Abstract
Objectives
This study aimed to determine whether SII on different days of admission is associated with severity and 180-day functional outcomes after basal ganglia ICH.
Methods
In this retrospective study, data on baseline CT imaging characteristics, mRS, hematoma volume, and laboratory variables were included. The SII and NLR, LMR, and PLR were calculated from laboratory data collected on admission day, day 1, and days 5–7. Both univariate and multivariable logistic regression analyses were used to assess the association between the SII and the outcome. The receiver operating characteristic (ROC) analysis and area under the curve (AUC) were also used to evaluate the ability of the SII to predict outcomes.
Result
A total of 245 patients were enrolled in the study. On different days, the NLR, PLR, and SII were significantly lower in patients with favorable outcomes than in those with poor outcomes, and the volume of hemorrhage was positively correlated with the SII. These parameters were associated with outcomes in the univariate logistic regression. In the adjusted analyses, the SII and PLR were independent predictors of basal ganglia ICH outcomes. ROC analysis revealed that the SII showed a stronger ability to predict the 6-month outcomes of patients after basal ganglia ICH than the PLR on different days (AUC = 0.642, 0.804, 0.827 vs. 0.592, 0.725, 0.757; all P < 0.001).
Conclusion
The SII independently and strongly predicts the outcome of basal ganglia ICH. A high SII was associated with poor 6-month outcomes in patients with basal ganglia ICH.
Keywords: Basal ganglia intracerebral hemorrhage (ICH), Prognostic marker, Systemic immune-inflammation index (SII), Biomarkers, Inflammation
1. Introduction
It is well-known that spontaneous intracerebral hemorrhage (ICH) is the common stroke subtype and leads to severe disability or death [1]. Basal ganglia ICH is the most common type of deep hemorrhage and a significant cause of patient dysfunction [2]. Therapeutic options remain limited because clinical trials for primary ICH injuries have failed to improve outcomes [3]. Crosstalk between the brain tissue and immune systems during ICH may be a potential mechanism contributing to brain injury, complications, and outcomes. Many studies have identified various indicators that can predict outcomes following ICH [[4], [5], [6]]; but only a limited number have included the use of biochemical tests in cases of basal ganglia ICH.
Blood extravasation into the brain is one of the leading causes of secondary brain injury (SBI) after ICH [7], and inflammatory cytokines are largely responsible for secondary damage after ICH [8]. Additionally, clinical laboratory test results that indicate inflammation, such as PLT, neutrophil-lymphocyte ratio (NLR), PLT-lymphocyte ratio (PLR), lymphocyte-monocyte ratio (LMR), and others, have been identified as potential predictors of ICH outcomes [[9], [10], [11]]. Recently, we found that levels of neurofilament light chain (NfL) and S100B in serum were linked to both disease severity and outcome among patients with aneurysmal subarachnoid hemorrhage (aSAH) [12]. In addition, the studies indicated that there is a strong correlation between inflammatory reactions and clinical outcomes after experiencing an ICH [13].
The systemic immune-inflammation index (SII) is a novel systemic inflammatory biomarker that reflects the inflammatory and immune status balance, which has not been sufficiently investigated in basal ganglia ICH. The SII has been identified as a predictive tool in oncology literature for various types of cancers, including hepatocellular carcinoma, pancreatic and bladder cancers, and endocrine tumors [[14], [15], [16], [17]]. In the central nervous system, the preoperative SII helps predict prognosis in high-grade gliomas [18], and the SII can independently predict the development of delayed cerebral vasospasm in aSAH [19]. More recently, the SII was only shown to predict short-term and three-month poor outcomes following ICH [20,21]. Nevertheless, the results of prior studies on ICH have not sufficiently demonstrated the usefulness of the SII as a potential predictor of prognosis or clinical outcome. The potential of SII as a predictor of long-term outcomes after basal ganglia ICH is currently unknown. The SII as a novel biomarker could better reflect the prognosis of patients with basal ganglia ICH than LMR, NLR, and PLR [22]. In the present study, we investigated the SII on the admission day, day 1, and day 5–7 outcomes of basal ganglia ICH during recovery. The correlation between them was also examined to determine whether the SII increase was associated with unfavorable outcomes in basal ganglia ICH. Moreover, we explored dynamic changes in the SII to identify a more precise method of predicting outcomes and to compare its effectiveness with other inflammatory biomarkers. The purpose of this study was to determine the relationship between high SII and the 6-month poor prognosis of patients after basal ganglia intracerebral hemorrhage, and the relationship between SII threshold and dynamic changes.
2. Methods
Dujiangyan Medical Center’s ethics committee reviewed and approved the study and concluded that obtaining informed consent was not necessary. All recorded data were anonymized to protect patient privacy. Moreover, this study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [23].
2.1. Patients and exclusion criteria
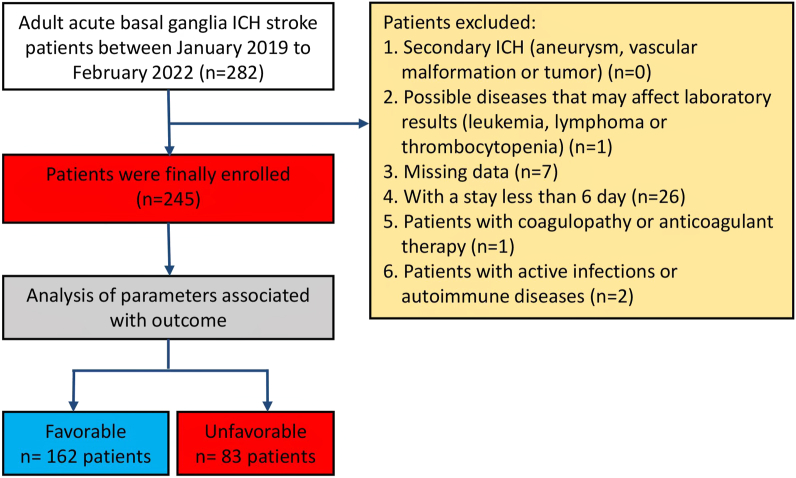
A total of 282 adult patients with acute basal ganglia ICH who were diagnosed by computerized tomography (CT) of the brain in the Department of Neurosurgery, Dujiangyan Medical Center, Chengdu, China, between January 2019 and March 2022, were retrospectively enrolled, and 245 of those patients met the inclusion criteria. Patients were included if they met the following criteria:
-
•
Over 18 years of age
-
•
Admission diagnosis of basal ganglia ICH based on brain CT scans
-
•
< 24 h between symptom onset and hospital admission
-
•
Available clinical data, including at least one laboratory test for PLT, ANC, ALC, and AMC
Exclusion criteria were as follows:
-
•
Pre-stroke disability (aneurysm, vascular malformation, tumor, or fistula)
-
•
Disease that may affect laboratory results (immune, hematologic, or infectious diseases)
-
•
Missing clinical outcome three months after discharge
-
•
Patients with coagulopathy or anticoagulant therapy
-
•
Patients with active infection or autoimmune disease
Thirty-seven patients were excluded from this study. Of these 37 patients, 1 patient had leukemia, 7 did not receive follow-ups and were thus missing data, 26 patients had spent less than six days in the hospital, 1 had anticoagulant therapy, and 2 had an infection or autoimmune disease.
2.2. Clinical and laboratory parameters
The recorded data included age, sex, clinical records, and previous medical history (hypertension, diabetes mellitus, smoking, hyperlipidemia, and drinking). ICH volume was measured using the A*B*C/2 method [24]. Additionally, we collected laboratory data, including absolute lymphocyte count (ALC), platelet count (PLT), absolute monocyte count (AMC), and absolute neutrophil count (ANC). The baseline CT imaging characteristics and surgical information were also collected. All laboratory test results were collected upon admission to the emergency department and during hospitalization in the neurosurgery department and ICU. The results were then analyzed using an autoanalyzer (XE-2100, Sysmex Company, Japan) within 1 h of venipuncture.
Functional outcomes were evaluated using a modified Rankin Scale (mRS) score at 6 months after the ictus of basal ganglia hemorrhage. The mRS was evaluated during outpatient or telephone interviews employing a structured interview format [25]. At 6 months, a mRS score of 0–3 was considered a favorable outcome, whereas a poor outcome was defined as a mRS score of 4–6.
NLR was calculated as (ANC/ALC), LMR was calculated as (ALC/AMC), and PLR was calculated as (PLT/ALC) [26]. The SII was calculated using the following equation: SII = PLT × ANC/ALC [27].
2.3. Statistical analysis
The Statistical Package for the Social Sciences software (Version 26, IBM® SPSS®, Chicago, Illinois, USA) and R language software (Version 4.0.2, R Core Team, Vienna, Austria) were used to conduct the analysis. Continuous variables were expressed as medians (interquartile range, IQR), while categorical variables are expressed as numbers (percentages). Continuous univariate analysis was performed using the Mann-Whitney U test, and categorical univariate analysis was performed using the χ2 test. Logistic regression analysis was used to determine predictors of laboratory tests for basal ganglia ICH [28]. A univariate logistic regression analysis was performed for each laboratory test variable. In the univariate analysis, variables with P < 0.001 were included in a multivariate logistic regression model, and each laboratory value was adjusted for covariates. The ability of the SII, NLR, LMR, PLR, and other factors to predict poor outcomes of basal ganglia ICH was estimated by generating receiver operating curves (ROCs) and calculating the area under the curve (AUC). The ROC was used to establish the optimal cutoff points for sensitivity and specificity. Unless otherwise indicated, two-side P-values < 0.05 were considered statistically significant.
3. Results
In total, 282 adult patients with acute basal ganglia ICH were enrolled in this study. Of these, 245 patients met the inclusion criteria (Fig. 1). Baseline characteristics of the enrolled patients are shown in Table 1 (mean age 59 years [range 51–70], female sex N = 74 [28.7%]) with different days of blood parameters and 180-day mRS. Fifty-two (20.5%) patients underwent surgical treatment on admission. Moreover, 162 (66.12%) patients had favorable outcomes (mRS 0–3).
Fig. 1.
Study design with inclusion and exclusion criteria for basal ganglia ICH patients. From these 282 patients, 245 (86.9%) patients met the criteria.
Table 1.
Baseline characteristics of the cohort.
| Variables | Baseline (N = 245) | Favorable (n = 162,66.12%) | Unfavorable (n = 83,33.88%) | P-value |
|---|---|---|---|---|
| Age (years) | 59 (51–70) | 57 (50–67) | 66 (53.5–74) | 0.005 |
| Sex (female) | 74 (28.7%) | 42 (25.3%) | 29 (33.3%) | 0.141 |
| GCS | 14 (10–15) | 14 (13–15) | 10 (6–14) | <0.0001 |
| Hypertension, n (%) | 237 (95.9%) | 147 (94.5%) | 80 (91.9%) | 0.826 |
| Diabetes mellitus, n (%) | 26 (10.5%) | 16 (9.6%) | 10 (11.4%) | 0.601 |
| Smoking, n (%) | 50 (20.2%) | 34 (20.4%) | 16 (18.3%) | 0.753 |
| Hyperlipidemia, n (%) | 29 (11.7%) | 17 (10.2%) | 12 (13.7%) | 0.363 |
| Drinking, n (%) | 46 (18.6%) | 27 (16.2%) | 19 (21.8%) | 0.238 |
| Surgery | 52 (20.5%) | 14 (9.7%) | 35 (40.2%) | <0.0001 |
| ICH parameters | ||||
| Volume (ml) | 12 (5–26) | 8 (3–16) | 28 (13.5–45) | <0.0001 |
| Intraventricular extension | 40 (16.1%) | 14 (8.4%) | 26 (29.8%) | <0.0001 |
| Admission value | ||||
| Neutrophils, ±109/L | 5.47 (3.78–8.08) | 4.72 (3.52–6.97) | 7.36 (4.48–9.42) | <0.0001 |
| Lymphocytes, ±109/L | 1.31 (0.94–2.07) | 1.34 (1.01–2.09) | 1.11 (0.73–1.92) | 0.003 |
| Platelets, ±109/L | 151 (120–194) | 150 (120.25–190) | 153 (118–197) | 0.911 |
| Monocytes, ±109/L | 0.4 (0.29–0.50) | 0.39 (0.3–0.49) | 0.4 (0.29–0.54) | 0.375 |
| LMR | 3.52 (2.48–5.38) | 3.67 (2.74–5.75) | 3.08 (1.89–4.55) | 0.002 |
| NLR | 3.67 (2.21–7.39) | 3.26 (1.98–5.78) | 5.65 (2.74–13.55) | <0.0001 |
| PLR | 111.45 (70.9–169.23) | 101.13 (71.26–149.55) | 124.12 (71.74–239.94) | 0.019 |
| SII index, ±109/L | 567.41 (296.14–122.17) | 486.05 (274.23–1005.82) | 869.60 (350.97–2207.28) | 0.0003 |
| Day 1 value | ||||
| Neutrophils, ±109/L | 6.61 (4.61–9.92) | 5.66 (4.01–7.76) | 9.93 (7.73–11.94) | <0.0001 |
| Lymphocytes, ±109/L | 1.06 (0.77–1.48) | 1.21 (0.90–1.69) | 0.78 (0.56–1.15) | <0.0001 |
| Platelets, ±109/L | 145 (118–181) | 143 (119–182.25) | 148 (105–180.5) | 0.938 |
| Monocytes, ±109/L | 0.44 (0.33–0.60) | 0.41 (0.33–0.55) | 0.52 (0.35–0.75) | 0.003 |
| LMR | 2.51 (1.49–3.77) | 3.09 (2.08–4.16) | 1.49 (1.00–2.25) | <0.0001 |
| NLR | 5.97 (3.35–11.95) | 4.29 (2.75–8.04) | 11.99 (7.12–17.77) | <0.0001 |
| PLR | 131.41 (96.97–194.17) | 120.17 (90.73–154.19) | 183.48 (125.72–266.67) | <0.0001 |
| SII index, ±109/L | 872.5 (487.64–1668.53) | 673.68 (386.69–1097.25) | 1706.95 (1062.74–2755.75) | <0.0001 |
| Day 5–7 value | ||||
| Neutrophils, ±109/L | 5.31 (4.08–7.22) | 4.66 (3.67–6.03) | 7.33 (5.80–9.81) | <0.0001 |
| Lymphocytes, ±109/L | 1.3 (0.86–1.70) | 1.45 (1.12–1.80) | 0.89 (0.57–1.33) | <0.0001 |
| Platelets, ±109/L | 165 (131–215) | 165 (131.25–213) | 164 (128.5–220) | 0.844 |
| Monocytes, ±109/L | 0.48 (0.38–0.61) | 0.44 (0.36–0.55) | 0.58 (0.47–0.82) | <0.0001 |
| LMR | 2.73 (1.52–3.94) | 3.25 (2.39–4.46) | 1.38 (0.99–2.01) | <0.0001 |
| NLR | 4.28 (2.66–7.37) | 3.18 (2.4–4.7) | 8.23 (5.27–12.38) | <0.0001 |
| PLR | 133.77 (94.80–192.23) | 115.84 (87.27–153.56) | 186.13 (134.16–280.26) | <0.0001 |
| SII index, ±109/L | 725.33 (415.12–1225.77) | 560.58 (364.86–801.44) | 1439.72 (897.44–2216.73) | <0.0001 |
Data are n (%), or median (interquartile range). ICH, intracerebral hemorrhage. GCS, Glasgow coma scale score.
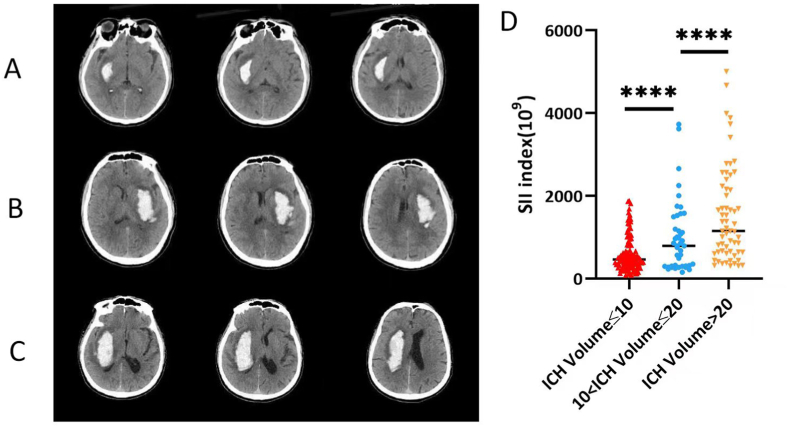
Our comparative analysis showed that patients with poor outcomes (mRS 4–6) after ICH had lower GCS scores (P < 0.01), larger hematoma volume at admission (P < 0.01), significant ventricular extension (P < 0.01), and most of them underwent surgery (P < 0.01), as shown in Table 1. Compared with those poor outcomes, patients who developed favorable outcomes had a lower ANC, AMC, median NLR, PLR, and SII (P < 0.01) on admission day, day 1, and day 5–7, while the ALC and LMR on day 1 and 5–7 showed the opposite results (P < 0.01), as shown in Table 1. Based on the CT imaging characteristics, the volume of cerebral hemorrhage was divided into less than 10 ml, 10–20 ml, and more than 20 ml (Fig. 2A–C). We found that the volume of cerebral hemorrhage was positively correlated with the SII (P < 0.001) (Fig. 2D). Univariate logistic regression analysis for patient-based variables and laboratory data variables indicated that these parameters were associated with the outcomes of basal ganglia ICH (Table 2). After adjusting for age, GCS score, hematoma volume, and surgical treatment and laboratory data, the SII and PLR on admission day, day 1, and day 5–7 were able to independently predict the outcomes of patients after basal ganglia ICH (all P < 0.05), as shown in Table 3. LMR was able to predict the outcomes on the admission day (95% CI 1.0000–1.0004, P = 0.014) and day 5–7 (95% CI 0.055–0.41, P = 0.0004) but not on day 1 (95% CI 0.48–1.77, P = 0.86). In contrast, NLR was only able to predict outcomes on the day of admission (95% CI 1.054–1.50, P = 0.024) but not on day 1 (95% CI 0.93–1.12, P = 0.82) or day 5–7 (95% CI 0.99–1.39, P = 0.094), as shown in Supplementary Table 1. Three other factors that remained independent predictors of the outcomes of patients with basal ganglia ICH across each model were age, GCS score, and hematoma volume (all P < 0.05; see Table 2, Table 3 and Supplementary Table 1).
Fig. 2.
Representative head computed tomography (CT) scan of patients with basal ganglia ICH. The volume of hemorrhage <10 ml (A), 10–20 ml (B), >20 ml (C). All of the head CT images showed perihematomal edema and without hydrocephalus (D). The volume of hemorrhage was positively correlated with SII (***P < 0.001).
Table 2.
The univariate and multivariate logistic analysis of predictors for patient outcome.
| Predictors |
Univariate analysis |
P-value | Variable |
Multivariate analysis |
P-value |
|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | ||||
| Age | 1.033 (1.01–1.06) | 0.003 | Age | 1.094 (1.058–1.14) | <0.001 |
| Sex (M) | 0.15 (0.86–2.72) | 0.14 | GCS | 0.84 (0.73–0.96) | 0.0097 |
| GCS | 0.71 (0.64–0.78) | <0.001 | Volume | 1.080 (1.043–1.12) | <0.001 |
| Hypertension | 0.85 (0.20–4.22) | 0.83 | Surgery | 1.42 (0.36–0.60) | 0.62 |
| Diabetes mellitus | 1.25 (0.52–2.86) | 0.60 | Interaventricular extension | 0.36 (0.12–1.091) | 0.074 |
| Smoking | 0.90 (0.45–1.72) | 0.75 | Admission | ||
| Dyslipidemia | 1.44 (0.64–3.16) | 0.37 | Neutrophils | 0.90 (0.73–1.099) | 0.31 |
| Drinking | 1.48 (0.76–2.86) | 0.24 | Lymphocytes | 1.60 (0.90–2.80) | 0.10 |
| Surgery | 0.15 (0.075–0.29) | <0.001 | SII index | 1.0011 (1.00046–1.0019) | 0.0026 |
| ICH Volume | 1.074 (1.054–1.098) | <0.001 | |||
| Intraventricular extension | 0.21 (0.099–0.42) | <0.001 | |||
| Admission | Age | 1.080 (1.047–1.12) | <0.001 | ||
| Neutrophils | 1.24 (1.13–1.36) | <0.001 | GCS | 0.85 (0.74–0.97) | 0.014 |
| Lymphocytes | 0.68 (0.48–0.95) | 0.028 | Volume | 1.068 (1.030–1.11) | <0.001 |
| Platelets | 1.00 (0.995–1.004) | 0.98 | Surgery | 2.00 (0.51–8.48) | 0.33 |
| Monocytes | 2.45 (0.51–11.96) | 0.26 | Interaventricular extension | 0.48 (0.16–1.40) | 0.18 |
| LMR | 1.00 (1.0–1.00)) | <0.001 | Day 1 | ||
| NLR | 1.14 (1.077–1.21) | <0.001 | Neutrophils | 1.10 (0.91–1.34) | 0.33 |
| PLR | 1.01 (1.00–1.01) | <0.001 | Lymphocytes | 0.88 (0.30–2.46) | 0.81 |
| SII index | 1.00061 (1.00034–1.00092) | <0.001 | Monocytes | 0.47 (0.074–3.02) | 0.43 |
| Day 1 | SII index | 1.00068 (1.000094–1.0013) | 0.038 | ||
| Neutrophils | 1.37 (1.25–1.52) | <0.001 | |||
| Lymphocytes | 0.13 (0.062–0.26) | <0.001 | |||
| Platelets | 1.00 (0.995–1.003) | 0.76 | |||
| Monocytes | 6.64 (2.32–20.63) | <0.001 | |||
| LMR | 0.44 (0.34–0.57) | <0.001 | Age | 1.093 (1.054–1.14) | <0.001 |
| NLR | 1.20 (1.14–1.27) | <0.001 | GCS | 0.87 (0.75–1.0028) | 0.048 |
| PLR | 1.010 (1.006–1.013) | <0.001 | Volume | 1.080 (1.043–1.12) | <0.001 |
| SII index | 1.0011 (1.00075–1.0014) | <0.001 | Surgery | 2.35 (0.54–11.15) | 0.30 |
| Day 5–7 | Interaventricular extension | 0.47 (0.15–1.44) | 0.20 | ||
| Neutrophils | 1.56 (1.37–1.80) | <0.001 | Day 5–7 | ||
| Lymphocytes | 0.21 (0.11–0.36) | <0.001 | Neutrophils | 1.15 (0.83–1.59) | 0.21 |
| Platelets | 1.00 (0.997–1.005) | <0.001 | Lymphocytes | 1.26 (0.475–3.85) | 0.82 |
| Monocytes | 23.75 (6.58–98.65) | <0.001 | Platelets | 1.00 (0.99–1.0068) | 0.64 |
| LMR | 0.29 (0.20–0.40) | <0.001 | Monocytes | 0.834 (0.088–8.90) | 0.83 |
| NLR | 1.40 (1.27–1.56) | <0.001 | SII index | 1.0016 (1.00034–1.0031) | 0.0039 |
| PLR | 1.012 (1.0077–1.016) | <0.001 | |||
| SII index | 1.0018 (1.0013–1.0023) | <0.001 |
Table 3.
Multivariable Logistic Regression Analysis of Admission Laboratory Values as Predictors of Spontaneous intracerebral hemorrhage.
| Models | Variable | Beta (SE) | OR (95%CI) | P value |
|---|---|---|---|---|
| 1 | Age (yesrs) | 0.085 (0.018) | 1.08 (1.05–1.13) | <0.0001 |
| GCS | −0.19 (0.069) | 0.83 (0.72–0.94) | 0.005 | |
| Surgery | 0.38 (0.69) | 1.46 (0.38–5.94) | 0.58 | |
| ICH Volume | 0.075 (0.018) | 1.08 (1.042–1.12) | <0.0001 | |
| Intraventricular extension | −0.88 (0.55) | 0.42 (0.14–1.21) | 0.11 | |
| Admission_Neutrophils | 0.082 (0.073) | 1.09 (0.94–1.25) | 0.26 | |
| Admission_Lymphocytes | 0.49 (0.31) | 1.64 (0.89–2.97) | 0.11 | |
| Admission_PLR | 0.0076(0.0028) | 1.01(1.002–1.013) | 0.006 | |
| 2 | Age (yesrs) | 0.077 (0.017) | 1.08 (1.047–1.12) | <0.0001 |
| GCS | −0.17 (0.068) | 0.84 (0.74–0.96) | 0.013 | |
| Surgery | 0.65 (0.71) | 1.92 (0.49–8.08) | 0.36 | |
| ICH Volume | 0.067 (0.019) | 1.069 (1.031–1.11) | 0.0005 | |
| Intraventricular extension | −0.65 (0.55) | 0.52 (0.18–1.53) | 0.23 | |
| Day1_Neutrophils | 0.19 (0.078) | 1.22 (1.047–1.43) | 0.012 | |
| Day1_Lymphocytes | 0.037 (0.56) | 1.038 (0.33–3.08) | 0.95 | |
| Day1_Monocytes | −0.79 (0.95) | 0.46 (0.069–2.93) | 0.41 | |
| Day1_PLR | 0.0067(0.0029) | 1.0068(1.0013–1.013) | 0.019 | |
| 3 | Age (yesrs) | 0.087 (0.019) | 1.09 (1.05–1.14) | <0.0001 |
| GCS | −0.15 (0.073) | 0.86 (0.75–0.99) | 0.042 | |
| Surgery | 0.70 (0.75) | 2.01 (0.47–9.20) | 0.35 | |
| ICH Volume | 0.073 (0.019) | 1.08 (1.04–1.12) | <0.0001 | |
| Intraventricular extension | −0.71 (0.57) | 4.92 (0.16–1.49) | 0.21 | |
| Day5-7_Neutrophils | 0.37 (0.11) | 1.45 (1.17–1.81) | 0.0008 | |
| Day5-7_Lymphocytes | 0.14 (0.54) | 1.15 (0.46–4.48) | 0.8 | |
| Day5-7_Platelets | −0.0021 (0.0047) | 0.998 (0.987–1.007) | 0.65 | |
| Day5-7_Monocytes | −0.30 (1.15) | 0.74 (0.083–7.41) | 0.79 | |
| Day5-7_PLR | 0.011(0.0044) | 1.01(1.003–1.02) | 0.016 | |
| 4 | Age (yesrs) | 0.090 (0.018) | 1.094 (1.058–1.14) | <0.0001 |
| GCS | −0.18 (0.070) | 0.84 (0.73–0.96) | 0.01 | |
| Surgery | 0.35 (0.72) | 1.42 (0.36–6.031) | 0.62 | |
| ICH Volume | 0.077 (0.019) | 1.08 (1.04–1.12) | <0.0001 | |
| Intraventricular extension | −1.02 (0.57) | 0.36 (0.12–1.09) | 0.074 | |
| Admission_Neutrophils | −0.10 (0.10) | 0.90 (0.73–1.09) | 0.31 | |
| Admission_Lymphocytes | 0.47 (0.29) | 1.60 (0.90–2.80) | 0.1 | |
| Admission_SII index | 0.0011(0.00038) | 1.001(1.0005–1.002) | 0.0026 | |
| 5 | Age (yesrs) | 0.077 (0.017) | 1.08 (1.047–1.12) | <0.0001 |
| GCS | −0.17 (0.068) | 0.85 (0.74–0.97) | 0.014 | |
| Surgery | 0.69 (0.71) | 2.01 (0.51–8.48) | 0.33 | |
| ICH Volume | 0.066 (0.019) | 1.068 (1.03–1.11) | 0.0005 | |
| Intraventricular extension | −0.74 (0.55) | 0.48 (0.16–1.40) | 0.18 | |
| Day1_Neutrophils | 0.095 (0.099) | 1.10 (0.91–1.34) | 0.33 | |
| Day1_Lymphocytes | −0.13 (0.54) | 0.88 (0.30–2.46) | 0.81 | |
| Day1_Monocytes | −0.75 (0.94) | 0.47 (0.074–3.02) | 0.43 | |
| Day1_SII index | 0.00068(0.00033) | 1.0007(1.0000–1.001) | 0.038 | |
| 6 | Age (yesrs) | 0.089 (0.019) | 1.09 (1.05–1.14) | <0.0001 |
| GCS | −0.14 (0.073) | 0.87 (0.75–1.003) | 0.056 | |
| Surgery | 0.85 (0.77) | 2.35 (0.54–11.15) | 0.27 | |
| ICH Volume | 0.077 (0.019) | 1.08 (1.04–1.12) | <0.0001 | |
| Intraventricular extension | −0.75 (0.57) | 0.47 (0.15–1.44) | 0.19 | |
| Day5-7_Neutrophils | 0.14 (0.16) | 1.15 (0.83–1.59) | 0.4 | |
| Day5-7_Lymphocytes | 0.23 (0.51) | 1.26 (0.47–3.85) | 0.64 | |
| Day5-7_Platelets | −0.0021 (0.0046) | 0.998 (0.988–1.007) | 0.64 | |
| Day5-7_Monocytes | −0.18 (1.19) | 0.83 (0.088–8.90) | 0.88 | |
| Day5-7_SII index | 0.0016(0.00069) | 1.002(1.0003–1.003) | 0.02 |
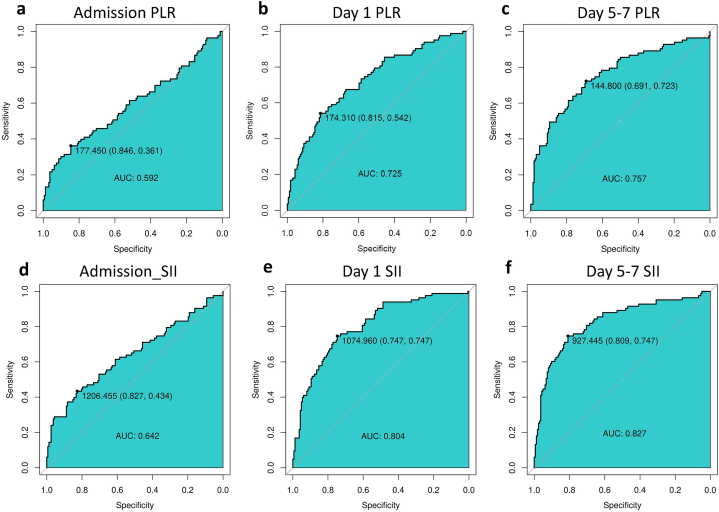
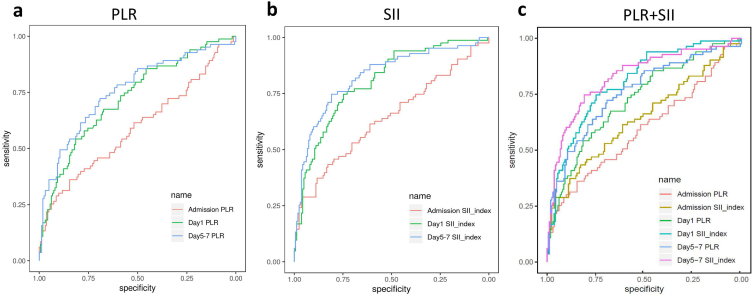
Next, we compared the ROCs of the SII and PLR on admission day, day 1, and day 5–7 with the AUC values (specificity/sensitivity) of the SII at 0.642 (0.827/0.434) (95% CI: 0.565–0.720, P = 0.00014), 0.804 (0.747/0.747) (95% CI: 0.746–0.861, P < 0.001), 0.827 (0.809/0.747) (95% CI: 0.769–0.885, P < 0.001), respectively; and the AUC values (specificity/sensitivity) of the PLR at 0.592 (0.846/0.361) (95% CI: 0.512–0.672, P = 0.0095), 0.725 (0.815/0.542) (95% CI: 0.657–0.793, P < 0.001), 0.757 (0.691/0.723) (95% CI: 0.690–0.824, P < 0.001), respectively (Fig. 3a–f). The AUC values of the SII and PLR increased gradually with ongoing hospitalization days and were highest on day 5–7, as shown in Fig. 4a–c, and Table 4. Moreover, the AUC value of the SII was significantly higher than that of the PLR at every time point (P < 0.01), as shown in Table 5. Based on these analyses, the SII showed a better ability to predict the 6-month outcomes of patients after basal ganglia ICH than the PLR, and with the increase in the number of days hospitalized, the better predictive ability of the SII gradually increased. On days 5–7, the SII exhibited the strongest predictive ability for the 6-month outcomes of patients with basal ganglia ICH. Finally, based on the better predictive power of the SII, we further divided the patients into two groups according to their SII threshold on different days. On admission day, the functional outcome at 6 months was comparatively worse in patients with SII ≥ 1206 than those with SII < 1206 (mRS score 0–3: SII <1206 = 134/181 (74.03%) vs. SII ≥ 1206 = 28/64 (43.75%); P < 0.001); similarly, on day 1, the optimal cut off value of SII was 1075 (mRS score 0–3: SII < 1075 = 121/142 (85.2%) vs. SII ≥ 1075 = 41/103 (39.8%); P < 0.001); finally, on day 5–7, the optimal cut off value of SII was 927 (mRS score 0–3: SII < 927 = 131/152 (86.2%) vs. SII ≥ 927 = 31/93 (43.75%); P < 0.001), as shown in Supplementary Table 2.
Fig. 3.
Discriminative ability of the SII and PLR on different days for patient outcome (AUC).
Fig. 4.
ROCs showing the discriminative ability using different features.
Table 4.
Associations of inflammatory markers with clinical outcome.
| Area under the curve | 95% CI | P value | |
|---|---|---|---|
| Admission PLR | 0.592 | 0.512–0.672 | 0.0095 |
| Day 1 PLR | 0.725 | 0.657–0.793 | <0.0001 |
| Day 5–7 PLR | 0.757 | 0.690–0.824 | <0.0001 |
| Admission SII_index, × 109/L | 0.642 | 0.565–0.720 | 0.00014 |
| Day 1 SII_index, × 109/L | 0.804 | 0.746–0.861 | <0.0001 |
| Day 5–7 SII_index, × 109/L | 0.827 | 0.769–0.885 | <0.0001 |
Table 5.
Diagnostic values of admission PLR and SII, Day 1 PLR and SII, Day 5–7 PLR and SII for ICH.
| Variable | AUC (95% CI) | P value |
|---|---|---|
| Admission | ||
| SII_index, × 109/L | 0.642 (0.565–0.720) | Reference |
| PLR | 0.592 (0.512–0.672) | 0.0025 |
| Day 1 | ||
| SII_index, × 109/L | 0.804 (0.746–0.861) | Reference |
| PLR | 0.725 (0.657–0.793) | 0.00019 |
| Day 5–7 | ||
| SII_index, × 109/L | 0.827 (0.769–0.885) | Reference |
| PLR | 0.757 (0.690–0.824) | 0.00050 |
4. Discussion
The change in the SII is dynamic in patients with basal ganglia ICH, and our study found that it peaked at day 5–7 after hemorrhage onset. We found that on admission day, day 1, and day 5–7, the SII was associated with a 6-month poor functional outcome. Furthermore, in multivariable logistic regression, the SII on admission day, day 1, and day 5–7 can predict the functional outcomes of patients after basal ganglia ICH (all P < 0.05), with an optimal cutoff of 1,206, 1,075, and 927*109/L, respectively. Although with the increase in the number of days hospitalized, both the SII and PLR were able to predict the outcomes of patients after basal ganglia ICH, the predictive ability of the SII was superior to that of the PLR on admission day, day 1, and day 5–7 at different times. These results suggest that the SII could be a crucial new indicator for predicting prognosis, stratifying risks, assessing disease severity, adjusting quality monitoring, and facilitating communication among clinicians, patients, and their family members. This was the first study to show an association between the dynamic change of the SII and the functional outcome after basal ganglia ICH. Our study is based on a single-center study, and the results are consistent with the reported association between SII and 90-day mortality after ICH [21]. Our results further found that SII on admission day, day 1, and days 5–7 may be a promising predictive biomarker of poor function at 6 months, and that the prognosis specificity on day 5–7 was the highest.
When ICH occurs, the hematoma first causes mechanical compression damage to the cerebral tissue. Hematoma volume is known to be an independent prognostic predictor of ICH, and we found that the volume of cerebral hemorrhage was positively correlated with the SII.
After ICH, the innate and adaptive immune systems activate the inflammatory response. Thrombin and vitronectin, that is, plasma-derived factors, as well as components released following erythrocyte lysis, act immediately on inflammatory cells and trigger an inflammatory cascade. Blood components, such as plasma proteins, red blood cells, macrophages, and leukocytes are released into the cerebral parenchyma and intensify local inflammatory processes [29,30]. An increased release of pattern recognition receptors (PRRs), pro-inflammatory cytokines (TNF-α and IL-1β), adhesion molecules, and matrix metalloproteinases (MMPs) disrupts the blood-brain barrier (BBB), leading to infiltration of inflammatory cells and exacerbating the formation of cerebral edema. The lysis of red blood cells leads to the release of pro-oxidative hemoglobin as well as its degradation products, heme and iron [31]. Macrophages and microglia can gradually eliminate these cytotoxic products through phagocytosis. However, secondary brain injury typically occurs as a result of brain edema, inflammation, free radicals, hematoma toxicity, and the resulting breakdown byproducts [32]. In general, the immune system plays a critical role in a variety of pathophysiological processes following ICH and represents potential targets for treatment and prognosis prediction.
Inflammatory cells were detected as early as 4 h after ICH, peaked around day 2–3, and attenuated by day 7 [33]. Clinical laboratory data demonstrated that increased leukocyte counts were associated with higher disease severity and worse outcomes in patients with stroke [34]. The hyperactivation of the sympathetic system and hypothalamic-pituitary-adrenal axis leads to increased levels of steroids and catecholamines, which trigger systemic immunosuppression. This, in turn, further induces functional inactivation of peripheral lymphocytes and apoptosis, substantially reducing the number of circulating lymphocytes [35]. In addition, previous studies have shown that elevated platelet levels induce a hypercoagulable state in the blood, which increases the risk of poor outcomes [36]. In brief, neutrophil, platelet, and lymphocyte counts are associated with the occurrence of complications and prognosis of ICH.
Increasing evidence suggests that serum inflammatory markers such as neutrophils, platelets, and lymphocytes are more readily available in clinical practice. These cellular changes have valuable prognostic implications for various diseases including ICH and other central nervous system diseases [[37], [38], [39], [40]]. However, individual blood cell parameters are susceptible to certain variables such as hydration, excessive dehydration, specimen handling, and race. Therefore, using the ratio of LMR, PLR, and NLR in blood cells to reduce the influence of the above confounding factors can more reliably predict the prognosis of patients [10,11,41]. In our study, we examined the relationship between these three indices and individual blood cell parameters, and the prognosis of patients with basal ganglia ICH. Compared to those with poor outcomes, patients who developed favorable outcomes had lower neutrophils, monocytes, median NLR, and PLR (P < 0.01) on admission, day 1 and day 5–7, while lymphocytes and LMR on days 1 and 5–7 showed the opposite results (P < 0.01). Similarly, this study showed SII to be a good predictor of basal ganglia ICH prognosis at 6 months.
Our results suggest that SII is a novel independent and integrated inflammatory biomarker in basal ganglia ICH. In our research, ROC analysis indicated the SII better predicted the poor outcomes than the PLR, and with the increase in the number of days hospitalized, the predictive ability of the SII gradually increased. NLR primarily reflects inflammatory injury, while PLR mainly indicates the effects of hemostasis and thrombosis. SII, on the other hand, offers comprehensive information about inflammation, hemostasis, immunity, and thrombosis throughout the body [42]. Thus, it was reasonable for the SII to show a stronger predictive ability than other indicators. Combining the SII with ANC, ALC, and PLT may provide a comprehensive biomarker for assessing the severity of basal ganglia ICH and predicting its 6-month outcomes. Studies have shown that the pathophysiological processes differ between acute and chronic ICH. Nevertheless, it remains unclear whether there is a difference in SII between the acute and chronic stages of basal ganglia ICH. Previous studies have suggested that the early inflammatory response acts as a protective mechanism for brain cells following an intracerebral hemorrhage, whereas a persistent inflammatory response can be detrimental to brain tissue [43]. Moreover, as basal ganglia ICH develops, patients may develop serious complications such as pneumonia, acute kidney injury, epilepsy, hydrocephalus, delayed cerebral ischemia (DCI), among others, which can lead to a poor prognosis. Prior research has demonstrated that MLR is an effective predictor of the onset of acute kidney injury. [44], while a high NLR can be a predictor of stroke-associated pneumonia [45]. In addition, perihematomal edema (PHE) in deep ICH typically occurs 3–10 days after admission, with the highest incidence rate at approximately one week [46]. Our results showed good predictive ability of the SII on day 5–7, which is consistent with the literature [20]. Given that SII is a more comprehensive indicator than MLR and NLR, it should be equally effective in predicting the onset of late complications that may arise following basal ganglia ICH.
To obtain specific serum indicators from patients in hospitals is easy, which we used to calculate the SII, and our research proved the reliable predictive ability of the SII. Therefore, it is reasonable to believe that the SII could be used to guide the risk stratification of basal ganglia ICH with poor prognosis and provide personalized treatment in the early stages of basal ganglia ICH. However, it remains to be determined whether there are variations in the cut-off values of SII among different regions and populations, and whether SII can serve as a useful monitoring indicator for patients with basal ganglia ICH during hospitalization.
Our study has some potential limitations. First, our sample size was small; therefore, it is necessary to conduct further, extensive studies in large, multi-center cohorts. Second, other inflammatory indicators such as edema volume and interleukin six were not collected, and the interaction with other infectious complications was not studied. Moreover, the cut-off values may vary with population and region. Finally, the level of peripheral blood inflammatory cells varies greatly during the acute attack and progression of basal ganglia ICH, and neuroinflammatory responses are often observed to be biphasic, protecting brain cells at the beginning but promoting brain injury in the later stage.
5. Conclusion
In summary, the SII is an easily calculated index, and a high SII was associated with a poor 6-month outcome in patients with basal ganglia ICH. SII could be a novel, independent, and noninvasive prognostic factor for patients with basal ganglia ICH. Further prospective studies on SII should be conducted.
Ethics statements
Studies involving patient participants were approved and reviewed by the Dujiangyan Medical Center Ethics Committee and written informed consent for participation was not required for this study in accordance with national legislation and institutional requirements.
Funding information
This study was supported by the Sichuan Provincial Health Commission Support Program (No. 140026).
Author contribution statement
Zhang Liang, He Liu, Li Xue: Performed the experiments; Wrote the paper.
Bin Ma: Analyzed and interpreted the data.
Ling-Zhi Yang: Contributed reagents, materials, analysis tools or data.
Qing-Le Liang, Zhang-Ming Zhou: Conceived and designed the experiments.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e16937.
Contributor Information
Qing-Le Liang, Email: liangqingle@126.com.
Zhang-Ming Zhou, Email: zhangmingzhou2010@126.com.
Abbreviations
- SII
systemic immune-inflammation index
- ICH
spontaneous intracerebral hemorrhage
- mRS
modified Rankin Scale
- ANC
absolute neutrophil count
- ALC
absolute lymphocyte count
- AMC
absolute monocyte count
- NLR
neutrophil-lymphocyte ratio
- LMR
lymphocyte count/monocyte count
- PLR
platelet count/lymphocyte count
- ROC
receiver operating characteristic
- AUC
area under the curve
- CNS
central nervous system
- BBB
blood brain barrier
- DCI
delayed cerebral vasospasm
- PHE
perihematomal edema
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Qureshi A.I., Mendelow A.D., Hanley D.F. Intracerebral haemorrhage. Lancet. 2009;373(9675):1632–1644. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain A., Malhotra A., Payabvash S. Imaging of spontaneous intracerebral hemorrhage, Neuroimaging. Clin. N. Am. 2021;31(2):193–203. doi: 10.1016/j.nic.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamborska A., Poon M.T.C., Al-Shahi Salman R. Characteristics of randomized trials focusing on stroke due to intracerebral hemorrhage: systematic Review. Stroke. 2018;49(3):594–600. doi: 10.1161/STROKEAHA.117.019227. [DOI] [PubMed] [Google Scholar]
- 4.Leasure A.C., Kuohn L.R., Vanent K.N., Bevers M.B., Kimberly W.T., Steiner T., Mayer S.A., Matouk C.C., Sansing L.H., Falcone G.J., Sheth K.N. Association of serum IL-6 (Interleukin 6) with functional outcome after intracerebral hemorrhage. Stroke. 2021;52(5):1733–1740. doi: 10.1161/STROKEAHA.120.032888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loan J.J., Kirby C., Emelianova K., Dando O.R., Poon M.T., Pimenova L., Hardingham G.E., McColl B.W., Klijn C.J., Al-Shahi Salman R., et al. Secondary injury and inflammation after intracerebral haemorrhage: a systematic review and meta-analysis of molecular markers in patient brain tissue. J. Neurol. Neurosurg. Psychiatry. 2022;93(2):126–132. doi: 10.1136/jnnp-2021-327098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turroni S., Pedersini P., Villafañe J.H. The human gut microbiome and its relationship with osteoarthritis pain. Pain Med. 2021;22(7):1467–1469. doi: 10.1093/pm/pnaa422. [DOI] [PubMed] [Google Scholar]
- 7.Shao L., Chen S., Ma L. Secondary brain injury by oxidative stress after cerebral hemorrhage: recent advances. Front. Cell. Neurosci. 2022;16 doi: 10.3389/fncel.2022.853589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu H., Wang Z., Yu J., Yang X., He F., Liu Z., Che F., Chen X., Ren H., Hong M., Wang J. Role and mechanisms of cytokines in the secondary brain injury after intracerebral hemorrhage. Prog. Neurobiol. 2019;178 doi: 10.1016/j.pneurobio.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Lattanzi S., Brigo F., Trinka E., Cagnetti C., Di Napoli M., Silvestrini M. Neutrophil-to-lymphocyte ratio in acute cerebral hemorrhage: a system review. Transl. Stroke Res. 2019;10(2):137–145. doi: 10.1007/s12975-018-0649-4. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J., Zhang C.H., Lin X.L., Zhang Q., Wang J., Shi S.L. Serum glial fibrillary acidic protein as a biomarker for differentiating intracerebral hemorrhage and ischemic stroke in patients with symptoms of acute stroke: a systematic review and meta-analysis. Neurol. Sci. 2013;34(11):1887–1892. doi: 10.1007/s10072-013-1541-3. [DOI] [PubMed] [Google Scholar]
- 11.Qi H., Wang D., Deng X., Pang X. Lymphocyte-to-monocyte ratio is an independent predictor for neurological deterioration and 90-Day mortality in spontaneous intracerebral hemorrhage. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2018;24:9282–9291. doi: 10.12659/MSM.911645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Z., Zeng J., Yu S., Zhao Y., Yang X., Zhou Y., Liang Q. Neurofilament light chain and S100B serum levels are associated with disease severity and outcome in patients with aneurysmal subarachnoid hemorrhage. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.956043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kearns K.N., Ironside N., Park M.S., Worrall B.B., Southerland A.M., Chen C.J., Ding D. Neuroprotective therapies for spontaneous intracerebral hemorrhage. Neurocritical Care. 2021;35(3):862–886. doi: 10.1007/s12028-021-01311-3. [DOI] [PubMed] [Google Scholar]
- 14.Wang B.L., Tian L., Gao X.H., Ma X.L., Wu J., Zhang C.Y., Zhou Y., Guo W., Yang X.R. Dynamic change of the systemic immune inflammation index predicts the prognosis of patients with hepatocellular carcinoma after curative resection. Clin. Chem. Lab. Med. 2016;54(12):1963–1969. doi: 10.1515/cclm-2015-1191. [DOI] [PubMed] [Google Scholar]
- 15.Zhang K., Hua Y.Q., Wang D., Chen L.Y., Wu C.J., Chen Z., Liu L.M., Chen H. Systemic immune-inflammation index predicts prognosis of patients with advanced pancreatic cancer. J. Transl. Med. 2019;17(1):30. doi: 10.1186/s12967-019-1782-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J., Cao D., Huang Y., Xiong Q., Tan D., Liu L., Lin T., Wei Q. The prognostic and clinicopathological significance of systemic immune-Inflammation index in bladder cancer. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.865643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marques P., de Vries F., Dekkers O.M., Korbonits M., Biermasz N.R., Pereira A.M. Serum inflammation-based scores in endocrine tumors. J. Clin. Endocrinol. Metab. 2021;106(10):e3796–e3819. doi: 10.1210/clinem/dgab238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang R., Li J., Tang X., Liu Y. The prognostic role of preoperative systemic immune-inflammation index and albumin/globulin ratio in patients with newly diagnosed high-grade glioma. Clin. Neurol. Neurosurg. 2019;184 doi: 10.1016/j.clineuro.2019.105397. [DOI] [PubMed] [Google Scholar]
- 19.Geraghty J.R., Lung T.J., Hirsch Y., Katz E.A., Cheng T., Saini N.S., Pandey D.K., Testai F.D. Systemic Immune-Inflammation index predicts delayed cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2021;89(6):1071–1079. doi: 10.1093/neuros/nyab354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trifan G., Testai F.D. Systemic Immune-Inflammation (SII) index predicts poor outcome after spontaneous supratentorial intracerebral hemorrhage. J. Stroke Cerebrovasc. Dis. 2020;29(9) doi: 10.1016/j.jstrokecerebrovasdis.2020.105057. [DOI] [PubMed] [Google Scholar]
- 21.Li Y., Wen D., Cui W., Chen Y., Zhang F., Yuan M., Xiao H., Li H., Ma L., Hu X., You C. The prognostic value of the acute phase systemic immune-inflammation index in patients with intracerebral hemorrhage. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.628557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trifan G., Testai F.D. Systemic Immune-Inflammation (SII) index predicts poor outcome after spontaneous supratentorial intracerebral hemorrhage. J. Stroke Cerebrovasc. Dis. 2020;29(9) doi: 10.1016/j.jstrokecerebrovasdis.2020.105057. [DOI] [PubMed] [Google Scholar]
- 23.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P., STROBE Initiative The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Webb A.J., Ullman N.L., Morgan T.C., Muschelli J., Kornbluth J., Awad I.A., Mayo S., Rosenblum M., Ziai W., Zuccarrello M., et al. Accuracy of the ABC/2 score for intracerebral hemorrhage: systematic review and analysis of MISTIE, CLEAR-IVH, and CLEAR III. Stroke. 2015;46(9):2470–2476. doi: 10.1161/STROKEAHA.114.007343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasner S.E. Clinical interpretation and use of stroke scales. Lancet Neurol. 2006;5(7):603–612. doi: 10.1016/S1474-4422(06)70495-1. [DOI] [PubMed] [Google Scholar]
- 26.Dupré A., Malik H.Z. Inflammation and cancer: what a surgical oncologist should know. Eur. J. Surg. Oncol. 2018;44(5):566–570. doi: 10.1016/j.ejso.2018.02.209. [DOI] [PubMed] [Google Scholar]
- 27.Hu B., Yang X.R., Xu Y., Sun Y.F., Sun C., Guo W., Zhang X., Wang W.M., Qiu S.J., Zhou J., Fan J. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 2014;20(23):6212–6222. doi: 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- 28.Seung W.L. Regression analysis for continuous independent variables in medical research: statistical standard and guideline of Life Cycle Committee. Life Cycle. 2022;2:e3. [Google Scholar]
- 29.Villafane J.H. Aging-mediated gastrointestinal-microbiome changes are postulated to affect osteoarthritis-related pain. Top. Geriatr. Rehabil. 2021;37(4):37. [Google Scholar]
- 30.Garton T., Keep R.F., Wilkinson D.A., Strahle J.M., Hua Y., Garton H.J., Xi G. Intraventricular hemorrhage: the role of blood components in secondary injury and hydrocephalus. Transl. Stroke Res. 2016;7(6):447–451. doi: 10.1007/s12975-016-0480-8. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Y., Wang Y., Wang J., Anne Stetler R., Yang Q.W. Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog. Neurobiol. 2014;115:25–44. doi: 10.1016/j.pneurobio.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Li Y., Liu H., Tian C., An N., Song K., Wei Y., Sun Y., Xing Y., Gao Y. Targeting the multifaceted roles of mitochondria in intracerebral hemorrhage and therapeutic prospects. Biomed. Pharmacother. 2022;148 doi: 10.1016/j.biopha.2022.112749. [DOI] [PubMed] [Google Scholar]
- 33.Lan X., Han X., Li Q., Yang Q.W., Wang J. Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat. Rev. Neurol. 2017;13(7):420–433. doi: 10.1038/nrneurol.2017.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMahon C.J., Hopkins S., Vail A., King A.T., Smith D., Illingworth K.J., Clark S., Rothwell N.J., Tyrrell P.J. Inflammation as a predictor for delayed cerebral ischemia after aneurysmal subarachnoid haemorrhage. J. Neurointerventional Surg. 2013;5(6):512–517. doi: 10.1136/neurintsurg-2012-010386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Mufti F., Misiolek K.A., Roh D., Alawi A., Bauerschmidt A., Park S., Agarwal S., Meyers P.M., Connolly E.S., Claassen J., Schmidt J.M. White blood cell count improves prediction of delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage. Neurosurgery. 2019;84(2):397–403. doi: 10.1093/neuros/nyy045. [DOI] [PubMed] [Google Scholar]
- 36.Sarrafzadeh A., Schlenk F., Meisel A., Dreier J., Vajkoczy P., Meisel C. Immunodepression after aneurysmal subarachnoid hemorrhage. Stroke. 2011;42(1):53–58. doi: 10.1161/STROKEAHA.110.594705. [DOI] [PubMed] [Google Scholar]
- 37.Meisel C., Schwab J.M., Prass K., Meisel A., Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat. Rev. Neurosci. 2005;6(10):775–786. doi: 10.1038/nrn1765. [DOI] [PubMed] [Google Scholar]
- 38.Tao C., Wang J., Hu X., Ma J., Li H., You C. Clinical value of neutrophil to lymphocyte and platelet to lymphocyte ratio after aneurysmal Subarachnoid hemorrhage. Neurocritical Care. 2017;26(3):393–401. doi: 10.1007/s12028-016-0332-0. [DOI] [PubMed] [Google Scholar]
- 39.Troiani Z., Ascanio L., Rossitto C.P., Ali M., Mohammadi N., Majidi S., Mocco J., Kellner C.P. Prognostic utility of serum biomarkers in intracerebral hemorrhage: a systematic review. Neurorehabilitation Neural Repair. 2021;35(11):946–959. doi: 10.1177/15459683211041314. [DOI] [PubMed] [Google Scholar]
- 40.Yan J., Zhai W., Li Z., Ding L., You J., Zeng J., Yang X., Wang C., Meng X., Jiang Y., et al. ICH-LR2S2: a new risk score for predicting stroke-associated pneumonia from spontaneous intracerebral hemorrhage. J. Transl. Med. 2022;20(1):193. doi: 10.1186/s12967-022-03389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mercier-Letondal P., Marton C., Godet Y., Galaine J. Validation of a method evaluating T cell metabolic potential in compliance with ICH Q2 (R1) J. Transl. Med. 2021;19(1):21. doi: 10.1186/s12967-020-02672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan Y.K., Huang H., Li D.P., Ai Z.Y., Li X., Sun Z. Prognostic value of the platelet-to-lymphocyte ratio for outcomes of stroke: a systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2021;25(21):6529–6538. doi: 10.26355/eurrev_202111_27095. [DOI] [PubMed] [Google Scholar]
- 43.Magid-Bernstein J., Girard R., Polster S., Srinath A., Romanos S., Awad I.A., Sansing L.H. Cerebral hemorrhage: pathophysiology, treatment, and future directions. Circ. Res. 2022;130(8):1204–1229. doi: 10.1161/CIRCRESAHA.121.319949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang F., Liu J., Yu X., Li R., Zhou R., Ren J., Liu X., Zhao S., Yang B. The monocyte-to-lymphocyte ratio predicts acute kidney injury after acute hemorrhagic stroke. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.904249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nam K.W., Kim T.J., Lee J.S., Kwon H.M., Lee Y.S., Ko S.B., Yoon B.W. High neutrophil-to-lymphocyte ratio predicts stroke-associated pneumonia. Stroke. 2018;49(8):1886–1892. doi: 10.1161/STROKEAHA.118.021228. [DOI] [PubMed] [Google Scholar]
- 46.Leasure A.C., Qureshi A.I., Murthy S.B., Kamel H., Goldstein J.N., Walsh K.B., Woo D., Shi F.D., Huttner H.B., Ziai W.C., et al. Intensive blood pressure reduction and perihematomal edema expansion in deep intracerebral hemorrhage. Stroke. 2019;50(8):2016–2022. doi: 10.1161/STROKEAHA.119.024838. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.