Abstract
Aims
Due to growing environmental focus, plant-based diets are increasing steadily in popularity. Uncovering the effect on well-established risk factors for cardiovascular diseases, the leading cause of death worldwide, is thus highly relevant. Therefore, a systematic review and meta-analysis were conducted to estimate the effect of vegetarian and vegan diets on blood levels of total cholesterol, low-density lipoprotein cholesterol, triglycerides, and apolipoprotein B.
Methods and results
Studies published between 1980 and October 2022 were searched for using PubMed, Embase, and references of previous reviews. Included studies were randomized controlled trials that quantified the effect of vegetarian or vegan diets vs. an omnivorous diet on blood lipids and lipoprotein levels in adults over 18 years. Estimates were calculated using a random-effects model. Thirty trials were included in the study. Compared with the omnivorous group, the plant-based diets reduced total cholesterol, low-density lipoprotein cholesterol, and apolipoprotein B levels with mean differences of −0.34 mmol/L (95% confidence interval, −0.44, −0.23; P = 1 × 10−9), −0.30 mmol/L (−0.40, −0.19; P = 4 × 10−8), and −12.92 mg/dL (−22.63, −3.20; P = 0.01), respectively. The effect sizes were similar across age, continent, duration of study, health status, intervention diet, intervention program, and study design. No significant difference was observed for triglyceride levels.
Conclusion
Vegetarian and vegan diets were associated with reduced concentrations of total cholesterol, low-density lipoprotein cholesterol, and apolipoprotein B—effects that were consistent across various study and participant characteristics. Plant-based diets have the potential to lessen the atherosclerotic burden from atherogenic lipoproteins and thereby reduce the risk of cardiovascular disease.
Keywords: Plant-based diet, Vegetarian, Vegan, Blood lipids, Lipoproteins, Meta-analysis
Structured Graphical Abstract
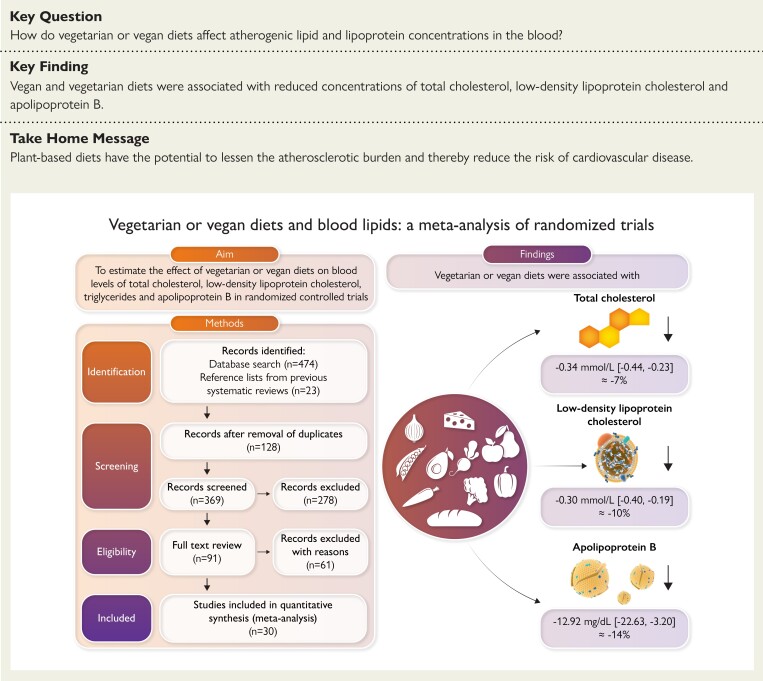
Structured Graphical Abstract.
Overall aim, methods, and findings of the study. The flowchart in left, lower corner visualizes the study selection process. The items on the right side visualize the meta-analysed effects of vegetarian and vegan diets on levels of total cholesterol, low-density lipoprotein cholesterol and apolipoprotein B.
Introduction
Each year 18 million people die from cardiovascular diseases (CVDs) making this the leading cause of mortality in the world.1 The main cause of CVD is atherosclerosis—a condition that progresses through life until the appearance of clinical disease. Medical costs for treating atherosclerotic cardiovascular disease (ASCVD) are increasing due to the growing elderly population globally.2 Improved prevention is therefore key to slowing down the progression of atherosclerosis, halting the presence of disease and diminishing medical costs.
In 2021 the European Society of Cardiology published guidelines for CVD prevention.3 Unhealthy lifestyles are excessively prevalent and cigarette smoking, high blood pressure, diabetes, and atherogenic apolipoprotein B (apoB) containing lipoprotein particles are primary risk factors for ASCVD.3 ApoB is the main apolipoprotein in low-density lipoprotein cholesterol (LDL-C) and triglyceride-rich lipoproteins. Numerous genetic, observational and interventional studies have shown a causal role of LDL-C and other apoB-containing lipoprotein particles for risk of ASCVD.4 These risk factors are modifiable, and shifting to a healthier and more plant-based diet can reduce CVD risk directly by lowering levels of atherogenic lipoproteins, blood pressure, and levels of blood glucose.3 The increased focus on the environment as a result of climate changes matches the present tendency to be vegetarian; omitting meat products but allowing eggs and/or dairy products, or vegan; and totally excluding all animal products. The effects of vegetarian and vegan diets on lipid and lipoprotein levels have previously been examined in two systematic reviews and meta-analyses.5,6 However, no meta-analysis including randomized controlled trials (RCTs) has been published since 2017, and no previous meta-analyses of RCTs have investigated the effect of vegetarian and vegan diets on apoB concentrations or stratified for a range of participant and study characteristics. Furthermore, because of the increased focus on the atherogenic potential of triglyceride-rich lipoproteins and the growing popularity of plant-based diets, the field warrants an update.
We therefore conducted a systematic review and meta-analysis of 30 RCTs with the aim of examining changes in blood levels of total cholesterol (TC), LDL-C, triglycerides (TG), and apoB after consumption of a plant-based intervention diet vs. an omnivorous diet.
Methods
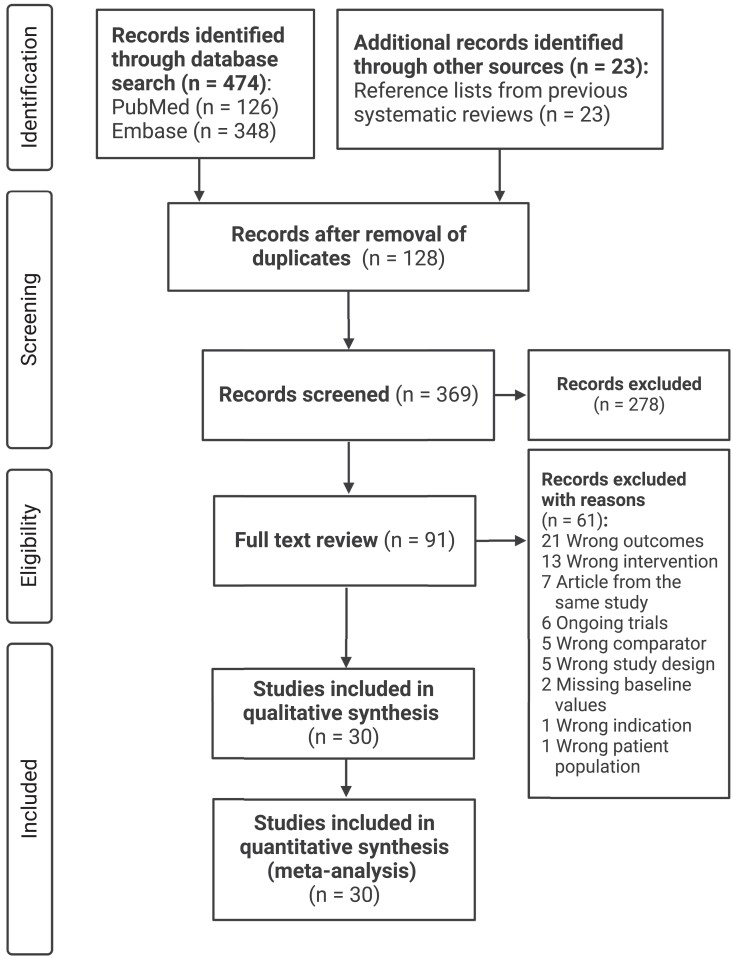
Plant-based diets are defined as dietary patterns with low or no intake of animal products.7 In this review, plant-based diets will refer to only vegetarian and vegan diets. This review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (Figure 1).
Figure 1.
Flowchart of study selection
Literature search
The online literature search was conducted from 16 September 2021 to 11 October 2022 using the PubMed and Embase databases. Studies between 1980 and 11 October 2022 were included. Additional studies were identified through references of prior original articles or systematic reviews.5,6 The overall keywords used for the screening process were: (‘Vegetarian’ OR ‘vegan’ OR ‘lacto-vegetarian’ OR ‘ovo-vegetarian’ OR ‘lacto-ovo-vegetarian’) AND (‘blood lipids’ OR ‘serum lipids’ OR ‘plasma lipids’ OR ‘total cholesterol’ OR ‘low-density lipoprotein’ OR ‘triglyceride’ OR ‘non-HDL lipoproteins’ OR ‘apolipoprotein B’) AND (‘clinical trial’ OR ‘randomized controlled trial’ OR ‘RCT’). Keywords were from titles and abstracts or by MeSH terms (see Supplementary data online, Appendix S1).
Eligibility criteria
The PICOS model (Population, Intervention, Comparison, Outcome, and Study design) was used to specify the eligibility criteria (see Supplementary data online, Figure S1). Studies were assessed as eligible if the population was human, aged ≥18 years, and if individuals were not pregnant; if the intervention included consumption of a vegan or vegetarian diet; if the comparison was an omnivorous control group (consuming all food groups); and if the outcomes were TC, LDL-C, TG, and/or apoB in blood, plasma, or serum. Outcomes had to be presented as means or medians at baseline and endpoint for both intervention and control groups. Lastly, the study design included only RCTs.
Study selection
The study selection was performed by using the Covidence systematic review software.8 Studies retrieved from the literature search were screened by title and abstract independently by C.A.K. and E.W.K. When there was conflict in the eligibility assessment between authors, all authors were involved in the inclusion or exclusion of the study in question. Only articles published in English, which met the eligibility criteria, went on to full text screening and data extraction. If studies lacked to present sufficient data needed for the meta-analysis, they were excluded from the review and meta-analysis. Unpublished or duplicate studies were also excluded.
Data extraction
We extracted study characteristics including author, year, country, duration of trial, number of individuals included in the trial, mean body mass index (BMI) at baseline, mean age at baseline, health status, lipid-lowering therapy, changes of this type of therapy during the trial period, intervention and control diet, intervention program, study design, and outcome analysis. We further included mean baseline levels of TC, LDL-C, TG, and apoB and if there were changes in blood lipid and lipoprotein levels between baseline and end of trial.
Data synthesis and statistical analysis
The mean and standard deviation (SD) of blood lipid and lipoprotein concentrations at baseline, and post-intervention were extracted from each trial in both intervention and control groups. Some studies reported post-intervention concentrations at several time points—only the last time point was extracted for further analysis. Obtaining SDs for group of means were calculated from standard error of the mean (SEM) or 95% confidence intervals (CIs) by using equations from the Cochrane Handbook chapter 6.5.2.2 when the group SDs were not provided directly [ or ].9 When concentrations were provided in medians and 25th–75th percentile, we converted these into means ± SD by using the equation by Wan et al. (Cochrane Handbook chapter 6.5.2.5).10 Furthermore, when not reported, change-from-baseline SDs were estimated using the equation by Follmann et al. assuming a correlation coefficient of 0.50 between baseline and post-intervention lipid and lipoprotein values [Cochrane Handbook chapter 6.5.2.8, 2: ].9,11 The correlation coefficient (Corr) of 0.50 was chosen based on previous, similar meta-analyses and calculations by Follmann et al.6,11 Sensitivity analyses testing different values of Corr (Corr = 0.2 and Corr = 0.8) were conducted, yielding similar results. Overall percentage change in lipid and lipoprotein concentrations was calculated from the weighted average change for the intervention groups minus the weighted average change for the control groups. We converted extracted data to international units. TC and LDL-C provided in mg/dL were converted to mmol/L by multiplying with 0.0259 and TG in mg/dL by multiplying with 0.0113. ApoB concentrations listed in g/L were converted to mg/dL by dividing with 0.01.
We used Stata/SE version 17.0 (Stata Corp, College Station, TX) for all statistical analyses. The random-effects model described by DerSimonian and Laird was used to take both within- and between-study variability into account.12I2 statistics assessed heterogeneity between studies. I2 values were considered as follows: 0%–40% might not be important, 30%–60% may represent moderate heterogeneity, 50%–90% may represent substantial heterogeneity, and 75%–100% presented considerable heterogeneity, where the latter three intervals depended on effect size and evidence of heterogeneity.13 Cochran’s statistic was used for calculating the test of group differences in subgroup analysis. This test investigated the difference between the group-specific overall effect sizes.14
In the meta-analysis, estimates of lipid and lipoprotein level differences were shown as means with 95% CI. Statistical significance was a 2-sided P < 0.05. We performed subgroup analyses that stratified outcomes of TC, LDL-C, and TG by mean age at baseline (≤50 or >50 years), mean BMI at baseline (normal: < 25 kg/m2; overweight: 25–29.9 kg/m2; and obese: > 29.9 kg/m2), continent, duration of trial (≤3 or >3 months), health status (healthy or with CVD risk), intervention diet (vegetarian or vegan), intervention program (dietary intervention or multi-interventional), inclusion of subjects treated with lipid-lowering therapy (none or some), outcome analysis [per protocol (PP) or intention to treat (ITT)], year of publication (before or after 2005), sample size (≤80 or >80 individuals), and study design (crossover or parallel). For LDL-C, we made an additional subgroup analysis stratifying for baseline LDL-C concentration (≤ or > mean LDL-C). The analyses for intervention program, outcome analysis, and baseline LDL-C level were conducted post hoc while the remaining were ad hoc.
We performed sensitivity analyses in the form of leave-one-out meta-analyses to assess whether the estimated effect on blood lipids and lipoproteins differed significantly when each study was excluded from the meta-analyses. Further, we checked for publication bias by examining funnel plots for each meta-analysis and tested for plot asymmetry by using Egger’s linear regression test.15 The trim and fill method was used to adjust for funnel plot asymmetry, as it ‘fills’ imputed missing studies in the plot where these would likely appear.
Risk of bias assessment
We used Cochrane’s risk of bias version 2 (RoB 2) tool to assess risk of bias in each included RCT.16 Specific versions of the tool were used for crossover and parallel trials, respectively. The tool consists of five domains, each containing a list of signalling questions linked to specific aspects of the RCT. An algorithm marks the risk of bias in each domain as ‘low’, ‘some concerns’, or ‘high’ depending on the answers to the signalling questions. The final judgement of risk of bias in each domain and in the overall study was assessed independently by C.A.K. and E.W.K. and discussed when conflicts between assessments occurred.
Results
We retrieved 497 studies from the literature search and additional sources. After removal of duplicates, 369 articles were screened by title and abstract, and 91 went on to full text review. After further exclusion of 61 studies due to specific reasons, a total of 30 studies were included in both the qualitative and quantitative synthesis (Figure 1).17–46
Study characteristics
The included studies were published between 1982 and 2022 and conducted in USA (18 studies), Sweden (2), Finland (2), South Korea (2), Australia (1), Brazil (1), Czech Republic (1), Italy (1), Iran (1), and New Zealand (1). The intervention period ranged from ten days to five years with a mean of 29 weeks (15 studies ≤ 3 months; 12 studies 3–12 months; and three studies > 1 year). Nine used a crossover design while 21 used a parallel design. In the parallel studies, participants followed only one diet, whereas participants in the crossover trials started with either the control or intervention diet and then crossed over to consume the other.47 Sample sizes varied from 11 to 291 participants (mean = 79) with mean BMI between 21.5 and 35.1 kg/m2 and mean age between 20 and 67 years. Five studies included solely healthy participants with a mean BMI < 30 kg/m2.17,20,23,33,38 The 25 remaining studies consisted of participants who were either overweight or obese and/or were diagnosed with a specific health condition; primarily type 2 diabetes and/or CVD. Thirteen studies reported that they included participants treated with lipid-lowering therapy at baseline. Of these, four reported changes in the use of this medication.26,27,32,46 The dietary intervention was vegetarian in 15 of the trials (three lacto-vegetarian and 12 lacto-ovo-vegetarian) and vegan in 15 of the trials. The intervention was solely dietary in 23 of the studies and part of a multi-interventional program in the remaining seven trials.19,21,27,29,30,34,38 All study characteristics are listed with references in Table 1.
Table 1.
Study characteristics: baseline participant characteristics of included randomized controlled trials
| Reference, year | Country | Duration | n (F/M) | Mean BMI, kg/m2 | Mean age, year | Health status | Lipid-lowering therapy (E/C) | Intervention | Control | Intervention program |
|---|---|---|---|---|---|---|---|---|---|---|
| Cooper et al., 198217 | USA | 3 weeks | 15 (5/10) | NR | 28.0 | Healthy subjects | None | Lacto-vegetarian | Omnivorous | Dietary |
| Kestin et al., 198918 | Australia | 6 weeks | 26 (0/26) | 25.5 | 44.0 | NR | None | Lacto-ovo-vegetarian | High-fat omnivorous | Dietary |
| Ornish et al., 199019 | USA | 12 months | 41 (5/36) | 27.5 | 57.8 | With CAD | None | Low-fat lacto-ovo-vegetarian | Omnivorous | Multi-interventional |
| Ling et al., 199220 | Finland | 4 weeks | 18 (14/4) | 26.6 | 42.8 | Healthy or with unrelated conditions | NR | Uncooked vegan | Omnivorous | Dietary |
| Ornish et al., 199821 | USA | 5 years | 35 (3/32) | 27.1 | 59.3 | With CAD | 9 (0/9) | Low-fat lacto-ovo-vegetarian (Ornish) | Omnivorous | Multi-interventional |
| Nicholson et al., 199922 | USA | 12 weeks | 11 (5/6) | NR | 54.3 | With non-insulin dependent DM | 4 (3/1) | Low-fat vegan | Omnivorous | Dietary |
| Barnard et al., 200023 | USA | 2 months | 35 (35/0) | 25.5 | 36.1 | Healthy menopausal women | None | Low-fat vegan | Omnivorous | Dietary |
| Ågren et al., 200124 | Finland | 3 months | 29 (28/1) | 24.3 | 50.8 | With rheumatoid arthritis | None | Uncooked vegan | Omnivorous | Dietary |
| Gardner et al., 200525 | USA | 4 weeks | 120 (60/60) | 26.5 | 48.5 | Without DM or heart diseases | None | Low-fat lacto-ovo-vegetarian | Low-fat omnivorous | Dietary |
| Barnard et al., 200626 | USA | 22 weeks | 99 (60/39) | 34.9 | 55.6 | With T2DM | 54 (27/27) | Vegan | Omnivorous (ADA diet) | Dietary |
| Burke et al., 200627 | USA | 6 months | 182 (159/23) | 34.1 | 44.1 | Overweight and obese | 10 (8/2) | Lacto-ovo-vegetarian | Omnivorous | Multi-interventional |
| de Mello et al., 200628 | Brazil | 4 weeks | 17 (3/14) | 26.2 | 59.0 | With T2DM and macroalbuminuria | None | Low-protein lacto-vegetarian | Omnivorous | Dietary |
| Aldana et al., 200729 | USA | 12 months | 93 (40/53) | 31.0 | 61.5 | With CAD | Yes (N subjects NR) | Low-fat lacto-ovo-vegetarian (Ornish) | Omnivorous | Multi-interventional |
| Burke et al., 200730 | USA | 18 months | 176 (153/23) | 34.0 | 44.0 | Overweight and obese | None | Low-fat lacto-ovo-vegetarian | Low-fat omnivorous | Multi-interventional |
| Elkan et al., 200831 | Sweden | 12 months | 58 (52/6) | 24.0 | 50.3 | With rheumatoid arthritis | None | Gluten-free vegan | Omnivorous | Dietary |
| Barnard et al., 200932 | USA | 74 weeks | 99 (60/39) | 34.9 | 55.6 | With T2DM | 54 (27/27) | Vegan | Omnivorous (ADA diet) | Dietary |
| Miller et al., 200933 | USA | 4 weeks | 18 (9/9) | 22.6 | 30.6 | Healthy with BMI < 30 and no history of metabolic, hepatic, renal, or systemic disease | None | Low-fat lacto-ovo-vegetarian (Ornish) | Omnivorous (Mediterranean/South Beach) | Dietary |
| Kahleova et al., 201134 | Czech | 24 weeks | 74 (39/35) | 35.1 | 56.2 | With T2DM | 38 (22/16) | Lacto-vegetarian | Omnivorous (EASD diet) | Multi-interventional |
| Mishra et al., 201335 | USA | 18 weeks | 291 (242/50) | 35.0 | 45.2 | BMI ≥ 25 and/or T2DM | NR | Low-fat vegan | Omnivorous | Dietary |
| Bunner et al., 201436 | USA | 16 weeks | 42 (39/3) | 27.5 | 45.7 | With prior migraine diagnosis | NR | Low-fat vegan | Omnivorous | Dietary |
| Lee et al., 201637 | South Korea | 12 weeks | 93 (75/18) | 23.5 | 57.9 | With T2DM | 49 (23/26) | Vegan | Omnivorous (conventional diet recommended by the Korean Diabetes Association) | Dietary |
| Lee et al., 201738 | South Korea | 10 days | 30 (30/0) | 21.5 | 20.0 | Healthy subjects with no physical and/or psychological disease | NR | Lacto-ovo-vegetarian | Omnivorous | Multi-interventional |
| Wright et al., 201739 | New Zealand | 6 months | 65 (39/26) | 34.4 | 56.0 | Obese or overweight and with T2DM, IHD, hypertension, and/or hypercholesterolaemia | Yes (N subjects NR) | Low-fat vegan | Omnivorous | Dietary |
| Kahleova et al., 201840 | USA | 16 weeks | 75 (67/8) | 33.3 | 53.4 | Overweight and obese without DM | 9 (5/4) | Low-fat vegan | Omnivorous | Dietary |
| Shah et al., 201841 | USA | 8 weeks | 100 (15/85) | 30.7 | 61.3 | With CAD | 95 (47/48) | Vegan | Omnivorous (AHA diet) | Dietary |
| Sofi et al., 201842 | Italy | 3 months | 118 (92/26) | 30.6 | 50.0 | Clinically healthy, BMI ≥ 25, and the presence of ≥1 of the following: TC > 190 mg/dL, LDL-C > 115 mg/dL, TG > 150 mg/dL, and glucose levels 110–126 mg/dL | None | Low-calorie lacto-ovo-vegetarian | Low-calorie omnivorous | Dietary |
| Djekic et al., 202043 | Sweden | 10 weeks | 31 (2/29) | 28.0 | 67.0 | With IHD | 31 (16/15) | Lacto-ovo-vegetarian | Omnivorous | Dietary |
| Kahleova et al., 202044 | USA | 16 weeks | 244 (211/33) | 33.4 | 55.0 | Overweight and obese | NR | Low-fat vegan | Omnivorous | Dietary |
| Garousi et al., 202145 | Iran | 3 months | 75 (39/36) | 31.0 | 43.2 | Overweight and obese adults with NAFLD | None | Lacto-ovo-vegetarian | Omnivorous (standard weight-loss diet) | Dietary |
| Barnard et al., 202246 | USA | 16 weeks | 62 (48/14) | 33.3 | 57.4 | Overweight and obese | 23 (11/12) | Low-fat vegan | Omnivorous (Mediterranean) | Dietary |
Overweight, BMI 25–29.9 kg/m2, obese ≥ 29.9 kg/m2; ADA, American Diabetes Association; AHA, American Heart Association; BMI, body mass index; CAD, coronary artery disease; DM, diabetes mellitus; E/C, experimental/control group; EASD, European Association for the Study of Diabetes; IHD, ischaemic heart disease; NAFLD, non-alcoholic fatty liver disease; NR, not reported; Omnivorous, contains all food groups; T2DM, type 2 diabetes mellitus.
Risk of bias in trials
In both crossover and parallel trials, risk of bias was highest in the domains concerning the randomization process and deviations from the intended interventions. Among the 30 included RCTs, none were participant blinded. In seven of the trials, all or some outcome assessors were blinded.26,30,32,39,41,44,46 Thirteen studies did not describe the randomization process.17–20,22,24,28,29,33,34,40,43,46 Thirteen followed the ITT principle while 17 followed PP. ITT studies include data from all participants enrolled in the trial, including excluded subjects and dropouts. PP trials only include data from subjects who finish the trial, which introduces risk of biases attributable to exclusion. Lastly, the participants’ adherence to the intervention diets was in most studies estimated through self-reported dietary records and questionnaires, which might have led to under- or overestimation of nutrient intake.
The crossover trials demonstrated higher risk of bias arising from period and carryover effects. Of the nine crossover trials, five reported a washout period of four weeks.28,33,36,43,46 Blood lipids stabilize after three to four weeks, wherefore it is encouraged to introduce a washout period of at least four weeks to avoid carryover effects.47 The remaining four studies did not report any washout period between the intervention and control period, although one reported no detection of carryover effects.42
Results of blood lipid and lipoprotein levels
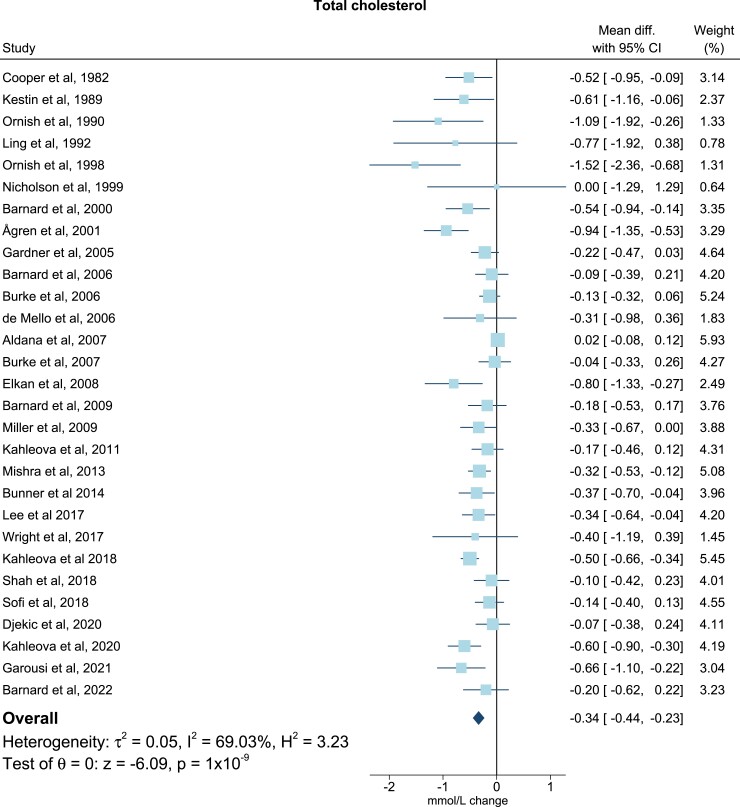
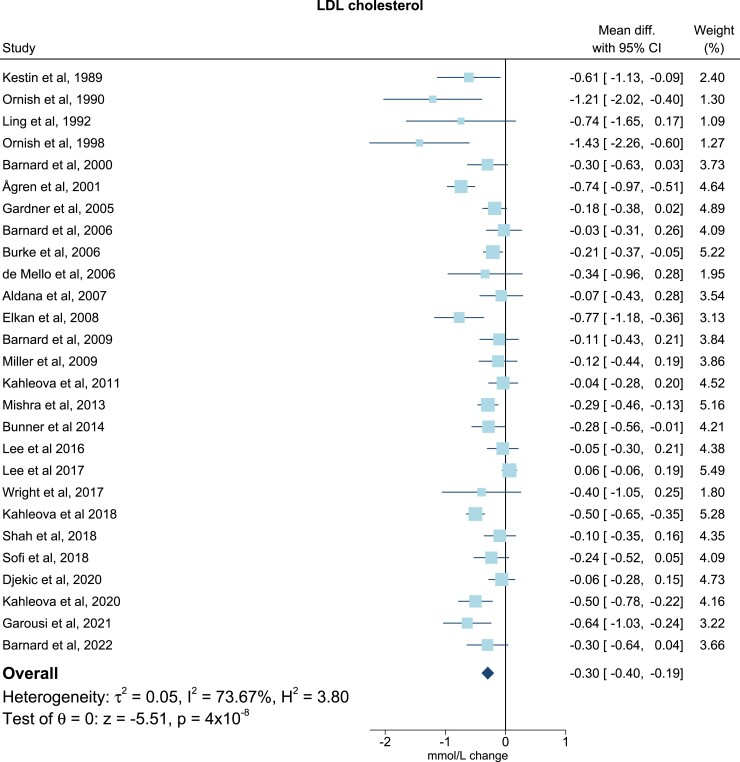
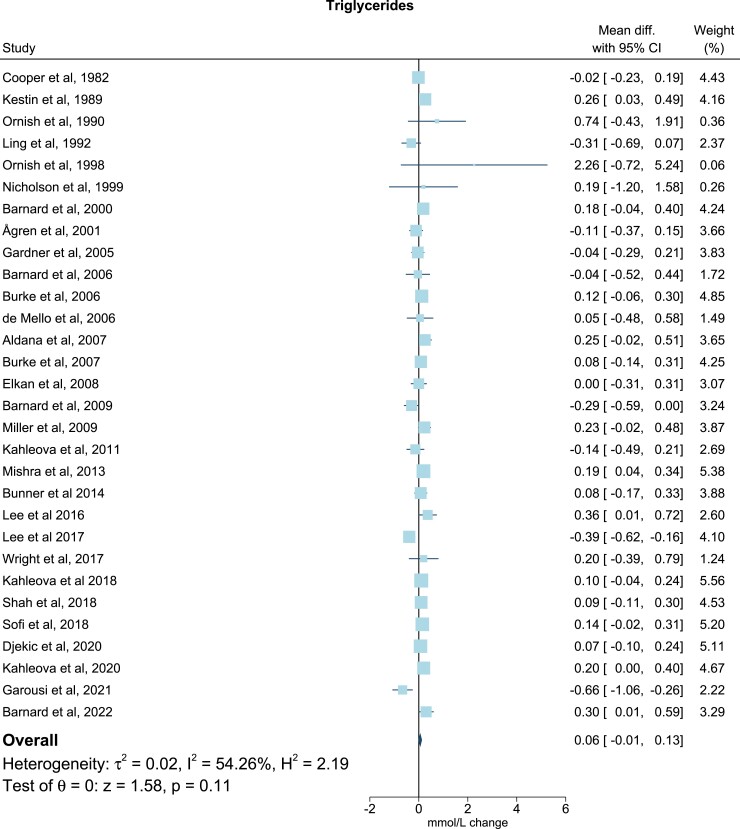
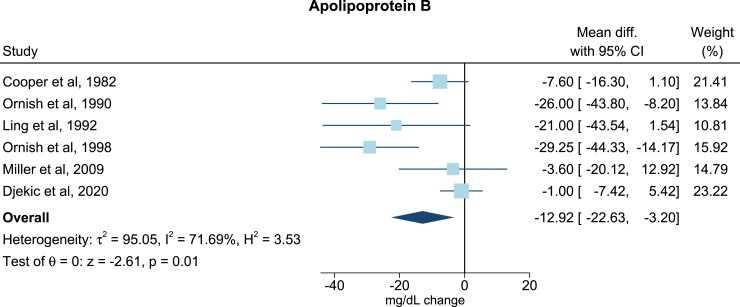
For TG all trials presented baseline levels, end of trial values, and/or changes in TG. One study did not include sufficient data on TC37, and three did not include on LDL-C (Table 2).17,22,30 Six studies included adequate values of apoB levels at baseline, end of trial, and/or changes of apoB levels (Table 2).17,19–21,33,43 All post-intervention lipid and lipoprotein concentrations were measured immediately after ended intervention. Only few studies reported follow-up periods after the finalized intervention. In the meta-analysis, compared with the omnivorous control group, the plant-based diet group showed a mean reduction in TC of −0.34 mmol/L (95% confidence interval, −0.44, −0.23; P = 1 × 10−9; I2 = 69.03%), equivalent to a reduction from baseline of 7% (Figure 2). For LDL-C levels the mean reduction was −0.30 mmol/L (−0.40, −0.19; P = 4 × 10−8; I2 = 73.67%) corresponding to a 10% reduction from baseline (Figure 3), while no changes were seen in TG levels (0.06 mmol/L; 7%; −0.01, 0.13; P = 0.11; I2 = 54.26) (Figure 4). Lastly, the apoB meta-analysis showed an overall decrease in apoB levels of −12.92 mg/dL (−22.63, −3.20; P = 0.01; I2 = 71.69%) and thereby a 14% reduction from baseline (Figure 5). The heterogeneity (I2) in the TG meta-analysis was characterized as moderate (54%) while the results for TC, LDL-C, and apoB were characterized as substantial heterogenic (69%, 74%, and 72%, respectively).
Table 2.
Baseline blood lipid and lipoprotein levels, outcomes, and study design
| Reference, year | Baseline TC, mmol/L (combined mean) | Baseline LDL-C, mmol/L (combined mean) | Baseline TG, mmol/L (combined mean) | Baseline apoB, mg/dL (combined mean) | CI | Effect on TC (yes/no) | Effect on LDL-C (yes/no) | Effect on TG (yes/no) | Effect on apoB (yes/no) | Report of changed lipid-lowering therapy [yes (E/C)/no] | Design | Outcome analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cooper et al., 198217 | 4.1 | 0.7 | 57.5 | 95% | Yes | Yes | Yes | No | CO | PP | ||
| Kestin et al., 198918 | 6.1 | 4.1 | 1.3 | 95% | Yes | Yes | Yes | No | CO | PP | ||
| Ornish et al., 199019 | 6.1 | 4.1 | 2.4 | 104 | 95% | Yes | Yes | Yes | Yes | No | PL | PP |
| Ling et al., 199220 | 5.6 | 3.7 | 1.2 | 87.5 | 95% | Yes | Yes | Yes | Yes | NR | PL | PP |
| Ornish et al., 199821 | 6.1 | 4.0 | 5.9 | 101.2 | 95% | Yes | Yes | Yes | Yes | No | PL | ITT |
| Nicholson et al., 199922 | 5.4 | 2.2 | 95% | Yes | Yes | No | PL | PP | ||||
| Barnard et al., 200023 | 4.2 | 2.5 | 0.9 | 95% | Yes | Yes | Yes | No | CO | PP | ||
| Ågren et al., 200124 | 4.9 | 3.3 | 1.2 | 95% | Yes | Yes | Yes | No | PL | PP | ||
| Gardner et al., 200525 | 5.8 | 3.9 | 1.5 | 95% | Yes | Yes | Yes | No | PL | PP | ||
| Barnard et al., 200626 | 5.0 | 2.9 | 1.8 | 95% | Yes | Yes | Yes | Yes (10/9) | PL | ITT | ||
| Burke et al., 200627 | 5.3 | 3.2 | 1.5 | 95% | Yes | Yes | Yes | Yes (4/2) | PL | ITT | ||
| de Mello et al., 200628 | 5.3 | 3.4 | 1.6 | 95% | Yes | Yes | Yes | No | CO | PP | ||
| Aldana et al., 200729 | 4.4 | 2.4 | 1.8 | 95% | Yes | Yes | Yes | NR | PL | ITT | ||
| Burke et al., 200730 | 5.3 | 1.5 | 95% | Yes | Yes | No | PL | ITT | ||||
| Elkan et al., 200831 | 5.0 | 3.1 | 1.1 | 95% | Yes | Yes | No | No | PL | PP | ||
| Barnard et al., 200932 | 5.0 | 2.9 | 1.3 | 95% | Yes | Yes | Yes | Yes (n subjects NR) | PL | ITT | ||
| Miller et al., 200933 | 4.8 | 2.8 | 0.9 | 80.7 | 95% | Yes | Yes | Yes | Yes | No | CO | PP |
| Kahleova et al., 201134 | 4.3 | 2.6 | 2.1 | 95% | Yes | Yes | Yes | NR | PL | ITT | ||
| Mishra et al., 201335 | 4.9 | 2.8 | 1.3 | 95% | Yes | Yes | Yes | NR | PL | ITT | ||
| Bunner et al., 201436 | 4.9 | 2.8 | 1.1 | 95% | Yes | Yes | Yes | NR | CO | ITT | ||
| Lee et al., 201637 | 2.6 | 1.6 | 95% | Yes | Yes | No | PL | PP | ||||
| Lee et al., 201738 | 4.5 | 2.6 | 0.9 | 95% | Yes | Yes | Yes | NR | PL | PP | ||
| Wright et al., 201739 | 5.4 | 3.4 | 1.5 | 95% | Yes | Yes | Yes | NR | PL | ITT | ||
| Kahleova et al., 201840 | 5.4 | 3.2 | 1.2 | 95% | Yes | Yes | Yes | No | PL | ITT | ||
| Shah et al., 201841 | 3.7 | 1.9 | 1.3 | 95% | Yes | Yes | Yes | NR | PL | ITT | ||
| Sofi et al., 201842 | 5.5 | 3.4 | 1.4 | 95% | Yes | Yes | Yes | No | CO | PP | ||
| Djekic et al., 202043 | 3.5 | 1.6 | 1.1 | 65.5 | 95% | Yes | Yes | Yes | Yes | No | CO | ITT |
| Kahleova et al., 202044 | 5.1 | 3.0 | 1.3 | 95% | Yes | Yes | Yes | No | PL | PP | ||
| Garousi et al., 202145 | 4.7 | 3.1 | 1.9 | 95% | Yes | Yes | Yes | No | PL | PP | ||
| Barnard et al., 202246 | 5.0 | 2.8 | 1.4 | 95% | Yes | Yes | Yes | Yes (7/3) | CO | PP |
Conversion factor from mmol/L to mg/dL is 0.0259 for TC and LDL-C and 0.0113 for TG. Conversion factor for mg/dL to g/L is 0.01 for apoB. Combined mean at baseline was 5.0 mmol/L for TC, 3.1 mmol/L for LDL-C, 1.6 mmol/L for TG, and 82.7 mg/dL for ApoB. ApoB, apolipoprotein B; CI, confidence interval; CO, crossover; E/C, experimental/control group; ITT, intention to treat; LDL-C, low-density lipoprotein cholesterol; NR, not reported; PL, parallel; PP, per protocol; TC, total cholesterol; TG, triglycerides.
Figure 2.
Meta-analysis: pooled mean effect sizes of vegetarian and vegan diets on total cholesterol. Based on 29 randomized controlled trials. Calculated by using a random-effects model. Overall P = 1 × 10−9; I2 = 69.03%. The squares demonstrate the weighted mean difference between intervention and control groups. Different sizes of squares illustrate the different weight of the studies’ sample sizes. The horizontal lines and parentheses demonstrate the 95% CI. CI, confidence interval; Diff., difference
Figure 3.
Meta-analysis: pooled mean effect sizes of vegetarian and vegan diets on LDL cholesterol. Based on 27 randomized controlled trials. Calculated by using a random-effects model. Overall P = 4 × 10−8; I2 = 73.67%. The squares demonstrate the weighted mean difference between intervention and control groups. Different sizes of squares illustrate the different weight of the studies’ sample sizes. The horizontal lines and parentheses demonstrate the 95% CI. CI, confidence interval; Diff., difference; LDL, low-density lipoprotein
Figure 4.
Meta-analysis: pooled mean effect sizes of vegetarian and vegan diets on triglycerides. Based on 30 randomized controlled trials. Calculated by using a random-effects model. Overall P = 0.11; I2 = 54.26%. The squares demonstrate the weighted mean difference between intervention and control groups. Different sizes of squares illustrate the different weight of the studies’ sample sizes. The horizontal lines and parentheses demonstrate the 95% CI. CI, confidence interval; Diff., difference
Figure 5.
Meta-analysis: pooled mean effect sizes of vegetarian and vegan diets on apolipoprotein B. Based on six randomized controlled trials. Calculated by using a random-effects model. Overall P = 0.01; I2 = 71.69%. The squares demonstrate the weighted mean difference between intervention and control groups. Different sizes of squares illustrate the different weight of the studies’ sample sizes. The horizontal lines and parentheses demonstrate the 95% CI. CI, confidence interval; Diff., difference
Subgroup analyses
Forest plots for subgroup analyses are presented in Supplementary data online, Figures S2–S38. For TC significant differences between subgroups of BMI (normal vs. overweight vs. obese) and outcome analysis (PP vs. ITT) were observed (between group differences P = 0.01) (see Supplementary data online, Figures S5 and S26). For LDL-C significant differences between subgroups of baseline LDL-C (>2.8 mmol/L vs. ≤2.8 mmol/L) were observed (see Supplementary data online, Figure S38, between group differences P ≤ 0.001). For TC and LDL-C significant differences between subgroups were observed in studies with no participants treated with lipid-lowering therapy vs. studies with some treated participants (between group differences, TC: P = 0.04; LDL-C: P = 0.03); in studies published before 2005 vs. after 2005 (between group differences, TC: P ≤ 0.001; LDL-C: P ≤ 0.001); and in studies with sample sizes under 80 individuals vs. over 80 individuals (between group differences, TC: P = 0.001; LDL-C: P = 0.03) (see Supplementary data online, Figures S23–S24, S29–S30, and S32–S33).
The remaining subgroup analyses regarding age, continent, duration of trial, health status, intervention diet, intervention program, BMI, outcome analysis (LDL-C and TG), and study design did not show any significant between group differences (see Supplementary data online, Figures S2–S4, S6–S22, S25, S27–S28, S31, and S34–S37). Subgroup analyses were not conducted for apoB due to few included studies.
Sensitivity analyses
The leave-one-out sensitivity analyses are shown in Supplementary data online, Figures S39–S42 and demonstrated no considerable changes in effect sizes of TC, LDL-C, and apoB when each study was left out of the analysis (see Supplementary data online, Figures S39–S40 and S42). After leaving each study out, changes in TC levels for plant-based diets vs. omnivorous diets ranged from −0.35 to −0.31 mmol/L (P < 0.001); from −0.31 to −0.27 mmol/L (P < 0.001) for LDL-C levels; and from −16.42 to −8.82 mg/dL (P-values 0.002–0.038) for apoB levels. For TG no effect of leave-one-out analysis was observed (see Supplementary data online, Figure S41).
Publication bias
Funnel plots examining publication bias illustrated missing studies on the right side of the plots for TC, LDL-C, and apoB, particularly in the bottom right corners (see Supplementary data online, Figures S43, S45, and S49). This was confirmed by Egger’s test (P = 1 × 10−3 for TC; P = 2 × 10−3 for LDL-C; P = 0.03 for apoB), suggesting the occurrence of small studies effects where estimates from smaller studies are overrepresented. The associated trim and fill plots imputed six studies for TC and five for LDL-C (see Supplementary data online, Figures S44 and S46). The funnel plot for TG showed a predominantly symmetrical distribution, however, the trim and fill method imputed four studies on the left side of the plot. Egger’s test did not confirm a small studies effect for TG (P = 0.85) (see Supplementary data online, Figure S48).
Discussion
The aim of this systematic review and meta-analysis was to estimate the effect of vegetarian and vegan diets on TC, LDL-C, TG, and apoB blood levels in 30 RCTs. We found that compared with omnivorous diets, consumption of vegetarian or vegan diets was associated with reduced levels of TC, LDL-C, and apoB. These effects were similar in a range of subgroup analyses stratified by participant and study characteristics (Structured Graphical Abstract).
Previous systematic reviews and meta-analyses conducted up until 2017 have shown similar associations between plant-based diets and decreased levels of TC and LDL-C.5,6 However, previous studies have neither included meta-analyses on apoB nor comprehensive subgroup analyses yielding novel knowledge on effect modification or robustness across participant and study characteristics. The importance of our findings is emphasized by the United Nations’ Sustainable Development Agenda stating that by 2030 premature mortality caused by non-communicable diseases (NCDs) should be reduced by one-third.48 CVDs are the biggest drivers among NCDs1 and apoB-containing lipoproteins, as LDL and TG-rich lipoproteins, are substantial risk factors for ASCVD.3 In fact, numerous Mendelian randomization studies have shown that alterations in absolute LDL-C concentrations are proportional to ASCVD risk.49 Identifying measures such as specific diets that could contribute to lowering apoB-containing lipoprotein particles are therefore of pivotal relevance for the prevention of CVD.3
To explain our findings, one should consider the nutritional composition of plant-based diets, as these, compared with omnivorous diets, are usually higher in poly-unsaturated fatty acids (PUFAs) while being lower in saturated fatty acids, cholesterol, and total fat.50 A reduced consumption of fat leads to lower intestinal absorption of triglycerides and cholesterol and subsequently decreased levels of cholesterol-containing lipoprotein particles in the blood. Moreover, the PUFAs that reduce LDL-C by increasing the expression of hepatic LDL receptors are omega-6 and especially linoleic acid.51 Importantly, omega-3 has no significant effect in this regard.
The TC and LDL-C findings are consistent with the present apoB results as the blood concentration of apoB is an estimate of the total amount of atherogenic lipoprotein particles in the blood. LDL is the most abundant apoB-containing lipoprotein particle in the blood, and a reduction in cholesterol, especially LDL-C, will therefore result in decreased levels of apoB as demonstrated by this meta-analysis. These are important and novel findings since other apoB-containing lipoprotein particles such as very-low-density lipoprotein, intermediate density lipoprotein, and lipoprotein(a) also exhibit atherogenic abilities. Quantifying the effect of different diets on apoB levels, therefore, gives us a more direct estimate of the ability of plant-based diets to reduce the atherosclerotic burden than measurements of specific lipids or lipoprotein particles. However, the findings on apoB are only based on six RCTs, two of which are multi-interventional,19,21 and these results should therefore be interpreted with care. Yet, this merely emphasizes that more trials investigating dietary effects on apoB are warranted.
Subgroup analyses cannot stand alone as evidence for a biological process but can be used to show tendencies and generate hypotheses. In these analyses, we observed that obese participants experienced a smaller decrease in TC compared with normal and overweight participants. This could be explained by the adverse effects of obesity on cholesterol metabolism, as the hepatic and intestinal cholesterol synthesis is known to increase in obese individuals.52 Obese individuals are therefore generally synthesizers, rather than absorbers, wherefore plant-based diets typically have a smaller impact on their cholesterol levels in plasma. Moreover, obesity predisposes to leptin tolerance and resistance, which diminishes the stimulating effect of leptin on hepatic cholesterol clearance.53,54 Together, these mechanisms can increase TC levels despite following a similar diet as normal and overweight participants. Further, the outcome analysis stratification for TC showed that the effect on TC levels was lower in trials done by ITT compared with trials following PP. This was however not found for LDL-C. ITT trials include data from all included subjects wherefore also results from dropouts and non-adherent participants are included in the final analysis. Consequently, ITT trials often demonstrate reduced effect estimates compared with PP trials. The lower effect observed in subjects being treated with lipid-lowering therapy plausibly depends on the fact that all lipid drugs activate the expression of LDL receptors. Consequently, the additional effect of diets may become weaker. By stratifying on lipid-lowering therapy in the present study, there were indeed significant differences, however, the effects in both groups remained significant. We observed a similar scenario for individuals with baseline LDL-C levels below vs. above the mean, and the same biological explanation as for lipid-lowering therapy may apply. Finally, the sample size stratification showed larger decreases in TC and LDL-C levels for studies under 80 individuals vs. studies with more than 80 individuals. The same was observed for studies published before 2005, all of which were categorized as small studies, vs. after 2005 where larger sample sizes were included. Smaller studies, which thus apply to the studies published before 2005, tend to follow their participants more closely and provide a higher degree of nursing compared with larger studies. This increases participant compliance and results in larger effect sizes. Additionally, small studies showing little or no effect of the intervention tend to not be published. Only small studies with larger effect sizes are hereby published, which altogether creates the small studies effect as shown in the tests for publication bias.
Our findings illustrate that lipid profiles improve when following a plant-based diet. However, changing to and maintaining a healthy plant-based diet can be a challenge, and methods to motivate and help people stick to this type of diet are warranted. A recent study showed that incorporating dietary assessment into ten-year risk charts for ASCVD presented similar risk estimates as when incorporating the routine non-high-density lipoprotein cholesterol (HDL-C),55 as done in SCORE2.56 Therefore, risk charts integrating dietary assessment could be a method for motivating individuals to improve or keep their adherence to dietary guidelines—a diet rich in plant-based foods.55 Furthermore, in another trial, more than 1000 subjects with known coronary heart disease were over seven years assigned to follow either a Mediterranean diet or a low-fat diet—both of which are high in complex fibres from fruits and vegetables and low in saturated fatty acids, especially from red or processed meats.57 Reoccurrence of a CVD event was substantially reduced with both diets; however, the Mediterranean diet was superior to the low-fat diet. The Mediterranean diet is not meat or animal-product free but focuses on a high intake of plant-based foods and the use of unsaturated fat. Nevertheless, this study found a notable reduction in the reoccurrence of cardiovascular events, implying that the beneficial effects of a diet can also be achieved by a reduced intake of animal products. This indicates that such diets may be easier for people to stick to than e.g. a low-fat diet or a vegan diet. Together, these studies and this meta-analysis emphasize the importance of adhering to a healthy, plant-based diet for both primary and secondary prevention of CVD and moreover, that specific diets and methods are crucial for the motivation and maintenance of healthy eating habits.
It should be considered whether our findings are attributed to the dietary composition of plant-based diets or whether they are due to confounders such as weight loss. Weight loss, however, tends to decrease TG levels which is in contrast to our findings.2 Moreover, statin treatment is superior to plant-based diets in reducing lipid and lipoprotein levels.6 However, one regimen does not exclude the other. Prevention of disease risk factors such as overweight, hypertension, and dyslipidaemia is key to slowing down the atherosclerotic process, wherefore consumption of plant-based diets could postpone or even diminish the need for statins, thus sparring individuals from side effects related to the treatment. Furthermore, combining statins and plant-based diets will likely have a synergistic effect resulting in an even larger, and more beneficial effect on lipid and lipoprotein levels. At last, this study did not investigate the effect of plant-based diets on HDL-C since we focused on established atherogenic lipids and lipoproteins. Elevated levels of HDL-C are not causally associated with a lower risk of CVD as established by Mendelian randomized studies58–61 and so far no studies have revealed significant results on HDL-C-increasing therapies and lower CVD risk where effects were attributed to HDL-C alone.62–67
This study has several strengths including a stringent design with clearly defined inclusion and exclusion criteria. To our knowledge, this systematic review is the first to include as many as 30 RCTs with a total sample size of 2372 participants while previous reviews included 832 and 1484 individuals.5,6 Furthermore, changes in apoB levels were assessed for the first time. The present field is highly relevant considering the United Nations’ establishment of the 2030 Sustainable Development Agenda and the increased focus on the environment.48 In fact, recent systematic reviews have shown that shifting to lacto-ovo-vegetarian or vegan diets, at a population level in high-income countries, can reduce the net emission of greenhouse gasses by respectively 35% and 49%; making these diets highly beneficial for the environment.68 Furthermore, populations are aging globally and as a consequence expenses for treatment of age-related diseases such as ASCVD are increasing.2 Plant-based diets are thus key instruments for changing food production to more sustainable forms while at the same time reducing the growing burden of CVD.
Several limitations should also be considered. First, the sample sizes of the individual RCTs were relatively small; and the present meta-analysis represents however the largest compilation of studies to date. The intervention period for most of the included studies lasted under one year, which emphasizes the need for more long-term trials. Previous studies found that after a few months the effect on LDL-C was halved as compared to that observed after a shorter follow-up.69,70 Short-term studies may therefore have a larger effect on the lipid profile (due to better compliance) and may lead to overestimating the effects obtainable in the long term. By stratifying for duration period over and at or under 3 months in the present analysis, we did however not observe attenuation of effects with longer duration.
Other limitations include that none of the RCTs were participant blinded, which could interfere with the participants’ motivation to adhere to the assigned diet. The randomization process was not described in all studies, therefore, making it difficult to assess whether subjects were 100% randomly assigned and, thus, if the observed effects were attributed to the intervention or confounders. Four of the nine crossover trials did not report any washout period between intervention periods, which increases the risk of carryover effects and overestimated effect sizes. This could have been prevented by treating the crossover studies as parallel and thus only extracting data from the first intervention period. However, this was not possible due to missing data.71 The subgroup analyses, nonetheless, did not show any differences between the estimated effects in the parallel vs. crossover trials. Moreover, handling crossover trials as we have done in this meta-analysis tends to widen the confidence intervals, resulting in crossover trials being under-weighted compared with parallel trials.71 The impact of potential carryover effects from crossover trials is thereby diminished. Lastly, it was not possible to adopt the ITT principle in the meta-analysis since this requires access to individual participant data from each trial.72 The findings should therefore be interpreted with caution as the potential impact of missing data may influence the results. Likewise, the results on publication bias should, in general, be treated with caution as the methods used to evaluate publication bias have several limitations.
In conclusion, consumption of vegetarian and vegan diets reduces blood levels of atherogenic lipoproteins. Shifting to plant-based diets at a populational level will reduce emissions of greenhouse gasses considerably—together making these diets efficient means towards a more sustainable development, while at the same time reducing the growing burden of ASCVD.
Supplementary Material
Acknowledgements
The Structured Graphical Abstract, Figure 1, and Supplementary data online, Figure S1 were created with the use of BioRender.
Contributor Information
Caroline A Koch, Department of Clinical Biochemistry, Copenhagen University Hospital—Rigshospitalet, Blegdamsvej 9, DK-2100 Copenhagen, Denmark.
Emilie W Kjeldsen, Department of Clinical Biochemistry, Copenhagen University Hospital—Rigshospitalet, Blegdamsvej 9, DK-2100 Copenhagen, Denmark; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, 2200 Copenhagen, Denmark.
Ruth Frikke-Schmidt, Department of Clinical Biochemistry, Copenhagen University Hospital—Rigshospitalet, Blegdamsvej 9, DK-2100 Copenhagen, Denmark; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, 2200 Copenhagen, Denmark.
Supplementary data
Supplementary data is available at European Heart Journal online.
Author contributions
All authors: aim of study, study design, conduction, data interpretation, and critical revision of the manuscript. C.A.K. and E.W.K.: literature search, data extraction, statistical analysis, and drafting of the manuscript. E.W.K. and R.F.S.: study supervision. R.F.S.: final responsibility for all matters of the study.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Funding
The study was funded by the Lundbeck Foundation, the Danish Heart Foundation, and the Leducq Foundation.
References
- 1. WHO . Cardiovascular Diseases. World Health Organization. https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (23 November 2021).
- 2. Abbafati C, Abbas KM, Abbasi-Kangevari M, Abd-Allah F, Abdelalim A, Abdollahi M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet 2020;396:1204–1222. 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021;42:3227–3337. 10.1093/eurheartj/ehab484 [DOI] [PubMed] [Google Scholar]
- 4. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2017;38:2459–2472. 10.1093/eurheartj/ehx144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang F, Zheng J, Yang B, Jiang J, Fu Y, Li D. Effects of vegetarian diets on blood lipids: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc 2015;4:e002408. 10.1161/JAHA.115.002408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yokoyama Y, Levin SM, Barnard ND. Association between plant-based diets and plasma lipids: a systematic review and meta-analysis. Nutr Rev 2017;75:683–698. 10.1093/nutrit/nux030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Satija A, Hu FB. Plant-based diets and cardiovascular health. Trends Cardiovasc Med 2018;28:437–441. 10.1016/j.tcm.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Veritas Health Innovation . Covidence systematic review software. https://www.covidence.org(11 October 2022).
- 9. Higgins JPT, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. Cochrane handbook for systematic reviews of interventions version 6.3. https://training.cochrane.org/handbook/current/chapter-06#section-6-5-1-2(12 November 2021).
- 10. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol 1992;45:769–773. 10.1016/0895-4356(92)90054-Q [DOI] [PubMed] [Google Scholar]
- 12. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 13. Deeks JJ, Higgins JPT, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. Cochrane handbook for systematic reviews of interventions version 6.3. http://www.training.cochrane.org/handbook(18 October 2022).
- 14. Cochran WG. The comparison of percentages in matched samples. Biometrika 1950;37:256–266. 10.1093/biomet/37.3-4.256 [DOI] [PubMed] [Google Scholar]
- 15. Higgins JPT, Churchill R, Chandler J, Cumpston M. Chapter 10: Addressing reporting biases. Cochrane handbook for systematic reviews of interventions version 5.1. https://www.handbook-5-1.cochrane.org/chapter_10/10_addressing_reporting_biases.htm(16 April 2022).
- 16. Sterne J, Savović J, Page MJ, Elbers R, Blencowe N, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 17. Cooper RS, Goldberg RB, Trevisan M, Tsong Y, Liu K, Stamler J, et al. The selective lipid-lowering effect of vegetarianism on low density lipoproteins in a cross-over experiment. Atherosclerosis 1982;44:293–305. 10.1016/0021-9150(82)90004-1 [DOI] [PubMed] [Google Scholar]
- 18. Kestin M, Rouse IL, Correll RA, Nestel PJ. Cardiovascular disease risk factors in free-living men: comparison of two prudent diets, one based on lactoovovegetarianism and the other allowing lean meat. Am J Clin Nutr 1989;50:280–287. 10.1093/ajcn/50.2.280 [DOI] [PubMed] [Google Scholar]
- 19. Ornish D, Brown S, Billings J, Scherwitz L, Armstrong W, Ports T, et al. Can lifestyle changes reverse coronary heart disease? The lifestyle heart trial. Lancet 1990;336:129–133. 10.1016/0140-6736(90)91656-U [DOI] [PubMed] [Google Scholar]
- 20. Ling WH, Laitinen M, Hänninen O. Shifting from conventional diet to an uncooked vegan diet reversibly alters serum lipid and apolipoprotein levels. Nutr Res 1992;12:1431–1440. 10.1016/S0271-5317(05)80185-X [DOI] [PubMed] [Google Scholar]
- 21. Ornish D, Scherwitz LW, Billings JH, Lance Gould K, Merritt TA, Sparler S, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA 1998;280:2001–2007. 10.1001/jama.280.23.2001 [DOI] [PubMed] [Google Scholar]
- 22. Nicholson AS, Sklar M, Barnard ND, Gore S, Sullivan R, Browning S. Toward improved management of NIDDM: a randomized, controlled, pilot intervention using a lowfat, vegetarian diet. Prev Med 1999;29:87–91. 10.1006/pmed.1999.0529 [DOI] [PubMed] [Google Scholar]
- 23. Barnard ND, Scialli AR, Bertron P, Hurlock D, Edmonds K, Talev L. Effectiveness of a low-fat vegetarian diet in altering serum lipids in healthy premenopausal women. Am J Cardiol 2000;85:969–972. 10.1016/S0002-9149(99)00911-X [DOI] [PubMed] [Google Scholar]
- 24. Ågren JJ, Tvrzicka E, Nenonen MT, Helve T, Hänninen O. Divergent changes in serum sterols during a strict uncooked vegan diet in patients with rheumatoid arthritis. Br J Nutr 2001;85:137–139. 10.1079/BJN2000234 [DOI] [PubMed] [Google Scholar]
- 25. Gardner CD, Coulston A, Chatterjee L, Rigby A, Spiller G, Farquhar JW. The effect of a plant-based diet on plasma lipids in hypercholesterolemic adults: a randomized trial. Ann Intern Med 2005;142:725–733. 10.7326/0003-4819-142-9-200505030-00007 [DOI] [PubMed] [Google Scholar]
- 26. Barnard ND, Cohen J, Jenkins DJA, Turner-McGrievy G, Gloede L, Jaster B, et al. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care 2006;29:1777–1783. 10.2337/dc06-0606 [DOI] [PubMed] [Google Scholar]
- 27. Burke LE, Styn MA, Steenkiste AR, Music E, Warziski M, Choo J. A randomized clinical trial testing treatment preference and two dietary options in behavioral weight management: preliminary results of the impact of diet at 6 months—PREFER study. Obesity 2006;14:2007–2017. 10.1038/oby.2006.235 [DOI] [PubMed] [Google Scholar]
- 28. de Mello VDF, Zelmanovitz T, Perassolo MS, Azevedo MJ, Gross JL. Withdrawal of red meat from the usual diet reduces albuminuria and improves serum fatty acid profile in type 2 diabetes patients with macroalbuminuria. Am J Clin Nutr 2006;83:1032–1038. 10.1093/ajcn/83.5.1032 [DOI] [PubMed] [Google Scholar]
- 29. Aldana SG, Greenlaw R, Salberg A, Merrill RM, Hager R, Jorgensen RB. The effects of an intensive lifestyle modification program on carotid artery intima-media thickness: a randomized trial. Am J Health Promot 2007;21:510–516. 10.4278/0890-1171-21.6.510 [DOI] [PubMed] [Google Scholar]
- 30. Burke LE, Hudson AG, Warziski MT, Styn MA, Music E, Elci OU, et al. Effects of a vegetarian diet and treatment preference on biochemical and dietary variables in overweight and obese adults: a randomized clinical trial. Am J Clin Nutr 2007;86:588–596. 10.1093/ajcn/86.3.588 [DOI] [PubMed] [Google Scholar]
- 31. Elkan AC, Sjöberg B, Kolsrud B, Ringertz B, Hafström I, Frostegård J. Gluten-free vegan diet induces decreased LDL and oxidized LDL levels and raised atheroprotective natural antibodies against phosphorylcholine in patients with rheumatoid arthritis: a randomized study. Arthritis Res Ther 2008;10:R34. 10.1186/ar2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barnard ND, Cohen J, Jenkins DJA, Turner-McGrievy G, Gloede L, Green A, et al. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am J Clin Nutr 2009;89:1588S–1596S. 10.3945/ajcn.2009.26736H [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miller M, Beach V, Sorkin JD, Mangano C, Dobmeier C, Novacic D, et al. Comparative effects of three popular diets on lipids, endothelial function, and C-reactive protein during weight maintenance. J Am Diet Assoc 2009;109:713–717. 10.1016/j.jada.2008.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kahleova H, Matoulek M, Malinska H, Oliyarnik O, Kazdova L, Neskudla T, et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with type 2 diabetes. Diabet Med 2011;28:549–559. 10.1111/j.1464-5491.2010.03209.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mishra S, Xu J, Agarwal U, Gonzales J, Levin S, Barnard ND. A multicenter randomized controlled trial of a plant-based nutrition program to reduce body weight and cardiovascular risk in the corporate setting: the GEICO study. Eur J Clin Nutr 2013;67:718–724. 10.1038/ejcn.2013.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bunner AE, Agarwal U, Gonzales JF, Valente F, Barnard ND. Nutrition intervention for migraine: a randomized crossover trial. J Headache Pain 2014;15:69. 10.1186/1129-2377-15-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee Y, Kim S, Lee I, Kim J, Park K, Jeong J, et al. Effect of a brown rice based vegan diet and conventional diabetic diet on glycemic control of patients with type 2 diabetes: a 12-week randomized clinical trial. PLoS One 2016;11:e0155918. 10.1371/journal.pone.0155918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee KS, Lee JK, Yeun YR. Effects of a 10-day intensive health promotion program combining diet and physical activity on body composition, physical fitness, and blood factors of young adults: a randomized pilot study. Med Sci Monit 2017;23:1759–1767. 10.12659/MSM.900515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wright N, Wilson L, Smith M, Duncan B, McHugh P. The BROAD study: a randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes 2017;7:e256. 10.1038/nutd.2017.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kahleova H, Tura A, Hill M, Holubkov R, Barnard N. A plant-based dietary intervention improves beta-cell function and insulin resistance in overweight adults: a 16-week randomized clinical trial. Nutrients 2018;10:189. 10.3390/nu10020189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shah B, Newman JD, Woolf K, Ganguzza L, Guo Y, Allen N, et al. Anti-inflammatory effects of a vegan diet versus the American Heart Association-recommended diet in coronary artery disease trial. J Am Heart Assoc 2018;7:e011367. 10.1161/JAHA.118.011367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sofi F, Dinu M, Pagliai G, Cesari F, Gori AM, Sereni A, et al. Low-calorie vegetarian versus Mediterranean diets for reducing body weight and improving cardiovascular risk profile. Circulation 2018;137:1103–1113. 10.1161/CIRCULATIONAHA.117.030088 [DOI] [PubMed] [Google Scholar]
- 43. Djekic D, Shi L, Brolin H, Carlsson F, Särnqvist C, Savolainen O, et al. Effects of a vegetarian diet on cardiometabolic risk factors, gut microbiota, and plasma metabolome in subjects with ischemic heart disease: a randomized, crossover study. J Am Heart Assoc 2020;9:e016518. 10.1161/JAHA.120.016518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kahleova H, Petersen KF, Shulman GI, Alwarith J, Rembert E, Tura A, et al. Effect of a low-fat vegan diet on body weight, insulin sensitivity, postprandial metabolism, and intramyocellular and hepatocellular lipid levels in overweight adults. JAMA Netw Open 2020;3:e2025454. 10.1001/jamanetworkopen.2020.25454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Garousi N, Tamizifar B, Pourmasoumi M, Feizi A, Askari G, Clark CCT, et al. Effects of lacto-ovo-vegetarian diet vs. standard-weight-loss diet on obese and overweight adults with non-alcoholic fatty liver disease: a randomised clinical trial. Arch Physiol Biochem 2021;9:1–9. 10.1080/13813455.2021.1890128 [DOI] [PubMed] [Google Scholar]
- 46. Barnard ND, Alwarith J, Rembert E, Brandon L, Nguyen M, Goergen A, et al. A Mediterranean diet and low-fat vegan diet to improve body weight and cardiometabolic risk factors: a randomized, cross-over trial. J Am Nutr Assoc 2022;41:127–139. 10.1080/07315724.2020.1869625 [DOI] [PubMed] [Google Scholar]
- 47. Kris-etherton PM, Dietschy J. Design criteria for studies examining individual fatty acid effects on cardiovascular disease risk factors: human and animal studies. Am J Clin Nutr 1997;65:1593S. 10.1093/ajcn/65.5.1590S [DOI] [PubMed] [Google Scholar]
- 48. United Nations . Transforming our world: The 2030 agenda for sustainable development. https://sdgs.un.org/publications/transforming-our-world-2030-agenda-sustainable-development-17981(9 May 2022).
- 49. Mach F, Baigent C, Catapano AL, Koskina KC, Casula M, Badimon L, et al. ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis 2019;2019:140–205. 10.1016/j.atherosclerosis.2019.08.014 [DOI] [PubMed] [Google Scholar]
- 50. Li D. Chemistry behind vegetarianism. J Agric Food Chem 2011;59:777–784. 10.1021/jf103846u [DOI] [PubMed] [Google Scholar]
- 51. Mustad VA, Etherton TD, Cooper AD, Mastro AM, Pearson TA, Jonnalagadda SS, et al. Reducing saturated fat intake is associated with increased levels of LDL receptors on mononuclear cells in healthy men and women. J Lipid Res 1997;38:459–468. 10.1016/S0022-2275(20)37254-0 [DOI] [PubMed] [Google Scholar]
- 52. Miettinen TA. Cholesterol production in obesity. Circulation 1971;44:842–850. 10.1161/01.CIR.44.5.842 [DOI] [PubMed] [Google Scholar]
- 53. Vanpatten S, Ranginani N, Shefer S, Nguyen LB, Rossetti L, Cohen DE. Impaired biliary lipid secretion in obese Zucker rats: leptin promotes hepatic cholesterol clearance. Am J Physiol Gastrointest Liver Physiol 2001;281:G393–G404. 10.1152/ajpgi.2001.281.2.G393 [DOI] [PubMed] [Google Scholar]
- 54. Scarpace PJ, Zhang Y. Leptin resistance: a prediposing factor for diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 2009;296:R493–R500. 10.1152/ajpregu.90669.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kjeldsen EW, Thomassen JQ, Rasmussen KL, Nordestgaard BG, Tybjærg-Hansen A, Frikke-Schmidt R. Impact of diet on ten-year absolute cardiovascular risk in a prospective cohort of 94 321 individuals: a tool for implementation of healthy diets. Lancet Reg Health Eur 2022;19:100419. 10.1016/j.lanepe.2022.100419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. SCORE2 working group and ESC Cardiovascular risk collaboration . SCORE2 risk prediction algorithms: new models to estimate 10-year risk of cardiovascular disease in Europe. Eur Heart J 2021;42:2439–2454. 10.1093/eurheartj/ehab309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Delgado-Lista J, Alcala-Diaz JF, Torres-Peña JD, Quintana-Navarro GM, Fuentes F, Garcia-Rios A, et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): a randomised controlled trial. Lancet 2022;399:1876–1885. 10.1016/S0140-6736(22)00122-2 [DOI] [PubMed] [Google Scholar]
- 58. Frikke-Schmidt R, Nordestgaard BG, Stene MCA, Sethi AA, Remaley AT, Schnohr P, et al. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA 2008;299:2524–2532. 10.1001/jama.299.21.2524 [DOI] [PubMed] [Google Scholar]
- 59. Haase CL, Tybjærg-Hansen A, Qayyum AA, Schou J, Nordestgaard BG, Frikke-Schmidt R. HDL Cholesterol and ischemic cardiovascular disease: a Mendelian randomization study of HDL cholesterol in 54,500 individuals. J Clin Endocrinol Metab 2012;97:E248–E256. 10.1210/jc.2011-1846 [DOI] [PubMed] [Google Scholar]
- 60. Haase CL, Tybjærg-Hansen A, Grande P, Frikke-Schmidt R. Genetically elevated apolipoprotein A-I, high-density lipoprotein cholesterol levels, and risk of ischemic heart disease. J Clin Endocrinol Metab 2010;95:E500–E510. 10.1210/jc.2010-0450 [DOI] [PubMed] [Google Scholar]
- 61. Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, et al. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet 2012;380:572–580. 10.1016/S0140-6736(12)60312-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bowman L, Hopewell JC, Chen F, Wallendszus K, Stevens W, Collins R, et al. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med 2017;377:1217–1227. 10.1056/NEJMoa1706444 [DOI] [PubMed] [Google Scholar]
- 63. Keene D, Price C, Shun-Shin MJ, Francis DP. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117 411 patients. BMJ 2014;349:g4379. 10.1136/bmj.g4379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJP, Komajda M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007;357:2109–2122. 10.1056/NEJMoa0706628 [DOI] [PubMed] [Google Scholar]
- 65. Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 2012;367:2089–2099. 10.1056/NEJMoa1206797 [DOI] [PubMed] [Google Scholar]
- 66. The HPS2-THRIVE Collaborative Group . Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014;371:203–212. 10.1056/NEJMoa1300955 [DOI] [PubMed] [Google Scholar]
- 67. Lincoff AM, Nicholls SJ, Riesmeyer JS, Barter PJ, Brewer HB, Fox KAA, et al. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med 2017;376:1933–1942. 10.1056/NEJMoa1609581 [DOI] [PubMed] [Google Scholar]
- 68. Fresán U, Sabaté J. Vegetarian diets: planetary health and its alignment with human health. Adv Nutr 2019;10:S380–S388. 10.1093/advances/nmz019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jenkins DJA, Kendall CWC, Marchie A, Faulkner DA, Wong JMW, De SR, et al. Effects of a dietary portfolio of cholesterol-lowering foods vs lovastatin on serum lipids and C-reactive protein. JAMA 2003;290:502–510. 10.1001/jama.290.4.502 [DOI] [PubMed] [Google Scholar]
- 70. Jenkins DJA, Kendall CWC, Faulkner DA, Nguyen T, Kemp T, Marchie A, et al. Assessment of the longer-term effects of a dietary portfolio of cholesterol-lowering foods in hypercholesterolemia. Am J Clin Nutr 2006;83:582–591. 10.1093/ajcn.83.3.582 [DOI] [PubMed] [Google Scholar]
- 71. Higgins JPT, Eldridge S, Li T. Chapter 23.2: Crossover trials. Cochrane handbook for systematic reviews of interventions version 6.3. https://training.cochrane.org/handbook/current/chapter-23#section-23-2(29 October 2022).
- 72. Higgins JPT, Green S. Chapter 16.2.3: Intention-to-treat issues for continuous data. Cochrane handbook for systematic reviews of interventions version 5.1. https://handbook-5-1.cochrane.org/chapter_16/16_2_3_intention_to_treat_issues_for_continuous_data.htm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.