Abstract
Objective
To explore the relationship between Tuberous sclerosis complex (TSC) and cardiac tumors at our institution over the past five years and to evaluate the value of imaging technologies and genetic testing in the prenatal diagnosis of TSC.
Methods
Fetal echocardiography (FE) was performed in the whole population between 2016 and 2020. Fetuses detected with cardiac tumor(s) were included. Fetal cranial magnetic resonance imaging (MRI) and gene mutation tests were further examined. Those who declined genetic testing were excluded in the final analysis.
Results
A total of 40 fetuses were included in our study. There were 27 cases performed cranial magnetic resonance imaging (MRI) and the rest of 13 cases refused. Among 10 fetuses with cranial lesions detected by MRI, all of them were eventually diagnosed with TSC. And for 17 fetuses without cranial lesions, none of them were identified with a pathogenic variation in gene TSC1/2. The prevalence of TSC was significantly higher in the multiple tumors group than in the solitary group (9/20 vs. 2/20, P = 0.034). 11 fetuses had TSC1 (n = 3) or TSC2 (n = 8) causative or suspected causative mutations, of which 9 were sporadic mutations and 2 were familial mutations.
Conclusion
Fetal cranial MRI should be recommended to evaluate brain lesions, and genetic mutation should be examined, if possible, especially for those with multiple heart tumors. When typical cardiac tumors and cranial lesions are detected, the diagnosis of TSC can almost be made even without genetic mutation results.
Keywords: Fetal cardiac tumor, Tuberous sclerosis complex, Fetal echocardiography, Genetic testing, Termination of pregnancy, Fetal cranial MRI
1. Introduction
Tuberous sclerosis complex (TSC) is an autosomal dominant disorder caused by mutations in the TSC1 and TSC2 genes, which are located on chromosomes 9q34 and 16p13.3, respectively. The prevalence of TSC is estimated to be 1 in 6000 live births [1]. Classical TSC is identified by the triad of facial angiofibroma, intellectual disability and epilepsy. TSC increases the psychological pressure and economic burden on patients and has a considerable impact on the quality of their lives, especially on patients with symptoms of epilepsy and mental retardation [2]. Diagnosing this disorder is often complicated and delayed due to diverse manifestations and varied ages at onset [3]. In addition to clinical manifestations, genetic criteria have been added to diagnose TSC in the second International TSC Consensus Conference [4]. Several studies showed the beneficial role of early intervention in TSC patients and the possibility of improving cognitive outcomes [[4], [5], [6], [7], [8]]. This strategy is currently applied in the multicenter European Commission, even worldwide [9,10]. Therefore, early recognition and diagnosis of TSC have become the focus of recent studies [11].
Cardiac rhabdomyoma, the most common type of cardiac tumor [12], may be the initial manifestation of TSC according to previous studies [[13], [14], [15]] and thus become a prenatal marker for TSC. Advances in fetal echocardiography technology have facilitated the prenatal diagnosis of fetal cardiac rhabdomyoma. However, antenatal consultation for fetal cardiac rhabdomyoma remains challenging because cardiac rhabdomyoma may occur alone or in association with TSC. Besides, prenatal ultrasound has poor specificity, and related specific brain or renal lesions may develop progressively after birth, making it difficult to diagnose TSC prenatally based on clinical features alone. Fetal cranial magnetic resonance imaging (MRI) can be used to detect the cortical tuber, which occurs in more than 90% of TSC patients. Therefore, MRI is useful in the prenatal diagnosis of TSC [4,16]. The primary aim of this study was to explore the incidence of TSC in fetuses detected with cardiac tumors at our institution over the past five years. An additional objective was to evaluate the value of fetal cranial MRI and genetic testing in the prenatal diagnosis of TSC.
2. Materials and methods
2.1. Subjects
From January 2016 to December 2020, a total of 17999 pregnant women and their families attended the Fetal Medicine Center of Women's Hospital, Zhejiang University School of Medicine. All women underwent fetal echocardiography, and those identified with fetal cardiac tumors were included in the study. After obtaining informed consent, further examinations, including fetal cranial MRI and genetic testing, were performed. Families who declined genetic examination were excluded.
3. Methods
3.1. Fetal echocardiography
According to the 2013 International Society of Ultrasound in Obstetrics and Gynecology guidelines, fetal echocardiography was performed by a maternal fetal medicine specialist and a Voluson E8 ultrasound system (GE Healthcare, Zipf, Austria) using a Philip iU22 color Doppler diagnostic ultrasound equipped with a transabdominal probe (model RAB4-8-D and C5-1). The number, size and location of all cardiac tumors were determined and recorded.
3.2. Fetal cranial MRI examination
A GE Medical Systems LLC 1.5T Signa HDxt MRI scanner with gradient field strength was used. Standard cross-sectional, coronal, and sagittal sweeps were performed during the fetal cranial examination, with all sequences taking less than 15 min.
3.3. TSC genetic testing and histological examination
For those who agreed to undergo the TSC genetic test, amniocentesis was performed from 16 weeks to 24 weeks of gestation, and paracentesis of umbilical cord blood was performed after 24 weeks of gestation or paracentesis of peripheral blood was performed after birth. If families chose to terminate the pregnancy, pathological autopsy was performed after the abortion, and fetal tissue samples were taken for histological examination. Among the families who agreed to undergo genetic analysis after the termination of the pregnancy, fetal tissue was obtained for DNA extraction. Peripheral blood samples of parents and other family members were also collected. Approximately 20 ml of amniotic fluids was collected in aseptic tubes. Blood was stored using a 4-ml ethylenediaminetetraacetic acid (EDTA) anticoagulation vacuum tube.
Genomic DNA was extracted from amniotic fluid, cord blood, aborted tissue, or peripheral blood using the Rapid Genetic DNA Blood Kit (Huada Gene Corporation). We amplified 21 exons of the TSC1 gene, 40 exons of the TSC2 gene and their intron flanking sequences by polymerase chain reaction (PCR)-mediated amplification using appropriate primers. All PCR products were sequenced and analyzed with Illumina HiSeq 2500 following standard protocols. Next-generation sequencing of the PCR amplicons revealed full coverage of all the exons and their intron flanking sequences. To detect the large deletions and duplications in both TSC genes, we perform multiplex linkage correlation probe amplification (MLPA) reactions with MLPA kits for TSC1 and TSC2 (Huada Medical Laboratory, Shenzhen). The pathogenicity of all variants was determined according to the sequence variant interpretation criteria and guidelines developed by the American College of Medical Genetics and Genomics [17].
When pathogenic variation was identified, the family history was investigated and genetic testing was performed in the parents. Clinicians and genetic counsellors analyzed and matched the biological information, clinical phenotype, clinical symptoms and treatment status of each patient's pedigree. Finally, a genetic diagnosis could be made based on the data.
3.4. Pathological autopsy and follow up of newborns
For those families who decided to terminate the pregnancy, informed consent of pathological autopsy was obtained. Neonates who were delivered successfully have been followed up till three years after birth. Epilepsy status, cognitive/behavioral outcome, growth and development,and other organ manifestations were followed and recorded every three months. This study was approved by the Ethics Committee of the Women's Hospital, Zhejiang University, School of Medicine (IRB-20210332-R).
3.5. Statistical methods
We divided all participants into 2 groups according to the number of cardiac tumors, and comparison of the prevalence of TSC was made. Categorical variables were expressed as frequencies (percentage) and were compared using the Pearson χ2 test or Fisher's exact test. Statistical analysis was performed using SPSS version 25 (IBM Corp., Armonk, NY, USA). A two-tailed P < 0.05 was considered statistically significant.
4. Results
4.1. Clinical characteristics
Over the past five years (2016–2020), we identified 58 fetuses with cardiac tumors. Eighteen cases were excluded due to declining genetic testing. Specific information of every included case is shown in Supplemental Table 1. The mean age of the pregnant women was 28.9 ± 6.4 years (range 20–40 years), and the mean gestational week when fetal cardiac tumor was identified was 29.2 ± 4.5 weeks (range 22–40 weeks). The clinical characteristics of our study population are presented in Table 1. In total, 18 women chose to continue the pregnancy and experienced successful deliveries. A total of 38.9% (7/18) of the women underwent cesarean section for various indications. The median week of gestation at delivery was 38.3 weeks, and the mean neonatal birth weight was 3105 g. The median length of stay in the neonatal intensive care unit (NICU) was six days, mainly due to TSC investigations and hospitalization for observation.
Table 1.
Clinical characteristics of the prenatally diagnosed cardiac tumor(s) with follow-up data (n = 40).
| characteristic | Value |
|---|---|
| Maternal age (years) | 28.9 (20–40) |
| GA at echocardiographic and ultrasound examinations (weeks) | 29.2(22–40) |
| GA at fetal cranial MRI (weeks) | 30 (23–40) |
| Number of echocardiographic examinations | 2(1–3) |
| Delivery | 18 |
| Abortion | 22 |
| Cesarean delivery | 7(38.9%) |
| Indication for Cesarean delivery | |
| Previous Cesarean delivery | 4(22.2%) |
| Failure to progress | 1(5.6%) |
| Fetal presentation (breech/transverse) | 2(11.1%) |
| Non-reassuring FHR pattern | 1(5.6%) |
| GA at delivery (weeks) | 38.3(35.0–40.4) |
| Live birth | 18(100.0%) |
| Birth weight (g) | 3105(2685–3750) |
| Apgar score | |
| 1-min | 8(5–10) |
| 5-min | 9(7–10) |
| Male-to-female ratio of neonates | 11:7 |
| NICU admission | 18(100.0%) |
| Duration of stay in NICU (days) | 6(4–10) |
| Diagnosed with tuberous sclerosis after birth | 3(16.7%) |
| Abnormalities of postnatal brain MRI in TSC cases | 3(16.7%) |
Data are presented as median (range) or frequencies (percentage).
GA, gestational age, MRI, magnetic resonance imaging, NICU, neonatal intensive care unit.
4.2. Flowchart of the study
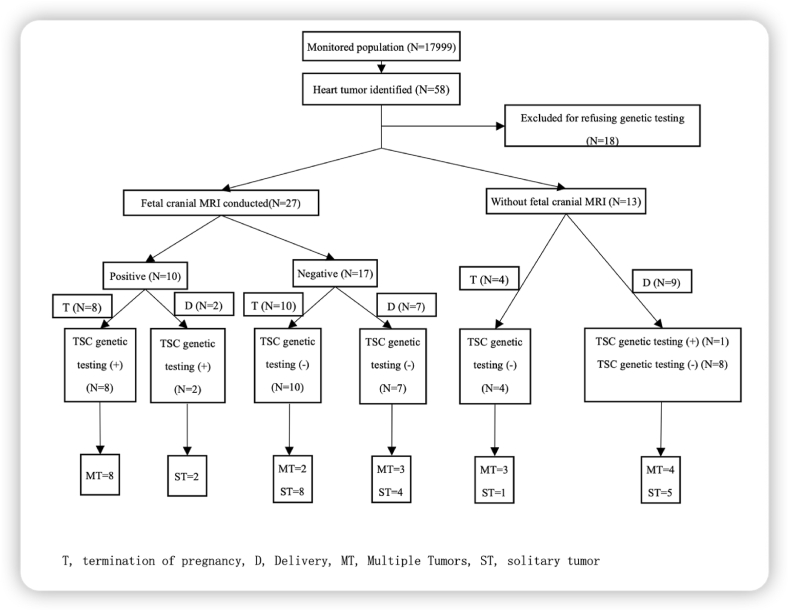
Fig. 1 depicts the overall flowchart of this study. Of 40 participants identified with fetal cardiac tumors, 27 fetuses underwent fetal cranial MRI, and the rest declined. There were 10 patients with fetal cranial lesions, 8 of whom chose to terminate the pregnancy. Among these aborted fetuses, pathogenic mutations were found in all samples. Two neonates with pathological lesions on cranial MRI harbored mutant TSC gene. Seventeen fetuses were identified without cranial lesions in cranial MRI: 7 survived and were found to be negative in the gene test and without the presence of clinical manifestations until three years after birth; 10 were aborted, and their genetic analysis indicated that no mutation was found. Of the 13 participants who refused fetal cranial MRI, 9 chose to continue the pregnancy and experience a successful delivery. Among these 9 newborns, only one harbored a pathogenic mutation of the TSC gene, and the other 8 children were genetically and clinically confirmed to not have TSC. In addition, 4 women chose to terminate the pregnancy, and no mutation was found in their fetuses.
Fig. 1.
Flow chart for confirming the diagnosis of tuberous sclerosis complex with fetal cardiac rhabdomyomas.
4.3. Fetal echocardiography
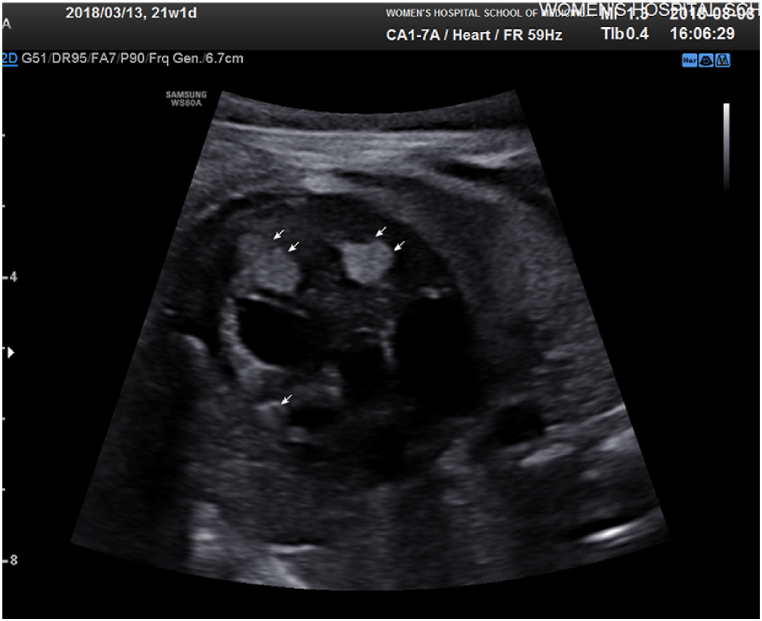
We divided 40 women into two groups according to the number of cardiac tumors (Table 2, Fig. 2), with half in the single tumor group (ST group) and the other half in the multiple tumors group (MT group). The prevalence of TSC in the ST group was significantly lower than that in the MT group (10.0% vs. 45.0%, P < 0.05). As Table 2 shows, the left ventricle (LV) was the most common location where tumors occurred (28 cases), followed by the right ventricle (RV, 20 cases). There was no cardiac tumor located in the pericardium. In the MT group, 13 women chose to terminate the pregnancy. Compared to the high abortion rate in the MT group (65.0%), there were only 9 women who terminated the pregnancy in the ST group (45.0%). However, this difference was not statistically significant.
Table 2.
Comparison of clinical characteristics between solitary cardiac tumor group and multiple cardiac tumors group.
| Total (N = 40) | Solitary (N = 20) | Multiple (N = 20) | |
|---|---|---|---|
| Diagnosis of TSC | 11 (27.50) | 2 (10.00) | 9 (45.00) * |
| Location | |||
| LV | 28 | 11 | 17 |
| RV | 20 | 6 | 14 |
| IVS | 8 | 3 | 5 |
| LA | 2 | 0 | 2 |
| RA | 1 | 0 | 1 |
| Pericardium | 0 | 0 | 0 |
| Abortion | 22 (55.00) | 9 (45.00) | 13 (65.00)# |
| Delivery | 18 (45.00) | 11 (55.00) | 7 (35.00)# |
Data are presented as frequencies (percentage).
LV, left ventricle; RV, right ventricle; IVS, interventricular septum; LA, left atrium; RA, right atrium.
*, P < 0.05; #, P > 0.05.
There was a statistically significant difference in the positive TSC gene mutation rate of 45.0% in the fetal heart tumor multiple group and 10.0% in the single group. (P = 0.034).
Fig. 2.
Fetal heart ultrasound showing cardiac tumors.
4.4. Fetal cranial MRI
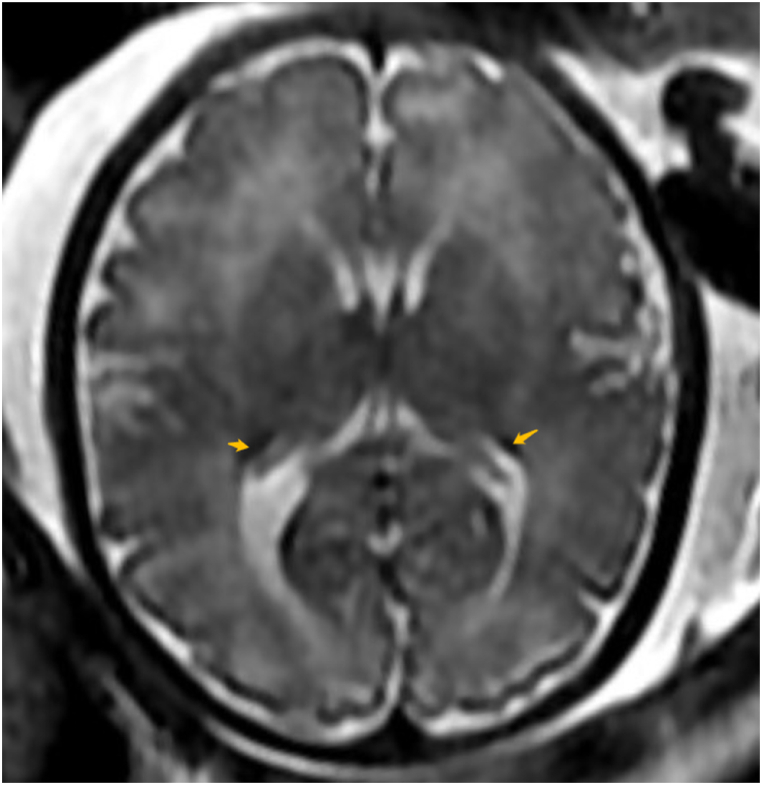
For those whose fetal cranial MRI results were positive, pathogenic changes were widely distributed within the brain (Fig. 3), including subventricular, paraventricular, paraventricular white matter areas, lateral ventricles, and brain parenchyma. There were no other combined abnormalities found in 5 cases and other abnormalities found in 5 patients (Supplemental Table 2).
Fig. 3.
Fetal cranial MRI showing pathological cranial lesions.
4.5. TSC gene detection
Genetic testing for TSC was successfully performed in 40 fetuses and their families. The results for 29 fetuses and their families were all negative. Among 11 fetuses with positive results, 3 harbored TSC1 mutations, and 8 harbored TSC2 mutations. All three TSC1 mutations were sporadic. Of the 8 TSC2 mutations, 6 were sporadic mutations, and 2 were familial mutations. The positive rate of TSC gene testing was 45.0% in the multiple tumors group and 10.0% in the single tumor group; this difference was statistically significant.
4.6. Pathological examination
There were totally 22 families choosing to terminate the pregnancy and all of them agree the autopsy. The results revealed that 21 cases had cardiac rhabdomyomas, including 13 cases of multiple rhabdomyomas and 8 cases of single rhabdomyomas. The rest one involved single fibroma.
4.7. Follow-up results of 18 children
Twenty-two women and their families decided to terminate the pregnancy, while 18 fetuses were successfully delivered. We followed up these children for three years. For the 18 children with fetal heart tumors, the tumors decreased or disappeared after birth in 16 children. Only two remained the same without progression, with no cases requiring surgical intervention. The 15 children with a negative genetic result showed no clinical symptoms during three-years following period. Three patients were diagnosed with TSC and treated with rapamycin after birth. One patient had central nervous system symptoms including epilepsy and growth retardation, and got a satisfactory seizure control after drug treatment. The remaining two families actively selected drug treatment for cortical tubers and the size of tubers remained stable.
5. Discussion
Advances in imaging technology and genetic analysis facilitate the diagnosis of TSC and prenatal consultation. Although a positive result of molecular analysis is sufficient to confirm the diagnosis, the cost of genetic testing is high, and there are 10–15% of TSC patients do not have mutations identified by conventional genetic testing [18,19]. Some specific clinical manifestations occur after birth or even later. Therefore, it is still difficult for experts in fetal medicine to provide a clear diagnosis. Epilepsy is the most common symptom appearing in 80–90% of patients, mainly in the first year of life. And there is a strong association between mental retardation and epilepsy. In the large series of 160 patients from Mayo Clinic Gomez found that all patients with mental retardation had history of epilepsy, and none without seizures had mental retardation [20,21]. Therefore, we followed for three years to ensure that out diagnosis were relatively reliable. And we excluded the 15 gene-negative children from the diagnosis of TSC because no clinical symptoms showed up during the follow-up period.
In our study, there were totally 22 families choosing to terminate the pregnancy for fetal cardiac tumor(s) and the percentage of termination was over 50% (22/40). There were 14 aborted fetuses without pathogenic variations in TSC1/2. Despite there is possibility that not all of these 22 cases are healthy, improvement of prenatal consulting is extremely needed to help parents make a better decision.
Our study showed that those with lesions found by fetal cranial MRI were eventually diagnosed as TSC by genetic testing. However, no one in the cranial MRI-negative group was confirmed as a TSC patient. This result again demonstrates the crucial value of fetal cranial MRI. A previous study concluded that fetal brain MRI appears to be more sensitive than fetal brain ultrasound in the prenatal diagnosis of TSC cerebral lesions because a proportion of cases diagnosed by MRI were initially reported as normal on US [22]. Considering its high diagnostic value and affordable cost, fetal cranial MRI is recommended for pregnant women with fetal cardiac tumors. Furthermore, simple cardiac tumors without other lesions symbolized a better prognosis, mentally and physically [23,24]. Most of the delivered children in this study had cardiac rhabdomyoma that shrank or even disappeared after birth. Therefore, when a single cardiac tumor is detected, if fetal cranial MRI and genetic testing are both negative, the risk of TSC is low.
Cranial cortical tuber can be detectable in ∼80–90% TSC patients and thus be classified another major feature of TSC [23]. Chances are that a TSC patient can have multiple cranial tubers in different size and location, which is speculated to be responsible for the neurological symptoms [25]. Focal epileptiform interictal discharges mapped by intracranial electroencephalogram (EEG) frequently superimpose with cortical tubers [26]. For pharmacologically refractory epilepsy, surgical resection of intracranial tubers could alleviate seizures in the majority of TSC patients [27]. However, some studies indicated that perituberal region was also playing an important role in epileptiform activity, besides tuber core [27,28]. Therefore, cerebral cortical tubers are the leading cause of seizures, which demonstrates the significance of cranial MRI in the evaluation of prognosis of TSC. When cranial lesion is detected prenatally, there is strong possibility that neurological manifestations will occur in the neonate sooner or later. However, there is also limitation in fetal MRI. Considering low myelinization of the fetal brain, some cranial lesions may not be detected by imaging technology prenatally and even in early neonatal period [11]. In our study, the median gestational age of MRI conducted is 30 weeks and there is possibility that some cortical tubers cannot be detected.
In our study, we found that the prevalence of TSC in the single tumor group was significantly lower than that in the multiple tumor group, which indicated that fetuses with multiple cardiac tumors were more likely to suffer from TSC. This result was consistent with previous studies [15,29]. In their study, approximately 86% (32/37) of the fetuses with multiple cardiac tumors and 31% (5/16) of the fetuses with a single cardiac tumor harbored TSC1 or TSC2 mutations and were genetically diagnosed with TSC [15]. Consequently, the number of heart tumors might be of great significance to evaluate the possibility of TSC. Besides, there is higher incidence of single heart tumor (50%) than that of other literature, the reason might be the exclusion of 18 cases who refused genetic testing. Among those 18 cases excluded, there were 15 cases with multiple heart tumors and 3 cases with solitary heart tumor.
TSC2 gene mutations are more common and are associated with more severe clinical manifestations. The incidence of TSC2 mutations is twice that of TSC1 in familial inheritance and 3.5 times that in sporadic cases [30,31]. J. Chen et al. [15] examined a total of 53 pregnant women with fetal cardiac tumor(s) and performed TSC gene tests. They found that 37 fetuses harbored pathogenetic TSC1 or TSC2 mutations, of which 25 were sporadic mutations and 12 were familial mutations. In addition, 6 mutations were located in TSC1, and 31 mutations were located in TSC2. The results of genetic testing in our study further confirmed the ratio of TSC1:TSC2 to some extent. The incidence of TSC gene mutations is lower than that in other studies, and the reason might be the difference in races and regions. The high detection rate of fetal cardiac tumors currently could also contribute to this bias. In addition, there exists mosaicism and deep intronic mutations in TSC1/TSC2 genes, which could not be identified by conventional testing [19].
The prenatal diagnosis of TSC is complicated, and fetal echocardiography is the preferred screening technology. If pathological variants in the TSC1 or TSC2 gene are identified, it is clear to diagnose the disease antenatally. However, high cost and long waiting time make the popularization of genetic testing difficult to achieve. Most pregnant women are already in the late stage of pregnancy when fetal cardiac tumors are detected. Definitive diagnosis and antenatal suggestions are required as soon as possible. Therefore, the application of fetal cardiac ultrasound and cranial MRI to clarify the coexistence of fetal heart tumors and intracranial nodules can improve the prenatal confirmation rate of TSC and optimize prenatal consultation for tuberous sclerosis.
Data from the surviving children in this study indicated that those with simple cardiac rhabdomyoma had a good prognosis and satisfactory mental and physical development. Most of the children in this study had cardiac rhabdomyoma that shrank or even disappeared after birth, which might be influenced by changes in maternal hormone levels and related to the pathological mechanism. Rhabdomyoma cells are glycogen-rich heterotypic cells, most of which undergo vacuolation degeneration and apoptosis after birth.
Some limitations of the study should be acknowledged. This was a retrospective study, and only fetuses with primary symptoms of cardiac tumors were included. For people with a positive family history, when they get pregnant, they could have been performed particular examinations such as non-invasive prenatal testing or amniocentesis even before fetal heart tumor detected. Therefore, they are not in the scope of our research. Those families who refused genetic tests were excluded from our study because the diagnosis of these fetuses could never be confirmed after they chose to terminate the pregnancy. This exclusion might influence the incidence rate of TSC. Besides, we excluded the 15 gene-negative children from the diagnosis of TSC because no clinical symptoms showed up on them during the follow-up period. However, TSC related manifestations can develop with ages, and thus the diagnosis could be missed. Considering that most of TSC symptoms develop with age, the incidence of TSC might be higher. In addition, the sample size was relatively small. There were only 40 cases included in the final analysis, and 18 fetuses were diagnosed with TSC. There were fewer infants with TSC who survived and the follow-up period was short, thus making the prognosis of TSC less convincing.
6. Conclusion
The disease of TSC has drawn more attention in clinical practice. For all cases where cardiac tumors are found, fetal cranial MRI is recommended, and TSC genes need to be tested if possible. Fetuses with simple cardiac tumors usually have a good prognosis, and such pregnancies should not be terminated before cranial lesions are detected. Although TSC gene pathogenic mutations have become an independent diagnostic criterion, this affordable prenatal diagnostic test to detect typical cardiac and cranial lesions could still help to confirm the disease.
Funding
This research has been funded by Zhejiang Province Medical and Health Science and Technology Program (2022KY391).
Data availability statement
The data that support the findings of this study are available upon reasonable request from the corresponding author.
Author contribution statement
Neng Jin: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Yan Wu: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Qing Meng: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Qiong Luo: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e16980.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Curatolo P., Bombardieri R., Jozwiak S. Tuberous sclerosis. Lancet. 2008;372(9639):657–668. doi: 10.1016/S0140-6736(08)61279-9. [DOI] [PubMed] [Google Scholar]
- 2.Curatolo P., Moavero R., de Vries P.J. Neurological and neuropsychiatric aspects of tuberous sclerosis complex. Lancet Neurol. 2015;14(7):733–745. doi: 10.1016/S1474-4422(15)00069-1. [DOI] [PubMed] [Google Scholar]
- 3.Hapsara S., Sutomo R., Danarti R., Diwasasri A. Long-term follow up of a tuberous sclerosis patient: evaluation of anti-epileptic drugs and self- management support therapy. Paediatr. Indones. 2020;60(1):53–60. [Google Scholar]
- 4.Northrup H., Krueger D.A. International tuberous sclerosis complex consensus G. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 iinternational tuberous sclerosis complex consensus conference. Pediatr. Neurol. 2013;49(4):243–254. doi: 10.1016/j.pediatrneurol.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cusmai R., Moavero R., Bombardieri R., Vigevano F., Curatolo P. Long-term neurological outcome in children with early-onset epilepsy associated with tuberous sclerosis. Epilepsy Behav. 2011;22(4):735–739. doi: 10.1016/j.yebeh.2011.08.037. [DOI] [PubMed] [Google Scholar]
- 6.Jozwiak S., Kotulska K., Domanska-Pakiela D., et al. Antiepileptic treatment before the onset of seizures reduces epilepsy severity and risk of mental retardation in infants with tuberous sclerosis complex. Eur. J. Paediatr. Neurol. 2011;15(5):424–431. doi: 10.1016/j.ejpn.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Jozwiak S., Slowinska M., Borkowska J., et al. Preventive antiepileptic treatment in tuberous sclerosis complex: a long-term, prospective trial. Pediatr. Neurol. 2019;101:18–25. doi: 10.1016/j.pediatrneurol.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Sancak O., Nellist M., Goedbloed M., et al. Mutational analysis of the TSC1 and TSC2 genes in a diagnostic setting: genotype--phenotype correlations and comparison of diagnostic DNA techniques in Tuberous Sclerosis Complex. Eur. J. Hum. Genet. 2005;13(6):731–741. doi: 10.1038/sj.ejhg.5201402. [DOI] [PubMed] [Google Scholar]
- 9.Curatolo P., Nabbout R., Lagae L., et al. Management of epilepsy associated with tuberous sclerosis complex: updated clinical recommendations. Eur. J. Paediatr. Neurol. 2018;22(5):738–748. doi: 10.1016/j.ejpn.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Slowinska M., Kotulska K., Szymanska S., Roberds S.L., Fladrowski C., Jozwiak S. Approach to preventive epilepsy treatment in tuberous sclerosis complex and current clinical practice in 23 countries. Pediatr. Neurol. 2021;115:21–27. doi: 10.1016/j.pediatrneurol.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Słowińska M., Jóźwiak S., Peron A., et al. Early diagnosis of tuberous sclerosis complex: a race against time. How to make the diagnosis before seizures? Orphanet J. Rare Dis. 2018;13(1):25. doi: 10.1186/s13023-018-0764-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Więckowska K., Piątek K., Respondek-Liberska M. Heart tumors in 33 fetuses - review of twenty-two years of the single-centre experience. Prenatal Cardiology. 2016;6(1):22–30. [Google Scholar]
- 13.Isaacs H., Jr. Fetal and neonatal cardiac tumors. Pediatr. Cardiol. 2004;25(3):252–273. doi: 10.1007/s00246-003-0590-4. [DOI] [PubMed] [Google Scholar]
- 14.DiMario F.J., Jr., Sahin M., Ebrahimi-Fakhari D. Tuberous sclerosis complex. Pediatr. Clin. 2015;62(3):633–648. doi: 10.1016/j.pcl.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Chen J., Wang J., Sun H., et al. Fetal cardiac tumor: echocardiography, clinical outcome and genetic analysis in 53 cases. Ultrasound Obstet. Gynecol. 2019;54(1):103–109. doi: 10.1002/uog.19108. [DOI] [PubMed] [Google Scholar]
- 16.Józwiak S., Schwartz R.A., Ck J., Jb C. Usefulness of diagnostic criteria of tuberous sclerosis complex in pediatric patients. J. Child Neurol. 2020;15(10):652–659. doi: 10.1177/088307380001501003. [DOI] [PubMed] [Google Scholar]
- 17.Richards S., Aziz N., Bale S., et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of medical genetics and Genomics and the association for molecular pathology. Genet. Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Northrup H., Aronow M.E., Bebin E.M., et al. Updated international tuberous sclerosis complex diagnostic criteria and surveillance and management recommendations. Pediatr. Neurol. 2021;123:50–66. doi: 10.1016/j.pediatrneurol.2021.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Tyburczy M.E., Dies K.A., Glass J., et al. Mosaic and intronic mutations in TSC1/TSC2 explain the majority of TSC patients with No mutation identified by conventional testing. PLoS Genet. 2015;11(11) doi: 10.1371/journal.pgen.1005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holmes G.L., Stafstrom C.E., Tuberous Sclerosis Study G. Tuberous sclerosis complex and epilepsy: recent developments and future challenges. Epilepsia. 2007;48(4):617–630. doi: 10.1111/j.1528-1167.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- 21.Jansen F.E.V.K., Algra A., Anbeek P., Braams O., Nellist M., Zonnenberg B.A., Jennekens-Schinkel A., van den Ouweland A., Halley D., van Huffelen A.C., van Nieuwenhuizen O. Cognitive impairment in tuberous sclerosis complex is a multifactorial condition. Neurology. 2008;70(12):916–923. doi: 10.1212/01.wnl.0000280579.04974.c0. [DOI] [PubMed] [Google Scholar]
- 22.Wortmann S.B., Reimer A., Creemers J.W., Mullaart R.A. Prenatal diagnosis of cerebral lesions in Tuberous sclerosis complex (TSC). Case report and review of the literature. Eur. J. Paediatr. Neurol. 2008;12(2):123–126. doi: 10.1016/j.ejpn.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Krueger D.A., Northrup H. International tuberous sclerosis complex consensus G. Tuberous sclerosis complex surveillance and management: recommendations of the 2012 international tuberous sclerosis complex consensus conference. Pediatr. Neurol. 2013;49(4):255–265. doi: 10.1016/j.pediatrneurol.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tworetzky W., McElhinney D.B., Margossian R., et al. Association between cardiac tumors and tuberous sclerosis in the fetus and neonate. Am. J. Cardiol. 2003;92(4):487–489. doi: 10.1016/s0002-9149(03)00677-5. [DOI] [PubMed] [Google Scholar]
- 25.Feliciano D.M. The neurodevelopmental pathogenesis of tuberous sclerosis complex (TSC) Front. Neuroanat. 2020;14:39. doi: 10.3389/fnana.2020.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohamed A.R., Bailey C.A., Freeman J.L., Maixner W., Jackson G.D., Harvey A.S. Intrinsic epileptogenicity of cortical tubers revealed by intracranial EEG monitoring. Neurology. 2012;79:2249–2257. doi: 10.1212/WNL.0b013e3182768923. [DOI] [PubMed] [Google Scholar]
- 27.Fallah A., Rodgers S.D., Weil A.G., et al. Resective epilepsy surgery for tuberous sclerosis in children: determining predictors of seizure outcomes in a multicenter retrospective cohort study. Neurosurgery. 2015;77(4):517–524. doi: 10.1227/NEU.0000000000000875. ; discussion 524. [DOI] [PubMed] [Google Scholar]
- 28.Major P., Rakowski S., Simon M.V., et al. Are cortical tubers epileptogenic? Evidence from electrocorticography. Epilepsia. 2009;50(1):147–154. doi: 10.1111/j.1528-1167.2008.01814.x. [DOI] [PubMed] [Google Scholar]
- 29.Bader R.S., Chitayat D., Kelly E., et al. Fetal rhabdomyoma: prenatal diagnosis, clinical outcome, and incidence of associated tuberous sclerosis complex. J. Pediatr. 2003;143(5):620–624. doi: 10.1067/S0022-3476(03)00494-3. [DOI] [PubMed] [Google Scholar]
- 30.Au K.S., Williams A.T., Roach E.S., et al. Genotype/phenotype correlation in 325 individuals referred for a diagnosis of tuberous sclerosis complex in the United States. Genet. Med. 2007;9(2):88–100. doi: 10.1097/gim.0b013e31803068c7. [DOI] [PubMed] [Google Scholar]
- 31.Dabora S.L., Jozwiak S., Franz D.N., et al. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am. J. Hum. Genet. 2001;68(1):64–80. doi: 10.1086/316951. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon reasonable request from the corresponding author.