Abstract
Importance
It is necessary to determine whether safety and efficacy of autologous skin tissue cells grafting for facial sunken or flat scars.
Objective
To identify autologous skin tissue cells grafting can reduce facial sunken or flat scars.
Design, setting, and participants
In this retrospective cross-sectional study, a total of 128 patients with scar (exclude pathological scar patients), who were receiving autologous skin tissue cells grafting therapy from January 1, 2016, to December 31, 2019.
Interventions
Autologous skin tissue cells grafting.
Main outcomes and measures
Changes in scar severity, color changes in the scar area, infection rate and patient satisfaction.
Results
A total of 128 patients with scar (89 females [69.5%]; mean [SD] age, 30.6 [13.12] years) received autologous skin tissue cells grafting therapy. SCAR (Scar Cosmesis Assessment and Rating), with scores ranging from 0 (best possible scar) to 15 (worst possible scar). After treatment 12 months, the mean [SD] of SCAR score went down from 9.85 [1.33] to 2.67 [1.21]. No infection was observed during treatment or recovery, and the main drawback after autologous skin tissue cells grafting is that the color recovery time is longer. The patient satisfaction 6 months after treatment was 85.2%, furthermore 12 months after treatment patient satisfaction was 94.7%.
Conclusions and relevance
In this study, autologous skin tissue cells grafting was safe and effective to treat facial scars. Therefore, autologous skin tissue cells grafting may be recommended as a reliable treatment for facial scar.
Keywords: Scar, Autologous skin tissue cells grafting, Safety and efficacy
1. Introduction
Skin is the largest organ in human body and it serves the key role of the barrier to a wide range of external insults, like bacteria and other pathogens [1], so any skin damage must be efficiently and rapidly mended to restore the barrier function. However, it also has a considerable flaw: severely damaged skin can heal, but it can not regenerate. Instead, it forms scars. A scar is the product of the body's repair mechanism after tissue injury. Compared to the normal tissue, the composition of the scar tissue is different,it is mainly composed of fibroblasts and collagenous fibers [2,3]. Four of the most common types are flat, hypertrophic, keloid scarring and sunken scar [[4], [5], [6]]. The scar tissue is actually weaker than normal skin, although scars seem to be thicker. These marks can not just inhibit a person's movement. It also can lead to cosmetic defects, and especially the scar occurs in the face [7,8].
There are many ways to treat scarring, such as chemical peels, laser treatment, radiotherapy, pressure dressing, steroids and surgery. Filler injections of collagen can be used to raise sunken scars to the level of surrounding skin. However, these measures may lead to other defects [[9], [10], [11]]. Although many new therapeutic approaches have made progress, there is still need for better methods to enhance healing and recovery, especially scar occurs in face [12,13]. There are increasingly studies have demonstrated that minced skin grafting can accelerate healing and re-epithelialization of fluid-treated skin wounds, moreover, minced skin grafting can also reduce scar formation [[14], [15], [16]]. In the process of split-thickness skin grafting, minced skin grafting can promote epithelialization of the donor site. In addition, minced skin grafting improved the appearance of the split-thickness skin grafting donor site [17].
However, the effect of minced autologous skin tissue cells grafting on the appearance of scar has not been described. Minced skin tissue cells can absorb nutrients as much as possible, and as the same time small skin tissue grafts also disrupts the normal protective barrier of the skin tissue and exposes viable dermal and epidermal cells within the grafts. Moreover, Adequate proliferation of dermal and epidermal cells can inhibit the proliferation of fibroblasts, that can inhibit scarring [18,19]. The aim of this study was to illustrate the safety and efficacy of autologous skin tissue cells grafting for facial scar.
2. Methods
2.1. Indications and contraindications
The indications of autologous skin tissue cells grafting include atrophic acne scars, superficial scar and scar contracture. Moreover, atrophic acne scars caused by varicella or acne can acquire better long-term outcomes. Hypertrophic scar and keloid have tumor-like properties considered as pathological scars, so they are categorized as contraindications. This experiment was approved by the Ethics Committee of Yong jia Jin xi institute for scar repair and Shanghai Hospital of Traditional Chinese Medicine (2022SHL-KYYS-24). The experiment was conducted according to established ethical guidelines, and informed consent obtained from the participants.
2.2. Autologous skin tissue cells harvest
The donor area of skin tissue is usually chosen around the scar. If the scar area is large, additional selection of donor area is required, such as forehead, cheek area and abdomen. The donor area of skin tissue and scar area were disinfected using iodine, then the skin tissue around scar area was cleaned thoroughly three times using 0.9% normal saline. The split-thickness flap was excised tangentially using electrical dermatome, including the entire epidermis and a part of the dermis, then minced split-thickness skin tissue utilizing kitchen blender into tiny particles that can allow the skin tissue cells to migrate and multiply better. Finally, the cells were placed in normal saline and the necrotic tissue in the upper layer was removed. The remaining cells were preserved in ringer's solution.
2.2.1. Autologous skin tissue cells grafting
Before autologous skin tissue cells grafting, the scar needs to be removed pigmentation from the surface and to make it even. For superficial sunken scars, the skin grinding machine should be used to grind until the surface is smooth and skin tissue fluid or blood is generated, which is conducive to the transplantation of cells. And For linear scars, abnormal scar tissue needs to be removed, and then broken up to fill the original scar site, so as to facilitate the proliferation of microvessels. The autologous skin tissue cells were based on macroscopic evaluation, and the approximate tiny particle diameter was <0.5 mm. The small pasty graft mass composed of a large number of minced graft particles and a small amount of ringer's solution was uniformly spread on the entire surface of the scar area with Adson forceps or small dressing forceps.
2.3. Statistical analysis
Data were analyzed from January 1, 2016, to December 31, 2019. Baseline covariates were summarized using means, SDs, frequencies, and percentages. Statistical analysis was performed using t-test to investigate whether the differences were significant between Preoperative and postoperative. Values of p<0.05 were regarded as statistically significant. All statistical analyses were performed using GraphPad Prism-5 software.
3. Results
3.1. Location and type of scar
In total, 144 patients received autologous skin tissue cells grafting, and 16 patients lost to follow-up. Of the 128 patients(39male [31.5%]; mean [SD] age, 30.6 [13.12] years), 76 had sunken scars and 52 had flat scars (Fig. 1). All of these scars were on the head and face, include 65 cheek scars (50.8%), 16 lip scars (12.5%), 8 nose scars (6.3%), and 39 forehead scars(30.5%)(Table 1). Due to the tumor-like nature of pathological scars, autologous skin tissue cells grafting is not recommended in the first place.
Fig. 1.
Flow diagram of patient selection process.
Table 1.
Characteristics of facial sunken or flat scars. The values are presented as the quantity (percentage) or the means (SD).
| Characteristic | Value (n = 128) |
|---|---|
| Sex | |
| male | 39 (31.5%) |
| female | 89 (69.5%) |
| Age | 30.6 [13.1] |
| Types of scars | |
| sunken scars | 76 (59.4%) |
| flat scars | 52 (40.6%) |
| Location | |
| cheek | 65 (50.8%) |
| lip | 16 (12.5) |
| nose | 8 (6.3%) |
| forehead | 39 (30.5%) |
Efficacy of Autologous Skin Tissue Cells Grafting.
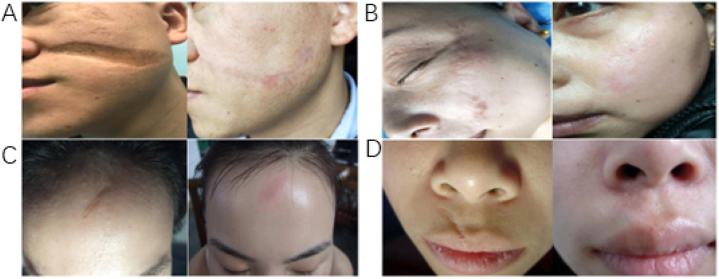
After autologous skin tissue cells grafting treatment 4 h, mild exudation can be seen on the wound gauze, and the exudation was light red, that peaked 8–12 h postoperatively. After 3 days, the gauze gradually dried. Generally, the wound healing takes about 10 days. After the gauze dressing was removed, the autologous skin tissue cells were alive, and the pink skin tissue was similar to normal, replacing the original scar. Moreover, all the donor sites healed without any postoperative complications including contamination from recipient site, discoloring, and hypertrophic scar [Fig. 2(A-D)].
Fig. 2.
Efficacy of autologous skin tissue cells grafting. A. A male patient with facial sunken scar, 12 months after autologous skin tissue cell transplantation. B. A female patient with facial flat scar, 9 months after autologous skin tissue cell transplantation. C. A female patient with facial flat scar, 6 months after autologous skin tissue cell transplantation. D. A male patient with facial flat scar, 8 months after autologous skin tissue cell transplantation.
SCAR (Scar Cosmesis Assessment and Rating) is a simple and effective scar assessment method, with scores ranging from 0 (best possible scar) to 15 (worst possible scar) [20]. Of the 128 patients the mean [SD] of SCAR score went down from 9.85 [1.33] to 2.67 [1.21] after treatment 12 months. Six months after treatment, 25 people needed treatment again. They were followed up to 12 months after first treatment, and only 6 needed minor treatment again. Hence, autologous skin tissue cells grafting for scar is effective method.
3.2. Safety of autologous skin tissue cells grafting
Autologous skin grafting currently represents the most widespread method for reconstructing significant skin defects. However, the safety of minced autologous skin tissue cells grafting for scar still need to observed. Of the 128 patients, there were no cases of infection or necrosis. Instead, autologous skin tissue cells grafting can promote wound healing. Therefore, the autologous skin tissue cells grafting is a safety method for scar. As the same time, it showed rapid re-epithelialization of the wounds grafted with the autologous skin tissue cells (Table 2).
Table 2.
Analysis of the safety and efficacy of autologous skin tissue cells grafting. The values are presented as the quantity (percentage) or the means (SD), **p<0.01 versus preoperative.
| Pre-operation | Postoperative 6 months | Postoperative 12 months | p | |
|---|---|---|---|---|
| SCAR score | 9.58 [1.33] | 3.9 [1.36] | 2.67 [1.21] | <0.01 |
| Satisfactory rate | 0 | 85.2% | 94.7% | <0.01 |
| Infection rate | 0 | 0 | 0 | |
| Necrosis rate | 0 | 0 | 0 | |
| Re-treatment | 128(100%) | 25(19.5%) | 6(4.69%) | <0.01 |
| Sunken | 76(59.4%) | 12(9.4%) | 3(2.3%) | <0.01 |
| Apophysis | 52(40.6%) | 8(6.3%) | 2(1.6%) | <0.01 |
| Pigmentation | 128(100%) | 0 | 0 | |
| Hypopigmentation | 0 | 128(100%) | 20(15.6%) |
4. Discussion
Scar is the product of the body's repair mechanism after tissue injury, and it consists of an over-proliferation of fibroblasts and collagen fibers. Compared to the normal tissue, the composition of the scar tissue is different. The scar tissue is actually weaker than normal skin, although scars seem to be thicker. These marks can't just inhibit movement of a person, it also can lead to cosmetic defects, and especially the scar occurs in the face [7,8].
There are many ways to treat scarring, such as chemical peels, laser treatment, radiotherapy, pressure dressing, steroids and surgery. However, these measures may lead to other defects. Although many new therapeutic approaches have made progress, there is still need for better methods to enhance healing and recovery, especially scar occurs in face [12].
The concept of making small particles of skin tissue for transplantation onto wound is relatively old. The pinch-grafting technique was, for example, described by Reverdin in 1869. And from then on, many other methods have been used for this purpose [21]. The advantages of the methods include theoretically be beneficial to the adequate amplification of skin tissue cells and in some cases simplicity. Enhanced epithelial regeneration, revascularization and reduced wound contracture were reported in micrograft-treated wounds [22]. In this study, we grind or cut the scar tissue that's not even, and then we chop up the scar tissue, at last, lay it flat over the original scar. If the scar area is large, additional selection of donor area is required, such as forehead, cheek area and abdomen.
Autologous skin tissue cells grafting is an effective and safe method for scar. After treatment 12 months, the mean [SD] of SCAR score went down from 9.85 [1.33] to 2.67 [1.21]. No infection was observed during treatment or recovery, and the main drawback after autologous skin tissue cells grafting is that the color recovery time is longer. The patient satisfaction 6 months after treatment was 85.2%, furthermore 12 months after treatment patient satisfaction was 94.7%. There was statistical difference before and after autologous skin tissue cells grafting.
The procedure of creating small skin tissue grafts also disrupts the normal protective barrier of the skin tissue and exposes viable dermal and epidermal cells within the grafts. As the same time, Adequate proliferation of dermal and epidermal cells can inhibit the proliferation of fibroblasts, that can inhibit scarring.
5. Conclusions
The autologous skin tissue cells grafting substantially reduces the healing time of the wound, and it can obviously improve scar shape. As the same time, there were no cases of infection or necrosis. Hence, the autologous skin tissue cells grafting is an effective and safe method for scar.
6. Limitation
In order to obtain enough skin tissue, it is usually necessary to enlarge the original scar area. And autologous skin tissue cells grafting recovery time is long, about 10–12 months.
Key points
Question is the autologous skin tissue cells grafting safety and efficacy for facial sunken or flat scars?
Findings In this cross-sectional study of 128 patients with facial sunken or flat scars, autologous skin tissue cells grafting can obviously improve the treatment effect of facial sunken or flat scars, and main drawback after autologous skin tissue cells grafting is that the color recovery time is longer, about 12 mouths.
Meaning Facial sunken or flat scars can seriously affect the patient's appearance, there are many ways to treat scar, but they are not satisfactory. Autologous skin tissue cells grafting is safety and efficacy for facial scar. It may become a revolutionary treatment for sunken or flat scars.
Declaration of competing interest
The authors state no conflict of interest.
Acknowledgments
This work was supported by Shanghai University of Traditional Chinese Medicine budget Project (No.2021LK072), Shanghai Foundation for Innovation and Development “Future Plan” (NO.WL-XJRY-2021010 K) and the project of Shanghai University of Medicine &Health Sciences (No.SSF-22-16-001).
Contributor Information
Fangfang Nie, Email: niefangfang@jdhospital.com.
Guanghui Zhu, Email: zhuguanghui@shutcm.edu.cn.
References
- 1.Boyko T.V., Longaker M.T., Yang G.P. Laboratory models for the study of normal and pathologic wound healing. Plast. Reconstr. Surg. 2017;139(3):654–662. doi: 10.1097/PRS.0000000000003077. [DOI] [PubMed] [Google Scholar]
- 2.Abbasi S., Sinha S., Labit E., Rosin N.L., Yoon G., Rahmani W., Jaffer A., Sharma N., Hagner A., Shah P., Arora R., Yoon J., Islam A., Uchida A., Chang C.K., Stratton J.A., Scott R.W., Rossi M.V., Underhill T.M., Biernaskie J. Distinct regulatory programs control the latent regenerative potential of dermal fibroblasts during wound healing. Cell Stem Cell. 2020;27(3):396–412.e6. doi: 10.1016/j.stem.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Mascharak S., desJardins-Park Heather E., Longaker Michael T. Fibroblast heterogeneity in wound healing: hurdles to clinical translation. Trends Mol. Med. 2020;26(12):1101–1106. doi: 10.1016/j.molmed.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Gauglitz G.G., Korting H.C., Pavicic T., Ruzicka T., Jeschke M.G. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol. Med. 2011;17:113–125. doi: 10.2119/molmed.2009.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mascharak S., Talbott H.E., Januszyk M., Griffin M., Chen K., Davitt M.F., Demeter J., Henn D., Bonham C.A., Foster D.S., Mooney N., Cheng R., Jackson P.K., Wan D.C., Gurtner G.C., Longaker M.T. Multi-omic analysis reveals divergent molecular events in scarring and regenerative wound healing. Cell Stem Cell. 2022;29(2):315–327.e6. doi: 10.1016/j.stem.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mascharak S., desJardins-Park H.E., Davitt M.F., Griffin M., Borrelli M.R., Moore A.L., Chen K., Duoto B., Chinta M., Foster D.S., Shen A.H., Januszyk M., Kwon S.H., Wernig G., Wan D.C., Lorenz H.P., Gurtner G.C., Longaker M.T. Engrailed-1Preventing activation in fibroblasts yields wound regeneration without scarring. Science. 2021;(6540):372. doi: 10.1126/science.aba2374. undefined. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276(5309):75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 8.Willyard C. Unlocking the secrets of scar-free skin healing. Nature. 2018;563(7732):S86–S88. doi: 10.1038/d41586-018-07430-w. [DOI] [PubMed] [Google Scholar]
- 9.Cooper J.S., Lee B.T. Treatment of facial scarring: lasers, filler, and nonoperative techniques. Facial Plast. Surg. 2009;25(5):311–315. doi: 10.1055/s-0029-1243079. [DOI] [PubMed] [Google Scholar]
- 10.Pekmezci E. The use of radio-wave surgery: an underestimated method for small hyperpigmented flat or macular lesions on face. J. Cosmet. Dermatol. 2022;1(10):4691–4696. doi: 10.1111/jocd.14995. [DOI] [PubMed] [Google Scholar]
- 11.Liu T., Ma X.R., Ouyang T.X., Chen H.P., Xiao Y., Huang Y.Y., Liu J., Xu M. Efficacy of 5-aminolevulinic acid-based photodynamic therapy against keloid compromised by downregulation of SIRT1-SIRT3-SOD2-mROS dependent autophagy pathway. Redox Biol. 2019;20:195–203. doi: 10.1016/j.redox.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hakkarainen T., Koivuniemi R., Kosonen M., Escobedo-Lucea C., Sanz-Garcia A., Vuola J., Valtonen J., Tammela P., Mäkitie A., Luukko K., Yliperttula M., Kavola H. Nanofibrillar cellulose wound dressing in skin graft donor site treatment. J. Contr. Release. 2016;244:292–301. doi: 10.1016/j.jconrel.2016.07.053. [DOI] [PubMed] [Google Scholar]
- 13.Kang B.Y., Ibrahim S.A., Weil A., Reynolds K.A., Johnson T., Wilson S., Lee M.H., Kim John Y.S., Dirr M.A., Poon E., Alam M. Treatment of surgical scars with combination pulsed dye and fractional nonablative laser: a randomized ControlledTrial. Ann. Surg. 2022;276(6):975–980. doi: 10.1097/SLA.0000000000005377. [DOI] [PubMed] [Google Scholar]
- 14.Miyanaga T., Kishibe M., Yamashita M., Kaneko T., Kinoshita F., Shimada K. Minced skin grafting for promoting wound healing and improving donor-site appearance after split-thickness skin grafting: a prospective half-side comparative trial. Plast. Reconstr. Surg. 2019;144(2):475–483. doi: 10.1097/PRS.0000000000005868. [DOI] [PubMed] [Google Scholar]
- 15.Radharaman, Kumar P., Ajai K.S., Kumar S.R. The role of recruited minced skin grafting in improving the quality of healing at the donor site of split-thickness skin graft-A comparative study. Burns. 2019;45(4):923–928. doi: 10.1016/j.burns.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Simizu Ruka, Kishi Kazuo, Okabe Keisuke, Uchikawa Yumiko, Sakamoto Yoshiaki, Hattori Noriko, Nobuaki Imanishi. Recruited minced skin grafting for improving the skin appearance of the donor site of a split-thickness skin graft. Dermatol. Surg. 2012;38(4):654–660. doi: 10.1111/j.1524-4725.2011.02266.x. [DOI] [PubMed] [Google Scholar]
- 17.Miyanaga T., Haseda Y., Sakagami A. Minced skin grafting for promoting epithelialization of the donor site after split-thickness skin grafting. Burns. 2017;43(4):819–823. doi: 10.1016/j.burns.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Fossum M., Zuhaili B., Hirsch T., Spielmann M., Reish R.G., Mehta P., Eriksson E. Minced skin for tissue engineering of epithelialized subcutaneous tunnels. Tissue Eng. 2009;15(8):2085–2092. doi: 10.1089/ten.tea.2008.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pertusi G., Tiberio R., Graziola F., Boggio P., Colombo E., Chiarella Bozzo. Selective release of cytokines, chemokines, and growth factors by minced skin in vitro supports the effectiveness of autologous minced micrografts technique for chronic ulcer repair. Wound Repair Regen. 2012;20(2):178–184. doi: 10.1111/j.1524-475X.2011.00762.x. [DOI] [PubMed] [Google Scholar]
- 20.Kantor Jonathan. Reliability and photographic equivalency of the scar Cosmesis assessment and rating (SCAR) scale, an outcome measure for postoperative scars. JAMA Dermatol. 2017;153(1):55–60. doi: 10.1001/jamadermatol.2016.3757. [DOI] [PubMed] [Google Scholar]
- 21.Biswas A., Bharara M., Hurst C., Armstrong D.G., Rilo H. The micrograft concept for wound healing: strategies and applications. J. Diabetes Sci. Technol. 2010;4(4):808–819. doi: 10.1177/193229681000400407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hackl F., Bergmann J., Granter S.R., Koyama T., Kiwanuka E., Zuhaili B., Pomahac B., Caterson E.J., Junker J.P.E., Eriksson E. Epidermal regeneration by micrograft transplantation with immediate 100-fold expansion. Plast. Reconstr. Surg. 2012;129(3):443e–452e. doi: 10.1097/PRS.0b013e318241289c. [DOI] [PubMed] [Google Scholar]