Abstract
It is hard for medical students to recognize and understand the clinical presentation of systemic connective tissue diseases (SCTDs). In this study, we aimed to review the immune mechanisms of the main SCTDs and to propose a classification system focused on the student and based on each immune dysfunction’s clinical phenotype. The search involved the MEDLINE database and included the terms “systemic lupus erythematosus,” “antiphospholipid syndrome,” “inflammatory myopathies,” “rheumatoid arthritis,” “Sjögren’s syndrome” or “systemic sclerosis” and “pathogenesis,” and “immunology” or “mechanism of disease.” Systemic lupus erythematosus (SLE) is a prototypic immune-complex disease with a tendency toward vascular injury. Antiphospholipid syndrome (APS) is a diffuse immune-mediated thrombotic vasculopathy. In inflammatory myopathies (IMs), muscle inflammation leading to muscle weakness is the cardinal manifestation. Rheumatoid arthritis (RA) is a unique form of erosive and destructive polyarthritis. Sjögren’s syndrome (SS) causes sicca symptoms due to infiltration of the exocrine glands. Disseminated fibrosis in systemic sclerosis (SSc) is caused by vascular injury with excessive fibroblast activation. After the review, we created a focus group involving all the authors to group the diseases according to their pathogenesis and clinical phenotype. Our group agreed that SCTDs can be divided in 3 groups based on the preferential clinical presentation and immune dysfunction: 1) vasculopathic features (SLE and APS), 2) tissue inflammation (IMs, RA, and SS), and 3) tissue fibrosis (SSc). In synthesis, we suggest that clustering SCTDs in groups based on clinical phenotype and presumptive immune dysfunction instead of ordering autoantibodies randomly can help students understand the diseases.
Keywords: Connective tissue diseases, Pathogenesis, Immunology, Teaching, Medical students
1. Introduction
Medical students regard systemic connective tissue diseases (SCTDs) as mysterious and poorly defined. For instance, an Irish study showed that up to 86% of the interns at a large teaching hospital were not confident enough to diagnose them [1]. Even in residency, doctors feel more comfortable dealing with diseases of the cardiorespiratory or gastrointestinal system than musculoskeletal ones [2]. Part of the problem may be a lack of training in clinical reasoning in these scenarios during formation, but the literature regarding teaching strategies is scarce.
We decided to conduct a poll among our interns to understand their perception in our institution. We collected 83 answers and observed that 23% of the students did not even know what SCTDs were. Also, 80.7% of them felt uncomfortable or very uncomfortable establishing these diagnoses, and no student felt very comfortable diagnosing STCDs.
Our results have no scientific value, but corroborate our feeling that SCTDs are somewhat confusing for young doctors and that efforts to integrate them could be welcome.
The purpose of our review was to gather the information available in the literature regarding the most common SCTDs as well as their pathogeneses and clinical phenotypes. Afterward, we propose a new classification method focused on the medical student. This framework is not intended to substitute the contemporary accepted classification criteria, but to facilitate the recognition of clinical patterns by the young students.
1.1. Search strategy and selection criteria
In our search, we utilized the MEDLINE database, including articles in English from 2000 to 2021. We included reviews of the respective diseases and its pathophysiology. The search terms were “systemic lupus erythematosus,” “antiphospholipid syndrome,” “inflammatory myopathies,” “rheumatoid arthritis,” “Sjögren’s syndrome” or “systemic sclerosis” and “pathogenesis,” or “immunology” or “mechanism of disease.” The diseases included on the study were based on the classification of systemic connective tissue diseases proposed by West and Kolfenbach [3].
Each author performed the literature research in one of the diseases, favoring the 20 first articles sorted by the “best match” tool provided by PubMed. If necessary, the author would explore any among all the articles found by the search strategy, favoring those published in the last 10 years.
After the review, we formed a focus group to discuss the clustering. When consensus was reached, we reviewed once again the evidence for each disease and produced a brief report focusing on pathogenesis and clinical phenotype. In the end, we developed the classification based on our findings and discussions, aiming to develop a framework for basic comprehension of the clinical diagnosis for medical students, focusing on the link between T-cell response and clinical phenotype.
2. Literature review
2.1. Systemic lupus erythematosus (SLE)
SLE is a prototypic immune complex disease [4], which means that most of the pathological processes derive from immune complex deposition. Many of the SLE autoantibodies will target the cell’s inner structures, mainly the nucleus, because apoptotic cells' clearance mechanisms are defective, leading to a higher chance of loss of immunotolerance [5]. Because many autoantibodies will find their antigens in circulation, immune complex formation frequently occurs and vascular deposition is a major pathological feature [[6], [7], [8]]. This deposition leads to complement activation, recruitment of inflammatory cells, thrombosis, and tissue damage [9], which is usually multisystemic, with a preference for kidneys, skin, and the central nervous system. Considering that antiphospholipid antibodies are often present, thrombosis of the microcirculation might be crucial [7].
The clinical picture of a patient with SLE is that of disseminated inflammation, with nonspecific signs, but also with organ-specific involvement, usually in a very multisystemic fashion [5]. Manifestations not closely related to vascular phenomena, such as musculoskeletal inflammation, are often present [5] but tend to be indistinguishable from other SCTDs. The cutaneous manifestations can be suggestive of SLE, such as malar rash, but erythematous lesions can be difficult to differentiate from the ones from other SCTDs, even in their pathology and immunofluorescence [10]. However, the magnitude of vascular involvement in SLE is remarkable [8,11]. The main clinical manifestations somehow involving vessel occlusion include some important SLE-associated renal syndromes (class III and IV glomerulonephritis, true renal vasculitis, and thrombotic microangiopathy) [6], the focal central nervous system manifestations (psychosis, seizures, movement disorders, and stroke syndromes) [12], diffuse alveolar hemorrhage [6], and some skin manifestations (palpable purpura, nail-fold ulcerations, palmar vasculitis, and urticaria) [4] among other, more infrequent situations. We propose that, when present, these vascular manifestations should prompt suspicion of SLE more than of other SCTDs.
Other than diffuse vasculopathy or vasculitis, another important feature is the presence of circulating autoantibodies against cell-surface proteins. These antibodies contribute to hematopoietic cell damage [9,13], leading to cytopenia.
2.2. Antiphospholipid syndrome (APS)
APS is a systemic prothrombotic autoimmune disease characterized by thrombotic events and presence of antiphospholipid antibodies (aPLAs). The main autoantibodies that play a major role in the disease’s pathogenesis are anticardiolipin (aCL), anti-β2-glycoprotein I (aβ2GPI), and the lupus anticoagulant (LA) [14,15].
Although it can occur without any underlying disease, APS has been linked to SLE and other autoimmune, malignant, and infectious entities [16].
The mechanism of disease involves the “two hit hypothesis,” which postulates that the pathogenesis includes two events: the first is the loss of immunotolerance and production of autoantibodies targeting the membrane phospholipids (PLs), and the second is an event that leads to the exposition of these PLs, such as infections, smoking, and pregnancy [17]. This theory tries to explain why patients with APS suffer only occasionally from thrombotic events despite being permanently exposed to aPLA.
The best studied aPLA is aβ2GPI, which is a natural anticoagulant that inhibits prothrombinase and tenase activity on platelets, inhibits factor XII activation, and can modulate platelets; it has also been shown to bind to factor XI directly to attenuate its activation [18]. However, oxidative stress can change the conformation of the protein, not only exposing the epitope target of aβ2GPI but also making it prone to binding PLs [19]. As such, the oxidized β2GPI acts as a link between the aPLA and the membrane PLs. In addition, this modified form is able to antagonize the effects of endothelial nitric oxide synthase (eNOS) [20], promoting cell adhesion and vasoconstriction.
The complex formatted by aPLA and its targets (i.e., membrane PLs) is able to activate the target cell, usually a platelet, endothelial cell, or monocyte [21], which in turn is capable of inducing thrombosis through complement and coagulation cascades activation, tissue factor expression, platelet aggregation, or even cytokine production [19,21,22].
The mechanisms underlying the loss of immunotolerance are less well-established, but molecular mimicry has been proposed [23], including with SARS-CoV-2 [24]. Because antibodies are necessary for the disease’s development, T-lymphocyte and B-lymphocyte dysfunction are probably involved in Th2 and Tfh polarization.
The clinical picture that mostly resembles APS is that of multiple thrombotic events in a person without risk factors or multiple pregnancy morbidities, but small-vessel disease is increasingly identified, and APS more likely represents a diffuse immune-mediated vasculopathy.
2.3. Systemic autoimmune myopathies (inflammatory myopathies)
Systemic autoimmune myopathies (SAMs) more properly represent a subfamily of diseases. Although all its counterparts involve some form of muscle pathology, the pattern of involvement and the extramuscular manifestations can differ significantly, making the clustering of these diseases difficult and somewhat imprecise. However, regarding the major pathogenic models proposed in this review, three distinct phenotypes emerge in SAM: 1) a complement-driven vasculopathy in dermatomyositis [25,26] (and possibly also in antisynthetase syndrome [27]), 2) a cytotoxic muscle infiltration in polymyositis and inclusion body myositis [28,29], and 3) a massive complement-driven muscle necrosis in necrotizing autoimmune myopathy [30]. In spite of the key mechanisms leading to damage, the uniting clinical phenotype of SAMs patients is muscle weakness [31].
2.3.1. Dermatomyositis (DM) and antisynthetase syndrome (ASyS)
The main pathogenic mechanism thought to mediate DM is the formation of the membrane attack complex (MAC) in the microvasculature of the perimysial region, triggered by the local deposition of antibodies directed against endothelial cells [32]. This attack of the capillaries leads to overexpression of adhesion molecules [33] and microvasculature necrosis [26], which in turn will lead to the influx of lymphocytes and plasmacytoid dendritic cells in the perifascicular regions [31] with muscle ischemia and dysfunction. Last, ischemic damage is sensed by RIG-1 signaling, promoting augmentation of cytokines related to innate immunity, such as IFN α and β, which also stimulate the cell invasion in muscle fibers [34], thus perpetuating the cycle.
Some of DM’s skin manifestations can also be explained by vasculopathy. The fingernails' capillary loops are usually dilated and the cuticle is thickened, suggesting hypoperfusion [35]. The distinct rash depicts perivascular lymphocytic infiltrate and interface dermatitis [36]. When ulcers are present, they are probably related to vasculopathy, vascular fibrin deposition, or true vasculitis [37,38].
In synthesis, DM is an autoimmune muscle disease prominently mediated by humoral and vascular mechanisms. Considering these hypotheses, Tfh-lymphocytes and B-lymphocytes probably play a major role [39].
The pathogenesis of ASyS is far more obscure but probably involves the production of autoantigenic forms of tRNA-synthetases (tRS) in the lungs [40]. Also, the secretion of these spliced variants of tRS extracellularly might explain the propagation of the disease to the muscle [41]. Independent of the inciting event, the histopathology of ASyS in the muscle somewhat resembles that of DM, predominantly affecting the perimysium but with necrotic features [25], predominantly CD8+ populations and actin myonuclear inclusions [27].
2.3.2. Polymyositis (PM) and sporadic inclusion body myositis (sIBM)
The pathogenesis of PM and sIBM is more cell-driven. The main effector of the disease is the invading CD8+ cytotoxic lymphocyte [25]. These lymphocytes are clonally expanded and are stimulated by CD4+ helper lymphocytes, mainly Th1, Th17, and CD28null cells [42]. Chemokines and cytokines, such as interleukin-1, 6, 8, and monocyte chemoattractant protein 1, are expressed in the targeted cells [25].
It is thought that, in sIBM, the intracellular accumulation of abnormal proteins due to impaired autophagic activity [43] also lead to muscle damage and dysfunction [42]. The mechanisms behind protein dyshomeostasis include aging, nitric oxid-induced cell stress, and long-standing inflammation [44,45].
In synthesis, PM and sIBM are systemic conditions but with a much more muscle-centered phenotype than DM or ASyS. The main mechanism of disease is cell-mediated toxicity with aberrant protein homeostasis concurring with muscle dysfunction and dystrophy in sIBM.
2.4. Rheumatoid arthritis (RA)
The most striking feature of RA is damage to the joint, causing inflammation, and destruction of its cartilage and peri-articular bone [[46], [47], [48], [49]]. It is a multifactorial disease, occurring in genetically predisposed individuals exposed to environmental factors that can cause loss of tolerance and activation of the immune system against modified autoantigens [48].
The excessive citrullination of proteins is considered the main inciting event in RA [48,50,51]. This posttranslational modification of the proteins makes them more susceptible to recognition by T-cells and further production of anti-citrullinated peptide antibodies (ACPA). These antibodies migrate to the synovium, where they promote cell damage, possibly of the osteoclasts of the subchondral bone [47], because these cells need citrullinating enzymes for their normal differentiation. The continuous lesion promoted by the ACPA serves as a permanent source of joint antigens for the resident DC. In genetically predisposed individuals, these antigens might break immunotolerance and initiate autoimmunity, with the synovium acting as a germinal center [52]. Dysfunctional T-lymphocytes within the synovial tissue produce cytokines that will enhance cell recruitment, such as TNF, and recruit B-lymphocytes that will participate in the production of autoantibodies, such as rheumatoid factor and ACPA [4,48]. Synovial macrophages also contribute to the cytokine profile, producing IL-6 and feeding the TNF loop [53]. Although the cytokine profile in RA is somewhat mixed, the predominant pattern seems to be the one related to Th1, Th17, and Tfh lymphocytes [48].
Osteoclasts are important in the pathogenesis of RA, not only serving as targets for the circulating ACPA but causing the characteristic bone demineralization and erosion adjacent to inflammation. Osteoclasts are activated through the receptor activator of the nuclear factor kappa-Β (RANK) ligand, expressed by activated T-lymphocytes and fibroblast-like synoviocytes [4,54]. Direct stimulation from TNF can also contribute to osteoclastogenesis [46].
Apart from macrophages, chondrocytes, and osteoclasts, all important and active cells promoting damage in RA stimulated by the cytokine and damage environment, fibroblast-like synoviocytes (FLSs) are particularly active in RA, different from any other SCTD. In fact, it is theorized that FLSs from RA patients present a more aggressive phenotype, probably promoted by abnormalities in transcription factors and intracellular signaling pathways [48]. FLSs are stimulated by TNF, IL-1, IL-6, and TGFβ [4] and are responsible for the hyperproliferation and hypervascularization of the synovial layers seen in RA, promoting cartilage destruction and expansion of the synovial tissue (pannus).
Tissue inflammation, pannus, and bone erosion all contribute to the singular phenotype of RA: a remarkably phlogistic and erosive polyarthritis.
2.5. Sjögren’s syndrome (SS)
The cardinal manifestations of SS are inflammation and destruction of salivary and lacrimal glands and consequential sicca symptoms [55]. Just as in RA synovium, salivary glands from patients with SS can act as ectopic germinal centers.
The immune dysfunction is believed to start in the salivary glands' epithelial cells (SGECs) after cell lesion and/or apoptosis. The inciting injury mostly recognized is of viral origin, especially Epstein-Barr virus (EBV) [56]. This virus is able to produce RNA-like molecules that are released by exosomes and stimulate DC response through type I IFN [[57], [58], [59]]. These RNA-like structures and other natural intracellular proteins (Ro52, Ro60, and La) are presented by DCs and can act as self-antigens that will make autoreactive T-lymphocytes emerge [55]. The type I IFN environment is also able to stimulate an immune response in the SGECs themselves, transforming them into competent antigen presenting cells and co-stimulators of the immune system [60]. This regional specificity (called “autoimmune epithelitis”) helps explain why the salivary glands are the main target tissue in SS albeit the autoimmunity is driven against ubiquitous proteins.
The profiles of T-cell response are Th1, Th17, and Tfh [4,55]. The cytokines involved in Th1 and Th17 signaling (such as IL-6, IL-17, and IFNγ) can induce lymphocytotoxicity [61], macrophage migration [62], and direct salivary gland disfunction [63]. On the other hand, Tfh cells work together with the innate immune system through cytokines such as IL-6, IL-7, IL-21, and BAFF to activate and maturate B-lymphocytes that will produce autoantibodies (like anti-Ro/SSA and anti-La/SSB) that will cause further destruction of the gland and autoantigen presentation.
The clinical picture of a patient with SS is primarily sicca syndrome and unspecific signs of inflammation. Patients with a marked humoral response might exhibit low levels of complement, hypergammaglobulinemia, cryoglobulinemia, and clinical vasculitis. These latest have a higher chance of developing lymphoma [55].
2.6. Systemic sclerosis (SSc)
It is known that the pathogenesis of SSc presents three crucial events: vasculopathy, dysregulation of innate and adaptive immunity, and fibrosis [64,65].
Microvascular damage is believed to be triggered by viral aggression, chemical, or oxidative products [65]. After the initial aggression and endothelial activation, the expression of adhesion molecules (VCAM-1, ICAM-1 and E-selectin) and activation of chemokines (MCP-1, MIP-1α and MIP-1β) occurs, resulting in the recruitment of inflammatory cells [65]. Endothelial dysfunction also promotes an imbalance in vasoconstrictor and vasodilator agents (eg, endothelin-1 and prostacyclin, respectively) and hence tissue hypoxia and increased oxidative stress [64] Endothelin-1, in addition to being a potent vasoconstrictor, induces the proliferation of fibroblasts and their differentiation into myofibroblasts, cells that play an important role in the fibrogenesis [64,65].
In response to the expression of adhesion molecules and the secretion of chemokines, inflammatory cells are recruited, especially macrophages and T-lymphocytes responsible for the production of proinflammatory and profibrotic mediators [65]. T-lymphocytes promote a predominantly type 2 response [64,66,67], producing IL-4 and IL-13. These cytokines can induce macrophages to release TGFβ (a stimulator for collagen production by fibroblasts) [64,67] and can directly activate fibroblasts [67]. Another cytokine of recent interest is IL-6, released by T-lymphocytes, B-lymphocytes, macrophages, and fibroblasts [68]. IL-6 is a key link between innate immunity, adaptive immunity, and fibrosis because it is released by macrophages, induces T-cell and B-cell responses, and is able to stimulate fibroblasts to produce an extracellular matrix (ECM). Although autoantibodies are usually present in patients with SSc, their role in the pathogenesis is still unknown [65,68].
The last step in the pathogenesis of SSc is the activation of fibroblasts and their differentiation into myofibroblasts by the previous mechanisms. After their activation, they release ROS (promoting a feedback mechanism) and undergo differentiation into myofibroblasts, responsible for the exaggerated production, assembly, and crosslinking of ECM [68].
The phenotype of a patient with SSc is that of diffuse fibrosis, manifested by skin thickening and organic dysfunction (especially gastrointestinal and pulmonary). The infiltration of the blood vessels by ECM, combined with vasomotor dysfunction, will also cause vasculopathy, with loss of capillary beds, ischemia (especially in the extremities), and hypertension, predominantly of the lung’s vasculature.
3. Discussion
This review focused on the pathogenesis of the SCTDs, focusing on the relationship between the predominant T-cell response and the clinical manifestations. The first challenge in this review was to select the target diseases. The study utilized the classification of rheumatic disorders proposed by West and Kolfenbach, in which SCTDs are described apart from the vasculitides and spondyloarthritis [3]. One could hypothesize that SCTDs have some features that allow the differentiation from its rheumatic counterparts such as a remarkable systemic behavior, with many organs simultaneously affected. This feature would help to differentiate from the systemic vasculitis, in which the target for the immune system is notoriously the blood vessels and all the main manifestations can be explained by that. Also, SCTDs bear a predominantly autoimmune dysfunction, rather than an autoinflammatory one [69]. This would permit the separation from the spondyloarthritis, entities in which the articular manifestations stand out and the pathogenesis mixes adaptative and innate immune system’s dysfunction [70].
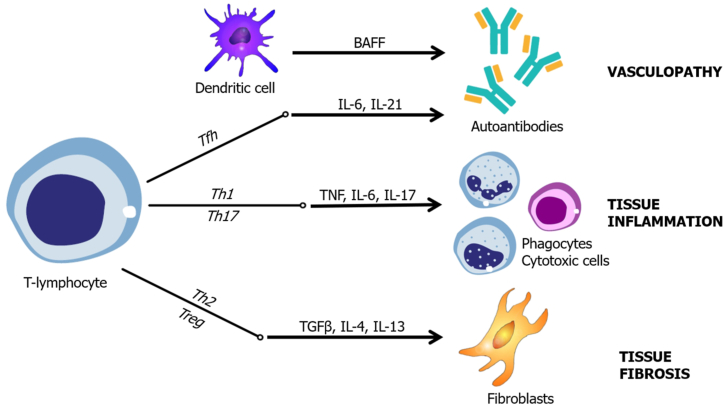
Regarding SCTDs, our group identified three basic pathogenic patterns: 1) vasculopathy and vasculitis, 2) tissue inflammation, and 3) tissue fibrosis. All STCDs can produce clinical manifestations within all “poles” of pathogenesis, but it is possible to determine a predominant pattern for each disease. Each pattern has a fairly constant lymphocyte T helper response, cytokine profile, and mechanism of lesion (Fig. 1).
Fig. 1.
Clinical phenotype according to T-helper predominant response and main cytokines.
3.1. Vasculopathy and vasculitis
Within this cluster, the clinical manifestations are related to vasculitis (i.e., infiltration of the vessel wall by inflammatory cells) and/or vasculopathy caused by vessel thrombosis. Because these mechanisms lead to vessel occlusion, the main clinical manifestations are due to tissue or organ ischemia [7]. Examples of the clinical syndromes related to vasculopathy include glomerular disease, ischemic central nervous system syndromes, cutaneous vasculitis, limb ischemia, pulmonary capillaritis, and large-vessel thrombosis.
The main pathogenic mechanism for vascular disease is immunocomplex deposition and the activation of the coagulation cascade [7,12,19,68]. Inflammatory cells also eventually infiltrate the vessel wall, driven by the immunocomplex deposition. Because a large quantity of antibodies is necessary for immunocomplex disease, cytokines related to this spectrum include those necessary for lymphocyte B activation, such as IL-6, IL-21, and BAFF [19,[71], [72], [73]]. Therefore, Tfh cells are most likely involved in this response [72].
Our group understood that SLE and APS were the clinical entities better suited for this cluster.
3.2. Tissue inflammation
In this cluster, the main clinical manifestations are related to the infiltration of organs and its apparatus by inflammatory cells. Examples of clinical syndromes include synovitis, myositis, dermatitis, adenitis, and serositis.
The main pathogenic mechanism for tissue inflammation is cytokine-driven inflammatory cell recruitment, directly targeting the respective tissue [25,46,74]. The clinical picture results from organic dysfunction promoted by inflammation and architecture destruction. Cytokines strongly participate in this clinical phenotype, especially TNF, IL-6, and IL-17 [42,47,48,74]. Therefore, lymphocytes Th1 and Th17 would most likely be predominant.
Our group considered the SAMs, RA, and SS more prone to this clinical pattern.
3.3. Tissue fibrosis
Some degree of fibrosis is expected in most if not all SCTDs. Clinicians will frequently find stigmata of tissue fibrosis in a large proportion of patients [75]. However, some patients' clinical pictures are dominated by fibrotic features. The deposition of fibrotic tissue in organs will impair their ability to maintain normal homeostasis, leading to organic and systemic failure. Examples of manifestations attributed to fibrosis include skin thickening, fibrotic vasculopathy in extremities, lungs, and kidneys, interstitial lung disease, and gastrointestinal fibrotic disease [68].
The main pathogenic mechanism for tissue fibrosis is fibroblast activation. It is thought that the trigger is an endothelial dysfunction, leading to ischemia and inflammatory inflow. The cytokines released from inflammatory stimuli lead to fibroblast hyperregulation, leading to exaggerated production of ECM [68]. The main cytokines related to this pathogenesis are TGF-β, IL-6, IL-10, IL-4, and IL-13 [[64], [65], [66], [67]]. Therefore, the T-helper phenotype in this cluster is somewhat polarized to Th2 and Treg.
Our focus group considered that SSc was the archetypal disease belonging to this cluster.
Following this approach, students might use their skills in semiology to classify the patient according to the dominant clinical picture. This strategy helps narrow the differential diagnosis, reduces the ordering of unhelpful exams, and might also have therapeutic value.
As expected from any unifying strategy, our group encountered many difficulties. For instance, some researchers pointed out that DM and SS have a large contribution from B-cells and autoantibodies in its pathogenesis, so they could be classified as belonging to the first cluster. It is true that many DM patients experience a vasculopathic Raynaud phenomenon and even skin ulcers due to vasculitis [75], and it is also very relevant that B-cell dysfunction is at the core of Sjögren’s pathogenesis [55].
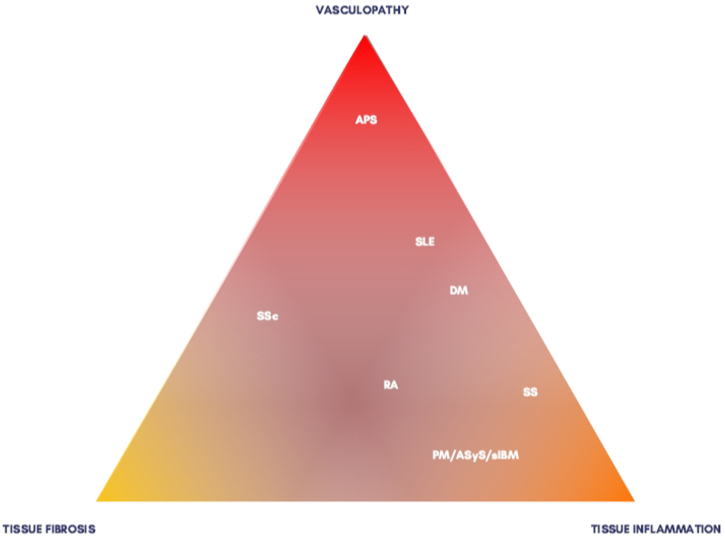
However, because our proposal was to develop a global vision, we thought that tissue inflammation would better represent the clinical findings that would make most clinicians consider SS or DM the final diagnosis. To call attention to these entities' possible humoral features, we developed Fig. 2, representing the spectrum of the SCTD.
Fig. 2.
The clinical spectrum of the systemic connective tissue diseases. Abbreviations: APS: antiphospholipid syndrome, SLE: systemic lupus erythematosus, DM: dermatomyositis, RA: rheumatoid arthritis, SS: Sjögren’s syndrome, PM: polymyositis, ASyS: antisynthetase syndrome, sIBM: sporadic inclusion body myositis, SSc: systemic sclerosis.
In a different extremity, SLE could be regarded as a predominantly inflammatory condition, because many students would often think of SLE when seeing a malar rash—or other cutaneous lesions—that is not necessarily accompanied by vasculopathy or vasculitis in the pathology. Indeed, 85% of SLE patients will present with mucocutaneous or musculoskeletal complaints [5]. Nevertheless, we thought that clustering SLE into the Tissue Inflammation group would neglect the renal manifestations—especially classes III and IV of lupus nephritis, which are essentially an immune complex-mediated injury of the glomerular capillaries—and the focal neuropsychiatric syndromes [12]. Neither condition is as common as skin and articular manifestations but, when present, strongly suggest SLE; on the other hand, synovitis is very common in SCTDs, and malar rash and photosensitivity can be found in DM and mixed connective tissue disease, among others.
Last, SSc is prominently a fibrotic condition, but vascular disease is a cornerstone of the pathogenesis. Therefore, one could consider including it in the first group. However, the vasculopathy of systemic sclerosis is not directly driven by inflammation itself but from oxidative stress and fibrosis. In fact, immunosuppression brings no benefit to the vasculopathy in SSc [76].
Again, to represent this conundrum less rigidly, we tried to highlight the mixed patterns in Fig. 2.
We understand that our review has many limitations, including the narrative design, the absence of newer mechanisms of pathogenesis, such as genetic profiles and intracellular signaling, and the limited usefulness for the postgraduate students. Also, the literature research was limited to the list of SCTD proposed by West and Kolfenbach [3], thus not mentioning systemic vasculitis for instance. However, in order to simplify and unify concepts for the students, we believe we had to lose some depth regarding the diseases individually. This does not mean that the framework proposed cannot be used by experienced doctors as a first approach in the daily practice though, but one must have in mind that it is not intended to be a diagnostic tool in isolation. In addition, this is just a first insight into the subject and the scientific community surely will greatly improve it in the future.
In summary, we conclude that the studying of SCTD can be overwhelming for young students. One explanation could be because it is centered in diagnostic criteria and autoantibodies, becoming an exercise of intensive memorization. Herein, we proposed a different approach, focusing on the clinical phenotype related to each immune mechanism. Future studies testing the performance of the methodology are being developed by our university.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author contribution statement
Ana Beatriz Lima Resende: Giovanna Paliares Monteiro: Carolina Carotenuto Ramos: Guilherme Santos Lopes: Livia Airoldi Broekman: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Jean Marcos de Souza: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
As a “Review” manuscript, no new data was gathered other than the articles already available in periodics.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
References
- 1.McCarthy E.M., Sheane B.J., Cunnane G. Greater focus on clinical rheumatology is required for training in internal medicine. Clin. Rheumatol. 2009;28:139–143. doi: 10.1007/s10067-008-0997-7. [DOI] [PubMed] [Google Scholar]
- 2.Katz S.J., Oswald A.E. How confident are internal medicine residents in rheumatology versus other common internal medicine clinical skills: an issue of training time or exposure? Clin. Rheumatol. 2011;30:1081–1093. doi: 10.1007/s10067-011-1715-4. [DOI] [PubMed] [Google Scholar]
- 3.West S.G., Kolfenbach J. fourth ed. Elsevier; Philadelphia: 2020. Rheumatology Secrets; pp. 118–218. [Google Scholar]
- 4.Hochberg M. seventh ed. Elsevier; Philadelphia: 2019. Rheumatology; pp. 1103–1127. [Google Scholar]
- 5.Kaul A., Gordon C., Crow M.K., et al. Systemic lupus erythematosus. Nat. Rev. Dis. Prim. 2016;2 doi: 10.1038/nrdp.2016.39. [DOI] [PubMed] [Google Scholar]
- 6.Leone P., Prete M., Malerba E., et al. Lupus vasculitis: an overview. Biomedicines. 2021;9:1626. doi: 10.3390/biomedicines9111626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen D., Rijnink E.C., Nabuurs R.J., et al. Brain histopathology in patients with systemic lupus erythematosus: identification of lesions associated with clinical neuropsychiatric lupus syndromes and the role of complement. Rheumatology (Oxford) 2017;56:77–86. doi: 10.1093/rheumatology/kew341. [DOI] [PubMed] [Google Scholar]
- 8.Seshan S.V. Lupus vasculopathy and vasculitis. Pathol. Case Rev. 2007;12:214–221. [Google Scholar]
- 9.Rahman A., Isenberg D.A. Systemic lupus erythematosus. N. Engl. J. Med. 2008;358:929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 10.Smith C.D., Marino C., Rothfield N.F. The clinical utility of the lupus band test. Arthritis Rheum. 1984;27:382–387. doi: 10.1002/art.1780270404. [DOI] [PubMed] [Google Scholar]
- 11.Radic M., Martinovic Kaliterna D., Radic J. Vascular manifestations of systemic lupus erythematosis. Neth. J. Med. 2013;71:10–16. [PubMed] [Google Scholar]
- 12.Jeltsch-David H., Muller S. Neuropsychiatric systemic lupus erythematosus: pathogenesis and biomarkers. Nat. Rev. Neurol. 2014;10:579–596. doi: 10.1038/nrneurol.2014.148. [DOI] [PubMed] [Google Scholar]
- 13.Winfield J.B., Mimura T. Pathogenetic significance of anti-lymphocyte autoantibodies in systemic lupus erythematosus. Clin. Immunol. Immunopathol. 1992;63:13–16. doi: 10.1016/0090-1229(92)90085-3. [DOI] [PubMed] [Google Scholar]
- 14.Ballanti E., Perricone C., Greco E., et al. Complement and autoimmunity. Immunol. Res. 2013;56:477–491. doi: 10.1007/s12026-013-8422-y. [DOI] [PubMed] [Google Scholar]
- 15.Pontara E., Cheng C., Cattini M.G., et al. An in vitro model to mimic the thrombotic occlusion of small vessels in catastrophic antiphospholipid syndrome (CAPS) Lupus. 2019;28:1663–1668. doi: 10.1177/0961203319886915. [DOI] [PubMed] [Google Scholar]
- 16.Brandt K.J., Kruithof E.K.O., Moerloose P. Receptors involved in cell activation by anti phospholipid antibodies. Thromb. Res. 2013;132:408–418. doi: 10.1016/j.thromres.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Meroni P.L., Tedesco F., Locati M., et al. Anti-phospholipid antibody mediated fetal loss: still an open question from a pathogenic point of view. Lupus. 2010;19:453–456. doi: 10.1177/0961203309361351. [DOI] [PubMed] [Google Scholar]
- 18.Matsuura E., Shen L., Matsunami Y., et al. Pathophysiology of β2-glycoprotein I in antiphospholipid syndrome. Lupus. 2010;19:379–384. doi: 10.1177/0961203310361352. [DOI] [PubMed] [Google Scholar]
- 19.Giannakopoulos B., Krilis S.A. The pathogenesis of the antiphospholipid syndrome. N. Engl. J. Med. 2013;368:1033–1044. doi: 10.1056/NEJMra1112830. [DOI] [PubMed] [Google Scholar]
- 20.Ramesh S., Morrell C.N., Tarango C., et al. Antiphospholipid antibodies promote leukocyte-endothelial cell adhesion and thrombosis in mice by antagonizing eNOS via β2GPI and apoER2. J. Clin. Invest. 2011;121:120–131. doi: 10.1172/JCI39828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schreiber K., Sciascia S., de Groot P.G., et al. Antiphospholipid syndrome. Nat. Rev. Dis. Prim. 2018;4 doi: 10.1038/nrdp.2017.103. [DOI] [PubMed] [Google Scholar]
- 22.Mulla M.J., Brosens J.J., Chamley L.W., et al. Antiphospholipid antibodies induce a pro-inflammatory response in first trimester trophoblast via the TLR4/MyD88 pathway. Am. J. Reprod. Immunol. 2009;62:96–111. doi: 10.1111/j.1600-0897.2009.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cruz-Tapias P., Blank M., Anaya J.M., Shoenfeld Y. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr. Opin. Rheumatol. 2012;24:389–393. doi: 10.1097/BOR.0b013e32835448b8. [DOI] [PubMed] [Google Scholar]
- 24.Harzallah I., Debliquis A., Drénou B. Lupus anticoagulant is frequent in patients with Covid-19. J. Thromb. Haemostasis. 2020;18:2064–2065. doi: 10.1111/jth.14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dalakas M.C. Inflammatory myopathies: update on diagnosis, pathogenesis and therapies, and COVID-19-related implications. Acta Myol. 2020;39:289–301. doi: 10.36185/2532-1900-032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emslie-Smith A.M., Engel A.G. Microvascular changes in early and advanced dermatomyositis: a quantitative study. Ann. Neurol. 1990;27:343–356. doi: 10.1002/ana.410270402. [DOI] [PubMed] [Google Scholar]
- 27.Stenzel W., Preuße C., Allenbach Y., et al. Nuclear actin aggregation is a hallmark of anti-synthetase syndrome-induced dysimmune myopathy. Neurology. 2015;84:1346–1354. doi: 10.1212/WNL.0000000000001422. [DOI] [PubMed] [Google Scholar]
- 28.Goebels N., Michaelis D., Engelhardt M., et al. Differential expression of perforin in muscle-infiltrating T cells in polymyositis and dermatomyositis. J. Clin. Invest. 1996;97:2905–2910. doi: 10.1172/JCI118749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orimo S., Koga R., Goto K., et al. Immunohistochemical analysis of perforin and granzyme A in inflammatory myopathies. Neuromuscul. Disord. 1994;4:219–226. doi: 10.1016/0960-8966(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 30.Allenbach Y., Arouche-Delaperche L., Preusse C., et al. Necrosis in anti-SRP+ and anti-HMGCR+myopathies: role of autoantibodies and complement. Neurology. 2018;90:e507–e517. doi: 10.1212/WNL.0000000000004923. [DOI] [PubMed] [Google Scholar]
- 31.Dalakas M.C. Pathophysiology of inflammatory and autoimmune myopathies. Presse Med. 2011;40:e237–e247. doi: 10.1016/j.lpm.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Kissel J.T., Mendell J.R., Rammohan K.W. Microvascular deposition of complement membrane attack complex in dermatomyositis. N. Engl. J. Med. 1986;314:329–334. doi: 10.1056/NEJM198602063140601. [DOI] [PubMed] [Google Scholar]
- 33.Figarella-Branger D., Civatte M., Bartoli C., Pellissier J.F. Cytokines, chemokines, and cell adhesion molecules in inflammatory myopathies. Muscle Nerve. 2003;28:659–682. doi: 10.1002/mus.10462. [DOI] [PubMed] [Google Scholar]
- 34.Suárez-Calvet X., Gallardo E., Nogales-Gadea G., et al. Altered RIG-I/DDX58-mediated innate immunity in dermatomyositis. J. Pathol. 2014;233:258–268. doi: 10.1002/path.4346. [DOI] [PubMed] [Google Scholar]
- 35.Dalakas M.C. Inflammatory muscle diseases. N. Engl. J. Med. 2015;372:1734–1747. doi: 10.1056/NEJMra1402225. [DOI] [PubMed] [Google Scholar]
- 36.Greenberg S.A., Fiorentino D. Similar topology of injury to keratinocytes and myofibres in dermatomyositis skin and muscle. Br. J. Dermatol. 2009;160:464–465. doi: 10.1111/j.1365-2133.2008.08967.x. [DOI] [PubMed] [Google Scholar]
- 37.Fiorentino D., Chung L., Zwerner J., Rosen A., Casciola-Rosen L. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J. Am. Acad. Dermatol. 2011;65:25–34. doi: 10.1016/j.jaad.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narang N.S., Casciola-Rosen L., Li S., Chung L., Fiorentino D.F. Cutaneous ulceration in dermatomyositis: association with anti-melanoma differentiation-associated gene 5 antibodies and interstitial lung disease. Arthritis Care Res. (Hoboken) 2015;67:667–672. doi: 10.1002/acr.22498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franco C., Gatto M., Iaccarino L., Ghirardello A., Doria A. Lymphocyte immunophenotyping in inflammatory myositis: a review. Curr. Opin. Rheumatol. 2021;33:522–528. doi: 10.1097/BOR.0000000000000831. [DOI] [PubMed] [Google Scholar]
- 40.Levine S.M., Raben N., Xie D., et al. Novel conformation of histidyl-transfer RNA synthetase in the lung: the target tissue in Jo-1 autoantibody-associated myositis. Arthritis Rheum. 2007;56:2729–2739. doi: 10.1002/art.22790. [DOI] [PubMed] [Google Scholar]
- 41.Gallay L., Gayed C., Hervier B. Antisynthetase syndrome pathogenesis: knowledge and uncertainties. Curr. Opin. Rheumatol. 2018;30:664–673. doi: 10.1097/BOR.0000000000000555. [DOI] [PubMed] [Google Scholar]
- 42.Miller F.W., Lamb J.A., Schmidt J., Nagaraju K. Risk factors and disease mechanisms in myositis. Nat. Rev. Rheumatol. 2018;14:255–268. doi: 10.1038/nrrheum.2018.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Güttsches A.K., Brady S., Krause K., et al. Proteomics of rimmed vacuoles define new risk allele in inclusion body myositis. Ann. Neurol. 2017;81:227–239. doi: 10.1002/ana.24847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmid J., Barthel K., Zschüntzsch J., et al. Nitric oxide stress in sIBM muscle fibres: inhibition of iNOS prevents IL-1β-induced accumulation of β-amyloid and cell death. Brain. 2012;135:1102–1114. doi: 10.1093/brain/aws046. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt J., Barthel K., Wrede A., et al. Interrelation of inflammation and APP in sIBM: IL-1β induces accumulation of β-amyloid in skeletal muscle. Brain. 2008;131:1228–1240. doi: 10.1093/brain/awn053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schett G., Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat. Rev. Rheumatol. 2012;8:656–664. doi: 10.1038/nrrheum.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Catrina A.I., Deane K.D., Scher J.U. Gene, environment, microbiome and mucosal immune tolerance in rheumatoid arthritis. Rheumatology (Oxford) 2016;55:391–402. doi: 10.1093/rheumatology/keu469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smolen J.S., Aletaha D., McInnes I.B. Rheumatoid arthritis. Lancet. 2016;388:2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 49.Derksen V.F.A.M., Huizinga T.W.J., van der Woude D. The role of autoantibodies in the pathophysiology of rheumatoid arthritis. Semin. Immunopathol. 2017;39:437–446. doi: 10.1007/s00281-017-0627-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dissick A., Redman R.S., Jones M., et al. Association of periodontitis with rheumatoid arthritis: a pilot study. J. Periodontol. 2010;81:223–230. doi: 10.1902/jop.2009.090309. [DOI] [PubMed] [Google Scholar]
- 51.Makrygiannakis D., Hermansson M., Ulfgren A.K., et al. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann. Rheum. Dis. 2008;67:1488–1492. doi: 10.1136/ard.2007.075192. [DOI] [PubMed] [Google Scholar]
- 52.Humby F., Bombardieri M., Manzo A., et al. Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLoS Med. 2009;6:e1. doi: 10.1371/journal.pmed.0060001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chu C.Q., Field M., Allard S., Abney E., Feldmann M., Maini R.N. Detection of cytokines at the cartilage/pannus junction in patients with rheumatoid arthritis: implications for the role of cytokines in cartilage destruction and repair. Br. J. Rheumatol. 1992;31:653–661. doi: 10.1093/rheumatology/31.10.653. [DOI] [PubMed] [Google Scholar]
- 54.Lacey D.L., Timms E., Tan H.L., et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 55.Brito-Zerón P., Baldini C., Bootsma H., et al. Sjögren syndrome. Nat. Rev. Dis. Prim. 2016;2 doi: 10.1038/nrdp.2016.47. [DOI] [PubMed] [Google Scholar]
- 56.Croia C., Astorri E., Murray-Brown W., et al. Implication of Epstein-Barr virus infection in disease-specific autoreactive B cell activation in ectopic lymphoid structures of Sjögren’s syndrome. Arthritis Rheumatol. 2014;66:2545–2557. doi: 10.1002/art.38726. [DOI] [PubMed] [Google Scholar]
- 57.Hall J.C., Baer A.N., Shah A.A., et al. Molecular subsetting of interferon pathways in Sjögren’s syndrome. Arthritis Rheumatol. 2015;67:2437–2446. doi: 10.1002/art.39204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iwakiri D., Zhou L., Samanta M., et al. Epstein-Barr virus (EBV)-encoded small RNA is released from EBV-infected cells and activates signaling from Toll-like receptor 3. J. Exp. Med. 2009;206:2091–2099. doi: 10.1084/jem.20081761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Båve U., Nordmark G., Lövgren T., et al. Activation of the type I interferon system in primary Sjögren’s syndrome: a possible etiopathogenic mechanism. Arthritis Rheum. 2005;52:1185–1195. doi: 10.1002/art.20998. [DOI] [PubMed] [Google Scholar]
- 60.Mitsias D.I., Kapsogeorgou E.K., Moutsopoulos H.M. The role of epithelial cells in the initiation and perpetuation of autoimmune lesions: lessons from Sjögren’s syndrome (autoimmune epithelitis) Lupus. 2006;15:255–261. doi: 10.1191/0961203306lu2290rr. [DOI] [PubMed] [Google Scholar]
- 61.Zhou H., Yang J., Tian J., Wang S. CD8+ T lymphocytes: crucial players in Sjögren’s syndrome. Front. Immunol. 2021;11 doi: 10.3389/fimmu.2020.602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou D., McNamara N.A. Macrophages: important players in primary Sjögren’s syndrome? Expet Rev. Clin. Immunol. 2014;10:513–520. doi: 10.1586/1744666X.2014.900441. [DOI] [PubMed] [Google Scholar]
- 63.Meehan S., Wu A.J., Kang E.C., et al. Interferon-gamma induces a decrease in the intracellular calcium pump in a human salivary gland cell line. Am. J. Physiol. 1997;273:C2030–C2036. doi: 10.1152/ajpcell.1997.273.6.C2030. [DOI] [PubMed] [Google Scholar]
- 64.Hua-Huy T., Dinh-Xuan A.T. Cellular and molecular mechanisms in the pathophysiology of systemic sclerosis. Pathol. Biol. (Paris) 2015;63:61–68. doi: 10.1016/j.patbio.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 65.Fuschiotti P. Current perspectives on the immunopathogenesis of systemic sclerosis. ImmunoTargets Ther. 2016;5:21–35. doi: 10.2147/ITT.S82037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Korman B. Evolving insights into the cellular and molecular pathogenesis of fibrosis in systemic sclerosis. Transl. Res. 2019;209:77–89. doi: 10.1016/j.trsl.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Truchetet M.E., Brembilla N.C., Chizzolini C. Current concepts on the pathogenesis of systemic sclerosis. Clin. Rev. Allergy Immunol. 2021 Sep 6 doi: 10.1007/s12016-021-08889-8. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Allanore Y., Simms R., Distler O., et al. Systemic sclerosis. Nat. Rev. Dis. Prim. 2015;1 doi: 10.1038/nrdp.2015.2. [DOI] [PubMed] [Google Scholar]
- 69.Doria A., Zen M., Bettio S., et al. Autoinflammation and autoimmunity: bridging the divide. Autoimmun. Rev. 2012;12:22–30. doi: 10.1016/j.autrev.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 70.Generali E., Bose T., Selmi C., Voncken J.W., Damoiseaux J.G.M.C. Nature versus nurture in the spectrum of rheumatic diseases: classification of spondyloarthritis as autoimmune or autoinflammatory. Autoimmun. Rev. 2018;17:935–941. doi: 10.1016/j.autrev.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 71.Lim W. Complement and the anti phospholipid syndrome. Curr. Opin. Hematol. 2011;18:361–365. doi: 10.1097/MOH.0b013e3283497f3e. [DOI] [PubMed] [Google Scholar]
- 72.Blanco P., Ueno H., Schmitt N. T follicular helper (Tfh) cells in lupus: activation and involvement in SLE pathogenesis. Eur. J. Immunol. 2016;46:281–290. doi: 10.1002/eji.201545760. [DOI] [PubMed] [Google Scholar]
- 73.Pan L., Lu M.P., Wang J.H., Xu M., Yang S.R. Immunological pathogenesis and treatment of systemic lupus erythematosus. World J. Pediatr. 2020;16:19–30. doi: 10.1007/s12519-019-00229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Srivastava A., Makarenkova H.P. Innate immunity and biological therapies for the treatment of Sjögren’s syndrome. Int. J. Mol. Sci. 2020;21:9172. doi: 10.3390/ijms21239172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sontheimer R.D. Skin manifestations of systemic autoimmune connective tissue disease: diagnostics and therapeutics. Best Pract. Res. Clin. Rheumatol. 2004;18:429–462. doi: 10.1016/j.berh.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 76.Sanchez O., Sitbon O., Jaïs X., Simonneau G., Humbert M. Immunosuppressive therapy in connective tissue diseases-associated pulmonary arterial hypertension. Chest. 2006;130:182–189. doi: 10.1378/chest.130.1.182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
As a “Review” manuscript, no new data was gathered other than the articles already available in periodics.