SUMMARY
A 57-year-old man with nonischemic cardiomyopathy who was dependent on venoarterial extracorporeal membrane oxygenation (ECMO) and was not a candidate for standard therapeutics, including a traditional allograft, received a heart from a genetically modified pig source animal that had 10 individual gene edits. Immunosuppression was based on CD40 blockade. The patient was weaned from ECMO, and the xenograft functioned normally without apparent rejection. Sudden diastolic thickening and failure of the xenograft occurred on day 49 after transplantation, and life support was withdrawn on day 60. On autopsy, the xenograft was found to be edematous, having nearly doubled in weight. Histologic examination revealed scattered myocyte necrosis, interstitial edema, and red-cell extravasation, without evidence of microvascular thrombosis — findings that were not consistent with typical rejection. Studies are under way to identify the mechanisms responsible for these changes. (Funded by the University of Maryland Medical Center and School of Medicine.)
A 57-year-old man with chronic mild thrombocytopenia, hyper-tension, nonischemic cardiomyopathy, and previous mitral valve repair was hospitalized for severe heart failure with a left ventricular ejection fraction (LVEF) of 10%. His care was escalated to include multiple intravenous inotropic agents, and the placement of an intraaortic balloon pump was added on hospital day 11. Despite these measures, he had multiple ventricular arrythmias with arrests leading to resuscitation and began to receive peripheral venoarterial extracorporeal membrane oxygenation (ECMO) on hospital day 23.
The patient was deemed to have poor adherence to treatment, which is an exclusion criterion for allotransplantation and mechanical circulatory support. At the time that his condition was assessed by our hospital selection committee for advanced circulatory support, he had a 3-week history of nonambulatory status. His case was reviewed by two regional and two prominent national heart-transplantation programs, and the request for a transplant was denied by all four programs. Our selection committee agreed to consider experimental xenotransplantation. To offset the patient’s history of poor adherence to treatment, enhanced postprocedure oversight was planned by the transplantation team. Although the patient favored a heart transplant from a human donor, he was informed of his options and agreed to undergo xenotransplantation.
Despite his biventricular heart failure, the patient had preserved renal function and received supplementary oxygen only intermittently through a nasal cannula for mild hypoxemia. His pretransplantation course was notable for adrenal insufficiency, gastrointestinal bleeding, bacteremia (which cleared with antimicrobial therapy), and drug-induced leukopenia. He underwent cardiac xenotransplantation from a genetically modified pig source animal on January 7, 2022.
METHODS
EXPANDED-ACCESS INVESTIGATIONAL NEW DRUG APPLICATION AND ETHICS APPROVALS
The basis of the Investigational New Drug application to the Food and Drug Administration (FDA) rested on our belief that the outcome of the experimental transplantation was not likely to be inferior to continuation of medical therapy and venoarterial ECMO. The request to the FDA was for the combined use of a 10-gene-edit pig donor (Revivicor), a humanized anti-CD40 monoclonal antibody (Kiniksa Pharmaceuticals), and an XVIVO heart perfusion system (XVIVO Perfusion) for cardiac preservation. The FDA evaluated our preclinical experience with cardiac xenotransplantation, the detailed consent form, and the plan for surveillance and for the prevention of zoonosis.
The patient was referred to our hospital ethics committee for an assessment of competency and his ability to give consent. Three institutional evaluations and one external independent psychiatric evaluation were performed to confirm his capacity for consent. Our entire institutional review board approved the plan, and the patient provided written informed consent.
GENETICALLY ENGINEERED PIG SOURCE ANIMAL
The pig was delivered from a biosecure Revivicor facility in Blacksburg, Virginia, and was isolated in our hospital-adjacent animal facility. The animal was clonally derived from fibroblasts, a cell line that included 10 gene edits (Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org) to render the cardiac xenograft more compatible for transplantation into a human, as described previously.1 The gene edits included three immunodominant xenoantigen carbohydrate knockouts — galactosealpha-1,3-galactose, Sda blood group antigen, and N-glycolylneuraminic acid. To reduce intrinsic xenograft growth, growth hormone receptor was knocked out.2–4 To mitigate antibody-dependent complement graft injury, human CD46 and decay-accelerating factor were expressed.5–7 The human thromboregulatory proteins thrombomodulin and endothelial cell protein C receptor were also expressed to augment the inefficiencies of porcine-derived blood factors for the activation of protein C.7–9 Additional human transgenic proteins included the antiinflammatory proteins CD47 and heme oxygenase 1.10,11 On the basis of the weight of the source animal (110 kg), it was assumed that the size of the heart would be adequate for the recipient, who weighed 85 kg.
IMMUNOSUPPRESSION AND MONITORING
The goal was to replicate the laboratory protocols we had used successfully with a nonhuman primate model, while balancing the patient’s preoperative leukopenia (white-cell count, 2200 to 3200 per microliter) and baseline thrombocytopenia (platelet count, 90,000 to 111,000 per microliter) before induction (Fig. S1 and Table S2).12,13 Rituximab and antithymocyte globulin were used for B-cell and T-cell depletion, respectively, and complement C1 esterase inhibitor (Berinert, CSL Behring) was used for complement inhibition. Humanized monoclonal antibody (KPL-404, Kiniksa Pharmaceuticals), used to block CD40 costimulation, was administered through repeated single dosing. A pulse dose of methylprednisolone (1000 mg on the day of xenotransplantation) was also administered.12 Maintenance immunosuppression included mycophenolate mofetil, KPL-404, and a rapid taper of methylprednisolone (from 125 mg daily to 30 mg daily).
XENOZOONOSIS MITIGATION STRATEGIES AND SURVEILLANCE
The rigorous husbandry of the source animal included early weaning, the use of biosecure facilities, and routine surveillance for pathogens. The source animal was derived from a porcine endogenous retrovirus (PERV)–C–negative line and was tested every 3 months for pathogens that affect porcine or human health, including PERV-A, PERV-B, PERV-C, porcine cytomegalovirus (pCMV), and porcine lymphotropic herpesvirus (pLHV). Polymerase-chain-reaction (PCR) testing for pathogens was performed at the University of Minnesota Veterinary Diagnostic Laboratory.
After xenotransplantation, the patient was tested for PERV-A, PERV-B, and PERV-C at predefined time points. Plasma, serum, and peripheral blood mononuclear cells (PBMCs) were obtained over time and stored for future testing for xenozoonoses. Unbiased testing for human pathogens with the use of a plasma microbial cell-free DNA (mcfDNA) test (KT, Karius) was performed weekly.14,15 Testing for mcfDNA was also performed under a research-use-only protocol for surveillance of a limited number of porcine viral pathogens (Table S4); the sequences of several porcine viral pathogens, used as reference genomes for these analyses, were obtained from the National Center for Biotechnology Information RefSeq and GenBank databases.
RESULTS
VIRAL SCREENING
The results of pathogen screening of the source animal before and after its arrival at the University of Maryland are provided in Table S3. PERV-A and PERV-B were detected, and PERV-C was not detected. Porcine circovirus 3 (PCV3) — a virus that has not been shown to infect human cells16 — was detected, albeit at a high cycle threshold (39) that was not suspected to indicate positivity. Other porcine viral pathogens, such as pCMV and pLHV, were not detected.
EARLY POSTOPERATIVE PERIOD
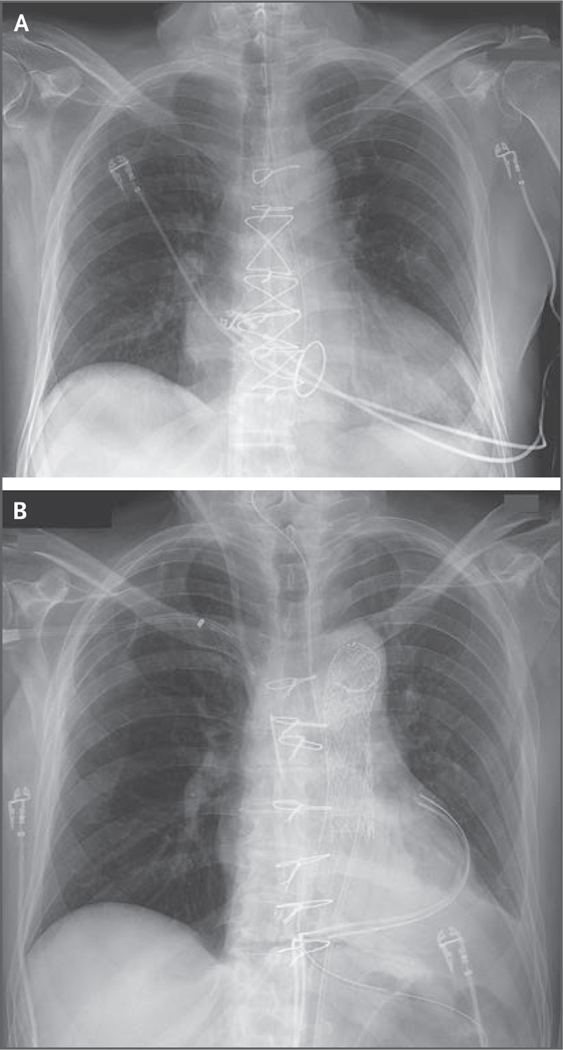
Venoarterial ECMO was continued after transplantation at 2 to 3 liters per minute to support the xenograft, to reduce the effect of any sudden ventricular arrythmia, and because after 46 days of support through the femoral vessels, decannulation would have required a lengthy addition to the index operation. The patient’s urine output decreased 8 hours after surgery, and the renal flow was reevaluated. An endovascular stent was placed for an occlusion of an upper-pole left renal artery by the false lumen of a residual dissection, which had been caused by the aortic cross clamp and had been diagnosed and repaired during the transplantation procedure. However, oligoanuric acute renal failure persisted throughout his postoperative course and led to the administration of renal replacement therapy. The patient’s trachea was extubated after chest closure on day 2 after transplantation. The chest radiograph showed clear lung fields (Fig. 1). Inotropic support was never warranted, and ECMO was discontinued on day 4.
Figure 1. Chest Radiography.
Panel A is a chest radiograph obtained before transplantation, showing cardiomegaly. Panel B is a chest radiograph obtained after transplantation, showing the xenograft in situ.
Before removal of the Swan–Ganz catheter on day 6, the patient’s systolic blood pressure averaged between 130 and 170 mm Hg and his diastolic blood pressure averaged between 40 and 60 mm Hg with low-dose nicardipine treatment; the systolic and diastolic pulmonary arterial pressures were 32 to 46 mm Hg and 18 to 25 mm Hg, respectively, and the central venous pressure ranged from 6 to 13 mm Hg. The cardiac output ranged from 5.0 to 6.0 liters per minute, and the stroke volume ranged from 65 to 70 ml per square meter of body-surface area. The xenograft remained in sinus rhythm at rates between 70 and 90 beats per minute. The LVEF was at least 55%.
The LVEF remained normal or hyperdynamic throughout the postoperative course (Figs. 2 and 3B). However, the left ventricular and right ventricular wall thickness, left ventricular chamber size (i.e., the left ventricular end-diastolic dimension), and global longitudinal strain changed. From transplantation until day 45, the left ventricular wall thickness measured between 1.2 and 1.4 cm along the basal interventricular septum and posterior wall, the right ventricular wall thickness measured 1.0 to 1.1 cm on shortaxis views, and the left ventricular chamber size was 3.4 to 4.6 cm. The global longitudinal strain indicated adequate compliance at baseline, with values ranging from −25 to −36%.
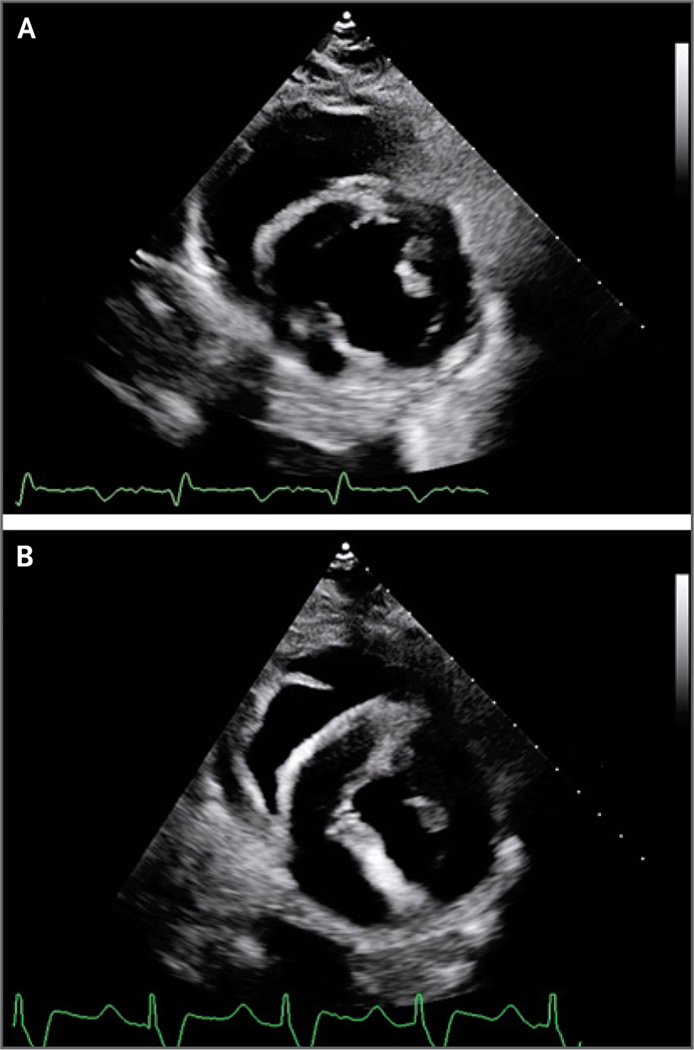
Figure 2. Transthoracic Echocardiography.
Transthoracic echocardiography of the transplanted xenograft obtained on day 19 after transplantation (Panel A, parasternal short axis) showed normal xenograft function, normal global longitudinal strain, and normal left ventricular posterior wall thickness in end diastole. Transthoracic echocardiography performed on day 49 (Panel B), before cannulation for venoarterial extracorporeal membrane oxygenation (ECMO), showed preserved systolic function, less negative (i.e., more abnormal) global longitudinal strain, and increased thickness of the left ventricular posterior wall in end diastole.
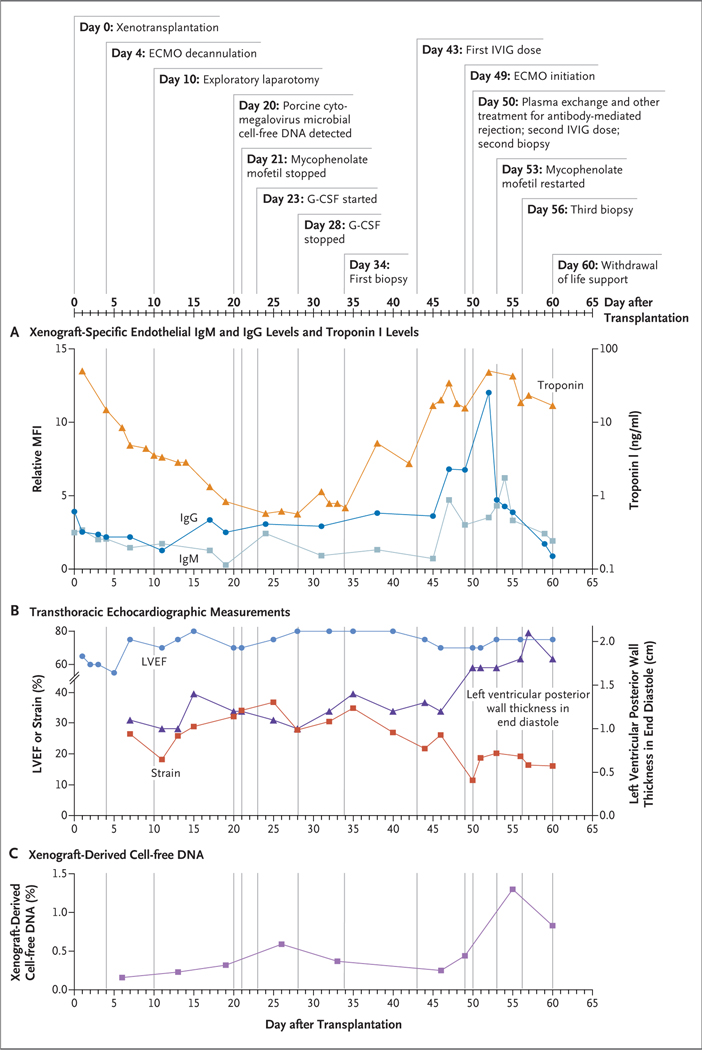
Figure 3. Clinical Details and Test Results during the Postoperative Course.
Mean fluorescence intensity (MFI) values were normalized to a positive control (assigned a value of 100%). Global longitudinal strain is expressed in Panel B as the absolute value; strain is typically reported as a negative percentage. G-CSF denotes granulocyte colony-stimulating factor, IVIG intravenous immune globulin, and LVEF left ventricular ejection fraction.
PERITONITIS AND FEEDING ISSUES
On day 12 after transplantation, the patient reported having abdominal pain. Computed tomography showed thickening of the small bowel and free fluid, which aroused concern about bowel ischemia or perforation. An exploratory laparotomy revealed purulent fluid that grew Escherichia coli and Candida tropicalis. The small bowel was thickened and contained a focal area of resolving ischemia but did not have signs of acute ischemia or perforation. Intravascular ultrasonography and mesenteric angiography confirmed that blood flow was adequate. After an abdominal washout, the patient’s abdomen was subsequently closed without additional intervention. His thrombocytopenia worsened to a nadir of 21,000 platelets per microliter and remained at that level until the fifth week after transplantation, when the platelet count rose to 40,000 to 60,000 per microliter (Fig. S1D). The patient recovered from the peritonitis, but caloric goals were unable to be met through enteric feeding; nonpathogenic diarrhea related to tube feeding developed and led to parenteral nutrition being administered. His bowel slowly readapted to enteral feeding and had adapted by day 40 after transplantation. During hospitalization, the patient’s cachexia worsened, and his weight declined from 85 kg at admission to a postoperative nadir of 62 kg.
IMMUNOSUPPRESSION AND INFECTION PROPHYLAXIS
After induction immunosuppression was administered, peripheral B cells (CD20+) and T cells (CD3+) were depleted. KPL-404 was administered as part of the maintenance immunosuppression regimen. In the immediate postoperative period, the patient began to receive ganciclovir for antiviral prophylaxis, isavuconazole for antifungal prophylaxis, and atovaquone for anti–Pneumocystis jirovecii prophylaxis. Treatment with mycophenolate mofetil was initiated on day 1 after transplantation but was discontinued on day 21 because of severe neutropenia (white-cell count, 200 per microliter; absolute neutrophil count, 100 per micro-liter) that responded to 5 days of treatment with granulocyte colony-stimulating factor (Fig. S1A and S1B). Tacrolimus treatment was initiated on day 35 with a low target level of 3 to 5 ng per milliliter in response to the discontinuation of mycophenolate mofetil. Levels of donor-specific antibodies remained below baseline levels after induction immunosuppression and stayed low until day 47 after transplantation, when a sharp increase in IgG levels and, to a lesser extent, in IgM levels occurred, which corresponded to the administration of intravenous immune globulin at day 43. Serum troponin I levels were increased after transplantation but returned to baseline by day 24 and then began to increase sharply by day 35 (Fig. 3A).
ENDOMYOCARDIAL BIOPSY
On day 34 after transplantation, an endomyocardial biopsy showed no evidence of rejection (Fig. 4A), and the heart had a right atrial pressure of 5 mm Hg, pulmonary-artery pressure of 25/15 mm Hg, a cardiac index (the cardiac output in liters per minute divided by the bodysurface area in square meters) of 2.7, and a mixed venous saturation of 65% (haemoglobin level, 8.1 g per deciliter). The patient was able to rehabilitate without any cardiovascular support, and the xenograft functioned normally without evidence of rejection (Fig. 2A and Video 1).
Figure 4. Histologic Assessments.
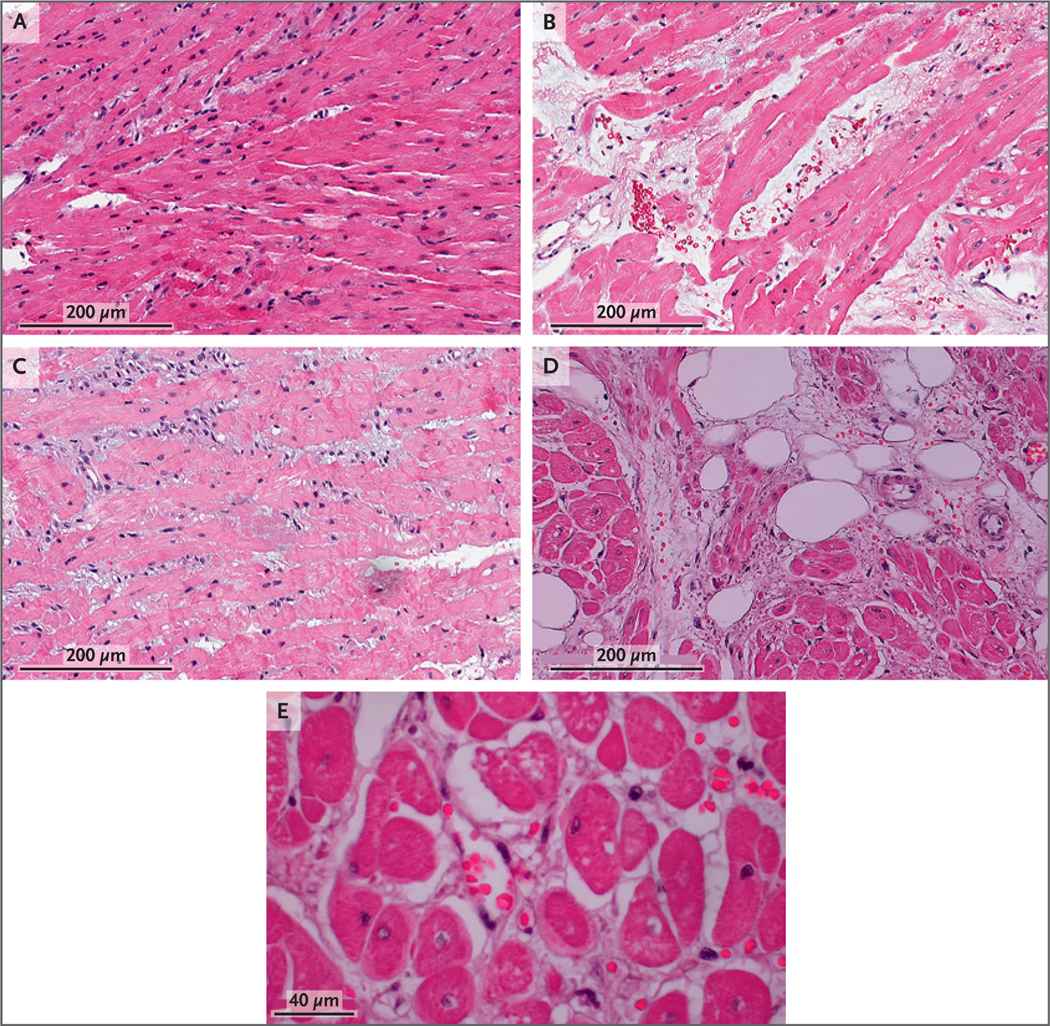
The first endomyocardial biopsy (Panel A, day 34 after transplantation) showed normal histologic characteristics. The second endomyocardial biopsy (Panel B, day 50) showed interstitial red-cell extravasation, fragmentation, and edema without cellular infiltrate or intravascular thrombosis. The third endomyocardial biopsy (Panel C, day 56) showed some resolution of interstitial edema and red-cell extravasation but also showed evidence of necrotic myocytes. The septum at autopsy (Panels D and E, day 60) showed interstitial edema and red-cell extravasation, myocardial necrosis, scant fibrosis, and central nuclei.
Video 1.
Day 19 after Transplantation. On day 19, transthoracic echocardiography showed normal xenograft function, normal global longitudinal strain, and normal left ventricular posterior wall thickness in end diastole
EVIDENCE OF INFECTION
On day 43 after transplantation, the patient became more somnolent, and his trachea was intubated; hypotension developed, which was responsive to administration of fluids and vasopressin. A chest radiograph suggested worsening soft infiltrates in both lungs. Bronchoscopy showed diffuse shallow ulcers throughout the right primary and secondary airways that were suggestive of viral or fungal infection, despite ongoing prophylaxis. The patient had hypogammaglobulinemia (total IgG level, 185 mg per deciliter). Antimicrobial coverage was broadened, and 1 g of intravenous immune globulin per kilogram of body weight (i.e., a dose of 80 g) was administered. In addition, the results of our weekly mcfDNA testing revealed a notable increase in pCMV (also called suid herpesvirus 2, first identified at day 20) (Fig. S2), which aroused concern about a possible viral infection.
To verify the mcfDNA results, quantitative PCR was performed on a spleen sample from the source animal and PBMCs from the patient. The donor spleen tested positive for pCMV, which indicated that the source animal was likely to have had a latent pCMV infection. The patient’s PBMCs were also positive. As a result, antiviral therapy was changed from ganciclovir to cidofovir. A biopsy of these airway lesions was performed and did not show any viral cytopathic effect or viral inclusions, and Grocott methenamine silver staining was negative. A repeat bronchoscopic examination performed approximately 5 days later showed abatement of the diffuse airway ulcerations. The patient’s trachea was extubated on day 47 after transplantation, and he resumed in-room rehabilitation.
Failure of the Xenograft
On day 48 after transplantation, the patient sat alone in a chair and waved to caregivers; he was free from bed for the first time in 109 days. On the evening of day 49, the serum lactate level rose from 4 mg per deciliter (0.4 mmol per liter) to 11.2 mg per deciliter (1.2 mmol per liter) over a period of 8 hours in the context of mild abdominal discomfort and distention. Hypotension developed, leading to treatment with vasopressors, and the patient’s trachea was intubated. Acrocyanosis developed, which suggested a lowered cardiac output for the first time since transplantation. He underwent an exploratory laparotomy, but no abnormalities were found. A newly placed pulmonary-artery catheter showed a mixed venous oxygen saturation of 33%. Although an echocardiogram showed an LVEF of 65 to 70%, dramatically increased left ventricular wall thickness (1.7 cm) and right ventricular wall thickness (1.4 cm) and reduced left ventricular chamber size (3.2 to 3.5 cm) were noted (Fig. 2B and Video 2). Both the left ventricular and right ventricular walls remained persistently thickened and the left ventricular chamber size remained persistently reduced from baseline, independent of left ventricular end-diastolic volume and loading conditions. The global longitudinal strain values became dramatically less negative (i.e., more abnormal) (Fig. 3B). His family was consulted and encouraged recannulation for venoarterial ECMO, which he underwent on the evening of day 49.
Video 2.
Day 49 after Transplantation. On day 49, before venoarterial cannulation for extracorporeal membrane oxygenation, transthoracic echocardiography showed preservation of systolic function, but with more abnormal global longitudinal strain, increased thickness of the left ventricular posterior wall in end diastole, and decreased left ventricular chamber size.
An endomyocardial biopsy (day 50) did not show antibody-mediated or acute cellular rejection (International Society for Heart and Lung Transplantation [ISHLT] grade 0) (Fig. 4B).17 However, focal capillary damage with extravasated erythrocytes and edema was present. Antibody staining indicated the presence of IgG and IgM (with the former present at higher levels) in capillaries but was negative for C3d or C4d. There was a single ischemic myocyte and no cellular infiltrates seen on either hematoxylin–eosin or immunohistochemical (CD3+ or CD68+) staining. The troponin I level was increasing. It was later determined that from that date, levels of xenograft-derived cell-free DNA (xdcfDNA) (Fig. 3C), as well as serum levels of xenograft-specific IgG and to a lesser extent IgM, were also peaking (Fig. 3A). We suspected an atypical manifestation of antibody-mediated rejection and initiated treatment. The treatment included therapeutic plasma-exchange sessions with 1.5 times the plasma volume over a period of 5 days, followed by three additional sessions of plasma exchange with 1.0 times the plasma volume, along with intravenous immune globulin (1 g per kilogram), complement inhibition (two doses of complement C1 esterase inhibitor and one dose of eculizumab), and B-cell depletion (rituximab [375 mg per square meter]). We continued to support the patient with ECMO because he was awake and encouraged us to do so.
On day 56, a repeat endomyocardial biopsy showed pathologic antibody-mediated rejection of ISHLT grade 1 (pAMR1; decreased IgG and IgM capillary staining, but with C4d staining now present) (Fig. 4C). Interstitial red-cell extravasation and edema had decreased since the previous biopsy, but the endomyocardial biopsy showed 40% myocyte necrosis. Again, there was no evidence of cellular rejection, but staining was weakly positive for C4d, IgG, and IgM and was more prominent in the areas of necrotic myocytes, which may have indicated nonspecific binding. There was focal capillary damage with extravasated erythrocytes and stromal cells within the prominent intermyocyte edema. A repeat echocardiogram showed an LVEF of greater than 70%, normal right ventricular function, and improved longitudinal strain (−19.2%). The biventricular wall thickening abated slightly, and the left ventricular chamber size remained reduced. The patient was able to be slowly weaned from venoarterial ECMO flows of 4.5 liters per minute down to 3 liters per minute without the need for catecholamine support. Further echocardiography did not show improvement in wall thickness, chamber size, or global longitudinal strain, and ECMO could not be decreased to below 2 liters per minute.
We concluded that an irreversible injury to the xenograft was present and, with the patient’s family at the bedside, compassionately withdrew life support on day 60 after transplantation. A preliminary postmortem examination of the heart showed an increase in heart weight to 600 g, up from 328 g at transplantation. Cardiac myocytes were widely spaced with central nuclei separated by thin bands of fibrosis. Necrosis of the myocytes was scattered and was associated with a loss of myocyte integrity. Endothelial changes ranged from prominent nuclei to cell swelling with areas of complete vascular dissolution. Erythrocytes were found to be scattered throughout the interstitial space between myocytes in a pattern consistent with vascular extravasation. The findings were not consistent with typical xenotransplant rejection (Fig. 4D and 4E). Additional studies are under way to characterize the pathophysiologic mechanisms that resulted in this damage.
DISCUSSION
The xenoheart was able to support the patient for 7 weeks. It appeared that our T-cell– and B-cell–depleting induction combined with CD40-based therapy prevented obvious rejection of the genetically modified xenograft. Endomyocardial biopsies of the xenograft did not show acute cellular or antibody-mediated rejection according to ISHLT criteria. No complement staining was identified until a week after the late dysfunction that led to ECMO support.17 The pronounced sudden diastolic failure and global pathologic myocardial thickening without systolic dysfunction remains unexplained. These findings, in combination with focal capillary injury in the virtual absence of complement deposition, are not normally seen in human allotransplantation.
Intravenous immune globulin was administered twice during the patient’s postoperative course. The first dose was given on day 43 after transplantation, for potential infection. The second dose was given on day 50 for the treatment of presumed antibody-mediated rejection after initial therapeutic plasma exchange. Both doses coincided with an increase in the recipient’s anti-pig IgG levels and to a lesser extent IgM levels. Although in vitro binding of intravenous immune globulin from the University of Maryland Medical Center pharmacy to donor-specific cells was observed in two separate laboratories, including ours, testing conducted by Revivicor did not show antibody-mediated, complement-dependent cytotoxicity.
The donor pig was maintained in a biosecure facility and was weaned early from its mother. Pathogen screening conducted before and after the arrival of the source animal at the University of Maryland showed the expected presence of PERV-A and PERV-B and an absence of PERV-C. PCV3 was also detected but was of unclear significance and was not suspected to indicate positivity, given the high cycle threshold (39). PCV3 has not been shown to be capable of infecting human cells.16 The absence of pCMV, pLHV, and other viruses supported published studies that have shown successful elimination of porcine viral pathogens through husbandry practices such as colostrum deprivation, early weaning, and the use of biosecure facilities.18 Therefore, active surveillance after transplantation was targeted to PERV-A and PERV-B.
PCR testing conducted 60 days after xenotransplantation did not show evidence of PERV-A, PERV-B, or PERV-C in the recipient’s PBMCs, which suggested that transmission to the recipient did not occur. Testing for mcfDNA did not show evidence of PCV3 in the recipient after transplantation. Testing for mcfDNA was positive for pCMV at low levels on day 20, and the levels increased over subsequent weeks. The detection of pCMV was unexpected, given the husbandry practices, negative surveillance PCR testing of nasal swab specimens from the donor animal before organ transplantation, and the use of antiviral prophylaxis. It is uncertain whether the detection of pCMV through plasma mcfDNA or PCR testing represents replicating virus in the xenograft, replicating virus in the recipient, or shedding of genetic material from the xenograft. The presence of pCMV in explanted xenografts from nonhuman primate recipients has been correlated with worse outcomes than an absence of pCMV, for reasons that are unclear.19 Further viral testing is warranted because human herpesvirus 6 (HHV-6) was also detected in a lunglavage specimen from this patient. HHV-6 has been shown to cross-react with pCMV and has been associated with allograft rejection.20,21 No obvious viral cytopathic changes were identified on preliminary hematoxylin–eosin examination of thoracic or abdominal organs.
The xenograft dysfunction, along with the patient’s severe deconditioning and complicated postoperative course, led to the compassionate withdrawal of further advanced supportive care on day 60 after transplantation.
Supplementary Material
Acknowledgments
Supported by the University of Maryland Medical Center and School of Medicine. The source animal was provided by Revivicor, and the KPL-404 antibody was provided by Kiniksa Pharmaceuticals, both in kind. CareDx and Karius analytics were provided in kind. No financial support was provided by Revivicor, United Therapeutics, Kiniksa Pharmaceuticals, or XVIVO. No federal funding was used for the experimental transplantation.
We thank the laboratory and clinical staff for their contribution to this case, referred to here as the University of Maryland Cardiac Xenotransplantation Working Group: members of the laboratory team not otherwise listed as authors (Tianshu Zhang, M.D., Ph.D., Ivan Tatarov, D.V.M., Alena Hershfeld, M.S., Gheorghe Braileanu, D.V.M., Ph.D., Faith Sentz, B.S., Billeta Lewis, M.S., and Sarah Mudd, B.S.R.N.), anesthesia staff (Patrick Odonkor, M.D., and Erik Strauss, M.D.), perfusion staff (Brian McCormick, C.C.P., Alyssa Druzgala, C.C.P., and Brian Donahue, C.C.P.), cardiology staff (Charles Hong, M.D., Ph.D., Albert Hicks, M.D., Manjula Ananthram, M.D., Gautam Ramani, M.D., Anuj Gupta, M.D., Mike Domanski, M.D., Timm Dickfield, M.D., Asadi Sadegh, M.D., and Peter Hanna, M.D.), nursing staff (Lauren Szostek, B.S.R.N., Sarah Cipriano, C.R.N.P., and Marco Oldsman, C.R.N.P.), ethics staff (Henry Silverman, M.D.), transplant pharmacy staff (Amanda Szczapanik, Pharm.D., and Myounghee Lee, Pharm.D.), surgical team (Jonathan Bromberg, M.D., Dan Maluf, M.D., Bradley Taylor, M.D., Chetan Pasrija, M.D., Shahab Toursavadkohi, M.D., Joseph Rabin, M.D., Barry Deatrick, M.D., Mehrdad Ghoreishi, M.D., and Laura DiChiacchio, M.D.), University of Maryland Transplant Pathology staff (Allen P. Burke, M.D., Kathryn Rice, M.D., and Ashley Celini, A.S.C.P.), staff of the National Institutes of Health Laboratory of Pathology (Stephen Hewitt, M.D., Ph.D., and Sabrina Ramelli, Ph.D.), staff of the University of Maryland School of Medicine Center for Innovative Biomedical Resources (including Veterinary Resources and the Flow Cytometry Facility), staff members at Kiniksa Pharmaceuticals (John F. Paolini, M.D., Ph.D., Randy Perrin, Ph.D., Manoj Samant, Ph.D., Moses Njenga, M.A., Mei Jiang, Ph.D., Becky Hamilton, B.Sc., Steve Schmitz, M.D., and Jennifer Ring, M.B.A.), staff members at CareDx (Robert Woodward, Ph.D., Alesha Luxon, B.S.N., C.C.T.C., and Alicia Griswold, B.S.R.N.), and staff members at Revivicor (Maria Kokkinaki, Ph.D., for porcine endogenous retrovirus polymerasechain-reaction analysis, and Amy Dandro, M.Sc., for cytotoxicity assays); and the staff of Karius for thoughtful analyses.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Eyestone W, Adams K, Ball S, et al. Gene-edited pigs for xenotransplantation. In: Cooper DKC, Byrne G, eds. Clinical xenotransplantation: pathways and progress in the transplantation of organs and tissues between species. Cham, Switzerland: Springer, 2020: 121–40. [Google Scholar]
- 2.Goerlich CE, Griffith B, Hanna P, et al. The growth of xenotransplanted hearts can be reduced with growth hormone receptor knockout pig donors. J Thorac Cardiovasc Surg 2021. September 4 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinrichs A, Riedel EO, Klymiuk N, et al. Growth hormone receptor knockout to reduce the size of donor pigs for preclinical xenotransplantation studies. Xenotransplantation 2021; 28: (2)e12664. [DOI] [PubMed] [Google Scholar]
- 4.Hinrichs A, Kessler B, Kurome M, et al. Growth hormone receptor-deficient pigs resemble the pathophysiology of human Laron syndrome and reveal altered activation of signaling cascades in the liver. Mol Metab 2018; 11: 113–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diamond LE, Quinn CM, Martin MJ, Lawson J, Platt JL, Logan JS. A human CD46 transgenic pig model system for the study of discordant xenotransplantation. Transplantation 2001; 71: 132–42. [DOI] [PubMed] [Google Scholar]
- 6.Dalmasso AP, Platt JL, Bach FH. Reaction of complement with endothelial cells in a model of xenotransplantation. Clin Exp Immunol 1991; 86: Suppl 1: 31–5. [PMC free article] [PubMed] [Google Scholar]
- 7.Chan JL, Singh AK, Corcoran PC, et al. Encouraging experience using multi-transgenic xenografts in a pig-to-baboon cardiac xenotransplantation model. Xenotransplantation 2017; 24(6):e12330. [DOI] [PubMed] [Google Scholar]
- 8.Singh AK, Chan JL, DiChiacchio L, et al. Cardiac xenografts show reduced survival in the absence of transgenic human thrombomodulin expression in donor pigs. Xenotransplantation 2019; 26(2): e12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwase H, Ekser B, Hara H, et al. Regulation of human platelet aggregation by genetically modified pig endothelial cells and thrombin inhibition. Xenotransplantation 2014; 21: 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper DKC, Hara H, Iwase H, et al. Justification of specific genetic modifications in pigs for clinical organ xenotransplantation. Xenotransplantation 2019; 26(4): e12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soares MP, Lin Y, Anrather J, et al. Ex-pression of heme oxygenase-1 can determine cardiac xenograft survival. Nat Med 1998; 4: 1073–7. [DOI] [PubMed] [Google Scholar]
- 12.Mohiuddin MM, Singh AK, Corcoran PC, et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat Commun 2016;7 : 11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohiuddin MM, Goerlich CE, Singh AK, et al. Progressive genetic modifications of porcine cardiac xenografts extend survival to 9 months. Xenotransplantation 2022. March 31 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blauwkamp TA, Thair S, Rosen MJ, et al. Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease. Nat Microbiol 2019; 4: 663–74. [DOI] [PubMed] [Google Scholar]
- 15.Benamu E, Gajurel K, Anderson JN, et al. Plasma microbial cell-free DNA nextgeneration sequencing in the diagnosis and management of febrile neutropenia. Clin Infect Dis 2022; 74: 1659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krüger L, Längin M, Reichart B, et al. Transmission of porcine circovirus 3 (PCV3) by xenotransplantation of pig hearts into baboons. Viruses 2019; 11:650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berry GJ, Burke MM, Andersen C, et al. The 2013 International Society for Heart and Lung Transplantation Working Formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. J Heart Lung Transplant 2013; 32: 1147–62. [DOI] [PubMed] [Google Scholar]
- 18.Egerer S, Fiebig U, Kessler B, et al. Early weaning completely eliminates porcine cytomegalovirus from a newly established pig donor facility for xenotransplantation. Xenotransplantation 2018; 25(4):e12449. [DOI] [PubMed] [Google Scholar]
- 19.Denner J, Längin M, Reichart B, et al. Impact of porcine cytomegalovirus on long-term orthotopic cardiac xenotransplant survival. Sci Rep 2020; 10: 17531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu W, Zeng N, Zhou L, Ge X, Guo X, Yang H. Genomic organization and molecular characterization of porcine cytomegalovirus. Virology 2014; 460–461: 16572. [DOI] [PubMed] [Google Scholar]
- 21.Fiebig U, Holzer A, Ivanusic D, et al. Antibody cross-reactivity between porcine cytomegalovirus (PCMV) and human herpesvirus-6 (HHV-6). Viruses 2017; 9: 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.