Abstract
Dolphins are marine mammals that often live in coastal habitats. Common causes of severe skeletal disorders among wild dolphins are congenital vertebral anomalities, collisions with sea vessels, trauma, hunting-related injury, infectious diseases, environmental pollution, and tumors.
A free-ranging male, 3-year-old common dolphin (Delphinus delphis) was found dead in the coast of Asturias in northern Spain. Postmortem examination revealed lordosis in the caudal vertebral column, while X-ray imaging and computer tomography showed well-organized palisade-like periosteal proliferation, appearing as florid-like accretions, along the spinous apophysis of 26 lumbar-caudal vertebrae. The transverse apophysis was affected on only a few caudal vertebrae. The cortical layer remained intact. Histology of vertebra tissue showed periosteal proliferation of cancellous bone. The animal was diagnosed with hypertrophic osteopathy. The lungs showed diffuse parasitic granulomatous bronchointerstitial pneumonia caused by Halocercus delphini, consolidation of the pulmonary tissue, congestion, and alveolar edema. The animal was also afflicted by parasitic granulomatous gastritis caused by Anisakis simplex sensu lato and tattoo skin disease.
The dolphin suffered from hypertrophic osteopathy associated with pulmonary Halocercus delphini infestation. This syndrome, known as hypertrophic pulmonary osteopathy, has been described in diverse terrestrial mammals, including domestic animals, wildlife and humans, but not in dolphins. This case reports the first description of hypertrophic osteopathy associated to a pulmonary disorder in dolphin, and it provides insights into factors that can induce column malformation in dolphins, suggesting the importance of taking thoracic lesions into account during differential diagnosis.
Keywords: Hypertrophic pulmonary osteopathy, Halocercus delphini, Common dolphin, Delphinus delphis
1. Introduction
Necropsy in stranded cetaceans is relevant for the detection and recognition of potential sanitary risks or other hazards [1]. Dolphins are marine mammals that often live in coastal habitats, where they face dangers due to heavy boat traffic, habitat destruction as well as contamination from industrial and agricultural pollutants. Dolphins in the wild can present vertebral column lesions, which include scoliosis, defined as lateral curvature of the vertebral column; lordosis, defined as concave curvature of the vertebral column; kyphosis, defined as convex curvature of the vertebral column; kyphoscoliosis, defined as backward, lateral curvature of the vertebral column; and lordoscoliosis, in which lordosis and scoliosis co-occur [[2], [3], [4]]. Dolphins can also present with spondylitic changes, lumpy dorsal masses, vertebral fractures and joint dislocations [[2], [3], [4]].
The causes of vertebral column deformities and lesions can be difficult to determine. The most frequent causes include congenital vertebral anomalities, collisions with sea vessels, trauma, hunting-related injury, infectious diseases, environmental pollution, and tumors [2,3,[5], [6], [7]].
Here we describe a free-ranging common dolphin (Delphinus delphis) in which skeletal disorder is associated with pulmonary Halocercus delphini infestation.
2. Material and methods
In Asturias (northern Spain) a cetacean passive surveillance program has been carried out since 2018 by the Government of the Principality of Asturias. As part of that program, well-preserved subjects - either stranded or found dead - are collected and necropsied to identify their cause of death. A free-ranging male common dolphin was found dead in January 2022 on the coast of Asturias (Cantabrian Sea). After the detection of the dead animal in the beach, a complete postmortem examination of the carcass was conducted at SERIDA (Government of the Principality of Asturias, Spain) in less than 24 hours. The animal was well-preserved, weighed 30.9 kg and appeared to be in poor health and body condition. The total length of the dolphin, from the mouth to the caudal fin, was 149.5 cm, and the chest circumference was 77 cm. Tissues were taken for evaluation using standard methods in histopathology. Briefly, they were fixed in 10% neutral buffered formalin and dehydrated through graded alcohols and xylol before being embedded in paraffin wax. Sections 4 μm were cut from each sample and stained with hematoxylin and eosin. A dental histological study was also performed in order to determine the age of the dolphin [8,9]. Additionally, analysis by X-ray imaging (Vet-Ray, Sedecal, Madrid, Spain) and computer tomography (Optima 540 Tang-3, General Electric, Madrid, Spain) were conducted.
3. Results
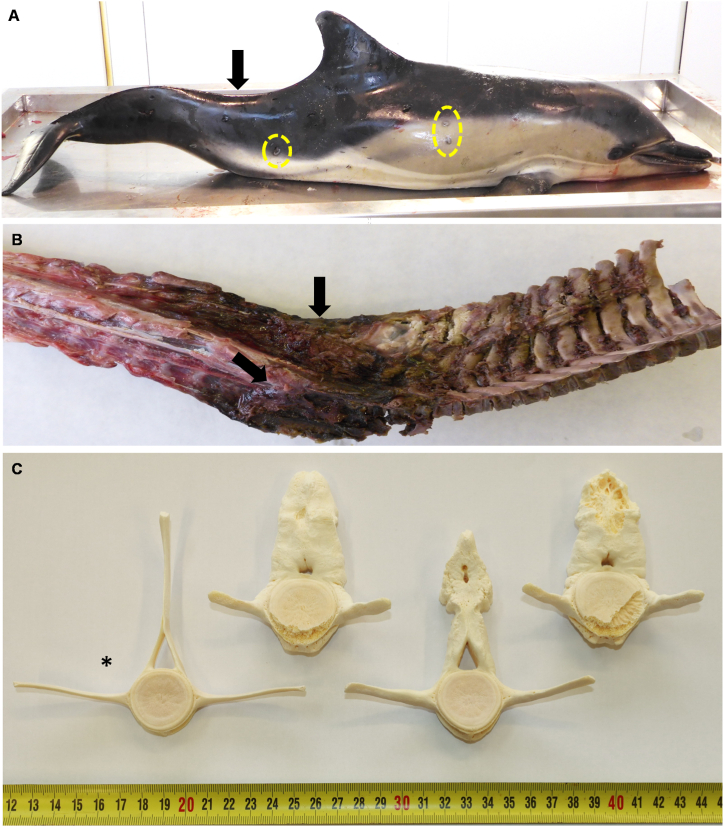
Postmortem examination revealed lordosis in the caudal vertebral column, which appeared to be rigid from the caudal of the dorsal fin to the peduncle (Fig. 1A). Gross bone examination showed irregular periosteal thickening of the last lumbar vertebrae and caudal vertebrae, as well as prominent congestion of periosteal and associated musculature (Fig. 1B). After manual cleaning with a scalpel followed by chemical cleaning with sodium perborate, further analysis of the bone revealed new periosteal bone formation along the spinous apophysis of 26 lumbar-caudal vertebrae (Fig. 1C). The transverse apophysis, in contrast, was affected on only a few caudal vertebrae.
Fig. 1.
Gross appearance of hypertrophic osteopathy in a free-ranging common dolphin (Delphinus delphis). (A) Lordosis was observed in the caudal vertebral column (arrow), as were tattoo skin lesions (yellow circles). (B) Prominent congestion in the periosteal and associated musculature (arrows) was observed in the caudal vertebral column. (C) Lumbar and caudal vertebrae after cleaning, showing different degrees of irregular periosteal thickening in the spinous and transverse apophysis. The asterisk marks a normal vertebra. From left to right: 12th lumbar vertebra, 5ht caudal vertebra, 18th lumbar vertebra and 6th caudal vertebra. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
X-ray imaging and computer tomography revealed well-organized, palisade-like periosteal proliferation (hyperostosis), which appeared as florid-like accretions (Fig. 2A–E). The cortical layer remained intact (Fig. 2D). None of the joint spaces was affected. Microscopically, periosteal proliferation of cancellous bone was observed in the vertebrae, with a clear distinction with the compact bone (Fig. 3A–C). On the basis of these findings, the animal was diagnosed with hypertrophic osteopathy. Other pathologies were excluded during differential diagnosis. For example, we excluded bone neoplasms because lesions were distributed across several vertebrae, neoplastic cells were not observed and because the cortical layer showed no signs of erosion. We also excluded osteitis based on the lack of inflammation events (i.e., either presence of inflammatory infiltrate or other events such as necrosis).
Fig. 2.
Imaging of hypertrophic osteopathy in a free-ranging common dolphin (Delphinus delphis). (A) X-ray imaging of three normal vertebrae. (B) X-ray imaging of three vertebrae with hypertrophic osteopathy. New periosteal bone formation was observed along the spinous apophysis. (C) Computer tomography of the vertebral column. Numerous vertebrae (inside the yellow circle) showed irregular bony periosteal proliferations along the length of the column. (D) Computer tomography of a vertebra with hypertrophic osteopathy, manifesting as florid-like accretions affecting mainly the spinous apophysis. Periosteal proliferation was observed over the intact cortical layer in the transverse apophysis (arrow). Inset, scan of a normal vertebra. (E) Three-dimensional reconstruction of vertebra showing extensive new periosteal bone growth, particularly on the spinous apophysis. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
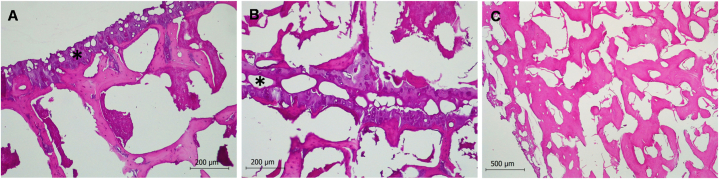
Fig. 3.
Histology of hypertrophic osteopathy in vertebrae in a free-ranging common dolphin (Delphinus delphis). (A) Histology of the transverse apophysis of a normal vertebra. Asterisk marks the compact bone. Hematoxylin-eosin stain. (B) Periosteal proliferation of cancellous bone was observed in the transverse apophysis of vertebrae, with a clear distinction with the compact bone (asterisk). Hematoxylin-eosin stain. (C) Histology of the spinous apophysis of a vertebra with proliferation of cancellous bone. Hematoxylin-eosin stain. Histology was performed after vertebral cleaning.
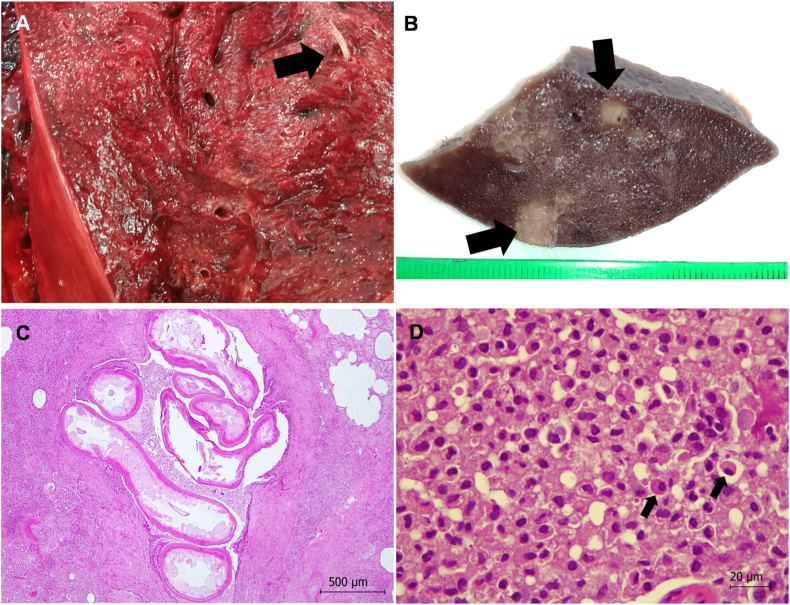
The lungs showed congestion, alveolar edema, consolidation of the pulmonary tissue and diffuse parasitic granulomatous bronchointerstitial pneumonia (Fig. 4A). Firm, white-gray foci with diameters of approximately 0.5 cm were present throughout the lung tissue (Fig. 4B). The lumen of bronchi and bronchioli contained numerous parasites, which were identified based on morphology as Halocercus delphini [10] (Fig. 4C). The inflammatory infiltrate consisted mainly of lymphocytes, plasma cells, macrophages, and eosinophils, with occasional neutrophils and multinucleated giant cells (Fig. 4D). Gram staining of lung tissue was negative.
Fig. 4.
Pathology findings in the lungs of a free-ranging common dolphin (Delphinus delphis) with hypertrophic osteopathy. (A) Diffuse parasitic bronchointerstitial pneumonia caused by Halocercus delphini (arrow) was observed. (B) Firm white-gray foci approximately 0.5 cm in diameter (arrows) were observed in tissues fixed in 10% formalin. (C) Parasitic granulomatous bronchointerstitial pneumonia was observed after hematoxyin-eosin staining, and at least seven Halocercus delphini within a bronchiolus were identified. Hematoxylin-eosin stain. (D) Higher magnification of the tissue in panel (C) shows an inflammatory infiltrate mainly formed by lymphocytes, macrophages and plasma cells (arrows). Hematoxylin-eosin stain.
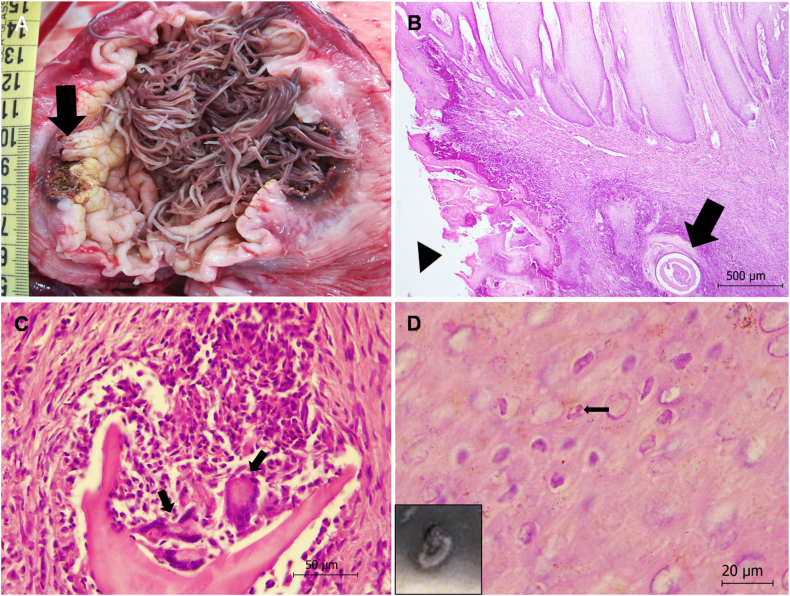
The stomachs were empty of food and the saccular forestomach was completely occupied by nematodes morphologically identified as Anisakis simplex sensu lato [11] (Fig. 5A). Histology indicated severe parasitic granulomatous gastritis and myositis in the forestomach (Fig. 5B). Parasitic granulomas consisted mainly of lymphocytes, macrophages, epithelioid cells and multinucleated giant cells (Fig. 5C). A gastric ulcer extended into the deep muscle, in association with loss of the stratified squamous epithelium and the presence of nematodes (Fig. 5A–B). The mesentery contained encapsulated and necrotic forms of A. simplex sensu lato with diameters of 1 cm.
Fig. 5.
Pathology findings in the stomach (A–C) and skin (D) of a free-ranging common dolphin (Delphinus delphis) with hypertrophic osteopathy. (A) The saccular forestomach was occupied completely by nematodes, identified as Anisakis simplex sensu lato, and an ulcer (arrow) was observed. (B) The saccular forestomach tissue after hematoxylin-eosin staining showed signs of parasitic granulomatous gastritis and myositis, as well as an ulcer with loss of the stratified squamous epithelial layer (arrowhead) and intralesional nematodes (arrow). (C) Parasitic granulomatous gastritis: detail of a parasitic granuloma made up of lymphocytes, macrophages, epithelioid cells and multinucleated giant cells (arrows). Hematoxyin-eosin stain. (D) Skin, stratum intermedium. Tattoo skin disease: hydropic keratinocyte degeneration was observed, as well as eosinophilic intracytoplasmic inclusion bodies (arrow) within infected cells. Hematoxyin-eosin stain. Inset, gross appearance of tattoo skin lesion.
We further diagnosed the animal with tattoo skin disease on the basis of irregular, dark gray-black marks with a stippled pattern over the animal's entire body (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5D). The affected skin showed mild thickening of the stratum corneum, hydropic keratinocyte degeneration of intermedium cells in the core of the lesion and eosinophilic intracytoplasmic inclusion bodies in infected stratum intermedium cells (Fig. 5D).
The animal also showed non-purulent tracheitis that was accompanied by congestion, as well as congestion of the kidneys, liver or brain. No other gross or microscopic abnormalities were observed. Dental histology indicated an age of 3 years.
4. Discussion and conclusions
Here we report hypertrophic osteopathy in a dolphin concurrent with parasitic granulomatous inflammatory disease involving the thoracic cavity. This syndrome is known as hypertrophic pulmonary osteopathy and it has been described in a wide variety of terrestrial mammals, including domestic animals (most often dogs), wildlife and humans [[12], [13], [14]]. It usually affects the forelimbs and hindlimbs, compromising the motor skills [[12], [13], [14]]. Similarly to terrestrial mammals, the motor region (lumbar and caudal vertebral column) in the dolphin was affected. Lung diseases are common among wild and captive populations of cetaceans [1], but the present case appears to be the first time that they have been linked with bone lesions. In a similar way that occurs in terrestrial mammals, few animals with pulmonary disorders would develope hyperthrophic osteopathy [12,13].
Although pulmonary and the bone related lesions could have been two independent pathologies happening concurrently in the present dolphin, hypertrophic osteopathy is linked to thoracic disorders [[12], [13], [14], [15], [16]]. Diverse thoracic lesions have been associated with hypertrophic osteopathy. Often these lesions are primary or secondary pulmonary neoplasms, but they can also be granulomatous pleuritis or lymphadenitis of bronchial or mediastinal lymph nodes, chronic bronchitis, bacterial endocarditis, Dirofilaria immitis infestation, esophageal granulomas, and Spirocerca lupi infestation [12,13,15,16]. The pathogenesis of the syndrome is poorly understood. It may involve hypoxia, arteriovenous shunting, humoral processes and neurogenic processes, such as vagus nerve-mediated reflex vasodilation in the limbs [13]. Through these or other processes, peripheral blood flow increases and the periosteum proliferates. The excess peripheral blood flow appears to be poorly oxygenated, passing through arteriovenous shunts and bypassing the capillary bed. This results in local passive congestion, poor tissue oxygenation, proliferation of connective tissue and finally production of osteophytes, which spread out from the cortex [12].
The severe and diffuse pulmonary Halocercus delphini infestation in the present case may be associated with hypertrophic osteopathy. It is unclear whether the vertebral column lesion in our dolphin made swimming or feeding difficult or shortened its lifespan, since the effects of such lesions can vary depending on their severity and comorbidities [2]. Vertebral column lesions have been associated with body stiffness and weight loss, which may help explain why our dolphin appeared to be underdeveloped for its age [8], and why it presented two pathologies (parasitic gastritis and tattoo skin disease) suggestive of immunodeficiency or weakness [17]. However, we cannot exclude that the animal had a preexisting column malformation that may have favored osteopathy. In this regard, the primary cause of the death of this dolphin could not be determined, although we hypothesize that starvation and weakness may have caused the death of the animal.
In conclusion, this case reports the first description of hypertrophic osteopathy associated to a pulmonary disorder in dolphin. It also provides insights into factors that can induce column malformation in dolphins, and it suggests the importance of taking thoracic lesions into account during differential diagnosis.
List of abbreviations
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Funding
This work was partially funded by the Principality of Asturias (PCTI 2021–2023,GRUPIN: IDI-2021-000102) and European Regional Development Fund.
Author contributions
AB, GH, LJR, JAA, JMM and JAPM participated in the necropsy. AB, JRA, JFGM, RB and JAPM analyzed and interpreted clinical and histology data. RVP identified the parasites. AB drafted the manuscript. All authors revised and approved the final version.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank the Dirección General del Medio Natural y Planificación Rural del Principado de Asturias (Oviedo, Spain). We also thank Francisco Javier Aznar from Instituto Cavanilles de Biodiversidad y Biología Evolutiva and personnel from SERIDA and TRAGSA for help during necropsy. We would also like to thank A. Chapin for critically reviewing the manuscript.
References
- 1.Díaz-Delgado J., Fernández A., Sierra E., Sacchini S., Andrada M., Vela A.I., Quesada-Canales Ó Paz Y., Zucca D., Groch K., Arbelo M. Pathologic findings and causes of death of stranded cetaceans in the Canary Islands (2006-2012) PLoS One. 2018;13 doi: 10.1371/journal.pone.02044441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berghan J., Visser N. Vertebral column malformations in New Zealand delphinids with a review of cases world wide. Aquat. Mamm. 2000;26(1):17–25. [Google Scholar]
- 3.Bertulli C.G., Galatius A., Kinze C.C., Rasmussen M.H., Deaville R., Jepson P., et al. Vertebral column deformities in white-beaked dolphins from the eastern North Atlantic. Dis. Aquat. Org. 2015;116:59–67. doi: 10.3354/dao02904. [DOI] [PubMed] [Google Scholar]
- 4.Weir C.R., Wang J.Y. Vertebral column anomalies in Indo-Pacific and Atlantic humpback dolphins Sousa spp. Dis. Aquat. Org. 2016;120:179–187. doi: 10.3354/dao03026. [DOI] [PubMed] [Google Scholar]
- 5.Sweeny M.M., Price J.M., Jones G.S., French T.W., Early G.A., Moore M.J. Spondylitic changes in long-finned pilot whales (Globicephala melas) stranded on Cape Cod, Massachusetts, USA, between 1982 and 2000. J. Wildl. Dis. 2005;41:717–727. doi: 10.7589/0090-3558-41.4.717. [DOI] [PubMed] [Google Scholar]
- 6.Martinez E., Stockin K.A. Blunt trauma observed in a common dolphin (Delphinussp.) likely caused by a vessel collision in the Hauraki Gulf, New Zealand. Pac. Conserv. Biol. 2013;19:19–27. [Google Scholar]
- 7.Robinson K.P. Agonistic behaviour in a North Sea bottlenose dolphin community: directed intraspecific aggression by adult males towards calves. Mar. Mamm. Sci. 2014;30:381–388. doi: 10.1111/mms.12023. [DOI] [Google Scholar]
- 8.Ferrero R.C., Walker W. Growth and reproduction of the common dolphin, Delphinus delphis Linnaeus, in the offshore waters of the North Pacific Ocean. Fish. Bull. 1995;93:483–494. [Google Scholar]
- 9.Cheng I., Watson A., Chou L.S. Insights from life history traits of Risso's dolphins (Grampus griseus) in Taiwanese waters: shorter body length characterizes northwest Pacific population. Mar. Mamm. Sci. 2011;27:E43–E64. doi: 10.1111/j.1748-7692.2010.00429.x. [DOI] [Google Scholar]
- 10.Pool R., Romero-Rubira C., Raga J.A., Fernández M., Aznar F.J. Determinants of lungworm specificity in five cetacean species in the western Mediterranean. Parasites Vectors. 2021;14:196. doi: 10.1186/s13071-021-04629-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abollo E., Paggi L., Pascual S., D'Amelio S. Occurrence of recombinant genotypes of Anisakis simplex s.s. and Anisakis pegreffii (Nematoda: Anisakidae) in an area of sympatry. Inf. Genet. Evol. 2003;3:175–181. doi: 10.1016/s1567-1348(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson N.M., Lévy M., Ramos-Vara J.A., Baird D.K., Wu C.C. Hypertrophic osteopathy associated with mycotic pneumonia in two juvenile elk (Cervus elaphus) J. Vet. Diagn. Invest. 2008;20:849–853. doi: 10.1177/104063870802000628. [DOI] [PubMed] [Google Scholar]
- 13.Craig L.E., Dittmer K.E., Thompson K.G. In: Jubb, Kennedy and Palmer's Pathology of Domestic Animals, 6th Ed. Maxie M.G., editor. Elsevier; London: 2016. Bones and joints; pp. 16–164. [Google Scholar]
- 14.Yasuda K., Kashu N., Yoshitake H., Mushiake Y., Takami T., Shintani H., et al. A case of lung adenocarcinoma with pulmonary hypertrophic osteoarthropathy. Gan To Kagaku Ryoho. 2021;48:297–299. [PubMed] [Google Scholar]
- 15.de Aguiar I., García R., Madriz D., Alfaro-Alarcón A., Montenegro V.M., Aizenberg I., et al. Esophageal spirocercosis with pulmonary egg deposition and secondary hypertrophic osteopathy in a dog from Costa Rica. Vet Parasitol Reg Stud Reports. 2021;23:100510. doi: 10.1016/j.vprsr.2020.100510. [DOI] [PubMed] [Google Scholar]
- 16.Griffith J.E., Stephenson T., McLelland D.J., Woolford L. Hypertrophic osteopathy in South Australian koalas (Phascolarctos cinereus) with concurrent pulmonary actinomycosis. Aust. Vet. J. 2021;99:172–177. doi: 10.1111/avj.13052. [DOI] [PubMed] [Google Scholar]
- 17.Van Bressem M.F., Van Waerebeek K., Montes D., Kennedy S., Reyes J.C., Garcia-Godos I.A., et al. Diseases, lesions and malformations in the long-beaked common dolphin Delphinus capensis from the Southeast Pacific. Dis. Aquat. Org. 2006;68:149–165. doi: 10.3354/dao068149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.