454. 10.1093/clinchem/hvz032 [DOI] [PubMed] [Google Scholar]
- 32. Aakre KM, Saenger AK, Body R, Collinson P, Hammarsten O, Jaffe AS, et al. Analytical considerations in deriving 99th percentile upper reference limits for high-sensitivity cardiac troponin assays: educational recommendations from the IFCC Committee on clinical application of cardiac bio-markers. Clin Chem 2022;68:1022–1030. 10.1093/clinchem/hvac092 [DOI] [PubMed] [Google Scholar]
- 33. Strasser B, Tomasits J, Fellner A, Lambert T. Troponin interference with special regard to macrocomplex formation. Clin Chem Lab Med 2021. 10.1515/cclm-2021-0841 [DOI] [PubMed] [Google Scholar]
- 34. Pretorius CJ, Dimeski G, O'Rourke PK, Marquart L, Tyack SA, Wilgen U, et al. Outliers as a cause of false cardiac troponin results: investigating the robustness of 4 contemporary assays. Clin Chem 2011;57:710–718. 10.1373/clinchem.2010.159830 [DOI] [PubMed] [Google Scholar]
- 35. Mair J, Giannitsis E, Mills NL, Mueller C. Study group on biomarkers of the European Society of Cardiology Association for Acute Cardiovascular C. How to deal with unexpected cardiac troponin results. Eur Heart J Acute Cardiovasc Care 2022;11:e1–e3. 10.1093/ehjacc/zuac023 [DOI] [PubMed] [Google Scholar]
- 36. deFilippi CR, Mills NL. Rapid cardiac troponin release after transient ischemia: implications for the diagnosis of myocardial infarction. Circulation 2021;143:1105–1108. 10.1161/CIRCULATIONAHA.120.052649 [DOI] [PubMed] [Google Scholar]
- 37. Katrukha AG, Bereznikova AV, Filatov VL, Esakova TV, Kolosova OV, Pettersson K, et al. Degradation of cardiac troponin I: implication for reliable immunodetection. Clin Chem 1998;44:2433–2440. 10.1093/clinchem/44.12.2433 [DOI] [PubMed] [Google Scholar]
- 38. Damen SAJ, Cramer GE, Dieker HJ, Gehlmann H, Ophuis T, Aengevaeren WRM, et al. Cardiac troponin composition characterization after non ST-elevation myocardial infarction: relation with culprit artery, ischemic time window, and severity of injury. Clin Chem 2021;67:227–236. 10.1093/clinchem/hvaa231 [DOI] [PubMed] [Google Scholar]
- 39. Gaze DC, Collinson PO. Multiple molecular forms of circulating cardiac troponin: analytical and clinical significance. Ann Clin Biochem 2008;45:349–355. 10.1258/acb.2007.007229 [DOI] [PubMed] [Google Scholar]
- 40. Basit M, Bakshi N, Hashem M, Allebban Z, Lawson N, Rosman HS, et al. The effect of freezing and long-term storage on the stability of cardiac troponin T. Am J Clin Pathol 2007;128:164–167. 10.1309/LR7FC0LUGLHT8X6J [DOI] [PubMed] [Google Scholar]
- 41. Agarwal SK, Avery CL, Ballantyne CM, Catellier D, Nambi V, Saunders J, et al. Sources of variability in measurements of cardiac troponin T in a community-based sample: the atherosclerosis risk in communities study. Clin Chem 2011;57:891–897. 10.1373/clinchem.2010.159350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kavsak PA, Worster A, Hill SA, MacRae AR, Jaffe AS. Analytical comparison of three different versions of a high-sensitivity cardiac troponin I assay over 10years. Clin Chim Acta 2017;475:51–55. 10.1016/j.cca.2017.10.006 [DOI] [PubMed] [Google Scholar]
- 43. Kavsak PA, Clark L, Caruso N, Bamford K, Lamers S, Hill S, et al. Macrocomplexes and high-sensitivity cardiac troponin assays in samples stored for over 15 years. Clin Chim Acta 2020;505:6–8. 10.1016/j.cca.2020.02.013 [DOI] [PubMed] [Google Scholar]
- 44. Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol 2004;57:1096–1103. 10.1016/j.jclinepi.2004.04.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The NHANES data, including cardiac troponin, underlying this article are available in the Center for Disease Control website (https://wwwn.cdc.gov/nchs/nhanes/).